User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
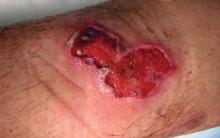
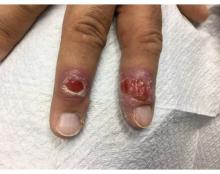
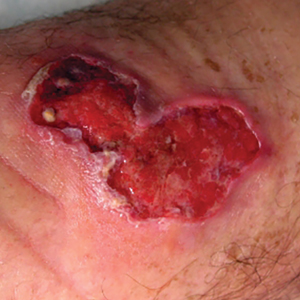
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
Buruli ulcer (BU) is a potentially disabling necrotizing skin and soft tissue disease caused by Mycobacterium ulcerans infection.1,2 Buruli ulcer is most common in hot and humid climates and has caused considerable morbidity in Western African countries (Côte d’Ivoire, Ghana, and Benin account for 73% of annual cases) and the temperate areas of Australia (283 reported cases in 2017).1-4 In fact, the first recognizable cases of BU were described in 6 Australian individuals living in a riverine area in 1948, although the term Buruli ulcer is derived from the increased number of cases reported from Buruli county in Uganda near the Nile River.1,3,4
From 2002 to 2017, 66,000 cases of BU were reported in 33 countries.1 While the focal distribution has been demonstrated in the tropical areas of Sri Lanka, Malaysia, Papua New Guinea, Peru, and Mexico,4 nontropical nations such as Japan also are affected. Since 1981, 66 cases have been reported in Japan with M ulcerans subspecies—primarily Shinshuense, which has adapted to higher latitudes.1 Herein, we provide an overview of the pathogenesis, clinical presentation, and treatment of BU and highlight aquatic insects and mosquitoes as possible vectors of transmission.
Pathogenesis
Mycobacterium ulcerans is a nontuberculous mycobacterium and ubiquitous acid-fast gram-positive bacillus that can be cultured using a Lowenstein-Jensen agar and has a doubling rate of 48 hours.1,5 It produces the small 174-kb plasmid pMUM001-encoded compound mycolactone, a pathogenic toxin that causes immunosuppression, analgesia, and cytotoxic-associated tissue necrosis.1,5-9
Mycolactone is a polyketide macrolide with a 12-membrane lactone with 2 attached acyl side chains.1,7 Mycolactone is synthesized by the giant polyketide synthetases of M ulcerans. Mycolactone post-transcriptionally inhibits the development of lipopolysaccharide-dependent proinflammatory mediators—specifically by blocking protein translocation from the cytosol into the endoplasmic reticulum by targeting the SEC61 translocon.1,6 The lack of translocation of 30% to 50% of proteins leads to cellular stress and apoptosis mediated by Bim/Bcl2. A single point mutation in the SEC61 translocon subunit alpha 1 gene (SEC61A1) is associated with resistance to the cytotoxic effects of mycolactone.1
There are divergent hypotheses regarding the relationship of mycolactone to the Wiskott-Aldrich syndrome protein, with some researchers suggesting that mycolactone can attach to this protein, leading to cell detachment and death.7 However, others have proposed that mycolactone inhibits mTOR, activating the Wiskott-Aldrich syndrome protein and leading to subsequent extensive cytoskeleton remodeling.1 Mycolactone also can cause hypoesthesia, either by activating type 2 angiotensin II receptors and creating downstream neuron hyperpolarization or by killing Schwann cells.1
Transmission
Buruli ulcer caused by M ulcerans has a poorly understood transmission mechanism, and further studies are required to understand the underlying pathophysiology to decrease transmission rates and associated morbidity. Buruli ulcer is widely accepted to be transmitted to humans via predominantly water-rich environments; most cases occur around slow-moving and still bodies of water such as swamps, ponds, and marshes.1Mycobacterium ulcerans DNA has been found in fish, water insects, and snails.1,4 It also has been present in samples from aquatic insects such as Hemiptera (water strider), Naucoridae (creeping water bugs and saucer bugs), and Belostomatidae (giant water bugs) in West Africa and also from Aulacodes feces and moss.1
Variations in geographic climate may lead to different modes of transmission of BU. For example, mosquitoes have been studied as a potential vector for BU in the temperate climate of Australia.2 However, more data are needed from other countries to support mosquitoes as possible vectors. Wallace et al10 performed a study that showed skin puncture from insect bites or other injuries increases the chance of transmitting M ulcerans in the environment to the skin.
Clinical Presentation
Buruli ulcer most often manifests in healthy children younger than 15 years.1,8 Potential risk factors include residing near a contaminated water source, swimming in a river, and being bitten by an insect in a river during the rainy season. Lack of protective clothing and mosquito nets also have been proposed as considerable risk factors for BU.2 Genetic polymorphism in the solute carrier family 11 member 1 gene (SLC11A1) may increase the risk for BU with M ulcerans transmission. It is essential to understand that infection with M ulcerans does not always lead to the development of BU.1
Buruli ulcer often begins as a painless nodule or papule that patients may confuse with an insect bite. Within a couple of weeks, the induration will grow into ill-defined edematous plaques that gradually turn into necrotic skin, which will eventually slough off to create painless to mildly painful irregular skin ulceration (Figure).11 The surrounding uninvolved skin often is edematous and pigmented. Unfortunately, deep ulcerations can lead to osteomyelitis with exposure of the underlying bone. A secondary bacterial infection may be involved if a foul smell accompanies the ulcer. The vast extension of the ulcer has been known to lead to amputations, contractures, or deformities.1-5 The nodule progression to ulceration varies and can occur within 3 weeks to 1 year of the initial exposure.1,8
skin, which will eventually slough off to create painless to mildly painful
irregular skin ulceration. The image is in the public domain. Ezzedine K,
Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal.
Emerg Infect Dis. 2009;15:118-119. doi:10.3201/eid1501.080123
The World Health Organization (WHO) classifies BU into 3 categories: category 1 includes ulcers less than 5 cm in diameter; category 2 involves ulcers that are 5 to 15 cm in diameter; and category 3 involves ulcers that are larger than 15 cm in diameter as well as those involving the breasts, genitals, eyes, bones, or joints.4,8
Diagnosis
Cultures and microscopic examination of M ulcerans acid-fast bacilli can be used to confirm the diagnosis of BU. However, polymerase chain reaction (PCR) is the best confirmatory test, as the WHO reports that 70% of reported cases of BU are confirmed by PCR detection of DNA.1,4 Unfortunately, many BU-endemic areas lack feasible access to perform confirmatory tests such as PCR. Antigen detection assays, loop-mediated isothermal amplification tests, and detection of mycolactone by thinlayer chromatography are being developed to create more rapid and sensitive testing for BU.12
The lack of diagnostic testing for BU means physicians must rely on clinical diagnosis.1 However, the differential diagnosis is extensive and includes ulcers due to diabetes and arterial and venous insufficiency, cutaneous leishmaniasis, and Haemophilus ducreyi ulcers.5 Despite the broad differential, Eddyani et al12 found that BU diagnosed clinically by physicians had a sensitivity of 92%.
Treatment
Surgery was the first-line treatment for BU before the introduction of antibiotics for this condition. Antibiotics have created better outcomes with increased cure rates and decreased amputation.4
Pharmacotherapy—In the early 2000s, the WHO recommended a treatment regimen of once-daily 10 mg/kg rifampin (oral) and 15 mg/kg streptomycin ( intramuscular) for 8 weeks. This treatment protocol is effective for lesions measuring less than 10 cm in diameter and has an average cure rate of 50%.5 Unfortunately, streptomycin is associated with ototoxicity and nephrotoxicity.1,4 Clinicians should be aware that 1.9% to 26% of patients may have paradoxical worsening of BU early during antibiotic use due to increased host inflammatory response, but it subsides with continued treatment.1,4
Researchers in Australia have begun testing and using rifampin plus oral clarithromycin, ciprofloxacin, or moxifloxacin for 3 months. A common combination is oncedaily 10 mg/kg rifampicin and 400 mg/kg moxifloxacin.1 After multiple randomized controlled trials showed the efficacy of rifampicin in combination with clarithromycin, many physicians now recommend 10 mg/kg of rifampicin once daily and 7.5 mg/kg of clarithromycin twice daily.1,5 When BU is severe, intravenous amikacin and oral rifampin can be used for 4 to 8 weeks.5
Wound Management and Surgical Considerations—Since BU can cause extensive widespread ulceration, daily wound care is recommended. Clinicians should note that patients often experience pain during wound dressing, as gauze impairs dermal regeneration and adheres to wounds. A second-line treatment to combat patients’ intolerance to gauze placement—especially for large BU lesions causing mobility issues—includes surgical debridement with wide margins and grafting 4 weeks after antibiotic therapy. This surgical procedure also can treat the releasing contractures that BU is known to cause.1 Severe cases of BU also can be treated with physiotherapy to prevent further disability.5
If histologic analysis of the margins reveals the presence of acid-fast bacilli and granulomas, the probability of future recurrence is high. In those instances, antibiotic therapy is given for prevention. In Australia, the Consensus Council Conference has recommended the removal of not only necrotic tissue but also a small margin of normal tissue to prevent the spread leading to recurrence.1,5,8
Prevention—Multiple prevention techniques have been suggested to combat BU. Long sleeves and pants should be worn outdoors along with insect repellents in BU-endemic areas. Comprehensive—but perhaps impractical—prevention measures include avoidance of swimming and aquatic activities such as boating and fishing in BU-endemic areas. In the event of a skin abrasion, the wound should be cleaned and covered promptly.
There is no vaccine currently available for BU. Bacillus Calmette—Guérin vaccination can provide minimal protection against disseminated BU but with a short-term response.5 Fortunately, M ulcerans–specific vaccines are being developed. Currently, tested vaccines target an enzyme called mycolyl transferase, which is essential for the stability of the mycobacterial cell wall and could have powerful implications in preventing these ulcers. These mycolyl transferase–directed vaccines need to be further explored in the plight against BU.1,5,8
Final Thoughts
Buruli ulcer remains a considerable public health challenge in endemic regions, with substantial morbidity and potential long-term disability. Hence, continued research into its transmission mechanisms, treatment options, and preventive measures is crucial for reducing the impact of this disease on affected populations.
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
- Yotsu RR, Suzuki K, Simmonds RE, et al. Buruli ulcer: a review of the current knowledge. Curr Trop Med Rep. 2018;5:247-256. doi:10.1007 /s40475-018-0166-2
- Muleta AJ, Lappan R, Stinear TP, et al. Understanding the transmission of Mycobacterium ulcerans: a step towards controlling Buruli ulcer. PLoS Negl Trop Dis. 2021;15:E0009678. doi:10.1371/journal.pntd.0009678
- MacCallum P, Tolhurst JC. A new mycobacterial infection in man. J Pathol Bacteriol. 1948;60:93-122.
- Van der Werf TS, Stienstra Y, Johnson RC, et al. Mycobacterium ulcerans disease. Bull World Health Organ. 2005;83:785-791.
