User login
Don’t discount sleep disturbance for children with atopic dermatitis
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
The itching associated with atopic dermatitis (AD) may interfere with children’s sleep, and sleep studies suggest that children with active disease are more restless at night, wrote Faustine D. Ramirez of the University of California, San Francisco, and her colleagues. Their report is in JAMA Pediatrics.
“Acute and chronic sleep disturbances have been associated with a wide range of cognitive, mood, and behavioral impairments and have been linked to poor educational performance,” the researchers noted.
To determine the impact of active AD on children’s sleep, the researchers reviewed data from 13,988 children followed for a median of 11 years. Of these, 4,938 children met the definition for AD between age 2 and 16 years.
Overall, children with active AD were approximately 50% more likely to experience poor sleep quality than were those without AD (adjusted odds ratio, 1.48). Sleep quality was even worse for children with severe active AD (aOR, 1.68), and active AD plus asthma or allergic rhinitis (aOR 2.15). Sleep quality was significantly worse in children reporting mild AD (aOR, 1.40) or inactive AD (aOR, 1.41), compared with children without AD. Nighttime sleep duration was similar throughout childhood for children with and without AD.
“In addition to increased nighttime awakenings and difficulty falling asleep, we found that children with active atopic dermatitis were more likely to report nightmares and early morning awakenings, which has not been previously studied,” Ms. Ramirez and her associates said.
Total sleep duration was statistically shorter overall for children with AD, compared with those without AD, but the difference was not clinically significant, they noted.
The participants were from a longitudinal study in the United Kingdom in which pregnant women were recruited between 1990 and 1992. For those with children alive at 1 year, their children were followed for approximately 16 years. Sleep quality was assessed at six time points with four standardized questionnaires between ages 2 and 10 years, and sleep duration was assessed at eight time points between ages 2 and 16 years with standardized questionnaires.
The study findings were limited by several factors, including some missing data and patient attrition, as well as possible misclassification bias because of the use of parent and patient self-reports, and a possible lack of generalizability to other populations, the researchers noted.
However, the results support the need for developing clinical outcome measures to address sleep quality in children with AD, they said. “Additional work should investigate interventions to improve sleep quality and examine the association between atopic dermatitis treatment and children’s sleep.”
The study was funded primarily by a grant from the National Eczema Association. Ms. Ramirez disclosed a grant from the National Institutes of Health. Two other investigators received grants, one from NIH and the other Wellcome Senior Clinical Fellowship in Science. One coauthor reported receiving multiple grants, as well as paid consulting for TARGETPharma, a company developing a prospective atopic dermatitis registry.
SOURCE: Ramirez FD al. JAMA Pediatr. 2019 Mar 4. doi: 10.1001/jamapediatrics.2019.0025.
FROM JAMA PEDIATRICS
Dermatologists name isobornyl acrylate contact allergen of the year
WASHINGTON – The American Contact Dermatitis Society has selected isobornyl acrylate the contact allergen of the year. It is an acrylic monomer used as an adhesive.

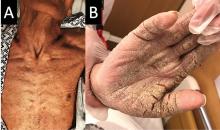
Among other applications, isobornyl acrylate is often used in medical devices. The selection was made based in part on multiple case reports of diabetes patients developing contact allergies to their diabetes devices, such as insulin pumps, explained Golara Honari, MD, of Stanford (Calif.) University, who presented the selection at the ACDS annual meeting.
The significance of this allergen is that testing through routine panels does not identify it, so clinician awareness is especially important, Dr. Honari noted in a video interview at the meeting.
Most of the reported contact allergen cases have been in patients with diabetes, but clinicians should think about other possible sources, such as acrylic nails, she said. As for treatment, clinicians and patients can consider alternative diabetes devices without isobornyl acrylate, she said.
In the future, close collaboration between clinicians and the medical device industry to develop appropriate labeling can help increase awareness of the potential for allergic reactions, she added.
Dr. Honari had no relevant financial conflicts to disclose.
WASHINGTON – The American Contact Dermatitis Society has selected isobornyl acrylate the contact allergen of the year. It is an acrylic monomer used as an adhesive.

Among other applications, isobornyl acrylate is often used in medical devices. The selection was made based in part on multiple case reports of diabetes patients developing contact allergies to their diabetes devices, such as insulin pumps, explained Golara Honari, MD, of Stanford (Calif.) University, who presented the selection at the ACDS annual meeting.
The significance of this allergen is that testing through routine panels does not identify it, so clinician awareness is especially important, Dr. Honari noted in a video interview at the meeting.
Most of the reported contact allergen cases have been in patients with diabetes, but clinicians should think about other possible sources, such as acrylic nails, she said. As for treatment, clinicians and patients can consider alternative diabetes devices without isobornyl acrylate, she said.
In the future, close collaboration between clinicians and the medical device industry to develop appropriate labeling can help increase awareness of the potential for allergic reactions, she added.
Dr. Honari had no relevant financial conflicts to disclose.
WASHINGTON – The American Contact Dermatitis Society has selected isobornyl acrylate the contact allergen of the year. It is an acrylic monomer used as an adhesive.

Among other applications, isobornyl acrylate is often used in medical devices. The selection was made based in part on multiple case reports of diabetes patients developing contact allergies to their diabetes devices, such as insulin pumps, explained Golara Honari, MD, of Stanford (Calif.) University, who presented the selection at the ACDS annual meeting.
The significance of this allergen is that testing through routine panels does not identify it, so clinician awareness is especially important, Dr. Honari noted in a video interview at the meeting.
Most of the reported contact allergen cases have been in patients with diabetes, but clinicians should think about other possible sources, such as acrylic nails, she said. As for treatment, clinicians and patients can consider alternative diabetes devices without isobornyl acrylate, she said.
In the future, close collaboration between clinicians and the medical device industry to develop appropriate labeling can help increase awareness of the potential for allergic reactions, she added.
Dr. Honari had no relevant financial conflicts to disclose.
AT ACDS 2019
Risk for Appendicitis, Cholecystitis, or Diverticulitis in Patients With Psoriasis
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.
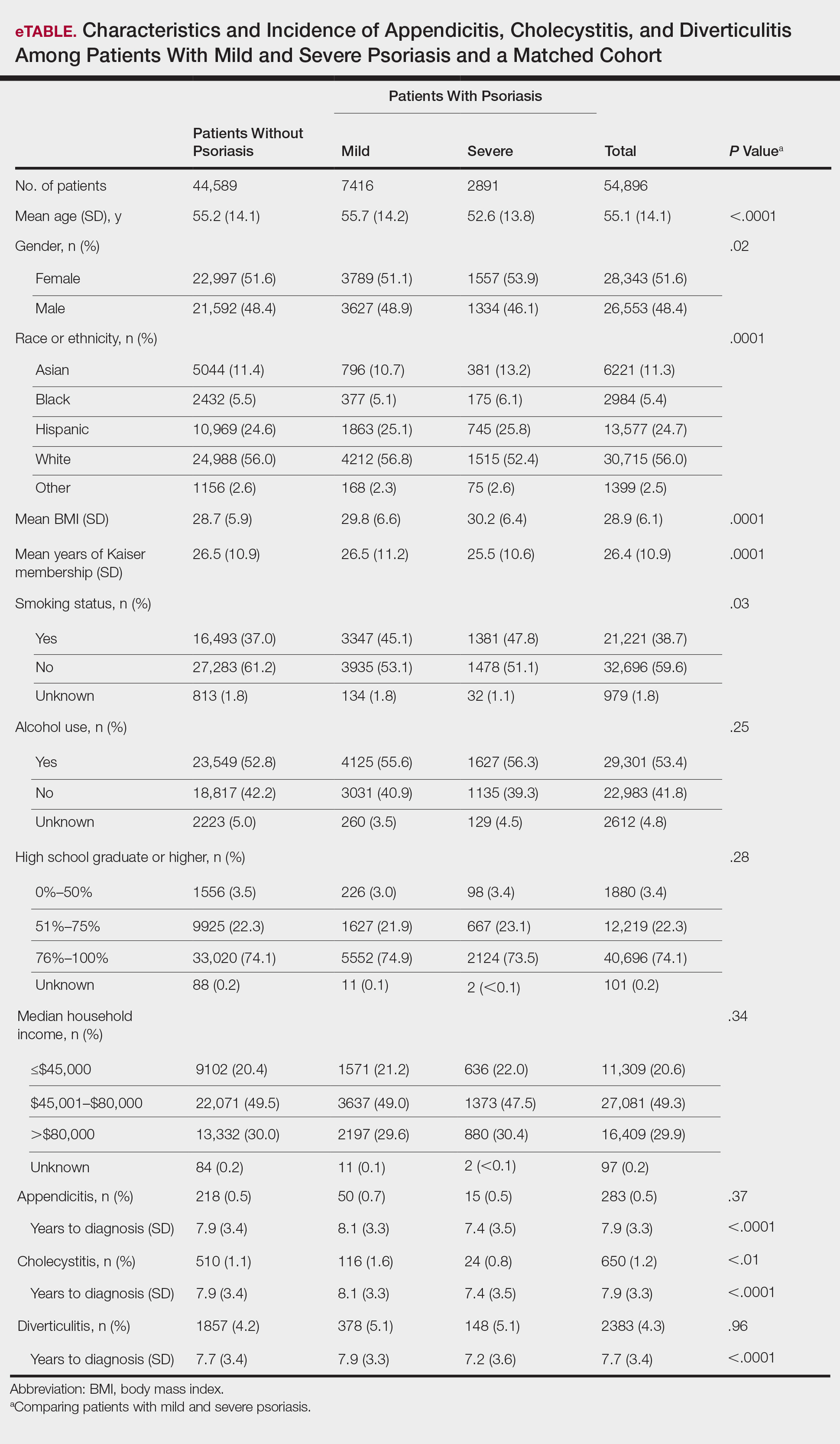
Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).
