User login
75-year-old man • recent history of hand-foot-mouth disease • discolored fingernails and toenails lifting from the proximal end • Dx?
THE CASE
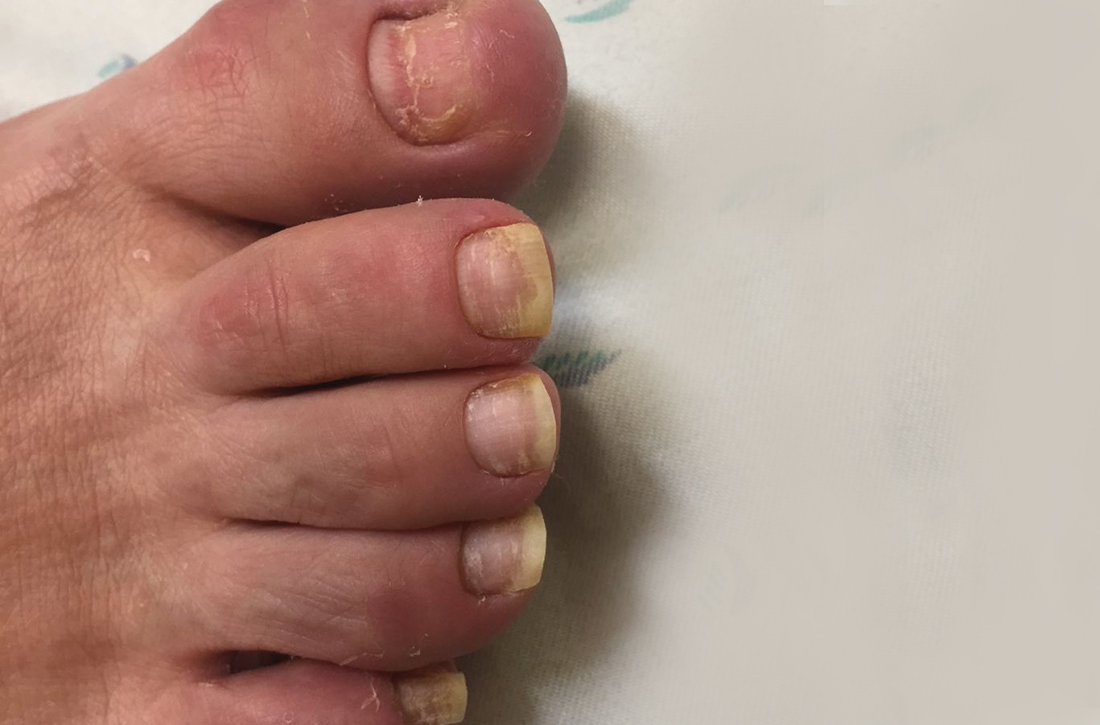
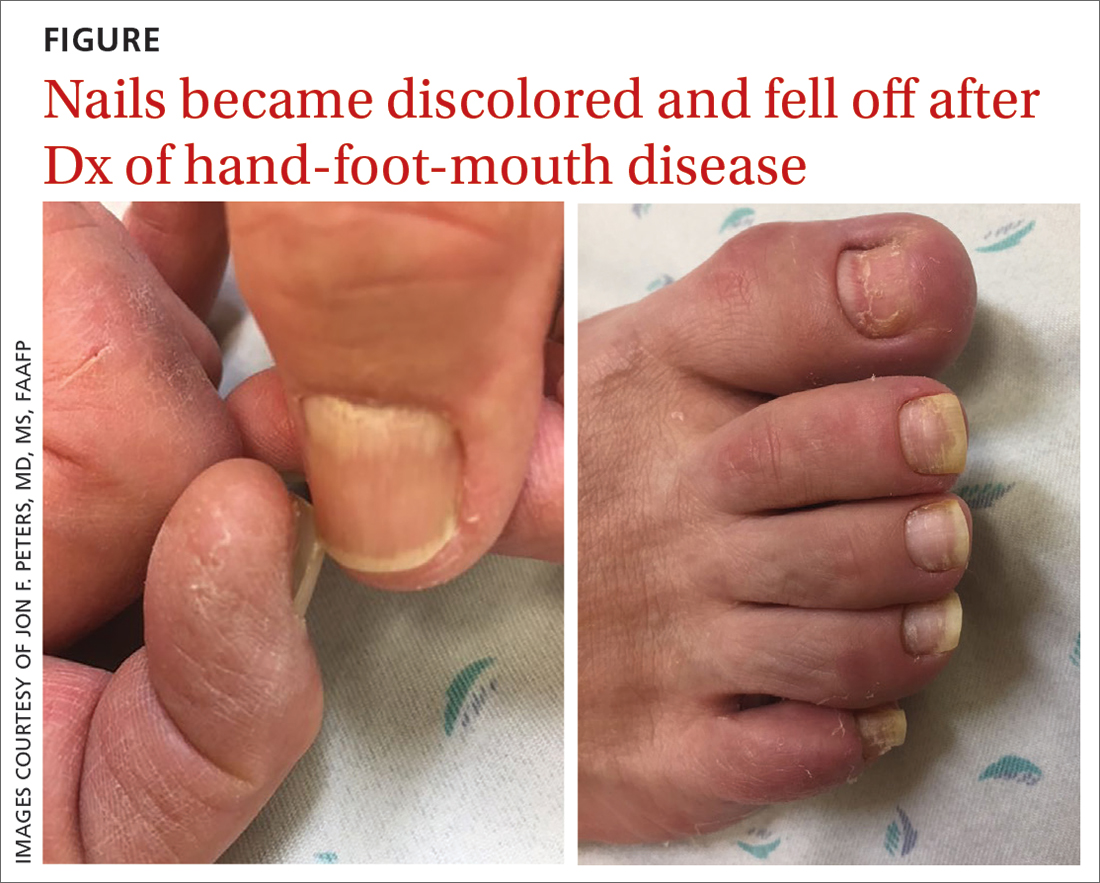
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.
The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
THE CASE
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.

The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
THE CASE
A 75-year-old man sought care from his primary care physician because his “fingernails and toenails [were] all falling off.” He did not feel ill and had no other complaints. His vital signs were unremarkable. He had no history of malignancies, chronic skin conditions, or systemic diseases. His fingernails and toenails were discolored and lifting from the proximal end of his nail beds (FIGURE). One of his great toenails had already fallen off, 1 thumb nail was minimally attached with the cuticle, and the rest of his nails were loose and in the process of separating from their nail beds. There was no nail pitting, rash, or joint swelling and tenderness.

The patient reported that while on vacation in Hawaii 3 weeks earlier, he had sought care at an urgent care clinic for a painless rash on his hands and the soles of his feet. At that time, he did not feel ill or have mouth ulcers, penile discharge, or arthralgia. There had been no recent changes to his prescription medications, which included finasteride, terazosin, omeprazole, and an albuterol inhaler. He denied taking over-the-counter medications or supplements.
The physical exam at the urgent care had revealed multiple blotchy, dark, 0.5- to 1-cm nonpruritic lesions that were desquamating. No oral lesions were seen. He had been given a diagnosis of hand-foot-mouth disease (HFMD) and reassured that it would resolve on its own in about 10 days.
THE DIAGNOSIS
Several possible diagnoses for nail disorders came to mind with this patient, including onychomycosis, onychoschizia, onycholysis, and onychomadesis.
Onychomycosis is a chronic fungal infection of the nail that affects toenails more often than fingernails.1 The most common form is distal subungual onychomycosis, which begins distally and slowly migrates proximally through the nail matrix.1 Often onychomycosis affects only a few nails unless the patient is elderly or has comorbid conditions, and the nails rarely separate from the nail bed.
Onychoschizia involves lamellar splitting and peeling of the dorsal surface of the nail plate.2 Usually white discolorations appear on the distal edges of the nail.3 It is more common in women than in men and is often caused by nail dehydration from repeated excessive immersion in water with detergents or recurrent application of nail polish.2 However, the nails do not separate from the nail bed, and usually only the fingernails are involved.
Onycholysis is a nail attachment disorder in which the nail plate distally separates from the nail bed. Areas of separation will appear white or yellow. There are many etiologies for onycholysis, including trauma, psoriasis, fungal infection, and contact irritant reactions.3 It also can be caused by medications and thyroid disease.3,4
Continue to: Onychomadesis
Onychomadesis, sometimes considered a severe form of Beau’s line,5,6 is defined by the spontaneous separation of the nail plate from the nail matrix. Although the nail will initially remain attached, proximal shedding will eventually occur.7 When several nails are involved, a systemic source—such as an acute infection, autoimmune disease, medication, malignancy (eg, cutaneous T-cell lymphoma), Kawasaki disease, skin disorders (eg, pemphigus vulgaris or keratosis punctata et planters), or chemotherapy—may be the cause.6-8 If only a few nails are involved, it may be associated with trauma, and in rare cases, onychomadesis can be idiopathic.5,7
In this case, all signs pointed to onychomadesis. All of the patient’s nails were affected (discolored and lifting), his nail loss involved spontaneous proximal separation of the nail plate from the nail matrix, and he had a recent previous infection: HFMD.
DISCUSSION
Onychomadesis is a rare nail-shedding disorder thought to be caused by the temporary arrest of the nail matrix.8 It is a potential late complication of infection, such as HFMD,9 and was first reported in children in Chicago in 2000.10 Since then, onychomadesis has been noted in children in many countries.8 Reports of onychomadesis following HFMD in adults are rare, but it may be underreported because HFMD is more common in children and symptoms are usually minor in adults.11
Molecular studies have associated onychomadesis with coxsackievirus (CV)A6 and CVA10.4 Other serotypes associated with onychomadesis include CVB1, CVB2, CVA5, CVA16, and enteroviruses 71 and 9.4 Most known outbreaks seem to be caused by CVA6.4
No treatment is needed for onychomadesis; physicians can reassure patients that normal nail growth will begin within 1 to 4 months. Because onychomadesis is rare, it does not have its own billing code, so one can use code L60.8 for “Other nail disorders.”12
Our patient was seen in the primary care clinic 3 months after his initial visit. At that time, his nails were no longer discolored and no other abnormalities were present. All of the nails on his fingers and toes were firmly attached and growing normally.
THE TAKEAWAY
The sudden asymptomatic loss of multiple fingernails and toenails—especially with proximal nail shedding—is a rare disorder known as onychomadesis. It can be caused by various etiologies and can be a late complication of HFMD or other viral infections. Onychomadesis should be considered when evaluating older patients, particularly when all of their nails are involved after a viral infection.
CORRESPONDENCE
Jon F. Peters, MD, MS, FAAFP, 14486 SE Lyon Court, Happy Valley, OR 97086; [email protected]
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
1. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677-678.
2. Sparavigna A, Tenconi B, La Penna L. Efficacy and tolerability of a biomineral formulation for treatment of onychoschizia: a randomized trial. Clin Cosmet Investig Dermatol. 2019:12:355-362. doi: 10.2147/CCID.S187305
3. Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74. doi: 10.4103/2229-5178.153002
4. Cleveland Clinic. Onycholysis. Accessed March 1, 2023. https://my.clevelandclinic.org/health/diseases/22903-onycholysis
5. Chiu H-H, Liu M-T, Chung W-H, et al. The mechanism of onychomadesis (nail shedding) and Beau’s lines following hand-foot-mouth disease. Viruses. 2019;11:522. doi: 10.3390/v11060522
6. Suchonwanit P, Nitayavardhana S. Idiopathic sporadic onychomadesis of toenails. Case Rep Dermatol Med. 2016;2016:6451327. doi: 10.1155/2016/6451327
7. Hardin J, Haber RM. Onychomadesis: literature review. Br J Dermatol. 2015;172:592-596. doi: 10.1111/bjd.13339
8. Li D, Yang W, Xing X, et al. Onychomadesis and potential association with HFMD outbreak in a kindergarten in Hubei providence, China, 2017. BMC Infect Dis. 2019:19:995. doi: 10.1186/s12879-019-4560-8
9. Chiu HH, Wu CS, Lan CE. Onychomadesis: a late complication of hand, foot, and mouth disease. J Emerg Med. 2017;52:243-245. doi: 10.1016/j.jemermed.2016.01.034
10. Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11. doi: 10.1046/j.1525-1470.2000.01702.x
11. Scarfi F, Arunachalam M, Galeone M, et al. An uncommon onychomadesis in adults. Int J Derm. 2014;53:1392-1394. doi: 10.1111/j.1365-4632.2012.05774.x
12. ICD10Data.com. 2023 ICD-10-CM codes. Accessed February 15, 2023. www.icd10data.com/ICD10CM/codes
► Recent history of hand-foot-mouth disease
► Discolored fingernails and toenails lifting from the proximal end
Racial disparities not found in chronic hepatitis B treatment initiation
Researchers studying differences in treatment initiation for chronic hepatitis B (CHB) among a large, multiracial cohort in North America did not find evidence of disparities by race or socioeconomic status.