- World Health Organization. Buruli ulcer (Mycobacterium ulcerans infection). January 12, 2023. Accessed November 7, 2024. https://www.who.int/news-room/fact-sheets/detail/buruli-ulcer-(mycobacterium-ulcerans-infection)
- Hall BS, Hill K, McKenna M, et al. The pathogenic mechanism of the Mycobacterium ulcerans virulence factor, mycolactone, depends on blockade of protein translocation into the ER. PLoS Pathog. 2014;10:E1004061. doi:10.1371/journal.ppat.1004061
- Sarfo FS, Phillips R, Wansbrough-Jones M, et al. Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease. Cell Microbiol. 2016;18:17-29. doi:10.1111/cmi.12547
- Guarner J. Buruli ulcer: review of a neglected skin mycobacterial disease. J Clin Microbiol. 2018;56:E01507- E01517. doi:10.1128 /JCM.01507-17
- Adusumilli S, Mve-Obiang A, Sparer T, et al. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M ulcerans in vitro and in vivo. Cell Microbiol. 2005;7:1295-1304. doi:10.1111/j.1462-5822.2005.00557
- Wallace JR, Mangas KM, Porter JL, et al. Mycobacterium ulcerans low infectious dose and mechanical transmission support insect bites and puncturing injuries in the spread of Buruli ulcer. PLoS Negl Trop Dis. 2017;11:E0005553. doi:10.1371/journal.pntd.0005553
- Ezzedine K, Pistone T, Cottin J, et al. Buruli ulcer in long-term traveler to Senegal. Emerg Infect Dis. 2009;15:118-119. doi:10.3201 /eid1501.080123
- Eddyani M, Sopoh GE, Ayelo G, et al. Diagnostic accuracy of clinical and microbiological signs in patients with skin lesions resembling Buruli ulcer in an endemic region. Clin Infect Dis. 2018;67:827-834. doi:10.1093/cid/ciy197
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
Buruli Ulcer Transmission: Environmental Pathways and Implications for Dermatologic Care
PRACTICE POINTS
- Buruli ulcer (BU) is a necrotizing cutaneous disease caused by Mycobacterium ulcerans with possible transmission from aquatic insects and mosquitoes.
- Buruli ulcer often manifests in children as painless induration that gradually progresses to painless or mildly painful irregular skin ulceration.
- Treatment options for BU include rifampin and streptomycin, but larger lesions may require surgical debridement.
- No vaccine currently exists for M ulcerans, but clinical trials targeting mycolyl transferase are underway.
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
Prurigo nodularis (PN) is a chronic inflammatory skin condition characterized by firm hyperkeratotic nodules that develop when patients persistently scratch or rub intensely itchy areas of the skin. This potent itch-scratch cycle can be traced back to a dysfunctional interplay between cutaneous nerve fibers and the local immune environment.1-3 Pruritis lasting at least 6 weeks is a hallmark symptom of PN and can be accompanied by pain and/or a burning sensation.4 The lesions are symmetrically distributed in areas that are easy to scratch (eg, arms, legs, trunk), typically sparing the face, palms, and soles; however, facial lesions have been reported in pediatric patients with PN, who also are more likely to have back, hand, and foot involvement.5,6
Prurigo nodularis can greatly affect patients’ quality of life, leading to increased rates of depression and anxiety.7-9 Patients with severe symptoms also report increased sleep disturbance, distraction from work, self-consciousness leading to social isolation, and missed days of work/school.9 In one study, patients with PN reported missing at least 1 day of work, school, training, or learning; giving up a leisure activity or sport; or refusing an invitation to dinner or a party in the past 3 months due to the disease.10
Epidemiology
Prurigo nodularis has a prevalence of 72 per 100,000 individuals in the United States,11 most commonly affecting adults aged 51 to 65 years and disproportionately affecting African American and female patients.12,13 Most patients with PN experience a 2-year delay in diagnosis after initial onset of symptoms.10 Adults with PN have an increased likelihood of having other dermatologic conditions, including atopic dermatitis (AD) and psoriasis.11 Nearly two-thirds of pediatric patients with PN present with AD, and those with AD showed more resistance to first-line treatment options.5
Key Clinical Features
Compared to White patients, who typically present with lesions that appear erythematous or pink, patients with darker skin tones may present with hyperpigmented nodules that are larger and darker.12 The pruritic nodules often show signs of scratching or picking (eg, excoriations, lichenification, and angulated erosions).4
Worth Noting
Diagnosis of PN is made clinically, but skin biopsy may be helpful to rule out alternative diseases. Histologically, the hairy palm sign may be present in addition to other histologic features commonly associated with excessive scratching or rubbing of the skin.
Patients with PN have a high risk for HIV, which is not suprising considering HIV is a known systemic cause of generalized chronic pruritus. Other associations include type 2 diabetes mellitus and thyroid, kidney, and liver disease.11,13 Work-up for patients with PN should include a complete blood count with differential; liver and renal function testing; and testing for C-reactive protein, thyroid-stimulating hormone, and lactate dehydrogenase.4,14 Hemoglobin A1c and HIV testing as well as a hepatitis panel also should be considered when appropriate. Because generalized pruritus may be a sign of malignancy, chest radiography and lymph node and abdominal ultrasonography should be performed in patients who have experienced itch for less than 1 year along with B symptoms (fever, night sweats, ≥10% weight loss over 6 months, fatigue).14 Frequent scratching can disrupt the skin barrier, contributing to the increased risk for skin infections.13 All patients with a suspected PN diagnosis also should undergo screening for depression and anxiety, as patients with PN are at an increased risk for these conditions.4
Treatment of PN starts with breaking the itch-scratch cycle by addressing the underlying cause of the pruritus. Therapies are focused on addressing the immunologic and neural components of the disease. Topical treatments include moderate to strong corticosteroids, calcineurin inhibitors (tacrolimus or pimecrolimus), capsaicin, and antipruritic emollients. Systemic agents include phototherapy (narrowband UVB or excimer laser), gabapentin, pregabalin, paroxetine, and amitriptyline to address the neural component of itch. Methotrexate or cyclosporine can be used to address the immunologic component of PN and diminish the itch. That said, methotrexate and cyclosporine often are inadequate to control pruritus.10 Of note, sedating antihistamines are not effective in treating itch in PN but can be used as an adjuvant therapy for sleep disturbances in these patients.15
The only drugs currently approved by the US Food and Drug Administration to treat PN are the biologics dupilumab (targeting the IL-4 receptor) approved in 2022 and nemolizumab (targeting the IL-31 receptor) approved in 2024.16-18 The evidence that these injectable biologics work is heartening in a condition that has historically been very challenging to treat.16,18 It should be noted that the high cost of these 2 medications can restrict access to care for patients who are uninsured or underinsured.
Resolution of a prurigo nodule may result in a hyperpigmented macule taking months to years to fade.
Health Disparity Highlight
Patients with PN have a considerable comorbidity burden, negative impact on quality of life, and increased health care utilization rates.12 Prurigo nodularis is 3.4 times more common in Black patients than White patients.13 Black patients with PN have increased mortality, higher health care utilization rates, and increased systemic inflammation compared to White patients.12,19,20
Social drivers of health (eg, socioeconomic challenges, education, access to high-quality health care) likely contribute to PN. Historically, there has been a paucity of research on PN, as with most conditions that disproportionately affect patients with skin of color. Several PN clinical trials currently are underway to explore additional therapeutic options.11
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
- Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448-460.
- Lou H, Lu J, Choi EB, et al. Expression of IL-22 in the skin causes Th2-biased immunity, epidermal barrier dysfunction, and pruritus via stimulating epithelial Th2 cytokines and the GRP pathway. J Immunol. 2017;198:2543-2555.
- Sutaria N, Adawi W, Goldberg R, et al. Itch: pathogenesis and treatment. J Am Acad Dermatol. 2022;86:17-34.
- Elmariah S, Kim B, Berger T, et al. Practical approaches for diagnosis and management of prurigo nodularis: United States expert panel consensus. J Am Acad Dermatol. 2021;84:747-760.
- Kyvayko R, Fachler-Sharp T, Greenberger S, et al. Characterization of paediatric prurigo nodularis: a multicentre retrospective, observational study. Acta Derm Venereol. 2024;104:adv15771.
- Aggarwal P, Choi J, Sutaria N, et al. Clinical characteristics and disease burden in prurigo nodularis. Clin Exp Dermatol. 2021;46:1277-1284.
- Whang KA, Le TK, Khanna R, et al. Health-related quality of life and economic burden of prurigo nodularis. J Am Acad Dermatol. 2022;86:573-580.
- Jørgensen KM, Egeberg A, Gislason GH, et al. Anxiety, depression and suicide in patients with prurigo nodularis. J Eur Acad Dermatol Venereol. 2017;31:E106-E107.
- Rodriguez D, Kwatra SG, Dias-Barbosa C, et al. Patient perspectives on living with severe prurigo nodularis. JAMA Dermatol. 2023;159:1205-1212.
- Misery L, Patras de Campaigno C, Taieb C, et al. Impact of chronic prurigo nodularis on daily life and stigmatization. J Eur Acad Dermatol Venereol. 2023;37:E908-E909.
- Huang AH, Canner JK, Khanna R, et al. Real-world prevalence of prurigo nodularis and burden of associated diseases. J Investigative Dermatol. 2020;140:480-483.e4.
- Sutaria N, Adawi W, Brown I, et al. Racial disparities in mortality among patients with prurigo nodularis: a multi-center cohort study. J Am Acad Dermatol. 2022;82:487-490.
- Boozalis E, Tang O, Patel S, et al. Ethnic differences and comorbidities of 909 prurigo nodularis patients. J Am Acad Dermatol. 2018; 79:714-719.e3.
- Müller S, Zeidler C, Ständer S. Chronic prurigo including prurigo nodularis: new insights and treatments. Am J Clin Dermatol. 2024;25:15-33.
- Williams KA, Roh YS, Brown I, et al. Pathophysiology, diagnosis, and pharmacological treatment of prurigo nodularis. Expert Rev Clin Pharmacol. 2021;14:67-77.
- Kwatra SG, Yosipovitch G, Legat FJ, et al. Phase 3 trial of nemolizumab in patients with prurigo nodularis. N Engl J Med. 2023;389:1579-1589.
- Beck KM, Yang EJ, Sekhon S, et al. Dupilumab treatment for generalized prurigo nodularis. JAMA Dermatol. 2019;155:118-120.
- Yosipovitch G, Mollanazar N, Ständer S, et al. Dupilumab in patients with prurigo nodularis: two randomized, double-blind, placebocontrolled phase 3 trials. Nat Med. 2023;29:1180-1190.
- Wongvibulsin S, Sutaria N, Williams KA, et al. A nationwide study of prurigo nodularis: disease burden and healthcare utilization in the United States. J Invest Dermatol. 2021;141:2530-2533.e1.
- Sutaria N, Alphonse MP, Marani M, et al. Cluster analysis of circulating plasma biomarkers in prurigo nodularis reveals a distinct systemic inflammatory signature in African Americans. J Invest Dermatol. 2022;142:1300-1308.e3.
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Break the Itch-Scratch Cycle to Treat Prurigo Nodularis
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
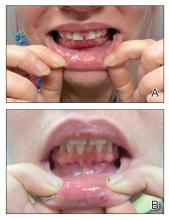
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
Cyclosporine is a calcineurin inhibitor and immunosuppressive medication with several indications, including prevention of parenchymal organ and bone marrow transplant rejection as well as treatment of numerous dermatologic conditions (eg, psoriasis, atopic dermatitis). Although it is an effective medication, there are many known adverse effects including nephrotoxicity, hypertension, and gingival hyperplasia.1 Addressing symptomatic cyclosporine-induced gingival hyperplasia can be challenging, especially if continued use of cyclosporine is necessary for adequate control of the underlying disease. We present a simplified approach for conservative management of cyclosporine-induced gingival hyperplasia that allows for continued use of cyclosporine.
Practice Gap
Cyclosporine-induced gingival hyperplasia is a fibrous overgrowth of the interdental papilla and labial gingiva that may lead to gum pain, difficulty eating, gingivitis, and/ or tooth decay or loss.2 The condition usually occurs 3 to 6 months after starting cyclosporine but may occur as soon as 1 month later.1,3 The pathophysiology of this adverse effect is incompletely understood, but several mechanisms have been implicated, including upregulation of the salivary proinflammatory cytokines IL-1α, IL-8, and IL-6.1 Additionally, patients with cyclosporine-induced gingival hyperplasia have increased bacterial colonization with species such as Porphyromonas gingivalis.4 Risk factors for cyclosporine- induced gingival hyperplasia include higher serum concentrations (>400 ng/mL) of cyclosporine, history of gingival hyperplasia, concomitant use of calcium channel blockers, and insufficient oral hygiene.2,3 A study by Seymour and Smith5 found that proper oral hygiene leads to less severe cases of cyclosporine-induced gingival hyperplasia but does not prevent gingival overgrowth. Treatment of cyclosporine-induced gingival hyperplasia traditionally involves targeting oral bacteria and reducing inflammation. Decreasing dental plaque through regular tooth-brushing and interdental cleaning may reduce symptoms such as bleeding and discomfort of the gums.
