User login
Strategies to reduce and prevent polypharmacy in older patients
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
CASE
Ronald Wa is a 74-year old man with an extensive medical history: diabetes, hypertension, heart failure, atrial fibrillation, pancreatitis, hyperlipidemia, gout, depression, generalized anxiety, obstructive sleep apnea, and benign prostatic hypertrophy. He arrives at the emergency department (ED) of the hospital by nonemergent ambulance from home for evaluation of lethargy and confusion over the past week.
In the ED, Mr. W is afebrile, normotensive, and oxygenating on room air. Mucous membranes are dry. On physical examination, he appears pale, fatigued, and modestly confused but is able to state his name and birthday, although not the location or date.
Laboratory testing reveals: blood glucose, 107 mg/dL; serum creatinine, 2.3 mg/dL; sodium, 127 mEq/L; and hemoglobin level and hematocrit, within normal limits. Urinalysis is negative. Renal ultrasonography is unremarkable, without evidence of urinary tract obstruction.
Mr. W is admitted to the general medical unit with hyponatremia. The pharmacy admission specialist begins reconciliation of the long list of the patient’s home medications.
Overprescribing: Often, more is not better
Some experts consider prescribing medication to be the most common form of medical intervention; beyond that, polypharmacy—often defined as the use of more medications than are medically necessary (see the next section on terminology)—is recognized as an increasingly serious problem in many medical specialties.1 Here are specifics about the extent of, and harm caused by, the problem2,3:
- The US General Accounting Office reports that inappropriate polypharmacy is associated with significant morbidity and mortality.2 Research has established a strong relationship between polypharmacy and harmful clinical consequences,3 to which the older patient population is most susceptible.
- Polypharmacy is also recognized as an expensive practice; the US Center for Medicare and Medicaid Services estimates that polypharmacy cost US health insurers more than $50 billion annually.2
- Worldwide, with more and more people older than 65 years, polypharmacy is becoming more prevalent, and a growing concern, in older adults; approximately 50% of them take ≥ 1 medications that are medically unnecessary.3
Despite many programs to help with deprescribing, drug–drug interactions and the so-called prescribing cascade (ie, when signs and symptoms of an adverse drug effect are misdiagnosed as a new medical condition) continue to affect patients, leading to comorbidities. It is important, therefore, for physicians to be aware of commonly used tools to prevent polypharmacy and its consequences.
What is “polypharmacy” understood to mean?
Despite the compelling association of polypharmacy with the presence of multiple morbidities in the older patient population, there is no consensus on its definition:
- Starting with the dictionary, “polypharmacy” derives from 2 words in Ancient Greek: poly, “more than one,” and “pharmakon, “drug.”3
- The definition can vary based on the number of drugs a patient has been prescribed, their safety, and the appropriateness of their use.1
- Another definition is the use of more medications than are medically necessary; such a grouping includes agents that are not indicated, are ineffective, or constitute a therapeutic duplication. Although this definition is more clinically relevant than the others, it is premised on undertaking a clinical review of a medication regimen.3
- A numerical definition is the most commonly reported category, a number that varies from study to study—from ≥ 2 to ≥ 11 medications. When applied to health care settings, accepted definitions are ≥ 5 medications at hospital discharge and ≥ 10 during a hospital stay.4 Numerical definitions of polypharmacy do not ascertain the clinical appropriateness of therapy nor the process of rationalizing those medications.1
aA composite, hypothetical patient, based on the authors' clinical experience.
Continue to: Appropriateness
Appropriateness
Polypharmacy is classified as appropriate or inappropriate:
- Appropriate polypharmacy is the optimization of medications for patients with complex or multiple conditions, when the use of medicine is in agreement with best evidence.
- Inappropriate polypharmacy can increase the risk of adverse drug effects and drug–drug interactions and can be characterized by medication underuse and duplication.4
There are subdefinitions of “appropriateness,” but these are beyond the scope of this article.
What variables contribute to polypharmacy?
Multimorbidity is common in the older population. The presence of multiple chronic conditions increases the complexity of therapeutic management for health professionals and patients; such complexity can have a harmful impact on health outcomes. Combinations of medications to treat chronic diseases automatically push many patients into polypharmacy. Few treatment guidelines provide recommendations on when to stop medications.
Consequences of polypharmacy, some of which are masked as syndromes in the older patient, include delirium and dementia, urinary incontinence, dizziness, falls, adverse drug reactions, increased length of hospital stay, readmission soon after discharge, and death.3-5 Relatively high rates of drug consumption and other variables (eg, decreased renal and hepatic function, decreased total body water and lean body mass, cognitive impairment, age-related decline in vision and hearing, frequency of chronic diseases and medical comorbidities, communication barriers, prescribing cascades, and health care delivery involving multiple prescribers) can contribute to an increased prevalence of medication-associated morbidity and mortality as the result of polypharmacy.
In a descriptive study6 that examined these variables, researchers explored whether general practitioners experience barriers to medication review in multimorbid patients with polypharmacy. They concluded that the primary barriers were (1) lack of communication and teamwork with specialists and (2) the challenge of handling polypharmacy in a culture that encourages adding medications and inhibits conversations about medication withdrawal.6
Continue to: Reducing consequences of polypharmacy
Reducing consequences of polypharmacy
Collaborative medication review
Interventions to help physicians reduce polypharmacy include reviewing medications with older patients at every office visit and during transitions of care into and out of the hospital or other care facility. A 2016 Cochrane review of 5 randomized trials of inpatient medication reviews led by pharmacists, physicians, and other health care professionals showed a 36% reduction in ED visits 30 days to 1 year after discharge.7
Patients can collaborate in this effort by bringing all medications to each appointment or upon hospital admission—not just a list but the actual supply, to ensure that a correct medication list is compiled and a thorough review conducted.8 Explicitly ask open-ended questions of the patient about over-the-counter medications, herbal products, and other home remedies that have not been prescribed; many patients may have trouble with recall or are uncertain what fits the definition of a nonprescription medication.8,9
Compare the medication list with the patient’s current problem list; consider removing medications that do not have a pertinent indication. (Physicians can help in this regard when prescribing by making note in the medical record of the indication for each medication they prescribe.)
Evaluate the patient’s signs and symptoms as a possible drug-related adverse effect, thus making an effort to minimize the chance of a prescribing cascade.9
Use Beers criteria,10 which list potentially inappropriate medications to be avoided in older adults. The criteria serve as a filter when considering starting a new medication and aiding in the review process.8
Continue to: The NO TEARS tool...
The NO TEARS tool11 can be useful for simplifying the medication review process. Components of this tool are:
- Need and indication: Does the patient still require each of his medications? Was long-term treatment anticipated?
- Open questions: Ask the patient for his views about his medications; for example, “Do you think the drugs you take work?”
- Tests and monitoring: Are any of the patient’s conditions undertreated, based on laboratory and clinical findings?
- Evidence and guidelines: Has the base of evidence been updated for each of the patient’s medications since they were started?
- Adverse events: Is the patient experiencing adverse effects of medication? Have possible adverse drug interactions been noted?
- Risk reduction or prevention: Does the patient face risks of treatment (eg, loss of appetite, urinary incontinence) that can be reduced by optimizing the medication plan?
- Simplification and switches: Can treatment be simplified while maintaining effectiveness?
There are strategies to promote patient advocacy, as well. Encourage patients to use a holistic approach by asking you, their other physicians, and their pharmacist about how their condition is being treated:
- What other treatment options exist, including nonpharmacotherapeutic options?
- What are the possible benefits and harms of medical therapy?
- Under what circumstances would discontinuing a medication be appropriate?12
CASE
Medication reconciliation identifies > 20 medications that had been prescribed for the patient to take at home (TABLE 1). A clinical pharmacist then performs a home medication review as part of routine patient care upon transition of care into the hospital.
Identifying polypharmacy
Implementing polypharmacy identification tools is a necessary first step in the process of mitigating the risk of multiple concurrent medications (TABLE 22,10,12-18). In addition to tools that are used to identify polypharmacy, there are steps that physicians and pharmacists can take to decrease the risk of polypharmacy.
For example, in a longitudinal, time-series cohort study measuring polypharmacy events, a pharmacist intervention was used as the means to decrease polypharmacy.19 Pharmacists intervened twice (each intervention separated by 1 year) to identify and manage 5 categories of high-risk drugs in patients whose care was provided by a managed care plan.19 During that time, pharmacists provided drug therapy reviews, education to physicians and patients about drug safety, and information for physicians on ways to correct problems with polypharmacy.19
Continue to: Over the course of the 2 interventions...
Over the course of the 2 interventions, the overall rate of polypharmacy events decreased 67% after the first intervention and 39% after the second. The practice of having pharmacists spearhead this task was shown to reduce the cost and number of prescriptions in patients at risk for polypharmacy. (In fact, some general practitioners report that they deem multidisciplinary decision-making with pharmacists a necessary component of managing polypharmacy effectively.6)
Screening for medications as a cause of signs and symptoms
As noted earlier, a prescribing cascade arises when a drug administered to a patient causes an adverse event that is then mistakenly identified as a new condition, resulting in a new medication being prescribed.9 The pattern of a cascade then repeats itself, resulting in inappropriate polypharmacy.
Erroneous treatment of an adverse drug event as a medical condition is often the result of a lack of pharmacologic knowledge—which is why it is necessary to evaluate each new symptom with the mindset that a medication might, in fact, be causing the sign or symptom and with the aim of reducing the risk of a prescribing cascade.8,9 Routinely update a patient’s medication list in the event that a medication no longer has an indication aligned with the patient’s problem list; then, ideally, the initial therapy can be adjusted instead of starting additional medications.9
CASE
A review of Mr. W’s home medications reveals 1 therapeutic duplication and 2 drugs that lacked an indication. Application of the Screening Tool of Older Persons’ potentially inappropriate Prescriptions (STOPP)15 and Beers criteria10 helped the pharmacist identify additional elements of inappropriate polypharmacy, including inappropriate medication use, drug–disease interactions, contraindications, and recommendations for dosage adjustment based on kidney function. Specifically:
- Aripiprazole and quetiapine: Present an increased risk of falls. (General recommendation: Avoid using Frutiger LT Std≥ 3 drugs that act on the central nervous system [CNS], due to an increased risk of falls.)
- Fluoxetine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Gabapentin: Presents an increased risk of CNS adverse effects. Reduce the dosage when the estimated creatinine clearance is < 60 mL/min.
- Hydrocodone–acetaminophen: Presents an increased risk of falls. (Again, avoid or minimize the number of drugs that act on the CNS.)
- Lorazepam: Indication is missing. Avoid use of this drug due to an increased risk of cognitive impairment and decreased metabolism of medication.
- Mirtazapine: Can cause the syndrome of inappropriate secretion of antidiuretic hormone. Use with caution.
- Pantoprazole: Avoid scheduled use for > 8 weeks, except in high-risk patients, due to the risk of Clostridium difficile infection and bone loss and fractures.
- Prazosin: Indication is missing. Avoid use of this drug as an antihypertensive due to the high risk of orthostatic hypotension.
- Ranitidine: Duplicates concurrent treatment with pantoprazole. Reduce the dosage when the estimated creatinine clearance is < 50 mL/min.
The value of deprescribing
Direct evidence of the efficacy and safety of deprescribing, and strategies for deprescribing, have been documented in the literature:
Observational study. Cessation of inappropriate antihypertensive agents was associated with fewer cardiovascular events and deaths over a 5-year follow-up period.20
Continue to: Deprescribing protocol
Deprescribing protocol. A method developed by Scott and co-workers21 is an additional resource to consider. Appropriate times to consider deprescribing are (1) when new symptoms suggest an adverse drug effect; (2) in the presence of end-stage disease, terminal illness, dementia, extreme frailty, or full dependence on others for all care; (3) upon receipt of high-risk medications or combinations; and (4) upon receipt of preventive medications for which risk outweighs benefit.21
This suggested method of deprescribing comprises several steps: (1) collecting all medications that the patient is taking and identifying the indication for each; (2) considering the overall risk of drug-induced harm to determine necessary intensity of deprescribing; (3) assessing each drug for its eligibility to be discontinued, such as no indication, part of a prescribing cascade, or lack of benefit; (4) prioritizing drugs for discontinuation; and (5) implementing and monitoring the drug discontinuation regimen.21
Drug-by-drug elimination trial. Reducing the dosage of, or stopping, only 1 medication at a time has been shown to be paramount to assessing development of medication-associated problems and then identifying a likely cause.14
Good Palliative-Geriatric Practice algorithm. This algorithm22 can be used to guide discontinuation of inappropriate medications and improve drug therapy in community-dwelling older adults. The algorithm has been shown to improve the overall well-being of patients studied; however, it has been tested only in patients in long-term care settings and community-dwelling palliative care patients, limiting its generalizability to a larger population. The algorithm is also difficult to apply to patients who have multiple comorbidities.
Risk vs. benefit of discontinuing chronic medical therapy. A systematic review of the effects of discontinuing chronic medication reveals that the risk of doing so might outweigh benefit14; this finding is thought to be due to potential relapse in the disease state being treated.11 The risks of discontinuation should be contemplated before removing the medication or reducing the dosage. Medications that can be considered to present a risk when discontinued include, but are not limited to, benzodiazepines, oral corticosteroids, antidepressants, acid suppressants, bisphosphonates, statins, and transdermal opioids.1
Continue to: CASE
CASE
After applying Beers criteria10 and STOPP15, the pharmacist makes several recommendations:
- Use aripiprazole and quetiapine with caution.
- Consider discontinuing fluoxetine, hydrocodone–acetaminophen, lorazepam, pantoprazole, and ranitidine.
- Reduce the dosage of gabapentin.
- Clarify the indication for prazosin. Consider discontinuing if being used as an antihypertensive.
In addition, the pharmacist recommends holding metformin because lactic acidosis can develop (however rarely) when a person taking metformin experiences acute kidney injury.
CORRESPONDENCE
Tracy Mahvan, PharmD, BCGP, University of Wyoming, School of Pharmacy, 1000 East University Avenue, Laramie, WY 82071; [email protected]
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
1. All Wales Medicines Strategy Group. Polypharmacy: Guidance for Prescribing. July 2014. http://awmsg.org/docs/awmsg/medman/Polypharmacy%20-%20Guidance%20for%20Prescribing.pdf. Accessed October 3, 2019.
2. Bushardt RL, Massey EB, Simpson TW, et al. Polypharmacy: misleading, but manageable. Clin Interv Aging. 2008;3:383-389.
3. Maher RL, Hanlon J, Hajjar ER. Clinical consequences of polypharmacy in elderly. Expert Opin Drug Saf. 2014;13:57-65.
4. Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230.
5. Milton JC, Hill-Smith I, Jackson SH. Prescribing for older people. BMJ. 2008;336:606-609.
6. Laursen J, Kornholt J, Betzer C, et al. General practitioners’ barriers toward medication reviews in polymedicated multimorbid patients: How can a focus on the pharmacotherapy in an outpatient clinic support GPs? Health Serv Res Manag Epidemiol. 2018;5:2333392818792169.
7. Christensen M, Lundh A. Medication review in hospitalized patients to reduce morbidity and mortality. Cochrane Database Syst Rev. 2016;2:CD008986.
8. Zurakowski T. The practicalities and pitfalls of polypharmacy. Nurse Pract. 2009;34:36-41.
9. Ponte ML, Wachs L, Wachs A, et al. Prescribing cascade. A proposed new way to evaluate it. Medicina (B Aires). 2017;77:13-16.
10. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc. 2015;63:2227-2246.
11. Lewis T. Using the NO TEARS tool for medication review. BMJ. 2004;329:434.
12. Hamilton HJ, Gallagher PF, O’Mahony D. Inappropriate prescribing and adverse events in older people. BMC Geriatr. 2009;9:5.
13. Skinner M. A literature review: polypharmacy protocol for primary care. Geriatr Nurs. 2015;36:367-371.
14. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31.
15. Gallagher P, O’Mahony D. STOPP (Screening Tool of Older Persons’ potentially inappropriate Prescriptions): application to acutely ill elderly patients and comparison with Beers criteria. Age Ageing. 2008;37:673-679.
16. Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol. 1992;45:1045-1051.
17. Samsa G, Hanlon JT, Schmader KE, et al. A summated score for the Medication Appropriateness Index: development and assessment of clinimetric properties including content validity. J Clin Epidemiol. 1994;47:891-896.
18. Carnahan RM, Lund BC, Perry PJ, et al. The Anticholinergic Drug Scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J Clin Pharmacol. 2006;46:1481-1486.
19. Zarowitz BJ, Stebelsky LA, Muma BK, et al. Reduction of high-risk polypharmacy drug combinations in patients in a managed care setting. Pharmacotherapy. 2005;25:1636-1645.
20. Thio SL, Nam J, van Driel ML, et al. Effects of discontinuation of chronic medication in primary care: a systematic review of deprescribing trials. Br J Gen Pract. 2018;68:e663-e672.
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175:827-834.
22. Garfinkel D, Mangin D. Feasibility study of a systematic approach for discontinuation of multiple medications in older adults: addressing polypharmacy. Arch Intern Med. 2010;170:1648-1654.
PRACTICE RECOMMENDATIONS
› Use one of the available tested and recommended screening tools to identify polypharmacy. C
› Engage in collaborative medication review to reduce the incidence of polypharmacy. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Aplastic Anemia: Diagnosis and Treatment
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
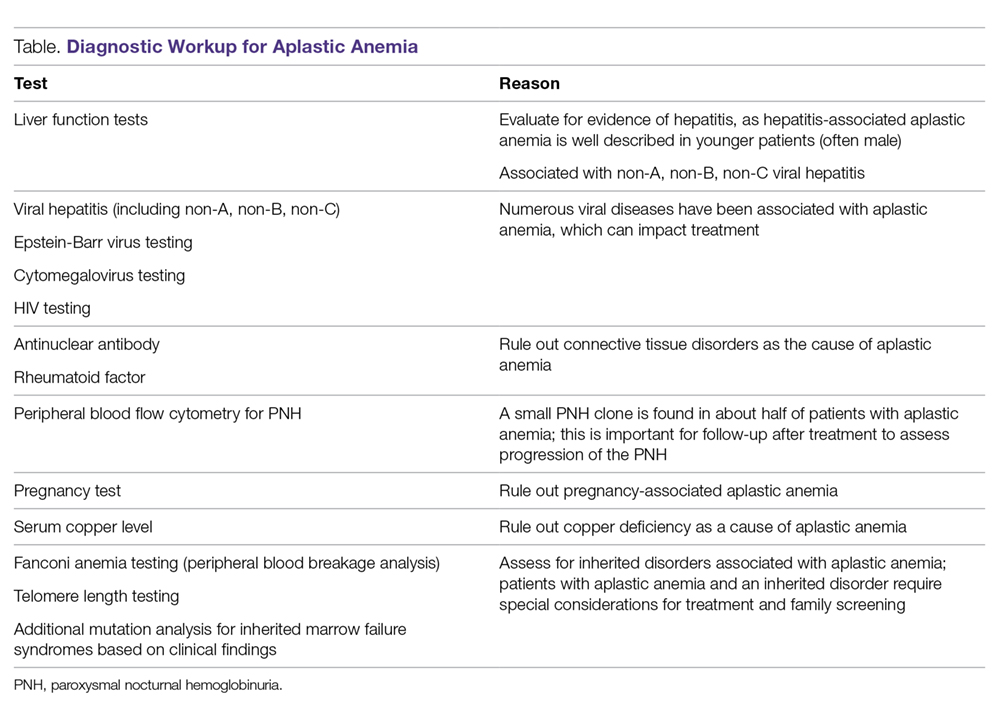
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7
Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.
Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68
Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7
Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.
Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68
Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
From the Oregon Health and Science University, Portland, OR.
Abstract
- Objective: To describe the current approach to diagnosis and treatment of aplastic anemia.
- Methods: Review of the literature.
- Results: Aplastic anemia can be acquired or associated with an inherited marrow failure syndrome (IMFS), and the treatment and prognosis vary dramatically between these 2 etiologies. Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to life-threatening neutropenic infections or bleeding. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia.
- Conclusion: Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marro
w transplant program to evaluate for early transplantation is the new standard of care for aplastic anemia.
Keywords: inherited marrow failure syndrome; Fanconi anemia; immunosuppression; transplant; stem cell.
