User login
Diversity in Dermatology: A Society Devoted to Skin of Color
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
The US Census Bureau predicts that more than half of the country’s population will identify as a race other than non-Hispanic white by the year 2044.In 2014, the US population was 62.2% non-Hispanic white, and the projected figure for 2060 is 43.6%.1 However, most physicians currently are informed by research that is generalized from a study population of primarily white males.2 Disparities also exist among the physician population where black individuals and Latinos are underrepresented.3 These differences have inspired dermatologists to develop methods to address the need for parity among patients with skin of color. Both ethnic skin centers and the Skin of Color Society (SOCS) have been established since the turn of the millennium to improve disparities and prepare for the future. The efforts and impact of SOCS are widening since its inception and chronicle one approach to broadening the scope of the specialty of dermatology.
Established in 2004 by dermatologist Susan C. Taylor, MD (Philadelphia, Pennsylvania), SOCS provides educational support to health care providers, the media, the legislature, third parties (eg, insurance organizations), and the general public on dermatologic health for patients with skin of color. The society is organized into committees that represent the multifaceted aspects of the organization. It also stimulates and endorses an increase in scientific knowledge through basic science and clinical, surgical, and cosmetic research.4
Scientific, research, mentorship, professional development, national and international outreach, patient education, and technology and media committees within SOCS, as well as a newly formed diversity in action task force, uphold the mission of the society. The scientific committee, one of the organization’s major committees, plans the annual symposium. The annual symposium, which immediately precedes the Annual Meeting of the American Academy of Dermatology, acts as a central educational symposium for dermatologists (both domestic and international), residents, students, and other scientists to present data on unique properties, statistics, and diseases associated with individuals with ethnic skin. New research, perspectives, and interests are shared with an audience of physicians, research fellows, residents, and students who are also the presenters of topics relevant to skin of color such as cutaneous T-cell lymphomas/mycosis fungoides in black individuals, central centrifugal cicatricial alopecia (CCCA), pigmentary disorders in Brazilians, and many others. There is an emphasis on allowing learners to present their research in a comfortable and constructive setting, and these shorter talks are interspersed with experts who deliver cutting-edge lectures in their specialty area.4
Each year during the SOCS symposium, the SOCS Research Award is endowed to a dermatology resident, fellow, or young dermatologist within the first 8 years of postgraduate training. The research committee oversees the selection of the SOCS Research Award. Prior recipients of the award have explored topics such as genetic causes of keloid formation or CCCA, epigenetic changes in ethnic skin during skin aging, and development of a vitiligo-specific quality-of-life scale.4
Another key mission of SOCS is to foster the growth of younger dermatologists interested in skin of color via mentorships; SOCS has a mentorship committee dedicated to engaging in this effort. Dermatology residents or young dermatologists who are within 3 years of finishing residency can work with a SOCS-approved mentor to develop knowledge, skills, and networking in the skin of color realm. Research is encouraged, and 3 to 4 professional development meetings (both in person or online) help set objectives. The professional development committee also coordinates efforts to offer young dermatologists opportunities to work with experienced mentors and further partnerships with existing members.4
The national and international outreach committee acts as a liaison between organizations abroad and those based in the United States. The patient education committee strives to improve public knowledge about dermatologic diseases that affect individuals with skin of color. Ethnic patients often have poor access to medical information, and sometimes adequate medical information does not exist in the current searchable medical literature. The SOCS website (http://skinofcolorsociety.org/) offers an entire section on dermatology education with succinct, patient-friendly prose on diseases such as acne in skin of color, CCCA, eczema, melanoma, melasma, sun protection, tinea capitis, and more; the website also includes educational videos, blogs, and a central location for useful links to other dermatology organizations that may be of interest to both members and patients who use the site. Maintenance of the website and the SOCS media day fall under the purview of the technology and media committee. There have been 2 media days thus far that have given voice to sun safety and skin cancer in individuals with skin of color as well as hair health and cosmetic treatments for patients with pigmented skin. The content for the media days is provided by SOCS experts to national magazine editors and beauty bloggers to raise awareness about these issues and get the message to the public.4
The diversity in action task force is a new committee that is tasked with addressing training for individuals of diverse ethnicities and backgrounds for health care careers at every level, ranging from middle school to dermatology residency. Resources to help those applying to medical school and current medical students interested in dermatology as well as those applying for dermatology residency are being developed for students at all stages of their academic careers. The middle school to undergraduate educational levels will encompass general guidelines for success; the medical school level will focus on students taking the appropriate steps to enter dermatology residency. The task force also will act as a liaison through existing student groups, such as the Student National Medical Association, Minority Association of Premedical Students, Latino Medical Student Association, Dermatology Interest Group Association, and more to reach learners at critical stages in their academic development.4The society plays an important role in the educational process for dermatologists at all levels. Although this organization is critical in increasing knowledge of treatment of individuals with skin of color in research, clinical practice, and the public domain, the hope is that SOCS will continue to reach new members of the dermatology community. As a group that embraces the onus to improve skin of color education, the members of SOCS know that there is still much to do to increase awareness among the public as well as dermatology residents and dermatologists practicing in geographical regions that are not ethnically diverse. There are many reasons that both cultural competence and knowledge of skin of color in dermatology will be important as the United States becomes increasingly diverse, and SOCS is at the forefront of this effort. Looking to the future, the goals of SOCS really are the goals of dermatology, which are to continue to deliver the best care to all patients and to continue to improve our specialty with new techniques and medications for all patients who need care.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
- Colby SL, Jennifer JO. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. Washington, DC: US Census Bureau; 2014.
- Oh SS, Galanter J, Thakur N, et al. Diversity in clinical and biomedical research: a promise yet to be fulfilled. PLoS Med. 2015;12:e1001918.
- Castillo-Page L. Diversity in the physician workforce facts & figures 2010. Washington, DC: Association of American Medical Colleges; 2010. https://www.aamc.org/download/432976/data/factsandfigures2010.pdf. Accessed April 12, 2017.
- Our committees. Skin of Color Society website. http://skinofcolorsociety.org/about-socs/our-committees/. Accessed April 19, 2017.
Practice Points
- The mission of the Skin of Color Society (SOCS) is to improve education of young dermatologists relevant to skin of color patients.
- Educational resources on many different diseases important to patients with skin of color are available to patients and providers on the SOCS website.
Skin Cancer Mortality in Patients With Skin of Color
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
Skin cancers in patients with skin of color are less prevalent but have a higher morbidity and mortality compared to white patients. Challenges to early detection, including clinical differences in presentation, low public awareness, lower index of suspicion among health care providers, and access to specialty care, likely contribute to observed differences in prognosis between skin of color and white populations.
Skin cancer is the most common malignancy in the United States, accounting for approximately 40% of all neoplasms in white patients but only 1% to 4% in Asian American and black patients.1,2 Largely due to the photoprotective effects of increased constitutive epidermal melanin, melanoma is approximately 10 to 20 times less frequent in black patients and 3 to 7 times less common in Hispanics than age-matched whites.1 Nonmelanoma skin cancers including squamous cell carcinoma (SCC) and basal cell carcinoma also are less prevalent in darker skin types.3,4
In the United States, Hispanic, American Indian
Similar to melanoma, the mortality from SCC is disproportionately increased in skin of color populations, ranging from 18% to 29% in black patients.3,10,11 There is a paucity of population-based studies in the United States looking at mortality rates of nonmelanoma skin cancers and their trends over time, but a 1993 study suggests that mortality rates are declining less consistently in black patients than white patients.11
Factors that may contribute to higher mortality rates in patients with skin of color include a greater propensity for inherently aggressive skin cancers (eg, higher risk of SCC) and delays in diagnosis (eg, late-stage diagnosis of melanoma).1,4 For melanoma, increased mortality has been attributed to a predominance of acral lentiginous melanomas, which are more frequently diagnosed at more advanced stages than other melanoma subtypes.6,12,13 Black patients, Hispanics, Asians, and Pacific Islanders are all more likely to present with thicker tumors and metastases on initial presentation than their white counterparts (P<.001).2,8,9,12-14 The higher risk of death from SCC results from the predominance of lesions on non–sun-exposed areas, particularly the legs and anogenital areas, and within sites of chronic scarring or inflammation.4 Unlike sun-induced SCC, the most commonly observed type of SCC in lighter skin types, SCCs that develop in association with chronic inflammatory or ulcerative processes are aggressive and invasive, and they metastasize to distant sites in 20% to 40% of cases (versus 1%–4% in sun-induced SCC).1,3,4 For all skin cancers, poor access to medical care, patients’ unawareness of their skin cancer risk, lack of adequate skin examinations, and prevalence of lesions on uncommon sites that may be inconspicuous or overlooked have all been suggested to delay diagnosis.1,15,16 Given that more advanced disease is associated with worse outcomes, the implications of this delay are enormous and remain a cause for concern.
The alarming skin cancer mortality rates in patients with skin of color are a call to action for the medical community. The consistent use of full-body skin examinations including close inspection of mucosal, acral, and genital areas for all patients independent of skin type and racial/ethnic background is paramount. Advancing skin cancer education in skin of color populations, such as through distribution of patient-directed educational materials produced by organizations such as the American Academy of Dermatology, Skin Cancer Foundation, and Skin of Color Society, is an important step toward increased public awareness.16 Use of social and traditional media outlets as well as community-directed health outreach campaigns also are important strategies to change the common misconception that darker-skinned individuals do not get skin cancer. We hope that with a multipronged approach, disparities in skin cancer mortality will steadily be eliminated.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
- Gloster HM Jr, Neal K. Skin cancer in skin of color. J Am Acad Dermatol. 2006;55:741-760; quiz 761-764.
- Cormier JN, Xing Y, Ding M, et al. Ethnic differences among patients with cutaneous melanoma. Arch Intern Med. 2006;166:1907-1914.
- Mora RG, Perniciaro C. Cancer of the skin in blacks: I. a review of 163 black patients with cutaneous squamous cell carcinoma. J Am Acad Dermatol. 1981;5:535-543.
- Halder RM, Bridgeman-Shah S. Skin cancer in African Americans. Cancer. 1995;75:667-673.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2013. Bethesda, MD: National Cancer Institute; April 2016. http://seer.cancer.gov/csr/1975_2013/. Updated September 12, 2016. Accessed April 7, 2017.
- Bellows CF, Belafsky P, Fortgang IS, et al. Melanoma in African-Americans: trends in biological behavior and clinical characteristics over two decades. J Surg Oncol. 2001;78:10-16.
- Chen L, Jin S. Trends in mortality rates of cutaneous melanoma in East Asian populations. Peer J. 2014;4:e2809.
- Cress RD, Holly EA. Incidence of cutaneous melanoma among non-Hispanic whites, Hispanics, Asians, and blacks: an analysis of California Cancer Registry data. Cancer Causes Control. 1997;8:246-252.
- Johnson DS, Yamane S, Morita S, et al. Malignant melanoma in non-Caucasians: experience from Hawaii. Surg Clin N Am. 2003;83:275-282.
- Fleming ID, Barnawell JR, Burlison PE, et al. Skin cancer in black patients. Cancer. 1975;35:600-605.
- Weinstock MA. Nonmelanoma skin cancer mortality in the United States, 1969 through 1988. Arch Dermatol. 1993;129:1286-1290.
- Byrd KM, Wilson DC, Hoyler SS. Advanced presentation of melanoma in African Americans. J Am Acad Dermatol. 2004;50:142-143.
- Hu S, Parmet Y, Allen G, et al. Disparity in melanoma: a trend analysis of melanoma incidence and stage at diagnosis among whites, Hispanics, and blacks in Florida. Arch Dermatol. 2009;145:1369-1374.
- Black WC, Goldhahn RT, Wiggins C. Melanoma within a southwestern Hispanic population. Arch Dermatol. 1987;123:1331-1334.
- Harvey VM, Oldfield CW, Chen JT, et al. Melanoma disparities among US Hispanics: use of the social ecological model to contextualize reasons for inequitable outcomes and frame a research agenda [published online August 29, 2016]. J Skin Cancer. 2016;2016:4635740.
- Robinson JK, Joshi KM, Ortiz S, et al. Melanoma knowledge, perception, and awareness in ethnic minorities in Chicago: recommendations regarding education. Psychooncology. 2011;20:313-320.
Serpentine Supravenous Hyperpigmentation Following Cisplatin and Pemetrexed Chemotherapy
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
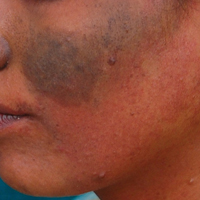
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
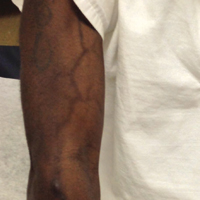
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5
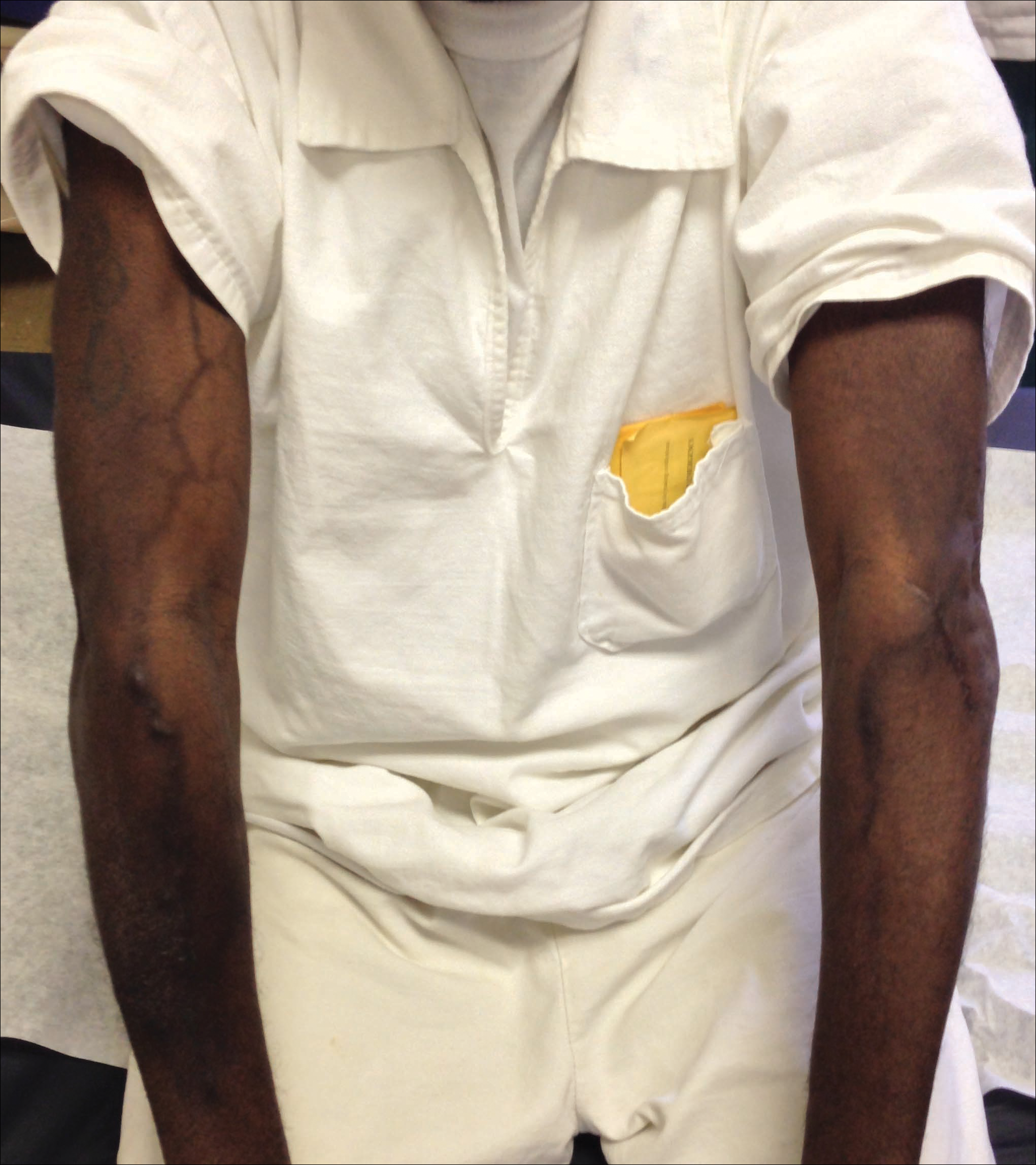
Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
To the Editor:
Serpentine supravenous hyperpigmentation (SSH) is a rare phenomenon characterized by linear hyperpigmentation of the skin overlying veins secondary to intravenous antineoplastic therapy. The term was first suggested by Hrushesky1 in 1976 as an uncommon side effect of administering intravenous 5-fluorouracil (5-FU). Although 5-FU is the most frequent offending agent, cases involving treatment with actinomycin, cyclophosphamide, docetaxel, fotemustine, nitrogen mustard, nitrosoureas, taxanes, and triazinate, as well as various combinations of chemotherapeutic agents, also have been observed.2,3 We present the case of SSH following a cisplatin and pemetrexed chemotherapy regimen.
A 52-year-old man with newly diagnosed inoperable adenocarcinoma in the left upper lung lobe received 2 cycles of treatment with cisplatin 138 mg and pemetrexed 920 mg 21 days apart. The first cycle of chemotherapy was delivered intravenously through the left forearm and the second cycle through the right forearm. Each infusion was followed by a 20-cc 0.9% saline flush. The patient developed nausea, vomiting, diarrhea, and hyperpigmentation tracing the path of infusion on the right arm as well as a slight darkness on the left arm that were noted by medical staff. At that time, cisplatin was discontinued from the chemotherapeutic regimen.
A port-a-cath was inserted into the patient’s right upper chest 4 weeks later and was used for subsequent infusions. Carboplatin 450 mg was initiated with pemetrexed thereafter. The patient was seen in the dermatology clinic 3 weeks after the insertion of the port-a-cath for evaluation of diffuse tinea versicolor of the trunk. Further examination of the arms revealed asymptomatic serpiginous hyperpigmentation overlying the superficial venous network tracing from the prior intravenous access points in the bilateral forearms to the upper arms (Figure). There was no evidence of extravasation or phlebitis prior to the hyperpigmentation. The patient was continued on pemetrexed and was subsequently lost to follow-up.
Cisplatin was the first member of the platinum-based chemotherapeutic agent class and is now one of the most potent and widely used in the treatment of solid malignancies. The cytotoxic mode of action is primarily mediated through interaction with DNA to form intrastrand cross-link adducts leading to aberrant mitosis and culminating in the activation of apoptosis. A variety of dermatologic complications have been reported with cisplatin chemotherapy including melanonychia, oral mucosal hyperpigmentation, hypersensitivity reactions, extravasation,4 Raynaud phenomenon, and flushing.5