The intensity of cyclosporine-induced gingival hyperplasia can be reduced with chlorhexidine or azithromycin. Individually, each therapy has been shown to clinically improve cyclosporine-induced gingival hyperplasia; however, to our knowledge the combination of these treatments has not been reported.1 We present a simplified approach to treating cyclosporine-induced gingival hyperplasia using both azithromycin and chlorhexidine. This conservative approach results in effective and sustained improvement of gingival hyperplasia while allowing patients to continue cyclosporine therapy to control underlying disease with minimal adverse effects.
Technique
Before initiating treatment, it is important to confirm that the etiology of gingival hyperplasia is due to cyclosporine use and rule out nutritional deficiencies and autoimmune conditions as potential causes. Be sure to inquire about nutritional intake, systemic symptoms, and family history of autoimmune conditions. Our approach includes the use of azithromycin 500 mg once daily for 7 days followed by chlorhexidine 0.12% oral solution 15 mL twice daily (swish undiluted for 30 seconds, then spit) for at least 3 months for optimal management of gingival hyperplasia. Chlorhexidine should be continued for at least 6 months to maintain symptom resolution. While cyclosporine therapy may be continued throughout the duration of this regimen, consider switching to other immunosuppressive medications that are not associated with gingival hyperplasia (eg, tacrolimus) if symptoms are severe and/or resistant to therapy.1,6
We applied this technique to treat cyclosporine-induced gingival hyperplasia in a 28-year-old woman with a 3-year history of primary aplastic anemia. The patient initially presented with pain and bleeding of the gums of several months’ duration and reported experiencing gum pain when eating solid foods. Her medications included cyclosporine 225 mg daily for aplastic anemia and dapsone 100 mg daily for pneumocystis pneumonia prophylaxis, both of which were taken for the past 6 months. Oral examination revealed pink to bright red hyperplastic gingivae (Figure). She had no other symptoms associated with aplastic anemia and no signs of vitamin or nutritional deficiencies. She denied pre-existing periodontitis prior to starting cyclosporine and reported that the symptoms started several months after initiating cyclosporine therapy. Thus, the clinical diagnosis of cyclosporine-induced gingival hyperplasia was made, and treatment with azithromycin and chlorhexidine was initiated with marked reduction in symptoms.
Conservative management of gingival hyperplasia with oral hygiene including regular tooth-brushing and flossing and antimicrobial therapies was preferred in this patient to reduce gum pain and minimize the risk for tooth loss while also limiting the use of surgically invasive interventions. Due to limited therapeutic options for aplastic anemia, continued administration of cyclosporine was necessary in our patient to prevent further complications.
Practice Implications
The precise mechanism by which azithromycin treats gingival hyperplasia is unclear but may involve its antimicrobial and anti-inflammatory properties. Small concentrations of azithromycin have been shown to persist in macrophages and fibroblasts of the gingiva even with short-term administration of 3 to 5 days.7 Chlorhexidine is another antimicrobial agent often used in oral rinse solutions to decrease plaque formation and prevent gingivitis. Chlorhexidine can reduce cyclosporine-induced gingival overgrowth when used twice daily.8 After rinsing with chlorhexidine, saliva exhibits antibacterial activity for up to 5 hours; however, tooth and gum discoloration may occur.8
Recurrence of gingival hyperplasia is likely if cyclosporine is not discontinued or maintained with treatment.3 Conventional gingivectomy should be considered for cases in which conservative treatment is ineffective, aesthetic concerns arise, or gingival hyperplasia persists for more than 6 to 12 months after discontinuing cyclosporine.1
We theorize that the microbial properties of azithromycin and chlorhexidine help reduce periodontal inflammation and bacterial overgrowth in patients with cyclosporine-induced gingival hyperplasia, which allows for restoration of gingival health. Our case highlights the efficacy of our treatment approach using a 7-day course of azithromycin followed by twice-daily use of chlorhexidine oral rinse in the treatment of cyclosporine-induced gingival hyperplasia with continued use of cyclosporine.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
- Chojnacka-Purpurowicz J, Wygonowska E, Placek W, et al. Cyclosporine-induced gingival overgrowth—review. Dermatol Ther. 2022;35:E15912.
- Greenburg KV, Armitage GC, Shiboski CH. Gingival enlargement among renal transplant recipients in the era of new-generation immunosuppressants. J Periodontol. 2008;79:453-460.
- Cyclosporine (ciclosporin)(systemic): drug information. UpToDate. Accessed December 19, 2023. https://www.uptodate.com/contents/table-of-contents/drug-information/general-drug-information
- Gong Y, Bi W, Cao L, et al. Association of CD14-260 polymorphisms, red-complex periodontopathogens and gingival crevicular fluid cytokine levels with cyclosporine A-induced gingival overgrowth in renal transplant patients. J Periodontal Res. 2013;48:203-212.
- Seymour RA, Smith DG. The effect of a plaque control programme on the incidence and severity of cyclosporin-induced gingival changes. J Clin Periodontol. 1991;18:107-110.
- Nash MM, Zaltzman JS. Efficacy of azithromycin in the treatment of cyclosporine-induced gingival hyperplasia in renal transplant recipients. Transplantation. 1998;65:1611-1615.
- Martín JM, Mateo E, Jordá E. Utilidad de la azitromicina en la hyperplasia gingival inducida por ciclosporina [azithromycin for the treatment of ciclosporin-induced gingival hyperplasia]. Actas Dermosifiliogr. 2016;107:780.
- Gau CH, Tu HS, Chin YT, et al. Can chlorhexidine mouthwash twice daily ameliorate cyclosporine-induced gingival overgrowth? J Formos Med Assoc. 2013;112:131-137.
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
Conservative Approach to Treatment of Cyclosporine-Induced Gingival Hyperplasia With Azithromycin and Chlorhexidine
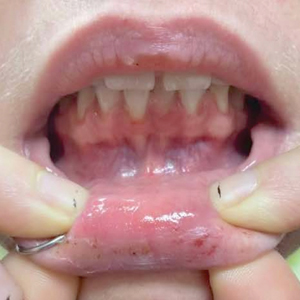
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
The recent Supreme Court ruling that struck down affirmative action1 has caused many initiatives aimed at promoting diversity, equity, and inclusion (DEI) to fall under scrutiny; however, the American Academy of Dermatology (AAD) published a statement of intent in 2022 recognizing and committing to DEI as a priority in the specialty.2 In this study, we used a formal survey to investigate the perceptions of dermatology program directors (PDs) on DEI programming from the AAD and how DEI is integrated into the resident selection process at varying institutions.
Methods
We conducted a cross-sectional study of dermatology PDs across the United States from April 2024 to July 2024. Program directors were contacted via the Association of Professors of Dermatology PD listserve, which includes all 103 PDs who are members of the organization. Personalized survey links were created and sent individually to each PD’s email address. Thirty responses were received. All survey responses were captured anonymously. The survey consisted of 17 questions focusing on dermatology PD demographics and opinions on DEI initiatives in the AAD and in the dermatology resident selection process. Data were collected using Qualtrics survey tools and analyzed using Qualtrics reports.
Results
Demographics—A total of 30 completed surveys were received. Thirty-three percent (10/30) of respondents were from the Midwest, and 23% (7/30) were from the Northeast. The next most represented region was the West, with 20% (6/30) of respondents. The Southeast and Southwest were the least represented regions captured in our survey, accounting for 13% (4/30) and 10% (3/30) of respondents, respectively. After answering this initial demographic question, 1 respondent stopped the survey, bringing our new total to 29 respondents.
Most (66% [19/29]) of the survey respondents had served as PDs for 5 years or less. Sixty-nine percent (20/29) identified as female, while 31% (9/29) identified as male. Seventy-two percent (21/29) identified as White, 17% (5/29) identified as Asian, 3% (1/29) identified as Black/African American, 3% (1/29) identified as Hispanic or Latinx, and 3% (1/29) identified as mixed race.
Opinions on DEI Initiatives—When asked about their satisfaction level with the current amount of DEI efforts within the AAD, 17% (5/29) of respondents said they were very satisfied, 59% (17/29) said they were satisfied, 17% (5/29) said they were neutral, and 7% (2/29) said they were dissatisfied. Given that none of the questions were mandatory to answer before proceeding with the survey, there were variable response rates to each of the remaining questions, which may have caused respondents to answer only questions they felt strongly about.
Twenty respondents answered when prompted to further classify their level of satisfaction: 70% (14/20) said there should be more DEI efforts through the AAD providing financial support, and 50% (10/20) wanted more nonfinancial support. When given the opportunity to specify which DEI initiatives should be enhanced, the majority (67% [14/21]) of PDs chose the AAD’s health disparities curriculum, followed by the Diversity Mentorship Program (52% [11/21]), AAD Diversity Toolkit (43% [9/21]), and the Skin of Color Curriculum (43% [9/21]). Thiry-three percent (7/30) of PDs wanted enhancement of Medicine Without Barriers: Overcoming Unintended Bias in Practice (an AAD educational resource), and 19% (4/21) of respondents did not think any of the AAD’s DEI initiatives needed to be enhanced. There were 14 responses to a question about choosing which DEI initiatives to reduce with singular votes (7% [1/14] each) to reduce Medicine Without Barriers: Overcoming Unintended Bias in Practice and the Skin of Color Curriculum.
Our survey also invited PDs to introduce ideas for new DEI initiatives or programs. The following were suggestions offered by respondents: education for senior members of the AAD on the importance of DEI in dermatology, professional development resources directed toward academic faculty members to prepare them for interacting with and teaching residents from different backgrounds, and more advertisements and support for the AAD’s Diversity Champion Workshop.
DEI in Resident Selection—When asked about the role that DEI plays in how programs develop their match lists for residency, 13% (3/23) of PDs responded that it plays a very large role, 52% (12/23) stated that it plays a large role, 26% (6/23) responded that it plays somewhat of a role, 4% (1/23) stated that it plays a small role, and 4% (1/23) stated that it plays no role. Twenty-four percent (4/17) of respondents were PDs in states that have legislation limiting or defunding DEI initiatives at institutions of higher education. Another 12% (2/17) were from states where such legislation was pending a vote, while 59% (10/17) of respondents indicated that their state had not introduced such legislation. Four percent (1/17) indicated that they were from a state that had introduced legislation to limit or defund DEI initiatives that failed to pass. Only 17 respondents answered this question, which may be due to a lack of awareness among respondents of state-specific legislation on limiting or defunding DEI initiatives.
Resident Selection Factors—Ninety-six percent (22/23) of PDs stated that their residency program uses a holistic review that takes into account factors such as experiences (eg, volunteer work, research endeavors), personal attributes, and metrics in a balanced manner. No PDs offered United States Medical Licensing Examination Step score cutoffs or medical school clerkship cutoff grades. When asked to rank the importance placed on individual factors in the residency application, the following were ranked from most to least important in the process: performance on clerkships/rotations, performance on interviews, letters of recommendation, clerkship grades, United States Medical Licensing Examination Step scores, research content/ quality, race/ethnicity, history of teaching and mentorship, volunteering, and research amount. When asked to indicate the most pertinent factors used to incorporate DEI in resident selection, the most popular factor was lived experience/life, which was chosen by 90% (18/20) of PDs followed by 75% (15/20) of respondents incorporating underrepresented in medicine (URM) status (including Black, Latinx, and Native American applicants) and 70% (4/20) incorporating socioeconomic status. Sexual orientation and geographic ties of the applicant to the region of the residency program was incorporated by 45% (9/20) of respondents, and other characteristics of race and sex each were incorporated by 30% (6/20) of respondents. Religion was the least incorporated, with 10% (2/20) of PDs selecting this classification. In considering URM status when choosing dermatology residents, 100% (11/11) of respondents indicated that their institution promotes diversity as a part of the recruitment process. Eighty-two percent (9/11) of respondents try to recruit URM applicants to reflect their patient population, 82% (9/11) try as part of a belief that a diverse group benefits everyone in their program, and 45% (5/11) try in order to address societal inequities and as a broader mission to diversify the health care workforce. Seventy-three percent (8/11) indicated that they pay attention to URM status throughout the application process.