.
That gap suggests that treatment guidelines need to be simplified and that efforts to increase hepatitis B virus (HBV) awareness and train more clinicians are needed to achieve the World Health Organization’s goal of eliminating HBV by 2030, the researchers write.
The Hepatitis B Research Network study was published online in JAMA Network Open.
The prevalence of CHB in the United States is estimated at 2.4 million. It disproportionately affects persons of Asian or African descent, the investigators note. Their study examined whether treatment initiation and outcomes differ between African American and Black, Asian, and White participants, as well as between African American and Black participants born in North America and East or West Africa.
The research involved 1,550 adult patients: 1,157 Asian American, 193 African American or Black (39 born in the United States, 90 in East Africa, 53 in West Africa, and 11 elsewhere), 157 White, and 43 who identified as being of “other races.” All had CHB but were not receiving antiviral treatment at enrollment.
Participants came from 20 centers in the United States and one in Canada. They underwent clinical and laboratory assessments and could receive anti-HBV treatment after they enrolled. Enrollment was from Jan. 14, 2011, to Jan. 28, 2018. Participants were followed at 12 and 24 weeks and every 24 weeks thereafter in the longitudinal cohort study by Mandana Khalili, MD, division of gastroenterology and hepatology, University of California, San Francisco, and colleagues.
Information on patients’ country of birth, duration of U.S. or Canadian residency, educational level, employment, insurance, prior antiviral treatment, family history of HBV or hepatocellular carcinoma (HCC), and mode of transmission were collected by research coordinators.
Treatment initiation
During the study period, slightly fewer than one-third (32.5%) of the participants initiated treatment. The incidences were 4.8 per 100 person-years in African American or Black participants, 9.9 per 100 person-years in Asian participants, 6.6 per 100 person-years in White participants, and 7.9 per 100 person-years in those of other races (P < .001).
A lower percentage of African American and Black participants (14%) met the American Association for the Study of Liver Diseases treatment criteria, compared with Asian (22%) and White (27%) participants (P = .01).
When the researchers compared cumulative probability of initiating treatment by race for those who met criteria for treatment, they found no significant differences by race.
At 72 weeks, initiation probability was 0.45 for African American and Black patients and 0.51 for Asian and White patients (P = .68). Similarly, among African American and Black participants who met treatment criteria, there were no significant differences in cumulative probability of treatment by region of birth.
The cumulative percentage of treatment initiation for those who met guideline-based criteria was 62%.
“Among participants with a treatment indication, treatment rates did not differ significantly by race, despite marked differences in educational level, income, and type of health care insurance across the racial groups,” the researchers write. “Moreover, race was not an independent estimator of treatment initiation when adjusting for known factors associated with a higher risk of adverse clinical outcomes, namely, HBV DNA, disease severity, sex, and age.”
Adverse liver outcomes (hepatic decompensation, HCC, liver transplant, and death) were rare and did not vary significantly by race, the researchers write.
One study limitation is that participants were linked to specialty liver clinics, so the findings may not be generalizable to patients who receive care in other settings, the authors note.
The results are “reassuring,” said senior author Anna S. Lok, MD, division of gastroenterology and hepatology at University of Michigan in Ann Arbor. However, she noted, study participants had already overcome barriers to receiving care at major academic centers.
“Once you get into the big academic liver centers, then maybe everything is equal, but in the real world, a lot of people don’t ever get to the big liver centers,” she said. The question becomes: “Are we serving only a portion of the patient population?”
Many factors drive the decision to undergo treatment, including the doctor’s opinion as to need and the patient’s desire to receive treatment, she said.
The study participants who were more likely to get treated were those with higher-level disease who had a stronger indication for treatment, Dr. Lok said.
Finding the disparities
Centers for Disease Control and Prevention statistics show that Black people are 3.9 times more likely to have CHB and 2.5 times more likely to die from it than White people, notes H. Nina Kim, MD, with the department of medicine, University of Washington, Seattle, in an accompanying invited commentary.
“The fact that we have not observed racial disparities in treatment initiation does not mean none exist; it means we have to look harder to find them,” she writes.
“We need to examine whether our guidelines for HBV treatment are so complex that it becomes the purview of specialists, thereby restricting access and deepening inequities,” Dr. Kim adds. “We should look closely at retention in care, the step that precedes treatment, and stratify this outcome by race and ethnicity.”
Primary care physicians in some regions might find it difficult to manage patients who have hepatitis B because they see so few of them, Dr. Lok noted.
Dr. Khalili has received grants and consulting fees from Gilead Sciences Inc and grants from Intercept Pharmaceuticals outside the submitted work. Dr. Lok has received grants from Target and consultant fees from Abbott, Ambys, Arbutus, Chroma, Clear B, Enanta, Enochian, GNI, GlaxoSmithKline, Eli Lilly, and Virion outside the submitted work. Coauthors have received grants, consulting fees, or personal fees from Bayer, Boston Scientific, Exact Sciences, Fujifilm Medical Sciences, Gilead Sciences, Glycotest, Redhill Biopharma, Target RWE, MedEd Design, Pontifax, Global Life, the Lynx Group, AstraZeneca, Eisai, Novartis Venture Fund, Grail, QED Therapeutics, Genentech, Hepion Pharmaceuticals, Roche, Abbott, AbbVie, and Pfizer. Dr. Kim has received grants from Gilead Sciences (paid to her institution) outside the submitted work.
A version of this article first appeared on Medscape.com.
Researchers studying differences in treatment initiation for chronic hepatitis B (CHB) among a large, multiracial cohort in North America did not find evidence of disparities by race or socioeconomic status.
.
That gap suggests that treatment guidelines need to be simplified and that efforts to increase hepatitis B virus (HBV) awareness and train more clinicians are needed to achieve the World Health Organization’s goal of eliminating HBV by 2030, the researchers write.
The Hepatitis B Research Network study was published online in JAMA Network Open.
The prevalence of CHB in the United States is estimated at 2.4 million. It disproportionately affects persons of Asian or African descent, the investigators note. Their study examined whether treatment initiation and outcomes differ between African American and Black, Asian, and White participants, as well as between African American and Black participants born in North America and East or West Africa.
The research involved 1,550 adult patients: 1,157 Asian American, 193 African American or Black (39 born in the United States, 90 in East Africa, 53 in West Africa, and 11 elsewhere), 157 White, and 43 who identified as being of “other races.” All had CHB but were not receiving antiviral treatment at enrollment.
Participants came from 20 centers in the United States and one in Canada. They underwent clinical and laboratory assessments and could receive anti-HBV treatment after they enrolled. Enrollment was from Jan. 14, 2011, to Jan. 28, 2018. Participants were followed at 12 and 24 weeks and every 24 weeks thereafter in the longitudinal cohort study by Mandana Khalili, MD, division of gastroenterology and hepatology, University of California, San Francisco, and colleagues.
Information on patients’ country of birth, duration of U.S. or Canadian residency, educational level, employment, insurance, prior antiviral treatment, family history of HBV or hepatocellular carcinoma (HCC), and mode of transmission were collected by research coordinators.
Treatment initiation
During the study period, slightly fewer than one-third (32.5%) of the participants initiated treatment. The incidences were 4.8 per 100 person-years in African American or Black participants, 9.9 per 100 person-years in Asian participants, 6.6 per 100 person-years in White participants, and 7.9 per 100 person-years in those of other races (P < .001).
A lower percentage of African American and Black participants (14%) met the American Association for the Study of Liver Diseases treatment criteria, compared with Asian (22%) and White (27%) participants (P = .01).
When the researchers compared cumulative probability of initiating treatment by race for those who met criteria for treatment, they found no significant differences by race.
At 72 weeks, initiation probability was 0.45 for African American and Black patients and 0.51 for Asian and White patients (P = .68). Similarly, among African American and Black participants who met treatment criteria, there were no significant differences in cumulative probability of treatment by region of birth.
The cumulative percentage of treatment initiation for those who met guideline-based criteria was 62%.
“Among participants with a treatment indication, treatment rates did not differ significantly by race, despite marked differences in educational level, income, and type of health care insurance across the racial groups,” the researchers write. “Moreover, race was not an independent estimator of treatment initiation when adjusting for known factors associated with a higher risk of adverse clinical outcomes, namely, HBV DNA, disease severity, sex, and age.”
Adverse liver outcomes (hepatic decompensation, HCC, liver transplant, and death) were rare and did not vary significantly by race, the researchers write.
One study limitation is that participants were linked to specialty liver clinics, so the findings may not be generalizable to patients who receive care in other settings, the authors note.
The results are “reassuring,” said senior author Anna S. Lok, MD, division of gastroenterology and hepatology at University of Michigan in Ann Arbor. However, she noted, study participants had already overcome barriers to receiving care at major academic centers.
“Once you get into the big academic liver centers, then maybe everything is equal, but in the real world, a lot of people don’t ever get to the big liver centers,” she said. The question becomes: “Are we serving only a portion of the patient population?”
Many factors drive the decision to undergo treatment, including the doctor’s opinion as to need and the patient’s desire to receive treatment, she said.
The study participants who were more likely to get treated were those with higher-level disease who had a stronger indication for treatment, Dr. Lok said.
Finding the disparities
Centers for Disease Control and Prevention statistics show that Black people are 3.9 times more likely to have CHB and 2.5 times more likely to die from it than White people, notes H. Nina Kim, MD, with the department of medicine, University of Washington, Seattle, in an accompanying invited commentary.
“The fact that we have not observed racial disparities in treatment initiation does not mean none exist; it means we have to look harder to find them,” she writes.
“We need to examine whether our guidelines for HBV treatment are so complex that it becomes the purview of specialists, thereby restricting access and deepening inequities,” Dr. Kim adds. “We should look closely at retention in care, the step that precedes treatment, and stratify this outcome by race and ethnicity.”
Primary care physicians in some regions might find it difficult to manage patients who have hepatitis B because they see so few of them, Dr. Lok noted.
Dr. Khalili has received grants and consulting fees from Gilead Sciences Inc and grants from Intercept Pharmaceuticals outside the submitted work. Dr. Lok has received grants from Target and consultant fees from Abbott, Ambys, Arbutus, Chroma, Clear B, Enanta, Enochian, GNI, GlaxoSmithKline, Eli Lilly, and Virion outside the submitted work. Coauthors have received grants, consulting fees, or personal fees from Bayer, Boston Scientific, Exact Sciences, Fujifilm Medical Sciences, Gilead Sciences, Glycotest, Redhill Biopharma, Target RWE, MedEd Design, Pontifax, Global Life, the Lynx Group, AstraZeneca, Eisai, Novartis Venture Fund, Grail, QED Therapeutics, Genentech, Hepion Pharmaceuticals, Roche, Abbott, AbbVie, and Pfizer. Dr. Kim has received grants from Gilead Sciences (paid to her institution) outside the submitted work.
A version of this article first appeared on Medscape.com.
Researchers studying differences in treatment initiation for chronic hepatitis B (CHB) among a large, multiracial cohort in North America did not find evidence of disparities by race or socioeconomic status.
.
That gap suggests that treatment guidelines need to be simplified and that efforts to increase hepatitis B virus (HBV) awareness and train more clinicians are needed to achieve the World Health Organization’s goal of eliminating HBV by 2030, the researchers write.
The Hepatitis B Research Network study was published online in JAMA Network Open.