Two cases of SSH have been reported following combination chemotherapy with cisplatin included in the regimen. A 61-year-old man with inoperable esophageal squamous cell carcinoma received cisplatin and 5-FU in addition to concurrent radiotherapy.6 After worsening renal function, cisplatin promptly was replaced with leucovorin. The patient developed SSH after the eighth infusion of 5-FU–leucovorin delivered through a peripheral catheter over a 24-hour period. The cutaneous side effect was attributed to the use of intravenous 5-FU.6 The second case involved a 48-year-old woman diagnosed with Paget disease of the breast who received adjuvant therapy with 12 courses of once-daily 5-FU and docetaxel for 5 years as well as 2 courses of vinorelbine and 1 course of cisplatin and etoposide for lung metastases.7 Serpentine supravenous hyperpigmentation lesions slowly developed over approximately 6 months. Based on the literature, the authors speculated that 5-FU and vinorelbine were most likely to be responsible. They noted, however, the inability to clarify the relationship between the onset of skin lesions and the time course of the chemotherapy.7 Although these cases do not directly implicate cisplatin as the cause of SSH, the possibility of a delayed reaction or augmentation of another drug’s effect cannot be excluded.
Pemetrexed, on the other hand, has not been associated with SSH. Several cutaneous adverse reactions have been reported, including acute generalized exanthematous pustulosis, alopecia, pityriasis lichenoides, radiation recall dermatitis, toxic epidermal necrolysis, and urticarial vasculitis.8 Three cases of pemetrexed-induced skin hyperpigmentation including the palms of the hands and soles of the feet as well as diffuse hyperpigmentation sparing only the palms and soles have been reported.8-10
Similar cases of SSH have demonstrated histopathologic findings with increased basal melanin synthesis and occasional melanophages in the papillary dermis without inflammatory changes.7,11 Although the unique serpentine pattern of hyperpigmentation is instantly recognizable, clinical differential diagnosis may include thrombophlebitis, cutis marmorata, erythema ab igne, livedo reticularis, and lichen planus.2,12
The exact mechanism of SSH has not been conclusively elucidated. Several studies postulate that direct cytotoxic damage causes loss of endothelial integrity permitting the extravasation of the agent to the overlying epidermis and interfering with melanogenesis.2,6,11 Other hypotheses include direct stimulation of melanocytes, depletion of reduced thioredoxin leading to tyrosinase stimulation, hyperthermia-related changes including reduced cytokine production and/or increased expression of melanocyte-stimulating hormone receptor, subclinical phlebitis leading to postinflammatory hyperpigmentation, or hyperpigmentation secondary to increased blood flow in certain areas and therefore increased drug deposition.12,13
Currently, there is no specific therapy recommended for SSH and the pigment may persist anywhere from a few months to more than a year after completing chemotherapy.2,7 Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.12 Several authors have suggested avoiding peripheral infusions of chemotherapeutic agents known to cause SSH or have recommended using a permanent central venous catheter.6,7 Another option, which needs further investigation, is the administration of an abundant flush following chemotherapy. This technique was described in a case report of a 47-year-old man who developed persistent SSH in the right forearm following docetaxel injection.13 Copious venous washing with 1000 mL of isotonic saline solution following the second infusion in the unaffected arm prevented discoloration. The lack of subsequent reaction may support the theory that direct toxic effect on the vascular endothelium results in hyperpigmentation of the supravenous skin.13
Serpentine supravenous hyperpigmentation is an uncommon cutaneous reaction secondary to antineoplastic therapies. Given the widespread use of chemotherapeutic regimens, dermatologists should be aware of the reaction. Additional studies are warranted to better elucidate the pathogenesis and investigate how infusion techniques might aid in the prevention of skin discoloration. Although this side effect originally was described in relation to 5-FU, subsequent observations have included other chemotherapeutic agents. In light of the findings presented in this report, cisplatin and pemetrexed should be considered on the list of offending agents. Ultimately, patients should be reassured that the lesions are benign, self-limiting, and gradually resolve on their own in most cases.12
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
- Hrushesky WJ. Letter: serpentine supravenous fluorouracil hyperpigmentation. JAMA. 1976;236:138.
- Ghosh SK, Bandyopadhyay D, Ghoshal L, et al. Letter: docetaxel-induced supravenous serpentine dermatitis. Dermatol Online J. 2011;17:16.
- Pujol RM, Rocamora V, Lopez-Pousa A, et al. Persistent supravenous erythematous eruption: a rare local complication of intravenous 5-fluorouracil therapy. J Am Acad Dermatol. 1998;39:839-842.
- Kufe DW, Pollock RE, Weichsebaum RR, et al, eds. Holland-Frei Cancer Medicine. 6th ed. Hamilton, Ontario, Canada: BC Decker Inc; 2000.
- Mann MW, Berk DR, Popkin DL, et al. Handbook of Dermatology: A Practical Manual. Hoboken, NJ: Wiley-Blackwell; 2009.
- Chan CC, Lin SJ. Serpentine supravenous hyperpigmentation. N Engl J Med. 2010;29:363.
- Ouyang Y-H, Chu C-Y, Hu S-L. Linear hyperpigmentation of the left hand following chemotherapy. Dermatol Sinica. 2004;22:262-263.
- Piérard-Franchimont C, Quatresooz P, Reginster MA, et al. Revisiting cutaneous adverse reactions to pemetrexed. Oncol Lett. 2011;2:769-772.
- Buchinger K, Stahel R, Niggemeier V, et al. Pemetrexed-induced neutropenic enteritis and severe cutaneous hyperpigmentation in a patient with malignant pleural mesothelioma. Lung Cancer. 2013;80:347-349.
- Schallier D, Decoster L, De Greve J. Pemetrexed-induced hyperpigmentation of the skin. Anticancer Res. 2011;31:1753-1755.
- Rao R, Balachandran C. Serpentine supravenous pigmentation. a rare vasculocutaneous effect induced by systemic 5-fluoruracil. Indian J Dermatol Venereol Leprol. 2010;76:714-715.
- Geddes ER, Cohen PR. Antineoplastic agent-associated serpentine supravenous hyperpigmentation: superficial venous system hyperpigmentation following intravenous chemotherapy. South Med J. 2010;103:231-235.
- Ayodogan I, Kavak A, Parlak AH, et al. Persistent serpentine supravenous hyperpigmented eruption associated with docetaxel. J Eur Acad Dermatol Venereol. 2005;19:345-347.
Practice Points
- A variety of dermatologic complications have been reported with cisplatin chemotherapy, including serpentine supravenous hyperpigmentation (SSH); however, pemetrexed has not been associated with SSH.
- Although discontinuing the offending agent would certainly prevent further development, due to the benign nature of the reaction, modifying therapy based on cutaneous findings alone is not recommended.
Eruptive Melanocytic Nevi During Azathioprine Therapy for Antisynthetase Syndrome
Case Report
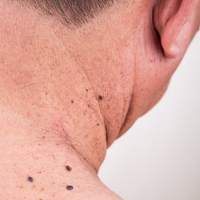
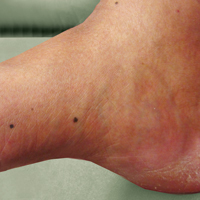
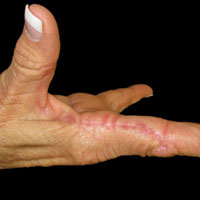
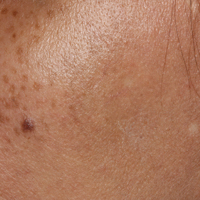
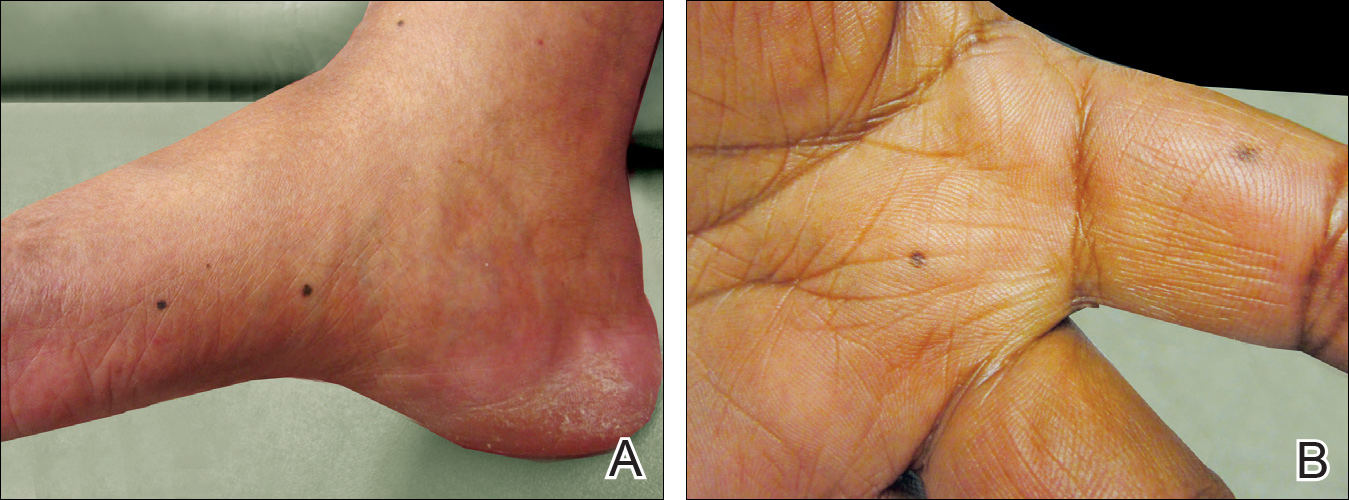
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.
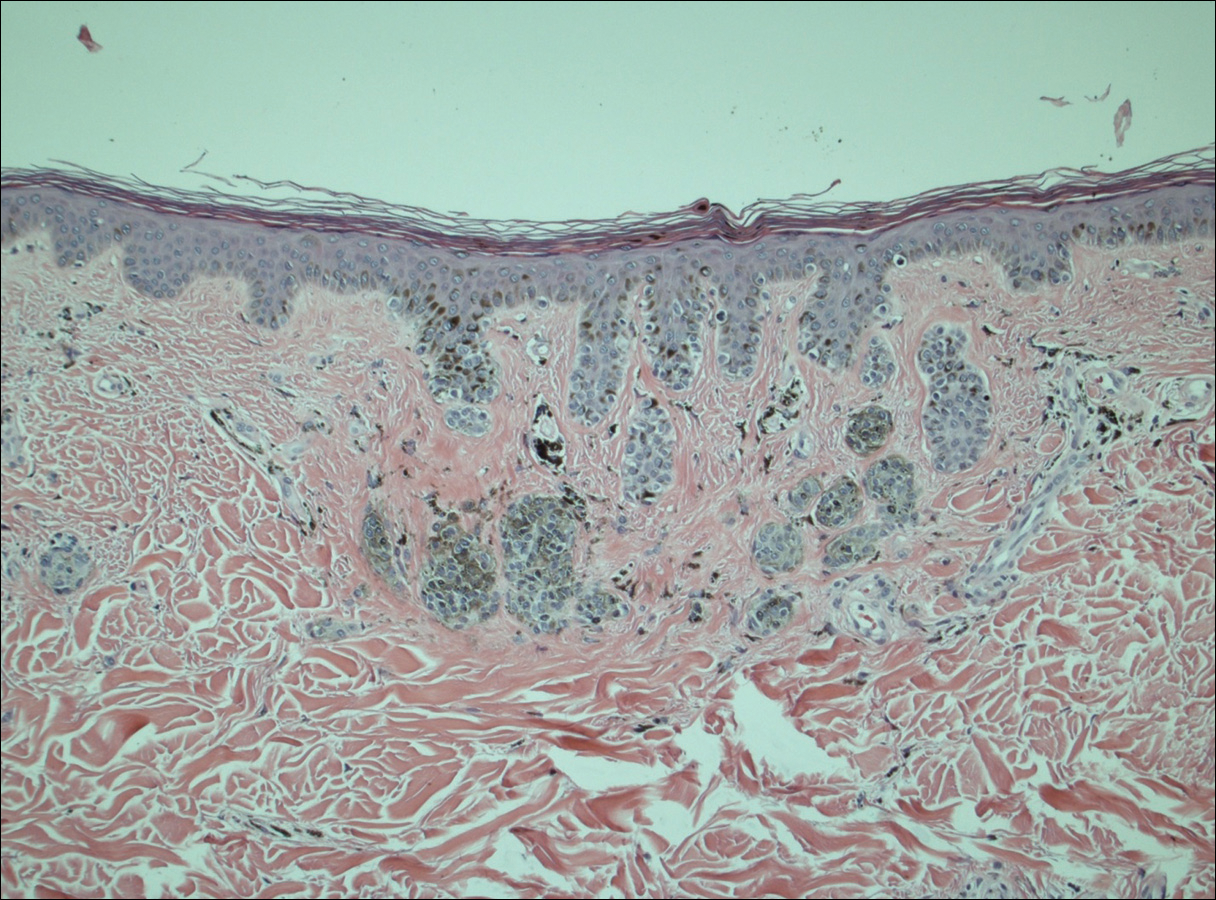
Comment
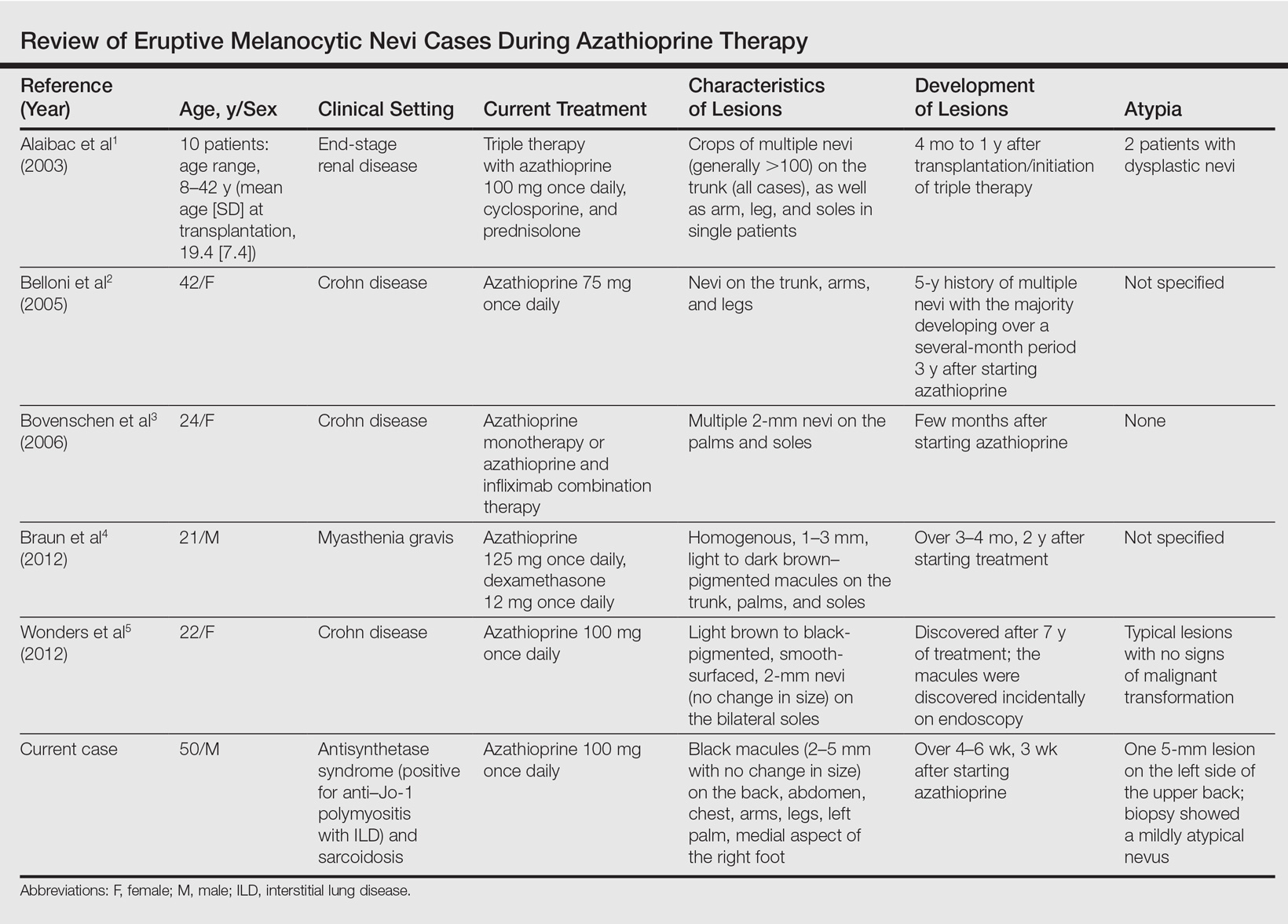
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.
Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
Case Report
A 50-year-old man with a history of antisynthetase syndrome (positive for anti–Jo-1 polymyositis with interstitial lung disease) and sarcoidosis presented for evaluation of numerous new moles. The lesions had developed on the trunk, arms, legs, hands, and feet approximately 3 weeks after starting azathioprine 100 mg once daily for pulmonary and muscular involvement of antisynthetase syndrome. He denied any preceding cutaneous inflammation or sunburns. He had no personal or family history of skin cancer, and no family members had multiple nevi. Physical examination revealed 30 to 40 benign-appearing, 2- to 5-mm, hyperpigmented macules scattered on the medial aspect of the right foot (Figure 1A), left palm (Figure 1B), back, abdomen, chest, arms, and legs. A larger, somewhat asymmetric, irregularly bordered, and irregularly pigmented macule was noted on the left side of the upper back. A punch biopsy of the lesion revealed a benign, mildly atypical lentiginous compound nevus (Figure 2). Pathology confirmed that the lesions represented eruptive melanocytic nevi (EMN). The patient continued azathioprine therapy and was followed with regular full-body skin examinations. Mycophenolate mofetil was suggested as an alternative therapy, if clinically appropriate, though this change has not been made by the patient’s rheumatologists.


Comment
A PubMed search of articles indexed for MEDLINE using the search terms eruptive melanocytic nevi and azathioprine revealed 14 cases of EMN in the setting of azathioprine therapy, either during azathioprine monotherapy or in combination with other immunosuppressants, including systemic corticosteroids, biologics, and cyclosporine (Table).1-5 The majority of these cases occurred in renal transplant patients,1 with 3 additional cases reported in the setting of Crohn disease,2,3,5 and another in a patient with myasthenia gravis.4 Patients ranged in age from 8 to 42 years (mean age, 22 years), with lesions developing a few months to up to 7 years after starting therapy. When specified, the reported lesions typically were small, ranging from 1 to 3 mm in size, and developed rapidly over a couple of months with a predilection for the palms, soles, and trunk. Although dysplastic nevi were described in only 2 patients, melanomas were not detected.