Comment
Diversity in the US population is steadily increasing. Within the past decade, the diversity index (the probability that 2 people chosen at random will be from different racial and ethnic groups) has grown from 54.9% in 2010 to 61.1% in 2020.3 There was a 24.9% increase in population groups other than non-Hispanic Whites from 2010 to 2020, an increase in diversity that was present in every region of the United States.4 The field of dermatology already does not reflect the racial distribution of the nation,4 with Black individuals accounting for 13.7% of the nation’s population but only 3% of dermatologists; similarly, Hispanic individuals account for 19.5% of the population but only comprise 4.2% of dermatologists.5,6 There is overwhelming evidence that patients prefer to be diagnosed and treated by physicians who reflect their own demographics.7 Furthermore, physicians who prescribe treatment plans that reflect and respect socioeconomic and religious beliefs of the populations they serve enable patients to meet treatment expectations and experience better outcomes.8 Direct action is required to ensure that the specialty more accurately represents the evolving demographics of the country. This can be accomplished in myriad ways, including but not limited to cultural humility training9 for current dermatologists and trainees and recruitment of a more diverse workforce. These measures can ultimately improve treatment approaches and outcomes for dermatologic conditions across various groups.10
There are efforts by various dermatologic organizations, including the AAD, Society for Pediatric Dermatology, Pediatric Dermatology Research Alliance, Skin of Color Society, Women’s Dermatologic Society, and American Society for Dermatologic Surgery, that are focused on promoting DEI through research, education, and mentorship of potential future dermatologists.11 However, the perceptions, opinions, and selection process instituted by PDs are most consequential in determining the diversity of the specialty, as PDs are at the forefront of establishing the next generation of dermatologists. Through this study, we have found that most PDs recognize the importance of diversity in residency education and recruitment without it being the only deciding factor.
The main limitation of this study was the small sample size, which may not adequately represent all dermatology residency programs accredited by the Accreditation Council for Graduate Medical Education as a result of selection bias toward respondents who were more likely to participate in survey-based research on topics of DEI.
Conclusion
This study revealed that, among dermatology residency PDs, there is interest in modifying the resources and initiatives surrounding DEI in the field. It also revealed that DEI remains a consideration in the resident selection process despite the recent Supreme Court ruling. In conclusion, there is an eagerness among dermatology PDs to incorporate DEI into resident selection even though gaps in knowledge and awareness remain.
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
- Supreme Court of the United States. Students for Fair Admissions, Inc v President and Fellows of Harvard College (No. 20–1199). Argued October 31, 2022. Decided June 29, 2023. https://www.supremecourt.gov/opinions/22pdf/20-1199_hgdj.pdf
- American Academy of Dermatology. AAD’s DEI Statement of Intent. Published March 28, 2022. Accessed November 18, 2024. https://www.aad.org/member/career/diversity/diversity-statement-of-intent
- Jensen E, Jones N, Rabe M, et al. The chance that two people chosen at random are of different race or ethnicity groups has increased since 2010. United States Census Bureau. August 12, 2021. Accessed November 5, 2024. https://www.census.gov/library/stories/2021/08/2020-united-states-population-more-racially-ethnically-diverse-than-2010.html
- Johnson K. New Census reflects growing U.S. population diversity, with children in the forefront. University of New Hampshire Carsey School of Public Policy. October 6, 2021. Accessed November 5, 2024. https://carsey.unh.edu/publication/new-census-reflects-growing-us-population-diversity-children-forefront
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74; 584-587. doi:10.1016/j.jaad.2015.10.044
- United States Census Bureau. QuickFacts: United States. Population estimates, July 1, 2023 (V2023). Accessed November 5, 2024. https://www.census.gov/quickfacts/fact/table/US/PST045222
- Saha S, Beach MC. Impact of physician race on patient decision-making and ratings of physicians: a randomized experiment using video vignettes. J Gen Intern Med. 2020;35:1084-1091. doi:10.1007/s11606-020-05646-z
- Nair L, Adetayo OA. Cultural competence and ethnic diversity in healthcare. Plast Reconstr Surg Glob Open. 2019;7:E2219. doi:10.1097/GOX.0000000000002219
- Yeager KA, Bauer-Wu S. Cultural humility: essential foundation for clinical researchers. Appl Nurs Res. 2013;26:251-256. doi:10.1016/j.apnr.2013.06.008
- Narla S, Heath CR, Alexis A, et al. Racial disparities in dermatology. Arch Dermatol Res. 2023;315:1215-1223. doi:10.1007/s00403-022- 02507-z
- Desai SR, Khanna R, Glass D, et al. Embracing diversity in dermatology: creation of a culture of equity and inclusion in dermatology. Int J Womens Dermatol. 2021;7:378-382. doi:10.1016/j.ijwd.2021.08.002
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
Program Director Perspectives on DEI Initiatives in the Dermatology Residency Selection Process
PRACTICE POINTS
- A majority of dermatology program directors (PDs) express support for increased diversity, equity, and inclusion (DEI) funding through the American Academy of Dermatology, including initiatives centered on education and mentorship.
- Dermatology PDs are invested in recruiting underrepresented in medicine applicants to create residency classes that are representative of their patient populations.
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
To the Editor:
Psoriasis is a chronic inflammatory skin condition that commonly affects the nail matrix and/or nail bed.1 Nail involvement is present in up to 50% of patients with cutaneous psoriasis and 80% of patients with psoriatic arthritis.1 Approximately 5% to 10% of patients with psoriasis demonstrate isolated nail involvement with no skin or joint manifestations.1 Nail psoriasis can cause severe pain and psychological distress, and extreme cases may cause considerable morbidity and functional impairment.2,3 Treatment often requires a long duration and may not result in complete recovery due to the slow rate of nail growth. Patients can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.1,2 Despite the availability of various treatment options, many cases remain refractory to standard interventions, which underscores the need for novel therapeutic approaches. Herein, we present a severe case of refractory isolated nail psoriasis that was successfully treated with deucravacitinib, an oral tyrosine kinase 2 (TYK2) inhibitor.
A 59-year-old woman presented with a progressive, yellow, hyperkeratotic lesion on the left thumbnail of 2 years’ duration. The patient noted initial discoloration and peeling at the distal end of the nail. Over time, the discoloration progressed to encompass the entire nail. Previous treatments performed by outside physicians including topical corticosteroids, calcineurin inhibitors, and 2 surgeries to remove the nail plate and nail bed all were unsuccessful. The patient also reported severe left thumbnail pain and pruritus that considerably impaired her ability to work. The rest of the nails were unaffected, and she had no personal or family history of psoriasis. Her medical history was notable for hypertension, gastroesophageal reflux disease, and osteomyelitis of the right thumb without nail involvement. Drug allergies included penicillin G benzathine, sulfonamides, amoxicillin, and ciprofloxacin.
Physical examination of the left thumbnail revealed severe yellow, hyperkeratotic, dystrophic changes with a large, yellow, crumbling hyperkeratotic plaque that extended from approximately 1 cm beyond the nail plate to the proximal end of the distal interphalangeal joint, to and along the lateral nail folds, with extensive distal onycholysis. The proximal and lateral nail folds demonstrated erythema as well as maceration that was extremely tender to minimal palpation (Figure 1). No cutaneous lesions were noted elsewhere on the body. The patient had no tenderness, swelling, or stiffness in any of the joints. The differential diagnosis at the time included squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
Radiography of the left thumb revealed irregular swelling and nonspecific soft tissue enlargement at the tip of the digit. A nail clipping from the left thumbnail and 3-mm punch biopsies of the lateral and proximal nail folds as well as the horn of the proximal nail fold (Figure 2) were negative for fungus and confirmed psoriasiform dermatitis of the nail.
The patient was started on vinegar soaks (1:1 ratio of vinegar to water) every other day as well as urea cream 10%, ammonium lactate 15%, and petrolatum twice daily for 2 months without considerable improvement. Due to lack of improvement during this 2-month period, the patient subsequently was started on oral deucravacitinib 6 mg/d along with continued use of petrolatum twice daily and vinegar soaks every other day. We selected a trial of deucravacitinib for our patient because of its convenient daily oral dosing and promising clinical evidence.4,5 After 2 months of treatment with deucravacitinib, the patient reported substantial improvement and satisfaction with the treatment results. Physical examination of the left thumbnail after 2 months of deucravacitinib treatment revealed mildly hyperkeratotic, yellow, dystrophic changes of the nail with notable improvement of the yellow hyperkeratotic plaque on the distal thumbnail. Normal-appearing nail growth was noted at the proximal nail fold, demonstrating considerable improvement from the initial presentation (Figure 3). However, the patient had developed multiple oral ulcers, generalized pruritus, and an annular urticarial plaque on the left arm. As such, deucravacitinib was discontinued after 2 months of treatment. These symptoms resolved within a week of discontinuing deucravacitinib.
While the etiology of nail psoriasis remains unclear, it is believed to be due to a combination of immunologic, genetic, and environmental factors.3 Classical clinical features include nail pitting, leukonychia, onycholysis, nail bed hyperkeratosis, and splinter hemorrhages.1,3 Our patient exhibited a severe form of nail psoriasis, encompassing the entire nail matrix and bed and extending to the distal interphalangeal joint and lateral nail folds. Previous surgical interventions may have triggered the Koebner phenomenon—which commonly is associated with psoriasis—and resulted in new skin lesions as a secondary response to the surgical trauma.6 The severity of the condition profoundly impacted her quality of life and considerably hindered her ability to work.
Treatment for nail psoriasis includes topical or systemic therapies such as corticosteroids, vitamin D analogs, tacrolimus, and tumor necrosis factor α inhibitors.1,3 Topical treatment is challenging because it is difficult to deliver medication effectively to the nail bed and nail matrix, and patient adherence may be poor.2 Although it has been shown to be effective, intralesional triamcinolone can be associated with pain as the most common adverse effect.7 Systemic medications such as oral methotrexate also may be effective but are contraindicated in pregnant patients and are associated with potential adverse events (AEs), including hepatotoxicity and acute kidney injury.8 The use of biologics may be challenging due to potential AEs and patient reluctance toward injection-based treatments.9
Deucravacitinib is a TYK2 inhibitor approved for treatment of plaque psoriasis.10 Tyrosine kinase 2 is an intracellular kinase that mediates the signaling of IL-23 and other cytokines involved in psoriasis pathogenesis.10 Deucravacitinib selectively binds to the regulatory domain of TYK2, leading to targeted allosteric inhibition of TYK2-mediated IL-23 and type I interferon signaling.4,5,10 Compared with biologics, deucravacitinib is advantageous because it can be administered as a daily oral pill, encouraging high patient compliance.
