User login
What does it mean to be a trustworthy male ally?
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
“If you want to be trusted, be trustworthy” – Stephen Covey
A few years ago, while working in my office, a female colleague stopped by for a casual chat. During the course of the conversation, she noticed that I did not have any diplomas or certificates hanging on my office walls. Instead, there were clusters of pictures drawn by my children, family photos, and a white board with my “to-do” list. The only wall art was a print of Banksy’s “The Thinker Monkey,” which depicts a monkey with its fist to its chin similar to Rodin’s famous sculpture, “Le Penseur.”
When asked why I didn’t hang any diplomas or awards, I replied that I preferred to keep my office atmosphere light and fun, and to focus on future goals rather than past accomplishments. I could see her jaw tense. Her frustration appeared deep, but it was for reasons beyond just my self-righteous tone. She said, “You know, I appreciate your focus on future goals, but it’s a pretty privileged position to not have to worry about sharing your accomplishments publicly.”
What followed was a discussion that was generative, enlightening, uncomfortable, and necessary. I had never considered what I chose to hang (or not hang) on my office walls as a privilege, and that was exactly the point. She described numerous episodes when her accomplishments were overlooked or (worse) attributed to a male colleague because she was a woman. I began to understand that graceful self-promotion is not optional for many women in medicine, it is a necessary skill.
This is just one example of how my privilege as a male in medicine contributed to my ignorance of the gender inequities that my female coworkers have faced throughout their careers. My colleague showed a lot of grace by taking the time to help me navigate my male privilege in a constructive manner. I decided to learn more about gender inequities, and eventually determined that I was woefully inadequate as a male ally, not by refusal but by ignorance. I wanted to start earning my colleague’s trust that I would be an ally that she could count on.
Trustworthiness
I wanted to be a trustworthy ally, but what does that entail? Perhaps we can learn from medical education. Trust is a complex construct that is increasingly used as a framework for assessing medical students and residents, such as with entrustable professional activities (EPAs).1,2 Multiple studies have examined the characteristics that make a learner “trustworthy” when determining how much supervision is required.3-8 Ten Cate and Chen performed an interpretivist, narrative review to synthesize the medical education literature on learner trustworthiness in the past 15 years,9 developing five major themes that contribute to trustworthiness: Humility, Capability, Agency, Reliability, and Integrity. Let’s examine each of these through the lens of male allyship.
Humility
Humility involves knowing one’s limits, asking for help, and being receptive to feedback.9 The first thing men need to do is to put their egos in check and recognize that women do not need rescuing; they need partnership. Systemic inequities have led to men holding the majority of leadership positions and significant sociopolitical capital, and correcting these inequities is more feasible when those in leadership and positions of power contribute. Women don’t need knights in shining armor, they need collaborative activism.
Humility also means a willingness to admit fallibility and to ask for help. Men often don’t know what they don’t know (see my foibles in the opening). As David G. Smith, PhD, and W. Brad Johnson, PhD, write in their book, “Good Guys,” “There are no perfect allies. As you work to become a better ally for the women around you, you will undoubtedly make a mistake.”10 Men must accept feedback on their shortcomings as allies without feeling as though they are losing their sociopolitical standing. Allyship for women does not mean there is a devaluing of men. We must escape a “zero-sum” mindset. Mistakes are where growth happens, but only if we approach our missteps with humility.
Capability
Capability entails having the necessary knowledge, skills, and attitudes to be a strong ally. Allyship is not intuitive for most men for several reasons. Many men do not experience the same biases or systemic inequities that women do, and therefore perceive them less frequently. I want to acknowledge that men can be victims of other systemic biases such as those against one’s race, ethnicity, gender identity, sexual orientation, religion, or any number of factors. Men who face inequities for these other reasons may be more cognizant of the biases women face. Even so, allyship is a skill that few men have been explicitly taught. Even if taught, few standard or organized mechanisms for feedback on allyship capability exist. How, then, can men become capable allies?
Just like in medical education, men must become self-directed learners who seek to build capability and receive feedback on their performance as allies. Men should seek allyship training through local women-in-medicine programs or organizations, or through the increasing number of national education options such as the recent ADVANCE PHM Gender Equity Symposium. As with learning any skill, men should go to the literature, seeking knowledge from experts in the field. I recommend starting with “Good Guys: How Men Can Be Better Allies for Women in the Workplace10 or “Athena Rising: How and Why Men Should Mentor Women.”11 Both books, by Dr. Smith and Dr. Johnson, are great entry points into the gender allyship literature. Seek out other resources from local experts on gender equity and allyship. Both aforementioned books were recommended to me by a friend and gender equity expert; without her guidance I would not have known where to start.
Agency
Agency involves being proactive and engaged rather than passive or apathetic. Men must be enthusiastic allies who seek out opportunities to mentor and sponsor women rather than waiting for others to ask. Agency requires being curious and passionate about improving. Most men in medicine are not openly and explicitly misogynistic or sexist, but many are only passive when it comes to gender equity and allyship. Trustworthy allyship entails turning passive support into active change. Not sure how to start? A good first step is to ask female colleagues questions such as, “What can I do to be a better ally for you in the workplace?” or “What are some things at work that are most challenging to you, but I might not notice because I’m a man?” Curiosity is the springboard toward agency.
Reliability
Reliability means being conscientious, accountable, and doing what we say we will do. Nothing undermines trustworthiness faster than making a commitment and not following through. Allyship cannot be a show or an attempt to get public plaudits. It is a longitudinal commitment to supporting women through individual mentorship and sponsorship, and to work toward institutional and systems change.
Reliability also means taking an equitable approach to what Dr. Smith and Dr. Johnson call “office housework.” They define this as “administrative work that is necessary but undervalued, unlikely to lead to promotion, and disproportionately assigned to women.”10 In medicine, these tasks include organizing meetings, taking notes, planning social events, and remembering to celebrate colleagues’ achievements and milestones. Men should take on more of these tasks and advocate for change when the distribution of office housework in their workplace is inequitably directed toward women.
Integrity
Integrity involves honesty, professionalism, and benevolence. It is about making the morally correct choice even if there is potential risk. When men see gender inequity, they have an obligation to speak up. Whether it is overtly misogynistic behavior, subtle sexism, use of gendered language, inequitable distribution of office housework, lack of inclusivity and recognition for women, or another form of inequity, men must act with integrity and make it clear that they are partnering with women for change. Integrity means being an ally even when women are not present, and advocating that women be “at the table” for important conversations.
Beyond the individual
Allyship cannot end with individual actions; systems changes that build trustworthy institutions are necessary. Organizational leaders must approach gender conversations with humility to critically examine inequities and agency to implement meaningful changes. Workplace cultures and institutional policies should be reviewed with an eye toward system-level integrity and reliability for promoting and supporting women. Ongoing faculty and staff development programs must provide men with the knowledge, skills, and attitudes (capability) to be strong allies. We have a long history of male-dominated institutions that are unfair or (worse) unsafe for women. Many systems are designed in a way that disadvantages women. These systems must be redesigned through an equity lens to start building trust with women in medicine.
Becoming trustworthy is a process
Even the best male allies have room to improve their trustworthiness. Many men (myself included) have a LOT of room to improve, but they should not get discouraged by the amount of ground to be gained. Steady, deliberate improvement in men’s humility, capability, agency, reliability, and integrity can build the foundation of trust with female colleagues. Trust takes time. It takes effort. It takes vulnerability. It is an ongoing, developmental process that requires deliberate practice, frequent reflection, and feedback from our female colleagues.
Dr. Kinnear is associate professor of internal medicine and pediatrics in the Division of Hospital Medicine at Cincinnati Children’s Hospital Medical Center and University of Cincinnati Medical Center. He is associate program director for the Med-Peds and Internal Medicine residency programs.
References
1. Ten Cate O. Nuts and bolts of entrustable professional activities. J Grad Med Educ. 2013 Mar;5(1):157-8. doi: 10.4300/JGME-D-12-00380.1.
