User login
Medical assistants identify strategies and barriers to clinic efficiency
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
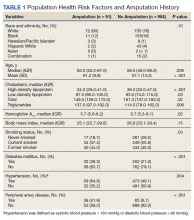
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
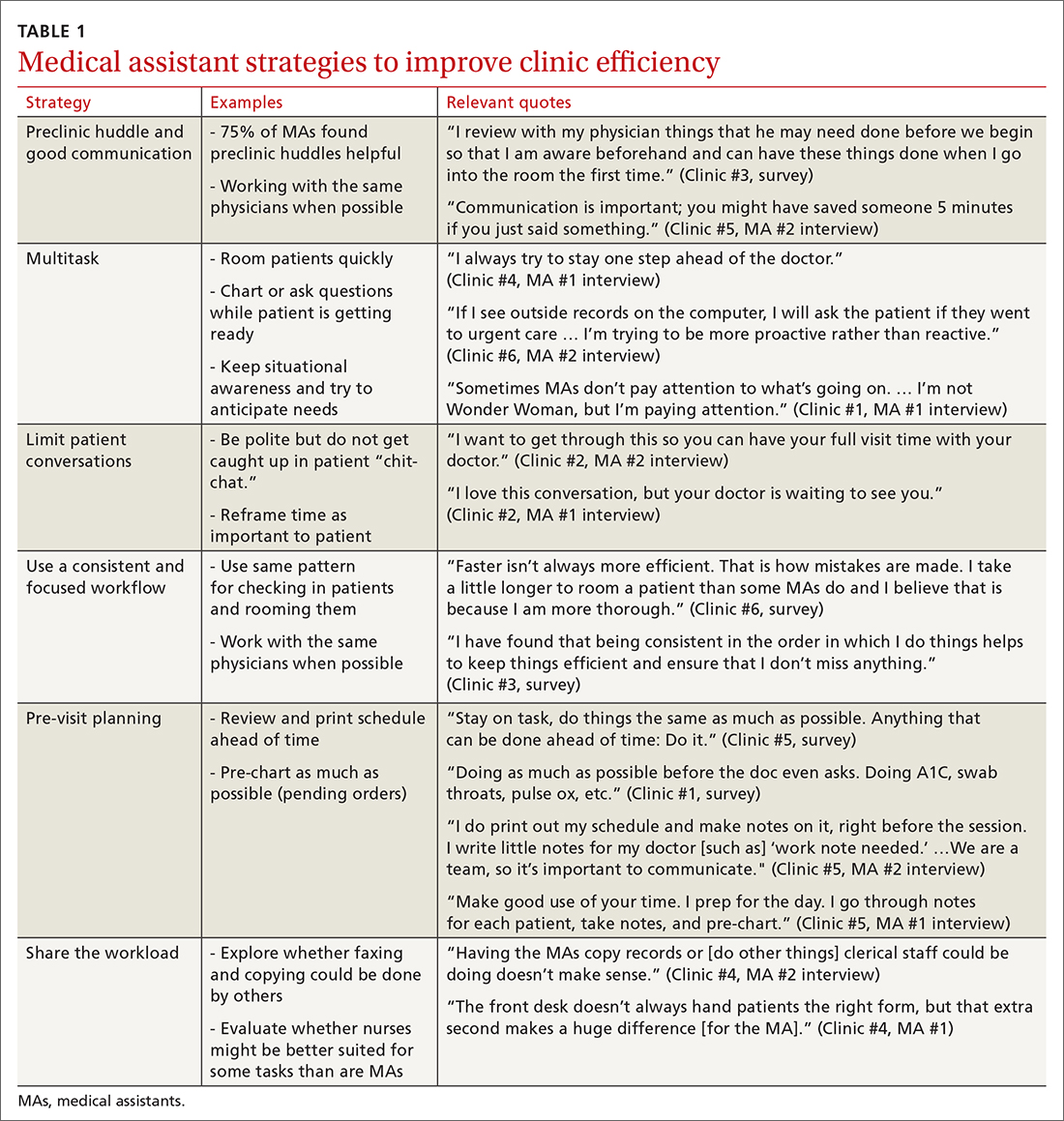
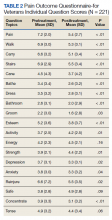
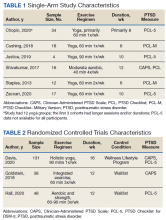
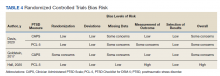
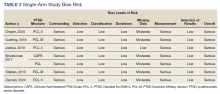
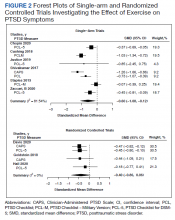
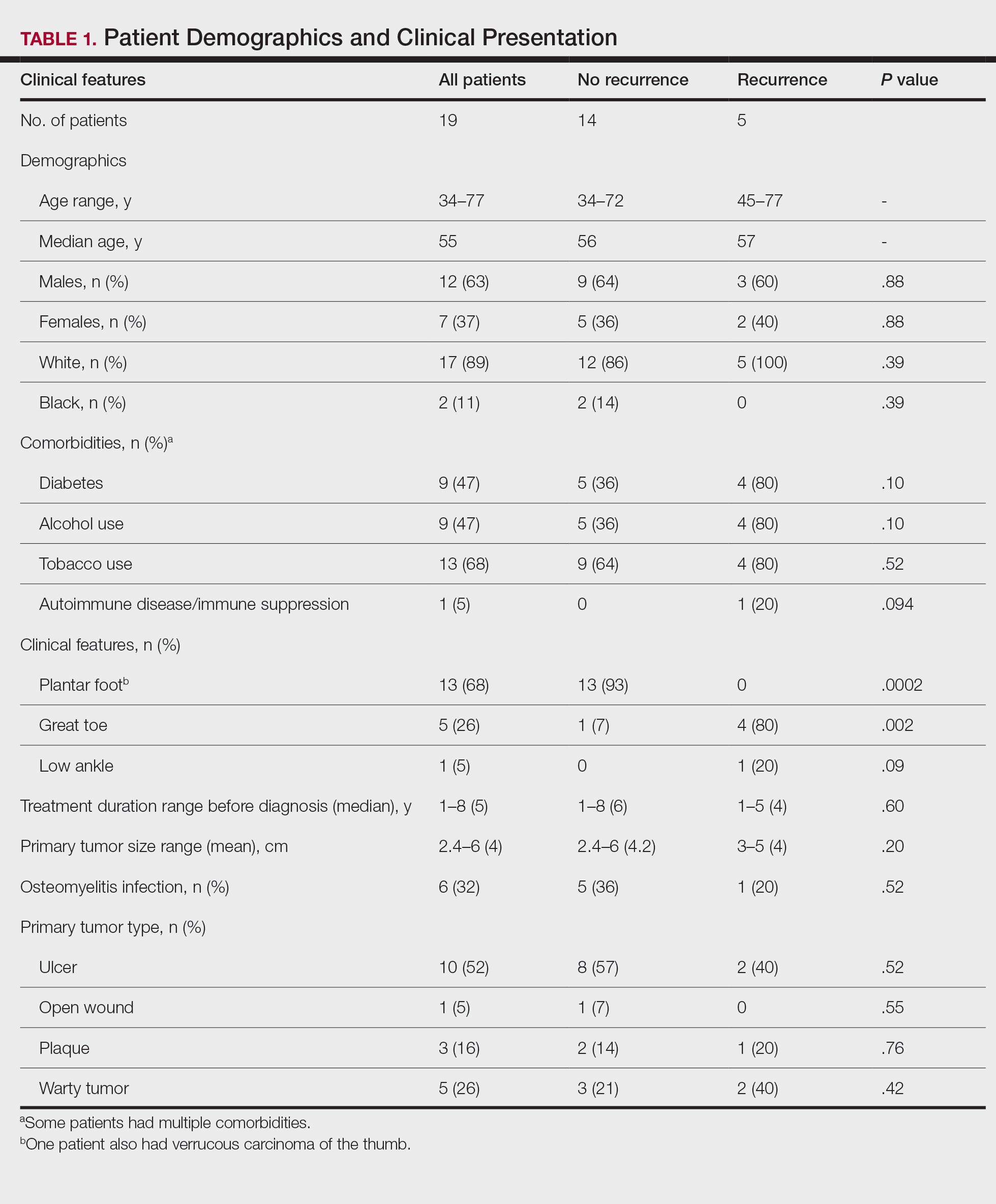
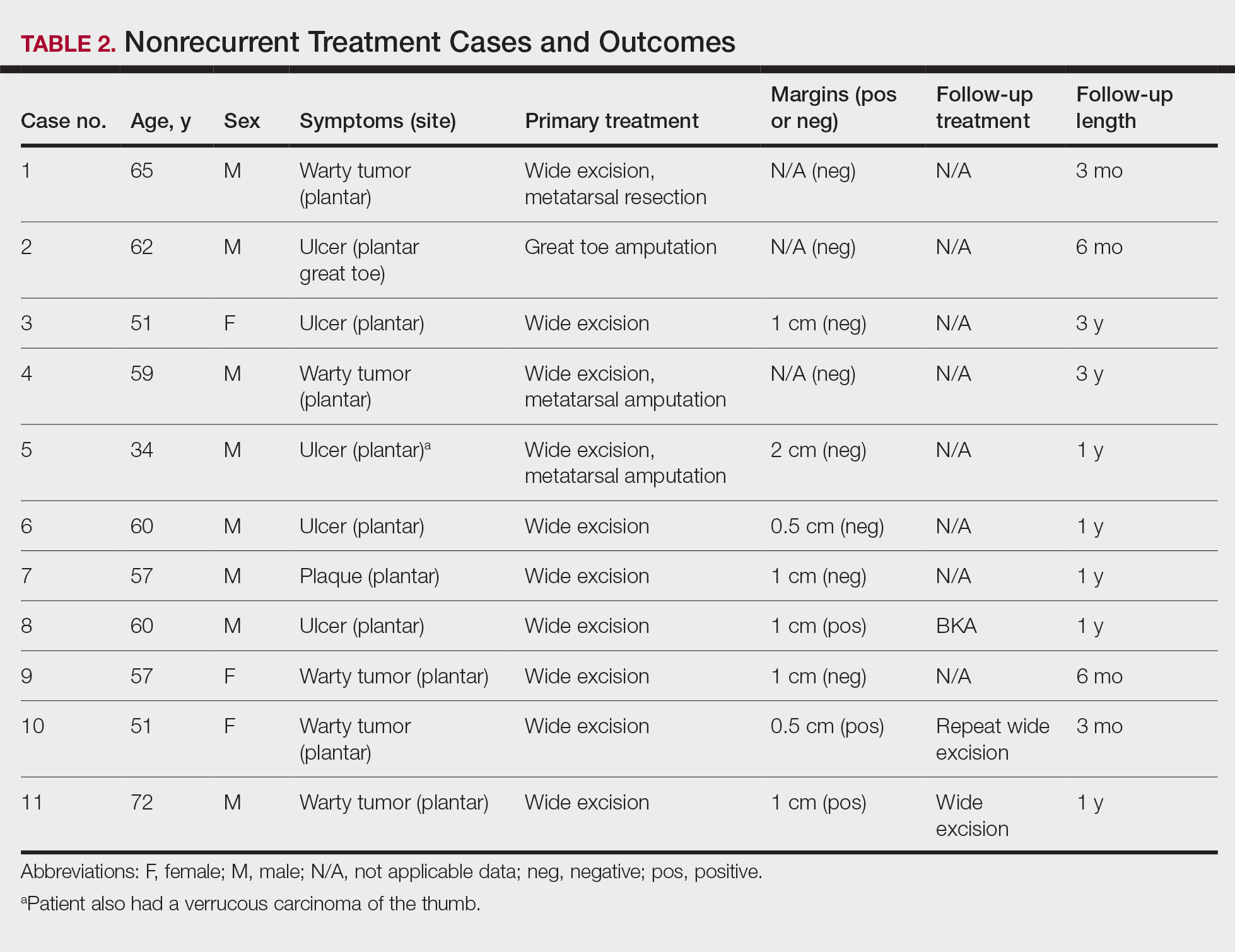
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
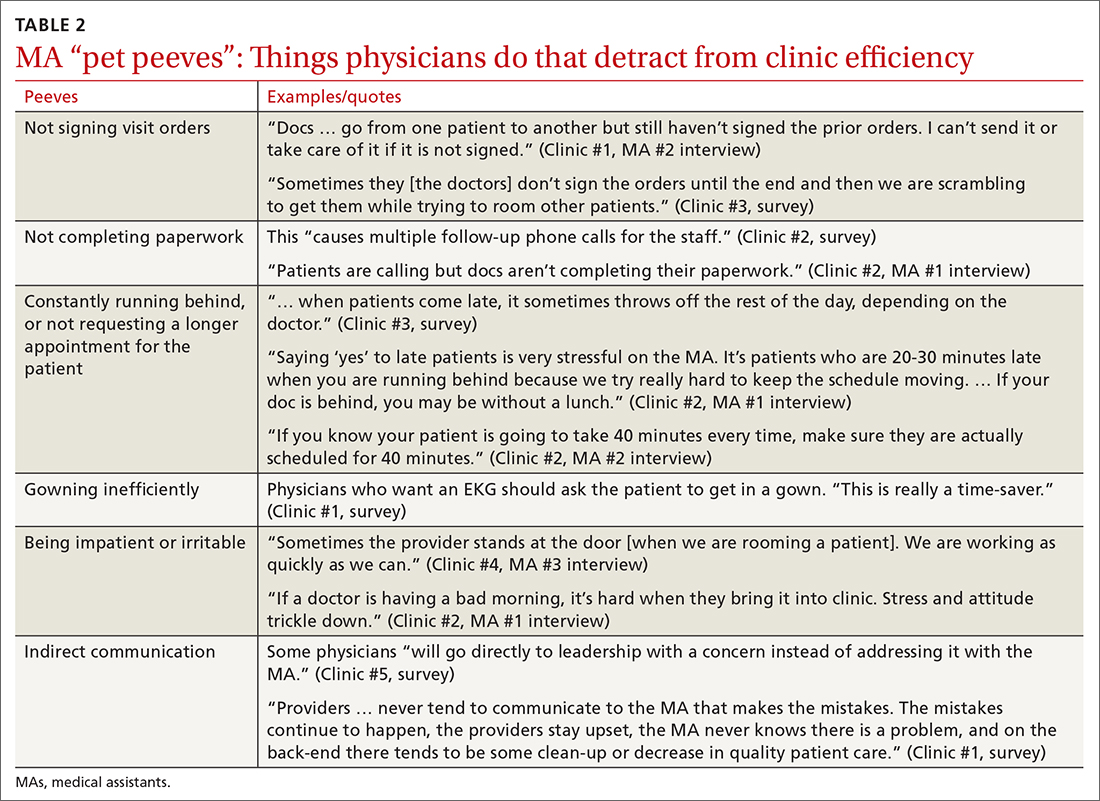
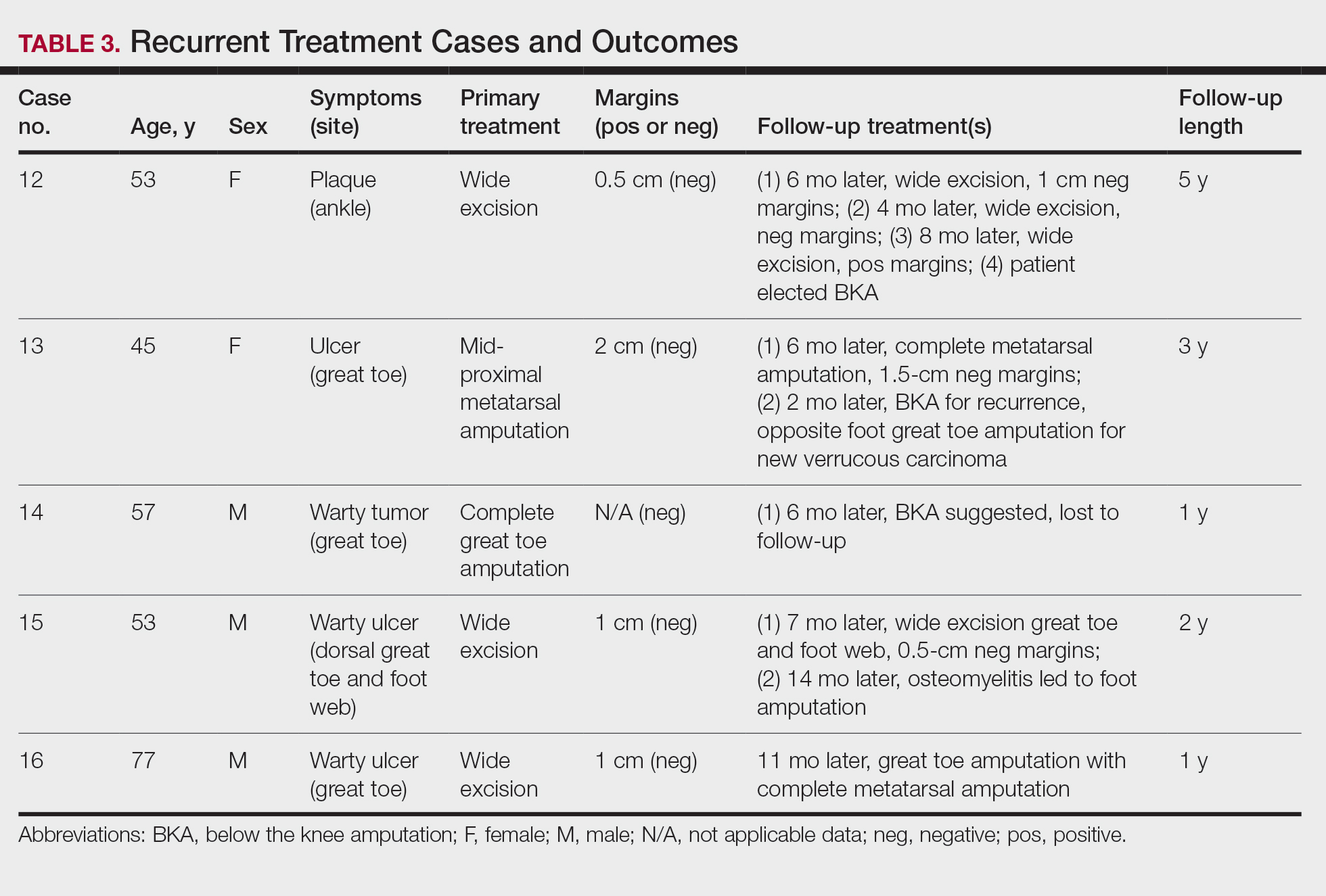
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
ABSTRACT
Background: Medical assistant (MA) roles have expanded rapidly as primary care has evolved and MAs take on new patient care duties. Research that looks at the MA experience and factors that enhance or reduce efficiency among MAs is limited.
Methods: We surveyed all MAs working in 6 clinics run by a large academic family medicine department in Ann Arbor, Michigan. MAs deemed by peers as “most efficient” were selected for follow-up interviews. We evaluated personal strategies for efficiency, barriers to efficient care, impact of physician actions on efficiency, and satisfaction.
Results: A total of 75/86 MAs (87%) responded to at least some survey questions and 61/86 (71%) completed the full survey. We interviewed 18 MAs face to face. Most saw their role as essential to clinic functioning and viewed health care as a personal calling. MAs identified common strategies to improve efficiency and described the MA role to orchestrate the flow of the clinic day. Staff recognized differing priorities of patients, staff, and physicians and articulated frustrations with hierarchy and competing priorities as well as behaviors that impeded clinic efficiency. Respondents emphasized the importance of feeling valued by others on their team.
Conclusions: With the evolving demands made on MAs’ time, it is critical to understand how the most effective staff members manage their role and highlight the strategies they employ to provide efficient clinical care. Understanding factors that increase or decrease MA job satisfaction can help identify high-efficiency practices and promote a clinic culture that values and supports all staff.
As primary care continues to evolve into more team-based practice, the role of the medical assistant (MA) has rapidly transformed.1 Staff may assist with patient management, documentation in the electronic medical record, order entry, pre-visit planning, and fulfillment of quality metrics, particularly in a Primary Care Medical Home (PCMH).2 From 2012 through 2014, MA job postings per graduate increased from 1.3 to 2.3, suggesting twice as many job postings as graduates.3 As the demand for experienced MAs increases, the ability to recruit and retain high-performing staff members will be critical.
MAs are referenced in medical literature as early as the 1800s.4 The American Association of Medical Assistants was founded in 1956, which led to educational standardization and certifications.5 Despite the important role that MAs have long played in the proper functioning of a medical clinic—and the knowledge that team configurations impact a clinic’s efficiency and quality6,7—few investigations have sought out the MA’s perspective.8,9 Given the increasing clinical demands placed on all members of the primary care team (and the burnout that often results), it seems that MA insights into clinic efficiency could be valuable.
METHODS
This cross-sectional study was conducted from February to April 2019 at a large academic institution with 6 regional ambulatory care family medicine clinics, each one with 11,000 to 18,000 patient visits annually. Faculty work at all 6 clinics and residents at 2 of them. All MAs are hired, paid, and managed by a central administrative department rather than by the family medicine department. The family medicine clinics are currently PCMH certified, with a mix of fee-for-service and capitated reimbursement.
Continue to: We developed and piloted...
We developed and piloted a voluntary, anonymous 39-question (29 closed-ended and 10 brief open-ended) online Qualtrics survey, which we distributed via an email link to all the MAs in the department. The survey included clinic site, years as an MA, perceptions of the clinic environment, perception of teamwork at their site, identification of efficient practices, and feedback for physicians to improve efficiency and flow. Most questions were Likert-style with 5 choices ranging from “strongly agree” to “strongly disagree” or short answer. Age and gender were omitted to protect confidentiality, as most MAs in the department are female. Participants could opt to enter in a drawing for three $25 gift cards. The survey was reviewed by the University of Michigan Institutional Review Board and deemed exempt.
We asked MAs to nominate peers in their clinic who were “especially efficient and do their jobs well—people that others can learn from.” The staff members who were nominated most frequently by their peers were invited to share additional perspectives via a 10- to 30-minute semi-structured interview with the first author. Interviews covered highly efficient practices, barriers and facilitators to efficient care, and physician behaviors that impaired efficiency. We interviewed a minimum of 2 MAs per clinic and increased the number of interviews through snowball sampling, as needed, to reach data saturation (eg, the point at which we were no longer hearing new content). MAs were assured that all comments would be anonymized. There was no monetary incentive for the interviews. The interviewer had previously met only 3 of the 18 MAs interviewed.
Analysis. Summary statistics were calculated for quantitative data. To compare subgroups (such as individual clinics), a chi-square test was used. In cases when there were small cell sizes (< 5 subjects), we used the Fisher’s Exact test. Qualitative data was collected with real-time typewritten notes during the interviews to capture ideas and verbatim quotes when possible. We also included open-ended comments shared on the Qualtrics survey. Data were organized by theme using a deductive coding approach. Both authors reviewed and discussed observations, and coding was conducted by the first author. Reporting followed the STROBE Statement checklist for cross-sectional studies.10 Results were shared with MAs, supervisory staff, and physicians, which allowed for feedback and comments and served as “member-checking.” MAs reported that the data reflected their lived experiences.
RESULTS
Surveys were distributed to all 86 MAs working in family medicine clinics. A total of 75 (87%) responded to at least some questions (typically just demographics). We used those who completed the full survey (n = 61; 71%) for data analysis. Eighteen MAs participated in face-to-face interviews. Among respondents, 35 (47%) had worked at least 10 years as an MA and 21 (28%) had worked at least a decade in the family medicine department.
Perception of role
All respondents (n = 61; 100%) somewhat or strongly agreed that the MA role was “very important to keep the clinic functioning” and 58 (95%) reported that working in health care was “a calling” for them. Only 7 (11%) agreed that family medicine was an easier environment for MAs compared to a specialty clinic; 30 (49%) disagreed with this. Among respondents, 32 (53%) strongly or somewhat agreed that their work was very stressful and just half (n = 28; 46%) agreed there were adequate MA staff at their clinic.
Continue to: Efficiency and competing priorities
Efficiency and competing priorities
MAs described important work values that increased their efficiency. These included clinic culture (good communication and strong teamwork), as well as individual strategies such as multitasking, limiting patient conversations, and doing tasks in a consistent way to improve accuracy. (See TABLE 1.) They identified ways physicians bolster or hurt efficiency and ways in which the relationship between the physician and the MA shapes the MA’s perception of their value in clinic.
Communication was emphasized as critical for efficient care, and MAs encouraged the use of preclinic huddles and communication as priorities. Seventy-five percent of MAs reported preclinic huddles to plan for patient care were helpful, but only half said huddles took place “always” or “most of the time.” Many described reviewing the schedule and completing tasks ahead of patient arrival as critical to efficiency.
Participants described the tension between their identified role of orchestrating clinic flow and responding to directives by others that disrupted the flow. Several MAs found it challenging when physicians agreed to see very late patients and felt frustrated when decisions that changed the flow were made by the physician or front desk staff without including the MA. MAs were also able to articulate how they managed competing priorities within the clinic, such as when a patient- or physician-driven need to extend appointments was at odds with maintaining a timely schedule. They were eager to share personal tips for time management and prided themselves on careful and accurate performance and skills they had learned on the job. MAs also described how efficiency could be adversely affected by the behaviors or attitudes of physicians. (See TABLE 2.)
Clinic environment
Thirty-six MAs (59%) reported that other MAs on their team were willing to help them out in clinic “a great deal” or “a lot” of the time, by helping to room a patient, acting as a chaperone for an exam, or doing a point-of-care lab. This sense of support varied across clinics (38% to 91% reported good support), suggesting that cultures vary by site. Some MAs expressed frustration at peers they saw as resistant to helping, exemplified by this verbatim quote from an interview:
“ Some don’t want to help out. They may sigh. It’s how they react—you just know.” (Clinic #1, MA #2 interview)
Efficient MAs stressed the need for situational awareness to recognize when co-workers need help:
“ [Peers often] are not aware that another MA is drowning. There’s 5 people who could have done that, and here I am running around and nobody budged.” (Clinic #5, MA #2 interview)
Continue to: A minority of staff...
A minority of staff used the open-ended survey sections to describe clinic hierarchy. When asked about “pet peeves,” a few advised that physicians should not “talk down” to staff and should try to teach rather than criticize. Another asked that physicians not “bark orders” or have “low gratitude” for staff work. MAs found micromanaging stressful—particularly when the physician prompted the MA about patient arrivals:
“[I don’t like] when providers will make a comment about a patient arriving when you already know this information. You then rush to put [the] patient in [a] room, then [the] provider ends up making [the] patient wait an extensive amount of time. I’m perfectly capable of knowing when a patient arrives.” (Clinic #6, survey)
MAs did not like physicians “talking bad about us” or blaming the MA if the clinic is running behind.
Despite these concerns, most MAs reported feeling appreciated for the job they do. Only 10 (16%) reported that the people they work with rarely say “thank you,” and 2 (3%) stated they were not well supported by the physicians in clinic. Most (n = 38; 62%) strongly agreed or agreed that they felt part of the team and that their opinions matter. In the interviews, many expanded on this idea:
“I really feel like I’m valued, so I want to do everything I can to make [my doctor’s] day go better. If you want a good clinic, the best thing a doc can do is make the MA feel valued.” (Clinic #1, MA #1 interview)
DISCUSSION
Participants described their role much as an orchestra director, with MAs as the key to clinic flow and timeliness.9 Respondents articulated multiple common strategies used to increase their own efficiency and clinic flow; these may be considered best practices and incorporated as part of the basic training. Most MAs reported their day-to-day jobs were stressful and believed this was underrecognized, so efficiency strategies are critical. With staff completing multiple time-sensitive tasks during clinic, consistent co-worker support is crucial and may impact efficiency.8 Proper training of managers to provide that support and ensure equitable workloads may be one strategy to ensure that staff members feel the workplace is fair and collegial.
Several comments reflected the power differential within medical offices. One study reported that MAs and physicians “occupy roles at opposite ends of social and occupational hierarchies.”11 It’s important for physicians to be cognizant of these patterns and clinic culture, as reducing a hierarchy-based environment will be appreciated by MAs.9 Prior research has found that MAs have higher perceptions of their own competence than do the physicians working with them.12 If there is a fundamental lack of trust between the 2 groups, this will undoubtedly hinder team-building. Attention to this issue is key to a more favorable work environment.
Continue to: Almost all respondents...
Almost all respondents reported health care was a “calling,” which mirrors physician research that suggests seeing work as a “calling” is protective against burnout.13,14 Open-ended comments indicated great pride in contributions, and most staff members felt appreciated by their teams. Many described the working relationships with physicians as critical to their satisfaction at work and indicated that strong partnerships motivated them to do their best to make the physician’s day easier. Staff job satisfaction is linked to improved quality of care, so treating staff well contributes to high-value care for patients.15 We also uncovered some MA “pet peeves” that hinder efficiency and could be shared with physicians to emphasize the importance of patience and civility.
One barrier to expansion of MA roles within PCMH practices is the limited pay and career ladder for MAs who adopt new job responsibilities that require advanced skills or training.1,2 The mean MA salary at our institution ($37,372) is higher than in our state overall ($33,760), which may impact satisfaction.16 In addition, 93% of MAs are women; thus, they may continue to struggle more with lower pay than do workers in male-dominated professions.17,18 Expected job growth from 2018-2028 is predicted at 23%, which may help to boost salaries.19 Prior studies describe the lack of a job ladder or promotion opportunities as a challenge1,20; this was not formally assessed in our study.
MAs see work in family medicine as much harder than it is in other specialty clinics. Being trusted with more responsibility, greater autonomy,21-23 and expanded patient care roles can boost MA self-efficacy, which can reduce burnout for both physicians and MAs.8,24 However, new responsibilities should include appropriate training, support, and compensation, and match staff interests.7
Study limitations. The study was limited to 6 clinics in 1 department at a large academic medical center. Interviewed participants were selected by convenience and snowball sampling and thus, the results cannot be generalized to the population of MAs as a whole. As the initial interview goal was simply to gather efficiency tips, the project was not designed to be formal qualitative research. However, the discussions built on open-ended comments from the written survey helped contextualize our quantitative findings about efficiency. Notes were documented in real time by a single interviewer with rapid typing skills, which allowed capture of quotes verbatim. Subsequent studies would benefit from more formal qualitative research methods (recording and transcribing interviews, multiple coders to reduce risk of bias, and more complex thematic analysis).
Our research demonstrated how MAs perceive their roles in primary care and the facilitators and barriers to high efficiency in the workplace, which begins to fill an important knowledge gap in primary care. Disseminating practices that staff members themselves have identified as effective, and being attentive to how staff members are treated, may increase individual efficiency while improving staff retention and satisfaction.
CORRESPONDENCE
Katherine J. Gold, MD, MSW, MS, Department of Family Medicine and Department of Obstetrics and Gynecology, University of Michigan, 1018 Fuller Street, Ann Arbor, MI 48104-1213; [email protected]
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
1. Chapman SA, Blash LK. New roles for medical assistants in innovative primary care practices. Health Serv Res. 2017;52(suppl 1):383-406.
2. Ferrante JM, Shaw EK, Bayly JE, et al. Barriers and facilitators to expanding roles of medical assistants in patient-centered medical homes (PCMHs). J Am Board Fam Med. 2018;31:226-235.
3. Atkins B. The outlook for medical assisting in 2016 and beyond. Accessed January 27, 2022. www.medicalassistantdegrees.net/articles/medical-assisting-trends/
4. Unqualified medical “assistants.” Hospital (Lond 1886). 1897;23:163-164.
5. Ameritech College of Healthcare. The origins of the AAMA. Accessed January 27, 2022. www.ameritech.edu/blog/medical-assisting-history/
6. Dai M, Willard-Grace R, Knox M, et al. Team configurations, efficiency, and family physician burnout. J Am Board Fam Med. 2020;33:368-377.
7. Harper PG, Van Riper K, Ramer T, et al. Team-based care: an expanded medical assistant role—enhanced rooming and visit assistance. J Interprof Care. 2018:1-7.
8. Sheridan B, Chien AT, Peters AS, et al. Team-based primary care: the medical assistant perspective. Health Care Manage Rev. 2018;43:115-125.
9. Tache S, Hill-Sakurai L. Medical assistants: the invisible “glue” of primary health care practices in the United States? J Health Organ Manag. 2010;24:288-305.
10. STROBE checklist for cohort, case-control, and cross-sectional studies. Accessed January 27, 2022. www.strobe-statement.org/fileadmin/Strobe/uploads/checklists/STROBE_checklist_v4_combined.pdf
11. Gray CP, Harrison MI, Hung D. Medical assistants as flow managers in primary care: challenges and recommendations. J Healthc Manag. 2016;61:181-191.
12. Elder NC, Jacobson CJ, Bolon SK, et al. Patterns of relating between physicians and medical assistants in small family medicine offices. Ann Fam Med. 2014;12:150-157.
13. Jager AJ, Tutty MA, Kao AC. Association between physician burnout and identification with medicine as a calling. Mayo Clinic Proc. 2017;92:415-422.
14. Yoon JD, Daley BM, Curlin FA. The association between a sense of calling and physician well-being: a national study of primary care physicians and psychiatrists. Acad Psychiatry. 2017;41:167-173.
15. Mohr DC, Young GJ, Meterko M, et al. Job satisfaction of primary care team members and quality of care. Am J Med Qual. 2011;26:18-25.
16. US Bureau of Labor Statistics. Occupational employment and wage statistics. Accessed January 27, 2022. https://www.bls.gov/oes/current/oes319092.htm
17. Chapman SA, Marks A, Dower C. Positioning medical assistants for a greater role in the era of health reform. Acad Med. 2015;90:1347-1352.