Aplastic anemia is a clinical and pathological entity of bone marrow failure that causes progressive loss of hematopoietic progenitor stem cells (HPSC), resulting in pancytopenia.1 Patients may present along a spectrum, ranging from being asymptomatic with incidental findings on peripheral blood testing to having life-threatening neutropenic infections or bleeding. Aplastic anemia results from either inherited or acquired causes, and the pathophysiology and treatment approach vary significantly between these 2 causes. Therefore, recognition of inherited marrow failure diseases, such as Fanconi anemia and telomere biology disorders, is critical to establishing the management plan.
Epidemiology
Aplastic anemia is a rare disorder, with an incidence of approximately 1.5 to 7 cases per million individuals per year.2,3 A recent Scandinavian study reported that the incidence of aplastic anemia among the Swedish population is 2.3 cases per million individuals per year, with a median age at diagnosis of 60 years and a slight female predominance (52% versus 48%, respectively).2 This data is congruent with prior observations made in Barcelona, where the incidence was 2.34 cases per million individuals per year, albeit with a slightly higher incidence in males compared to females (2.54 versus 2.16, respectively).4 The incidence of aplastic anemia varies globally, with a disproportionate increase in incidence seen among Asian populations, with rates as high as 8.8 per million individuals per year.3-5 This variation in incidence in Asia versus other countries has not been well explained. There appears to be a bimodal distribution, with incidence peaks seen in young adults and in older adults.2,3,6
Pathophysiology
Acquired Aplastic Anemia
The leading hypothesis as to the cause of most cases of acquired aplastic anemia is that a dysregulated immune system destroys HPSCs. Inciting etiologies implicated in the development of acquired aplastic anemia include pregnancy, infection, medications, and exposure to certain chemicals, such as benzene.1,7 The historical understanding of acquired aplastic anemia implicates cytotoxic T-lymphocyte–mediated destruction of CD34+ hematopoietic stem cells.1,8,9 This hypothesis served as the basis for treatment of acquired aplastic anemia with immunosuppressive therapy, predominantly anti-thymocyte globulin (ATG) combined with cyclosporine A.1,8 More recent work has focused on cytokine interactions, particularly the suppressive role of interferon (IFN)-γ on hematopoietic stem cells independent of T-lymphocyte–mediated destruction, which has been demonstrated in a murine model.8 The interaction of IFN-γ with the hematopoietic stem cell pool is dynamic. IFN-γ levels are elevated during an acute inflammatory response, such as a viral infection, providing further basis for the immune-mediated nature of the acquired disease.10 Specifically, in vitro studies suggest the effects of IFN-γ on HPSC may be secondary to interruption of thrombopoietin and its respective signaling pathways, which play a key role in hematopoietic stem cell renewal.11 Eltrombopag, a thrombopoietin receptor antagonist, has shown promise in the treatment of refractory aplastic anemia, with studies indicating that its effectiveness is independent of IFN-γ levels.11,12
Inherited Aplastic Anemia
The inherited marrow failure syndromes (IMFSs) are a group of disorders characterized by cellular maintenance and repair defects, leading to cytopenias, increased cancer risk, structural defects, and risk of end organ damage, such as liver cirrhosis and pulmonary fibrosis.13-15 The most common diseases include Fanconi anemia, dyskeratosis congenita/telomere biology disorders, Diamond-Blackfan anemia, and Shwachman-Diamond syndrome, but with the advent of whole exome sequencing, new syndromes continue to be discovered. While classically these disorders present in children, adult presentations are now commonplace. Broadly, the pathophysiology of inherited aplastic anemia relates to the defective HPSCs and an accelerated decline of the hematopoietic stem cell compartment.
The most common IMFSs, Fanconi anemia and telomere biology disorders, are associated with numerous mutations in DNA damage repair pathways and telomere maintenance pathways. TERT, DKC, and TERC mutations are most commonly associated with dyskeratosis congenita, but may also be found infrequently in patients with aplastic anemia presenting at an older age in the absence of the classic phenotypical features.1,16,17 The recognition of an underlying genetic disorder or telomere biology disorder leading to constitutional aplastic anemia is significant, as these conditions are associated not only with marrow failure, but also with endocrinopathies, organ fibrosis, and and hematopoietic and solid organ malignancies.13-15 In particular, TERT and TERC gene mutations have been associated with dyskeratosis congenita as well as pulmonary fibrosis and cirrhosis.18,19 The implications of early diagnosis of an IMFS lie in the approach to treatment and prognosis.
Clonal Disorders and Secondary Malignancies
Myelodysplastic syndrome (MDS) and secondary acute myeloid leukemia (AML) are 2 clonal disorders that may arise from a background of aplastic anemia.9,20,21 Hypoplastic MDS can be difficult to differentiate from aplastic anemia at diagnosis based on morphology alone, although recent work has demonstrated that molecular testing for somatic mutations in ASXL1, DNMT3A, and BCOR can aid in differentiating a subset of aplastic anemia patients who are more likely to progress to MDS.21 Clonal populations of cells harboring 6p uniparental disomy are seen in more than 10% of patients with aplastic anemia on cytogenetic analysis, which can help differentiate the diseases.9 Yoshizato and colleagues found lower rates of ASXL1 and DNMT3A mutations in patients with aplastic anemia as compared with patients with MDS or AML. In this study, patients with aplastic anemia had higher rates of mutations in PIGA (reflecting the increased paroxysmal nocturnal hemoglobinuria [PNH] clonality seen in aplastic anemia) and BCOR.9 Mutations were also found in genes commonly mutated in MDS and AML, including TET2, RUNX1, TP53, and JAK2, albeit at lower frequencies.9 These mutations as a whole have not predicted response to therapy or prognosis. However, when performing survival analysis in patients with specific mutations, those commonly encountered in MDS/AML (ASXL1, DNMT3A, TP53, RUNX1, CSMD1) are associated with faster progression to overt MDS/AML and decreased overall survival (OS),20,21 suggesting these mutations may represent early clonality that can lead to clonal evolution and the development of secondary malignancies. Conversely, mutations in BCOR and BCORL appear to identify patients who may have a favorable outcome in response to immunosuppressive therapy and, similar to patients with PIGA mutations, improved OS.9
Paroxysmal Nocturnal Hemoglobinuria
In addition to having an increased risk of myelodysplasia and malignancy due to the development of a dominant pre-malignant clone, patients with aplastic anemia often harbor progenitor cell clones associated with PNH.1,17 PNH clones have been identified in more than 50% of patients with aplastic anemia.22,23 PNH represents a clonal disorder of hematopoiesis in which cells harbor X-linked somatic mutations in the PIGA gene; this gene encodes a protein responsible for the synthesis of glycosylphosphatidylinositol anchors on the cell surface.22,24 The lack of these cell surface proteins, specifically CD55 (also known as decay accelerating factor) and CD59 (also known as membrane inhibitor of reactive lysis), predisposes red cells to increased complement-mediated lysis.25 The exact mechanism for the development of these clones in patients with aplastic anemia is not fully understood. Current theories hypothesize that the clones are protected from the immune-mediated destruction of normal hematopoietic stem cells due to the absence of the cell surface proteins.1,20 The role of these clones over time in patients with aplastic anemia is less clear, though recent work demonstrated that despite differences in clonality over the disease course, aplastic anemia patients with small PNH clones are less likely to develop overt hemolysis and larger PNH clones compared to patients harboring larger (≥ 50%) PNH clones at diagnosis.23,26,27 Additionally, PNH clones in patients with aplastic anemia infrequently become clinically significant.27 It should be noted that these conditions exist along a continuum; that is, patients with aplastic anemia may develop PNH clones, while conversely patients with PNH may develop aplastic anemia.20 Patients with PNH clones should be followed via peripheral blood flow cytometry and complete blood count to track clonal stability and identify clinically significant PNH among aplastic anemia patients.28
Clinical Presentation
Patients with aplastic anemia typically are diagnosed either due to asymptomatic cytopenias found on peripheral blood sampling, symptomatic anemia, bleeding secondary to thrombocytopenia, or wound healing and infectious complications related to neutropenia.29 A thorough history to understand the timing of symptoms, recent infectious symptoms/exposure, habits, and chemical or toxin exposures (including medications, travel, and supplements) helps guide diagnostic testing. Family history is also critical, with attention given to premature graying; pulmonary, renal, and liver disease; and blood disorders.
Patients with an IMFS (eg, Fanconi anemia or dyskeratosis congenita) may have associated phenotypical findings such as urogenital abnormalities or short stature; in addition, those with dyskeratosis congenita may present with the classic triad of oral leukoplakia, lacy skin pigmentation, and dystrophic nails.7 However, classic phenotypical findings may be lacking in up to 30% to 40% of patients with an IMFS.7 As described previously, while congenital malformations are common in Fanconi anemia and dyskeratosis congenita, a third of patients may have no or only subtle phenotypical abnormalities, including alterations in skin or hair pigmentation, skeletal and growth abnormalities, and endocrine disorders.30 The International Fanconi Anemia Registry identified central nervous system, genitourinary, skin and musculoskeletal, ophthalmic, and gastrointestinal system malformations among children with Fanconi anemia.31,32 Patients with dyskeratosis congenita may present with pulmonary fibrosis, hepatic cirrhosis, or premature graying, as highlighted in a recent study by DiNardo and colleagues.33 Therefore, physicians must have a heightened index of suspicion in patients with subtle phenotypical findings and associated cytopenias.
Diagnosis
The diagnosis of aplastic anemia should be suspected in any patient presenting with pancytopenia. Aplastic anemia is a diagnosis of exclusion.34 Other conditions associated with peripheral blood pancytopenia should be considered, including infections (HIV, hepatitis, parvovirus B19, cytomegalovirus, Epstein-Barr virus, varicella-zoster virus), nutritional deficiencies (vitamin B12, folate, copper, zinc), autoimmune disease (systemic lupus erythematosus, rheumatoid arthritis, hemophagocytic lymphohistiocytosis), hypersplenism, marrow-occupying diseases (eg, leukemia, lymphoma, MDS), solid malignancies, and fibrosis (Table).7
Diagnostic Evaluation
The workup for aplastic anemia should include a thorough history and physical exam to search simultaneously for alternative diagnoses and clues pointing to potential etiologic agents.7 Diagnostic tests to be performed include a complete blood count with differential, reticulocyte count, immature platelet fraction, flow cytometry (to rule out lymphoproliferative disorders and atypical myeloid cells and to evaluate for PNH), and bone marrow biopsy with subsequent cytogenetic, immunohistochemical, and molecular testing.35 Typical findings in aplastic anemia include peripheral blood pancytopenia without dysplastic features and bone marrow biopsy demonstrating a hypocellular marrow.7 A relative lymphocytosis in the peripheral blood is common.7 In patients with a significant PNH clone, a macrocytosis along with elevated lactate dehydrogenase and elevated reticulocyte and granulocyte counts may be present.36
The diagnosis (based on the Camitta criteria37 and modified Camitta criteria38 for severe aplastic anemia) requires 2 of the following findings on peripheral blood samples:
- Absolute neutrophil count (ANC) < 500 cells/µL
- Platelet count < 20,000 cells/µL
- Reticulocyte count < 1% corrected or < 20,000 cells/µL.35
In addition to peripheral blood findings, bone marrow biopsy is essential for the diagnosis, and should demonstrate a markedly hypocellular marrow (cellularity < 25%), occasionally with an increase in T lymphocytes.7,39 Because marrow cellularity varies with age and can be challenging to assess, additional biopsies may be needed to confirm the diagnosis.29 A 1- to 2-cm core biopsy is necessary to confirm hypocellularity, as small areas of residual hematopoiesis may be present and obscure the diagnosis.35
Excluding Hypocellular MDS and IMFS
Excluding hypocellular MDS is challenging, especially in the older adult presenting with aplastic anemia, as patients with aplastic anemia may have some degree of erythroid dysplasia on bone marrow morphology.36 The presence of a PNH clone on flow cytometry can aid in diagnosing aplastic anemia and excluding MDS,34 although PNH clones can be present in refractory anemia MDS. Patients with aplastic anemia have a lower ratio of CD34+ cells compared to those with hypoplastic MDS, with 1 study demonstrating a mean CD34+ percentage of < 0.5% in aplastic anemia versus 3.7% in hypoplastic MDS.40 Cytogenetic and molecular testing can also aid in making this distinction by identifying mutations commonly implicated in MDS.7 The presence of monosomy 7 (-7) in aplastic anemia patients is associated with a poor overall prognosis.34,41
Peripheral blood screening using chromosome breakage analysis (done using either mitomycin C or diepoxybutane as in vitro DNA-crosslinking agents)42 and telomere length testing (of peripheral blood leukocytes) is necessary to exclude the main IMFSs, Fanconi anemia and telomere biology disorders, respectively. Ruling out these conditions is imperative, as the approach to treatment varies significantly between IMFS and aplastic anemia. Patients with shortened telomeres should undergo genetic screening for mutations in the telomere maintenance genes to evaluate the underlying defect leading to shortened telomeres. Patients with increased peripheral blood breakage should have genetic testing to detect mutations associated with Fanconi anemia.
Classification
Once the diagnosis of aplastic anemia has been made, the patient should be classified according to the severity of their disease. Disease severity is determined based on peripheral blood ANC: non-severe aplastic anemia (NSAA), ANC > 500 polymorphonuclear neutrophils (PMNs)/µL; severe aplastic anemia (SAA), 200–500 PMNs/µL; and very severe aplastic anemia (VSAA), 0–200 PMNs/µL.4,34 Disease classification is important, as VSAA is associated with a decreased OS compared to SAA.2 Disease classification may affect treatment decisions, as patients with NSAA may be observed for a short period of time, while, conversely, patients with SAA have a worse prognosis with delays in therapy.43-45
Treatment of Inherited Aplastic Anemia
First-line treatment options for patients with IMFS are androgen therapy and hematopoietic stem cell transplant (HSCT). When evaluating patients for HSCT, it is critical to identify the presence of an IMFS, as the risk and mortality associated with the conditioning regimen, stem cell source, graft-versus-host disease (GVHD), and secondary malignancies differ between patients with IMFS and those with acquired marrow failure syndromes or hematologic malignancies.
Potential sibling donors need to be screened for donor candidacy as well as for the inherited defect. Among patients with Fanconi anemia or a telomere biology disorder, the stem cell source must be considered, with multiple studies in IMFSs and SAA showing superior outcomes with a bone marrow product compared to peripheral blood stem cells.46-48 In IMFS patients, the donor cell type may affect the choice of conditioning regimen.5,6 Reduced-intensity conditioning in lieu of myeloablative conditioning without total body irradiation has proved feasible in patients with Fanconi anemia, and is associated with a reduced risk of secondary malignancies.49,50 Incorporation of fludarabine in the conditioning regimen of patients without a matched sibling donor is associated with superior engraftment and survival46,49,51 compared to cyclophosphamide conditioning, which was historically used in matched related donors.50,52 Adding fludarabine appears to be especially beneficial in older patients, in whom its use is associated with lower rates of graft failure, likely due to increased immunosuppression at the time of engraftment.51,53 Fludarabine has also been incorporated into conditioning regimens for patients with a telomere biology disorder, but outcomes data are limited.5
For patients presenting with AML or a high-risk MDS who are subsequently diagnosed with an IMFS, treatment can be more complex, as these patients are at high risk for toxicity from standard chemotherapy. Limited data suggest that induction therapy and transplantation are feasible in this group of patients, and this approach is associated with increased OS, despite lower OS rates than those of IMFS patients who present prior to the development of MDS or AML.54,55 Further work is needed to determine the optimal induction regimen that balances the risks of treatment-related mortality and complications associated with conditioning regimens, risk of relapse, and risk of secondary malignancies, especially in the cohort of patients diagnosed at an older age.
Treatment of Acquired Aplastic Anemia
Supportive Care
While the workup and treatment plan are being established, attention should be directed at supportive care for prevention of complications. The most common complications leading to death in patients with significant pancytopenia and neutropenia are opportunistic infections and hemorrhagic complications.2
Transfusion support is critical to avoid symptomatic anemia and hemorrhagic complications related to thrombocytopenia, which typically occur with platelet counts lower than 10,000 cells/µL. However, transfusion carries the risk of alloimmunization (which may persist for years following transfusion) and transfusion-related graft versus host disease (trGVHD), and thus use of transfusion should be minimized when possible.56,57 All blood products given to patients with aplastic anemia should be irradiated and leukoreduced to reduce the risk of both alloimmunization and trGVHD. Guidelines from the British Society for Haematology recommend routine screening for Rh and Kell antibodies to reduce the risk of alloimmunization.58 Infectious complications remain a common cause of morbidity and mortality in patients with aplastic anemia who have prolonged neutropenia (defined as an ANC < 500 cells/µL).59-62 Therefore, patients should receive broad-spectrum antibiotics with antipseudomonal coverage. In a study evaluating the role of granulocyte-colony stimulating factor (G-CSF) in patients with SAA receiving immunosuppressive therapy, 55% of all patient deaths were secondary to infection.63 There was no OS benefit seen in patients who received G-CSF, though a significantly lower rate of infection was observed in the G-CSF arm compared to those not receiving G-CSF (56% versus 81%, P = 0.006). This difference was largely driven by a decrease in infectious episodes in patients with VSAA treated with G-CSF as compared to those who did not receive this therapy (22% versus 48%, P = 0.014).63
Angio-invasive pulmonary aspergillosis and Zygomycetes (eg, Rhizopus, Mucor species) remain major causes of mortality related to opportunistic mycotic infections in patients with aplastic anemia.18 The infectious risk is directly related to the duration and severity of neutropenia, with one study demonstrating a significant increase in risk in AML patients with neutropenia lasting longer than 3 weeks.64 Invasive fungal infections carry a high mortality in patients with severe neutropenia, though due to earlier recognition and empiric antifungal therapy with extended-spectrum azoles, overall mortality secondary to invasive fungal infections is declining.62,65
While neutropenia related to cytotoxic chemotherapy is commonly associated with gram-negative bacteria due to disruption of mucosal barriers, patients with aplastic anemia have an increased incidence of gram-positive bacteremia with staphylococcal species compared to other neutropenic populations.61,62 This appears to be changing with time. Valdez et al demonstrated a decrease in prevalence of coagulase-negative staphylococcal infections, increased prevalence of gram-positive bacilli bacteremia, and no change in prevalence of gram-negative bacteremia in patients with aplastic anemia treated between 1989 and 2008.65 Gram-negative bacteremia caused by Stenotrophomonas maltophila, Escherichia coli, Klebsiella pneumoniae, Citrobacter, and Proteus has also been reported.62 Despite a lack of clinical trials investigating the role of antifungal and antibacterial prophylaxis for patients with aplastic anemia, most centers initiate antifungal prophylaxis in patients with SAA or VSAA with an anti-mold agent such as voriconazole or posaconazole (which has the additional benefit compared to voriconazole of covering Mucor species).60,66 This is especially true for patients who have received ATG or undergone HSCT. For antimicrobial prophylaxis, a fluoroquinolone antibiotic with a spectrum of activity against Pseudomonas should be considered for patients with an ANC < 500 cells/µL.60 Acyclovir or valacyclovir prophylaxis is recommended for varicella-zoster virus and herpes simplex virus. Cytomegalovirus reactivation is minimal in patients with aplastic anemia, unless multiple courses of ATG are used.
Iron overload is another complication the provider must be aware of in the setting of increased transfusions in aplastic anemia patients. Lee and colleagues showed that iron chelation therapy using deferasirox is effective at reducing serum ferritin levels in patients with aplastic anemia (median ferritin level of 3254 ng/mL prior to therapy, 1854 ng/mL following), and is associated with no serious adverse events (most common adverse events included nausea, diarrhea, vomiting, and rash).67 Approximately 25% of patients in this trial had an increase in creatinine, with patients taking concomitant cyclosporine affected to a greater degree than those on chelation therapy alone. For patients following HSCT or with improved hematopoiesis following immunosuppressive therapy, phlebotomy can be used to treat iron overload in lieu of chelation therapy.58
Approach to Therapy
The main treatment options for SAA and VSAA include allogeneic bone marrow transplant and immunosuppression. The deciding factors as to which treatment is best initially depends on the availability of HLA-matched related donors and age (Figure 1 and Figure 2). Survival is decreased in patients with SAA or VSAA who delay initiation of therapy, and therefore prompt referral for HLA typing and evaluation for bone marrow transplant is a very important first step in managing aplastic anemia.
