User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Docs fervently hope federal ban on noncompete clauses goes through
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
Teledermatology follow-up after Mohs surgery gets a thumbs up from patients
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – The , according to new findings.
In addition, nearly all patients surveyed (91.4%) were willing to go through electronic follow-up again.
“A big takeaway from our study is that streamlining this process is really essential for successful implementation,” said study author Laura Rezac, MD, a PGY IV dermatology resident at the University of Mississippi, Jackson. “This study demonstrated the flexibility and convenience for both patients and surgeons and can serve as a prototype for future innovation.”
The study results were presented at the annual meeting of the American College of Mohs Surgery.
The role of telehealth has rapidly expanded over the past decade, with its use accelerating during the COVID-19 pandemic and transforming into an indispensable resource. It can be synchronous, Dr. Rezac explained, which is when telehealth happens in live, real-time settings where the patient interacts with a clinician. This usually occurs via phone or video, and providers and patients communicate directly.
Conversely, asynchronous telehealth, also known as “store-and-forward,” is often used for patient intake or follow-up care. For example, in dermatology, a patient can send a photo of a skin condition that is then reviewed by a dermatologist later.
“A pilot survey regarding the adoption of telemedicine in Mohs surgery found that, although most dermatologic surgeons felt that it can play a role, most said that they didn’t plan on using it after the pandemic,” said Dr. Rezac.
The survey, which was reported by this news organization, found that 80% of surveyed surgeons said that they turned to telemedicine during the pandemic, compared with just 23% who relied on the technology prior to the pandemic.
There were numerous perceived barriers to the use of telemedicine, and the one most commonly cited was the uncertainty of how telemedicine fits in the workflow of clinical practice. Other limitations reported were for physical exams (88%), patient response and training (57%), reimbursement concerns (50%), implementation of the technology (37%), regulations such as HIPAA (24%), training of staff (17%), and licensing (8%).
“The survey did identify one key use of telemedicine in Mohs and that was for [postoperative] visits,” she said. “But thus far, a postoperative evaluation after Mohs via an integrated asynchronous ‘store and forward’ teledermatology platform has not yet been evaluated.”
In the study, Dr. Rezac and colleagues sought to evaluate feasibility and efficacy, as well as patient attitudes, using a telemedicine platform for postoperative follow-up. A total of 163 patients who were treated with Mohs at a single academic institution during the 9-month study period (December 2021 through August 2022) responded to a survey and elected to participate in postoperative follow-up using telemedicine.
Dr. Rezac explained how their procedure was implemented for the patient. “On the day of the follow-up, the patient receives a text with a link that takes them to the MyChart website or app on their phone,” she said. “Once they log in, they see that they have a message telling them that they have a teledermatology message waiting for them. When they view it, they are taken to the curated message with instructions and a phone call if they need assistance, and then at the bottom, it shows they have a task to complete, which is the questionnaire.”
The patient will then be prompted to upload photos, which can be taken with their phone camera. The next step is to answer questions regarding the surgical site or pain concerns, and finally, patients are asked to respond to a few short questions about this type of follow-up. Once submitted, then they wait to be contacted by the surgeon.
On the surgeon’s side, these answers come into their EPIC inbox, and they can respond via a MyChart message.
Patient response was overwhelmingly positive, Dr. Rezac noted. Of the patients, 80.4% found the electronic surgery follow-up process to be “easy” or “very easy,” while only 4% found it “difficult” or “very difficult,” she said. “Also, 75.5% preferred electronic follow-up while 17.2% preferred in-person follow-up.”
There were limitations to this study, primarily that the asynchronous method does reduce live interaction, which could be an issue, depending on person’s needs, she pointed out. “But it is easy to schedule a phone call or video call or office visit.”
“The universal barrier is how to adopt it into the workflow, which includes training of staff,” she continued, “But this was a very streamlined process and gave very detailed instructions to the staff. Additionally, widespread use is limited to dermatological proficiency and access, and patients have to be amenable to it, so there is a selection bias since these patients chose to participate.”
Asked to comment on the study, Vishal Patel, MD, director of cutaneous oncology at George Washington University in Washington, said: “The COVID pandemic changed how practices and providers considered follow-up visits for small routine matters. Postoperative visits are often simple and do not require an in-depth, in-person evaluation.” Dr. Patel was not involved with this research.
“This study highlights the comfort of the vast majority of patients to have follow-up postoperative visits conducted via teledermatology – an approach that can help cut overall costs and also increase access for patients who are more in need of in-office care,” he added.
No external funding of the study was reported. Dr. Rezac reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
Persistent Wounds Refractory to Broad-Spectrum Antibiotics
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.