18. Mandel H. The role of occupational attributes in gender earnings inequality, 1970-2010. Soc Sci Res. 2016;55:122-138.
19. US Bureau of Labor Statistics. Occupational outlook handbook: medical assistants. Accessed January 27, 2022. www.bls.gov/ooh/healthcare/medical-assistants.htm
20. Skillman SM, Dahal A, Frogner BK, et al. Frontline workers’ career pathways: a detailed look at Washington state’s medical assistant workforce. Med Care Res Rev. 2018:1077558718812950.
21. Morse G, Salyers MP, Rollins AL, et al. Burnout in mental health services: a review of the problem and its remediation. Adm Policy Ment Health. 2012;39:341-352.
22. Dubois CA, Bentein K, Ben Mansour JB, et al. Why some employees adopt or resist reorganization of work practices in health care: associations between perceived loss of resources, burnout, and attitudes to change. Int J Environ Res Pub Health. 2014;11:187-201.
23. Aronsson G, Theorell T, Grape T, et al. A systematic review including meta-analysis of work environment and burnout symptoms. BMC Public Health. 2017;17:264.
24. O’Malley AS, Gourevitch R, Draper K, et al. Overcoming challenges to teamwork in patient-centered medical homes: a qualitative study. J Gen Intern Med. 2015;30:183-192.
Developing and Measuring Effectiveness of a Distance Learning Dermatology Course: A Prospective Observational Study
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
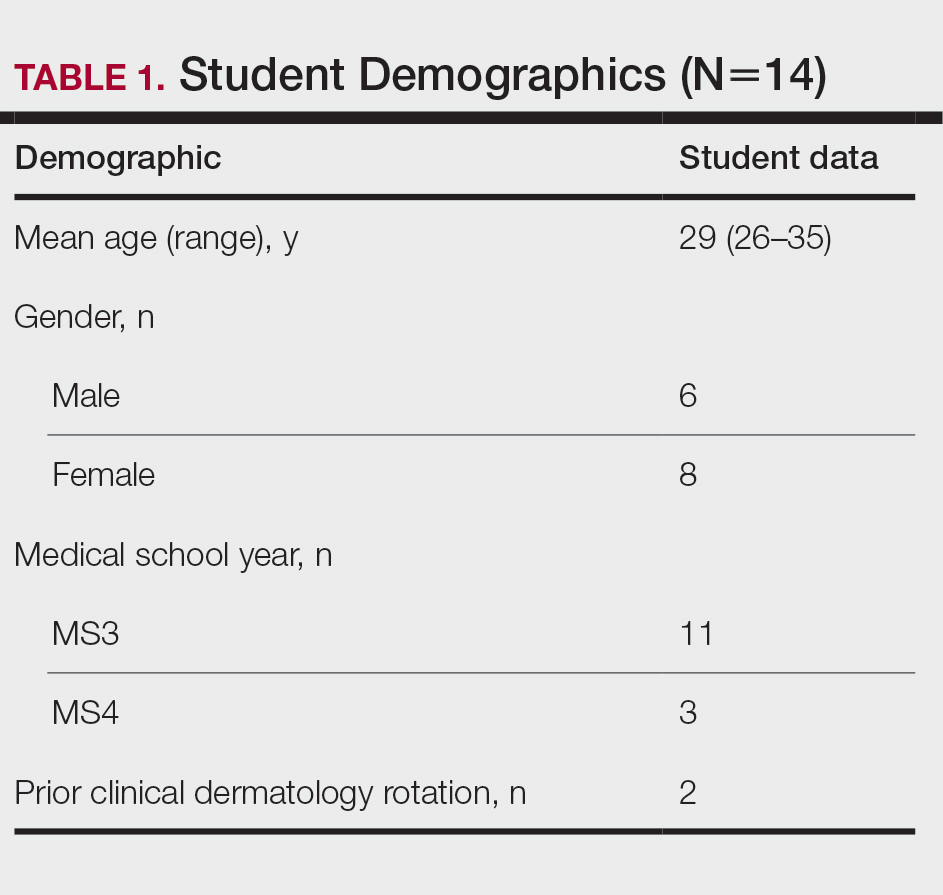
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.
An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
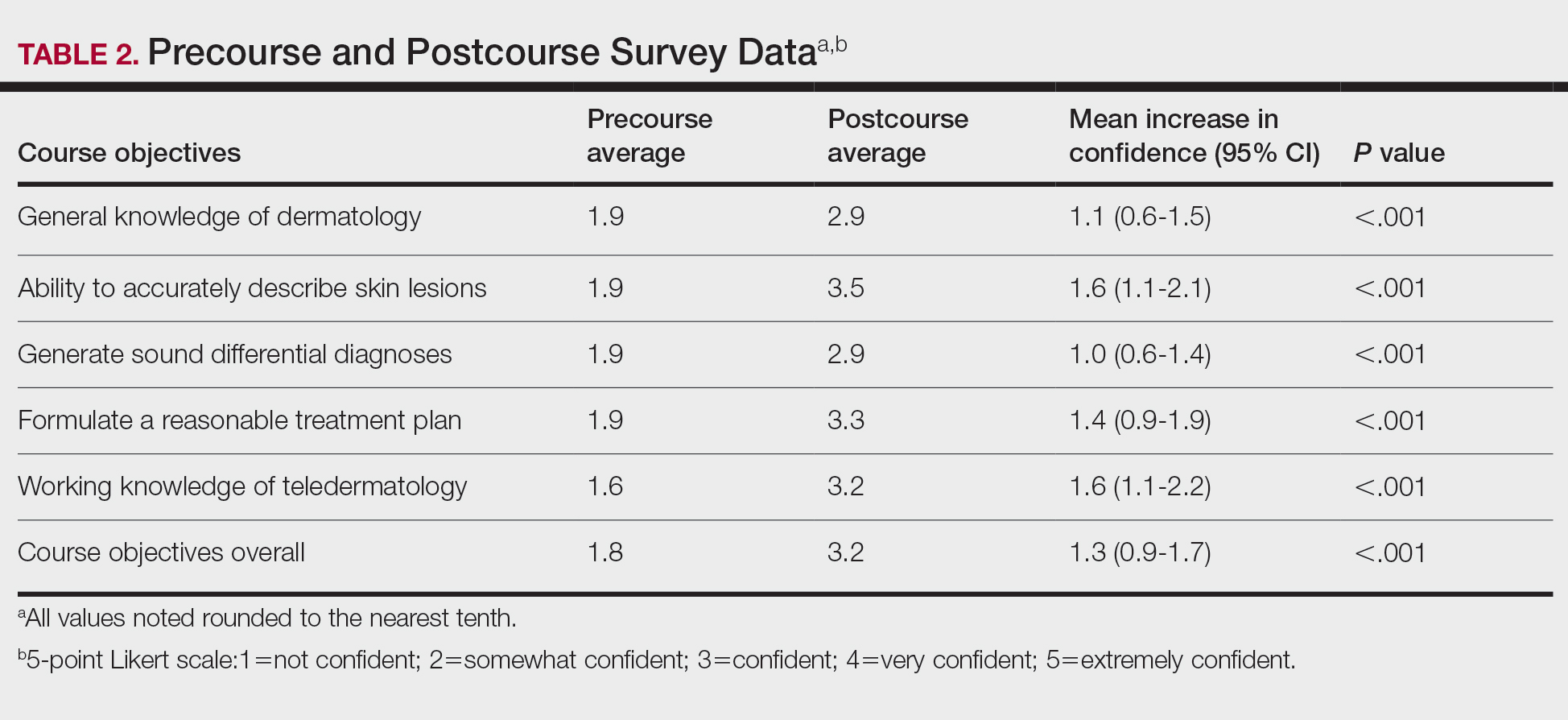
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.
Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.
An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.
Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
Medical education has seen major changes over the last decade. The allotted time for preclinical education has decreased from 24 months to 18 months or less at most institutions, with an increased focus on content associated with health care delivery and health system science.1,2 Many schools now include at least some blended learning with online delivery of preclinical education.3 On the other hand, the clinical portion of medical education has remained largely unchanged prior to the COVID-19 pandemic, with the apprenticeship framework allowing the experienced physician to observe, mentor, and pass on practical knowledge so that the apprentice can one day gain independence after demonstrating adequate proficiency.4
With respect to dermatology education, skin disorders are in the top 5 reported reasons for visits to primary care5; however, a 2009 survey found that only 0.24% to 0.30% of medical schools’ curricula are spent on dermatology.6 Moreover, one institution found that fourth-year medical students received an average of 46.6% on a 15-item quiz designed to assess the ability to diagnose and treat common dermatologic conditions, and within that same cohort, 87.6% of students felt that they received inadequate training in dermatology during medical school.7
COVID-19 caused an unprecedented paradigm shift when medical schools throughout the country, including our own, canceled clinical rotations at the end of March 2020 to protect students and control the spread of infection. To enable clinical and preclinical learning to continue, institutions around the globe turned to either online learning or participation in telehealth as a substitute for clinical rotations.8-10 At the Uniformed Services University of the Health Sciences (Bethesda, Maryland), one of the many online clinical courses offered included a distance learning (DL) dermatology course. Herein, we describe the results of a prospective study evaluating short-term information recall and comprehension as well as students’ confidence in their ability to apply course objectives over 3 months of an online DL dermatology course.
Methods
Between April and July 2020, 14 students at the Uniformed Services University of the Health Sciences (Table 1) enrolled in 1 of 3 four-week DL dermatology classes. The students independently completed the Basic Dermatology Curriculum, a set of online modules with demonstrated efficacy from the American Academy of Dermatology, over 4 weeks.11 Additionally, students were instructed to review an hour of clinical dermatology images daily from online dermatology atlases and e-books accessed through our medical school’s virtual library. Optional Free Open Access Meducation resources also were provided. The course syllabus provided the students with clear expectations, links to the resources, and a recommended daily schedule.
An online video conferencing platform was utilized for an orientation session and 4 subsequent weekly 1.5-hour virtual meetings. The weekly DL meetings focused on a discussion of clinical images pertinent to the American Academy of Dermatology modules covered for the week. These interactive analytic sessions were referred to as Clinpic sessions. With instructor guidance, the students learned to describe images, and they provided differential diagnoses, workup, and treatments for various skin diseases. The virtual meetings included supplemental lectures detailing the use of teledermatology and laser therapy in the Military Health System and a journal review on the cutaneous manifestations of COVID-19.
A 40-question, image-based pretest and posttest utilized during clinical rotations evaluated knowledge recall and comprehension. A precourse and postcourse survey using a 5-point Likert scale (1=not confident; 5=extremely confident) assessed students’ confidence levels across course objectives: general knowledge of dermatology, working knowledge of teledermatology, ability to accurately describe skin lesions, generate sound differential diagnoses, and formulate a reasonable treatment plan. Statistical analysis was performed using free online statistical software at statskingdom.com.12
Results
All 14 student enrollees completed the precourse and postcourse tests and surveys. Pretest and posttest scores followed a normal distribution and therefore met criteria for utilization of a parametric test. The precourse test average of 67% (range, 40%–90%) improved to 84% postcourse (range, 70%–98%; P<.001; 95% CI, 11-23 by paired t test). Not surprisingly, the 2 students who had completed a dermatology rotation had higher average pretest and posttest scores (pretest, 87%; posttest, 94%). Students’ confidence with the course objectives were mostly at the somewhat confident level on the 5-point Likert scale precourse survey. By the end of the course, student survey responses increased to confident and very confident levels, corresponding to an overall improvement of 1.3 points (P<.001 by paired t test)(Table 2) when the mean of the survey results was aggregated across every question. Instructor evaluation of student performance mirrored student assessments.
Comment
The DL dermatology course succeeded in helping the enrolled students attain course objectives and offered a reasonable solution when in-person interaction was restricted. The students in the DL course made notable improvements in their dermatology knowledge and improved their communication, diagnosis, and management skills. Although a blended dermatology curriculum with e-learning combined with clinical experience has been shown to increase knowledge acquisition,13,14 our results suggest that an online-only program also can increase comprehension as well as students’ confidence in their abilities.
A major challenge for the DL course was the lack of opportunity to perform common dermatology procedures. The addition of a hands-on skin procedure module would have been a great supplement to the course but was not possible due to social distancing guidelines during the COVID-19 pandemic. The small sample size and voluntary enrollment were limitations to this study.
Conclusion
Although the traditional dermatology rotation remains the gold standard for clinical instruction, a well-organized DL teaching environment allowed for a more controlled learning experience with a broader coverage of topics to include potentially greater exposure to rare skin disorders not typically encountered in everyday practice. A DL dermatology course may serve as an enduring curriculum for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure (eg, those pursuing non–primary care specialties, pathology, or radiology). It also may be attractive to students who have had a prior clinical dermatology rotation and desire a different learning experience with a wide coverage of topics.
Acknowledgments—The authors thank Thomas Darling, MD, PhD (Bethesda, Maryland), for coining the term Clinpic and providing critical feedback throughout the course. The authors also thank Sorana Raiciulescu, MS (Bethesda, Maryland), for assistance with the statistical analysis.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
- Emanuel EJ. The inevitable reimagining of medical education. JAMA. 2020;323:1127-1128.
- Skochelak SE, Stack SJ. Creating the medical schools of the future. Acad Med. 2017;92:16-19.
- Vallée A, Blacher J, Cariou A, et al. Blended learning compared to traditional learning in medical education: systematic review and meta-analysis. J Med Internet Res. 2020;22:E16504.
- Rangachari D, Brown LE, Kern DE, et al. Clinical coaching: evolving the apprenticeship model for modern housestaff. Med Teach. 2017;39:780-782.
- Finley CR, Chan DS, Garrison S, et al. What are the most common conditions in primary care? Can Fam Physician. 2018;64:832-840.
- McCleskey PE, Gilson RT, DeVillez RL. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.e4.
- Ulman CA, Binder SB, Borges NJ. Assessment of medical students’ proficiency in dermatology: are medical students adequately prepared to diagnose and treat common dermatologic conditions in the United States? J Educ Eval Health Prof. 2015;12:18.
- Loh TY, Hsiao JL, Shi VY. COVID-19 and its effect on medical student education in dermatology. J Am Acad Dermatol. 2020;83:E163-E164.
- Hilburg R, Patel N, Ambruso S, et al. Medical education during the coronavirus disease-2019 pandemic: learning from a distance. Adv Chronic Kidney Dis. 2020;27:412-417.
- Rose S. Medical student education in the time of COVID-19. JAMA. 2020;323:2131-2132.
- McCleskey PE. Clinic teaching made easy: a prospective study of the American Academy of Dermatology core curriculum in primary care learners. J Am Acad Dermatol. 2013;69:273-279.e1.
- Paired T Test calculator. Statistics Kingdom website. Accessed February 7, 2022. http://www.statskingdom.com/160MeanT2pair.html
- Fransen F, Martens H, Nagtzaam I, et al. Use of e-learning in clinical clerkships: effects on acquisition of dermatological knowledge and learning processes. Int J Med Educ. 2018;9:11-17.
- Silva CS, Souza MB, Silva Filho RS, et al. E-learning program for medical students in dermatology. Clinics. 2011;66:619-622.
Practice Points
- An e-learning distance learning (DL) dermatology course can substantially improve clinically relevant skills and knowledge in dermatology.
- A DL dermatology course may serve as an alternative to clinical rotations for those who wish to learn dermatology more broadly and are not interested in performing skin procedures or direct patient exposure.
Outcomes After Injection-Based Therapy: A Pain Outcomes Questionnaire for Veterans Univariate Analysis
Chronic pain is persistent or recurring pain lasting more than 3 months past normal healing time. Primary care professionals usually refer patients experiencing chronic pain to pain specialists to better identify, treat, and manage the pain. Chronic noncancer-related pain affects more Americans than diabetes mellitus, cardiac disease, and cancer combined.1 Veterans are no exception. The prevalence of severe pain was significantly higher in veterans compared with that of nonveterans who had back pain (21.6 vs 16.7%, respectively), jaw pain (37.5 vs 22.9%, respectively), severe headaches or migraine (26.4 vs 15.9%, respectively), and neck pain (27.7 vs 21.4%, respectively).2 At an individual level, those who experience chronic pain can expect impaired functional capacity, reduced ability to work, sleep disturbance, reduced social interactions, and considerable psychological distress. At a societal level, the cost of treating chronic pain is exorbitant, exceeding $600 billion annually, yet treatment outcomes remain variable at best.3 Greater efforts are needed to improve and standardize patient outcomes.
Interventional pain procedures performed under fluoroscopic or ultrasound guidance by specialist physicians have shown mixed responses in previous studies. Past systematic reviews demonstrate reductions in pain scores after lumbar or caudal epidural steroid injections (ESIs) and radiofrequency ablation of nerves supplying lumbar and thoracic facet joints.4-7 However, one review found insufficient evidence to support injection therapy for chronic low back pain.8 Unfortunately, the majority of the included studies evaluated outcomes using the visual analogue scale (VAS) or other limited factors, such as physical examination findings. Current biopsychosocial conceptualizations of chronic pain are beginning to recognize the complex nature of the experience of pain and highlighting the significance of multimodal management.9 It is vital that our assessment of chronic pain, like our treatment options, be multidimensional and reflect these underpinning principles.
The Pain Outcomes Questionnaire-For Veterans (POQ-VA) was developed within the Veterans Health Administration (VHA) by Clark and colleagues in 2003. It represents a brief but psychometrically sound pain outcomes instrument that assesses all key domains and meets accreditation body standards. The POQ-VA is valid and reliable for evaluating effectiveness of treatment of chronic noncancer pain in veterans in routine clinical practice.10 This review is the first study to use the POQ-VA to assess the impact of interventional pain procedures on veterans with chronic noncancer pain.
The aim of this study was to perform a retrospective review of POQ-VA scores before and after injection-based interventional treatment for chronic pain to determine whether the procedure affected patient outcomes. We hypothesized that POQ-VA scores would improve across multiple domains in the veteran population postprocedure. This study was approved by the Institutional Review Board (IRB-2018-053) at the Providence Veterans Affairs Medical Center (VAMC) in Rhode Island.
Methods
Using the Computerized Patient Record System, all adult veteran patients who had attended at least 2 appointments between April 1, 2009, and April 1, 2019 at the Providence VAMC interventional pain clinic were identified. POQ-VA reports were extracted provided the following criteria were met: (1) the veteran received an injection-based interventional treatment for chronic pain, including trigger point injections, ESIs, nerve blocks, and radiofrequency ablations; (2) the veteran completed POQ-VA both pre- and posttreatment; and (3) posttreatment POQ-VA reports were completed within 6 months of treatment. All patients who did not fit these criteria were excluded from the study.
After deidentification, 112 pre- and posttreatment POQ-VA reports were identified. All subsequent statistical analyses were conducted using Stata SE version 15. Descriptive statistics including mean, range, SD, and percent change were computed for POQ-VA domain—pain, mobility, activities of daily living (ADL), vitality, negative affect, fear, and total raw score—as well as for each POQ-VA question. Given that POQ-VA domain scores were found to be approximately normally distributed without outliers, domain scores were treated as continuous variables, and a paired samples t test was conducted to compare means among POQ-VA domains. Individual question responses were analyzed using nonparametric testing methods to account for the lack of normal distribution in each question, treating the range of 0 to 10 as an ordinal variable. A Wilcoxon matched-pairs signed-rank test was conducted to compare means among individual question responses before and after treatment.
Results
Of 112 included patients, 102 (91%) were male and 10 (9%) were female. The mean age was 62 years (range, 35-90). Diagnosis and procedures varied due to patient symptoms varying from muscle pain, nerve pain, degenerative disc disease, and osteoarthritis.
POQ-VA scores across all domains, including total raw score, showed statistically significant improvement after treatment (Table 1). Directionally, the POQ-VA scores for all 20 questions reflect a positive treatment response and 17 had statistically significant changes (P < .05) (Table 2). The changes in self-perceived energy level, safety, and feelings of tension were not statistically significant. Esteem had the greatest magnitude decrease, falling from 5.2 preprocedure to 3.8 postprocedure (P < .001). Other similarly significant magnitudes of improvement were seen from pre- to postprocedure in questions pertaining to grooming (2.2 to 1.6, P = .003) and the ability to use the bathroom (3.4 to 2.6, P < .001).
Discussion
The most important finding of this study was the ability of the POQ-VA to detect statistically significant positive responses to injection therapy across all domains. The largest improvements were in self-reported pain intensity, pain-related impairment in mobility and ADLs, and self-reported dysphoric effects. The single largest improvement posttreatment was a reduction in scores related to low self-esteem.
Chronic pain can be assessed in a variety of ways ranging from physical examination findings and subjective numerical ratings to extensive patient-reported questionnaires. The International Association for the Study of Pain acknowledges that pain is a complex experience and recommends assessment should be comprehensive.11 Many patient-reported questionnaires are available to clinicians, including some that address pain in a specific body part, such as the Oswestry Low Back Pain Disability Questionnaire, or those that focus on depression or quality-of-life measures, such as the SF-36.12,13
One major benefit of using the POQ-VA is its potential to demonstrate benefits across multiple domains, reflecting the complex nature of chronic pain. The POQ-VA also separates domain or scale scores, allowing clinicians to identify individuals with different patterns of dysfunction across domains.10 This separation also provides insight into which treatment options are best for chronic pain patients with predominant patterns or lower scores in certain domains. The use of a single summary score, as seen in other questionnaires such as the Roland-Morris Activity Scale, may conceal treatment-induced changes in specific outcome domains.14 Additionally, like many other similar instruments, the POQ-VA is easy to understand and use, requires no special training, takes little time to complete, and can be completed in person or over the phone.
As chronic pain has been studied further and its complexity recognized, more instruments have been developed and modified to reflect these new elements. There is no one scale applicable to all populations. A discussion about the strengths and weaknesses of each available assessment tool is outside the scope of this review. However, to date, the POQ-VA is the only instrument that has been validated to detect change following treatment of chronic pain in an exclusively veteran population.10 This validation emphasizes the importance of this study as it supports the use of this outcome measure to monitor treatment of pain in VA facilities.
One of the secondary findings indicated that injection therapy improved veterans’ physical activity levels and self-esteem and lowered pain scores as well as kinesiophobia and anxiety. The role of interventional procedures has been well established in the field of chronic pain, but their efficacy has been less clear. Injections are costly and not without risk, and these factors relegate them to fourth-line treatment options in most situations.15 Several meta-analyses have demonstrated small improvements in pain scores and patient-reported questionnaires after medial branch blocks, and lumbar or caudal ESIs for chronic back pain.5-7 However, an updated Cochrane Review concluded that there was insufficient evidence to support the use of injection therapy in subacute and chronic low back pain.8 The review acknowledged the limited methodologic quality of the trials and could not definitively report that injection therapy did not have benefits for certain subgroups of patients. The ability of researchers to detect benefit from an intervention is intrinsically linked to how outcomes are determined. The most interesting finding of our study was the patient-reported improved self-esteem scores. Many trials included in the systematic reviews discussed used outcome measures that did not have the multidimensional scope to demonstrate such a potential benefit.
Limitations
Our relatively small sample size represents the main shortcoming of this study. Because many posttreatment questionnaires were never collected, unfortunately, much potential data was lost. Most procedures performed were corticosteroid injections for the treatment of low back pain. This represented a combination of lumbar ESI, caudal ESI, medial branch blocks, and sacroiliac joint injections. The limited numbers meant that a further regression analysis of each injection type was not possible. Since few interventions treated pain in other areas of the body, it is difficult to determine whether procedures such as hip joint injections and ilioinguinal nerve blocks provided overall benefit. In the same vein, there is an inability to comment on which injection for chronic low back pain was the most efficacious.
The veteran population, while similar to the general population experiencing chronic pain, is more likely to experience PTSD and other mental health conditions.2 According to medical literature, no randomized controlled trials have been published examining pain interventions exclusively in veterans, so the applicability of these results needs further investigation. This study suggests there are potential benefits for the veteran population, not solely perhaps from receiving injection therapy, but to having access to an interventional pain clinic led by a pain physician within a network of other specialties. While limited by the inherent biases of a retrospective review, this study highlights the potential value in continuing to study this subgroup of patients, especially in the setting of an interdisciplinary approach.
Recent literature suggests interdisciplinary chronic pain management represents the best outcomes for patients’ physical, emotional, and social health, though these kinds of focused outpatient programs have not been studied on a large scale.16 The evolution of pain management in recent years to incorporating a biopsychosocial model has revolutionized how pain is treated and assessed, with multiple studies suggesting the greatest benefits lie in a multipronged approach.16,17 Past studies assessing individual interventions for chronic pain tend not to show strongly positive results, further reinforcing the idea that the answer does not lie in a specific treatment. Many veterans who were included in this study possibly had received or were receiving adjunct therapies such as physical therapy, cognitive behavioral therapy, and acupuncture for pain management, as well as oral and topical medications. Unfortunately, due to the selected methodology, it was not possible for us to gather those data. In turn, we were unable to determine how much these additional factors played a role in changing patient scores, alongside injection therapy. This inability to control variables in this type of research continues to present a challenge to data interpretation, even in the highest quality of research, as acknowledged by Staal and colleagues.8
Future research may be best focused by expanding our knowledge of outpatient interdisciplinary pain management programs. Some interventions may be more relevant for a particular group within a program, and this information can be useful to direct resources.18 Future prospects will require an appropriate multidimensional assessment tool, and the POQ-VA is an example of a valid and reliable option for monitoring progress in pain management in the veteran population.
Conclusions
The POQ-VA is the only instrument to date that has been validated to detect change following treatment of chronic pain in an exclusively veteran population. Our study is the first univariate analysis since the instrument’s validation in 2003. Our descriptive and inferential statistics suggest that the majority of veterans undergoing injection therapy for chronic pain had statistically significant improvements in POQ-VA measures within a 6-month period following treatment. In order to conduct more rigorous, multivariate studies, continued and more widespread use of the POQ-VA instrument is warranted.
1. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230-1239. doi:10.1016/j.jpain.2010.07.002
2. Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Witkin LR, Farrar JT, Ashburn MA. Can assessing chronic pain outcomes data improve outcomes?. Pain Med. 2013;14(6):779-791. doi:10.1111/pme.12075
4. Benyamin RM, Manchikanti L, Parr AT, et al. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician. 2012;15(4):E363-E404.
5. Zhai J, Zhang L, Li M, et al. Epidural injection with or without steroid in managing chronic low-back and lower extremity pain: a meta-analysis of 10 randomized controlled trials. Am J Ther. 2017;24(3):e259-e269. doi:10.1097/MJT.0000000000000265
6. Parr AT, Manchikanti L, Hameed H, et al. Caudal epidural injections in the management of chronic low back pain: a systematic appraisal of the literature. Pain Physician. 2012;15(3):E159-E198.
7. Lee CH, Chung CK, Kim CH. The efficacy of conventional radiofrequency denervation in patients with chronic low back pain originating from the facet joints: a meta-analysis of randomized controlled trials. Spine J. 2017;17(11):1770-1780. doi:10.1016/j.spinee.2017.05.006
8. Staal JB, de Bie R, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2008;2008(3):CD001824. Published 2008 Jul 16. doi:10.1002/14651858.CD001824.pub3
9. Gironda RJ, Clark ME. Cluster analysis of the pain outcomes questionnaire. Pain Med. 2008;9(7):813-823. doi:10.1111/j.1526-4637.2007.00397.x
10. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395. doi:10.1682/jrrd.2003.09.0381
11. Watt-Watson J, McGillion M, Lax L, et al. Evaluating an Innovative eLearning Pain Education Interprofessional Resource: A Pre-Post Study. Pain Med. 2019;20(1):37-49. doi:10.1093/pm/pny105
12. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
13. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
14. Jensen MP, Strom SE, Turner JA, Romano JM. Validity of the Sickness Impact Profile Roland scale as a measure of dysfunction in chronic pain patients. Pain. 1992;50(2):157-162. doi:10.1016/0304-3959(92)90156-6
15. Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37(1):29-42. doi:10.1007/s00296-016-3481-8
16. Bujak BK, Regan E, Beattie PF, Harrington S. The effectiveness of interdisciplinary intensive outpatient programs in a population with diverse chronic pain conditions: a systematic review and meta-analysis. Pain Manag. 2019;9(4):417-429. doi:10.2217/pmt-2018-0087
17. Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2002;(1):CD000963. doi:10.1002/14651858.CD000963
18. Wilson IR. Management of chronic pain through pain management programmes. Br Med Bull. 2017;124(1):55-64. doi:10.1093/bmb/ldx032
Chronic pain is persistent or recurring pain lasting more than 3 months past normal healing time. Primary care professionals usually refer patients experiencing chronic pain to pain specialists to better identify, treat, and manage the pain. Chronic noncancer-related pain affects more Americans than diabetes mellitus, cardiac disease, and cancer combined.1 Veterans are no exception. The prevalence of severe pain was significantly higher in veterans compared with that of nonveterans who had back pain (21.6 vs 16.7%, respectively), jaw pain (37.5 vs 22.9%, respectively), severe headaches or migraine (26.4 vs 15.9%, respectively), and neck pain (27.7 vs 21.4%, respectively).2 At an individual level, those who experience chronic pain can expect impaired functional capacity, reduced ability to work, sleep disturbance, reduced social interactions, and considerable psychological distress. At a societal level, the cost of treating chronic pain is exorbitant, exceeding $600 billion annually, yet treatment outcomes remain variable at best.3 Greater efforts are needed to improve and standardize patient outcomes.
Interventional pain procedures performed under fluoroscopic or ultrasound guidance by specialist physicians have shown mixed responses in previous studies. Past systematic reviews demonstrate reductions in pain scores after lumbar or caudal epidural steroid injections (ESIs) and radiofrequency ablation of nerves supplying lumbar and thoracic facet joints.4-7 However, one review found insufficient evidence to support injection therapy for chronic low back pain.8 Unfortunately, the majority of the included studies evaluated outcomes using the visual analogue scale (VAS) or other limited factors, such as physical examination findings. Current biopsychosocial conceptualizations of chronic pain are beginning to recognize the complex nature of the experience of pain and highlighting the significance of multimodal management.9 It is vital that our assessment of chronic pain, like our treatment options, be multidimensional and reflect these underpinning principles.
The Pain Outcomes Questionnaire-For Veterans (POQ-VA) was developed within the Veterans Health Administration (VHA) by Clark and colleagues in 2003. It represents a brief but psychometrically sound pain outcomes instrument that assesses all key domains and meets accreditation body standards. The POQ-VA is valid and reliable for evaluating effectiveness of treatment of chronic noncancer pain in veterans in routine clinical practice.10 This review is the first study to use the POQ-VA to assess the impact of interventional pain procedures on veterans with chronic noncancer pain.
The aim of this study was to perform a retrospective review of POQ-VA scores before and after injection-based interventional treatment for chronic pain to determine whether the procedure affected patient outcomes. We hypothesized that POQ-VA scores would improve across multiple domains in the veteran population postprocedure. This study was approved by the Institutional Review Board (IRB-2018-053) at the Providence Veterans Affairs Medical Center (VAMC) in Rhode Island.
Methods
Using the Computerized Patient Record System, all adult veteran patients who had attended at least 2 appointments between April 1, 2009, and April 1, 2019 at the Providence VAMC interventional pain clinic were identified. POQ-VA reports were extracted provided the following criteria were met: (1) the veteran received an injection-based interventional treatment for chronic pain, including trigger point injections, ESIs, nerve blocks, and radiofrequency ablations; (2) the veteran completed POQ-VA both pre- and posttreatment; and (3) posttreatment POQ-VA reports were completed within 6 months of treatment. All patients who did not fit these criteria were excluded from the study.
After deidentification, 112 pre- and posttreatment POQ-VA reports were identified. All subsequent statistical analyses were conducted using Stata SE version 15. Descriptive statistics including mean, range, SD, and percent change were computed for POQ-VA domain—pain, mobility, activities of daily living (ADL), vitality, negative affect, fear, and total raw score—as well as for each POQ-VA question. Given that POQ-VA domain scores were found to be approximately normally distributed without outliers, domain scores were treated as continuous variables, and a paired samples t test was conducted to compare means among POQ-VA domains. Individual question responses were analyzed using nonparametric testing methods to account for the lack of normal distribution in each question, treating the range of 0 to 10 as an ordinal variable. A Wilcoxon matched-pairs signed-rank test was conducted to compare means among individual question responses before and after treatment.
Results
Of 112 included patients, 102 (91%) were male and 10 (9%) were female. The mean age was 62 years (range, 35-90). Diagnosis and procedures varied due to patient symptoms varying from muscle pain, nerve pain, degenerative disc disease, and osteoarthritis.
POQ-VA scores across all domains, including total raw score, showed statistically significant improvement after treatment (Table 1). Directionally, the POQ-VA scores for all 20 questions reflect a positive treatment response and 17 had statistically significant changes (P < .05) (Table 2). The changes in self-perceived energy level, safety, and feelings of tension were not statistically significant. Esteem had the greatest magnitude decrease, falling from 5.2 preprocedure to 3.8 postprocedure (P < .001). Other similarly significant magnitudes of improvement were seen from pre- to postprocedure in questions pertaining to grooming (2.2 to 1.6, P = .003) and the ability to use the bathroom (3.4 to 2.6, P < .001).
Discussion
The most important finding of this study was the ability of the POQ-VA to detect statistically significant positive responses to injection therapy across all domains. The largest improvements were in self-reported pain intensity, pain-related impairment in mobility and ADLs, and self-reported dysphoric effects. The single largest improvement posttreatment was a reduction in scores related to low self-esteem.
