User login
Metformin use may curb BCC risk
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
in Iceland.
“In addition to general anticarcinogenic effects, metformin has also been shown to directly inhibit the sonic hedgehog pathway, a key pathway in basal cell carcinoma (BCC) pathogenesis,” Jonas A. Adalsteinsson, MD, of the University of Iceland, Reykjavik, and colleagues wrote. “The relationship between metformin and keratinocyte carcinoma has not been well-characterized but is of importance considering that metformin is a commonly prescribed medication.”
They added that the hedgehog pathway inhibitors vismodegib (Erivedge) and sonidegib (Odomzo), approved for treating BCC, “are highly effective for BCC prevention, but their broad use for BCC prophylaxis is limited due to numerous side effects.”
In the study, published in the Journal of the American Academy of Dermatology, the researchers identified 6,880 first-time cancer patients with BCC, squamous cell carcinoma in situ (SCCis), or invasive SCC, and 69,620 population controls using data from the Icelandic Cancer Registry and the Icelandic Prescription Medicine Register between 2003 and 2017. Metformin exposure was defined as having filled at least one prescription of metformin more than 2 years prior to cancer diagnosis. They used grams and daily dose units of metformin in their analysis; one DDU of metformin, “or its average daily maintenance dose when used for its primary indication, is 2 grams,” they noted.
Overall, metformin use was associated with a significantly lower risk of developing BCC, compared with nonuse (adjusted odds ratio, 0.71; 95% confidence interval, 0.61-0.83).
The reduced risk occurred similarly across age and gender subgroups, with the exception of individuals younger than 60 years, the researchers said. “This might signify that metformin has less of a protective effect in younger individuals, but we might also have lacked power in this category.” The association with reduced BCC risk remained significant at all three cumulative dose levels measured: 1-500 DDUs, 501-1,500 DDUs, and more than 1,500 DDUs.
Metformin use was not significantly associated with reduced risk of invasive SCC (aOR, 1.01) and in most cases of SCCis. However, the 501-1,500 DDU dose category was associated with a slight increase in risk of SCCis (aOR, 1.40; 95% CI, 1.00-1.96), “showing a possible increased risk of SCCis,” the authors wrote.
The decrease in BCC risk was seen across all metformin dosing levels, but the reason for this remains unclear, and might be related to a confounding factor that was not considered in this study, the researchers said. “It could also be that metformin’s BCC risk-lowering effect is immediate, with only a low dose being needed to see a clinical benefit.”
The study findings were limited by several factors, including the retrospective design and the inability to adjust for factors including ultraviolet exposure, Fitzpatrick skin type, and comorbidities. The frequent use of metformin by people with type 2 diabetes suggests diabetes itself or other diabetes medications could be possible confounding factors, the researchers wrote.
However, the results were strengthened by the large study population, and the data suggest an association between reduced risk of first-time BCC and metformin use, they added.
“Randomized, prospective trials are required to fully understand the effect metformin has on BCC and SCC risk,” the researchers concluded.
“There is a dire need to reduce incidence of skin cancers in general, and consequently a need for new non-surgical treatment options for keratinocytic nonmelanoma skin cancers,” Amor Khachemoune, MD, a dermatologist at the State University of New York, Brooklyn, and the department of dermatology of the Veteran Affairs NY Harbor Healthcare System, also in Brooklyn, said in an interview.
Dr. Khachemoune, who was not involved with the study, said that he was not surprised by the findings. “Like other well-studied sonic hedgehog inhibitors, vismodegib and sonidegib, metformin has a demonstrated effect on this pathway. The medical community outside of dermatology has extensive experience with the use of metformin for a host of other indications, including its role as anticarcinogenic, so it seemed natural that one would consider widening its use to quell the ever-expanding cases of basal cell carcinomas.”
However, complications from long-term use, though likely rare, could be a limitation in using metformin as a chemoprotective agent, Dr. Khachemoune said. Metformin-associated lactic acidosis is one example of a rare, but potentially life-threatening adverse event.
“Finding the right dosage and having an algorithm for follow up monitoring of side effects would certainly need to be put in place in a standardized way,” he emphasized. “As stated by the authors of this study, more inclusive research involving other groups with nonkeratinocytic malignancies in larger cohorts is needed.”
The study received no outside funding. The researchers and Dr. Khachemoune had no financial conflicts to disclose.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY
Latest FDA pembrolizumab approval expands label to cutaneous SCCs
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
The
The July 6 approval for the programmed death–1 inhibitor follows a June FDA approval for pembrolizumab monotherapy in patients with recurrent or metastatic cSCC disease not curable by surgery or radiation. Both approvals, pembrolizumab’s first for cSCC, are based on findings from the second interim analysis of the phase 2, multicenter, open-label KEYNOTE-629 trial.
The objective response rate in the cohort of 54 patients with locally advanced disease was 50%, including a complete response rate of 17% and a partial response rate of 33%. Duration of response was 6 months or longer in 81% of the 27 responders, and 12 months or longer in 37% of responders. After a median follow-up of 13.4 months, median duration of response had not yet been reached.
Pembrolizumab has previously received FDA approvals, either as monotherapy or in combination with other agents, for the treatment of numerous cancer types, including certain melanomas, non–small cell lung cancers, head and neck SCCs, classical Hodgkin lymphomas, primary mediastinal large B-cell lymphomas, urothelial carcinomas, microsatellite instability–high or mismatch repair–deficient cancers, and gastric, esophageal, cervical, hepatocellular, Merkel cell, renal cell, tumor mutational burden–high, and triple-negative breast cancers.
Patients in the KEYNOTE-629 trial received pembrolizumab at a dose of 200 mg IV every 3 weeks for 24 months or until documented disease progression or unacceptable toxicity.
Adverse reactions occurring in patients with recurrent or metastatic cSCC or locally advanced cSCC in KEYNOTE-629 were similar to those observed in patients with melanoma or non–small cell lung cancer who were treated with pembrolizumab monotherapy in previous trials.
The checkpoint inhibitor can cause immune-mediated adverse reactions, which may be severe or fatal, according to Merck, the drug’s manufacturer. The reactions can occur in any organ system or tissue and can affect more than one body system simultaneously.
“Immune-mediated adverse reactions can occur at any time during or after treatment with Keytruda, including pneumonitis, colitis, hepatitis, endocrinopathies, nephritis, dermatologic reactions, solid organ transplant rejection, and complications of allogeneic hematopoietic stem cell transplantation,” Merck explained in a press release, noting that “early identification and management of immune-mediated adverse reactions are essential to ensure safe use of Keytruda.”
Depending on the severity of any reaction, treatment should be withheld or permanently discontinued, and corticosteroids administered if appropriate, Merck stated.
Etanercept-Induced Squamous Proliferations in a Patient With Porokeratosis
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
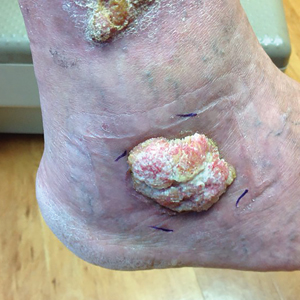
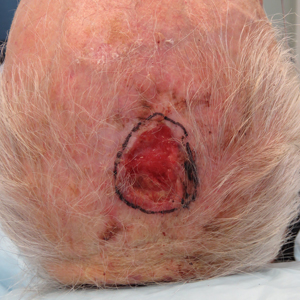
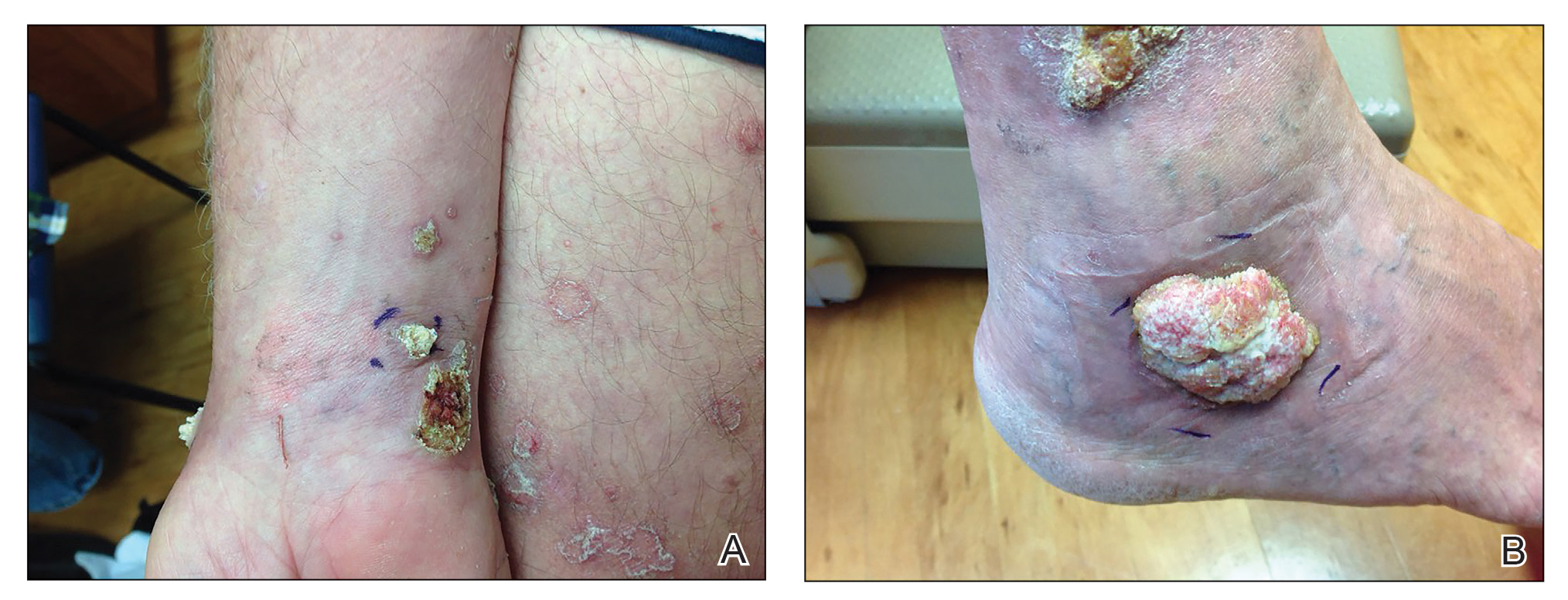
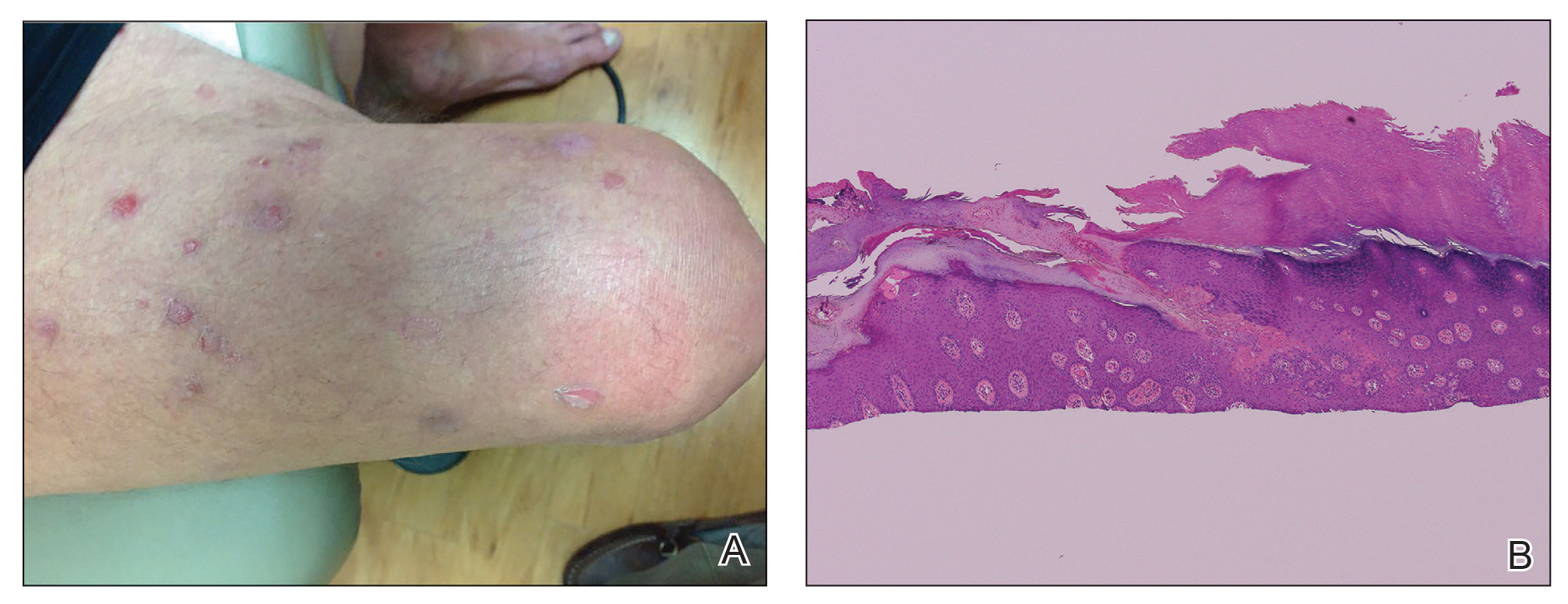
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).
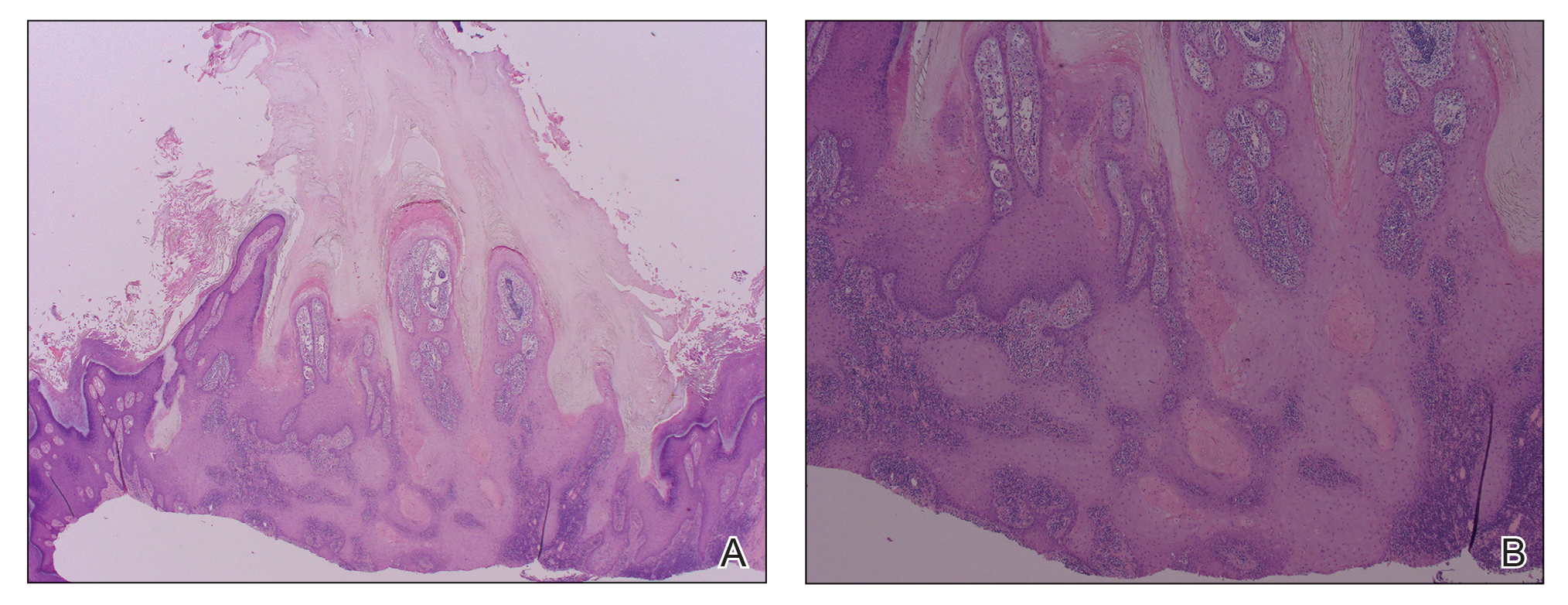
The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).