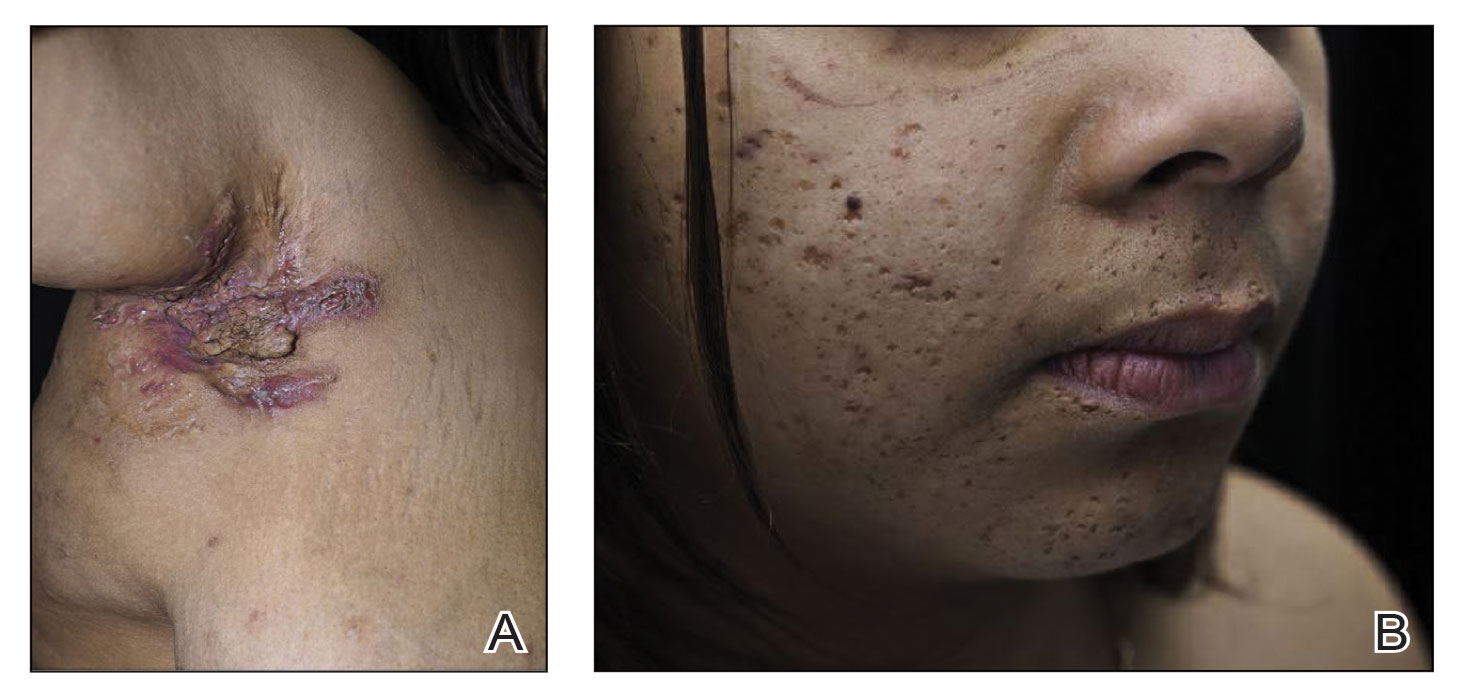
Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
The Diagnosis: PASH (Pyoderma Gangrenosum, Acne, Hidradenitis Suppurativa) Syndrome
Obtaining our patient’s history of hidradenitis suppurativa (HS), a hallmark sterile neutrophilic dermatosis, was key to making the correct diagnosis of PASH (pyoderma gangrenosum, acne, HS) syndrome. In our patient, the history of HS increased the consideration of pyoderma gangrenosum (PG) due to the persistent breast and leg wounds. Additionally, it was important to consider a diagnosis of PG in lesions that were not responding to broad-spectrum antimicrobial treatment. In our patient, the concurrent presentation of draining abscesses in the axillae (Figure, A) and inflammatory nodulocystic facial acne (Figure, B) were additional diagnostic clues that suggested the triad of PASH syndrome.

Although SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome also can present with cutaneous features of acne and HS, the lack of bone and joint involvement in our patient made this diagnosis less likely. Calciphylaxis can present as ulcerations on the lower extremities, but it usually presents with a livedolike pattern with overlying black eschar and is unlikely in the absence of underlying metabolic or renal disease. PAPA (pyogenic arthritis, PG, acne) syndrome is characterized by recurrent joint involvement and lacks features of HS. Lastly, our patient was immunocompetent with no risk factors for mycobacterial infection.