Various hypotheses have sought to explain the largely unknown etiology of EMN. Bovenschen et al3 suggested that immunocompromised patients have diminished immune surveillance in the skin, which allows for unchecked proliferation of melanocytes. Specifically, immune suppression may induce melanocyte-stimulating hormone or melanoma growth stimulatory activity, with composition-specific growth in skin at the palms and soles.3,4 The preferential growth on the palms and soles suggests that those regions may have special sensitivity to melanocyte-stimulating hormone.4 Woodhouse and Maytin6 postulated that the increased density of eccrine sweat glands in the palms and soles as well as the absence of pilosebaceous units and apocrine glands and plentiful Pacinian and Meissner corpuscles may allow for a unique response to circulating melanocytic growth factors. Another hypothesis suggests the presence of genetic factors that allow subclinical nests of nevus cells to form, which become clinical eruptions following chemotherapy or immunosuppressive therapy.3 Azathioprine also has been suggested to induce various transcription factors that play a critical role in differentiation and proliferation of melanocytic stem cells, which leads to the formation of nevi.4 Our case and others similar to it implore that further studies be done to determine the molecular mechanism driving this phenomenon and whether a specific genetic predisposition exists that lowers the threshold for rapid proliferation of melanocytes given an immunosuppressed status.2
The risk for melanoma development in cases of EMN is unknown. Although our review of the literature did not reveal any melanomas reported in cases attributed to azathioprine, a theoretical risk exists given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.6 Accordingly, these patients should be followed with regular skin examinations and biopsies of atypical-appearing lesions as indicated.2,3,5 Braun et al4 also suggested the discontinuance of azathioprine and switch to mycophenolic acid, which has not been noted to cause such eruptions; this drug was recommended in our case.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
- Alaibac M, Piaserico S, Rossi CR, et al. Eruptive melanocytic nevi in patients with renal allografts: report of 10 cases with dermoscopic findings. J Am Acad Dermatol. 2003;49:1020-1022.
- Belloni FA, Piaserico S, Zattra E, et al. Dermoscopic features of eruptive melanocytic naevi in an adult patient receiving immunosuppressive therapy for Crohn’s disease. Melanoma Res. 2005;15:223-224.
- Bovenschen HJ, Tjioe M, Vermaat H, et al. Induction of eruptive benign melanocytic naevi by immune suppressive agents, including biologicals. Br J Dermatol. 2006;154:880-884.
- Braun SA, Helbig D, Frank J, et al. Eruptive melanocytic nevi during azathioprine therapy in myasthenia gravis [in German]. Hautarzt. 2012;63:756-759.
- Wonders J, De Boer N, Van Weyenberg S. Spot diagnosis: eruptive melanocytic naevi during azathioprine therapy in Crohn’s disease [published online March 6, 2012]. J Crohns Colitis. 2012;6:636.
- Woodhouse J, Maytin EV. Eruptive nevi of the palms and soles. J Am Acad Dermatol. 2005;52(5 suppl 1):S96-S100.
Practice Points
- A theoretical risk exists in the setting of eruptive melanocytic nevi (EMN) given the established associations between melanoma and immunosuppression as well as increased numbers of nevi.
- Follow patients with EMN with regular skin examinations and biopsies of atypical-appearing lesions given the increased risk for melanoma in this population.
Electronic Collaboration in Dermatology Resident Training Through Social Networking
More than 1.8 billion individuals utilize social media, a number that continues to grow as the social media market expands.1 Social media enables individuals, groups, and organizations to efficiently disperse and access information2-4 and also provides a structure that encourages collaboration between patients, staff, and physicians that cannot be achieved by other communication modalities.4-6 Expert opinions and related educational materials can be shared globally, improving collaboration between dermatologists.6 A structured social networking site for sharing training materials, research, and ideas can help bring the national dermatology community together in a new way.
Other professions have employed social networking tools to accomplish similar goals of organizing training resources; radiology has an electronic database that allows sharing of training materials and incorporates social networking capabilities.7 Their Web software provides functionality for individual file uploading and supports collaboration and sharing, all while maintaining the security of uploaded information. General surgery has already addressed similar concerns via a task force that incorporates all the essential organizations in surgical education.8 Increased satisfaction and academic abilities have been demonstrated with their collaborative curriculum.9 Gastroenterologists also utilize electronic resources; one study showed that using videos to educate patients prior to colonoscopies was superior to face-to-face education.10 In addition, video education may free up time for office staff to accomplish other tasks.
As a specialty, dermatology has not been a leader in the implementation of social networking for collaboration and training purposes. Every dermatologist is an educator. To maintain a successful practice, dermatologists must keep up-to-date on their own clinical knowledge, provide training to their staff, and educate their patients. Although there are numerous educational resources available to dermatologists, an informal survey of 30 dermatology faculty members revealed a practice gap in awareness and utilization of these expanding electronic resources.11
To better understand the needs of the specialty as a whole, we chose to focus on one aspect of dermatology education: resident training. The goal of our study was to survey dermatology residents and faculty to gain a better understanding of how they currently provide education and what online resources and social networking sites they currently use or would be willing to use. The study included 3 central hypotheses: First, residents would be less satisfied with their current curriculum and residents would report greater contributions to the curriculum relative to faculty. Second, both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Lastly, residents would be more willing than faculty to participate in social networking for educational purposes.
Methods
This study was granted institutional review board exemption. Two surveys were developed by the authors to assess the current structure and satisfaction of dermatology residency curriculum and the willingness to participate in social networking to use and share educational materials. The surveys were evaluated for relevance by the survey evaluation team of the Association of Professors of Dermatology (APD). The instrument was not pilot tested.
The surveys were electronically distributed using an online service to dermatology faculty via the APD listserve, which comprised the entirety of the APD membership in 2014. The resident survey was distributed to the dermatology residents via the American Society for Dermatologic Surgery listserve, which included all residents in training (2013-2014 academic year). Second and third invitations to complete the surveys were distributed 3 and 5 weeks later, respectively.
Resident and faculty responses were compared. Additionally, responses were stratified for large (>9 residents) and small programs (≤9 residents) for comparison. Descriptive statistics including means and medians for continuous variables and frequency tables for categorical variables were generated using research and spreadsheet software.
Results
There were 137 survey respondents; 52 of 426 (12.2%) dermatology faculty and 85 of 1539 (5.5%) dermatology residents responded to the survey. Small programs accounted for 24% of total survey responses and 76% were from large programs.
Current Curriculum
The majority of dermatology faculty (44%) and residents (35%) identified 1 to 2 faculty members as contributing to the creation and organization of their respective curricula; however, a notable percentage of residents (9%) reported that no faculty contributed to the organization of the curriculum. Residents noted that senior residents carry twice the responsibility for structuring the curriculum compared to faculty (61% vs 32% of the workload), but faculty described an even split between senior residents and faculty (47% vs 49% of the workload). Faculty believed their residents spend a similar amount of time in resident- and faculty-led instruction (38% vs 35% of their time); however, the majority of residents reported spending too little time in faculty-led instruction (53%). When residents ranked their preference for learning modes, faculty-led and self-study learning were ranked first and second by 48% and 45% of residents, respectively. Resident-led instruction was ranked last by 66% of residents. Likewise, a majority of residents (53%) described their amount of time in faculty-led instruction as too little.
When asked what subjects in dermatology were lacking at their programs, residents reported clinical trials (47%), skin of color (46%), cosmetic dermatology (34%), and aggressive skin cancer/multidisciplinary tumor board (32%). Although 11% of residents reported lacking inpatient dermatology in their curriculum, 0% of faculty reported the same. A notable percentage of faculty reported nothing was lacking compared to residents (25% vs 7%). Despite these different views between residents and faculty on their contributions to and structure of their curriculums, both faculty and residents claimed overall satisfaction (satisfied or very satisfied) with their program’s ability to optimally cover the field of dermatology in 3 years (100% and 91%, respectively).
Large Versus Small Residency Programs
When stratifying the resident responses for small versus large programs, both program sizes reported more time in resident-led instruction than faculty-led instruction. Likewise, residents in both program sizes equally preferred self-study or faculty-led instruction to resident-led instruction. Residents at small programs more often reported lacking instruction in rheumatology, immunobullous diseases, and basic science/skin biology compared to large-program residents. Compared to large-program faculty, small-program faculty reported lacking instruction in cosmetic dermatology.
Faculty at small programs reported spending too little time preparing for their faculty-led instruction compared to faculty at large programs (44% vs 12%). All (100%) of the faculty at small programs were likely to seek out study materials shared by top educators, while 77% of faculty at large programs were likely to do the same. When asked if faculty would translate what their program does well into an electronic format for sharing, 30% of large-program faculty were likely to do so compared to 11% of small-program faculty (Figure 1).
Use of Online Educational Materials and Interest in Collaboration
A majority of faculty and residents stated that they use online educational materials as supplements to traditional classroom lecture and print materials (81% vs 86%); however, almost twice as many residents stated that online educational materials were essential to their current study routines compared to faculty (39% vs 21%).
The majority of faculty (92%) and residents (84%) were either interested or very interested in a collaborative online curriculum. Both residents (85%) and faculty (81%) stated they would be likely to seek out online educational materials shared by top educators. Although both residents and faculty reported many aspects of their curriculums they thought could be beneficial to other dermatology programs (Table 1), only 27% of faculty and 19% of residents were likely to translate those strengths into a shareable electronic format. Several reasons were reported for not contributing to an online curriculum, with lack of time being the most common reason (Table 2).
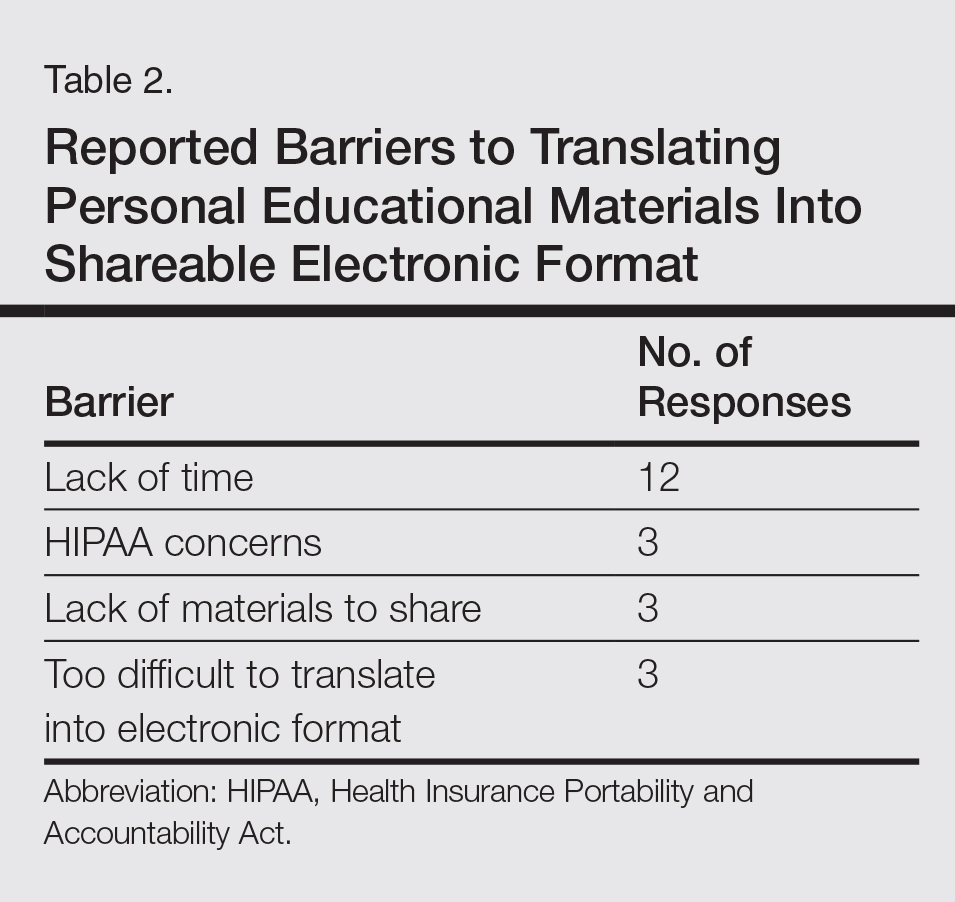
Eighty percent of residents and 88% of faculty reported they were either interested or very interested in being more connected/interactive with their dermatology peers nationally (Figure 2). Likewise, 94% of residents and 87% of faculty agreed that the dermatology community could benefit from a social networking site for educational collaboration. Four times as many residents versus faculty currently use social networking sites (eg, Facebook, LinkedIn, Google Groups) as a primary mode of communication with distant professional peers. The majority of residents (52%) reported they would be likely to participate in a professional social networking site, while the majority of faculty (50%) stated they were neutral on their likelihood of participating. Both residents and faculty reported lack of time as a common reason for being unlikely to utilize a professional social networking site. Other barriers to participation are listed in Table 3.
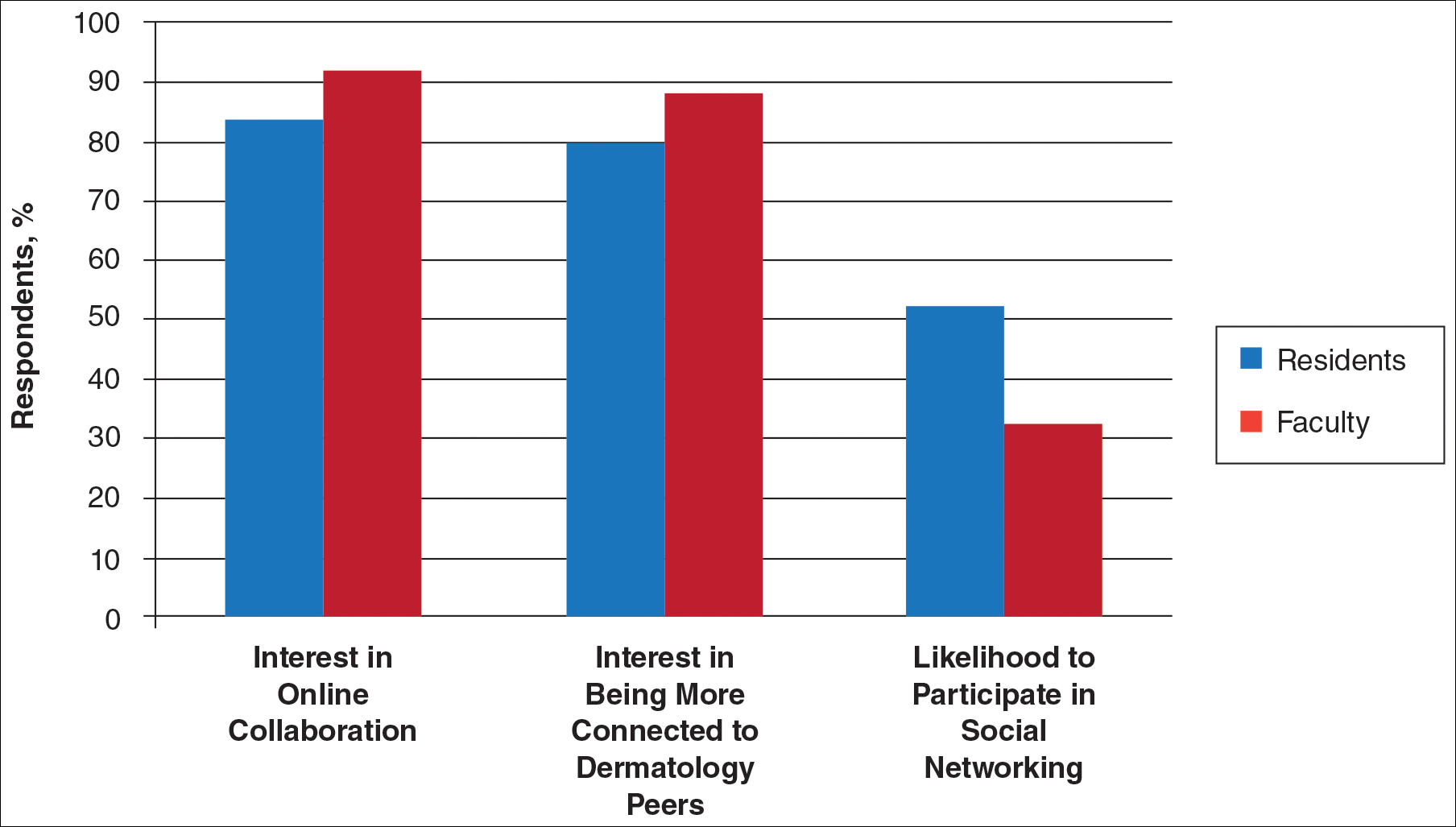
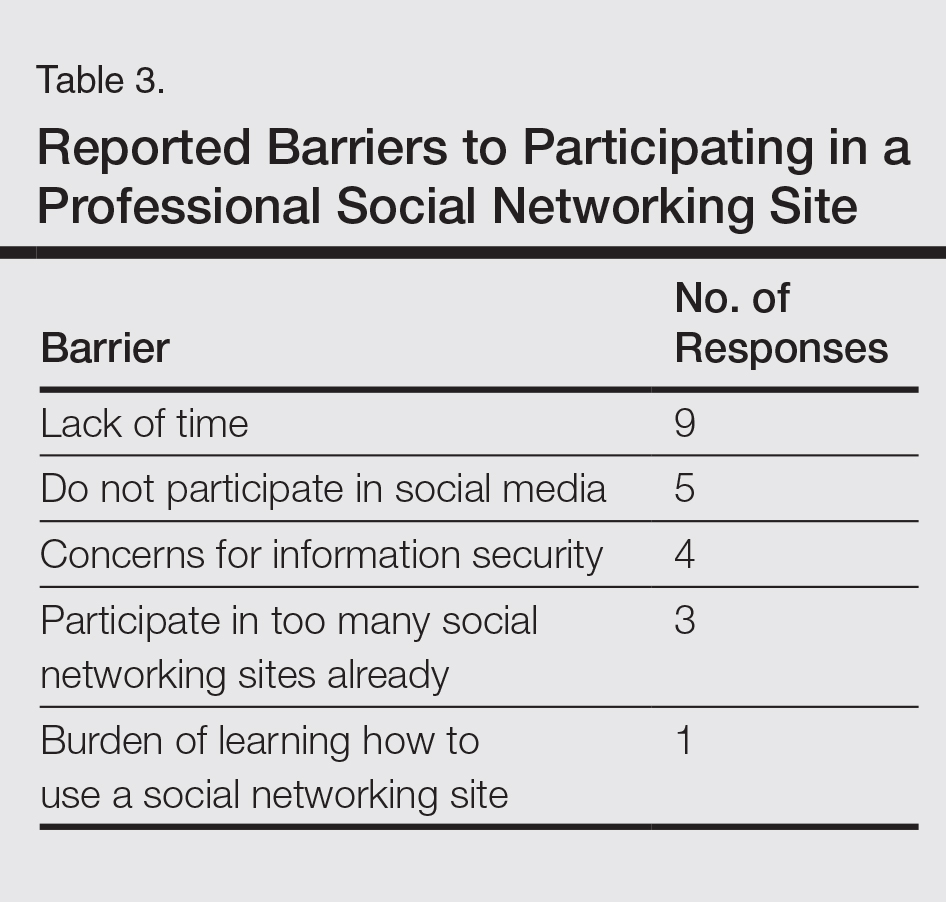
Comment
This study showed how dermatology faculty and residents currently provide training and what online resources and social networking sites they currently use or would be willing to use. The generalizability of the conclusions is limited by the low response rate for the surveys. The results demonstrated the different views between faculty and residents and between large and small residency programs on various topics. This microcosm of dermatology training can likely be applied to other training scenarios in dermatology, including patient education; training of nurses, physician extenders, and office staff; continuing medical education for physicians; and peer-to-peer collaboration.
Hypothesis 1: Partially Proven
We hypothesized that residents would report less satisfaction with their current curriculum and would report greater resident contributions to the curriculum relative to faculty. Overall, residents and faculty reported satisfaction with their curriculums to provide up-to-date information and breadth in the field of dermatology. Despite their overall satisfaction, more residents reported lacking instruction in several dermatology subtopics compared to faculty. Additionally, residents believed they spend twice as much time structuring their curriculum compared to faculty, with some residents reporting no faculty involvement. Although residents preferred faculty-led instruction, a majority of residents reported they do not have enough faculty-led didactics. The preference for faculty-led training is likely due to the expertise of faculty compared to residents.
Hypothesis 2: Partially Proven
We also hypothesized that both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Although there was no difference in interest between residents at small versus large programs, there was a difference between faculty at small versus large programs. Small-program faculty were more interested in using shared materials than larger programs, while large-program faculty were more likely to share their educational materials. Small-program faculty reported spending too little time preparing their lectures, which is possibly due to a lack of time for preparation. Additionally, residents and faculty at smaller programs report their curriculum was lacking specific dermatology topics compared to large programs. These disparities between program sizes indicate a need for a social networking site for training collaboration in dermatology. Large programs have the ability to share what they do well, which small programs are eager to utilize.
Hypothesis 3: Not Proven
We hypothesized that residents would be more willing than faculty to participate in social networking for educational purposes. The majority of faculty and residents were interested in participating in a collaborative online curriculum and using the shared materials from top educators; however, even though such large majorities favored collaboration and sharing, only 27% of faculty and 19% of residents were likely to translate their own materials into a shareable format. Although lack of time was the most common reason for not sharing materials, electronic methods may have the potential to ultimately save time and remove the burden of content creation. The time it would take to translate selected personal training materials into a shareable form would be made up for by the time saved using another educators’ materials. Updating and customizing shared online educational materials can be much quicker and easier than educators creating materials on their own. Dermatologists would be more efficient facilitators of training via high-quality shared materials while decreasing the time burden associated with resident education.5 Another concern for not sharing or participating in a social networking site was skepticism of information security on such a network. The poor organization and information overload of online resources can compound the already existing time constraints on dermatologists, which may limit their ability to utilize such valuable resources. In addition, quality of online resources is not always guaranteed, and determining the sources that are high quality is sometimes a difficult task.6 For online materials to remain useful, there should be a peer-review process to evaluate quality and assess satisfaction.5
Solution: Create a Dermatology Task Force
A dermatology task force could facilitate the resolution of these challenges of online materials. In addition, a task force could cover the administrative support needed to ensure security and provide maintenance on social networks.
The main limitation to implementing a social network is the presence of the administrative infrastructure to jumpstart its creation. A task force incorporating the essential stakeholders in dermatology training is the first step. With inclusive representation from all of the smaller professional dermatology societies, the American Academy of Dermatology is optimally positioned to create this task force. With existing information technologies, a task force could address the concerns revealed in our survey as well as any future concerns that may arise.
The goal is a single social network for dermatologists that has the capability of improving communication and collaboration between professional peers regardless of their practice setting. Such a network is ideal for the practicing dermatologist for the purposes of staff training, patient education, and obtaining continuing medical education credit. Additionally, peer group collaboration would facilitate the understanding and completion of the evolving requirements for Maintenance of Certification from the American Board of Dermatology. The availability of quality shared materials would save time and increase efficiency of an entire dermatology practice. Materials that aid in patient education would allow office staff to dedicate their time to other tasks, thereby increasing productivity. Shared training materials would decrease the burden of staff education, providing more time for advanced hands-on training. This method of collaborative effort is capable of advancing the field of dermatology as a whole. It can overcome geographical and institutional barriers to connect dermatologists with similar interests worldwide; disseminate advances in diagnosis and treatment; and improve the quality of dermatology training of dermatologists, staff, and patients.
- Statistics and facts about social networks. Statista website. http://www.statista.com/topics/1164/social-networks/. Accessed March 22, 2017.
- Baker RC, Klein M, Samaan Z, et al. Effectiveness of an online pediatric primary care curriculum. Acad Pediatr. 2010;10:131-137.
- Dolev JC, O’Sullivan P, Berger T. The eDerm online curriculum: a randomized study of effective skin cancer teaching to medical students. J Am Acad Dermatol. 2011;65:e165-e171.
- Amir M, Sampson BP, Endly D, et al. Social networking sites: emerging and essential tools for communication in dermatology. JAMA Dermatol. 2014;150:56-60.
- Ruiz JG, Mintzer MJ, Leipzig RM. The impact of e-learning in medical education. Acad Med. 2006;81:207-212.
- Hanson AH, Krause LK, Simmons RN, et al. Dermatology education and the internet: traditional and cutting-edge resources. J Am Acad Dermatol. 2011;65:836-842.
- Rowe SP, Siddiqui A, Bonekamp D. The key image and case log application: new radiology software for teaching file creation and case logging that incorporates elements of a social network. Acad Radiol. 2014;21:916-930.
- Bell RH. Surgical council on resident education: a new organization devoted to graduate surgical education. J Am Coll Surg. 2007;204:341-346.
- Kirton OC, Reilly P, Staff I, et al. Development and implementation of an interactive, objective, and simulation-based curriculum for general surgery residents. J Surg Educ. 2012;69:718-723.
- Prakash S, Verma S, McGowan J, et al. Improving the quality of colonoscopy bowel preparation using an educational video. Can J Gastroenterol. 2013;27:696-700.
- Carroll BT. eTools for teaching dermatologic surgery. Paper presented at the Association of Professors of Dermatology 2014 Annual Meeting; September 12-13, 2014; Chicago, IL.
More than 1.8 billion individuals utilize social media, a number that continues to grow as the social media market expands.1 Social media enables individuals, groups, and organizations to efficiently disperse and access information2-4 and also provides a structure that encourages collaboration between patients, staff, and physicians that cannot be achieved by other communication modalities.4-6 Expert opinions and related educational materials can be shared globally, improving collaboration between dermatologists.6 A structured social networking site for sharing training materials, research, and ideas can help bring the national dermatology community together in a new way.
Other professions have employed social networking tools to accomplish similar goals of organizing training resources; radiology has an electronic database that allows sharing of training materials and incorporates social networking capabilities.7 Their Web software provides functionality for individual file uploading and supports collaboration and sharing, all while maintaining the security of uploaded information. General surgery has already addressed similar concerns via a task force that incorporates all the essential organizations in surgical education.8 Increased satisfaction and academic abilities have been demonstrated with their collaborative curriculum.9 Gastroenterologists also utilize electronic resources; one study showed that using videos to educate patients prior to colonoscopies was superior to face-to-face education.10 In addition, video education may free up time for office staff to accomplish other tasks.
As a specialty, dermatology has not been a leader in the implementation of social networking for collaboration and training purposes. Every dermatologist is an educator. To maintain a successful practice, dermatologists must keep up-to-date on their own clinical knowledge, provide training to their staff, and educate their patients. Although there are numerous educational resources available to dermatologists, an informal survey of 30 dermatology faculty members revealed a practice gap in awareness and utilization of these expanding electronic resources.11
To better understand the needs of the specialty as a whole, we chose to focus on one aspect of dermatology education: resident training. The goal of our study was to survey dermatology residents and faculty to gain a better understanding of how they currently provide education and what online resources and social networking sites they currently use or would be willing to use. The study included 3 central hypotheses: First, residents would be less satisfied with their current curriculum and residents would report greater contributions to the curriculum relative to faculty. Second, both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Lastly, residents would be more willing than faculty to participate in social networking for educational purposes.
Methods
This study was granted institutional review board exemption. Two surveys were developed by the authors to assess the current structure and satisfaction of dermatology residency curriculum and the willingness to participate in social networking to use and share educational materials. The surveys were evaluated for relevance by the survey evaluation team of the Association of Professors of Dermatology (APD). The instrument was not pilot tested.
The surveys were electronically distributed using an online service to dermatology faculty via the APD listserve, which comprised the entirety of the APD membership in 2014. The resident survey was distributed to the dermatology residents via the American Society for Dermatologic Surgery listserve, which included all residents in training (2013-2014 academic year). Second and third invitations to complete the surveys were distributed 3 and 5 weeks later, respectively.
Resident and faculty responses were compared. Additionally, responses were stratified for large (>9 residents) and small programs (≤9 residents) for comparison. Descriptive statistics including means and medians for continuous variables and frequency tables for categorical variables were generated using research and spreadsheet software.
Results
There were 137 survey respondents; 52 of 426 (12.2%) dermatology faculty and 85 of 1539 (5.5%) dermatology residents responded to the survey. Small programs accounted for 24% of total survey responses and 76% were from large programs.
Current Curriculum
The majority of dermatology faculty (44%) and residents (35%) identified 1 to 2 faculty members as contributing to the creation and organization of their respective curricula; however, a notable percentage of residents (9%) reported that no faculty contributed to the organization of the curriculum. Residents noted that senior residents carry twice the responsibility for structuring the curriculum compared to faculty (61% vs 32% of the workload), but faculty described an even split between senior residents and faculty (47% vs 49% of the workload). Faculty believed their residents spend a similar amount of time in resident- and faculty-led instruction (38% vs 35% of their time); however, the majority of residents reported spending too little time in faculty-led instruction (53%). When residents ranked their preference for learning modes, faculty-led and self-study learning were ranked first and second by 48% and 45% of residents, respectively. Resident-led instruction was ranked last by 66% of residents. Likewise, a majority of residents (53%) described their amount of time in faculty-led instruction as too little.
When asked what subjects in dermatology were lacking at their programs, residents reported clinical trials (47%), skin of color (46%), cosmetic dermatology (34%), and aggressive skin cancer/multidisciplinary tumor board (32%). Although 11% of residents reported lacking inpatient dermatology in their curriculum, 0% of faculty reported the same. A notable percentage of faculty reported nothing was lacking compared to residents (25% vs 7%). Despite these different views between residents and faculty on their contributions to and structure of their curriculums, both faculty and residents claimed overall satisfaction (satisfied or very satisfied) with their program’s ability to optimally cover the field of dermatology in 3 years (100% and 91%, respectively).
Large Versus Small Residency Programs
When stratifying the resident responses for small versus large programs, both program sizes reported more time in resident-led instruction than faculty-led instruction. Likewise, residents in both program sizes equally preferred self-study or faculty-led instruction to resident-led instruction. Residents at small programs more often reported lacking instruction in rheumatology, immunobullous diseases, and basic science/skin biology compared to large-program residents. Compared to large-program faculty, small-program faculty reported lacking instruction in cosmetic dermatology.
Faculty at small programs reported spending too little time preparing for their faculty-led instruction compared to faculty at large programs (44% vs 12%). All (100%) of the faculty at small programs were likely to seek out study materials shared by top educators, while 77% of faculty at large programs were likely to do the same. When asked if faculty would translate what their program does well into an electronic format for sharing, 30% of large-program faculty were likely to do so compared to 11% of small-program faculty (Figure 1).

Use of Online Educational Materials and Interest in Collaboration
A majority of faculty and residents stated that they use online educational materials as supplements to traditional classroom lecture and print materials (81% vs 86%); however, almost twice as many residents stated that online educational materials were essential to their current study routines compared to faculty (39% vs 21%).
The majority of faculty (92%) and residents (84%) were either interested or very interested in a collaborative online curriculum. Both residents (85%) and faculty (81%) stated they would be likely to seek out online educational materials shared by top educators. Although both residents and faculty reported many aspects of their curriculums they thought could be beneficial to other dermatology programs (Table 1), only 27% of faculty and 19% of residents were likely to translate those strengths into a shareable electronic format. Several reasons were reported for not contributing to an online curriculum, with lack of time being the most common reason (Table 2).


Eighty percent of residents and 88% of faculty reported they were either interested or very interested in being more connected/interactive with their dermatology peers nationally (Figure 2). Likewise, 94% of residents and 87% of faculty agreed that the dermatology community could benefit from a social networking site for educational collaboration. Four times as many residents versus faculty currently use social networking sites (eg, Facebook, LinkedIn, Google Groups) as a primary mode of communication with distant professional peers. The majority of residents (52%) reported they would be likely to participate in a professional social networking site, while the majority of faculty (50%) stated they were neutral on their likelihood of participating. Both residents and faculty reported lack of time as a common reason for being unlikely to utilize a professional social networking site. Other barriers to participation are listed in Table 3.