The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
To the Editor:
Etanercept is an immune-modulating drug used for the treatment of a variety of diseases including psoriasis, rheumatoid arthritis, and ankylosing spondylitis. It is an anti–tumor necrosis factor (TNF) fusion protein consisting of an extracellular domain of the p75 TNF receptor and the Fc portion of human IgG.1 Etanercept is well known for its immunosuppressive side effects. A handful of case reports have provided evidence of squamous cell cancers in the setting of etanercept therapy. The most comprehensive description was a case series by Brewer et al2 describing 4 patients with squamous cell carcinoma (SCC) that developed 1 to 17 months after the initiation of etanercept therapy. We present a case of a patient diagnosed with psoriasis and concomitant porokeratosis who developed multiple SCCs and squamous proliferations after initiation of etanercept therapy.
A 66-year-old man was referred to our clinic for treatment of psoriasis, as noted on a biopsy of the right ankle diagnosed several years prior. He was being treated with etanercept 50 mg twice weekly. Other treatments included calcipotriene–betamethasone dipropionate, salicylic acid gel, intralesional triamcinolone, clobetasol, and urea 40%. Physical examination revealed multiple erythematous tender nodules with hyperkeratotic scale distributed on the right arm and leg (Figure 1) that were concerning for SCC. Biopsies from 6 lesions revealed multiple SCC/keratoacanthomas (KAs) with verrucous features (Figure 2). Primers for human papillomavirus (HPV) 6, 11, 16, 18, 31, 33, and 51 were all negative. At that time, etanercept was discontinued. The patient was referred for Mohs micrographic surgery and underwent excision of several SCC lesions including an approximately 7-cm SCC on the right ankle (Figure 1B). Positron emission tomography/computed tomography found hypermetabolic lymphadenopathy. A follow-up biopsy of the inguinal nodes identified no malignant cells. Given their multiplicity, the patient was initiated on a prolonged course of a retinoid with acitretin 35 mg daily. The clearance of the large 7-cm lesion with a single stage of Mohs micrographic surgery directed suspicion to a pseudoepitheliomatous or HPV-induced cause for the lesions. Rereview of the original 6 biopsies indicated 1 definitive SCC on the right wrist, 2 KAs, and 3 that were most consistent with verruca vulgaris. At 1-year follow-up, most of the hyperkeratotic lesions had resolved with continued acitretin. Baseline porokeratosis lesions that were abundantly present on the arms and legs resolved by 1-year follow-up (Figure 3A).



