User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Untreatable, drug-resistant fungus found in Texas and Washington, D.C.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The CDC has reported two clusters of Candida auris infections resistant to all antifungal medications in long-term care facilities in 2021. Because these panresistant infections occurred without any exposure to antifungal drugs, the cases are even more worrisome. These clusters are the first time such nosocomial transmission has been detected.
In the District of Columbia, three panresistant isolates were discovered through screening for skin colonization with resistant organisms at a long-term acute care facility (LTAC) that cares for patients who are seriously ill, often on mechanical ventilation.
In Texas, the resistant organisms were found both by screening and in specimens from ill patients at an LTAC and a short-term acute care hospital that share patients. Two were panresistant, and five others were resistant to fluconazole and echinocandins.
These clusters occurred simultaneously and independently of each other; there were no links between the two institutions.
Colonization of skin with C. auris can lead to invasive infections in 5%-10% of affected patients. Routine skin surveillance cultures are not commonly done for Candida, although perirectal cultures for vancomycin-resistant enterococci and nasal swabs for MRSA have been done for years. Some areas, like Los Angeles, have recommended screening for C. auris in high-risk patients – defined as those who were on a ventilator or had a tracheostomy admitted from an LTAC or skilled nursing facility in Los Angeles County, New York, New Jersey, or Illinois.
In the past, about 85% of C. auris isolates in the United States have been resistant to azoles (for example, fluconazole), 33% to amphotericin B, and 1% to echinocandins. Because of generally strong susceptibility, an echinocandin such as micafungin or caspofungin has been the drug of choice for an invasive Candida infection.
C. auris is particularly difficult to deal with for several reasons. First, it can continue to live in the environment, on both dry or moist surfaces, for up to 2 weeks. Outbreaks have occurred both from hand (person-to-person) transmission or via inanimate surfaces that have become contaminated. Equally troublesome is that people become colonized with the yeast indefinitely.
Meghan Lyman, MD, of the fungal diseases branch of the CDC’s National Center for Emerging and Zoonotic Infectious Diseases, said in an interview that facilities might be slow in recognizing the problem and in identifying the organism. “We encounter problems in noninvasive specimens, especially urine,” Dr. Lyman added.
“Sometimes ... they consider Candida [to represent] colonization so they will often not speciate it.” She emphasized the need for facilities that care for ventilated patients to consider screening. “Higher priority ... are places in areas where there’s a lot of C. auris transmission or in nearby areas that are likely to get introductions.” Even those that do speciate may have difficulty identifying C. auris.
Further, Dr. Lyman stressed “the importance of antifungal susceptibility testing and testing for resistance. Because that’s also something that’s not widely available at all hospitals and clinical labs ... you can send it to the [CDC’s] antimicrobial resistance lab network” for testing.
COVID-19 has brought particular challenges. Rodney E. Rohde, PhD, MS, professor and chair, clinical lab science program, Texas State University, San Marcos, said in an interview that he is worried about all the steroids and broad-spectrum antibiotics patients receive.
They’re “being given medical interventions, whether it’s ventilators or [extracorporeal membrane oxygenation] or IVs or central lines or catheters for UTIs and you’re creating highways, right for something that may be right there,” said Dr. Rohde, who was not involved in the CDC study. “It’s a perfect storm, not just for C. auris, but I worry about bacterial resistance agents, too, like MRSA and so forth, having kind of a spike in those types of infections with COVID. So, it’s kind of a doubly dangerous time, I think.”
Multiresistant bacteria are a major health problem, causing illnesses in 2.8 million people annually in the United States, and causing about 35,000 deaths.
Dr. Rohde raised another, rarely mentioned concern. “We’re in crisis mode. People are leaving our field more than they ever had before. The medical laboratory is being decimated because people have burned out after these past 14 months. And so I worry just about competent medical laboratory professionals that are on board to deal with these types of other crises that are popping up within hospitals and long-term care facilities. It kind of keeps me awake.”
Dr. Rohde and Dr. Lyman shared their concern that COVID caused a decrease in screening for other infections and drug-resistant organisms. Bare-bones staffing and shortages of personal protective equipment have likely fueled the spread of these infections as well.
In an outbreak of C. auris in a Florida hospital’s COVID unit in 2020, 35 of 67 patients became colonized, and 6 became ill. The epidemiologists investigating thought that contaminated gowns or gloves, computers, and other equipment were likely sources of transmission.