2. Ten Cate O. Entrustment decisions: Bringing the patient into the assessment equation. Acad Med. 2017 Jun;92(6):736-8. doi: 10.1097/ACM.0000000000001623.
3. Kennedy TJT et al. Point-of-care assessment of medical trainee competence for independent clinical work. Acad Med. 2008 Oct;83(10 Suppl):S89-92. doi: 10.1097/ACM.0b013e318183c8b7.
4. Choo KJ et al. How do supervising physicians decide to entrust residents with unsupervised tasks? A qualitative analysis. J Hosp Med. 2014 Mar;9(3):169-75. doi: 10.1002/jhm.2150.
5. Hauer KE et al. How clinical supervisors develop trust in their trainees: A qualitative study. Med Educ. 2015 Aug;49(8):783-95. doi: 10.1111/medu.12745.
6. Sterkenburg A et al. When do supervising physicians decide to entrust residents with unsupervised tasks? Acad Med. 2010 Sep;85(9):1408-17. doi: 10.1097/ACM.0b013e3181eab0ec.
7. Sheu L et al. How supervisor experience influences trust, supervision, and trainee learning: A qualitative study. Acad Med. 2017 Sep;92(9):1320-7. doi: 10.1097/ACM.0000000000001560.
8. Pingree EW et al. Encouraging entrustment: A qualitative study of resident behaviors that promote entrustment. Acad Med. 2020 Nov;95(11):1718-25. doi: 10.1097/ACM.0000000000003487.
9. Ten Cate O, Chen HC. The ingredients of a rich entrustment decision. Med Teach. 2020 Dec;42(12):1413-20. doi: 10.1080/0142159X.2020.1817348.
10. Smith DG, Johnson WB. Good guys: How men can be better allies for women in the workplace: Harvard Business School Publishing Corporation 2020.
11. Johnson WB, Smith D. Athena rising: How and why men should mentor women: Routledge 2016.
Hormone blocker sticker shock – again – as patients lose cheaper drug option
In 2020, he’d fought to get insurance to cover a lower-priced version of a drug his then-8-year-old needed. She’d been diagnosed with central precocious puberty, a rare condition marked by early onset of sexual development – often years earlier than one’s peers. KHN and NPR wrote about Dr. Taksali and his family as part of the Bill of the Month series.
The girl’s doctors and the Taksalis decided to put her puberty on pause with a hormone-blocking drug implant that would be placed under the skin in her arm and release a little bit of the medication each day.
Dr. Taksali, an orthopedic surgeon, learned there were two nearly identical drug products made by Endo Pharmaceuticals, both containing 50 mg of the hormone blocker histrelin. One cost more than eight times more than the other. He wanted to use the cheaper one, Vantas, which costs about $4,800 per implant. But his insurer would not initially cover it, instead preferring Supprelin LA, which is approved by the Food and Drug Administration to treat central precocious puberty, and costs about $43,000.
Vantas can be prescribed off label for the condition, and after much back-and-forth dialogue, Dr. Taksali finally got the insurer to cover it.
Then this summer, it was time to replace the implant.
“I thought we would just get a Vantas replacement,” Dr. Taksali said. “In my mind, I was like: ‘Well, she got it the first time, and we’ve already kind of fought the battle with the insurance company and, you know, got it approved.”
But during a virtual appointment with his daughter’s doctor, he learned they couldn’t get Vantas. No one could. There was a Vantas shortage.
Endo cited a manufacturing problem. Batches of Vantas weren’t coming out right and couldn’t be released to the public, the company’s vice president of corporate affairs, Heather Zoumas Lubeski, said in an email. Vantas and Supprelin were made in the same facility, but the problem affected only Vantas, she wrote, stressing that the drugs are “not identical products.”
In August, Endo’s president and CEO, Blaise Coleman, told investors Supprelin was doing particularly well for the company. Revenue had grown by 79%, compared with the same quarter the year before. The growth was driven in part, Mr. Coleman explained, “by stronger-than-expected demand resulting from expanded patient awareness and a competitor product shortage,” he said.
What competitor product shortage? Could that be Vantas?
Asked about this, Ms. Zoumas Lubeski said Mr. Coleman wasn’t referring to Vantas. Since Vantas isn’t approved to treat central precocious puberty, it can’t technically be considered a competitor to Supprelin. Mr. Coleman was referring to the rival product Lupron Depot-Ped, not an implant, but an injection made by AbbVie, Ms. Zoumas Lubeski said.
Dr. Taksali was skeptical.
“It’s all very curious, like, huh, you know, when this particular option went away and your profits went up nearly 80% from the more expensive drug,” he said.
Then, in September, Endo told the FDA it stopped making Vantas for good.
Ms. Zoumas Lubeski said that, when Endo investigated its Vantas manufacturing problem, it wasn’t able to find “a suitable corrective action that resolves the issue.”
“As a result, and after analysis of the market for the availability of alternative therapies, we made the difficult decision to discontinue the supply of this product,” she said via email. “Endo is committed to maintaining the highest quality standards for all of its products.”
Dr. Taksali said he felt resigned to giving his daughter Supprelin even before the shortage turned into a discontinuation. Ultimately, he won’t pay much more out-of-pocket, but his insurance will pay the rest. And that could raise his business’s premiums.
The FDA cannot force Endo to keep making the drug or set a lower price for the remaining one. It doesn’t have the authority. That decision lies with Endo Pharmaceuticals. A drugmaker discontinuing a product isn’t anything new, said Erin Fox, who directs drug information and support services at University of Utah Health hospitals.
“The FDA has very little leverage because there is no requirement for any company to make any drug, no matter how lifesaving,” she said. “We have a capitalist society. We have a free market. And so any company can discontinue anything ... at any time for any reason.”
Still, companies are supposed to tell the FDA about potential shortages and discontinuations ahead of time so it can minimize the impact on public health. It can help a firm resolve a manufacturing issue, decide whether it’s safe to extend an expiration date or help a company making an alternative product to ramp up production.
“The FDA expects that manufacturers will notify the agency before a meaningful disruption in their own supply occurs,” FDA spokesperson Jeremy Kahn wrote in an email. “When the FDA does not receive timely, informative notifications, the agency’s ability to respond appropriately is limited.”
But the rules are somewhat flexible. A company is required to notify the FDA of an upcoming drug supply disruption 6 months before it affects consumers or “as soon as practicable” after that. But their true deadline is 5 business days after manufacturing stops, according to the FDA website.
“They’re supposed to tell the FDA, but even if they don’t, there’s no penalty,” Ms. Fox said. “There’s no teeth in that law. ... Their name can go on the FDA naughty list. That’s pretty much it.”
In rare cases, the FDA will send a noncompliance letter to the drugmaker and require it to explain itself. This has happened only five times since 2015. There is no such letter about Vantas, suggesting that Endo met the FDA’s requirements for notification.
Concerned about potential drug shortages caused by COVID-19 in March 2020, a bipartisan group of legislators introduced the Preventing Drug Shortages Act, which aimed to increase transparency around shortages. But the legislation gained no traction.
As a result of limited FDA power, the intricacies of drug shortages remain opaque, Ms. Fox said. Companies don’t have to make the reasons for shortages public. That sets the Vantas shortage and discontinuation apart from many others. The company is saying more about what happened than most do.
“Many companies will actually just put drugs on temporarily unavailable or long-term backorder, and sometimes that can last years before the company finally makes a decision” on whether to discontinue a product, she said. “It can take a long time, and so it can be frustrating to not know – or to kind of stake your hopes on a product coming back to the market once it’s been in shortage for so long.”
It’s hard to know exactly how many children will be affected by the Vantas discontinuation because data about off-label use is hard to come by.
Erica Eugster, MD, a professor of pediatrics at Indiana University, Indianapolis, said central precocious puberty patients weren’t her first thought when she learned of the Vantas discontinuation.
“I immediately thought about our transgender population,” she said. “They’re the ones that are really going to suffer from this.”