The prevalence of CHB in the United States is estimated at 2.4 million. It disproportionately affects persons of Asian or African descent, the investigators note. Their study examined whether treatment initiation and outcomes differ between African American and Black, Asian, and White participants, as well as between African American and Black participants born in North America and East or West Africa.
The research involved 1,550 adult patients: 1,157 Asian American, 193 African American or Black (39 born in the United States, 90 in East Africa, 53 in West Africa, and 11 elsewhere), 157 White, and 43 who identified as being of “other races.” All had CHB but were not receiving antiviral treatment at enrollment.
Participants came from 20 centers in the United States and one in Canada. They underwent clinical and laboratory assessments and could receive anti-HBV treatment after they enrolled. Enrollment was from Jan. 14, 2011, to Jan. 28, 2018. Participants were followed at 12 and 24 weeks and every 24 weeks thereafter in the longitudinal cohort study by Mandana Khalili, MD, division of gastroenterology and hepatology, University of California, San Francisco, and colleagues.
Information on patients’ country of birth, duration of U.S. or Canadian residency, educational level, employment, insurance, prior antiviral treatment, family history of HBV or hepatocellular carcinoma (HCC), and mode of transmission were collected by research coordinators.
Treatment initiation
During the study period, slightly fewer than one-third (32.5%) of the participants initiated treatment. The incidences were 4.8 per 100 person-years in African American or Black participants, 9.9 per 100 person-years in Asian participants, 6.6 per 100 person-years in White participants, and 7.9 per 100 person-years in those of other races (P < .001).
A lower percentage of African American and Black participants (14%) met the American Association for the Study of Liver Diseases treatment criteria, compared with Asian (22%) and White (27%) participants (P = .01).
When the researchers compared cumulative probability of initiating treatment by race for those who met criteria for treatment, they found no significant differences by race.
At 72 weeks, initiation probability was 0.45 for African American and Black patients and 0.51 for Asian and White patients (P = .68). Similarly, among African American and Black participants who met treatment criteria, there were no significant differences in cumulative probability of treatment by region of birth.
The cumulative percentage of treatment initiation for those who met guideline-based criteria was 62%.
“Among participants with a treatment indication, treatment rates did not differ significantly by race, despite marked differences in educational level, income, and type of health care insurance across the racial groups,” the researchers write. “Moreover, race was not an independent estimator of treatment initiation when adjusting for known factors associated with a higher risk of adverse clinical outcomes, namely, HBV DNA, disease severity, sex, and age.”
Adverse liver outcomes (hepatic decompensation, HCC, liver transplant, and death) were rare and did not vary significantly by race, the researchers write.
One study limitation is that participants were linked to specialty liver clinics, so the findings may not be generalizable to patients who receive care in other settings, the authors note.
The results are “reassuring,” said senior author Anna S. Lok, MD, division of gastroenterology and hepatology at University of Michigan in Ann Arbor. However, she noted, study participants had already overcome barriers to receiving care at major academic centers.
“Once you get into the big academic liver centers, then maybe everything is equal, but in the real world, a lot of people don’t ever get to the big liver centers,” she said. The question becomes: “Are we serving only a portion of the patient population?”
Many factors drive the decision to undergo treatment, including the doctor’s opinion as to need and the patient’s desire to receive treatment, she said.
The study participants who were more likely to get treated were those with higher-level disease who had a stronger indication for treatment, Dr. Lok said.
Finding the disparities
Centers for Disease Control and Prevention statistics show that Black people are 3.9 times more likely to have CHB and 2.5 times more likely to die from it than White people, notes H. Nina Kim, MD, with the department of medicine, University of Washington, Seattle, in an accompanying invited commentary.
“The fact that we have not observed racial disparities in treatment initiation does not mean none exist; it means we have to look harder to find them,” she writes.
“We need to examine whether our guidelines for HBV treatment are so complex that it becomes the purview of specialists, thereby restricting access and deepening inequities,” Dr. Kim adds. “We should look closely at retention in care, the step that precedes treatment, and stratify this outcome by race and ethnicity.”
Primary care physicians in some regions might find it difficult to manage patients who have hepatitis B because they see so few of them, Dr. Lok noted.
Dr. Khalili has received grants and consulting fees from Gilead Sciences Inc and grants from Intercept Pharmaceuticals outside the submitted work. Dr. Lok has received grants from Target and consultant fees from Abbott, Ambys, Arbutus, Chroma, Clear B, Enanta, Enochian, GNI, GlaxoSmithKline, Eli Lilly, and Virion outside the submitted work. Coauthors have received grants, consulting fees, or personal fees from Bayer, Boston Scientific, Exact Sciences, Fujifilm Medical Sciences, Gilead Sciences, Glycotest, Redhill Biopharma, Target RWE, MedEd Design, Pontifax, Global Life, the Lynx Group, AstraZeneca, Eisai, Novartis Venture Fund, Grail, QED Therapeutics, Genentech, Hepion Pharmaceuticals, Roche, Abbott, AbbVie, and Pfizer. Dr. Kim has received grants from Gilead Sciences (paid to her institution) outside the submitted work.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
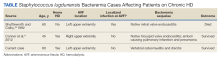
High-Grade Staphylococcus lugdunensis Bacteremia in a Patient on Home Hemodialysis
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
Staphylococcus lugdunensis (S lugdunensis) is a species of coagulase-negative Staphylococcus (CoNS) and a constituent of human skin flora. Unlike other strains of CoNS, however, S lugdunensis has gained notoriety for virulence that resembles Staphylococcus aureus (S aureus). S lugdunensis is now recognized as an important nosocomial pathogen and cause of prosthetic device infections, including vascular catheter infections. We present a case of persistent S lugdunensis bacteremia occurring in a patient on hemodialysis (HD) without any implanted prosthetic materials.
Case Presentation
A 60-year-old man with a history of uncontrolled type 2 diabetes mellitus (T2DM) and end-stage renal disease on home HD via arteriovenous fistula (AVF) presented to the emergency department (ED) for evaluation of subacute progressive low back pain. His symptoms began abruptly 2 weeks prior to presentation without any identifiable trigger or trauma. His pain localized to the lower thoracic spine, radiating anteriorly into his abdomen. He reported tactile fever for several days before presentation but no chills, night sweats, paresthesia, weakness, or bowel/bladder incontinence. He had no recent surgeries, implanted hardware, or invasive procedures involving the spine. HD was performed 5 times a week at home with a family member cannulating his AVF via buttonhole technique. He initially sought evaluation in a community hospital several days prior, where he underwent magnetic resonance imaging (MRI) of the thoracic spine. He was discharged from the community ED with oral opioids prior to the MRI results. He presented to West Los Angeles Veterans Affairs Medical Center (WLAVAMC) ED when MRI results came back indicating abnormalities and he reported recalcitrant pain.
On arrival at WLAVAMC, the patient was afebrile with a heart rate of 107 bpm and blood pressure of 152/97 mm Hg. The remainder of his vital signs were normal. The physical examination revealed midline tenderness on palpation of the distal thoracic and proximal lumbar spine. Muscle strength was 4 of 5 in the bilateral hip flexors, though this was limited by pain. The remainder of his neurologic examination was nonfocal. The cardiac examination was unremarkable with no murmurs auscultated. His left upper extremity AVF had an audible bruit and palpable thrill. The skin examination was notable for acanthosis nigricans but no areas of skin erythema or induration and no obvious stigmata of infective endocarditis.
The initial laboratory workup was remarkable for a white blood cell (WBC) count of 10.0 × 103/µL with left shift, blood urea nitrogen level of 59 mg/dL, and creatinine level of 9.3 mg/dL. The patient’s erythrocyte sedimentation rate (ESR) was 45 mm/h (reference range, ≤ 20 mm/h) and C-reactive protein level was > 8.0 mg/L (reference range, ≤ 0.74 mg/L). Two months prior the hemoglobin A1c had been recorded at 9.9%.
Given his intractable low back pain and elevated inflammatory markers, the patient underwent an MRI of the thoracic and lumbar spine with contrast while in the ED. This MRI revealed abnormal marrow edema in the T11-T12 vertebrae with abnormal fluid signal in the T11-T12 disc space. Subjacent paravertebral edema also was noted. There was no well-defined fluid collection or abnormal signal in the spinal cord. Taken together, these findings were concerning for T11-T12 discitis with osteomyelitis.
Two sets of blood cultures were obtained, and empiric IV vancomycin and ceftriaxone were started. Interventional radiology was consulted for consideration of vertebral biopsy but deferred while awaiting blood culture data. Neurosurgery also was consulted and recommended nonoperative management given his nonfocal neurologic examination and imaging without evidence of abscess. Both sets of blood cultures collected on admission later grew methicillin-sensitive S lugdunensis, a species of CoNS. A transthoracic and later transesophageal echocardiogram did not show any valvular vegetations. The patient’s antibiotic regimen was narrowed to IV oxacillin based on susceptibility data. It was later discovered that both blood cultures obtained during his outside ED encounter were also growing S lugdunensis.
The patient’s S lugdunensis bacteremia persisted for the first 8 days of his admission despite appropriate dosing of oxacillin. During this time, the patient remained afebrile with stable vital signs and a normal WBC count. Positron emission tomography was obtained to evaluate for potential sources of his persistent bacteremia. Aside from tracer uptake in the T11-T12 vertebral bodies and intervertebral disc space, no other areas showed suspicious uptake. Neurosurgery reevaluated the patient and again recommended nonoperative management. Blood cultures cleared and based on recommendations from an infectious disease specialist, the patient was transitioned to IV cefazolin dosed 3 times weekly after HD, which was transitioned to an outpatient dialysis center. The patient continued taking cefazolin for 6 weeks with subsequent improvement in back pain and normalization of inflammatory markers at outpatient follow-up.
Discussion
CoNS are a major contributor to human skin flora, a common contaminant of blood cultures, and an important cause of nosocomial bloodstream infections.1,2 These species have a predilection for forming biofilms, making CoNS a major cause of prosthetic device infections.3 S lugdunensis is a CoNS species that was first described in 1988.4 In addition to foreign body–related infections, S lugdunensis has been implicated in bone/joint infections, native valve endocarditis, toxic shock syndrome, and brain abscesses.5-8 Infections due to S lugdunensis are notorious for their aggressive and fulminant courses. With its increased virulence that is atypical of other CoNS, S lugdunensis has understandably been likened more to S aureus.
Prior cases have been reported of S lugdunensis bacteremia in patients using HD. However, the suspected source of bacteremia in these cases has generally been central venous catheters.9-12
Notably, our patient’s AVF was accessed using the buttonhole technique for his home HD sessions, which involves cannulating the same site along the fistula until an epithelialized track has formed from scar tissue. At later HD sessions, duller needles can then be used to cannulate this same track. In contrast, the rope-ladder technique involves cannulating a different site along the fistula until the entire length of the fistula has been used. Patients report higher levels of satisfaction with the buttonhole technique, citing decreased pain, decreased oozing, and the perception of easier cannulation by HD nurses.14 However, the buttonhole technique also appears to confer a higher risk of vascular access-related bloodstream infection when compared with the rope-ladder technique.13,15,16
The buttonhole technique is hypothesized to increase infection risk due to the repeated use of the same site for needle entry. Skin flora, including CoNS, may colonize the scab that forms after dialysis access. If proper sterilization techniques are not rigorously followed, the bacteria colonizing the scab and adjacent skin may be introduced into a patient’s bloodstream during needle puncture. Loss of skin integrity due to frequent cannulation of the same site may also contribute to this increased infection risk. It is relevant to recall that our patient received HD 5 times weekly using the buttonhole technique. The use of the buttonhole technique, frequency of his HD sessions, unclear sterilization methods, and immune dysfunction related to his uncontrolled T2DM and renal disease all likely contributed to our patient’s bacteremia.