Low pay, especially in nursing homes, is another problem Dr. Rohde mentioned. It’s an additional problem in both acute and long-term care that “some of the lowest-paid people are the environmental services people, and so the turnover is crazy.” Yet, we rely on them to keep everyone safe. He added that, in addition to pay, he “tries to give them the appreciation and the recognition that they really deserve.”
There are a few specific measures that can be taken to protect patients. Dr. Lyman concluded. “The best way is identifying cases and really ensuring good infection control to prevent the spread.” It’s back to basics – limiting broad-spectrum antibiotics and invasive medical devices, and especially good handwashing and thorough cleaning.
Dr. Lyman and Dr. Rohde have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Persistent Panniculitis in Dermatomyositis
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
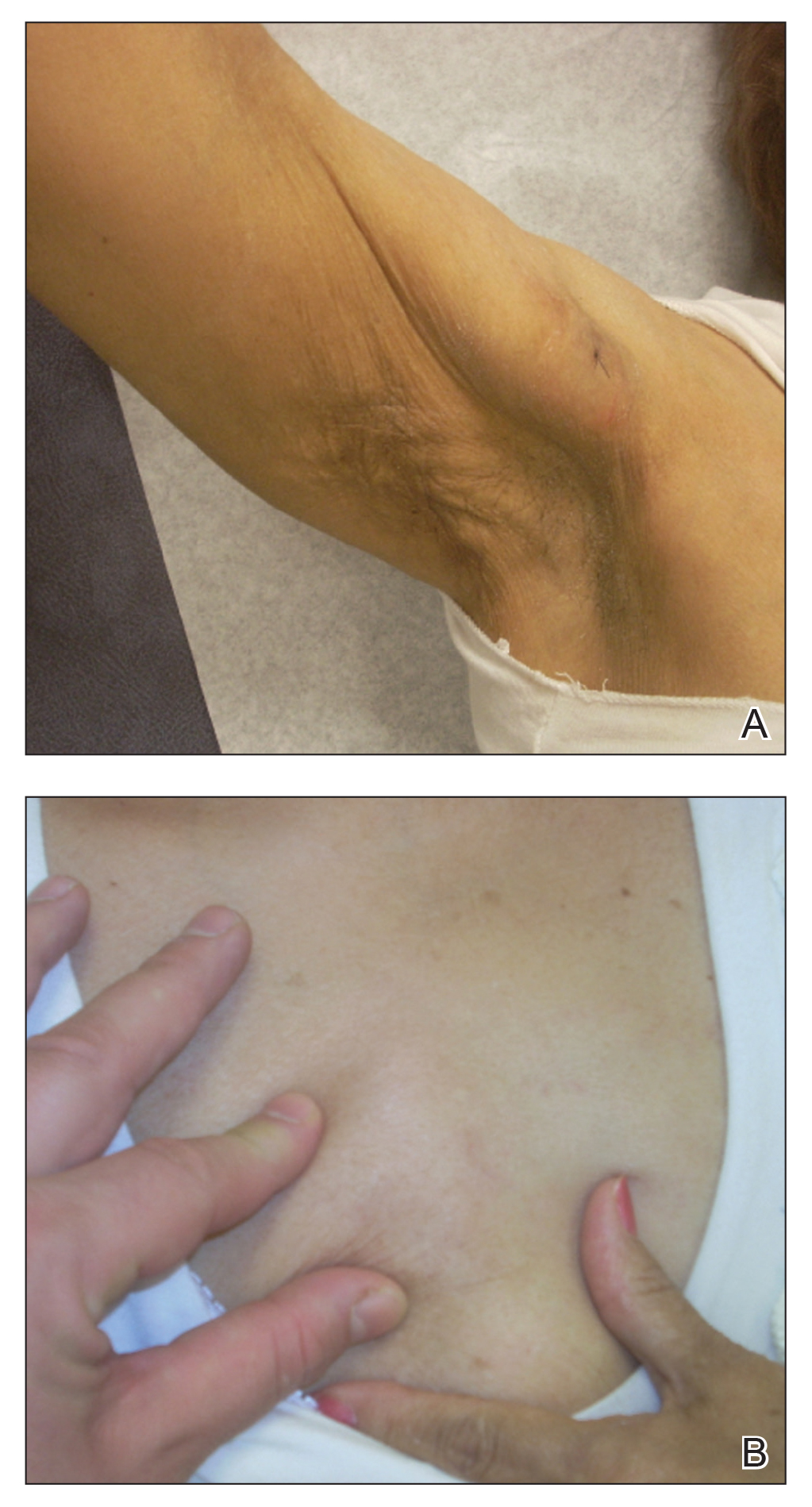
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.