No medications have been FDA approved to treat patients with gender dysphoria, the medical term for when the sex assigned at birth doesn’t match someone’s gender identity, causing them psychological distress. As a result, any drug to stop puberty in this population would be off label, making it difficult for families to get health insurance coverage. Vantas had been a lower-cost option.
The number of transgender patients receiving histrelin implants rose significantly from 2004 to 2016, according to a study published in the Journal of Pediatric Endocrinology and Metabolism.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In 2020, he’d fought to get insurance to cover a lower-priced version of a drug his then-8-year-old needed. She’d been diagnosed with central precocious puberty, a rare condition marked by early onset of sexual development – often years earlier than one’s peers. KHN and NPR wrote about Dr. Taksali and his family as part of the Bill of the Month series.
The girl’s doctors and the Taksalis decided to put her puberty on pause with a hormone-blocking drug implant that would be placed under the skin in her arm and release a little bit of the medication each day.
Dr. Taksali, an orthopedic surgeon, learned there were two nearly identical drug products made by Endo Pharmaceuticals, both containing 50 mg of the hormone blocker histrelin. One cost more than eight times more than the other. He wanted to use the cheaper one, Vantas, which costs about $4,800 per implant. But his insurer would not initially cover it, instead preferring Supprelin LA, which is approved by the Food and Drug Administration to treat central precocious puberty, and costs about $43,000.
Vantas can be prescribed off label for the condition, and after much back-and-forth dialogue, Dr. Taksali finally got the insurer to cover it.
Then this summer, it was time to replace the implant.
“I thought we would just get a Vantas replacement,” Dr. Taksali said. “In my mind, I was like: ‘Well, she got it the first time, and we’ve already kind of fought the battle with the insurance company and, you know, got it approved.”
But during a virtual appointment with his daughter’s doctor, he learned they couldn’t get Vantas. No one could. There was a Vantas shortage.
Endo cited a manufacturing problem. Batches of Vantas weren’t coming out right and couldn’t be released to the public, the company’s vice president of corporate affairs, Heather Zoumas Lubeski, said in an email. Vantas and Supprelin were made in the same facility, but the problem affected only Vantas, she wrote, stressing that the drugs are “not identical products.”
In August, Endo’s president and CEO, Blaise Coleman, told investors Supprelin was doing particularly well for the company. Revenue had grown by 79%, compared with the same quarter the year before. The growth was driven in part, Mr. Coleman explained, “by stronger-than-expected demand resulting from expanded patient awareness and a competitor product shortage,” he said.
What competitor product shortage? Could that be Vantas?
Asked about this, Ms. Zoumas Lubeski said Mr. Coleman wasn’t referring to Vantas. Since Vantas isn’t approved to treat central precocious puberty, it can’t technically be considered a competitor to Supprelin. Mr. Coleman was referring to the rival product Lupron Depot-Ped, not an implant, but an injection made by AbbVie, Ms. Zoumas Lubeski said.
Dr. Taksali was skeptical.
“It’s all very curious, like, huh, you know, when this particular option went away and your profits went up nearly 80% from the more expensive drug,” he said.
Then, in September, Endo told the FDA it stopped making Vantas for good.
Ms. Zoumas Lubeski said that, when Endo investigated its Vantas manufacturing problem, it wasn’t able to find “a suitable corrective action that resolves the issue.”
“As a result, and after analysis of the market for the availability of alternative therapies, we made the difficult decision to discontinue the supply of this product,” she said via email. “Endo is committed to maintaining the highest quality standards for all of its products.”
Dr. Taksali said he felt resigned to giving his daughter Supprelin even before the shortage turned into a discontinuation. Ultimately, he won’t pay much more out-of-pocket, but his insurance will pay the rest. And that could raise his business’s premiums.
The FDA cannot force Endo to keep making the drug or set a lower price for the remaining one. It doesn’t have the authority. That decision lies with Endo Pharmaceuticals. A drugmaker discontinuing a product isn’t anything new, said Erin Fox, who directs drug information and support services at University of Utah Health hospitals.
“The FDA has very little leverage because there is no requirement for any company to make any drug, no matter how lifesaving,” she said. “We have a capitalist society. We have a free market. And so any company can discontinue anything ... at any time for any reason.”
Still, companies are supposed to tell the FDA about potential shortages and discontinuations ahead of time so it can minimize the impact on public health. It can help a firm resolve a manufacturing issue, decide whether it’s safe to extend an expiration date or help a company making an alternative product to ramp up production.
“The FDA expects that manufacturers will notify the agency before a meaningful disruption in their own supply occurs,” FDA spokesperson Jeremy Kahn wrote in an email. “When the FDA does not receive timely, informative notifications, the agency’s ability to respond appropriately is limited.”
But the rules are somewhat flexible. A company is required to notify the FDA of an upcoming drug supply disruption 6 months before it affects consumers or “as soon as practicable” after that. But their true deadline is 5 business days after manufacturing stops, according to the FDA website.
“They’re supposed to tell the FDA, but even if they don’t, there’s no penalty,” Ms. Fox said. “There’s no teeth in that law. ... Their name can go on the FDA naughty list. That’s pretty much it.”
In rare cases, the FDA will send a noncompliance letter to the drugmaker and require it to explain itself. This has happened only five times since 2015. There is no such letter about Vantas, suggesting that Endo met the FDA’s requirements for notification.
Concerned about potential drug shortages caused by COVID-19 in March 2020, a bipartisan group of legislators introduced the Preventing Drug Shortages Act, which aimed to increase transparency around shortages. But the legislation gained no traction.
As a result of limited FDA power, the intricacies of drug shortages remain opaque, Ms. Fox said. Companies don’t have to make the reasons for shortages public. That sets the Vantas shortage and discontinuation apart from many others. The company is saying more about what happened than most do.
“Many companies will actually just put drugs on temporarily unavailable or long-term backorder, and sometimes that can last years before the company finally makes a decision” on whether to discontinue a product, she said. “It can take a long time, and so it can be frustrating to not know – or to kind of stake your hopes on a product coming back to the market once it’s been in shortage for so long.”
It’s hard to know exactly how many children will be affected by the Vantas discontinuation because data about off-label use is hard to come by.
Erica Eugster, MD, a professor of pediatrics at Indiana University, Indianapolis, said central precocious puberty patients weren’t her first thought when she learned of the Vantas discontinuation.
“I immediately thought about our transgender population,” she said. “They’re the ones that are really going to suffer from this.”
No medications have been FDA approved to treat patients with gender dysphoria, the medical term for when the sex assigned at birth doesn’t match someone’s gender identity, causing them psychological distress. As a result, any drug to stop puberty in this population would be off label, making it difficult for families to get health insurance coverage. Vantas had been a lower-cost option.
The number of transgender patients receiving histrelin implants rose significantly from 2004 to 2016, according to a study published in the Journal of Pediatric Endocrinology and Metabolism.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
In 2020, he’d fought to get insurance to cover a lower-priced version of a drug his then-8-year-old needed. She’d been diagnosed with central precocious puberty, a rare condition marked by early onset of sexual development – often years earlier than one’s peers. KHN and NPR wrote about Dr. Taksali and his family as part of the Bill of the Month series.
The girl’s doctors and the Taksalis decided to put her puberty on pause with a hormone-blocking drug implant that would be placed under the skin in her arm and release a little bit of the medication each day.
Dr. Taksali, an orthopedic surgeon, learned there were two nearly identical drug products made by Endo Pharmaceuticals, both containing 50 mg of the hormone blocker histrelin. One cost more than eight times more than the other. He wanted to use the cheaper one, Vantas, which costs about $4,800 per implant. But his insurer would not initially cover it, instead preferring Supprelin LA, which is approved by the Food and Drug Administration to treat central precocious puberty, and costs about $43,000.
Vantas can be prescribed off label for the condition, and after much back-and-forth dialogue, Dr. Taksali finally got the insurer to cover it.
Then this summer, it was time to replace the implant.
“I thought we would just get a Vantas replacement,” Dr. Taksali said. “In my mind, I was like: ‘Well, she got it the first time, and we’ve already kind of fought the battle with the insurance company and, you know, got it approved.”