Chronic pain can be assessed in a variety of ways ranging from physical examination findings and subjective numerical ratings to extensive patient-reported questionnaires. The International Association for the Study of Pain acknowledges that pain is a complex experience and recommends assessment should be comprehensive.11 Many patient-reported questionnaires are available to clinicians, including some that address pain in a specific body part, such as the Oswestry Low Back Pain Disability Questionnaire, or those that focus on depression or quality-of-life measures, such as the SF-36.12,13
One major benefit of using the POQ-VA is its potential to demonstrate benefits across multiple domains, reflecting the complex nature of chronic pain. The POQ-VA also separates domain or scale scores, allowing clinicians to identify individuals with different patterns of dysfunction across domains.10 This separation also provides insight into which treatment options are best for chronic pain patients with predominant patterns or lower scores in certain domains. The use of a single summary score, as seen in other questionnaires such as the Roland-Morris Activity Scale, may conceal treatment-induced changes in specific outcome domains.14 Additionally, like many other similar instruments, the POQ-VA is easy to understand and use, requires no special training, takes little time to complete, and can be completed in person or over the phone.
As chronic pain has been studied further and its complexity recognized, more instruments have been developed and modified to reflect these new elements. There is no one scale applicable to all populations. A discussion about the strengths and weaknesses of each available assessment tool is outside the scope of this review. However, to date, the POQ-VA is the only instrument that has been validated to detect change following treatment of chronic pain in an exclusively veteran population.10 This validation emphasizes the importance of this study as it supports the use of this outcome measure to monitor treatment of pain in VA facilities.
One of the secondary findings indicated that injection therapy improved veterans’ physical activity levels and self-esteem and lowered pain scores as well as kinesiophobia and anxiety. The role of interventional procedures has been well established in the field of chronic pain, but their efficacy has been less clear. Injections are costly and not without risk, and these factors relegate them to fourth-line treatment options in most situations.15 Several meta-analyses have demonstrated small improvements in pain scores and patient-reported questionnaires after medial branch blocks, and lumbar or caudal ESIs for chronic back pain.5-7 However, an updated Cochrane Review concluded that there was insufficient evidence to support the use of injection therapy in subacute and chronic low back pain.8 The review acknowledged the limited methodologic quality of the trials and could not definitively report that injection therapy did not have benefits for certain subgroups of patients. The ability of researchers to detect benefit from an intervention is intrinsically linked to how outcomes are determined. The most interesting finding of our study was the patient-reported improved self-esteem scores. Many trials included in the systematic reviews discussed used outcome measures that did not have the multidimensional scope to demonstrate such a potential benefit.
Limitations
Our relatively small sample size represents the main shortcoming of this study. Because many posttreatment questionnaires were never collected, unfortunately, much potential data was lost. Most procedures performed were corticosteroid injections for the treatment of low back pain. This represented a combination of lumbar ESI, caudal ESI, medial branch blocks, and sacroiliac joint injections. The limited numbers meant that a further regression analysis of each injection type was not possible. Since few interventions treated pain in other areas of the body, it is difficult to determine whether procedures such as hip joint injections and ilioinguinal nerve blocks provided overall benefit. In the same vein, there is an inability to comment on which injection for chronic low back pain was the most efficacious.
The veteran population, while similar to the general population experiencing chronic pain, is more likely to experience PTSD and other mental health conditions.2 According to medical literature, no randomized controlled trials have been published examining pain interventions exclusively in veterans, so the applicability of these results needs further investigation. This study suggests there are potential benefits for the veteran population, not solely perhaps from receiving injection therapy, but to having access to an interventional pain clinic led by a pain physician within a network of other specialties. While limited by the inherent biases of a retrospective review, this study highlights the potential value in continuing to study this subgroup of patients, especially in the setting of an interdisciplinary approach.
Recent literature suggests interdisciplinary chronic pain management represents the best outcomes for patients’ physical, emotional, and social health, though these kinds of focused outpatient programs have not been studied on a large scale.16 The evolution of pain management in recent years to incorporating a biopsychosocial model has revolutionized how pain is treated and assessed, with multiple studies suggesting the greatest benefits lie in a multipronged approach.16,17 Past studies assessing individual interventions for chronic pain tend not to show strongly positive results, further reinforcing the idea that the answer does not lie in a specific treatment. Many veterans who were included in this study possibly had received or were receiving adjunct therapies such as physical therapy, cognitive behavioral therapy, and acupuncture for pain management, as well as oral and topical medications. Unfortunately, due to the selected methodology, it was not possible for us to gather those data. In turn, we were unable to determine how much these additional factors played a role in changing patient scores, alongside injection therapy. This inability to control variables in this type of research continues to present a challenge to data interpretation, even in the highest quality of research, as acknowledged by Staal and colleagues.8
Future research may be best focused by expanding our knowledge of outpatient interdisciplinary pain management programs. Some interventions may be more relevant for a particular group within a program, and this information can be useful to direct resources.18 Future prospects will require an appropriate multidimensional assessment tool, and the POQ-VA is an example of a valid and reliable option for monitoring progress in pain management in the veteran population.
Conclusions
The POQ-VA is the only instrument to date that has been validated to detect change following treatment of chronic pain in an exclusively veteran population. Our study is the first univariate analysis since the instrument’s validation in 2003. Our descriptive and inferential statistics suggest that the majority of veterans undergoing injection therapy for chronic pain had statistically significant improvements in POQ-VA measures within a 6-month period following treatment. In order to conduct more rigorous, multivariate studies, continued and more widespread use of the POQ-VA instrument is warranted.
Chronic pain is persistent or recurring pain lasting more than 3 months past normal healing time. Primary care professionals usually refer patients experiencing chronic pain to pain specialists to better identify, treat, and manage the pain. Chronic noncancer-related pain affects more Americans than diabetes mellitus, cardiac disease, and cancer combined.1 Veterans are no exception. The prevalence of severe pain was significantly higher in veterans compared with that of nonveterans who had back pain (21.6 vs 16.7%, respectively), jaw pain (37.5 vs 22.9%, respectively), severe headaches or migraine (26.4 vs 15.9%, respectively), and neck pain (27.7 vs 21.4%, respectively).2 At an individual level, those who experience chronic pain can expect impaired functional capacity, reduced ability to work, sleep disturbance, reduced social interactions, and considerable psychological distress. At a societal level, the cost of treating chronic pain is exorbitant, exceeding $600 billion annually, yet treatment outcomes remain variable at best.3 Greater efforts are needed to improve and standardize patient outcomes.
Interventional pain procedures performed under fluoroscopic or ultrasound guidance by specialist physicians have shown mixed responses in previous studies. Past systematic reviews demonstrate reductions in pain scores after lumbar or caudal epidural steroid injections (ESIs) and radiofrequency ablation of nerves supplying lumbar and thoracic facet joints.4-7 However, one review found insufficient evidence to support injection therapy for chronic low back pain.8 Unfortunately, the majority of the included studies evaluated outcomes using the visual analogue scale (VAS) or other limited factors, such as physical examination findings. Current biopsychosocial conceptualizations of chronic pain are beginning to recognize the complex nature of the experience of pain and highlighting the significance of multimodal management.9 It is vital that our assessment of chronic pain, like our treatment options, be multidimensional and reflect these underpinning principles.
The Pain Outcomes Questionnaire-For Veterans (POQ-VA) was developed within the Veterans Health Administration (VHA) by Clark and colleagues in 2003. It represents a brief but psychometrically sound pain outcomes instrument that assesses all key domains and meets accreditation body standards. The POQ-VA is valid and reliable for evaluating effectiveness of treatment of chronic noncancer pain in veterans in routine clinical practice.10 This review is the first study to use the POQ-VA to assess the impact of interventional pain procedures on veterans with chronic noncancer pain.
The aim of this study was to perform a retrospective review of POQ-VA scores before and after injection-based interventional treatment for chronic pain to determine whether the procedure affected patient outcomes. We hypothesized that POQ-VA scores would improve across multiple domains in the veteran population postprocedure. This study was approved by the Institutional Review Board (IRB-2018-053) at the Providence Veterans Affairs Medical Center (VAMC) in Rhode Island.
Methods
Using the Computerized Patient Record System, all adult veteran patients who had attended at least 2 appointments between April 1, 2009, and April 1, 2019 at the Providence VAMC interventional pain clinic were identified. POQ-VA reports were extracted provided the following criteria were met: (1) the veteran received an injection-based interventional treatment for chronic pain, including trigger point injections, ESIs, nerve blocks, and radiofrequency ablations; (2) the veteran completed POQ-VA both pre- and posttreatment; and (3) posttreatment POQ-VA reports were completed within 6 months of treatment. All patients who did not fit these criteria were excluded from the study.
After deidentification, 112 pre- and posttreatment POQ-VA reports were identified. All subsequent statistical analyses were conducted using Stata SE version 15. Descriptive statistics including mean, range, SD, and percent change were computed for POQ-VA domain—pain, mobility, activities of daily living (ADL), vitality, negative affect, fear, and total raw score—as well as for each POQ-VA question. Given that POQ-VA domain scores were found to be approximately normally distributed without outliers, domain scores were treated as continuous variables, and a paired samples t test was conducted to compare means among POQ-VA domains. Individual question responses were analyzed using nonparametric testing methods to account for the lack of normal distribution in each question, treating the range of 0 to 10 as an ordinal variable. A Wilcoxon matched-pairs signed-rank test was conducted to compare means among individual question responses before and after treatment.
Results
Of 112 included patients, 102 (91%) were male and 10 (9%) were female. The mean age was 62 years (range, 35-90). Diagnosis and procedures varied due to patient symptoms varying from muscle pain, nerve pain, degenerative disc disease, and osteoarthritis.
POQ-VA scores across all domains, including total raw score, showed statistically significant improvement after treatment (Table 1). Directionally, the POQ-VA scores for all 20 questions reflect a positive treatment response and 17 had statistically significant changes (P < .05) (Table 2). The changes in self-perceived energy level, safety, and feelings of tension were not statistically significant. Esteem had the greatest magnitude decrease, falling from 5.2 preprocedure to 3.8 postprocedure (P < .001). Other similarly significant magnitudes of improvement were seen from pre- to postprocedure in questions pertaining to grooming (2.2 to 1.6, P = .003) and the ability to use the bathroom (3.4 to 2.6, P < .001).
Discussion
The most important finding of this study was the ability of the POQ-VA to detect statistically significant positive responses to injection therapy across all domains. The largest improvements were in self-reported pain intensity, pain-related impairment in mobility and ADLs, and self-reported dysphoric effects. The single largest improvement posttreatment was a reduction in scores related to low self-esteem.
Chronic pain can be assessed in a variety of ways ranging from physical examination findings and subjective numerical ratings to extensive patient-reported questionnaires. The International Association for the Study of Pain acknowledges that pain is a complex experience and recommends assessment should be comprehensive.11 Many patient-reported questionnaires are available to clinicians, including some that address pain in a specific body part, such as the Oswestry Low Back Pain Disability Questionnaire, or those that focus on depression or quality-of-life measures, such as the SF-36.12,13
One major benefit of using the POQ-VA is its potential to demonstrate benefits across multiple domains, reflecting the complex nature of chronic pain. The POQ-VA also separates domain or scale scores, allowing clinicians to identify individuals with different patterns of dysfunction across domains.10 This separation also provides insight into which treatment options are best for chronic pain patients with predominant patterns or lower scores in certain domains. The use of a single summary score, as seen in other questionnaires such as the Roland-Morris Activity Scale, may conceal treatment-induced changes in specific outcome domains.14 Additionally, like many other similar instruments, the POQ-VA is easy to understand and use, requires no special training, takes little time to complete, and can be completed in person or over the phone.
As chronic pain has been studied further and its complexity recognized, more instruments have been developed and modified to reflect these new elements. There is no one scale applicable to all populations. A discussion about the strengths and weaknesses of each available assessment tool is outside the scope of this review. However, to date, the POQ-VA is the only instrument that has been validated to detect change following treatment of chronic pain in an exclusively veteran population.10 This validation emphasizes the importance of this study as it supports the use of this outcome measure to monitor treatment of pain in VA facilities.
One of the secondary findings indicated that injection therapy improved veterans’ physical activity levels and self-esteem and lowered pain scores as well as kinesiophobia and anxiety. The role of interventional procedures has been well established in the field of chronic pain, but their efficacy has been less clear. Injections are costly and not without risk, and these factors relegate them to fourth-line treatment options in most situations.15 Several meta-analyses have demonstrated small improvements in pain scores and patient-reported questionnaires after medial branch blocks, and lumbar or caudal ESIs for chronic back pain.5-7 However, an updated Cochrane Review concluded that there was insufficient evidence to support the use of injection therapy in subacute and chronic low back pain.8 The review acknowledged the limited methodologic quality of the trials and could not definitively report that injection therapy did not have benefits for certain subgroups of patients. The ability of researchers to detect benefit from an intervention is intrinsically linked to how outcomes are determined. The most interesting finding of our study was the patient-reported improved self-esteem scores. Many trials included in the systematic reviews discussed used outcome measures that did not have the multidimensional scope to demonstrate such a potential benefit.
Limitations
Our relatively small sample size represents the main shortcoming of this study. Because many posttreatment questionnaires were never collected, unfortunately, much potential data was lost. Most procedures performed were corticosteroid injections for the treatment of low back pain. This represented a combination of lumbar ESI, caudal ESI, medial branch blocks, and sacroiliac joint injections. The limited numbers meant that a further regression analysis of each injection type was not possible. Since few interventions treated pain in other areas of the body, it is difficult to determine whether procedures such as hip joint injections and ilioinguinal nerve blocks provided overall benefit. In the same vein, there is an inability to comment on which injection for chronic low back pain was the most efficacious.
The veteran population, while similar to the general population experiencing chronic pain, is more likely to experience PTSD and other mental health conditions.2 According to medical literature, no randomized controlled trials have been published examining pain interventions exclusively in veterans, so the applicability of these results needs further investigation. This study suggests there are potential benefits for the veteran population, not solely perhaps from receiving injection therapy, but to having access to an interventional pain clinic led by a pain physician within a network of other specialties. While limited by the inherent biases of a retrospective review, this study highlights the potential value in continuing to study this subgroup of patients, especially in the setting of an interdisciplinary approach.
Recent literature suggests interdisciplinary chronic pain management represents the best outcomes for patients’ physical, emotional, and social health, though these kinds of focused outpatient programs have not been studied on a large scale.16 The evolution of pain management in recent years to incorporating a biopsychosocial model has revolutionized how pain is treated and assessed, with multiple studies suggesting the greatest benefits lie in a multipronged approach.16,17 Past studies assessing individual interventions for chronic pain tend not to show strongly positive results, further reinforcing the idea that the answer does not lie in a specific treatment. Many veterans who were included in this study possibly had received or were receiving adjunct therapies such as physical therapy, cognitive behavioral therapy, and acupuncture for pain management, as well as oral and topical medications. Unfortunately, due to the selected methodology, it was not possible for us to gather those data. In turn, we were unable to determine how much these additional factors played a role in changing patient scores, alongside injection therapy. This inability to control variables in this type of research continues to present a challenge to data interpretation, even in the highest quality of research, as acknowledged by Staal and colleagues.8
Future research may be best focused by expanding our knowledge of outpatient interdisciplinary pain management programs. Some interventions may be more relevant for a particular group within a program, and this information can be useful to direct resources.18 Future prospects will require an appropriate multidimensional assessment tool, and the POQ-VA is an example of a valid and reliable option for monitoring progress in pain management in the veteran population.
Conclusions
The POQ-VA is the only instrument to date that has been validated to detect change following treatment of chronic pain in an exclusively veteran population. Our study is the first univariate analysis since the instrument’s validation in 2003. Our descriptive and inferential statistics suggest that the majority of veterans undergoing injection therapy for chronic pain had statistically significant improvements in POQ-VA measures within a 6-month period following treatment. In order to conduct more rigorous, multivariate studies, continued and more widespread use of the POQ-VA instrument is warranted.
1. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230-1239. doi:10.1016/j.jpain.2010.07.002
2. Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Witkin LR, Farrar JT, Ashburn MA. Can assessing chronic pain outcomes data improve outcomes?. Pain Med. 2013;14(6):779-791. doi:10.1111/pme.12075
4. Benyamin RM, Manchikanti L, Parr AT, et al. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician. 2012;15(4):E363-E404.
5. Zhai J, Zhang L, Li M, et al. Epidural injection with or without steroid in managing chronic low-back and lower extremity pain: a meta-analysis of 10 randomized controlled trials. Am J Ther. 2017;24(3):e259-e269. doi:10.1097/MJT.0000000000000265
6. Parr AT, Manchikanti L, Hameed H, et al. Caudal epidural injections in the management of chronic low back pain: a systematic appraisal of the literature. Pain Physician. 2012;15(3):E159-E198.
7. Lee CH, Chung CK, Kim CH. The efficacy of conventional radiofrequency denervation in patients with chronic low back pain originating from the facet joints: a meta-analysis of randomized controlled trials. Spine J. 2017;17(11):1770-1780. doi:10.1016/j.spinee.2017.05.006
8. Staal JB, de Bie R, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2008;2008(3):CD001824. Published 2008 Jul 16. doi:10.1002/14651858.CD001824.pub3
9. Gironda RJ, Clark ME. Cluster analysis of the pain outcomes questionnaire. Pain Med. 2008;9(7):813-823. doi:10.1111/j.1526-4637.2007.00397.x
10. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395. doi:10.1682/jrrd.2003.09.0381
11. Watt-Watson J, McGillion M, Lax L, et al. Evaluating an Innovative eLearning Pain Education Interprofessional Resource: A Pre-Post Study. Pain Med. 2019;20(1):37-49. doi:10.1093/pm/pny105
12. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
13. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
14. Jensen MP, Strom SE, Turner JA, Romano JM. Validity of the Sickness Impact Profile Roland scale as a measure of dysfunction in chronic pain patients. Pain. 1992;50(2):157-162. doi:10.1016/0304-3959(92)90156-6
15. Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37(1):29-42. doi:10.1007/s00296-016-3481-8
16. Bujak BK, Regan E, Beattie PF, Harrington S. The effectiveness of interdisciplinary intensive outpatient programs in a population with diverse chronic pain conditions: a systematic review and meta-analysis. Pain Manag. 2019;9(4):417-429. doi:10.2217/pmt-2018-0087
17. Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2002;(1):CD000963. doi:10.1002/14651858.CD000963
18. Wilson IR. Management of chronic pain through pain management programmes. Br Med Bull. 2017;124(1):55-64. doi:10.1093/bmb/ldx032
1. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an Internet-based survey. J Pain. 2010;11(11):1230-1239. doi:10.1016/j.jpain.2010.07.002
2. Nahin RL. Severe Pain in Veterans: The effect of age and sex, and comparisons with the general population. J Pain. 2017;18(3):247-254. doi:10.1016/j.jpain.2016.10.021
3. Witkin LR, Farrar JT, Ashburn MA. Can assessing chronic pain outcomes data improve outcomes?. Pain Med. 2013;14(6):779-791. doi:10.1111/pme.12075
4. Benyamin RM, Manchikanti L, Parr AT, et al. The effectiveness of lumbar interlaminar epidural injections in managing chronic low back and lower extremity pain. Pain Physician. 2012;15(4):E363-E404.
5. Zhai J, Zhang L, Li M, et al. Epidural injection with or without steroid in managing chronic low-back and lower extremity pain: a meta-analysis of 10 randomized controlled trials. Am J Ther. 2017;24(3):e259-e269. doi:10.1097/MJT.0000000000000265
6. Parr AT, Manchikanti L, Hameed H, et al. Caudal epidural injections in the management of chronic low back pain: a systematic appraisal of the literature. Pain Physician. 2012;15(3):E159-E198.
7. Lee CH, Chung CK, Kim CH. The efficacy of conventional radiofrequency denervation in patients with chronic low back pain originating from the facet joints: a meta-analysis of randomized controlled trials. Spine J. 2017;17(11):1770-1780. doi:10.1016/j.spinee.2017.05.006
8. Staal JB, de Bie R, de Vet HC, Hildebrandt J, Nelemans P. Injection therapy for subacute and chronic low-back pain. Cochrane Database Syst Rev. 2008;2008(3):CD001824. Published 2008 Jul 16. doi:10.1002/14651858.CD001824.pub3
9. Gironda RJ, Clark ME. Cluster analysis of the pain outcomes questionnaire. Pain Med. 2008;9(7):813-823. doi:10.1111/j.1526-4637.2007.00397.x
10. Clark ME, Gironda RJ, Young RW. Development and validation of the Pain Outcomes Questionnaire-VA. J Rehabil Res Dev. 2003;40(5):381-395. doi:10.1682/jrrd.2003.09.0381
11. Watt-Watson J, McGillion M, Lax L, et al. Evaluating an Innovative eLearning Pain Education Interprofessional Resource: A Pre-Post Study. Pain Med. 2019;20(1):37-49. doi:10.1093/pm/pny105
12. Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66(8):271-273.
13. Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473-483.
14. Jensen MP, Strom SE, Turner JA, Romano JM. Validity of the Sickness Impact Profile Roland scale as a measure of dysfunction in chronic pain patients. Pain. 1992;50(2):157-162. doi:10.1016/0304-3959(92)90156-6
15. Hylands-White N, Duarte RV, Raphael JH. An overview of treatment approaches for chronic pain management. Rheumatol Int. 2017;37(1):29-42. doi:10.1007/s00296-016-3481-8
16. Bujak BK, Regan E, Beattie PF, Harrington S. The effectiveness of interdisciplinary intensive outpatient programs in a population with diverse chronic pain conditions: a systematic review and meta-analysis. Pain Manag. 2019;9(4):417-429. doi:10.2217/pmt-2018-0087
17. Guzmán J, Esmail R, Karjalainen K, Malmivaara A, Irvin E, Bombardier C. Multidisciplinary bio-psycho-social rehabilitation for chronic low back pain. Cochrane Database Syst Rev. 2002;(1):CD000963. doi:10.1002/14651858.CD000963
18. Wilson IR. Management of chronic pain through pain management programmes. Br Med Bull. 2017;124(1):55-64. doi:10.1093/bmb/ldx032
Prevalence and Predictors of Lower Limb Amputation in the Spinal Cord Injury Population
At the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, the prevalence of amputations among patients at the spinal cord injury (SCI) center seems high. Despite limited data demonstrating altered hemodynamics in the lower extremities (LEs) among the SCI population and increased frequency of peripheral arterial disease (PAD), amputations among patients with SCI have received little attention in research.1-3
In the United States, most amputations are caused by vascular disease related to peripheral arterial disease (PAD) and diabetes mellitus (DM).4 PAD primarily affects the LEs and is caused by atherosclerotic obstruction leading to insufficient blood flow. PAD can present clinically as LE pain, nonhealing ulcers, nonpalpable distal pulses, shiny or cold skin, absence of hair on the LE, or distal extremity pallor when the affected extremity is elevated. However, PAD is often asymptomatic. The diagnosis of PAD is typically made with an ankle-brachial index (ABI) ≤ 0.9.5 The prevalence of PAD is about 4.3% in Americans aged ≥ 40 years, increases with age, and is almost twice as common among Black Americans compared with that of White Americans.6 Many studies in SCI populations have documented an increased prevalence of DM, dyslipidemia, obesity, hypertension (HTN), and cigarette smoking.7-9 PAD shares these risk factors with coronary artery disease (CAD), but relative to CAD, tobacco smoking was a more substantial causative factor for PAD.10 Given the preponderance of associated risk factors in this population, PAD is likely more prevalent among patients with SCI than in the population without disabilities. Beyond these known risk factors, researchers hypothesized that SCI contributes to vascular disease by altering arterial function. However, this is still a topic of debate.11-13 Trauma also is a common cause of amputation, accounting for 45% of amputations in 2005.4 Patients with SCI may experience traumatic amputations simultaneously as their SCI, but they may also be predisposed to traumatic amputations related to osteopenia and impaired sensation.
Since amputation is an invasive surgery, knowing the severity of this issue is important in the SCI population. This study quantifies the prevalence of amputations of the LEs among the patients at our SCI center. It then characterizes these amputations’ etiology, their relationship with medical comorbidities, and certain SCI classifications.
Methods
This retrospective cohort study used the US Department of Veterans Affairs (VA) Computerized Patient Record System. The cohort was defined as all patients who received an annual examination at our SCI center over 4 years from October 1, 2009 to September 30, 2013. Annual examination includes a physical examination, relevant surveillance laboratory tests, and imaging, such as renal ultrasound for those with indwelling urinary catheters. One characteristic of the patient population in the VA system is that diagnoses, such as multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS), that involve spinal cord lesions causing symptoms are included in the registry, besides those with other traumatic or nontraumatic SCI. October 1 to September 30 was chosen based on the VA fiscal year (FY).
During this period, 1678 patients had an annual examination. Of those, 299 patients had an SCI etiology of ALS or MS, and 41 had nonfocal SCI etiology that could not be assessed using the American Spinal Injury Association Impairment Scale (AIS) and were excluded. Also excluded were 283 patients who did not have an annual examination during the specified time span. Some patients do not have an annual examination every year; for those with multiple annual examinations during that time frame, the most recent was used.
One thousand fifty-five patients were included in the statistical analysis. Date of birth, sex, race, ethnicity, date of death, smoking status, DM diagnosis, HTN diagnosis, use of an antiplatelet, antihypertensive, or lipid-lowering agent, blood pressure, hemoglobin A1c, and lipid panel were collected. The amputation level and etiology were noted. The levels of amputation were classified as toe/partial foot,
Statistical Analysis
Descriptive data were summarized as the median and IQR for continuous variables or the number and percentage for categorical variables. The χ2 test was used to analyze the association between categorical variables and amputation status. A nonparametric Wilcoxon test was used to investigate the distribution of continuous variables across patients with amputation and patients without amputation. Binary logistic regression analysis was used to investigate amputation risk factors. We report goodness of fit using the Hosmer and Lemeshow test and the area under the curve (AUC) for the multivariate model. Statistical significance was prespecified at a 2-sided P < .05. SAS version 9.4 was used for all statistical analyses.
Results
Mean age was approximately 61 years for the 91 patients at the time of the most recent amputation (Table 1). Among those with amputation, 63% were paraplegic and 37% were tetraplegic.
Of 1055 patients with SCI, 91 (8.6%) patients had an amputation. Of those, 70 (76.1%) were from nontraumatic causes (dysvascular), 17 (18.5%) were traumatic, 4 (4.3%) were from other causes (ie, cancer), and only 1 (1.1%) was of unknown cause.
Of the 91 patients with amputation, 64 (69.6%) had at least 1 TFA—33 were unilateral and 31 were bilateral. Two patients had a TFA on one side and a TTA on the other. Partial foot/toe and TTA were less common amputation levels with 14 (15.4%) and 13 (14.3%), respectively. Most amputations (86.8%) occurred over 6 months from the day of initial SCI, and were most commonly dysvascular (Table 2). Traumatic amputation occurred more evenly at various stages, pre-SCI, during acute SCI, subacute SCI, and chronic SCI.
Injury by Impairment Scale Level
Forty-nine (11.5%) of 426 patients with AIS level A SCI had undergone amputation. In order of prevalence, 23 (46.9%) were unilateral TFA, 17 (34.6%) were bilateral TFA, 10.2% were partial foot/toe, 4.1% were unilateral TTA, and 4.1% were a TTA/TFA combination. Both hip and knee disarticulations were classified in the TFA category.
Sixteen (13.0%) of 123 patients with AIS level B SCI had undergone amputation; 5 (31.3%) of those amputations were unilateral TFA, 6 (37.5%) were bilateral TFA, 3 (18.8%) were partial toe or foot, and 1 (6.3%) was for unilateral and bilateral TTA each.
Twelve (8.4%) of 143 patients with AIS level C SCI had undergone amputation: 6 (50.0%) were bilateral TFA; 3 (25.0%) were unilateral TFA; and 3 (25.0%) were unilateral TTA.
Fourteen (3.9%) of 356 patients with AIS level D SCI had undergone amputation. Of those 6 (42.9%) underwent a partial foot/toe amputation; 5 (35.7%) had undergone a unilateral TTA, and 1 (7.1%) underwent amputation in each of the following categories: bilateral TTA, unilateral TFA, and bilateral TFA each.
None of the 7 individuals with AIS E level SCI had undergone amputation.
Health Risk Factors
Of the 91 patients with amputation, the majority (81.3%) were either former or current smokers. Thirty-six percent of those who had undergone amputation had a diagnosis of DM, while only 21% of those who had not undergone amputation had a diagnosis of DM.
At the time of their annual examination 532 patients had a diagnosis of HTN while 523 patients did not. Among patients with amputations, 59 (64.8%) had HTN, while 32 (35.2%) did not. Of the 964 patients without amputation, the prevalence of HTN was 50.9%
.Of 1055 patients with SCI, only 103 (9.8%) had a PAD diagnosis, including 38 (41.9%) patients with amputation. Just 65 (6.7%) patients with SCI without amputation had PAD (P < .001). PAD is highly correlated with dysvascular causes of amputation. Among those with amputations due to dysvascular etiology, 50.0% (35/70) had PAD, but for the 21 amputations due to nondysvascular etiology, only 3 (14.3%) had PAD (P = .004).
Amputation Predictive Model
A multivariate logistic regression analysis was used to build a predictive model for amputation among patients with SCI while controlling for covariates. In our multivariate analysis, high-density lipoprotein cholesterol (HDL-C), tetraplegia, and PAD were predictive factors for amputation. Patients with SCI who had PAD were 8.6 times more likely to undergo amputation compared to those without PAD (odds ratio [OR], 9.8; P < .001; 95% CI, 5.9-16.3). Every unit of HDL-C decreased the odds of amputation by 5% (OR, 0.95; P < .001; 95% CI, 0.93-0.98).
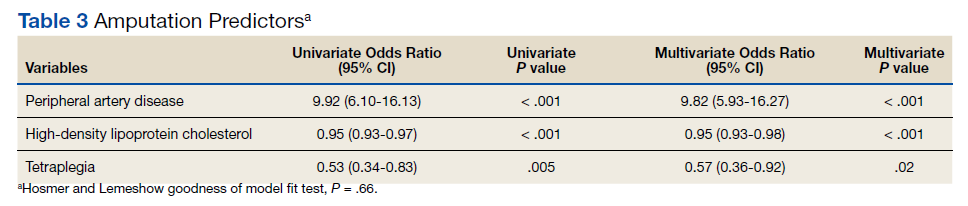
Having tetraplegia decreased the odds of amputation by 43%, compared with those with paraplegia (OR, 0.57; P = .02; 95% CI, 0.36 - 0.92). AUC was 0.76, and the Hosmer and Lemeshow goodness of model fit test P value was .66, indicating the good predictive power of the model (Table 3).
Discussion
In the US, 54 to 82% of amputations occur secondary to chronic vascular disease. Our study showed similar results: 76.1% of amputations were dysvascular.4,16 Even in a 2019 systematic review, the most recent prevalence of amputation data was in 2005.17 The study concluded that among the general population in the US, prevalence of amputation was estimated to be 1 in 190 people, or about 0.5% of the population.4 We found that the prevalence of amputation among the SCI population in this study was 8.7%. This result is consistent with our initial hypothesis that the prevalence of amputation would be higher among the people with SCI. Using a different case acquisition method, Svircev and colleagues reported that about a 4% prevalence of LE amputation among veterans with chronic SCI (over 1 year from the initial SCI), with an emphasis that it was not a study of amputation incidence.18 In comparison, we calculated a 7.5% prevalence of amputation during the chronic SCI stage, which showed institutional variation and a consistent observation that LE amputations occurred more frequently in the SCI population.
Our results showed a positive correlation between the completeness of injury and the prevalence of amputation. Those individuals with a motor complete injury, AIS A (40.3%) or AIS B (11.7%) account for approximately half of all amputations in our population with SCI. Another finding was that proximal amputations were more frequent with more neurologically complete SCIs. Of those with an injury classified as AIS A and an amputation, 42 of 49 subjects underwent at least 1 TFA (23 were unilateral TFA, 17 were bilateral TFA, 2 were a TFA/TTA combination). Of those with an AIS B injury and an amputation, 11 of 16 subjects (68.8%) had at least 1 TFA (5 unilateral TFA and 6 bilateral TFA). Among patients with AIS C injury and amputation, 75% had a TFA. At the same time, only 13.3% of all amputations were at the transfemoral level in those with an AIS D injury. None of the participants with an injury classified as AIS E had undergone an amputation.
Given a paucity of literature available regarding amputation levels in patients with SCI, a discussion with a JAHVH vascular surgeon helped explain the rationale behind different levels of amputation among the SCI population—TFA was performed in 64 of 91 cases (70%). Institutionally, TFAs were performed more often because this level had the greatest chance of healing, avoiding infection, and eliminating knee contracture issues, which may affect quality of life. This was believed to be the best option in those individuals who were already nonambulatory. Although this study did not collect data on ambulatory status, this helps explain why those with an SCI classification of AIS D were more likely to have had a more distal amputation to preserve current or a future chance of ambulation, provided that whether the limb is salvageable is the priority of surgical decision.
The prevalence of PAD among veterans is generally higher than it is in the nonveteran population. Studies show that the prevalence of PAD risk factors in the veteran population exceeds national estimates. Nearly two-thirds of veterans have HTN, 1 in 4 has DM, and 1 in 4 is a current smoker, placing veterans at a significantly increased risk of PADand, therefore, amputation.19,20 These rates were about the same or greater in our SCI population: 50.4% had HTN, 22.3% had a diagnosis of DM, and 71.8% smoked previously or currently smoked. In 3 large studies, HTN was second only to current smoking as the most attributable risk factor for PAD.21
Ongoing research by JAHVH vascular surgeons suggests that patients with SCI were younger and less likely to have HTN, PAD, and/or CAD compared with patients undergoing TFA without SCI. Additionally, patients with SCI had better postoperative outcomes in terms of 30-day mortality, 3-year mortality, and had no increased rate of surgical revisions, strokes, or wound-healing complications. This supports the previous thought that the AIS classification plays a large role in determining amputation levels.