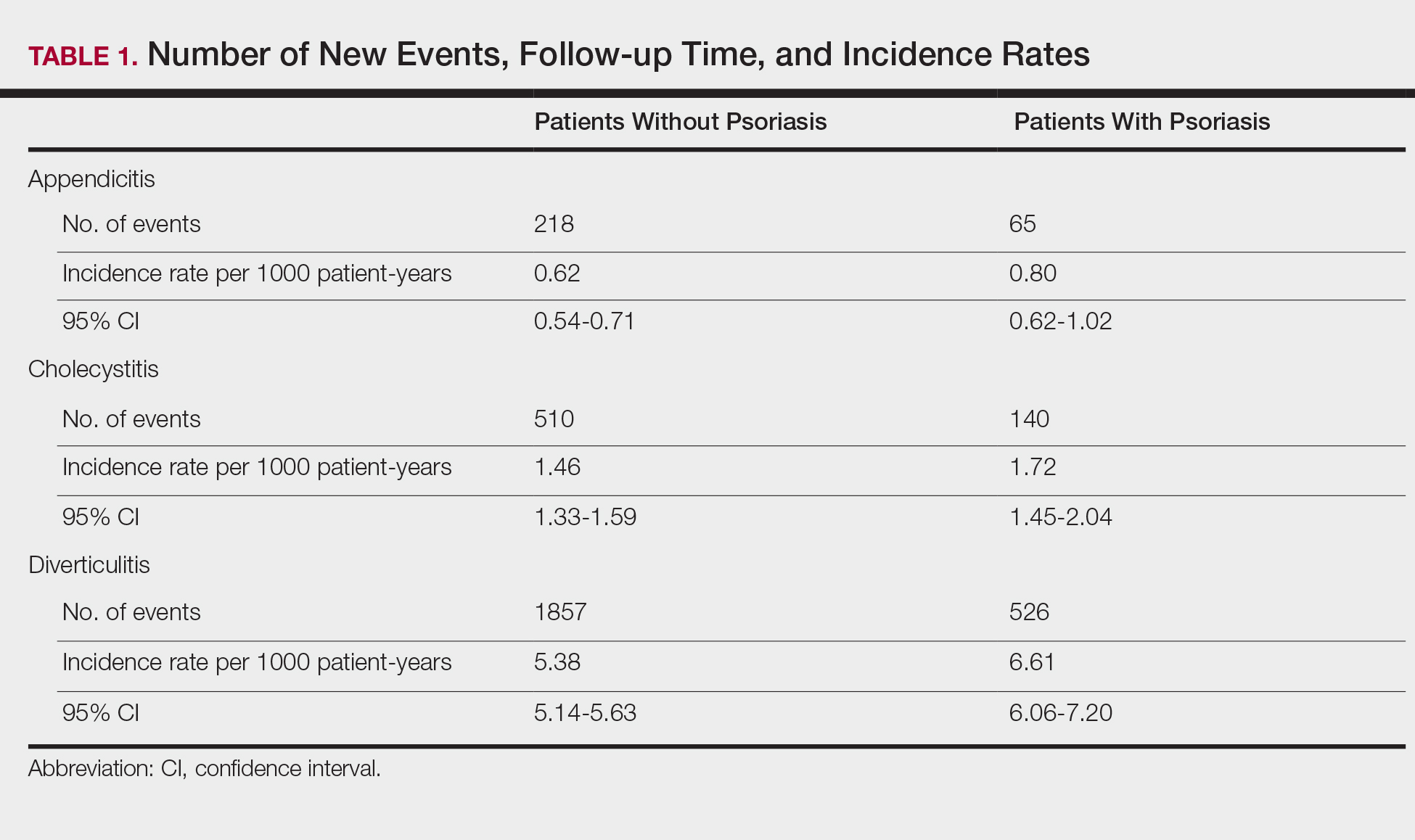
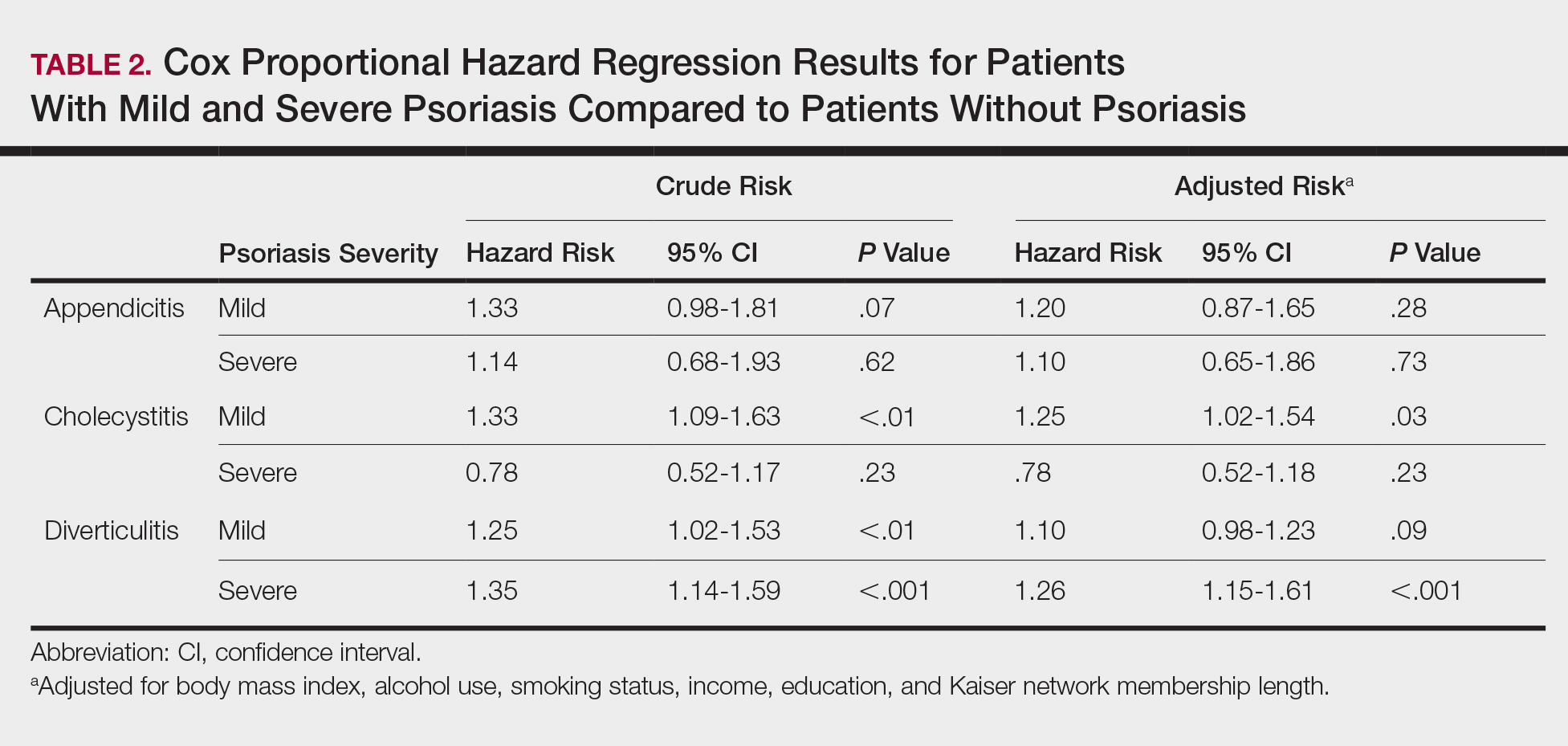
Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
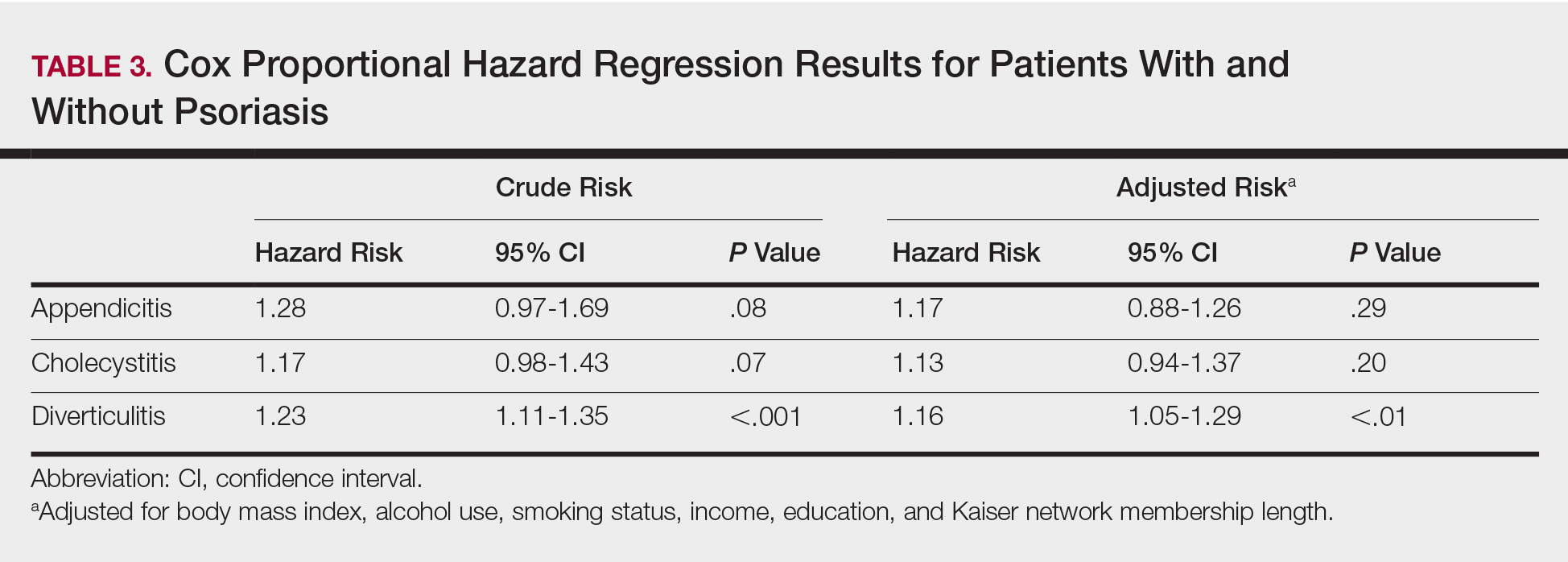
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).
Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.

Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).


Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).

Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
Psoriasis is a chronic skin condition affecting approximately 2% to 3% of the population.1,2 Beyond cutaneous manifestations, psoriasis is a systemic inflammatory state that is associated with an increased risk for cardiovascular disease, including obesity,3,4 type 2 diabetes mellitus,5,6 hypertension,5 dyslipidemia,3,7 metabolic syndrome,7 atherosclerosis,8 peripheral vascular disease,9 coronary artery calcification,10 myocardial infarction,11-13 stroke,9,14 and cardiac death.15,16
Psoriasis also has been associated with inflammatory bowel disease (IBD), possibly because of similar autoimmune mechanisms in the pathogenesis of both diseases.17,18 However, there is no literature regarding the risk for acute gastrointestinal pathologies such as appendicitis, cholecystitis, or diverticulitis in patients with psoriasis.
The primary objective of this study was to examine if patients with psoriasis are at increased risk for appendicitis, cholecystitis, or diverticulitis compared to the general population. The secondary objective was to determine if patients with severe psoriasis (ie, patients treated with phototherapy or systemic therapy) are at a higher risk for these conditions compared to patients with mild psoriasis.
Methods
Patients and Tools
A descriptive, population-based cohort study design with controls from a matched cohort was used to ascertain the effect of psoriasis status on patients’ risk for appendicitis, cholecystitis, or diverticulitis. Our cohort was selected using administrative data from Kaiser Permanente Southern California (KPSC) during the study period (January 1, 2004, through December 31, 2016).
Kaiser Permanente Southern California is a large integrated health maintenance organization that includes approximately 4 million patients as of December 31, 2016, and includes roughly 20% of the region’s population. The geographic area served extends from Bakersfield in the lower California Central Valley to San Diego on the border with Mexico. Membership demographics, socioeconomic status, and ethnicity composition are representative of California.