Comment
This study showed how dermatology faculty and residents currently provide training and what online resources and social networking sites they currently use or would be willing to use. The generalizability of the conclusions is limited by the low response rate for the surveys. The results demonstrated the different views between faculty and residents and between large and small residency programs on various topics. This microcosm of dermatology training can likely be applied to other training scenarios in dermatology, including patient education; training of nurses, physician extenders, and office staff; continuing medical education for physicians; and peer-to-peer collaboration.
Hypothesis 1: Partially Proven
We hypothesized that residents would report less satisfaction with their current curriculum and would report greater resident contributions to the curriculum relative to faculty. Overall, residents and faculty reported satisfaction with their curriculums to provide up-to-date information and breadth in the field of dermatology. Despite their overall satisfaction, more residents reported lacking instruction in several dermatology subtopics compared to faculty. Additionally, residents believed they spend twice as much time structuring their curriculum compared to faculty, with some residents reporting no faculty involvement. Although residents preferred faculty-led instruction, a majority of residents reported they do not have enough faculty-led didactics. The preference for faculty-led training is likely due to the expertise of faculty compared to residents.
Hypothesis 2: Partially Proven
We also hypothesized that both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Although there was no difference in interest between residents at small versus large programs, there was a difference between faculty at small versus large programs. Small-program faculty were more interested in using shared materials than larger programs, while large-program faculty were more likely to share their educational materials. Small-program faculty reported spending too little time preparing their lectures, which is possibly due to a lack of time for preparation. Additionally, residents and faculty at smaller programs report their curriculum was lacking specific dermatology topics compared to large programs. These disparities between program sizes indicate a need for a social networking site for training collaboration in dermatology. Large programs have the ability to share what they do well, which small programs are eager to utilize.
Hypothesis 3: Not Proven
We hypothesized that residents would be more willing than faculty to participate in social networking for educational purposes. The majority of faculty and residents were interested in participating in a collaborative online curriculum and using the shared materials from top educators; however, even though such large majorities favored collaboration and sharing, only 27% of faculty and 19% of residents were likely to translate their own materials into a shareable format. Although lack of time was the most common reason for not sharing materials, electronic methods may have the potential to ultimately save time and remove the burden of content creation. The time it would take to translate selected personal training materials into a shareable form would be made up for by the time saved using another educators’ materials. Updating and customizing shared online educational materials can be much quicker and easier than educators creating materials on their own. Dermatologists would be more efficient facilitators of training via high-quality shared materials while decreasing the time burden associated with resident education.5 Another concern for not sharing or participating in a social networking site was skepticism of information security on such a network. The poor organization and information overload of online resources can compound the already existing time constraints on dermatologists, which may limit their ability to utilize such valuable resources. In addition, quality of online resources is not always guaranteed, and determining the sources that are high quality is sometimes a difficult task.6 For online materials to remain useful, there should be a peer-review process to evaluate quality and assess satisfaction.5
Solution: Create a Dermatology Task Force
A dermatology task force could facilitate the resolution of these challenges of online materials. In addition, a task force could cover the administrative support needed to ensure security and provide maintenance on social networks.
The main limitation to implementing a social network is the presence of the administrative infrastructure to jumpstart its creation. A task force incorporating the essential stakeholders in dermatology training is the first step. With inclusive representation from all of the smaller professional dermatology societies, the American Academy of Dermatology is optimally positioned to create this task force. With existing information technologies, a task force could address the concerns revealed in our survey as well as any future concerns that may arise.
The goal is a single social network for dermatologists that has the capability of improving communication and collaboration between professional peers regardless of their practice setting. Such a network is ideal for the practicing dermatologist for the purposes of staff training, patient education, and obtaining continuing medical education credit. Additionally, peer group collaboration would facilitate the understanding and completion of the evolving requirements for Maintenance of Certification from the American Board of Dermatology. The availability of quality shared materials would save time and increase efficiency of an entire dermatology practice. Materials that aid in patient education would allow office staff to dedicate their time to other tasks, thereby increasing productivity. Shared training materials would decrease the burden of staff education, providing more time for advanced hands-on training. This method of collaborative effort is capable of advancing the field of dermatology as a whole. It can overcome geographical and institutional barriers to connect dermatologists with similar interests worldwide; disseminate advances in diagnosis and treatment; and improve the quality of dermatology training of dermatologists, staff, and patients.
More than 1.8 billion individuals utilize social media, a number that continues to grow as the social media market expands.1 Social media enables individuals, groups, and organizations to efficiently disperse and access information2-4 and also provides a structure that encourages collaboration between patients, staff, and physicians that cannot be achieved by other communication modalities.4-6 Expert opinions and related educational materials can be shared globally, improving collaboration between dermatologists.6 A structured social networking site for sharing training materials, research, and ideas can help bring the national dermatology community together in a new way.
Other professions have employed social networking tools to accomplish similar goals of organizing training resources; radiology has an electronic database that allows sharing of training materials and incorporates social networking capabilities.7 Their Web software provides functionality for individual file uploading and supports collaboration and sharing, all while maintaining the security of uploaded information. General surgery has already addressed similar concerns via a task force that incorporates all the essential organizations in surgical education.8 Increased satisfaction and academic abilities have been demonstrated with their collaborative curriculum.9 Gastroenterologists also utilize electronic resources; one study showed that using videos to educate patients prior to colonoscopies was superior to face-to-face education.10 In addition, video education may free up time for office staff to accomplish other tasks.
As a specialty, dermatology has not been a leader in the implementation of social networking for collaboration and training purposes. Every dermatologist is an educator. To maintain a successful practice, dermatologists must keep up-to-date on their own clinical knowledge, provide training to their staff, and educate their patients. Although there are numerous educational resources available to dermatologists, an informal survey of 30 dermatology faculty members revealed a practice gap in awareness and utilization of these expanding electronic resources.11
To better understand the needs of the specialty as a whole, we chose to focus on one aspect of dermatology education: resident training. The goal of our study was to survey dermatology residents and faculty to gain a better understanding of how they currently provide education and what online resources and social networking sites they currently use or would be willing to use. The study included 3 central hypotheses: First, residents would be less satisfied with their current curriculum and residents would report greater contributions to the curriculum relative to faculty. Second, both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Lastly, residents would be more willing than faculty to participate in social networking for educational purposes.
Methods
This study was granted institutional review board exemption. Two surveys were developed by the authors to assess the current structure and satisfaction of dermatology residency curriculum and the willingness to participate in social networking to use and share educational materials. The surveys were evaluated for relevance by the survey evaluation team of the Association of Professors of Dermatology (APD). The instrument was not pilot tested.
The surveys were electronically distributed using an online service to dermatology faculty via the APD listserve, which comprised the entirety of the APD membership in 2014. The resident survey was distributed to the dermatology residents via the American Society for Dermatologic Surgery listserve, which included all residents in training (2013-2014 academic year). Second and third invitations to complete the surveys were distributed 3 and 5 weeks later, respectively.
Resident and faculty responses were compared. Additionally, responses were stratified for large (>9 residents) and small programs (≤9 residents) for comparison. Descriptive statistics including means and medians for continuous variables and frequency tables for categorical variables were generated using research and spreadsheet software.
Results
There were 137 survey respondents; 52 of 426 (12.2%) dermatology faculty and 85 of 1539 (5.5%) dermatology residents responded to the survey. Small programs accounted for 24% of total survey responses and 76% were from large programs.
Current Curriculum
The majority of dermatology faculty (44%) and residents (35%) identified 1 to 2 faculty members as contributing to the creation and organization of their respective curricula; however, a notable percentage of residents (9%) reported that no faculty contributed to the organization of the curriculum. Residents noted that senior residents carry twice the responsibility for structuring the curriculum compared to faculty (61% vs 32% of the workload), but faculty described an even split between senior residents and faculty (47% vs 49% of the workload). Faculty believed their residents spend a similar amount of time in resident- and faculty-led instruction (38% vs 35% of their time); however, the majority of residents reported spending too little time in faculty-led instruction (53%). When residents ranked their preference for learning modes, faculty-led and self-study learning were ranked first and second by 48% and 45% of residents, respectively. Resident-led instruction was ranked last by 66% of residents. Likewise, a majority of residents (53%) described their amount of time in faculty-led instruction as too little.
When asked what subjects in dermatology were lacking at their programs, residents reported clinical trials (47%), skin of color (46%), cosmetic dermatology (34%), and aggressive skin cancer/multidisciplinary tumor board (32%). Although 11% of residents reported lacking inpatient dermatology in their curriculum, 0% of faculty reported the same. A notable percentage of faculty reported nothing was lacking compared to residents (25% vs 7%). Despite these different views between residents and faculty on their contributions to and structure of their curriculums, both faculty and residents claimed overall satisfaction (satisfied or very satisfied) with their program’s ability to optimally cover the field of dermatology in 3 years (100% and 91%, respectively).
Large Versus Small Residency Programs
When stratifying the resident responses for small versus large programs, both program sizes reported more time in resident-led instruction than faculty-led instruction. Likewise, residents in both program sizes equally preferred self-study or faculty-led instruction to resident-led instruction. Residents at small programs more often reported lacking instruction in rheumatology, immunobullous diseases, and basic science/skin biology compared to large-program residents. Compared to large-program faculty, small-program faculty reported lacking instruction in cosmetic dermatology.
Faculty at small programs reported spending too little time preparing for their faculty-led instruction compared to faculty at large programs (44% vs 12%). All (100%) of the faculty at small programs were likely to seek out study materials shared by top educators, while 77% of faculty at large programs were likely to do the same. When asked if faculty would translate what their program does well into an electronic format for sharing, 30% of large-program faculty were likely to do so compared to 11% of small-program faculty (Figure 1).

Use of Online Educational Materials and Interest in Collaboration
A majority of faculty and residents stated that they use online educational materials as supplements to traditional classroom lecture and print materials (81% vs 86%); however, almost twice as many residents stated that online educational materials were essential to their current study routines compared to faculty (39% vs 21%).
The majority of faculty (92%) and residents (84%) were either interested or very interested in a collaborative online curriculum. Both residents (85%) and faculty (81%) stated they would be likely to seek out online educational materials shared by top educators. Although both residents and faculty reported many aspects of their curriculums they thought could be beneficial to other dermatology programs (Table 1), only 27% of faculty and 19% of residents were likely to translate those strengths into a shareable electronic format. Several reasons were reported for not contributing to an online curriculum, with lack of time being the most common reason (Table 2).


Eighty percent of residents and 88% of faculty reported they were either interested or very interested in being more connected/interactive with their dermatology peers nationally (Figure 2). Likewise, 94% of residents and 87% of faculty agreed that the dermatology community could benefit from a social networking site for educational collaboration. Four times as many residents versus faculty currently use social networking sites (eg, Facebook, LinkedIn, Google Groups) as a primary mode of communication with distant professional peers. The majority of residents (52%) reported they would be likely to participate in a professional social networking site, while the majority of faculty (50%) stated they were neutral on their likelihood of participating. Both residents and faculty reported lack of time as a common reason for being unlikely to utilize a professional social networking site. Other barriers to participation are listed in Table 3.


Comment
This study showed how dermatology faculty and residents currently provide training and what online resources and social networking sites they currently use or would be willing to use. The generalizability of the conclusions is limited by the low response rate for the surveys. The results demonstrated the different views between faculty and residents and between large and small residency programs on various topics. This microcosm of dermatology training can likely be applied to other training scenarios in dermatology, including patient education; training of nurses, physician extenders, and office staff; continuing medical education for physicians; and peer-to-peer collaboration.
Hypothesis 1: Partially Proven
We hypothesized that residents would report less satisfaction with their current curriculum and would report greater resident contributions to the curriculum relative to faculty. Overall, residents and faculty reported satisfaction with their curriculums to provide up-to-date information and breadth in the field of dermatology. Despite their overall satisfaction, more residents reported lacking instruction in several dermatology subtopics compared to faculty. Additionally, residents believed they spend twice as much time structuring their curriculum compared to faculty, with some residents reporting no faculty involvement. Although residents preferred faculty-led instruction, a majority of residents reported they do not have enough faculty-led didactics. The preference for faculty-led training is likely due to the expertise of faculty compared to residents.
Hypothesis 2: Partially Proven
We also hypothesized that both residents and faculty of smaller programs would be more interested in collaborative educational resources relative to larger programs. Although there was no difference in interest between residents at small versus large programs, there was a difference between faculty at small versus large programs. Small-program faculty were more interested in using shared materials than larger programs, while large-program faculty were more likely to share their educational materials. Small-program faculty reported spending too little time preparing their lectures, which is possibly due to a lack of time for preparation. Additionally, residents and faculty at smaller programs report their curriculum was lacking specific dermatology topics compared to large programs. These disparities between program sizes indicate a need for a social networking site for training collaboration in dermatology. Large programs have the ability to share what they do well, which small programs are eager to utilize.
Hypothesis 3: Not Proven
We hypothesized that residents would be more willing than faculty to participate in social networking for educational purposes. The majority of faculty and residents were interested in participating in a collaborative online curriculum and using the shared materials from top educators; however, even though such large majorities favored collaboration and sharing, only 27% of faculty and 19% of residents were likely to translate their own materials into a shareable format. Although lack of time was the most common reason for not sharing materials, electronic methods may have the potential to ultimately save time and remove the burden of content creation. The time it would take to translate selected personal training materials into a shareable form would be made up for by the time saved using another educators’ materials. Updating and customizing shared online educational materials can be much quicker and easier than educators creating materials on their own. Dermatologists would be more efficient facilitators of training via high-quality shared materials while decreasing the time burden associated with resident education.5 Another concern for not sharing or participating in a social networking site was skepticism of information security on such a network. The poor organization and information overload of online resources can compound the already existing time constraints on dermatologists, which may limit their ability to utilize such valuable resources. In addition, quality of online resources is not always guaranteed, and determining the sources that are high quality is sometimes a difficult task.6 For online materials to remain useful, there should be a peer-review process to evaluate quality and assess satisfaction.5
Solution: Create a Dermatology Task Force
A dermatology task force could facilitate the resolution of these challenges of online materials. In addition, a task force could cover the administrative support needed to ensure security and provide maintenance on social networks.
The main limitation to implementing a social network is the presence of the administrative infrastructure to jumpstart its creation. A task force incorporating the essential stakeholders in dermatology training is the first step. With inclusive representation from all of the smaller professional dermatology societies, the American Academy of Dermatology is optimally positioned to create this task force. With existing information technologies, a task force could address the concerns revealed in our survey as well as any future concerns that may arise.
The goal is a single social network for dermatologists that has the capability of improving communication and collaboration between professional peers regardless of their practice setting. Such a network is ideal for the practicing dermatologist for the purposes of staff training, patient education, and obtaining continuing medical education credit. Additionally, peer group collaboration would facilitate the understanding and completion of the evolving requirements for Maintenance of Certification from the American Board of Dermatology. The availability of quality shared materials would save time and increase efficiency of an entire dermatology practice. Materials that aid in patient education would allow office staff to dedicate their time to other tasks, thereby increasing productivity. Shared training materials would decrease the burden of staff education, providing more time for advanced hands-on training. This method of collaborative effort is capable of advancing the field of dermatology as a whole. It can overcome geographical and institutional barriers to connect dermatologists with similar interests worldwide; disseminate advances in diagnosis and treatment; and improve the quality of dermatology training of dermatologists, staff, and patients.
- Statistics and facts about social networks. Statista website. http://www.statista.com/topics/1164/social-networks/. Accessed March 22, 2017.
- Baker RC, Klein M, Samaan Z, et al. Effectiveness of an online pediatric primary care curriculum. Acad Pediatr. 2010;10:131-137.
- Dolev JC, O’Sullivan P, Berger T. The eDerm online curriculum: a randomized study of effective skin cancer teaching to medical students. J Am Acad Dermatol. 2011;65:e165-e171.
- Amir M, Sampson BP, Endly D, et al. Social networking sites: emerging and essential tools for communication in dermatology. JAMA Dermatol. 2014;150:56-60.
- Ruiz JG, Mintzer MJ, Leipzig RM. The impact of e-learning in medical education. Acad Med. 2006;81:207-212.
- Hanson AH, Krause LK, Simmons RN, et al. Dermatology education and the internet: traditional and cutting-edge resources. J Am Acad Dermatol. 2011;65:836-842.
- Rowe SP, Siddiqui A, Bonekamp D. The key image and case log application: new radiology software for teaching file creation and case logging that incorporates elements of a social network. Acad Radiol. 2014;21:916-930.
- Bell RH. Surgical council on resident education: a new organization devoted to graduate surgical education. J Am Coll Surg. 2007;204:341-346.
- Kirton OC, Reilly P, Staff I, et al. Development and implementation of an interactive, objective, and simulation-based curriculum for general surgery residents. J Surg Educ. 2012;69:718-723.
- Prakash S, Verma S, McGowan J, et al. Improving the quality of colonoscopy bowel preparation using an educational video. Can J Gastroenterol. 2013;27:696-700.
- Carroll BT. eTools for teaching dermatologic surgery. Paper presented at the Association of Professors of Dermatology 2014 Annual Meeting; September 12-13, 2014; Chicago, IL.
- Statistics and facts about social networks. Statista website. http://www.statista.com/topics/1164/social-networks/. Accessed March 22, 2017.
- Baker RC, Klein M, Samaan Z, et al. Effectiveness of an online pediatric primary care curriculum. Acad Pediatr. 2010;10:131-137.
- Dolev JC, O’Sullivan P, Berger T. The eDerm online curriculum: a randomized study of effective skin cancer teaching to medical students. J Am Acad Dermatol. 2011;65:e165-e171.
- Amir M, Sampson BP, Endly D, et al. Social networking sites: emerging and essential tools for communication in dermatology. JAMA Dermatol. 2014;150:56-60.
- Ruiz JG, Mintzer MJ, Leipzig RM. The impact of e-learning in medical education. Acad Med. 2006;81:207-212.
- Hanson AH, Krause LK, Simmons RN, et al. Dermatology education and the internet: traditional and cutting-edge resources. J Am Acad Dermatol. 2011;65:836-842.
- Rowe SP, Siddiqui A, Bonekamp D. The key image and case log application: new radiology software for teaching file creation and case logging that incorporates elements of a social network. Acad Radiol. 2014;21:916-930.
- Bell RH. Surgical council on resident education: a new organization devoted to graduate surgical education. J Am Coll Surg. 2007;204:341-346.
- Kirton OC, Reilly P, Staff I, et al. Development and implementation of an interactive, objective, and simulation-based curriculum for general surgery residents. J Surg Educ. 2012;69:718-723.
- Prakash S, Verma S, McGowan J, et al. Improving the quality of colonoscopy bowel preparation using an educational video. Can J Gastroenterol. 2013;27:696-700.
- Carroll BT. eTools for teaching dermatologic surgery. Paper presented at the Association of Professors of Dermatology 2014 Annual Meeting; September 12-13, 2014; Chicago, IL.
Practice Points
- Educational collaboration between residency programs via social media can result in more well-rounded dermatologists, which will enhance patient care.
- Social media can connect dermatologists nationwide to improve patient care via collaboration.
Collagenous and Elastotic Marginal Plaques of the Hands
To the Editor:
Collagenous and elastotic marginal plaques of the hands (CEMPHs) has several names including degenerative collagenous plaques of the hands, keratoelastoidosis marginalis, and digital papular calcific elastosis. This rare disorder is an acquired, slowly progressive, asymptomatic, dermal connective tissue abnormality that is underrecognized and underdiagnosed. Clinical presentation includes hyperkeratotic translucent papules arranged linearly on the radial aspect of the hands.
A 74-year-old woman described having "rough hands" of more than 20 years' duration. She presented with 4-cm wide longitudinal, erythematous, firm, depressed plaques along the lateral edge of the second finger and extending to the medial thumb in both hands (Figure 1). She had attempted multiple treatments by her primary care physician, including topical and oral medications unknown to the patient and light therapy, all without benefit over a period of several years. We have attempted salicylic acid 40%, clobetasol cream 0.05%, and emollient creams containing α-hydroxy acid. At best the condition fluctuated between a subtle raised scale at the edge to smooth and occasionally more red-pink, seemingly unrelated to any treatments.
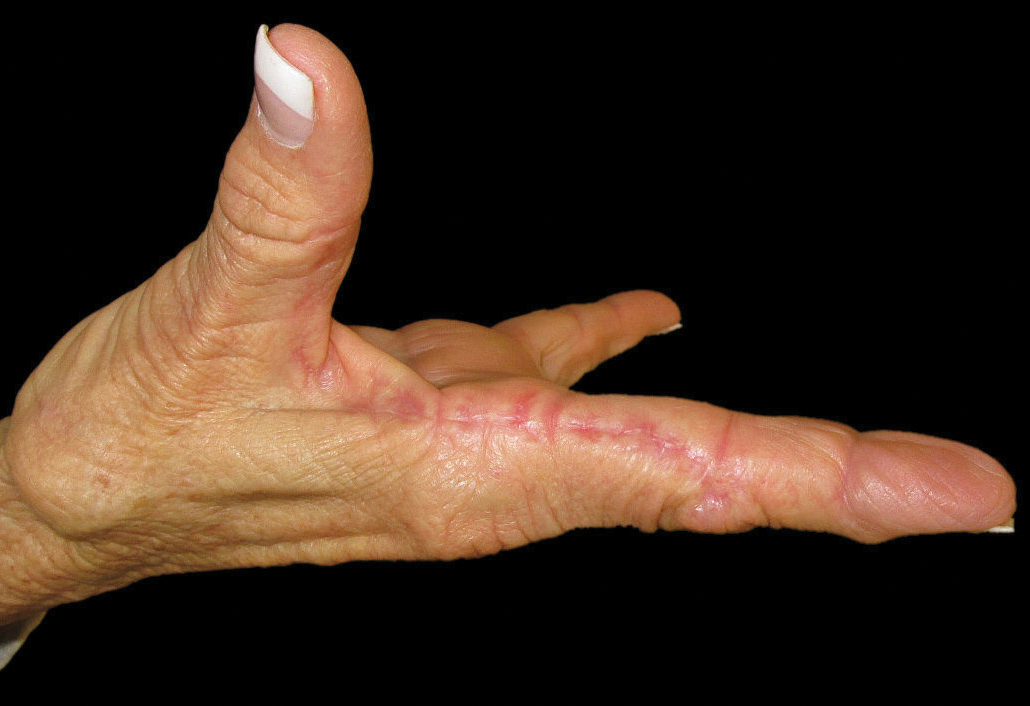
The patient did not have plaques elsewhere on the body, and notably, the feet were clear. She did not have a history of repeated trauma to the hands and did not engage in manual labor. She denied excessive sun exposure, though she had Fitzpatrick skin type III and a history of multiple precancers and nonmelanoma skin cancers 7 years prior to presentation.
Histology of CEMPH reveals a hyperkeratotic epidermis with an avascular and acellular replacement of the superficial reticular dermis by haphazardly arranged, thickened collagen fibers (Figure 2A-2C). Collagen fibers were oriented perpendicularly to the epidermal surface. Intervening amorphous basophilic elastotic masses were present in the upper dermis with occasional calcification and degenerative elastic fibers (Figure 2D).

Collagenous and elastotic marginal plaques of the hands is a chronic, asymptomatic, sclerotic skin disorder described in a 1960 case series of 5 patients reported by Burks et al.1 Although it has many names, the most common is CEMPH. Collagenous and elastotic marginal plaques of the hands most often presents in white men aged 50 to 60 years.2 Patients typically are asymptomatic with plaques limited to the junction of the palmar and dorsal surfaces of the hands with only minimal intermittent stiffness around the flexor creases. Lesions begin as discrete yellow papules that coalesce to form hyperkeratotic linear plaques with occasional telangiectasia.3
The etiology of CEMPH is attributed to collagen and elastin degeneration by chronic actinic damage, pressure, or trauma.4,5 The 3 stages of degeneration include an initial linear padded stage, an intermediate padded plaque stage, and an advanced padded hyperkeratotic plaque stage.4 Vascular compromise is seen from the enlarged and fused thickened collagen and elastic fibers that in turn lead to ischemic changes, hyperkeratosis with epidermal atrophy, and papillary dermis telangiectasia. Absence or weak expression of keratins 14 and 10 and strong expression of keratin 16 have been reported in the epidermis of CEMPH patients.4
Collagenous and elastotic marginal plaques of the hands do not have a specific treatment, as it is a benign, slowly progressive condition. Several treatments such as laser therapy, high-potency topical corticosteroids, topical tazarotene and tretinoin, oral isotretinoin, and cryotherapy have been tried with little long-term success.4 Moisturizing may help reduce fissuring, and patients are advised to avoid the sun and repeated trauma to the hands.
The differential diagnosis of CEMPH is summarized in the Table. Two genodermatoses—acrokeratoelastoidosis of Costa and focal acral hyperkeratosis—clinically resemble CEMPH. Acrokeratoelastoidosis of Costa is an autosomal-dominant condition that occurs without trauma in children and young adults. Histopathology shows orthokeratotic hyperkeratosis due to an overproduction of filaggrin in the granular layer of the epidermis. The reticular dermis shows basophilic, thick, curled and fragmented elastic fibers with dilated capillaries that can be seen with Weigert elastic, Verhoeff-van Gieson, or orcein stains. Focal acral hyperkeratosis occurs on the hands and feet, predominantly in black patients. On histology, the epidermis shows a characteristic orthohyperkeratosis, moderate acanthosis, and slight hypergranulosis with no dermal involvment.6
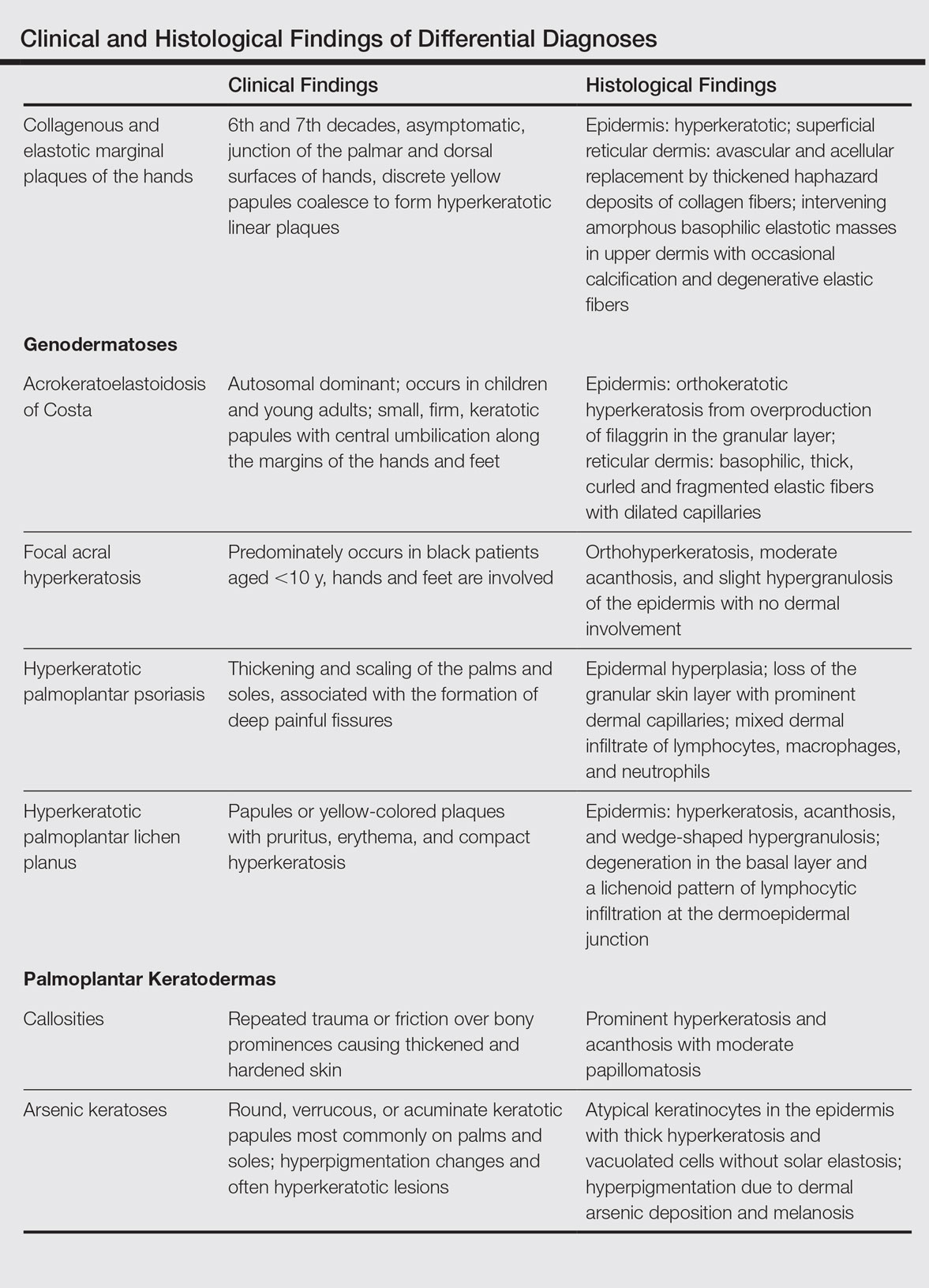
Chronic hyperkeratotic eczematous dermatitis is another common entity in the differential characterized by hyperkeratotic plaques that scale and fissure. Biopsy demonstrates a spongiotic acanthotic epidermis.7,8
Psoriasis of the hands, specifically hyperkeratotic palmoplantar psoriasis, is associated with manual labor, similar to CEMPH. Histology shows epidermal hyperplasia; regular acanthosis; loss of the granular skin layer with prominent dermal capillaries; and a mixed dermal infiltrate of lymphocytes, macrophages, and neutrophils.9 Hyperkeratotic palmoplantar lichen planus presents with pruritic papules in the third and fifth decades of life. Histologically, hyperkeratosis, acanthosis, and wedge-shaped hypergranulosis with a lichenoid lymphocytic infiltration at the dermoepidermal junction is seen.10
Palmoplantar keratodermas due to inflammatory reactive dermatoses include callosities that develop in response to repeated trauma or friction on the skin. On histology, there is prominent hyperkeratosis and acanthosis with moderate papillomatosis.11 Drug-related palmoplantar keratodermas such as those from arsenic exposure can lead to multiple, irregular, verrucous, keratotic, and pigmented lesions on the palms and soles. Histologically, atypical keratinocytes are seen in the epidermis with thick hyperkeratosis and vacuolated cells without solar elastosis.12
In conclusion, CEMPH is an underdiagnosed and underrecognized condition characterized by asymptomatic hyperkeratotic linear plaques along the medial aspect of the thumb and radial aspect of the index finger. It is important to keep CEMPH in mind when dealing with occupational cases of repeated long-term trauma or pressure to the hands as well as excessive sun exposure. It also is imperative to separate it from other diseases and avoid misdiagnosing this degenerative collagenous and elastotic disease as a malignant lesion.
- Burks JW, Wise LJ, Clark WH. Degenerative collagenous plaques of the hands. Arch Dermatol. 1960;82:362-366.
- Jordaan HF, Rossouw DJ. Digital papular calcific elastosis: a histopathological, histochemical and ultrastructural study of 20 patients. J Cutan Pathol. 1990;17:358-370.
- Mortimore RJ, Conrad RJ. Collagenous and elastotic marginal plaques of the hands. Australas J Dermatol. 2001;42:211-213.
- Tieu KD, Satter EK. Thickened plaques on the hands. Collagenous and elastotic marginal plaques of the hands (CEMPH). Arch Dermatol. 2011;147:499-504.
- Todd D, Al-Aboosi M, Hameed O, et al. The role of UV light in the pathogenesis of digital papular calcific elastosis. Arch Dermatol. 2001;137:379-381.
- Mengesha YM, Kayal JD, Swerlick RA. Keratoelastoidosis marginalis. J Cutan Med Surg. 2002;6:23-25.
- MacKee MG, Lewis MG. Keratolysis exfoliativa and the mosaic fungus. Arch Dermatol. 1931;23:445-447.
- Walling HW, Swick BL, Storrs FJ, et al. Frictional hyperkeratotic hand dermatitis responding to Grenz ray therapy. Contact Dermatitis. 2008;58:49-51.
- Farley E, Masrour S, McKey J, et al. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024-1031.
- Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
- Unal VS, Sevin A, Dayican A. Palmar callus formation as a result of mechanical trauma during sailing. Plast Reconstr Surg. 2005;115:2161-2162.
- Cöl M, Cöl C, Soran A, et al. Arsenic-related Bowen's disease, palmar keratosis, and skin cancer. Environ Health Perspect. 1999;107:687-689.
To the Editor:
Collagenous and elastotic marginal plaques of the hands (CEMPHs) has several names including degenerative collagenous plaques of the hands, keratoelastoidosis marginalis, and digital papular calcific elastosis. This rare disorder is an acquired, slowly progressive, asymptomatic, dermal connective tissue abnormality that is underrecognized and underdiagnosed. Clinical presentation includes hyperkeratotic translucent papules arranged linearly on the radial aspect of the hands.
A 74-year-old woman described having "rough hands" of more than 20 years' duration. She presented with 4-cm wide longitudinal, erythematous, firm, depressed plaques along the lateral edge of the second finger and extending to the medial thumb in both hands (Figure 1). She had attempted multiple treatments by her primary care physician, including topical and oral medications unknown to the patient and light therapy, all without benefit over a period of several years. We have attempted salicylic acid 40%, clobetasol cream 0.05%, and emollient creams containing α-hydroxy acid. At best the condition fluctuated between a subtle raised scale at the edge to smooth and occasionally more red-pink, seemingly unrelated to any treatments.