One result in this study is that paraplegia is one of the predictors of future amputation compared with tetraplegia. To our knowledge, there is no literature that supports or explains this finding. A hypothetical factor that could explain this observation is the difference in duration of survival—those with paraplegia who live longer are more likely to experience end-stage consequence of vascular diseases. Another proposed factor is that those with paraplegia are generally more active and have a higher likelihood of sustaining a traumatic cause of amputation, even though this etiology of amputation is minor.An unexpected finding in our study was that of 1055 patients with SCI, only 9.8% had a PAD diagnosis. In contrast, 41.3% of those with amputation had a PAD diagnosis. JAHVH does not screen for PAD, so this likely represents only the symptomatic cases.
Diagnosing PAD in patients with SCI is challenging as they may lack classic clinical symptoms, such as pain with ambulation and impotence, secondary to their neurologic injury. Instead, the health care practitioner must rely on physical signs, such as necrosis.22 Of note given the undetermined utility of diagnosing PAD in patients with SCI, early endovascular interventions are not typically performed. We could not find literature regarding when intervention for PAD in patients with SCI should be performed or how frequently those with SCI should be assessed for PAD. One study showed impaired ambulation prior to limb salvage procedures was associated with poor functional outcomes in terms of survival, independent living, and ambulatory status.23 This could help explain why endovascular procedures are done relatively infrequently in this population. With the lack of studies regarding PAD in the SCI population, outcomes analysis of these patients, including the rate of initial interventions, re-intervention for re-amputation (possibly at a higher level), or vascular inflow procedures, are needed.
It would be beneficial for future studies to examine whether inflammatory markers, such as C-reactive protein (CRP), were more elevated in patients with SCI who underwent amputation compared with those who did not. Chronic underlying inflammation has been shown to be a risk factor for PAD. One study showed that, independently of other risk factors, elevated CRP levels roughly tripled the risk of developing PAD.24 This study suggested that there is an increased risk of dysvascular amputation among the SCI population at this center. This information is significant because it can help influence JAHVH clinical practice for veterans with SCI and vascular diseases.
Limitations
As a single-center study carried out at an SCI specialized center of a VA hospital, this study's finding may not be generalizable. Incomplete documentation in the health record may have led to underreporting of amputations and other information. The practice of the vascular surgeons at JAHVH may not represent the approach of vascular surgeons nationwide. Another limitation of this study is that the duration of SCI was not considered when looking at health risk factors associated with amputation in the SCI population (ie, total cholesterol, hemoglobin A1c, etc). Finally, the medication regimens were not reviewed to determine whether they meet the standard of care in relation to eventual diagnosis of PAD.
A prospective study comparing the prevalence of amputation between veterans with SCI vs veterans without SCI could better investigate the difference in amputation risks. This study only compared our veterans with SCI in reference to the general population. Veterans are more likely to be smokers than the general population, contributing to PAD.17 In addition, data regarding patients’ functional status in regard to transferring and ambulation before and after amputation were not collected, which would have contributed to an understanding of how amputation affects functional status in this population.
Conclusions
There is an increased prevalence of amputation among veterans with SCI compared with that of the nationwide population and a plurality were TFAs. This data suggest that those with a motor complete SCI are more likely to undergo a more proximal amputation. This is likely secondary to a lower likelihood of ambulation with more neurologically complete injuries along with a greater chance of healing with a more proximal amputation. It is challenging to correlate any variables specific to SCI (ie, immobility, time since injury, level of injury, etc) with an increased risk of amputation as the known comorbidities associated with PAD are highly prevalent in this population. Having PAD, low HDL-C (< 40 mg/dL), and paraplegia instead of tetraplegia were independent predictors of amputation.
Health care professionals need to be aware of the high prevalence of amputation in the SCI population. Comorbidities should be aggressively treated as PAD, in addition to being associated with amputation, has been linked with increased mortality.25 Studies using a larger population and multiple centers are needed to confirm such a concerning finding.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital (JAHVH). Authors gratefully acknowledge the inputs and support of Dr. James Brooks, MD, RPVI, assistant professor of surgery, University of South Florida (USF), and attending surgeon, vascular surgery service, medical director of the peripheral vascular laboratory, JAHVH; and Dr. Kevin White, MD, assistant professor, USF, and Chief of Spinal Cord Injury Center, JAHVH.
1. Hopman MT, Nommensen E, van Asten WN, Oeseburg B, Binkhorst RA. Properties of the venous vascular system in the lower extremities of individuals with paraplegia. Paraplegia. 1994;32(12):810-816. doi:10.1038/sc.1994.128
2. Theisen D, Vanlandewijck Y, Sturbois X, Francaux M. Central and peripheral haemodynamics in individuals with paraplegia during light and heavy exercise. J Rehabil Med. 2001;33(1):16-20. doi:10.1080/165019701300006489
3. Bell JW, Chen D, Bahls M, Newcomer SC. Evidence for greater burden of peripheral arterial disease in lower extremity arteries of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2011;301(3):H766-H772. doi:10.1152/ajpheart.00507.2011
4. Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
5. Hennion DR, Siano KA. Diagnosis and treatment of peripheral arterial disease. Am Fam Physician. 2013;88(5):306-310.
6. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738-743. doi:10.1161/01.CIR.0000137913.26087.F0
7. Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749-756. doi:10.1016/0026-0495(94)90126-0
8. Jörgensen S, Hill M, Lexell J. Cardiovascular risk factors among older adults with long-term spinal cord injury. PM R. 2019;11(1):8-16. doi:10.1016/j.pmrj.2018.06.008
9. Wu JC, Chen YC, Liu L, et al. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology. 2012;78(14):1051-1057. doi:10.1212/WNL.0b013e31824e8eaa
10. Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20(5):344-353. doi:10.1053/euhj.1998.1194
11. Bell JW, Chen D, Bahls M, Newcomer SC. Altered resting hemodynamics in lower-extremity arteries of individuals with spinal cord injury. J Spinal Cord Med. 2013;36(2):104-111. doi:10.1179/2045772312Y.0000000052
12. Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med. 2009;32(1):72-78. doi:10.1080/10790268.2009.11760755
13. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23(4):554-566. doi:10.1161/01.ATV.0000060460.52916.D6
14. Ephraim PL, Dillifngham TR, Sector M, Pezzin LE, MacKenzie EJ. Epidemiology of limb loss and congenital limb deficiency: a review of the literature. Arch Phys Med Rehabil. 2003;84(5): 747-761. doi:10.1016/s0003-9993(02)04932-8.15. Levin ME. Preventing amputation in the patient with diabetes. Diabetes Care. 1995;18(10)1383-1394. doi:10.2337/diacare.18.10.1383
16. Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95(8):875-883. doi:10.1097/00007611- 200208000-00018
17. Lo J, Chan L, Flynn S. A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: a 2019 update. Arch Phys Med Rehabil. 2021;102:115-131. doi:10.1016/j.apmr.2020.04.001
18. Svircev, J, Tan D, Garrison A, Pennelly, B, Burns SP. Limb loss in individuals with chronic spinal cord injury. J Spinal Cord Med. doi:10.1080/10790268.2020.1800964
19. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
20. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276. doi:10.1111/j.1532-5415.2004.52355.x
21. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509-1526. doi:10.1161/CIRCRESAHA.116.303849
22. Yokoo KM, Kronon M, Lewis VL Jr, McCarthy WJ, McMillan WD, Meyer PR Jr. Peripheral vascular disease in spinal cord injury patients: a difficult diagnosis. Ann Plast Surg. 1996;37(5):495-499. doi:10.1097/00000637-199611000-00007
23. Taylor SM, Kalbaugh CA, Blackhurst DW, Cass, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44(4):747–756. doi:10.1016/j.jvs.2006.06.015
24. Abdellaoui A, Al-Khaffaf H. C-reactive protein (CRP) as a marker in peripheral vascular disease. Eur J Vasc Endovasc Surg. 2007;34(1):18-22. doi:10.1016/j.ejvs.2006.10.040
25. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord. 2005;5:14. doi:10.1186/1471-2261-5-14
At the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, the prevalence of amputations among patients at the spinal cord injury (SCI) center seems high. Despite limited data demonstrating altered hemodynamics in the lower extremities (LEs) among the SCI population and increased frequency of peripheral arterial disease (PAD), amputations among patients with SCI have received little attention in research.1-3
In the United States, most amputations are caused by vascular disease related to peripheral arterial disease (PAD) and diabetes mellitus (DM).4 PAD primarily affects the LEs and is caused by atherosclerotic obstruction leading to insufficient blood flow. PAD can present clinically as LE pain, nonhealing ulcers, nonpalpable distal pulses, shiny or cold skin, absence of hair on the LE, or distal extremity pallor when the affected extremity is elevated. However, PAD is often asymptomatic. The diagnosis of PAD is typically made with an ankle-brachial index (ABI) ≤ 0.9.5 The prevalence of PAD is about 4.3% in Americans aged ≥ 40 years, increases with age, and is almost twice as common among Black Americans compared with that of White Americans.6 Many studies in SCI populations have documented an increased prevalence of DM, dyslipidemia, obesity, hypertension (HTN), and cigarette smoking.7-9 PAD shares these risk factors with coronary artery disease (CAD), but relative to CAD, tobacco smoking was a more substantial causative factor for PAD.10 Given the preponderance of associated risk factors in this population, PAD is likely more prevalent among patients with SCI than in the population without disabilities. Beyond these known risk factors, researchers hypothesized that SCI contributes to vascular disease by altering arterial function. However, this is still a topic of debate.11-13 Trauma also is a common cause of amputation, accounting for 45% of amputations in 2005.4 Patients with SCI may experience traumatic amputations simultaneously as their SCI, but they may also be predisposed to traumatic amputations related to osteopenia and impaired sensation.
Since amputation is an invasive surgery, knowing the severity of this issue is important in the SCI population. This study quantifies the prevalence of amputations of the LEs among the patients at our SCI center. It then characterizes these amputations’ etiology, their relationship with medical comorbidities, and certain SCI classifications.
Methods
This retrospective cohort study used the US Department of Veterans Affairs (VA) Computerized Patient Record System. The cohort was defined as all patients who received an annual examination at our SCI center over 4 years from October 1, 2009 to September 30, 2013. Annual examination includes a physical examination, relevant surveillance laboratory tests, and imaging, such as renal ultrasound for those with indwelling urinary catheters. One characteristic of the patient population in the VA system is that diagnoses, such as multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS), that involve spinal cord lesions causing symptoms are included in the registry, besides those with other traumatic or nontraumatic SCI. October 1 to September 30 was chosen based on the VA fiscal year (FY).
During this period, 1678 patients had an annual examination. Of those, 299 patients had an SCI etiology of ALS or MS, and 41 had nonfocal SCI etiology that could not be assessed using the American Spinal Injury Association Impairment Scale (AIS) and were excluded. Also excluded were 283 patients who did not have an annual examination during the specified time span. Some patients do not have an annual examination every year; for those with multiple annual examinations during that time frame, the most recent was used.
One thousand fifty-five patients were included in the statistical analysis. Date of birth, sex, race, ethnicity, date of death, smoking status, DM diagnosis, HTN diagnosis, use of an antiplatelet, antihypertensive, or lipid-lowering agent, blood pressure, hemoglobin A1c, and lipid panel were collected. The amputation level and etiology were noted. The levels of amputation were classified as toe/partial foot,
Statistical Analysis
Descriptive data were summarized as the median and IQR for continuous variables or the number and percentage for categorical variables. The χ2 test was used to analyze the association between categorical variables and amputation status. A nonparametric Wilcoxon test was used to investigate the distribution of continuous variables across patients with amputation and patients without amputation. Binary logistic regression analysis was used to investigate amputation risk factors. We report goodness of fit using the Hosmer and Lemeshow test and the area under the curve (AUC) for the multivariate model. Statistical significance was prespecified at a 2-sided P < .05. SAS version 9.4 was used for all statistical analyses.
Results
Mean age was approximately 61 years for the 91 patients at the time of the most recent amputation (Table 1). Among those with amputation, 63% were paraplegic and 37% were tetraplegic.
Of 1055 patients with SCI, 91 (8.6%) patients had an amputation. Of those, 70 (76.1%) were from nontraumatic causes (dysvascular), 17 (18.5%) were traumatic, 4 (4.3%) were from other causes (ie, cancer), and only 1 (1.1%) was of unknown cause.
Of the 91 patients with amputation, 64 (69.6%) had at least 1 TFA—33 were unilateral and 31 were bilateral. Two patients had a TFA on one side and a TTA on the other. Partial foot/toe and TTA were less common amputation levels with 14 (15.4%) and 13 (14.3%), respectively. Most amputations (86.8%) occurred over 6 months from the day of initial SCI, and were most commonly dysvascular (Table 2). Traumatic amputation occurred more evenly at various stages, pre-SCI, during acute SCI, subacute SCI, and chronic SCI.
Injury by Impairment Scale Level
Forty-nine (11.5%) of 426 patients with AIS level A SCI had undergone amputation. In order of prevalence, 23 (46.9%) were unilateral TFA, 17 (34.6%) were bilateral TFA, 10.2% were partial foot/toe, 4.1% were unilateral TTA, and 4.1% were a TTA/TFA combination. Both hip and knee disarticulations were classified in the TFA category.
Sixteen (13.0%) of 123 patients with AIS level B SCI had undergone amputation; 5 (31.3%) of those amputations were unilateral TFA, 6 (37.5%) were bilateral TFA, 3 (18.8%) were partial toe or foot, and 1 (6.3%) was for unilateral and bilateral TTA each.
Twelve (8.4%) of 143 patients with AIS level C SCI had undergone amputation: 6 (50.0%) were bilateral TFA; 3 (25.0%) were unilateral TFA; and 3 (25.0%) were unilateral TTA.
Fourteen (3.9%) of 356 patients with AIS level D SCI had undergone amputation. Of those 6 (42.9%) underwent a partial foot/toe amputation; 5 (35.7%) had undergone a unilateral TTA, and 1 (7.1%) underwent amputation in each of the following categories: bilateral TTA, unilateral TFA, and bilateral TFA each.
None of the 7 individuals with AIS E level SCI had undergone amputation.
Health Risk Factors
Of the 91 patients with amputation, the majority (81.3%) were either former or current smokers. Thirty-six percent of those who had undergone amputation had a diagnosis of DM, while only 21% of those who had not undergone amputation had a diagnosis of DM.
At the time of their annual examination 532 patients had a diagnosis of HTN while 523 patients did not. Among patients with amputations, 59 (64.8%) had HTN, while 32 (35.2%) did not. Of the 964 patients without amputation, the prevalence of HTN was 50.9%
.Of 1055 patients with SCI, only 103 (9.8%) had a PAD diagnosis, including 38 (41.9%) patients with amputation. Just 65 (6.7%) patients with SCI without amputation had PAD (P < .001). PAD is highly correlated with dysvascular causes of amputation. Among those with amputations due to dysvascular etiology, 50.0% (35/70) had PAD, but for the 21 amputations due to nondysvascular etiology, only 3 (14.3%) had PAD (P = .004).
Amputation Predictive Model
A multivariate logistic regression analysis was used to build a predictive model for amputation among patients with SCI while controlling for covariates. In our multivariate analysis, high-density lipoprotein cholesterol (HDL-C), tetraplegia, and PAD were predictive factors for amputation. Patients with SCI who had PAD were 8.6 times more likely to undergo amputation compared to those without PAD (odds ratio [OR], 9.8; P < .001; 95% CI, 5.9-16.3). Every unit of HDL-C decreased the odds of amputation by 5% (OR, 0.95; P < .001; 95% CI, 0.93-0.98).

Having tetraplegia decreased the odds of amputation by 43%, compared with those with paraplegia (OR, 0.57; P = .02; 95% CI, 0.36 - 0.92). AUC was 0.76, and the Hosmer and Lemeshow goodness of model fit test P value was .66, indicating the good predictive power of the model (Table 3).
Discussion
In the US, 54 to 82% of amputations occur secondary to chronic vascular disease. Our study showed similar results: 76.1% of amputations were dysvascular.4,16 Even in a 2019 systematic review, the most recent prevalence of amputation data was in 2005.17 The study concluded that among the general population in the US, prevalence of amputation was estimated to be 1 in 190 people, or about 0.5% of the population.4 We found that the prevalence of amputation among the SCI population in this study was 8.7%. This result is consistent with our initial hypothesis that the prevalence of amputation would be higher among the people with SCI. Using a different case acquisition method, Svircev and colleagues reported that about a 4% prevalence of LE amputation among veterans with chronic SCI (over 1 year from the initial SCI), with an emphasis that it was not a study of amputation incidence.18 In comparison, we calculated a 7.5% prevalence of amputation during the chronic SCI stage, which showed institutional variation and a consistent observation that LE amputations occurred more frequently in the SCI population.
Our results showed a positive correlation between the completeness of injury and the prevalence of amputation. Those individuals with a motor complete injury, AIS A (40.3%) or AIS B (11.7%) account for approximately half of all amputations in our population with SCI. Another finding was that proximal amputations were more frequent with more neurologically complete SCIs. Of those with an injury classified as AIS A and an amputation, 42 of 49 subjects underwent at least 1 TFA (23 were unilateral TFA, 17 were bilateral TFA, 2 were a TFA/TTA combination). Of those with an AIS B injury and an amputation, 11 of 16 subjects (68.8%) had at least 1 TFA (5 unilateral TFA and 6 bilateral TFA). Among patients with AIS C injury and amputation, 75% had a TFA. At the same time, only 13.3% of all amputations were at the transfemoral level in those with an AIS D injury. None of the participants with an injury classified as AIS E had undergone an amputation.
Given a paucity of literature available regarding amputation levels in patients with SCI, a discussion with a JAHVH vascular surgeon helped explain the rationale behind different levels of amputation among the SCI population—TFA was performed in 64 of 91 cases (70%). Institutionally, TFAs were performed more often because this level had the greatest chance of healing, avoiding infection, and eliminating knee contracture issues, which may affect quality of life. This was believed to be the best option in those individuals who were already nonambulatory. Although this study did not collect data on ambulatory status, this helps explain why those with an SCI classification of AIS D were more likely to have had a more distal amputation to preserve current or a future chance of ambulation, provided that whether the limb is salvageable is the priority of surgical decision.
The prevalence of PAD among veterans is generally higher than it is in the nonveteran population. Studies show that the prevalence of PAD risk factors in the veteran population exceeds national estimates. Nearly two-thirds of veterans have HTN, 1 in 4 has DM, and 1 in 4 is a current smoker, placing veterans at a significantly increased risk of PADand, therefore, amputation.19,20 These rates were about the same or greater in our SCI population: 50.4% had HTN, 22.3% had a diagnosis of DM, and 71.8% smoked previously or currently smoked. In 3 large studies, HTN was second only to current smoking as the most attributable risk factor for PAD.21
Ongoing research by JAHVH vascular surgeons suggests that patients with SCI were younger and less likely to have HTN, PAD, and/or CAD compared with patients undergoing TFA without SCI. Additionally, patients with SCI had better postoperative outcomes in terms of 30-day mortality, 3-year mortality, and had no increased rate of surgical revisions, strokes, or wound-healing complications. This supports the previous thought that the AIS classification plays a large role in determining amputation levels.
One result in this study is that paraplegia is one of the predictors of future amputation compared with tetraplegia. To our knowledge, there is no literature that supports or explains this finding. A hypothetical factor that could explain this observation is the difference in duration of survival—those with paraplegia who live longer are more likely to experience end-stage consequence of vascular diseases. Another proposed factor is that those with paraplegia are generally more active and have a higher likelihood of sustaining a traumatic cause of amputation, even though this etiology of amputation is minor.An unexpected finding in our study was that of 1055 patients with SCI, only 9.8% had a PAD diagnosis. In contrast, 41.3% of those with amputation had a PAD diagnosis. JAHVH does not screen for PAD, so this likely represents only the symptomatic cases.
Diagnosing PAD in patients with SCI is challenging as they may lack classic clinical symptoms, such as pain with ambulation and impotence, secondary to their neurologic injury. Instead, the health care practitioner must rely on physical signs, such as necrosis.22 Of note given the undetermined utility of diagnosing PAD in patients with SCI, early endovascular interventions are not typically performed. We could not find literature regarding when intervention for PAD in patients with SCI should be performed or how frequently those with SCI should be assessed for PAD. One study showed impaired ambulation prior to limb salvage procedures was associated with poor functional outcomes in terms of survival, independent living, and ambulatory status.23 This could help explain why endovascular procedures are done relatively infrequently in this population. With the lack of studies regarding PAD in the SCI population, outcomes analysis of these patients, including the rate of initial interventions, re-intervention for re-amputation (possibly at a higher level), or vascular inflow procedures, are needed.
It would be beneficial for future studies to examine whether inflammatory markers, such as C-reactive protein (CRP), were more elevated in patients with SCI who underwent amputation compared with those who did not. Chronic underlying inflammation has been shown to be a risk factor for PAD. One study showed that, independently of other risk factors, elevated CRP levels roughly tripled the risk of developing PAD.24 This study suggested that there is an increased risk of dysvascular amputation among the SCI population at this center. This information is significant because it can help influence JAHVH clinical practice for veterans with SCI and vascular diseases.
Limitations
As a single-center study carried out at an SCI specialized center of a VA hospital, this study's finding may not be generalizable. Incomplete documentation in the health record may have led to underreporting of amputations and other information. The practice of the vascular surgeons at JAHVH may not represent the approach of vascular surgeons nationwide. Another limitation of this study is that the duration of SCI was not considered when looking at health risk factors associated with amputation in the SCI population (ie, total cholesterol, hemoglobin A1c, etc). Finally, the medication regimens were not reviewed to determine whether they meet the standard of care in relation to eventual diagnosis of PAD.
A prospective study comparing the prevalence of amputation between veterans with SCI vs veterans without SCI could better investigate the difference in amputation risks. This study only compared our veterans with SCI in reference to the general population. Veterans are more likely to be smokers than the general population, contributing to PAD.17 In addition, data regarding patients’ functional status in regard to transferring and ambulation before and after amputation were not collected, which would have contributed to an understanding of how amputation affects functional status in this population.
Conclusions
There is an increased prevalence of amputation among veterans with SCI compared with that of the nationwide population and a plurality were TFAs. This data suggest that those with a motor complete SCI are more likely to undergo a more proximal amputation. This is likely secondary to a lower likelihood of ambulation with more neurologically complete injuries along with a greater chance of healing with a more proximal amputation. It is challenging to correlate any variables specific to SCI (ie, immobility, time since injury, level of injury, etc) with an increased risk of amputation as the known comorbidities associated with PAD are highly prevalent in this population. Having PAD, low HDL-C (< 40 mg/dL), and paraplegia instead of tetraplegia were independent predictors of amputation.
Health care professionals need to be aware of the high prevalence of amputation in the SCI population. Comorbidities should be aggressively treated as PAD, in addition to being associated with amputation, has been linked with increased mortality.25 Studies using a larger population and multiple centers are needed to confirm such a concerning finding.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital (JAHVH). Authors gratefully acknowledge the inputs and support of Dr. James Brooks, MD, RPVI, assistant professor of surgery, University of South Florida (USF), and attending surgeon, vascular surgery service, medical director of the peripheral vascular laboratory, JAHVH; and Dr. Kevin White, MD, assistant professor, USF, and Chief of Spinal Cord Injury Center, JAHVH.
At the James A. Haley Veterans’ Hospital (JAHVH) in Tampa, Florida, the prevalence of amputations among patients at the spinal cord injury (SCI) center seems high. Despite limited data demonstrating altered hemodynamics in the lower extremities (LEs) among the SCI population and increased frequency of peripheral arterial disease (PAD), amputations among patients with SCI have received little attention in research.1-3
In the United States, most amputations are caused by vascular disease related to peripheral arterial disease (PAD) and diabetes mellitus (DM).4 PAD primarily affects the LEs and is caused by atherosclerotic obstruction leading to insufficient blood flow. PAD can present clinically as LE pain, nonhealing ulcers, nonpalpable distal pulses, shiny or cold skin, absence of hair on the LE, or distal extremity pallor when the affected extremity is elevated. However, PAD is often asymptomatic. The diagnosis of PAD is typically made with an ankle-brachial index (ABI) ≤ 0.9.5 The prevalence of PAD is about 4.3% in Americans aged ≥ 40 years, increases with age, and is almost twice as common among Black Americans compared with that of White Americans.6 Many studies in SCI populations have documented an increased prevalence of DM, dyslipidemia, obesity, hypertension (HTN), and cigarette smoking.7-9 PAD shares these risk factors with coronary artery disease (CAD), but relative to CAD, tobacco smoking was a more substantial causative factor for PAD.10 Given the preponderance of associated risk factors in this population, PAD is likely more prevalent among patients with SCI than in the population without disabilities. Beyond these known risk factors, researchers hypothesized that SCI contributes to vascular disease by altering arterial function. However, this is still a topic of debate.11-13 Trauma also is a common cause of amputation, accounting for 45% of amputations in 2005.4 Patients with SCI may experience traumatic amputations simultaneously as their SCI, but they may also be predisposed to traumatic amputations related to osteopenia and impaired sensation.
Since amputation is an invasive surgery, knowing the severity of this issue is important in the SCI population. This study quantifies the prevalence of amputations of the LEs among the patients at our SCI center. It then characterizes these amputations’ etiology, their relationship with medical comorbidities, and certain SCI classifications.
Methods
This retrospective cohort study used the US Department of Veterans Affairs (VA) Computerized Patient Record System. The cohort was defined as all patients who received an annual examination at our SCI center over 4 years from October 1, 2009 to September 30, 2013. Annual examination includes a physical examination, relevant surveillance laboratory tests, and imaging, such as renal ultrasound for those with indwelling urinary catheters. One characteristic of the patient population in the VA system is that diagnoses, such as multiple sclerosis (MS) and amyotrophic lateral sclerosis (ALS), that involve spinal cord lesions causing symptoms are included in the registry, besides those with other traumatic or nontraumatic SCI. October 1 to September 30 was chosen based on the VA fiscal year (FY).
During this period, 1678 patients had an annual examination. Of those, 299 patients had an SCI etiology of ALS or MS, and 41 had nonfocal SCI etiology that could not be assessed using the American Spinal Injury Association Impairment Scale (AIS) and were excluded. Also excluded were 283 patients who did not have an annual examination during the specified time span. Some patients do not have an annual examination every year; for those with multiple annual examinations during that time frame, the most recent was used.
One thousand fifty-five patients were included in the statistical analysis. Date of birth, sex, race, ethnicity, date of death, smoking status, DM diagnosis, HTN diagnosis, use of an antiplatelet, antihypertensive, or lipid-lowering agent, blood pressure, hemoglobin A1c, and lipid panel were collected. The amputation level and etiology were noted. The levels of amputation were classified as toe/partial foot,
Statistical Analysis
Descriptive data were summarized as the median and IQR for continuous variables or the number and percentage for categorical variables. The χ2 test was used to analyze the association between categorical variables and amputation status. A nonparametric Wilcoxon test was used to investigate the distribution of continuous variables across patients with amputation and patients without amputation. Binary logistic regression analysis was used to investigate amputation risk factors. We report goodness of fit using the Hosmer and Lemeshow test and the area under the curve (AUC) for the multivariate model. Statistical significance was prespecified at a 2-sided P < .05. SAS version 9.4 was used for all statistical analyses.
Results
Mean age was approximately 61 years for the 91 patients at the time of the most recent amputation (Table 1). Among those with amputation, 63% were paraplegic and 37% were tetraplegic.
Of 1055 patients with SCI, 91 (8.6%) patients had an amputation. Of those, 70 (76.1%) were from nontraumatic causes (dysvascular), 17 (18.5%) were traumatic, 4 (4.3%) were from other causes (ie, cancer), and only 1 (1.1%) was of unknown cause.
Of the 91 patients with amputation, 64 (69.6%) had at least 1 TFA—33 were unilateral and 31 were bilateral. Two patients had a TFA on one side and a TTA on the other. Partial foot/toe and TTA were less common amputation levels with 14 (15.4%) and 13 (14.3%), respectively. Most amputations (86.8%) occurred over 6 months from the day of initial SCI, and were most commonly dysvascular (Table 2). Traumatic amputation occurred more evenly at various stages, pre-SCI, during acute SCI, subacute SCI, and chronic SCI.
Injury by Impairment Scale Level
Forty-nine (11.5%) of 426 patients with AIS level A SCI had undergone amputation. In order of prevalence, 23 (46.9%) were unilateral TFA, 17 (34.6%) were bilateral TFA, 10.2% were partial foot/toe, 4.1% were unilateral TTA, and 4.1% were a TTA/TFA combination. Both hip and knee disarticulations were classified in the TFA category.
Sixteen (13.0%) of 123 patients with AIS level B SCI had undergone amputation; 5 (31.3%) of those amputations were unilateral TFA, 6 (37.5%) were bilateral TFA, 3 (18.8%) were partial toe or foot, and 1 (6.3%) was for unilateral and bilateral TTA each.
Twelve (8.4%) of 143 patients with AIS level C SCI had undergone amputation: 6 (50.0%) were bilateral TFA; 3 (25.0%) were unilateral TFA; and 3 (25.0%) were unilateral TTA.
Fourteen (3.9%) of 356 patients with AIS level D SCI had undergone amputation. Of those 6 (42.9%) underwent a partial foot/toe amputation; 5 (35.7%) had undergone a unilateral TTA, and 1 (7.1%) underwent amputation in each of the following categories: bilateral TTA, unilateral TFA, and bilateral TFA each.
None of the 7 individuals with AIS E level SCI had undergone amputation.
Health Risk Factors
Of the 91 patients with amputation, the majority (81.3%) were either former or current smokers. Thirty-six percent of those who had undergone amputation had a diagnosis of DM, while only 21% of those who had not undergone amputation had a diagnosis of DM.
At the time of their annual examination 532 patients had a diagnosis of HTN while 523 patients did not. Among patients with amputations, 59 (64.8%) had HTN, while 32 (35.2%) did not. Of the 964 patients without amputation, the prevalence of HTN was 50.9%
.Of 1055 patients with SCI, only 103 (9.8%) had a PAD diagnosis, including 38 (41.9%) patients with amputation. Just 65 (6.7%) patients with SCI without amputation had PAD (P < .001). PAD is highly correlated with dysvascular causes of amputation. Among those with amputations due to dysvascular etiology, 50.0% (35/70) had PAD, but for the 21 amputations due to nondysvascular etiology, only 3 (14.3%) had PAD (P = .004).
Amputation Predictive Model
A multivariate logistic regression analysis was used to build a predictive model for amputation among patients with SCI while controlling for covariates. In our multivariate analysis, high-density lipoprotein cholesterol (HDL-C), tetraplegia, and PAD were predictive factors for amputation. Patients with SCI who had PAD were 8.6 times more likely to undergo amputation compared to those without PAD (odds ratio [OR], 9.8; P < .001; 95% CI, 5.9-16.3). Every unit of HDL-C decreased the odds of amputation by 5% (OR, 0.95; P < .001; 95% CI, 0.93-0.98).

Having tetraplegia decreased the odds of amputation by 43%, compared with those with paraplegia (OR, 0.57; P = .02; 95% CI, 0.36 - 0.92). AUC was 0.76, and the Hosmer and Lemeshow goodness of model fit test P value was .66, indicating the good predictive power of the model (Table 3).
Discussion
In the US, 54 to 82% of amputations occur secondary to chronic vascular disease. Our study showed similar results: 76.1% of amputations were dysvascular.4,16 Even in a 2019 systematic review, the most recent prevalence of amputation data was in 2005.17 The study concluded that among the general population in the US, prevalence of amputation was estimated to be 1 in 190 people, or about 0.5% of the population.4 We found that the prevalence of amputation among the SCI population in this study was 8.7%. This result is consistent with our initial hypothesis that the prevalence of amputation would be higher among the people with SCI. Using a different case acquisition method, Svircev and colleagues reported that about a 4% prevalence of LE amputation among veterans with chronic SCI (over 1 year from the initial SCI), with an emphasis that it was not a study of amputation incidence.18 In comparison, we calculated a 7.5% prevalence of amputation during the chronic SCI stage, which showed institutional variation and a consistent observation that LE amputations occurred more frequently in the SCI population.
Our results showed a positive correlation between the completeness of injury and the prevalence of amputation. Those individuals with a motor complete injury, AIS A (40.3%) or AIS B (11.7%) account for approximately half of all amputations in our population with SCI. Another finding was that proximal amputations were more frequent with more neurologically complete SCIs. Of those with an injury classified as AIS A and an amputation, 42 of 49 subjects underwent at least 1 TFA (23 were unilateral TFA, 17 were bilateral TFA, 2 were a TFA/TTA combination). Of those with an AIS B injury and an amputation, 11 of 16 subjects (68.8%) had at least 1 TFA (5 unilateral TFA and 6 bilateral TFA). Among patients with AIS C injury and amputation, 75% had a TFA. At the same time, only 13.3% of all amputations were at the transfemoral level in those with an AIS D injury. None of the participants with an injury classified as AIS E had undergone an amputation.
Given a paucity of literature available regarding amputation levels in patients with SCI, a discussion with a JAHVH vascular surgeon helped explain the rationale behind different levels of amputation among the SCI population—TFA was performed in 64 of 91 cases (70%). Institutionally, TFAs were performed more often because this level had the greatest chance of healing, avoiding infection, and eliminating knee contracture issues, which may affect quality of life. This was believed to be the best option in those individuals who were already nonambulatory. Although this study did not collect data on ambulatory status, this helps explain why those with an SCI classification of AIS D were more likely to have had a more distal amputation to preserve current or a future chance of ambulation, provided that whether the limb is salvageable is the priority of surgical decision.