Matched Sibling Donor Transplant. Current standards of care recommend HLA-matched sibling donor transplant for patients with SAA or VSAA who are younger than 50 years, with the caveat that integration of fludarabine and reduced cyclophosphamide dosing along with ATG shows the best overall outcomes. Locasciulli and colleagues examined outcomes in patients given either immunosuppressive therapy or sibling HSCT between 1991-1996 and 1997-2002, respectively, and found that sibling HSCT was associated with a superior 10-year OS compared to immunosuppressive therapy (73% versus 68%).43 Interestingly in this study, there was no OS improvement seen with immunosuppressive therapy alone (69% versus 73%) between the 2 time periods, despite increased OS in both sibling HSCT (74% and 80%) and MUD HSCT (38% and 65%).43 Though total body irradiation has been used in the past, it is typically not included in current conditioning regimens for matched related donor transplants.68
Current conditioning regimens typically use a combination of cyclophosphamide and ATG,69,70 with or without fludarabine. Fludarabine-based conditioning regimens have shown promise in patients undergoing sibling HSCT. Maury and colleagues evaluated the role of fludarabine in addition to low-dose cyclophosphamide and ATG compared to cyclophosphamide alone or in combination with ATG in patients over age 30 undergoing sibling HSCT.53 There was a nonsignificant improvement in 5-year OS in the fludarabine arm compared to controls (77% ± 8% versus 60% ± 3%, P = 0.14) in the pooled analysis, but when adjusted for age the fludarabine arm had a significantly lower relative risk (RR) of death (0.44; P = 0.04) compared to the control arm. Shin et al reported outcomes with fludarabine/cyclophosphamide/ATG, with excellent overall outcomes and no difference in patients older or younger than 40 years.71
Kim et al evaluated their experience with patients older than 40 years receiving matched related donors, finding comparable outcomes in those ages 41 to 50 years compared to younger patients. Outcomes declined in those over the age of 50 years.72 Long-term data for matched related donor transplant for aplastic anemia show excellent long-term outcomes, with minimal chronic GVHD and good performance status.73 Hence, these factors support the role of matched related donor transplant as the initial treatment in SAA and VSAA.
Regarding the role of transplant for patients who lack a matched related donor, a growing body of literature demonstrating identical outcomes between matched related and MUD transplants for pediatric patients74,75 supports recent recommendations for upfront unrelated donor transplantation for aplastic anemia.76,77
Immunosuppressive Therapy. For patients without an HLA-matched sibling donor or those who are older than 50 years of age, immunosuppressive therapy is the first-line therapy. ATG and cyclosporine A are the treatments of choice.78 The potential effectiveness of immunosuppressive therapy in treating aplastic anemia was initially observed in patients in whom autologous transplant failed but who still experienced hematopoietic reconstitution despite the failed graft; this observation led to the hypothesis that the conditioning regimen may have an effect on hematopoiesis.59,78,79
Immunosuppressive therapy with ATG has been used for the treatment of aplastic anemia since the 1980s.80 Historically, rabbit ATG had been used, but a 2011 study of horse ATG demonstrated superior hematological response at 6 months compared to rabbit ATG (68% versus 37%).59 Superior survival was also seen with horse ATG compared to rabbit ATG (3-year OS, 96% versus 76%). Due to these results, horse ATG is preferred over rabbit ATG. ATG should be used in combination with cyclosporine A to optimize outcomes.
Early studies also demonstrated the efficacy of cyclosporine A in the treatment of aplastic anemia, with response rates equivalent to that of ATG monotherapy.81 Recent publications still note the efficacy of cyclosporine A in the treatment of aplastic anemia. Its role as an affordable option for single-agent therapy in developing countries is intriguing.81 The combination of ATG and cyclosporine A was proven superior to either agent alone in a study by Frickhofen et al.79 In this study, patients were randomly assigned to a control arm that received ATG plus methylprednisolone or to an arm that received ATG plus cyclosporine A and methylprednisolone. At 6 months, 70% of patients in the cyclosporine A arm had a complete remission (CR) or partial remission compared to 46% in the control arm.82 Further work confirmed the long-term efficacy of this regimen, reporting a 7-year OS of 55%.83 Among a pediatric population, immunosuppressive therapy was associated with an 83% 10-year OS.84
It is recommended that patients remain on cyclosporine therapy for a minimum of 6 months, after which a gradual taper may be considered, although there is variation among practitioners, with some continuing immunosuppressive therapy for a minimum of 12 months due to a proportion of patients being cyclosporine dependent.34,84 A study found that within a population of patients who responded to immunosuppressive therapy, 18% became cyclosporine dependent.84 The median duration of cyclosporine A treatment at full dose was 12 months, with tapering completed over a median of 19 months after patients had been in a stable CR for a minimum of 3 months. Relapse occurred more often when patients were tapered quickly (decrease ≥ 0.8 mg/kg/month) compared to slowly (0.4-0.7 mg/kg/month) or very slowly (< 0.3 mg/kg/month).
Townsley and colleagues recently investigated incorporating the use of the thrombopoietin receptor agonist eltrombopag with immunosuppressive therapy as first-line therapy in aplastic anemia.85 When given at a dose of 150 mg daily in patients ages 12 years and older or 75 mg daily in patients younger than 12 years, in conjunction with cyclosporine A and ATG, patients demonstrated markedly improved hematological response compared to historical treatment with standard immunosuppressive therapy alone.45 In the patient cohort administered eltrombopag starting on day 1 and continuing for 6 months, the complete response rate was 58%. Eltrombopag led to improvement in all cell lines among all treatment subgroups, and OS (censored for patients who proceeded to transplant) was 99% at 2 years.12 Overall, toxicities associated with this therapy were low, with liver enzyme elevations most commonly observed.85 Recently, a phase 2 trial of immunosuppressive therapy with or without eltrombopag was reported. Of the 38 patients enrolled, overall response, complete response, and time to response were not statistically different.86 With this recent finding, the role of eltrombopag in addition to immunosuppressive therapy is not clearly defined, and further studies are warranted.
OS for patients who do not respond to immunosuppressive therapy is approximately 57% at 5 years, largely due to improved supportive measures among this patient population.48,65 Therefore, it is important to recognize those patients who have a low chance of response so that second-line therapy can be pursued to improve outcomes.
Matched Unrelated Donor Transplant. For patients with refractory disease following immunosuppressive therapy who lack a matched sibling donor, MUD HSCT is considered standard therapy given the marked improvement in overall outcomes with modulating conditioning regimens and high-resolution HLA typing. A European Society for Blood and Marrow Transplantation (EBMT) analysis comparing matched sibling HSCT to MUD HSCT noted significantly higher rates of acute grade II-IV and grade III-IV GVHD (grade II-IV 13% versus 25%, grade III-IV 5% versus 10%) among patients undergoing MUD transplant.47 Chronic GVHD rates were 14% in the sibling group, as compared to 26% in the MUD group. Factors associated with improved survival in this analysis include transplant under age 20 years (84% versus 72%), transplant within 6 months of diagnosis (85% versus 72%), the use of ATG in the conditioning regimen (81% versus 73%), and cytomegalovirus-negative donor and recipient as compared to other combinations (82% versus 76%).87 Interestingly, this study demonstrated that OS was not significantly increased when using a sibling HSCT compared to a MUD HSCT, likely as a result of improved understanding of conditioning regimens, GVHD prophylaxis, and supportive care.
Additional studies of MUD HSCT have shown outcomes similar to those seen in sibling HSCT.34,48 A French study found a significant increase in survival in patients undergoing MUD HSCT compared to historical cohorts (2000-2005: OS 52%; 2006-2012: OS 74%).75 The majority of patients underwent conditioning with cyclophosphamide or a combination of busulfan and cyclophosphamide, with or without fludarabine; 81% of patients underwent in vivo T-cell depletion, and a bone marrow donor source was utilized. OS was significantly lower in patients over age 30 years undergoing MUD HSCT (57%) compared to those under age 30 years (70%). Improved OS was also seen when patients underwent transplant within 1 year of diagnosis and when a 10/10 matched donor (compared to a 9/10 mismatched donor) was utilized.48
A 2015 study investigated the role of MUD HSCT as frontline therapy instead of immunosuppressive therapy in patients without a matched sibling donor.75 The 2-year OS was 96% in the MUD HSCT cohort compared to 91%, 94%, and 74% in historical cohorts of sibling HSCT, frontline immunosuppressive therapy, and second-line MUD HSCT following failed immunosuppressive therapy, respectively. Additionally, event-free survival in the MUD HSCT cohort (defined by the authors as death, lack of response, relapse, occurrence of clonal evolution/clinical PNH, malignancies developing over follow‐up, and transplant for patients receiving immunosuppressive therapy frontline) was similar compared to sibling HSCT and superior to frontline immunosuppressive therapy and second-line MUD HSCT. Furthermore, Samarasinghe et al highlighted the importance of in vivo T-cell depletion with either ATG or alemtuzumab (anti-CD52 monoclonal antibody) in the prevention of acute and chronic GVHD in both sibling HSCT and MUD HSCT.88
With continued improvement of less toxic and more immunomodulating conditioning regimens,utilization of bone marrow as a donor cell source, in vivo T-cell depletion, and use of GVHD and antimicrobial prophylaxis, more clinical evidence supports elevating MUD HSCT in the treatment plan for patients without a matched sibling donor.89 However, there is still a large population of patients without matched sibling or unrelated donor options. Given the need to expand the transplant pool and thus avoid clonal hematopoiesis, clinically significant PNH, and relapsed aplastic anemia, more work continues to recognize the expanding role of alternative donor transplants (cord blood and haploidentical) as another viable treatment strategy for aplastic anemia after immunosuppressive therapy failure.90
Summary
Aplastic anemia is a rare but potentially life-threatening disorder with pancytopenia and a marked reduction in the HSC compartment. It can be acquired or associated with an IMFS, and the treatment and prognosis vary dramatically between these 2 etiologies. Workup and diagnosis involves investigating IMFSs and ruling out malignant or infectious etiologies for pancytopenia. Treatment outcomes are excellent with modern supportive care and the current approach to allogeneic transplantation, and therefore referral to a bone marrow transplant program to evaluate for early transplantation is the new standard of care.
Corresponding author: Gabrielle Meyers, MD, 3181 SW Sam Jackson Park Road, Mail Code UHN73C, Portland, OR 97239.
Financial disclosures: None.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
1. Young NS, Calado RT, Scheinberg P. Current concepts in the pathophysiology and treatment of aplastic anemia. Blood. 2006;108:2509-2519.
2. Vaht K, Göransson M, Carlson K, et al. Incidence and outcome of acquired aplastic anemia: real-world data from patients diagnosed in Sweden from 2000–2011. Haematologica. 2017;102:1683-1690.
3. Incidence of aplastic anemia: the relevance of diagnostic criteria. By the International Agranulocytosis and Aplastic Anemia Study. Blood. 1987;70:1718-1721.
4. Montané E, Ibanez L, Vidal X, et al. Epidemiology of aplastic anemia: a prospective multicenter study. Haematologica. 2008;93:518-523.
5. Ohta A, Nagai M, Nishina M, et al. Incidence of aplastic anemia in Japan: analysis of data from a nationwide registration system. Int J Epidemiol. 2015; 44(suppl_1):i178.
6. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
7. Weinzierl EP, Arber DA. The differential diagnosis and bone marrow evaluation of new-onset pancytopenia. Am J Clin Pathol. 2013;139:9-29.
8. Lin FC, Karwan M, Saleh B, et al. IFN-γ causes aplastic anemia by altering hematopoiesis stem/progenitor cell composition and disrupting lineage differentiation. Blood. 2014;124:3699-3708.
9. Yoshizato T, Dumitriu B, Hosokawa K, et al. Somatic mutations and clonal hematopoiesis in aplastic anemia. N Engl J Med. 2015;373:35-47.
10. de Bruin AM, Voermans C, Nolte MA. Impact of interferon-γ on hematopoiesis. Blood. 2014;124:2479-2486.
11. Cheng H, Cheruku PS, Alvarado L, et al. Interferon-γ perturbs key signaling pathways induced by thrombopoietin, but not eltrombopag, in human hematopoietic stem/progenitor cells. Blood. 2016;128:3870.
12. Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367:11-19.
13. Townsley DM, Dumitriu B, Young NS, et al. Danazol treatment for telomere diseases. N Engl J Med. 2016;374:1922-1931.
14. Feurstein S, Drazer MW, Godley LA. Genetic predisposition to leukemia and other hematologic malignancies. Semin Oncol. 2016;43:598-608.
15. Townsley DM, Dumitriu B, Young NS. Bone marrow failure and the telomeropathies. Blood. 2014;124:2775-2783.
16. Young NS, Bacigalupo A, Marsh JC. Aplastic anemia: pathophysiology and treatment. Biol Blood Marrow Transplant. 2010;16:S119-S125.
17. Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446-4455.
18. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
19. Borie R, Tabèze L, Thabut G, et al. Prevalence and characteristics of TERT and TERC mutations in suspected genetic pulmonary fibrosis. Eur Respir J. 2016;48:1721-1731.
20. Ogawa S. Clonal hematopoiesis in acquired aplastic anemia. Blood. 2016;128:337-347.
21. Kulasekararaj AG, Jiang J, Smith AE, et al. Somatic mutations identify a sub-group of aplastic anemia patients that progress to myelodysplastic syndrome. Blood. 2014;124:2698-2704.
22. Mukhina GL, Buckley JT, Barber JP, et al. Multilineage glycosylphosphatidylinositol anchor‐deficient haematopoiesis in untreated aplastic anaemia. Br J Haematol. 2001;115:476-482.
23. Pu JJ, Mukhina G, Wang H, et al. Natural history of paroxysmal nocturnal hemoglobinuria clones in patients presenting as aplastic anemia. Eur J Haematol. 2011;87:37-45.
24. Hall SE, Rosse WF. The use of monoclonal antibodies and flow cytometry in the diagnosis of paroxysmal nocturnal hemoglobinuria. Blood. 1996;87:5332-5340.
25. Devalet B, Mullier F, Chatelain B, et al. Pathophysiology, diagnosis, and treatment of paroxysmal nocturnal hemoglobinuria: a review. Eur J Haematol. 2015;95:190-198.
26. Sugimori C, Chuhjo T, Feng X, et al. Minor population of CD55-CD59-blood cells predicts response to immunosuppressive therapy and prognosis in patients with aplastic anemia. Blood. 2006;107:1308-1314.
27. Scheinberg P, Marte M, Nunez O, Young NS. Paroxysmal nocturnal hemoglobinuria clones in severe aplastic anemia patients treated with horse anti-thymocyte globulin plus cyclosporine. Haematologica. 2010;95:1075-1080.
28. Parker C, Omine M, Richards S, et al. Diagnosis and management of paroxysmal nocturnal hemoglobinuria. Blood. 2005;106:3699-3709.
29. Guinan EC. Diagnosis and management of aplastic anemia. Hematology Am Soc Hematol Educ Program. 2011;2011:76-81.
30. Giampietro PF, Verlander PC, Davis JG, Auerbach AD. Diagnosis of Fanconi anemia in patients without congenital malformations: an international Fanconi Anemia Registry Study. Am J Med Genetics. 1997;68:58-61.
31. Auerbach AD. Fanconi anemia and its diagnosis. Mutat Res. 2009;668:4-10.
32. Giampietro PF, Davis JG, Adler-Brecher B, et al. The need for more accurate and timely diagnosis in Fanconi anemia: a report from the International Fanconi Anemia Registry. Pediatrics. 1993;91:1116-1120.
33. DiNardo CD, Bannon SA, Routbort M, et al. Evaluation of patients and families with concern for predispositions to hematologic malignancies within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin Lymphoma Myeloma Leuk. 2016;16:417-428.
34. Bacigalupo A. How I treat acquired aplastic anemia. Blood. 2017;129:1428-1436.
35. DeZern AE, Brodsky RA. Clinical management of aplastic anemia. Expert Rev Hematol. 2011;4:221-230.
36. Tichelli A, Gratwohl A, Nissen C, et al. Morphology in patients with severe aplastic anemia treated with antilymphocyte globulin. Blood. 1992;80:337-345.
37. Camitta BM, Storb R, Thomas ED. Aplastic anemia: pathogenesis, diagnosis, treatment, and prognosis. N Engl J Med. 1982;306:645-652.
38. Bacigalupo A, Hows J, Gluckman E, et al. Bone marrow transplantation (BMT) versus immunosuppression for the treatment of severe aplastic anaemia (SAA): a report of the EBMT SAA working party. Br J Haematol. 1988;70:177-182.
39. Brodsky RA, Chen AR, Dorr D, et al. High-dose cyclophosphamide for severe aplastic anemia: long-term follow-up. Blood. 2010;115:2136-2141.
40. Matsui WH, Brodsky RA, Smith BD, et al. Quantitative analysis of bone marrow CD34 cells in aplastic anemia and hypoplastic myelodysplastic syndromes. Leukemia. 2006;20:458-462.
41. Maciejewski JP, Risitano AM, Nunez O, Young NS. Distinct clinical outcomes for cytogenetic abnormalities evolving from aplastic anemia. Blood. 2002;99:3129-3135.
42. Auerbach AD. Diagnosis of Fanconi anemia by diepoxybutane analysis. Curr Protoc Hum Genet. 2015;85:8.7.1-17.
43. Locasciulli A, Oneto R, Bacigalupo A, et al. Outcome of patients with acquired aplastic anemia given first line bone marrow transplantation or immunosuppressive treatment in the last decade: a report from the European Group for Blood and Marrow Transplantation. Haematologica. 2007;92:11-18.
44. Passweg JR, Socié G, Hinterberger W, et al. Bone marrow transplantation for severe aplastic anemia: has outcome improved? Blood. 1997;90:858-864.
45. Gupta V, Eapen M, Brazauskas R, et al. Impact of age on outcomes after transplantation for acquired aplastic anemia using HLA-identical sibling donors. Haematologica. 2010;95:2119-2125.
46. Peffault de Latour R, Le Rademacher J, Antin JH, et al. Allogeneic hematopoietic stem cell transplantation in Fanconi anemia: the European Group for Blood and Marrow Transplantation experience. Blood. 2013;122:4279-4286.
47. Eapen M, Le Rademacher J, Antin JH, et al. Effect of stem cell source on outcomes after unrelated donor transplantation in severe aplastic anemia. Blood. 2011;118:2618-2621.
48. Devillier R, Dalle JH, Kulasekararaj A, et al. Unrelated alternative donor transplantation for severe acquired aplastic anemia: a study from the French Society of Bone Marrow Transplantation and Cell Therapies and the Severe Aplastic Anemia Working Party of EBMT. Haematologica. 2016;101:884-890.
49. Peffault de Latour R, Peters C, Gibson B, et al. Recommendations on hematopoietic stem cell transplantation for inherited bone marrow failure syndromes. Bone Marrow Transplant. 2015;50:1168-1172.
50. De Medeiros CR, Zanis-Neto J, Pasquini R. Bone marrow transplantation for patients with Fanconi anemia: reduced doses of cyclophosphamide without irradiation as conditioning. Bone Marrow Transplant. 1999;24:849-852.
51. Mohanan E, Panetta JC, Lakshmi KM, et al. Population pharmacokinetics of fludarabine in patients with aplastic anemia and Fanconi anemia undergoing allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 2017;52:977-983.
52. Gluckman E, Auerbach AD, Horowitz MM, et al. Bone marrow transplantation for Fanconi anemia. Blood. 1995;86:2856-2862.
53. Maury S, Bacigalupo A, Anderlini P, et al. Improved outcome of patients older than 30 years receiving HLA-identical sibling hematopoietic stem cell transplantation for severe acquired aplastic anemia using fludarabine-based conditioning: a comparison with conventional conditioning regimen. Haematologica. 2009;94:1312-1315.
54. Talbot A, Peffault de Latour R, Raffoux E, et al. Sequential treatment for allogeneic hematopoietic stem cell transplantation in Fanconi anemia with acute myeloid leukemia. Haematologica. 2014;99:e199-e200.
55. Ayas M, Saber W, Davies SM, et al. Allogeneic hematopoietic cell transplantation for Fanconi anemia in patients with pretransplantation cytogenetic abnormalities, myelodysplastic syndrome, or acute leukemia. J Clin Oncol. 2013;31:1669-1676.