PASH syndrome is a rare inherited syndrome, but its constituent inflammatory conditions are ubiquitous. They share a common underlying mechanism consisting of overactivation of the innate immune systems driven by increased production of the inflammatory cytokines IL-1, IL-17, and tumor necrosis factor α, resulting in sterile neutrophilic dermatoses.1 The diagnosis is based on the clinical presentation, as laboratory investigations are nondiagnostic. Biopsies and cultures can be performed to rule out infectious etiologies. Additionally, PASH syndrome is considered part of a larger spectrum of syndromes including PAPA and PAPASH (pyogenic arthritis, acne, PG, HS) syndromes. The absence of pyogenic arthritis distinguishes PASH syndrome from PAPA and PAPASH syndromes.2 Clinically, PASH syndrome and the related sterile neutrophilic dermatoses share the characteristic of pronounced cutaneous involvement that substantially alters the patient’s quality of life. Cigarette smoking is an exacerbating factor and has a well-established association with HS.3 Therefore, smoking cessation should be encouraged in these patients to avoid exacerbation of the disease process.
Maintaining adequate immunosuppression is key to managing the underlying disease processes. Classic immunosuppressive agents such as systemic glucocorticoids and methotrexate may fail to satisfactorily control the disease.4 Treatment options currently are somewhat limited and are aimed at targeting the inflammatory cytokines that propagate the disease. The most consistent responses have been observed with anti–tumor necrosis factor α antagonists such as adalimumab, infliximab, and etanercept.5 Additionally, there is varied response to anakinra, suggesting the importance of selectively targeting IL-1β.6 Unfortunately, misdiagnosis for an infectious etiology is common, and antibiotics and debridement are of limited use for the underlying pathophysiology of PASH syndrome. Importantly, biopsy and debridement often are discouraged due to the risk of pathergy.7
Our case demonstrates the importance of maintaining a high clinical suspicion for immune-mediated lesions that are refractory to antimicrobial agents. Additionally, prior history of multiple neutrophilic dermatoses should prompt consideration for the PASH/PAPA/PAPASH disease spectrum. Early and accurate identification of neutrophilic dermatoses such as PG and HS are crucial to initiating proper cytokine-targeting treatment and achieving disease remission.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
- Cugno M, Borghi A, Marzano AV. PAPA, PASH and PAPASH syndromes: pathophysiology, presentation and treatment. Am J Clin Dermatol. 2017;18:555-562.
- Genovese G, Moltrasio C, Garcovich S, et al. PAPA spectrum disorders. G Ital Dermatol Venereol. 2020;155:542-550.
- König A, Lehmann C, Rompel R, et al. Cigarette smoking as a triggering factor of hidradenitis suppurativa. Dermatology. 1999;198:261-264.
- Ahn C, Negus D, Huang W. Pyoderma gangrenosum: a review of pathogenesis and treatment. Expert Rev Clin Immunol. 2018;14:225-233.
- Saint-Georges V, Peternel S, Kaštelan M, et al. Tumor necrosis factor antagonists in the treatment of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) syndrome. Acta Dermatovenerol Croat. 2018;26:173-178.
- Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66:409-415.
- Patel DK, Locke M, Jarrett P. Pyoderma gangrenosum with pathergy: a potentially significant complication following breast reconstruction. J Plast Reconstr Aesthet Surg. 2017;70:884-892.
A 28-year-old Black woman presented to the hospital for evaluation of worsening leg wounds as well as a similar eroding plaque on the left breast of 1 month’s duration. Broad-spectrum antibiotics prescribed during a prior emergency department visit resulted in no improvement. Her medical history was notable for hidradenitis suppurativa that previously was well controlled on adalimumab prior to discontinuation 1 year prior. A review of systems was negative for fever, chills, shortness of breath, chest pain, night sweats, and arthralgia. The patient had discontinued the antibiotics and was not taking any other medications at the time of presentation. She reported a history of smoking cigarettes (5 pack years). Physical examination revealed hyperkeratotic eroded plaques with violaceous borders circumferentially around the left breast (top) and legs with notable undermining (bottom). Inflammatory nodulocystic acne of the face as well as sinus tract formation with purulent drainage in the axillae also were present. Laboratory workup revealed an elevated erythrocyte sedimentation rate (116 mm/h [reference range, <20 mm/h]). Computed tomography of the leg wound was negative for soft-tissue infection. Aerobic and anaerobic tissue cultures demonstrated no growth.

Foot ulcers red flag for eye disease in diabetes
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Sores on the feet can signal problems with the eyes in patients with diabetes.
Prior research and anecdotal experience show that diabetic foot ulcers and diabetic retinopathy frequently co-occur.
David J. Ramsey, MD, PhD, MPH, director of ophthalmic research at Lahey Hospital & Medical Center, Burlington, Mass., said when clinicians detect either condition, they should involve a team that can intervene to help protect a patient’s vision and mobility.
For example, they should ensure patients receive comprehensive eye and foot evaluations and help them optimize diabetes management.