The patient did not have plaques elsewhere on the body, and notably, the feet were clear. She did not have a history of repeated trauma to the hands and did not engage in manual labor. She denied excessive sun exposure, though she had Fitzpatrick skin type III and a history of multiple precancers and nonmelanoma skin cancers 7 years prior to presentation.
Histology of CEMPH reveals a hyperkeratotic epidermis with an avascular and acellular replacement of the superficial reticular dermis by haphazardly arranged, thickened collagen fibers (Figure 2A-2C). Collagen fibers were oriented perpendicularly to the epidermal surface. Intervening amorphous basophilic elastotic masses were present in the upper dermis with occasional calcification and degenerative elastic fibers (Figure 2D).

Collagenous and elastotic marginal plaques of the hands is a chronic, asymptomatic, sclerotic skin disorder described in a 1960 case series of 5 patients reported by Burks et al.1 Although it has many names, the most common is CEMPH. Collagenous and elastotic marginal plaques of the hands most often presents in white men aged 50 to 60 years.2 Patients typically are asymptomatic with plaques limited to the junction of the palmar and dorsal surfaces of the hands with only minimal intermittent stiffness around the flexor creases. Lesions begin as discrete yellow papules that coalesce to form hyperkeratotic linear plaques with occasional telangiectasia.3
The etiology of CEMPH is attributed to collagen and elastin degeneration by chronic actinic damage, pressure, or trauma.4,5 The 3 stages of degeneration include an initial linear padded stage, an intermediate padded plaque stage, and an advanced padded hyperkeratotic plaque stage.4 Vascular compromise is seen from the enlarged and fused thickened collagen and elastic fibers that in turn lead to ischemic changes, hyperkeratosis with epidermal atrophy, and papillary dermis telangiectasia. Absence or weak expression of keratins 14 and 10 and strong expression of keratin 16 have been reported in the epidermis of CEMPH patients.4
Collagenous and elastotic marginal plaques of the hands do not have a specific treatment, as it is a benign, slowly progressive condition. Several treatments such as laser therapy, high-potency topical corticosteroids, topical tazarotene and tretinoin, oral isotretinoin, and cryotherapy have been tried with little long-term success.4 Moisturizing may help reduce fissuring, and patients are advised to avoid the sun and repeated trauma to the hands.
The differential diagnosis of CEMPH is summarized in the Table. Two genodermatoses—acrokeratoelastoidosis of Costa and focal acral hyperkeratosis—clinically resemble CEMPH. Acrokeratoelastoidosis of Costa is an autosomal-dominant condition that occurs without trauma in children and young adults. Histopathology shows orthokeratotic hyperkeratosis due to an overproduction of filaggrin in the granular layer of the epidermis. The reticular dermis shows basophilic, thick, curled and fragmented elastic fibers with dilated capillaries that can be seen with Weigert elastic, Verhoeff-van Gieson, or orcein stains. Focal acral hyperkeratosis occurs on the hands and feet, predominantly in black patients. On histology, the epidermis shows a characteristic orthohyperkeratosis, moderate acanthosis, and slight hypergranulosis with no dermal involvment.6

Chronic hyperkeratotic eczematous dermatitis is another common entity in the differential characterized by hyperkeratotic plaques that scale and fissure. Biopsy demonstrates a spongiotic acanthotic epidermis.7,8
Psoriasis of the hands, specifically hyperkeratotic palmoplantar psoriasis, is associated with manual labor, similar to CEMPH. Histology shows epidermal hyperplasia; regular acanthosis; loss of the granular skin layer with prominent dermal capillaries; and a mixed dermal infiltrate of lymphocytes, macrophages, and neutrophils.9 Hyperkeratotic palmoplantar lichen planus presents with pruritic papules in the third and fifth decades of life. Histologically, hyperkeratosis, acanthosis, and wedge-shaped hypergranulosis with a lichenoid lymphocytic infiltration at the dermoepidermal junction is seen.10
Palmoplantar keratodermas due to inflammatory reactive dermatoses include callosities that develop in response to repeated trauma or friction on the skin. On histology, there is prominent hyperkeratosis and acanthosis with moderate papillomatosis.11 Drug-related palmoplantar keratodermas such as those from arsenic exposure can lead to multiple, irregular, verrucous, keratotic, and pigmented lesions on the palms and soles. Histologically, atypical keratinocytes are seen in the epidermis with thick hyperkeratosis and vacuolated cells without solar elastosis.12
In conclusion, CEMPH is an underdiagnosed and underrecognized condition characterized by asymptomatic hyperkeratotic linear plaques along the medial aspect of the thumb and radial aspect of the index finger. It is important to keep CEMPH in mind when dealing with occupational cases of repeated long-term trauma or pressure to the hands as well as excessive sun exposure. It also is imperative to separate it from other diseases and avoid misdiagnosing this degenerative collagenous and elastotic disease as a malignant lesion.
To the Editor:
Collagenous and elastotic marginal plaques of the hands (CEMPHs) has several names including degenerative collagenous plaques of the hands, keratoelastoidosis marginalis, and digital papular calcific elastosis. This rare disorder is an acquired, slowly progressive, asymptomatic, dermal connective tissue abnormality that is underrecognized and underdiagnosed. Clinical presentation includes hyperkeratotic translucent papules arranged linearly on the radial aspect of the hands.
A 74-year-old woman described having "rough hands" of more than 20 years' duration. She presented with 4-cm wide longitudinal, erythematous, firm, depressed plaques along the lateral edge of the second finger and extending to the medial thumb in both hands (Figure 1). She had attempted multiple treatments by her primary care physician, including topical and oral medications unknown to the patient and light therapy, all without benefit over a period of several years. We have attempted salicylic acid 40%, clobetasol cream 0.05%, and emollient creams containing α-hydroxy acid. At best the condition fluctuated between a subtle raised scale at the edge to smooth and occasionally more red-pink, seemingly unrelated to any treatments.

The patient did not have plaques elsewhere on the body, and notably, the feet were clear. She did not have a history of repeated trauma to the hands and did not engage in manual labor. She denied excessive sun exposure, though she had Fitzpatrick skin type III and a history of multiple precancers and nonmelanoma skin cancers 7 years prior to presentation.
Histology of CEMPH reveals a hyperkeratotic epidermis with an avascular and acellular replacement of the superficial reticular dermis by haphazardly arranged, thickened collagen fibers (Figure 2A-2C). Collagen fibers were oriented perpendicularly to the epidermal surface. Intervening amorphous basophilic elastotic masses were present in the upper dermis with occasional calcification and degenerative elastic fibers (Figure 2D).

Collagenous and elastotic marginal plaques of the hands is a chronic, asymptomatic, sclerotic skin disorder described in a 1960 case series of 5 patients reported by Burks et al.1 Although it has many names, the most common is CEMPH. Collagenous and elastotic marginal plaques of the hands most often presents in white men aged 50 to 60 years.2 Patients typically are asymptomatic with plaques limited to the junction of the palmar and dorsal surfaces of the hands with only minimal intermittent stiffness around the flexor creases. Lesions begin as discrete yellow papules that coalesce to form hyperkeratotic linear plaques with occasional telangiectasia.3
The etiology of CEMPH is attributed to collagen and elastin degeneration by chronic actinic damage, pressure, or trauma.4,5 The 3 stages of degeneration include an initial linear padded stage, an intermediate padded plaque stage, and an advanced padded hyperkeratotic plaque stage.4 Vascular compromise is seen from the enlarged and fused thickened collagen and elastic fibers that in turn lead to ischemic changes, hyperkeratosis with epidermal atrophy, and papillary dermis telangiectasia. Absence or weak expression of keratins 14 and 10 and strong expression of keratin 16 have been reported in the epidermis of CEMPH patients.4
Collagenous and elastotic marginal plaques of the hands do not have a specific treatment, as it is a benign, slowly progressive condition. Several treatments such as laser therapy, high-potency topical corticosteroids, topical tazarotene and tretinoin, oral isotretinoin, and cryotherapy have been tried with little long-term success.4 Moisturizing may help reduce fissuring, and patients are advised to avoid the sun and repeated trauma to the hands.
The differential diagnosis of CEMPH is summarized in the Table. Two genodermatoses—acrokeratoelastoidosis of Costa and focal acral hyperkeratosis—clinically resemble CEMPH. Acrokeratoelastoidosis of Costa is an autosomal-dominant condition that occurs without trauma in children and young adults. Histopathology shows orthokeratotic hyperkeratosis due to an overproduction of filaggrin in the granular layer of the epidermis. The reticular dermis shows basophilic, thick, curled and fragmented elastic fibers with dilated capillaries that can be seen with Weigert elastic, Verhoeff-van Gieson, or orcein stains. Focal acral hyperkeratosis occurs on the hands and feet, predominantly in black patients. On histology, the epidermis shows a characteristic orthohyperkeratosis, moderate acanthosis, and slight hypergranulosis with no dermal involvment.6

Chronic hyperkeratotic eczematous dermatitis is another common entity in the differential characterized by hyperkeratotic plaques that scale and fissure. Biopsy demonstrates a spongiotic acanthotic epidermis.7,8
Psoriasis of the hands, specifically hyperkeratotic palmoplantar psoriasis, is associated with manual labor, similar to CEMPH. Histology shows epidermal hyperplasia; regular acanthosis; loss of the granular skin layer with prominent dermal capillaries; and a mixed dermal infiltrate of lymphocytes, macrophages, and neutrophils.9 Hyperkeratotic palmoplantar lichen planus presents with pruritic papules in the third and fifth decades of life. Histologically, hyperkeratosis, acanthosis, and wedge-shaped hypergranulosis with a lichenoid lymphocytic infiltration at the dermoepidermal junction is seen.10
Palmoplantar keratodermas due to inflammatory reactive dermatoses include callosities that develop in response to repeated trauma or friction on the skin. On histology, there is prominent hyperkeratosis and acanthosis with moderate papillomatosis.11 Drug-related palmoplantar keratodermas such as those from arsenic exposure can lead to multiple, irregular, verrucous, keratotic, and pigmented lesions on the palms and soles. Histologically, atypical keratinocytes are seen in the epidermis with thick hyperkeratosis and vacuolated cells without solar elastosis.12
In conclusion, CEMPH is an underdiagnosed and underrecognized condition characterized by asymptomatic hyperkeratotic linear plaques along the medial aspect of the thumb and radial aspect of the index finger. It is important to keep CEMPH in mind when dealing with occupational cases of repeated long-term trauma or pressure to the hands as well as excessive sun exposure. It also is imperative to separate it from other diseases and avoid misdiagnosing this degenerative collagenous and elastotic disease as a malignant lesion.
- Burks JW, Wise LJ, Clark WH. Degenerative collagenous plaques of the hands. Arch Dermatol. 1960;82:362-366.
- Jordaan HF, Rossouw DJ. Digital papular calcific elastosis: a histopathological, histochemical and ultrastructural study of 20 patients. J Cutan Pathol. 1990;17:358-370.
- Mortimore RJ, Conrad RJ. Collagenous and elastotic marginal plaques of the hands. Australas J Dermatol. 2001;42:211-213.
- Tieu KD, Satter EK. Thickened plaques on the hands. Collagenous and elastotic marginal plaques of the hands (CEMPH). Arch Dermatol. 2011;147:499-504.
- Todd D, Al-Aboosi M, Hameed O, et al. The role of UV light in the pathogenesis of digital papular calcific elastosis. Arch Dermatol. 2001;137:379-381.
- Mengesha YM, Kayal JD, Swerlick RA. Keratoelastoidosis marginalis. J Cutan Med Surg. 2002;6:23-25.
- MacKee MG, Lewis MG. Keratolysis exfoliativa and the mosaic fungus. Arch Dermatol. 1931;23:445-447.
- Walling HW, Swick BL, Storrs FJ, et al. Frictional hyperkeratotic hand dermatitis responding to Grenz ray therapy. Contact Dermatitis. 2008;58:49-51.
- Farley E, Masrour S, McKey J, et al. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024-1031.
- Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
- Unal VS, Sevin A, Dayican A. Palmar callus formation as a result of mechanical trauma during sailing. Plast Reconstr Surg. 2005;115:2161-2162.
- Cöl M, Cöl C, Soran A, et al. Arsenic-related Bowen's disease, palmar keratosis, and skin cancer. Environ Health Perspect. 1999;107:687-689.
- Burks JW, Wise LJ, Clark WH. Degenerative collagenous plaques of the hands. Arch Dermatol. 1960;82:362-366.
- Jordaan HF, Rossouw DJ. Digital papular calcific elastosis: a histopathological, histochemical and ultrastructural study of 20 patients. J Cutan Pathol. 1990;17:358-370.
- Mortimore RJ, Conrad RJ. Collagenous and elastotic marginal plaques of the hands. Australas J Dermatol. 2001;42:211-213.
- Tieu KD, Satter EK. Thickened plaques on the hands. Collagenous and elastotic marginal plaques of the hands (CEMPH). Arch Dermatol. 2011;147:499-504.
- Todd D, Al-Aboosi M, Hameed O, et al. The role of UV light in the pathogenesis of digital papular calcific elastosis. Arch Dermatol. 2001;137:379-381.
- Mengesha YM, Kayal JD, Swerlick RA. Keratoelastoidosis marginalis. J Cutan Med Surg. 2002;6:23-25.
- MacKee MG, Lewis MG. Keratolysis exfoliativa and the mosaic fungus. Arch Dermatol. 1931;23:445-447.
- Walling HW, Swick BL, Storrs FJ, et al. Frictional hyperkeratotic hand dermatitis responding to Grenz ray therapy. Contact Dermatitis. 2008;58:49-51.
- Farley E, Masrour S, McKey J, et al. Palmoplantar psoriasis: a phenotypical and clinical review with introduction of a new quality-of-life assessment tool. J Am Acad Dermatol. 2009;60:1024-1031.
- Rotunda AM, Craft N, Haley JC. Hyperkeratotic plaques on the palms and soles. palmoplantar lichen planus, hyperkeratotic variant. Arch Dermatol. 2004;140:1275-1280.
- Unal VS, Sevin A, Dayican A. Palmar callus formation as a result of mechanical trauma during sailing. Plast Reconstr Surg. 2005;115:2161-2162.
- Cöl M, Cöl C, Soran A, et al. Arsenic-related Bowen's disease, palmar keratosis, and skin cancer. Environ Health Perspect. 1999;107:687-689.
Practice Points
- The etiology of collagenous and elastotic marginal plaques of the hands (CEMPHs) is attributed to collagen and elastin degeneration by chronic actinic damage, pressure, or trauma.
- It is important to keep CEMPH in mind when dealing with occupational cases of repeated long-term trauma or pressure to the hands as well as excessive sun exposure. It should be separated from other diseases and avoid being misdiagnosed as a malignant lesion.
Successful Treatment of Ota Nevus With the 532-nm Solid-State Picosecond Laser
Ota nevus is a dermal melanocytosis that is typically characterized by blue, gray, or brown pigmented patches in the periorbital region.1 The condition has a prevalence of 0.04% in a Philadelphia study of 6915 patients and is most notable in patients with skin of color, affecting up to 0.6% of Asians,2 0.038% of white individuals, and 0.014% of black individuals.3,4 The appearance of an Ota nevus often imparts a negative psychosocial impact on the patient, prompting requests for treatment and/or removal.5 Laser treatment of Ota nevi must be carefully implemented, especially in Fitzpatrick skin types IV through VI. Although 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,5,6 typically only moderate improvement is seen; further treatment at higher fluences will only increase the risk for dyspigmentation and scarring.6
We report a case of successful treatment of an Ota nevus following 2 treatment sessions with the 532-nm solid-state picosecond laser, which is a novel application in patients with skin of color (Fitzpatrick skin types IV-VI). The Q-switched nanosecond laser has been shown to be moderately effective at treating Ota nevi.6
Case Report
An 18-year-old woman with Fitzpatrick skin type IV presented for cosmetic removal of an 8×5-cm dark brown-blue patch on the right temple and malar and buccal cheek present since birth that had failed to respond to an unknown laser treatment that was administered outside of the United States (Figure, A). To ascertain the diagnosis, a biopsy was performed, showing histology consistent with Ota nevus. Initially, the 755-nm Q-switched nanosecond laser was recommended for treatment. Over the course of 7 months (1 treatment session per month [Table]), the patient saw improvement but not to the desired extent. The patient then underwent 2 treatments at 4-week intervals with the 1064-nm solid-state picosecond and nanosecond lasers; however, no improvement was seen following these 2 sessions (Table).
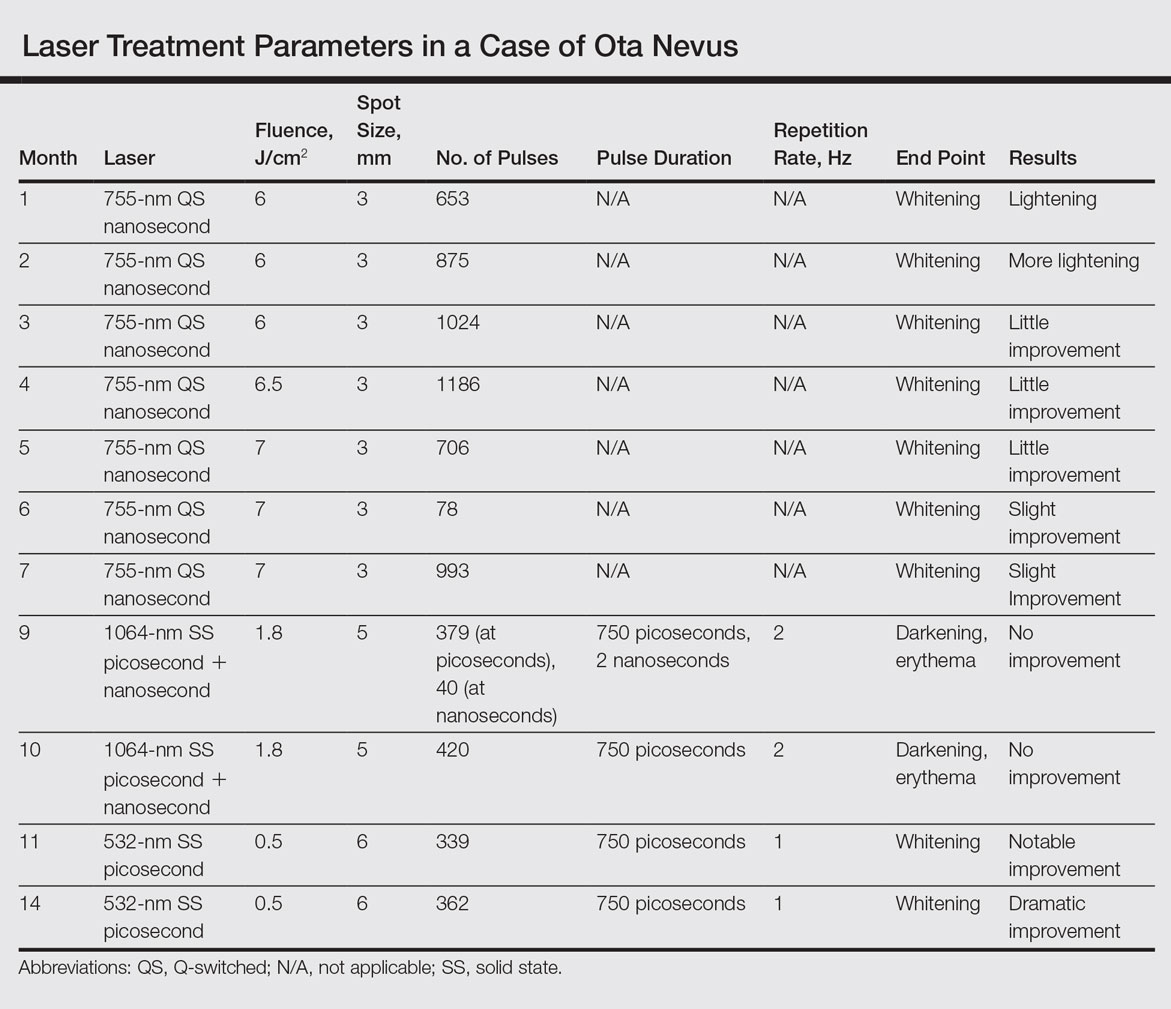
The next month the patient received treatment with a novel 532-nm solid-state picosecond laser using the following parameters: fluence, 0.5 J/cm2; spot size, 6 mm; repetition rate, 1 Hz; pulse duration, 750 picoseconds; 339 pulses. The end point was whitening. A remarkable clinical response was demonstrated 6 weeks later (Figure, B). A second treatment with the 532-nm solid-state picosecond laser was then performed at 14 months. On a return visit 2 months after the second treatment, the patient showed dramatic improvement, almost to the degree of complete resolution (Figure, C).
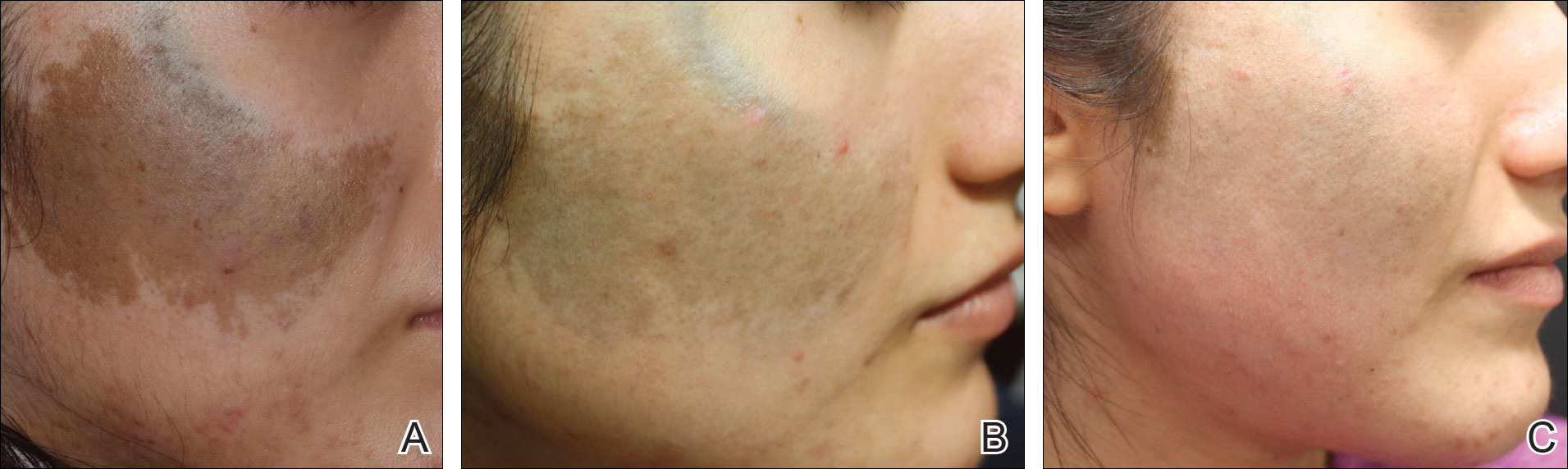
Comment
Pigmentation disorders are more common in patients with skin of color, and those affected may experience psychological effects secondary to these dermatoses, prompting requests for treatment and/or removal.7 Although the 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,3 the challenge remains for patients with skin of color, as these lasers work through photothermolysis, which generates heat and may cause thermal damage by targeting melanin. Because more melanin is present in skin of color patients, the threshold for too much heat is lower and these patients are at a higher risk for adverse events such as scarring and hyperpigmentation.6,8
By delivering energy in shorter pulses, the novel 532-nm solid-state picosecond laser shows greater fragmentation of melanosomes into melanin particles that are eventually phagocytosed.8 In our patient, dramatic improvement was noted after only 2 treatments, as evidenced by other picosecond treatments on Ota nevi,6,8 suggesting that fewer treatments are necessary when using the 532-nm solid-state picosecond laser for Ota nevi.
Although the 532-nm solid-state picosecond laser was cleared by the US Food and Drug Administration for tattoo removal, this laser shows potential use in other pigmentary disorders, particularly in patients with skin of color, as demonstrated in our case. With continued understanding through further studies, this picosecond laser with a shorter pulse duration may prove to be a safer and more effective alternative to the Q-switched nanosecond laser.
Conclusion
As shown in our case, the 532-nm solid-state picosecond laser appears to be a safe and effective modality for treating Ota nevi. This case demonstrates the potential utility of this laser in patients desiring more complete clearing, as it removes pigment more rapidly with lower risk for serious adverse effects. The 9th Cosmetic Surgery Forum will be held November 29-December 2, 2017, in Las Vegas, Nevada. Get more information at www.cosmeticsurgeryforum.com.
- Kim JY, Lee HG, Kim MJ, et al. The efficacy and safety of episcleral pigmentation removal from pig eyes: using a 532-nm quality-switched Nd: YAG laser. Cornea. 2012;31:1449-1454.
- Watanabe S, Takahashi H. Treatment of nevus of Ota with the Q-switched ruby laser. N Engl J Med. 1994;331:1745-1750.
- Yates B, Que SK, D'Souza L, et al. Laser treatment of periocular skin conditions. Clin Dermatol. 2015;33:197-206.
- Gonder JR, Ezell PC, Shields JA, et al. Ocular melanocytosis. a study to determine the prevalence rate of ocular melanocytosis. Ophthalmology. 1982;89:950-952.
- Chesnut C, Diehl J, Lask G. Treatment of nevus of Ota with a picosecond 755-nm alexandrite laser. Dermatol Surg. 2015;41:508-510.
- Moreno-Arias GA, Camps-Fresneda A. Treatment of nevus of Ota with the Q-switched alexandrite laser. Lasers Surg Med. 2001;28:451-455.
- Manuskiatti W, Eimpunth S, Wanitphakdeedecha R. Effect of cold air cooling on the incidence of postinflammatory hyperpigmentation after Q-switched Nd:YAG laser treatment of acquired bilateral nevus of Ota like macules. Arch Dermatol. 2007;143:1139-1143.
- Levin MK, Ng E, Bae YS, et al. Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: a retrospective photographic review. Lasers Surg Med. 2016;48:181-187.
Ota nevus is a dermal melanocytosis that is typically characterized by blue, gray, or brown pigmented patches in the periorbital region.1 The condition has a prevalence of 0.04% in a Philadelphia study of 6915 patients and is most notable in patients with skin of color, affecting up to 0.6% of Asians,2 0.038% of white individuals, and 0.014% of black individuals.3,4 The appearance of an Ota nevus often imparts a negative psychosocial impact on the patient, prompting requests for treatment and/or removal.5 Laser treatment of Ota nevi must be carefully implemented, especially in Fitzpatrick skin types IV through VI. Although 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,5,6 typically only moderate improvement is seen; further treatment at higher fluences will only increase the risk for dyspigmentation and scarring.6
We report a case of successful treatment of an Ota nevus following 2 treatment sessions with the 532-nm solid-state picosecond laser, which is a novel application in patients with skin of color (Fitzpatrick skin types IV-VI). The Q-switched nanosecond laser has been shown to be moderately effective at treating Ota nevi.6
Case Report
An 18-year-old woman with Fitzpatrick skin type IV presented for cosmetic removal of an 8×5-cm dark brown-blue patch on the right temple and malar and buccal cheek present since birth that had failed to respond to an unknown laser treatment that was administered outside of the United States (Figure, A). To ascertain the diagnosis, a biopsy was performed, showing histology consistent with Ota nevus. Initially, the 755-nm Q-switched nanosecond laser was recommended for treatment. Over the course of 7 months (1 treatment session per month [Table]), the patient saw improvement but not to the desired extent. The patient then underwent 2 treatments at 4-week intervals with the 1064-nm solid-state picosecond and nanosecond lasers; however, no improvement was seen following these 2 sessions (Table).

