User login
High-dose prophylactic anticoagulation benefits patients with COVID-19 pneumonia
High-dose prophylactic anticoagulation or therapeutic anticoagulation reduced de novo thrombosis in patients with hypoxemic COVID-19 pneumonia, based on data from 334 adults.
Patients with hypoxemic COVID-19 pneumonia are at increased risk of thrombosis and anticoagulation-related bleeding, therefore data to identify the lowest effective anticoagulant dose are needed, wrote Vincent Labbé, MD, of Sorbonne University, Paris, and colleagues.
Previous studies of different anticoagulation strategies for noncritically ill and critically ill patients with COVID-19 pneumonia have shown contrasting results, but some institutions recommend a high-dose regimen in the wake of data showing macrovascular thrombosis in patients with COVID-19 who were treated with standard anticoagulation, the authors wrote.
However, no previously published studies have compared the effectiveness of the three anticoagulation strategies: high-dose prophylactic anticoagulation (HD-PA), standard dose prophylactic anticoagulation (SD-PA), and therapeutic anticoagulation (TA), they said.
In the open-label Anticoagulation COVID-19 (ANTICOVID) trial, published in JAMA Internal Medicine, the researchers identified consecutively hospitalized adults aged 18 years and older being treated for hypoxemic COVID-19 pneumonia in 23 centers in France between April 2021 and December 2021.
The patients were randomly assigned to SD-PA (116 patients), HD-PA (111 patients), and TA (112 patients) using low-molecular-weight heparin for 14 days, or until either hospital discharge or weaning from supplemental oxygen for 48 consecutive hours, whichever outcome occurred first. The HD-PA patients received two times the SD-PA dose. The mean age of the patients was 58.3 years, and approximately two-thirds were men; race and ethnicity data were not collected. Participants had no macrovascular thrombosis at the start of the study.
The primary outcomes were all-cause mortality and time to clinical improvement (defined as the time from randomization to a 2-point improvement on a 7-category respiratory function scale).
The secondary outcome was a combination of safety and efficacy at day 28 that included a composite of thrombosis (ischemic stroke, noncerebrovascular arterial thrombosis, deep venous thrombosis, pulmonary artery thrombosis, and central venous catheter–related deep venous thrombosis), major bleeding, or all-cause death.
For the primary outcome, results were similar among the groups; HD-PA had no significant benefit over SD-PA or TA. All-cause death rates for SD-PA, HD-PA, and TA patients were 14%, 12%, and 13%, respectively. The time to clinical improvement for the three groups was approximately 8 days, 9 days, and 8 days, respectively. Results for the primary outcome were consistent across all prespecified subgroups.
However, HD-PA was associated with a significant fourfold reduced risk of de novo thrombosis compared with SD-PA (5.5% vs. 20.2%) with no observed increase in major bleeding. TA was not associated with any significant improvement in primary or secondary outcomes compared with HD-PA or SD-PA.
The current study findings of no improvement in survival or disease resolution in patients with a higher anticoagulant dose reflects data from previous studies, the researchers wrote in their discussion. “Our study results together with those of previous RCTs support the premise that the role of microvascular thrombosis in worsening organ dysfunction may be narrower than estimated,” they said.
The findings were limited by several factors including the open-label design and the relatively small sample size, the lack of data on microvascular (vs. macrovascular) thrombosis at baseline, and the predominance of the Delta variant of COVID-19 among the study participants, which may have contributed to a lower mortality rate, the researchers noted.
However, given the significant reduction in de novo thrombosis, the results support the routine use of HD-PA in patients with severe hypoxemic COVID-19 pneumonia, they concluded.
Results inform current clinical practice
Over the course of the COVID-19 pandemic, “Patients hospitalized with COVID-19 manifested the highest risk for thromboembolic complications, especially patients in the intensive care setting,” and early reports suggested that standard prophylactic doses of anticoagulant therapy might be insufficient to prevent thrombotic events, Richard C. Becker, MD, of the University of Cincinnati, and Thomas L. Ortel, MD, of Duke University, Durham, N.C., wrote in an accompanying editorial.
“Although there have been several studies that have investigated the role of anticoagulant therapy in hospitalized patients with COVID-19, this is the first study that specifically compared a standard, prophylactic dose of low-molecular-weight heparin to a ‘high-dose’ prophylactic regimen and to a full therapeutic dose regimen,” Dr. Ortel said in an interview.
“Given the concerns about an increased thrombotic risk with prophylactic dose anticoagulation, and the potential bleeding risk associated with a full therapeutic dose of anticoagulation, this approach enabled the investigators to explore the efficacy and safety of an intermediate dose between these two extremes,” he said.
In the current study, , a finding that was not observed in other studies investigating anticoagulant therapy in hospitalized patients with severe COVID-19,” Dr. Ortel told this news organization. “Much initial concern about progression of disease in patients hospitalized with severe COVID-19 focused on the role of microvascular thrombosis, which appears to be less important in this process, or, alternatively, less responsive to anticoagulant therapy.”
The clinical takeaway from the study, Dr. Ortel said, is the decreased risk for venous thromboembolism with a high-dose prophylactic anticoagulation strategy compared with a standard-dose prophylactic regimen for patients hospitalized with hypoxemic COVID-19 pneumonia, “leading to an improved net clinical outcome.”
Looking ahead, “Additional research is needed to determine whether a higher dose of prophylactic anticoagulation would be beneficial for patients hospitalized with COVID-19 pneumonia who are not in an intensive care unit setting,” Dr. Ortel said. Studies are needed to determine whether therapeutic interventions are equally beneficial in patients with different coronavirus variants, since most patients in the current study were infected with the Delta variant, he added.
The study was supported by LEO Pharma. Dr. Labbé disclosed grants from LEO Pharma during the study and fees from AOP Health unrelated to the current study.
Dr. Becker disclosed personal fees from Novartis Data Safety Monitoring Board, Ionis Data Safety Monitoring Board, and Basking Biosciences Scientific Advisory Board unrelated to the current study. Dr. Ortel disclosed grants from the National Institutes of Health, Instrumentation Laboratory, Stago, and Siemens; contract fees from the Centers for Disease Control and Prevention; and honoraria from UpToDate unrelated to the current study.
A version of this article originally appeared on Medscape.com.
High-dose prophylactic anticoagulation or therapeutic anticoagulation reduced de novo thrombosis in patients with hypoxemic COVID-19 pneumonia, based on data from 334 adults.
Patients with hypoxemic COVID-19 pneumonia are at increased risk of thrombosis and anticoagulation-related bleeding, therefore data to identify the lowest effective anticoagulant dose are needed, wrote Vincent Labbé, MD, of Sorbonne University, Paris, and colleagues.
Previous studies of different anticoagulation strategies for noncritically ill and critically ill patients with COVID-19 pneumonia have shown contrasting results, but some institutions recommend a high-dose regimen in the wake of data showing macrovascular thrombosis in patients with COVID-19 who were treated with standard anticoagulation, the authors wrote.
However, no previously published studies have compared the effectiveness of the three anticoagulation strategies: high-dose prophylactic anticoagulation (HD-PA), standard dose prophylactic anticoagulation (SD-PA), and therapeutic anticoagulation (TA), they said.
In the open-label Anticoagulation COVID-19 (ANTICOVID) trial, published in JAMA Internal Medicine, the researchers identified consecutively hospitalized adults aged 18 years and older being treated for hypoxemic COVID-19 pneumonia in 23 centers in France between April 2021 and December 2021.
The patients were randomly assigned to SD-PA (116 patients), HD-PA (111 patients), and TA (112 patients) using low-molecular-weight heparin for 14 days, or until either hospital discharge or weaning from supplemental oxygen for 48 consecutive hours, whichever outcome occurred first. The HD-PA patients received two times the SD-PA dose. The mean age of the patients was 58.3 years, and approximately two-thirds were men; race and ethnicity data were not collected. Participants had no macrovascular thrombosis at the start of the study.
The primary outcomes were all-cause mortality and time to clinical improvement (defined as the time from randomization to a 2-point improvement on a 7-category respiratory function scale).
The secondary outcome was a combination of safety and efficacy at day 28 that included a composite of thrombosis (ischemic stroke, noncerebrovascular arterial thrombosis, deep venous thrombosis, pulmonary artery thrombosis, and central venous catheter–related deep venous thrombosis), major bleeding, or all-cause death.
For the primary outcome, results were similar among the groups; HD-PA had no significant benefit over SD-PA or TA. All-cause death rates for SD-PA, HD-PA, and TA patients were 14%, 12%, and 13%, respectively. The time to clinical improvement for the three groups was approximately 8 days, 9 days, and 8 days, respectively. Results for the primary outcome were consistent across all prespecified subgroups.
However, HD-PA was associated with a significant fourfold reduced risk of de novo thrombosis compared with SD-PA (5.5% vs. 20.2%) with no observed increase in major bleeding. TA was not associated with any significant improvement in primary or secondary outcomes compared with HD-PA or SD-PA.
The current study findings of no improvement in survival or disease resolution in patients with a higher anticoagulant dose reflects data from previous studies, the researchers wrote in their discussion. “Our study results together with those of previous RCTs support the premise that the role of microvascular thrombosis in worsening organ dysfunction may be narrower than estimated,” they said.
The findings were limited by several factors including the open-label design and the relatively small sample size, the lack of data on microvascular (vs. macrovascular) thrombosis at baseline, and the predominance of the Delta variant of COVID-19 among the study participants, which may have contributed to a lower mortality rate, the researchers noted.
However, given the significant reduction in de novo thrombosis, the results support the routine use of HD-PA in patients with severe hypoxemic COVID-19 pneumonia, they concluded.
Results inform current clinical practice
Over the course of the COVID-19 pandemic, “Patients hospitalized with COVID-19 manifested the highest risk for thromboembolic complications, especially patients in the intensive care setting,” and early reports suggested that standard prophylactic doses of anticoagulant therapy might be insufficient to prevent thrombotic events, Richard C. Becker, MD, of the University of Cincinnati, and Thomas L. Ortel, MD, of Duke University, Durham, N.C., wrote in an accompanying editorial.
“Although there have been several studies that have investigated the role of anticoagulant therapy in hospitalized patients with COVID-19, this is the first study that specifically compared a standard, prophylactic dose of low-molecular-weight heparin to a ‘high-dose’ prophylactic regimen and to a full therapeutic dose regimen,” Dr. Ortel said in an interview.
“Given the concerns about an increased thrombotic risk with prophylactic dose anticoagulation, and the potential bleeding risk associated with a full therapeutic dose of anticoagulation, this approach enabled the investigators to explore the efficacy and safety of an intermediate dose between these two extremes,” he said.
In the current study, , a finding that was not observed in other studies investigating anticoagulant therapy in hospitalized patients with severe COVID-19,” Dr. Ortel told this news organization. “Much initial concern about progression of disease in patients hospitalized with severe COVID-19 focused on the role of microvascular thrombosis, which appears to be less important in this process, or, alternatively, less responsive to anticoagulant therapy.”
The clinical takeaway from the study, Dr. Ortel said, is the decreased risk for venous thromboembolism with a high-dose prophylactic anticoagulation strategy compared with a standard-dose prophylactic regimen for patients hospitalized with hypoxemic COVID-19 pneumonia, “leading to an improved net clinical outcome.”
Looking ahead, “Additional research is needed to determine whether a higher dose of prophylactic anticoagulation would be beneficial for patients hospitalized with COVID-19 pneumonia who are not in an intensive care unit setting,” Dr. Ortel said. Studies are needed to determine whether therapeutic interventions are equally beneficial in patients with different coronavirus variants, since most patients in the current study were infected with the Delta variant, he added.
The study was supported by LEO Pharma. Dr. Labbé disclosed grants from LEO Pharma during the study and fees from AOP Health unrelated to the current study.
Dr. Becker disclosed personal fees from Novartis Data Safety Monitoring Board, Ionis Data Safety Monitoring Board, and Basking Biosciences Scientific Advisory Board unrelated to the current study. Dr. Ortel disclosed grants from the National Institutes of Health, Instrumentation Laboratory, Stago, and Siemens; contract fees from the Centers for Disease Control and Prevention; and honoraria from UpToDate unrelated to the current study.
A version of this article originally appeared on Medscape.com.
High-dose prophylactic anticoagulation or therapeutic anticoagulation reduced de novo thrombosis in patients with hypoxemic COVID-19 pneumonia, based on data from 334 adults.
Patients with hypoxemic COVID-19 pneumonia are at increased risk of thrombosis and anticoagulation-related bleeding, therefore data to identify the lowest effective anticoagulant dose are needed, wrote Vincent Labbé, MD, of Sorbonne University, Paris, and colleagues.
Previous studies of different anticoagulation strategies for noncritically ill and critically ill patients with COVID-19 pneumonia have shown contrasting results, but some institutions recommend a high-dose regimen in the wake of data showing macrovascular thrombosis in patients with COVID-19 who were treated with standard anticoagulation, the authors wrote.
However, no previously published studies have compared the effectiveness of the three anticoagulation strategies: high-dose prophylactic anticoagulation (HD-PA), standard dose prophylactic anticoagulation (SD-PA), and therapeutic anticoagulation (TA), they said.
In the open-label Anticoagulation COVID-19 (ANTICOVID) trial, published in JAMA Internal Medicine, the researchers identified consecutively hospitalized adults aged 18 years and older being treated for hypoxemic COVID-19 pneumonia in 23 centers in France between April 2021 and December 2021.
The patients were randomly assigned to SD-PA (116 patients), HD-PA (111 patients), and TA (112 patients) using low-molecular-weight heparin for 14 days, or until either hospital discharge or weaning from supplemental oxygen for 48 consecutive hours, whichever outcome occurred first. The HD-PA patients received two times the SD-PA dose. The mean age of the patients was 58.3 years, and approximately two-thirds were men; race and ethnicity data were not collected. Participants had no macrovascular thrombosis at the start of the study.
The primary outcomes were all-cause mortality and time to clinical improvement (defined as the time from randomization to a 2-point improvement on a 7-category respiratory function scale).
The secondary outcome was a combination of safety and efficacy at day 28 that included a composite of thrombosis (ischemic stroke, noncerebrovascular arterial thrombosis, deep venous thrombosis, pulmonary artery thrombosis, and central venous catheter–related deep venous thrombosis), major bleeding, or all-cause death.
For the primary outcome, results were similar among the groups; HD-PA had no significant benefit over SD-PA or TA. All-cause death rates for SD-PA, HD-PA, and TA patients were 14%, 12%, and 13%, respectively. The time to clinical improvement for the three groups was approximately 8 days, 9 days, and 8 days, respectively. Results for the primary outcome were consistent across all prespecified subgroups.
However, HD-PA was associated with a significant fourfold reduced risk of de novo thrombosis compared with SD-PA (5.5% vs. 20.2%) with no observed increase in major bleeding. TA was not associated with any significant improvement in primary or secondary outcomes compared with HD-PA or SD-PA.
The current study findings of no improvement in survival or disease resolution in patients with a higher anticoagulant dose reflects data from previous studies, the researchers wrote in their discussion. “Our study results together with those of previous RCTs support the premise that the role of microvascular thrombosis in worsening organ dysfunction may be narrower than estimated,” they said.
The findings were limited by several factors including the open-label design and the relatively small sample size, the lack of data on microvascular (vs. macrovascular) thrombosis at baseline, and the predominance of the Delta variant of COVID-19 among the study participants, which may have contributed to a lower mortality rate, the researchers noted.
However, given the significant reduction in de novo thrombosis, the results support the routine use of HD-PA in patients with severe hypoxemic COVID-19 pneumonia, they concluded.
Results inform current clinical practice
Over the course of the COVID-19 pandemic, “Patients hospitalized with COVID-19 manifested the highest risk for thromboembolic complications, especially patients in the intensive care setting,” and early reports suggested that standard prophylactic doses of anticoagulant therapy might be insufficient to prevent thrombotic events, Richard C. Becker, MD, of the University of Cincinnati, and Thomas L. Ortel, MD, of Duke University, Durham, N.C., wrote in an accompanying editorial.
“Although there have been several studies that have investigated the role of anticoagulant therapy in hospitalized patients with COVID-19, this is the first study that specifically compared a standard, prophylactic dose of low-molecular-weight heparin to a ‘high-dose’ prophylactic regimen and to a full therapeutic dose regimen,” Dr. Ortel said in an interview.
“Given the concerns about an increased thrombotic risk with prophylactic dose anticoagulation, and the potential bleeding risk associated with a full therapeutic dose of anticoagulation, this approach enabled the investigators to explore the efficacy and safety of an intermediate dose between these two extremes,” he said.
In the current study, , a finding that was not observed in other studies investigating anticoagulant therapy in hospitalized patients with severe COVID-19,” Dr. Ortel told this news organization. “Much initial concern about progression of disease in patients hospitalized with severe COVID-19 focused on the role of microvascular thrombosis, which appears to be less important in this process, or, alternatively, less responsive to anticoagulant therapy.”
The clinical takeaway from the study, Dr. Ortel said, is the decreased risk for venous thromboembolism with a high-dose prophylactic anticoagulation strategy compared with a standard-dose prophylactic regimen for patients hospitalized with hypoxemic COVID-19 pneumonia, “leading to an improved net clinical outcome.”
Looking ahead, “Additional research is needed to determine whether a higher dose of prophylactic anticoagulation would be beneficial for patients hospitalized with COVID-19 pneumonia who are not in an intensive care unit setting,” Dr. Ortel said. Studies are needed to determine whether therapeutic interventions are equally beneficial in patients with different coronavirus variants, since most patients in the current study were infected with the Delta variant, he added.
The study was supported by LEO Pharma. Dr. Labbé disclosed grants from LEO Pharma during the study and fees from AOP Health unrelated to the current study.
Dr. Becker disclosed personal fees from Novartis Data Safety Monitoring Board, Ionis Data Safety Monitoring Board, and Basking Biosciences Scientific Advisory Board unrelated to the current study. Dr. Ortel disclosed grants from the National Institutes of Health, Instrumentation Laboratory, Stago, and Siemens; contract fees from the Centers for Disease Control and Prevention; and honoraria from UpToDate unrelated to the current study.
A version of this article originally appeared on Medscape.com.
Meet the JCOM Author with Dr. Barkoudah: A Multidisciplinary Team–Based Clinical Care Pathway for Infective Endocarditis
Progressive Primary Cutaneous Nocardiosis in an Immunocompetent Patient
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
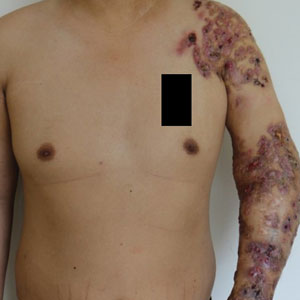
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