In the POETYK PSO-1 and PSO-2 phase 3 randomized controlled trials, 20.9% (n=332) and 20.3% (n=510) of deucravacitinib-treated patients with moderate to severe nail involvement achieved a Physician’s Global Assessment of Fingernail score of 0/1 compared with 8.8% (n=165) and 7.9% (n=254) of patients in the placebo group, respectively. All patients in these trials had a diagnosis of plaque psoriasis with at least 10% body surface area involvement; none of the patients had isolated nail psoriasis.4,5
The phase 3 POETYK PSO-1 and PSO-2 trials demonstrated deucravacitinib to be safe and well tolerated with minimal AEs.4,5 However, the development of AEs in our patient, including oral ulcers and generalized pruritus, underscores the need for close monitoring and consideration of potential risks of treatment. Common AEs associated with deucravacitinib include upper respiratory infections (19.2% [n=840]), increased blood creatine phosphokinase levels (2.7% [n=840]), herpes simplex virus (2.0% [n=840]), and mouth ulcers (1.9% [n=840]).11
Patient education also is a crucial component in the treatment of nail psoriasis. Physicians should emphasize the slow growth of nails and need for prolonged treatment. Clear communication and realistic expectations are essential for ensuring patient adherence to treatment.
Our case highlights the potential efficacy and safety of deucravacitinib for treatment of nail psoriasis, potentially laying the groundwork for future clinical studies. Our patient had a severe case of nail psoriasis that involved the entire nail bed and nail plate, resulting in extreme pain, pruritus, and functional impairment. Her case was unique because involvement was isolated to the nail without any accompanying skin or joint manifestations. She showed a favorable response to deucravacitinib within only 2 months of treatment and exhibited considerable improvement of nail psoriasis, with a reported high level of satisfaction with the treatment. We plan to continue to monitor the patient for long-term results. Future randomized clinical trials with longer follow-up periods are crucial to further establish the efficacy and safety of deucravacitinib for treatment of nail psoriasis.
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
- Hwang JK, Grover C, Iorizzo M, et al. Nail psoriasis and nail lichen planus: updates on diagnosis and management. J Am Acad Dermatol. 2024;90:585-596. doi:10.1016/j.jaad.2023.11.024
- Ji C, Wang H, Bao C, et al. Challenge of nail psoriasis: an update review. Clin Rev Allergy Immunol. 2021;61:377-402. doi:10.1007/s12016-021-08896-9
- Muneer H, Sathe NC, Masood S. Nail psoriasis. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated March 1, 2024. Accessed October 24, 2024. https://www.ncbi.nlm.nih.gov/books/NBK559260/
- Armstrong AW, Gooderham M, Warren RB, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol. 2023;88:29-39. doi:10.1016/j.jaad.2022.07.002
- Strober B, Thaçi D, Sofen H, et al. Deucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, phase 3 Program fOr Evaluation of TYK2 inhibitor psoriasis second trial. J Am Acad Dermatol. 2023;88:40-51. doi:10.1016/j.jaad.2022.08.061
- Sanchez DP, Sonthalia S. Koebner phenomenon. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated November 14, 2022. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK553108/
- Grover C, Kharghoria G, Bansal S. Triamcinolone acetonide injections in nail psoriasis: a pragmatic analysis. Skin Appendage Disord. 2024;10:50-59. doi:10.1159/000534699
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. StatPearls Publishing; 2024 Jan-. Updated August 16, 2023. Accessed April 11, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Singh JA, Wells GA, Christensen R, et al. Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Syst Rev. 2011;2011:Cd008794. doi:10.1002/14651858.CD008794.pub2
- Thaçi D, Strober B, Gordon KB, et al. Deucravacitinib in moderate to severe psoriasis: clinical and quality-of-life outcomes in a phase 2 trial. Dermatol Ther (Heidelb). 2022;12:495-510. doi:10.1007/s13555-021-00649-y
- Week 0-16: demonstrated safety profile. Bristol-Myers Squibb. 2024. Accessed October 24, 2024. https://www.sotyktuhcp.com/safety-profile?cid=sem_2465603&gclid=CjwKCAiA9ourBhAVEiwA3L5RFnyYqmxbqkz1_zBNPz3dcyHKCSFf1XQ-7acznV0XbR5DDJHYkZcKJxoCWN0QAvD_BwE&gclsrc=aw.ds
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
Successful Treatment of Severe Dystrophic Nail Psoriasis With Deucravacitinib
PRACTICE POINTS
- Nail psoriasis can masquerade as other dermatologic conditions, including squamous cell carcinoma of the nail bed and acrodermatitis continua of Hallopeau.
- Nail psoriasis can progress to permanent nail loss if not treated properly, making early recognition and treatment crucial.
- Deucravacitinib, an oral tyrosine kinase 2 inhibitor approved for the treatment of plaque psoriasis, has shown promise as an effective treatment for nail psoriasis in cases that are refractory to standard therapies.
25TH Annual Las Vegas Dermatology Seminar Abstract Compendium; Las Vegas, Nevada; September 19-21, 2024
US Study Pinpoints Merkel Cell Risk Factors
TOPLINE:
in the United States.
METHODOLOGY:
- Researchers evaluated 38,020 MCC cases (38% women; 93% non-Hispanic White, 4% Hispanic, 1% non-Hispanic Black) diagnosed in the United States from 2001 to 2019 to estimate the contribution of potentially modifiable risk factors to the burden of MCC.
- Population-based cancer registries and linkages with HIV and transplant registries were utilized to identify MCC cases in patients with HIV, solid organ transplant recipients, and patients with chronic lymphocytic leukemia (CLL).
- Data on cloud-adjusted daily ambient UVR irradiance were merged with cancer registry information on the county of residence at diagnosis to assess UVR exposure. Studies reporting the prevalence of MCPyV in MCC specimens collected in the United States were combined via a meta-analysis.
- The study assessed population attributable fractions of MCC cases that were attributable to major immunosuppressive conditions (HIV, solid organ transplant, and chronic CLL), ambient UVR exposure, and MCPyV.
TAKEAWAY:
- The incidence of MCC was higher in people with HIV (standardized incidence ratio [SIR], 2.78), organ transplant recipients (SIR, 13.1), and patients with CLL (SIR, 5.75) than in the general US population. However, only 2.5% of MCC cases were attributable to these immunosuppressive conditions.
- Non-Hispanic White individuals showed elevated MCC incidence at both lower and higher ambient UVR exposure levels, with incidence rate ratios of 4.05 and 4.91, respectively, for MCC on the head and neck.
- A meta-analysis of 19 case series revealed that 63.8% of MCC cases were attributable to MCPyV, with a similar prevalence observed between immunocompromised and immunocompetent patients.
- Overall, 65.1% of MCC cases were attributable to ambient UVR exposure, with higher attribution for cases diagnosed on the head and neck than those diagnosed on other sites (72.1% vs 60.2%).
IN PRACTICE:
“The results of this study suggest that most MCC cases in the US are attributable to MCPyV and/or ambient UVR [UV radiation] exposure, with a smaller fraction attributable to three major immunosuppressive conditions,” the authors wrote. “Future studies should investigate UVR mutational signature, TMB [tumor mutational burden], and MCPyV prevalence according to race and ethnicity and patient immune status to help clarify the overlap between MCC risk factors.”
SOURCE:
The study was led by Jacob T. Tribble, BA, Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), Rockville, Maryland. It was published online on November 27, 2024, in JAMA Dermatology.
LIMITATIONS:
Incidences of MCC may have been inflated because of increased medical surveillance in immunosuppressed populations. The analysis assumed that only cases among non-Hispanic White individuals were associated with UVR. Additionally, the meta-analysis of MCPyV prevalence primarily included studies from large academic institutions, which may not be representative of the entire US population.
DISCLOSURES:
This study was supported in part by the Intramural Research Program of the NCI and the National Institutes of Health Medical Research Scholars Program. Additional funding was provided through a public-private partnership with contributions from the American Association for Dental Research and the Colgate-Palmolive Company to the Foundation for the National Institutes of Health. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
in the United States.
METHODOLOGY:
- Researchers evaluated 38,020 MCC cases (38% women; 93% non-Hispanic White, 4% Hispanic, 1% non-Hispanic Black) diagnosed in the United States from 2001 to 2019 to estimate the contribution of potentially modifiable risk factors to the burden of MCC.
- Population-based cancer registries and linkages with HIV and transplant registries were utilized to identify MCC cases in patients with HIV, solid organ transplant recipients, and patients with chronic lymphocytic leukemia (CLL).
- Data on cloud-adjusted daily ambient UVR irradiance were merged with cancer registry information on the county of residence at diagnosis to assess UVR exposure. Studies reporting the prevalence of MCPyV in MCC specimens collected in the United States were combined via a meta-analysis.
- The study assessed population attributable fractions of MCC cases that were attributable to major immunosuppressive conditions (HIV, solid organ transplant, and chronic CLL), ambient UVR exposure, and MCPyV.
TAKEAWAY:
- The incidence of MCC was higher in people with HIV (standardized incidence ratio [SIR], 2.78), organ transplant recipients (SIR, 13.1), and patients with CLL (SIR, 5.75) than in the general US population. However, only 2.5% of MCC cases were attributable to these immunosuppressive conditions.
- Non-Hispanic White individuals showed elevated MCC incidence at both lower and higher ambient UVR exposure levels, with incidence rate ratios of 4.05 and 4.91, respectively, for MCC on the head and neck.
- A meta-analysis of 19 case series revealed that 63.8% of MCC cases were attributable to MCPyV, with a similar prevalence observed between immunocompromised and immunocompetent patients.
- Overall, 65.1% of MCC cases were attributable to ambient UVR exposure, with higher attribution for cases diagnosed on the head and neck than those diagnosed on other sites (72.1% vs 60.2%).
IN PRACTICE:
“The results of this study suggest that most MCC cases in the US are attributable to MCPyV and/or ambient UVR [UV radiation] exposure, with a smaller fraction attributable to three major immunosuppressive conditions,” the authors wrote. “Future studies should investigate UVR mutational signature, TMB [tumor mutational burden], and MCPyV prevalence according to race and ethnicity and patient immune status to help clarify the overlap between MCC risk factors.”
SOURCE:
The study was led by Jacob T. Tribble, BA, Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), Rockville, Maryland. It was published online on November 27, 2024, in JAMA Dermatology.
LIMITATIONS:
Incidences of MCC may have been inflated because of increased medical surveillance in immunosuppressed populations. The analysis assumed that only cases among non-Hispanic White individuals were associated with UVR. Additionally, the meta-analysis of MCPyV prevalence primarily included studies from large academic institutions, which may not be representative of the entire US population.
DISCLOSURES:
This study was supported in part by the Intramural Research Program of the NCI and the National Institutes of Health Medical Research Scholars Program. Additional funding was provided through a public-private partnership with contributions from the American Association for Dental Research and the Colgate-Palmolive Company to the Foundation for the National Institutes of Health. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
in the United States.
METHODOLOGY:
- Researchers evaluated 38,020 MCC cases (38% women; 93% non-Hispanic White, 4% Hispanic, 1% non-Hispanic Black) diagnosed in the United States from 2001 to 2019 to estimate the contribution of potentially modifiable risk factors to the burden of MCC.
- Population-based cancer registries and linkages with HIV and transplant registries were utilized to identify MCC cases in patients with HIV, solid organ transplant recipients, and patients with chronic lymphocytic leukemia (CLL).
- Data on cloud-adjusted daily ambient UVR irradiance were merged with cancer registry information on the county of residence at diagnosis to assess UVR exposure. Studies reporting the prevalence of MCPyV in MCC specimens collected in the United States were combined via a meta-analysis.
- The study assessed population attributable fractions of MCC cases that were attributable to major immunosuppressive conditions (HIV, solid organ transplant, and chronic CLL), ambient UVR exposure, and MCPyV.
TAKEAWAY:
- The incidence of MCC was higher in people with HIV (standardized incidence ratio [SIR], 2.78), organ transplant recipients (SIR, 13.1), and patients with CLL (SIR, 5.75) than in the general US population. However, only 2.5% of MCC cases were attributable to these immunosuppressive conditions.