The link between classic porokeratosis and the development of squamous cell proliferations is well established. Ninomiya et al3 noted a possible mechanism of p53 overexpression in the epidermis of porokeratotic lesions that may make the lesions particularly susceptible to the development of immunosuppression-induced SCC. Etanercept is an immune-modulating drug with well-known immunosuppressive side effects including reactivation of HPV as well as the development of SCCs.
Our patient initially was diagnosed with psoriasis and etanercept was initiated. The presence of coexistent porokeratosis likely predisposed him to etanercept-induced squamous proliferations including 2 SCCs and verrucous lesions, with histologic features suggesting SCC/KA. Histopathology revealed a cornoid lamella in SCC (Figure 3B), suggesting development of malignancy within epithelial clones, as noted by Lee et al.4
Targeted systemic therapies may lead to the formation of SCCs. The association between epidermal growth factor receptor (EGFR) kinase inhibitors and SCC formation is well known. For instance, sorafenib—a multikinase inhibitor that is downstream in the EGFR pathway—has been noted to induce epidermal growths including KAs and SCCs.5 There has been no definitive causal relationship identified between the development of SCC and TNF-α inhibitors. It has been suggested that perhaps there is an unmasking effect, as subclinical SCC manifests after TNF-α inhibition that leads to SCC development. Discontinuation of etanercept and resolution of lesions highlights a potential role of TNF-α inhibition and tumorigenesis of SCCs, especially in the background of porokeratosis. Vigilance for development of immunosuppression-induced malignancy, especially squamous cell proliferations, has become exceedingly important with exponentially increasing use of biologic therapies in medicine.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
- Feldmann M, Charles P, Taylor P, et al. Biological insights from clinical trials with anti-TNF therapy. Springer Semin Immunopathol Springer Sem Immunopathol. 1998;20:211-228.
- Brewer JD, Schott ARH, Roenigk RK. Multiple squamous cell carcinomas in the setting of psoriasis treated with etanercept: a report of four cases and review of the literature. Int J Dermatol. 2011;50:1555-1559.
- Ninomiya Y, Urano Y, Yoshimoto K, et al. p53 gene mutation analysis in porokeratosis and porokeratosis-associated squamous cell carcinoma. J Dermatol Sci. 1997;14:173-178.
- Lee HR, Han TY, Son S-J, et al. Squamous cell carcinoma developing within lesions of disseminated superficial actinic porokeratosis. Ann Dermatol. 2011;23:536.
- Kwon EJ, Kish LS, Jaworsky C. The histologic spectrum of epithelial neoplasms induced by sorafenib. J Am Acad Dermatol. 2009;61:522-527.
Practice Points
- The use of biologics, particularly tumor necrosis factor α blockers, rarely are reported to induce skin cancer.
- Squamous cell carcinoma in the setting of biologic treatment would warrant a change of systemic medication.
Cutaneous Carcinomatous Arteriopathy and Retiform Purpura Secondary to Metastatic Penile Carcinoma
To the Editor:
A 56-year-old man with a history of stage IV metastatic penile squamous cell carcinoma treated with penectomy and chemotherapy with 5-fluorouracil and cisplatin presented with several painful ulcerations in the groin, abdomen, and thighs. The lesions initially appeared in the groin and were treated as bacterial abscesses with antibiotics. Over the next few weeks, new lesions appeared on the abdomen and thighs. An additional cycle of chemotherapy led to a reduction in number; however, they again increased within a few weeks. Medications included enoxaparin followed by 3 weeks of warfarin use due to a right leg deep vein thrombosis.
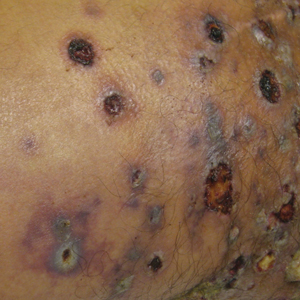
Physical examination revealed multiple 1- to 4-cm, firm, ulcerated nodules on the bilateral inguinal folds, abdomen, and upper thighs, as well as areas of livedo racemosa and noninflammatory retiform purpura with central ulceration (Figures 1 and 2). This retiform purpura was both perilesional and in areas without ulcerations. Laboratory values included the following: sodium, 127 mmol/L (reference range, 136–145 mmol/L); prothrombin time, 16.1 seconds (reference range, 11–15 seconds); white blood cell count, 20.69×109/L (reference range, 4.5–11.0×109/L) with 87% neutrophils (reference range, 54%–62%); hemoglobin, 6.1 g/dL (reference range, 13.5–17.5 g/dL); hematocrit, 18.8% (reference range, 41%–53%); platelets, 474×109/L (reference range, 150–400×109/L); D-dimer, 0.77 mg/L (reference range, ≤0.50 mg/L); fibrinogen, 489 mg/dL (reference range, 150–400 mg/dL); prior urine culture positive for Pseudomonas aeruginosa. He was negative for hepatitis B and hepatitis C viruses as well as HIV, and the lesions were not clinically consistent with herpes simplex virus, as they were not scalloped or circinate. Punch biopsies were obtained from a nodule on the left leg and a purpuric patch on the right leg.
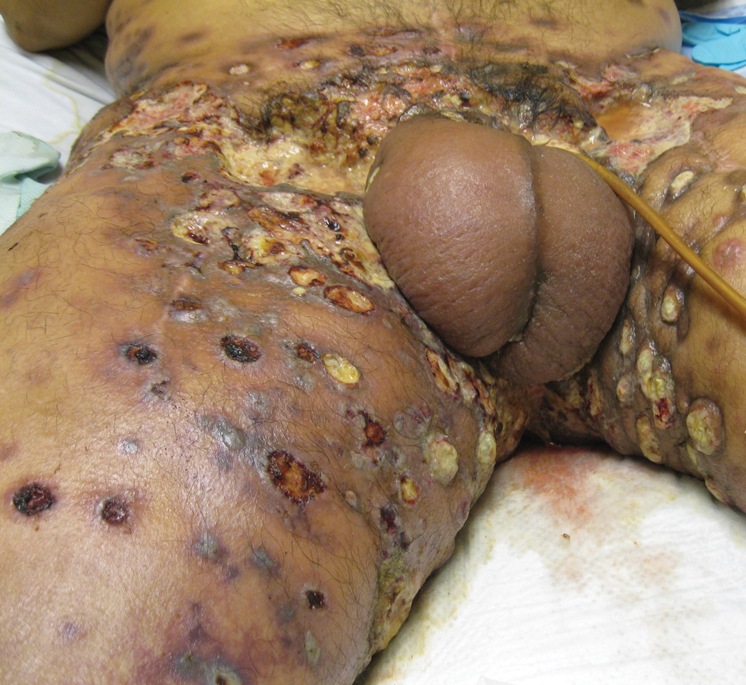
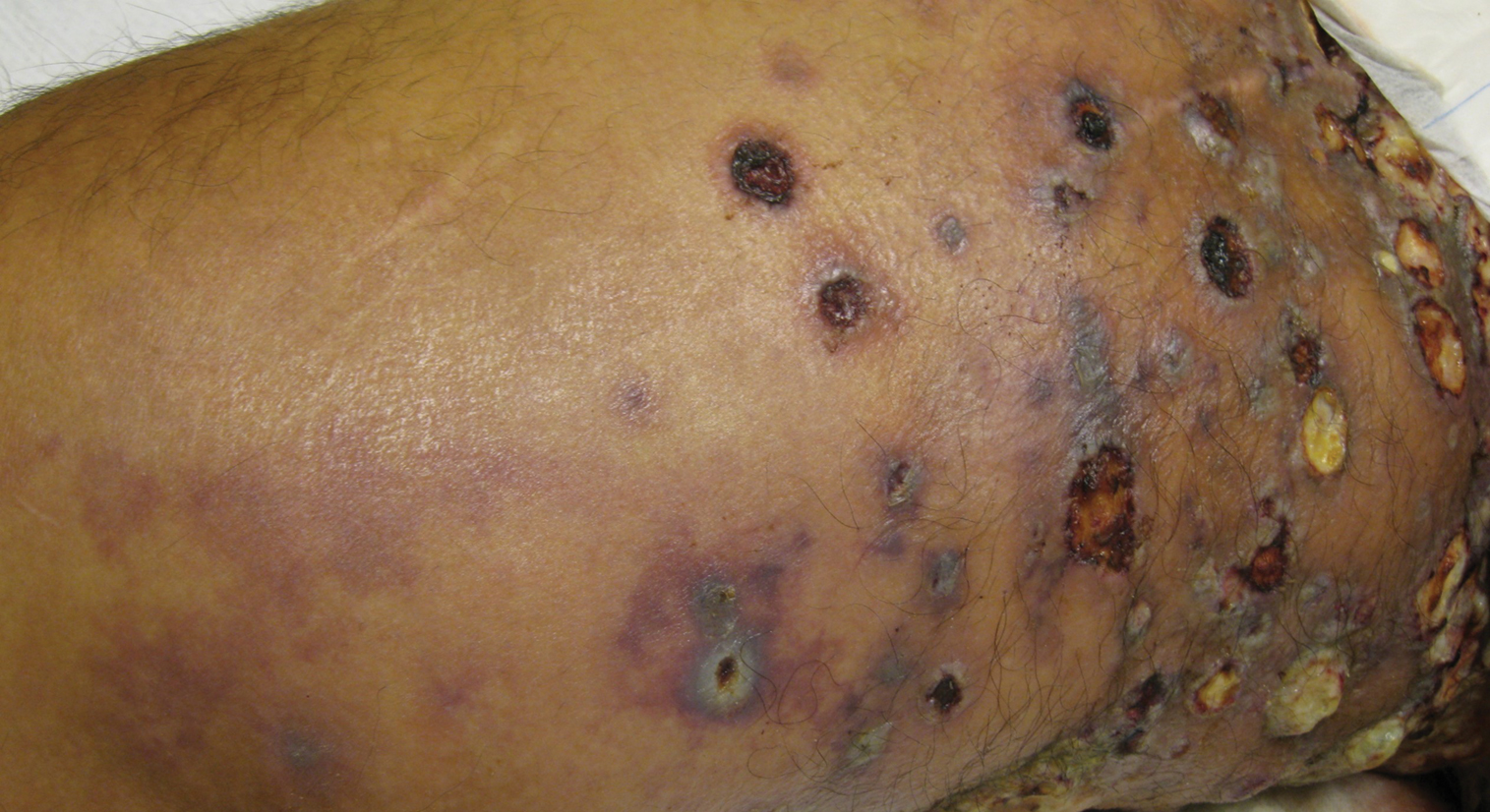
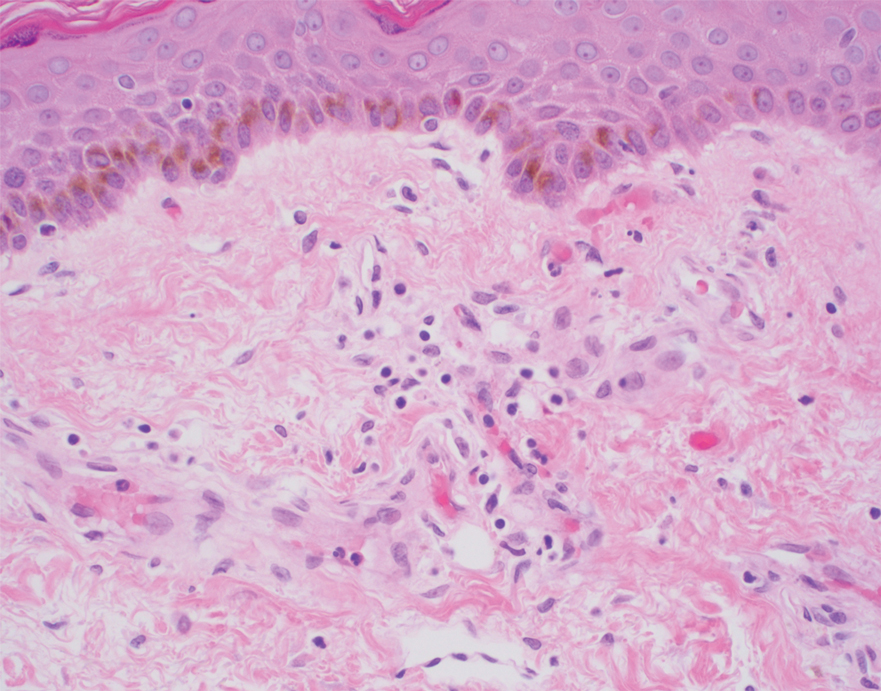
Histopathology of the ulcerated nodule revealed a proliferation of atypical keratinocytes with hyperchromatic and pleomorphic nuclei in the dermis without involvement of the overlying epidermis, consistent with metastatic squamous cell carcinoma (Figure 3). Histopathology of the purpuric patch demonstrated a thrombotic vasculopathy with numerous fibrin thrombi in the lumina of superficial dermal capillaries (Figure 4). No atypical cells, calcifications, or organisms were seen in the vessels. Periodic acid–Schiff, Fite, and Gram stains also were negative. The extent of the disease portended a poor prognosis, and additional vasculopathic workup was not pursued. Following antibiotic treatment and palliative care consultation, he died from subsequent infectious complications 1 month after presentation.
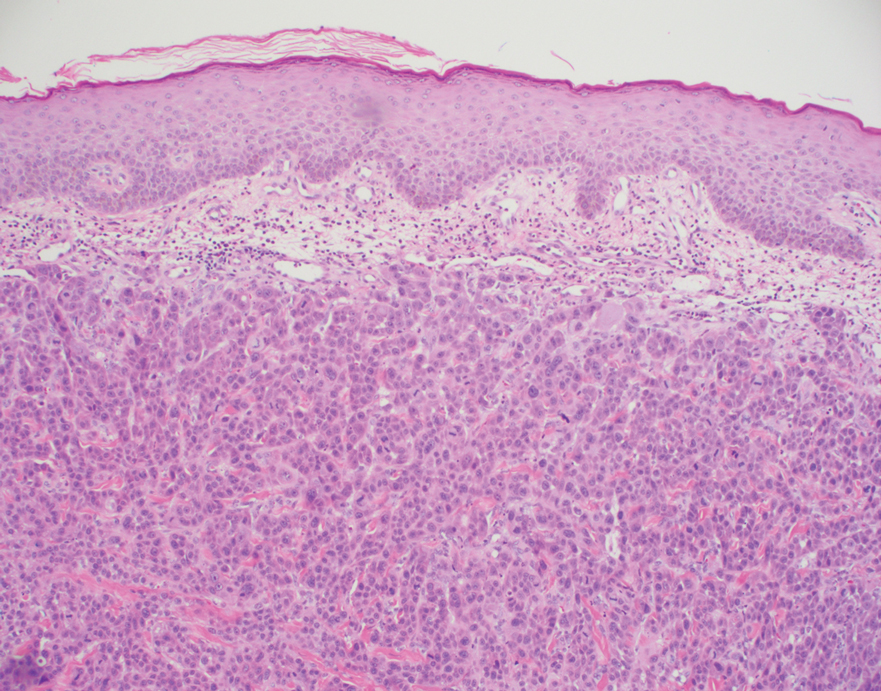
Cutaneous metastases may occur in the setting of multiple malignancies including breast, lung, melanoma, and various gastrointestinal cancers.1 These may present in multiple ways, including firm nontender nodules or as plaques with one of the following morphologies: carcinoma erysipeloides: erythematous, occasionally tender areas resembling cellulitis due to lymphatic obstruction by tumor cells2; carcinoma en cuirasse: indurated sclerotic scarlike plaques due to collagen infiltration3; or carcinoma telangiectoides: telangiectatic, thin erythematous plaques due to dermal capillary infiltration by malignant cells.3
Ischemic cutaneous lesions less commonly occur in the setting of malignancy and can be the result of both direct and indirect systemic effects from the cancer. Malignancies are known to directly trigger vasculopathies in other organs, most commonly the lungs, through 2 primary mechanisms. First, in carcinomatous arteriopathy, metastatic cells promote fibrocellular intimal proliferation of small pulmonary arteries and arterioles leading to stenosis, thrombosis, and obliteration. This mechanism has been described in pulmonary thrombotic microangiopathy secondary to lung carcinoma.4 This pathophysiology likely is also what underlies paraneoplastic acral vascular syndromes, which culminate in digital ischemia. Hypothesized mechanisms for this ischemia also range from vasospasm to thromboembolism.5 Secondly, in vasculitis carcinomatosa, metastatic tumor cells damage or block vessel walls, resulting in end-organ ischemia. Vasculitis carcinomatosa is a well-known phenomenon in angiocentric and intravascular lymphoid malignancies (typically of B-T or natural killer/T-cell origin) but also has been reported in a case of gastric adenocarcinoma with arterial invasion.6 This process is different than carcinoma telangiectoides where malignant cells may be present in the vasculature on histopathology but not trigger thrombosis and ischemic skin necrosis.
Systemic coagulopathies such as disseminated intravascular coagulation (DIC), thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome can occur in the setting of malignancies.7 Clinically, all may present with livedo racemosa, noninflammatory retiform purpura, and widespread skin necrosis. In adult patients, purpura fulminans most often is seen in the setting of sepsis and DIC, with accompanying evidence of microangiopathy.8 Catastrophic antiphospholipid antibody syndrome can be triggered by malignancy and is characterized by central nervous system, renal, pulmonary, and gastrointestinal complications. Skin involvement such as ulcers, livedo reticularis, and gangrene have been reported.9 Other causes of thrombotic vasculopathy include warfarin necrosis, heparin-induced thrombotic thrombocytopenia, calciphylaxis, and angioinvasive infections.8 Warfarin necrosis and heparin-induced thrombotic thrombocytopenia typically present days after initiating therapy with the respective medication. Calciphylaxis typically occurs in patients on dialysis, though it may occur in nonuremic patients including those with malignancy.8 Patients with malignancies on chemotherapy can become neutropenic and are at risk for ecthyma gangrenosum due to P aeruginosa and other gram-negative rods, Staphylococcus aureus, and angioinvasive fungi.10
Based on clinical, histopathological, and laboratory data, we favored a diagnosis of cutaneous carcinomatous arteriopathy. Vasculitis carcinomatosa was a possibility despite the lack of vasculotropism on histopathology, which may have been due to biopsy site selection. Systemic coagulopathies such as DIC, thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome were unlikely, as the ischemic skin lesions and livedo racemosa were limited to areas adjacent to cutaneous metastases, and the patient lacked other common multiorgan manifestations or laboratory findings. Although our patient was on warfarin, he was on a stable dose for weeks and histopathologic features of subcutaneous thrombosis were not seen. The biopsy also was not consistent with calciphylaxis. Ecthyma gangrenosum was unlikely given the lack of organisms on histopathology and negative skin and blood cultures. Although additional laboratory testing in this patient may have included cryoglobulins and cryofibrinogens, both entities were unlikely due to a lack of ischemic acral lesions.
In conclusion, we present a case of localized thrombotic vasculopathy that likely was secondary to cutaneous carcinomatous arteriopathy in the setting of cutaneous metastatic penile squamous cell carcinoma. The differential diagnosis of retiform purpura, livedo racemosa, and other signs of cutaneous ischemia in patients with metastatic cancer is broad and can be the result of both direct and indirect systemic effects from the cancer. Appropriate workup in these cases should include skin biopsies for histopathology and culture, medication review, and laboratory evaluation for systemic coagulopathies.
- Alcaraz I, Cerroni L, Ruetten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Prat L, Chouaid C, Kettaneh A, et al. Cutaneous lymphangitis carcinomatosa in a patient with lung adenocarcinoma: case report and literature review. Lung Cancer. 2013;79:91-93.
- Marneros AG, Blanco F, Husain S, et al. Classification of cutaneous intravascular breast cancer metastases based on immunolabeling for blood and lymph vessels. J Am Acad Dermatol. 2009;60:633-638.
- von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587-592.
- Besnerais ML, Miranda S, Cailleux N, et al. Digital ischemia associated with cancer. Medicine. 2014;93:E47.
- Sweeney S, Utzschneider R, Fraire AE. Vasculitis carcinomatosa occurring in association with adenocarcinoma of the stomach. Ann Diagn Pathol. 1998;2:247-249.
- Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126-136.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miesbach W, Asherson RA, Cervera R, et al; CAPS Registry Group. The role of malignancies in patients with catastrophic anti-phospholipid (Asherson’s) syndrome. Clin Rheumatol. 2007;26:2109-2114.
- Pozo D. Ecthyma gangrenosum‐like eruption associated with Morganella morganii infection. Br J Dermatol. 1998;139:520-521.
To the Editor:
A 56-year-old man with a history of stage IV metastatic penile squamous cell carcinoma treated with penectomy and chemotherapy with 5-fluorouracil and cisplatin presented with several painful ulcerations in the groin, abdomen, and thighs. The lesions initially appeared in the groin and were treated as bacterial abscesses with antibiotics. Over the next few weeks, new lesions appeared on the abdomen and thighs. An additional cycle of chemotherapy led to a reduction in number; however, they again increased within a few weeks. Medications included enoxaparin followed by 3 weeks of warfarin use due to a right leg deep vein thrombosis.
Physical examination revealed multiple 1- to 4-cm, firm, ulcerated nodules on the bilateral inguinal folds, abdomen, and upper thighs, as well as areas of livedo racemosa and noninflammatory retiform purpura with central ulceration (Figures 1 and 2). This retiform purpura was both perilesional and in areas without ulcerations. Laboratory values included the following: sodium, 127 mmol/L (reference range, 136–145 mmol/L); prothrombin time, 16.1 seconds (reference range, 11–15 seconds); white blood cell count, 20.69×109/L (reference range, 4.5–11.0×109/L) with 87% neutrophils (reference range, 54%–62%); hemoglobin, 6.1 g/dL (reference range, 13.5–17.5 g/dL); hematocrit, 18.8% (reference range, 41%–53%); platelets, 474×109/L (reference range, 150–400×109/L); D-dimer, 0.77 mg/L (reference range, ≤0.50 mg/L); fibrinogen, 489 mg/dL (reference range, 150–400 mg/dL); prior urine culture positive for Pseudomonas aeruginosa. He was negative for hepatitis B and hepatitis C viruses as well as HIV, and the lesions were not clinically consistent with herpes simplex virus, as they were not scalloped or circinate. Punch biopsies were obtained from a nodule on the left leg and a purpuric patch on the right leg.