Patients were included if they had a diagnosis of psoriasis (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] code 696.1; International Classification of Diseases, Tenth Revision, Clinical Modification [ICD-10-CM] codes L40.0, L40.4, L40.8, or L40.9) for at least 3 visits between January 1, 2004, and December 31, 2016. Patients were not excluded if they also had a diagnosis of psoriatic arthritis (ICD-9-CM code 696.0; ICD-10-CM code L40.5x). Patients also must have been continuously enrolled for at least 1 year before and 1 year after the index date, which was defined as the date of the third psoriasis diagnosis.
Each patient with psoriasis was assigned to 1 of 2 cohorts: (1) severe psoriasis: patients who received UVB phototherapy, psoralen plus UVA phototherapy, methotrexate, acitretin, cyclosporine, apremilast, etanercept, adalimumab, infliximab, ustekinumab, efalizumab, alefacept, secukinumab, or ixekizumab during the study period; and (2) mild psoriasis: patients who had a diagnosis of psoriasis who did not receive one of these therapies during the study period.
Patients were excluded if they had a history of appendicitis, cholecystitis, or diverticulitis at any time before the index date. Only patients older than 18 years were included.
Patients with psoriasis were frequency matched (1:5) with healthy patients, also from the KPSC network. Individuals were matched by age, sex, and ethnicity.
Statistical Analysis
Baseline characteristics were described with means and SD for continuous variables as well as percentages for categorical variables. Chi-square tests for categorical variables and the Mann-Whitney U Test for continuous variables were used to compare the patients’ characteristics by psoriasis status. Cox proportional hazards regression models were used to examine the risk for appendicitis, cholecystitis, or diverticulitis among patients with and without psoriasis and among patients with mild and severe psoriasis. Proportionality assumption was validated using Pearson product moment correlation between the scaled Schoenfeld residuals and log transformed time for each covariate.
Results were presented as crude (unadjusted) hazard ratios (HRs) and adjusted HRs, where confounding factors (ie, age, sex, ethnicity, body mass index [BMI], alcohol use, smoking status, income, education, and membership length) were adjusted. All tests were performed with SAS EG 5.1 and R software. P<.05 was considered statistically significant. Results are reported with the 95% confidence interval (CI), when appropriate.
Results
A total of 1,690,214 KPSC patients were eligible for the study; 10,307 (0.6%) met diagnostic and inclusion criteria for the psoriasis cohort. Patients with psoriasis had a significantly higher mean BMI (29.9 vs 28.7; P<.0001) as well as higher mean rates of alcohol use (56% vs 53%; P<.0001) and smoking (47% vs 38%; P<.01) compared to controls. Psoriasis patients had a shorter average duration of membership within the Kaiser network (P=.0001) compared to controls.
A total of 7416 patients met criteria for mild psoriasis and 2891 patients met criteria for severe psoriasis (eTable). Patients with severe psoriasis were significantly younger and had significantly higher mean BMI compared to patients with mild psoriasis (P<.0001 and P=.0001, respectively). No significant difference in rates of alcohol or tobacco use was detected among patients with mild and severe psoriasis.

Appendicitis
The prevalence of appendicitis was not significantly different between patients with and without psoriasis or between patients with mild and severe psoriasis, though the incidence rate was slightly higher among patients with psoriasis (0.80 per 1000 patient-years compared to 0.62 per 1000 patient-years among patients without psoriasis)(Table 1). However, there was not a significant difference in risk for appendicitis between healthy patients, patients with severe psoriasis, and patients with mild psoriasis after adjusting for potential confounding factors (Table 2). Interestingly, patients with severe psoriasis who had a diagnosis of appendicitis had a significantly shorter time to diagnosis of appendicitis compared to patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001).


Cholecystitis
Psoriasis patients also did not have an increased prevalence of cholecystitis compared to healthy patients. However, patients with severe psoriasis had a significantly higher prevalence of cholecystitis compared to patients with mild psoriasis (P=.0038). Overall, patients with psoriasis had a slightly higher incidence rate (1.72 per 1000 patient-years) compared to healthy patients (1.46 per 1000 patient-years). Moreover, the time to diagnosis of cholecystitis was significantly shorter for patients with severe psoriasis than for patients with mild psoriasis (7.4 years vs 8.1 years; P<.0001). Mild psoriasis was associated with a significantly increased risk (HR, 1.33; 95% CI, 1.09-1.63; P<.01) for cholecystitis compared to individuals without psoriasis in both the crude and adjusted models (Table 2). There was no difference between mild psoriasis patients and severe psoriasis patients in risk for cholecystitis.
Diverticulitis
Patients with psoriasis had a significantly greater prevalence of diverticulitis compared to the control cohort (5.1% vs 4.2%; P<.0001). There was no difference in prevalence between the severe psoriasis group and the mild psoriasis group (P=.96), but the time to diagnosis of diverticulitis was shorter in the severe psoriasis group than in the mild psoriasis group (7.2 years vs 7.9 years; P<.0001). Psoriasis patients had an incidence rate of diverticulitis of 6.61 per 1000 patient-years compared to 5.38 per 1000 patient-years in the control group. Psoriasis conferred a higher risk for diverticulitis in both the crude and adjusted models (HR, 1.23; 95% CI, 1.11-1.35 [P<.001] and HR, 1.16; 95% CI, 1.05-1.29; [P<.01], respectively)(Table 3); however, when stratified by disease severity, only patients with severe psoriasis were found to be at higher risk (HR, 1.26; 95% CI, 1.15-1.61; P<.001 for the adjusted model).

Comment
The objective of this study was to examine the background risks for specific gastrointestinal pathologies in a large cohort of patients with psoriasis compared to the general population. After adjusting for measured confounders, patients with severe psoriasis had a significantly higher risk of diverticulitis compared to the general population. Although more patients with severe psoriasis developed appendicitis or cholecystitis, the difference was not significant.
The pathogenesis of diverticulosis and diverticulitis has been thought to be related to increased intracolonic pressure and decreased dietary fiber intake, leading to formation of diverticula in the colon.19 Our study did not correct for differences in diet between the 2 groups, making it a possible confounding variable. Studies evaluating dietary habits of psoriatic patients have found that adult males with psoriasis might consume less fiber compared to healthy patients,20 and psoriasis patients also might consume less whole-grain fiber.21 Furthermore, fiber deficiency also might affect gut flora, causing low-grade chronic inflammation,18 which also has been supported by response to anti-inflammatory medications such as mesalazine.22 Given the autoimmune association between psoriasis and IBD, it is possible that psoriasis also might create an environment of chronic inflammation in the gut, predisposing patients with psoriasis to diverticulitis. However, further research is needed to better evaluate this possibility.
Our study also does not address any potential effects on outcomes of specific treatments for psoriasis. Brandl et al23 found that patients on immunosuppressive therapy for autoimmune diseases had longer hospital and intensive care unit stays, higher rates of emergency operations, and higher mortality while hospitalized. Because our results suggest that patients with severe psoriasis, who are therefore more likely to require treatment with an immunomodulator, are at higher risk for diverticulitis, these patients also might be at risk for poorer outcomes.
There is no literature evaluating the relationship between psoriasis and appendicitis. Our study found a slightly lower incidence rate compared to the national trend (9.38 per 10,000 patient-years in the United States in 2008) in both healthy patients and psoriasis patients.24 Of note, this statistic includes children, whereas our study did not, which might in part account for the lower rate. However, Cheluvappa et al25 hypothesized a relationship between appendicitis and subsequent appendectomy at a young age and protection against IBD. They also found that the mechanism for protection involves downregulation of the helper T cell (TH17) pathway,25 which also has been found to play a role in psoriasis pathogenesis.26,27 Although our results suggest that the risk for appendicitis is not increased for patients with psoriasis, further research might be able to determine if appendicitis and subsequent appendectomy also can offer protection against development of psoriasis.
We found that patients with severe psoriasis had a higher incidence rate of cholecystitis compared to patients with mild psoriasis. Egeberg et al28 found an increased risk for cholelithiasis among patients with psoriasis, which may contribute to a higher rate of cholecystitis. Although both acute and chronic cholecystitis were incorporated in this study, a Russian study found that chronic cholecystitis may be a predictor of progression of psoriasis.29 Moreover, patients with severe psoriasis had a shorter duration to diagnosis of cholecystitis than patients with mild psoriasis. It is possible that patients with severe psoriasis are in a state of greater chronic inflammation than those with mild psoriasis, and therefore, when combined with other risk factors for cholecystitis, may progress to disease more quickly. Alternatively, this finding could be treatment related, as there have been reported cases of cholecystitis related to etanercept use in patients treated for psoriasis and juvenile polyarticular rheumatoid arthritis.30,31 The relationship is not yet well defined, however, and further research is necessary to evaluate this association.
Study Strengths
Key strengths of this study include the large sample size and diversity of the patient population. Kaiser Permanente Southern California membership generally is representative of the broader community, making our results fairly generalizable to populations with health insurance. Use of a matched control cohort allows the results to be more specific to the disease of interest, and the population-based design minimizes bias.
Study Limitations
This study has several limitations. Although the cohorts were categorized based on type of treatment received, exact therapies were not specified. As a retrospective study, it is difficult to control for potential confounding variables that are not included in the electronic medical record. The results of this study also demonstrated significantly shorter durations to diagnosis of all 3 conditions, indicating that surveillance bias may be present.
Conclusion
Patients with psoriasis may be at an increased risk for diverticulitis compared to patients without psoriasis, which could be due to the chronic inflammatory state induced by psoriasis. Therefore, it may be beneficial for clinicians to evaluate psoriasis patients for other risk factors for diverticulitis and subsequently provide counseling to these patients to minimize their risk for diverticulitis. Psoriasis patients do not appear to be at an increased risk for appendicitis or cholecystitis compared to controls; however, further research is needed for confirmation.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385.
- Channual J, Wu JJ, Dann FJ. Effects of tumor necrosis factor-α blockade on metabolic syndrome in psoriasis and psoriatic arthritis and additional lessons learned from rheumatoid arthritis. Dermatol Ther. 2009;22:61-73.
- Koebnick C, Black MH, Smith N, et al. The association of psoriasis and elevated blood lipids in overweight and obese children. J Pediatr. 2011;159:577-583.
- Herron MD, Hinckley M, Hoffman MS, et al. Impact of obesity and smoking on psoriasis presentation and management. Arch Dermatol. 2005;141:1527-1534.
- Qureshi AA, Choi HK, Setty AR, et al. Psoriasis and the risk of diabetes and hypertension: a prospective study of US female nurses. Arch Dermatol. 2009;145:379-382.
- Shapiro J, Cohen AD, David M, et al. The association between psoriasis, diabetes mellitus, and atherosclerosis in Israel: a case-control study. J Am Acad Dermatol. 2007;56:629-634.
- Love TJ, Qureshi AA, Karlson EW, et al. Prevalence of the metabolic syndrome in psoriasis: results from the National Health and Nutrition Examination Survey, 2003-2006. Arch Dermatol. 2011;147:419-424.
- El-Mongy S, Fathy H, Abdelaziz A, et al. Subclinical atherosclerosis in patients with chronic psoriasis: a potential association. J Eur Acad Dermatol Venereol. 2010;24:661-666.
- Prodanovich S, Kirsner RS, Kravetz JD, et al. Association of psoriasis with coronary artery, cerebrovascular, and peripheral vascular diseases and mortality. Arch Dermatol. 2009;145:700-703.