56. Passweg JR, Marsh JC. Aplastic anemia: first-line treatment by immunosuppression and sibling marrow transplantation. Hematology Am Soc Hematol Educ Program. 2010;2010:36-42.
57. Laundy GJ, Bradley BA, Rees BM, et al. Incidence and specificity of HLA antibodies in multitransfused patients with acquired aplastic anemia. Transfusion. 2004;44:814-825.
58. Killick SB, Bown N, Cavenagh J, et al. Guidelines for the diagnosis and management of adult aplastic anaemia. Br J Haematol. 2016;172:187-207.
59. Scheinberg P, Nunez O, Weinstein B, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430-438.
60. Höchsmann B, Moicean A, Risitano A, et al. Supportive care in severe and very severe aplastic anemia. Bone Marrow Transplant. 2013;48:168-173.
61. Valdez JM, Scheinberg P, Young NS, Walsh TJ. Infections in patients with aplastic anemia. Semin Hematol. 2009;46:269-276.
62. Torres HA, Bodey GP, Rolston KV, et al. Infections in patients with aplastic anemia: experience at a tertiary care cancer center. Cancer. 2003;98:86-93.
63. Tichelli A, Schrezenmeier H, Socié G, et al. A randomized controlled study in patients with newly diagnosed severe aplastic anemia receiving antithymocyte globulin (ATG), cyclosporine, with or without G-CSF: a study of the SAA Working Party of the European Group for Blood and Marrow Transplantation. Blood. 2011;117:4434-4441.
64. Gerson SL, Talbot GH, Hurwitz S, et al. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345-351.
65. Valdez JM, Scheinberg P, Nunez O, et al. Decreased infection-related mortality and improved survival in severe aplastic anemia in the past two decades. Clin Infect Dis. 2011;52:726-735.
66. Robenshtok E, Gafter-Gvili A, Goldberg E, et al. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: systematic review and meta-analysis. J Clin Oncol. 2007;25:5471-5489.
67. Lee JW, Yoon SS, Shen ZX, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448-2454.
68. Deeg HJ, Amylon MD, Harris RE, et al. Marrow transplants from unrelated donors for patients with aplastic anemia: minimum effective dose of total body irradiation. Biol Blood Marrow Transplant. 2001;7:208-215.
69. Kahl C, Leisenring W, Joachim Deeg H, et al. Cyclophosphamide and antithymocyte globulin as a conditioning regimen for allogeneic marrow transplantation in patients with aplastic anaemia: a long‐term follow‐up. Br J Haematol. 2005;130:747-751.
70. Socié G. Allogeneic BM transplantation for the treatment of aplastic anemia: current results and expanding donor possibilities. Hematology Am Soc Hematol Educ Program. 2013;2013:82-86.
71. Shin SH, Jeon YW, Yoon JH, et al. Comparable outcomes between younger (<40 years) and older (>40 years) adult patients with severe aplastic anemia after HLA-matched sibling stem cell transplantation using fludarabine-based conditioning. Bone Marrow Transplant. 2016;51:1456-1463.
72. Kim H, Lee KH, Yoon SS, et al; Korean Society of Blood and Marrow Transplantation. Allogeneic hematopoietic stem cell transplant for adults over 40 years old with acquired aplastic anemia. Biol Blood Marrow Transplant. 2012;18:1500-1508.
73. Mortensen BK, Jacobsen N, Heilmann C, Sengelov H. Allogeneic hematopoietic cell transplantation for severe aplastic anemia: similar long-term overall survival after transplantation with related donors compared to unrelated donors. Bone Marrow Transplant. 2016;51:288-290.
74. Dufour C, Svahn J, Bacigalupo A. Front-line immunosuppressive treatment of acquired aplastic anemia. Bone Marrow Transplant. 2013;48:174-177.
75. Dufour C, Veys P, Carraro E, et al. Similar outcome of upfront-unrelated and matched sibling stem cell transplantation in idiopathic paediatric aplastic anaemia. A study on the behalf of the UK Paediatric BMT Working Party, Paediatric Diseases Working Party and Severe Aplastic Anaemia Working Party of the EBMT. Br J Haematol. 2015;171:585-594.
76. Georges GE, Doney K, Storb R. Severe aplastic anemia: allogeneic bone marrow transplantation as first-line treatment. Blood Adv. 2018;2:2020-2028.
77. Yoshida N, Kojima S. Updated guidelines for the treatment of acquired aplastic anemia in children. Curr Oncol Rep. 2018;20:67.
78. Mathe G, Amiel JL, Schwarzenberg L, et al. Bone marrow graft in man after conditioning by antilymphocytic serum. Br Med J. 1970;2:131-136.
79. Frickhofen N, Kaltwasser JP, Schrezenmeier H, et al; German Aplastic Anemia Study Group. Treatment of aplastic anemia with antilymphocyte globulin and methylprednisolone with or without cyclosporine. N Engl J Med. 1991;324:1297-1304.
80. Speck B, Gratwohl A, Nissen C, et al. Treatment of severe aplastic anaemia with antilymphocyte globulin or bone-marrow transplantation. Br Med J. 1981;282:860-863.
81. Al-Ghazaly J, Al-Dubai W, Al-Jahafi AK, et al. Cyclosporine monotherapy for severe aplastic anemia: a developing country experience. Ann Saudi Med. 2005;25:375-379.
82. Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120:1185-1196.
83. Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289:1130-1135.
84. Saracco P, Quarello P, Iori AP, et al. Cyclosporin A response and dependence in children with acquired aplastic anaemia: a multicentre retrospective study with long‐term observation follow‐up. Br J Haematol. 2008;140:197-205.
85. Townsley DM, Scheinberg P, Winkler T, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376:1540-1550.
86. Assi R, Garcia-Manero G, Ravandi F, et al. Addition of eltrombopag to immunosuppressive therapy in patients with newly diagnosed aplastic anemia. Cancer. 2018;124:4192-4201.
87. Bacigalupo A, Socié G, Hamladji RM, et al. Current outcome of HLA identical sibling vs. unrelated donor transplants in severe aplastic anemia: an EBMT analysis. Haematologica. 2015;100:696-702.
88. Samarasinghe S, Iacobelli S, Knol C, et al. Impact of different in vivo T cell depletion strategies on outcomes following hematopoietic stem cell transplantation for idiopathic aplastic anaemia: a study on behalf of the EBMT SAA Working Party. Am J Hematol. 2019; 94:80-86.
89. Clesham K, Dowse R, Samarasinghe S. Upfront matched unrelated donor transplantation in aplastic anemia. Hematol Oncol Clin North Am. 2018;32:619-628.
90. DeZern AE, Brodsky RA. Haploidentical donor bone marrow transplantation for severe aplastic anemia. Hematol Oncol Clin North Am. 2018;32:629-642.
AUGMENT: Lenalidomide/Rituximab vs Placebo/Rituximab in Relapsed or Refractory Indolent Lymphoma
Study Overview
Objective. To compare the efficacy and safety of lenalidomide in combination with rituximab (known as the R2 regimen) to rituximab plus placebo in patients with relapsed or refractory follicular lymphoma or marginal zone lymphoma (MZL).
Design. Phase 3, multicenter, international, placebo controlled randomized trial.
Setting and participants. 358 patients with rituximab-sensitive relapsed or refractory grade 1-3a follicular lymphoma or MZL.
Intervention. Patients were randomly assigned 1:1 to receive lenalidomide or placebo for 12 cycles plus rituximab once per week for 4 weeks in cycle 1 and day 1 of cycles 2 through 5.
Main outcome measures. The primary endpoint was progression-free survival (PFS) as determined by independent radiology reviewers using intent-to-treat analysis. Secondary end points included overall response rate, complete response rate, duration of response, overall survival, event-free survival, and time to next anti-lymphoma therapy. Time to next chemotherapy treatment and histologic transformation were exploratory endpoints. Responses were assessed by participating investigators and independent reviewers. Computed tomography or magnetic resonance imaging was used to obtain tumor measurements. Positron emission tomography was not used. Complete remissions were confirmed by bone marrow biopsy, as bone marrow involvement is exceedingly common in these lymphomas. Gastrointestinal endoscopy was performed to obtain disease status if there was involvement by lymphoma initially.
Improvement in primary and secondary endpoints as well as extrapolatory endpoints were reported in the R2 group. Primary efficacy analyses were conducted in the intention-to-treat population primary endpoint of PFS at 1-sided α = 0.025 level.
Main results. PFS was significantly improved for patients treated with the R2 regimen compared to those who recieved placebo plus rituximab, with a hazard ratio of 0.46 (95% confidence interval [CI], 0.34-0.62; P < 0.001). Median duration of PFS in the R2 group was 39.4 months (95% CI, 22.9 months to not reached) versus 14.1 months (95% CI, 11.4 to 16.7 months) in the rituximab/placebo group. Overall response in the R2 group was 78% (95% CI, 71%-83%) versus 53% (95% CI, 46%-61%; P < 0.0001) in the rituximab/placebo group, with 34% (95% CI, 27%-41%) versus 18% (95% CI, 13%-25%) of patients achieving complete remission (P = 0.001). There were 15 deaths in the R2 group versus 26 deaths in the rituximab/placebo group. Overall survival data is not mature yet.
Conclusion. The R2 regimen was superior to rituximab and placebo in relapsed or recurrent follicular lymphomas. The regimen’s safety profile was acceptable, with higher events of usual and expected but manageable toxicities in the R2 regimen compared to rituximab/placebo.
Commentary
Nearly half of non-Hodgkins lymphomas (NHLs) diagnosed in the United States are classified as indolent B-cell lymphomas.1 Follicular lymphomas constitute about 50% of all indolent NHLs, while MZLs comprise less than 15%.1 These slowly progressive B-cell lymphomas are currently considered treatable but have very low cure rates. Cure is primarily limited to early stage I/II disease and may be possible in less than half of patients by applying involved-field radiation therapy with curative intent.
More than two thirds of indolent lymphomas present in advanced stages (III-IV). Despite an advanced stage at presentation, initial chemoimmunotherapy can induce complete remission in nearly 60% of patients. Unfortunately, nearly all patients relapse over the next 10 years.2 The wait-and-watch approach is a common strategy, and most patients are administered initial therapy or subsequent lines of therapy if they are symptomatic.2 As such, for the majority of these patients, the goal of therapy is to minimize toxicities, preserve quality of life, treat symptoms, and achieve a long PFS without an attempt to cure. Following each line of therapy, patients often revert to watchful surveillance, sometimes for more than a decade. With additional subsequent lines of therapy, lymphoma tends to get more refractory to treatment.
A median survival of nearly 2 decades has been achieved in advanced follicular lymphomas2,3 and MZL.4 However, wide variation in overall response, duration of response, and survival is reported based on the individual risk profile.
The drug of interest in the present study by Leonard and colleagues, lenalidomide, has immunomodulatory properties and antiproliferative effects, possibly related to its binding of the E3 ligase protein cereblon and subsequent ubiquitination of the transcription factors Aiolos and Ikaros.5 The benefits of combination lenalidomide/rituximab against follicular lymphoma in preclinical settings have been attributed to mechanisms mediated by tumor-infiltrating lymphocytes, natural killer cells, monocytes, and antibody-dependent cell-mediated toxicity.5 The combination has now been studied in first-line and subsequent lines of therapy for follicular lymphoma and MZL.6
RELEVANCE, a phase 3 trial, compared the R2 regimen in the upfront setting in advanced follicular lymphoma with rituximab and chemotherapy combination (including CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], and bendamustine).7 Efficacy outcomes were similar between the comparators and R2 was noninferior. MAGNIFY, a phase 3b trial involving rituximab-sensitive and rituximab-refractory patients with previously treated follicular lymphoma and MZL, demonstrated an overall response rate of 73%, complete response rate of 45%, and median PFS of 36 months in patients who received the R2 regimen and who entered a plan to receive maintenance with rituximab.8
The AUGMENT trial was conducted at 97 centers in the United States and 14 Asian and European countries; it enrolled 358 patients, 82% of whom had a follicular lymphoma, between February 13, 2014 and January 26, 2017. The study was well conducted. The R2 regimen was compared to the often used second-line therapy of rituximab alone, and 1:1 randomization was done with stratification factors of prior rituximab use, marginal versus follicular histology, and time lapse of less than or greater than 2 years since last therapy. A limitation of this study is that it selected individuals with a better prognosis, as the study patients were not rituximab refractory and 57% had received only a single prior therapy.
As observed in other R2 regimen trials in follicular or marginal zone lymphomas, the most common adverse reactions (occurring in at least 20% of patients) were neutropenia, fatigue, and constipation. These were manageable with dose adjustments and interruptions, and, in the opinion of authors, did not take away from the overall benefits seen.
The authors acknowledge that a limitation of this study was a lower assessment of median PFS in both arms by investigators than by independent reviewers. The independent review committee assessed PFS for R2 at 39.4 months, whereas investigators assessed it at 25.4 months. The median PFS benefit remained at 14.1 months by both methods of assessment. This may highlight the differences of radiographic measurements in a central setting versus at individual centers.
Histologic transformation to a higher-grade aggressive lymphoma occurred in 2 patients in the R2 arm and 10 patients in the placebo/rituximab arm. After transformation, 1 patient in the R2 arm and 6 in the placebo plus rituximab arm died. A plausible mechanism for this variation has not been provided. If confirmed across a wider population, this may be one of the most significant benefits of the R2 regimen.
Applications for Clinical Practice
Therapy for relapsed and refractory indolent B-cell lymphomas continues to evolve. While chemotherapy remains an effective option, immunomodulation using non-chemotherapeutic intervention has emerged as an attractive strategy. The AUGMENT trial further solidifies adoption of the non-chemotherapy doublet option of rituximab/lenalidomide based on the premise of immunomodulation. Both the agents have been commercially available for more than a decade and are being used for other indications beyond the study population for this trial.
Based on the AUGMENT and MAGNIFY trials, lenalidomide combined with rituximab was approved by the Food and Drug Administration for use in relapsed and refractory follicular or marginal zone lymphomas soon after the AUGMENT study results were published. The recommended lenalidomide dose for both lymphomas is 20 mg once daily orally on days 1 to 21 of repeated 28-day cycles for up to 12 cycles.
The evidence from this trial has yielded what is likely to be a practice changing regimen, with R2 replacing single-agent rituximab for treating follicular lymphoma in the second line or beyond. The response rates and PFS periods were slightly lower in MZL. R2 offers advantages associated with a chemotherapy-free regimen and improved PFS. Also, in the AUGEMENT trial the secondary and exploratory endpoints of time to next therapy, overall response rates, and overall survival rates were improved in patients treated with R2.
Practitioners may choose lenalidomide plus rituximab over rituximab alone based on the AUGMENT study. When considering this regimen, several points should be kept in mind. A very careful selection of patients would be prudent, considering that the study’s follow-up of less than 4 years is short for a disease with long overall survival rates. The study was not powered to compare overall survival benefit. Also, practitioners are reminded to limit the use of lenalidomide to a maximum of 12 months, with planned interruptions and 8 doses of rituximab, replicating the trial schema. Additionally, as per the clinical trial design, the regimen is not intended for rituximab-refractory patients. Patients with MZL constituted only 18% of the study, and conclusions of superiority in this subgroup were not statistically significant. Lenalidomide is not approved for other indolent B cell lymphoproliferative malignancies, such as small lymphocytic lymphoma and chronic lymphocytic leukemia. The conclusion of the published study abstract suggests acceptable use in recurrent indolent lymphomas, but no such conclusion can be made due to lack of inclusion of all indolent lymphoma subtypes in this study.
Longer-term use of lenalidomide has been associated with a marginally increased risk of secondary hematologic malignancies in patients with multiple myeloma who were prescribed lenalidomide maintenance therapy for up to 2 years following high-dose chemotherapy and autologous hematopoietic stem cell transplant.9 Interestingly, in the AUGMENT study and other trials using lenalidomide/rituximab, no significant increase in secondary hematologic malignancies has been reported. The absence of prior myeloablative chemotherapy and a shorter duration of use (1 year) in this group of patients may be factors in why no additional risk of secondary hematologic malignancies was observed. Longer-term follow-up may be needed to evaluate this risk.
In the R2 arm of this study, 55% patients experienced grades 3 and 4 neutropenia. With a median age of presentation for both follicular lymphoma and MZL of over 60 years, oncologists should remain aware of this potentially fatal complication, especially in the frail, the elderly, and previously treated individuals who may have a high risk of myelosuppression. Clinicians should be prepared to rapidly adopt strategies of dose interruption, dose reduction, and growth factor use, as implemented in the trial. Of note, despite the high rates of severe neutropenia, only 3% of the participants experienced febrile neutropenia, and 71% patients in R2 group and 61% in rituximab group completed planned protocol therapy. Growth factor use was high at 36% in the R2 group, which may have been responsible for a lower incidence of febrile neutropenia.
Increased toxicities of tumor flare, rash, and constipation were observed in the R2 arm. Patients with greater than grade 1 neuropathy were excluded. For those at risk of thromboembolism, prophylactic anticoagulation or antiplatelet therapy was recommended in the trial. Lenalidomide dose was reduced to 10 mg for those with creatinine clearance of 30 to 59 mL/min.
The cost-effectiveness of lenalidomide/rituximab combination has not been fully studied against a sequential approach of using rituximab and lenalidomide for a limited number of cycles. The cost of a Revlimid 10-mg pill may be over $700.10 Costs associated with supportive care due to additional toxicities have not been quantified. For those with cost concerns or lack of insurance coverage, the R2 regimen may be cost prohibitive without financial assistance from charities.
Indolent NHL remains mostly incurable. The R2 approach is still not a curative one, and resources should be directed to investigate a cure for this population. Whenever feasible, participation in a clinical trial should be encouraged. Parameters have not been reported based on prognostic groups, and the study did not identify any biomarkers that may correlate with improved outcome. Perhaps a biomarker-based trial design may be most suitable in explaining the heterogeneity in follicular and marginal zone lymphomas.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
1. Perry AM, Diebold J, Nathwani BN, et al. Classification of non-Hodgkin lymphoma in seven geographic regions around the world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244-1250.
2. Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127:2804-2808.
3. Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood. 2013;122:981-987.
4. Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119:629-638.
5. Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811-821.
6. Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33:3635-3640.
7. Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934-947.
8. Andorsky DJ, Coleman M, Yacoubeman A, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37 (suppl; abstr 7513).
9. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
10. Revlimid prices, coupons and patient assistance programs. www.drugs.com/price-guide/revlimid. Accessed August 27, 2019.
Study Overview
Objective. To compare the efficacy and safety of lenalidomide in combination with rituximab (known as the R2 regimen) to rituximab plus placebo in patients with relapsed or refractory follicular lymphoma or marginal zone lymphoma (MZL).
Design. Phase 3, multicenter, international, placebo controlled randomized trial.
Setting and participants. 358 patients with rituximab-sensitive relapsed or refractory grade 1-3a follicular lymphoma or MZL.
Intervention. Patients were randomly assigned 1:1 to receive lenalidomide or placebo for 12 cycles plus rituximab once per week for 4 weeks in cycle 1 and day 1 of cycles 2 through 5.
Main outcome measures. The primary endpoint was progression-free survival (PFS) as determined by independent radiology reviewers using intent-to-treat analysis. Secondary end points included overall response rate, complete response rate, duration of response, overall survival, event-free survival, and time to next anti-lymphoma therapy. Time to next chemotherapy treatment and histologic transformation were exploratory endpoints. Responses were assessed by participating investigators and independent reviewers. Computed tomography or magnetic resonance imaging was used to obtain tumor measurements. Positron emission tomography was not used. Complete remissions were confirmed by bone marrow biopsy, as bone marrow involvement is exceedingly common in these lymphomas. Gastrointestinal endoscopy was performed to obtain disease status if there was involvement by lymphoma initially.
Improvement in primary and secondary endpoints as well as extrapolatory endpoints were reported in the R2 group. Primary efficacy analyses were conducted in the intention-to-treat population primary endpoint of PFS at 1-sided α = 0.025 level.