The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.

The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
To the Editor:
A 62-year-old woman with a history of dermatomyositis (DM) presented to dermatology clinic for evaluation of multiple subcutaneous nodules. Two years prior to the current presentation, the patient was diagnosed by her primary care physician with DM based on clinical presentation. She initially developed body aches, muscle pain, and weakness of the upper extremities, specifically around the shoulders, and later the lower extremities, specifically around the thighs. The initial physical examination revealed pain with movement, tenderness to palpation, and proximal extremity weakness. The patient also noted a 50-lb weight loss. Over the next year, she noted dysphagia and developed multiple subcutaneous nodules on the right arm, chest, and left axilla. Subsequently, she developed a violaceous, hyperpigmented, periorbital rash and erythema of the anterior chest. She did not experience hair loss, oral ulcers, photosensitivity, or joint pain.
Laboratory testing in the months following the initial presentation revealed a creatine phosphokinase level of 436 U/L (reference range, 20–200 U/L), an erythrocyte sedimentation rate of 60 mm/h (reference range, <31 mm/h), and an aldolase level of 10.4 U/L (reference range, 1.0–8.0 U/L). Lactate dehydrogenase and thyroid function tests were within normal limits. Antinuclear antibodies, anti–double-stranded DNA, anti-Smith antibodies, anti-ribonucleoprotein, anti–Jo-1 antibodies, and anti–smooth muscle antibodies all were negative. Total blood complement levels were elevated, but complement C3 and C4 were within normal limits. Imaging demonstrated normal chest radiographs, and a modified barium swallow confirmed swallowing dysfunction. A right quadricep muscle biopsy confirmed the diagnosis of DM. A malignancy work-up including mammography, colonoscopy, and computed tomography of the chest, abdomen, and pelvis was negative aside from nodular opacities in the chest. She was treated with prednisone (60 mg, 0.9 mg/kg) daily and methotrexate (15–20 mg) weekly for several months. While the treatment attenuated the rash and improved weakness, the nodules persisted, prompting a referral to dermatology.
Physical examination at the dermatology clinic demonstrated the persistent subcutaneous nodules were indurated and bilaterally located on the arms, axillae, chest, abdomen, buttocks, and thighs with no pain or erythema (Figure). Laboratory tests demonstrated a normal creatine phosphokinase level, elevated erythrocyte sedimentation rate (70 mm/h), and elevated aldolase level (9.3 U/L). Complement levels were elevated, though complement C3 and C4 remained within normal limits. Histopathology of nodules from the medial right upper arm and left thigh showed lobular panniculitis with fat necrosis, calcification, and interface changes. The patient was treated for several months with daily mycophenolate mofetil (1 g increased to 3 g) and daily hydroxychloroquine (200 mg) without any effect on the nodules.

The histologic features of panniculitis in lupus and DM are similar and include multifocal hyalinization of the subcuticular fat and diffuse lobular infiltrates of mature lymphocytes without nuclear atypia.1 Though clinical panniculitis is a rare finding in DM, histologic panniculitis is a relatively common finding.2 Despite the similar histopathology of lupus and DM, the presence of typical DM clinical and laboratory features in our patient (body aches, muscle pain, proximal weakness, cutaneous manifestations, elevated creatine phosphokinase, normal complement C3 and C4) made a diagnosis of DM more likely.
Clinical panniculitis is a rare subcutaneous manifestation of DM with around 50 cases reported in the literature (Table). A PubMed search of articles indexed for MEDLINE was conducted using the terms dermatomyositis and panniculitis through July 2019. Additionally, a full-text review and search of references within these articles was used to identify all cases of patients presenting with panniculitis in the setting of DM. Exclusion criteria were cases in which another etiology was considered likely (infectious panniculitis and lupus panniculitis) as well as those without an English translation. We identified 43 cases; the average age of the patients was 39.6 years, and 36 (83.7%) of the cases were women. Patients typically presented with persistent, indurated, painful, erythematous, nodular lesions localized to the arms, abdomen, buttocks, and thighs.