- Ludwig RJ, Herzog C, Rostock A, et al. Psoriasis: a possible risk factor for development of coronary artery calcification. Br J Dermatol. 2007;156:271-276.
- Kaye JA, Li L, Jick SS. Incidence of risk factors for myocardial infarction and other vascular diseases in patients with psoriasis. Br J Dermatol. 2008;159:895-902.
- Kimball AB, Robinson D Jr, Wu Y, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27-37.
- Gelfand JM, Neimann AL, Shin DB, et al. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735-1741.
- Gelfand JM, Dommasch ED, Shin DB, et al. The risk of stroke in patients with psoriasis. J Invest Dermatol. 2009;129:2411-2418.
- Mehta NN, Azfar RS, Shin DB, et al. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000-1006.
- Abuabara K, Azfar RS, Shin DB, et al. Cause-specific mortality in patients with severe psoriasis: a population-based cohort study in the United Kingdom. Br J Dermatol. 2010;163:586-592.
- Christophers E. Comorbidities in psoriasis. Clin Dermatol. 2007;25:529-534.
- Wu JJ, Nguyen TU, Poon KY, et al. The association of psoriasis with autoimmune diseases. J Am Acad Dermatol. 2012;67:924-930.
- Floch MH, Bina I. The natural history of diverticulitis: fact and theory. Clin Gastroenterol. 2004;38(5, suppl 1):S2-S7.
- Barrea L, Macchia PE, Tarantino G, et al. Nutrition: a key environmental dietary factor in clinical severity and cardio-metabolic risk in psoriatic male patients evaluated by 7-day food-frequency questionnaire. J Transl Med. 2015;13:303.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. National Survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Matrana MR, Margolin DA. Epidemiology and pathophysiology of diverticular disease. Clin Colon Rectal Surg. 2009;22:141-146.
- Brandl A, Kratzer T, Kafka-Ritsch R, et al. Diverticulitis in immunosuppressed patients: a fatal outcome requiring a new approach? Can J Surg. 2016;59:254-261.
- Buckius MT, McGrath B, Monk J, et al. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res. 2012;175:185-190.
- Cheluvappa R, Luo AS, Grimm MC. T helper type 17 pathway suppression by appendicitis and appendectomy protects against colitis. Clin Exp Immunol. 2014;175:316-322.
- Lynde CW, Poulin Y, Vender R, et al. Interleukin 17A: toward a new understanding of psoriasis pathogenesis. J Am Acad Dermatol. 2014;71:141-150.
- Arican O, Aral M, Sasmaz S, et al. Serum levels of TNF-α, IFN-γ, IL6, IL-8, IL-12, IL-17, and IL-18 in patients with active psoriasis and correlation with disease severity. Mediators Inflamm. 2005:2005;273-279.
- Egeberg A, Anderson YMF, Gislason GH, et al. Gallstone risk in adult patients with atopic dermatitis and psoriasis: possible effect of overweight and obesity. Acta Derm Venereol. 2017;97:627-631.
- Smirnova SV, Barilo AA, Smolnikova MV. Hepatobiliary system diseases as the predictors of psoriasis progression [in Russian]. Vestn Ross Akad Med Nauk. 2016:102-108.
- Bagel J, Lynde C, Tyring S, et al. Moderate to severe plaque psoriasis with scalp involvement: a randomized, double-blind, placebo-controlled study of etanercept. J Am Acad Dermatol. 2012;67:86-92.
- Foeldvari I, Krüger E, Schneider T. Acute, non-obstructive, sterile cholecystitis associated with etanercept and infliximab for the treatment of juvenile polyarticular rheumatoid arthritis. Ann Rheum Dis. 2003;62:908-909.
Practice Points
- Patients with psoriasis may have elevated risk of diverticulitis compared to healthy patients. However, psoriasis patients do not appear to have increased risk of appendicitis or cholecystitis.
- Clinicians treating psoriasis patients should consider assessing for other risk factors of diverticulitis at regular intervals.
Click for Credit: Endometriosis surgery benefits; diabetes & aging; more
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Here are 5 articles from the March issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Endometriosis surgery: Women can expect years-long benefits
To take the posttest, go to: https://bit.ly/2Ez8mdu
Expires January 3, 2019
2. Cerebral small vessel disease progression linked to MCI in hypertensive patients
To take the posttest, go to: https://bit.ly/2ExDV7o
Expires January 4, 2019
3. Adult atopic dermatitis is fraught with dermatologic comorbidities
To take the posttest, go to: https://bit.ly/2Vl7E9a
Expires January 11, 2019
4. Antidepressants tied to greater hip fracture incidence in older adults
To take the posttest, go to: https://bit.ly/2GRfMeH
Expires January 4, 2019
5. Researchers exploring ways to mitigate aging’s impact on diabetes
To take the posttest, go to: https://bit.ly/2tFxF7v
Expires January 8, 2019
Pediatric pruritus requires distinct approach to assessment and management
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Using special scales to measure itch in children and understanding that quality of life concerns may be different for children should also be kept in mind, said Dr. Chen, professor of dermatology at Emory University, Atlanta. Furthermore, several psychiatric comorbidities that have been associated with pediatric pruritus, such as ADHD and suicidal thoughts. Another consideration is that a child’s pruritus can have significant effects on his or her parents.
Measuring itch is challenging in children, who may have difficulty responding to visual analogue scales, verbal rating scales, and numerical rating scales. Dr. Chen and her colleagues developed the ItchyQuant scale as a self-report measure of itch severity (J Invest Dermatol. 2017 Jan;137[1]:57-61). It is a scale from 0 to 10 with cartoon illustrations depicting increasing itch severity, from no itch to the “worst itch imaginable.” Currently it is validated only in adults, but they are working towards getting it validated in children.
Another itch assessment scale for children, Itch Man, is available, but has only been studied in children who have survived burns.
The ItchyQOL scale measures the extent to which itch affects quality of life in adults. It examines the symptoms associated with itch, as well as its functional and emotional effects. But children’s concerns about quality of life are not the same as those of adults, so Dr. Chen and her colleagues used ItchyQOL as a basis for the “Tween ItchyQOL,” which is intended for children aged 8-17 years, and the “Kids ItchyQOL,” which is intended for children aged 6-7 years. The Tween ItchyQOL includes items (such as being made fun of) that are not in the ItchyQOL and eliminates items (such as working and spending money) that do not apply to children. The Kids ItchyQOL includes cartoons to help children understand the questions.
Dermatologists often use parents as proxies to measure their children’s itch, assuming that the latter’s responses might be unreliable. But when Dr. Chen and her colleagues administered the ItchyQuant to children with pruritus, parents, and their medical providers to evaluate the extent of agreement among assessors, they found that parents’ scores were higher than their children’s scores, although the difference was not significant.
Providers’ scores, however, were significantly lower than those of children and parents. All scores were in the moderate range. Dr. Chen and colleagues also found that, for each 1-point increase in the difference between children’s and parents’ responses, parents were 1.25 times less likely to have experience with chronic pruritus, outside of their children. This finding provides a gauge of how well a parent can serve as a proxy to characterize his or her child’s itch.
Adolescents are in general a troubled group, and caregivers have concerns about suicide in the adolescent population, Dr. Chen said. She referred to a large study of adolescents published in 2012, which indicated that the prevalence of suicidal ideation was 8.4% among adolescents with no itch, compared with 21.1% among adolescents with severe itch (Acta Derm Venereol. 2012 Sep;92[5]:543-6).
In the study, those with severe itch were three times more likely to have suicidal ideation than the general population, which Dr. Chen noted was comparable with that of suicidal ideation in patients with chronic pain in the study (odds ratio, 3.8).
Cross-sectional data suggest a link between itching and ADHD, but “it’s a chicken-and-egg phenomenon,” she said. “If you’re so itchy and squirmy, you’re not going to pay attention. Then again, if you’re not paying attention, maybe you’re that much more prone to scratch.” Longitudinal data indicate that improving itch correlates with improvement in ADHD symptoms.
In addition, pruritus affects the genders disproportionately. Girls report a significantly greater impact on quality of life than boys when itching is severe, with much of the difference in emotional impact, said Dr. Chen. Boys may report more functional impact than girls.
Chronic pruritus also affects parents, who may have disturbed sleep, feel stress about their own parenting, and have difficulty enforcing discipline. “They feel an incredible amount of guilt and blame for giving this to their child,” she commented. “As more and more places develop itch centers, it would be good to have a multidisciplinary approach bringing in mental health providers and social workers, because the impact of itch on parents can be quite profound.”
Dr. Chen reported disclosures with several companies, including BioPharmX, Dermecular Therapeutics, Leo Pharma, and Unilever.
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Using special scales to measure itch in children and understanding that quality of life concerns may be different for children should also be kept in mind, said Dr. Chen, professor of dermatology at Emory University, Atlanta. Furthermore, several psychiatric comorbidities that have been associated with pediatric pruritus, such as ADHD and suicidal thoughts. Another consideration is that a child’s pruritus can have significant effects on his or her parents.
Measuring itch is challenging in children, who may have difficulty responding to visual analogue scales, verbal rating scales, and numerical rating scales. Dr. Chen and her colleagues developed the ItchyQuant scale as a self-report measure of itch severity (J Invest Dermatol. 2017 Jan;137[1]:57-61). It is a scale from 0 to 10 with cartoon illustrations depicting increasing itch severity, from no itch to the “worst itch imaginable.” Currently it is validated only in adults, but they are working towards getting it validated in children.
Another itch assessment scale for children, Itch Man, is available, but has only been studied in children who have survived burns.
The ItchyQOL scale measures the extent to which itch affects quality of life in adults. It examines the symptoms associated with itch, as well as its functional and emotional effects. But children’s concerns about quality of life are not the same as those of adults, so Dr. Chen and her colleagues used ItchyQOL as a basis for the “Tween ItchyQOL,” which is intended for children aged 8-17 years, and the “Kids ItchyQOL,” which is intended for children aged 6-7 years. The Tween ItchyQOL includes items (such as being made fun of) that are not in the ItchyQOL and eliminates items (such as working and spending money) that do not apply to children. The Kids ItchyQOL includes cartoons to help children understand the questions.
Dermatologists often use parents as proxies to measure their children’s itch, assuming that the latter’s responses might be unreliable. But when Dr. Chen and her colleagues administered the ItchyQuant to children with pruritus, parents, and their medical providers to evaluate the extent of agreement among assessors, they found that parents’ scores were higher than their children’s scores, although the difference was not significant.
Providers’ scores, however, were significantly lower than those of children and parents. All scores were in the moderate range. Dr. Chen and colleagues also found that, for each 1-point increase in the difference between children’s and parents’ responses, parents were 1.25 times less likely to have experience with chronic pruritus, outside of their children. This finding provides a gauge of how well a parent can serve as a proxy to characterize his or her child’s itch.
Adolescents are in general a troubled group, and caregivers have concerns about suicide in the adolescent population, Dr. Chen said. She referred to a large study of adolescents published in 2012, which indicated that the prevalence of suicidal ideation was 8.4% among adolescents with no itch, compared with 21.1% among adolescents with severe itch (Acta Derm Venereol. 2012 Sep;92[5]:543-6).