The next month the patient received treatment with a novel 532-nm solid-state picosecond laser using the following parameters: fluence, 0.5 J/cm2; spot size, 6 mm; repetition rate, 1 Hz; pulse duration, 750 picoseconds; 339 pulses. The end point was whitening. A remarkable clinical response was demonstrated 6 weeks later (Figure, B). A second treatment with the 532-nm solid-state picosecond laser was then performed at 14 months. On a return visit 2 months after the second treatment, the patient showed dramatic improvement, almost to the degree of complete resolution (Figure, C).

Comment
Pigmentation disorders are more common in patients with skin of color, and those affected may experience psychological effects secondary to these dermatoses, prompting requests for treatment and/or removal.7 Although the 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,3 the challenge remains for patients with skin of color, as these lasers work through photothermolysis, which generates heat and may cause thermal damage by targeting melanin. Because more melanin is present in skin of color patients, the threshold for too much heat is lower and these patients are at a higher risk for adverse events such as scarring and hyperpigmentation.6,8
By delivering energy in shorter pulses, the novel 532-nm solid-state picosecond laser shows greater fragmentation of melanosomes into melanin particles that are eventually phagocytosed.8 In our patient, dramatic improvement was noted after only 2 treatments, as evidenced by other picosecond treatments on Ota nevi,6,8 suggesting that fewer treatments are necessary when using the 532-nm solid-state picosecond laser for Ota nevi.
Although the 532-nm solid-state picosecond laser was cleared by the US Food and Drug Administration for tattoo removal, this laser shows potential use in other pigmentary disorders, particularly in patients with skin of color, as demonstrated in our case. With continued understanding through further studies, this picosecond laser with a shorter pulse duration may prove to be a safer and more effective alternative to the Q-switched nanosecond laser.
Conclusion
As shown in our case, the 532-nm solid-state picosecond laser appears to be a safe and effective modality for treating Ota nevi. This case demonstrates the potential utility of this laser in patients desiring more complete clearing, as it removes pigment more rapidly with lower risk for serious adverse effects. The 9th Cosmetic Surgery Forum will be held November 29-December 2, 2017, in Las Vegas, Nevada. Get more information at www.cosmeticsurgeryforum.com.
Ota nevus is a dermal melanocytosis that is typically characterized by blue, gray, or brown pigmented patches in the periorbital region.1 The condition has a prevalence of 0.04% in a Philadelphia study of 6915 patients and is most notable in patients with skin of color, affecting up to 0.6% of Asians,2 0.038% of white individuals, and 0.014% of black individuals.3,4 The appearance of an Ota nevus often imparts a negative psychosocial impact on the patient, prompting requests for treatment and/or removal.5 Laser treatment of Ota nevi must be carefully implemented, especially in Fitzpatrick skin types IV through VI. Although 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,5,6 typically only moderate improvement is seen; further treatment at higher fluences will only increase the risk for dyspigmentation and scarring.6
We report a case of successful treatment of an Ota nevus following 2 treatment sessions with the 532-nm solid-state picosecond laser, which is a novel application in patients with skin of color (Fitzpatrick skin types IV-VI). The Q-switched nanosecond laser has been shown to be moderately effective at treating Ota nevi.6
Case Report
An 18-year-old woman with Fitzpatrick skin type IV presented for cosmetic removal of an 8×5-cm dark brown-blue patch on the right temple and malar and buccal cheek present since birth that had failed to respond to an unknown laser treatment that was administered outside of the United States (Figure, A). To ascertain the diagnosis, a biopsy was performed, showing histology consistent with Ota nevus. Initially, the 755-nm Q-switched nanosecond laser was recommended for treatment. Over the course of 7 months (1 treatment session per month [Table]), the patient saw improvement but not to the desired extent. The patient then underwent 2 treatments at 4-week intervals with the 1064-nm solid-state picosecond and nanosecond lasers; however, no improvement was seen following these 2 sessions (Table).

The next month the patient received treatment with a novel 532-nm solid-state picosecond laser using the following parameters: fluence, 0.5 J/cm2; spot size, 6 mm; repetition rate, 1 Hz; pulse duration, 750 picoseconds; 339 pulses. The end point was whitening. A remarkable clinical response was demonstrated 6 weeks later (Figure, B). A second treatment with the 532-nm solid-state picosecond laser was then performed at 14 months. On a return visit 2 months after the second treatment, the patient showed dramatic improvement, almost to the degree of complete resolution (Figure, C).