While panniculitis has been reported preceding and concurrent with a diagnosis of DM, a number of cases described presentation as late as 5 years following onset of classic DM symptoms.12,13,31 In some cases (3/43 [7.0%]), panniculitis was the only cutaneous manifestation of DM.15,33,36 However, it occurred more commonly with other characteristic skin findings, such as heliotrope rash or Gottron sign.Some investigators have recommended that panniculitis be included as a diagnostic feature of DM and that DM be considered in the differential diagnosis in isolated cases of panniculitis.25,33
Though it seems panniculitis in DM may correlate with a better prognosis, we identified underlying malignancies in 3 cases. Malignancies associated with panniculitis in DM included ovarian adenocarcinoma, nasopharyngeal carcinoma, and parotid carcinoma, indicating that appropriate cancer screening still is critical in the diagnostic workup.2,11,22
A majority of the reported panniculitis cases in DM have responded to treatment with prednisone; however, treatment with prednisone has been more recalcitrant in other cases. Reports of successful additional therapies include methotrexate, cyclosporine, azathioprine, hydroxychloroquine, intravenous immunoglobulin, mepacrine, or a combination of these entities.19,22 In most cases, improvement of the panniculitis and other DM symptoms occurred simultaneously.25 It is noteworthy that the muscular symptoms often resolved more rapidly than cutaneous manifestations.33 Few reported cases (6 including the current case) found a persistent panniculitis despite improvement and remission of the myositis.3,5,10,11,30
Our patient was treated with both prednisone and methotrexate for several months, leading to remission of muscular symptoms (along with return to baseline of creatine phosphokinase), yet the panniculitis did not improve. The subcutaneous nodules also did not respond to treatment with mycophenolate mofetil and hydroxychloroquine.
Recent immunohistochemical studies have suggested that panniculitic lesions show better outcomes with immunosuppressive therapy when compared with other DM-related skin lesions.40 However, this was not the case for our patient, who after months of immunosuppressive therapy showed complete resolution of the periorbital and chest rashes with persistence of multiple indurated subcutaneous nodules.
Our case adds to a number of reports of DM presenting with panniculitis. Our patient fit the classic demographic of previously reported cases, as she was an adult woman without evidence of underlying malignancy; however, our case remains an example of the therapeutic challenge that exists when encountering a persistent, treatment-resistant panniculitis despite resolution of all other features of DM.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Wick MR. Panniculitis: a summary. Semin Diagn Pathol. 2017;34:261-272.
- Girouard SD, Velez NF, Penson RT, et al. Panniculitis associated with dermatomyositis and recurrent ovarian cancer. Arch Dermatol. 2012;148:740-744.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:E187-E188.
- Choi YJ, Yoo WH. Panniculitis, a rare presentation of onset and exacerbation of juvenile dermatomyositis: a case report and literature review. Arch Rheumatol. 2018;33:367-371.
- Azevedo PO, Castellen NR, Salai AF, et al. Panniculitis associated with amyopathic dermatomyositis. An Bras Dermatol. 2018;93:119-121.
- Agulló A, Hinds B, Larrea M, et al. Livedo racemosa, reticulated ulcerations, panniculitis and violaceous plaques in a 46-year-old woman. Indian Dermatol Online J. 2018;9:47-49.
- Hattori Y, Matsuyama K, Takahashi T, et al. Anti-MDA5 antibody-positive dermatomyositis presenting with cellulitis-like erythema on the mandible as an initial symptom. Case Rep Dermatol. 2018;10:110-114.
- Hasegawa A, Shimomura Y, Kibune N, et al. Panniculitis as the initial manifestation of dermatomyositis with anti-MDA5 antibody. Clin Exp Dermatol. 2017;42:551-553.
- Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol. 2016;43:951-953.
- Carroll M, Mellick N, Wagner G. Dermatomyositis panniculitis: a case report. Australas J Dermatol. 2015;56:224‐226.
- Chairatchaneeboon M, Kulthanan K, Manapajon A. Calcific panniculitis and nasopharyngeal cancer-associated adult-onset dermatomyositis: a case report and literature review. Springerplus. 2015;4:201.
- Otero Rivas MM, Vicente Villa A, González Lara L, et al. Panniculitis in juvenile dermatomyositis. Clin Exp Dermatol. 2015;40:574-575.
- Yanaba K, Tanito K, Hamaguchi Y, et al. Anti‐transcription intermediary factor‐1γ/α/β antibody‐positive dermatomyositis associated with multiple panniculitis lesions. Int J Rheum Dis. 2015;20:1831-1834.