In the study, those with severe itch were three times more likely to have suicidal ideation than the general population, which Dr. Chen noted was comparable with that of suicidal ideation in patients with chronic pain in the study (odds ratio, 3.8).
Cross-sectional data suggest a link between itching and ADHD, but “it’s a chicken-and-egg phenomenon,” she said. “If you’re so itchy and squirmy, you’re not going to pay attention. Then again, if you’re not paying attention, maybe you’re that much more prone to scratch.” Longitudinal data indicate that improving itch correlates with improvement in ADHD symptoms.
In addition, pruritus affects the genders disproportionately. Girls report a significantly greater impact on quality of life than boys when itching is severe, with much of the difference in emotional impact, said Dr. Chen. Boys may report more functional impact than girls.
Chronic pruritus also affects parents, who may have disturbed sleep, feel stress about their own parenting, and have difficulty enforcing discipline. “They feel an incredible amount of guilt and blame for giving this to their child,” she commented. “As more and more places develop itch centers, it would be good to have a multidisciplinary approach bringing in mental health providers and social workers, because the impact of itch on parents can be quite profound.”
Dr. Chen reported disclosures with several companies, including BioPharmX, Dermecular Therapeutics, Leo Pharma, and Unilever.
WASHINGTON – Suephy C. Chen, MD, said at the annual meeting of the American Academy of Dermatology.
Using special scales to measure itch in children and understanding that quality of life concerns may be different for children should also be kept in mind, said Dr. Chen, professor of dermatology at Emory University, Atlanta. Furthermore, several psychiatric comorbidities that have been associated with pediatric pruritus, such as ADHD and suicidal thoughts. Another consideration is that a child’s pruritus can have significant effects on his or her parents.
Measuring itch is challenging in children, who may have difficulty responding to visual analogue scales, verbal rating scales, and numerical rating scales. Dr. Chen and her colleagues developed the ItchyQuant scale as a self-report measure of itch severity (J Invest Dermatol. 2017 Jan;137[1]:57-61). It is a scale from 0 to 10 with cartoon illustrations depicting increasing itch severity, from no itch to the “worst itch imaginable.” Currently it is validated only in adults, but they are working towards getting it validated in children.
Another itch assessment scale for children, Itch Man, is available, but has only been studied in children who have survived burns.
The ItchyQOL scale measures the extent to which itch affects quality of life in adults. It examines the symptoms associated with itch, as well as its functional and emotional effects. But children’s concerns about quality of life are not the same as those of adults, so Dr. Chen and her colleagues used ItchyQOL as a basis for the “Tween ItchyQOL,” which is intended for children aged 8-17 years, and the “Kids ItchyQOL,” which is intended for children aged 6-7 years. The Tween ItchyQOL includes items (such as being made fun of) that are not in the ItchyQOL and eliminates items (such as working and spending money) that do not apply to children. The Kids ItchyQOL includes cartoons to help children understand the questions.
Dermatologists often use parents as proxies to measure their children’s itch, assuming that the latter’s responses might be unreliable. But when Dr. Chen and her colleagues administered the ItchyQuant to children with pruritus, parents, and their medical providers to evaluate the extent of agreement among assessors, they found that parents’ scores were higher than their children’s scores, although the difference was not significant.
Providers’ scores, however, were significantly lower than those of children and parents. All scores were in the moderate range. Dr. Chen and colleagues also found that, for each 1-point increase in the difference between children’s and parents’ responses, parents were 1.25 times less likely to have experience with chronic pruritus, outside of their children. This finding provides a gauge of how well a parent can serve as a proxy to characterize his or her child’s itch.
Adolescents are in general a troubled group, and caregivers have concerns about suicide in the adolescent population, Dr. Chen said. She referred to a large study of adolescents published in 2012, which indicated that the prevalence of suicidal ideation was 8.4% among adolescents with no itch, compared with 21.1% among adolescents with severe itch (Acta Derm Venereol. 2012 Sep;92[5]:543-6).
In the study, those with severe itch were three times more likely to have suicidal ideation than the general population, which Dr. Chen noted was comparable with that of suicidal ideation in patients with chronic pain in the study (odds ratio, 3.8).
Cross-sectional data suggest a link between itching and ADHD, but “it’s a chicken-and-egg phenomenon,” she said. “If you’re so itchy and squirmy, you’re not going to pay attention. Then again, if you’re not paying attention, maybe you’re that much more prone to scratch.” Longitudinal data indicate that improving itch correlates with improvement in ADHD symptoms.
In addition, pruritus affects the genders disproportionately. Girls report a significantly greater impact on quality of life than boys when itching is severe, with much of the difference in emotional impact, said Dr. Chen. Boys may report more functional impact than girls.
Chronic pruritus also affects parents, who may have disturbed sleep, feel stress about their own parenting, and have difficulty enforcing discipline. “They feel an incredible amount of guilt and blame for giving this to their child,” she commented. “As more and more places develop itch centers, it would be good to have a multidisciplinary approach bringing in mental health providers and social workers, because the impact of itch on parents can be quite profound.”
Dr. Chen reported disclosures with several companies, including BioPharmX, Dermecular Therapeutics, Leo Pharma, and Unilever.
REPORTING FROM AAD 2019
Bermekimab reduces lesions, cuts pain in patients with hidradenitis suppurativa
WASHINGTON – It was nearly as effective in patients refractory to anti–tumor necrosis factor (TNF) therapy as it was to those naive to the treatment.
The antibody, which is derived directly from healthy human volunteers and then lab expanded, also improved patients’ quality of life in a “clinically meaningful way,” Alice Gottlieb, MD, PhD said at the annual meeting of the American Academy of Dermatology.
“These are sick people, and improvement of this kind is really something very important,” said Dr. Gottlieb of Mount Sinai Medical Center in New York. “I think what we see here supports the movement of bermekimab into phase 3 studies for HS [hidradenitis suppurativa].”
Bermekimab is the first inhibitor of IL-1 alpha to be investigated in HS. An overabundance of the cytokine produces several potentially problematic effects. IL-1 alpha induces inflammatory cells to migrate into the skin, drives neoangiogenesis, potentiates pain, and induces matrix metalloproteinase. The last two are particularly an issue in patients with HS. The abscesses and fistulas cause severe pain, which Dr. Gottlieb said is an undertreated and an underappreciated driver of disease disability. The tissue breakdown characteristic of the disease can also be highly disfiguring. “Many patients, especially my female patients, look as if they are basically autodigesting.”
IL-1 alpha also induces procollagen type I and III and fibroblast proliferation, contributing to the scarring many patients experience.
“Ten years ago, I thought it would be a potential target for HS,” Dr, Gottlieb said, and the idea has finally come to fruition through studies by biopharmaceutical company XBiotech in Austin, Tex. Dr. Gottlieb designed the treatment protocol and was a principal investigator on the study.
A similarly positive 2018 study employed twice-weekly intravenous infusions; this study utilized a more-concentrated form of the antibody delivered subcutaneously from prefilled syringes.
The study comprised 42 patients, 24 of whom had failed a course of anti-TNF therapy and 18 of whom were anti-TNF naive. Each group received bermekimab 400 mg subcutaneous once a week for 12 weeks. The primary endpoint was change on the Hidradenitis Suppurativa Clinical Response Score; good response was deemed at least a 50% reduction in abscesses and inflammatory nodules, with no new abscesses or draining fistulas. Secondary endpoints included pain scores, patient quality of life, and the physicians global clinical assessment.
At baseline, subjects in the anti-TNF–refractory group had a worse clinical profile than the naive patients, with more abscesses and inflammatory nodules (mean, 14 vs. 6) and worse scores on the Physicians Global Assessment. But they reported similar pain on a 10-point scale (around 8) and negative quality of life (17/30). Both groups experienced anxiety and depression.
Eight patients dropped out before finishing the trial for a variety of reasons, including family and transportation issues and comorbid illness. Only one discontinued for a reaction to the study drug (injection site redness). These patients were included in the final analysis in a last observation carried forward.
About 10% of patients began to experience improvement as soon as 2 weeks after the first injection. By week 6, 40% of the refractory patients and 10% of the naive patents had experienced a lesion reduction of at least 50%. By the end of the study, however, about 62% of patients in each group achieved that goal.
By week 12, the mean improvement in the Physicians Global Assessment was about 23% in the refractory group and 53% in the naive group. Both results were significant improvements over baseline.
The mean improvement in the pain score was about 54% in the refractory group and 65% in the naive group. In a scale that measured patients’ view of their disease severity, refractory patients reported a mean 40% improvement, and naive patients, a mean 67% improvement.
There were 57 adverse events recorded; 94% were grade 1 or 2. There were two serious adverse events requiring hospitalization – a fall and an admission for HS pain. Neither were judged related to the study drug. Two patients experienced injection site reactions and one patient experienced six bouts of nausea. There were no serious infections, no major cardiovascular events, and no neoplasms.
Dr. Gottlieb designed the study protocol and was a principal investigator. She did not receive financial compensation from the company.
WASHINGTON – It was nearly as effective in patients refractory to anti–tumor necrosis factor (TNF) therapy as it was to those naive to the treatment.
The antibody, which is derived directly from healthy human volunteers and then lab expanded, also improved patients’ quality of life in a “clinically meaningful way,” Alice Gottlieb, MD, PhD said at the annual meeting of the American Academy of Dermatology.
“These are sick people, and improvement of this kind is really something very important,” said Dr. Gottlieb of Mount Sinai Medical Center in New York. “I think what we see here supports the movement of bermekimab into phase 3 studies for HS [hidradenitis suppurativa].”
Bermekimab is the first inhibitor of IL-1 alpha to be investigated in HS. An overabundance of the cytokine produces several potentially problematic effects. IL-1 alpha induces inflammatory cells to migrate into the skin, drives neoangiogenesis, potentiates pain, and induces matrix metalloproteinase. The last two are particularly an issue in patients with HS. The abscesses and fistulas cause severe pain, which Dr. Gottlieb said is an undertreated and an underappreciated driver of disease disability. The tissue breakdown characteristic of the disease can also be highly disfiguring. “Many patients, especially my female patients, look as if they are basically autodigesting.”
IL-1 alpha also induces procollagen type I and III and fibroblast proliferation, contributing to the scarring many patients experience.
“Ten years ago, I thought it would be a potential target for HS,” Dr, Gottlieb said, and the idea has finally come to fruition through studies by biopharmaceutical company XBiotech in Austin, Tex. Dr. Gottlieb designed the treatment protocol and was a principal investigator on the study.
A similarly positive 2018 study employed twice-weekly intravenous infusions; this study utilized a more-concentrated form of the antibody delivered subcutaneously from prefilled syringes.
The study comprised 42 patients, 24 of whom had failed a course of anti-TNF therapy and 18 of whom were anti-TNF naive. Each group received bermekimab 400 mg subcutaneous once a week for 12 weeks. The primary endpoint was change on the Hidradenitis Suppurativa Clinical Response Score; good response was deemed at least a 50% reduction in abscesses and inflammatory nodules, with no new abscesses or draining fistulas. Secondary endpoints included pain scores, patient quality of life, and the physicians global clinical assessment.