Using topical mupirocin for prophylaxis at the intended buttonhole puncture site has shown promising results in decreasing rates of S aureus bacteremia.17 It is unclear whether this intervention also would be effective against S lugdunensis. Increasing rates of mupirocin resistance have been reported among S lugdunensis isolates in dialysis settings, but further research in this area is warranted.18
There are no established treatment guidelines for S lugdunensis infections. In vitro studies suggest that S lugdunensis is susceptible to a wide variety of antibiotics. The mecA gene is a major determinant of methicillin resistance that is commonly observed among CoNS but is uncommonly seen with S lugdunensis.5 In a study by Tan and colleagues of 106 S lugdunensis isolates, they found that only 5 (4.7%) were mecA positive.19
Vancomycin is generally reasonable for empiric antibiotic coverage of staphylococci while speciation is pending. However, if S lugdunensis is isolated, its favorable susceptibility pattern typically allows for de-escalation to an antistaphylococcal β-lactam, such as oxacillin or nafcillin. In cases of bloodstream infections caused by methicillin-sensitive S aureus, treatment with a β-lactam has demonstrated superiority over vancomycin due to the lower rates of treatment failure and mortality with β-lactams.20,21 It is unknown whether β-lactams is superior for treating bacteremia with methicillin-sensitive S lugdunensis.
Our patient’s isolate of S lugdunensis was pansensitive to all antibiotics tested, including penicillin. These susceptibility data were used to guide the de-escalation of his empiric vancomycin and ceftriaxone to oxacillin on hospital day 1.
Due to their virulence, bloodstream infections caused by S aureus and S lugdunensis often require more than timely antimicrobial treatment to ensure eradication. Consultation with an infectious disease specialist to manage patients with S aureus bacteremia has been proven to reduce mortality.25 A similar mortality benefit is seen when infectious disease specialists are consulted for S lugdunensis bacteremia.26 This mortality benefit is likely explained by S lugdunensis’ propensity to cause aggressive, metastatic infections. In such cases, infectious disease consultants may recommend additional imaging (eg, transthoracic echocardiogram) to evaluate for occult sources of infection, advocate for appropriate source control, and guide the selection of an appropriate antibiotic course to ensure resolution of the bacteremia.
Conclusions
S lugdunensis is an increasingly recognized cause of nosocomial bloodstream infections. Given the commonalities in virulence that S lugdunensis shares with S aureus, treatment of bacteremia caused by either species should follow similar management principles: prompt initiation of IV antistaphylococcal therapy, a thorough evaluation for the source(s) of bacteremia as well as metastatic complications, and consultation with an infectious disease specialist. This case report also highlights the importance of considering a patient’s AVF as a potential source for infection even in the absence of localized signs of infection. The buttonhole method of AVF cannulation was thought to be a major contributor to the development and persistence of our patient’s bacteremia. This risk should be discussed with patients using a shared decision-making approach when developing a dialysis treatment plan.
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
1. Huebner J, Goldmann DA. Coagulase-negative staphylococci: role as pathogens. Annu Rev Med. 1999;50(1):223-236. doi:10.1146/annurev.med.50.1.223
2. Beekmann SE, Diekema DJ, Doern GV. Determining the clinical significance of coagulase-negative staphylococci isolated from blood cultures. Infect Control Hosp Epidemiol. 2005;26(6):559-566. doi:10.1086/502584
3. Arrecubieta C, Toba FA, von Bayern M, et al. SdrF, a Staphylococcus epidermidis surface protein, contributes to the initiation of ventricular assist device driveline–related infections. PLoS Pathog. 2009;5(5):e1000411. doi.10.1371/journal.ppat.1000411
4. Freney J, Brun Y, Bes M, et al. Staphylococcus lugdunensis sp. nov. and Staphylococcus schleiferi sp. nov., two species from human clinical specimens. Int J Syst Bacteriol. 1988;38(2):168-172. doi:10.1099/00207713-38-2-168
5. Frank KL, del Pozo JL, Patel R. From clinical microbiology to infection pathogenesis: how daring to be different works for Staphylococcus lugdunensis. Clin Microbiol Rev. 2008;21(1):111-133. doi:10.1128/CMR.00036-07
6. Anguera I, Del Río A, Miró JM; Hospital Clinic Endocarditis Study Group. Staphylococcus lugdunensis infective endocarditis: description of 10 cases and analysis of native valve, prosthetic valve, and pacemaker lead endocarditis clinical profiles. Heart. 2005;91(2):e10. doi:10.1136/hrt.2004.040659
7. Pareja J, Gupta K, Koziel H. The toxic shock syndrome and Staphylococcus lugdunensis bacteremia. Ann Intern Med. 1998;128(7):603-604. doi:10.7326/0003-4819-128-7-199804010-00029
8. Woznowski M, Quack I, Bölke E, et al. Fulminant Staphylococcus lugdunensis septicaemia following a pelvic varicella-zoster virus infection in an immune-deficient patient: a case report. Eur J Med Res. 201;15(9):410-414. doi:10.1186/2047-783x-15-9-410
9. Mallappallil M, Salifu M, Woredekal Y, et al. Staphylococcus lugdunensis bacteremia in hemodialysis patients. Int J Microbiol Res. 2012;4(2):178-181. doi:10.9735/0975-5276.4.2.178-181
10. Shuttleworth R, Colby W. Staphylococcus lugdunensis endocarditis. J Clin Microbiol. 1992;30(8):5. doi:10.1128/jcm.30.8.1948-1952.1992
11. Conner RC, Byrnes TJ, Clough LA, Myers JP. Staphylococcus lugdunensis tricuspid valve endocarditis associated with home hemodialysis therapy: report of a case and review of the literature. Infect Dis Clin Pract. 2012;20(3):182-183. doi:1097/IPC.0b013e318245d4f1
12. Kamaraju S, Nelson K, Williams D, Ayenew W, Modi K. Staphylococcus lugdunensis pulmonary valve endocarditis in a patient on chronic hemodialysis. Am J Nephrol. 1999;19(5):605-608. doi:1097/IPC.0b013e318245d4f1
13. Lok C, Sontrop J, Faratro R, Chan C, Zimmerman DL. Frequent hemodialysis fistula infectious complications. Nephron Extra. 2014;4(3):159-167. doi:10.1159/000366477
14. Hashmi A, Cheema MQ, Moss AH. Hemodialysis patients’ experience with and attitudes toward the buttonhole technique for arteriovenous fistula cannulation. Clin Nephrol. 2010;74(5):346-350. doi:10.5414/cnp74346
15. Lyman M, Nguyen DB, Shugart A, Gruhler H, Lines C, Patel PR. Risk of vascular access infection associated with buttonhole cannulation of fistulas: data from the National Healthcare Safety Network. Am J Kidney Dis. 2020;76(1):82-89. doi:10.1053/j.ajkd.2019.11.006
16. MacRae JM, Ahmed SB, Atkar R, Hemmelgarn BR. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7(10):1632-1638. doi:10.2215/CJN.02730312
17. Nesrallah GE, Cuerden M, Wong JHS, Pierratos A. Staphylococcus aureus bacteremia and buttonhole cannulation: long-term safety and efficacy of mupirocin prophylaxis. Clin J Am Soc Nephrol. 2010;5(6):1047-1053. doi:10.2215/CJN.00280110
18. Ho PL, Liu MCJ, Chow KH, et al. Emergence of ileS2 -carrying, multidrug-resistant plasmids in Staphylococcus lugdunensis. Antimicrob Agents Chemother. 2016;60(10):6411-6414. doi:10.1128/AAC.00948-16
19. Tan TY, Ng SY, He J. Microbiological characteristics, presumptive identification, and antibiotic susceptibilities of Staphylococcus lugdunensis. J Clin Microbiol. 2008;46(7):2393-2395. doi:10.1128/JCM.00740-08
20. Chang FY, Peacock JE, Musher DM, et al. Staphylococcus aureus bacteremia: recurrence and the impact of antibiotic treatment in a prospective multicenter study. Medicine (Baltimore). 2003;82(5):333-339. doi:10.1097/01.md.0000091184.93122.09
21. Shurland S, Zhan M, Bradham DD, Roghmann MC. Comparison of mortality risk associated with bacteremia due to methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Infect Control Hosp Epidemiol. 2007;28(3):273-279. doi:10.1086/512627
22. Levine DP, Fromm BS, Reddy BR. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115(9):674. doi:10.7326/0003-4819-115-9-674
23. Fowler VG, Karchmer AW, Tally FP, et al; S. aureus Endocarditis and Bacteremia Study Group. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. 2006;355(7):653-665 . doi:10.1056/NEJMoa053783
24. Duhon B, Dallas S, Velasquez ST, Hand E. Staphylococcus lugdunensis bacteremia and endocarditis treated with cefazolin and rifampin. Am J Health Syst Pharm. 2015;72(13):1114-1118. doi:10.2146/ajhp140498
25. Lahey T, Shah R, Gittzus J, Schwartzman J, Kirkland K. Infectious diseases consultation lowers mortality from Staphylococcus aureus bacteremia. Medicine (Baltimore). 2009;88(5):263-267. doi:10.1097/MD.0b013e3181b8fccb
26. Forsblom E, Högnäs E, Syrjänen J, Järvinen A. Infectious diseases specialist consultation in Staphylococcus lugdunensis bacteremia. PLoS ONE. 2021;16(10):e0258511. doi:10.1371/journal.pone.0258511
Antimicrobial resistance requires a manifold response
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
Lack of food for thought: Starve a bacterium, feed an infection
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
, according to a study published online today in Pediatrics .
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD, with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Seizures started on day 1 of life
The babies began to seize from the first day of life. They had profound low tone (hypotonia) in their clinical exam, Dr. Benny explained.
“We had absolutely no good explanation for the early seizures and the degree of brain injury we saw,” Dr. Duara said.
Dr. Benny said that as their bodies grew, they had very small head circumference. Unlike some babies born with the Zika virus, these babies were not microcephalic at birth. Brain imaging on the two babies indicated significant brain atrophy, and neurodevelopment exams showed significant delay.
Discussions began with the center’s multidisciplinary team including neurologists, pathologists, neuroradiologists, and obstetricians who cared for both the mothers and the babies.
The experts examined the placentas and found some characteristic COVID changes and presence of the COVID virus. This was accompanied by increased markers for inflammation and a severe reduction in a hormone critical for placental health and brain development.
Examining the infant’s autopsy findings further raised suspicions of maternal transmission, something that had not been documented before.
Coauthor Ali G. Saad, MD, pediatric and perinatal pathology director at Miami, said, “I have seen literally thousands of brains in autopsies over the last 14 years, and this was the most dramatic case of leukoencephalopathy or loss of white matter in a patient with no significant reason. That’s what triggered the investigation.”
Mothers had very different presentations
Coauthor Michael J. Paidas, MD, with the department of obstetrics, gynecology, and reproductive sciences at Miami, pointed out that the circumstances of the two mothers, who were in their 20s, were very different.
One mother delivered at 32 weeks and had a very severe COVID presentation and spent a month in the intensive care unit. The team decided to deliver the child to save the mother, Dr. Paidas said.
In contrast, the other mother had asymptomatic COVID infection in the second trimester and delivered at full term.
He said one of the early suspicions in the babies’ presentations was hypoxic ischemic encephalopathy. “But it wasn’t lack of blood flow to the placenta that caused this,” he said. “As best we can tell, it was the viral infection.”
Instances are rare
The researchers emphasized that these instances are rare and have not been seen before or since the period of this study to their knowledge.
Dr. Duara said, “This is something we want to alert the medical community to more than the general public. We do not want the lay public to be panicked. We’re trying to understand what made these two pregnancies different, so we can direct research towards protecting vulnerable babies.”
Previous data have indicated a relatively benign status in infants who test negative for the COVID virus after birth. Dr. Benny added that COVID vaccination has been found safe in pregnancy and both vaccination and breastfeeding can help passage of antibodies to the infant and help protect the baby. Because these cases happened in the early days of the pandemic, no vaccines were available.
Dr. Paidas received funding from BioIncept to study hypoxic-ischemic encephalopathy with Preimplantation Factor, is a scientific advisory board member, and has stock options. Dr. Paidas and coauthor Dr. Jayakumar are coinventors of SPIKENET, University of Miami, patent pending 2023. The other authors have no conflicts of interest to disclose.
FROM PEDIATRICS
Likely cause of mysterious hepatitis outbreak in children identified
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
Coinfection with AAV2 and a human adenovirus (HAdV), in particular, appears to leave some children more vulnerable to this acute hepatitis of unknown origin, researchers reported in three studies published online in Nature. Coinfection with Epstein-Barr virus (EBV), herpes, and enterovirus also were found. Adeno-associated viruses are not considered pathogenic on their own and require a “helper” virus for productive infection.