But during a virtual appointment with his daughter’s doctor, he learned they couldn’t get Vantas. No one could. There was a Vantas shortage.
Endo cited a manufacturing problem. Batches of Vantas weren’t coming out right and couldn’t be released to the public, the company’s vice president of corporate affairs, Heather Zoumas Lubeski, said in an email. Vantas and Supprelin were made in the same facility, but the problem affected only Vantas, she wrote, stressing that the drugs are “not identical products.”
In August, Endo’s president and CEO, Blaise Coleman, told investors Supprelin was doing particularly well for the company. Revenue had grown by 79%, compared with the same quarter the year before. The growth was driven in part, Mr. Coleman explained, “by stronger-than-expected demand resulting from expanded patient awareness and a competitor product shortage,” he said.
What competitor product shortage? Could that be Vantas?
Asked about this, Ms. Zoumas Lubeski said Mr. Coleman wasn’t referring to Vantas. Since Vantas isn’t approved to treat central precocious puberty, it can’t technically be considered a competitor to Supprelin. Mr. Coleman was referring to the rival product Lupron Depot-Ped, not an implant, but an injection made by AbbVie, Ms. Zoumas Lubeski said.
Dr. Taksali was skeptical.
“It’s all very curious, like, huh, you know, when this particular option went away and your profits went up nearly 80% from the more expensive drug,” he said.
Then, in September, Endo told the FDA it stopped making Vantas for good.
Ms. Zoumas Lubeski said that, when Endo investigated its Vantas manufacturing problem, it wasn’t able to find “a suitable corrective action that resolves the issue.”
“As a result, and after analysis of the market for the availability of alternative therapies, we made the difficult decision to discontinue the supply of this product,” she said via email. “Endo is committed to maintaining the highest quality standards for all of its products.”
Dr. Taksali said he felt resigned to giving his daughter Supprelin even before the shortage turned into a discontinuation. Ultimately, he won’t pay much more out-of-pocket, but his insurance will pay the rest. And that could raise his business’s premiums.
The FDA cannot force Endo to keep making the drug or set a lower price for the remaining one. It doesn’t have the authority. That decision lies with Endo Pharmaceuticals. A drugmaker discontinuing a product isn’t anything new, said Erin Fox, who directs drug information and support services at University of Utah Health hospitals.
“The FDA has very little leverage because there is no requirement for any company to make any drug, no matter how lifesaving,” she said. “We have a capitalist society. We have a free market. And so any company can discontinue anything ... at any time for any reason.”
Still, companies are supposed to tell the FDA about potential shortages and discontinuations ahead of time so it can minimize the impact on public health. It can help a firm resolve a manufacturing issue, decide whether it’s safe to extend an expiration date or help a company making an alternative product to ramp up production.
“The FDA expects that manufacturers will notify the agency before a meaningful disruption in their own supply occurs,” FDA spokesperson Jeremy Kahn wrote in an email. “When the FDA does not receive timely, informative notifications, the agency’s ability to respond appropriately is limited.”
But the rules are somewhat flexible. A company is required to notify the FDA of an upcoming drug supply disruption 6 months before it affects consumers or “as soon as practicable” after that. But their true deadline is 5 business days after manufacturing stops, according to the FDA website.
“They’re supposed to tell the FDA, but even if they don’t, there’s no penalty,” Ms. Fox said. “There’s no teeth in that law. ... Their name can go on the FDA naughty list. That’s pretty much it.”
In rare cases, the FDA will send a noncompliance letter to the drugmaker and require it to explain itself. This has happened only five times since 2015. There is no such letter about Vantas, suggesting that Endo met the FDA’s requirements for notification.
Concerned about potential drug shortages caused by COVID-19 in March 2020, a bipartisan group of legislators introduced the Preventing Drug Shortages Act, which aimed to increase transparency around shortages. But the legislation gained no traction.
As a result of limited FDA power, the intricacies of drug shortages remain opaque, Ms. Fox said. Companies don’t have to make the reasons for shortages public. That sets the Vantas shortage and discontinuation apart from many others. The company is saying more about what happened than most do.
“Many companies will actually just put drugs on temporarily unavailable or long-term backorder, and sometimes that can last years before the company finally makes a decision” on whether to discontinue a product, she said. “It can take a long time, and so it can be frustrating to not know – or to kind of stake your hopes on a product coming back to the market once it’s been in shortage for so long.”
It’s hard to know exactly how many children will be affected by the Vantas discontinuation because data about off-label use is hard to come by.
Erica Eugster, MD, a professor of pediatrics at Indiana University, Indianapolis, said central precocious puberty patients weren’t her first thought when she learned of the Vantas discontinuation.
“I immediately thought about our transgender population,” she said. “They’re the ones that are really going to suffer from this.”
No medications have been FDA approved to treat patients with gender dysphoria, the medical term for when the sex assigned at birth doesn’t match someone’s gender identity, causing them psychological distress. As a result, any drug to stop puberty in this population would be off label, making it difficult for families to get health insurance coverage. Vantas had been a lower-cost option.
The number of transgender patients receiving histrelin implants rose significantly from 2004 to 2016, according to a study published in the Journal of Pediatric Endocrinology and Metabolism.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Drug combo at outset of polyarticular JIA benefits patients most
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
Initiating treatment of polyarticular juvenile idiopathic arthritis (polyJIA) with both a conventional synthetic disease-modifying antirheumatic drug and a biologic DMARD resulted in more patients achieving clinical inactive disease 2 years later than did starting with only a csDMARD and stepping up to a biologic, according to data presented at the virtual annual meeting of the American College of Rheumatology.
“The 24-month results support the 12-month primary results that suggested that the early-combination group was superior and that, at 24 months, more early combination CTP [consensus treatment plan] patients achieve CID [clinical inactive disease], compared to step up,” Yukiko Kimura, MD, division chief of pediatric rheumatology at HMH Hackensack (N.J.) University Medical Center, told attendees. “This suggests that starting biologics early in polyJIA may lead to better long-term outcomes in many patients.”
Dr. Kimura noted that polyarticular JIA patients are already at risk for poor outcomes, and initial therapy can especially impact outcomes. Further, little evidence exists to suggest when the best time is to start biologics, a gap this study aimed to address.
Diane Brown, MD, PhD, a pediatric rheumatologist at Children’s Hospital Los Angeles who was not involved in the study, was pleased to see the results, which she said support her own preferences and practice patterns.
“Starting sooner with combination therapy, taking advantage of the advances with biologics and our long history with methotrexate at the same time, gives better outcomes for the long run,” Dr. Brown said in an interview. “Having studies like this to back up my own recommendations can be very powerful when talking to families, and it is absolutely invaluable when battling with insurance companies who always want you to take the cheapest road.”
Study details
The findings were an update of 12-month results in the CARRA STOP-JIA study that enrolled 400 untreated patients with polyJIA and compared three Childhood Arthritis and Rheumatology Research Alliance (CARRA) CTPs. Overall, 49.5% of participants received biologics within 3 months of starting the study. For these updated results, 275 participants had complete data at 24 months for the three CTPs:
- A step-up group of 177 patients who started therapy with a csDMARD and added a biologic if needed at least 3 months later
- An early-combination group of 73 patients who started therapy with a csDMARD and biologic together
- A biologic-first group of 25 patients who started with biologic monotherapy, adding a csDMARD only if needed at least 3 months later.
The primary outcome was the percentage of participants who reached CID without taking glucocorticoids at 24 months. Since the participants were not randomized, the researchers made adjustments to account for baseline differences between the groups, including differences in JIA categories, number of active joints, physician global assessment of disease activity, and the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10).
At 24 months in an intention to treat analysis, 59.4% of the early-combination group had achieved CID, compared with 48% of the biologic-first group and 40.1% of the step-up group (P = .009 for early combination vs. step up). All three groups had improved since the 12-month time point, when 37% of the early-combination group, 24% of the biologic-first group, and 32% of the step-up group had reached CID.