Histopathology of the ulcerated nodule revealed a proliferation of atypical keratinocytes with hyperchromatic and pleomorphic nuclei in the dermis without involvement of the overlying epidermis, consistent with metastatic squamous cell carcinoma (Figure 3). Histopathology of the purpuric patch demonstrated a thrombotic vasculopathy with numerous fibrin thrombi in the lumina of superficial dermal capillaries (Figure 4). No atypical cells, calcifications, or organisms were seen in the vessels. Periodic acid–Schiff, Fite, and Gram stains also were negative. The extent of the disease portended a poor prognosis, and additional vasculopathic workup was not pursued. Following antibiotic treatment and palliative care consultation, he died from subsequent infectious complications 1 month after presentation.


Cutaneous metastases may occur in the setting of multiple malignancies including breast, lung, melanoma, and various gastrointestinal cancers.1 These may present in multiple ways, including firm nontender nodules or as plaques with one of the following morphologies: carcinoma erysipeloides: erythematous, occasionally tender areas resembling cellulitis due to lymphatic obstruction by tumor cells2; carcinoma en cuirasse: indurated sclerotic scarlike plaques due to collagen infiltration3; or carcinoma telangiectoides: telangiectatic, thin erythematous plaques due to dermal capillary infiltration by malignant cells.3
Ischemic cutaneous lesions less commonly occur in the setting of malignancy and can be the result of both direct and indirect systemic effects from the cancer. Malignancies are known to directly trigger vasculopathies in other organs, most commonly the lungs, through 2 primary mechanisms. First, in carcinomatous arteriopathy, metastatic cells promote fibrocellular intimal proliferation of small pulmonary arteries and arterioles leading to stenosis, thrombosis, and obliteration. This mechanism has been described in pulmonary thrombotic microangiopathy secondary to lung carcinoma.4 This pathophysiology likely is also what underlies paraneoplastic acral vascular syndromes, which culminate in digital ischemia. Hypothesized mechanisms for this ischemia also range from vasospasm to thromboembolism.5 Secondly, in vasculitis carcinomatosa, metastatic tumor cells damage or block vessel walls, resulting in end-organ ischemia. Vasculitis carcinomatosa is a well-known phenomenon in angiocentric and intravascular lymphoid malignancies (typically of B-T or natural killer/T-cell origin) but also has been reported in a case of gastric adenocarcinoma with arterial invasion.6 This process is different than carcinoma telangiectoides where malignant cells may be present in the vasculature on histopathology but not trigger thrombosis and ischemic skin necrosis.
Systemic coagulopathies such as disseminated intravascular coagulation (DIC), thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome can occur in the setting of malignancies.7 Clinically, all may present with livedo racemosa, noninflammatory retiform purpura, and widespread skin necrosis. In adult patients, purpura fulminans most often is seen in the setting of sepsis and DIC, with accompanying evidence of microangiopathy.8 Catastrophic antiphospholipid antibody syndrome can be triggered by malignancy and is characterized by central nervous system, renal, pulmonary, and gastrointestinal complications. Skin involvement such as ulcers, livedo reticularis, and gangrene have been reported.9 Other causes of thrombotic vasculopathy include warfarin necrosis, heparin-induced thrombotic thrombocytopenia, calciphylaxis, and angioinvasive infections.8 Warfarin necrosis and heparin-induced thrombotic thrombocytopenia typically present days after initiating therapy with the respective medication. Calciphylaxis typically occurs in patients on dialysis, though it may occur in nonuremic patients including those with malignancy.8 Patients with malignancies on chemotherapy can become neutropenic and are at risk for ecthyma gangrenosum due to P aeruginosa and other gram-negative rods, Staphylococcus aureus, and angioinvasive fungi.10
Based on clinical, histopathological, and laboratory data, we favored a diagnosis of cutaneous carcinomatous arteriopathy. Vasculitis carcinomatosa was a possibility despite the lack of vasculotropism on histopathology, which may have been due to biopsy site selection. Systemic coagulopathies such as DIC, thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome were unlikely, as the ischemic skin lesions and livedo racemosa were limited to areas adjacent to cutaneous metastases, and the patient lacked other common multiorgan manifestations or laboratory findings. Although our patient was on warfarin, he was on a stable dose for weeks and histopathologic features of subcutaneous thrombosis were not seen. The biopsy also was not consistent with calciphylaxis. Ecthyma gangrenosum was unlikely given the lack of organisms on histopathology and negative skin and blood cultures. Although additional laboratory testing in this patient may have included cryoglobulins and cryofibrinogens, both entities were unlikely due to a lack of ischemic acral lesions.
In conclusion, we present a case of localized thrombotic vasculopathy that likely was secondary to cutaneous carcinomatous arteriopathy in the setting of cutaneous metastatic penile squamous cell carcinoma. The differential diagnosis of retiform purpura, livedo racemosa, and other signs of cutaneous ischemia in patients with metastatic cancer is broad and can be the result of both direct and indirect systemic effects from the cancer. Appropriate workup in these cases should include skin biopsies for histopathology and culture, medication review, and laboratory evaluation for systemic coagulopathies.
To the Editor:
A 56-year-old man with a history of stage IV metastatic penile squamous cell carcinoma treated with penectomy and chemotherapy with 5-fluorouracil and cisplatin presented with several painful ulcerations in the groin, abdomen, and thighs. The lesions initially appeared in the groin and were treated as bacterial abscesses with antibiotics. Over the next few weeks, new lesions appeared on the abdomen and thighs. An additional cycle of chemotherapy led to a reduction in number; however, they again increased within a few weeks. Medications included enoxaparin followed by 3 weeks of warfarin use due to a right leg deep vein thrombosis.
Physical examination revealed multiple 1- to 4-cm, firm, ulcerated nodules on the bilateral inguinal folds, abdomen, and upper thighs, as well as areas of livedo racemosa and noninflammatory retiform purpura with central ulceration (Figures 1 and 2). This retiform purpura was both perilesional and in areas without ulcerations. Laboratory values included the following: sodium, 127 mmol/L (reference range, 136–145 mmol/L); prothrombin time, 16.1 seconds (reference range, 11–15 seconds); white blood cell count, 20.69×109/L (reference range, 4.5–11.0×109/L) with 87% neutrophils (reference range, 54%–62%); hemoglobin, 6.1 g/dL (reference range, 13.5–17.5 g/dL); hematocrit, 18.8% (reference range, 41%–53%); platelets, 474×109/L (reference range, 150–400×109/L); D-dimer, 0.77 mg/L (reference range, ≤0.50 mg/L); fibrinogen, 489 mg/dL (reference range, 150–400 mg/dL); prior urine culture positive for Pseudomonas aeruginosa. He was negative for hepatitis B and hepatitis C viruses as well as HIV, and the lesions were not clinically consistent with herpes simplex virus, as they were not scalloped or circinate. Punch biopsies were obtained from a nodule on the left leg and a purpuric patch on the right leg.


Histopathology of the ulcerated nodule revealed a proliferation of atypical keratinocytes with hyperchromatic and pleomorphic nuclei in the dermis without involvement of the overlying epidermis, consistent with metastatic squamous cell carcinoma (Figure 3). Histopathology of the purpuric patch demonstrated a thrombotic vasculopathy with numerous fibrin thrombi in the lumina of superficial dermal capillaries (Figure 4). No atypical cells, calcifications, or organisms were seen in the vessels. Periodic acid–Schiff, Fite, and Gram stains also were negative. The extent of the disease portended a poor prognosis, and additional vasculopathic workup was not pursued. Following antibiotic treatment and palliative care consultation, he died from subsequent infectious complications 1 month after presentation.