At baseline, subjects in the anti-TNF–refractory group had a worse clinical profile than the naive patients, with more abscesses and inflammatory nodules (mean, 14 vs. 6) and worse scores on the Physicians Global Assessment. But they reported similar pain on a 10-point scale (around 8) and negative quality of life (17/30). Both groups experienced anxiety and depression.
Eight patients dropped out before finishing the trial for a variety of reasons, including family and transportation issues and comorbid illness. Only one discontinued for a reaction to the study drug (injection site redness). These patients were included in the final analysis in a last observation carried forward.
About 10% of patients began to experience improvement as soon as 2 weeks after the first injection. By week 6, 40% of the refractory patients and 10% of the naive patents had experienced a lesion reduction of at least 50%. By the end of the study, however, about 62% of patients in each group achieved that goal.
By week 12, the mean improvement in the Physicians Global Assessment was about 23% in the refractory group and 53% in the naive group. Both results were significant improvements over baseline.
The mean improvement in the pain score was about 54% in the refractory group and 65% in the naive group. In a scale that measured patients’ view of their disease severity, refractory patients reported a mean 40% improvement, and naive patients, a mean 67% improvement.
There were 57 adverse events recorded; 94% were grade 1 or 2. There were two serious adverse events requiring hospitalization – a fall and an admission for HS pain. Neither were judged related to the study drug. Two patients experienced injection site reactions and one patient experienced six bouts of nausea. There were no serious infections, no major cardiovascular events, and no neoplasms.
Dr. Gottlieb designed the study protocol and was a principal investigator. She did not receive financial compensation from the company.
WASHINGTON – It was nearly as effective in patients refractory to anti–tumor necrosis factor (TNF) therapy as it was to those naive to the treatment.
The antibody, which is derived directly from healthy human volunteers and then lab expanded, also improved patients’ quality of life in a “clinically meaningful way,” Alice Gottlieb, MD, PhD said at the annual meeting of the American Academy of Dermatology.
“These are sick people, and improvement of this kind is really something very important,” said Dr. Gottlieb of Mount Sinai Medical Center in New York. “I think what we see here supports the movement of bermekimab into phase 3 studies for HS [hidradenitis suppurativa].”
Bermekimab is the first inhibitor of IL-1 alpha to be investigated in HS. An overabundance of the cytokine produces several potentially problematic effects. IL-1 alpha induces inflammatory cells to migrate into the skin, drives neoangiogenesis, potentiates pain, and induces matrix metalloproteinase. The last two are particularly an issue in patients with HS. The abscesses and fistulas cause severe pain, which Dr. Gottlieb said is an undertreated and an underappreciated driver of disease disability. The tissue breakdown characteristic of the disease can also be highly disfiguring. “Many patients, especially my female patients, look as if they are basically autodigesting.”
IL-1 alpha also induces procollagen type I and III and fibroblast proliferation, contributing to the scarring many patients experience.
“Ten years ago, I thought it would be a potential target for HS,” Dr, Gottlieb said, and the idea has finally come to fruition through studies by biopharmaceutical company XBiotech in Austin, Tex. Dr. Gottlieb designed the treatment protocol and was a principal investigator on the study.
A similarly positive 2018 study employed twice-weekly intravenous infusions; this study utilized a more-concentrated form of the antibody delivered subcutaneously from prefilled syringes.
The study comprised 42 patients, 24 of whom had failed a course of anti-TNF therapy and 18 of whom were anti-TNF naive. Each group received bermekimab 400 mg subcutaneous once a week for 12 weeks. The primary endpoint was change on the Hidradenitis Suppurativa Clinical Response Score; good response was deemed at least a 50% reduction in abscesses and inflammatory nodules, with no new abscesses or draining fistulas. Secondary endpoints included pain scores, patient quality of life, and the physicians global clinical assessment.
At baseline, subjects in the anti-TNF–refractory group had a worse clinical profile than the naive patients, with more abscesses and inflammatory nodules (mean, 14 vs. 6) and worse scores on the Physicians Global Assessment. But they reported similar pain on a 10-point scale (around 8) and negative quality of life (17/30). Both groups experienced anxiety and depression.
Eight patients dropped out before finishing the trial for a variety of reasons, including family and transportation issues and comorbid illness. Only one discontinued for a reaction to the study drug (injection site redness). These patients were included in the final analysis in a last observation carried forward.
About 10% of patients began to experience improvement as soon as 2 weeks after the first injection. By week 6, 40% of the refractory patients and 10% of the naive patents had experienced a lesion reduction of at least 50%. By the end of the study, however, about 62% of patients in each group achieved that goal.
By week 12, the mean improvement in the Physicians Global Assessment was about 23% in the refractory group and 53% in the naive group. Both results were significant improvements over baseline.
The mean improvement in the pain score was about 54% in the refractory group and 65% in the naive group. In a scale that measured patients’ view of their disease severity, refractory patients reported a mean 40% improvement, and naive patients, a mean 67% improvement.
There were 57 adverse events recorded; 94% were grade 1 or 2. There were two serious adverse events requiring hospitalization – a fall and an admission for HS pain. Neither were judged related to the study drug. Two patients experienced injection site reactions and one patient experienced six bouts of nausea. There were no serious infections, no major cardiovascular events, and no neoplasms.
Dr. Gottlieb designed the study protocol and was a principal investigator. She did not receive financial compensation from the company.
REPORTING FROM AAD 2019
Eczema increases the risk of impaired mental health among children
WASHINGTON – according to an analysis described at the annual meeting of the American Academy of Dermatology. Eczema appears to influence several domains of mental health, and the association remains in the absence of other atopic illnesses.
Estimates of the prevalence of eczema in children have ranged as high as 20%. European and Japanese studies have suggested that children with eczema have greater mental health impairments overall, but researchers have not evaluated this association among U.S. children. Although it has been established that children with eczema consult health care providers more often than children without eczema, data on health care utilization among children with eczema and impaired mental health are limited.
Joy Wan, MD, a dermatologist at Children’s Hospital of Philadelphia, and her colleagues performed a cross-sectional analysis of data obtained from 2013 to 2017 by the National Health Interview Survey. The Centers for Disease Control and Prevention administers the survey to a representative sample of the U.S. population. Children in each household are randomly sampled, and adult caregivers provide detailed health information about them.
Dr. Wan and her colleagues included children aged between 4 and 17 years in their analysis. The exposure of interest was eczema. Caregivers reported eczema in response to the question, “During the past 12 months, has the child had eczema or any kind of skin allergy?” The study’s primary outcome was mental health impairment. Using the Strengths and Difficulties Questionnaire (SDQ), the investigators categorized mental health impairment as none, mild, or severe. The SDQ is a validated instrument that assesses symptoms of mental health in children in domains such as conduct, emotion, peer relationships, and attention, which the researchers chose as secondary outcomes of interest. Dr. Wan’s group also examined the utilization of mental health and other health and social services among children with eczema.
The researchers performed logistic regression analysis to obtain odds ratios for mental health impairment among children with eczema, adjusting the analysis for potential socioeconomic and demographic confounders. Furthermore, they stratified the primary model by other atopic and behavioral disorders to assess for potential effects modification by these concomitant illnesses.
Approximately 12% of the children in the sample had eczema. Children with eczema tended to be female, non-Hispanic, or black; they also were more likely to report good, fair, or poor health, compared with children without eczema. Asthma, allergic rhinitis, and ADHD were more common among children with eczema than those without.
About 27% of children with eczema had any mental health impairment, compared with approximately 18% of children without eczema. About 11% of children with eczema had severe impairment; this rate was almost twice as high as that in children without eczema, Dr. Wan said. The adjusted odds of mental health impairment were 52% per year among children with eczema, compared with those without.
When the researchers examined specific domains of mental health, they found that children with eczema were significantly less likely to be reported to be well behaved or to have good attention spans. They also were significantly more likely to worry often, be unhappy or depressed, and to get along better with adults than their peers.
When Dr. Wan and his colleagues stratified the primary model by other atopic illnesses, they found that, among children without any other atopic illness, eczema remained independently associated with mental health impairment (OR, 1.52). The effect remained similar among children with asthma alone, but was attenuated among children with allergic rhinitis alone or with asthma and allergic rhinitis.
In the absence of ADHD, the investigators found a statistically significant effect of eczema on mental health impairment (OR, 1.46). In the presence of ADHD, the effect remained significant, but was attenuated.
Finally, approximately 20% of children with mildly impaired mental health had seen a mental health professional in the past year. In addition, 54% of children with severe mental health symptoms had seen a mental health professional in the past year. Among children with severe impairment, about 80% had consulted a general practitioner in the past year; 45% of them reported emotional or behavioral issues as the reason for the visit. Use of special education and early intervention services were more prevalent among children with increasing degrees of mental health impairment.
The study’s strengths include its population-based design, the use of a validated psychometric instrument, and the adjustment of data for socioeconomic factors and other comorbid illnesses, Dr. Wan said. The study is cross sectional, however, which precludes conclusions about the directionality of the relationship between eczema and mental health. In addition, the SDQ may not capture all mental health symptoms that eczema affects.
It is imperative that clinicians and caregivers recognize how common mental health impairment is among children with eczema so that children can be appropriately screened and referred for care, Dr. Wan said. “Our study suggests that there may be a critical gap in mental health services utilization by children who have eczema and concomitant mental health impairment. Some of the future directions in this area may be to understand the potential barriers to mental health care in children with eczema, and certainly to identify potentially effective interventions to reduce the mental health burden in pediatric eczema.”
Dr. Wan reported receiving research fellowship funding from Pfizer.
WASHINGTON – according to an analysis described at the annual meeting of the American Academy of Dermatology. Eczema appears to influence several domains of mental health, and the association remains in the absence of other atopic illnesses.
Estimates of the prevalence of eczema in children have ranged as high as 20%. European and Japanese studies have suggested that children with eczema have greater mental health impairments overall, but researchers have not evaluated this association among U.S. children. Although it has been established that children with eczema consult health care providers more often than children without eczema, data on health care utilization among children with eczema and impaired mental health are limited.
Joy Wan, MD, a dermatologist at Children’s Hospital of Philadelphia, and her colleagues performed a cross-sectional analysis of data obtained from 2013 to 2017 by the National Health Interview Survey. The Centers for Disease Control and Prevention administers the survey to a representative sample of the U.S. population. Children in each household are randomly sampled, and adult caregivers provide detailed health information about them.
Dr. Wan and her colleagues included children aged between 4 and 17 years in their analysis. The exposure of interest was eczema. Caregivers reported eczema in response to the question, “During the past 12 months, has the child had eczema or any kind of skin allergy?” The study’s primary outcome was mental health impairment. Using the Strengths and Difficulties Questionnaire (SDQ), the investigators categorized mental health impairment as none, mild, or severe. The SDQ is a validated instrument that assesses symptoms of mental health in children in domains such as conduct, emotion, peer relationships, and attention, which the researchers chose as secondary outcomes of interest. Dr. Wan’s group also examined the utilization of mental health and other health and social services among children with eczema.