“I am quite confident that we have identified the key viruses involved because we used a comprehensive metagenomic sequencing approach to look for potential infections from any virus or non-viral pathogen,” Charles Chiu, MD, PhD, senior author and professor of laboratory medicine and medicine/infectious diseases at the University of California, San Francisco, said in an interview.
Dr. Chiu and colleagues propose that lockdowns and social isolation during the COVID-19 pandemic left more children susceptible. A major aspect of immunity in childhood is the adaptive immune response – both cell-mediated and humoral – shaped in part by exposure to viruses and other pathogens early in life, Dr. Chiu said.
“Due to COVID-19, a large population of children did not experience this, so it is possible once restrictions were lifted, they were suddenly exposed over a short period of time to multiple viruses that, in a poorly trained immune system, would have increased their risk of developing severe disease,” he said.
This theory has been popular, especially because cases of unexplained acute hepatitis peaked during the height of the COVID-19 pandemic when isolation was common, William F. Balistreri, MD, who was not affiliated with the study, told this news organization. Dr. Balistreri is professor of pediatrics and director emeritus of the Pediatric Liver Care Center at Cincinnati Children’s Hospital Medical Center.
Identifying the culprits
Determining what factors might be involved was the main aim of the etiology study by Dr. Chiu and colleagues published online in Nature.
The journal simultaneously published a genomic study confirming the presence of AAV2 and other suspected viruses and a genomic and laboratory study further corroborating the results.
More than 1,000 children worldwide had been diagnosed with unexplained acute pediatric hepatitis as of August 2022. In the United States, there have been 358 cases, including 22 in which the child required a liver transplant and 13 in which the child died.
This new form of hepatitis, first detected in October 2021, does not fit into existing classifications of types A through E, so some researchers refer to the condition as acute non–A-E hepatitis of unknown etiology.
The investigators started with an important clue based on previous research: the role adenovirus might play. Dr. Chiu and colleagues assessed 27 blood, stool, and other samples from 16 affected children who each previously tested positive for adenoviruses. The researchers included cases of the condition identified up until May 22, 2022. The median age was 3 years, and approximately half were boys.
They compared viruses present in these children with those in 113 controls without the mysterious hepatitis. The control group consisted of 15 children who were hospitalized with a nonhepatitis inflammatory condition, 27 with a noninflammatory condition, 30 with acute hepatitis of known origin, 12 with acute gastroenteritis and an HAdV-positive stool sample, and 11 with acute gastroenteritis and an HAdV-negative stool sample, as well as 18 blood donors. The median age was 7 years.
The researchers assessed samples using multiple technologies, including metagenomic sequencing, tiling multiplex polymerase chain reaction (PCR) amplicon sequencing, metagenomic sequencing with probe capture viral enrichment, and virus-specific PCR. Many of these advanced techniques were not even available 5-10 years ago, Dr. Chiu said.
Key findings
Blood samples were available for 14 of the 16 children with acute hepatitis of unknown origin. Among this study group, AAV2 was found in 13 (93%). No other adeno-associated viruses were found. HAdV was detected in all 14 children: HAdV-41 in 11 children and HAdV-40, HAdV-2, and an untypeable strain in one child each. This finding was not intuitive because HAdVs are not commonly associated with hepatitis, according to the study.
AAV2 was much less common in the control group. For example, it was found in none of the children with hepatitis of known origin and in only four children (3.5%) with acute gastroenteritis and HAdV-positive stool. Of note, neither AAV2 nor HAdV-41 was detected among the 30 pediatric controls with acute hepatitis of defined etiology nor 42 of the hospitalized children without hepatitis, the researchers wrote.
In the search for other viruses in the study group, metagenomic sequencing detected EBV, also known as human herpesvirus (HHV)–4, in two children, cytomegalovirus (CMV) in one child, and HAdV type C in one child.
Analysis of whole blood revealed enterovirus A71 in one patient. HAdV type C also was detected in one child on the basis of a nasopharyngeal swab, and picobirnavirus was found in a stool sample from another patient.
Researchers conducted virus-specific PCR tests on both patient groups to identify additional viruses that may be associated with the unexplained acute hepatitis. EBV/HHV-4 was detected in 11 children (79%) in the study group vs. in 1 child (0.88%) in the control group. HHV-6 was detected in seven children (50%) in the study group, compared with one case in the control group. CMV was not detected in any of the children in the study group versus vs. two children (1.8%) in the control group.
“Although we found significant differences in the relative proportions of EBV and HHV-6 in cases compared to controls, we do not believe that these viruses are the primary cause of acute severe hepatitis,” the researchers wrote. The viral load of the two herpes viruses were very low, so the positive results could represent integrated proviral DNA rather than bona fide low-level herpesvirus. In addition, herpesvirus can be reactivated by an inflammatory condition.
“Nevertheless, it is striking that among the 16 cases (in the study group), dual, triple, or quadruple infections with AAV2, adenovirus, and one or both herpesviruses were detected in whole blood from at least 12 cases (75%),” the researchers wrote.
Management of suspected hepatitis
The study’s key messages for parents and health care providers “are awareness and reassurance,” Dr. Balistreri said in an interview.
Vigilance also is warranted if a child develops prodromal symptoms including respiratory and/or gastrointestinal signs such as nausea, vomiting, diarrhea, and abdomen pain, he said. If jaundice or scleral icterus is noted, then hepatitis should be suspected.
Some patients need hospitalization and quickly recover. In very rare instances, the inflammation may progress to liver failure and transplantation, Dr. Balistreri said.
“Reassurance is based on the good news that most children with acute hepatitis get better. If a case arises, it is good practice to keep the child well hydrated, offer a normal diet, and avoid medications that may be cleared by the liver,” Dr. Balistreri added.
“Of course, COVID-19 vaccination is strongly suggested,” he said.
Some existing treatments could help against unexplained acute hepatitis, Dr. Chiu said. “The findings suggest that antiviral therapy might be effective in these cases.”
Cidofovir can be effective against adenovirus, according to a report in The Lancet . Similarly, ganciclovir or valganciclovir may have activity against EBV/HHV-4 or HHV-6, Dr. Chiu said. “However, antiviral therapy is not available for AAV2.”
The three studies published in Nature “offer compelling evidence, from disparate centers, of a linkage of outbreak cases to infection by AAV2,” Dr. Balistreri said. The studies also suggest that liver injury was related to abnormal immune responses. This is an important clinical distinction, indicating a potential therapeutic approach to future cases – immunosuppression rather than anti-adenoviral agents, he said.
“We await further studies of this important concept,” Dr. Balistreri said.
Many unanswered questions remain about the condition’s etiology, he added. Is there a synergy or shared susceptibility related to SARS-CoV-2? Is the COVID-19 virus helping to trigger these infections, or does it increase the risk once infected? Also, are other epigenetic factors or viruses involved?
Moving forward
The next steps in the research could go beyond identifying presence of these different viruses and determining which one(s) are contributing the most to the acute pediatric hepatitis, Dr. Chiu said.
The researchers also would like to test early results from the United Kingdom that identified a potential association of acute severe hepatitis with the presence of human leukocyte antigen genotype DRB1*04:01, he added.
They also might investigate other unintended potential clinical consequences of the COVID-19 pandemic, including long COVID and resurgence of infections from other viruses, such as respiratory syncytial virus, influenza, and enterovirus D68.
The study was supported by the Centers for Disease Control and Prevention, the National Institutes of Health, the Department of Homeland Security, and other grants. Dr. Chiu is a founder of Delve Bio and on the scientific advisory board for Delve Bio, Mammoth Biosciences, BiomeSense, and Poppy Health. Dr. Balistreri had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM NATURE
Deadly bacteria in recalled eye drops can spread person-to-person
according to a new report.
Scientists are concerned that the once-rare treatment-resistant bacteria found in the eyedrops can spread person-to-person, posing a risk of becoming a recurrent problem in the United States, The New York Times reported.
In January, EzriCare and Delsam Pharma artificial tears and ointment products were recalled after being linked to the bacterium P. aeruginosa. The bacteria have caused at least 68 infections, including three deaths and at least eight cases of blindness. The eyedrops were imported to the United States from India, and many of the cases occurred after the bacteria spread person-to-person at a long-term care facility in Connecticut, according to the Times, which cited FDA and Centers for Disease Control and Prevention lead investigator Maroya Walters, PhD.
Dr. Walters said the cases that caused death or blindness were traced to the EzriCare artificial tears product.
“It’s very hard to get rid of,” University of North Carolina at Chapel Hill infectious disease specialist David van Duin, MD, PhD, told the Times, noting that the bacteria cling to sink drains, water faucets, and other moist places.
The FDA said it had halted the import of the recalled products and has since visited the plant in India where they were made, which is owned by Global Pharma Healthcare. In a citation to the company dated March 2, the FDA listed nearly a dozen problems, such as dirty equipment and the absence of safety procedures and tests.
A version of this article originally appeared on WebMD.com.
according to a new report.
Scientists are concerned that the once-rare treatment-resistant bacteria found in the eyedrops can spread person-to-person, posing a risk of becoming a recurrent problem in the United States, The New York Times reported.
In January, EzriCare and Delsam Pharma artificial tears and ointment products were recalled after being linked to the bacterium P. aeruginosa. The bacteria have caused at least 68 infections, including three deaths and at least eight cases of blindness. The eyedrops were imported to the United States from India, and many of the cases occurred after the bacteria spread person-to-person at a long-term care facility in Connecticut, according to the Times, which cited FDA and Centers for Disease Control and Prevention lead investigator Maroya Walters, PhD.
Dr. Walters said the cases that caused death or blindness were traced to the EzriCare artificial tears product.
“It’s very hard to get rid of,” University of North Carolina at Chapel Hill infectious disease specialist David van Duin, MD, PhD, told the Times, noting that the bacteria cling to sink drains, water faucets, and other moist places.
The FDA said it had halted the import of the recalled products and has since visited the plant in India where they were made, which is owned by Global Pharma Healthcare. In a citation to the company dated March 2, the FDA listed nearly a dozen problems, such as dirty equipment and the absence of safety procedures and tests.
A version of this article originally appeared on WebMD.com.
according to a new report.
Scientists are concerned that the once-rare treatment-resistant bacteria found in the eyedrops can spread person-to-person, posing a risk of becoming a recurrent problem in the United States, The New York Times reported.
In January, EzriCare and Delsam Pharma artificial tears and ointment products were recalled after being linked to the bacterium P. aeruginosa. The bacteria have caused at least 68 infections, including three deaths and at least eight cases of blindness. The eyedrops were imported to the United States from India, and many of the cases occurred after the bacteria spread person-to-person at a long-term care facility in Connecticut, according to the Times, which cited FDA and Centers for Disease Control and Prevention lead investigator Maroya Walters, PhD.
Dr. Walters said the cases that caused death or blindness were traced to the EzriCare artificial tears product.
“It’s very hard to get rid of,” University of North Carolina at Chapel Hill infectious disease specialist David van Duin, MD, PhD, told the Times, noting that the bacteria cling to sink drains, water faucets, and other moist places.
The FDA said it had halted the import of the recalled products and has since visited the plant in India where they were made, which is owned by Global Pharma Healthcare. In a citation to the company dated March 2, the FDA listed nearly a dozen problems, such as dirty equipment and the absence of safety procedures and tests.
A version of this article originally appeared on WebMD.com.