The new study, presented at the annual meeting of the Association for Research in Vision and Ophthalmology, “adds an important dimension” to understanding the association between the conditions, said Dr. Ramsey, who recently reviewed correlations between diabetic foot ulcers and diabetic retinopathy and their underlying causes.
“Patients with diabetic foot ulcers appear to receive less attention to their diabetic retinopathy and may receive fewer treatments with eye injections targeting vascular endothelial growth factor (VEGF), an important driver of progression of diabetic retinopathy,” said Dr. Ramsey, who is also an associate professor of ophthalmology at Tufts University School of Medicine, Boston. He was not involved in the study presented at ARVO 2023.
In the new study, Christopher T. Zhu, a medical student at UT Health San Antonio, and colleagues analyzed data from 426 eyes of 213 patients with type 2 diabetes who had had at least two eye exams between 2012 and 2022; 72 of the patients had diabetic foot ulcers. Patients were followed for about 4 years on average.
Patients with diabetic foot ulcers had a higher percentage of eyes with macular edema on their initial exam (32.6% vs. 28%). By the final exam, the percentage of eyes with macular edema was significantly greater in the group with diabetic foot ulcers (64.6% vs. 37.6%; P < .0001), Mr. Zhu’s group reported.
Eyes with nonproliferative diabetic retinopathy progressed to proliferative diabetic retinopathy, the worst grade, at a higher rate in the group with foot ulcers (50.6% vs. 35.6%; P = .03). In addition, patients with foot ulcers were more likely to experience vitreous hemorrhage (55.6% vs. 38.7%), the researchers found.
Despite patients with foot ulcers tending to have worse disease, they received fewer treatments for retinopathy. Those without ulcers received an average of 6.9 anti-VEGF injections per eye, while those with ulcers averaged 4.3.
Foot ulcers may hinder the ability of patients to get to appointments to receive the injections, Mr. Zhu and colleagues wrote. “For many patients in our part of the country [South Texas], a lack of transportation is a particular barrier to health care access,” Mr. Zhu told this news organization.
Mr. Zhu’s team conducted their study after noticing that patients with diabetes and foot ulcers who presented to their eye clinics “appeared to progress faster to worse grades of retinopathy” than patients with diabetes who did not have ulcers.
“Similar to how foot ulcers develop due to a severe disruption in blood flow [vascular] and a loss of sensation [neurologic], diabetic retinopathy may have a relation to microvascular disease, neurologic degeneration, and inflammation,” he said.
The findings confirm “that poor perfusion of the eye and foot are linked and can cause ischemic retinopathy leading to the development of proliferative diabetic retinopathy and vitreous hemorrhages, both serious, vision-threatening conditions,” Dr. Ramsey said.
To some extent, fewer treatments with anti-VEGF agents may account for why patients with foot ulcers have more eye complications, Dr. Ramsey added. “Additional research needs to be done to further dissect the cause and the effect, but it’s a very important finding that we need to increase awareness about,” he said.
Dr. Ramsey and Mr. Zhu reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ARVO 2023
Overcoming death anxiety: Understanding our lives and legacies
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Mohs surgery workforce continues to increase
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
SEATTLE – At least for now, and that has been the case for the past 5 years.
Using CMS billing codes as a surrogate, the researchers found that there was a steady increase in the number of physicians who billed from 2015 to 2020. With the exception of 2020, which was the height of the COVID-19 pandemic, the number of times that a specific code was billed for increased on average by 4.7% annually.
“Thus, if the attrition rate remains stable, even with changes in board certification and potential payer eligibility restrictions, the number of physicians will continue to increase,” study author Ji Won Ahn, MD, who specializes in dermatology and Mohs surgery at University of Pittsburgh Medical Center, said at the annual meeting of the American College of Mohs Surgery, where she presented the results.
The growth in the number of Mohs surgeons has been fueled by several factors, including a rising incidence of skin cancer as well as the superior cure rates and cosmetic outcomes with the procedure. Reimbursement has been favorable and training pathways have expanded. A 2019 retrospective study reported that there were 2,240 dermatologists who performed Mohs surgery in the United States, with nearly all of them (94.6%) residing in metropolitan areas.
Dr. Ahn explained that it was important to define the workforce because of several new factors that will be affecting it in the future. “With the establishment of Micrographic Surgery and Dermatologic Oncology [MSDO] board certification that went into effect 2 years ago, potential future payer eligibility restrictions may be coming,” she said. “The adequacy of the Mohs surgery workforce is an important consideration.”
Another issue is that new board certification will be limited to fellowship-trained physicians after the first 5 years. “We wanted to compare these numbers with the fellowship numbers,” she said. “Although fellowship numbers are something that the college potentially has the power to change.”
Dr. Ahn and colleagues used the Centers for Medicare & Medicaid Services database to evaluate the use of the Current Procedural Terminology (CPT) code 17311, which is one of the most common billing codes for Mohs micrographic technique. Looking at data from 2015-2020, they found that there was an annual increase in the number of unique national provider identifiers (NPIs) billing for 17311, at an average rate of 75.6 per year.