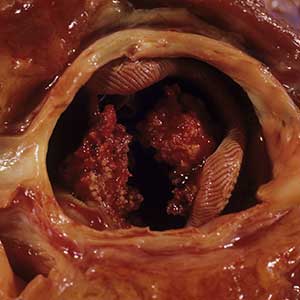
Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

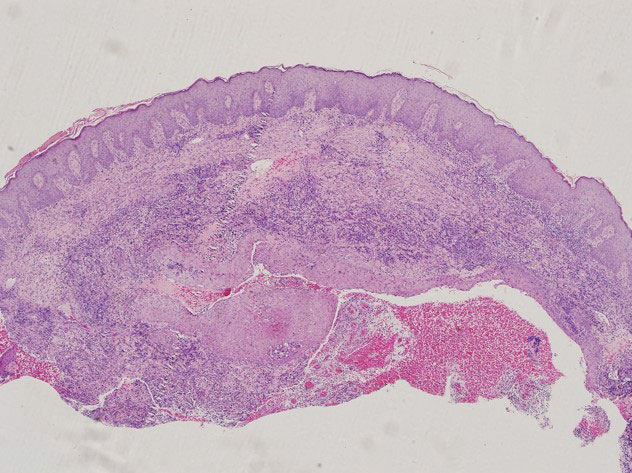
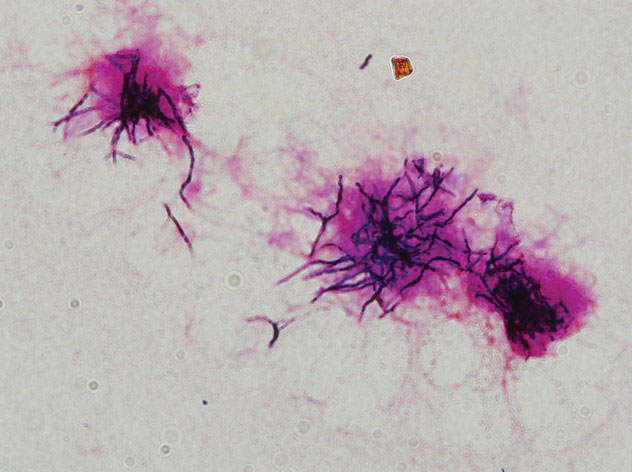
Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.
Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.
Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.

Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.

Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
To the Editor:
The organisms of the genus Nocardia are gram-positive, ubiquitous, aerobic actinomycetes found worldwide in soil, decaying organic material, and water.1 The genus Nocardia includes more than 50 species; some species, such as Nocardia asteroides, Nocardia farcinica, Nocardia nova, and Nocardia brasiliensis, are the cause of nocardiosis in humans and animals.2,3 Nocardiosis is a rare and opportunistic infection that predominantly affects immunocompromised individuals; however, up to 30% of infections can occur in immunocompetent hosts.4 Nocardiosis can manifest in 3 disease forms: cutaneous, pulmonary, or disseminated. Cutaneous nocardiosis commonly develops in immunocompetent individuals who have experienced a predisposing traumatic injury to the skin,5 and it can exhibit a diverse variety of clinical manifestations, making diagnosis difficult. We describe a case of serious progressive primary cutaneous nocardiosis with an unusual presentation in an immunocompetent patient.
A 26-year-old immunocompetent man presented with pain, swelling, nodules, abscesses, ulcers, and sinus drainage of the left arm. The left elbow lesion initially developed at the site of a trauma 6 years prior that was painless but was contaminated with mossy soil. The condition slowly progressed over the next 2 years, and the patient experienced increased swelling and eventually developed multiple draining sinus tracts. Over the next 4 years, the lesions multiplied, spreading to the forearm and upper arm; associated severe pain and swelling at the elbow and wrist joint developed. The patient sought medical care at a local hospital and subsequently was diagnosed with suspected cutaneous tuberculosis. The patient was empirically treated with a 6-month course of isoniazid, rifampicin, pyrazinamide, and ethambutol; however, the lesions continued to progress and worsen. The patient had to stop antibiotic treatment because of substantially elevated alanine aminotransferase and aspartate aminotransferase levels.
He subsequently was evaluated at our hospital. He had no notable medical history and was afebrile. Physical examination revealed multiple erythematous nodules, abscesses, and ulcers on the left arm. There were several nodules with open sinus tracts and seropurulent crusts along with numerous atrophic, ovoid, stellate scars. Other nodules and ulcers with purulent drainage were located along the lymphatic nodes extending up the patient’s left forearm (Figure 1A). The yellowish-white pus discharge from several active sinuses contained no apparent granules. The lesions were densely distributed along the elbow, wrist, and shoulder, which resulted in associated skin swelling and restricted joint movement. The left axillary lymph nodes were enlarged.