The researchers performed logistic regression analysis to obtain odds ratios for mental health impairment among children with eczema, adjusting the analysis for potential socioeconomic and demographic confounders. Furthermore, they stratified the primary model by other atopic and behavioral disorders to assess for potential effects modification by these concomitant illnesses.
Approximately 12% of the children in the sample had eczema. Children with eczema tended to be female, non-Hispanic, or black; they also were more likely to report good, fair, or poor health, compared with children without eczema. Asthma, allergic rhinitis, and ADHD were more common among children with eczema than those without.
About 27% of children with eczema had any mental health impairment, compared with approximately 18% of children without eczema. About 11% of children with eczema had severe impairment; this rate was almost twice as high as that in children without eczema, Dr. Wan said. The adjusted odds of mental health impairment were 52% per year among children with eczema, compared with those without.
When the researchers examined specific domains of mental health, they found that children with eczema were significantly less likely to be reported to be well behaved or to have good attention spans. They also were significantly more likely to worry often, be unhappy or depressed, and to get along better with adults than their peers.
When Dr. Wan and his colleagues stratified the primary model by other atopic illnesses, they found that, among children without any other atopic illness, eczema remained independently associated with mental health impairment (OR, 1.52). The effect remained similar among children with asthma alone, but was attenuated among children with allergic rhinitis alone or with asthma and allergic rhinitis.
In the absence of ADHD, the investigators found a statistically significant effect of eczema on mental health impairment (OR, 1.46). In the presence of ADHD, the effect remained significant, but was attenuated.
Finally, approximately 20% of children with mildly impaired mental health had seen a mental health professional in the past year. In addition, 54% of children with severe mental health symptoms had seen a mental health professional in the past year. Among children with severe impairment, about 80% had consulted a general practitioner in the past year; 45% of them reported emotional or behavioral issues as the reason for the visit. Use of special education and early intervention services were more prevalent among children with increasing degrees of mental health impairment.
The study’s strengths include its population-based design, the use of a validated psychometric instrument, and the adjustment of data for socioeconomic factors and other comorbid illnesses, Dr. Wan said. The study is cross sectional, however, which precludes conclusions about the directionality of the relationship between eczema and mental health. In addition, the SDQ may not capture all mental health symptoms that eczema affects.
It is imperative that clinicians and caregivers recognize how common mental health impairment is among children with eczema so that children can be appropriately screened and referred for care, Dr. Wan said. “Our study suggests that there may be a critical gap in mental health services utilization by children who have eczema and concomitant mental health impairment. Some of the future directions in this area may be to understand the potential barriers to mental health care in children with eczema, and certainly to identify potentially effective interventions to reduce the mental health burden in pediatric eczema.”
Dr. Wan reported receiving research fellowship funding from Pfizer.
WASHINGTON – according to an analysis described at the annual meeting of the American Academy of Dermatology. Eczema appears to influence several domains of mental health, and the association remains in the absence of other atopic illnesses.
Estimates of the prevalence of eczema in children have ranged as high as 20%. European and Japanese studies have suggested that children with eczema have greater mental health impairments overall, but researchers have not evaluated this association among U.S. children. Although it has been established that children with eczema consult health care providers more often than children without eczema, data on health care utilization among children with eczema and impaired mental health are limited.
Joy Wan, MD, a dermatologist at Children’s Hospital of Philadelphia, and her colleagues performed a cross-sectional analysis of data obtained from 2013 to 2017 by the National Health Interview Survey. The Centers for Disease Control and Prevention administers the survey to a representative sample of the U.S. population. Children in each household are randomly sampled, and adult caregivers provide detailed health information about them.
Dr. Wan and her colleagues included children aged between 4 and 17 years in their analysis. The exposure of interest was eczema. Caregivers reported eczema in response to the question, “During the past 12 months, has the child had eczema or any kind of skin allergy?” The study’s primary outcome was mental health impairment. Using the Strengths and Difficulties Questionnaire (SDQ), the investigators categorized mental health impairment as none, mild, or severe. The SDQ is a validated instrument that assesses symptoms of mental health in children in domains such as conduct, emotion, peer relationships, and attention, which the researchers chose as secondary outcomes of interest. Dr. Wan’s group also examined the utilization of mental health and other health and social services among children with eczema.
The researchers performed logistic regression analysis to obtain odds ratios for mental health impairment among children with eczema, adjusting the analysis for potential socioeconomic and demographic confounders. Furthermore, they stratified the primary model by other atopic and behavioral disorders to assess for potential effects modification by these concomitant illnesses.
Approximately 12% of the children in the sample had eczema. Children with eczema tended to be female, non-Hispanic, or black; they also were more likely to report good, fair, or poor health, compared with children without eczema. Asthma, allergic rhinitis, and ADHD were more common among children with eczema than those without.
About 27% of children with eczema had any mental health impairment, compared with approximately 18% of children without eczema. About 11% of children with eczema had severe impairment; this rate was almost twice as high as that in children without eczema, Dr. Wan said. The adjusted odds of mental health impairment were 52% per year among children with eczema, compared with those without.
When the researchers examined specific domains of mental health, they found that children with eczema were significantly less likely to be reported to be well behaved or to have good attention spans. They also were significantly more likely to worry often, be unhappy or depressed, and to get along better with adults than their peers.
When Dr. Wan and his colleagues stratified the primary model by other atopic illnesses, they found that, among children without any other atopic illness, eczema remained independently associated with mental health impairment (OR, 1.52). The effect remained similar among children with asthma alone, but was attenuated among children with allergic rhinitis alone or with asthma and allergic rhinitis.
In the absence of ADHD, the investigators found a statistically significant effect of eczema on mental health impairment (OR, 1.46). In the presence of ADHD, the effect remained significant, but was attenuated.
Finally, approximately 20% of children with mildly impaired mental health had seen a mental health professional in the past year. In addition, 54% of children with severe mental health symptoms had seen a mental health professional in the past year. Among children with severe impairment, about 80% had consulted a general practitioner in the past year; 45% of them reported emotional or behavioral issues as the reason for the visit. Use of special education and early intervention services were more prevalent among children with increasing degrees of mental health impairment.
The study’s strengths include its population-based design, the use of a validated psychometric instrument, and the adjustment of data for socioeconomic factors and other comorbid illnesses, Dr. Wan said. The study is cross sectional, however, which precludes conclusions about the directionality of the relationship between eczema and mental health. In addition, the SDQ may not capture all mental health symptoms that eczema affects.
It is imperative that clinicians and caregivers recognize how common mental health impairment is among children with eczema so that children can be appropriately screened and referred for care, Dr. Wan said. “Our study suggests that there may be a critical gap in mental health services utilization by children who have eczema and concomitant mental health impairment. Some of the future directions in this area may be to understand the potential barriers to mental health care in children with eczema, and certainly to identify potentially effective interventions to reduce the mental health burden in pediatric eczema.”
Dr. Wan reported receiving research fellowship funding from Pfizer.
REPORTING FROM AAD 2019
Norwegian scabies
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
DIAGNOSIS, TREATMENT, CONTROL
The differential diagnosis of Norwegian scabies includes psoriasis, eczema, contact dermatitis, insect bites, seborrheic dermatitis, lichen planus, systemic infection, palmoplantar keratoderma, and cutaneous lymphoma.2
Treatment involves eradicating the infestation with a topical ointment consisting of permethrin, crotamiton, lindane, benzyl benzoate, and sulfur, applied directly to the skin. However, topical treatments often cannot penetrate the crusted and thickened skin, leading to treatment failure. A dose of oral ivermectin 200 µg/kg on days 1, 2, and 8 is a safe, effective, first-line treatment for Norwegian scabies, rapidly reducing scabies symptoms.3 Adverse effects of oral ivermectin are rare and usually minor.
Norwegian scabies is extremely contagious, spread by close physical contact and sharing of contaminated items such as clothing, bedding, towels, and furniture. Scabies mites can survive off the skin for 48 to 72 hours at room temperature.4 Potentially contaminated items should be decontaminated by washing in hot water and drying in a drying machine or by dry cleaning. Body contact with other contaminated items should be avoided for at least 72 hours.
Outbreaks can spread among patients, visitors, and medical staff in institutions such as nursing homes, day care centers, long-term-care facilities, and hospitals.5 Early identification facilitates appropriate management and treatment, thereby preventing infection and community-wide scabies outbreaks.
Acknowledgment: The authors would like to sincerely thank Paul Williams for his editing of the article.
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
- Leone PA. Scabies and pediculosis pubis: an update of treatment regimens and general review. Clin Infect Dis 2007; 44(suppl 3):S153–S159. doi:10.1086/511428
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med 2015; 4(5):884–917. doi:10.3390/jcm4050884
- Salavastru CM, Chosidow O, Boffa MJ, Janier M, Tiplica GS. European guideline for the management of scabies. J Eur Acad Dermatol Venereol 2017; 31(8):1248–1253. doi:10.1111/jdv.14351
- Khalil S, Abbas O, Kibbi AG, Kurban M. Scabies in the age of increasing drug resistance. PLoS Negl Trop Dis 2017; 11(11):e0005920. doi:10.1371/journal.pntd.0005920
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med 2017; 30(1):78–84. doi:10.3122/jabfm.2017.01.160190
The Case of the Disappearing Eyebrows
When this 45-year-old man was in third grade, he began to notice areas of hair loss in his scalp. The affected area was always round and the hair loss complete—but it would grow back entirely within weeks to months. There was never any rash or discomfort associated with these changes.
Since then, he has experienced numerous similar episodes of focal hair loss, sometimes in the beard, sometimes on the arms or legs, and most recently, in his eyebrows. Again, no symptoms accompany the process.
Although his personal health history is relatively uneventful, his family has not been as fortunate. There are numerous cases of lupus, rheumatoid arthritis, diabetes, and thyroid disease.
EXAMINATION
There are sharply defined, crescent-shaped, 2.5-cm divots at the superior borders of both eyebrows in which every hair is gone. No redness, swelling, or scaling are seen or felt, and there is no detectable adenopathy in the region.
Examination of hair-bearing regions reveals no other areas of hair loss.
What’s the diagnosis?
DISCUSSION
This case illustrates several variants of an extremely common condition: alopecia areata (AA), literally translated as “hair loss confined to a particular localized area or areas.” Although AA is more common in adults, it often affects children. And though the scalp is by far the most commonly affected area, AA can cause hair loss anywhere on the body.
In general, the earlier the onset, the more likely the problem is to become recurrent or even progressive. In rare instances, a patient can develop alopecia totalis, in which there is permanent and total scalp hair loss, or even alopecia universalis, the permanent loss of every hair on the entire body. Other predictors of a poor prognosis include extensive involvement of the scalp, especially the periphery (termed ophiasis), and a history of atopy.
Much research has been done on the underlying pathology of AA, as well as potential remedies. This has proven, beyond any doubt, that the problem is autoimmune and tends to run in families (as with other autoimmune diseases), suggesting a hereditary basis. We also know that medications that dampen this autoimmune process, such as steroids and biologics, are useful but not always safe or practical.
What we don’t really know is what triggers an actual attack. My observation, based on 35 years of dermatology practice, is that stress often plays a part—but I’d be hard pressed to prove that, and it wouldn’t be very useful even if I could. This patient adamantly agreed that stress was the trigger for his AA.