- Non-Hispanic White individuals showed elevated MCC incidence at both lower and higher ambient UVR exposure levels, with incidence rate ratios of 4.05 and 4.91, respectively, for MCC on the head and neck.
- A meta-analysis of 19 case series revealed that 63.8% of MCC cases were attributable to MCPyV, with a similar prevalence observed between immunocompromised and immunocompetent patients.
- Overall, 65.1% of MCC cases were attributable to ambient UVR exposure, with higher attribution for cases diagnosed on the head and neck than those diagnosed on other sites (72.1% vs 60.2%).
IN PRACTICE:
“The results of this study suggest that most MCC cases in the US are attributable to MCPyV and/or ambient UVR [UV radiation] exposure, with a smaller fraction attributable to three major immunosuppressive conditions,” the authors wrote. “Future studies should investigate UVR mutational signature, TMB [tumor mutational burden], and MCPyV prevalence according to race and ethnicity and patient immune status to help clarify the overlap between MCC risk factors.”
SOURCE:
The study was led by Jacob T. Tribble, BA, Division of Cancer Epidemiology and Genetics, National Cancer Institute (NCI), Rockville, Maryland. It was published online on November 27, 2024, in JAMA Dermatology.
LIMITATIONS:
Incidences of MCC may have been inflated because of increased medical surveillance in immunosuppressed populations. The analysis assumed that only cases among non-Hispanic White individuals were associated with UVR. Additionally, the meta-analysis of MCPyV prevalence primarily included studies from large academic institutions, which may not be representative of the entire US population.
DISCLOSURES:
This study was supported in part by the Intramural Research Program of the NCI and the National Institutes of Health Medical Research Scholars Program. Additional funding was provided through a public-private partnership with contributions from the American Association for Dental Research and the Colgate-Palmolive Company to the Foundation for the National Institutes of Health. The authors reported no relevant conflicts of interest.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
A 58-year-old White male presented with lesions on his index and middle finger for 3 months
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
Syphilis
Two biopsies by punch technique were performed; one for pathology and one for tissue culture (fungal and atypical mycobacteria). Tissue cultures showed no growth at 4 and 6 weeks, respectively. The lesions were swabbed for bacterial and viral cultures. Bacterial culture was positive for methicillin-resistant Staphylococcus aureus (MRSA), Pseudomonas aeruginosa, and group C Streptococcus. Viral culture for herpes simplex virus (HSV) and varicella zoster virus (VZV) was negative. Histopathology confirmed the diagnosis of syphilis. Immunoperoxidase stain was positive for Treponema pallidum, and negative for HSV-1, HSV-2, and VZV. Special stains for PAS, GMS, Fite, and AFB were negative for organisms.
Syphilis, also known as Lues disease, is a contagious, sexually acquired disease caused by the spirochete T pallidum. The skin and mucous membranes are primarily infected. There are primary, secondary, and tertiary stages. In the primary or initial stage of syphilis, a chancre appears, usually 3-4 weeks after infection. The chancre is a painless papule or erosion that progresses to a firm ulceration. Lymphadenopathy may be present. Less often, multiple chancres may be present. Primary chancre on the finger has been reported in the literature, although it is far less common to have extragenital primary syphilis. The incidence ranges from 2% to 10%. Other extragenital areas that can be affected include lips, intraoral lesions, and the anus. Atypical chancres can be formed when other microbial agents are also present. Generally, an untreated chancre will heal spontaneously within a few months.
The patient referred to the department of health for treatment with penicillin G and further workup of sexually transmitted diseases. He was also seen by infectious disease for treatment of the superimposed bacterial infections and treated with an antibiotic regimen.
The case and photo were submitted by Dr. Bilu Martin.
Dr Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Florida. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
Ramoni S et al. Sex Transm Dis. 2010 Jul;37(7):468. doi: 10.1097/OLQ.0b013e3181e2cfac.
Starzycki Z. Br J Vener Dis. 1983 Jun;59(3):169-71. doi: 10.1136/sti.59.3.169.
A 58-year-old White male with no significant past medical history presented with lesions on his right index and middle fingers, which had been present for 3 months. The lesions were painless. The patient has a history of hand dermatitis. Upon questioning, the patient said he had not fished or cleaned fish tanks. He did garden occasionally (no roses). He has been using Neosporin on the lesions. He denied any fever or systemic symptoms and had no lymphadenopathy.
What's your diagnosis?
What’s Eating You? Hookworm and Cutaneous Larva Migrans
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
It is estimated that the prevalence of human hookworm infection is approximately 450 million individuals worldwide, representing a substantial global disease burden.1 The annual global public health burden ranges from approximately 2 million to 4 million disability-adjusted life-years and $10 billion to $140 billion in hookwormrelated costs.2 In this article, we discuss the lifecycle, transmission, and disease burden of cutaneous larva migrans (CLM) as well as prevention and treatment strategies.
Background
The Ancylostomatidae nematode family comprises at least 68 known species of hookworm that infect more than 110 different species of mammals.3 Many of these parasites are able to infect more than 1 primary host species, but from a disease perspective they can be classified as either anthropophilic, with humans as the intended host, or zoonotic, with humans as an incidental host. It is important to make this distinction because, though the lifecycles and biology of hookworm species generally are similar, the manifestations of incidental human infection from zoonotic hookworms are different from those of anthropophilic hookworms. Of the anthropophilic species, Necator americanus and Ancylostoma duodenale predominate. In the instance of zoonotic hookworm, dog-infecting A caninum and cat- and doginfecting A braziliense and Uncinaria stenocephala are common causes of incidental human disease.3
The life cycle of Ancylostomatidae organisms is astounding. Through millions of years of co-evolution with mammals,4 these parasitic worms have developed perhaps one of the most circuitous paths to propagate themselves in the natural world. Hookworms start their arduous journey as eggs deposited in soil, sand, and ground vegetation from the feces of infected animals.5 Approximately 1 day after the eggs are deposited, they hatch and begin the larval stage, during which they become infective 1 to 5 weeks later. At this point, the larvae become sensitive to their environment, responding to rising temperatures, increasing carbon dioxide levels, and vibrations in the soil—all of which suggest the presence of a potential host and contribute to a concordant increase in undulatory movement of the larvae.5,6 Here, the most vulnerable tissues include the uncovered soles, palms, and buttocks of host mammals that come into contact with contaminated soil. In an undulating fashion and guided by temperature cues, the larvae locate the skin of the host and utilize a mixture of enzymes including hyaluronidases, metalloprotease, and other proteases to penetrate the epidermis.7 Anthropophilic hookworms such as N americanus and A duodenale will enter the circulatory system; from there, the hookworms migrate through the right-sided cardiopulmonary circuit and eventually ascend into the pulmonary vasculature.8 They then penetrate the lung capillary beds and parenchyma to reach the alveoli, ascend the respiratory tree, and, with the help of the mucociliary escalator, reach the esophagus, where they are swallowed by the host. In the gastrointestinal tract, adult hookworms consume host blood, mate, and lay eggs over a period of approximately 1 to 3 years if left untreated.9 Eggs are laid into the lower gastrointestinal tract, and the journey begins again in feces contacting ground or soil.
Geographic Distribution
Hookworms are found in almost all regions of the world, with species-specific distributions that highlight tropical and subtropical regions. Necator americanus and A duodenale are the most common hookworm species, with the former found predominantly in Southeast Asia and Latin America and the latter in Asia-Pacific regions.10 The highest prevalence of hookworms is in Southeast Asia followed by Sub-Saharan Africa, and the unique climate and soil composition of a region help determine the best environments for specific species of hookworm to thrive.11 In addition, socioeconomics and social determinants of health play a big role in the spread of hookworms, as hygiene practices (eg, wearing clean shoes and clothing, bathing), infrastructure (eg, clean water and streets), and anthelmintic campaigns help reduce transmission.12 Soil-transmitted helminths were once endemic to the southeastern United States, with some reports of approximately 40% of individuals infected in the south in the early 1900s.13 Anthelmintic campaigns such as water, sanitation, and hygiene programs as well as deworming of humans and livestock have proven effective in reducing the prevalence of helminth disease in industrialized nations.13,14 However, zoonotic infections remain a problem in these regions, and in some parts of the United States more than 40% of sampled cats and dogs harbored species such as A braziliense.15
Clinical Manifestation
Initial hookworm infection often goes unnoticed because symptoms can range in severity, but it is characterized by transient ground itch—a local pruritic, erythematous, and papular eruption that develops in response to epidermal penetration.16 Because the larvae must traverse the host from skin to target organs for reproduction over several weeks, iron-deficiency anemia will manifest much later than signs of the initial penetration. In the case of incidental infection from zoonotic Ancylostomatidae organisms, the misguided larvae result in CLM, an often intensely pruritic skin condition that will self-resolve in 2 to 8 weeks with eventual death of the larvae.5
Diagnosis and Pathology of Disease
Zoonotic Hookworm—The major presenting sign of zoonotic hookworm infection is CLM. The diagnosis of CLM usually is made clinically, as the larvae themselves are 0.5 mm thick to 10 mm long (Figure 1) and usually extend several centimeters beyond the dermal lesion, with dermoscopy having limited utility.17 Patients may begin to experience itching as little as 1 hour after hookworm penetration of the skin.18 Once in contact with the skin, the hookworms’ hyaluronidases and proteases are capable of breaking through the epidermis, but zoonotic hookworms typically are unable to penetrate the basal layer of the human epidermis and remain entombed between the stratum granulosum and stratum corneum. With the exception of rare cases of direct or indirect pulmonary involvement resulting in Löffler syndrome,19 the larvae will die within weeks to months, and symptoms will subsequently resolve.
Although the infection generally is self-limiting, the dermatologic manifestations of CLM can be severe and warrant intervention. The lesions start as small reddish papules at the site of penetration (Figure 2), then the hallmark elevated, migrating, serpiginous, urticarial rash develops (Figure 3). Cutaneous larva migrans generally manifests unilaterally and is both erythematous and intensely pruritic. As the larvae migrate, they leave behind 1- to 5-cm tunneled creeping eruptions in their wake. The lesions, which can manifest with pain or be painless, may develop eczematous, bullous, follicular, or impetiginized appearances.20 Atypical manifestations include folliculitis and urticarial plaques.17
cutaneous larval migrans on the palm.