Cutaneous metastases may occur in the setting of multiple malignancies including breast, lung, melanoma, and various gastrointestinal cancers.1 These may present in multiple ways, including firm nontender nodules or as plaques with one of the following morphologies: carcinoma erysipeloides: erythematous, occasionally tender areas resembling cellulitis due to lymphatic obstruction by tumor cells2; carcinoma en cuirasse: indurated sclerotic scarlike plaques due to collagen infiltration3; or carcinoma telangiectoides: telangiectatic, thin erythematous plaques due to dermal capillary infiltration by malignant cells.3
Ischemic cutaneous lesions less commonly occur in the setting of malignancy and can be the result of both direct and indirect systemic effects from the cancer. Malignancies are known to directly trigger vasculopathies in other organs, most commonly the lungs, through 2 primary mechanisms. First, in carcinomatous arteriopathy, metastatic cells promote fibrocellular intimal proliferation of small pulmonary arteries and arterioles leading to stenosis, thrombosis, and obliteration. This mechanism has been described in pulmonary thrombotic microangiopathy secondary to lung carcinoma.4 This pathophysiology likely is also what underlies paraneoplastic acral vascular syndromes, which culminate in digital ischemia. Hypothesized mechanisms for this ischemia also range from vasospasm to thromboembolism.5 Secondly, in vasculitis carcinomatosa, metastatic tumor cells damage or block vessel walls, resulting in end-organ ischemia. Vasculitis carcinomatosa is a well-known phenomenon in angiocentric and intravascular lymphoid malignancies (typically of B-T or natural killer/T-cell origin) but also has been reported in a case of gastric adenocarcinoma with arterial invasion.6 This process is different than carcinoma telangiectoides where malignant cells may be present in the vasculature on histopathology but not trigger thrombosis and ischemic skin necrosis.
Systemic coagulopathies such as disseminated intravascular coagulation (DIC), thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome can occur in the setting of malignancies.7 Clinically, all may present with livedo racemosa, noninflammatory retiform purpura, and widespread skin necrosis. In adult patients, purpura fulminans most often is seen in the setting of sepsis and DIC, with accompanying evidence of microangiopathy.8 Catastrophic antiphospholipid antibody syndrome can be triggered by malignancy and is characterized by central nervous system, renal, pulmonary, and gastrointestinal complications. Skin involvement such as ulcers, livedo reticularis, and gangrene have been reported.9 Other causes of thrombotic vasculopathy include warfarin necrosis, heparin-induced thrombotic thrombocytopenia, calciphylaxis, and angioinvasive infections.8 Warfarin necrosis and heparin-induced thrombotic thrombocytopenia typically present days after initiating therapy with the respective medication. Calciphylaxis typically occurs in patients on dialysis, though it may occur in nonuremic patients including those with malignancy.8 Patients with malignancies on chemotherapy can become neutropenic and are at risk for ecthyma gangrenosum due to P aeruginosa and other gram-negative rods, Staphylococcus aureus, and angioinvasive fungi.10
Based on clinical, histopathological, and laboratory data, we favored a diagnosis of cutaneous carcinomatous arteriopathy. Vasculitis carcinomatosa was a possibility despite the lack of vasculotropism on histopathology, which may have been due to biopsy site selection. Systemic coagulopathies such as DIC, thrombotic thrombocytopenia purpura, and catastrophic antiphospholipid antibody syndrome were unlikely, as the ischemic skin lesions and livedo racemosa were limited to areas adjacent to cutaneous metastases, and the patient lacked other common multiorgan manifestations or laboratory findings. Although our patient was on warfarin, he was on a stable dose for weeks and histopathologic features of subcutaneous thrombosis were not seen. The biopsy also was not consistent with calciphylaxis. Ecthyma gangrenosum was unlikely given the lack of organisms on histopathology and negative skin and blood cultures. Although additional laboratory testing in this patient may have included cryoglobulins and cryofibrinogens, both entities were unlikely due to a lack of ischemic acral lesions.
In conclusion, we present a case of localized thrombotic vasculopathy that likely was secondary to cutaneous carcinomatous arteriopathy in the setting of cutaneous metastatic penile squamous cell carcinoma. The differential diagnosis of retiform purpura, livedo racemosa, and other signs of cutaneous ischemia in patients with metastatic cancer is broad and can be the result of both direct and indirect systemic effects from the cancer. Appropriate workup in these cases should include skin biopsies for histopathology and culture, medication review, and laboratory evaluation for systemic coagulopathies.
- Alcaraz I, Cerroni L, Ruetten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Prat L, Chouaid C, Kettaneh A, et al. Cutaneous lymphangitis carcinomatosa in a patient with lung adenocarcinoma: case report and literature review. Lung Cancer. 2013;79:91-93.
- Marneros AG, Blanco F, Husain S, et al. Classification of cutaneous intravascular breast cancer metastases based on immunolabeling for blood and lymph vessels. J Am Acad Dermatol. 2009;60:633-638.
- von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587-592.
- Besnerais ML, Miranda S, Cailleux N, et al. Digital ischemia associated with cancer. Medicine. 2014;93:E47.
- Sweeney S, Utzschneider R, Fraire AE. Vasculitis carcinomatosa occurring in association with adenocarcinoma of the stomach. Ann Diagn Pathol. 1998;2:247-249.
- Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126-136.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miesbach W, Asherson RA, Cervera R, et al; CAPS Registry Group. The role of malignancies in patients with catastrophic anti-phospholipid (Asherson’s) syndrome. Clin Rheumatol. 2007;26:2109-2114.
- Pozo D. Ecthyma gangrenosum‐like eruption associated with Morganella morganii infection. Br J Dermatol. 1998;139:520-521.
- Alcaraz I, Cerroni L, Ruetten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Prat L, Chouaid C, Kettaneh A, et al. Cutaneous lymphangitis carcinomatosa in a patient with lung adenocarcinoma: case report and literature review. Lung Cancer. 2013;79:91-93.
- Marneros AG, Blanco F, Husain S, et al. Classification of cutaneous intravascular breast cancer metastases based on immunolabeling for blood and lymph vessels. J Am Acad Dermatol. 2009;60:633-638.
- von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer. 1990;66:587-592.
- Besnerais ML, Miranda S, Cailleux N, et al. Digital ischemia associated with cancer. Medicine. 2014;93:E47.
- Sweeney S, Utzschneider R, Fraire AE. Vasculitis carcinomatosa occurring in association with adenocarcinoma of the stomach. Ann Diagn Pathol. 1998;2:247-249.
- Zwicker JI, Furie BC, Furie B. Cancer-associated thrombosis. Crit Rev Oncol Hematol. 2007;62:126-136.
- Thornsberry LA, LoSicco KI, English JC. The skin and hypercoagulable states. J Am Acad Dermatol. 2013;69:450-462.
- Miesbach W, Asherson RA, Cervera R, et al; CAPS Registry Group. The role of malignancies in patients with catastrophic anti-phospholipid (Asherson’s) syndrome. Clin Rheumatol. 2007;26:2109-2114.
- Pozo D. Ecthyma gangrenosum‐like eruption associated with Morganella morganii infection. Br J Dermatol. 1998;139:520-521.
Practice Points
- Cutaneous metastases may present in multiple ways, including carcinoma erysipeloides, carcinoma en cuirasse, or carcinoma telangiectoides.
- Ischemic cutaneous lesions, characterized by livedoid skin changes and retiform purpura, occur less commonly in the setting of malignancy.
- Direct mechanisms include carcinomatous arteriopathy and vasculitis carcinomatosa. Indirect systemic processes include coagulopathies such as disseminated intravascular coagulation, thrombotic thrombocytopenia purpura, catastrophic antiphospholipid antibody syndrome, calciphylaxis, and cryoglobulinemia.
Indoor tanning ICD-10 codes may be underused, study finds
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
according to a study presented at the annual meeting of the Society for Investigative Dermatology.
“Since indoor tanning ICD-10 codes were only recently universally implemented in 2015, and providers may still be using other codes that cover similar services, we think our data likely underestimate the number of encounters and sequelae associated with indoor tanning,” Alexandria M. Brown, BSA, of Baylor College of Medicine, Houston, said in her presentation. “We think increased usage of these indoor tanning exposure codes in coming years will strengthen this body of indoor tanning literature and data.”
Using insurance claims data on about 43 million patients from Truven Health MarketScan, Ms. Brown and colleagues analyzed patient encounters with ICD-10 indoor tanning codes W89.1, W89.1XXA, W89.1XXD, and W89.1XXS between 2016 and 2018 for about 43 million patients. Overall, there were 4,550 patient encounters where these codes had been recorded, with most (99%) occurring in an outpatient setting. The majority of providers at these encounters were dermatologists (72%). Patients were mostly women (85%); and most were ages 25-34 years (19.4%), 35-44 years (20.6%), 45-54 years (22.7%), and 55-64 years (19%). Almost 5% were 65 and over, 11.7% were ages 18-24, and 1.6% were under age 18.
The use of indoor tanning codes were most common in the Midwest (55 per 100,000 encounters with dermatologists), compared with 16 per 100,000 in the Northeast, 21 per 100,000 in the West, and 28 per 100,000 in the South. CPT codes for “destruction of a premalignant lesion” and “biopsy” were the most frequently used codes entered at visits where indoor tanning codes were also entered, and were present in 15.1% of encounters and 18.4% of encounters, respectively.
“This suggests that many of these encounters may have been for skin cancer surveillance and that indoor tanning exposure may have been coded as part of a patient’s skin cancer risk profile,” Ms. Brown noted.
The study shows how these codes are being used and could help determine health care use patterns for these patients as well as their comorbidities, behaviors, and risk factors, according to the authors, who believe this is the first study to look at the use of ICD-10 indoor tanning codes.
“Any effort to reduce indoor tanning requires knowledge of the population at risk. It has been shown that the ability to recognize and provide counseling to at-risk patients can improve sun protective behaviors and reduce indoor tanning,” Ms. Brown said. Claims databases can be a “valuable tool to better understand patients who have been exposed to indoor tanning and their associated risk factors, comorbidities, behaviors, and health care utilization.”
In an interview, Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said the study was interesting and “provides some guidance with respect to who, when, and where in the U.S. to target educational initiatives on the harms of tanning beds.”
Dr. Friedman, who was not involved with the research, agreed with the authors’ assertion that their study was underestimating the use of indoor tanning beds. “Using a large database provides the means to better generalize one’s dataset; however in this case, it relies on proper coding by the practitioner,” or even using the code for tanning bed use at all.
“There also could be some inherent bias given most of the cases for which the code was used was for skin cancer surveillance, and therefore tanning bed use was top of mind,” he said.
While he believes this study may not be most efficient way of determining demographics of at-risk individuals using tanning beds, Dr. Friedman said the results “should serve as the impetus to develop public health campaigns around this information, following which research can be conducted to evaluate if the intervention had an impact.”
Ms. Brown and Dr. Friedman reported no relevant financial disclosures.
FROM SID 2021
Rate of cutaneous toxicities from ICIs may be lower than previously reported
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
FROM SID 2021
EC approves cemiplimab for advanced or metastatic BCC after HHI therapy
The
The programmed death-1 (PD-1) inhibitor, which is being jointly developed by Regeneron and Sanofi under a global collaboration agreement, was approved by the Food and Drug Administration for this indication in the United States in February; the FDA granted full approval for its use in patients with locally advanced BCC and accelerated approval for use in patients with metastatic BCC.
The EC’s thumbs-up for cemiplimab as a treatment for BCC marks the third such approval for an advanced cancer in the European Union: The immunotherapy was concurrently approved by the EC for the first-line treatment of adults with advanced non–small cell lung cancer (NSCLC) whose tumor cells have ≥ 50% PD-L1 expression and no EGFR, ALK or ROS1 aberrations, and was approved in 2019 for the treatment of adults with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or curative radiation.
The FDA granted approval of cemiplimab for NSCLC in February, and for CSCC in 2018.
The latest BCC approval is based on data from an ongoing, open-label, prospective phase 2 clinical trial of 119 patients with advanced BCC who were previously treated with an HHI. The objective response rates in cemiplimab-treated patients were 32% (partial responses in 25%; complete responses in 7%) in those with locally advanced BCC, and 29% (partial responses in 26%; complete responses in 3%) in those with metastatic BCC.
About 90% of all patients had a duration of response (DOR) of 6 months or longer. Median DOR was not reached in either group at median follow-up of 16 months for locally advanced BCC and 9 months for metastatic BCC.
The safety profile of cemiplimab has been generally consistent across approved indications. Serious adverse events have been reported in 30% of 816 patients from all four cemiplimab monotherapy pivotal trials, and these led to permanent discontinuation of treatment in 8% of patients.
Immune-related adverse reactions occurred in 22% of patients, and led to permanent discontinuation in 4%. The most common such reactions were hypothyroidism (8%), hyperthyroidism (3%), pneumonitis (3%), hepatitis (2%), colitis (2%) and immune-related skin adverse reactions (2%).
Cemiplimab is administered by intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. The recommended dose is 350 mg.
A press release from Regeneron notes that research efforts with respect to cemiplimab – both as monotherapy and in combination with other agents – are focused on difficult-to-treat cancers, including advanced NSCLC, cervical cancer, and other solid tumors and blood cancers.
The
The programmed death-1 (PD-1) inhibitor, which is being jointly developed by Regeneron and Sanofi under a global collaboration agreement, was approved by the Food and Drug Administration for this indication in the United States in February; the FDA granted full approval for its use in patients with locally advanced BCC and accelerated approval for use in patients with metastatic BCC.
The EC’s thumbs-up for cemiplimab as a treatment for BCC marks the third such approval for an advanced cancer in the European Union: The immunotherapy was concurrently approved by the EC for the first-line treatment of adults with advanced non–small cell lung cancer (NSCLC) whose tumor cells have ≥ 50% PD-L1 expression and no EGFR, ALK or ROS1 aberrations, and was approved in 2019 for the treatment of adults with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or curative radiation.
The FDA granted approval of cemiplimab for NSCLC in February, and for CSCC in 2018.
The latest BCC approval is based on data from an ongoing, open-label, prospective phase 2 clinical trial of 119 patients with advanced BCC who were previously treated with an HHI. The objective response rates in cemiplimab-treated patients were 32% (partial responses in 25%; complete responses in 7%) in those with locally advanced BCC, and 29% (partial responses in 26%; complete responses in 3%) in those with metastatic BCC.
About 90% of all patients had a duration of response (DOR) of 6 months or longer. Median DOR was not reached in either group at median follow-up of 16 months for locally advanced BCC and 9 months for metastatic BCC.
The safety profile of cemiplimab has been generally consistent across approved indications. Serious adverse events have been reported in 30% of 816 patients from all four cemiplimab monotherapy pivotal trials, and these led to permanent discontinuation of treatment in 8% of patients.
Immune-related adverse reactions occurred in 22% of patients, and led to permanent discontinuation in 4%. The most common such reactions were hypothyroidism (8%), hyperthyroidism (3%), pneumonitis (3%), hepatitis (2%), colitis (2%) and immune-related skin adverse reactions (2%).
Cemiplimab is administered by intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. The recommended dose is 350 mg.
A press release from Regeneron notes that research efforts with respect to cemiplimab – both as monotherapy and in combination with other agents – are focused on difficult-to-treat cancers, including advanced NSCLC, cervical cancer, and other solid tumors and blood cancers.
The
The programmed death-1 (PD-1) inhibitor, which is being jointly developed by Regeneron and Sanofi under a global collaboration agreement, was approved by the Food and Drug Administration for this indication in the United States in February; the FDA granted full approval for its use in patients with locally advanced BCC and accelerated approval for use in patients with metastatic BCC.
The EC’s thumbs-up for cemiplimab as a treatment for BCC marks the third such approval for an advanced cancer in the European Union: The immunotherapy was concurrently approved by the EC for the first-line treatment of adults with advanced non–small cell lung cancer (NSCLC) whose tumor cells have ≥ 50% PD-L1 expression and no EGFR, ALK or ROS1 aberrations, and was approved in 2019 for the treatment of adults with metastatic or locally advanced cutaneous squamous cell carcinoma (CSCC) who are not candidates for curative surgery or curative radiation.
The FDA granted approval of cemiplimab for NSCLC in February, and for CSCC in 2018.
The latest BCC approval is based on data from an ongoing, open-label, prospective phase 2 clinical trial of 119 patients with advanced BCC who were previously treated with an HHI. The objective response rates in cemiplimab-treated patients were 32% (partial responses in 25%; complete responses in 7%) in those with locally advanced BCC, and 29% (partial responses in 26%; complete responses in 3%) in those with metastatic BCC.
About 90% of all patients had a duration of response (DOR) of 6 months or longer. Median DOR was not reached in either group at median follow-up of 16 months for locally advanced BCC and 9 months for metastatic BCC.
The safety profile of cemiplimab has been generally consistent across approved indications. Serious adverse events have been reported in 30% of 816 patients from all four cemiplimab monotherapy pivotal trials, and these led to permanent discontinuation of treatment in 8% of patients.
Immune-related adverse reactions occurred in 22% of patients, and led to permanent discontinuation in 4%. The most common such reactions were hypothyroidism (8%), hyperthyroidism (3%), pneumonitis (3%), hepatitis (2%), colitis (2%) and immune-related skin adverse reactions (2%).
Cemiplimab is administered by intravenous infusion over 30 minutes every 3 weeks until disease progression or unacceptable toxicity. The recommended dose is 350 mg.
A press release from Regeneron notes that research efforts with respect to cemiplimab – both as monotherapy and in combination with other agents – are focused on difficult-to-treat cancers, including advanced NSCLC, cervical cancer, and other solid tumors and blood cancers.
Pigmented Basal Cell Carcinoma With Annular Leukoderma
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.
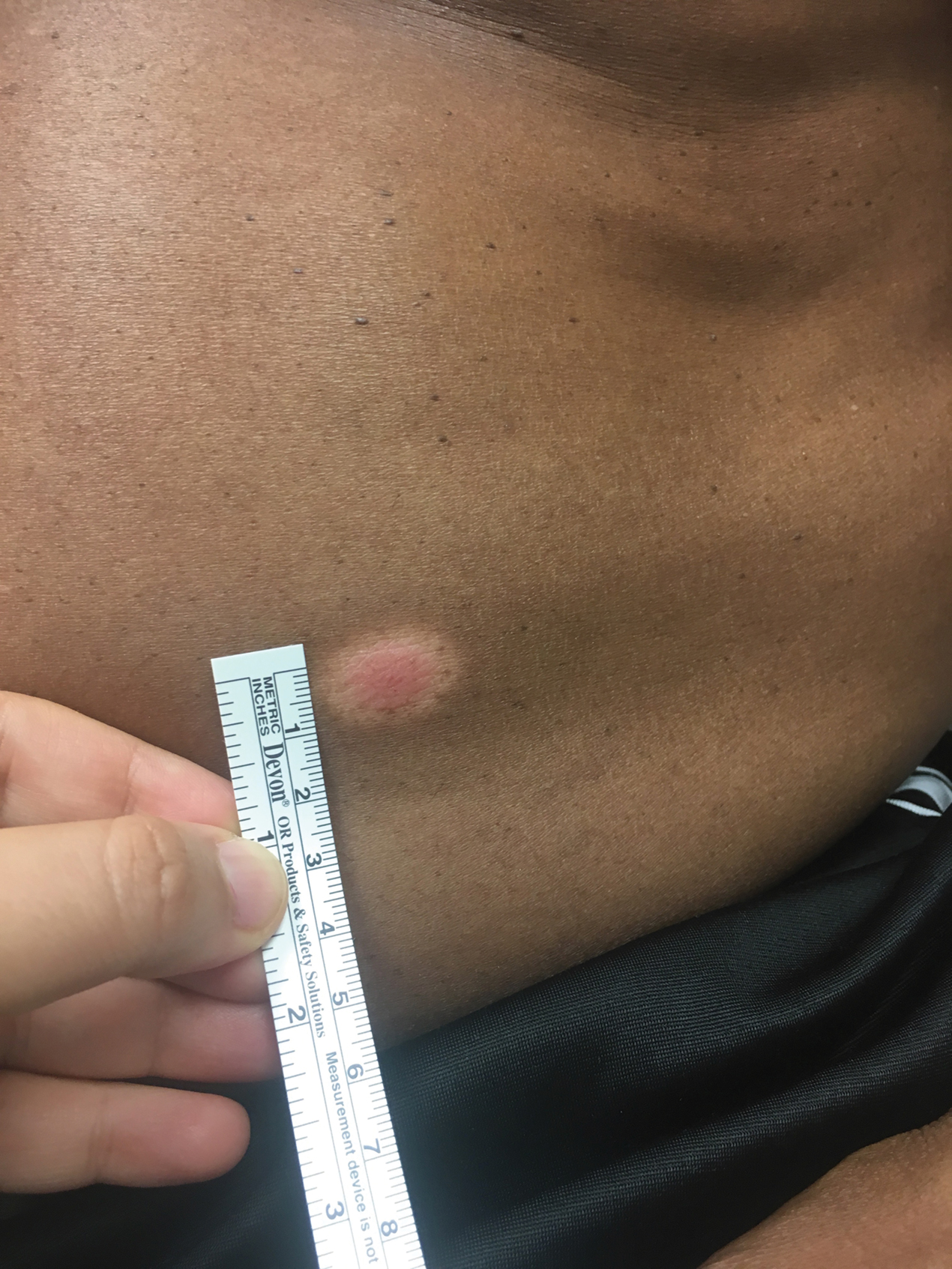
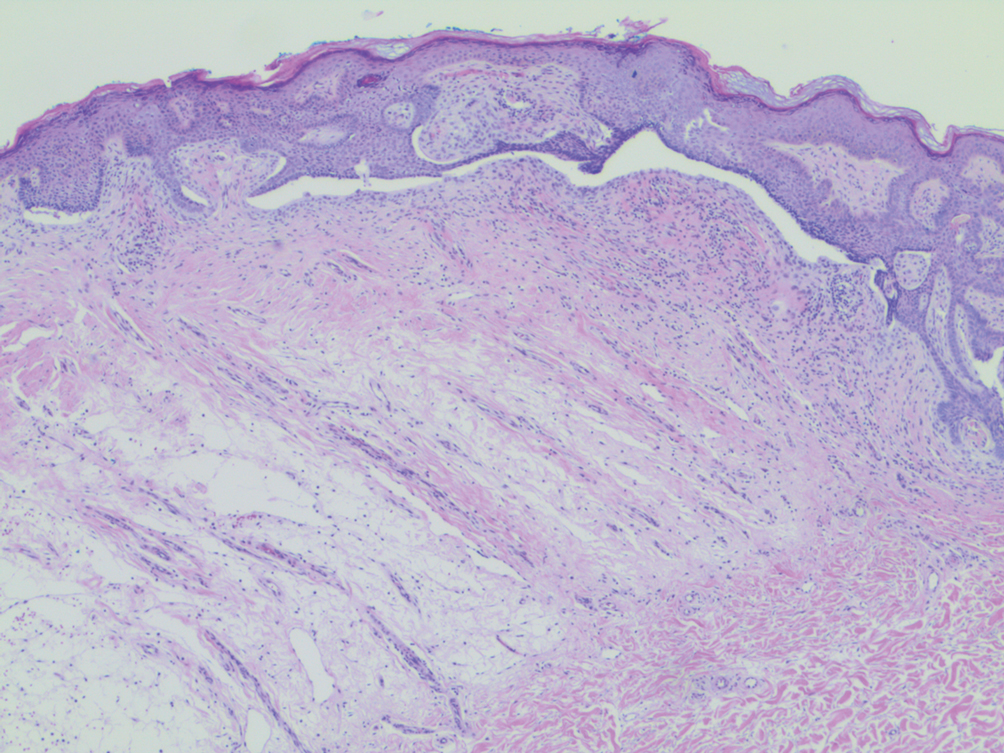
The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.