Comment
Pigmentation disorders are more common in patients with skin of color, and those affected may experience psychological effects secondary to these dermatoses, prompting requests for treatment and/or removal.7 Although the 532- and 755-nm Q-switched nanosecond lasers have been used to treat Ota nevi,3 the challenge remains for patients with skin of color, as these lasers work through photothermolysis, which generates heat and may cause thermal damage by targeting melanin. Because more melanin is present in skin of color patients, the threshold for too much heat is lower and these patients are at a higher risk for adverse events such as scarring and hyperpigmentation.6,8
By delivering energy in shorter pulses, the novel 532-nm solid-state picosecond laser shows greater fragmentation of melanosomes into melanin particles that are eventually phagocytosed.8 In our patient, dramatic improvement was noted after only 2 treatments, as evidenced by other picosecond treatments on Ota nevi,6,8 suggesting that fewer treatments are necessary when using the 532-nm solid-state picosecond laser for Ota nevi.
Although the 532-nm solid-state picosecond laser was cleared by the US Food and Drug Administration for tattoo removal, this laser shows potential use in other pigmentary disorders, particularly in patients with skin of color, as demonstrated in our case. With continued understanding through further studies, this picosecond laser with a shorter pulse duration may prove to be a safer and more effective alternative to the Q-switched nanosecond laser.
Conclusion
As shown in our case, the 532-nm solid-state picosecond laser appears to be a safe and effective modality for treating Ota nevi. This case demonstrates the potential utility of this laser in patients desiring more complete clearing, as it removes pigment more rapidly with lower risk for serious adverse effects. The 9th Cosmetic Surgery Forum will be held November 29-December 2, 2017, in Las Vegas, Nevada. Get more information at www.cosmeticsurgeryforum.com.
- Kim JY, Lee HG, Kim MJ, et al. The efficacy and safety of episcleral pigmentation removal from pig eyes: using a 532-nm quality-switched Nd: YAG laser. Cornea. 2012;31:1449-1454.
- Watanabe S, Takahashi H. Treatment of nevus of Ota with the Q-switched ruby laser. N Engl J Med. 1994;331:1745-1750.
- Yates B, Que SK, D'Souza L, et al. Laser treatment of periocular skin conditions. Clin Dermatol. 2015;33:197-206.
- Gonder JR, Ezell PC, Shields JA, et al. Ocular melanocytosis. a study to determine the prevalence rate of ocular melanocytosis. Ophthalmology. 1982;89:950-952.
- Chesnut C, Diehl J, Lask G. Treatment of nevus of Ota with a picosecond 755-nm alexandrite laser. Dermatol Surg. 2015;41:508-510.
- Moreno-Arias GA, Camps-Fresneda A. Treatment of nevus of Ota with the Q-switched alexandrite laser. Lasers Surg Med. 2001;28:451-455.
- Manuskiatti W, Eimpunth S, Wanitphakdeedecha R. Effect of cold air cooling on the incidence of postinflammatory hyperpigmentation after Q-switched Nd:YAG laser treatment of acquired bilateral nevus of Ota like macules. Arch Dermatol. 2007;143:1139-1143.
- Levin MK, Ng E, Bae YS, et al. Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: a retrospective photographic review. Lasers Surg Med. 2016;48:181-187.
- Kim JY, Lee HG, Kim MJ, et al. The efficacy and safety of episcleral pigmentation removal from pig eyes: using a 532-nm quality-switched Nd: YAG laser. Cornea. 2012;31:1449-1454.
- Watanabe S, Takahashi H. Treatment of nevus of Ota with the Q-switched ruby laser. N Engl J Med. 1994;331:1745-1750.
- Yates B, Que SK, D'Souza L, et al. Laser treatment of periocular skin conditions. Clin Dermatol. 2015;33:197-206.
- Gonder JR, Ezell PC, Shields JA, et al. Ocular melanocytosis. a study to determine the prevalence rate of ocular melanocytosis. Ophthalmology. 1982;89:950-952.
- Chesnut C, Diehl J, Lask G. Treatment of nevus of Ota with a picosecond 755-nm alexandrite laser. Dermatol Surg. 2015;41:508-510.
- Moreno-Arias GA, Camps-Fresneda A. Treatment of nevus of Ota with the Q-switched alexandrite laser. Lasers Surg Med. 2001;28:451-455.
- Manuskiatti W, Eimpunth S, Wanitphakdeedecha R. Effect of cold air cooling on the incidence of postinflammatory hyperpigmentation after Q-switched Nd:YAG laser treatment of acquired bilateral nevus of Ota like macules. Arch Dermatol. 2007;143:1139-1143.
- Levin MK, Ng E, Bae YS, et al. Treatment of pigmentary disorders in patients with skin of color with a novel 755 nm picosecond, Q-switched ruby, and Q-switched Nd:YAG nanosecond lasers: a retrospective photographic review. Lasers Surg Med. 2016;48:181-187.
Resident Pearl
The Q-switched 532-nm picosecond laser delivers energy in short pulses, creating fragmentation of melanosomes into melanin particles that eventually become phagocytosed. This process may be safer for patients with Fitzpatrick skin types IV to VI, as it decreases the risk for dyschromia and scarring.
Antioxidants plus sunscreens may be two-punch knockout for melasma
ORLANDO – A two-step regimen of high-potency topical antioxidants followed by a mineral-based sunscreen may help repair light-induced skin damage and protect against new damage in patients with melasma.
Recent studies suggest that the antioxidants tamp down inflammatory cytokines and damage of oxidative stress, Maria Ivonne Arellano-Mendoza, MD, said during a special focus session on Latino skin held at the annual meeting of the American Academy of Dermatology.
“Many compounds are being studied for this purpose,” said Dr. Arellano-Mendoza, head of the dermatology department of the General Hospital of Mexico, Mexico City. “One combination is vitamin C, vitamin E, ubiquinone, and grape-seed extract. This effectively prevented infrared-A radiation–induced matrix metalloproteinase-1 messenger RNA expression in human skin. This combination can be found now in some sunscreens and daily care products.”
Another effective combination seems to be a mixture of ferulic acid, tocopherol, and vitamin C, she said.
The two-step process of regularly using a topical product with antioxidants before applying a sunscreen is all there is for now, she added, because so far it’s been impossible to combine the agents in a single product.
“The challenge will be how to create a product that stays on the surface of the skin to protect it from light, while liberating the antioxidants to penetrate the skin,” she commented.
The antioxidants’ benefits, however, will be obliterated by the continued effects of some light wavelengths that aggravate melasma unless they are used in sequence with a light-scattering sunscreen.
Sunscreens are critical components of a melasma treatment regimen, Dr. Arellano-Mendoza said. “Sunscreens are a cornerstone of treatment. We clearly tell our patients that sunscreens have to be used every day, forever, and if they are not used properly, they will have no improvement.”
Patients with hyperpigmentation disorders are susceptible to longer wavelengths that aren’t covered by chemically derived sunscreens. Longer wavelengths, including infrared light and visible light, have been shown to increase expression of matrix metalloproteinase (MMP) -1 and -9, decrease expression of type 1 procollagen, and can induce macrophage infiltration. These wavelengths also increase reactive oxygen species and proinflammatory cytokines in vitro, Dr. Arellano-Mendoza noted.
Visible light can cause erythema, transient and long-lasting hyperpigmentation, thermal damage, free radical production, and premature photoaging. It also can stimulate the production of reactive oxygen species that can damage DNA.
Mineral-based, inorganic sunscreens, however – like those with titanium dioxide, zinc oxide, and iron oxide – scatter all wavelengths.
“These micronized forms of metal oxides not only scatter and reflect light, they also absorb ultraviolet radiation. The compounds aren’t new,” she said. In 1991, Dr. Elaine Kaye of Harvard University, Boston, and associates described (Arch Dermatol. 1991;127:351-5) opaque physical sunscreens that were useful blockers of visible light and found that transmittance of light can be lowered by adding iron oxide, Dr. Arellano-Mendoza pointed out.
The inorganic sunscreens have never been widely adopted because they are highly pigmented with white or, in the case of iron oxide, with red. “Not many people accepted [the iron-containing compounds] because of the redness, but now different shades are going to be hitting the market soon,” and the hope is that consumers will find them more appealing, she said.
This is good news, as the data emerging around iron oxide are intriguingly positive. A 2015 study showed that a sunscreen with iron oxides prevented melasma relapse during the summer months. Patients were randomized to the same ultraviolet filter topical sunscreen, but for one group, micronized iron oxide was added to it. After 6 months, the median melasma area severity index score was significantly better in the group using the iron oxide compound (J Am Acad Dermatol. 2015 Jan;72[1]:189-90.e1).
Support for the two-step regimen appeared in 2014, when a small study randomized 30 healthy volunteers to an SPF 30 sunscreen or the same sunscreen supplemented with an antioxidant cocktail of grape seed extract, vitamin E, ubiquinone, and vitamin C. The endpoint was MMP-1 upregulation after exposure to infrared-A light. Skin treated with the combination regimen showed significantly lower MMP-1 activation, leading the authors to conclude that the combination of topical antioxidants conferred protection against the irradiation (Photochem Photobiol. 2015 Jan-Feb;91[1]:248-50).
Those same authors published a companion article (Photodermatol Photoimmunol Photomed. 2014;30:167-74) suggesting that another antioxidant mixture (ferulic acid, tocopherol, and vitamin C) was similarly effective.
Using a combination of antioxidants is important, Dr. Arellano-Mendoza said, because different antioxidants work differently. Some (catalase, glutathione peroxidase, and superoxide dismutase) are enzymatic, catalyzing reactions that convert free radicals to oxygen and water. Others terminate free radicals by preventing the propagation of oxidative chain reactions (vitamins A and C, flavonoids, uric acid, bilirubin, albumin, and members of the thiol group). A third group consists of metal-binding proteins that sequester free iron or copper to prevent free radical production (ferritin, transferrin, lactoferrin, and ceruloplasmin).
“I think we can now consider antioxidants a part of the tools we use in treating some pigmentary disorders as melasma,” she said. “We need to choose the compounds carefully, and we definitely need more in vivo research, but the findings are very encouraging.”
Dr. Arellano-Mendoza had no relevant financial disclosures.
This article was updated 3/20/17.
[email protected]
On Twitter @Alz_Gal
ORLANDO – A two-step regimen of high-potency topical antioxidants followed by a mineral-based sunscreen may help repair light-induced skin damage and protect against new damage in patients with melasma.
Recent studies suggest that the antioxidants tamp down inflammatory cytokines and damage of oxidative stress, Maria Ivonne Arellano-Mendoza, MD, said during a special focus session on Latino skin held at the annual meeting of the American Academy of Dermatology.
“Many compounds are being studied for this purpose,” said Dr. Arellano-Mendoza, head of the dermatology department of the General Hospital of Mexico, Mexico City. “One combination is vitamin C, vitamin E, ubiquinone, and grape-seed extract. This effectively prevented infrared-A radiation–induced matrix metalloproteinase-1 messenger RNA expression in human skin. This combination can be found now in some sunscreens and daily care products.”
Another effective combination seems to be a mixture of ferulic acid, tocopherol, and vitamin C, she said.
The two-step process of regularly using a topical product with antioxidants before applying a sunscreen is all there is for now, she added, because so far it’s been impossible to combine the agents in a single product.
“The challenge will be how to create a product that stays on the surface of the skin to protect it from light, while liberating the antioxidants to penetrate the skin,” she commented.
The antioxidants’ benefits, however, will be obliterated by the continued effects of some light wavelengths that aggravate melasma unless they are used in sequence with a light-scattering sunscreen.
Sunscreens are critical components of a melasma treatment regimen, Dr. Arellano-Mendoza said. “Sunscreens are a cornerstone of treatment. We clearly tell our patients that sunscreens have to be used every day, forever, and if they are not used properly, they will have no improvement.”
Patients with hyperpigmentation disorders are susceptible to longer wavelengths that aren’t covered by chemically derived sunscreens. Longer wavelengths, including infrared light and visible light, have been shown to increase expression of matrix metalloproteinase (MMP) -1 and -9, decrease expression of type 1 procollagen, and can induce macrophage infiltration. These wavelengths also increase reactive oxygen species and proinflammatory cytokines in vitro, Dr. Arellano-Mendoza noted.
Visible light can cause erythema, transient and long-lasting hyperpigmentation, thermal damage, free radical production, and premature photoaging. It also can stimulate the production of reactive oxygen species that can damage DNA.
Mineral-based, inorganic sunscreens, however – like those with titanium dioxide, zinc oxide, and iron oxide – scatter all wavelengths.
“These micronized forms of metal oxides not only scatter and reflect light, they also absorb ultraviolet radiation. The compounds aren’t new,” she said. In 1991, Dr. Elaine Kaye of Harvard University, Boston, and associates described (Arch Dermatol. 1991;127:351-5) opaque physical sunscreens that were useful blockers of visible light and found that transmittance of light can be lowered by adding iron oxide, Dr. Arellano-Mendoza pointed out.
The inorganic sunscreens have never been widely adopted because they are highly pigmented with white or, in the case of iron oxide, with red. “Not many people accepted [the iron-containing compounds] because of the redness, but now different shades are going to be hitting the market soon,” and the hope is that consumers will find them more appealing, she said.
This is good news, as the data emerging around iron oxide are intriguingly positive. A 2015 study showed that a sunscreen with iron oxides prevented melasma relapse during the summer months. Patients were randomized to the same ultraviolet filter topical sunscreen, but for one group, micronized iron oxide was added to it. After 6 months, the median melasma area severity index score was significantly better in the group using the iron oxide compound (J Am Acad Dermatol. 2015 Jan;72[1]:189-90.e1).
Support for the two-step regimen appeared in 2014, when a small study randomized 30 healthy volunteers to an SPF 30 sunscreen or the same sunscreen supplemented with an antioxidant cocktail of grape seed extract, vitamin E, ubiquinone, and vitamin C. The endpoint was MMP-1 upregulation after exposure to infrared-A light. Skin treated with the combination regimen showed significantly lower MMP-1 activation, leading the authors to conclude that the combination of topical antioxidants conferred protection against the irradiation (Photochem Photobiol. 2015 Jan-Feb;91[1]:248-50).
Those same authors published a companion article (Photodermatol Photoimmunol Photomed. 2014;30:167-74) suggesting that another antioxidant mixture (ferulic acid, tocopherol, and vitamin C) was similarly effective.
Using a combination of antioxidants is important, Dr. Arellano-Mendoza said, because different antioxidants work differently. Some (catalase, glutathione peroxidase, and superoxide dismutase) are enzymatic, catalyzing reactions that convert free radicals to oxygen and water. Others terminate free radicals by preventing the propagation of oxidative chain reactions (vitamins A and C, flavonoids, uric acid, bilirubin, albumin, and members of the thiol group). A third group consists of metal-binding proteins that sequester free iron or copper to prevent free radical production (ferritin, transferrin, lactoferrin, and ceruloplasmin).
“I think we can now consider antioxidants a part of the tools we use in treating some pigmentary disorders as melasma,” she said. “We need to choose the compounds carefully, and we definitely need more in vivo research, but the findings are very encouraging.”
Dr. Arellano-Mendoza had no relevant financial disclosures.
This article was updated 3/20/17.
[email protected]
On Twitter @Alz_Gal
ORLANDO – A two-step regimen of high-potency topical antioxidants followed by a mineral-based sunscreen may help repair light-induced skin damage and protect against new damage in patients with melasma.
Recent studies suggest that the antioxidants tamp down inflammatory cytokines and damage of oxidative stress, Maria Ivonne Arellano-Mendoza, MD, said during a special focus session on Latino skin held at the annual meeting of the American Academy of Dermatology.
“Many compounds are being studied for this purpose,” said Dr. Arellano-Mendoza, head of the dermatology department of the General Hospital of Mexico, Mexico City. “One combination is vitamin C, vitamin E, ubiquinone, and grape-seed extract. This effectively prevented infrared-A radiation–induced matrix metalloproteinase-1 messenger RNA expression in human skin. This combination can be found now in some sunscreens and daily care products.”
Another effective combination seems to be a mixture of ferulic acid, tocopherol, and vitamin C, she said.
The two-step process of regularly using a topical product with antioxidants before applying a sunscreen is all there is for now, she added, because so far it’s been impossible to combine the agents in a single product.
“The challenge will be how to create a product that stays on the surface of the skin to protect it from light, while liberating the antioxidants to penetrate the skin,” she commented.
The antioxidants’ benefits, however, will be obliterated by the continued effects of some light wavelengths that aggravate melasma unless they are used in sequence with a light-scattering sunscreen.
Sunscreens are critical components of a melasma treatment regimen, Dr. Arellano-Mendoza said. “Sunscreens are a cornerstone of treatment. We clearly tell our patients that sunscreens have to be used every day, forever, and if they are not used properly, they will have no improvement.”
Patients with hyperpigmentation disorders are susceptible to longer wavelengths that aren’t covered by chemically derived sunscreens. Longer wavelengths, including infrared light and visible light, have been shown to increase expression of matrix metalloproteinase (MMP) -1 and -9, decrease expression of type 1 procollagen, and can induce macrophage infiltration. These wavelengths also increase reactive oxygen species and proinflammatory cytokines in vitro, Dr. Arellano-Mendoza noted.
Visible light can cause erythema, transient and long-lasting hyperpigmentation, thermal damage, free radical production, and premature photoaging. It also can stimulate the production of reactive oxygen species that can damage DNA.
Mineral-based, inorganic sunscreens, however – like those with titanium dioxide, zinc oxide, and iron oxide – scatter all wavelengths.
“These micronized forms of metal oxides not only scatter and reflect light, they also absorb ultraviolet radiation. The compounds aren’t new,” she said. In 1991, Dr. Elaine Kaye of Harvard University, Boston, and associates described (Arch Dermatol. 1991;127:351-5) opaque physical sunscreens that were useful blockers of visible light and found that transmittance of light can be lowered by adding iron oxide, Dr. Arellano-Mendoza pointed out.
The inorganic sunscreens have never been widely adopted because they are highly pigmented with white or, in the case of iron oxide, with red. “Not many people accepted [the iron-containing compounds] because of the redness, but now different shades are going to be hitting the market soon,” and the hope is that consumers will find them more appealing, she said.
This is good news, as the data emerging around iron oxide are intriguingly positive. A 2015 study showed that a sunscreen with iron oxides prevented melasma relapse during the summer months. Patients were randomized to the same ultraviolet filter topical sunscreen, but for one group, micronized iron oxide was added to it. After 6 months, the median melasma area severity index score was significantly better in the group using the iron oxide compound (J Am Acad Dermatol. 2015 Jan;72[1]:189-90.e1).
Support for the two-step regimen appeared in 2014, when a small study randomized 30 healthy volunteers to an SPF 30 sunscreen or the same sunscreen supplemented with an antioxidant cocktail of grape seed extract, vitamin E, ubiquinone, and vitamin C. The endpoint was MMP-1 upregulation after exposure to infrared-A light. Skin treated with the combination regimen showed significantly lower MMP-1 activation, leading the authors to conclude that the combination of topical antioxidants conferred protection against the irradiation (Photochem Photobiol. 2015 Jan-Feb;91[1]:248-50).
Those same authors published a companion article (Photodermatol Photoimmunol Photomed. 2014;30:167-74) suggesting that another antioxidant mixture (ferulic acid, tocopherol, and vitamin C) was similarly effective.
Using a combination of antioxidants is important, Dr. Arellano-Mendoza said, because different antioxidants work differently. Some (catalase, glutathione peroxidase, and superoxide dismutase) are enzymatic, catalyzing reactions that convert free radicals to oxygen and water. Others terminate free radicals by preventing the propagation of oxidative chain reactions (vitamins A and C, flavonoids, uric acid, bilirubin, albumin, and members of the thiol group). A third group consists of metal-binding proteins that sequester free iron or copper to prevent free radical production (ferritin, transferrin, lactoferrin, and ceruloplasmin).
“I think we can now consider antioxidants a part of the tools we use in treating some pigmentary disorders as melasma,” she said. “We need to choose the compounds carefully, and we definitely need more in vivo research, but the findings are very encouraging.”
Dr. Arellano-Mendoza had no relevant financial disclosures.
This article was updated 3/20/17.
[email protected]
On Twitter @Alz_Gal
EXPERT ANALYSIS FROM AAD 2017
EXPERT ANALYSIS FROM AAD 2017
Bluish Gray Hyperpigmentation on the Face and Neck
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.
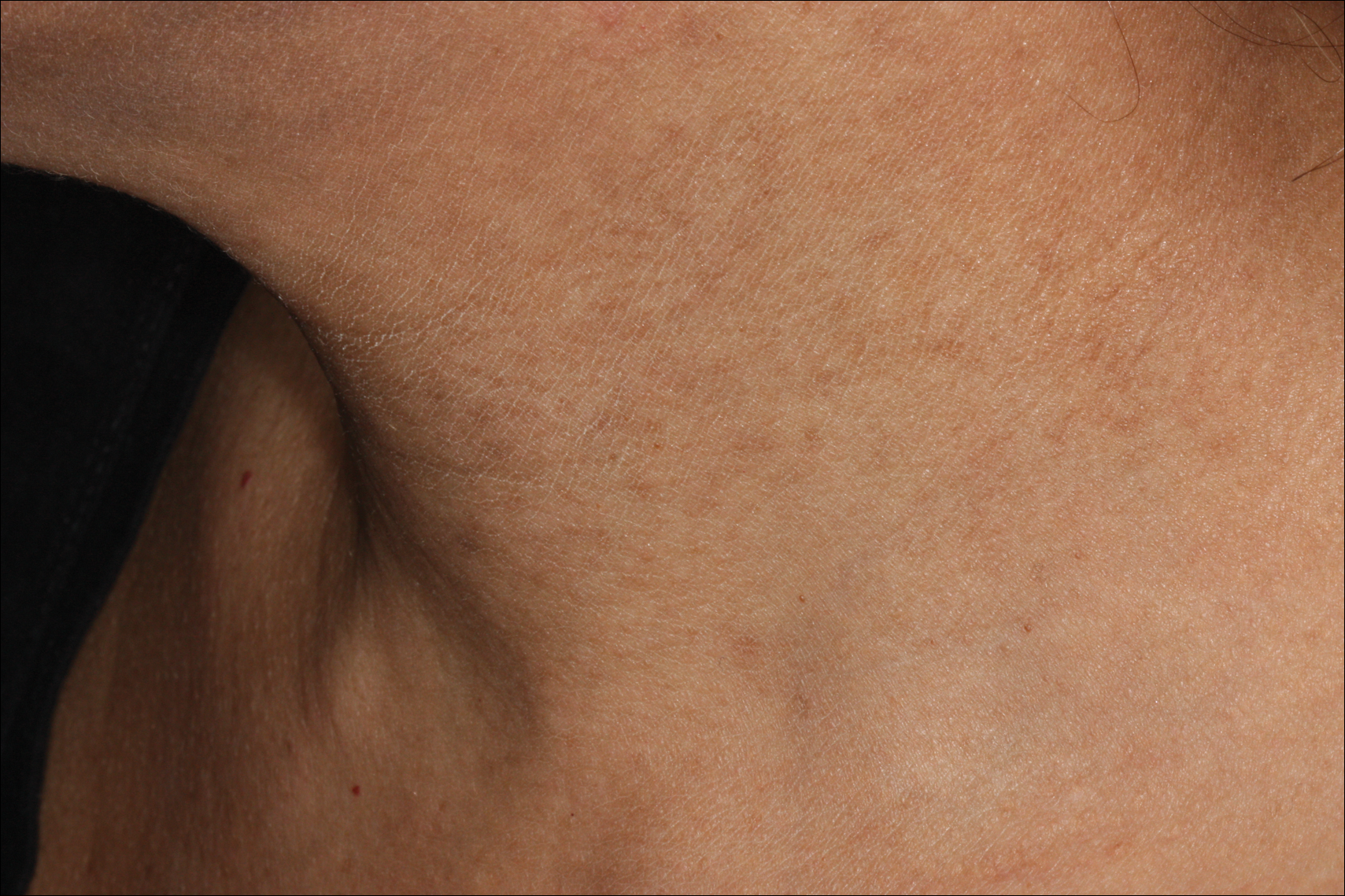
Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11
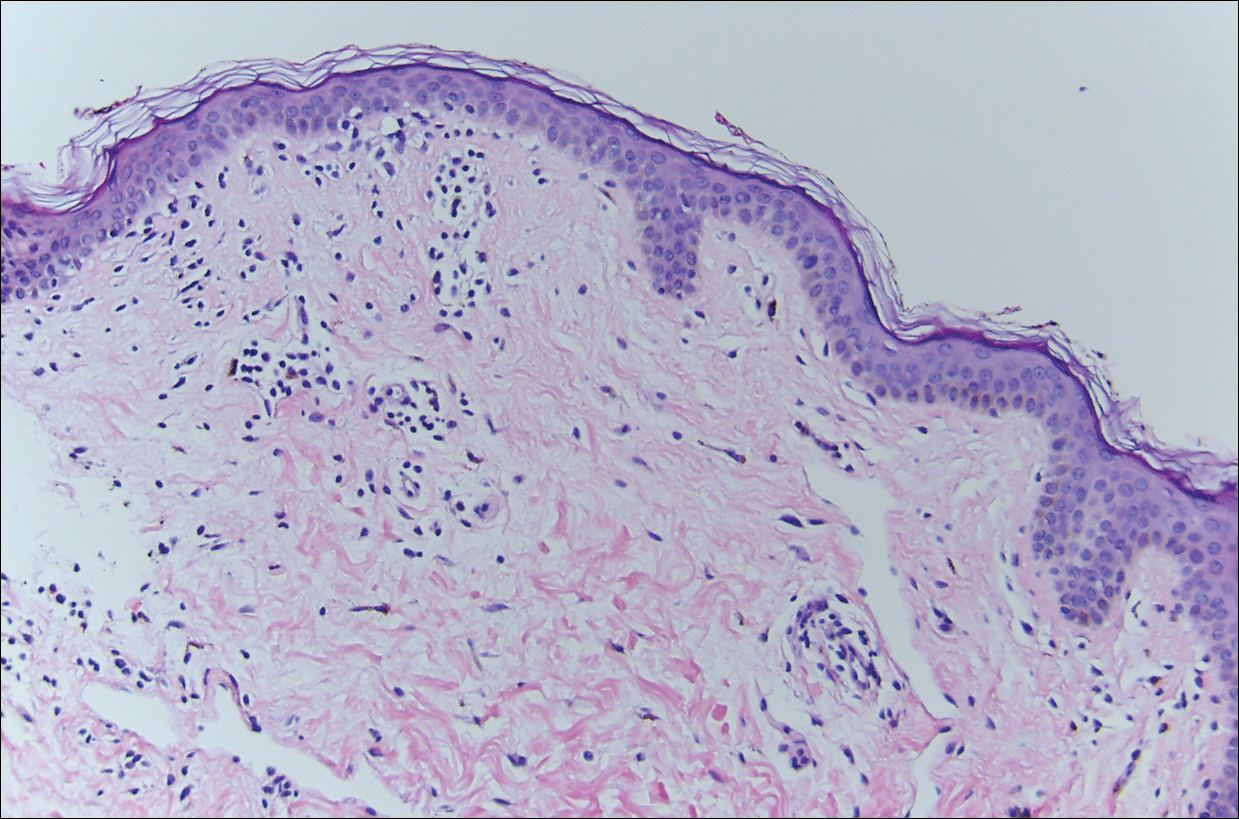
Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.


Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11

Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
The Diagnosis: Erythema Dyschromicum Perstans
Erythema dyschromicum perstans (EDP), also referred to as ashy dermatosis, was first described by Ramirez1 in 1957 who labeled the patients los cenicientos (the ashen ones). It preferentially affects women in the second decade of life; however, patients of all ages can be affected, with reported cases occurring in children as young as 2 years of age.2 Most patients have Fitzpatrick skin type IV, mainly Amerindian, Hispanic South Asian, and Southwest Asian; however, there are cases reported worldwide.3 A genetic predisposition is proposed, as major histocompatibility complex genes associated with HLA-DR4⁎0407 are frequent in Mexican patients with ashy dermatosis and in the Amerindian population.4
The etiology of EDP is unknown. Various contributing factors have been reported including alimentary, occupational, and climatic factors,5,6 yet none have been conclusively demonstrated. High expression of CD36 (thrombospondin receptor not found in normal skin) in spinous and granular layers, CD94 (cytotoxic cell marker) in the basal cell layer and in the inflammatory dermal infiltrate,7 and focal keratinocytic expression of intercellular adhesion molecule I (CD54) in the active lesions of EDP, as well as the absence of these findings in normal skin, suggests an immunologic role in the development of the disease.8
Erythema dyschromicum perstans presents clinically with blue-gray hyperpigmented macules varying in size and shape and developing symmetrically in both sun-exposed and sun-protected areas of the face, neck, trunk, arms, and sometimes the dorsal hands (Figures 1 and 2). Notable sparing of the palms, soles, scalp, and mucous membranes occurs.


Occasionally, in the early active stage of the disease, elevated erythematous borders are noted surrounding the hyperpigmented macules. Eventually a hypopigmented halo develops after a prolonged duration of disease.9 The eruption typically is chronic and asymptomatic, though some cases may be pruritic.10
Histopathologically, the early lesions of EDP with an erythematous active border reveal lichenoid dermatitis with basal vacuolar change and occasional Civatte bodies. A mild to moderate perivascular lymphohistiocytic infiltrate admixed with melanophages can be seen in the papillary dermis (Figure 3). In older lesions, the inflammatory infiltrate is sparse, and pigment incontinence consistent with postinflammatory pigmentation is prominent, though melanophages extending deep into the reticular dermis may aid in distinguishing EDP from other causes of postinflammatory pigment alteration.7,11