Mpox (Monkeypox) Clinical Pearls
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13
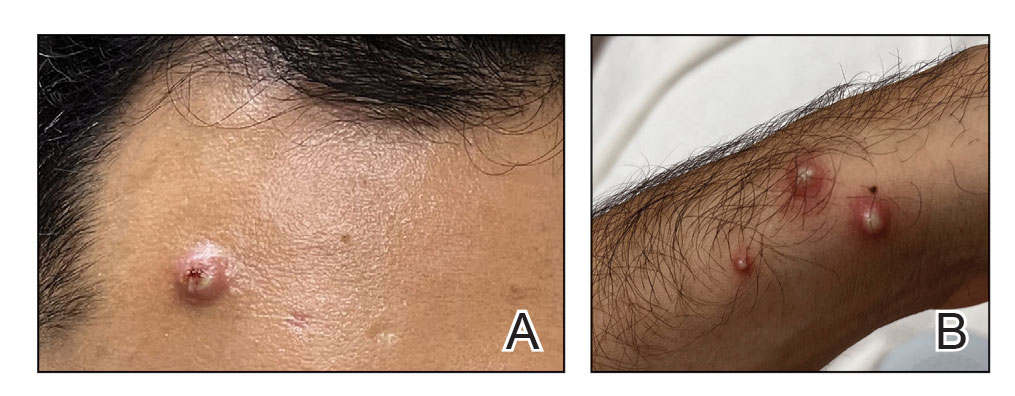
A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
The 2022 mpox (monkeypox) virus outbreak represents the latest example of how infectious diseases with previously limited reach can spread in a globalized society. More than 86,000 cases have been reported worldwide, with more than 30,000 cases in the United States as of March 15, 2023.1 Herein, we summarize the key features of mpox infection for the dermatologist.
Mpox Transmission
The mpox virus is a double-stranded DNA virus of the Orthopoxvirus genus and Poxviridae family.2,3 There are 2 types of the mpox virus: clade I (formerly the Congo Basin clade) and clade II (formerly the West African clade). Clade I causes more severe disease (10% mortality rate), while clade II is associated with lower mortality (1%–3%) and has been split into subclades of IIa (exhibits zoonotic transmission) and IIb (exhibits human-to-human spread).3,4 The current outbreak is caused by clade IIb, and patients typically have no travel history to classic endemic regions.5,6
In endemic countries, mpox transmission is zoonotic from small forest animals. In nonendemic countries, sporadic cases rarely have been reported, including a cluster in the United States in 2003 related to pet prairie dogs. In stark contrast, human-to-human transmission is occurring in the current epidemic mainly via intimate skin-to-skin contact and possibly via sexual fluids, meeting the criteria for a sexually transmitted infection. However, nonsexual transmission does still occur, though it is less common.7 Many of the reported cases so far are in young to middle-aged men who have sex with men (MSM).2,8 However, it is crucial to understand that mpox is not exclusive to the MSM population; the virus has been transmitted to heterosexual males, females, children, and even household pets of infected individuals.2,9,10 Labeling mpox as exclusive to the MSM community is both inaccurate and inappropriately stigmatizing.
Cutaneous Presentation and Diagnosis of Mpox
Mpox has an incubation time of approximately 9 days (range, 7–21 days), after which affected persons develop macular lesions that evolve over 2 to 4 weeks into papules, vesicles, and deep-seated pustules before crusting over and resolving with possible residual scarring.2,3,5,9,11,12 Palmoplantar involvement is a key feature.11 Although in some cases there will be multiple lesions with centrifugal progression, the lesions also may be few in number, with some patients presenting with a single lesion in the anogenital region or on the face, hand, or foot (Figure).6,9 Systemic symptoms such as prodromal fever, lymphadenopathy, and headache are common but not universal.9,13 Potential complications include penile edema, proctitis, bacterial superinfection, tonsillitis, conjunctivitis, encephalitis, and pneumonia.5,9,13

A high index of suspicion is needed to diagnose mpox infection. The differential diagnosis includes smallpox; varicella-zoster virus (primary or reactivation); secondary syphilis; measles; herpes simplex virus; molluscum contagiosum; hand, foot, and mouth disease; and disseminated gonococcal infection.2,3 For lesions confined to the genital area, sexually transmitted infections (eg, chancroid, lymphogranuloma venereum) as well as non–sexually related acute genital ulcers (Lipschütz ulcers) should be considered.2
Certain clinical features may help in distinguishing mpox from other diseases. Mpox exhibits synchronous progression and centrifugal distribution when multiple lesions are present; in contrast, the lesions of primary varicella (chickenpox) appear in multiple different stages, and those of localized herpes zoster (shingles) exhibit a dermatomal distribution. When these features are present, mpox causes a greater degree of lymphadenopathy and systemic symptoms than primary varicella.3Clinical diagnosis of mpox is more than 90% sensitive but only 9% to 26% specific.3 To confirm the diagnosis, a viral swab vigorously obtained from active skin lesions should be sent in viral transport media for mpox DNA-specific polymerase chain reaction testing, which is available from major laboratories.2,3 Other supportive tests include serum studies for anti–mpox virus immunoglobulins and immunohistochemical staining for viral antigens on skin biopsy specimens.2 When evaluating suspected and confirmed mpox cases, dermatologists should wear a gown, gloves, a fitted N95 mask, and eye protection to prevent infection.5
Treating Mpox
Symptomatic mpox infection can last for up to 2 to 5 weeks.3 The patient is no longer infectious once the lesions have crusted over.3,11 The majority of cases require supportive care only.2,3,5,14 However, mpox remains a potentially fatal disease, with 38 deaths to date in the current outbreak.1 High-risk populations include children younger than 8 years, pregnant women, and individuals who are immunocompromised.15 Tecovirimat, an antiviral medication approved by the US Food and Drug Administration (FDA) for smallpox, is available via the expanded access Investigational New Drug (EA-IND) protocol to treat severe mpox cases but is not widely available in the United States.6,16-18 Brincidofovir, a prodrug of the antiviral cidofovir, possesses single-patient emergency use Investigational New Drug (e-IND) status for treatment of mpox but also is not widely available in the United States.17 Intravenous vaccinia immune globulin is under consideration for high-risk individuals, but little is known regarding its efficacy against mpox.5,16,17
Two smallpox vaccines—JYNNEOS (Bavarian Nordic) and ACAM2000 (Emergent Bio Solutions)—are available for both preexposure and postexposure prophylaxis against mpox virus.19 At this time, only JYNNEOS is FDA approved for the prevention of mpox; ACAM2000 can be used against mpox under the FDA’s EA-IND protocol, which involves additional requirements, including informed consent from the patient.20 ACAM2000 is a live, replication-competent vaccine that carries a warning of increased risk for side effects in patients with cardiac disease, pregnancy, immunocompromise, and a history or presence of eczema and other skin conditions.3,21,22 JYNNEOS is a live but replication-deficient virus and therefore does not carry these warnings.3,21,22
Final Thoughts
Mpox is no longer an obscure illness occurring in limited geographic areas. Dermatologists must remain highly vigilant when evaluating any patient for new-onset vesicular or pustular eruptions to combat this ongoing public health threat. This issue of Cutis® also features a thorough mpox update on the clinical presentation, vaccine guidance, and management.23
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
- Centers for Disease Control and Prevention. Mpox: 2022 Outbreak Cases and Data. Updated March 15, 2023. Accessed March 121, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/
- Srivastava G. Human monkeypox disease [published online August 10, 2022]. Clin Dermatol. doi:10.1016/j.clindermatol.2022.08.009
- Bryer J, Freeman EE, Rosenbach M. Monkeypox emerges on a global scale: a historical review and dermatologic primer [published online July 8, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.07.007
- Americo JL, Earl PL, Moss B. Virulence differences of mpox (monkeypox) virus clades I, IIa, and IIb.1 in a small animal model. Proc Natl Acad Sci U S A. 2023;120:E2220415120. doi:10.1073 /pnas.2220415120
- Guarner J, Del Rio C, Malani PN. Monkeypox in 2022—what clinicians need to know. JAMA. 2022;328:139-140. doi:10.1001/jama.2022.10802
- Looi MK. Monkeypox: what we know about the 2022 outbreak so far [published online August 23, 2022]. BMJ. doi:10.1136/bmj.o2058
- Allan-Blitz LT, Gandhi M, Adamson P, et al. A position statement on mpox as a sexually transmitted disease [published online December 22, 2022]. Clin Infect Dis. doi:10.1093/cid/ciac960
- Cabanillas B, Murdaca G, Guemari A, et al. A compilation answering 50 questions on monkeypox virus and the current monkeypox outbreak. Allergy. 2023;78:639-662. doi:10.1111/all.15633
- Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study [published online August 8, 2022]. Lancet. doi:10.1016/S0140-6736(22)01436-2
- Seang S, Burrel S, Todesco E, et al. Evidence of human-to-dog transmission of monkeypox virus. Lancet. 2022;400:658-659. doi:10.1016 /s0140-6736(22)01487-8
- Ramdass P, Mullick S, Farber HF. Viral skin diseases. Prim Care. 2015;42:517-67. doi:10.1016/j.pop.2015.08.006
- Centers for Disease Control and Prevention. Mpox: Clinical Recognition. Updated August 23, 2022. Accessed March 21, 2023. https://www.cdc .gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Mpox Cases by Age and Gender, Race/Ethnicity, and Symptoms. Centers for Disease Control and Prevention. Updated March 15, 2023. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox /response/2022/demographics.html
- Kawsar A, Hussain K, Roberts N. The return of monkeypox: key pointers for dermatologists [published online July 29, 2022]. Clin Exp Dermatol. doi:10.1111/ced.15357
- Khanna U, Bishnoi A, Vinay K. Current outbreak of monkeypox— essentials for the dermatologist [published online June 23, 2022]. J Am Acad Dermatol. doi:10.1016/j.jaad.2022.06.1170
- Fox T, Gould S, Princy N, et al. Therapeutics for treating mpox in humans. Cochrane Database Syst Rev. 2023;3:CD015769. doi:10.1002/14651858 .CD015769
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 24, 2023. https://www.cdc.gov/poxvirus/mpox/clinicians /treatment.html#anchor_1666886364947
- Centers for Disease Control and Prevention. Guidance for tecovirimat use. Updated February 23, 2023. Accessed March 24, 2023. https://www .cdc.gov/poxvirus/mpox/clinicians/Tecovirimat.html
- Interim Clinical Considerations for Use of JYNNEOS and ACAM2000 Vaccines During the 2022 U.S. Monkeypox Outbreak. Centers for Disease Control and Prevention. Updated October 19, 2022. Accessed March 21, 2023. https://www.cdc.gov/poxvirus/monkeypox/health-departments/vaccine-considerations.html
- Key Facts About Vaccines to Prevent Monkeypox Disease. US Food and Drug Administration. Updated August 18, 2022. Accessed March 21, 2023. https://www.fda.gov/vaccines-blood-biologics/vaccines/key-facts-aboutvaccines-prevent-monkeypox-disease
- Smallpox: Vaccines. Centers for Disease Control and Prevention. Updated August 8, 2022. Accessed March 21, 2023. https://www.cdc.gov/smallpox/clinicians/vaccines.html
- ACAM2000. Package insert. Emergent Product Development Gaithersburg Inc; 2019.