The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
To the Editor:
Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that most commonly is observed alongside congenital nevi, acquired melanocytic nevi, blue nevi, Spitz nevi, vitiligo, and rarely melanoma.1 There is limited literature on the mechanism of the halo phenomenon. Most of the literature proposes a T cell–mediated immune response to antigens, which causes not only surrounding pigment loss but also heralds the regression of central lesions.2 Others have suggested a vascular mechanism, with blood shunted away from the lesions.3 Because guidelines discourage biopsy of typical halo nevi, it becomes important to evaluate lesions for worrisome features such as ulceration or asymmetry, especially in older patients. We present a case of a pigmented basal cell carcinoma (BCC) that exhibited the halo phenomenon. Four other cases have been described in the literature.3-6
A 53-year-old man presented for evaluation of an asymptomatic lesion on the left side of the abdomen of approximately 8 months’ duration. He had no personal or family history of skin cancer. Physical examination revealed a central 1-cm, pink, verrucous papule surrounded by a 2×1.2-cm, depigmented, circular patch on the left side of the inferior abdomen (Figure 1). Upon questioning, the patient produced cell phone photographs of the trunk from 3 years prior, which did not show any lesions present. Full-body skin examination did not reveal any other concerning pigmented lesions. Excisional biopsy was performed due to concern for amelanotic melanoma, and histopathology revealed a superficial and pigmented BCC (Figure 2). Immunohistochemistry with Melan-A was negative for atypical melanocytes, with no uptake in the leukoderma areas.


The clinical presentation initially was concerning for amelanotic melanoma. All melanoma subtypes may appear as hypomelanotic lesions, though these most commonly are observed in the desmoplastic or nodular subtypes. Amelanotic melanomas may present as well-defined red or pink macules, plaques, or nodules, with some tumors presenting with light brown pigmentation.7
The differential diagnosis for lesions with the halo phenomenon is large. In adults, the halo phenomenon may be concerning for malignant or regressing melanoma. As an immunogenic tumor, melanoma’s immunogenic melanocytes may incite a cell-mediated immune response to antigens common to neoplastic and normal melanocytes, which can clinically manifest not only as local annular leukoderma but also as distant vitiligo or halo nevi.7 The halo phenomenon more commonly is associated with benign processes such as vitiligo and halo nevi in children. In most children, halo nevi occur as an isolated phenomenon but still warrant a complete skin examination for melanoma and vitiligo. The presence of halo nevi has been associated with distant vitiligo—possibly through shared immunologic mechanisms—especially if patients present with the Koebner phenomenon, multiple halo nevi, or a family history of vitiligo.8 A prospective study also found that the presence of halo nevi was an independent risk factor for the progression of segmental vitiligo to mixed vitiligo.9 Hormones also may play a role in the leukoderma acquisitum centrifugum, or halo, nevi. Halo nevi most commonly affect adolescents and pregnant women. It has been postulated that congenital nevi may be unique in their response to altered estrogen levels, increasing the rate not only of halo nevi but also of melanoma in pregnant women.10
Our patient’s final histologic diagnosis was pigmented BCC, which comprises only 6% of all BCCs.3 The proposed mechanism is that melanocytes colonize the tumor in the surrounding stroma and produce excess melanin. Basal cell carcinoma with halo phenomenon is a rare presentation. As in our case, 2 prior BCC reports also involved patients older than 50 years,3,5 with the 2 other cases describing women in their late twenties and early thirties.4,6 Additionally, 2 of 4 reports described patients with a history of multiple BCCs.3,5
In summary, the seemingly benign halo phenomenon may accompany malignant processes such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
- Mooney MA, Barr RJ, Buxton MG. Halo nevus or halo phenomenon? a study of 142 cases. J Cutan Pathol. 1995;22:342-348.
- Zeff RA, Freitag A, Grin CM, et al. The immune response in halo nevi. J Am Acad Dermatol. 1997;37:620-624.
- Johnson DB, Ceilley RI. Basal cell carcinoma with annular leukoderma mimicking leukoderma acquisitum centrifugum. Arch Dermatol. 1980;116:352-353.
- Basak PY, Meric G, Ciris M. Basal cell carcinoma with halo phenomenon in a young female: significance of dermatoscopy in early diagnosis. Indian J Dermatol. 2015;60:214.
- Pembroke AC, Liddell K. Basal cell epithelioma with a hypopigmented halo. Arch Dermatol. 1981;117:317.
- Rustemeyer J, Günther L, Deichert L. A rare association: basal cell carcinoma in a vitiliginous macula. Oral Maxillofac Surg. 2011;15:175-177.
- Naveh HP, Rao UN, Butterfield LH. Melanoma‐associated leukoderma—immunology in black and white? Pigment Cell Melanoma Res. 2013;26:796-804.
- Zhou H, Wu L-C, Chen M-K, et al. Factors associated with development of vitiligo in patients with halo nevus. Chinese Med J. 2017;130:2703.
- Ezzedine K, Diallo A, Léauté‐Labrèze C, et al. Halo naevi and leukotrichia are strong predictors of the passage to mixed vitiligo in a subgroup of segmental vitiligo. Br J Dermatol. 2012;166:539-544.
- Nading MA, Nanney LB, Ellis DL. Pregnancy and estrogen receptor β expression in a large congenital nevus. Arch Dermatol. 2009;145:691-694.
Practice Points
- Annular leukoderma, or the halo phenomenon, is a circular reaction of hypopigmentation that more commonly is associated with benign processes such as halo nevi.
- The halo phenomenon may accompany malignant processes, such as nonmelanoma skin cancer. Careful consideration of lesion time course and atypia is imperative for proper clinical suspicion in such cases.
Americans’ sun protection practices fall short of intentions
commissioned by the American Academy of Dermatology.
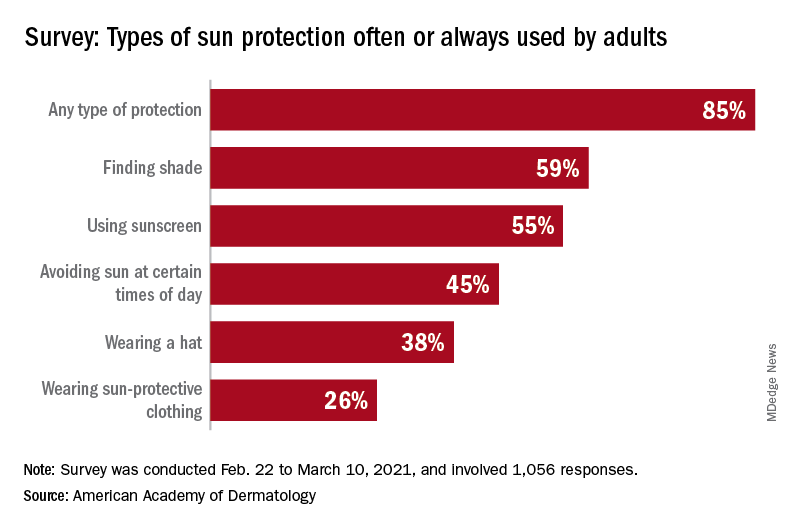
With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
commissioned by the American Academy of Dermatology.