The total number of times that 17311 was billed also increased from 2015 to 2019 at an average rate of 4.7% per year but declined in 2020 by 8.4%. “Overall, there was an average of 135 new NPIs that appeared and an average of 59.4 NPIs that stopped billing for 17311,” thus, an attrition rate of 59 surgeons, Dr. Ahn explained.
She emphasized that notably, the number of approved MSDO fellowship spots has remained stable since 2016 and is about 92 to 93 per year. “There are about 135 new surgeons and about two-thirds are new fellowship graduates,” she said.
The researchers were also interested in seeing how saturated each surgeon was and looked at the approximate number of cases that they were handling.
Of the physicians who billed 17311 through CMS, over 26% billed less than 100 times and more than 45% billed less than 200 times, and over 80% billed less than 500 times.
“One might be able to conclude that there might be some potential flexibility depending on the future need for surgeons,” she said.
The study was limited by several factors, one being that the researchers looked only at CPT code 17311 and not other designated codes for Mohs surgery. Other factors such as staff and space limitations were not accounted for since only billing data were used.
Dr. Ahn and her team are going to continue their work, and the next steps are to look at geographic trends and monitor for insurance network eligibility changes. “We are currently doing a workforce survey so we can better understand our current workforce rather than just historical data,” she concluded.
Asked to comment on the results, Vishal Patel, MD, assistant professor of dermatology and director of the cutaneous oncology program at George Washington University, Washington, who was not involved with the study, noted that the increase in the “billing rates of the first stage of Mohs micrographic surgery highlights not only the growing skin cancer epidemic, but also the number of providers who are providing these services. This underscores the importance of standardized training guidelines and board certifications of Mohs micrographic surgeons to assure high levels of patient care and the appropriate use of Mohs micrographic surgery,” he said.
No external funding of the study was reported. Dr. Ahn reported no relevant financial relationships. Dr. Patel is a consultant for Sanofi, Regeneron, and Almirall.
A version of this article originally appeared on Medscape.com.
AT ACMS 2023
CDC: Drug-resistant ringworm reported in New York
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
BY ALICIA AULT
The in New York.
Tinea, or ringworm, one of the most common fungal infections, is responsible for almost 5 million outpatient visits and 690 hospitalizations annually, according to the CDC.
Over the past 10 years, severe, antifungal-resistant tinea has spread in South Asia, in part because of the rise of a new dermatophyte species known as Trichophyton indotineae, wrote the authors of a report on the two patients with the drug-resistant strain. This epidemic “has likely been driven by misuse and overuse of topical antifungals and corticosteroids,” added the authors, in Morbidity and Mortality Weekly Report.
The cases were detected by a New York City dermatologist. In the first case, a 28-year-old woman developed a widespread pruritic eruption in the summer of 2021. She did not consult a dermatologist until December, when she was in the third trimester of pregnancy. She had large, annular, scaly, pruritic plaques on her neck, abdomen, pubic region, and buttocks, but had no underlying medical conditions, no known exposures to someone with a similar rash, and no recent international travel history.
After she gave birth in January, she started oral terbinafine therapy but had no improvement after 2 weeks. Clinicians administered a 4-week course of itraconazole, which resolved the infection.
The second patient, a 47-year-old woman with no medical conditions, developed a rash while in Bangladesh in the summer of 2022. Other family members had a similar rash. She was treated with topical antifungal and steroid combination creams but had no resolution. Back in the United States, she was prescribed hydrocortisone 2.5% ointment and diphenhydramine, clotrimazole cream, and terbinafine cream in three successive emergency department visits. In December 2022, dermatologists, observing widespread, discrete, scaly, annular, pruritic plaques on the thighs and buttocks, prescribed a 4-week course of oral terbinafine. When the rash did not resolve, she was given 4 weeks of griseofulvin. The rash persisted, although there was 80% improvement. Clinicians are now considering itraconazole. The woman’s son and husband are also being evaluated, as they have similar rashes.
In both cases, skin culture isolates were initially identified as Trichophyton mentagrophytes. Further analysis at the New York State Department of Health’s lab, using Sanger sequencing of the internal transcribed spacer region of the ribosomal gene, followed by phylogenetic analysis, identified the isolates as T. indotineae.
The authors note that culture-based techniques used by most clinical laboratories typically misidentify T. indotineae as T. mentagrophytes or T. interdigitale. Genomic sequencing must be used to properly identify T. indotineae, they wrote.
Clinicians should consider T. indotineae in patients with widespread ringworm, especially if they do not improve with topical antifungals or oral terbinafine, said the authors. If T. indotineae is suspected, state or local public health departments can direct clinicians to testing.