- Cices A, Prasad S, Akselrad M, et al. Mpox update: clinical presentation, vaccination guidance, and management. Cutis. 2023;111:197-202. doi:10.12788/cutis.0745
Mpox Update: Clinical Presentation, Vaccination Guidance, and Management
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19
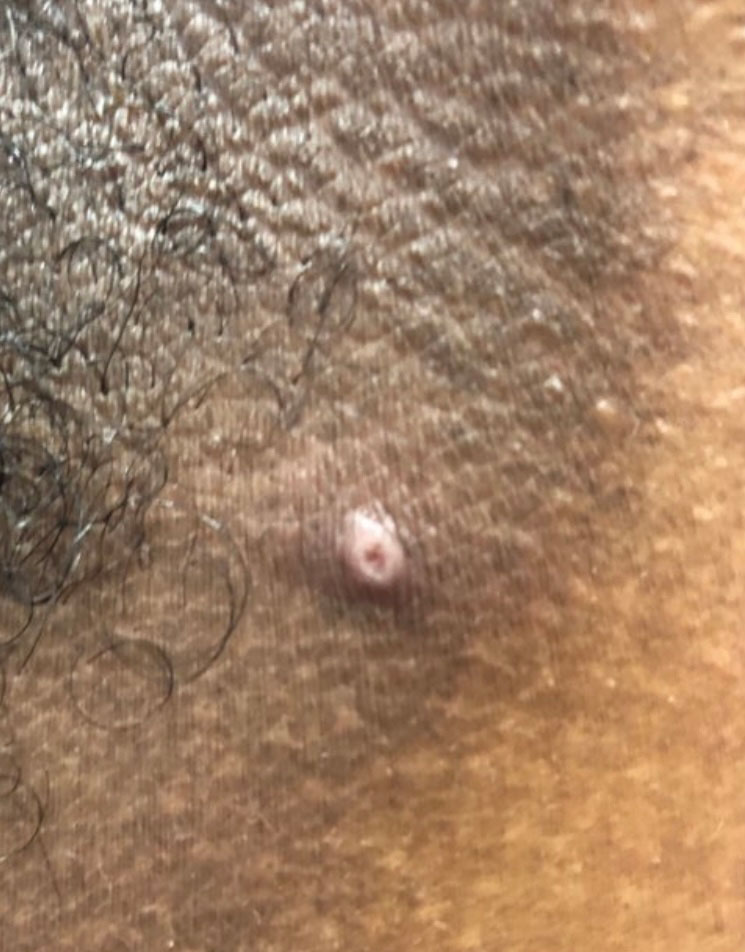
Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
The mpox (monkeypox) virus is a zoonotic orthopox DNA virus that results in a smallpoxlike illness.1 Vaccination against smallpox protects against other orthopox infections, including mpox; however, unlike smallpox, mpox is notable for a variety of not-yet-confirmed animal reservoirs.2 Mpox was first identified in Denmark in 1959 among nonhuman primates imported from Singapore, and the first case of human infection was diagnosed in 1970 in a 9-month-old child in the Democratic Republic of Congo.3 Endemic regions of Africa have had sporadic outbreaks with increasing frequency over time since the cessation of smallpox vaccination in 1980.2,4 Infections in nonendemic countries have occurred intermittently, including in 2003 in the Midwest United States. This outbreak was traced back to prairie dogs infected by exotic animals imported from the Republic of Ghana.5
Two genetic clades of mpox that differ in mortality rates have been identified: clade II (formerly the West African clade) generally is self-limited with an estimated mortality of 1% to 6%, whereas clade I (formerly the Congo Basin clade) is more transmissible, with a mortality of approximately 10%.2,6,7 Notably, as of May 2, 2022, all polymerase chain reaction–confirmed cases of mpox in nonendemic countries were identified as clade II.7 Following the continued international spread of mpox, the Director-General of the World Health Organization (WHO) declared the global outbreak a public health emergency of international concern on July 23, 2022.8 As of March 1, 2023, the Centers for Disease Control and Prevention (CDC) reports that there have been more than 86,000 cases of laboratory-confirmed mpox worldwide and 105 deaths, 89 of which occurred in nonendemic regions.9
Transmission of Mpox
In endemic countries, cases have been largely reported secondary to zoonotic spillover from contact with an infected animal.6 However, in nonendemic countries, mpox often results from human-to-human transmission, primarily via skin-to-skin contact with infected skin, but also may occur indirectly via contaminated fomites such as bedding or clothing, respiratory secretions, or vertical transmission.6,10 The indirect transmission of mpox via contaminated fomites is controversial, though some studies have shown the virus can survive on surfaces for up to 15 days.11 In the current outbreak, human-to-human transmission has been strongly associated with close contact during sexual activity, particularly among men who have sex with men (MSM), with notable physical concentration of initial lesions in the genital region.12 Anyone can acquire mpox—infections are not exclusive to MSM populations, and cases have been reported in all demographic groups, including women and children. It is important to avoid stigmatization of MSM to prevent the propagation of homophobia as well as a false sense of complacency in non-MSM populations.13
Clinical Presentation of Mpox
The incubation period of mpox has been reported to last up to 21 days and is posited to depend on the mode of transmission, with complex invasive exposures having a shorter duration of approximately 9 days compared to noninvasive exposures, which have a duration of approximately 13 days.14 In a recent report from the Netherlands, the average incubation time was 8.5 days in 18 men with exposure attributed to sexual encounters with men.12 Following the incubation period, mpox infection typically presents with nonspecific systemic symptoms such as fever, malaise, sore throat, cough, and headache for approximately 2 days, followed by painful generalized or localized lymphadenopathy 1 to 2 days prior to the onset of skin lesions.1,15 In a recent report from Portugal of more than 20 confirmed cases of mpox, approximately half of patients denied symptoms or had mild systemic symptoms, suggesting that many patients in the current outbreak do not endorse systemic symptoms.16
Classic cutaneous lesions are the hallmark feature of mpox.17 Over a period of 1 to 2 weeks, each lesion progresses through morphologic stages of macule, papule (Figure), vesicle, and pustule, which then crusts over, forming a scab that falls off after another 1 to 2 weeks and can result in dyspigmented or pitted scars.1,15 Lesions may be deep-seated or umbilicated; previously they were noted to typically start on the face and spread centrifugally, but recent cases have been notable for a predominance of anogenital lesions, often with the anogenital area as the sole or primary area of involvement.18 Given the high proportion of anogenital lesions in 2022, symptoms such as anogenital pain, tenesmus, and diarrhea are not uncommon.19 A recent study describing 528 international cases of mpox revealed that 95% of patients presented with a rash; nearly 75% had anogenital lesions; and 41%, 25%, and 10% had involvement of mucosae, the face, and palms/soles, respectively. More than half of patients had fewer than 10 lesions, and 10% presented with a single genital lesion.19

Given the recent predilection of lesions for the anogenital area, the differential diagnosis of mpox should include other common infections localized to these areas. Unlike herpes simplex and varicella-zoster infections, mpox does not exhibit the classic herpetiform clustering of vesicles, and unlike the painless chancre of syphilis, the lesions of mpox are exquisitely painful. Similar to chancroid, mpox presents with painful genital lesions and lymphadenopathy, and the umbilicated papules of molluscum could easily be confused with mpox lesions. Proctitis caused by many sexually transmitted infections (STIs), including chlamydia and gonorrhea, may be difficult to differentiate from proctitis symptoms of mpox. Co-infection with HIV and other STIs is common among patients developing mpox in 2022, which is not surprising given that the primary mechanism of transmission of mpox at this time is through sexual contact, and cases are more common in patients with multiple recent sexual partners.19 Considering these shared risk factors and similar presentation of multiple STIs, patients suspected of having an mpox infection should be tested for other STIs, including HIV.
Complications of Mpox
Although mpox generally is characterized by a mild disease course, there is concern for adverse outcomes, particularly in more vulnerable populations, including immunocompromised, pregnant, and pediatric populations. Complications of infection can include sepsis, encephalitis, bronchopneumonia, and ophthalmic complications that can result in loss of vision.6,17 The most common complications requiring hospitalization in a recent international report of 528 mpox cases were pain management, which was primarily due to severe anogenital pain, followed by soft-tissue superinfection, with other complications including severe pharyngitis limiting oral intake and infection control practices.19 In addition to severe rectal pain, proctitis and even rectal perforation have been reported.19,20
Vertical transmission has been described with devastating outcomes in a case series from the Democratic Republic of Congo, where 4 cases of mpox were identified in pregnant women; 3 of these pregnancies resulted in fetal demise.10 The only fetus to survive was born to a mother with mild infection. In comparison, 2 of 3 mothers with moderate to severe disease experienced spontaneous abortion in the first trimester, and 1 pregnancy ended due to intrauterine demise during the eighteenth week of gestation, likely a complication of mpox. These cases suggest that more severe disease may be linked to worse fetal outcomes.10 Further epidemiologic studies will be crucial, given the potential implications.
Diagnosis
When considering a diagnosis of mpox, clinicians should inquire about recent travel, living arrangements, sexual history, and recent sick contacts.6 A complete skin examination should include the oral and genital areas, given the high prevalence of lesions in these areas. A skin biopsy is not recommended for the diagnosis of mpox, as nonspecific viral changes cannot be differentiated from other viral exanthems, but it often is useful to rule out other differential diagnoses.21 Additionally, immunohistochemistry and electron microscopy can be utilized to aid in a histologic diagnosis of mpox.
Polymerase chain reaction detection of orthopox or mpox DNA is the gold standard for diagnosis.6 Two swabs should be collected from each lesion by swabbing vigorously using sterile swabs made of a synthetic material such as polyester, nylon, or Dacron and placed into a sterile container or viral transport medium.22 Some laboratories may have different instructions for collection of samples, so clinicians are advised to check for instructions from their local laboratory. Deroofing lesions prior to swabbing is not necessary, and specimens can include lesional material or crust. Collection of specimens from 2 to 3 lesions is recommended, preferably from different body areas or lesions with varying morphologies. Anal or rectal swabs can be considered in patients presenting with anal pain or proctitis with clinical suspicion for mpox based on history.19
Infection Prevention
Interim guidance from the WHO on November 16, 2022, reiterated the goal of outbreak control primarily via public health measures, which includes targeted use of vaccines for at-risk populations or postexposure prophylactic vaccination within 4 days, but heavily relies on surveillance and containment techniques, such as contact tracing with monitoring of contacts for onset of symptoms and isolation of cases through the complete infectious period.23 Patients are considered infectious from symptom onset until all cutaneous lesions are re-epithelized and should remain in isolation, including from household contacts and domestic and wildlife animals, for the duration of illness.24,25 Individuals exposed to humans or animals with confirmed mpox should be monitored for the development of symptoms for 21 days following last known exposure, regardless of vaccination status, and should be instructed to measure their temperature twice daily.26 Pets exposed to mpox should be isolated from other animals and humans for 21 days following last known contact.24 Vaccination strategies for preexposure and postexposure prophylaxis (PEP) are discussed below in further detail. Postinfection, the WHO suggests use of condoms for all oral, vaginal, and anal sexual activity for 12 weeks after recovery.7
Patients with suspected or confirmed mpox in a hospital should be in a single private room on special droplet and contact precautions.27 No special air handling or negative pressure isolation is needed unless the patient is undergoing an aerosol-generating procedure (eg, intubation, endoscopy, bronchoscopy). When hospitalized, patients should have a dedicated bathroom, if possible, and at-home patients should be isolated from household members until contagion risk resolves; this includes the use of a separate bathroom, when possible. Health care personnel entering the room of a patient should don appropriate personal protective equipment (PPE), including a disposable gown, gloves, eye protection, and N95 respirator or equivalent. Recommendations include standard practices for cleaning, with wet cleaning methods preferred over dry methods, using a disinfectant that covers emerging viral pathogens, and avoidance of shaking linens to prevent the spread of infectious particles.27 A variety of Environmental Protection Agency–registered wipes with virucidal activity against emerging viruses, including those with active ingredients such as quaternary ammonium, hydrogen peroxide, and hypochlorous acid, should be used for disinfecting surfaces.28
Vaccination
ACAM2000 (Emergent Bio Solutions) and JYNNEOS (Bavarian Nordic)(also known as Imvamune or Imvanex) are available in the United States for the prevention of mpox infection.29 ACAM2000, a second-generation, replication-competent, live smallpox vaccine administered as a single percutaneous injection, is contraindicated in immunocompromised populations, including patients with HIV or on immunosuppressive or biologic therapy, pregnant individuals, people with a history of atopic dermatitis or other exfoliative skin diseases with impaired barrier function, and patients with a history of cardiac disease due to the risk of myocarditis and pericarditis.30
JYNNEOS is a nonreplicating live vaccine approved by the US Food and Drug Administration (FDA) for the prevention of mpox in individuals older than 18 years administered as 2 subcutaneous doses 4 weeks apart. Patients are considered fully vaccinated 2 weeks after the second dose, and JYNNEOS is available to pediatric patients with a single patient expanded access use authorization from the FDA.29,30 More recently, the FDA issued an emergency use authorization (EUA) for administration of the vaccine to patients younger than 18 years who are at high risk of infection after exposure.31 More importantly, the FDA also issued an EUA for the intradermal administration of JYNNEOS at one-fifth of the subcutaneous dose to expand the current vaccine supply. This EUA is based on research by Frey et al,32 which showed that intradermal administration, even at a lower dose, elicited similar immune responses among study participants as the higher dose administered subcutaneously.