With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
commissioned by the American Academy of Dermatology.

With the pandemic seemingly behind it, the United States enters the summer months facing the paradox of sun protection. Four out of five adults know that sunscreen should be reapplied every 2 hours when they’re outdoors, but only one in three make the actual effort, and 77% are likely to use sunscreen at the beach or a pool, compared with 41% when they’re gardening or working outside on their homes, the AAD reported.
“These findings are surprising and seem to suggest that many people do not take skin cancer seriously or perhaps believe skin cancer won’t happen to them,” Robert T. Brodell, MD, professor of dermatology at the University of Mississippi Medical Center, Jackson, said in a written statement from the AAD, adding that “unprotected exposure to ultraviolet rays is the most preventable risk factor for skin cancer, including melanoma.”
A quarter of all survey respondents reported getting sunburned in 2020, with the youngest adults most likely to feel the wrath of the sun. Sunburn was reported by 43% of those aged 18-23 years, 37% of those aged 24-39, 25% of the 40- to 55-year-olds, 12% of the 56- to 74-year-olds, and 7% of those aged 75 and older. More than a quarter of those who got sunburned said that it was bad enough to make their clothes feel uncomfortable, the academy said.
“Americans see the damaging effects of the sun on their skin as they get older, and two out of three look back and wish they had been more careful. But when it comes to cancer, specifically, most feel unconcerned in spite of their own risk,” according to a statement from Versta Research, which conducted the poll on behalf of the AAD. The survey was conducted from Feb. 22 to March 10, 2021, and involved 1,056 respondents, with a ±3% margin of error.
The lack of concern for skin cancer looks like this: More than two-thirds of the respondents (69%) have at least one possible risk factor – lighter skin tone, blue or green eyes, more than 50 moles, family history – but only 36% expressed concern about developing it. “Indeed, half of survey respondents (49%) say they are more worried about avoiding sunburn than they are about preventing skin cancer, and a third (32%) are more worried about avoiding premature wrinkles than they are about preventing cancer,” the AAD said.
The AAD is considering the creation of a social media quiz or interactive tool, and if the results of this survey were recast as a potential “Knowledge and Awareness Quiz” and graded with a traditional scheme (A = 90%-100%, B = 80%-89%, etc.), then 34% of the respondents would have failed, 15% would have gotten a D, and only 5% would have earned an A, the academy noted.
Squamoid Eccrine Ductal Carcinoma
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).
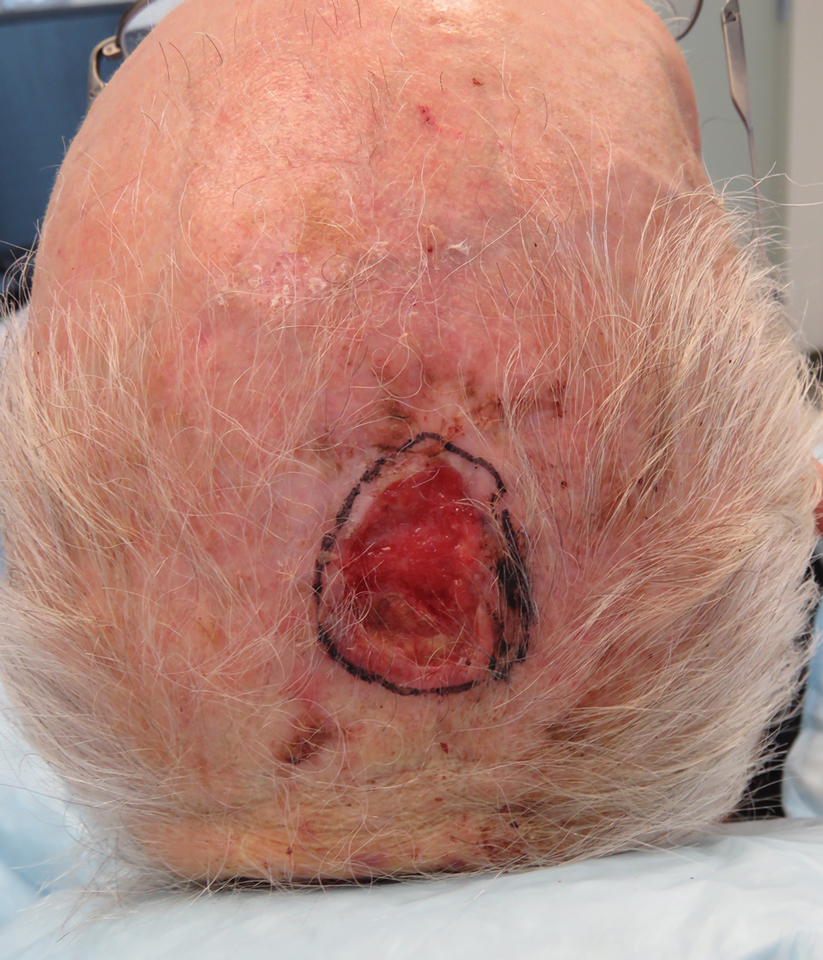
Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.
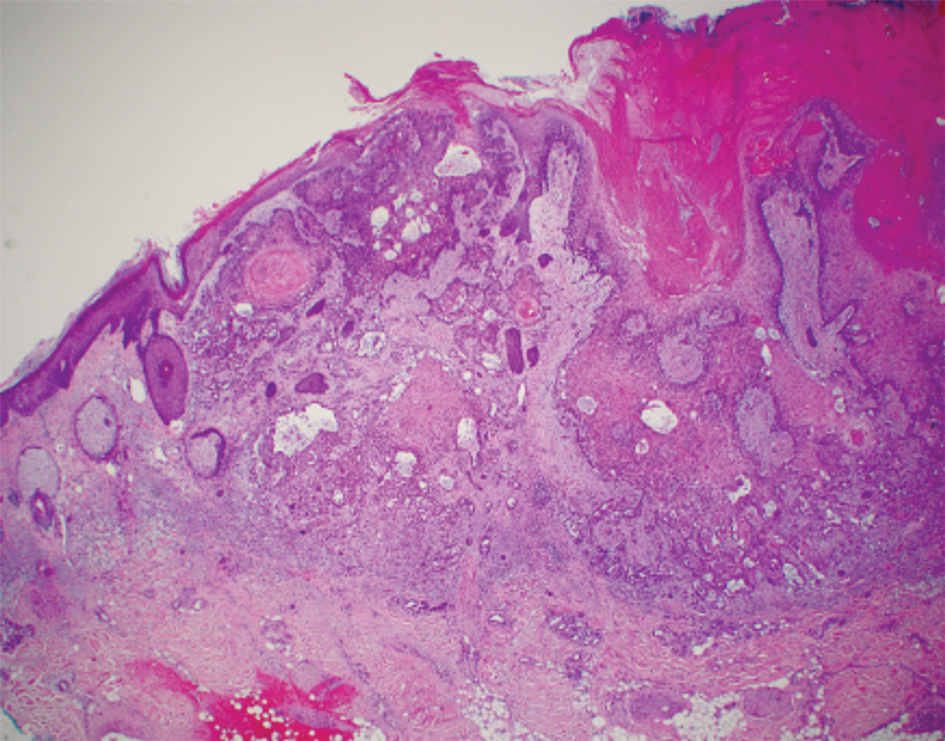


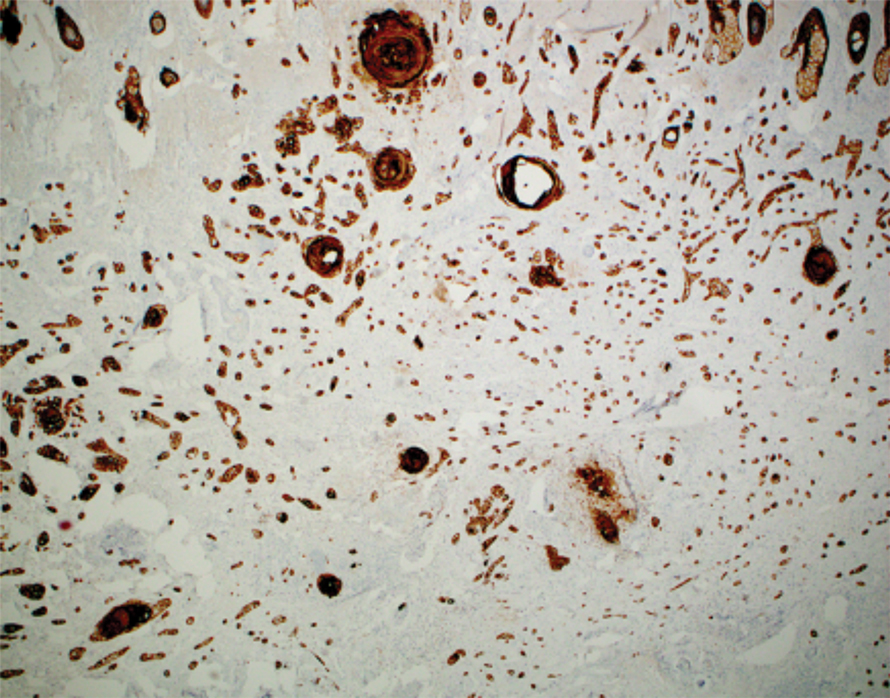
The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).

Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.




The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
Squamoid eccrine ductal carcinoma (SEDC) is an aggressive underrecognized cutaneous malignancy of unknown etiology.1 It is most likely to occur in sun-exposed areas of the body, most commonly the head and neck. Risk factors include male sex, increased age, and chronic immunosuppression.1-4 Current reports suggest that SEDC is likely a high-grade subtype of squamous cell carcinoma (SCC) with a high risk for local recurrence (25%) and metastasis (13%).1,3,5,6 There are as few as 56 cases of SEDC reported in the literature; however, the number of cases may be closer to 100 due to SEDC being classified as either adenosquamous carcinoma of the skin or ductal eccrine carcinoma with squamous differentiation.1
Clinically, SEDC mimics keratinocyte carcinomas. Histologically, SEDC is biphasic, with a superficial portion resembling well-differentiated SCC and a deeply invasive portion having infiltrative irregular cords with ductal differentiation. Perineural invasion (PNI) frequently is present. Multiple connections to the overlying epidermis also can be seen, serving as a subtle clue to the diagnosis on broad superficial specimens.1-3 Due to superficial sampling, approximately 50% of reported cases are misdiagnosed as SCC during the initial biopsy.4 The diagnosis of SEDC often is made during complete excision when deeper tissue is sampled. Establishing an accurate diagnosis is important given the more aggressive nature of SEDC compared with SCC and its proclivity for PNI.1,3,6 The purpose of this review is to increase awareness of this underrecognized entity and describe the histologic findings that help distinguish SEDC from SCC.
Patient Chart Review
We reviewed chart notes as well as frozen and formalin-fixed paraffin-embedded tissue sections from all 5 patients diagnosed with SEDC at a single institution between November 2018 and May 2020. The mean age of patients was 81 years, and 4 were male. Four of the patients presented for MMS with a preoperative diagnosis of SCC per the original biopsy results. Only 1 patient had a preoperative diagnosis of SEDC. The details of each case are recorded in the Table. All tumors were greater than 2 cm in diameter on initial presentation, were located on the head, and clinically resembled keratinocyte carcinoma with either a nodular or plaquelike appearance (Figure 1).

Intraoperative histologic examination of the excised tissue revealed a biphasic pattern consisting of superficial SCC features overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation in all 5 patients (Figures 2–4). Immunohistochemical staining with cytokeratin AE1/AE3 revealed thin strands of carcinoma in the mid to deeper dermis with squamous differentiation and eccrine ductal differentiation (Figure 5), thus confirming the diagnosis in all 5 patients.