- Pau-Charles I, Moreno PJ, Ortiz-Ibanez K, et al. Anti-MDA5 positive clinically amyopathic dermatomyositis presenting with severe cardiomyopathy. J Eur Acad Dermatol Venereol. 2014;28:1097-1102.
- Lamb R, Digby S, Stewart W, et al. Cutaneous ulceration: more than skin deep? Clin Exp Dermatol. 2013;38:443-445.
- Arias M, Hernández MI, Cunha LG, et al. Panniculitis in a patient with dermatomyositis. An Bras Dermatol. 2011;86:146-148.
- Hemmi S, Kushida R, Nishimura H, et al. Magnetic resonance imaging diagnosis of panniculitis in dermatomyositis. Muscle Nerve. 2010;41:151-153.
- Geddes MR, Sinnreich M, Chalk C. Minocycline-induced dermatomyositis. Muscle Nerve. 2010;41:547-549.
- Abdul‐Wahab A, Holden CA, Harland C, et al Calcific panniculitis in adult‐onset dermatomyositis. Clin Exp Dermatol. 2009;34:E854-E856.
- Carneiro S, Alvim G, Resende P, et al. Dermatomyositis with panniculitis. Skinmed. 2007;6:46-47.
- Carrera E, Lobrinus JA, Spertini O, et al. Dermatomyositis, lobarpanniculitis and inflammatory myopathy with abundant macrophages. Neuromuscul Disord. 2006;16:468-471.
- Lin JH, Chu CY, Lin RY. Panniculitis in adult onset dermatomyositis: report of two cases and review of the literature. Dermatol Sinica. 2006;24:194-200.
- Chen GY, Liu MF, Lee JY, et al. Combination of massive mucinosis, dermatomyositis, pyoderma gangrenosum-like ulcer, bullae and fatal intestinal vasculopathy in a young female. Eur J Dermatol. 2005;15:396-400.
- Nakamori A, Yamaguchi Y, Kurimoto I, et al. Vesiculobullous dermatomyositis with panniculitis without muscle disease. J Am Acad Dermatol. 2003;49:1136-1139.
- Solans R, Cortés J, Selva A, et al. Panniculitis: a cutaneous manifestation of dermatomyositis. J Am Acad Dermatol. 2002;46:S148-S150.
- Chao YY, Yang LJ. Dermatomyositis presenting as panniculitis. Int J Dermatol. 2000;39:141-144.
- Lee MW, Lim YS, Choi JH, et al. Panniculitis showing membranocystic changes in the dermatomyositis. J Dermatol. 1999;26:608‐610.
- Ghali FE, Reed AM, Groben PA, et al. Panniculitis in juvenile dermatomyositis. Pediatr Dermatol. 1999;16:270-272.
- Molnar K, Kemeny L, Korom I, et al. Panniculitis in dermatomyositis: report of two cases. Br J Dermatol. 1998;139:161‐163.
- Ishikawa O, Tamura A, Ryuzaki K, et al. Membranocystic changes in the panniculitis of dermatomyositis. Br J Dermatol. 1996;134:773-776.
- Sabroe RA, Wallington TB, Kennedy CT. Dermatomyositis treated with high-dose intravenous immunoglobulins and associated with panniculitis. Clin Exp Dermatol. 1995;20:164-167.
- Neidenbach PJ, Sahn EE, Helton J. Panniculitis in juvenile dermatomyositis. J Am Acad Dermatol. 1995;33:305-307.
- Fusade T, Belanyi P, Joly P, et al. Subcutaneous changes in dermatomyositis. Br J Dermatol. 1993;128:451-453.
- Winkelmann WJ, Billick RC, Srolovitz H. Dermatomyositis presenting as panniculitis. J Am Acad Dermatol. 1990;23:127-128.
- Commens C, O’Neill P, Walker G. Dermatomyositis associated with multifocal lipoatrophy. J Am Acad Dermatol. 1990;22:966-969.
- Raimer SS, Solomon AR, Daniels JC. Polymyositis presenting with panniculitis. J Am Acad Dermatol. 1985;13(2 pt 2):366‐369.
- Feldman D, Hochberg MC, Zizic TM, et al. Cutaneous vasculitis in adult polymyositis/dermatomyositis. J Rheumatol. 1983;10:85-89.
- Kimura S, Fukuyama Y. Tubular cytoplasmic inclusions in a case of childhood dermatomyositis with migratory subcutaneous nodules. Eur J Pediatr. 1977;125:275-283.