Anthropophilic Hookworm—The lifecycles of N americanus and A duodenale are completed in human infection. Dermatologic manifestations are transient with the development of ground itch at the site of epidermal penetration. The hookworms employ collagenases that allow penetration of the basal layer of the skin, and eosinophilia develops as the parasites travel from the skin to the small intestine. Once attached to the gastrointestinal lumen, blood meals and proteolytic enzymes result in iron-deficiency anemia in the host and may lead to weakness, fatigue, and low birth weights in pregnant patients. With prolonged infection or heavy parasitic burden, patients can develop hypoproteinemia, anasarca, and yellowing of the skin known as chlorosis.11 A clinical diagnosis can be made by examining patient stool samples for eggs, and definitive characterization can be made using molecular tools such as polymerase chain reaction.21,22
Common to hookworm infections is the immune reaction, which promotes inflammation with localized eosinophilia and mastocytosis.11 In a clinical biopsy specimen of gut—usually obtained through esophagogastroduodenoscopy— T-helper (Th) 2–type immune (IL-4, IL-5, IL-9 and IL-13), regulatory Th10 (IL-10 and transcription growth factor β), and some evidence of Th1 (interferon gamma and IL-2) cytokines are present, but little evidence of Th17-type immune response was found.23 It is believed that in zoonotic infections, antiparasitic IgE from basophils are somewhat successful at trapping the helminths in the epidermis, but in the anthropophilic species, IgE and Th2 responses are ineffective at clearing the parasite from the gut, and the defeated immune system transitions to a host-tolerance approach of limiting infection.11 It is now believed that this natural armistice can be manipulated into a potential therapy against autoimmune and inflammatory conditions. Intentional infection with zoonotic whipworm or hookworm has been proposed as a mechanism of switching Th1 and Th2 responses to host-tolerant mechanisms in conditions such as Crohn disease and celiac disease,24 and it has even been hypothesized that prior hookworm infection may reduce the chance of developing allergic conditions such as eczema.25
Treatment and Prevention
The World Health Organization and Centers for Disease Control and Prevention recommend a single oral dose of 400 mg albendazole for adults or 10 to 15 mg/kg in children for CLM. A single dose of ivermectin at 12 mg in adults or 150 μg/kg in children can be used as an alternative where albendazole is not available.11 Topical applications of thiabendazole 10% to 15% under occlusion or 3 times daily for 15 days without occlusion also can manage CLM, and pruritus can be treated with topical corticosteroids for symptomatic relief. Oral albendazole 400 mg twice daily or mebendazole 100 mg twice daily for 3 days or a single 500-mg dose, as well as 11 mg/kg (up to a maximum of 1 g) oral pyrantel pamoate once daily for 3 days can be used to treat intestinal hookworm infection, though it should be avoided in pregnancy. Iron deficiency should be managed with supplementation.11
Prevention of hookworm infection is focused around 2 broad public health efforts: mass drug administration programs and the water, sanitation, and hygiene program. In mass drug administration, treatments such as benzimidazoles are given in mass to communities affected by endemic hookworm as a single dose to reduce the burden of disease. Together, these strategies effectively eliminated hookworms in many developed nations, but areas of resurgence are beginning to surface worldwide. With changes in climate, emerging drug resistance, and socioeconomic disparities, particularly affecting the southeast, a resurgence of hookworm has occurred in the United States.26 One recent study demonstrated that almost one-third (19/55) of children sampled in an impoverished area of rural Alabama had hookworm eggs in their stool.27 Furthermore, pets serve not only as zoonotic reservoirs for CLM recurrence but also as vehicles for the evolution of drug-resistant strains, leading some to call for a ban of animals from beaches and playgrounds as well as tightly controlled veterinary programs.5,28 Ubiquitous benzimidazole use in livestock has led to bendazole-resistant strains, and it is likely that with continued and poorly adherent drug use, more zoonotic and anthropophilic drug-resistant strains of hookworm will emerge.29,30
Conclusion
The burden of hookworm infection and CLM is substantial in parts of the United States. Dermatologists play a critical role in the recognition and management of hookworm infection for both treatment of affected patients and the subsequent prevention of its spread. As drug-resistant strains evolve, clinicians, public health officials, and scientists need to continue to work together to prevent and treat hookworm infection.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
- Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211-1259.
- Bartsch SM, Hotez PJ, Asti L, et al. The global economic and health burden of human hookworm infection. PLoS Negl Trop Dis. 2016;10:E0004922.
- Seguel M, Gottdenker N. The diversity and impact of hookworm infections in wildlife. Int J Parasitol Parasites Wildl. 2017;6:177-194.
- Adams BJ, Peat SM, Dillman AR. Phylogeny and evolution. In: Entomopathogenic Nematodes: Systematics, Phylogeny and Bacterial Symbionts. Brill; 2010:693-733.
- Heukelbach J, Feldmeier H. Epidemiological and clinical characteristics of hookworm-related cutaneous larva migrans. Lancet Infect Dis. 2008;8:302-309.
- Haas W, Haberl B, Idris I, et al. Infective larvae of the human hookworms Necator americanus and Ancylostoma duodenale differ in their orientation behaviour when crawling on surfaces. Parasitol Res. 2005;95:25-29.
- Hotez P, Narasimhan S, Haggerty J, et al. Hyaluronidase from infective Ancylostoma hookworm larvae and its possible function as a virulence factor in tissue invasion and in cutaneous larva migrans. Infect Immun. 1992;60:1018-1023.
- Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st century. Adv Parasitol. 2004;58:197-288.
- Hoagland K, Schad G. Necator americanus and Ancylostoma duodenale: life history parameters and epidemiological implications of two sympatric hookworms of humans. Exp Parasitol. 1978;44:36-49.
- Clements ACA, Alene KA. Global distribution of human hookworm species and differences in their morbidity effects: a systematic review. Lancet Microbe. 2022;3:E72-E79.
- Loukas A, Hotez PJ, Diemert D, et al. Hookworm infection. Nat Rev Dis Primers. 2016;2:1-18.
- Gazzinelli A, Correa-Oliveira R, Yang GJ, et al. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6:E1603.
- Starr MC, Montgomery SP. Soil-transmitted helminthiasis in the United States: a systematic review—1940-2010. Am J Trop Med Hyg. 2011;85:680-684.
- Strunz EC, Addiss DG, Stocks ME, et al. Water, sanitation, hygiene, and soil-transmitted helminth infection: a systematic review and metaanalysis. PLoS Med. 2014;11:E1001620.
- Liotta JL, Youn H, Aksel S, et al. Prevalence of Ancylostoma braziliense in dogs from Alachua and Marion Counties, Florida, United States. J Parasitol. 2012;98:1039-1040.
- Hotez PJ, Brooker S, Bethony JM, et al. Hookworm infection. N Engl J Med. 2004;351:799-807.
- Prickett KA, Ferringer TC. What’s eating you? cutaneous larva migrans. Cutis. 2015;95:126-128.
- Feldmeier H, Schuster A. Mini review: hookworm-related cutaneous larva migrans. Eur J Clin Microbiol Infect Dis. 2012;31:915-918.
- Tan SK, Liu TT. Cutaneous larva migrans complicated by Löffler syndrome. Arch Dermatol. 2010;146:210-212.
- Eksomtramage T, Aiempanakit K. Bullous and pustular cutaneous larva migrans: two case reports and a literature review. IDCases. 2018;12:130-132.
- Utzinger J, Rinaldi L, Lohourignon LK, et al. FLOTAC: a new sensitive technique for the diagnosis of hookworm infections in humans. Trans R Soc Trop Med Hyg. 2008;102:84-90.
- Chidambaram M, Parija SC, Toi PC, et al. Evaluation of the utility of conventional polymerase chain reaction for detection and species differentiation in human hookworm infections. Trop Parasitol. 2017;7:111-116.
- Gaze S, McSorley HJ, Daveson J, et al. Characterising the mucosal and systemic immune responses to experimental human hookworm infection. PLoS Pathog. 2012;8:E1002520.
- Croese J, O’Neil J, Masson J, et al. A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors. Gut. 2006;55:136-137.
- Mpairwe H, Amoah AS. Parasites and allergy: observations from Africa. Parasite Immunol. 2019;41:E12589.
- Albonico M, Savioli L. Hookworm: a neglected resurgent infection. Editorial. BMJ. 2017;359:j4813.
- McKenna ML, McAtee S, Bryan PE, et al. Human intestinal parasite burden and poor sanitation in rural Alabama. Am J Trop Med Hyg. 2017;97:1623-1628.
- Traversa D. Pet roundworms and hookworms: a continuing need for global worming. Parasit Vectors. 2012;5:1-19.
- Geerts S, Gryseels B. Drug resistance in human helminths: current situation and lessons from livestock. Clin Microbiol Rev. 2000;13:207-222.
- Jimenez Castro PD, Howell SB, Schaefer JJ, et al. Multiple drug resistance in the canine hookworm Ancylostoma caninum: an emerging threat? Parasit Vectors. 2019;12:1-15.
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
What’s Eating You? Hookworm and
Cutaneous Larva Migrans
PRACTICE POINTS
- Anthropophilic hookworm infection should be considered with evidence of either transient ground itch or iron-deficient anemia in individuals who go barefoot, permitting ground-to-skin transmission.
- Zoonotic hookworm infection manifests as cutaneous larva migrans, an elevated serpiginous rash that, while usually self-resolving, can be intensely pruritic and should be treated accordingly.
- Considered a neglected tropical disease, hookworm infection still represents an enormous global disease burden. In addition to ongoing afflicted regions, hookworms are making a resurgence in developed nations, and drug-resistant strains have evolved.
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
Approximately 25% of Americans have at least one skin condition, and 20% are estimated to develop skin cancer during their lifetime.1,2 However, 40% of the US population lives in areas underserved by dermatologists. 3 The severity and mortality of skin cancers such as melanoma and mycosis fungoides have been positively associated with minoritized race, lack of health insurance, and unstable housing status.4-6 Patients who receive health care at free clinics often are of a racial or ethnic minoritized social group, are uninsured, and/or lack stable housing; this underserved group also includes recent immigrants to the United States who have limited English proficiency (LEP).7 Only 25% of free clinics offer specialty care services such as dermatology.7,8
Of the 42 free clinics and Federally Qualified Health Centers in Pittsburgh, Pennsylvania, the Birmingham Free Clinic (BFC) is one of the few that offers specialty care services including dermatology.9 Founded in 1994, the BFC serves as a safety net for Pittsburgh’s medically underserved population, offering primary and acute care, medication access, and social services. From January 2020 to May 2022, the BFC offered 27 dermatology clinics that provided approximately 100 people with comprehensive care including full-body skin examinations, dermatologic diagnoses and treatments, minor procedures, and dermatopathology services.
In this study, we compared the BFC dermatology patient care model with that of the dermatology department at the University of Pittsburgh Medical Center (UPMC), an insurance-based tertiary referral health care system in western Pennsylvania. By analyzing the demographics, dermatologic diagnoses, and management strategies of both the BFC and UPMC, we gained an understanding of how these patient care models differ and how they can be improved to care for diverse patient populations.
Methods
A retrospective chart review of dermatology patients seen in person at the BFC and UPMC during the period from January 2020 to May 2022 was performed. The UPMC group included patients seen by 3 general dermatologists (including A.J.J.) at matched time points. Data were collected from patients’ first in-person visit during the study period. Variables of interest included patient age, sex, race, ethnicity, primary language, zip code, health insurance status, distance to clinic (estimated using Google Maps to calculate the shortest driving distance from the patient’s zip code to the clinic), history of skin cancer, dermatologic diagnoses, and management strategies. These variables were not collected for patients who cancelled or noshowed their first in-person appointments. All patient charts and notes corresponding to the date and visit of interest were accessed through the electronic medical record (EMR). Patient data were de-identified and stored in a password-protected spreadsheet. Comparisons between the BFC and UPMC patient populations were performed using X2 tests of independence, Fisher exact tests, and Mann-Whitney U tests via SPSS software (IBM). Statistical significance was set at P<.05.
Results
Patient Characteristics—Our analysis included 76 initial appointments at the BFC and 322 at UPMC (Table 1). The mean age for patients at the BFC and UPMC was 39.6 years and 47.8 years, respectively (P=.001). Males accounted for 39 (51.3%) and 112 (34.8%) of BFC and UPMC patients, respectively (P=.008); 2 (0.6%) patients from UPMC were transgender. Of the BFC and UPMC patients, 44.7% (34/76) and 0.9% (3/322) were Hispanic, respectively (P<.001). With regard to race, 52.6% (40/76) of BFC patients were White, 19.7% (15/76) were Black, 6.6% (5/76) were Asian/Pacific Islander (Chinese, 1.3% [1/76]; other Asian, 5.3% [4/76]), and 21.1% (16/76) were American Indian/other/unspecified (American Indian, 1.3% [1/76]; other, 13.2% [10/76]; unspecified, 6.6% [5/76]). At UPMC, 61.2% (197/322) of patients were White, 28.0% (90/322) were Black, 5.3% (17/322) were Asian/Pacific Islander (Chinese, 1.2% [4/322]; Indian [Asian], 1.9% [6/322]; Japanese, 0.3% [1/322]; other Asian, 1.6% [5/322]; other Asian/American Indian, 0.3% [1/322]), and 5.6% (18/322) were American Indian/other/ unspecified (American Indian, 0.3% [1/322]; other, 0.3% [1/322]; unspecified, 5.0% [16/322]). Overall, the BFC patient population was more ethnically and racially diverse than that of UPMC (P<.001).