There were no significant differences between the groups in secondary outcomes of achieving cJADAS10 inactive disease of 2.5 or less or 70% improvement in pediatric ACR response criteria at 24 months. All groups improved in PROMIS pain interference or mobility measures from baseline. Most of the 17 severe adverse events were infections.
Moving from step-up therapy to early-combination treatment
Dr. Brown said that she spent many years in her practice using the step-up therapy because it was difficult to get insurance companies to pay for biologics without first showing that methotrexate was insufficient.
”But methotrexate takes so long to control the disease that you need a lot of steroids, with all of their side effects, at least temporarily, or you must simply accept a longer period of active and symptomatic disease before you get to that desired state of clinically inactive disease,” Dr. Brown said. “And during that time, you can be accumulating what may be permanent damage to joints, as well as increase in risk of contractures and deconditioning for that child who is too uncomfortable to move and exercise and play normally.”
Dr. Brown is also wary of using a biologic as an initial therapy by itself because the actions of biologics are so specific. ”I like to back up the powerful, rapid, and specific actions of a biologic with the broader, if slower, action of methotrexate to minimize chances that the immune system is going to find a way around blockade of a single cytokine by your biologic,” she said.
While patient preference will also play a role in what CTP patients with polyJIA start with, Dr. Brown said that she believes more medication upfront can result in less medication and better outcomes in the long run, as the findings of this study suggest. The results here are helpful when speaking with families who are anxious about “so much medicine” or “such powerful medicines,” she said. ”I hope it will also help ease the fears of other providers who share the same concerns about ‘so much medicine.’ ”
The study’s biggest limitation is not being a randomized, controlled trial, but Dr. Brown said the researchers demonstrated effectively that the disease burden remains similar across the groups at baseline.
”It would also be useful to have a clear breakdown of adverse events and opportunistic infections because an excess of opportunistic infections would be a key concern with early combination therapy,” she said, although she added that the study overall was a ”beautiful example of the value of registry data.”
Dr. Kimura emphasized that polyJIA remains a challenging disease to treat, with 40%-60% of participants not reaching CID at 24 months. The registry follow-up will continue for up to 10 years to hopefully provide more information about longer-term outcomes from different treatments.
The research was funded by a grant from Genentech to CARRA. Dr. Kimura reported royalties from UpToDate and salary support from CARRA. Dr. Brown had no disclosures.
FROM ACR 2021
Children and COVID: New cases up again after dropping for 8 weeks
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.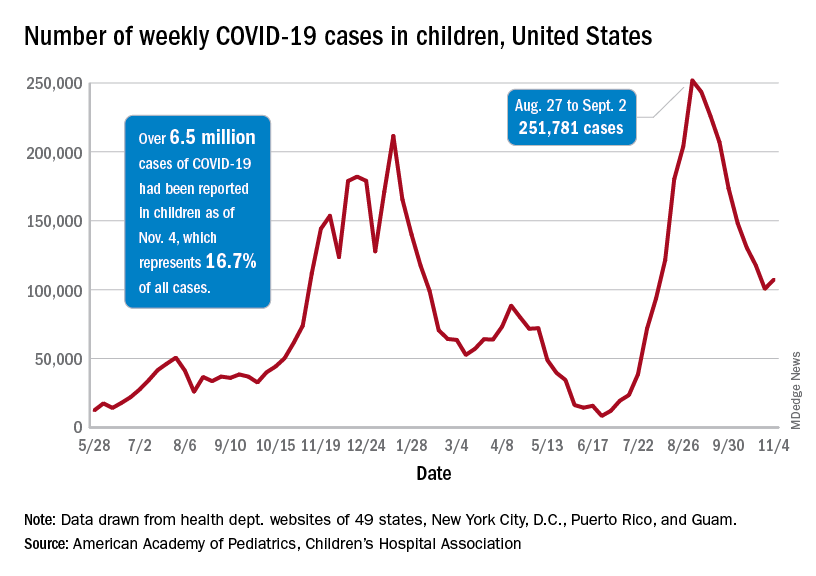
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
As children aged 5-11 years began to receive the first officially approved doses of COVID-19 vaccine, new pediatric cases increased after 8 consecutive weeks of declines, according to the American Academy of Pediatrics and the Children’s Hospital Association.
Weekly cases peaked at almost 252,000 in early September and then dropped for 8 straight weeks before this latest rise, the AAP and the CHA said in their weekly COVID report, which is based on data reported by 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The end of that 8-week drop, unfortunately, allowed another streak to continue: New cases have been above 100,000 for 13 consecutive weeks, the AAP and CHA noted.
The cumulative COVID count in children as of Nov. 4 was 6.5 million, the AAP/CHA said, although that figure does not fully cover Alabama, Nebraska, and Texas, which stopped public reporting over the summer. The Centers for Disease Control and Prevention, with input from all states and territories, puts the total through Nov. 8 at almost 5.7 million cases in children under 18 years of age, while most states define a child as someone aged 0-19 years.
As for the newest group of vaccinees, the CDC said that “updated vaccination data for 5-11 year-olds will be added to COVID Data Tracker later this week,” meaning the week of Nov. 7-13. Currently available data, however, show that almost 157,000 children under age 12 initiated vaccination in the 14 days ending Nov. 8, which was more than those aged 12-15 and 16-17 years combined (127,000).
Among those older groups, the CDC reports that 57.1% of 12- to 15-year-olds have received at least one dose and 47.9% are fully vaccinated, while 64.0% of those aged 16-17 have gotten at least one dose and 55.2% are fully vaccinated. Altogether, about 13.9 million children under age 18 have gotten at least one dose and almost 11.6 million are fully vaccinated, according to the CDC.
Breast milk of COVID-19–infected mothers helps build infant’s immune defenses
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
It’s rare for mothers with COVID-19 to transfer the infection to their newborns, according to a new small study.
The research, published in JAMA Network Open, found that newborns of mothers infected with the COVID-19 virus were able to develop their own immune defenses via their mother’s breast milk. Researchers detected antibodies in the infants’ saliva.
“It is the first time that this mechanism has been demonstrated,” said study author Rita Carsetti, MD, head of immunology diagnostics for Bambino Gesù Children’s Hospital in Rome. “We now know how breast milk can help babies develop their immune defenses. The system could work the same way for many other pathogens, which are present in the mother during breastfeeding.”
Dr. Carsetti and colleagues examined data from 28 pregnant women who tested positive for COVID-19 and who gave birth at Policlinico Umberto I in Rome between November 2020 and May 2021, and their newborns. They investigated the immune responses of the mothers and their newborns by detecting spike-specific antibodies in serum, and the mucosal immune response was assessed by measuring specific antibodies in maternal breast milk and infant saliva 48 hours after delivery and 2 months later.
Twenty-one mothers and their newborns completed the 2 months of follow-up. Researchers found that the majority of the mothers had mild symptoms of COVID-19, while only three of them were admitted for worsening condition. There was only one reported case of a possible vertical transmission – transmitted in utero – and one case of a horizontal infection through droplets or respiratory secretions, which occurred when the newborn was taken home.
The results of the study showed that antibodies specific to the virus were present in the mothers’ blood at 2 months after delivery, but not at 48 hours. However, in milk, specific antibodies were already present 48 hours after delivery.
Therefore, after 48 hours, the breastfed babies had specific mucosal antibodies against COVID-19 in their saliva that the other newborns did not have. Two months later, these antibodies continued to be present even though the mothers had stopped producing them.
The findings suggest that breast milk offers protection by transferring the antibodies produced by the mother to the baby, but also by helping them to produce their own immune defenses.
“I am not surprised that infants of mothers who had COVID-19 infection in the peripartum period pass anti-spike protein IgA to their infants,” J. Howard Smart, MD, FAAP, who was not involved with the study, said in an interview. “This confirmation is good news for breastfeeding mothers.
“I wonder whether we really know these infants did not become infected, and produce their own antibodies,” said Dr. Smart, chairman of the department of pediatrics at Sharp Rees-Stealy Medical Group in San Diego.