JYNNEOS is the preferred vaccine for the prevention of mpox because of its poor ability to replicate in human cells and resultant safety for use in populations that are immunocompromised, pregnant, or have skin barrier defects such as atopic dermatitis, without the risk of myocarditis or pericarditis. However, current supplies are limited. JYNNEOS was specifically studied in patients with atopic dermatitis and has been shown to be safe and effective in patients with a history of atopic dermatitis and active disease with a SCORAD (SCORing Atopic Dermatitis) score of 30 or lower.33 Of note, JYNNEOS is contraindicated in patients allergic to components of the vaccine, including egg, gentamicin, and ciprofloxacin. Although JYNNEOS is safe to administer to persons with immunocompromising conditions, the CDC reports that such persons might be at increased risk for severe disease if an occupational infection occurs, and in the setting of immunocompromise, such persons may be less likely to mount an effective response to vaccination. Therefore, the risk-benefit ratio should be considered to determine if an immunocompromised person should be vaccinated with JYNNEOS.30
The WHO and the CDC do not recommended mass vaccination of the general public for outbreaks of mpox in nonendemic countries, with immunization reserved for appropriate PEP and pre-exposure prophylaxis in intermediate- to high-risk individuals.23,26 The CDC recommends PEP vaccination for individuals with a high degree of exposure that includes unprotected contact of the skin or mucous membranes of an individual to the skin, lesions, body fluids, or contaminated fomites from a patient with mpox, as well as being within 6 feet of a patient during an aerosolization procedure without proper PPE. Following an intermediate degree of exposure, which includes being within 6 feet for 3 or more hours wearing at minimum a surgical mask or contact with fomites while wearing incomplete PPE, the CDC recommends monitoring and shared decision-making regarding risks and benefits of PEP vaccination. Monitoring without PEP is indicated for low and uncertain degrees of exposure, including entering a room without full PPE such as eye protection, regardless of the duration of contact.23,26
Postexposure prophylaxis vaccination should be administered within 4 days of a known high-level exposure to mpox to prevent infection.29 If administered within 4 to 14 days postexposure, vaccination may reduce disease severity but will not prevent infection.34
Pre-exposure prophylaxis is recommended for individuals at high risk for exposure to mpox, including health care workers such as laboratory personnel who handle mpox specimens and health care workers who administer ACAM2000 vaccinations or anticipate providing care for many patients with mpox.34
Management
Most cases of mpox are characterized by mild to moderate disease with a self-limited course. Most commonly, medical management of mpox involves supportive care such as fluid resuscitation, supplemental oxygen, and pain management.6 Treatment of superinfected skin lesions may require antibiotics. In the event of ophthalmologic involvement, patients should be referred to an ophthalmologist for further management.
Currently, there are no FDA-approved therapies for mpox; however, tecovirimat, cidofovir, brincidofovir, and vaccinia immune globulin intravenous are available under expanded access Investigational New Drug protocols.6,35 Human data for cidofovir, brincidofovir, and vaccinia immune globulin intravenous in the treatment of mpox are lacking, while cidofovir and brincidofovir have shown efficacy against orthopoxviruses in in vitro and animal studies, but are available therapeutic options.35
Tecovirimat is an antiviral that is FDA approved for smallpox with efficacy data against mpox in animal studies. It is the first-line treatment for patients with severe disease requiring hospitalization or 1 or more complications, including dehydration or secondary skin infections, as well as for populations at risk for severe disease, which includes immunocompromised patients, pediatric patients younger than 8 years, pregnant or breastfeeding individuals, or patients with a history of atopic dermatitis or active exfoliative skin conditions.36 In this current outbreak, both intravenous and oral tecovirimat are weight based in adult and pediatric patients for 14 days, with the intravenous form dosed every 12 hours by infusion over 6 hours, and the oral doses administered every 8 to 12 hours based on patient weight.37 Tecovirimat generally is well tolerated with mild side effects but is notably contraindicated in patients with severe renal impairment with a creatinine clearance less than 30 mL/min, and renal monitoring is indicated in pediatric patients younger than 2 years and in all patients receiving intravenous treatment.
Conclusion
Given that cutaneous lesions are the most specific presenting sign of mpox infection, dermatologists will play an integral role in identifying future cases and managing future outbreaks. Mpox should be considered in the differential diagnosis for all patients presenting with umbilicated or papulovesicular lesions, particularly in an anogenital distribution. The classic presentation of mpox may be more common among patients who are not considered high risk and have not been exposed via sexual activity. All patients with suspicious lesions should be managed following appropriate infection control precautions and should undergo molecular diagnostic assay of swabbed lesions to confirm the diagnosis. JYNNEOS is the only vaccine that is currently being distributed in the United States and is safe to administer to immunocompromised populations. The risks and benefits of vaccination should be considered on an individual basis between a patient and their provider. Taking into consideration that patients with atopic dermatitis are at risk for severe disease if infected with mpox, vaccination should be strongly encouraged if indicated based on patient risk factors. For atopic dermatitis patients treated with dupilumab, shared decision-making is essential given the FDA label, which recommends avoiding the use of live vaccines.38
The mpox epidemic occurring amidst the ongoing COVID-19 pandemic should serve as a wake-up call to the importance of pandemic preparedness and the global health response strategies in the modern era of globalization. Looking forward, widespread vaccination against mpox may be necessary to control the spread of the disease and to protect vulnerable populations, including pregnant individuals. In the current climate of hesitancy surrounding vaccines and the erosion of trust in public health agencies, it is incumbent upon health care providers to educate patients regarding the role of vaccines and public health measures to control this developing global health crisis.
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
- Di Giulio DB, Eckburg PB. Human monkeypox: an emerging zoonosis. Lancet Infect Dis. 2004;4:15-25. doi:10.1016/s1473-3099(03)00856-9
- Simpson K, Heymann D, Brown CS, et al. Human monkeypox—after 40 years, an unintended consequence of smallpox eradication. Vaccine. 2020;38:5077-5081. doi:10.1016/j.vaccine.2020.04.062
- Ladnyj ID, Ziegler P, Kima E. A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull World Health Organ. 1972;46:593-597.
- Alakunle EF, Okeke MI. Monkeypox virus: a neglected zoonotic pathogen spreads globally. Nat Rev Microbiol. 2022;20:507-508. doi:10.1038/s41579-022-00776-z
- Ligon BL. Monkeypox: a review of the history and emergence in the Western hemisphere. Semin Pediatr Infect Dis. 2004;15:280-287. doi:10.1053/j.spid.2004.09.001
- Titanji BK, Tegomoh B, Nematollahi S, et al. Monkeypox: a contemporary review for healthcare professionals. Open Forum Infect Dis. 2022;9:ofac310. doi:10.1093/ofid/ofac310
- Gigante CM, Korber B, Seabolt MH, et al. Multiple lineages of monkeypox virus detected in the United States, 2021-2022. Science. 2022;378:560-565. doi:10.1126/science.add4153
- World Health Organization. WHO Director-General’s statement at the press conference following IHR Emergency Committee regarding the multi-country outbreak of monkeypox—23 July 2022. July 23, 2022. Accessed March 10, 2023. https://www.who.int/director-general/speeches/detail/who-director-general-s-statement-on-the-press-conference-following-IHR-emergency-committee-regarding-the-multi--country-outbreak-of-monkeypox--23-july-2022
- Centers for Disease Control and Prevention. 2022 mpox outbreak global map. Updated March 1, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824-828. doi:10.1093/infdis/jix260
- Centers for Disease Control and Prevention. How to protect yourself. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/prevention/protect-yourself.html
- Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi:10.2807/1560-7917.Es.2022.27.24.2200448
- Treisman R. As monkeypox spreads, know the difference between warning and stigmatizing people. NPR. July 26, 2022. Accessed March 10, 2023. https://www.npr.org/2022/07/26/1113713684/monkeypox-stigma-gay-community
- Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773-780. doi:10.1086/505880
- Centers for Disease Control and Prevention. Clinical recognition. Updated August 23, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/clinical-recognition.html
- Alpalhão M, Frade JV, Sousa D, et al. Monkeypox: a new (sexuallytransmissible) epidemic? J Eur Acad Dermatol Venereol. 2022;36:e1016-e1017. doi:10.1111/jdv.18424
- Reynolds MG, McCollum AM, Nguete B, et al. Improving the care and treatment of monkeypox patients in low-resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017;9:380. doi:10.3390/v9120380
- Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764-769. doi:10.15585/mmwr.mm7123e1
- Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April-June 2022. N Engl J Med. 2022;387:679-691. doi:10.1056/NEJMoa2207323
- Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi:10.1136/bmj-2022-072410
- Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. doi:10.1111/j.0303-6987.2005.00254.x
- Centers for Disease Control and Prevention. Guidelines for collecting and handling of specimens for mpox testing. Updated September 20, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/prep-collection-specimens.html
- Vaccines and immunization for monkeypox: interim guidance, 16 November 2022. Accessed March 15, 2023. https://www.who.int/publications/i/item/WHO-MPX-Immunization
- Centers for Disease Control and Prevention. Pets in the home. Updated December 8, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/specific-settings/pets-in-homes.html
- Centers for Disease Control and Prevention. Isolation andprevention practices for people with monkeypox. Updated February 2, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
- Centers for Disease Control and Prevention. Monitoring people who have been exposed. Updated November 25, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/monitoring.html
- Centers for Disease Control and Prevention. Infection prevention and control of monkeypox in healthcare settings. Updated October 31, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/infection-control-healthcare.html
- United States Environmental Protection Agency. EPA releases list of disinfectants for emerging viral pathogens (EVPs) including monkeypox. May 26, 2022. Accessed March 10, 2023. https://www.epa.gov/pesticides/epa-releases-list-disinfectants-emerging-viral-pathogens-evps-including-monkeypox
- Centers for Disease Control and Prevention. Interim clinical considerations for use of JYNNEOS and ACAM2000 vaccines during the 2022 U.S. mpox outbreak. Updated October 19, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/considerations-for-monkeypox-vaccination.html
- Rao AK, Petersen BW, Whitehill F, et al. Use of JYNNEOS (smallpox and monkeypox vaccine, live, nonreplicating) for preexposure vaccination of persons at risk for occupational exposure to orthopoxviruses: recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:734-742. doi: http://dx.doi.org/10.15585/mmwr.mm7122e1
- US Food and Drug Administration. Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. August 9, 2022. Accessed March 10, 2023. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply#:~:text=Today%2C%20the%20U.S.%20Food%20and,high%20risk%20for%20monkeypox%20infection
- Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225-5234. doi:10.1016/j.vaccine.2015.06.075
- Greenberg RN, Hurley MY, Dinh DV, et al. A multicenter, open-label, controlled phase II study to evaluate safety and immunogenicity of MVA smallpox vaccine (IMVAMUNE) in 18-40 year old subjects with diagnosed atopic dermatitis. PLoS One. 2015;10:e0138348. doi:10.1371/journal.pone.0138348
- Centers for Disease Control and Prevention. Monkeypox and smallpox vaccine guidance. Accessed March 16, 2023. https://www.cdc.gov/poxvirus/mpox/interim-considerations/overview.html
- Centers for Disease Control and Prevention. Treatment information for healthcare professionals. Updated March 3, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- Centers for Disease Control and Prevention. Guidance for tecovirimat use: expanded access investigational new drug protocol during 2022 U.S. mpox outbreak. Updated February 23, 2023. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/clinicians/Tecovirimat.html
- Expanded access IND protocol: use of tecovirimat (TPOXX®) for treatment of human non-variola orthopoxvirus infections in adults and children. October 24, 2022. Accessed March 10, 2023. https://www.cdc.gov/poxvirus/monkeypox/pdf/tecovirimat-ind-protocol-cdc-irb.pdf
- Dupixent (dupilumab). Prescribing information. Regeneron Pharmaceuticals, Inc; 2017. Accessed March 10, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761055lbl.pdf
Practice Points
- Mpox (monkeypox) lesions typically present as well-circumscribed, painful, umbilicated papules, vesicles, or pustules, with recent cases having a predilection for an anogenital distribution accompanied by systemic viral symptoms.
- Health care workers treating suspected or confirmed cases of mpox should be familiar with current guidelines for controlling the spread of mpox, including proper personal protective equipment (gloves, disposable gowns, N95 or equivalent respirators, and eye protection) and indications for vaccination.