The prevalence of PAD among veterans is generally higher than it is in the nonveteran population. Studies show that the prevalence of PAD risk factors in the veteran population exceeds national estimates. Nearly two-thirds of veterans have HTN, 1 in 4 has DM, and 1 in 4 is a current smoker, placing veterans at a significantly increased risk of PADand, therefore, amputation.19,20 These rates were about the same or greater in our SCI population: 50.4% had HTN, 22.3% had a diagnosis of DM, and 71.8% smoked previously or currently smoked. In 3 large studies, HTN was second only to current smoking as the most attributable risk factor for PAD.21
Ongoing research by JAHVH vascular surgeons suggests that patients with SCI were younger and less likely to have HTN, PAD, and/or CAD compared with patients undergoing TFA without SCI. Additionally, patients with SCI had better postoperative outcomes in terms of 30-day mortality, 3-year mortality, and had no increased rate of surgical revisions, strokes, or wound-healing complications. This supports the previous thought that the AIS classification plays a large role in determining amputation levels.
One result in this study is that paraplegia is one of the predictors of future amputation compared with tetraplegia. To our knowledge, there is no literature that supports or explains this finding. A hypothetical factor that could explain this observation is the difference in duration of survival—those with paraplegia who live longer are more likely to experience end-stage consequence of vascular diseases. Another proposed factor is that those with paraplegia are generally more active and have a higher likelihood of sustaining a traumatic cause of amputation, even though this etiology of amputation is minor.An unexpected finding in our study was that of 1055 patients with SCI, only 9.8% had a PAD diagnosis. In contrast, 41.3% of those with amputation had a PAD diagnosis. JAHVH does not screen for PAD, so this likely represents only the symptomatic cases.
Diagnosing PAD in patients with SCI is challenging as they may lack classic clinical symptoms, such as pain with ambulation and impotence, secondary to their neurologic injury. Instead, the health care practitioner must rely on physical signs, such as necrosis.22 Of note given the undetermined utility of diagnosing PAD in patients with SCI, early endovascular interventions are not typically performed. We could not find literature regarding when intervention for PAD in patients with SCI should be performed or how frequently those with SCI should be assessed for PAD. One study showed impaired ambulation prior to limb salvage procedures was associated with poor functional outcomes in terms of survival, independent living, and ambulatory status.23 This could help explain why endovascular procedures are done relatively infrequently in this population. With the lack of studies regarding PAD in the SCI population, outcomes analysis of these patients, including the rate of initial interventions, re-intervention for re-amputation (possibly at a higher level), or vascular inflow procedures, are needed.
It would be beneficial for future studies to examine whether inflammatory markers, such as C-reactive protein (CRP), were more elevated in patients with SCI who underwent amputation compared with those who did not. Chronic underlying inflammation has been shown to be a risk factor for PAD. One study showed that, independently of other risk factors, elevated CRP levels roughly tripled the risk of developing PAD.24 This study suggested that there is an increased risk of dysvascular amputation among the SCI population at this center. This information is significant because it can help influence JAHVH clinical practice for veterans with SCI and vascular diseases.
Limitations
As a single-center study carried out at an SCI specialized center of a VA hospital, this study's finding may not be generalizable. Incomplete documentation in the health record may have led to underreporting of amputations and other information. The practice of the vascular surgeons at JAHVH may not represent the approach of vascular surgeons nationwide. Another limitation of this study is that the duration of SCI was not considered when looking at health risk factors associated with amputation in the SCI population (ie, total cholesterol, hemoglobin A1c, etc). Finally, the medication regimens were not reviewed to determine whether they meet the standard of care in relation to eventual diagnosis of PAD.
A prospective study comparing the prevalence of amputation between veterans with SCI vs veterans without SCI could better investigate the difference in amputation risks. This study only compared our veterans with SCI in reference to the general population. Veterans are more likely to be smokers than the general population, contributing to PAD.17 In addition, data regarding patients’ functional status in regard to transferring and ambulation before and after amputation were not collected, which would have contributed to an understanding of how amputation affects functional status in this population.
Conclusions
There is an increased prevalence of amputation among veterans with SCI compared with that of the nationwide population and a plurality were TFAs. This data suggest that those with a motor complete SCI are more likely to undergo a more proximal amputation. This is likely secondary to a lower likelihood of ambulation with more neurologically complete injuries along with a greater chance of healing with a more proximal amputation. It is challenging to correlate any variables specific to SCI (ie, immobility, time since injury, level of injury, etc) with an increased risk of amputation as the known comorbidities associated with PAD are highly prevalent in this population. Having PAD, low HDL-C (< 40 mg/dL), and paraplegia instead of tetraplegia were independent predictors of amputation.
Health care professionals need to be aware of the high prevalence of amputation in the SCI population. Comorbidities should be aggressively treated as PAD, in addition to being associated with amputation, has been linked with increased mortality.25 Studies using a larger population and multiple centers are needed to confirm such a concerning finding.
Acknowledgments
This material is based on work supported (or supported in part) with resources and the use of facilities at the James A. Haley Veterans’ Hospital (JAHVH). Authors gratefully acknowledge the inputs and support of Dr. James Brooks, MD, RPVI, assistant professor of surgery, University of South Florida (USF), and attending surgeon, vascular surgery service, medical director of the peripheral vascular laboratory, JAHVH; and Dr. Kevin White, MD, assistant professor, USF, and Chief of Spinal Cord Injury Center, JAHVH.
1. Hopman MT, Nommensen E, van Asten WN, Oeseburg B, Binkhorst RA. Properties of the venous vascular system in the lower extremities of individuals with paraplegia. Paraplegia. 1994;32(12):810-816. doi:10.1038/sc.1994.128
2. Theisen D, Vanlandewijck Y, Sturbois X, Francaux M. Central and peripheral haemodynamics in individuals with paraplegia during light and heavy exercise. J Rehabil Med. 2001;33(1):16-20. doi:10.1080/165019701300006489
3. Bell JW, Chen D, Bahls M, Newcomer SC. Evidence for greater burden of peripheral arterial disease in lower extremity arteries of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2011;301(3):H766-H772. doi:10.1152/ajpheart.00507.2011
4. Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
5. Hennion DR, Siano KA. Diagnosis and treatment of peripheral arterial disease. Am Fam Physician. 2013;88(5):306-310.
6. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738-743. doi:10.1161/01.CIR.0000137913.26087.F0
7. Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749-756. doi:10.1016/0026-0495(94)90126-0
8. Jörgensen S, Hill M, Lexell J. Cardiovascular risk factors among older adults with long-term spinal cord injury. PM R. 2019;11(1):8-16. doi:10.1016/j.pmrj.2018.06.008
9. Wu JC, Chen YC, Liu L, et al. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology. 2012;78(14):1051-1057. doi:10.1212/WNL.0b013e31824e8eaa
10. Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20(5):344-353. doi:10.1053/euhj.1998.1194
11. Bell JW, Chen D, Bahls M, Newcomer SC. Altered resting hemodynamics in lower-extremity arteries of individuals with spinal cord injury. J Spinal Cord Med. 2013;36(2):104-111. doi:10.1179/2045772312Y.0000000052
12. Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med. 2009;32(1):72-78. doi:10.1080/10790268.2009.11760755
13. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23(4):554-566. doi:10.1161/01.ATV.0000060460.52916.D6
14. Ephraim PL, Dillifngham TR, Sector M, Pezzin LE, MacKenzie EJ. Epidemiology of limb loss and congenital limb deficiency: a review of the literature. Arch Phys Med Rehabil. 2003;84(5): 747-761. doi:10.1016/s0003-9993(02)04932-8.15. Levin ME. Preventing amputation in the patient with diabetes. Diabetes Care. 1995;18(10)1383-1394. doi:10.2337/diacare.18.10.1383
16. Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95(8):875-883. doi:10.1097/00007611- 200208000-00018
17. Lo J, Chan L, Flynn S. A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: a 2019 update. Arch Phys Med Rehabil. 2021;102:115-131. doi:10.1016/j.apmr.2020.04.001
18. Svircev, J, Tan D, Garrison A, Pennelly, B, Burns SP. Limb loss in individuals with chronic spinal cord injury. J Spinal Cord Med. doi:10.1080/10790268.2020.1800964
19. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
20. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276. doi:10.1111/j.1532-5415.2004.52355.x
21. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509-1526. doi:10.1161/CIRCRESAHA.116.303849
22. Yokoo KM, Kronon M, Lewis VL Jr, McCarthy WJ, McMillan WD, Meyer PR Jr. Peripheral vascular disease in spinal cord injury patients: a difficult diagnosis. Ann Plast Surg. 1996;37(5):495-499. doi:10.1097/00000637-199611000-00007
23. Taylor SM, Kalbaugh CA, Blackhurst DW, Cass, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44(4):747–756. doi:10.1016/j.jvs.2006.06.015
24. Abdellaoui A, Al-Khaffaf H. C-reactive protein (CRP) as a marker in peripheral vascular disease. Eur J Vasc Endovasc Surg. 2007;34(1):18-22. doi:10.1016/j.ejvs.2006.10.040
25. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord. 2005;5:14. doi:10.1186/1471-2261-5-14
1. Hopman MT, Nommensen E, van Asten WN, Oeseburg B, Binkhorst RA. Properties of the venous vascular system in the lower extremities of individuals with paraplegia. Paraplegia. 1994;32(12):810-816. doi:10.1038/sc.1994.128
2. Theisen D, Vanlandewijck Y, Sturbois X, Francaux M. Central and peripheral haemodynamics in individuals with paraplegia during light and heavy exercise. J Rehabil Med. 2001;33(1):16-20. doi:10.1080/165019701300006489
3. Bell JW, Chen D, Bahls M, Newcomer SC. Evidence for greater burden of peripheral arterial disease in lower extremity arteries of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2011;301(3):H766-H772. doi:10.1152/ajpheart.00507.2011
4. Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008;89(3):422-429. doi:10.1016/j.apmr.2007.11.005
5. Hennion DR, Siano KA. Diagnosis and treatment of peripheral arterial disease. Am Fam Physician. 2013;88(5):306-310.
6. Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738-743. doi:10.1161/01.CIR.0000137913.26087.F0
7. Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749-756. doi:10.1016/0026-0495(94)90126-0
8. Jörgensen S, Hill M, Lexell J. Cardiovascular risk factors among older adults with long-term spinal cord injury. PM R. 2019;11(1):8-16. doi:10.1016/j.pmrj.2018.06.008
9. Wu JC, Chen YC, Liu L, et al. Increased risk of stroke after spinal cord injury: a nationwide 4-year follow-up cohort study. Neurology. 2012;78(14):1051-1057. doi:10.1212/WNL.0b013e31824e8eaa
10. Price JF, Mowbray PI, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relationship between smoking and cardiovascular risk factors in the development of peripheral arterial disease and coronary artery disease: Edinburgh Artery Study. Eur Heart J. 1999;20(5):344-353. doi:10.1053/euhj.1998.1194
11. Bell JW, Chen D, Bahls M, Newcomer SC. Altered resting hemodynamics in lower-extremity arteries of individuals with spinal cord injury. J Spinal Cord Med. 2013;36(2):104-111. doi:10.1179/2045772312Y.0000000052
12. Miyatani M, Masani K, Oh PI, Miyachi M, Popovic MR, Craven BC. Pulse wave velocity for assessment of arterial stiffness among people with spinal cord injury: a pilot study. J Spinal Cord Med. 2009;32(1):72-78. doi:10.1080/10790268.2009.11760755
13. Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23(4):554-566. doi:10.1161/01.ATV.0000060460.52916.D6
14. Ephraim PL, Dillifngham TR, Sector M, Pezzin LE, MacKenzie EJ. Epidemiology of limb loss and congenital limb deficiency: a review of the literature. Arch Phys Med Rehabil. 2003;84(5): 747-761. doi:10.1016/s0003-9993(02)04932-8.15. Levin ME. Preventing amputation in the patient with diabetes. Diabetes Care. 1995;18(10)1383-1394. doi:10.2337/diacare.18.10.1383
16. Dillingham TR, Pezzin LE, MacKenzie EJ. Limb amputation and limb deficiency: epidemiology and recent trends in the United States. South Med J. 2002;95(8):875-883. doi:10.1097/00007611- 200208000-00018
17. Lo J, Chan L, Flynn S. A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: a 2019 update. Arch Phys Med Rehabil. 2021;102:115-131. doi:10.1016/j.apmr.2020.04.001
18. Svircev, J, Tan D, Garrison A, Pennelly, B, Burns SP. Limb loss in individuals with chronic spinal cord injury. J Spinal Cord Med. doi:10.1080/10790268.2020.1800964
19. Brown DW. Smoking prevalence among US veterans. J Gen Intern Med. 2010;25(2):147-149. doi:10.1007/s11606-009-1160-0
20. Selim AJ, Berlowitz DR, Fincke G, et al. The health status of elderly veteran enrollees in the Veterans Health Administration. J Am Geriatr Soc. 2004;52(8):1271-1276. doi:10.1111/j.1532-5415.2004.52355.x
21. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509-1526. doi:10.1161/CIRCRESAHA.116.303849
22. Yokoo KM, Kronon M, Lewis VL Jr, McCarthy WJ, McMillan WD, Meyer PR Jr. Peripheral vascular disease in spinal cord injury patients: a difficult diagnosis. Ann Plast Surg. 1996;37(5):495-499. doi:10.1097/00000637-199611000-00007
23. Taylor SM, Kalbaugh CA, Blackhurst DW, Cass, et al. Determinants of functional outcome after revascularization for critical limb ischemia: an analysis of 1000 consecutive vascular interventions. J Vasc Surg. 2006;44(4):747–756. doi:10.1016/j.jvs.2006.06.015
24. Abdellaoui A, Al-Khaffaf H. C-reactive protein (CRP) as a marker in peripheral vascular disease. Eur J Vasc Endovasc Surg. 2007;34(1):18-22. doi:10.1016/j.ejvs.2006.10.040
25. Caro J, Migliaccio-Walle K, Ishak KJ, Proskorovsky I. The morbidity and mortality following a diagnosis of peripheral arterial disease: long-term follow-up of a large database. BMC Cardiovasc Disord. 2005;5:14. doi:10.1186/1471-2261-5-14
Exercise to Reduce Posttraumatic Stress Disorder Symptoms in Veterans
Physical exercise offers preventative and therapeutic benefits for a range of chronic health conditions, including cardiovascular disease, type 2 diabetes mellitus, Alzheimer disease, and depression.1,2 Exercise has been well studied for its antidepressant effects, its ability to reduce risk of aging-related dementia, and favorable effects on a range of cognitive functions.2 Lesser evidence exists regarding the impact of exercise on other mental health concerns. Therefore, an accurate understanding of whether physical exercise may ameliorate other conditions is important.
A small meta-analysis by Rosenbaum and colleagues found that exercise interventions were superior to control conditions for symptom reduction in study participants with posttraumatic stress disorder (PTSD).3 This meta-analysis included 4 randomized clinical trials representing 200 cases. The trial included a variety of physical activities (eg, yoga, aerobic, and strength-building exercises) and control conditions, and participants recruited from online, community, inpatient, and outpatient settings. The standardized mean difference (SMD) produced by the analysis indicated a small-to-medium effect (Hedges g, -0.35), with the authors reporting no evidence of publication bias, although an assessment of potential bias associated with individual trial design characteristics was not conducted. Of note, a meta-analysis by Watts and colleagues found that effect sizes for PTSD treatments tend to be smaller in veteran populations.4 Therefore, how much the mean effect size estimate in the study is applicable to veterans with PTSD is unknown.3
Veterans represent a unique subpopulation in which PTSD is common, although no meta-analysis yet published has synthesized the effects of exercise interventions from trials of veterans with PTSD.5 A recent systematic review by Whitworth and Ciccolo concluded that exercise may be associated with reduced risk of PTSD, a briefer course of PTSD symptoms, and/or reduced sleep- and depression-related difficulties.6 However, that review primarily included observational, cross-sectional, and qualitative works. No trials included in our meta-analysis were included in that review.6
Evidence-based psychotherapies like cognitive processing therapy and prolonged exposure have been shown to be effective for treating PTSD in veterans; however, these modalities are accompanied by high rates of dropout (eg, 40-60%), thereby limiting their clinical utility.7 The use of complementary and alternative approaches for treatment in the United States has increased in recent years, and exercise represents an important complementary treatment option.8 In a study by Baldwin and colleagues, nearly 50% of veterans reported using complementary or alternative approaches, and veterans with PTSD were among those likely to use such approaches.9 However, current studies of the effects of exercise interventions on PTSD symptom reduction are mostly small and varied, making determinations difficult regarding the potential utility of exercise for treating this condition in veterans.
Literature Search
No previous research has synthesized the literature on the effects of exercise on PTSD in the veteran population. The current meta-analysis aims to provide a synthesis of systematically selected studies on this topic to determine whether exercise-based interventions are effective at reducing veterans’ symptoms of PTSD. Our hypothesis was that, when used as a primary or adjuvant intervention for PTSD, physical exercise would be associated with a reduction of PTSD symptom scale scores. We planned a priori to produce separate estimates for single-arm and multi-arm trials. We also wanted to conduct a careful risk of bias assessment—or evaluation of study features that may have systematically influenced results—for included trials, not only to provide context for interpretation of results, but also to inform suggestions for research to advance this field of inquiry.10
Methods
This study was preregistered on PROSPERO and followed PRISMA guidelines for meta-analyses and systematic reviews.11 Supplementary materials, such as the PRISMA checklist, study data, and funnel plots, are available online (doi.org/10.6084/m9.figshare.c.5618437.v1). Conference abstracts were omitted due to a lack of necessary information. We decided early in the planning process to include both randomized and single-arm trials, expecting the number of completed studies in the area of exercise for PTSD symptom reduction in veterans, and particularly randomized trials of such, would be relatively small.
Studies were included if they met the following criteria: (1) the study was a single- or multi-arm interventional trial; (2) participants were veterans; (3) participants had a current diagnosis of PTSD or exhibited subthreshold PTSD symptoms, as established by authors of the individual studies and supported by a structured clinical interview, semistructured interview, or elevated scores on PTSD symptom self-report measures; (4) the study included an intervention in which exercise (physical activity that is planned, structured, repetitive, and purposive in the sense that improvement or maintenance of physical fitness or health is an objective) was the primary component; (5) PTSD symptom severity was by a clinician-rated or self-report measure; and (6) the study was published in a peer-reviewed journal.12 Studies were excluded if means, standard deviations, and sample sizes were not available or the full text of the study was not available in English.
The systematic review was conducted using PubMed, PsycINFO, and Cochrane Library databases, from the earliest record to February 2021. The following search phrase was used, without additional limits, to acquire a list of potential studies: (“PTSD” or “post-traumatic stress disorder” or “posttraumatic stress disorder” or “post traumatic stress disorder”) and (“veteran” or “veterans”) and (“exercise” or “aerobic” or “activity” or “physical activity”). The references of identified publications also were searched for additional studies. Then, study titles and abstracts were evaluated and finally, full texts were evaluated to determine study inclusion. All screening, study selection, and risk of bias and data extraction activities were performed by 2 independent reviewers (DR and MJ) with disagreements resolved through discussion and consensus (Figure 1). A list of studies excluded during full-text review and rationales can be viewed online (doi.org/10.6084/m9.figshare.c.5618437.v1).
Data Collection
Data were extracted from included studies using custom forms and included the following information based on PRISMA guidelines: (1) study design characteristics; (2) intervention details; and (3) PTSD outcome information.11 PTSD symptom severity was the primary outcome of interest. Outcome data were included if they were derived from a measure of PTSD symptoms—equivalency across measures was assumed for meta-analyses. Potential study bias for each outcome was evaluated using the ROBINS-I and Cochrane Collaboration’s RoB 2 tools for single-arm and multi-arm trials, respectively.13,14 These tools evaluate domains related to the design, conduct, and analysis of studies that are associated with bias (ie, systematic error in findings, such as under- or overestimation of results).10 Examples include how well authors performed and concealed randomization procedures, addressed missing data, and measured study outcomes.13,14 The risk of bias (eg, low, moderate, serious) associated with each domain is rated and, based on the domain ratings, each study is then given an overall rating regarding how much risk influences bias.13,14 Broadly, lower risk of bias corresponds to higher confidence in the validity of results.
Finally, 4 authors (associated with 2 single- and 2 multi-arm studies) were contacted and asked to provide further information. Data for 1 additional multi-arm study were obtained from these communications and included in the final study selection.15 These authors were also asked for information about any unpublished works of which they were aware, although no additional works were identified.
Statistical Analyses
Analyses were performed with R Studio R 3.6.0 software.16 An SMD (also known as Hedges g) was calculated for each study outcome: for single-arm trials, this was the SMD between pre- and postintervention scores, whereas for multi-arm trials, this was the SMD between postintervention outcome scores across groups. CIs for each SMD were calculated using a standard normal distribution. Combined SMDs were estimated separately for single- and multi-arm studies, using random-effects meta-analyses. In order to include multiple relevant outcomes from a single trial (ie, for studies using multiple PTSD symptom measures), robust variance estimation was used.17 Precision was used to weight SMDs.
Correlations between pre- and postintervention scores were not available for 1 single-arm study.18 A correlation coefficient of 0.8 was imputed to calculate the standard error of the of the SMDs for the Clinician-Administered PTSD Scale (CAPS) and the PTSD Checklist (PCL), as this value is consistent with past findings regarding the test-retest reliability of these measures.19-22 A sensitivity analysis, using several alternative correlational values, revealed that the choice of correlation coefficient did not impact the overall results of the meta-analysis.
I2 was used to evaluate between-study heterogeneity. Values of I2 > 25%, 50%, and 75% were selected to reflect low, moderate, and high heterogeneity, respectively, in accordance with guidelines described by Higgins and colleagues.23 Potential publication bias was assessed via funnel plot and Egger test.24 Finally, although collection of depressive symptom scores was proposed as a secondary outcome in the study protocol, such data were available only for 1 multi-arm study. As a result, this outcome was not evaluated.
Results
Six studies with 101 total participants were included in the single-arm analyses (Table 1).18,25-29 Participants consisted of veterans with chronic pain, post-9/11 veterans, female veterans of childbearing age, veterans with a history of trauma therapy, and other veterans. Types of exercise included moderate aerobic exercise and yoga. PTSD symptom measures included the CAPS and the PCL (PCL-5 or PCL-M versions). Reported financial sources for included studies included federal grant funding, nonprofit material support, outside organization support, use of US Department of Veterans Affairs (VA) resources, and no reported financial support.
With respect to individual studies, Shivakumar and colleagues found that completion of an aerobic exercise program was associated with reduced scores on 2 different PTSD symptom scales (PCL and CAPS) in 16 women veterans.18 A trauma-informed yoga intervention study with 18 participants by Cushing and colleagues demonstrated veteran participation to be associated with large reductions in PTSD, anxiety, and depression scale scores.25 In a study with 34 veterans, Chopin and colleagues found that a trauma-informed yoga intervention was associated with a statistically significant reduction in PTSD symptoms, as did a study by Zaccari and colleagues with 17 veterans.26,29 Justice and Brems also found some evidence that trauma-informed yoga interventions helped PTSD symptoms in a small sample of 4 veterans, although these results were not quantitatively analyzed.27 In contrast, a small pilot study (n = 12) by Staples and colleagues testing a biweekly, 6-week yoga program did not show a significant effect on PTSD symptoms.28
Three studies with 217 total veteran participants were included in the multi-arm analyses (Table 2).15,30,31 As all multi-arm trials incorporated randomization, they will be referred to as randomized controlled trials (RCTs). On contact, Davis and colleagues provided veteran-specific results for their trial; as such, our data differ from those within the published article.15 Participants from all included studies were veterans currently experiencing symptoms of PTSD. Types of exercise included yoga and combined methods (eg, aerobic and strength training).15,30,31 PTSD symptom measures included the CAPS or the PCL-5.15,30,31 Reported financial sources for included studies included federal grant funding, as well as nonprofit support, private donations, and VA and Department of Defense resources.
Davis and colleagues conducted a recently concluded RCT with > 130 veteran participants and found that a novel manualized yoga program was superior to an attention control in reducing PTSD symptom scale scores for veterans.15 Goldstein and colleagues found that a program consisting of both aerobic and resistance exercises reduced PTSD symptoms to a greater extent than a waitlist control condition, with 47 veterans randomized in this trial.30 Likewise, Hall and colleagues conducted a pilot RCT in which an intervention that integrated exercise and cognitive behavioral techniques was compared to a waitlist control condition.31 For the 48 veterans included in the analyses, the authors reported greater PTSD symptom reduction associated with integrated exercise than that of the control condition; however, the study was not powered to detect statistically significant differences between groups.
Bias Assessment
Results for the risk of bias assessments can be viewed in Tables 3 and 4. For single-arm studies, overall risk of bias was serious for all included trials. Serious risk of bias was found in 2 domains: confounding, due to a lack of accounting for potential preexisting baseline trends (eg, regression to the mean) that could have impacted study results; and measurement, due to the use of a self-report symptom measure (PCL) or CAPS with unblinded assessors. Multiple studies also showed moderate risk in the missing data domain due to participant dropout without appropriate analytic methods to address potential bias.
For RCTs, overall risk of bias ranged from some concerns to high risk. High risk of bias was found in 1 domain, measurement of outcome, due to use of a self-report symptom measure (PCL) with unblinded groups.31 The other 2 studies all had some concern of bias in at least 1 of the following domains: randomization, missing data, and measurement of outcome.
Pooled Standardized Mean Differences
Meta-analytic results can be viewed in Figure 2. The pooled SMD for the 6 single-arm studies was -0.60 (df = 4.41, 95% CI, -1.08 to -0.12, P = .03), indicating a statistically significant reduction in PTSD symptoms over the course of an exercise intervention. Combining SMDs for the 3 included RCTs revealed a pooled SMD of -0.40 (df = 1.57, 95% CI, -0.86 to 0.06, P = .06), indicating that exercise did not result in a statistically significant reduction in PTSD symptoms compared with control conditions.
Publication Bias and Heterogeneity
Visual inspection funnel plots and Egger test did not suggest the presence of publication bias for RCTs (t = 1.21, df = 2, P = .35) or single-arm studies (t = -0.36, df = 5, P = .73).
Single-arm studies displayed a high degree of heterogeneity (I2 = 81.5%). Including sample size or exercise duration as variables in meta-regressions did not reduce heterogeneity (I2 = 85.2% and I2 = 83.8%, respectively). Performing a subgroup analysis only on studies using yoga as an intervention also did not reduce heterogeneity (I2 = 79.2%). Due to the small number of studies, no further exploration of heterogeneity was conducted on single-arm studies. RCTs did not display any heterogeneity (I2 = 0%).
Discussion
Our report represents an early synthesis of the first prospective studies of physical exercise interventions for PTSD in veterans. Results from meta-analyses of 6 single-arm studies (101 participants) and 3 RCTs (217 participants) provide early evidence that exercise may reduce PTSD symptoms in veterans. Yoga was the most common form of exercise used in single-arm studies, whereas RCTs used a wider range of interventions. The pooled SMD of -0.60 for single-arm longitudinal studies suggest a medium decrease in PTSD symptoms for veterans who engage in exercise interventions. Analysis of the RCTs supported this finding, with a pooled SMD of -0.40 reflecting a small-to-medium effect of exercise on PTSD symptoms over control conditions, although this result did not achieve statistical significance. Of note, while the nonsignificant finding for RCTs may have been due to insufficient power caused by the limited number of included studies, possibly exercise was not more efficacious than were the control conditions.
Although RCTs represented a variety of exercise types, PTSD symptom measures, and veteran subgroups, statistical results were not indicative of heterogeneity. However, only the largest and most comprehensive study of exercise for PTSD in veterans to date by Davis and colleagues had a statistically significant SMD.15 Of note, one of the other 2 RCTs displayed an SMD of a similar magnitude, but this study had a much smaller sample size and was underpowered to detect significance.30 Additionally, risk of bias assessments for single-arm studies and RCTs revealed study characteristics that suggest possible inflation of absolute effect sizes for individual studies. Therefore, the pooled SMDs we report are interpretable but may exceed the true effect of exercise for PTSD symptom reduction in veterans.
Based on results of our analyses, it is reasonable, albeit preliminary, to conclude that exercise interventions may result in reduced PTSD symptoms among veterans. At the very least, these findings support the continued investigation of such interventions for veterans. Given the unique and salubrious characteristics of physical exercise, such results, if supported by further research, suggest that exercise-based interventions may be particularly valuable within the trauma treatment realm. For example, exercise can be less expensive and more convenient than attending traditional treatment, and for veterans reluctant to engage in standard treatment approaches such as psychiatric and psychosocial modalities, complementary approaches entailing exercise may be viewed as particularly acceptable or enjoyable.32 In addition to possibly reducing PTSD symptoms, exercise is a well-established treatment for conditions commonly comorbid with PTSD, including depression, anxiety disorders, cognitive difficulties, and certain chronic pain conditions.6 As such, exercise represents a holistic treatment option that has the potential to augment standard PTSD care.
Limitations
The present study has several important limitations. First, few studies were found that met the broad eligibility criteria and those that did often had a small sample size. Besides highlighting a gap in the extant research, the limited studies available for meta-analysis means that caution must be taken when interpreting results. Fortunately, this issue will likely resolve once additional studies investigating the impact of exercise on PTSD symptoms in veterans are available for synthesis.
Relatedly, the included study interventions varied considerably, both in the types of exercise used and the characteristics of the exercises (eg, frequency, duration, and intensity), which is relevant as different exercise modalities are associated with differential physical effects.33 Including such a mixture of exercises may have given an incomplete picture of their potential therapeutic effects. Also, none of the RCTs compared exercise against first-line treatments for PTSD, such as prolonged exposure or cognitive processing therapy, which would have provided further insight into the role exercise could play in clinical settings.7
Another limitation is the elevated risk of bias found in most studies, particularly present in the longitudinal single-arm studies, all of which were rated at serious risk. For instance, no single-arm study controlled for preexisting baseline trends: without such (and lacking a comparison control group like in RCTs), it is possible that the observed effects were due to extraneous factors, rather than the exercise intervention. Although not as severe, the multi-arm RCTs also displayed at least moderate risk of bias. Therefore, SMDs may have been overestimated for each group of studies.
Finally, the results of the single-arm meta-analysis displayed high statistical heterogeneity, reducing the generalizability of the results. One possible cause of this heterogeneity may have been the yoga interventions, as a separate analysis removing the only nonyoga study did not reduce heterogeneity. This result was surprising, as the included yoga interventions seemed similar across studies. While the presence of high heterogeneity does require some caution when applying these results to outside interventions, the present study made use of random-effects meta-analysis, a technique that incorporates study heterogeneity into the statistical model, thereby strengthening the findings compared with that of a traditional fixed-effects approach.10
Future Steps
Several future steps are warranted to improve knowledge of exercise as a treatment for PTSD in veterans and in the general population. With current meta-analyses limited to small numbers of studies, additional studies of the efficacy of exercise for treating PTSD could help in several ways. A larger pool of studies would enable future meta-analyses to explore related questions, such as those regarding the impact of exercise on quality of life or depressive symptom reduction among veterans with PTSD. A greater number of studies also would enable meta-analysts to explore potentially critical moderators. For example, the duration, frequency, or type of exercise may moderate the effect of exercise on PTSD symptom reduction. Moderators related to patient or study design characteristics also should be explored in future studies.
Future work also should evaluate the impact that specific features of exercise regimens have on PTSD. Knowing whether the type or structure of exercise affects its clinical use would be invaluable in developing and implementing efficient exercise-based interventions. For example, if facilitated exercise was found to be significantly more effective at reducing PTSD symptoms than exercise completed independently, the development of exercise intervention programs in the VA and other facilities that commonly treat PTSD may be warranted. Additionally, it may be useful to identify specific mechanisms through which exercise reduces PTSD symptoms. For example, in addition to its beneficial biological effects, exercise also promotes psychological health through behavioral activation and alterations within reinforcement/reward systems, suggesting that exercise regularity may be more important than intensity.34,35 Understanding which mechanisms contribute most to change will aid in the development of more efficient interventions.
Given that veterans are demonstrating considerable interest in complementary and alternative PTSD treatments, it is critical that researchers focus on high-quality randomized tests of these interventions. Therefore, in addition to greater quality of exercise intervention studies, future efforts should be focused on RCTs that are designed in such a way as to limit potential introduction of bias. For example, assessment data should be completed by blinded assessors using standardized measures, and analyses should account for missing data and unequal participant attrition between groups. Ideally, pre-intervention trends across multiple baseline datapoints also would be collected in single-arm studies to avoid confounding related to regression to the mean. It is also recommended that future meta-analyses use risk of bias assessments and consider how the results of such assessments may impact the interpretation of results.
Conclusions
Findings from both single-arm studies and RCTs suggest possible benefit of exercise on PTSD symptom reduction, although confirmation of findings is needed. No study found increased symptoms following exercise intervention. Thus, it is reasonable to consider physical exercise, such as yoga, as an adjunct, whole-health consistent treatment. HCPs working with veterans with past traumatic experiences should consider incorporating exercise into patient care. Enhanced educational efforts emphasizing the psychotherapeutic impact of exercise may also have value for the veteran population. Furthermore, the current risk of bias assessments highlights the need for additional high-quality RCTs evaluating the specific impact of exercise on PTSD symptom reduction in veterans. In particular, this field of inquiry would benefit from larger samples and design characteristics to reduce bias (eg, blinding when possible, use of CAPS vs only self-report symptom measures, reducing problematic attrition, corrections for missing data, etc).
Acknowledgments
This research is the result of work supported with resources and the use of facilities at the VA Eastern Kansas Healthcare System (Dwight D. Eisenhower VA Medical Center). It was also supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, as well as the Rocky Mountain Mental Illness Research, Education, and Clinical Center. Since Dr. Reis and Dr. Gaddy are employees of the US Government and contributed to this manuscript as part of their official duties, the work is not subject to US copyright. This study was preregistered on PROSPERO (https://www.crd.york.ac.uk/prospero/; ID: CRD42020153419).
1. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity—a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi:10.1186/1471-2458-13-813
2. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592. doi:10.1037/a0021769
3. Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of posttraumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res. 2015;230(2):130-136. doi:10.1016/j.psychres.2015.10.017
4. Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541-550. doi:10.4088/JCP.12r08225
5. Tanielian T, Jaycox L, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation; 2008
6. Whitworth JW, Ciccolo JT. Exercise and post-traumatic stress disorder in military veterans: a systematic review. Mil Med. 2016;181(9):953-960. doi:10.7205/MILMED-D-15-00488
7. Rutt BT, Oehlert ME, Krieshok TS, Lichtenberg JW. Effectiveness of cognitive processing therapy and prolonged exposure in the Department of Veterans Affairs. Psychol Rep. 2018;121(2):282-302. doi:10.1177/0033294117727746
8. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
9. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: Implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704. doi:10.1001/archinte.162.15.1697
10. Higgins JPT, Thomas J, Chanlder J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane; 2021.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
12. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-131.
13. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919
14. Sterne JAC, Savovic´ J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
15. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
16. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2019.
17. Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods .2015;20(3):375-393. doi:10.1037/met0000011
18. Shivakumar G, Anderson EH, Surís AM, North CS. Exercise for PTSD in women veterans: a proof-of-concept study. Mil Med. 2017;182(11):e1809-e1814. doi:10.7205/MILMED-D-16-00440
19. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
20. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34(8):669-673. doi:10.1016/0005-7967(96)00033-2
21. Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician- Administered PTSD Scale for DSM-5 (CAPS- 5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383-395.doi:10.1037/pas0000486
22. Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596-606. doi:10.1002/da.20837
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi:10.1136/bmj.327.7414.557
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi:10.1136/bmj.315.7109.629
25. Cushing RE, Braun KL, Alden CISW, Katz AR. Military- tailored yoga for veterans with post-traumatic stress disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. Justice L, Brems C. Bridging body and mind: case series of a 10-week trauma-informed yoga protocol for veterans. Int J Yoga Therap. 2019;29(1):65-79. doi:10.17761/D-17-2019-00029
28. Staples JK, Hamilton MF, Uddo M. A yoga program for the symptoms of post-traumatic stress disorder in veterans. Mil Med. 2013;178(8):854-860. doi:10.7205/MILMED-D-12-00536
29. Zaccari B, Callahan ML, Storzbach D, McFarlane N, Hudson R, Loftis JM. Yoga for veterans with PTSD: Cognitive functioning, mental health, and salivary cortisol. Psychol Trauma. 2020;12(8):913-917. doi:10.1037/tra0000909
30. Goldstein LA, Mehling WE, Metzler TJ, et al. Veterans Group Exercise: A randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J Affect Disord. 2018;227:345-352. doi:10.1016/j.jad.2017.11.002
31. Hall KS, Morey MC, Bosworth HB, et al. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J Behav Med. 2020;43(4):648-659. doi:10.1007/s10865-019-00073-w
32. Gaddy MA. Implementation of an integrative medicine treatment program at a Veterans Health Administration residential mental health facility. Psychol Serv. 2018;15(4):503- 509. doi:10.1037/ser0000189
33. Werner CM, Hecksteden A, Morsch A, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40(1):34- 46. doi:10.1093/eurheartj/ehy585
34. Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040. doi:10.1098/rsfs.2014.0040
35. Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. 2021;72:45-62. doi:10.1146/annurev-med-060619-022943.
Physical exercise offers preventative and therapeutic benefits for a range of chronic health conditions, including cardiovascular disease, type 2 diabetes mellitus, Alzheimer disease, and depression.1,2 Exercise has been well studied for its antidepressant effects, its ability to reduce risk of aging-related dementia, and favorable effects on a range of cognitive functions.2 Lesser evidence exists regarding the impact of exercise on other mental health concerns. Therefore, an accurate understanding of whether physical exercise may ameliorate other conditions is important.
A small meta-analysis by Rosenbaum and colleagues found that exercise interventions were superior to control conditions for symptom reduction in study participants with posttraumatic stress disorder (PTSD).3 This meta-analysis included 4 randomized clinical trials representing 200 cases. The trial included a variety of physical activities (eg, yoga, aerobic, and strength-building exercises) and control conditions, and participants recruited from online, community, inpatient, and outpatient settings. The standardized mean difference (SMD) produced by the analysis indicated a small-to-medium effect (Hedges g, -0.35), with the authors reporting no evidence of publication bias, although an assessment of potential bias associated with individual trial design characteristics was not conducted. Of note, a meta-analysis by Watts and colleagues found that effect sizes for PTSD treatments tend to be smaller in veteran populations.4 Therefore, how much the mean effect size estimate in the study is applicable to veterans with PTSD is unknown.3
Veterans represent a unique subpopulation in which PTSD is common, although no meta-analysis yet published has synthesized the effects of exercise interventions from trials of veterans with PTSD.5 A recent systematic review by Whitworth and Ciccolo concluded that exercise may be associated with reduced risk of PTSD, a briefer course of PTSD symptoms, and/or reduced sleep- and depression-related difficulties.6 However, that review primarily included observational, cross-sectional, and qualitative works. No trials included in our meta-analysis were included in that review.6
Evidence-based psychotherapies like cognitive processing therapy and prolonged exposure have been shown to be effective for treating PTSD in veterans; however, these modalities are accompanied by high rates of dropout (eg, 40-60%), thereby limiting their clinical utility.7 The use of complementary and alternative approaches for treatment in the United States has increased in recent years, and exercise represents an important complementary treatment option.8 In a study by Baldwin and colleagues, nearly 50% of veterans reported using complementary or alternative approaches, and veterans with PTSD were among those likely to use such approaches.9 However, current studies of the effects of exercise interventions on PTSD symptom reduction are mostly small and varied, making determinations difficult regarding the potential utility of exercise for treating this condition in veterans.
Literature Search
No previous research has synthesized the literature on the effects of exercise on PTSD in the veteran population. The current meta-analysis aims to provide a synthesis of systematically selected studies on this topic to determine whether exercise-based interventions are effective at reducing veterans’ symptoms of PTSD. Our hypothesis was that, when used as a primary or adjuvant intervention for PTSD, physical exercise would be associated with a reduction of PTSD symptom scale scores. We planned a priori to produce separate estimates for single-arm and multi-arm trials. We also wanted to conduct a careful risk of bias assessment—or evaluation of study features that may have systematically influenced results—for included trials, not only to provide context for interpretation of results, but also to inform suggestions for research to advance this field of inquiry.10
Methods
This study was preregistered on PROSPERO and followed PRISMA guidelines for meta-analyses and systematic reviews.11 Supplementary materials, such as the PRISMA checklist, study data, and funnel plots, are available online (doi.org/10.6084/m9.figshare.c.5618437.v1). Conference abstracts were omitted due to a lack of necessary information. We decided early in the planning process to include both randomized and single-arm trials, expecting the number of completed studies in the area of exercise for PTSD symptom reduction in veterans, and particularly randomized trials of such, would be relatively small.
Studies were included if they met the following criteria: (1) the study was a single- or multi-arm interventional trial; (2) participants were veterans; (3) participants had a current diagnosis of PTSD or exhibited subthreshold PTSD symptoms, as established by authors of the individual studies and supported by a structured clinical interview, semistructured interview, or elevated scores on PTSD symptom self-report measures; (4) the study included an intervention in which exercise (physical activity that is planned, structured, repetitive, and purposive in the sense that improvement or maintenance of physical fitness or health is an objective) was the primary component; (5) PTSD symptom severity was by a clinician-rated or self-report measure; and (6) the study was published in a peer-reviewed journal.12 Studies were excluded if means, standard deviations, and sample sizes were not available or the full text of the study was not available in English.
The systematic review was conducted using PubMed, PsycINFO, and Cochrane Library databases, from the earliest record to February 2021. The following search phrase was used, without additional limits, to acquire a list of potential studies: (“PTSD” or “post-traumatic stress disorder” or “posttraumatic stress disorder” or “post traumatic stress disorder”) and (“veteran” or “veterans”) and (“exercise” or “aerobic” or “activity” or “physical activity”). The references of identified publications also were searched for additional studies. Then, study titles and abstracts were evaluated and finally, full texts were evaluated to determine study inclusion. All screening, study selection, and risk of bias and data extraction activities were performed by 2 independent reviewers (DR and MJ) with disagreements resolved through discussion and consensus (Figure 1). A list of studies excluded during full-text review and rationales can be viewed online (doi.org/10.6084/m9.figshare.c.5618437.v1).
Data Collection
Data were extracted from included studies using custom forms and included the following information based on PRISMA guidelines: (1) study design characteristics; (2) intervention details; and (3) PTSD outcome information.11 PTSD symptom severity was the primary outcome of interest. Outcome data were included if they were derived from a measure of PTSD symptoms—equivalency across measures was assumed for meta-analyses. Potential study bias for each outcome was evaluated using the ROBINS-I and Cochrane Collaboration’s RoB 2 tools for single-arm and multi-arm trials, respectively.13,14 These tools evaluate domains related to the design, conduct, and analysis of studies that are associated with bias (ie, systematic error in findings, such as under- or overestimation of results).10 Examples include how well authors performed and concealed randomization procedures, addressed missing data, and measured study outcomes.13,14 The risk of bias (eg, low, moderate, serious) associated with each domain is rated and, based on the domain ratings, each study is then given an overall rating regarding how much risk influences bias.13,14 Broadly, lower risk of bias corresponds to higher confidence in the validity of results.
Finally, 4 authors (associated with 2 single- and 2 multi-arm studies) were contacted and asked to provide further information. Data for 1 additional multi-arm study were obtained from these communications and included in the final study selection.15 These authors were also asked for information about any unpublished works of which they were aware, although no additional works were identified.
Statistical Analyses
Analyses were performed with R Studio R 3.6.0 software.16 An SMD (also known as Hedges g) was calculated for each study outcome: for single-arm trials, this was the SMD between pre- and postintervention scores, whereas for multi-arm trials, this was the SMD between postintervention outcome scores across groups. CIs for each SMD were calculated using a standard normal distribution. Combined SMDs were estimated separately for single- and multi-arm studies, using random-effects meta-analyses. In order to include multiple relevant outcomes from a single trial (ie, for studies using multiple PTSD symptom measures), robust variance estimation was used.17 Precision was used to weight SMDs.
Correlations between pre- and postintervention scores were not available for 1 single-arm study.18 A correlation coefficient of 0.8 was imputed to calculate the standard error of the of the SMDs for the Clinician-Administered PTSD Scale (CAPS) and the PTSD Checklist (PCL), as this value is consistent with past findings regarding the test-retest reliability of these measures.19-22 A sensitivity analysis, using several alternative correlational values, revealed that the choice of correlation coefficient did not impact the overall results of the meta-analysis.
I2 was used to evaluate between-study heterogeneity. Values of I2 > 25%, 50%, and 75% were selected to reflect low, moderate, and high heterogeneity, respectively, in accordance with guidelines described by Higgins and colleagues.23 Potential publication bias was assessed via funnel plot and Egger test.24 Finally, although collection of depressive symptom scores was proposed as a secondary outcome in the study protocol, such data were available only for 1 multi-arm study. As a result, this outcome was not evaluated.
Results
Six studies with 101 total participants were included in the single-arm analyses (Table 1).18,25-29 Participants consisted of veterans with chronic pain, post-9/11 veterans, female veterans of childbearing age, veterans with a history of trauma therapy, and other veterans. Types of exercise included moderate aerobic exercise and yoga. PTSD symptom measures included the CAPS and the PCL (PCL-5 or PCL-M versions). Reported financial sources for included studies included federal grant funding, nonprofit material support, outside organization support, use of US Department of Veterans Affairs (VA) resources, and no reported financial support.
With respect to individual studies, Shivakumar and colleagues found that completion of an aerobic exercise program was associated with reduced scores on 2 different PTSD symptom scales (PCL and CAPS) in 16 women veterans.18 A trauma-informed yoga intervention study with 18 participants by Cushing and colleagues demonstrated veteran participation to be associated with large reductions in PTSD, anxiety, and depression scale scores.25 In a study with 34 veterans, Chopin and colleagues found that a trauma-informed yoga intervention was associated with a statistically significant reduction in PTSD symptoms, as did a study by Zaccari and colleagues with 17 veterans.26,29 Justice and Brems also found some evidence that trauma-informed yoga interventions helped PTSD symptoms in a small sample of 4 veterans, although these results were not quantitatively analyzed.27 In contrast, a small pilot study (n = 12) by Staples and colleagues testing a biweekly, 6-week yoga program did not show a significant effect on PTSD symptoms.28
Three studies with 217 total veteran participants were included in the multi-arm analyses (Table 2).15,30,31 As all multi-arm trials incorporated randomization, they will be referred to as randomized controlled trials (RCTs). On contact, Davis and colleagues provided veteran-specific results for their trial; as such, our data differ from those within the published article.15 Participants from all included studies were veterans currently experiencing symptoms of PTSD. Types of exercise included yoga and combined methods (eg, aerobic and strength training).15,30,31 PTSD symptom measures included the CAPS or the PCL-5.15,30,31 Reported financial sources for included studies included federal grant funding, as well as nonprofit support, private donations, and VA and Department of Defense resources.
Davis and colleagues conducted a recently concluded RCT with > 130 veteran participants and found that a novel manualized yoga program was superior to an attention control in reducing PTSD symptom scale scores for veterans.15 Goldstein and colleagues found that a program consisting of both aerobic and resistance exercises reduced PTSD symptoms to a greater extent than a waitlist control condition, with 47 veterans randomized in this trial.30 Likewise, Hall and colleagues conducted a pilot RCT in which an intervention that integrated exercise and cognitive behavioral techniques was compared to a waitlist control condition.31 For the 48 veterans included in the analyses, the authors reported greater PTSD symptom reduction associated with integrated exercise than that of the control condition; however, the study was not powered to detect statistically significant differences between groups.
Bias Assessment
Results for the risk of bias assessments can be viewed in Tables 3 and 4. For single-arm studies, overall risk of bias was serious for all included trials. Serious risk of bias was found in 2 domains: confounding, due to a lack of accounting for potential preexisting baseline trends (eg, regression to the mean) that could have impacted study results; and measurement, due to the use of a self-report symptom measure (PCL) or CAPS with unblinded assessors. Multiple studies also showed moderate risk in the missing data domain due to participant dropout without appropriate analytic methods to address potential bias.
For RCTs, overall risk of bias ranged from some concerns to high risk. High risk of bias was found in 1 domain, measurement of outcome, due to use of a self-report symptom measure (PCL) with unblinded groups.31 The other 2 studies all had some concern of bias in at least 1 of the following domains: randomization, missing data, and measurement of outcome.
Pooled Standardized Mean Differences
Meta-analytic results can be viewed in Figure 2. The pooled SMD for the 6 single-arm studies was -0.60 (df = 4.41, 95% CI, -1.08 to -0.12, P = .03), indicating a statistically significant reduction in PTSD symptoms over the course of an exercise intervention. Combining SMDs for the 3 included RCTs revealed a pooled SMD of -0.40 (df = 1.57, 95% CI, -0.86 to 0.06, P = .06), indicating that exercise did not result in a statistically significant reduction in PTSD symptoms compared with control conditions.
Publication Bias and Heterogeneity
Visual inspection funnel plots and Egger test did not suggest the presence of publication bias for RCTs (t = 1.21, df = 2, P = .35) or single-arm studies (t = -0.36, df = 5, P = .73).
Single-arm studies displayed a high degree of heterogeneity (I2 = 81.5%). Including sample size or exercise duration as variables in meta-regressions did not reduce heterogeneity (I2 = 85.2% and I2 = 83.8%, respectively). Performing a subgroup analysis only on studies using yoga as an intervention also did not reduce heterogeneity (I2 = 79.2%). Due to the small number of studies, no further exploration of heterogeneity was conducted on single-arm studies. RCTs did not display any heterogeneity (I2 = 0%).
Discussion
Our report represents an early synthesis of the first prospective studies of physical exercise interventions for PTSD in veterans. Results from meta-analyses of 6 single-arm studies (101 participants) and 3 RCTs (217 participants) provide early evidence that exercise may reduce PTSD symptoms in veterans. Yoga was the most common form of exercise used in single-arm studies, whereas RCTs used a wider range of interventions. The pooled SMD of -0.60 for single-arm longitudinal studies suggest a medium decrease in PTSD symptoms for veterans who engage in exercise interventions. Analysis of the RCTs supported this finding, with a pooled SMD of -0.40 reflecting a small-to-medium effect of exercise on PTSD symptoms over control conditions, although this result did not achieve statistical significance. Of note, while the nonsignificant finding for RCTs may have been due to insufficient power caused by the limited number of included studies, possibly exercise was not more efficacious than were the control conditions.
Although RCTs represented a variety of exercise types, PTSD symptom measures, and veteran subgroups, statistical results were not indicative of heterogeneity. However, only the largest and most comprehensive study of exercise for PTSD in veterans to date by Davis and colleagues had a statistically significant SMD.15 Of note, one of the other 2 RCTs displayed an SMD of a similar magnitude, but this study had a much smaller sample size and was underpowered to detect significance.30 Additionally, risk of bias assessments for single-arm studies and RCTs revealed study characteristics that suggest possible inflation of absolute effect sizes for individual studies. Therefore, the pooled SMDs we report are interpretable but may exceed the true effect of exercise for PTSD symptom reduction in veterans.
Based on results of our analyses, it is reasonable, albeit preliminary, to conclude that exercise interventions may result in reduced PTSD symptoms among veterans. At the very least, these findings support the continued investigation of such interventions for veterans. Given the unique and salubrious characteristics of physical exercise, such results, if supported by further research, suggest that exercise-based interventions may be particularly valuable within the trauma treatment realm. For example, exercise can be less expensive and more convenient than attending traditional treatment, and for veterans reluctant to engage in standard treatment approaches such as psychiatric and psychosocial modalities, complementary approaches entailing exercise may be viewed as particularly acceptable or enjoyable.32 In addition to possibly reducing PTSD symptoms, exercise is a well-established treatment for conditions commonly comorbid with PTSD, including depression, anxiety disorders, cognitive difficulties, and certain chronic pain conditions.6 As such, exercise represents a holistic treatment option that has the potential to augment standard PTSD care.
Limitations
The present study has several important limitations. First, few studies were found that met the broad eligibility criteria and those that did often had a small sample size. Besides highlighting a gap in the extant research, the limited studies available for meta-analysis means that caution must be taken when interpreting results. Fortunately, this issue will likely resolve once additional studies investigating the impact of exercise on PTSD symptoms in veterans are available for synthesis.
Relatedly, the included study interventions varied considerably, both in the types of exercise used and the characteristics of the exercises (eg, frequency, duration, and intensity), which is relevant as different exercise modalities are associated with differential physical effects.33 Including such a mixture of exercises may have given an incomplete picture of their potential therapeutic effects. Also, none of the RCTs compared exercise against first-line treatments for PTSD, such as prolonged exposure or cognitive processing therapy, which would have provided further insight into the role exercise could play in clinical settings.7
Another limitation is the elevated risk of bias found in most studies, particularly present in the longitudinal single-arm studies, all of which were rated at serious risk. For instance, no single-arm study controlled for preexisting baseline trends: without such (and lacking a comparison control group like in RCTs), it is possible that the observed effects were due to extraneous factors, rather than the exercise intervention. Although not as severe, the multi-arm RCTs also displayed at least moderate risk of bias. Therefore, SMDs may have been overestimated for each group of studies.
Finally, the results of the single-arm meta-analysis displayed high statistical heterogeneity, reducing the generalizability of the results. One possible cause of this heterogeneity may have been the yoga interventions, as a separate analysis removing the only nonyoga study did not reduce heterogeneity. This result was surprising, as the included yoga interventions seemed similar across studies. While the presence of high heterogeneity does require some caution when applying these results to outside interventions, the present study made use of random-effects meta-analysis, a technique that incorporates study heterogeneity into the statistical model, thereby strengthening the findings compared with that of a traditional fixed-effects approach.10
Future Steps
Several future steps are warranted to improve knowledge of exercise as a treatment for PTSD in veterans and in the general population. With current meta-analyses limited to small numbers of studies, additional studies of the efficacy of exercise for treating PTSD could help in several ways. A larger pool of studies would enable future meta-analyses to explore related questions, such as those regarding the impact of exercise on quality of life or depressive symptom reduction among veterans with PTSD. A greater number of studies also would enable meta-analysts to explore potentially critical moderators. For example, the duration, frequency, or type of exercise may moderate the effect of exercise on PTSD symptom reduction. Moderators related to patient or study design characteristics also should be explored in future studies.
Future work also should evaluate the impact that specific features of exercise regimens have on PTSD. Knowing whether the type or structure of exercise affects its clinical use would be invaluable in developing and implementing efficient exercise-based interventions. For example, if facilitated exercise was found to be significantly more effective at reducing PTSD symptoms than exercise completed independently, the development of exercise intervention programs in the VA and other facilities that commonly treat PTSD may be warranted. Additionally, it may be useful to identify specific mechanisms through which exercise reduces PTSD symptoms. For example, in addition to its beneficial biological effects, exercise also promotes psychological health through behavioral activation and alterations within reinforcement/reward systems, suggesting that exercise regularity may be more important than intensity.34,35 Understanding which mechanisms contribute most to change will aid in the development of more efficient interventions.
Given that veterans are demonstrating considerable interest in complementary and alternative PTSD treatments, it is critical that researchers focus on high-quality randomized tests of these interventions. Therefore, in addition to greater quality of exercise intervention studies, future efforts should be focused on RCTs that are designed in such a way as to limit potential introduction of bias. For example, assessment data should be completed by blinded assessors using standardized measures, and analyses should account for missing data and unequal participant attrition between groups. Ideally, pre-intervention trends across multiple baseline datapoints also would be collected in single-arm studies to avoid confounding related to regression to the mean. It is also recommended that future meta-analyses use risk of bias assessments and consider how the results of such assessments may impact the interpretation of results.
Conclusions
Findings from both single-arm studies and RCTs suggest possible benefit of exercise on PTSD symptom reduction, although confirmation of findings is needed. No study found increased symptoms following exercise intervention. Thus, it is reasonable to consider physical exercise, such as yoga, as an adjunct, whole-health consistent treatment. HCPs working with veterans with past traumatic experiences should consider incorporating exercise into patient care. Enhanced educational efforts emphasizing the psychotherapeutic impact of exercise may also have value for the veteran population. Furthermore, the current risk of bias assessments highlights the need for additional high-quality RCTs evaluating the specific impact of exercise on PTSD symptom reduction in veterans. In particular, this field of inquiry would benefit from larger samples and design characteristics to reduce bias (eg, blinding when possible, use of CAPS vs only self-report symptom measures, reducing problematic attrition, corrections for missing data, etc).
Acknowledgments
This research is the result of work supported with resources and the use of facilities at the VA Eastern Kansas Healthcare System (Dwight D. Eisenhower VA Medical Center). It was also supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, as well as the Rocky Mountain Mental Illness Research, Education, and Clinical Center. Since Dr. Reis and Dr. Gaddy are employees of the US Government and contributed to this manuscript as part of their official duties, the work is not subject to US copyright. This study was preregistered on PROSPERO (https://www.crd.york.ac.uk/prospero/; ID: CRD42020153419).
Physical exercise offers preventative and therapeutic benefits for a range of chronic health conditions, including cardiovascular disease, type 2 diabetes mellitus, Alzheimer disease, and depression.1,2 Exercise has been well studied for its antidepressant effects, its ability to reduce risk of aging-related dementia, and favorable effects on a range of cognitive functions.2 Lesser evidence exists regarding the impact of exercise on other mental health concerns. Therefore, an accurate understanding of whether physical exercise may ameliorate other conditions is important.
A small meta-analysis by Rosenbaum and colleagues found that exercise interventions were superior to control conditions for symptom reduction in study participants with posttraumatic stress disorder (PTSD).3 This meta-analysis included 4 randomized clinical trials representing 200 cases. The trial included a variety of physical activities (eg, yoga, aerobic, and strength-building exercises) and control conditions, and participants recruited from online, community, inpatient, and outpatient settings. The standardized mean difference (SMD) produced by the analysis indicated a small-to-medium effect (Hedges g, -0.35), with the authors reporting no evidence of publication bias, although an assessment of potential bias associated with individual trial design characteristics was not conducted. Of note, a meta-analysis by Watts and colleagues found that effect sizes for PTSD treatments tend to be smaller in veteran populations.4 Therefore, how much the mean effect size estimate in the study is applicable to veterans with PTSD is unknown.3
Veterans represent a unique subpopulation in which PTSD is common, although no meta-analysis yet published has synthesized the effects of exercise interventions from trials of veterans with PTSD.5 A recent systematic review by Whitworth and Ciccolo concluded that exercise may be associated with reduced risk of PTSD, a briefer course of PTSD symptoms, and/or reduced sleep- and depression-related difficulties.6 However, that review primarily included observational, cross-sectional, and qualitative works. No trials included in our meta-analysis were included in that review.6
Evidence-based psychotherapies like cognitive processing therapy and prolonged exposure have been shown to be effective for treating PTSD in veterans; however, these modalities are accompanied by high rates of dropout (eg, 40-60%), thereby limiting their clinical utility.7 The use of complementary and alternative approaches for treatment in the United States has increased in recent years, and exercise represents an important complementary treatment option.8 In a study by Baldwin and colleagues, nearly 50% of veterans reported using complementary or alternative approaches, and veterans with PTSD were among those likely to use such approaches.9 However, current studies of the effects of exercise interventions on PTSD symptom reduction are mostly small and varied, making determinations difficult regarding the potential utility of exercise for treating this condition in veterans.
Literature Search
No previous research has synthesized the literature on the effects of exercise on PTSD in the veteran population. The current meta-analysis aims to provide a synthesis of systematically selected studies on this topic to determine whether exercise-based interventions are effective at reducing veterans’ symptoms of PTSD. Our hypothesis was that, when used as a primary or adjuvant intervention for PTSD, physical exercise would be associated with a reduction of PTSD symptom scale scores. We planned a priori to produce separate estimates for single-arm and multi-arm trials. We also wanted to conduct a careful risk of bias assessment—or evaluation of study features that may have systematically influenced results—for included trials, not only to provide context for interpretation of results, but also to inform suggestions for research to advance this field of inquiry.10
Methods
This study was preregistered on PROSPERO and followed PRISMA guidelines for meta-analyses and systematic reviews.11 Supplementary materials, such as the PRISMA checklist, study data, and funnel plots, are available online (doi.org/10.6084/m9.figshare.c.5618437.v1). Conference abstracts were omitted due to a lack of necessary information. We decided early in the planning process to include both randomized and single-arm trials, expecting the number of completed studies in the area of exercise for PTSD symptom reduction in veterans, and particularly randomized trials of such, would be relatively small.
Studies were included if they met the following criteria: (1) the study was a single- or multi-arm interventional trial; (2) participants were veterans; (3) participants had a current diagnosis of PTSD or exhibited subthreshold PTSD symptoms, as established by authors of the individual studies and supported by a structured clinical interview, semistructured interview, or elevated scores on PTSD symptom self-report measures; (4) the study included an intervention in which exercise (physical activity that is planned, structured, repetitive, and purposive in the sense that improvement or maintenance of physical fitness or health is an objective) was the primary component; (5) PTSD symptom severity was by a clinician-rated or self-report measure; and (6) the study was published in a peer-reviewed journal.12 Studies were excluded if means, standard deviations, and sample sizes were not available or the full text of the study was not available in English.
The systematic review was conducted using PubMed, PsycINFO, and Cochrane Library databases, from the earliest record to February 2021. The following search phrase was used, without additional limits, to acquire a list of potential studies: (“PTSD” or “post-traumatic stress disorder” or “posttraumatic stress disorder” or “post traumatic stress disorder”) and (“veteran” or “veterans”) and (“exercise” or “aerobic” or “activity” or “physical activity”). The references of identified publications also were searched for additional studies. Then, study titles and abstracts were evaluated and finally, full texts were evaluated to determine study inclusion. All screening, study selection, and risk of bias and data extraction activities were performed by 2 independent reviewers (DR and MJ) with disagreements resolved through discussion and consensus (Figure 1). A list of studies excluded during full-text review and rationales can be viewed online (doi.org/10.6084/m9.figshare.c.5618437.v1).
Data Collection
Data were extracted from included studies using custom forms and included the following information based on PRISMA guidelines: (1) study design characteristics; (2) intervention details; and (3) PTSD outcome information.11 PTSD symptom severity was the primary outcome of interest. Outcome data were included if they were derived from a measure of PTSD symptoms—equivalency across measures was assumed for meta-analyses. Potential study bias for each outcome was evaluated using the ROBINS-I and Cochrane Collaboration’s RoB 2 tools for single-arm and multi-arm trials, respectively.13,14 These tools evaluate domains related to the design, conduct, and analysis of studies that are associated with bias (ie, systematic error in findings, such as under- or overestimation of results).10 Examples include how well authors performed and concealed randomization procedures, addressed missing data, and measured study outcomes.13,14 The risk of bias (eg, low, moderate, serious) associated with each domain is rated and, based on the domain ratings, each study is then given an overall rating regarding how much risk influences bias.13,14 Broadly, lower risk of bias corresponds to higher confidence in the validity of results.
Finally, 4 authors (associated with 2 single- and 2 multi-arm studies) were contacted and asked to provide further information. Data for 1 additional multi-arm study were obtained from these communications and included in the final study selection.15 These authors were also asked for information about any unpublished works of which they were aware, although no additional works were identified.
Statistical Analyses
Analyses were performed with R Studio R 3.6.0 software.16 An SMD (also known as Hedges g) was calculated for each study outcome: for single-arm trials, this was the SMD between pre- and postintervention scores, whereas for multi-arm trials, this was the SMD between postintervention outcome scores across groups. CIs for each SMD were calculated using a standard normal distribution. Combined SMDs were estimated separately for single- and multi-arm studies, using random-effects meta-analyses. In order to include multiple relevant outcomes from a single trial (ie, for studies using multiple PTSD symptom measures), robust variance estimation was used.17 Precision was used to weight SMDs.
Correlations between pre- and postintervention scores were not available for 1 single-arm study.18 A correlation coefficient of 0.8 was imputed to calculate the standard error of the of the SMDs for the Clinician-Administered PTSD Scale (CAPS) and the PTSD Checklist (PCL), as this value is consistent with past findings regarding the test-retest reliability of these measures.19-22 A sensitivity analysis, using several alternative correlational values, revealed that the choice of correlation coefficient did not impact the overall results of the meta-analysis.
I2 was used to evaluate between-study heterogeneity. Values of I2 > 25%, 50%, and 75% were selected to reflect low, moderate, and high heterogeneity, respectively, in accordance with guidelines described by Higgins and colleagues.23 Potential publication bias was assessed via funnel plot and Egger test.24 Finally, although collection of depressive symptom scores was proposed as a secondary outcome in the study protocol, such data were available only for 1 multi-arm study. As a result, this outcome was not evaluated.
Results
Six studies with 101 total participants were included in the single-arm analyses (Table 1).18,25-29 Participants consisted of veterans with chronic pain, post-9/11 veterans, female veterans of childbearing age, veterans with a history of trauma therapy, and other veterans. Types of exercise included moderate aerobic exercise and yoga. PTSD symptom measures included the CAPS and the PCL (PCL-5 or PCL-M versions). Reported financial sources for included studies included federal grant funding, nonprofit material support, outside organization support, use of US Department of Veterans Affairs (VA) resources, and no reported financial support.
With respect to individual studies, Shivakumar and colleagues found that completion of an aerobic exercise program was associated with reduced scores on 2 different PTSD symptom scales (PCL and CAPS) in 16 women veterans.18 A trauma-informed yoga intervention study with 18 participants by Cushing and colleagues demonstrated veteran participation to be associated with large reductions in PTSD, anxiety, and depression scale scores.25 In a study with 34 veterans, Chopin and colleagues found that a trauma-informed yoga intervention was associated with a statistically significant reduction in PTSD symptoms, as did a study by Zaccari and colleagues with 17 veterans.26,29 Justice and Brems also found some evidence that trauma-informed yoga interventions helped PTSD symptoms in a small sample of 4 veterans, although these results were not quantitatively analyzed.27 In contrast, a small pilot study (n = 12) by Staples and colleagues testing a biweekly, 6-week yoga program did not show a significant effect on PTSD symptoms.28
Three studies with 217 total veteran participants were included in the multi-arm analyses (Table 2).15,30,31 As all multi-arm trials incorporated randomization, they will be referred to as randomized controlled trials (RCTs). On contact, Davis and colleagues provided veteran-specific results for their trial; as such, our data differ from those within the published article.15 Participants from all included studies were veterans currently experiencing symptoms of PTSD. Types of exercise included yoga and combined methods (eg, aerobic and strength training).15,30,31 PTSD symptom measures included the CAPS or the PCL-5.15,30,31 Reported financial sources for included studies included federal grant funding, as well as nonprofit support, private donations, and VA and Department of Defense resources.
Davis and colleagues conducted a recently concluded RCT with > 130 veteran participants and found that a novel manualized yoga program was superior to an attention control in reducing PTSD symptom scale scores for veterans.15 Goldstein and colleagues found that a program consisting of both aerobic and resistance exercises reduced PTSD symptoms to a greater extent than a waitlist control condition, with 47 veterans randomized in this trial.30 Likewise, Hall and colleagues conducted a pilot RCT in which an intervention that integrated exercise and cognitive behavioral techniques was compared to a waitlist control condition.31 For the 48 veterans included in the analyses, the authors reported greater PTSD symptom reduction associated with integrated exercise than that of the control condition; however, the study was not powered to detect statistically significant differences between groups.