Laboratory analyses revealed a hemoglobin level of 9.6 g/dL (reference range, 13–17.5 g/dL), platelet count of 621×109/L (reference range, 125–350×109/L), and leukocyte count of 14.3×109/L (reference range, 3.5–9.5 ×109/L). C-reactive protein level was 88.4 mg/L (reference range, 0–10 mg/L). Blood, renal, and liver tests, as well as tumor marker, peripheral blood lymphocyte subset, immunoglobulin, and complement results were within reference ranges. Results for Treponema pallidum and HIV antibody tests were negative. Hepatitis B virus markers were positive for hepatitis B surface antigen, hepatitis B e antigen, and hepatitis B core antibody, and the serum concentration of hepatitis B virus DNA was 3.12×107 IU/mL (reference range, <5×102 IU/mL). Computed tomography of the chest and cranium were unremarkable. Ultrasonography of the left arm revealed multiple vertical sinus tracts and several horizontal communicating branches that were accompanied by worm-eaten bone destruction (Figure 2).

Additional testing included histopathologic staining of a skin tissue specimen—hematoxylin and eosin, periodic acid–Schiff, and acid-fast staining—showed nonspecific, diffuse, inflammatory cell infiltration suggestive of chronic suppurative granuloma (Figure 3) but failed to reveal any special strains or organisms. Gram stain examination of the purulent fluid collected from the subcutaneous tissue showed no apparent positive bacillus or filamentous granules. The specimen was then inoculated on Sabouraud dextrose agar and Lowenstein-Jensen medium for fungus and mycobacteria culture, respectively. After 5 days, chalky, yellow, adherent colonies were observed on the Löwenstein-Jensen medium, and after 26 days, yellow crinkled colonies were observed on Sabouraud dextrose agar. The colonies were then inoculated on Columbia blood agar and incubated for 1 week to aid in the identification of organisms. Growth of yellow colonies that were adherent to the agar, moist, and smooth with a velvety surface, as well as a characteristic moldy odor resulted. Gram staining revealed gram-positive, thin, and beaded branching filaments (Figure 4). Based on colony characteristics, physiological properties, and biochemical tests, the isolate was identified as Nocardia. Results of further investigations employing polymerase chain reaction analysis of the skin specimen and bacterial colonies using a Nocardia genus 596-bp fragment of 16S ribosomal RNA primer (forward primer NG1: 5’-ACCGACCACAAGGGG-3’, reverse primer NG2: 5’-GGTTGTAACCTCTTCGA-3’)6 were completely consistent with the reference for identification of N brasiliensis. Evaluation of these results led to a diagnosis of cutaneous nocardiosis after traumatic inoculation.

Because there was a high suspicion of actinophytosis or nocardiosis at admission, the patient received a combination antibiotic treatment with intravenous aqueous penicillin (4 million U every 4 hours) and oral trimethoprim-sulfamethoxazole (160/800 mg twice daily). Subsequently, treatment was changed to a combination of oral trimethoprim-sulfamethoxazole (160/800 mg twice daily) and moxifloxacin (400 mg once daily) based on pathogen identification and antibiotic sensitivity testing. After 1 month of treatment, the cutaneous lesions and left limb swelling dramatically improved and purulent drainage ceased, though some scarring occurred during the healing process. In addition, the mobility of the affected shoulder, elbow, and wrist joints slightly improved. Notable improvement in the mobility and swelling of the joints was observed at 6-month follow-up (Figure 1B). The patient continues to be monitored on an outpatient basis.