The median depth of tumor invasion was 4.1 mm (range, 2.2–5.45 mm). Ulceration was seen in 3 of the patients, and PNI of large-caliber nerves was observed in all 5 patients. A connection with the overlying epidermis was present in all 5 patients. All 5 patients required more than 1 Mohs stage for complete tumor clearance (Table).
In 4 of the patients, nodal imaging performed at the time of diagnosis revealed no evidence of metastasis. Two patients received adjuvant radiation therapy, and none demonstrated evidence of recurrence. The mean follow-up time was 11 months (range, 6.5–18 months) for the 4 cases with available follow-up data (Table).
Literature Review
A PubMed review of the literature using the search term squamoid eccrine ductal carcinoma resulted in 28 articles, 19 of which were included in the review based on inclusion criteria (original articles available in English, in full text, and pertained to SEDC). Our review yielded 56 cases of SEDC.1-19 The mean age of patients with SEDC was 72 years. The number of male and female cases was 52% (29/56) and 48% (27/56), respectively. The most common location of SEDC was on the head or neck (71% [40/56]), followed by the extremities (19% [11/56]). Immunosuppression was noted in 9% (5/56) of cases. Wide local excision was the most commonly employed treatment modality (91% [51/56]), with MMS being used in 4 patients (7%). Adjuvant radiation was reported in 5% (3/56) of cases. Perineural invasion was reported in 34% (19/56) of cases. Recurrence was seen in 23% (13/56) of cases, with a mean time to recurrence of 10.4 months. Metastasis to regional lymph nodes was observed in 13% (7/56) of cases, with 7% (4/56) of those cases having distant metastases.
Comment
Squamoid eccrine ductal carcinoma was successfully treated with MMS in all 5 of the patients we reviewed. Recognition of a distinct biphasic pattern consisting of squamous differentiation superficially with epidermal connection overlying deeper dermal and subcutaneous infiltrative malignant ductal elements with gland formation should lead to consideration of this diagnosis. A thorough inspection for PNI also should be performed, as this finding was present in all of 5 cases and in 34% of reported cases in our literature review.
The differential diagnosis for SEDC includes SCC, metastatic adenocarcinoma with squamoid features, and eccrine tumors, including eccrine poroma, microcystic adnexal carcinoma (MAC), and porocarcinoma with squamous differentiation. The combination of histologic features with the immunoexpression profile of carcinoembryonic antigen (CEA), epithelial membrane antigen (EMA), cytokeratin (CK) 5/6, and p63 can effectively exclude the other entities in the differential and confirm the diagnosis of SEDC.1,3,4 While the diagnosis of SEDC relies on the specific histologic features of multiple surface attachments and superficial squamoid changes with deep ductular elements, immunohistochemistry can nonetheless be adjunctive in difficult cases. Positive immunohistochemical staining for CEA and EMA can help to highlight and delineate true glandular elements, whereas CK5/6 highlights the overall contour of the tumor, displaying more clearly the multiple epidermal attachments and the subtle infiltrative nature of the deeper components of invasive cords and ducts. In addition, the combination of CK5/6 and p63 positivity supports the primary cutaneous nature of the lesion rather than metastatic adenocarcinoma.13,20 Other markers of eccrine secretory coils, such as CK7, CAM5.2, and S100, also are sometimes used for confirmation, some of which can aid in distinction from noneccrine sweat gland differentiation, as CK7 and CAM5.2 are negative in both luminal and basal cells of the dermal duct while being positive within the secretory coil, and S100 protein is expressed within eccrine secretory coil but negative within the apocrine sweat glands.2,4,21
The clinical findings from our chart review corroborated those reported in the literature. The mean age of SEDC in the 5 patients we reviewed was 81 years, and all cases presented on the head, consistent with the findings observed in the literature. Although 4 of our cases were male, there may not be a difference in risk based on sex as previously thought.1 Our literature review revealed an almost equivalent percentage of male and female cases, with 52% being male.
Immunosuppression has been associated with an increased risk for SEDC. Our literature review revealed that approximately 9% (5/56) of cases occurred in immunosuppressed individuals. Two of these reported cases were in the setting of underlying chronic lymphocytic leukemia, 2 in individuals with a history of organ transplant, and 1 treated with azathioprine for myasthenia gravis.2,4,10,12,13 Our chart review supported this correlation, as all 5 patients had a medical history potentially consistent with being in an immunocompromised state (Table). Notably, patient 5 represents a unique case of SEDC occurring in the setting of HIV. The patient had HIV for 33 years, with his most recent CD4+ count of 794 mm3 and HIV-1 RNA load of 35 copies/mL. Given that HIV-positive individuals may have more than a 2-fold increased risk of SCC, a greater degree of suspicion for SEDC should be maintained for these patients.22,23
The etiology of SEDC is controversial but is thought to be either an SCC arising from eccrine glands or a variant of eccrine carcinoma with extensive squamoid differentiation.4,6,13,14,17,24 While SEDC certainly appears to share the proclivity for PNI with the malignant eccrine tumor MAC, it is simultaneously quite distinct, demonstrating nuclear pleomorphism and mitotic activity, both of which are lacking in the bland nature of MACs.12,25
The exact prevalence of SEDC is difficult to ascertain because of its frequent misdiagnosis and variable nomenclature used within the literature. Most reported cases of SEDC are mistakenly diagnosed as SCC on the initial shave or punch biopsy because of superficial sampling. This also was the case in 4 of the patients we reviewed. In addition, there are reported cases of SEDC that were referred to by the investigators as cutaneous adenosquamous carcinoma (cASC), among other descriptors, such as ductal eccrine carcinoma with squamous differentiation, adnexal carcinoma with squamous and ductal differentiation, and syringoid eccrine carcinoma.26-32 While the World Health Organization classifies SEDC as a distinct variant of cASC, which is a rare variant of SCC in itself, the 2 can be differentiated. Despite the similar clinical and histologic features shared between cASC and SEDC, the neoplastic aggregates in SEDC exhibit ductal differentiation containing lumina positive for CEA and EMA.4 Overall, we favor the term squamoid eccrine ductal carcinoma, as there has recently been more uniformity for the designation of this disease entity as such.
It is unclear whether the high incidence of local recurrence (23% [13/56]) of SEDC reported in the literature is related to the treatment modality employed (ie, wide local excision) or due to the innate aggressiveness of SEDC.1,3,5 The literature has shown that MMS has lower recurrence rates than other treatments at 5-year follow-up for SCC (3.1%–5%) and eccrine carcinomas (0%–5%).33,34 Although studies assessing tumor behavior or comparing treatment modalities are limited because of the rarity and underrecognition of SEDC, MMS has been used several times for SEDC with only 1 recurrence reported.4,13,17,24 Given that all 5 of the patients we reviewed required more than 1 Mohs stage for complete tumor clearance and none demonstrated evidence of recurrence or metastasis (Table), we recommend MMS as the treatment of choice for SEDC.
Conclusion
Squamoid eccrine ductal carcinoma is a rare but likely underdiagnosed cutaneous tumor of uncertain etiology. Because of its propensity for recurrence and metastasis, excision of SEDC with complete circumferential peripheral and deep margin assessment with close follow-up is recommended.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Jacob J, Kugelman L. Squamoid eccrine ductal carcinoma. Cutis. 2018;101:378-380, 385.
- Yim S, Lee YH, Chae SW, et al. Squamoid eccrine ductal carcinoma of the ear helix. Clin Case Rep. 2019;7:1409-1411.
- Terushkin E, Leffell DJ, Futoryan T, et al. Squamoid eccrine ductal carcinoma: a case report and review of the literature. Am J Dermatopathol. 2010;32:287-292.
- Jung YH, Jo HJ, Kang MS. Squamoid eccrine ductal carcinoma of the scalp. Korean J Pathol. 2012;46:278-281.
- Saraiva MI, Vieira MA, Portocarrero LK, et al. Squamoid eccrine ductal carcinoma. An Bras Dermatol. 2016;91:799-802.
- Phan K, Kim L, Lim P, et al. A case report of temple squamoid eccrine ductal carcinoma: a diagnostic challenge beneath the tip of the iceberg. Dermatol Ther. 2020;33:E13213.
- McKissack SS, Wohltmann W, Dalton SR, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Lobo-Jardim MM, Souza BdCE, Kakizaki P, et al. Dermoscopy of squamoid eccrine ductal carcinoma: an aid for early diagnosis. An Bras Dermatol. 2018;93:893-895.
- Chan H, Howard V, Moir D, et al. Squamoid eccrine ductal carcinoma of the scalp. Aust J Dermatol. 2016;57:E117-E119.
- Wang B, Jarell AD, Bingham JL, et al. PET/CT imaging of squamoid eccrine ductal carcinoma. Clin Nucl Med. 2015;40:322-324.
- Frouin E, Vignon-Pennamen MD, Balme B, et al. Anatomoclinical study of 30 cases of sclerosing sweat duct carcinomas (microcystic adnexal carcinoma, syringomatous carcinoma and squamoid eccrine ductal carcinoma). J Eur Acad Dermatol Venereol. 2015;29:1978-1994.
- Clark S, Young A, Piatigorsky E, et al. Mohs micrographic surgery in the setting of squamoid eccrine ductal carcinoma: addressing a diagnostic and therapeutic challenge. J Clin Aesthet Dermatol. 2013;6:33-36.
- Pusiol T, Morichetti D, Zorzi MG, et al. Squamoid eccrine ductal carcinoma: inappropriate diagnosis. Dermatol Surg. 2011;37:1819-1820.
- Kavand S, Cassarino DS. “Squamoid eccrine ductal carcinoma”: an unusual low-grade case with follicular differentiation. are these tumors squamoid variants of microcystic adnexal carcinoma? Am J Dermatopathol. 2009;31:849-852.
- Wasserman DI, Sack J, Gonzalez-Serva A, et al. Sentinel lymph node biopsy for a squamoid eccrine carcinoma with lymphatic invasion. Dermatol Surg. 2007;33:1126-1129.
- Kim YJ, Kim AR, Yu DS. Mohs micrographic surgery for squamoid eccrine ductal carcinoma. Dermatol Surg. 2005;31:1462-1464.
- Herrero J, Monteagudo C, Jorda E, et al. Squamoid eccrine ductal carcinoma. Histopathology. 1998;32:478-480.
- Wong TY, Suster S, Mihm MC. Squamoid eccrine ductal carcinoma. Histopathology. 1997;30:288-293.
- Qureshi HS, Ormsby AH, Lee MW, et al. The diagnostic utility of p63, CK5/6, CK 7, and CK 20 in distinguishing primary cutaneous adnexal neoplasms from metastatic carcinomas. J Cutan Pathol. 2004;31:145-152.
- Dabbs DJ. Diagnostic Immunohistochemistry: Theranostic and Genomic Applications. 4th ed. Elsevier/Saunders; 2014.
- Silverberg MJ, Leyden W, Warton EM, et al. HIV infection status, immunodeficiency, and the incidence of non-melanoma skin cancer. J Natl Cancer Inst. 2013;105:350-360.
- Asgari MM, Ray GT, Quesenberry CP Jr, et al. Association of multiple primary skin cancers with human immunodeficiency virus infection, CD4 count, and viral load. JAMA Dermatol. 2017;153:892-896.
- Tolkachjov SN. Adnexal carcinomas treated with Mohs micrographic surgery: a comprehensive review. Dermatol Surg. 2017;43:1199-1207.
- Kazakov DV. Cutaneous Adnexal Tumors. Wolters Kluwer Health/ Lippincott Williams & Wilkins; 2012.
- Weidner N, Foucar E. Adenosquamous carcinoma of the skin. an aggressive mucin- and gland-forming squamous carcinoma. Arch Dermatol. 1985;121:775-779.
- Banks ER, Cooper PH. Adenosquamous carcinoma of the skin: a report of 10 cases. J Cutan Pathol. 1991;18:227-234.
- Ko CJ, Leffell DJ, McNiff JM. Adenosquamous carcinoma: a report of nine cases with p63 and cytokeratin 5/6 staining. J Cutan Pathol. 2009;36:448-452.
- Patel V, Squires SM, Liu DY, et al. Cutaneous adenosquamous carcinoma: a rare neoplasm with biphasic differentiation. Cutis. 2014;94:231-233.
- Chhibber V, Lyle S, Mahalingam M. Ductal eccrine carcinoma with squamous differentiation: apropos a case. J Cutan Pathol. 2007;34:503-507.
- Sidiropoulos M, Sade S, Al-Habeeb A, et al. Syringoid eccrine carcinoma: a clinicopathological and immunohistochemical study of four cases. J Clin Pathol. 2011;64:788-792.
- Azorín D, López-Ríos F, Ballestín C, et al. Primary cutaneous adenosquamous carcinoma: a case report and review of the literature. J Cutan Pathol. 2001;28:542-545.
- Wildemore JK, Lee JB, Humphreys TR. Mohs surgery for malignant eccrine neoplasms. Dermatol Surg. 2004;30(12 pt 2):1574-1579.
- Garcia-Zuazaga J, Olbricht SM. Cutaneous squamous cell carcinoma. Adv Dermatol. 2008;24:33-57.
PRACTICE POINTS
- Squamoid eccrine ductal carcinoma is an aggressive underrecognized cutaneous malignancy that often is misdiagnosed as squamous cell carcinoma (SCC) during initial biopsy.
- Squamoid eccrine ductal carcinoma has a biphasic histologic appearance with a superficial portion resembling well-differentiated SCC and a deeply invasive portion comprised of infiltrative irregular cords with ductal differentiation.
- Excision with complete circumferential peripheral and deep margin assessment with close follow-up is recommended for these patients because of the high risk for recurrence and metastasis.