The authors report no relevant financial relationships.
Evolve your website
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The past few years have seen major transformations in the way health care websites operate and interact with patients. .
In mid-2018, a major Google algorithm change, known to the IT community as the “Medic Update,” significantly changed search criteria for most health and wellness websites. Another big update went live in late 2021. Websites that have not evolved with these changes have dropped in search rankings and provide a poorer user experience all around.
Many potential patients are searching for your services online, so your website cannot be an afterthought. Not only does it need to be designed with your target audience in mind, but it is also important to consider the metrics Google and other search engines now use when assessing the quality of your website so that patients will find it in the first place.
Here are some features that you (or your website company) need to prioritize to keep your site current and atop search results in 2023 and beyond.
Begin with an understandable URL. Search engines use URLs to determine how well your site, or a portion of it, matches search criteria. URLs also need to make sense to searchers, especially when they link specific areas of expertise (more on that in a minute). For example, a URL like “jonesdermatology.com/?p=89021” is meaningless to anyone except programmers; but “jonesdermatology.com/psoriasistreatments” obviously leads to a page about psoriasis treatments. Search engines look for not only the most relevant, but also the most helpful and user-friendly answers to a user’s query.
Incidentally, if the URL for your site is not your own name, you should register your name as a separate domain name – even if you never use it – to be sure that a trickster or troll, or someone with the same name but a bad reputation, doesn’t get it.
Continue with a good meta description. That’s the grayish text that follows the title and URL in search results. Searchers will read it to confirm that your site is what they seek, so make sure it describes exactly what you do, including any areas of special expertise.
Make your practice approachable with photos. New patients are more comfortable when they know what you look like, so real photos of you and your staff are always more effective than stock photos of models. Photos or a video tour of your office will reassure prospective patients that they will be visiting a clean, modern, professional facility.
Describe your principal services in detail. You never know which specific service a prospective patient is searching for, so describe everything you offer. Don’t try to summarize everything on a single page; relevance is determined by how deeply a topic is covered, so each principal service should have a detailed description on its own page. Not only will your skills become more visible to search engines, but you can also use the space to enumerate your qualifications and expertise in each area. Whenever possible, write your descriptions in question-and-answer form. Searchers tend to ask questions (“what is the best ... ?”), particularly in voice searches. Search engines increasingly value sites that ask and answer common questions.
Make your site interactive. “Interactivity” is a major buzzword in modern search engine parlance. Once searchers make an appointment, they stop searching. If they have to wait until the next day to call your office, they may keep looking – and might find a competitor with online scheduling. HIPAA-compliant chatbots, secure messaging, and online patient portals to access medical records, lab results, and other important information will also set your site apart.
Testimonials are essential. Amazon.com taught us that candid reviews from customers go a long way toward building the trust necessary to buy products and services, and nowhere is that truer than for medical services. According to one study, when it comes to finding a doctor, 88% of people trust online reviews as much as a personal recommendation. Loyal patients will be happy to write you glowing reviews; feature them prominently.
How does your site look on small screens? More than half of all searches are now made on smartphones, so the more mobile-friendly your site is, the higher it will be ranked. Prospective patients who are forced to scroll forever, or zoom in to tap a link, are likely to become frustrated and move on. Mobile searchers prefer sites that provide the best experience for the least amount of effort, and rankings tend to reflect that preference. You can test how easily a visitor can use your website on a mobile device with Google’s free Mobile-Friendly Test..
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Men underrepresented in clinical trials of laser hair removal, review finds
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
PHOENIX – .
To characterize the sex of patients in trials evaluating hair removal with energy-based devices, Dr. Lee, an internal medicine intern at Beth Israel Deaconess Medical Center, Boston, and Jessica Labadie, MD, director of lasers and cosmetic surgery at the Icahn School of Medicine at Mount Sinai, New York, conducted a systematic review using PubMed with the search query hair AND laser AND removal AND (dermatology OR skin OR cutaneous). They limited the analysis to English-language clinical trials that investigated a laser and light-based therapy as an intervention and if hair reduction was an outcome, and excluded studies that did not include the face as a treatment area and laser hair removal for diseases with disproportionate occurrence in females or males, such as polycystic ovarian syndrome or pseudofolliculitis barbae.
Of 121 articles identified from the PubMed search, 28 studies involving 3,882 patients treated with lasers or intense pulsed light (IPL) for hair removal were included in the final analysis. Of these 28 articles, 22 (79%) reported the sex of trial participants. The population of these 22 studies included 3,104 (88.7%) females, 384 (11.0%) males, and 11 (0.003%) nonbinary identifying patients. None of the studies evaluated laser hair removal outcomes by sex.