Cutaneous nocardiosis is a disfiguring granulomatous infection involving cutaneous and subcutaneous tissue that can progress to cause injury to viscera and bone.7 It has been called one of the great imitators because cutaneous nocardiosis can present in multiple forms,8,9 including mycetoma, sporotrichoid infection, superficial skin infection, and disseminated infection with cutaneous involvement. The differential diagnoses of cutaneous nocardiosis are broad and include tuberculosis; actinomycosis; deep fungal infections such as sporotrichosis, blastomycosis, phaeohyphomycosis, histoplasmosis, and coccidioidomycosis; other bacterial causes of cellulitis, abscess, or ecthyma; and malignancies.10 The principle method of diagnosis is the identification of Nocardia from the infection site.
Our patient ultimately was diagnosed with primary cutaneous nocardiosis resulting from a traumatic injury to the skin that was contaminated with soil. The clinical manifestation pattern was a compound type, including both mycetoma and sporotrichoid infections. Initially, Nocardia mycetoma occurred with subcutaneous infection by direct extension10,11 and appeared as dense, predominantly painless, swollen lesions. After 4 years, the skin lesions continued to spread linearly to the patient’s upper arm and forearm and manifested as the sporotrichoid infection type with painful swollen lesions at the site of inoculation and painful enlargement of the ipsilateral axillary lymph node.
Although nocardiosis is found worldwide, it is endemic to tropical and subtropical regions such as India, Africa, Southeast Asia, and Latin America.12 Nocardiosis most often is observed in individuals aged 20 to 40 years. It affects men more than women, and it commonly occurs in field laborers and cultivators whose occupations involve direct contact with the soil.13 Most lesions are found on the lower extremities, though localized nocardiosis infections can occur in other areas such as the neck, breasts, back, buttocks, and elbows.
Our patient initially was misdiagnosed, and treatment was delayed for several reasons. First, nocardiosis is not common in China, and most clinicians are unfamiliar with the disease. Second, the related lesions do not have specific features, and our patient had a complex clinical presentation that included mycetoma and sporotrichoid infection. Third, the characteristic grain of Nocardia species is small but that of N brasiliensis is even smaller (approximately 0.1–0.2 mm in diameter), which makes visualization difficult in both histopathologic and microbiologic examinations.14 The histopathologic examination results of our patient in the local hospital were nonspecific. Fourth, our patient did not initially go to the hospital but instead purchased some over-the-counter antibiotic ointments for external application because the lesions were painless. Moreover, microbiologic smear and culture examinations were not conducted in the local hospital before administering antituberculosis treatment to the patient. Instead, a polymerase chain reaction examination of skin lesion tissue for tubercle bacilli and atypical mycobacteria was negative. These findings imply that the traditional microbial smear and culture evaluations cannot be omitted. Furthermore, culture examinations should be conducted on multiple skin tissue and purulent fluid specimens to increase the likelihood of detection. These cultures should be monitored for at least 2 to 4 weeks because Nocardia is a slow-growing organism.10
The optimal antimicrobial treatment regimens for nocardiosis have not been firmly established.15 Trimethoprim-sulfamethoxazole is regarded as the first-line antimicrobial agent for treatment of nocardial infections. The optimal duration of antimicrobial therapy for nocardiosis also has not been determined, and the treatment regimen depends on the severity and extent of the infection as well as on the presence of infection-related complications. The main complication is bone involvement. Notable bony changes include periosteal thickening, osteoporosis, and osteolysis.
We considered the severity of skin lesions and bone marrow invasion in our patient and planned to treat him continually with oral trimethoprim-sulfamethoxazole according to the in vitro drug susceptibility test. The patient showed clinical improvement after 1 month of treatment, and he continued to improve after 6 months of treatment. To prevent recurrence, we found it necessary to treat the patient with a long-term antibiotic course over 6 to 12 months.16
Cutaneous nocardiosis remains a diagnostic challenge because of its nonspecific and diverse clinical and histopathological presentations. Diagnosis is further complicated by the inherent difficulty of cultivating and identifying the clinical isolate in the laboratory. A high degree of clinical suspicion followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
- McNeil MM, Brown JM. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357-417.
- Brown-Elliott BA, Brown JM, Conville PS, et al. Clinical and laboratory features of the Nocardia spp. based on current molecular taxonomy. Clin Microbiol Rev. 2006;19:259-282.
- Fatahi-Bafghi M. Nocardiosis from 1888 to 2017. Microb Pathog. 2018;114:369-384.
- Beaman BL, Burnside J, Edwards B, et al. Nocardial infections in the United States, 1972-1974. J Infect Dis. 1976;134:286-289.
- Lerner PI. Nocardiosis. Clin Infect Dis. 1996;22:891-903.
- Laurent FJ, Provost F, Boiron P. Rapid identification of clinically relevant Nocardia species to genus level by 16S rRNA gene PCR. J Clin Microbiol. 1999;37:99-102.
- Nguyen NM, Sink JR, Carter AJ, et al. Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. 2018;4:33-35.
- Sharna NL, Mahajan VK, Agarwal S, et al. Nocardial mycetoma: diverse clinical presentations. Indian J Dermatol Venereol Leprol. 2008;74:635-640.
- Huang L, Chen X, Xu H, et al. Clinical features, identification, antimicrobial resistance patterns of Nocardia species in China: 2009-2017. Diagn Microbiol Infect Dis. 2019;94:165-172.
- Bonifaz A, Tirado-Sánchez A, Calderón L, et al. Mycetoma: experience of 482 cases in a single center in Mexico. PLoS Negl Trop Dis. 2014;8:E3102.
- Welsh O, Vero-Cabrera L, Salinas-Carmona MC. Mycetoma. Clin Dermatol. 2007;25:195-202.
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883.
- Emmanuel P, Dumre SP, John S, et al. Mycetoma: a clinical dilemma in resource limited settings. Ann Clin Microbiol Antimicrob. 2018;17:35.
- Reis CMS, Reis-Filho EGM. Mycetomas: an epidemiological, etiological, clinical, laboratory and therapeutic review. An Bras Dermatol. 2018;93:8-18.
- Wilson JW. Nocardiosis: updates and clinical overview. Mayo Clin Proc. 2012;87:403-407.
- Welsh O, Vera-Cabrera L, Salinas-Carmona MC. Current treatment for Nocardia infections. Expert Opin Pharmacother. 2013;14:2387-2398.
Practice Points
- Although unusual, cutaneous nocardiosis can present with both mycetoma and sporotrichoid infection, which should be treated based on pathogen identification and antibiotic sensitivity testing.
- A high degree of clinical suspicion by clinicians followed by successful identification of the organism by a laboratory technologist will aid in the early diagnosis and treatment of the infection, ultimately reducing the risk for complications and morbidity.
Spotting STIs: Vaginal swabs work best
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
Vaginal swabs are more effective than urine analysis in detecting certain types of sexually transmitted infections, researchers have found.
In the study, which was published online in the Annals of Family Medicine, investigators found that the diagnostic sensitivity of commercially available vaginal swabs was significantly greater than that of urine tests in detecting certain infections, including those caused by Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis.
Researchers studied chlamydia and gonorrhea, which are two of the most frequently reported STIs in the United States. Trichomoniasis is the most curable nonviral STI globally, with 156 million cases worldwide in 2016.
The Centers for Disease Control and Prevention has long recommended that vaginal swabs be used to produce optimal samples.
But despite the CDC’s recommendation, urine analysis for these STIs is more commonly used than vaginal swabs among U.S. health care providers.
“We’re using a poor sample type, and we can do better,” said Barbara Van Der Pol, PhD, a professor of medicine and public health at the University of Alabama at Birmingham and an author of the new study, a meta-analysis of 97 studies published between 1995 and 2021.
Vaginal swabs for chlamydia trachomatis had a diagnostic sensitivity of 94.1% (95% confidence interval, 93.2%-94.9%; P < .001), higher than urine testing (86.9%; 95% CI, 85.6%-88.0%; P < .001). The pooled sensitivity estimates for Neisseria gonorrhoeae were 96.5% (95% CI, 94.8%-97.7%; P < .001) for vaginal swabs and 90.7% (95% CI, 88.4%-92.5%; P < .001) for urine specimens.
The difference in pooled sensitivity estimates between vaginal swabs and urine analyses for Trichomonas vaginalis was 98% (95% CI, 97.0%-98.7%; P < .001) for vaginal swabs and 95.1% (95% CI, 93.6%-96.3%) for urine specimens.
STIs included in the study are not typically found in the urethra and appear in urine analyses only if cervical or vaginal cells have dripped into a urine sample. Dr. Van Der Pol and her colleagues estimated that the use of urine samples rather than vaginal swabs may result in more than 400,000 undiagnosed infections annually.
Undiagnosed and untreated STIs can lead to transmissions of the infection as well as infertility and can have negative effects on romantic relationships, according to Dr. Van Der Pol.
Sarah Wood, MD, an attending physician at Children’s Hospital of Philadelphia, said some health care providers may use urine analysis because patients may be more comfortable with this method. The approach also can be more convenient for medical offices: All they must do is hand a specimen container to the patient.
Conversations between clinicians and patients about vaginal swabbing may be considered “sensitive” and the swabbing more invasive, Dr. Wood, an author of an editorial accompanying the journal article, said. Clinicians may also lack awareness that the swab is a more sensitive method of detecting these STIs.
“We all want to do what’s right for our patient, but we often don’t know what’s right for the patient,” Dr. Wood said. “I don’t think people are really aware of a potential real difference in outcomes with one method over the other.”
Dr. Wood advised making STI screening using vaginal swabs more common by “offering universal opt-out screening, so not waiting until you find out if someone’s having sex but just sort of saying, ‘Hey, across our practice, we screen everybody for chlamydia. Is that something that you want to do today?’ That approach sort of takes out the piece of talking about sex, talking about sexual activity.”
Dr. Van Der Pol, who said she has worked in STI diagnostics for 40 years, said she was not surprised by the results and hopes the study changes how samples are collected and used.
“I really hope that it influences practice so that we really start using vaginal swabs, because it gives us better diagnostics for chlamydia and gonorrhea,” Dr. Van Der Pol said.
“Also, then starting to think about comprehensive women’s care in such a way that they actually order other tests on that same sample if a woman is presenting with complaints.”
A version of this article originally appeared on Medscape.com.
eNose knows S. aureus in children with cystic fibrosis
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
An electronic nose effectively detected Staphylococcus aureus in children with cystic fibrosis, based on data from 100 individuals.
Staphylococcus aureus is the most common pathogen found in children with cystic fibrosis (CF), but current detection strategies are based on microbiology cultures, wrote Johann-Christoph Licht, a medical student at the University of Toronto, and colleagues.
Noninvasive tools are needed to screen children with CF early for respiratory infections, the researchers said.
The electronic Nose (eNose) is a technology that detects volatile organic compounds (VOCs). Although exhaled breath can be used to create distinct profiles, the ability of eNose to identify S. aureus (SA) in the breath of children with CF remains unclear, they wrote.
In a study published in the Journal of Cystic Fibrosis, the researchers analyzed breath profiles data from 100 children with CF. The study population included children aged 5-18 years with clinically stable CF who were recruited from CF clinics during routine visits. Patients with a CF pulmonary exacerbation were excluded.
The children’s median predicted FEV1 was 91%. The researchers collected sputum from 67 patients and throat cultures for 33 patients. A group of 25 age-matched healthy controls served for comparison.
Eighty patients were positive for CF pathogens. Of these, 67 were positive for SA (44 with SA only and 23 with SA and at least one other pathogen).
Overall, patients with any CF pathogen on airway cultures were identified compared to airway cultures with no CF pathogens with an area under the curve accuracy of 79.0%.
Previous studies have shown a high rate of accuracy using eNose to detect Pseudomonas aeruginosa (PA). In the current study, the area under the curve accuracy for PA infection compared to no CF pathogens was 78%. Both SA-specific and PA-specific signatures were driven by different sensors in the eNose, which suggests pathogen-specific breath signatures, the researchers wrote.
The study findings were limited by several factors including the small number of patients with positive airway cultures for PA and the lack of data on variability of measures over time or treatment-induced changes, the researchers noted.
However, the results confirm the value of the eNose in real-time point-of-care detection of airway infection in children with CF, and this is the first study known to suggest the potential of an eNose to detect SA infection in particular in a routine clinical setting, the researchers wrote in their discussion.
Other points in favor of eNose compared to current practice include “low cost, ease of use and portability to the point-of-care,” they said. The eNose provides an opportunity for early detection of pathogens that challenges conventional microbiology testing, they concluded.
The study received no outside funding. Two coauthors disclosed fees and/or an interest in the company Breathomix BV.
FROM THE JOURNAL OF CYSTIC FIBROSIS
Implementation of a Multidisciplinary Team–Based Clinical Care Pathway Is Associated With Increased Surgery Rates for Infective Endocarditis
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
From the University of Missouri School of Medicine, Columbia, MO (Haley Crosby); Department of Clinical Family and Community Medicine, University of Missouri, Columbia, MO (Dr. Pierce); and Department of Medicine, Divisions of Infectious Diseases and Pulmonary, Critical Care and Environmental Medicine, University of Missouri, Columbia, MO, and Divisions of Pulmonary and Critical Care Medicine and Infectious Diseases, University of Maryland Baltimore Washington Medical Center, Glen Burnie, MD (Dr. Regunath).
ABSTRACT
Objective: Multidisciplinary teams (MDTs) improve outcomes for patients with infective endocarditis (IE), but methods of implementation vary. In our academic medical center, we developed an MDT approach guided by a clinical care pathway and assessed outcomes of patients with IE.
Methods: We compared outcomes of patients with IE and indications for surgery between December 2018 and June 2020 with our prior published data for the period January to December 2016. MDT interventions involved recurring conferences with infectious diseases physicians in team meetings and promoting a clinical care pathway to guide providers on steps in management. Primary outcomes were surgery and in-hospital death.
Results: Prior to the intervention, 6 of 21 (28.6%) patients with indications for surgery underwent surgery or were transferred to higher centers for surgery, and 6 (28.6%) patients died. Post intervention, 17 of 31 (54.8%) patients underwent or were transferred for surgery, and 5 (16.1%) died. After adjusting for age and gender, the odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) compared with the pre-intervention period. The odds ratio for death among patients in the postintervention period was 0.40 (95% CI, 0.09-1.69; P = .21).
Conclusion: An MDT team approach using a clinical pathway was associated with an increased number of surgeries performed for IE and may lower rates of in-hospital mortality.
Keywords: infective endocarditis, clinical pathway, quality improvement, multidisciplinary team, valve surgery.
Infective endocarditis (IE) is associated with significant morbidity and mortality.1 Rates of IE due to Staphylococcus aureus are increasing in the United States.2 Reported in-hospital mortality from IE ranges from 15% to 20%.3
Clinical pathways are defined as “structured, multidisciplinary plans of care used by health services to detail essential steps in the care of patients with a specific clinical problem.”12 In the modern era, these pathways are often developed and implemented via the electronic health record (EHR) system. Studies of clinical pathways generally demonstrate improvements in patient outcomes, quality of care, or resource utilization.13,14 Clinical pathways represent 1 possible approach to the implementation of a MDT in the care of patients with IE.15
In our earlier work, we used quality improvement principles in the design of an MDT approach to IE care at our institution.16 Despite having indications for surgery, 12 of 21 (57.1%) patients with IE did not undergo surgery, and we identified these missed opportunities for surgery as a leverage point for improvement of outcomes. With input from the various specialties and stakeholders, we developed a clinical pathway (algorithm) for the institutional management of IE that guides next steps in clinical care and their timelines, aiming to reduce by 50% (from 57.1% to 28.6%) the number of patients with IE who do not undergo surgery despite guideline indications for early surgical intervention. In this report, we describe the implementation of this clinical pathway as our MDT approach to the care of patients with IE at our institution.
Methods
The University of Missouri, Columbia, is a tertiary care academic health system with 5 hospitals and more than 60 clinic locations across central Missouri. In the spring of 2018, an MDT was developed, with support from administrative leaders, to improve the care of patients with IE at our institution. The work group prioritized one leverage point to improve IE outcomes, which was improving the number of surgeries performed on those IE patients who had guideline indications for surgery. A clinical pathway was developed around this leverage point (Figure 1). The pathway leveraged the 6 T’s (Table 1) to guide providers through the evaluation and management of IE.17 The pathway focused on improving adherence to standards of care and reduction in practice variation by defining indications for referrals and diagnostic interventions, helping to reduce delays in consultation and diagnosis. The pathway also clearly outlined the surgical indications and timing for patients with IE and provided the basis for decisions to proceed with surgery.

Starting in late 2018, in collaboration with cardiology and CTS teams, ID specialists socialized the clinical pathway to inpatient services that cared for patients with IE. Infectious diseases physicians also provided recurring conferences on the effectiveness of MDTs in IE management and participated in heart-valve team case discussions. Finally, in May 2019, an electronic version of the pathway was embedded in the EHR system using a Cerner PowerChart feature known as Care Pathways. The feature presents the user with algorithm questions in the EHR and provides recommendations, relevant orders, timelines, and other decision support in the clinical pathway. The feature is available to all providers in the health system.