Bias Assessment
Results for the risk of bias assessments can be viewed in Tables 3 and 4. For single-arm studies, overall risk of bias was serious for all included trials. Serious risk of bias was found in 2 domains: confounding, due to a lack of accounting for potential preexisting baseline trends (eg, regression to the mean) that could have impacted study results; and measurement, due to the use of a self-report symptom measure (PCL) or CAPS with unblinded assessors. Multiple studies also showed moderate risk in the missing data domain due to participant dropout without appropriate analytic methods to address potential bias.
For RCTs, overall risk of bias ranged from some concerns to high risk. High risk of bias was found in 1 domain, measurement of outcome, due to use of a self-report symptom measure (PCL) with unblinded groups.31 The other 2 studies all had some concern of bias in at least 1 of the following domains: randomization, missing data, and measurement of outcome.
Pooled Standardized Mean Differences
Meta-analytic results can be viewed in Figure 2. The pooled SMD for the 6 single-arm studies was -0.60 (df = 4.41, 95% CI, -1.08 to -0.12, P = .03), indicating a statistically significant reduction in PTSD symptoms over the course of an exercise intervention. Combining SMDs for the 3 included RCTs revealed a pooled SMD of -0.40 (df = 1.57, 95% CI, -0.86 to 0.06, P = .06), indicating that exercise did not result in a statistically significant reduction in PTSD symptoms compared with control conditions.
Publication Bias and Heterogeneity
Visual inspection funnel plots and Egger test did not suggest the presence of publication bias for RCTs (t = 1.21, df = 2, P = .35) or single-arm studies (t = -0.36, df = 5, P = .73).
Single-arm studies displayed a high degree of heterogeneity (I2 = 81.5%). Including sample size or exercise duration as variables in meta-regressions did not reduce heterogeneity (I2 = 85.2% and I2 = 83.8%, respectively). Performing a subgroup analysis only on studies using yoga as an intervention also did not reduce heterogeneity (I2 = 79.2%). Due to the small number of studies, no further exploration of heterogeneity was conducted on single-arm studies. RCTs did not display any heterogeneity (I2 = 0%).
Discussion
Our report represents an early synthesis of the first prospective studies of physical exercise interventions for PTSD in veterans. Results from meta-analyses of 6 single-arm studies (101 participants) and 3 RCTs (217 participants) provide early evidence that exercise may reduce PTSD symptoms in veterans. Yoga was the most common form of exercise used in single-arm studies, whereas RCTs used a wider range of interventions. The pooled SMD of -0.60 for single-arm longitudinal studies suggest a medium decrease in PTSD symptoms for veterans who engage in exercise interventions. Analysis of the RCTs supported this finding, with a pooled SMD of -0.40 reflecting a small-to-medium effect of exercise on PTSD symptoms over control conditions, although this result did not achieve statistical significance. Of note, while the nonsignificant finding for RCTs may have been due to insufficient power caused by the limited number of included studies, possibly exercise was not more efficacious than were the control conditions.
Although RCTs represented a variety of exercise types, PTSD symptom measures, and veteran subgroups, statistical results were not indicative of heterogeneity. However, only the largest and most comprehensive study of exercise for PTSD in veterans to date by Davis and colleagues had a statistically significant SMD.15 Of note, one of the other 2 RCTs displayed an SMD of a similar magnitude, but this study had a much smaller sample size and was underpowered to detect significance.30 Additionally, risk of bias assessments for single-arm studies and RCTs revealed study characteristics that suggest possible inflation of absolute effect sizes for individual studies. Therefore, the pooled SMDs we report are interpretable but may exceed the true effect of exercise for PTSD symptom reduction in veterans.
Based on results of our analyses, it is reasonable, albeit preliminary, to conclude that exercise interventions may result in reduced PTSD symptoms among veterans. At the very least, these findings support the continued investigation of such interventions for veterans. Given the unique and salubrious characteristics of physical exercise, such results, if supported by further research, suggest that exercise-based interventions may be particularly valuable within the trauma treatment realm. For example, exercise can be less expensive and more convenient than attending traditional treatment, and for veterans reluctant to engage in standard treatment approaches such as psychiatric and psychosocial modalities, complementary approaches entailing exercise may be viewed as particularly acceptable or enjoyable.32 In addition to possibly reducing PTSD symptoms, exercise is a well-established treatment for conditions commonly comorbid with PTSD, including depression, anxiety disorders, cognitive difficulties, and certain chronic pain conditions.6 As such, exercise represents a holistic treatment option that has the potential to augment standard PTSD care.
Limitations
The present study has several important limitations. First, few studies were found that met the broad eligibility criteria and those that did often had a small sample size. Besides highlighting a gap in the extant research, the limited studies available for meta-analysis means that caution must be taken when interpreting results. Fortunately, this issue will likely resolve once additional studies investigating the impact of exercise on PTSD symptoms in veterans are available for synthesis.
Relatedly, the included study interventions varied considerably, both in the types of exercise used and the characteristics of the exercises (eg, frequency, duration, and intensity), which is relevant as different exercise modalities are associated with differential physical effects.33 Including such a mixture of exercises may have given an incomplete picture of their potential therapeutic effects. Also, none of the RCTs compared exercise against first-line treatments for PTSD, such as prolonged exposure or cognitive processing therapy, which would have provided further insight into the role exercise could play in clinical settings.7
Another limitation is the elevated risk of bias found in most studies, particularly present in the longitudinal single-arm studies, all of which were rated at serious risk. For instance, no single-arm study controlled for preexisting baseline trends: without such (and lacking a comparison control group like in RCTs), it is possible that the observed effects were due to extraneous factors, rather than the exercise intervention. Although not as severe, the multi-arm RCTs also displayed at least moderate risk of bias. Therefore, SMDs may have been overestimated for each group of studies.
Finally, the results of the single-arm meta-analysis displayed high statistical heterogeneity, reducing the generalizability of the results. One possible cause of this heterogeneity may have been the yoga interventions, as a separate analysis removing the only nonyoga study did not reduce heterogeneity. This result was surprising, as the included yoga interventions seemed similar across studies. While the presence of high heterogeneity does require some caution when applying these results to outside interventions, the present study made use of random-effects meta-analysis, a technique that incorporates study heterogeneity into the statistical model, thereby strengthening the findings compared with that of a traditional fixed-effects approach.10
Future Steps
Several future steps are warranted to improve knowledge of exercise as a treatment for PTSD in veterans and in the general population. With current meta-analyses limited to small numbers of studies, additional studies of the efficacy of exercise for treating PTSD could help in several ways. A larger pool of studies would enable future meta-analyses to explore related questions, such as those regarding the impact of exercise on quality of life or depressive symptom reduction among veterans with PTSD. A greater number of studies also would enable meta-analysts to explore potentially critical moderators. For example, the duration, frequency, or type of exercise may moderate the effect of exercise on PTSD symptom reduction. Moderators related to patient or study design characteristics also should be explored in future studies.
Future work also should evaluate the impact that specific features of exercise regimens have on PTSD. Knowing whether the type or structure of exercise affects its clinical use would be invaluable in developing and implementing efficient exercise-based interventions. For example, if facilitated exercise was found to be significantly more effective at reducing PTSD symptoms than exercise completed independently, the development of exercise intervention programs in the VA and other facilities that commonly treat PTSD may be warranted. Additionally, it may be useful to identify specific mechanisms through which exercise reduces PTSD symptoms. For example, in addition to its beneficial biological effects, exercise also promotes psychological health through behavioral activation and alterations within reinforcement/reward systems, suggesting that exercise regularity may be more important than intensity.34,35 Understanding which mechanisms contribute most to change will aid in the development of more efficient interventions.
Given that veterans are demonstrating considerable interest in complementary and alternative PTSD treatments, it is critical that researchers focus on high-quality randomized tests of these interventions. Therefore, in addition to greater quality of exercise intervention studies, future efforts should be focused on RCTs that are designed in such a way as to limit potential introduction of bias. For example, assessment data should be completed by blinded assessors using standardized measures, and analyses should account for missing data and unequal participant attrition between groups. Ideally, pre-intervention trends across multiple baseline datapoints also would be collected in single-arm studies to avoid confounding related to regression to the mean. It is also recommended that future meta-analyses use risk of bias assessments and consider how the results of such assessments may impact the interpretation of results.
Conclusions
Findings from both single-arm studies and RCTs suggest possible benefit of exercise on PTSD symptom reduction, although confirmation of findings is needed. No study found increased symptoms following exercise intervention. Thus, it is reasonable to consider physical exercise, such as yoga, as an adjunct, whole-health consistent treatment. HCPs working with veterans with past traumatic experiences should consider incorporating exercise into patient care. Enhanced educational efforts emphasizing the psychotherapeutic impact of exercise may also have value for the veteran population. Furthermore, the current risk of bias assessments highlights the need for additional high-quality RCTs evaluating the specific impact of exercise on PTSD symptom reduction in veterans. In particular, this field of inquiry would benefit from larger samples and design characteristics to reduce bias (eg, blinding when possible, use of CAPS vs only self-report symptom measures, reducing problematic attrition, corrections for missing data, etc).
Acknowledgments
This research is the result of work supported with resources and the use of facilities at the VA Eastern Kansas Healthcare System (Dwight D. Eisenhower VA Medical Center). It was also supported by the Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship Program in Mental Illness Research and Treatment, as well as the Rocky Mountain Mental Illness Research, Education, and Clinical Center. Since Dr. Reis and Dr. Gaddy are employees of the US Government and contributed to this manuscript as part of their official duties, the work is not subject to US copyright. This study was preregistered on PROSPERO (https://www.crd.york.ac.uk/prospero/; ID: CRD42020153419).
1. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity—a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi:10.1186/1471-2458-13-813
2. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592. doi:10.1037/a0021769
3. Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of posttraumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res. 2015;230(2):130-136. doi:10.1016/j.psychres.2015.10.017
4. Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541-550. doi:10.4088/JCP.12r08225
5. Tanielian T, Jaycox L, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation; 2008
6. Whitworth JW, Ciccolo JT. Exercise and post-traumatic stress disorder in military veterans: a systematic review. Mil Med. 2016;181(9):953-960. doi:10.7205/MILMED-D-15-00488
7. Rutt BT, Oehlert ME, Krieshok TS, Lichtenberg JW. Effectiveness of cognitive processing therapy and prolonged exposure in the Department of Veterans Affairs. Psychol Rep. 2018;121(2):282-302. doi:10.1177/0033294117727746
8. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
9. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: Implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704. doi:10.1001/archinte.162.15.1697
10. Higgins JPT, Thomas J, Chanlder J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane; 2021.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
12. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-131.
13. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919
14. Sterne JAC, Savovic´ J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
15. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
16. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2019.
17. Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods .2015;20(3):375-393. doi:10.1037/met0000011
18. Shivakumar G, Anderson EH, Surís AM, North CS. Exercise for PTSD in women veterans: a proof-of-concept study. Mil Med. 2017;182(11):e1809-e1814. doi:10.7205/MILMED-D-16-00440
19. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
20. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34(8):669-673. doi:10.1016/0005-7967(96)00033-2
21. Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician- Administered PTSD Scale for DSM-5 (CAPS- 5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383-395.doi:10.1037/pas0000486
22. Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596-606. doi:10.1002/da.20837
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi:10.1136/bmj.327.7414.557
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi:10.1136/bmj.315.7109.629
25. Cushing RE, Braun KL, Alden CISW, Katz AR. Military- tailored yoga for veterans with post-traumatic stress disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. Justice L, Brems C. Bridging body and mind: case series of a 10-week trauma-informed yoga protocol for veterans. Int J Yoga Therap. 2019;29(1):65-79. doi:10.17761/D-17-2019-00029
28. Staples JK, Hamilton MF, Uddo M. A yoga program for the symptoms of post-traumatic stress disorder in veterans. Mil Med. 2013;178(8):854-860. doi:10.7205/MILMED-D-12-00536
29. Zaccari B, Callahan ML, Storzbach D, McFarlane N, Hudson R, Loftis JM. Yoga for veterans with PTSD: Cognitive functioning, mental health, and salivary cortisol. Psychol Trauma. 2020;12(8):913-917. doi:10.1037/tra0000909
30. Goldstein LA, Mehling WE, Metzler TJ, et al. Veterans Group Exercise: A randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J Affect Disord. 2018;227:345-352. doi:10.1016/j.jad.2017.11.002
31. Hall KS, Morey MC, Bosworth HB, et al. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J Behav Med. 2020;43(4):648-659. doi:10.1007/s10865-019-00073-w
32. Gaddy MA. Implementation of an integrative medicine treatment program at a Veterans Health Administration residential mental health facility. Psychol Serv. 2018;15(4):503- 509. doi:10.1037/ser0000189
33. Werner CM, Hecksteden A, Morsch A, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40(1):34- 46. doi:10.1093/eurheartj/ehy585
34. Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040. doi:10.1098/rsfs.2014.0040
35. Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. 2021;72:45-62. doi:10.1146/annurev-med-060619-022943.
1. Reiner M, Niermann C, Jekauc D, Woll A. Long-term health benefits of physical activity—a systematic review of longitudinal studies. BMC Public Health. 2013;13:813. doi:10.1186/1471-2458-13-813
2. Walsh R. Lifestyle and mental health. Am Psychol. 2011;66(7):579-592. doi:10.1037/a0021769
3. Rosenbaum S, Vancampfort D, Steel Z, Newby J, Ward PB, Stubbs B. Physical activity in the treatment of posttraumatic stress disorder: a systematic review and meta-analysis. Psychiatry Res. 2015;230(2):130-136. doi:10.1016/j.psychres.2015.10.017
4. Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541-550. doi:10.4088/JCP.12r08225
5. Tanielian T, Jaycox L, eds. Invisible Wounds of War: Psychological and Cognitive Injuries, Their Consequences, and Services to Assist Recovery. RAND Corporation; 2008
6. Whitworth JW, Ciccolo JT. Exercise and post-traumatic stress disorder in military veterans: a systematic review. Mil Med. 2016;181(9):953-960. doi:10.7205/MILMED-D-15-00488
7. Rutt BT, Oehlert ME, Krieshok TS, Lichtenberg JW. Effectiveness of cognitive processing therapy and prolonged exposure in the Department of Veterans Affairs. Psychol Rep. 2018;121(2):282-302. doi:10.1177/0033294117727746
8. Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002-2012. Natl Health Stat Report. 2015(79):1-16.
9. Baldwin CM, Long K, Kroesen K, Brooks AJ, Bell IR. A profile of military veterans in the southwestern United States who use complementary and alternative medicine: Implications for integrated care. Arch Intern Med. 2002;162(15):1697-1704. doi:10.1001/archinte.162.15.1697
10. Higgins JPT, Thomas J, Chanlder J, et al, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 6.2 (updated February 2021). Cochrane; 2021.
11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100
12. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126-131.
13. Sterne JAC, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi:10.1136/bmj.i4919
14. Sterne JAC, Savovic´ J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
15. Davis LW, Schmid AA, Daggy JK, et al. Symptoms improve after a yoga program designed for PTSD in a randomized controlled trial with veterans and civilians. Psychol Trauma. 2020;12(8):904-912. doi:10.1037/tra0000564
16. R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2019.
17. Tipton E. Small sample adjustments for robust variance estimation with meta-regression. Psychol Methods .2015;20(3):375-393. doi:10.1037/met0000011
18. Shivakumar G, Anderson EH, Surís AM, North CS. Exercise for PTSD in women veterans: a proof-of-concept study. Mil Med. 2017;182(11):e1809-e1814. doi:10.7205/MILMED-D-16-00440
19. Blake DD, Weathers FW, Nagy LM, et al. The development of a Clinician-Administered PTSD Scale. J Trauma Stress. 1995;8(1):75-90. doi:10.1007/BF02105408
20. Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL). Behav Res Ther. 1996;34(8):669-673. doi:10.1016/0005-7967(96)00033-2
21. Weathers FW, Bovin MJ, Lee DJ, et al. The Clinician- Administered PTSD Scale for DSM-5 (CAPS- 5): Development and initial psychometric evaluation in military veterans. Psychol Assess. 2018;30(3):383-395.doi:10.1037/pas0000486
22. Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depress Anxiety. 2011;28(7):596-606. doi:10.1002/da.20837
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi:10.1136/bmj.327.7414.557
24. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629-634. doi:10.1136/bmj.315.7109.629
25. Cushing RE, Braun KL, Alden CISW, Katz AR. Military- tailored yoga for veterans with post-traumatic stress disorder. Mil Med. 2018;183(5-6):e223-e231. doi:10.1093/milmed/usx071
26. Chopin SM, Sheerin CM, Meyer BL. Yoga for warriors: An intervention for veterans with comorbid chronic pain and PTSD. Psychol Trauma. 2020;12(8):888-896. doi:10.1037/tra0000649
27. Justice L, Brems C. Bridging body and mind: case series of a 10-week trauma-informed yoga protocol for veterans. Int J Yoga Therap. 2019;29(1):65-79. doi:10.17761/D-17-2019-00029
28. Staples JK, Hamilton MF, Uddo M. A yoga program for the symptoms of post-traumatic stress disorder in veterans. Mil Med. 2013;178(8):854-860. doi:10.7205/MILMED-D-12-00536
29. Zaccari B, Callahan ML, Storzbach D, McFarlane N, Hudson R, Loftis JM. Yoga for veterans with PTSD: Cognitive functioning, mental health, and salivary cortisol. Psychol Trauma. 2020;12(8):913-917. doi:10.1037/tra0000909
30. Goldstein LA, Mehling WE, Metzler TJ, et al. Veterans Group Exercise: A randomized pilot trial of an Integrative Exercise program for veterans with posttraumatic stress. J Affect Disord. 2018;227:345-352. doi:10.1016/j.jad.2017.11.002
31. Hall KS, Morey MC, Bosworth HB, et al. Pilot randomized controlled trial of exercise training for older veterans with PTSD. J Behav Med. 2020;43(4):648-659. doi:10.1007/s10865-019-00073-w
32. Gaddy MA. Implementation of an integrative medicine treatment program at a Veterans Health Administration residential mental health facility. Psychol Serv. 2018;15(4):503- 509. doi:10.1037/ser0000189
33. Werner CM, Hecksteden A, Morsch A, et al. Differential effects of endurance, interval, and resistance training on telomerase activity and telomere length in a randomized, controlled study. Eur Heart J. 2019;40(1):34- 46. doi:10.1093/eurheartj/ehy585
34. Silverman MN, Deuster PA. Biological mechanisms underlying the role of physical fitness in health and resilience. Interface Focus. 2014;4(5):20140040. doi:10.1098/rsfs.2014.0040
35. Smith PJ, Merwin RM. The role of exercise in management of mental health disorders: an integrative review. Annu Rev Med. 2021;72:45-62. doi:10.1146/annurev-med-060619-022943.
Review of Ethnoracial Representation in Clinical Trials (Phases 1 Through 4) of Atopic Dermatitis Therapies
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).
Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).
Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
To the Editor:
Atopic dermatitis (AD) affects an estimated 7.2% of adults and 10.7% of children in the United States; however, AD might affect different races at a varying rate.1 Compared to their European American counterparts, Asian/Pacific Islanders and African Americans are 7 and 3 times more likely, respectively, to be given a diagnosis of AD.2
Despite being disproportionately affected by AD, minority groups might be underrepresented in clinical trials of AD treatments.3 One explanation for this imbalance might be that ethnoracial representation differs across regions in the United States, perhaps in regions where clinical trials are conducted. Price et al3 investigated racial representation in clinical trials of AD globally and found that patients of color are consistently underrepresented.
Research on racial representation in clinical trials within the United States—on national and regional scales—is lacking from the current AD literature. We conducted a study to compare racial and ethnic disparities in AD clinical trials across regions of the United States.
Using the ClinicalTrials.gov database (www.clinicaltrials.gov) of the National Library of Medicine, we identified clinical trials of AD treatments (encompassing phases 1 through 4) in the United States that were completed before March 14, 2021, with the earliest data from 2013. Search terms included atopic dermatitis, with an advanced search for interventional (clinical trials) and with results.
In total, 95 completed clinical trials were identified, of which 26 (27.4%) reported ethnoracial demographic data. One trial was excluded due to misrepresentation regarding the classification of individuals who identified as more than 1 racial category. Clinical trials for systemic treatments (7 [28%]) and topical treatments (18 [72%]) were identified.
All ethnoracial data were self-reported by trial participants based on US Food and Drug Administration guidelines for racial and ethnic categorization.4 Trial participants who identified ethnically as Hispanic or Latino might have been a part of any racial group. Only 7 of the 25 included clinical trials (28%) provided ethnic demographic data (Hispanic [Latino] or non-Hispanic); 72% of trials failed to report ethnicity. Ethnic data included in our analysis came from only the 7 clinical trials that included these data. International multicenter trials that included a US site were excluded.
Ultimately, the number of trials included in our analysis was 25, comprised of 2443 participants. Data were further organized by US geographic region (Northeast, Midwest, South, West, and multiregion trials [ie, conducted in ≥2 regions]). No AD clinical trials were conducted solely in the Midwest; it was only included within multiregion trials.
Compared to their representation in the 2019 US Census, most minority groups were overrepresented in clinical trials, while White individuals were underrepresented (eTable). The percentages of our findings on representation for race are as follows (US Census data are listed in parentheses for comparison5):
- White: 56.8% (72.5%)
- Black/African American: 28.3% (12.7%)
- Asian: 10.3% (5.5%)
- Multiracial: 1.1% (3.3%)
- Native Hawaiian or other Pacific Islander: 0.3% (0.2%)
- American Indian or Alaska Native: 0.2% (0.8%)
- Other: 0.5% (4.9%).
Our findings on representation for ethnicity are as follows (US Census data is listed in parentheses for comparison5):
- Hispanic or Latino: 21.4% (18.0%)
Although representation of Black/African American and Asian participants in clinical trials was higher than their representation in US Census data and representation of White participants was lower in clinical trials than their representation in census data, equal representation among all racial and ethnic groups is still lacking. A potential explanation for this finding might be that requirements for trial inclusion selected for more minority patients, given the propensity for greater severity of AD among those racial groups.2 Another explanation might be that efforts to include minority patients in clinical trials are improving.
There were great differences in ethnoracial representation in clinical trials when regions within the United States were compared. Based on census population data by region, the West had the highest percentage (29.9%) of Hispanic or Latino residents; however, this group represented only 11.7% of participants in AD clinical trials in that region.5
The South had the greatest number of participants in AD clinical trials of any region, which was consistent with research findings on an association between severity of AD and heat.6 With a warmer climate correlating with an increased incidence of AD, it is possible that more people are willing to participate in clinical trials in the South.
The Midwest was the only region in which region-specific clinical trials were not conducted. Recent studies have shown that individuals with AD who live in the Midwest have comparatively less access to health care associated with AD treatment and are more likely to visit an emergency department because of AD than individuals in any other US region.7 This discrepancy highlights the need for increased access to resources and clinical trials focused on the treatment of AD in the Midwest.
In 1993, the National Institutes of Health Revitalization Act established a federal legislative mandate to encourage inclusion of women and people of color in clinical trials.8 During the last 2 decades, there have been improvements in ethnoracial reporting. A 2020 global study found that 81.1% of randomized controlled trials (phases 2 and 3) of AD treatments reported ethnoracial data.3
Equal representation in clinical trials allows for further investigation of the connection between race, AD severity, and treatment efficacy. Clinical trials need to have equal representation of ethnoracial categories across all regions of the United States. If one group is notably overrepresented, ethnoracial associations related to the treatment of AD might go undetected.9 Similarly, if representation is unequal, relationships of treatment efficacy within ethnoracial groups also might go undetected. None of the clinical trials that we analyzed investigated treatment efficacy by race, suggesting that there is a need for future research in this area.
It also is important to note that broad classifications of race and ethnicity are limiting and therefore overlook differences within ethnoracial categories. Although representation of minority patients in clinical trials for AD treatments is improving, we conclude that there remains a need for greater and equal representation of minority groups in clinical trials of AD treatments in the United States.
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
- Avena-Woods C. Overview of atopic dermatitis. Am J Manag Care. 2017;23(8 suppl):S115-S123.
- Kaufman BP, Guttman‐Yassky E, Alexis AF. Atopic dermatitis in diverse racial and ethnic groups—variations in epidemiology, genetics, clinical presentation and treatment. Exp Dermatol. 2018;27:340-357. doi:10.1111/exd.13514
- Price KN, Krase JM, Loh TY, et al. Racial and ethnic disparities in global atopic dermatitis clinical trials. Br J Dermatol. 2020;183:378-380. doi:10.1111/bjd.18938
- Collection of race and ethnicity data in clinical trials: guidance for industry and Food and Drug Administration staff. US Food and Drug Administration; October 26, 2016. Accessed February 20, 2022. https://www.fda.gov/media/75453/download
- United States Census Bureau. 2019 Population estimates by age, sex, race and Hispanic origin. Published June 25, 2020. Accessed March 22, 2022. https://www.census.gov/newsroom/press-kits/2020/population-estimates-detailed.html
- Fleischer AB Jr. Atopic dermatitis: the relationship to temperature and seasonality in the United States. Int J Dermatol. 2019;58:465-471. doi:10.1111/ijd.14289
- Wu KK, Nguyen KB, Sandhu JK, et al. Does location matter? geographic variations in healthcare resource use for atopic dermatitis in the United States. J Dermatolog Treat. 2021;32:314-320. doi:10.1080/09546634.2019.1656796
- National Institutes of Health Revitalization Act of 1993, 42 USC 201 (1993). Accessed February 20, 2022. https://www.govinfo.gov/content/pkg/STATUTE-107/pdf/STATUTE-107-Pg122.pdf
- Hirano SA, Murray SB, Harvey VM. Reporting, representation, and subgroup analysis of race and ethnicity in published clinical trials of atopic dermatitis in the United States between 2000 and 2009. Pediatr Dermatol. 2012;29:749-755. doi:10.1111/j.1525-1470.2012.01797.x
Practice Points
- Although minority groups are disproportionally affected by atopic dermatitis (AD), they may be underrepresented in clinical trials for AD in the United States.
- Equal representation among ethnoracial groups in clinical trials is important to allow for a more thorough investigation of the efficacy of treatments for AD.
Verrucous Carcinoma of the Foot: A Retrospective Study of 19 Cases and Analysis of Prognostic Factors Influencing Recurrence
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
Verrucous carcinoma is a rare cancer with the greatest predilection for the foot. Multiple case reports with only a few large case series have been published. 1-3 Plantar verrucous carcinoma is characterized as a slowly but relentlessly enlarging warty tumor with low metastatic potential and high risk for local invasion. The tumor occurs most frequently in patients aged 60 to 70 years, predominantly in White males. 1 It often is misdiagnosed for years as an ulcer or wart that is highly resistant to therapy. Size typically ranges from 1 to 12 cm in greatest dimension. 1
The pathogenesis of plantar verrucous carcinoma remains unclear, but some contributing factors have been proposed, including trauma, chronic irritation, infection, and poor local hygiene.2 This tumor has been reported to occur in chronic foot ulcerations, particularly in the diabetic population.4 It has been proposed that abnormal expression of the p53 tumor suppressor protein and several types of human papillomavirus (HPV) may have a role in the pathogenesis of verrucous carcinoma.5
The pathologic hallmarks of this tumor include a verrucous/hyperkeratotic surface with a deeply endophytic, broad, pushing base. Tumor cells are well differentiated, and atypia is either absent or confined to 1 or 2 layers at the base of the tumor. Overt invasion at the base is lacking, except in cases with a component of conventional invasive squamous cell carcinoma. Human papillomavirus viropathic changes are classically absent.1,3 Studies of the histopathology of verrucous carcinoma have been complicated by similar entities, nomenclatural uncertainty, and variable diagnostic criteria. For example, epithelioma cuniculatum variously has been defined as being synonymous with verrucous carcinoma, a distinct clinical verrucous carcinoma subtype occurring on the soles, a histologic subtype (characterized by prominent burrowing sinuses), or a separate entity entirely.1,2,6,7 Furthermore, in the genital area, several different types of carcinomas have verruciform features but display distinct microscopic findings and outcomes from verrucous carcinoma.8
Verrucous carcinoma represents an unusual variant of squamous cell carcinoma and is treated as such. Treatments have included laser surgery; immunotherapy; retinoid therapy; and chemotherapy by oral, intralesional, or iontophoretic routes in select patients.9 Radiotherapy presents another option, though reports have described progression to aggressive squamous cell carcinoma in some cases.9 Surgery is the best course of treatment, and as more case reports have been published, a transition from radical resection to wide excision with tumor-free margins is the treatment of choice.2,3,10,11 To minimize soft-tissue deficits, Mohs micrographic surgery has been discussed as a treatment option for verrucous carcinoma.11-13
Few studies have described verrucous carcinoma recurrence, and none have systematically examined recurrence rate, risk factors, or prognosis
Methods
Patient cases were
Of the 19 cases, 16 were treated at the University of Michigan and are included in the treatment analyses. Specific attention was then paid to the cases with a clinical recurrence despite negative surgical margins. We compared the clinical and surgical differences between recurrent cases and nonrecurrent cases.
Pathology was rereviewed for selected cases, including 2 cases with recurrence and matched primary, 2 cases with recurrence (for which the matched primary was unavailable for review), and 5 representative primary cases that were not complicated by recurrence. Pathology review was conducted in a blinded manner by one of the authors (P.W.H) who is a board-certified dermatopathologist for approximate depth of invasion from the granular layer, perineural invasion, bone invasion, infiltrative growth, presence of conventional squamous cell carcinoma, and margin status.
Statistical analysis was performed when appropriate using an N1 χ2 test or Student t test.
Results
Demographics and Comorbidities—The median age of the patients at the time of diagnosis was 55 years (range, 34–77 years). There were 12 males and 7 females (Table 1). Two patients were Black and 17 were White. Almost all patients had additional comorbidities including tobacco use (68%), alcohol use (47%), and diabetes (47%). Only 1 patient had an autoimmune disease and was on chronic steroids. No significant difference was found between the demographics of patients with recurrent lesions and those without recurrence.
Tumor Location and Clinical Presentation—The most common clinical presentation included a nonhealing ulceration with warty edges, pain, bleeding, and lowered mobility. In most cases, there was history of prior treatment over a duration ranging from 1 to 8 years, with a median of 5 years prior to biopsy-based diagnosis (Table 1). Six patients had a history of osteomyelitis, diagnosed by imaging or biopsy, within a year before tumor diagnosis. The size of the primary tumor ranged from 2.4 to 6 cm, with a mean of 4 cm (P=.20). The clinical presentation, time before diagnosis, and size of the tumors did not differ significantly between recurrent and nonrecurrent cases.
The tumor location for the recurrent cases differed significantly compared to nonrecurrent cases. All 5 of the patients with a recurrence presented with a tumor on the nonglabrous part of the foot. Four patients (80%) had lesions on the dorsal or lateral aspect of the great toe (P=.002), and 1 patient (20%) had a lesion on the low ankle (P=.09)(Table 1). Of the nonrecurrent cases, 1 patient (7%) presented with a tumor on the plantar surface of the great toe (P=.002), 13 patients (93%) presented with tumors on the distal plantar surface of the foot (P=.0002), and 1 patient with a plantar foot tumor (Figure 1) also had verrucous carcinoma on the thumb (Table 1 and Figure 2).
Histopathology—Available pathology slides for recurrent cases of verrucous carcinoma were reviewed alongside representative cases of verrucous carcinomas that did not progress to recurrence. The diagnosis of verrucous carcinoma was confirmed in all cases, with no evidence of conventional squamous cell carcinoma, perineural invasion, extension beyond the dermis, or bone invasion in any case. The median size of the tumors was 4.2 cm and 4 cm for nonrecurrent and recurrent specimens, respectively. Recurrences displayed a trend toward increased depth compared to primary tumors without recurrence (average depth, 5.5 mm vs 3.7 mm); however, this did not reach statistical significance (P=.24). Primary tumors that progressed to recurrence (n=2) displayed similar findings to the other cases, with invasive depths of 3.5 and 5.5 mm, and there was no evidence of conventional squamous cell carcinoma, perineural invasion, or extension beyond the dermis.
Treatment of Nonrecurrent Cases—Of the 16 total cases treated at the University of Michigan, surgery was the primary mode of therapy in every case (Tables 2 and 3). Of the 11 nonrecurrent cases, 7 patients had wide local excision with a dermal regeneration template, and delayed split-thickness graft reconstruction. Three cases had wide local excision with metatarsal resection, dermal regeneration template, and delayed skin grafting. One case had a great toe amputation
Treatment of Recurrent Cases—For the 5 patients with recurrence, surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm (4/5 [80%] reported). On average, follow-up for this group of patients was 29 months, with a range of 12 to 60 months (Table 3).
The first case with a recurrence (patient 12) initially presented with a chronic calluslike growth of the medial ankle. The lesion initially was treated with wide local excision with negative margins. Reconstruction was performed in a staged fashion with use of a dermal regenerative template followed later by split-thickness skin grafting. Tumor recurrence with negative margins occurred 3 times over the next 2 years despite re-resections with negative pathologic margins. Each recurrence presented as graft breakdown and surrounding hyperkeratosis (Figure 3). After the third graft placement failed, the patient elected for a BKA. There has not been recurrence since the BKA after 5 years total follow-up from the time of primary tumor resection. Of note, this was the only patient in our cohort who was immunosuppressed and evaluated for regional nodal involvement by positron emission tomography.