Main results. PFS was significantly improved for patients treated with the R2 regimen compared to those who recieved placebo plus rituximab, with a hazard ratio of 0.46 (95% confidence interval [CI], 0.34-0.62; P < 0.001). Median duration of PFS in the R2 group was 39.4 months (95% CI, 22.9 months to not reached) versus 14.1 months (95% CI, 11.4 to 16.7 months) in the rituximab/placebo group. Overall response in the R2 group was 78% (95% CI, 71%-83%) versus 53% (95% CI, 46%-61%; P < 0.0001) in the rituximab/placebo group, with 34% (95% CI, 27%-41%) versus 18% (95% CI, 13%-25%) of patients achieving complete remission (P = 0.001). There were 15 deaths in the R2 group versus 26 deaths in the rituximab/placebo group. Overall survival data is not mature yet.
Conclusion. The R2 regimen was superior to rituximab and placebo in relapsed or recurrent follicular lymphomas. The regimen’s safety profile was acceptable, with higher events of usual and expected but manageable toxicities in the R2 regimen compared to rituximab/placebo.
Commentary
Nearly half of non-Hodgkins lymphomas (NHLs) diagnosed in the United States are classified as indolent B-cell lymphomas.1 Follicular lymphomas constitute about 50% of all indolent NHLs, while MZLs comprise less than 15%.1 These slowly progressive B-cell lymphomas are currently considered treatable but have very low cure rates. Cure is primarily limited to early stage I/II disease and may be possible in less than half of patients by applying involved-field radiation therapy with curative intent.
More than two thirds of indolent lymphomas present in advanced stages (III-IV). Despite an advanced stage at presentation, initial chemoimmunotherapy can induce complete remission in nearly 60% of patients. Unfortunately, nearly all patients relapse over the next 10 years.2 The wait-and-watch approach is a common strategy, and most patients are administered initial therapy or subsequent lines of therapy if they are symptomatic.2 As such, for the majority of these patients, the goal of therapy is to minimize toxicities, preserve quality of life, treat symptoms, and achieve a long PFS without an attempt to cure. Following each line of therapy, patients often revert to watchful surveillance, sometimes for more than a decade. With additional subsequent lines of therapy, lymphoma tends to get more refractory to treatment.
A median survival of nearly 2 decades has been achieved in advanced follicular lymphomas2,3 and MZL.4 However, wide variation in overall response, duration of response, and survival is reported based on the individual risk profile.
The drug of interest in the present study by Leonard and colleagues, lenalidomide, has immunomodulatory properties and antiproliferative effects, possibly related to its binding of the E3 ligase protein cereblon and subsequent ubiquitination of the transcription factors Aiolos and Ikaros.5 The benefits of combination lenalidomide/rituximab against follicular lymphoma in preclinical settings have been attributed to mechanisms mediated by tumor-infiltrating lymphocytes, natural killer cells, monocytes, and antibody-dependent cell-mediated toxicity.5 The combination has now been studied in first-line and subsequent lines of therapy for follicular lymphoma and MZL.6
RELEVANCE, a phase 3 trial, compared the R2 regimen in the upfront setting in advanced follicular lymphoma with rituximab and chemotherapy combination (including CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], and bendamustine).7 Efficacy outcomes were similar between the comparators and R2 was noninferior. MAGNIFY, a phase 3b trial involving rituximab-sensitive and rituximab-refractory patients with previously treated follicular lymphoma and MZL, demonstrated an overall response rate of 73%, complete response rate of 45%, and median PFS of 36 months in patients who received the R2 regimen and who entered a plan to receive maintenance with rituximab.8
The AUGMENT trial was conducted at 97 centers in the United States and 14 Asian and European countries; it enrolled 358 patients, 82% of whom had a follicular lymphoma, between February 13, 2014 and January 26, 2017. The study was well conducted. The R2 regimen was compared to the often used second-line therapy of rituximab alone, and 1:1 randomization was done with stratification factors of prior rituximab use, marginal versus follicular histology, and time lapse of less than or greater than 2 years since last therapy. A limitation of this study is that it selected individuals with a better prognosis, as the study patients were not rituximab refractory and 57% had received only a single prior therapy.
As observed in other R2 regimen trials in follicular or marginal zone lymphomas, the most common adverse reactions (occurring in at least 20% of patients) were neutropenia, fatigue, and constipation. These were manageable with dose adjustments and interruptions, and, in the opinion of authors, did not take away from the overall benefits seen.
The authors acknowledge that a limitation of this study was a lower assessment of median PFS in both arms by investigators than by independent reviewers. The independent review committee assessed PFS for R2 at 39.4 months, whereas investigators assessed it at 25.4 months. The median PFS benefit remained at 14.1 months by both methods of assessment. This may highlight the differences of radiographic measurements in a central setting versus at individual centers.
Histologic transformation to a higher-grade aggressive lymphoma occurred in 2 patients in the R2 arm and 10 patients in the placebo/rituximab arm. After transformation, 1 patient in the R2 arm and 6 in the placebo plus rituximab arm died. A plausible mechanism for this variation has not been provided. If confirmed across a wider population, this may be one of the most significant benefits of the R2 regimen.
Applications for Clinical Practice
Therapy for relapsed and refractory indolent B-cell lymphomas continues to evolve. While chemotherapy remains an effective option, immunomodulation using non-chemotherapeutic intervention has emerged as an attractive strategy. The AUGMENT trial further solidifies adoption of the non-chemotherapy doublet option of rituximab/lenalidomide based on the premise of immunomodulation. Both the agents have been commercially available for more than a decade and are being used for other indications beyond the study population for this trial.
Based on the AUGMENT and MAGNIFY trials, lenalidomide combined with rituximab was approved by the Food and Drug Administration for use in relapsed and refractory follicular or marginal zone lymphomas soon after the AUGMENT study results were published. The recommended lenalidomide dose for both lymphomas is 20 mg once daily orally on days 1 to 21 of repeated 28-day cycles for up to 12 cycles.
The evidence from this trial has yielded what is likely to be a practice changing regimen, with R2 replacing single-agent rituximab for treating follicular lymphoma in the second line or beyond. The response rates and PFS periods were slightly lower in MZL. R2 offers advantages associated with a chemotherapy-free regimen and improved PFS. Also, in the AUGEMENT trial the secondary and exploratory endpoints of time to next therapy, overall response rates, and overall survival rates were improved in patients treated with R2.
Practitioners may choose lenalidomide plus rituximab over rituximab alone based on the AUGMENT study. When considering this regimen, several points should be kept in mind. A very careful selection of patients would be prudent, considering that the study’s follow-up of less than 4 years is short for a disease with long overall survival rates. The study was not powered to compare overall survival benefit. Also, practitioners are reminded to limit the use of lenalidomide to a maximum of 12 months, with planned interruptions and 8 doses of rituximab, replicating the trial schema. Additionally, as per the clinical trial design, the regimen is not intended for rituximab-refractory patients. Patients with MZL constituted only 18% of the study, and conclusions of superiority in this subgroup were not statistically significant. Lenalidomide is not approved for other indolent B cell lymphoproliferative malignancies, such as small lymphocytic lymphoma and chronic lymphocytic leukemia. The conclusion of the published study abstract suggests acceptable use in recurrent indolent lymphomas, but no such conclusion can be made due to lack of inclusion of all indolent lymphoma subtypes in this study.
Longer-term use of lenalidomide has been associated with a marginally increased risk of secondary hematologic malignancies in patients with multiple myeloma who were prescribed lenalidomide maintenance therapy for up to 2 years following high-dose chemotherapy and autologous hematopoietic stem cell transplant.9 Interestingly, in the AUGMENT study and other trials using lenalidomide/rituximab, no significant increase in secondary hematologic malignancies has been reported. The absence of prior myeloablative chemotherapy and a shorter duration of use (1 year) in this group of patients may be factors in why no additional risk of secondary hematologic malignancies was observed. Longer-term follow-up may be needed to evaluate this risk.
In the R2 arm of this study, 55% patients experienced grades 3 and 4 neutropenia. With a median age of presentation for both follicular lymphoma and MZL of over 60 years, oncologists should remain aware of this potentially fatal complication, especially in the frail, the elderly, and previously treated individuals who may have a high risk of myelosuppression. Clinicians should be prepared to rapidly adopt strategies of dose interruption, dose reduction, and growth factor use, as implemented in the trial. Of note, despite the high rates of severe neutropenia, only 3% of the participants experienced febrile neutropenia, and 71% patients in R2 group and 61% in rituximab group completed planned protocol therapy. Growth factor use was high at 36% in the R2 group, which may have been responsible for a lower incidence of febrile neutropenia.
Increased toxicities of tumor flare, rash, and constipation were observed in the R2 arm. Patients with greater than grade 1 neuropathy were excluded. For those at risk of thromboembolism, prophylactic anticoagulation or antiplatelet therapy was recommended in the trial. Lenalidomide dose was reduced to 10 mg for those with creatinine clearance of 30 to 59 mL/min.
The cost-effectiveness of lenalidomide/rituximab combination has not been fully studied against a sequential approach of using rituximab and lenalidomide for a limited number of cycles. The cost of a Revlimid 10-mg pill may be over $700.10 Costs associated with supportive care due to additional toxicities have not been quantified. For those with cost concerns or lack of insurance coverage, the R2 regimen may be cost prohibitive without financial assistance from charities.
Indolent NHL remains mostly incurable. The R2 approach is still not a curative one, and resources should be directed to investigate a cure for this population. Whenever feasible, participation in a clinical trial should be encouraged. Parameters have not been reported based on prognostic groups, and the study did not identify any biomarkers that may correlate with improved outcome. Perhaps a biomarker-based trial design may be most suitable in explaining the heterogeneity in follicular and marginal zone lymphomas.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
Study Overview
Objective. To compare the efficacy and safety of lenalidomide in combination with rituximab (known as the R2 regimen) to rituximab plus placebo in patients with relapsed or refractory follicular lymphoma or marginal zone lymphoma (MZL).
Design. Phase 3, multicenter, international, placebo controlled randomized trial.
Setting and participants. 358 patients with rituximab-sensitive relapsed or refractory grade 1-3a follicular lymphoma or MZL.
Intervention. Patients were randomly assigned 1:1 to receive lenalidomide or placebo for 12 cycles plus rituximab once per week for 4 weeks in cycle 1 and day 1 of cycles 2 through 5.
Main outcome measures. The primary endpoint was progression-free survival (PFS) as determined by independent radiology reviewers using intent-to-treat analysis. Secondary end points included overall response rate, complete response rate, duration of response, overall survival, event-free survival, and time to next anti-lymphoma therapy. Time to next chemotherapy treatment and histologic transformation were exploratory endpoints. Responses were assessed by participating investigators and independent reviewers. Computed tomography or magnetic resonance imaging was used to obtain tumor measurements. Positron emission tomography was not used. Complete remissions were confirmed by bone marrow biopsy, as bone marrow involvement is exceedingly common in these lymphomas. Gastrointestinal endoscopy was performed to obtain disease status if there was involvement by lymphoma initially.
Improvement in primary and secondary endpoints as well as extrapolatory endpoints were reported in the R2 group. Primary efficacy analyses were conducted in the intention-to-treat population primary endpoint of PFS at 1-sided α = 0.025 level.
Main results. PFS was significantly improved for patients treated with the R2 regimen compared to those who recieved placebo plus rituximab, with a hazard ratio of 0.46 (95% confidence interval [CI], 0.34-0.62; P < 0.001). Median duration of PFS in the R2 group was 39.4 months (95% CI, 22.9 months to not reached) versus 14.1 months (95% CI, 11.4 to 16.7 months) in the rituximab/placebo group. Overall response in the R2 group was 78% (95% CI, 71%-83%) versus 53% (95% CI, 46%-61%; P < 0.0001) in the rituximab/placebo group, with 34% (95% CI, 27%-41%) versus 18% (95% CI, 13%-25%) of patients achieving complete remission (P = 0.001). There were 15 deaths in the R2 group versus 26 deaths in the rituximab/placebo group. Overall survival data is not mature yet.
Conclusion. The R2 regimen was superior to rituximab and placebo in relapsed or recurrent follicular lymphomas. The regimen’s safety profile was acceptable, with higher events of usual and expected but manageable toxicities in the R2 regimen compared to rituximab/placebo.
Commentary
Nearly half of non-Hodgkins lymphomas (NHLs) diagnosed in the United States are classified as indolent B-cell lymphomas.1 Follicular lymphomas constitute about 50% of all indolent NHLs, while MZLs comprise less than 15%.1 These slowly progressive B-cell lymphomas are currently considered treatable but have very low cure rates. Cure is primarily limited to early stage I/II disease and may be possible in less than half of patients by applying involved-field radiation therapy with curative intent.
More than two thirds of indolent lymphomas present in advanced stages (III-IV). Despite an advanced stage at presentation, initial chemoimmunotherapy can induce complete remission in nearly 60% of patients. Unfortunately, nearly all patients relapse over the next 10 years.2 The wait-and-watch approach is a common strategy, and most patients are administered initial therapy or subsequent lines of therapy if they are symptomatic.2 As such, for the majority of these patients, the goal of therapy is to minimize toxicities, preserve quality of life, treat symptoms, and achieve a long PFS without an attempt to cure. Following each line of therapy, patients often revert to watchful surveillance, sometimes for more than a decade. With additional subsequent lines of therapy, lymphoma tends to get more refractory to treatment.
A median survival of nearly 2 decades has been achieved in advanced follicular lymphomas2,3 and MZL.4 However, wide variation in overall response, duration of response, and survival is reported based on the individual risk profile.
The drug of interest in the present study by Leonard and colleagues, lenalidomide, has immunomodulatory properties and antiproliferative effects, possibly related to its binding of the E3 ligase protein cereblon and subsequent ubiquitination of the transcription factors Aiolos and Ikaros.5 The benefits of combination lenalidomide/rituximab against follicular lymphoma in preclinical settings have been attributed to mechanisms mediated by tumor-infiltrating lymphocytes, natural killer cells, monocytes, and antibody-dependent cell-mediated toxicity.5 The combination has now been studied in first-line and subsequent lines of therapy for follicular lymphoma and MZL.6
RELEVANCE, a phase 3 trial, compared the R2 regimen in the upfront setting in advanced follicular lymphoma with rituximab and chemotherapy combination (including CHOP [cyclophosphamide, doxorubicin, vincristine, prednisone], CVP [cyclophosphamide, vincristine, prednisone], and bendamustine).7 Efficacy outcomes were similar between the comparators and R2 was noninferior. MAGNIFY, a phase 3b trial involving rituximab-sensitive and rituximab-refractory patients with previously treated follicular lymphoma and MZL, demonstrated an overall response rate of 73%, complete response rate of 45%, and median PFS of 36 months in patients who received the R2 regimen and who entered a plan to receive maintenance with rituximab.8
The AUGMENT trial was conducted at 97 centers in the United States and 14 Asian and European countries; it enrolled 358 patients, 82% of whom had a follicular lymphoma, between February 13, 2014 and January 26, 2017. The study was well conducted. The R2 regimen was compared to the often used second-line therapy of rituximab alone, and 1:1 randomization was done with stratification factors of prior rituximab use, marginal versus follicular histology, and time lapse of less than or greater than 2 years since last therapy. A limitation of this study is that it selected individuals with a better prognosis, as the study patients were not rituximab refractory and 57% had received only a single prior therapy.
As observed in other R2 regimen trials in follicular or marginal zone lymphomas, the most common adverse reactions (occurring in at least 20% of patients) were neutropenia, fatigue, and constipation. These were manageable with dose adjustments and interruptions, and, in the opinion of authors, did not take away from the overall benefits seen.
The authors acknowledge that a limitation of this study was a lower assessment of median PFS in both arms by investigators than by independent reviewers. The independent review committee assessed PFS for R2 at 39.4 months, whereas investigators assessed it at 25.4 months. The median PFS benefit remained at 14.1 months by both methods of assessment. This may highlight the differences of radiographic measurements in a central setting versus at individual centers.
Histologic transformation to a higher-grade aggressive lymphoma occurred in 2 patients in the R2 arm and 10 patients in the placebo/rituximab arm. After transformation, 1 patient in the R2 arm and 6 in the placebo plus rituximab arm died. A plausible mechanism for this variation has not been provided. If confirmed across a wider population, this may be one of the most significant benefits of the R2 regimen.
Applications for Clinical Practice
Therapy for relapsed and refractory indolent B-cell lymphomas continues to evolve. While chemotherapy remains an effective option, immunomodulation using non-chemotherapeutic intervention has emerged as an attractive strategy. The AUGMENT trial further solidifies adoption of the non-chemotherapy doublet option of rituximab/lenalidomide based on the premise of immunomodulation. Both the agents have been commercially available for more than a decade and are being used for other indications beyond the study population for this trial.
Based on the AUGMENT and MAGNIFY trials, lenalidomide combined with rituximab was approved by the Food and Drug Administration for use in relapsed and refractory follicular or marginal zone lymphomas soon after the AUGMENT study results were published. The recommended lenalidomide dose for both lymphomas is 20 mg once daily orally on days 1 to 21 of repeated 28-day cycles for up to 12 cycles.
The evidence from this trial has yielded what is likely to be a practice changing regimen, with R2 replacing single-agent rituximab for treating follicular lymphoma in the second line or beyond. The response rates and PFS periods were slightly lower in MZL. R2 offers advantages associated with a chemotherapy-free regimen and improved PFS. Also, in the AUGEMENT trial the secondary and exploratory endpoints of time to next therapy, overall response rates, and overall survival rates were improved in patients treated with R2.
Practitioners may choose lenalidomide plus rituximab over rituximab alone based on the AUGMENT study. When considering this regimen, several points should be kept in mind. A very careful selection of patients would be prudent, considering that the study’s follow-up of less than 4 years is short for a disease with long overall survival rates. The study was not powered to compare overall survival benefit. Also, practitioners are reminded to limit the use of lenalidomide to a maximum of 12 months, with planned interruptions and 8 doses of rituximab, replicating the trial schema. Additionally, as per the clinical trial design, the regimen is not intended for rituximab-refractory patients. Patients with MZL constituted only 18% of the study, and conclusions of superiority in this subgroup were not statistically significant. Lenalidomide is not approved for other indolent B cell lymphoproliferative malignancies, such as small lymphocytic lymphoma and chronic lymphocytic leukemia. The conclusion of the published study abstract suggests acceptable use in recurrent indolent lymphomas, but no such conclusion can be made due to lack of inclusion of all indolent lymphoma subtypes in this study.
Longer-term use of lenalidomide has been associated with a marginally increased risk of secondary hematologic malignancies in patients with multiple myeloma who were prescribed lenalidomide maintenance therapy for up to 2 years following high-dose chemotherapy and autologous hematopoietic stem cell transplant.9 Interestingly, in the AUGMENT study and other trials using lenalidomide/rituximab, no significant increase in secondary hematologic malignancies has been reported. The absence of prior myeloablative chemotherapy and a shorter duration of use (1 year) in this group of patients may be factors in why no additional risk of secondary hematologic malignancies was observed. Longer-term follow-up may be needed to evaluate this risk.
In the R2 arm of this study, 55% patients experienced grades 3 and 4 neutropenia. With a median age of presentation for both follicular lymphoma and MZL of over 60 years, oncologists should remain aware of this potentially fatal complication, especially in the frail, the elderly, and previously treated individuals who may have a high risk of myelosuppression. Clinicians should be prepared to rapidly adopt strategies of dose interruption, dose reduction, and growth factor use, as implemented in the trial. Of note, despite the high rates of severe neutropenia, only 3% of the participants experienced febrile neutropenia, and 71% patients in R2 group and 61% in rituximab group completed planned protocol therapy. Growth factor use was high at 36% in the R2 group, which may have been responsible for a lower incidence of febrile neutropenia.
Increased toxicities of tumor flare, rash, and constipation were observed in the R2 arm. Patients with greater than grade 1 neuropathy were excluded. For those at risk of thromboembolism, prophylactic anticoagulation or antiplatelet therapy was recommended in the trial. Lenalidomide dose was reduced to 10 mg for those with creatinine clearance of 30 to 59 mL/min.
The cost-effectiveness of lenalidomide/rituximab combination has not been fully studied against a sequential approach of using rituximab and lenalidomide for a limited number of cycles. The cost of a Revlimid 10-mg pill may be over $700.10 Costs associated with supportive care due to additional toxicities have not been quantified. For those with cost concerns or lack of insurance coverage, the R2 regimen may be cost prohibitive without financial assistance from charities.