To evaluate the effectiveness of our intervention, we recorded outcomes for patients with IE with surgical indications between December 2018 and June 2020 and compared them with our prior published data from January to December 2016. Cases of IE for the current study period were identified via retrospective chart review. Records from December 2018 to June 2020 were searched using International Statistical Classification of Diseases, Tenth Revision (ICD-10) discharge codes for IE (I33, I33.0, I33.9, I38, I39, M32.11). To select those patients with definitive IE and indications for surgery, the following criteria were applied: age ≥ 18 years; fulfilled modified Duke criteria for definite IE18; and met ≥ 1 American Heart Association (AHA)/Infection Diseases Society of America criteria for recommendation for surgery. Indications for surgery were ≥ 1 of the following: left-sided endocarditis caused by S aureus, fungal, or highly resistant organism; new heart block; annular or aortic abscess; persistent bacteremia or fever despite 5 days of appropriate antimicrobials; vegetation size ≥ 10 mm and evidence of embolic phenomena; recurrence of prosthetic valve infection; recurrent emboli and persistent vegetation despite antimicrobials; and increase in vegetation size despite antimicrobials.16
Age was treated as a categorical variable, using the age groups 18 to 44 years, 45 to 64 years, and 65 years and older. Gender was self-reported. Primary outcomes were surgery or transfer to a higher center for surgery and in-hospital death. Secondary outcomes included consults to teams involved in multidisciplinary care of patients with IE, including ID, cardiology, and CTS. Bivariate analyses were performed using Pearson χ2 tests. Odds ratios for surgery and death were calculated using a multivariate logistic regression model including age and gender covariates. Statistical significance was defined at α = 0.05, and statistical analysis was performed using Stata/IC v16.1 (StataCorp LLC). Our university institutional review board (IRB) reviewed the project (#2010858-QI) and determined that the project was quality-improvement activity, not human subject research, and therefore did not require additional IRB review.
Results
We identified 21 patients in the pre-intervention period and 31 patients in the postintervention period with definitive IE who had guideline indications for surgery. The postintervention cohort was older and had more male patients; this difference was not statistically significant. No differences were noted between the groups for race, gender, or intravenous (IV) drug use (Table 2). Chi-square tests of independence were performed to assess the relationship between age and our primary outcomes. There was a significant relationship between age and the likelihood of receiving or being transferred for surgery (59.3% vs 50% vs 7.7% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 [2, N = 52] = 9.67; P = .008), but not between age and mortality (14.8% vs 25.0% vs 30.8% for 18-44 y, 45-64 y, and ≥ 65 y, respectively; χ2 = 1.48 [2, N = 52; P = .478]. The electronic version of the clinical pathway was activated and used in only 3 of the 31 patients in the postintervention period. Consultations to ID, cardiology, and CTS teams were compared between the study periods (Table 2). Although more consultations were seen in the postintervention period, differences were not statistically significant.

The unadjusted primary outcomes are shown in Table 2. More surgeries were performed per guideline indications, and fewer deaths were noted in the postintervention period than in the pre-intervention period, but the differences were not statistically significant (Table 2).
Because the postintervention period had more male patients and older patients, we evaluated the outcomes using a logistic regression model controlling for both age and gender. The odds of surgery or transfer for surgery for patients in the postintervention period were 4.88 (95% CI, 1.20-19.79; P = .027) as compared with the pre-intervention period, and the odds ratio for death among patients in the postintervention period compared with the pre-intervention period was 0.40 (95% CI, 0.09-1.69; P = .21) (Figure 2).