Forty-six percent (35/76) of BFC patients and 4.3% (14/322) of UPMC patients had LEP (P<.001). Primary languages among BFC patients were 53.9% (41/76) English, 40.8% (31/76) Spanish, and 5.2% (4/76) other/ unspecified (Chinese, 1.3% [1/76]; Indonesian, 2.6% [2/76]; unspecified, 1.3% [1/76]). Primary languages among UPMC patients were 95.7% (308/322) English and 4.3% (14/322) other/unspecified (Chinese, 0.6% [2/322]; Nepali, 0.6% [2/322]; Pali, 0.3% [1/322]; Russian, 0.3% [1/322]; unspecified, 2.5% [8/322]). There were notable differences in insurance status at the BFC vs UPMC (P<.001), with more UPMC patients having private insurance (52.8% [170/322] vs 11.8% [9/76]) and more BFC patients being uninsured (52.8% [51/76] vs 1.9% [6/322]). There was no significant difference in distance to clinic between the 2 groups (P=.183). More UPMC patients had a history of skin cancer (P=.003). More patients at the BFC were no-shows for their appointments (P<.001), and UPMC patients more frequently canceled their appointments (P<.001).
Dermatologic Diagnoses—The most commonly diagnosed dermatologic conditions at the BFC were dermatitis (23.7% [18/76]), neoplasm of uncertain behavior (15.8% [12/76]), alopecia (11.8% [9/76]), and acne (10.5% [8/76]) (Table 2). The most commonly diagnosed conditions at UPMC were nevi (26.4% [85/322]), dermatitis (22.7% [73/322]), seborrheic keratosis (21.7% [70/322]), and skin cancer screening (21.4% [70/322]). Neoplasm of uncertain behavior was more common in BFC vs UPMC patients (P=.040), while UPMC patients were more frequently diagnosed with nevi (P<.001), seborrheic keratosis (P<.001), and skin cancer screening (P<.001). There was no significant difference between the incidence of skin cancer diagnoses in the BFC (1.3% [1/76]) and UPMC (0.6% [2/76]) patient populations (P=.471). Among the biopsied neoplasms, there was also no significant difference in malignant (BFC, 50.0% [5/10]; UPMC, 32.0% [8/25]) and benign (BFC, 50.0% [5/10]; UPMC, 36.0% [9/25]) neoplasms diagnosed at each clinic (P=.444).
Management Strategies—Systemic antibiotics were more frequently prescribed (P<.001) and laboratory testing/ imaging were more frequently ordered (P=.005) at the BFC vs UPMC (Table 3). Patients at the BFC also more frequently required emergency insurance (P=.036). Patients at UPMC were more frequently recommended sunscreen (P=.003) and received education about skin cancer signs by review of the ABCDEs of melanoma (P<.001), sun-protective behaviors (P=.001), and skin examination frequency (P<.001). Notes in the EMR for UPMC patients more frequently specified patient followup instructions (P<.001).
Comment
As of 2020, the city of Pittsburgh had an estimated population of nearly 303,000 based on US Census data.10 Its population is predominantly White (62.7%) followed by Black/African American (22.8%) and Asian (6.5%); 5.9% identify as 2 or more races. Approximately 3.8% identify as Hispanic or Latino. More than 11% of the Pittsburgh population aged 5 years and older speaks a language other than English as their primary language, including Spanish (2.3%), other Indo-European languages (3.9%), and Asian and Pacific Island languages (3.5%).11 More than 5% of the Pittsburgh population does not have health insurance.12
The BFC is located in Pittsburgh’s South Side area, while one of UPMC’s primary dermatology clinics is located in the Oakland district; however, most patients who seek care at these clinics live outside these areas. Our study results indicated that the BFC and UPMC serve distinct groups of people within the Pittsburgh population. The BFC patient population was younger with a higher percentage of patients who were male, Hispanic, racially diverse, and with LEP compared with the UPMC patient population. In this clinical setting, the BFC health care team engages with people from diverse backgrounds and requires greater interpreter and medical support services.
The BFC largely is supported by volunteers, UPMC, grants, and philanthropy. Dermatology clinics are staffed by paid and volunteer team members. Paid team members include 1 nurse and 1 access lead who operates the front desk and registration. Volunteer team members include 1 board-certified dermatologist from UPMC (A.J.J.), or an affiliate clinic and 1 or 2 of each of the following: UPMC dermatology residents, medical or undergraduate students from the University of Pittsburgh, AmeriCorps national service members, and student or community medical interpreters. The onsite pharmacy is run by volunteer faculty, resident, and student pharmacists from the University of Pittsburgh. Dermatology clinics are half-day clinics that occur monthly. Volunteers for each clinic are recruited approximately 1 month in advance.
Dermatology patients at the BFC are referred from the BFC general medicine clinic and nearby Federally Qualified Health Center s for simple to complex medical and surgical dermatologic skin conditions. Each BFC dermatology clinic schedules an average of 7 patients per clinic and places other patients on a wait-list unless more urgent triage is needed. Patients are notified when they are scheduled via phone or text message, and they receive a reminder call or text 1 or 2 days prior to their appointment that also asks them to confirm attendance. Patients with LEP are called with an interpreter and also may receive text reminders that can be translated using Google Translate. Patients are instructed to notify the BFC if they need to cancel or reschedule their appointment. At the end of each visit, patients are given an after-visit summary that lists follow-up instructions, medications prescribed during the visit, and upcoming appointments. The BFC offers bus tickets to help patients get to their appointments. In rare cases, the BFC may pay for a car service to drive patients to and from the clinic.
Dermatology clinics at UPMC use scheduling and self-scheduling systems through which patients can make appointments at a location of their choice with any available board-certified dermatologist or physician assistant. Patients receive a reminder phone call 3 days prior to their appointment instructing them to call the office if they are unable to keep their appointment. Patients signed up for the online portal also receive a reminder message and an option to confirm or cancel their appointment. Patients with cell phone numbers in the UPMC system receive a text message approximately 2 days prior to their appointment that allows them to preregister and pay their copayment in advance. They receive another text 20 minutes prior to their appointment with an option for contactless check-in. At the conclusion of their visit, patients can schedule a follow-up appointment and receive a printed copy of their after-visit summary that provides information about follow-up instructions, prescribed medications, and upcoming visits. They may alternatively access this summary via the online patient portal. Patients are not provided transportation to UPMC clinics, but they are offered parking validation.
Among the most common dermatologic diagnoses for each group, BFC patients presented for treatment of more acute dermatologic conditions, while UPMC patients presented for more benign and preventive-care conditions. This difference may be attributable to the BFC’s referral and triage system, wherein patients with more urgent problems are given scheduling priority. This patient care model contrasts with UPMC’s scheduling process in which no known formal triage system is utilized. Interestingly, there was no difference in skin cancer incidence despite a higher percentage of preventive skin cancer screenings at UPMC.
Patients at the BFC more often required emergency insurance for surgical interventions, which is consistent with the higher percentage of uninsured individuals in this population. Patients at UPMC more frequently were recommended sunscreen and were educated about skin cancer, sun protection, and skin examination, in part due to this group’s more extensive history of skin cancer and frequent presentation for skin cancer screenings. At the same time, educational materials for skin care at both the BFC and UPMC are populated into the EMR in English, whereas materials in other languages are less readily available.
Our retrospective study had several limitations. Demographic information that relied on clinic-dependent intake questionnaires may be limited due to variable intake processes and patients opting out of self-reporting. By comparing patient populations between 2 clinics, confounding variables such as location and hours of operation may impact the patient demographics recorded at the BFC vs UPMC. Resources and staff availability may affect the management strategies and follow-up care offered by each clinic. Our study period also was unique in that COVID-19 may have affected resources, staffing, scheduling, and logistics at both clinics.
Based on the aforementioned differences between the BFC and UPMC patient characteristics, care models should be strategically designed to support the needs of diverse populations. The BFC patient care model appropriately focuses on communication skills with patients with LEP by using interpreter services. Providing more skin care education and follow-up instructions in patients’ primary languages will help them develop a better understanding of their skin conditions. Another key asset of the BFC patient care model is its provision of social services such as transportation and insurance assistance.
To improve the UPMC patient care model, providing patients with bus tickets and car services may potentially reduce appointment cancellations. Using interpreter services to call and text appointment reminders, as well as interpreter resources to facilitate patient visits and patient instructions, also can mitigate language barriers for patients with LEP. Implementing a triage system into the UPMC scheduling system may help patients with more urgent skin conditions to be seen in a timely manner.
Other investigators have analyzed costs of care and proven the value of dermatologic services at free clinics to guide allocation of supplies and resources, demonstrating an area for future investigation at the BFC.13 A cost analysis of care provided at the BFC compared to UPMC could inform us about the value of the BFC’s services.
Conclusion
The dermatology clinics at the BFC and UPMC have distinct demographics, diagnoses, and management strategies to provide an inclusive patient care model. The services provided by both clinics are necessary to ensure that people in Pittsburgh have access to dermatologic care regardless of social barriers (eg, lack of health insurance, LEP). To achieve greater accessibility and health equity, dermatologic care at the BFC and UPMC can be improved by strengthening communication with people with LEP, providing skin care education, and offering social and scheduling services.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2. doi:10.1016/j.jaad.2016.12.043
- American Academy of Dermatology. Skin cancer. Accessed October 7, 2024. https://www.aad.org/media/stats-skin-cancer
- Suneja T, Smith ED, Chen GJ, et al. Waiting times to see a dermatologist are perceived as too long by dermatologists: implications for the dermatology workforce. Arch Dermatol. 2001;137:1303-1307. doi:10.1001/archderm.137.10.1303
- Grossberg AL, Carranza D, Lamp K, et al. Dermatologic care in the homeless and underserved populations: observations from the Venice Family Clinic. Cutis. 2012;89:25-32.
- Amini A, Rusthoven CG, Waxweiler TV, et al. Association of health insurance with outcomes in adults ages 18 to 64 years with melanoma in the United States. J Am Acad Dermatol. 2016;74:309-316. doi:10.1016/j.jaad.2015.09.054
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.e2. doi:10.1016/j.jaad .2017.04.1137
- Darnell JS. Free clinics in the United States: a nationwide survey. Arch Intern Med. 2010;170:946-953. doi:10.1001/archinternmed .2010.107
- Madray V, Ginjupalli S, Hashmi O, et al. Access to dermatology services at free medical clinics: a nationwide cross-sectional survey. J Am Acad Dermatol. 2019;81:245-246. doi:10.1016/j.jaad.2018.12.011
- Pennsylvania free and income-based clinics. Accessed October 7, 2024. https://www.freeclinics.com/sta/pennsylvania
- United States Census Bureau. Decennial census. P1: race. Accessed October 7, 2024. https://data.census.gov/table/DECENNIALPL2020.P1?g=160XX00US4261000
- United States Census Bureau. American community survey. S1601: language spoken at home. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020S1601?g=160XX00US4261000
- United States Census Bureau. S2701: selected characteristics of health insurance coverage in the United States. Accessed October 7, 2024. https://data.census.gov/table/ACSST5Y2020.S2701?g=160XX00US4261000
- Lin CP, Loy S, Boothe WD, et al. Value of Dermatology Nights at a student-run free clinic. Proc (Bayl Univ Med Cent). 2020;34:260-261. doi:10.1080/08998280.2020.1834771
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
Comparing Patient Care Models at a Local Free Clinic vs an Insurance- Based University Medical Center
PRACTICE POINTS
- Both free clinics and insurance-based health care systems serve dermatology patients with diverse characteristics, necessitating inclusive health care models.
- Dermatologic care can be improved at both free and insurance-based clinics by strengthening communication with individuals with limited English proficiency, providing skin care education, and offering social and scheduling services such as transportation, insurance assistance, and triage.