Indolent NHL remains mostly incurable. The R2 approach is still not a curative one, and resources should be directed to investigate a cure for this population. Whenever feasible, participation in a clinical trial should be encouraged. Parameters have not been reported based on prognostic groups, and the study did not identify any biomarkers that may correlate with improved outcome. Perhaps a biomarker-based trial design may be most suitable in explaining the heterogeneity in follicular and marginal zone lymphomas.
—Rakesh Gaur, MD, MPH, FACP, Cancer and Blood Center at Kansas Institute of Medicine, Lenexa, KS
1. Perry AM, Diebold J, Nathwani BN, et al. Classification of non-Hodgkin lymphoma in seven geographic regions around the world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244-1250.
2. Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127:2804-2808.
3. Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood. 2013;122:981-987.
4. Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119:629-638.
5. Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811-821.
6. Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33:3635-3640.
7. Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934-947.
8. Andorsky DJ, Coleman M, Yacoubeman A, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37 (suppl; abstr 7513).
9. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
10. Revlimid prices, coupons and patient assistance programs. www.drugs.com/price-guide/revlimid. Accessed August 27, 2019.
1. Perry AM, Diebold J, Nathwani BN, et al. Classification of non-Hodgkin lymphoma in seven geographic regions around the world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101:1244-1250.
2. Armitage JO, Longo DL. Is watch and wait still acceptable for patients with low-grade follicular lymphoma? Blood. 2016;127:2804-2808.
3. Tan D, Horning SJ, Hoppe RT, et al. Improvements in observed and relative survival in follicular grade 1-2 lymphoma during 4 decades: The Stanford University experience. Blood. 2013;122:981-987.
4. Olszewski AJ, Castillo JJ. Survival of patients with marginal zone lymphoma: Analysis of the Surveillance, Epidemiology, and End Results database. Cancer. 2013;119:629-638.
5. Gandhi AK, Kang J, Havens CG, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol. 2014;164:811-821.
6. Leonard JP, Jung SH, Johnson J, et al. Randomized trial of lenalidomide alone versus lenalidomide plus rituximab in patients with recurrent follicular lymphoma: CALGB 50401 (Alliance). J Clin Oncol. 2015;33:3635-3640.
7. Morschhauser F, Fowler NH, Feugier P, et al. Rituximab plus lenalidomide in advanced untreated follicular lymphoma. N Engl J Med. 2018;379:934-947.
8. Andorsky DJ, Coleman M, Yacoubeman A, et al. MAGNIFY: Phase IIIb interim analysis of induction R2 followed by maintenance in relapsed/refractory indolent non-Hodgkin lymphoma. J Clin Oncol. 2019;37 (suppl; abstr 7513).
9. McCarthy PL, Holstein SA, Petrucci MT, et al. Lenalidomide maintenance after autologous stem-cell transplantation in newly diagnosed multiple myeloma: a meta-analysis. J Clin Oncol. 2017;35:3279-3289.
10. Revlimid prices, coupons and patient assistance programs. www.drugs.com/price-guide/revlimid. Accessed August 27, 2019.
One-third of patients with severe asthma are overusing corticosteroids
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
MADRID – if data from a Dutch study presented at the annual congress of the European Respiratory Society are representative of practice elsewhere.
“The main message from our study is that OCS overuse is common and unnecessary in the majority of asthma patients,” reported Katrien A.B. Eger, MD, Amsterdam University Medical Centre.
In this study, 5,002 patients on high doses of inhaled corticosteroids (ICS), defined as at least 500 mcg/day, were identified in a pharmacy database in the Netherlands. These patients were asked to complete a questionnaire to determine how many had severe asthma and had received rescue or maintenance OCS in the past year.
Drawing from the pharmacy database, it could be determined that 29% of the 2,312 patients who responded to the questionnaire were taking harmfully high doses of OCS as well as high doses of ICS. For this study, harmful exposure was defined as a cumulative intake of 420 mg of prednisone-equivalent OCS over a 1-year period. The median cumulative 1-year exposure, according to Dr. Eger, was 750 mg of prednisone equivalent.
In this population, the investigators then calculated ICS medication adherence based on prescription refills. In addition, a subset of this population was evaluated for inhaler technique.
On the basis of these calculations, 47.4% of patients with harmful OCS exposure were found not to be adherent to their prescribed ICS. Of those who were adherent, 53.9% were found not be taking their inhaled steroids appropriately,
When these numbers are put together, the data suggest “78.1% of high OCS users are either nonadherent or using poor inhalation techniques, which means there is a big potential for treatment optimization,” Dr. Eger said.
Yet even among the 21.9% who were adherent and using good inhaler technique, identifying a group who presumably require OCS for exacerbations, the study found that only 46.1% had been prescribed a biologic, which Dr. Eger considers an important steroid-sparing option. She conceded that many of those not on a biologic might not be candidates, but she believes this is another missed opportunity for reducing OCS exposure.
“In the Netherlands, we have very good access to health care, and biologics are available to anyone who needs them,” said Dr. Eger, explaining that access to these drugs is not a barrier.
The evidence overall is that not enough is being done to ensure that asthma patients are being protected from the risks of OCS, according to Dr. Eger. Citing evidence that adverse events associated with OCS begin with a cumulative lifetime prednisone-equivalent exposure of only 500 mg, she believes that clinicians should be more aggressive in intervening.
“We know that there are both acute and chronic complications associated with OCS that involve a range of organ systems,” Dr. Eger said. She listed osteoporosis, diabetes mellitus, hypertension, and adrenal insufficiency as examples. Rescue OCS, even if used sparingly, can drive risk of OCS complications attributable to the importance of cumulative exposure.
In the session where these data were presented, the moderator, Guy Brusselle, MD, professor of asthma and immunology, Ghent (Belgium) University, labeled them “important.” However, he quibbled with Dr. Eger’s assertion that biologics represent a major opportunity to reduce OCS exposure.
“By suggesting that biologics are not being used often enough, there is an assumption that all of these patients have type 2 inflammatory asthma,” Dr. Brusselle said. “I think it makes more sense to emphasize steroid-sparing strategies, not just biologics.”
Dr. Eger did not disagree, but she emphasized that steroid-sparing alternatives are just one strategy to reduce OCS exposure, and ensuring that patients are adherent to prescribed ICS therapies and are using them correctly might have an even greater impact.
Dr. Eger reports no potential conflicts of interest.
REPORTING FROM ERS 2019
A few pearls can help prepare the mind
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
We need to recognize the diverse problems that patients with potential multisystem disease can develop, lobby when necessary for them to be seen promptly by the relevant specialists, and initiate appropriate diagnostic testing and management in less-urgent scenarios. Most of us need frequent refreshers on the clinical manifestations of these disorders so that we can recognize them when they appear unannounced in our exam rooms.
The caregiver with a prepared mind is more likely to experience the diagnostic epiphany, and then use point-of-care references to hone in on the details. With many patients and clinical conundrums, the basics matter.
Dr. Chester Oddis, in this issue of the Journal, reviews the basics of several primary muscle disorders. He discusses, in a case-based format extracted from his recent Medicine Grand Rounds presentation at Cleveland Clinic, nuances of specific diagnoses and the clinical progression of diseases that are critical to be aware of in order to recognize and manage them, and expeditiously refer the patient to our appropriate subspecialty colleagues.
Major challenges exist in recognizing the inflammatory myopathies and their mimics early in their course. These are serious but uncommon entities, and in part because patients and physicians often attribute their early symptoms to more-common causes, diagnosis can be elusive—until the possibility is considered. We hope that Dr. Oddis’s article will make it easier to rapidly recognize these muscle disorders.
Patients often struggle to explain their symptoms of early muscle dysfunction. Since patients often verbalize their fatigue as “feeling weak,” we often misconstrue complaints of true muscle weakness (like difficulty walking up steps) as being due to fatigue. Add in some anemia from chronic inflammation and some “liver test” abnormalities, and it is easy to see how the recognition of true muscle weakness can be delayed.
We can tease muscle weakness from fatigue or dyspnea by asking the patient to specifically and functionally describe their “weakness,” and then by asking pointed questions: “Do you have difficulty getting up from the toilet without using your arms? Do you have trouble brushing your hair or teeth?” Physical examination can clearly help here, but without routine examination of muscle strength in normal fragile elderly patients, the degree of muscle weakness can be difficult to assess. Likewise challenging is detecting the early onset of weakness by examination in a 280-lb power-lifter.
Obtaining an accurate functional and behavioral history is often critical to the early recognition of muscle disease. Muscle pain, as Dr. Oddis notes, is not a characteristic feature of many myopathies, whereas, paradoxically, the coexistence of new-onset symmetrical small-joint pain (especially with arthritis) along with muscle weakness can be a powerful clue to the diagnosis of an inflammatory myopathy.
An elevated creatine kinase (CK) level generally points directly to a muscle disease, although some neurologic disorders are associated with elevations in CK, and the entity of benign “hyperCKemia” must be recognized and not overmanaged. The latter becomes a problem when laboratory tests are allowed to drive the diagnostic evaluation in a vacuum of clinical details.
A more common scenario is the misinterpretation of common laboratory test abnormalities in the setting of a patient with “fatigue” or generalized weakness who has elevations in aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Although AST and ALT are often called “liver function tests,” these enzymes are also abundant in skeletal muscle, and since they are included on routine biochemical panels, their elevation often leads to liver imaging and sometimes even biopsy before anyone recognizes muscle disease as the cause of the patient’s symptoms and laboratory test abnormalities. Hence, a muscle source (or hemolysis) should at least be considered when AST and ALT are elevated in the absence of elevated alkaline phosphatase or gamma-glutamyl transferase.
When evaluating innumerable clinical scenarios, experienced clinicians can most certainly generate similar principles of diagnostic reasoning, based on having a few fundamental facts at their fingertips. Increasing the chances of having a prepared mind when confronted with a patient with a less-than-straightforward set of symptoms is one of my major arguments in support of continuing to read and generate internal medicine teaching literature and to attend and participate in clinical teaching conferences such as Medicine Grand Rounds. It is also why we will continue to appreciate and publish presentations like this one in the Journal.
I don’t expect to retain all the details from these and similar papers, and I know we all carry virtually infinite databases in our pockets. But keeping a few clinical pearls outside of my specialty in my head comes in handy. Having a prepared mind makes it much easier to converse with patients, to promptly initiate appropriate testing, plans, and consultations, and to then decide what to search for on my smartphone between patients.
A complication of enoxaparin injection
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
A 78-year-old woman presented to the emergency department with shortness of breath and palpitations and was found to have atrial fibrillation with rapid ventricular response. Medical therapy with drug therapy and cardioversion proved ineffective. She then underwent atrioventricular node ablation and placement of a pacemaker.
At the time of admission, anticoagulation was started with full-dose enoxaparin, injected subcutaneously on the left side of the abdominal wall, as her CHA2DS2-VASc score (http://chadvasc.org) was 5, due to age, female sex, and history of heart failure and hypertension.
Four days after admission, she reported lower abdominal pain, and her urine output was minimal. A bladder scan showed more than 500 mL of residual urine. She was hemodynamically stable, but physical examination revealed mild abdominal distention and tenderness in the suprapubic region. Laboratory testing showed a sharp rise in serum creatinine and a drop in hematocrit.
The patient was initially managed conservatively with serial physical examinations, monitoring of the hematocrit, serial imaging studies, and discontinuation of anticoagulation, but the pain and anuria persisted. Repeat computed tomography 15 days after admission showed that the hematoma had expanded, and she now had hydronephrosis on the right side as well, requiring urologic intervention with bilateral nephrostomy tube placement.
The size of the hematoma was evaluated with serial abdominal and pelvic examinations. After several days, her urine output had improved, the nephrostomy tubes were removed, and she was discharged.
RECTUS SHEATH HEMATOMA
Our patient had a giant pelvic hematoma, probably arising from the rectus sheath. This uncommon problem can arise from trauma, anticoagulation, or increased intra-abdominal pressure, but it can also occur spontaneously.1
In rectus sheath hematoma, a branch of the inferior epigastric artery is injured at its insertion into the rectus abdominis muscle. Symptoms arise if bleeding does not stop spontaneously from a tamponade effect.2
We speculate that in our patient, deep injection of enoxaparin into the abdominal wall injured the inferior epigastric artery, which started the hematoma, and the bleeding was exacerbated by the anticoagulation effect of the enoxaparin.
Another form of pelvic hematoma is retroperitoneal. It is most commonly caused by trauma but can occur due to rupture of the aorta, compression from tumors, or, infrequently, anticoagulation therapy.3
The role of anticoagulation
Spontaneous pelvic hematoma is usually missed as a cause of abdominal pain in patients on anticoagulation therapy and is mistaken for common acute conditions such as ulcer, diverticulitis, appendicitis, ovarian cyst torsion, and tumor.4 It usually develops within 5 days of starting anticoagulation therapy. Symptoms vary depending on the location of the hematoma and are best diagnosed with abdominal computed tomography, with sensitivity as high as 100%.
MANAGEMENT
Conservative management, reserved for patients in stable condition, includes temporarily stopping and reevaluating the risks and benefits of anticoagulation and antiplatelet agents, giving blood transfusions, and controlling pain. If conservative measures fail, options are arterial embolization, stent grafting, and blood vessel ligation.5 If these measures fail, patients should undergo surgical evacuation of the hematoma and ligation of bleeding vessels.6
TAKE-HOME MESSAGE
Subcutaneous injections, especially of anticoagulants, into the abdominal wall can increase the risk of hematoma. Other risk factors are older age, female sex, and thin body habitus with less abdominal fat.7 Healthcare professionals should avoid deep injections into the abdomen and should counsel patients and their caregivers about this, as well. The deltoid region could be a safer alternative.
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
- Cherry WB, Mueller PS. Rectus sheath hematoma: review of 126 cases at a single institution. Medicine (Baltimore) 2006; 85(2):105–110. doi:10.1097/01.md.0000216818.13067.5a
- Hatjipetrou A, Anyfantakis D, Kastanakis M. Rectus sheath hematoma: a review of the literature. Int J Surg 2015; 13:267–271. doi:10.1016/j.ijsu.2014.12.015
- Haq MM, Taimur SDM, Khan SR, Rahman MA. Retroperitoneal hematoma following enoxaparin treatment in an elderly woman—a case report. Cardiovasc J 2010; 3(1):94–97. doi:10.3329/cardio.v3i1.6434
- Luhmann A, Williams EV. Rectus sheath hematoma: a series of unfortunate events. World J Surg 2006; 30(11):2050–2055. doi:10.1007/s00268-005-0702-9
- Pace F, Colombo GM, Del Vecchio LR, et al. Low molecular weight heparin and fatal spontaneous extraperitoneal hematoma in the elderly. Geriatr Gerontol Int 2012; 12(1):172–174. doi:10.1111/j.1447-0594.2011.00742.x
- Velicki L, Cemerlic-Adic N, Bogdanovic D, Mrdanin T. Rectus sheath haematoma: enoxaparin-related complication. Acta Clin Belg 2013; 68(2):147–149. doi:10.2143/ACB.68.2.3213
- Sheth HS, Kumar R, DiNella J, Janov C, Kaldas H, Smith RE. Evaluation of risk factors for rectus sheath hematoma. Clin Appl Thromb Hemost 2016; 22(3):292–296. doi:10.1177/1076029614553024
Myopathy for the general internist: Statins and much more
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
Myopathies can present with a wide variety of symptoms, so patients with muscle weakness are often seen initially by a general practitioner. Nonrheumatologists should be able to evaluate a patient presenting with muscle weakness or myalgia and be aware of red flags indicating potentially dangerous syndromes that require a prompt, thorough investigation.
This article reviews selected causes of muscle weakness, such as statin-induced and autoimmune disorders, and systemic features of inflammatory myopathies beyond myositis, such as dermatologic and pulmonary manifestations.
FOCUSING THE EVALUATION
The evaluation of a patient presenting with muscle weakness should include several assessments:
Temporal progression. Was the onset of symptoms rapid or insidious? Patterns of onset may give clues to etiology, including the possibility of an associated autoimmune condition.
Location of muscle weakness. Are symptoms global or localized? And if localized, are they proximal or distal? Proximal weakness can be manifested by difficulty rising from a chair (hip muscles) or combing one’s hair (shoulder muscles), whereas distal weakness can involve difficulty standing on toes (gastrocnemius and soleus muscles) or performing fine motor activities (intrinsic hand muscles).
Symmetry. A focal or asymmetric pattern often has a neurologic etiology, but this could also be consistent with inclusion body myositis.
Other symptoms. Arthritis, rash, and swallowing problems point to a possible underlying rheumatologic disease. Weight gain or loss may indicate a thyroid disorder.
Family history. Some patients report that others in their family have this pattern of weakness, indicating a likely genetic myopathy. If the patient reports a relative with multiple sclerosis, lupus erythematosus, rheumatoid arthritis, or another autoimmune disease, then an immune-mediated myopathy should be considered.
Medications should be reviewed, particularly statins.
CASE 1: SLOWLY PROGRESSIVE WEAKNESS
A 65-year-old man presented with the insidious onset of muscle weakness and episodes of falling. On review of his medical record, his serum creatine kinase (CK) levels were elevated at various periods at 2 to 4 times the upper limit of normal. Electromyography (EMG) previously showed a myopathic pattern, and a muscle biopsy was abnormal, consistent with endomysial inflammation (term is consistent with “polymyositis”). He was treated for polymyositis for several years with prednisone alone, with steroids plus methotrexate, and with combined immunosuppression including methotrexate and azathioprine, but with no improvement. Eventually, another muscle biopsy revealed inclusion bodies with rimmed vacuoles, consistent with inclusion body myositis.
Inclusion body myositis
Inclusion body myositis is the most common myopathy in middle-aged to elderly people, especially men. These patients are often told “You are just getting old,” but they have a defined condition. It should also be considered in patients failing to respond to treatment or with those with “refractory” polymyositis.
The onset of muscle weakness is insidious and painless, and the weakness progresses slowly. The pattern is distal and asymmetric (eg, foot drop), and muscle atrophy typically affects the forearm flexors, quadriceps, and intrinsic muscles of the hands.1
Magnetic resonance imaging may show marked muscle atrophy. Unfortunately, no treatment has shown efficacy, and most neuromuscular and rheumatology experts do not treat inclusion body myositis with immunosuppressive drugs.
CASE 2: MILD MYALGIA WITHOUT WEAKNESS
A black 52-year-old man was referred because of myalgia and a CK level of 862 U/L (reference range < 200). His physician wanted to start him on a statin but was hesitant to do so without first consulting a rheumatologist.
The patient had a long history of mild arthralgias and myalgias without muscle weakness. He had dyslipidemia and hypertension. He reported no family history of myopathy and no illicit drug use. He was formerly an athlete. Medications included a thiazide diuretic and a beta-blocker. On examination, his muscles were strong (rated 5 on a scale of 5) in the upper and lower extremities, without atrophy.
His records showed that his CK levels had risen and fallen repeatedly over the past few years, ranging from 600 to 1,100 U/L. On further questioning, he reported that when he had joined the army 30 years previously, a physician had recommended he undergo a liver biopsy in view of elevated liver function tests, but that he had refused because he felt fine.
Currently, his gamma-glutamyl transpeptidase levels were normal.
Idiopathic ‘hyperCKemia’
So-called idiopathic hyperCKemia is not a form of myositis but merely a laboratory result outside the “normal” range. Reference ranges are based predominantly on measurements in white people and on an assumption that the distribution is Gaussian (bell-shaped). A normal CK level is usually defined as less than 200 U/L. Using this standard, up to 20% of men and 5% of women have hyperCKemia.2
However, CK levels vary by sex and ethnicity, with mean levels highest in black men, followed by black women, white men, and white women. The mean level in black men is higher than the standard cutoff point for normal, and especially in this population, there is wide fluctuation around the mean, leading to hyperCKemia quite frequently in black men. Exercise and manual labor also drive up CK levels.3–5
Idiopathic hyperCKemia is benign. D’Adda et al6 followed 55 patients for a mean of 7.5 years. CK levels normalized in 12 patients or at least decreased in 24. Most remained symptom-free or had minimal symptoms.