The American College of Obstetricians and Gynecologists said having COVID-19 should not stop mothers from giving their children breast milk. The organization also said that the chance of COVID-19 passing through the breast milk and causing infection in the newborn infant is slim.
“Breast milk also helps protect babies from infections, including infections of the ears, lungs, and digestive system. For these reasons, having COVID-19 should not stop you from giving your baby breast milk,” according to ACOG’s website.
Similar studies on mothers who received the COVID-19 vaccination rather than being infected would be interesting, Dr. Smart added.
The authors of the current study plan to broaden their research by evaluating the response of pregnant mothers vaccinated against SARS-CoV-2 for the presence of antibodies in the milk and the immunity of their newborns. Dr. Carsetti said her team plans to expand the study to other infections, such as cytomegalovirus and respiratory syncytial virus.
None of the researchers or commentators had financial disclosures.
FROM JAMA NETWORK OPEN
More eczema in children exposed to toxic metals in utero
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
published Oct. 27, 2021, in JAMA Network Open.
In this multicenter cohort study, led by epidemiologist Shu-Li Wang, PhD, of the National Institute of Environmental Health Sciences, in Taiwan, each twofold increase in prenatal arsenic level correlated with a 2.4-fold higher rate of atopic dermatitis in 4-year-olds.
Atopic diseases have been on the rise. Eczema (atopic dermatitis) is the first stage of the so-called atopic march, followed by food allergies, allergic rhinitis, and asthma later in childhood. Previous research has linked heavy metal exposure to allergic diseases in adults. In another study by Dr. Wang and colleagues that was published in 2021, prenatal and early-life arsenic exposure was found to correlate with higher rates of allergic rhinitis and asthma in children. In that study, the participants were followed every 2-3 years through the age of 14 as part of the Taiwan Maternal and Infant Cohort Study.
The new study included 370 mother and child pairs who were enrolled in that birth cohort study between October 2012 and May 2015. During their third trimester of pregnancy, women completed questionnaires about their lifestyle, diet, and living environment. In addition, their height, weight, and blood pressure were recorded, and urine samples were taken. In follow-up interviews 3-4 years later, the mothers were asked whether their child had ever been diagnosed with atopic dermatitis.
The researchers used an inductively coupled plasma mass spectrometer to analyze the participants’ urine samples. They assessed for exposures in utero to eight metals: arsenic, cadmium, lead, cobalt, copper, nickel, thallium, and zinc.
Each unit increase of an index that estimates the combined exposure to these metals during pregnancy was associated with 63% higher odds of atopic dermatitis in the children by age 4. The researchers adjusted for parental allergies (yes or no), mother’s educational level (<12 years, 13-16 years, or >16 years), geographic area (central or eastern Taiwan), exposure to tobacco smoke during pregnancy, and the child’s gender. Arsenic (40.1%) and cadmium (20.5%) accounted for most of the metal coexposure index.
A wealth of previous research links arsenic exposure during adulthood to skin disease and immune dysfunction. Early-life arsenic exposure has been linked with elevated risk for various adult disorders, including cancer, diabetes, and heart disease, years later. In light of such research, “the findings in this paper are not surprising,” J. Christopher States, PhD, director of the Center for Integrative Environmental Health Science at the University of Louisville (Ky.), told this news organization. “Low-level arsenic exposure does not cause disease immediately, but it does appear to have long-lasting effects, making individuals susceptible to ‘second hits’ with another environmental agent.”
Research into the molecular mechanisms for these links has shown that arsenic and cadmium exposure can promote allergic phenotypes in immune cells. “We think the toxic metals activate the alarmin pathway, thus inducing innate lymphoid cells, then activating T-helper 2 cells, which drive immunoglobulin E production and breakdown of the epithelium and promotion of allergies,” said Kari Nadeau, MD, PhD, director of the Sean N. Parker Center for Allergy and Asthma Research at Stanford University. Dr. Nadeau led that study, published in 2017 in PLOS One, along with epidemiologist Margaret Karagas, PhD, of Geisel School of Medicine at Dartmouth, Hanover, N.H.
As for what pregnant women can do to minimize their exposure to heavy metals, “that is a difficult problem and primarily a function of where one lives,” said Dr. States.
Drinking water and food are major sources of arsenic exposure. Groundwater is naturally contaminated with arsenic deposits that seep in from bedrock, said Dr. States. The U.S. Environmental Protection Agency regulates arsenic levels in public drinking water that is supplied to more than a few thousand people. However, small water supplies and private wells are unregulated, he said, and having these water sources tested for arsenic or fitted with systems to reduce arsenic can be very expensive.
Among foods, rice can have high concentrations of arsenic, Dr. Karagas told this news organization. To minimize arsenic exposure through the diet, women can limit rice-based foods, according to a web-based tool developed by her and coworkers.
In addition, tobacco smoke is a major source of cadmium exposure and a moderate source of arsenic exposure, Dr. States noted. Women can reduce their exposure to these metals by avoiding tobacco and secondhand smoke.
The study was supported by grants from the National Health Research Institutes, Chung Shan Medical University Hospital, Taiwan Ministry of Science and Technology, and the Taiwan Environmental Protection Administration. The authors and quoted experts report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Early peanut feeding guidelines still not reaching families
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Four years after new infant feeding guidelines were issued to prevent allergies to peanut and other foods, 70% of surveyed parents and caregivers in the United States said they had never heard about the new recommendation.
Food allergies in developed countries have doubled in each of the last decades and now affect 7.6% of U.S. children. About 1 in 50 are allergic to peanut. Data from the 2015 LEAP study and other research has convincingly shown that early, sustained feeding of peanuts, eggs, and other allergens can prevent babies from developing allergies to these foods.
Based on those findings, the National Institute of Allergy and Infectious Diseases (NIAID) updated its feeding guidelines in 2017, urging parents to introduce these foods to babies around 4-6 months of age rather than wait until 1-3 years of age, as previously recommended. The American Academy of Pediatrics approved those guidelines too, and in 2019 changed its own feeding recommendations.
To assess awareness of this new guidance and to what extent these recommendations are being translated into clinical practice, researchers surveyed a demographically representative U.S. sample of 3,062 parents and caregivers with children between 7 months and 3½ years old. The survey was conducted in English and Spanish over the web or by phone.
More than one-third reported that their child’s primary care physician never discussed when to start feeding peanut-containing foods. And among those whose doctors did offer guidance, fewer than 1 in 4 specifically recommended introducing peanut by 6 months of age.
These data show that “despite strong evidence that early introduction of peanut within the first year of life can prevent the development of peanut allergy, this evidence is simply not making its way to parents of infants,” said Christopher Warren, PhD, assistant professor of preventive medicine at the Northwestern University Feinberg School of Medicine, Chicago. Dr. Warren led the study and presented the findings on a poster at this year’s American College of Allergy, Asthma & Immunology annual meeting in New Orleans.
In addition to caregivers, the Northwestern team surveyed U.S. allergists and pediatricians about the new feeding guidelines. Uptake was fairly good among allergists, with 65% reporting full implementation. On the other hand, while most pediatricians seemed familiar with the 2017 recommendations, fewer than one-third said they were following them.
“What’s unique about this challenge is that it’s not just a guideline change – it’s a guideline reversal,” said Wendy Sue Swanson, MD, chief medical officer for SpoonfulONE, a company that makes mix-ins and other products for multi-allergen feeding. After telling families for years to avoid these allergens in early life because food allergies were rising, “it’s harder advice to say, actually, we were wrong. Not only should you not wait, you should get peanut in while your baby’s immune system has this critical moment to learn and develop, and you should keep getting it in,” Dr. Swanson said in an interview.
Making matters worse, pediatricians are time pressed. Typically, at 4- to 6-month-old well-check visits, “they’re talking about sleep and development and feeding and milestones,” said Ruchi Gupta, MD, MPH, professor of pediatrics and medicine at Northwestern Feinberg, who led the allergist and pediatrician analyses.