Discussion
In our study, patients with IE with guideline indications for surgery had significantly higher rates of surgery in the postintervention period than in the pre-intervention period. The implementation of an MDT, recurring educational sessions, and efforts to implement and familiarize team members with the clinical pathway approach are the most likely reasons for this change. The increased rates of surgery in the postintervention period were the likely proximate cause of the 60% reduction in in-hospital mortality. This improvement in mortality, while not statistically significant, is very likely to be clinically significant and helps reinforce the value of the MDT intervention used.
Our findings are consistent with existing and mounting literature on the use of MDTs to improve outcomes for patients with IE, including 2 studies that noted an increased rate of surgery for patients with indications.8,19 Several other studies in both Europe and North America have found significant decreases in mortality,6-11,20,21 rates of complications,9 time to diagnosis and treatment,11 and length of stay9,20 for patients with IE managed with an MDT strategy. Although current AHA guidelines for care of patients with IE do suggest an MDT approach, the strategy for this approach is not well established.22 Only 1 study that has implemented a new MDT protocol for care of IE has been conducted in the United States.8
While effective MDTs certainly improve outcomes in patients with IE, there are reported differences in implementation of such an approach. With the MDT model as the core, various implementations included regular case conferences,10,11,19,21,23 formation of a consulting team,6,8 or establishment of a new protocol or algorithm for care.8,9,20 Our approach used a clinical pathway as a basis for improved communication among consulting services, education of learning providers via regular case conferences, and implementation of an electronic clinical care pathway to guide them step by step. Our pathway followed the institutionally standardized algorithm (Figure 1), using what we called the 6 T’s approach (Table 1), that guides providers to evaluate critical cases in a fast track.17
To the best of our knowledge, ours is the first report of an MDT that used an electronic clinical care pathway embedded within the EHR. The electronic version of our clinical pathway went live for only the second half of the postintervention study period, which is the most likely reason for its limited utilization. It is also possible that educational efforts in the first half of the intervention period were sufficient to familiarize providers with the care pathway such that the electronic version was seldom needed. We are exploring other possible ways of improving electronic pathway utilization, such as improving the feature usability and further systemwide educational efforts.
Our study has other limitations. Quasi-experimental before-and-after comparisons are subject to confounding from concurrent interventions. We had a substantial change in cardiothoracic faculty soon after the commencement of our efforts to form the MDT, and thus cannot rule out differences related to their comfort level in considering or offering surgery. We also cannot rule out a Hawthorne effect, where knowledge of the ongoing quality-improvement project changed provider behavior, making surgery more likely. We did not evaluate rates of right- versus left-sided endocarditis, which have been linked to mortality.24 Our study also was performed across a single academic institution, which may limit its generalizability. Finally, our study may not have been adequately powered to detect differences in mortality due to implementation of the MDT approach.
Conclusion
Our work suggests that an MDT for IE can be successfully designed and implemented with a clinical pathway using quality-improvement tools in centers where subspecialty services are available. Our approach was associated with a higher rate of surgery among patients with guideline indications for surgery and may reduce in-hospital mortality. An electronic clinical care pathway embedded in the EHR is feasible and may have a role in MDT implementation.
These data were also accepted as a poster at IDWeek 2022, Washington, DC. The poster abstract is published in an online supplement of Open Forum Infectious Diseases as an abstract publication.
Corresponding author: Haley Crosby; [email protected]
Disclosures: None reported.
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
1. Baddour LM, Wilson WR, Bayer AS, et al. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2015;132(15):1435-1486. doi:10.1161/cir.0000000000000296
2. Federspiel JJ, Stearns SC, Peppercorn AF, et al. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363-365. doi:10.1001/archinternmed.2011.1027
3. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(23):e521-e643. doi:10.1161/cir.0000000000000031
4. Chambers J, Sandoe J, Ray S, et al. The infective endocarditis team: recommendations from an international working group. Heart. 2014;100(7):524-527. doi:10.1136/heartjnl-2013-304354
5. Habib G, Lancellotti P, Antunes MJ, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36(44):3075-3128. doi:10.1093/eurheartj/ehv319
6. Chirillo F, Scotton P, Rocco F, et al. Impact of a multidisciplinary management strategy on the outcome of patients with native valve infective endocarditis. Am J Cardiol. 2013;112(8):1171-1176. doi:10.1016/j.amjcard.2013.05.060
7. Botelho-Nevers E, Thuny F, Casalta JP, et al. Dramatic reduction in infective endocarditis-related mortality with a management-based approach. Arch Intern Med. 2009;169(14):1290-1298. doi:10.1001/archinternmed.2009.192
8. El-Dalati S, Cronin D, Riddell IV J, et al. The clinical impact of implementation of a multidisciplinary endocarditis team. Ann Thorac Surg. 2022;113(1):118-124.
9. Carrasco-Chinchilla F, Sánchez-Espín G, Ruiz-Morales J, et al. Influence of a multidisciplinary alert strategy on mortality due to left-sided infective endocarditis. Rev Esp Cardiol (Engl Ed). 2014;67(5):380-386. doi:10.1016/j.rec.2013.09.010
10. Issa N, Dijos M, Greib C, et al. Impact of an endocarditis team in the management of 357 infective endocarditis [abstract]. Open Forum Infect Dis. 2016;3(suppl 1):S201. doi:10.1093/ofid/ofw172.825
11. Kaura A, Byrne J, Fife A, et al. Inception of the ‘endocarditis team’ is associated with improved survival in patients with infective endocarditis who are managed medically: findings from a before-and-after study. Open Heart. 2017;4(2):e000699. doi:10.1136/openhrt-2017-000699
12. Rotter T, Kinsman L, James E, et al. Clinical pathways: effects on professional practice, patient outcomes, length of stay and hospital costs. Cochrane Database Syst Rev. 2010;(3):Cd006632. doi:10.1002/14651858.CD006632.pub2
13. Neame MT, Chacko J, Surace AE, et al. A systematic review of the effects of implementing clinical pathways supported by health information technologies. J Am Med Inform Assoc. 2019;26(4):356-363. doi:10.1093/jamia/ocy176
14. Trimarchi L, Caruso R, Magon G, et al. Clinical pathways and patient-related outcomes in hospital-based settings: a systematic review and meta-analysis of randomized controlled trials. Acta Biomed. 2021;92(1):e2021093. doi:10.23750/abm.v92i1.10639
15. Gibbons EF, Huang G, Aldea G, et al. A multidisciplinary pathway for the diagnosis and treatment of infectious endocarditis. Crit Pathw Cardiol. 2020;19(4):187-194. doi:10.1097/hpc.0000000000000224
16. Regunath H, Vasudevan A, Vyas K, et al. A quality improvement initiative: developing a multi-disciplinary team for infective endocarditis. Mo Med. 2019;116(4):291-296.
17. Regunath H, Whitt SP. Multidisciplinary service delivery for the endocarditis patient. In: Infective Endocarditis: A Multidisciplinary Approach. 1st ed. Kilic A, ed. Academic Press; 2022.
18. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. 1994;96(3):200-209. doi:10.1016/0002-9343(94)90143-0
19. Tan C, Hansen MS, Cohen G, et al. Case conferences for infective endocarditis: a quality improvement initiative. PLoS One. 2018;13(10):e0205528. doi:10.1371/journal.pone.0205528
20. Ruch Y, Mazzucotelli JP, Lefebvre F, et al. Impact of setting up an “endocarditis team” on the management of infective endocarditis. Open Forum Infect Dis. 2019;6(9):ofz308. doi:10.1093/ofid/ofz308
21. Camou F, Dijos M, Barandon L, et al. Management of infective endocarditis and multidisciplinary approach. Med Mal Infect. 2019;49(1):17-22. doi:10.1016/j.medmal.2018.06.007
22. Pettersson GB, Hussain ST. Current AATS guidelines on surgical treatment of infective endocarditis. Ann Cardiothorac Surg. 2019;8(6):630-644. doi:10.21037/acs.2019.10.05
23. Mestres CA, Paré JC, Miró JM. Organization and functioning of a multidisciplinary team for the diagnosis and treatment of infective endocarditis: a 30-year perspective (1985-2014). Rev Esp Cardiol (Engl Ed). 2015;68(5):363-368. doi:10.1016/j.rec.2014.10.006
24. Stavi V, Brandstaetter E, Sagy I, et al. Comparison of clinical characteristics and prognosis in patients with right- and left-sided infective endocarditis. Rambam Maimonides Med J. 2019;10(1):e00003. doi:10.5041/rmmj.10338
Expert shares her tips for diagnosing, treating onychomycosis
NEW ORLEANS – .
“The PAS [periodic acid-Schiff] stain is very popular because it can identify the presence or absence of fungal elements, but a fungal culture will identify the organism living in the nail,” Dr. Elewski, professor and chair of dermatology at the University of Alabama, Birmingham, said at the annual meeting of the American Academy of Dermatology. “You also could do a PCR to identify the organism, with or without a KOH or PAS stain. It is often helpful to know what organism is causing the infection.”
While waiting for lab results, there are three clinical clues to look for – the first being that an infection likely resides in the toenail. “You almost never see dermatophyte onychomycosis in the fingernails without it being in the toenails, too,” Dr. Elewski said.
The presence of tinea pedis is a second clinical clue. “Sometimes it’s subtle, so I will ask the patient, ‘Have you been treating yourself for athlete’s foot?’ If they say ‘no, I’ve never had it,’ put down on your list that it’s unlikely they have onychomycosis. How is the fungus going to jump from the floor into the nail without taking a little vacation on the bottom of the foot? It just isn’t going to happen.”
The presence of dermatophytoma is the third clinical clue. “These are dermatophyte abscesses encased in a biofilm, and they’re really hard to treat,” she said.
Treatments
Clinicians typically turn to one of three oral drugs for treating onychomycosis: terbinafine, itraconazole, and fluconazole, Dr. Elewski noted. Referring to terbinafine as “the gold standard,” she said that she typically writes a prescription for 90 250-mg pills. “When I give terbinafine, I often do baseline liver profiling, depending on the patient’s age, their state of health, their comorbidities, and other medications they’re taking,” she said. “If they’re 18 years old and otherwise healthy, I probably don’t.” While she generally prescribes 90 pills, she added, “keep in mind that 90 pills are not going to cure everybody. I see the patient 4 months later because the drug should stay in the nail for 30 days or more at therapeutic levels after you take that 90-day course.”
Another option is itraconazole, which can be taken at a dose of 200 mg a day for 12 weeks, or at a pulse dose, where patients take 400 mg every day for 1 week, 1 week a month, for 4 consecutive months. “I’ll often do a baseline liver profile with itraconazole, too,” Dr. Elewski said. “I don’t think you have to, but it makes sense if it’s feasible for you. Decide that based on each patient.”
Itraconazole can’t be given concomitantly with statins because of the potential for rhabdomyolysis. For patients taking statins, she consults with their physicians to make sure it’s safe to stop the statin a couple of days before and after their scheduled pulse dose of itraconazole. “This involves 1 week per month of taking itraconazole without the statin,” she said. “Or they could stop statins for the time you treat, if cleared by their doctor.”
As for fluconazole, Dr. Elewski usually prescribes 200 mg once or twice per week until the nail is normal. She offers patients the mnemonic for “Fungal Fridays” or Toesdays” as a way for them to remember which day to take the fluconazole.
According to data in the package inserts, rates of complete and mycologic cures are 38% and 70% for terbinafine, respectively, 14% and 54% for itraconazole, and 37% to 48% and 47% to 62% for fluconazole. “These cures are not 100% based on the standard course [of the drug],” Dr. Elewski noted. “I don’t use the standard course. I believe in treating to terminate. You want to kill the fungus.”
Resistant dermatophytes ‘are coming’
Halting treatment with an oral drug at a particular time point instead of when the nail is fungal-free likely contributes to resistant strains, she added, noting that she has at least two dozen patients in her practice with dermatophyte resistance documented in labs. “We need to be antifungal stewards, because resistant dermatophytes are coming to us,” she said. “They’re here already, and we don’t want it to be endemic in the U.S.”
In a published study from 2020, researchers from India enrolled 200 patients with relapsing tinea corporis, tinea cruris, and tinea faciei and allocated 50 each to treatment with either fluconazole, griseofulvin, itraconazole, or terbinafine. At week 4, all treatment arms had cure rates of less than 8%. At week 8, the cure rates were 42% for fluconazole, 16% for griseofulvin, 28% for terbinafine, and 66% for itraconazole.
Based in part on these study findings, Dr. Elewski said that she has become more aggressive in her therapeutic approach, including treating some of her patients on terbinafine for a minimum of 6 months. “If that’s not enough, I keep treating,” she said. “But, patients may not respond to terbinafine; we see resistance. So, itraconazole may be our best drug going forward for treating onychomycosis. You just have to watch out for side effects of itraconazole, mainly drug-drug interactions.”
Dr. Elewski reported having no relevant financial disclosures related to her presentation.
NEW ORLEANS – .
“The PAS [periodic acid-Schiff] stain is very popular because it can identify the presence or absence of fungal elements, but a fungal culture will identify the organism living in the nail,” Dr. Elewski, professor and chair of dermatology at the University of Alabama, Birmingham, said at the annual meeting of the American Academy of Dermatology. “You also could do a PCR to identify the organism, with or without a KOH or PAS stain. It is often helpful to know what organism is causing the infection.”
While waiting for lab results, there are three clinical clues to look for – the first being that an infection likely resides in the toenail. “You almost never see dermatophyte onychomycosis in the fingernails without it being in the toenails, too,” Dr. Elewski said.
The presence of tinea pedis is a second clinical clue. “Sometimes it’s subtle, so I will ask the patient, ‘Have you been treating yourself for athlete’s foot?’ If they say ‘no, I’ve never had it,’ put down on your list that it’s unlikely they have onychomycosis. How is the fungus going to jump from the floor into the nail without taking a little vacation on the bottom of the foot? It just isn’t going to happen.”
The presence of dermatophytoma is the third clinical clue. “These are dermatophyte abscesses encased in a biofilm, and they’re really hard to treat,” she said.
Treatments
Clinicians typically turn to one of three oral drugs for treating onychomycosis: terbinafine, itraconazole, and fluconazole, Dr. Elewski noted. Referring to terbinafine as “the gold standard,” she said that she typically writes a prescription for 90 250-mg pills. “When I give terbinafine, I often do baseline liver profiling, depending on the patient’s age, their state of health, their comorbidities, and other medications they’re taking,” she said. “If they’re 18 years old and otherwise healthy, I probably don’t.” While she generally prescribes 90 pills, she added, “keep in mind that 90 pills are not going to cure everybody. I see the patient 4 months later because the drug should stay in the nail for 30 days or more at therapeutic levels after you take that 90-day course.”
Another option is itraconazole, which can be taken at a dose of 200 mg a day for 12 weeks, or at a pulse dose, where patients take 400 mg every day for 1 week, 1 week a month, for 4 consecutive months. “I’ll often do a baseline liver profile with itraconazole, too,” Dr. Elewski said. “I don’t think you have to, but it makes sense if it’s feasible for you. Decide that based on each patient.”
Itraconazole can’t be given concomitantly with statins because of the potential for rhabdomyolysis. For patients taking statins, she consults with their physicians to make sure it’s safe to stop the statin a couple of days before and after their scheduled pulse dose of itraconazole. “This involves 1 week per month of taking itraconazole without the statin,” she said. “Or they could stop statins for the time you treat, if cleared by their doctor.”
As for fluconazole, Dr. Elewski usually prescribes 200 mg once or twice per week until the nail is normal. She offers patients the mnemonic for “Fungal Fridays” or Toesdays” as a way for them to remember which day to take the fluconazole.
According to data in the package inserts, rates of complete and mycologic cures are 38% and 70% for terbinafine, respectively, 14% and 54% for itraconazole, and 37% to 48% and 47% to 62% for fluconazole. “These cures are not 100% based on the standard course [of the drug],” Dr. Elewski noted. “I don’t use the standard course. I believe in treating to terminate. You want to kill the fungus.”
Resistant dermatophytes ‘are coming’
Halting treatment with an oral drug at a particular time point instead of when the nail is fungal-free likely contributes to resistant strains, she added, noting that she has at least two dozen patients in her practice with dermatophyte resistance documented in labs. “We need to be antifungal stewards, because resistant dermatophytes are coming to us,” she said. “They’re here already, and we don’t want it to be endemic in the U.S.”
In a published study from 2020, researchers from India enrolled 200 patients with relapsing tinea corporis, tinea cruris, and tinea faciei and allocated 50 each to treatment with either fluconazole, griseofulvin, itraconazole, or terbinafine. At week 4, all treatment arms had cure rates of less than 8%. At week 8, the cure rates were 42% for fluconazole, 16% for griseofulvin, 28% for terbinafine, and 66% for itraconazole.
Based in part on these study findings, Dr. Elewski said that she has become more aggressive in her therapeutic approach, including treating some of her patients on terbinafine for a minimum of 6 months. “If that’s not enough, I keep treating,” she said. “But, patients may not respond to terbinafine; we see resistance. So, itraconazole may be our best drug going forward for treating onychomycosis. You just have to watch out for side effects of itraconazole, mainly drug-drug interactions.”
Dr. Elewski reported having no relevant financial disclosures related to her presentation.
NEW ORLEANS – .
“The PAS [periodic acid-Schiff] stain is very popular because it can identify the presence or absence of fungal elements, but a fungal culture will identify the organism living in the nail,” Dr. Elewski, professor and chair of dermatology at the University of Alabama, Birmingham, said at the annual meeting of the American Academy of Dermatology. “You also could do a PCR to identify the organism, with or without a KOH or PAS stain. It is often helpful to know what organism is causing the infection.”
While waiting for lab results, there are three clinical clues to look for – the first being that an infection likely resides in the toenail. “You almost never see dermatophyte onychomycosis in the fingernails without it being in the toenails, too,” Dr. Elewski said.
The presence of tinea pedis is a second clinical clue. “Sometimes it’s subtle, so I will ask the patient, ‘Have you been treating yourself for athlete’s foot?’ If they say ‘no, I’ve never had it,’ put down on your list that it’s unlikely they have onychomycosis. How is the fungus going to jump from the floor into the nail without taking a little vacation on the bottom of the foot? It just isn’t going to happen.”
The presence of dermatophytoma is the third clinical clue. “These are dermatophyte abscesses encased in a biofilm, and they’re really hard to treat,” she said.
Treatments
Clinicians typically turn to one of three oral drugs for treating onychomycosis: terbinafine, itraconazole, and fluconazole, Dr. Elewski noted. Referring to terbinafine as “the gold standard,” she said that she typically writes a prescription for 90 250-mg pills. “When I give terbinafine, I often do baseline liver profiling, depending on the patient’s age, their state of health, their comorbidities, and other medications they’re taking,” she said. “If they’re 18 years old and otherwise healthy, I probably don’t.” While she generally prescribes 90 pills, she added, “keep in mind that 90 pills are not going to cure everybody. I see the patient 4 months later because the drug should stay in the nail for 30 days or more at therapeutic levels after you take that 90-day course.”
Another option is itraconazole, which can be taken at a dose of 200 mg a day for 12 weeks, or at a pulse dose, where patients take 400 mg every day for 1 week, 1 week a month, for 4 consecutive months. “I’ll often do a baseline liver profile with itraconazole, too,” Dr. Elewski said. “I don’t think you have to, but it makes sense if it’s feasible for you. Decide that based on each patient.”
Itraconazole can’t be given concomitantly with statins because of the potential for rhabdomyolysis. For patients taking statins, she consults with their physicians to make sure it’s safe to stop the statin a couple of days before and after their scheduled pulse dose of itraconazole. “This involves 1 week per month of taking itraconazole without the statin,” she said. “Or they could stop statins for the time you treat, if cleared by their doctor.”
As for fluconazole, Dr. Elewski usually prescribes 200 mg once or twice per week until the nail is normal. She offers patients the mnemonic for “Fungal Fridays” or Toesdays” as a way for them to remember which day to take the fluconazole.
According to data in the package inserts, rates of complete and mycologic cures are 38% and 70% for terbinafine, respectively, 14% and 54% for itraconazole, and 37% to 48% and 47% to 62% for fluconazole. “These cures are not 100% based on the standard course [of the drug],” Dr. Elewski noted. “I don’t use the standard course. I believe in treating to terminate. You want to kill the fungus.”
Resistant dermatophytes ‘are coming’
Halting treatment with an oral drug at a particular time point instead of when the nail is fungal-free likely contributes to resistant strains, she added, noting that she has at least two dozen patients in her practice with dermatophyte resistance documented in labs. “We need to be antifungal stewards, because resistant dermatophytes are coming to us,” she said. “They’re here already, and we don’t want it to be endemic in the U.S.”
In a published study from 2020, researchers from India enrolled 200 patients with relapsing tinea corporis, tinea cruris, and tinea faciei and allocated 50 each to treatment with either fluconazole, griseofulvin, itraconazole, or terbinafine. At week 4, all treatment arms had cure rates of less than 8%. At week 8, the cure rates were 42% for fluconazole, 16% for griseofulvin, 28% for terbinafine, and 66% for itraconazole.
Based in part on these study findings, Dr. Elewski said that she has become more aggressive in her therapeutic approach, including treating some of her patients on terbinafine for a minimum of 6 months. “If that’s not enough, I keep treating,” she said. “But, patients may not respond to terbinafine; we see resistance. So, itraconazole may be our best drug going forward for treating onychomycosis. You just have to watch out for side effects of itraconazole, mainly drug-drug interactions.”
Dr. Elewski reported having no relevant financial disclosures related to her presentation.
AT AAD 2023
Celebrity death finally solved – with locks of hair
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.
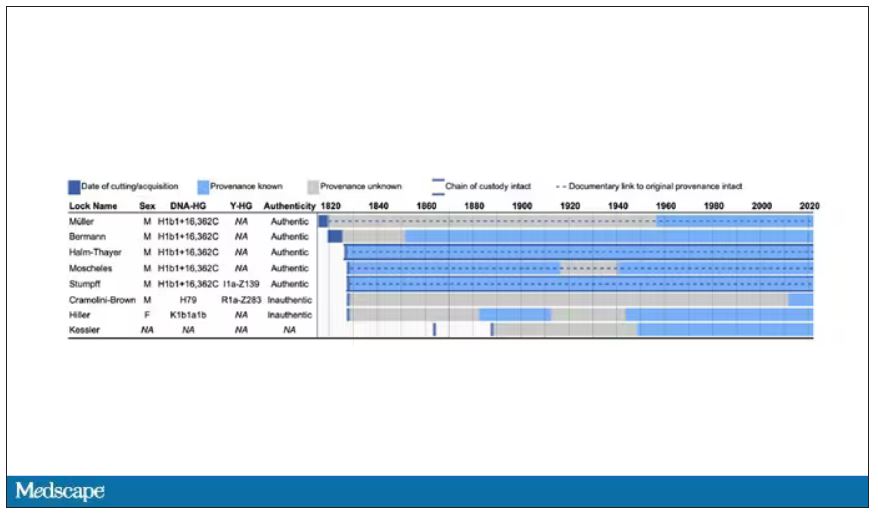
The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.
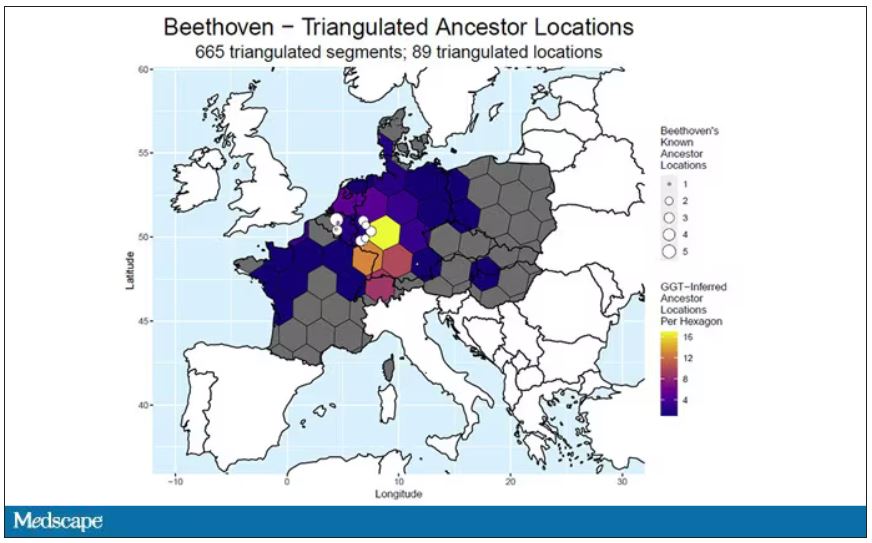
In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.
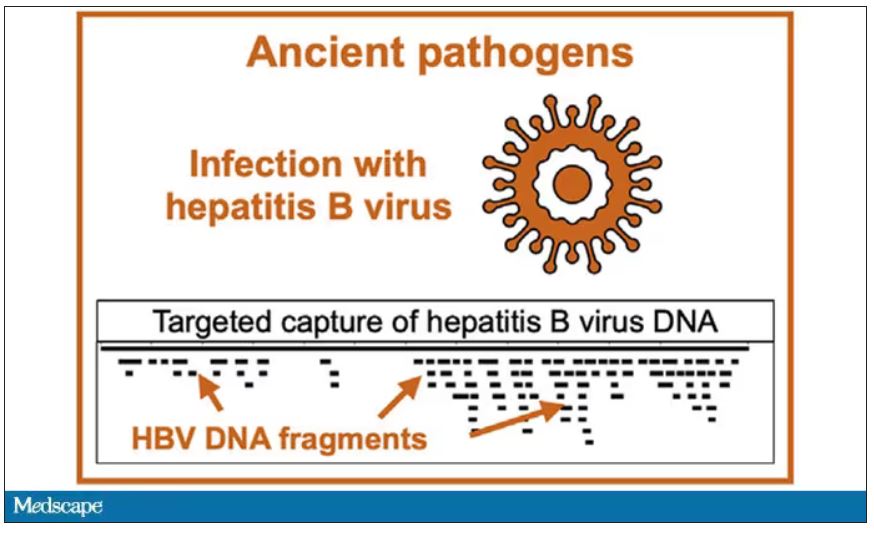
It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.