Erythema dyschromicum perstans and lichen planus pigmentosus (LPP) may be indistinguishable histopathologically and may both be variants of lichen planus actinicus. Lichen planus pigmentosus often differs from EDP in that it presents with brown-black macules and patches often on the face and flexural areas. A subset of cases of LPP also may have mucous membrane involvement. The erythematous border that characterizes the active lesion of EDP is characteristically absent in LPP. In addition, pruritus often is reported with LPP. Direct immunofluorescence is not a beneficial tool in distinguishing the entities.12
Other differential diagnoses of predominantly facial hyperpigmentation include a lichenoid drug eruption; drug-induced hyperpigmentation (deposition disorder); postinflammatory hyperpigmentation following atopic dermatitis; contact dermatitis or photosensitivity reaction; early pinta; and cutaneous findings of systemic diseases manifesting with diffuse hyperpigmentation such as lupus erythematosus, dermatomyositis, hemochromatosis, and Addison disease. A detailed history including medication use, thorough clinical examination, and careful histopathologic evaluation will help distinguish these conditions.
Chrysiasis is a rare bluish to slate gray discoloration of the skin that predominantly occurs in sun-exposed areas. It is caused by chronic use of gold salts, which have been used to treat rheumatoid arthritis. UV light may contribute to induce the uptake of gold and subsequently stimulate tyrosinase activity.13 Histologic features of chrysiasis include dermal and perivascular gold deposition within the macrophages and endothelial cells as well as extracellular granules. It demonstrates an orange-red birefringence on fluorescent microscopy.14,15
Minocycline-induced hyperpigmentation is a well-recognized side effect of this drug. It is dose dependent and appears as a blue-black pigmentation that most frequently affects the shins, ankles, and arms.16 Three distinct types were documented: abnormal discoloration of the skin that has been linked to deposition of pigmented metabolites of minocycline producing blue-black pigmentation at the site of scarring or prior inflammation (type 1); blue-gray pigmentation affecting normal skin, mainly the legs (type 2); and elevated levels of melanin on the sun-exposed areas producing dirty skin syndrome (type 3).17,18
Topical and systemic corticosteroids, UV light therapy, oral dapsone, griseofulvin, retinoids, and clofazimine are reported as treatment options for ashy dermatosis, though results typically are disappointing.7
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
- Ramirez CO. Los cenicientos: problema clinica. In: Memoria del Primer Congresso Centroamericano de Dermatologica, December 5-8, 1957. San Salvador, El Salvador; 1957:122-130.
- Lee SJ, Chung KY. Erythema dyschromicum perstans in early childhood. J Dermatol. 1999;26:119-121.
- Homez-Chacin, Barroso C. On the etiopathogenic of the erythema dyschromicum perstans: possibility of a melanosis neurocutaneous. Dermatol Venez. 1996;4:149-151.
- Correa MC, Memije EV, Vargas-Alarcon G, et al. HLA-DR association with the genetic susceptibility to develop ashy dermatosis in Mexican Mestizo patients [published online November 20, 2006]. J Am Acad Dermatol. 2007;56:617-620.
- Jablonska S. Ingestion of ammonium nitrate as a possible cause of erythema dyschromicum perstans (ashy dermatosis). Dermatologica. 1975;150:287-291.
- Stevenson JR, Miura M. Erythema dyschromicum perstans (ashy dermatosis). Arch Dermatol. 1966;94:196-199.
- Baranda L, Torres-Alvarez B, Cortes-Franco R, et al. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). the effect of clofazimine therapy. Arch Dermatol. 1997;133:325-329.
- Vasquez-Ochoa LA, Isaza-Guzman DM, Orozco-Mora B, et al. Immunopathologic study of erythema dyschromicum perstans (ashy dermatosis). Int J Dermatol. 2006;45:937-941.
- Convit J, Kerdel-Vegas F, Roderiguez G. Erythema dyschromicum perstans: a hiltherto undescribed skin disease. J Invest Dermatol. 1961;36:457-462.
- Ono S, Miyachi Y, Kabashima K. Ashy dermatosis with prior pruritic and scaling skin lesions. J Dermatol. 2012;39:1103-1104.
- Sanchez NP, Pathak MA, Sato SS, et al. Circumscribed dermal melaninoses: classification, light, histochemical, and electron microscopic studies on three patients with the erythema dyschromicum perstans type. Int J Dermatol. 1982;21:25-32.
- Vega ME, Waxtein L, Arenas R, et al. Ashy dermatosis and lichen planus pigmentosus: a clinicopathologic study of 31 cases. Int J Dermatol. 1992;31:90-94.
- Ahmed SV, Sajjan R. Chrysiasis: a gold "curse!" [published online May 21, 2009]. BMJ Case Rep. 2009;2009.
- Fiscus V, Hankinson A, Alweis R. Minocycline-induced hyperpigmentation. J Community Hosp Intern Med Perspect. 2014;4. doi:10.3402/jchimp.v4.24063.
- Cox AJ, Marich KW. Gold in the dermis following gold therapy for rheumatoid arthritis. Arch Dermatol. 1973;108:655-657.
- al-Talib RK, Wright DH, Theaker JM. Orange-red birefringence of gold particles in paraffin wax embedded sections: an aid to the diagnosis of chrysiasis. Histopathology. 1994;24:176-178.
- Meyer AJ, Nahass GT. Hyperpigmented patches on the dorsa of the feet. minocycline pigmentation. Arch Dermatol. 1995;131:1447-1450.
- Bayne-Poorman M, Shubrook J. Bluish pigmentation of face and sclera. J Fam Pract. 2010;59:519-522.
A middle-aged woman with Fitzpatrick skin type IV was evaluated for progressive hyperpigmentation of several months' duration involving the neck, jawline, both sides of the face, and forehead. The lesions were mildly pruritic. She denied contact with any new substance and there was no history of an eruption preceding the hyperpigmentation. Medical history included chronic anemia that was managed with iron supplementation. On physical examination, blue-gray nonscaly macules and patches were observed distributed symmetrically on the neck, jawline, sides of the face, and forehead. Microscopic examination of 2 shave biopsies revealed subtle vacuolar interface dermatitis with mild perivascular lymphocytic infiltrate and dermal melanophages (inset).
Phacomatosis Cesioflammea in Association With von Recklinghausen Disease (Neurofibromatosis Type I)
To the Editor:
Vascular lesions associated with melanocytic nevi were first described by Ota et al1 in 1947 and given the name phacomatosis pigmentovascularis. In 2005, Happle2 reclassified phacomatosis pigmentovascularis into 3 well-defined types: (1) phacomatosis cesioflammea: blue spots (caesius means bluish gray in Latin) and nevus flammeus; (2) phacomatosis spilorosea: nevus spilus coexisting with a pale pink telangiectatic nevus; and (3) phacomatosis cesiomarmorata: blue spots and cutis marmorata telangiectatica congenita. In 2011 Joshi et al3 described a case of a 31-year-old woman who had a port-wine stain in association with neurofibromatosis type I (NF-1). We present a case of phacomatosis cesioflammea in association with NF-1.
A 20-year-old woman presented to our outpatient section with a bluish black birthmark on the left side of the face since birth with the onset of multiple painless flesh-colored nodules on the trunk and arms of 1 year’s duration. She reported having occasional pruritus over the nodular lesions. Cutaneous examination showed multiple well-defined café au lait macules (0.5–3.0 cm) with regular margins. Multiple flesh-colored nodules were evident on the upper arms (Figure 1) and trunk. The nodules were firm in consistency and showed buttonholing phenomenon with some of the lesions demonstrating bag-of-worms consistency on palpation. Both palms showed multiple brownish frecklelike macules (Figure 2). A single bluish patch extended from the left ala of the nose to the sideburns. Adjoining the bluish patch was a subtle, ill-defined, nonblanchable red patch extending from the lower margin of the bluish patch to the mandibular ridge (Figure 3). Ocular examination showed melanosis bulbi of the left sclera and a few iris hamartomas (Lisch nodules) in both eyes. A biopsy of the skin nodule was obtained under local anesthesia after obtaining the patient’s informed consent; the specimen was fixed in 10% buffered formalin. A hematoxylin and eosin–stained section showed a well-circumscribed nonencapsulated tumor in the dermis composed of loosely spaced spindle cells and wavy collagenous strands (Figure 4). Routine hemogram and blood biochemistry including urinalysis were within reference range. Radiologic examination of the long bones was unremarkable. Our patient had 3 of 6 criteria defined by the National Institutes of Health for diagnosis of NF-1.4 On clinicopathological correlation we made a diagnosis of phacomatosis cesioflammea in association with NF-1. We have reassured the patient about the benign nature of vascular nevus. She was informed that the skin nodules could increase in size during pregnancy and to regularly follow-up with an eye specialist if any visual abnormalities occur.
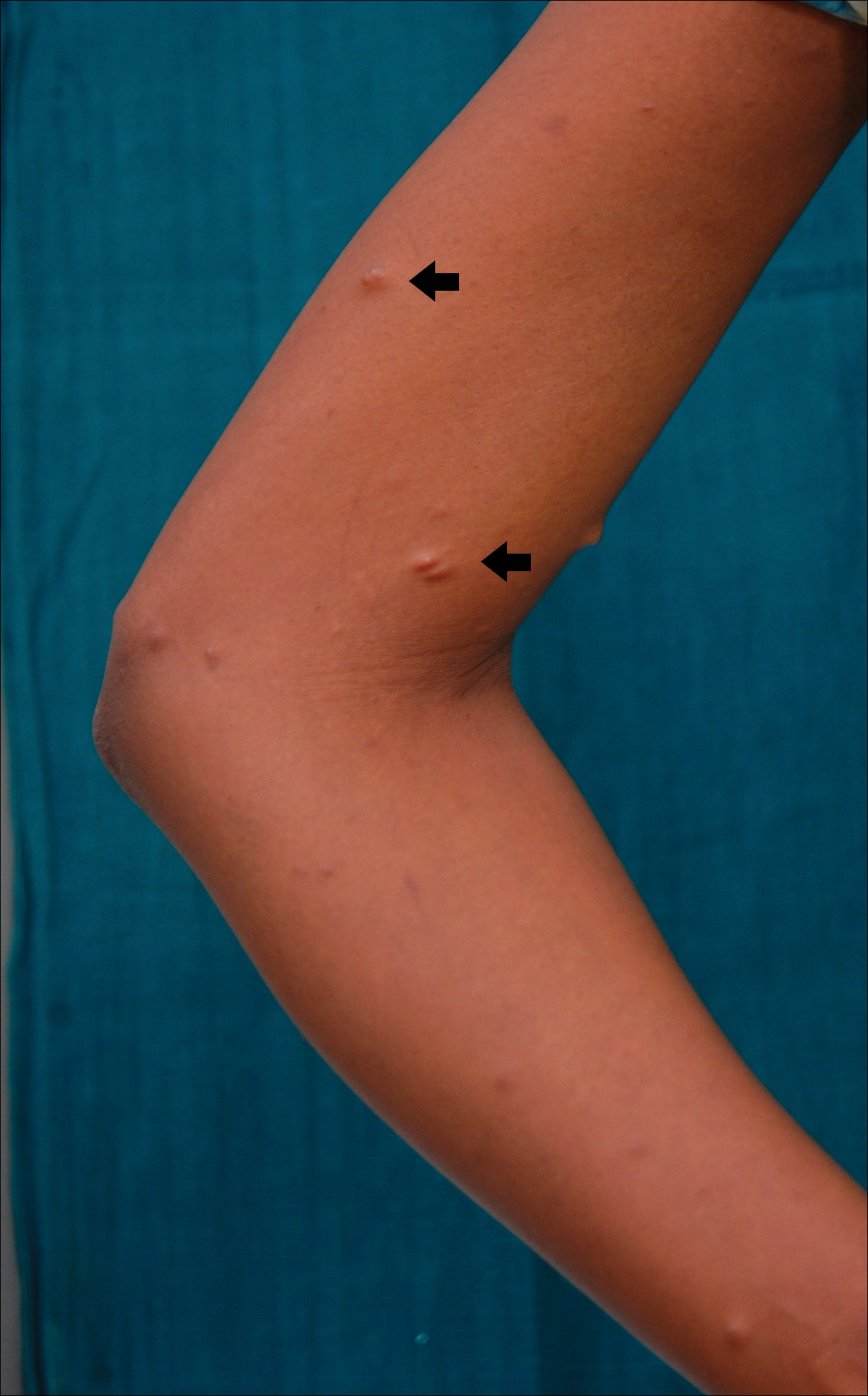
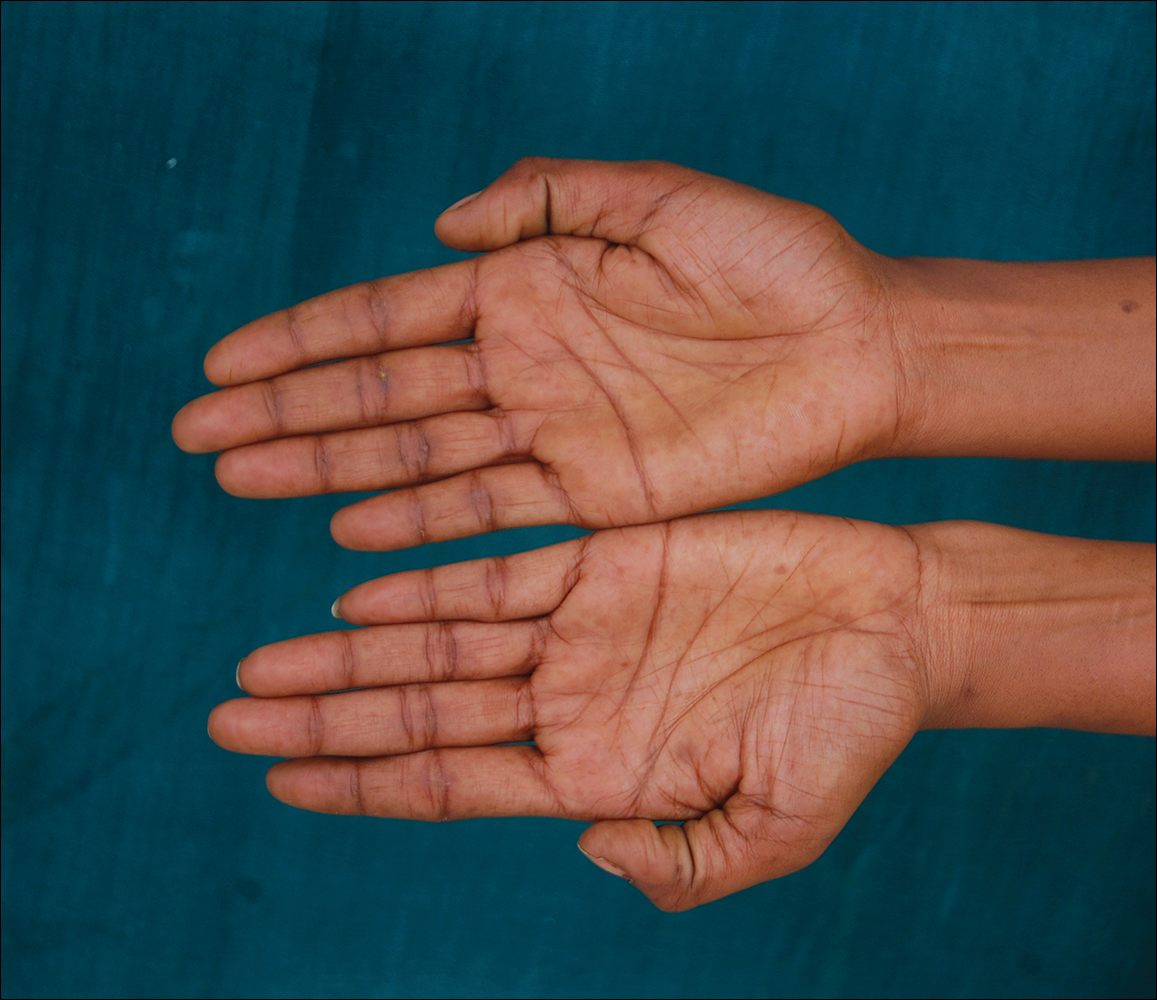
The term phacomatosis is applied to genetically determined disorders of tissue derived from ectodermal origin (eg, skin, central nervous system, eyes) and commonly includes NF-1, tuberous sclerosis, and von Hippel-Lindau syndrome. Neurofibromatosis type I was first described by German pathologist Friedrich Daniel von Recklinghausen.5 Phacomatosis pigmentovascularis has been defined as the association of vascular nevus with a pigmentary nevus. Its pathogenesis can be explained by the twin spotting phenomenon.6 Twin spots are paired patches of mutant tissue that differ from each other and from the surrounding normal background skin. They can occur as 2 clinical types: allelic and nonallelic twin spotting. Our patient had nonallelic twin spots for 2 nevoid conditions: vascular (nevus flammeus) and pigmentary (nevus of Ota). Nevus of Ota was distributed in the V2 segment (maxillary nerve) of the fifth cranial nerve along with classical melanosis bulbi, which is considered a characteristic clinical feature of nevus of Ota (nevus cesius).7 Nevus flammeus (port-wine stain) is a vascular malformation presenting with flat lesions that persists throughout a patient’s life. The phenomenon of twin spotting, or didymosis (didymos means twin in Greek), has been proposed for co-occurrence of vascular and pigmented nevi.8 The association of NF-1 along with phacomatosis cesioflammea (a twin spot) could be explained from mosaicism of tissues derived from neuroectodermal and mesenchymal elements. Neurofibromatosis type I can occur as a mosaic disorder due to either postzygotic germ line or somatic mutations in the NF1 gene located on the proximal long arm of chromosome 17.9 Irrespective of the mutational event, a mosaic patient has a mixture of cells, some have normal copies of a particular gene and others have an abnormal copy of the same gene. Somatic mutation can lead to segmental (localized), generalized, or gonadal mosaicism. Somatic mutations occurring early during embryonic development produce generalized mosaicism, and generalized mosaics clinically appear similar to nonmosaic NF-1 cases.10,11 However, due to a lack of adequate facilities for mutation analysis and financial constraints, we were unable to confirm our case as generalized somatic mosaic for NF1 gene.
Several morphologic abnormalities have been reported with phacomatosis cesioflammea. Wu et al12 reported a single case of phacomatosis cesioflammea associated with pectus excavatum in a 9-month-old infant. Shields et al13 suggested that a thorough ocular examination on a periodic basis is essential to rule out melanoma of ocular tissues in patients with nevus flammeus and ocular melanosis.
Phacomatosis cesioflammea can occur in association with NF-1. The exact incidence of association is not known. The nevoid condition can be treated with appropriate lasers.
- Ota M, Kawamura T, Ito N. Phacomatosis pigmentovascularis (Ota). Jpn J Dermatol. 1947;52:1-3.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Joshi A, Manchanda Y, Rijhwani M. Port-wine-stain with rare associations in two cases from Kuwait: phakomatosis pigmentovascularis redefined. Gulf J Dermatol Venereol. 2011;18:59-64.
- Neurofibromatosis. Conference Statement. National Institutes of Health Consensus. Arch Neurol. 1988;45:575-578.
- Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
- Goyal T, Varshney A. Phacomatosis cesioflammea: first case report from India. Indian J Dermatol Venereol Leprol. 2010;76:307.
- Happle R. Didymosis cesioanemica: an unusual counterpart of phakomatosis cesioflammea. Eur J Dermatol. 2011;21:471.
- Happle R, Steijlen PM. Phacomatosis pigmentovascularis interpreted as a phenomenon of twin spots [in German]. Hautarzt. 1989;40:721-724.
- Adigun CG, Stein J. Segmental neurofibromatosis. Dermatol Online J. 2011;17:25.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:309-310.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
To the Editor:
Vascular lesions associated with melanocytic nevi were first described by Ota et al1 in 1947 and given the name phacomatosis pigmentovascularis. In 2005, Happle2 reclassified phacomatosis pigmentovascularis into 3 well-defined types: (1) phacomatosis cesioflammea: blue spots (caesius means bluish gray in Latin) and nevus flammeus; (2) phacomatosis spilorosea: nevus spilus coexisting with a pale pink telangiectatic nevus; and (3) phacomatosis cesiomarmorata: blue spots and cutis marmorata telangiectatica congenita. In 2011 Joshi et al3 described a case of a 31-year-old woman who had a port-wine stain in association with neurofibromatosis type I (NF-1). We present a case of phacomatosis cesioflammea in association with NF-1.
A 20-year-old woman presented to our outpatient section with a bluish black birthmark on the left side of the face since birth with the onset of multiple painless flesh-colored nodules on the trunk and arms of 1 year’s duration. She reported having occasional pruritus over the nodular lesions. Cutaneous examination showed multiple well-defined café au lait macules (0.5–3.0 cm) with regular margins. Multiple flesh-colored nodules were evident on the upper arms (Figure 1) and trunk. The nodules were firm in consistency and showed buttonholing phenomenon with some of the lesions demonstrating bag-of-worms consistency on palpation. Both palms showed multiple brownish frecklelike macules (Figure 2). A single bluish patch extended from the left ala of the nose to the sideburns. Adjoining the bluish patch was a subtle, ill-defined, nonblanchable red patch extending from the lower margin of the bluish patch to the mandibular ridge (Figure 3). Ocular examination showed melanosis bulbi of the left sclera and a few iris hamartomas (Lisch nodules) in both eyes. A biopsy of the skin nodule was obtained under local anesthesia after obtaining the patient’s informed consent; the specimen was fixed in 10% buffered formalin. A hematoxylin and eosin–stained section showed a well-circumscribed nonencapsulated tumor in the dermis composed of loosely spaced spindle cells and wavy collagenous strands (Figure 4). Routine hemogram and blood biochemistry including urinalysis were within reference range. Radiologic examination of the long bones was unremarkable. Our patient had 3 of 6 criteria defined by the National Institutes of Health for diagnosis of NF-1.4 On clinicopathological correlation we made a diagnosis of phacomatosis cesioflammea in association with NF-1. We have reassured the patient about the benign nature of vascular nevus. She was informed that the skin nodules could increase in size during pregnancy and to regularly follow-up with an eye specialist if any visual abnormalities occur.




The term phacomatosis is applied to genetically determined disorders of tissue derived from ectodermal origin (eg, skin, central nervous system, eyes) and commonly includes NF-1, tuberous sclerosis, and von Hippel-Lindau syndrome. Neurofibromatosis type I was first described by German pathologist Friedrich Daniel von Recklinghausen.5 Phacomatosis pigmentovascularis has been defined as the association of vascular nevus with a pigmentary nevus. Its pathogenesis can be explained by the twin spotting phenomenon.6 Twin spots are paired patches of mutant tissue that differ from each other and from the surrounding normal background skin. They can occur as 2 clinical types: allelic and nonallelic twin spotting. Our patient had nonallelic twin spots for 2 nevoid conditions: vascular (nevus flammeus) and pigmentary (nevus of Ota). Nevus of Ota was distributed in the V2 segment (maxillary nerve) of the fifth cranial nerve along with classical melanosis bulbi, which is considered a characteristic clinical feature of nevus of Ota (nevus cesius).7 Nevus flammeus (port-wine stain) is a vascular malformation presenting with flat lesions that persists throughout a patient’s life. The phenomenon of twin spotting, or didymosis (didymos means twin in Greek), has been proposed for co-occurrence of vascular and pigmented nevi.8 The association of NF-1 along with phacomatosis cesioflammea (a twin spot) could be explained from mosaicism of tissues derived from neuroectodermal and mesenchymal elements. Neurofibromatosis type I can occur as a mosaic disorder due to either postzygotic germ line or somatic mutations in the NF1 gene located on the proximal long arm of chromosome 17.9 Irrespective of the mutational event, a mosaic patient has a mixture of cells, some have normal copies of a particular gene and others have an abnormal copy of the same gene. Somatic mutation can lead to segmental (localized), generalized, or gonadal mosaicism. Somatic mutations occurring early during embryonic development produce generalized mosaicism, and generalized mosaics clinically appear similar to nonmosaic NF-1 cases.10,11 However, due to a lack of adequate facilities for mutation analysis and financial constraints, we were unable to confirm our case as generalized somatic mosaic for NF1 gene.
Several morphologic abnormalities have been reported with phacomatosis cesioflammea. Wu et al12 reported a single case of phacomatosis cesioflammea associated with pectus excavatum in a 9-month-old infant. Shields et al13 suggested that a thorough ocular examination on a periodic basis is essential to rule out melanoma of ocular tissues in patients with nevus flammeus and ocular melanosis.
Phacomatosis cesioflammea can occur in association with NF-1. The exact incidence of association is not known. The nevoid condition can be treated with appropriate lasers.
To the Editor:
Vascular lesions associated with melanocytic nevi were first described by Ota et al1 in 1947 and given the name phacomatosis pigmentovascularis. In 2005, Happle2 reclassified phacomatosis pigmentovascularis into 3 well-defined types: (1) phacomatosis cesioflammea: blue spots (caesius means bluish gray in Latin) and nevus flammeus; (2) phacomatosis spilorosea: nevus spilus coexisting with a pale pink telangiectatic nevus; and (3) phacomatosis cesiomarmorata: blue spots and cutis marmorata telangiectatica congenita. In 2011 Joshi et al3 described a case of a 31-year-old woman who had a port-wine stain in association with neurofibromatosis type I (NF-1). We present a case of phacomatosis cesioflammea in association with NF-1.
A 20-year-old woman presented to our outpatient section with a bluish black birthmark on the left side of the face since birth with the onset of multiple painless flesh-colored nodules on the trunk and arms of 1 year’s duration. She reported having occasional pruritus over the nodular lesions. Cutaneous examination showed multiple well-defined café au lait macules (0.5–3.0 cm) with regular margins. Multiple flesh-colored nodules were evident on the upper arms (Figure 1) and trunk. The nodules were firm in consistency and showed buttonholing phenomenon with some of the lesions demonstrating bag-of-worms consistency on palpation. Both palms showed multiple brownish frecklelike macules (Figure 2). A single bluish patch extended from the left ala of the nose to the sideburns. Adjoining the bluish patch was a subtle, ill-defined, nonblanchable red patch extending from the lower margin of the bluish patch to the mandibular ridge (Figure 3). Ocular examination showed melanosis bulbi of the left sclera and a few iris hamartomas (Lisch nodules) in both eyes. A biopsy of the skin nodule was obtained under local anesthesia after obtaining the patient’s informed consent; the specimen was fixed in 10% buffered formalin. A hematoxylin and eosin–stained section showed a well-circumscribed nonencapsulated tumor in the dermis composed of loosely spaced spindle cells and wavy collagenous strands (Figure 4). Routine hemogram and blood biochemistry including urinalysis were within reference range. Radiologic examination of the long bones was unremarkable. Our patient had 3 of 6 criteria defined by the National Institutes of Health for diagnosis of NF-1.4 On clinicopathological correlation we made a diagnosis of phacomatosis cesioflammea in association with NF-1. We have reassured the patient about the benign nature of vascular nevus. She was informed that the skin nodules could increase in size during pregnancy and to regularly follow-up with an eye specialist if any visual abnormalities occur.




The term phacomatosis is applied to genetically determined disorders of tissue derived from ectodermal origin (eg, skin, central nervous system, eyes) and commonly includes NF-1, tuberous sclerosis, and von Hippel-Lindau syndrome. Neurofibromatosis type I was first described by German pathologist Friedrich Daniel von Recklinghausen.5 Phacomatosis pigmentovascularis has been defined as the association of vascular nevus with a pigmentary nevus. Its pathogenesis can be explained by the twin spotting phenomenon.6 Twin spots are paired patches of mutant tissue that differ from each other and from the surrounding normal background skin. They can occur as 2 clinical types: allelic and nonallelic twin spotting. Our patient had nonallelic twin spots for 2 nevoid conditions: vascular (nevus flammeus) and pigmentary (nevus of Ota). Nevus of Ota was distributed in the V2 segment (maxillary nerve) of the fifth cranial nerve along with classical melanosis bulbi, which is considered a characteristic clinical feature of nevus of Ota (nevus cesius).7 Nevus flammeus (port-wine stain) is a vascular malformation presenting with flat lesions that persists throughout a patient’s life. The phenomenon of twin spotting, or didymosis (didymos means twin in Greek), has been proposed for co-occurrence of vascular and pigmented nevi.8 The association of NF-1 along with phacomatosis cesioflammea (a twin spot) could be explained from mosaicism of tissues derived from neuroectodermal and mesenchymal elements. Neurofibromatosis type I can occur as a mosaic disorder due to either postzygotic germ line or somatic mutations in the NF1 gene located on the proximal long arm of chromosome 17.9 Irrespective of the mutational event, a mosaic patient has a mixture of cells, some have normal copies of a particular gene and others have an abnormal copy of the same gene. Somatic mutation can lead to segmental (localized), generalized, or gonadal mosaicism. Somatic mutations occurring early during embryonic development produce generalized mosaicism, and generalized mosaics clinically appear similar to nonmosaic NF-1 cases.10,11 However, due to a lack of adequate facilities for mutation analysis and financial constraints, we were unable to confirm our case as generalized somatic mosaic for NF1 gene.
Several morphologic abnormalities have been reported with phacomatosis cesioflammea. Wu et al12 reported a single case of phacomatosis cesioflammea associated with pectus excavatum in a 9-month-old infant. Shields et al13 suggested that a thorough ocular examination on a periodic basis is essential to rule out melanoma of ocular tissues in patients with nevus flammeus and ocular melanosis.
Phacomatosis cesioflammea can occur in association with NF-1. The exact incidence of association is not known. The nevoid condition can be treated with appropriate lasers.
- Ota M, Kawamura T, Ito N. Phacomatosis pigmentovascularis (Ota). Jpn J Dermatol. 1947;52:1-3.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Joshi A, Manchanda Y, Rijhwani M. Port-wine-stain with rare associations in two cases from Kuwait: phakomatosis pigmentovascularis redefined. Gulf J Dermatol Venereol. 2011;18:59-64.
- Neurofibromatosis. Conference Statement. National Institutes of Health Consensus. Arch Neurol. 1988;45:575-578.
- Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
- Goyal T, Varshney A. Phacomatosis cesioflammea: first case report from India. Indian J Dermatol Venereol Leprol. 2010;76:307.
- Happle R. Didymosis cesioanemica: an unusual counterpart of phakomatosis cesioflammea. Eur J Dermatol. 2011;21:471.
- Happle R, Steijlen PM. Phacomatosis pigmentovascularis interpreted as a phenomenon of twin spots [in German]. Hautarzt. 1989;40:721-724.
- Adigun CG, Stein J. Segmental neurofibromatosis. Dermatol Online J. 2011;17:25.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:309-310.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
- Ota M, Kawamura T, Ito N. Phacomatosis pigmentovascularis (Ota). Jpn J Dermatol. 1947;52:1-3.
- Happle R. Phacomatosis pigmentovascularis revisited and reclassified. Arch Dermatol. 2005;141:385-388.
- Joshi A, Manchanda Y, Rijhwani M. Port-wine-stain with rare associations in two cases from Kuwait: phakomatosis pigmentovascularis redefined. Gulf J Dermatol Venereol. 2011;18:59-64.
- Neurofibromatosis. Conference Statement. National Institutes of Health Consensus. Arch Neurol. 1988;45:575-578.
- Gerber PA, Antal AS, Neumann NJ, et al. Neurofibromatosis. Eur J Med Res. 2009;14:102-105.
- Goyal T, Varshney A. Phacomatosis cesioflammea: first case report from India. Indian J Dermatol Venereol Leprol. 2010;76:307.
- Happle R. Didymosis cesioanemica: an unusual counterpart of phakomatosis cesioflammea. Eur J Dermatol. 2011;21:471.
- Happle R, Steijlen PM. Phacomatosis pigmentovascularis interpreted as a phenomenon of twin spots [in German]. Hautarzt. 1989;40:721-724.
- Adigun CG, Stein J. Segmental neurofibromatosis. Dermatol Online J. 2011;17:25.
- Ruggieri M, Huson SM. The clinical and diagnostic implications of mosaicism in the neurofibromatoses. Neurology. 2001;56:1433-1443.
- Boyd KP, Korf BR, Theos A. Neurofibromatosis type 1. J Am Acad Dermatol. 2009;61:1-14.
- Wu CY, Chen PH, Chen GS. Phacomatosis cesioflammea associated with pectus excavatum. Acta Derm Venereol. 2009;89:309-310.
- Shields CL, Kligman BE, Suriano M, et al. Phacomatosis pigmentovascularis of cesioflammea type in 7 patients: combination of ocular pigmentation (melanocytosis or melanosis) and nevus flammeus with risk for melanoma. Arch Ophthalmol. 2011;129:746-750.
Practice Points
- Phacomatosis cesioflammea can be associated with neurofibromatosis type I.
- The port-wine stain component of phacomatosis cesioflammea may develop nodularity in long-standing cases.
- The Nd:YAG laser is beneficial for treating blue spots of phacomatosis cesioflammea.