Another challenge: Guidelines differ depending on the child’s level of food allergy risk, so it’s hard to explain them clearly and quickly. Babies at highest risk – as judged by having severe eczema, egg allergy, or both – should get peanut IgE blood testing and, if negative, begin regular consumption of peanut by 4-6 months. Intermediate-risk babies who have mild-to-moderate eczema are recommended to start peanut-containing foods by 6 months. And for low-risk babies with no eczema or known food allergies, the guidance is simply to introduce peanut-containing foods “in accordance with family preferences and cultural practices.”
As for pediatricians who say it’s hard to distinguish mild-to-moderate from severe eczema, “any eczema puts you at some risk,” Dr. Gupta told this news organization. “If they’ve required steroid creams to clear up their skin, or if you look at their skin, and you think it’s severe, don’t hesitate. Go ahead and draw the IgE and send them to an allergist.”
Australia, which has the highest rate of confirmed food allergy, has had more success implementing early feeding guidelines, said Dr. Swanson. Unlike the United States’ tiered approach, she said, they “had a national guideline that very crisply, years ago, told parents what to do.” Australia also has nurse educators that follow up with new moms to make sure they understand and follow the recommendations.
Dr. Gupta receives research support from the National Institutes of Health, Food Allergy Research and Education, the Melchiorre Family Foundation, the Sunshine Charitable Foundation, the Walder Foundation, the UnitedHealth Group, Thermo Fisher Scientific, and Genentech. She serves as a medical consultant/advisor for Genentech, Novartis, and Food Allergy Research and Education. Dr. Swanson serves as chief medical officer for SpoonfulONE.
A version of this article first appeared on Medscape.com.
Expected spike in acute flaccid myelitis did not occur in 2020
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
suggested researchers at the Centers for Disease Control and Prevention.

Acute flaccid myelitis (AFM) is an uncommon but serious complication of some viral infections, including West Nile virus and nonpolio enteroviruses. It is “characterized by sudden onset of limb weakness and lesions in the gray matter of the spinal cord,” they said, and more than 90% of cases occur in young children.
Cases of AFM, which can lead to respiratory insufficiency and permanent paralysis, spiked during the late summer and early fall in 2014, 2016, and 2018 and were expected to do so again in 2020, Sarah Kidd, MD, and associates at the division of viral diseases at the CDC’s National Center for Immunization and Respiratory Diseases, Atlanta, said in the Morbidity and Mortality Weekly Report.
Monthly peaks in those previous years – each occurring in September – reached 51 cases in 2014, 43 cases in 2016, and 88 cases in 2018, but in 2020 there was only 1 case reported in September, with a high of 4 coming in May, CDC data show. The total number of cases for 2020 (32) was, in fact, lower than in 2019, when 47 were reported.
The investigators’ main objective was to see if there were any differences between the 2018 and 2019-2020 cases. Reports from state health departments to the CDC showed that, in 2019-2020, “patients were older; more likely to have lower limb involvement; and less likely to have upper limb involvement, prodromal illness, [cerebrospinal fluid] pleocytosis, or specimens that tested positive for EV [enterovirus]-D68” than patients from 2018, Dr. Kidd and associates said.
Mask wearing and reduced in-school attendance may have decreased circulation of EV-D68 – the enterovirus type most often detected in the stool and respiratory specimens of AFM patients – as was seen with other respiratory viruses, such as influenza and respiratory syncytial virus, in 2020. Previous studies have suggested that EV-D68 drives the increases in cases during peak years, the researchers noted.
The absence of such an increase “in 2020 reflects a deviation from the previously observed biennial pattern, and it is unclear when the next increase in AFM should be expected. Clinicians should continue to maintain vigilance and suspect AFM in any child with acute flaccid limb weakness, particularly in the setting of recent febrile or respiratory illness,” they wrote.
FROM MMWR
Firm Digital Papulonodules in an Infant
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.
A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).
A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.
Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.

A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).

A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.

Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

The Diagnosis: Infantile Digital Fibromatosis
Infantile digital fibromatosis (IDF) is a rare benign neoplasm of infancy prone to recurrence after resection but not to metastasis. It usually is limited to the fingers and toes.1 One-third of cases occur at birth. Most patients develop clinical symptoms within the first year of life, but presentation can occur in adolescents and adults. The exact etiology and pathogenesis of IDF remain unclear, but trauma is thought to be a trigger.
Physical examination reveals single or multiple smooth, round, pink papules or nodules confined to the sides and backs of the fingers, sparing the thumb and first toe.2,3 The nodules typically are firm, less than 2 cm in diameter, and often painless. Infantile digital fibromatosis exhibits an indolent progression followed by a rapid growth phase during several months, which may lead to functional impairment and joint deformities.4,5 Histopathology displays spindle cells with eosinophilic cytoplasmic inclusions that range from round to oval with uneven distribution, lack of refraction, and a large size difference (3–15 μm).6 The inclusions are deep red with Masson trichrome staining and can express smooth muscle actin and calponin. Tumor cells usually express vimentin, smooth muscle actin, calponin, and desmin but fail to express S-100 protein. The Ki67 proliferation index is 2% to 15%.6,7
Nonsurgical treatments for IDF include topical imiquimod, topical or intradermal injection of glucocorticoids, and intradermal injection of 5-fluorouracil. Complete resection should be reserved for cases with invasive growth that may lead to joint deformities, tendon or ligament involvement, digit or contracture deformity, and complications such as decreased joint mobility. Although there is a recurrence rate of up to 50% after excision, most lesions eventually will spontaneously regress and will leave no scar.8-10
The clinical and histopathologic differential diagnoses of IDF include other cutaneous diseases that occur in the digits. A dermatofibroma is a round, firm, fibrohistiocytic nodule that mainly occurs on the extensor limbs. Histopathology includes both fibrous and cellular types.11 Histologic analysis shows an ill-defined dermal proliferation of spindled fibroblasts with pale eosinophilic cytoplasm and bland fusiform nuclei growing in bands or fascicles that trap collagen fibers at the periphery (Figure 1). Generally, dermatofibromas have marked epidermal hyperplasia, which differs from IDF.

A digital myxoid cyst is characterized by a fleshcolored, hemispherical, and translucent cystic nodule that arises from the dorsum of the distal interphalangeal joint.12 It commonly is associated with injury and chronic pressure. Translucent viscous liquid may flow out when the cyst is punctured, a hallmark feature of this entity. Clinical variants of myxoid cyst include myxomatous and ganglion types. Histopathology reveals excessive mucin deposited in the dermis, and the surrounding collagen is compressed to form the pseudocyst (Figure 2).

A giant cell tumor of the tendon sheath presents with asymptomatic nodules or lumps. Lesions frequently are localized to the tendon sheath, especially on the fingers and wrists, with no malignant tendency or propensity for spontaneous regression.13 The local recurrence rate is as high as 45%, which is related to surgical resection insufficiency.14 Histopathologic examination shows lobulated tumor tissue surrounded by dense fibrosis. The tumor cells are histiocytic with scattered giant cells (Figure 3). The characteristic osteoclastlike giant cells have eosinophilic cytoplasm and irregularly arranged nuclei in varying numbers.

Keloids are connective tissue hyperplasias caused by skin injury. Histopathologically, keloids are characterized by nodules of thick hyalinized collagen bundles and whorled fibroblasts (Figure 4). No inclusions in the fibroblasts and a history of trauma can differentiate keloids from IDF.

- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
- Marks E, Ewart M. Infantile digital fibroma: a rare fibromatosis. Arch Pathol Lab Med. 2016;140:1153‐1156.
- Botelho LF, Matsushigue T, Enokihara MM, et al. Case for diagnosis. An Bras Dermatol. 2012;87:493-494.
- Paloni G, Mattei I, Salmaso R, et al. Infantile digital fibromatosis. Arch Dis Child. 2013;98:308.
- Girgenti V, Restano L, Arcangeli F, et al. Infantile digital fibromatosis: a rare tumour of infancy. report of five cases. Australas J Dermatol. 2012;53:285-287.