Another patient with recurrence (patient 13) presented with a chronic great toe ulcer of 5 years’ duration that formed on the dorsal aspect of the great toe after a previously excised wart (Figure 4A). This patient underwent mid-proximal metatarsal amputation with 2-cm margins and subsequent skin graft. Pathologic margins were negative. Within 6 months, there was hyperkeratosis and a draining wound (Figure 4B). Biopsy results confirmed recurrent disease that was treated with re-resection, including complete metatarsal amputation with negative margins and skin graft placement. Verrucous carcinoma recurred at the edges of the graft within 8 months, and the patient elected for a BKA. In addition, this patient also presented with a verrucous carcinoma of the contralateral great toe. The tumor presented as a warty ulcer of 4 months’ duration in the setting of osteomyelitis and was resected by great toe amputation that was performed concurrently with the opposite leg BKA; there has been no recurrence. Of note, this was the only patient to have right inguinal sentinel lymph node tissue sampled and HPV testing conducted, which were negative for verrucous carcinoma and high or low strains of HPV.
Another recurrent case (patient 14) presented with a large warty lesion on the dorsal great toe positive for verrucous carcinoma. He underwent a complete great toe amputation with skin graft placement. Verrucous carcinoma recurred on the edges of the graft within 6 months, and the patient was lost to follow-up when a BKA was suggested.
The fourth recurrent case (patient 15) initially had been treated for 1 year as a viral verruca of the dorsal aspect of the great toe. He had an exophytic mass positive for verrucous carcinoma growing on the dorsal aspect of the great toe around the prior excision site. After primary wide excision with negative 1-cm margins and graft placement, the tumor was re-excised twice within the next 2 years with pathologic negative margins. The patient underwent a foot amputation due to a severe osteomyelitis infection at the reconstruction site.
The final recurrent case (patient 16) presented with a mass on the lateral great toe that initially was treated as a viral verruca (for unknown duration) that had begun to ulcerate. The patient underwent wide excision with 1-cm margins and graft placement. Final pathology was consistent with verrucous carcinoma with negative margins. Recurrence occurred within 11 months on the edge of the graft, and a great toe amputation through the metatarsal phalangeal joint was performed.
Comment
Our series of 19 cases of verrucous carcinoma adds to the limited number of reported cases in the literature. We sought to evaluate the potential risk factors for early recurrence. Consistent with prior studies, our series found verrucous carcinoma of the foot to occur most frequently in patients aged 50 to 70 years, predominantly in White men.1 These tumors grew in the setting of chronic inflammation, tissue regeneration, multiple comorbidities, and poor wound hygiene. Misdiagnosis of verrucous carcinoma often leads to ineffective treatments and local invasion of nerves, muscle, and bone tissue.9,15,16 Our case series also clearly demonstrated the diagnostic challenge verrucous carcinoma presents, with an average delay in diagnosis of 5 years; correct diagnosis often did not occur until the tumor was 4 cm in size (average) and more than 50% had chronic ulceration.
The histologic features of the tumors showed striking uniformity. Within the literature, there is confusion regarding the use of the terms verrucous carcinoma and carcinoma (epithelioma) cuniculatum and the possible pathologic differences between the two. The World Health Organization’s classification of skin tumors describes epithelioma cuniculatum as verrucous carcinoma located on the sole of the foot.7 Kubik and Rhatigan6 pointed out that carcinoma cuniculatum does not have a warty or verrucous surface, which is a defining feature of verrucous carcinoma. Multiple authors have further surmised that the deep burrowing sinus tracts of epithelioma cuniculatum are different than those seen in verrucous carcinoma formed by the undulations extending from the papillomatous and verrucous surface.1,6 We did not observe these notable pathologic differences in recurrent or nonrecurrent primary tumors or differences between primary and recurrent cases. Although our cohort was small, the findings suggest that standard histologic features do not predict aggressive behavior in verrucous carcinomas. Furthermore, our observations support a model wherein recurrence is an inherent property of certain verrucous carcinomas rather than a consequence of histologic progression to conventional squamous cell carcinoma. The lack of overt malignant features in such cases underscores the need for distinction of verrucous carcinoma from benign mimics such as viral verruca or reactive epidermal hyperplasia.
Our recurrent cases showed a greater predilection for nonplantar surfaces and the great toe (P=.002). Five of 6 cases on the nonplantar surface—1 on the ankle and 5 on the great toe—recurred despite negative pathologic margins. There was no significant difference in demographics, pathogenesis, tumor size, chronicity, phenotype, or metastatic spread in recurrent and nonrecurrent cases in our cohort.
The tumor has only been described in rare instances at extrapedal cutaneous sites including the hand, scalp, and abdomen.14,17,18 Our series did include a case of synchronous presentation with a verrucous carcinoma on the thumb. Given the rarity of this presentation, thus far there are no data supporting any atypical locations of verrucous carcinoma having greater instances of recurrence. Our recurrent cases displaying atypical location on nonglabrous skin could suggest an underlying pathologic mechanism distinct from tumors on glabrous skin and relevant to increased recurrence risk. Such a mechanism might relate to distinct genetic insults, tumor-microenvironment interactions, or field effects. There are few studies regarding physiologic differences between the plantar surface and the nonglabrous surface and how that influences cancer genesis. Within acral melanoma studies, nonglabrous skin of more sun-exposed surfaces has a higher burden of genetic insults including BRAF mutations.19 Genetic testing of verrucous carcinoma is highly limited, with abnormal expression of the p53 tumor suppressor protein and possible association with several types of HPV. Verrucous carcinoma in general has been found to contain HPV types 6 and 11, nononcogenic forms, and higher risk from HPV types 16 and 18.9,20 However, only a few cases of HPV type 16 as well as 1 case each of HPV type 2 and type 11 have been found within verrucous carcinoma of the foot.21,22 In squamous cell carcinoma of the head and neck, HPV-positive tumors have shown better response to treatment. Further investigation of HPV and genetic contributors in verrucous carcinoma is warranted.
There is notable evidence that surgical resection is the best mode of treatment of verrucous carcinoma.2,3,10,11 Our case series was treated with wide local excision, with partial metatarsal amputation or great toe amputation, in cases with bone invasion or osteomyelitis. Surgical margins were not reported in all the cases but ranged from 0.5 to 2 cm with no significant differences between the recurrent and nonrecurrent groups. After excision, closure was conducted by incorporating primary, secondary, and delayed closure techniques, along with skin grafts for larger defects. Lymph node biopsy traditionally has not been recommended due to reported low metastatic potential. In all 5 recurrent cases, the tumors recurred after multiple attempts at wide excision and greater resection of bone and tissue, with negative margins. The tumors regrew quickly, within months, on the edges of the new graft or in the middle of the graft. The sites of recurrent tumor growth would suggest regrowth in the areas of greatest tissue stress and proliferation. We recommend a low threshold for biopsy and aggressive retreatment in the setting of exophytic growth at reconstruction sites.
Recurrence is uncommon in the setting of verrucous carcinoma, with our series being the first to analyze prognostic factors.3,9,14 Our findings indicate that
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- McKee PH, Wilkinson JD, Black M, et al. Carcinoma (epithelioma) cuniculatum: a clinic-pathologic study of nineteen cases and review of the literature. Histopathology. 1981;5:425-436.
- Penera KE, Manji KA, Craig AB, et al. Atypical presentation of verrucous carcinoma: a case study and review of the literature. Foot Ankle Spec. 2013;6:318-322.
- Rosales MA, Martin BR, Armstrong DG, et al. Verrucous hyperplasia: a common and problematic finding in the high-risk diabetic foot. J Am Podiatr Assoc. 2006:4:348-350.
- Noel JC, Peny MO, De Dobbeleer G, et al. p53 Protein overexpression in verrucous carcinoma of the skin. Dermatology. 1996;192:12-15.
- Kubik MJ, Rhatigan RM. Carcinoma cuniculatum: not a verrucous carcinoma. J Cutan Pathol. 2012;39:1083-1087
- Elder D, Massi D, Scolver R, et al. Verrucous squamous cell carcinoma. WHO Classification of Tumours (Medicine). Vol 11. 4th ed. International Agency for Research on Cancer: 2018;35-57.
- Chan MP. Verruciform and condyloma-like squamous proliferations in the anogenital region. Arch Pathol Lab Med. 2019;143:821-831
- Schwartz RA. Verrucous carcinoma of the skin and mucosa. J Am Acad Dermatol. 1995;32:1-21.
- Flynn K, Wiemer D. Treatment of an epithelioma cuniculatum plantare by local excision and a plantar skin flap. J Dermatol Surg Oncol. 1978;4:773-775.
- Spyriounis P, Tentis D, Sparveri I, et al. Plantar epithelioma cuniculatum: a case report with review of the literature. Eur J Plast Surg. 2004;27:253-256.
- Swanson NA, Taylor WB. Plantar verrucous carcinoma: literature review and treatment by the Moh’s chemosurgery technique. Arch Dermatol. 1980;116:794-797.
- Alkalay R, Alcalay J, Shiri J. Plantar verrucous carcinoma treated with Mohs micrographic surgery: a case report and literature review. J Drugs Dermatol. 2006:5:68-73.
- Kotwal M, Poflee S, Bobhate, S. Carcinoma cuniculatum at various anatomical sites. Indian J Dermatol. 2005;50:216-220.
- Nagarajan D, Chandrasekhar M, Jebakumar J, et al. Verrucous carcinoma of foot at an unusual site: lessons to be learnt. South Asian J Cancer. 2017;6:63.
- Pempinello C, Bova A, Pempinello R, et al Verrucous carcinoma of the foot with bone invasion: a case report. Case Rep Oncol Med. 2013;2013:135307.
- Vandeweyer E, Sales F, Deramaecker R. Cutaneous verrucous carcinoma. Br J Plastic Surg. 2001;54:168-170.
- Joybari A, Azadeh P, Honar B. Cutaneous verrucous carcinoma superimposed on chronically inflamed ileostomy site skin. Iran J Pathol. 2018;13:285-288.
- Davis EJ, Johnson DB, Sosman JA, et al. Melanoma: what do all the mutations mean? Cancer. 2018;124:3490-3499.
- Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A. 1983;80:560-563.
- Knobler RM, Schneider S, Neumann RA, et al. DNA dot-blot hybridization implicates human papillomavirus type 11-DNA in epithelioma cuniculatum. J Med Virol. 1989;29:33-37.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
Practice Points
- Clinicians should have a high suspicion for verrucous carcinoma in the setting of a chronic ulceration or warty lesion that is resistant to traditional treatment. Early biopsy with tissue collection of the raised ulcer borders and the deep dermis layer of warty lesions is imperative for diagnosis.
- Verrucous carcinoma originating on the nonglabrous surface of the foot may have a higher rate of recurrence often occurring within months of previous treatment. Patients presenting with nonhealing surgical sites in this area should be treated with a high level of suspicion for recurrence.
Inpatient Dermatology Consultations for Suspected Skin Cancer: A Retrospective Review
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.
In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.
In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.
In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.
In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
To the Editor:
Dermatologists sometimes are consulted in the inpatient setting to rule out possible skin cancer. This scenario provides an opportunity to facilitate the diagnosis and treatment of cutaneous malignancy, often in patients who might not have sought regular outpatient dermatology care. Few studies have described the outcomes of inpatient biopsies to identify skin cancer.1,2
Seeking to better understand the nature of these patient encounters, we reviewed all consultations at a medical center for which the referring physician suspected skin cancer rather than only those lesions that were biopsied by the dermatologist. We also collected data about subsequent treatment to better understand the outcomes of these patient encounters.
We conducted a retrospective review of inpatient dermatology referrals at an academic-affiliated tertiary medical center. We identified all patients who were provided with an inpatient dermatology consultation for suspected skin cancer or what was identified as a “skin lesion” between July 1, 2013, and July 1, 2019. We collected information on each patient’s sex, age at time of consultation, and race, as well as the specialty of the referring provider, lesion location, maximum diameter of the lesion, whether a biopsy was performed, where the biopsy was performed (inpatient or outpatient setting), clinical diagnosis, histopathologic diagnosis, and subsequent treatment.
The institutional review board at Eastern Virginia Medical School (Norfolk, Virginia) approved this study, and all protocol conformed to the ethical guidelines of the Declaration of Helsinki.
Thirty-eight patients met the inclusion criteria. Their characteristics are listed in the Table. Consultations for possible skin cancer accounted for 4% (38/950) of all inpatient dermatology consultations over the study period. Outcomes of the referrals are shown in the Figure. Consultations were received from 12 different physician specialties.
In the 38 patients, 47 lesions were identified; most (66% [31/47]) were on the head and neck. Twenty of 38 patients were found to have at least 1 biopsy-confirmed cutaneous malignancy (23 total tumors). Of those 23 identified malignancies, 10 were basal cell carcinoma, 11 squamous cell carcinoma, 1 malignant melanoma, and 1 anaplastic T-cell lymphoma. Of note, 17 of 23 (74%) identified cutaneous malignancies were 2.0 cm in diameter at biopsy or larger. Subsequently performed treatments for these patients included wide local excision (n=3), Mohs micrographic surgery (n=5), radiation therapy (n=3), topical fluorouracil (n=1), electrodesiccation and curettage (n=4), and chemotherapy or immunotherapy (n=2). Two patients who were diagnosed with skin cancer died of unrelated causes before treatment was completed.
In 10 of 38 patients, only nonmalignant entities were diagnosed, including seborrheic keratosis (n=6), benign melanocytic nevus (n=1), epidermal inclusion cyst (n=1), actinic keratosis (n=1), and radiation-induced necrosis (n=1). Of the 8 remaining patients, 4 were ultimately lost to follow-up before planned outpatient biopsy could be completed; 1 opted to follow up for biopsy at an unaffiliated outpatient dermatology provider. For 2 patients, the decision was made to forgo biopsy despite clinical suspicion of skin cancer because of overall poor health status, and 1 additional patient died before a planned outpatient biopsy could be performed.
In summary, approximately half of the inpatient dermatology consultations for suspected cutaneous malignancy resulted in a diagnosis of skin cancer. The patients in this population were admitted for a range of diagnoses, most unrelated to their cutaneous malignancy, suggesting that the inpatient setting offers the opportunity for physicians in a variety of specialties to help identify skin cancer that might otherwise be unaddressed and then facilitate management, whether ultimately in an inpatient or outpatient setting.
In many of these cases, it might be most appropriate to arrange subsequent outpatient dermatology follow-up after hospitalization, rather than making an inpatient consultation, as these situations usually are nonurgent and not directly related to hospitalization. However, in cases in which the lesion is directly related to admission, the lesion is advanced, there is concern for metastatic disease, or extenuating circumstances make outpatient follow-up difficult, inpatient dermatology consultation may be reasonable. There sometimes can be compelling reasons to expedite diagnosis and treatment as an inpatient.
In hospitalized, medically complex patients, in whom a new cutaneous malignancy is identified, dermatologists should discuss the situation thoughtfully with the patient, the patient’s family (when appropriate), and other physicians on the treatment team to determine the most appropriate course of action. In some cases, the most appropriate course might be to delay biopsy or treatment until the outpatient setting or to even defer further action completely when the prognosis is very limited. Consulting dermatologists must be mindful of patients’ overall medical situation in planning care for a cutaneous malignancy in these inpatient situations.
This study also highlights the surprising number of large-diameter, high-risk tumors identified in these scenarios. Limitations of this study include a relatively small sample size from a single facility that might not be representative of other practice settings and locations. Future multicenter studies could further explore the impact of inpatient dermatologic consultation on the diagnosis and management of skin cancer.
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
- Bauer J, Maroon M. Dermatology inpatient consultations: a retrospective study. J Am Acad Dermatol. 2010;62:518-519. doi:10.1016/j.jaad.2009.06.030
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:E116-E118. doi:10.1111/bjd.15401
Practice Points
- Dermatologists who perform inpatient consultations should be prepared to be consulted for cutaneous malignancies.
- Relatively large skin tumors may be identified, often incidentally, in the inpatient population.
- Careful consideration should be involved when deciding how to diagnose and manage cutaneous malignancies identified in the inpatient setting, taking the overall medical and social context into account.
Acute STEMI During the COVID-19 Pandemic at a Regional Hospital: Incidence, Clinical Characteristics, and Outcomes
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
From the Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, Athens, GA (Syed H. Ali, Syed Hyder, and Dr. Murrow), and the Department of Cardiology, Piedmont Heart Institute, Piedmont Athens Regional, Athens, GA (Dr. Murrow and Mrs. Davis).
Abstract
Objectives: The aim of this study was to describe the characteristics and in-hospital outcomes of patients with acute ST-segment elevation myocardial infarction (STEMI) during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods: A retrospective study was conducted at PAR to evaluate patients with acute STEMI admitted over an 8-week period during the initial COVID-19 outbreak. This study group was compared to patients admitted during the corresponding period in 2019. The primary endpoint of this study was defined as a composite of sustained ventricular arrhythmia, congestive heart failure (CHF) with pulmonary congestion, and/or in-hospital mortality.
Results: This study cohort was composed of 64 patients with acute STEMI; 30 patients (46.9%) were hospitalized during the COVID-19 pandemic. Patients with STEMI in both the COVID-19 and control groups had similar comorbidities, Killip classification score, and clinical presentations. The median (interquartile range) time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (84.8-132) in 2019 to 149 minutes (96.3-231.8; P = .032) in 2020. Hospitalization during the COVID-19 period was associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046).
Conclusion: Patients with STEMI admitted during the first wave of the COVID-19 outbreak experienced longer total ischemic time and increased risk for combined in-hospital outcomes compared to patients admitted during the corresponding period in 2019.
Keywords: myocardial infarction, acute coronary syndrome, hospitalization, outcomes.

The emergence of the SARS-Cov-2 virus in December 2019 caused a worldwide shift in resource allocation and the restructuring of health care systems within the span of a few months. With the rapid spread of infection, the World Health Organization officially declared a pandemic in March 2020. The pandemic led to the deferral and cancellation of in-person patient visits, routine diagnostic studies, and nonessential surgeries and procedures. This response occurred secondary to a joint effort to reduce transmission via stay-at-home mandates and appropriate social distancing.1
Alongside the reduction in elective procedures and health care visits, significant reductions in hospitalization rates due to decreases in acute ST-segment elevation myocardial infarction (STEMI) and catheterization laboratory utilization have been reported in many studies from around the world.2-7 Comprehensive data demonstrating the impact of the COVID-19 pandemic on acute STEMI patient characteristics, clinical presentation, and in-hospital outcomes are lacking. Although patients with previously diagnosed cardiovascular disease are more likely to encounter worse outcomes in the setting of COVID-19, there may also be an indirect impact of the pandemic on high-risk patients, including those without the infection.8 Several theories have been hypothesized to explain this phenomenon. One theory postulates that the fear of contracting the virus during hospitalization is great enough to prevent patients from seeking care.2 Another theory suggests that the increased utilization of telemedicine prevents exacerbation of chronic conditions and the need for hospitalization.9 Contrary to this trend, previous studies have shown an increased incidence of acute STEMI following stressful events such as natural disasters.10
The aim of this study was to describe trends pertaining to clinical characteristics and in-hospital outcomes of patients with acute STEMI during the early COVID-19 pandemic at Piedmont Athens Regional (PAR), a 330-bed tertiary referral center in Northeast Georgia.
Methods
A retrospective cohort study was conducted at PAR to evaluate patients with STEMI admitted to the cardiovascular intensive care unit over an 8-week period (March 5 to May 5, 2020) during the COVID-19 outbreak. COVID-19 was declared a national emergency on March 13, 2020, in the United States. The institutional review board at PAR approved the study; the need for individual consent was waived under the condition that participant data would undergo de-identification and be strictly safeguarded.
Data Collection
Because there are seasonal variations in cardiovascular admissions, patient data from a control period (March 9 to May 9, 2019) were obtained to compare with data from the 2020 period. The number of patients with the diagnosis of acute STEMI during the COVID-19 period was recorded. Demographic data, clinical characteristics, and primary angiographic findings were gathered for all patients. Time from symptom onset to hospital admission and time from hospital admission to reperfusion (defined as door-to-balloon time) were documented for each patient. Killip classification was used to assess patients’ clinical status on admission. Length of stay was determined as days from hospital admission to discharge or death (if occurring during the same hospitalization).
Adverse in-hospital complications were also recorded. These were selected based on inclusion of the following categories of acute STEMI complications: ischemic, mechanical, arrhythmic, embolic, and inflammatory. The following complications occurred in our patient cohort: sustained ventricular arrhythmia, congestive heart failure (CHF) defined as congestion requiring intravenous diuretics, re-infarction, mechanical complications (free-wall rupture, ventricular septal defect, or mitral regurgitation), second- or third-degree atrioventricular block, atrial fibrillation, stroke, mechanical ventilation, major bleeding, pericarditis, cardiogenic shock, cardiac arrest, and in-hospital mortality. The primary outcome of this study was defined as a composite of sustained ventricular arrhythmia, CHF with congestion requiring intravenous diuretics, and/or in-hospital mortality. Ventricular arrythmia and CHF were included in the composite outcome because they are defined as the 2 most common causes of sudden cardiac death following acute STEMI.11,12
Statistical Analysis
Normally distributed continuous variables and categorical variables were compared using the paired t-test. A 2-sided P value <.05 was considered to be statistically significant. Mean admission rates for acute STEMI hospitalizations were determined by dividing the number of admissions by the number of days in each time period. The daily rate of COVID-19 cases per 100,000 individuals was obtained from the Centers for Disease Control and Prevention COVID-19 database. All data analyses were performed using Microsoft Excel.
Results
The study cohort consisted of 64 patients, of whom 30 (46.9%) were hospitalized between March 5 and May 5, 2020, and 34 (53.1%) who were admitted during the analogous time period in 2019. This reflected a 6% decrease in STEMI admissions at PAR in the COVID-19 cohort.
Acute STEMI Hospitalization Rates and COVID-19 Incidence
The mean daily acute STEMI admission rate was 0.50 during the study period compared to 0.57 during the control period. During the study period in 2020 in the state of Georgia, the daily rate of newly confirmed COVID-19 cases ranged from 0.194 per 100,000 on March 5 to 8.778 per 100,000 on May 5. Results of COVID-19 testing were available for 9 STEMI patients, and of these 0 tests were positive.
Baseline Characteristics
Baseline characteristics of the acute STEMI cohorts are presented in Table 1. Approximately 75% were male; median (interquartile range [IQR]) age was 60 (51-72) years. There were no significant differences in age and gender between the study periods. Three-quarters of patients had a history of hypertension, and 87.5% had a history of dyslipidemia. There was no significant difference in baseline comorbidity profiles between the 2 study periods; therefore, our sample populations shared similar characteristics.

Clinical Presentation
Significant differences were observed regarding the time intervals of STEMI patients in the COVID-19 period and the control period (Table 2). Median time from symptom onset to hospital admission (patient delay) was extended from 57.5 minutes (IQR, 40.3-106) in 2019 to 93 minutes (IQR, 48.8-132) in 2020; however, this difference was not statistically significant (P = .697). Median time from hospital admission to reperfusion (system delay) was prolonged from 45 minutes (IQR, 28-61) in 2019 to 78 minutes (IQR, 50-110) in 2020 (P < .001). Overall time from symptom onset to reperfusion (total ischemic time) increased from 99.5 minutes (IQR, 84.8-132) in 2019 to 149 minutes (IQR, 96.3-231.8) in 2020 (P = .032).

Regarding mode of transportation, 23.5% of patients in 2019 were walk-in admissions to the emergency department. During the COVID-19 period, walk-in admissions decreased to 6.7% (P = .065). There were no significant differences between emergency medical service, transfer, or in-patient admissions for STEMI cases between the 2 study periods.
Killip classification scores were calculated for all patients on admission; 90.6% of patients were classified as Killip Class 1. There was no significant difference between hemodynamic presentations during the COVID-19 period compared to the control period.
Angiographic Data
Overall, 53 (82.8%) patients admitted with acute STEMI underwent coronary angiography during their hospital stay. The proportion of patients who underwent primary reperfusion was greater in the control period than in the COVID-19 period (85.3% vs 80%; P = .582). Angiographic characteristics and findings were similar between the 2 study groups (Table 2).
In-Hospital Outcomes
In-hospital outcome data were available for all patients. As shown in Table 3, hospitalization during the COVID-19 period was independently associated with an increased risk for combined in-hospital outcome (odds ratio, 3.96; P = .046). The rate of in-hospital mortality was greater in the COVID-19 period (P = .013). We found no significant difference when comparing secondary outcomes from admissions during the COVID-19 period and the control period in 2019. For the 5 patients who died during the study period, the primary diagnosis at death was acute STEMI complicated by CHF (3 patients) or cardiogenic shock (2 patients).

Discussion
This single-center retrospective study at PAR looks at the impact of COVID-19 on hospitalizations for acute STEMI during the initial peak of the pandemic. The key findings of this study show a significant increase in ischemic time parameters (symptom onset to reperfusion, hospital admission to reperfusion), in-hospital mortality, and combined in-hospital outcomes.
There was a 49.5-minute increase in total ischemic time noted in this study (P = .032). Though there was a numerical increase in time of symptom onset to hospital admission by 23.5 minutes, this difference was not statistically significant (P = .697). However, this study observed a statistically significant 33-minute increase in ischemic time from hospital admission to reperfusion (P < .001). Multiple studies globally have found a similar increase in total ischemic times, including those conducted in China and Europe.13-15 Every level of potential delay must be considered, including pre-hospital, triage and emergency department, and/or reperfusion team. Pre-hospital sources of delays that have been suggested include “stay-at-home” orders and apprehension to seek medical care due to concern about contracting the virus or overwhelming the health care facilities. There was a clinically significant 4-fold decrease in the number of walk-in acute STEMI cases in the study period. In 2019, there were 8 walk-in cases compared to 2 cases in 2020 (P = .065). However, this change was not statistically significant. In-hospital/systemic sources of delays have been mentioned in other studies; they include increased time taken to rule out COVID-19 (nasopharyngeal swab/chest x-ray) and increased time due to the need for intensive gowning and gloving procedures by staff. It was difficult to objectively determine the sources of system delay by the reperfusion team due to a lack of quantitative data.
In the current study, we found a significant increase in in-hospital mortality during the COVID-19 period compared to a parallel time frame in 2019. This finding is contrary to a multicenter study from Spain that reported no difference in in-hospital outcomes or mortality rates among all acute coronary syndrome cases.16 The worsening outcomes and prognosis may simply be a result of increased ischemic time; however, the virus that causes COVID-19 itself may play a role as well. Studies have found that SARS-Cov-2 infection places patients at greater risk for cardiovascular conditions such as hypercoagulability, myocarditis, and arrhythmias.17 In our study, however, there were no acute STEMI patients who tested positive for COVID-19. Therefore, we cannot discuss the impact of increased thrombus burden in patients with COVID-19. Piedmont Healthcare published a STEMI treatment protocol in May 2020 that advised increased use of tissue plasminogen activator (tPA) in COVID-19-positive cases; during the study period, however, there were no occasions when tPA use was deemed appropriate based on clinical judgment.
Our findings align with previous studies that describe an increase in combined in-hospital adverse outcomes during the COVID-19 era. Previous studies detected a higher rate of complications in the COVID-19 cohort, but in the current study, the adverse in-hospital course is unrelated to underlying infection.18,19 This study reports a higher incidence of major in-hospital outcomes, including a 65% increase in the rate of combined in-hospital outcomes, which is similar to a multicenter study conducted in Israel.19 There was a 2.3-fold numerical increase in sustained ventricular arrhythmias and a 2.5-fold numerical increase in the incidence of cardiac arrest in the study period. This phenomenon was observed despite a similar rate of reperfusion procedures in both groups.
Acute STEMI is a highly fatal condition with an incidence of 8.5 in 10,000 annually in the United States. While studies across the world have shown a 25% to 40% reduction in the rate of hospitalized acute coronary syndrome cases during the COVID-19 pandemic, the decrease from 34 to 30 STEMI admissions at PAR is not statistically significant.20 Possible reasons for the reduction globally include increased out-of-hospital mortality and decreased incidence of acute STEMI across the general population as a result of improved access to telemedicine or decreased levels of life stressors.20
In summary, there was an increase in ischemic time to reperfusion, in-hospital mortality, and combined in-hospital outcomes for acute STEMI patients at PAR during the COVID period.
Limitations
This study has several limitations. This is a single-center study, so the sample size is small and may not be generalizable to a larger population. This is a retrospective observational study, so causation cannot be inferred. This study analyzed ischemic time parameters as average rates over time rather than in an interrupted time series. Post-reperfusion outcomes were limited to hospital stay. Post-hospital follow-up would provide a better picture of the effects of STEMI intervention. There is no account of patients who died out-of-hospital secondary to acute STEMI. COVID-19 testing was not introduced until midway in our study period. Therefore, we cannot rule out the possibility of the SARS-Cov-2 virus inciting acute STEMI and subsequently leading to worse outcomes and poor prognosis.
Conclusions
This study provides an analysis of the incidence, characteristics, and clinical outcomes of patients presenting with acute STEMI during the early period of the COVID-19 pandemic. In-hospital mortality and ischemic time to reperfusion increased while combined in-hospital outcomes worsened.
Acknowledgment: The authors thank Piedmont Athens Regional IRB for approving this project and allowing access to patient data.
Corresponding author: Syed H. Ali; Department of Medicine, Medical College of Georgia at the Augusta University-University of Georgia Medical Partnership, 30606, Athens, GA; [email protected]
Disclosures: None reported.
doi:10.12788/jcom.0085
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046
1. Bhatt AS, Moscone A, McElrath EE, et al. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
2. Metzler B, Siostrzonek P, Binder RK, Bauer A, Reinstadler SJR. Decline of acute coronary syndrome admissions in Austria since the outbreak of Covid-19: the pandemic response causes cardiac collateral damage. Eur Heart J. 2020;41:1852-1853. doi:10.1093/eurheartj/ehaa314
3. De Rosa S, Spaccarotella C, Basso C, et al. Reduction of hospitalizations for myocardial infarction in Italy in the Covid-19 era. Eur Heart J. 2020;41(22):2083-2088.
4. Wilson SJ, Connolly MJ, Elghamry Z, et al. Effect of the COVID-19 pandemic on ST-segment-elevation myocardial infarction presentations and in-hospital outcomes. Circ Cardiovasc Interv. 2020; 13(7):e009438. doi:10.1161/CIRCINTERVENTIONS.120.009438
5. Mafham MM, Spata E, Goldacre R, et al. Covid-19 pandemic and admission rates for and management of acute coronary syndromes in England. Lancet. 2020;396 (10248):381-389. doi:10.1016/S0140-6736(20)31356-8
6. Bhatt AS, Moscone A, McElrath EE, et al. Fewer Hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(3):280-288. doi:10.1016/j.jacc.2020.05.038
7. Tam CF, Cheung KS, Lam S, et al. Impact of Coronavirus disease 2019 (Covid-19) outbreak on ST-segment elevation myocardial infarction care in Hong Kong, China. Circ Cardiovasc Qual Outcomes. 2020;13(4):e006631. doi:10.1161/CIRCOUTCOMES.120.006631
8. Clerkin KJ, Fried JA, Raikhelkar J, et al. Coronavirus disease 2019 (COVID-19) and cardiovascular disease. Circulation. 2020;141:1648-1655. doi:10.1161/CIRCULATIONAHA.120.046941
9. Ebinger JE, Shah PK. Declining admissions for acute cardiovascular illness: The Covid-19 paradox. J Am Coll Cardiol. 2020;76(3):289-291. doi:10.1016/j.jacc.2020.05.039
10 Leor J, Poole WK, Kloner RA. Sudden cardiac death triggered by an earthquake. N Engl J Med. 1996;334(7):413-419. doi:10.1056/NEJM199602153340701
11. Hiramori K. Major causes of death from acute myocardial infarction in a coronary care unit. Jpn Circ J. 1987;51(9):1041-1047. doi:10.1253/jcj.51.1041
12. Bui AH, Waks JW. Risk stratification of sudden cardiac death after acute myocardial infarction. J Innov Card Rhythm Manag. 2018;9(2):3035-3049. doi:10.19102/icrm.2018.090201
13. Xiang D, Xiang X, Zhang W, et al. Management and outcomes of patients with STEMI during the COVID-19 pandemic in China. J Am Coll Cardiol. 2020;76(11):1318-1324. doi:10.1016/j.jacc.2020.06.039
14. Hakim R, Motreff P, Rangé G. COVID-19 and STEMI. [Article in French]. Ann Cardiol Angeiol (Paris). 2020;69(6):355-359. doi:10.1016/j.ancard.2020.09.034
15. Soylu K, Coksevim M, Yanık A, Bugra Cerik I, Aksan G. Effect of Covid-19 pandemic process on STEMI patients timeline. Int J Clin Pract. 2021;75(5):e14005. doi:10.1111/ijcp.14005
16. Salinas P, Travieso A, Vergara-Uzcategui C, et al. Clinical profile and 30-day mortality of invasively managed patients with suspected acute coronary syndrome during the COVID-19 outbreak. Int Heart J. 2021;62(2):274-281. doi:10.1536/ihj.20-574
17. Hu Y, Sun J, Dai Z, et al. Prevalence and severity of corona virus disease 2019 (Covid-19): a systematic review and meta-analysis. J Clin Virol. 2020;127:104371. doi:10.1016/j.jcv.2020.104371
18. Rodriguez-Leor O, Cid Alvarez AB, Perez de Prado A, et al. In-hospital outcomes of COVID-19 ST-elevation myocardial infarction patients. EuroIntervention. 2021;16(17):1426-1433. doi:10.4244/EIJ-D-20-00935
19. Fardman A, Zahger D, Orvin K, et al. Acute myocardial infarction in the Covid-19 era: incidence, clinical characteristics and in-hospital outcomes—A multicenter registry. PLoS ONE. 2021;16(6): e0253524. doi:10.1371/journal.pone.0253524
20. Pessoa-Amorim G, Camm CF, Gajendragadkar P, et al. Admission of patients with STEMI since the outbreak of the COVID-19 pandemic: a survey by the European Society of Cardiology. Eur Heart J Qual Care Clin Outcomes. 2020;6(3):210-216. doi:10.1093/ehjqcco/qcaa046