The differential for AA includes tinea capitis, discoid lupus, and lichen planopilaris. This patient’s multiple episodes over decades made the correct diagnosis clear. However, when the diagnosis is in doubt—other items in the differential commonly affect the scalp, although they may also manifest with redness, scaling, or swelling—a punch biopsy may be necessary to sort through the possibilities. Care must be taken to enter the skin parallel to hair follicles when the sample is removed.
Many treatments have been tried for AA, but none are reliably effective. In the vast majority of patients, the problem resolves itself. Treatments to try when needed include topical steroids, intralesional steroids, and topical immune stimulators (eg, squaric acid or dinitrochlorobenzene)—all of which have their limitations.
TAKE-HOME LEARNING POINTS
- Alopecia areata (AA) is more common in adults but can affect children as well.
- In rare cases, the patient can experience permanent total hair loss on the scalp (alopecia totalis) or the entire body (alopecia universalis).
- Because AA is an autoimmune disease, steroids and biologics may be useful treatments—but they are not always safe or practical.
- In a majority of patients with AA, the problem will resolve on its own.
When this 45-year-old man was in third grade, he began to notice areas of hair loss in his scalp. The affected area was always round and the hair loss complete—but it would grow back entirely within weeks to months. There was never any rash or discomfort associated with these changes.
Since then, he has experienced numerous similar episodes of focal hair loss, sometimes in the beard, sometimes on the arms or legs, and most recently, in his eyebrows. Again, no symptoms accompany the process.
Although his personal health history is relatively uneventful, his family has not been as fortunate. There are numerous cases of lupus, rheumatoid arthritis, diabetes, and thyroid disease.

EXAMINATION
There are sharply defined, crescent-shaped, 2.5-cm divots at the superior borders of both eyebrows in which every hair is gone. No redness, swelling, or scaling are seen or felt, and there is no detectable adenopathy in the region.
Examination of hair-bearing regions reveals no other areas of hair loss.
What’s the diagnosis?
DISCUSSION
This case illustrates several variants of an extremely common condition: alopecia areata (AA), literally translated as “hair loss confined to a particular localized area or areas.” Although AA is more common in adults, it often affects children. And though the scalp is by far the most commonly affected area, AA can cause hair loss anywhere on the body.
In general, the earlier the onset, the more likely the problem is to become recurrent or even progressive. In rare instances, a patient can develop alopecia totalis, in which there is permanent and total scalp hair loss, or even alopecia universalis, the permanent loss of every hair on the entire body. Other predictors of a poor prognosis include extensive involvement of the scalp, especially the periphery (termed ophiasis), and a history of atopy.
Much research has been done on the underlying pathology of AA, as well as potential remedies. This has proven, beyond any doubt, that the problem is autoimmune and tends to run in families (as with other autoimmune diseases), suggesting a hereditary basis. We also know that medications that dampen this autoimmune process, such as steroids and biologics, are useful but not always safe or practical.
What we don’t really know is what triggers an actual attack. My observation, based on 35 years of dermatology practice, is that stress often plays a part—but I’d be hard pressed to prove that, and it wouldn’t be very useful even if I could. This patient adamantly agreed that stress was the trigger for his AA.
The differential for AA includes tinea capitis, discoid lupus, and lichen planopilaris. This patient’s multiple episodes over decades made the correct diagnosis clear. However, when the diagnosis is in doubt—other items in the differential commonly affect the scalp, although they may also manifest with redness, scaling, or swelling—a punch biopsy may be necessary to sort through the possibilities. Care must be taken to enter the skin parallel to hair follicles when the sample is removed.
Many treatments have been tried for AA, but none are reliably effective. In the vast majority of patients, the problem resolves itself. Treatments to try when needed include topical steroids, intralesional steroids, and topical immune stimulators (eg, squaric acid or dinitrochlorobenzene)—all of which have their limitations.
TAKE-HOME LEARNING POINTS
- Alopecia areata (AA) is more common in adults but can affect children as well.
- In rare cases, the patient can experience permanent total hair loss on the scalp (alopecia totalis) or the entire body (alopecia universalis).
- Because AA is an autoimmune disease, steroids and biologics may be useful treatments—but they are not always safe or practical.
- In a majority of patients with AA, the problem will resolve on its own.
When this 45-year-old man was in third grade, he began to notice areas of hair loss in his scalp. The affected area was always round and the hair loss complete—but it would grow back entirely within weeks to months. There was never any rash or discomfort associated with these changes.
Since then, he has experienced numerous similar episodes of focal hair loss, sometimes in the beard, sometimes on the arms or legs, and most recently, in his eyebrows. Again, no symptoms accompany the process.
Although his personal health history is relatively uneventful, his family has not been as fortunate. There are numerous cases of lupus, rheumatoid arthritis, diabetes, and thyroid disease.

EXAMINATION
There are sharply defined, crescent-shaped, 2.5-cm divots at the superior borders of both eyebrows in which every hair is gone. No redness, swelling, or scaling are seen or felt, and there is no detectable adenopathy in the region.
Examination of hair-bearing regions reveals no other areas of hair loss.
What’s the diagnosis?
DISCUSSION
This case illustrates several variants of an extremely common condition: alopecia areata (AA), literally translated as “hair loss confined to a particular localized area or areas.” Although AA is more common in adults, it often affects children. And though the scalp is by far the most commonly affected area, AA can cause hair loss anywhere on the body.
In general, the earlier the onset, the more likely the problem is to become recurrent or even progressive. In rare instances, a patient can develop alopecia totalis, in which there is permanent and total scalp hair loss, or even alopecia universalis, the permanent loss of every hair on the entire body. Other predictors of a poor prognosis include extensive involvement of the scalp, especially the periphery (termed ophiasis), and a history of atopy.
Much research has been done on the underlying pathology of AA, as well as potential remedies. This has proven, beyond any doubt, that the problem is autoimmune and tends to run in families (as with other autoimmune diseases), suggesting a hereditary basis. We also know that medications that dampen this autoimmune process, such as steroids and biologics, are useful but not always safe or practical.
What we don’t really know is what triggers an actual attack. My observation, based on 35 years of dermatology practice, is that stress often plays a part—but I’d be hard pressed to prove that, and it wouldn’t be very useful even if I could. This patient adamantly agreed that stress was the trigger for his AA.
The differential for AA includes tinea capitis, discoid lupus, and lichen planopilaris. This patient’s multiple episodes over decades made the correct diagnosis clear. However, when the diagnosis is in doubt—other items in the differential commonly affect the scalp, although they may also manifest with redness, scaling, or swelling—a punch biopsy may be necessary to sort through the possibilities. Care must be taken to enter the skin parallel to hair follicles when the sample is removed.
Many treatments have been tried for AA, but none are reliably effective. In the vast majority of patients, the problem resolves itself. Treatments to try when needed include topical steroids, intralesional steroids, and topical immune stimulators (eg, squaric acid or dinitrochlorobenzene)—all of which have their limitations.
TAKE-HOME LEARNING POINTS
- Alopecia areata (AA) is more common in adults but can affect children as well.
- In rare cases, the patient can experience permanent total hair loss on the scalp (alopecia totalis) or the entire body (alopecia universalis).
- Because AA is an autoimmune disease, steroids and biologics may be useful treatments—but they are not always safe or practical.
- In a majority of patients with AA, the problem will resolve on its own.
Growing spot on nose

The FP was concerned that this could be melanoma.
He used his dermatoscope and saw suspicious patterns that included polygonal lines and circle-within-circle patterns. He informed the patient about his concerns for melanoma and discussed the need for a biopsy. After obtaining informed consent, the FP injected the patient’s nose with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. (See “Biopsies for skin cancer detection: Dispelling the myths”). The FP used a Dermablade to perform a broad shave biopsy, which revealed a lentigo maligna melanoma in situ (also known as lentigo maligna). (See the Watch & Learn video on “Shave biopsy”)
During the follow-up visit, the FP presented the patient with 2 options for treatment: topical imiquimod for 3 months or Mohs surgery. The FP recommended Mohs surgery because the data for topical imiquimod in the treatment of lentigo maligna indicate that it is less effective on the nose than other areas of the face. The patient agreed to surgery, and the FP sent the referral and the photo of the original lesion to the Mohs surgeon. The outcome was good, and the need for ongoing sun safety and regular skin surveillance was explained to the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine, 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP was concerned that this could be melanoma.
He used his dermatoscope and saw suspicious patterns that included polygonal lines and circle-within-circle patterns. He informed the patient about his concerns for melanoma and discussed the need for a biopsy. After obtaining informed consent, the FP injected the patient’s nose with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. (See “Biopsies for skin cancer detection: Dispelling the myths”). The FP used a Dermablade to perform a broad shave biopsy, which revealed a lentigo maligna melanoma in situ (also known as lentigo maligna). (See the Watch & Learn video on “Shave biopsy”)
During the follow-up visit, the FP presented the patient with 2 options for treatment: topical imiquimod for 3 months or Mohs surgery. The FP recommended Mohs surgery because the data for topical imiquimod in the treatment of lentigo maligna indicate that it is less effective on the nose than other areas of the face. The patient agreed to surgery, and the FP sent the referral and the photo of the original lesion to the Mohs surgeon. The outcome was good, and the need for ongoing sun safety and regular skin surveillance was explained to the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine, 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com

The FP was concerned that this could be melanoma.
He used his dermatoscope and saw suspicious patterns that included polygonal lines and circle-within-circle patterns. He informed the patient about his concerns for melanoma and discussed the need for a biopsy. After obtaining informed consent, the FP injected the patient’s nose with 1% lidocaine and epinephrine for anesthesia and to prevent bleeding. Remember, it is safe to use injectable epinephrine along with lidocaine when doing surgery on the nose. (See “Biopsies for skin cancer detection: Dispelling the myths”). The FP used a Dermablade to perform a broad shave biopsy, which revealed a lentigo maligna melanoma in situ (also known as lentigo maligna). (See the Watch & Learn video on “Shave biopsy”)
During the follow-up visit, the FP presented the patient with 2 options for treatment: topical imiquimod for 3 months or Mohs surgery. The FP recommended Mohs surgery because the data for topical imiquimod in the treatment of lentigo maligna indicate that it is less effective on the nose than other areas of the face. The patient agreed to surgery, and the FP sent the referral and the photo of the original lesion to the Mohs surgeon. The outcome was good, and the need for ongoing sun safety and regular skin surveillance was explained to the patient.
Photos and text for Photo Rounds Friday courtesy of Richard P. Usatine, MD. This case was adapted from: Karnes J, Usatine R. Melanoma. In: Usatine R, Smith M, Mayeaux EJ, et al. Color Atlas and Synopsis of Family Medicine, 3rd ed. New York, NY: McGraw-Hill; 2019:1112-1123.
To learn more about the newest 3rd edition of the Color Atlas and Synopsis of Family Medicine, see: https://www.amazon.com/Color-Atlas-Synopsis-Family-Medicine/dp/1259862046/
You can get the Color Atlas of Family Medicine app by clicking on this link: usatinemedia.com