- Weber FP, Gray AMH. Chronic relapsing polydermatomyositis with predominant involvement of the subcutaneous fat. Br J Dermatol. 1924;36:544-560.
- Santos‐Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
Practice Points
- Clinical panniculitis is a rare subcutaneous manifestation of dermatomyositis (DM) that dermatologists must consider when evaluating patients with this condition.
- Panniculitis can precede, occur simultaneously with, or develop up to 5 years after onset of DM.
- Many patients suffer from treatment-resistant panniculitis in DM, suggesting that therapeutic management of this condition may require long-term and more aggressive treatment modalities.
COVID-19: Delta variant is raising the stakes
Empathetic conversations with unvaccinated people desperately needed
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Empathetic conversations with unvaccinated people desperately needed
Empathetic conversations with unvaccinated people desperately needed
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
Like many colleagues, I have been working to change the minds and behaviors of acquaintances and patients who are opting to forgo a COVID vaccine. The large numbers of these unvaccinated Americans, combined with the surging Delta coronavirus variant, are endangering the health of us all.
When I spoke with the 22-year-old daughter of a family friend about what was holding her back, she told me that she would “never” get vaccinated. I shared my vaccination experience and told her that, except for a sore arm both times for a day, I felt no side effects. Likewise, I said, all of my adult family members are vaccinated, and everyone is fine. She was neither moved nor convinced.
Finally, I asked her whether she attended school (knowing that she was a college graduate), and she said “yes.” So I told her that all 50 states require children attending public schools to be vaccinated for diseases such as diphtheria, tetanus, polio, and the chickenpox – with certain religious, philosophical, and medical exemptions. Her response was simple: “I didn’t know that. Anyway, my parents were in charge.” Suddenly, her thinking shifted. “You’re right,” she said. She got a COVID shot the next day. Success for me.
When I asked another acquaintance whether he’d been vaccinated, he said he’d heard people were getting very sick from the vaccine – and was going to wait. Another gentleman I spoke with said that, at age 45, he was healthy. Besides, he added, he “doesn’t get sick.” When I asked another acquaintance about her vaccination status, her retort was that this was none of my business. So far, I’m batting about .300.
But as a physician, I believe that we – and other health care providers – must continue to encourage the people in our lives to care for themselves and others by getting vaccinated. One concrete step advised by the Centers for Disease Control and Prevention is to help people make an appointment for a shot. Some sites no longer require appointments, and New York City, for example, offers in-home vaccinations to all NYC residents.
Also, NYC Mayor Bill de Blasio announced Aug. 3 the “Key to NYC Pass,” which he called a “first-in-the-nation approach” to vaccination. Under this new policy, vaccine-eligible people aged 12 and older in New York City will need to prove with a vaccination card, an app, or an Excelsior Pass that they have received at least one dose of vaccine before participating in indoor venues such as restaurants, bars, gyms, and movie theaters within the city. Mayor de Blasio said the new initiative, which is still being finalized, will be phased in starting the week of Aug. 16. I see this as a major public health measure that will keep people healthy – and get them vaccinated.
The medical community should support this move by the city of New York and encourage people to follow CDC guidance on wearing face coverings in public settings, especially schools. New research shows that physicians continue to be among the most trusted sources of vaccine-related information.
Another strategy we might use is to point to the longtime practices of surgeons. We could ask: Why do surgeons wear face masks in the operating room? For years, these coverings have been used to protect patients from the nasal and oral bacteria generated by operating room staff. Likewise, we can tell those who remain on the fence that, by wearing face masks, we are protecting others from all variants, but specifically from Delta – which the CDC now says can be transmitted by people who are fully vaccinated.
Why did the CDC lift face mask guidance for fully vaccinated people in indoor spaces in May? It was clear to me and other colleagues back then that this was not a good idea. Despite that guidance, I continued to wear a mask in public places and advised anyone who would listen to do the same.
The development of vaccines in the 20th and 21st centuries has saved millions of lives. The World Health Organization reports that 4 million to 5 million lives a year are saved by immunizations. In addition, research shows that, before the emergence of SARS-CoV-2, vaccinations led to the eradication of smallpox and polio, and a 74% drop in measles-related deaths between 2004 and 2014.