Idiopathic hyperCKemia: Bottom line
Before prescribing a statin, determine the baseline CK level. If slightly elevated (ie, up to 3 to 5 times the upper limit of normal, or even higher) in the setting of normal muscle strength, there is no need for electromyography or muscle biopsy, and the patient can certainly receive a statin. Most of these patients do not need to see a rheumatologist but can simply have their CK and muscle strength monitored.
CLASSIFYING MYOSITIS
Myositis (idiopathic inflammatory myopathy) is a heterogeneous group of autoimmune syndromes of unknown cause characterized by chronic muscle weakness and inflammation of striated muscle. These syndromes likely arise as a result of genetic predisposition and an environmental or infectious “hit.”
Myositis is rare, with an incidence of 5 to 10 cases per million per year and an estimated prevalence of 50 to 90 cases per million. It has 2 incidence peaks: 1 in childhood (age 5–15) and another in adult midlife (age 30–50). Women are affected 2 to 3 times more often than men, with black women most commonly affected.
Myositis is traditionally classified as follows:
- Adult polymyositis
- Adult dermatomyositis
- Juvenile myositis (dermatomyositis much more frequent than polymyositis)
- Malignancy-associated myositis (usually dermatomyositis)
- Myositis overlapping with another autoimmune disease
- Inclusion body myositis.
However, polymyositis is less common than we originally thought, and the term necrotizing myopathy is now used in many patients, as noted in the case studies below. Further, myositis overlap syndromes are being increasingly diagnosed, likely related to the emergence of autoantibodies and clinical “syndromes” associated with these autoantibody subsets (discussed in cases below).
Dermatomyositis
Dermatomyositis is characterized by muscle weakness and a rash that can be obvious or subtle. Classic skin lesions are Gottron papules, which are raised, flat-topped red or purplish lesions over the knuckles, elbows, or knees.
Lesions may be confused with those of psoriasis. There can also be a V-neck rash over the anterior chest or upper back (“shawl sign”) or a rash over the lateral thigh (“holster sign”). A facial rash may occur, but unlike lupus, dermatomyositis does not spare the nasolabial area. However, the V-neck rash can be similar to that seen in lupus.
Dermatomyositis may cause muscle pain, perhaps related to muscle ischemia, whereas polymyositis and necrotizing myopathy are often painless. However, pain is also associated with fibromyalgia, which may be seen in many autoimmune conditions. It is important not to overtreat rheumatologic diseases with immunosuppression to try to control pain if the pain is actually caused by fibromyalgia.
Polymyositis mimics
Hypothyroid myopathy can present as classic polymyositis. The serum CK may be elevated, and there may be myalgias, muscle hypertrophy with stiffness, weakness, cramps, and even features of a proximal myopathy, and rhabdomyolysis. The electromyogram can be normal or myopathic. Results of muscle biopsy are often normal but may show focal necrosis and mild inflammatory infiltrates, thus mimicking that seen with inflammatory myopathy.7
Drug-induced or toxic myopathies can also mimic polymyositis. Statins are among the most commonly prescribed drugs in the United States, with more than 35 million people taking them. Statins are generally well tolerated but have a broad spectrum of toxicity, ranging from myalgias to life-threatening rhabdomyolysis. Myalgias lead to about 5% to 10% of patients refusing to take a statin or stopping it on their own.
Myalgias affect up to 20% of statin users in clinical practice.8,9 A small cross-sectional study10 of 1,000 patients in a primary care setting found that the risk of muscle complaints in statin users was 1.5 times higher than in nonstatin users, similar to findings in other studies.
My strategy for managing a patient with possible statin-induced myopathy is illustrated in Figure 1.
CASE 3: WEAKNESS, VERY HIGH CK ON A STATIN
In March 2010, a 67-year-old woman presented with muscle weakness. She had a history of hypertension, hyperlipidemia, and, more than 10 years previously, uterine cancer. In 2004, she was given atorvastatin for dyslipidemia. Four years later, she developed lower-extremity weakness, which her doctor attributed to normal aging. A year after that, she found it difficult to walk up steps and lift her arms overhead. In June 2009, she stopped taking the atorvastatin on her own, but the weakness did not improve.
In September 2009, she returned to her doctor, who found her CK level was 6,473 U/L but believed it to be an error, so the test was repeated, with a result of 9,375 U/L. She had no rash or joint involvement.
She was admitted to the hospital and underwent muscle biopsy, which showed myonecrosis with no inflammation or vasculitis. She was treated with prednisone 60 mg/day, and her elevated CK level and weakness improved.
Immune-mediated necrotizing myopathy associated with statins
The hallmark of necrotizing myopathy is myonecrosis without significant inflammation.12 This pattern contrasts with that of polymyositis, which is characterized by lymphocytic inflammation.
Although statins became available in the United States in 1987, immune-mediated necrotizing myopathy associated with statins was first described only in 2010. In that report, Grable-Esposito et al13 described 25 patients from 2 neuromuscular centers seen between 2000 and 2008 who had elevated CK and proximal weakness during or after statin use, both of which persisted despite stopping the statin. Patients improved with immunosuppressive agents but had a relapse when steroids were stopped or tapered, a pattern typical in autoimmune disease.
Autoantibody defines subgroup of necrotizing myopathy
Also in 2010, Christopher-Stine et al14 reported an antibody associated with necrotizing myopathy. Of 38 patients with the condition, 16 were found to have an abnormal “doublet” autoantibody recognizing 200- and 100-kDa proteins. All patients had weakness and a high CK level, and 63% had statin exposure before the weakness (this percentage increased to 83% in patients older than 50). All responded to immunosuppressive therapy, and many had a relapse when it was withdrawn.
Statins lower cholesterol by inhibiting 3-hydroxy-3-methylglutaryl-Co A reductase (HMGCR), and paradoxically, they also upregulate it. HMGCR has a molecular weight of 97 kDa. Mammen et al15 identified HMGCR as the 100-kDa target of the identified antibody and developed an enzyme-linked immunosorbent assay for it. Of 750 patients presenting to one center, only 45 (6%) had anti-HMGCR autoantibodies, but all 16 patients who had the abnormal doublet antibody tested positive for anti-HMGCR. Regenerating muscle cells express high levels of HMGCR, which may sustain the immune response after statins are discontinued.
Case 3 continued: Intravenous immunoglobulin brings improvement
In March 2010, when the 67-year-old patient presented to our myositis center, her CK level was 5,800 U/L, which increased as prednisone was tapered. She still felt weak. On examination, her muscle strength findings were deltoids 4+/5, neck flexors 4/5, and iliopsoas 3+/5. She was treated with methotrexate and azathioprine without benefit. She was next treated with intravenous immunoglobulin, and after 3 months, her strength normalized for the first time in years. Her CK level decreased but did not normalize. Testing showed that she was positive for anti-HMGCR autoantibody, as this test had become commercially available.
In 2015, Mammen and Tiniakou16 suggested using intravenous immunoglobulin as first-line therapy for statin-associated autoimmune necrotizing myopathy, based on experience at a single center with 3 patients who declined glucocorticoid treatment.
Necrotizing myopathy: Bottom line
Myositis overlap syndromes
Heterogeneity is the rule in myositis, and it can present with a wide variety of signs and symptoms as outlined in Table 2.
CASE 4: FEVER, NEW ‘RHEUMATOID ARTHRITIS,’ AND LUNG DISEASE
A 52-year-old woman with knee osteoarthritis saw her primary care physician in November 2013 for dyspnea and low-grade fever. The next month, she presented with polyarthritis, muscle weakness, and Raynaud phenomenon.
In January 2014, she developed acrocyanosis of her fingers. Examination revealed hyperkeratotic, cracked areas of her fingers. Her oxygen saturation by pulse oximetry was low. She was admitted to the hospital. Her doctor suspected new onset of rheumatoid arthritis, but blood tests revealed a negative antinuclear antibody, so an autoimmune condition was deemed unlikely. Her CK was mildly elevated at 350 U/L.
Because of her dyspnea, an open-lung biopsy was performed. High-resolution computed tomography (CT) revealed infiltrates and ground-glass opacities, leading to the diagnosis of nonspecific interstitial pneumonia. A rheumatologist was consulted and recommended pulse methylprednisolone, followed by prednisone 60 mg/day and mycophenolate mofetil. Testing for Jo-1 antibodies was positive.
Antisynthetase syndrome
The antisynthetase syndrome is a clinically heterogeneous condition that can occur with any or all of the following:
- Fever
- Myositis
- Arthritis (often misdiagnosed as rheumatoid arthritis)
- Raynaud phenomenon
- Mechanic’s hands (hyperkeratotic roughness with fissures on the lateral aspects of the fingers and finger pads)
- Interstitial lung disease.
The skin rashes and myositis may be subtle, making the presentation “lung-dominant,” and nonrheumatologists should be aware of this syndrome. Although in our patient the condition developed in a classic manner, with all of the aforementioned features of the antisynthetase syndrome, some patients will manifest one or a few of the features.
Clinically, patients with the Jo-1 antisynthetase syndrome often present differently than those with non-Jo-1 antisynthetase autoantibodies. When we compared 122 patients with Jo-1 vs 80 patients with a non-Jo-1 antisynthetase autoantibody, patients with Jo-1 antibodies were more likely to have initially received a diagnosis of myositis (83%), while myositis was the original diagnosis in only 17% of those possessing non-Jo-1 antisynthetase autoantibodies. In fact, many patients (approximately 50%) were diagnosed as having undifferentiated connective tissue disease or an overlap syndrome, and 13% had scleroderma as their first diagnosis.17
We also found that the survival rate was higher in patients with Jo-1 syndrome compared with patients with non-Jo-1 antisynthetase syndromes. We attributed the difference in survival rates to a delayed diagnosis in the non-Jo-1 group, perhaps due to their “nonclassic” presentations of the antisynthetase syndrome, delaying appropriate treatment. Patients received a diagnosis of Jo-1 antibody syndrome after a mean of 0.4 year (range 0.2–0.8), while those with a non-Jo-1 antisynthetase autoantibody had a delay in diagnosis of 1.0 year (range 0.4–5.1) (P < .01).17
In nearly half the cases in this cohort, pulmonary fibrosis was the cause of death, with primary pulmonary hypertension being the second leading cause (11%).
Antisynthetase syndrome: Bottom line
Antisynthetase syndrome is an often fatal disease that does not always present in a typical fashion with symptoms of myositis, as lung disease may be the predominant feature. A negative antinuclear antibody test result does not imply antibody negativity, as the autoantigen in these diseases is not located in the nucleus. Prompt diagnosis and appropriate immunosuppressive therapy are critical to improving outcomes.
CASE 5: FEVER, UNDIAGNOSED LUNG DISEASE, NO MYOSITIS
In January 2001, a 39-year-old woman was admitted to the hospital after 5 weeks of fever (temperatures 103°–104°F) and myalgias. An extensive workup was negative except for low-titer antinuclear antibody and for mild basilar fibrosis noted on chest radiography. She left the hospital against medical advice because of frustration with a lack of a specific diagnosis (“fever of unknown origin”).
Two months later, at a follow-up rheumatology consult, she reported more myalgias and arthralgias, as well as fever. Chest radiography now showed pleural effusions. Her fingers had color changes consistent with Raynaud phenomenon. At that time, I diagnosed an undifferentiated connective tissue disease and told her that I suspected an autoimmune condition that would need time to reveal itself. In the meantime, I treated her empirically with prednisone.
In April, she returned, much more short of breath and with more prominent diffuse pulmonary infiltrates. Physical examination revealed subtle Gottron changes. Testing revealed poor pulmonary function: forced vital capacity (FVC) 56%, forced expiratory volume in 1 second (FEV1) 52%, and diffusing capacity for carbon monoxide (Dlco) 40%. Blood testing was positive for anti-PL-12 antibody, one of the non-Jo-1 antisynthetase antibodies. At this time, we treated her with glucocorticoids and tacrolimus.
More than 15 years later, this patient is doing well. Her skin rash, joint symptoms, and fever have not returned, and interestingly, she never developed myositis. Her Raynaud symptoms are mild. Her most recent pulmonary function test results (January 2018) were FVC 75%, FEV1 87%, and Dlco 78%. Although these results are not normal, they are much improved and allow her to be completely functional without supplemental oxygen. Echocardiography showed normal pulmonary artery systolic pressure (25 mm Hg). She was still taking tacrolimus and prednisone. When we tried to stop tacrolimus after she had done well for many years, her condition flared.
Non-Jo-1 antisynthetase syndrome: Bottom line
Patients with a non-Jo-1 antisynthetase syndrome often present without myositis symptoms and may never manifest myositis symptoms. Likely because of this presentation, diagnosis of a specific connective tissue disorder is delayed, perhaps leading to increased mortality risk from pulmonary disease. Chronic immunosuppression is often required for these autoimmune conditions.
CASE 6: DERMATOMYOSITIS, RAPIDLY PROGRESSIVE INTERSTITIAL LUNG DISEASE
A 58-year-old woman presented in the summer of 2012 with a photosensitive rash. The following January, she returned with polyarthritis, mild muscle weakness, and a dermatomyositis-pattern rash. Her CK level was normal, and her antinuclear antibody and Sjögren syndrome antibody test results were negative. She improved on low-dose prednisone and methotrexate.
She was originally referred to me in May of that year for worsening rash and mild weakness. She denied pulmonary symptoms, but examination revealed faint basilar crackles. I increased her prednisone dosage to 20 mg/day and started mycophenolate mofetil mainly for the mild cutaneous and myositis features. I also recommended high-resolution CT of the lungs and pulmonary function tests, which she underwent in early June. High-resolution CT showed nonspecific mild infiltrates with minimal ground-glass opacities.
On July 1, she presented to her local emergency department with severe shortness of breath, requiring oxygen 12 L/min. She had a palmar rash. Repeat high-resolution CT showed dramatic worsening compared with the scan the previous month. Because of continued inadequate oxygenation, she was transferred to our center. A blood test later was positive for antimelanoma differentiation-associated gene 5 (MDA-5) autoantibody, previously known as anticlinically amyopathic dermatomyositis (anti-CADM)-140 antibody (based on immunoprecipitation results).
She died on the third day after transfer, just 2 months after I had originally seen her, at which time she had had no pulmonary symptoms.
Clinically amyopathic dermatomyositis
Anti-CADM-140, first reported from Asia,18–20 is an autoantibody-associated disease but not an antisynthetase. It is associated with dermatomyositis; patients often have a “vasculopathy” with cutaneous ulcerations and palmar papules.
MDA-5 is a cytoplasmic protein that “senses” viral RNA and induces production of type 1 interferon. It is involved in the innate immune defense against viruses.
Anti-MDA-5 positivity is associated with a poor pulmonary outcome.21 In our cohort from the University of Pittsburgh, many patients died within 3 years, compared with about a 40% survival rate in patients with dermatomyositis who tested negative for this antibody. That being said, many patients with anti-MDA-5 do not develop rapidly progressive interstitial lung disease.
Autoimmune interstitial lung disease: Bottom line
Autoimmune interstitial lung disease is easy to miss, especially in the case of a non-Jo-1 syndrome, for 3 important reasons:
- The autoimmune features may initially be subtle (eg, Raynaud phenomena, mild dermatomyositis rash, undifferentiated connective tissue disease)
- Autoantibody testing is not often ordered, is not standardized, or may be unavailable
- Providers are mistakenly reassured that a patient who tests negative for antinuclear antibody does not have an autoimmune condition.
To emphasize the last point, in a cohort of 202 patients who tested positive for an antisynthetase antibody, only half were antinuclear antibody-positive, but nearly three-quarters demonstrated anticytoplasmic staining on indirect immunofluorescence (due to the location of the autoantigen in the cytoplasm), making the latter a better screening test for an antisynthetase antibody. For scleroderma, 99% were antinculear antibody-positive, but for myositis, this test is much less sensitive.22
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
- Felice KJ, North WA. Inclusion body myositis in Connecticut: observations in 35 patients during an 8-year period. Medicine (Baltimore) 2001; 80(5):320–327. doi:10.1097/00005792-200109000-00006
- Lev EI, Tur-Kaspa I, Ashkenazy I, et al. Distribution of serum creatine kinase activity in young healthy persons. Clin Chim Acta 1999; 279(1-2):107–115. doi:10.1016/S0009-8981(98)00180-6
- Lilleng H, Abeler K, Johnsen SH, et al. Variation of serum creatine kinase (CK) levels and prevalence of persistent hyperCKemia in a Norwegian normal population. The Tromsø Study. Neuromuscul Disord 2011; 21(7):494–500. doi:10.1016/j.nmd.2011.04.007
- Johnston JD, Lloyd M, Mathews JA, Hawthorne SW. Racial variation in serum creatine kinase levels. J R Soc Med 1996; 89(8):462-464. pmid:8795501
- Prelle A, Tancredi L, Sciacco M, et al. Retrospective study of a large population of patients with asymptomatic or minimally symptomatic raised serum creatine kinase levels. J Neurol 2002; 249(3):305–311. pmid:11993531
- D’Adda E, Sciacco M, Fruguglietti ME, et al. Follow-up of a large population of asymptomatic/oligosymptomatic hyperckemic subjects. J Neurol 2006; 253(11):1399–1403. doi:10.1007/s00415-006-0223-y
- Madariaga MG. Polymyositis-like syndrome in hypothyroidism: review of cases reported over the past twenty-five years. Thyroid 2002; 12(4):331–336. doi:10.1089/10507250252949478
- de Sauvage Nolting PR, Buirma RJ, Hutten BA, Kastelein JJ; Dutch ExPRESS Investigator Group. Two-year efficacy and safety of simvastatin 80 mg in familial hypercholesterolemia (the Examination of Probands and Relatives in Statin Studies With Familial Hypercholesterolemia [ExPRESS FH]). Am J Cardiol 2002; 90(2):181–184. doi:10.1016/s0002-9149(02)02449-9
- Bruckert E, Hayem G, Dejager S, Yau C, Bégaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients--the PRIMO study. Cardiovasc Drugs Ther 2005; 19(6):403–414. doi:10.1007/s10557-005-5686-z
- Mosshammer D, Lorenz G, Meznaric S, Schwarz J, Muche R, Mörike K. Statin use and its association with musculoskeletal symptoms—a cross-sectional study in primary care settings. Fam Pract 2009; 26(2):88–95. doi:10.1093/fampra/cmp006
- Nichols GA, Koro CE. Does statin therapy initiation increase the risk for myopathy? An observational study of 32,225 diabetic and nondiabetic patients. Clin Ther 2007; 29(8):1761–1770. doi:10.1016/j.clinthera.2007.08.022
- Kassardjian CD, Lennon VA, Alfugham NB, Mahler M, Milone M. Clinical features and treatment outcomes of necrotizing autoimmune myopathy. JAMA Neurol 2015; 72(9):996–1003. doi:10.1001/jamaneurol.2015.1207
- Grable-Esposito P, Katzberg HD, Greenberg SA, Srinivasan J, Katz J, Amato AA. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010; 41(2):185–190. doi:10.1002/mus.21486
- Christopher-Stine L, Casciola-Rosen LA, Hong G, Chung T, Corse AM, Mammen AL. A novel autoantibody recognizing 200-kd and 100-kd proteins is associated with an immune-mediated necrotizing myopathy. Arthritis Rheum 2010; 62(9):2757–2766. doi:10.1002/art.27572
- Mammen AL, Chung T, Christopher-Stine L, et al. Autoantibodies against 3-hydroxy-3-methylglutaryl-coenzyme A reductase in patients with statin-associated autoimmune myopathy. Arthritis Rheum 2011; 63(3):713–721. doi:10.1002/art.30156
- Mammen AL, Tiniakou E. Intravenous immune globulin for statin-triggered autoimmune myopathy. N Engl J Med 2015; 373(17):1680–1682. doi:10.1056/NEJMc1506163
- Aggarwal R, Cassidy E, Fertig N, et al. Patients with non-Jo-1 anti-tRNA-synthetase autoantibodies have worse survival than Jo-1 positive patients. Ann Rheum Dis 2014; 73(1):227–232. doi:10.1136/annrheumdis-2012-201800
- Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 2005; 52(5):1571–1576. doi:10.1002/art.21023
- Sato S, Hoshino K, Satoh T, et al. RNA helicase encoded by melanoma differentiation-associated gene 5 is a major autoantigen in patients with clinically amyopathic dermatomyositis: association with rapidly progressive interstitial lung disease. Arthritis Rheum 2009; 60(7):2193–2200. doi:10.1002/art.24621
- Chen F, Wang D, Shu X, Nakashima R, Wang G. Anti-MDA5 antibody is associated with A/SIP and decreased T cells in peripheral blood and predicts poor prognosis of ILD in Chinese patients with dermatomyositis. Rheumatol Int 2012; 32(12):3909–3915. doi:10.1007/s00296-011-2323-y
- Moghadam-Kia S, Oddis CV, Sato S, Kuwana M, Aggarwal R. Anti-melanoma differentiation-associated gene 5 is associated with rapidly progressive lung disease and poor survival in US patients with amyopathic and myopathic dermatomyositis. Arthritis Care Res (Hoboken) 2016; 68(5):689–694. doi:10.1002/acr.22728
- Aggarwal R, Dhillon N, Fertig N, Koontz D, Qi Z, Oddis CV. A negative antinuclear antibody does not indicate autoantibody negativity in myositis: role of anticytoplasmic antibody as a screening test for antisynthetase syndrome. J Rheumatol 2017; 44(2):223–229. doi:10.3899/jrheum.160618
KEY POINTS
- Inclusion body myositis affects older men more than women and is characterized by slowly progressive, asymmetric, distal and proximal weakness and atrophy.