The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.

In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.

It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
I’m going to open this week with a case.
A 56-year-old musician presents with diffuse abdominal pain, cramping, and jaundice. His medical history is notable for years of diffuse abdominal complaints, characterized by disabling bouts of diarrhea.
In addition to the jaundice, this acute illness was accompanied by fever as well as diffuse edema and ascites. The patient underwent several abdominal paracenteses to drain excess fluid. One consulting physician administered alcohol to relieve pain, to little avail.
The patient succumbed to his illness. An autopsy showed diffuse liver injury, as well as papillary necrosis of the kidneys. Notably, the nerves of his auditory canal were noted to be thickened, along with the bony part of the skull, consistent with Paget disease of the bone and explaining, potentially, why the talented musician had gone deaf at such a young age.
An interesting note on social history: The patient had apparently developed some feelings for the niece of that doctor who prescribed alcohol. Her name was Therese, perhaps mistranscribed as Elise, and it seems that he may have written this song for her.
We’re talking about this paper in Current Biology, by Tristan Begg and colleagues, which gives us a look into the very genome of what some would argue is the world’s greatest composer.
The ability to extract DNA from older specimens has transformed the fields of anthropology, archaeology, and history, and now, perhaps, musicology as well.
The researchers identified eight locks of hair in private and public collections, all attributed to the maestro.
Four of the samples had an intact chain of custody from the time the hair was cut. DNA sequencing on these four and an additional one of the eight locks came from the same individual, a male of European heritage.

The three locks with less documentation came from three other unrelated individuals. Interestingly, analysis of one of those hair samples – the so-called Hiller Lock – had shown high levels of lead, leading historians to speculate that lead poisoning could account for some of Beethoven’s symptoms.

DNA analysis of that hair reveals it to have come from a woman likely of North African, Middle Eastern, or Jewish ancestry. We can no longer presume that plumbism was involved in Beethoven’s death. Beethoven’s ancestry turns out to be less exotic and maps quite well to ethnic German populations today.

In fact, there are van Beethovens alive as we speak, primarily in Belgium. Genealogic records suggest that these van Beethovens share a common ancestor with the virtuoso composer, a man by the name of Aert van Beethoven.
But the DNA reveals a scandal.
The Y-chromosome that Beethoven inherited was not Aert van Beethoven’s. Questions of Beethoven’s paternity have been raised before, but this evidence strongly suggests an extramarital paternity event, at least in the generations preceding his birth. That’s right – Beethoven may not have been a Beethoven.
With five locks now essentially certain to have come from Beethoven himself, the authors could use DNA analysis to try to explain three significant health problems he experienced throughout his life and death: his hearing loss, his terrible gastrointestinal issues, and his liver failure.
Let’s start with the most disappointing results, explanations for his hearing loss. No genetic cause was forthcoming, though the authors note that they have little to go on in regard to the genetic risk for otosclerosis, to which his hearing loss has often been attributed. Lead poisoning is, of course, possible here, though this report focuses only on genetics – there was no testing for lead – and as I mentioned, the lock that was strongly lead-positive in prior studies is almost certainly inauthentic.
What about his lifelong GI complaints? Some have suggested celiac disease or lactose intolerance as explanations. These can essentially be ruled out by the genetic analysis, which shows no risk alleles for celiac disease and the presence of the lactase-persistence gene which confers the ability to metabolize lactose throughout one’s life. IBS is harder to assess genetically, but for what it’s worth, he scored quite low on a polygenic risk score for the condition, in just the 9th percentile of risk. We should probably be looking elsewhere to explain the GI distress.
The genetic information bore much more fruit in regard to his liver disease. Remember that Beethoven’s autopsy showed cirrhosis. His polygenic risk score for liver cirrhosis puts him in the 96th percentile of risk. He was also heterozygous for two variants that can cause hereditary hemochromatosis. The risk for cirrhosis among those with these variants is increased by the use of alcohol. And historical accounts are quite clear that Beethoven consumed more than his share.
But it wasn’t just Beethoven’s DNA in these hair follicles. Analysis of a follicle from later in his life revealed the unmistakable presence of hepatitis B virus. Endemic in Europe at the time, this was a common cause of liver failure and is likely to have contributed to, if not directly caused, Beethoven’s demise.