“This study adds to the current knowledge of laser hair removal as a part of gender-affirming care by characterizing the representation of assigned sexes of patients in clinical trials evaluating the effectiveness of laser hair removal,” Dr. Lee told this news organization. “It highlights the underrepresentation of people assigned to male sex at birth in these clinical trials, despite this population’s potential interest in laser hair removal as a part of gender-affirming care.”
She acknowledged certain limitations of the review, including the absence of reporting on sex in the demographic sections of many trials and the exclusion of trials that did not include treatment of the face. “Clinicians need to be aware of the underrepresentation of men in clinical trials evaluating laser hair removal, and this may limit their understanding of treatment outcomes in this particular cohort,” she concluded. “Clinicians should emphasize inclusivity in future laser hair removal clinical trials and include outcomes by sex.”
The study “looks at an important aspect of clinical trials in the device-based space,” said Omar A. Ibrahimi, MD, PhD, medical director of the Connecticut Skin Institute, Stamford, who was not involved in the study and was asked to comment on the results. “Laser hair removal is the most commonly performed procedure in aesthetic energy-based device dermatology. While these trials are often very small compared to drug trials, it highlights that men are a very underrepresented cohort in laser hair removal trials,” he said. “More recently, there is an increased interest in gender-affirming procedures, and this has highlighted the need to ensure we include a diverse spectrum of patients in devices-based research studies. This is a very challenging mandate but certainly one we should strive for to make efforts to be more inclusive when designing these clinical studies so that the information we gain from these studies is more broadly applicable.”
The researchers reported having no financial disclosures. Dr. Ibrahimi disclosed that he is a member of the advisory board for Accure Acne, AbbVie, Cutera, Lutronic, Blueberry Therapeutics, Cytrellis, and Quthero, and holds stock in many device and pharmaceutical companies.
AT ASLMS 2023
Facial, hand, and foot dermatitis: Lebrikizumab and dupilumab show efficacy in new studies
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
in a secondary analysis of randomized, double-blind, placebo-controlled phase 3 trials of the drug, Jenny E. Murase, MD, reported at the annual Revolutionizing Atopic Dermatitis conference.
At week 16 in the ADvocate 1, ADvocate 2, and ADhere trials, with and without concomitant topical corticosteroid (TCS) use, at least 58% of treated patients experienced improvement in facial dermatitis, and 62% or more experienced improvement in hand dermatitis – statistically significant differences over placebo.
“Lebrikizumab was efficacious in clearing and improving facial and hand dermatitis, burdensome and difficult-to-treat areas, in most patients with moderate to severe AD,” said Dr. Murase, of the department of dermatology at the University of California, San Francisco, and director of medical dermatology consultative services and patch testing for the Palo Alto (Calf.) Foundation Medical Group.
In another late-breaking abstract presented at the RAD conference, the injectable biologic dupilumab – now in its 6th year on the market – was reported by Jonathan I. Silverberg, MD, PhD, MPH, to “rapidly and significantly” improve the signs, symptoms, and quality of life in some adults and adolescents with moderate to severe hand and foot AD in a recently completed phase 3 trial of dupilumab.
Lebrikizumab results for facial, hand dermatitis
The ADvocate 1 and ADvocate 2 trials evaluated lebrikizumab monotherapy and randomized patients to receive 250 mg subcutaneously every 2 weeks (after a 500-mg loading dose at baseline and week 2) or placebo. (Patients who received any corticosteroid as a rescue medication were considered nonresponders.) The ADhere trial compared low to mid–potency TCS plus lebrikizumab, using the same dosing of lebrikizumab as in the ADvocate studies, versus TCS plus placebo.
In all three trials, with a total of more than 1,000 participants, clinicians assessed for the presence or absence of facial or hand dermatitis at baseline. At week 16, they then assessed the change from baseline based on a 4-point scale of cleared, improved, no change, and worsened. “Improvement” was defined as cleared or improved.
Both facial and hand dermatitis were identified in a majority of patients at baseline. For instance, in ADvocate 1, facial dermatitis was identified in 71.4% of patients in the lebrikizumab group and 80.9% of those in the placebo group. Hand dermatitis was identified in 72% and 73% of the treatment and placebo groups, respectively.
Across the trials, at 16 weeks, 58%-69% of adult and adolescent patients receiving lebrikizumab had improvement in facial dermatitis, compared with 22%-46% on placebo. For hand dermatitis, 62%-73% experienced improvement, compared with 19%-43% on placebo, respectively. Proportions of improved patients in both the lebrikizumab and placebo groups were highest in the ADhere trial, Dr. Murase reported.
In the ADvocate trials, 16 weeks marked the end of the induction phase and the start of a 36-week maintenance period. The ADhere trial was a 16-week study. Overall results from ADhere were published in January in JAMA Dermatology, and results from the 16-week induction period of the ADvocate trials were published in March in the New England Journal of Medicine.