- Statin-associated muscle complaints are common, whereas necrotizing myopathy, characterized by a very high CK plus weakness, is rare but must be recognized.
- Elevated CK does not necessarily indicate myositis, especially in African Americans or after heavy exercise.
- Dermatomyositis is characterized by muscle weakness and raised red or purple Gottron papules over the knuckles, elbows, or knees.
- Autoimmune interstitial lung disease may be caused by a variety of antibodies, the most common being anti-Jo-1 (directed against histidyl tRNA synthetase).
- The rarer non-Jo-1 antisynthetase autoantibodies may be associated with rapidly progressive interstitial lung disease, which is a challenge to recognize because associated rheumatologic symptoms may be minimal.
Q&A: Drug costs and value in cancer
Skyrocketing drug costs are a key issue facing physicians, patients, and policymakers, but an even thornier problem may be determining a drug’s value.
In this Q&A, Richard L. Schilsky, MD, senior vice president and chief medical officer at the American Society of Clinical Oncology (ASCO), weighs in on the value proposition for cancer drugs and the implications for physicians.
Q: What tools exist for determining a drug’s value?
A: A number of organizations have developed tools to try to determine the value of cancer drug treatments. ASCO, the European Society for Medical Oncology (ESMO), the Institute for Clinical and Economic Review, Memorial Sloan Kettering Cancer Center, and the National Comprehensive Cancer Network have all developed tools for this purpose.
Our tool, the ASCO Value Framework, assesses the value of new cancer drug treatments based on clinical benefit, side effects, and improvements in patient symptoms or quality of life in the context of cost. While it’s hard to directly compare frameworks – given differences in methodology and the many nuances of evaluating clinical trial results – in 2018, ASCO and ESMO published a joint analysis of our value frameworks in the Journal of Clinical Oncology (2018; 37[4]:336-49).
The analysis found that the frameworks produce comparable measures of the clinical benefits of new therapies in approximately two-thirds of the more than 100 treatment comparisons that were examined. It also identified a number of factors that may contribute to the discordant scores, revealing potential ways for both of our organizations to refine our frameworks in the future.
That said, ASCO’s Value Framework is just one part of our broader, multifaceted effort to achieve high-quality, high-value care for all patients with cancer. Other efforts include ASCO’s proposed Patient-Centered Oncology Payment model, the Choosing Wisely campaign to identify low-value clinical strategies, and CancerLinQ and the Quality Oncology Practice Initiative to implement quality measurement and improvement.
Q: How can the issues around drug price and value be addressed earlier in the context of clinical trials?
A: The definition of value ultimately comes down to the price that must be paid to achieve meaningfully improved health outcomes for individual patients or the broader population of affected individuals. Optimizing the value of a new cancer drug treatment begins with an innovation to address an unmet medical need, followed by defining and achieving clinically meaningful improvements in health outcomes through well-designed and efficiently conducted clinical trials. Effectiveness research is also essential to determining how well new treatments perform compared with available alternatives and how they perform in more diverse populations than those typically included in the clinical trials used to establish efficacy.
Patient goals, preferences, and choices shape the real-world experience of a new product, and the direct and indirect costs of a treatment to patients and their families significantly affect whether it is adopted widely. Until their value is clearly established, new and costly products should be deployed judiciously and after careful consideration of the goals of treatment, available options, and the unique needs, preferences, and goals of individual patients.
More research is needed to improve how we assess the value of new cancer drug treatments. New clinical efficacy endpoints – both provider- and patient-reported ones – that accurately describe how a patient feels and functions must be developed and should reflect outcomes of value to patients other than survival, particularly in noncurative settings.
Better predictive biomarkers can transform a drug of modest efficacy in an unselected population to one of high efficacy in a biomarker-defined subgroup and thereby contribute to improving the value of a treatment.
Regulatory and policy initiatives such as adaptive licensing, value-based insurance, and indication-specific pricing that affect marketing approval, reimbursement, or price, respectively, based on treatment effectiveness, also deserve careful consideration and further research to determine their effects on aligning cost with benefit while ensuring patient access to potentially life-extending therapies and continued innovation in drug development.
Q: Aside from the policy options, what’s the role of the oncologist in discussing the value of drugs with patients when determining a treatment plan?
A: Since oncologists don’t control drug prices, our role in improving the value of cancer care involves appropriately managing how resources are used and guiding patients during discussions around the right treatment plan for their particular diagnosis, prognosis, and treatment goals.
Adopting and adhering to high-quality oncology clinical pathways is an important way to improve the quality, efficiency, and value of cancer care. High-quality oncology pathways are detailed, evidence-based treatment protocols for delivering cancer care to patients with specific disease types and stages. When properly designed and implemented, oncology pathways serve as an important tool in appropriately managing cancer care resources and improving the quality of care that patients with cancer receive, while also reducing costs.
Dr. Schilsky is the senior vice president and chief medical officer of ASCO. Formerly the chief of hematology/oncology in the department of medicine and deputy director of the University of Chicago Comprehensive Cancer Center, he is a leader in the field of clinical oncology, specializing in new drug development and the treatment of gastrointestinal cancers. Dr. Schilsky reported research funding from several pharmaceutical companies to ASCO for the Targeted Agent and Profiling Utilization Registry (TAPUR) clinical trial. He also reported travel/accommodation/expense support from Varian.
Skyrocketing drug costs are a key issue facing physicians, patients, and policymakers, but an even thornier problem may be determining a drug’s value.
In this Q&A, Richard L. Schilsky, MD, senior vice president and chief medical officer at the American Society of Clinical Oncology (ASCO), weighs in on the value proposition for cancer drugs and the implications for physicians.
Q: What tools exist for determining a drug’s value?
A: A number of organizations have developed tools to try to determine the value of cancer drug treatments. ASCO, the European Society for Medical Oncology (ESMO), the Institute for Clinical and Economic Review, Memorial Sloan Kettering Cancer Center, and the National Comprehensive Cancer Network have all developed tools for this purpose.
Our tool, the ASCO Value Framework, assesses the value of new cancer drug treatments based on clinical benefit, side effects, and improvements in patient symptoms or quality of life in the context of cost. While it’s hard to directly compare frameworks – given differences in methodology and the many nuances of evaluating clinical trial results – in 2018, ASCO and ESMO published a joint analysis of our value frameworks in the Journal of Clinical Oncology (2018; 37[4]:336-49).
The analysis found that the frameworks produce comparable measures of the clinical benefits of new therapies in approximately two-thirds of the more than 100 treatment comparisons that were examined. It also identified a number of factors that may contribute to the discordant scores, revealing potential ways for both of our organizations to refine our frameworks in the future.
That said, ASCO’s Value Framework is just one part of our broader, multifaceted effort to achieve high-quality, high-value care for all patients with cancer. Other efforts include ASCO’s proposed Patient-Centered Oncology Payment model, the Choosing Wisely campaign to identify low-value clinical strategies, and CancerLinQ and the Quality Oncology Practice Initiative to implement quality measurement and improvement.
Q: How can the issues around drug price and value be addressed earlier in the context of clinical trials?
A: The definition of value ultimately comes down to the price that must be paid to achieve meaningfully improved health outcomes for individual patients or the broader population of affected individuals. Optimizing the value of a new cancer drug treatment begins with an innovation to address an unmet medical need, followed by defining and achieving clinically meaningful improvements in health outcomes through well-designed and efficiently conducted clinical trials. Effectiveness research is also essential to determining how well new treatments perform compared with available alternatives and how they perform in more diverse populations than those typically included in the clinical trials used to establish efficacy.
Patient goals, preferences, and choices shape the real-world experience of a new product, and the direct and indirect costs of a treatment to patients and their families significantly affect whether it is adopted widely. Until their value is clearly established, new and costly products should be deployed judiciously and after careful consideration of the goals of treatment, available options, and the unique needs, preferences, and goals of individual patients.
More research is needed to improve how we assess the value of new cancer drug treatments. New clinical efficacy endpoints – both provider- and patient-reported ones – that accurately describe how a patient feels and functions must be developed and should reflect outcomes of value to patients other than survival, particularly in noncurative settings.
Better predictive biomarkers can transform a drug of modest efficacy in an unselected population to one of high efficacy in a biomarker-defined subgroup and thereby contribute to improving the value of a treatment.
Regulatory and policy initiatives such as adaptive licensing, value-based insurance, and indication-specific pricing that affect marketing approval, reimbursement, or price, respectively, based on treatment effectiveness, also deserve careful consideration and further research to determine their effects on aligning cost with benefit while ensuring patient access to potentially life-extending therapies and continued innovation in drug development.
Q: Aside from the policy options, what’s the role of the oncologist in discussing the value of drugs with patients when determining a treatment plan?
A: Since oncologists don’t control drug prices, our role in improving the value of cancer care involves appropriately managing how resources are used and guiding patients during discussions around the right treatment plan for their particular diagnosis, prognosis, and treatment goals.
Adopting and adhering to high-quality oncology clinical pathways is an important way to improve the quality, efficiency, and value of cancer care. High-quality oncology pathways are detailed, evidence-based treatment protocols for delivering cancer care to patients with specific disease types and stages. When properly designed and implemented, oncology pathways serve as an important tool in appropriately managing cancer care resources and improving the quality of care that patients with cancer receive, while also reducing costs.
Dr. Schilsky is the senior vice president and chief medical officer of ASCO. Formerly the chief of hematology/oncology in the department of medicine and deputy director of the University of Chicago Comprehensive Cancer Center, he is a leader in the field of clinical oncology, specializing in new drug development and the treatment of gastrointestinal cancers. Dr. Schilsky reported research funding from several pharmaceutical companies to ASCO for the Targeted Agent and Profiling Utilization Registry (TAPUR) clinical trial. He also reported travel/accommodation/expense support from Varian.
Skyrocketing drug costs are a key issue facing physicians, patients, and policymakers, but an even thornier problem may be determining a drug’s value.
In this Q&A, Richard L. Schilsky, MD, senior vice president and chief medical officer at the American Society of Clinical Oncology (ASCO), weighs in on the value proposition for cancer drugs and the implications for physicians.
Q: What tools exist for determining a drug’s value?
A: A number of organizations have developed tools to try to determine the value of cancer drug treatments. ASCO, the European Society for Medical Oncology (ESMO), the Institute for Clinical and Economic Review, Memorial Sloan Kettering Cancer Center, and the National Comprehensive Cancer Network have all developed tools for this purpose.
Our tool, the ASCO Value Framework, assesses the value of new cancer drug treatments based on clinical benefit, side effects, and improvements in patient symptoms or quality of life in the context of cost. While it’s hard to directly compare frameworks – given differences in methodology and the many nuances of evaluating clinical trial results – in 2018, ASCO and ESMO published a joint analysis of our value frameworks in the Journal of Clinical Oncology (2018; 37[4]:336-49).
The analysis found that the frameworks produce comparable measures of the clinical benefits of new therapies in approximately two-thirds of the more than 100 treatment comparisons that were examined. It also identified a number of factors that may contribute to the discordant scores, revealing potential ways for both of our organizations to refine our frameworks in the future.
That said, ASCO’s Value Framework is just one part of our broader, multifaceted effort to achieve high-quality, high-value care for all patients with cancer. Other efforts include ASCO’s proposed Patient-Centered Oncology Payment model, the Choosing Wisely campaign to identify low-value clinical strategies, and CancerLinQ and the Quality Oncology Practice Initiative to implement quality measurement and improvement.
Q: How can the issues around drug price and value be addressed earlier in the context of clinical trials?
A: The definition of value ultimately comes down to the price that must be paid to achieve meaningfully improved health outcomes for individual patients or the broader population of affected individuals. Optimizing the value of a new cancer drug treatment begins with an innovation to address an unmet medical need, followed by defining and achieving clinically meaningful improvements in health outcomes through well-designed and efficiently conducted clinical trials. Effectiveness research is also essential to determining how well new treatments perform compared with available alternatives and how they perform in more diverse populations than those typically included in the clinical trials used to establish efficacy.
Patient goals, preferences, and choices shape the real-world experience of a new product, and the direct and indirect costs of a treatment to patients and their families significantly affect whether it is adopted widely. Until their value is clearly established, new and costly products should be deployed judiciously and after careful consideration of the goals of treatment, available options, and the unique needs, preferences, and goals of individual patients.
More research is needed to improve how we assess the value of new cancer drug treatments. New clinical efficacy endpoints – both provider- and patient-reported ones – that accurately describe how a patient feels and functions must be developed and should reflect outcomes of value to patients other than survival, particularly in noncurative settings.
Better predictive biomarkers can transform a drug of modest efficacy in an unselected population to one of high efficacy in a biomarker-defined subgroup and thereby contribute to improving the value of a treatment.
Regulatory and policy initiatives such as adaptive licensing, value-based insurance, and indication-specific pricing that affect marketing approval, reimbursement, or price, respectively, based on treatment effectiveness, also deserve careful consideration and further research to determine their effects on aligning cost with benefit while ensuring patient access to potentially life-extending therapies and continued innovation in drug development.
Q: Aside from the policy options, what’s the role of the oncologist in discussing the value of drugs with patients when determining a treatment plan?
A: Since oncologists don’t control drug prices, our role in improving the value of cancer care involves appropriately managing how resources are used and guiding patients during discussions around the right treatment plan for their particular diagnosis, prognosis, and treatment goals.
Adopting and adhering to high-quality oncology clinical pathways is an important way to improve the quality, efficiency, and value of cancer care. High-quality oncology pathways are detailed, evidence-based treatment protocols for delivering cancer care to patients with specific disease types and stages. When properly designed and implemented, oncology pathways serve as an important tool in appropriately managing cancer care resources and improving the quality of care that patients with cancer receive, while also reducing costs.
Dr. Schilsky is the senior vice president and chief medical officer of ASCO. Formerly the chief of hematology/oncology in the department of medicine and deputy director of the University of Chicago Comprehensive Cancer Center, he is a leader in the field of clinical oncology, specializing in new drug development and the treatment of gastrointestinal cancers. Dr. Schilsky reported research funding from several pharmaceutical companies to ASCO for the Targeted Agent and Profiling Utilization Registry (TAPUR) clinical trial. He also reported travel/accommodation/expense support from Varian.
Vitamin D does not improve bone density, structure in healthy patients
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
ORLANDO – after 2 years of daily use, according to data presented at the annual meeting of the American Society for Bone and Mineral Research.
“Participants may have already reached the vitamin D level needed for bone health,” Meryl S. LeBoff, MD, of Brigham and Women’s Hospital in Boston, said in her presentation.
Dr. LeBoff presented results from 771 patients (mean age, 63.8 years) in the Bone Health Subcohort of VITAL (Vitamin D and OmegA-3 TriaL) who were not on any bone active medications and were randomized to receive daily vitamin D3 at a dose of 2,000 IU or placebo. Patients received bone imaging at baseline and at 2 years; areal bone mineral density (aBMD) of the whole body, femoral neck, total hip, and spine was assessed via dual x-ray absorptiometry scan. Total 25-hydroxyvitamin D (25[OH]D) levels were measured via liquid chromatography tandem mass spectrometry, and free 25(OH)D levels were measured via the ELISA assay. The baseline characteristics of the vitamin D3 supplementation and placebo groups were similar. Overall, 52% of patients had osteopenia and 10.4% had osteoporosis.
Between baseline and 2 years, the vitamin D group’s total 25(OH)D levels increased from a mean 27.0 ng/mL to 39.5 ng/mL (46%) and the free 25(OH)D levels increased from 5.8 pg/mL to 9.0 pg/mL (55%), whereas levels in the placebo stayed the same. The researchers found no significant absolute percentage changes over 2 years in aBMD of the whole body (P = .60), femoral neck (P = .16), total hip (P = .23) and spine (P = .55), compared with patients in the placebo group.
In a secondary analysis, Dr. LeBoff and colleagues found no benefit to volumetric BMD (vBMD) of the radius and the tibia at 2 years, and the results persisted after they performed a sensitivity analysis. Adverse events, such as hypercalciuria, kidney stones, and gastrointestinal symptoms, were not significantly different in the vitamin D group, compared with the placebo group.
Dr. LeBoff noted among the limitations of the study that it evaluated one dose level of vitamin D and was not designed to determine whether vitamin D supplementation was effective in people with vitamin D insufficiency, and the results are not generalizable to patients with osteoporosis or osteomalacia. Future studies should also examine whether free 25(OH)D levels can be used to detect which patients can benefit from vitamin D supplementation, she added.
Risk of falls
In a separate abstract, which Dr. LeBoff presented in a different session, 12,927 patients who received vitamin D supplementation for 5 years, were studied for risk of falls, compared with 12,994 individuals in a placebo group. At baseline, 33.3% of patients had fallen at least once in the previous year, and overall 6,605 patients reported 13,235 falls. At 5.3 years of follow-up, there were no significant differences in number of falls between groups, falls leading to injury, and falls leading to a doctor or a hospital visit.
There are ongoing parallel studies examining the incidence of fractures between groups in the total population of the VITAL study (25,871 participants); bone turnover markers; bone microarchitecture measurements through high-resolution peripheral quantitative computed tomography; and examining the connection between free 25(OH)D, parathyroid hormone, and vitamin D binding protein, said Dr. LeBoff.
The study was funded in part by grants from the National Cancer Institute, the National Heart, Lung and Blood Institute, the Office of Dietary Supplements, the National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. Dr. LeBoff reported receiving grants from the National Institute of Arthritis Musculoskeletal and Skin Diseases. Two authors reported nonfinancial support Pharmavite LLC of Northridge, Calif., Pronova BioPharma of Norway and BASF, and Quest Diagnostics. The remaining authors reported no conflicts of interest.
SOURCE: LeBoff M et al. ASBMR 2019, Abstracts 1046 and 1057.
REPORTING FROM ASBMR 2019
FDA approves oral semaglutide for HbA1c management in type 2 diabetes
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.
The Food and Drug Administration has approved semaglutide (Rybelsus) tablets for the treatment of type 2 diabetes in adults who have not met their hemoglobin A1c goal. It is the first glucagonlike peptide–1 (GLP-1) analogue to be approved in pill form in the United States.
The approval was based on results from the PIONEER trials, a series of 10 studies that assessed semaglutide against sitagliptin, empagliflozin, and liraglutide in a total of 9,543 patients with type 2 diabetes. Patients who received semaglutide had reduced hemoglobin A1c levels as well as reduced body weight.
The most common adverse events reported during the PIONEER trials were nausea, abdominal pain, diarrhea, decreased appetite, vomiting, and constipation. The rate of adverse events were similar across trials.
“GLP-1 receptor agonists are effective medications for people with type 2 diabetes but have been underutilized, in part because until now, they have been available only as an injectable treatment. The availability of an oral GLP-1 receptor agonist represents a significant development, and primary care providers, specialists, and patients alike may now be more receptive to the use of a GLP-1 therapy to help them achieve their blood sugar goals,” said Vanita R. Aroda, MD, director of diabetes clinical research at Brigham and Women’s Hospital in Boston and PIONEER clinical trial researcher.
Semaglutide is approved for once-daily use, at doses of 7 mg and 14 mg. Find the full press release on the Novo Nordisk website.