It’s hard to read these results and not marvel at the fact that, two centuries after his death, our fascination with Beethoven has led us to probe every corner of his life – his letters, his writings, his medical records, and now his very DNA. What are we actually looking for? Is it relevant to us today what caused his hearing loss? His stomach troubles? Even his death? Will it help any patients in the future? I propose that what we are actually trying to understand is something ineffable: Genius of magnitude that is rarely seen in one or many lifetimes. And our scientific tools, as sharp as they may have become, are still far too blunt to probe the depths of that transcendence.
In any case, friends, no more of these sounds. Let us sing more cheerful songs, more full of joy.
For Medscape, I’m Perry Wilson.
Dr. Wilson is associate professor, department of medicine, and director, Clinical and Translational Research Accelerator, at Yale University, New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
Tyrosine kinase inhibitors – a new weapon against respiratory viruses?
Five different nonreceptor tyrosine kinase inhibitors were effective against viral replication of pandemic viruses and seasonal influenza viruses in an ex vivo lung model.
Influenza viruses remain a high cause of morbidity and mortality worldwide as viral mutations outwit vaccine efficacy, Robert Meineke, PhD, of the University of Veterinary Medicine in Hannover, Germany, and colleagues wrote.
“As with previous influenza pandemics and the current SARS-CoV-2 pandemic, effective vaccines are not readily available at early stages of a pandemic,” they noted. To help manage the limitations of timing and effectiveness of current vaccines, the researchers proposed repurposing nonreceptor tyrosine kinase inhibitors (NRTKIs) to block seasonal flu and COVID-19 viral replication.
In a study published in iScience, the researchers identified six NRTKIs currently approved by the U.S. Food and Drug Administration that showed in vitro inhibition of both pandemic viruses (H1N1) and seasonal influenza viruses (H3N2). These included defactinib, acalabrutinib, saracatinib, and bosutinib, all of which reduced hPCLS infectivity by approximately 50%. In addition, ibrutinib and bosutinib had the largest impact on viral titers. The antiviral effects of NRTKIs appeared to be independent of multiplicity of infection.
The researchers then tested the NRIKIs on an ex vivo model of human precision-cut lung slices to validate the effects of NRTKIs as antivirals against influenza A viruses (IAVs).
In this model, the highest peak titers were achieved at 48 hpi following infection with virus strains NL09 and NL11. The hPCLS models also showed consistent tolerability to 1x concentrations. “Our cytotoxicity cut-off was 20% of the positive control treatment; none of the NRTKIs surpassed this cutoff at [1x] max,” the researchers wrote.
Five of the six identified NRTKIs were validated in the ex vivo setting. All five reduced viral titers by at least 10-fold to more than 1,000-fold. Of these, ibrutinib, bosutinib, and bosutinib showed a significant effect at all concentrations, while treatments with acalabrutinib and defactinib were significant at 24 hpi and 48 hpi. The NRTKs also showed a high genetic barrier against emerging resistant virus mutations.
The study demonstrates the ability of NRTKIs to target kinases required for replication of IAV, the researchers wrote, and that NRTKIs “represent promising drugs for the development of the next generation of antivirals.”
More research is needed to determine the therapeutic window given that NRTKIs are targeting host factors versus virus-targeted antivirals, but the advantages of NRTKIs include localized delivery that can limit possible cytotoxic effects, and their safety and bioavailability are well established, they said.
The findings were limited by several factors including the use of lung tissue mainly from older donors with lung cancer, the researchers noted. However, this population could be considered at increased risk for IAVs and therefore the data are more clinically applicable.
In addition, “because many viruses utilize the same (or related) host kinases to facilitate replication and transmission, our studies have broader implications for the potential use of these SMKIs to treat infections by other viruses,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Five different nonreceptor tyrosine kinase inhibitors were effective against viral replication of pandemic viruses and seasonal influenza viruses in an ex vivo lung model.
Influenza viruses remain a high cause of morbidity and mortality worldwide as viral mutations outwit vaccine efficacy, Robert Meineke, PhD, of the University of Veterinary Medicine in Hannover, Germany, and colleagues wrote.
“As with previous influenza pandemics and the current SARS-CoV-2 pandemic, effective vaccines are not readily available at early stages of a pandemic,” they noted. To help manage the limitations of timing and effectiveness of current vaccines, the researchers proposed repurposing nonreceptor tyrosine kinase inhibitors (NRTKIs) to block seasonal flu and COVID-19 viral replication.
In a study published in iScience, the researchers identified six NRTKIs currently approved by the U.S. Food and Drug Administration that showed in vitro inhibition of both pandemic viruses (H1N1) and seasonal influenza viruses (H3N2). These included defactinib, acalabrutinib, saracatinib, and bosutinib, all of which reduced hPCLS infectivity by approximately 50%. In addition, ibrutinib and bosutinib had the largest impact on viral titers. The antiviral effects of NRTKIs appeared to be independent of multiplicity of infection.
The researchers then tested the NRIKIs on an ex vivo model of human precision-cut lung slices to validate the effects of NRTKIs as antivirals against influenza A viruses (IAVs).
In this model, the highest peak titers were achieved at 48 hpi following infection with virus strains NL09 and NL11. The hPCLS models also showed consistent tolerability to 1x concentrations. “Our cytotoxicity cut-off was 20% of the positive control treatment; none of the NRTKIs surpassed this cutoff at [1x] max,” the researchers wrote.
Five of the six identified NRTKIs were validated in the ex vivo setting. All five reduced viral titers by at least 10-fold to more than 1,000-fold. Of these, ibrutinib, bosutinib, and bosutinib showed a significant effect at all concentrations, while treatments with acalabrutinib and defactinib were significant at 24 hpi and 48 hpi. The NRTKs also showed a high genetic barrier against emerging resistant virus mutations.
The study demonstrates the ability of NRTKIs to target kinases required for replication of IAV, the researchers wrote, and that NRTKIs “represent promising drugs for the development of the next generation of antivirals.”
More research is needed to determine the therapeutic window given that NRTKIs are targeting host factors versus virus-targeted antivirals, but the advantages of NRTKIs include localized delivery that can limit possible cytotoxic effects, and their safety and bioavailability are well established, they said.
The findings were limited by several factors including the use of lung tissue mainly from older donors with lung cancer, the researchers noted. However, this population could be considered at increased risk for IAVs and therefore the data are more clinically applicable.
In addition, “because many viruses utilize the same (or related) host kinases to facilitate replication and transmission, our studies have broader implications for the potential use of these SMKIs to treat infections by other viruses,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Five different nonreceptor tyrosine kinase inhibitors were effective against viral replication of pandemic viruses and seasonal influenza viruses in an ex vivo lung model.
Influenza viruses remain a high cause of morbidity and mortality worldwide as viral mutations outwit vaccine efficacy, Robert Meineke, PhD, of the University of Veterinary Medicine in Hannover, Germany, and colleagues wrote.
“As with previous influenza pandemics and the current SARS-CoV-2 pandemic, effective vaccines are not readily available at early stages of a pandemic,” they noted. To help manage the limitations of timing and effectiveness of current vaccines, the researchers proposed repurposing nonreceptor tyrosine kinase inhibitors (NRTKIs) to block seasonal flu and COVID-19 viral replication.
In a study published in iScience, the researchers identified six NRTKIs currently approved by the U.S. Food and Drug Administration that showed in vitro inhibition of both pandemic viruses (H1N1) and seasonal influenza viruses (H3N2). These included defactinib, acalabrutinib, saracatinib, and bosutinib, all of which reduced hPCLS infectivity by approximately 50%. In addition, ibrutinib and bosutinib had the largest impact on viral titers. The antiviral effects of NRTKIs appeared to be independent of multiplicity of infection.
The researchers then tested the NRIKIs on an ex vivo model of human precision-cut lung slices to validate the effects of NRTKIs as antivirals against influenza A viruses (IAVs).
In this model, the highest peak titers were achieved at 48 hpi following infection with virus strains NL09 and NL11. The hPCLS models also showed consistent tolerability to 1x concentrations. “Our cytotoxicity cut-off was 20% of the positive control treatment; none of the NRTKIs surpassed this cutoff at [1x] max,” the researchers wrote.
Five of the six identified NRTKIs were validated in the ex vivo setting. All five reduced viral titers by at least 10-fold to more than 1,000-fold. Of these, ibrutinib, bosutinib, and bosutinib showed a significant effect at all concentrations, while treatments with acalabrutinib and defactinib were significant at 24 hpi and 48 hpi. The NRTKs also showed a high genetic barrier against emerging resistant virus mutations.
The study demonstrates the ability of NRTKIs to target kinases required for replication of IAV, the researchers wrote, and that NRTKIs “represent promising drugs for the development of the next generation of antivirals.”
More research is needed to determine the therapeutic window given that NRTKIs are targeting host factors versus virus-targeted antivirals, but the advantages of NRTKIs include localized delivery that can limit possible cytotoxic effects, and their safety and bioavailability are well established, they said.
The findings were limited by several factors including the use of lung tissue mainly from older donors with lung cancer, the researchers noted. However, this population could be considered at increased risk for IAVs and therefore the data are more clinically applicable.
In addition, “because many viruses utilize the same (or related) host kinases to facilitate replication and transmission, our studies have broader implications for the potential use of these SMKIs to treat infections by other viruses,” they concluded.
The study received no outside funding. The researchers had no financial conflicts to disclose.
FROM ISCIENCE
Cases of potentially deadly fungus jump 200%: CDC
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.
prompting the Centers for Disease Control and Prevention to issue a warning to health care facilities about the rising threat.
C. auris is a yeast that spreads easily from touching it on a surface like a countertop. It can also spread from person to person. It isn’t a threat to healthy people, but people in hospitals and nursing homes are at a heightened risk because they might have weakened immune systems or be using invasive medical devices that can introduce the fungus inside their bodies. When C. auris progresses to causing an infection that reaches the brain, blood, or lungs, more than one in three people die.
The worrying increase was detailed in the journal Annals of Internal Medicine. In 2021, cases reached a count of 3,270 with an active infection, and 7,413 cases showed the fungus was present but hadn’t caused an infection. Infection counts were up 95% over the previous year, and the fungus showed up on screenings three times as often. The number of cases resistant to medication also tripled.
The CDC called the figures “alarming,” noting that the fungus was only detected in the United States in 2016.
“The timing of this increase and findings from public health investigations suggest C. auris spread may have worsened due to strain on health care and public health systems during the COVID-19 pandemic,” the CDC explained in a news release.
Another potential reason for the jump could be that screening for C. auris has simply increased and it’s being found more often because it’s being looked for more often. But researchers believe that, even with the increase in testing, the reported counts are underestimated. That’s because even though screening has increased, health care providers still aren’t looking for the presence of the fungus as often as the CDC would like.
“The rapid rise and geographic spread of cases is concerning and emphasizes the need for continued surveillance, expanded lab capacity, quicker diagnostic tests, and adherence to proven infection prevention and control,” said study author Meghan Lyman, MD, a CDC epidemiologist in Atlanta, in a statement.
Cases of C. auris continued to rise in 2022, the CDC said. A map on the agency’s website of reported cases from 2022 shows it was found in more than half of U.S. states, with the highest counts occurring in California, Florida, Illinois, Nevada, New York, and Texas. The fungus is a problem worldwide and is listed among the most threatening treatment-resistant fungi by the World Health Organization.
The study authors concluded that screening capacity for the fungus needs to be expanded nationwide so that when C. auris is detected, measures can be taken to prevent its spread.
A version of this article originally appeared on WebMD.com.