Lebrikizumab received fast-track designation for AD by the Food and Drug Administration in 2019. Regulatory decisions in the United States and the European Union are expected later this year, according to a press release from Eli Lilly, the drug’s developer.
Asked to comment on the study results, Zelma Chiesa Fuxench, MD, MSCE, assistant professor of dermatology at the University of Pennsylvania, Philadelphia, called the post-hoc results promising. “While newer, more targeted treatments for AD offer the possibility of overall improvement and long-term disease control, we do not have sufficient data to help guide us when it comes to selecting treatment based on which area of the body is affected,” she explained. Most published findings have used “overall scores and not scores stratified by body region.”
The new findings, “help expand our current understanding of how the drug works for different areas of the body,” which can help inform treatment discussions with patients, she added.
AD can be especially challenging to treat when it involves “what are considered to be more sensitive areas such as the face or hands,” said Dr. Chiesa Fuxench. Challenges may include poor tolerance to topical medications, concerns for safety with long-term use, and the need for constant reapplication.
“Those of us who treat a large number of AD patients suspect that the impact and/or burden of AD may be different depending on what areas of the body are affected,” but more data are needed, she added. Limitations of the study, she noted, include “that the study may not have been adequately powered and that the sample size was small.”
Dupilumab result for hand, foot dermatitis
The phase 3 LIBERTY-AD-HAFT trial randomized 133 patients with moderate to severe atopic hand and/or foot dermatitis to a 16-week course of dupilumab (Dupixent) monotherapy, 300 mg every 2 weeks in adults and 200 or 300 mg every 2 weeks in adolescents, or placebo. Patients were then followed during a 12-week safety follow-up period.
Significantly more patients in the dupilumab group achieved the primary endpoint of a hand and foot Investigator Global Assessment (IGA) score of 0/1 at 16 weeks: 40.3% vs. 16.7% in the placebo group (P = .003). Statistical significance was reached at week 8, reported Dr. Silverberg, professor of dermatology and director of clinical research at George Washington University, Washington. Dupilumab, a human monoclonal IgG4 antibody that inhibits IL-4 and IL-13 signaling, is FDA approved for treating moderate to severe AD in patients age 6 months and older, among other indications.
In addition, the proportion of patients achieving a 4-point or greater improvement in the weekly average of daily hand and foot Peak Pruritus Numerical Rating Scale (PPNRS), the key secondary endpoint, was about fourfold greater with dupilumab: 52.2%, compared with 13.6% on placebo (P < .0001). This reduction in itch reached statistical significance by week 1. Dupilumab-treated patients also experienced significant improvement in other lesion measures and in Quality of Life in Hand Eczema Questionnaire scores, Dr. Silverberg noted.
The patients had a mean age in their 30s and a mean duration of atopic hand and/or foot dermatitis of 15-16 years. For more than one-quarter of patients, morphology was hyperkeratotic, which “has to be one of the toughest subsets to affect positive change in,” he said.
About 40% of patients had lesions on the hands only, and more than half had lesions on both hands and feet. “This is pretty realistic – we generally don’t see much isolated foot dermatitis in the AD population,” Dr. Silverberg said.
About 70%-75% had concomitant AD outside of the hands and feet, mostly of moderate severity. Patients with positive patch tests or whose hand and foot eczema was believed to be driven by irritants were excluded from the trial, as were patients who had used TCS or other topical treatments within 2 weeks of the baseline visit.
Rescue medication use was low (3% with dupilumab vs. 21% with placebo), and adverse events were “pretty consistent with everything we’ve seen with dupilumab,” said Dr. Silverberg.
Commenting on this study, Dr. Chiesa Fuxench said she was “excited to see [the findings], as hand and foot AD can often be quite challenging to treat in clinic.” The improvements in overall disease scores, itch, and quality of life scores – with fairly good tolerance – are “reassuring and what we would expect based on our current experience with dupilumab,” she said.
The lebrikizumab study was funded by Dermira, a wholly owned subsidiary of Eli Lilly. The dupilumab study was sponsored by Sanofi and Regeneron Pharmaceuticals. Some of the data were also reported by lead investigator Eric Simpson, MD, of Oregon Health and Science University at the annual meeting of the American Academy of Dermatology in March 2023.
Dr. Murase reported consulting/advising for Eli Lilly, Leo Pharma, UCB, Sanofi-Genzyme, and non-CME speaking/honoraria for UCB and Regeneron. Dr. Silverberg reported consulting fees and fees for non-CME services from Sanofi Genzyme, Regeneron, Pfizer, and other companies. Dr. Chiesa Fuxench, who was a speaker at the RAD meeting but was not involved in the studies, disclosed receiving honoraria for CME work in AD sponsored by education grants from Regeneron/Sanofi, and grant/research support from Lilly, Regeneron, and Sanofi, among other disclosures.
AT RAD 2023