- Eypper EH, Lee JC, Tarasen AJ, et al. An algorithmic approach to the management of infantile digital fibromatosis: review of literature and a case report. Eplasty. 2018;18:E19.
- Laskin WB, Miettinen M, Fetsch JF. Infantile digital fibroma /fibromatosis: a clinicopathologic and immunohistochemical study of 69 tumors from 57 patients with long-term follow-up. Am J Surg Pathol. 2009;33:1-13.
- Henderson H, Peng YJ, Salter DM. Anti-calponin 1 antibodies highlight intracytoplasmic inclusions of infantile digital fibromatosis. Histopathology. 2014,64:752-755.
- Campbell LB, Petrick MG. Mohs micrographic surgery for a problematic infantile digital fibroma. Dermatol Surg. 2007;33:385-387.
- Ochi H, Puhaindran ME, Tan KW. Firm digital papulonodules in a young boy. Int J Dermatol. 2019;58:91-92.
- Albertini JG, Welsch MJ, Conger LA, et al. Infantile digital fibroma treated with Mohs micrography surgery. Dermatol Surg. 2002;28:959-961.
- Alves JV, Matos DM, Barreiros HF, et al. Variants of dermatofibroma— a histopathological study. An Bras Dermatol. 2014;89:472-477.
- Meyers AL, Fallahi AKM. Digital Mucous Cyst. StatPearls Publishing; 2020.
- Zhao Q, Lu H. Giant cell tumor of tendon sheath in the wrist that damaged the extensor indicis proprius tendon: a case report and literature review. BMC Cancer. 2019;19:1057.
- DiGrazia S, Succi G, Fragetta F, et al. Giant cell tumor of tendon sheath: study of 64 cases and review of literature. G Chir. 2013;34:149-152.
A 3-month-old girl presented with papulonodules on the distal left ring finger. Initially the lesions were thought to be insect bites but became firm over the course of 3 weeks and then gradually increased in size over 2 months. Physical examination revealed a 0.5×0.5-cm firm nodule and a 0.2×0.3-cm firm papule on the radial aspect of the left ring finger over the distal interphalangeal joint. There was no deformity or dysfunction of the finger. Radiography showed soft tissue swelling without bony abnormalities. The lesions were excised; however, a new fleshy nodule reappeared 1 month postoperatively on the radial aspect of the left ring finger over the distal interphalangeal joint. The patient did not seem bothered by the lesions and was in good general health.
Radiologically Isolated Syndrome: A condition that often precedes an MS diagnosis in children
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
Naila Makhani, MD completed medical school training at the University of British Columbia (Vancouver, Canada). This was followed by a residency in child neurology and fellowship in MS and other demyelinating diseases at the University of Toronto and The Hospital for Sick Children (Toronto, Canada). Concurrent with fellowship training, Dr. Makhani obtained a Masters’ degree in public health from Harvard University. Dr. Makhani is the Director of the Pediatric MS Program at Yale and the lead investigator of a multi-center international study examining outcomes following the radiologically isolated syndrome in children.
Q1. Could you please provide an overview of Radiologically Isolated Syndrome ?
A1. Radiologically Isolated Syndrome (RIS) was first described in adults in 2009. Since then it has also been increasingly recognized and diagnosed in children. RIS is diagnosed after an MRI of the brain that the patient has sought for reasons other than suspected multiple sclerosis-- for instance, for evaluation of head trauma or headache. However, unexpectedly or incidentally, the patient’s MRI shows the typical findings that we see in multiple sclerosis, even in the absence of any typical clinical symptoms. RIS is generally considered a rare syndrome.
Q2. You created Yale Medicine’s Pediatric Multiple Sclerosis program which advocates for the eradication of MS. What criteria defines a rare disease? Does RIS meet these criteria? And if so, how?
A2. The criteria for a rare disease vary, depending on the reference. In the US, a rare disease is defined as a condition that affects fewer than 200,000 people, in total, across the country. By contrast, in Europe, a disease is considered rare if it affects fewer than one in every 2,000 people within the country’s population.
In the case of RIS, especially in children, we suspect that this is a rare condition, but we don't know for sure, as there have been very few population-based studies. There is one large study that was conducted in Europe that found one case of RIS among approximately 5,000 otherwise healthy children, who were between 7 and 14 years of age. I think that's our best estimate of the overall prevalence of RIS in children. Using that finding, it likely would qualify as a rare condition, although, as I said, we really don't know for sure, as the prevalence may vary among different populations or age groups.
Q3. How do you investigate and manage RIS in children? What are some of the challenges?
A3. For children with radiologically isolated syndrome, we usually undertake a comprehensive workup. This includes a detailed clinical neurological exam to ensure that there are no abnormalities that would, for instance, suggest a misdiagnosis of multiple sclerosis or an alternative diagnosis. In addition to the brain MRI, we usually obtain an MRI of the spinal cord to determine whether there is any spinal cord involvement. We also obtain blood tests. We often analyze spinal fluid as well, primarily to exclude other alternative processes that may explain the MRI findings. A key challenge in this field is that there are currently no formal guidelines for the investigation and management of children with RIS. Collaborations within the pediatric MS community are needed to develop such consensus approaches to standardize care.
Q4. What are the most significant risk factors that indicate children with RIS could one day develop multiple sclerosis?
A4.This is an area of active research within our group. So far, we've found that approximately 42% of children with RIS develop multiple sclerosis in the future; on average, two years following their first abnormal MRI. Therefore, this is a high-risk group for developing multiple sclerosis in the future. Thus far, we've determined that in children with RIS, it is the presence of abnormal spinal cord imaging and an abnormality in spinal fluid – namely, the presence of oligoclonal bands – that are likely the predictors of whether these children could develop MS in the future. a child’s possible development
Q5. Based on your recent studies, are there data in children highlighting the potential for higher prevalence in one population over another?
A5. Thus far, population-based studies assessing RIS, especially in children, have been rare and thus far have not identified particular subgroups with increased prevalence. We do know that the prevalence of multiple sclerosis varies across different age groups and across gender. Whether such associations are also present for RIS is an area of active research.
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227
de Mol CL, Bruijstens AL, Jansen PR, Dremmen M, Wong Y, van der Lugt A, White T, Neuteboom RF.Mult Scler. 2021 Oct;27(11):1790-1793. doi: 10.1177/1352458521989220. Epub 2021 Jan 22.PMID: 33480814
2. Radiologically isolated syndrome in children: Clinical and radiologic outcomes.
Makhani N, Lebrun C, Siva A, Brassat D, Carra Dallière C, de Seze J, Du W, Durand Dubief F, Kantarci O, Langille M, Narula S, Pelletier J, Rojas JI, Shapiro ED, Stone RT, Tintoré M, Uygunoglu U, Vermersch P, Wassmer E, Okuda DT, Pelletier D.Neurol Neuroimmunol Neuroinflamm. 2017 Sep 25;4(6):e395. doi: 10.1212/NXI.0000000000000395. eCollection 2017 Nov.PMID: 28959703
Makhani N, Lebrun C, Siva A, Narula S, Wassmer E, Brassat D, Brenton JN, Cabre P, Carra Dallière C, de Seze J, Durand Dubief F, Inglese M, Langille M, Mathey G, Neuteboom RF, Pelletier J, Pohl D, Reich DS, Ignacio Rojas J, Shabanova V, Shapiro ED, Stone RT, Tenembaum S, Tintoré M, Uygunoglu U, Vargas W, Venkateswaren S, Vermersch P, Kantarci O, Okuda DT, Pelletier D; Observatoire Francophone de la Sclérose en Plaques (OFSEP), Société Francophone de la Sclérose en Plaques (SFSEP), the Radiologically Isolated Syndrome Consortium (RISC) and the Pediatric Radiologically Isolated Syndrome Consortium (PARIS).Mult Scler J Exp Transl Clin. 2019 Mar 20;5(1):2055217319836664. doi: 10.1177/2055217319836664. eCollection 2019 Jan-Mar.PMID: 30915227