Protecting the most vulnerable
With COVID cases surging, particularly in parts of the South and Midwest, I am concerned about children under age 12 who do not yet qualify for a vaccine. Certainly, unvaccinated parents could spread the virus to their young children, and unvaccinated children could transmit the illness to immediate and extended family. Now that the CDC has said that there is a risk of SARS-CoV-2 breakthrough infection among fully vaccinated people in areas with high community transmission, should we worry about unvaccinated young children with vaccinated parents? I recently spoke with James C. Fagin, MD, a board-certified pediatrician and immunologist, to get his views on this issue.
Dr. Fagin, who is retired, said he is in complete agreement with the Food and Drug Administration when it comes to approving medications for children. However, given the seriousness of the pandemic and the need to get our children back to in-person learning, he would like to see the approval process safely expedited. Large numbers of unvaccinated people increase the pool for the Delta variant and could increase the likelihood of a new variant that is more resistant to the vaccines, said Dr. Fagin, former chief of academic pediatrics at North Shore University Hospital and a former faculty member in the allergy/immunology division of Cohen Children’s Medical Center, both in New York.
Meanwhile, I agree with the American Academy of Pediatrics’ recommendations that children, teachers, and school staff and other adults in school settings should wear masks regardless of vaccination status. Kids adjust well to masks – as my grandchildren and their friends have.
The bottom line is that we need to get as many people as possible vaccinated as soon as possible, and while doing so, we must continue to wear face coverings in public spaces. As clinicians, we have a special responsibility to do all that we can to change minds – and behaviors.
Dr. London is a practicing psychiatrist who has been a newspaper columnist for 35 years, specializing in and writing about short-term therapy, including cognitive-behavioral therapy and guided imagery. He is author of “Find Freedom Fast” (New York: Kettlehole Publishing, 2019). He has no conflicts of interest.
FDA approves anifrolumab (Saphnelo) as first new lupus treatment in more than 10 years
Anifrolumab, an inhibitor of type 1 interferons, received approval from the Food and Drug Administration for the treatment of adults with moderate to severe systemic lupus erythematosus (SLE) who are receiving standard therapy, according to a statement released Aug. 2 from its manufacturer, AstraZeneca.
Anifrolumab will be marketed as Saphnelo. It is a fully human monoclonal antibody against subunit 1 of the type 1 interferon receptor, and its approval represents the only new treatment approved for patients with SLE in a decade. The recommended dosage is 300 mg as an intravenous infusion over a 30-minute period every 4 weeks, according to its prescribing information, and it will be sold in a single-dose vial containing 300 mg/2 mL (150 mg/mL).
Increased type I interferon (IFN) signaling is associated with increased disease activity in patients with SLE, and the option of a type I IFN receptor antagonist may allow physicians to treat patients with fewer corticosteroids, according to the statement.
The approval was based on data from three trials. The TULIP (Treatment of Uncontrolled Lupus via the Interferon Pathway) phase 3 research included two randomized, double-blind, placebo-controlled studies, TULIP-1 and TULIP-2. The TULIP trials each enrolled seropositive patients with moderate to severe active disease despite standard-of-care therapy (SOC), which included oral corticosteroids, antimalarials, and immunosuppressants (methotrexate, azathioprine, or mycophenolate mofetil). All patients met American College of Rheumatology criteria and had an SLE Disease Activity Index (SLEDAI)-2K of 6 or greater, as well as British Isles Lupus Assessment Group (BILAG) index scoring showing one or more organ systems with grade A involvement or two or more with grade B. Both trials required stable SOC therapy throughout the study except for mandatory attempts at oral corticosteroid tapering for patients who were receiving 10 mg/day or more of prednisone or its equivalent at study entry.
TULIP-1 failed to meet its primary endpoint of SLE Responder Index (SRI) at 52 weeks, but investigators determined after the trial that some patients taking anifrolumab had been inappropriately labeled as nonresponders because the trial automatically required any patient who used a restricted drug, including NSAIDs, to be classified as a nonresponder even if they used the medication for something unrelated to SLE. When these rules were amended in a post hoc analysis, differences between the groups treated with anifrolumab and placebo widened in secondary endpoints for oral corticosteroid dose reduction, Cutaneous Lupus Erythematosus Disease Activity Severity Index response, and BILAG-Based Composite Lupus Assessment (BICLA) response.
The TULIP-2 trial included 362 patients who received a fixed dose of 300 mg anifrolumab or a placebo intravenously every 4 weeks for 48 weeks. In this study, anifrolumab patients showed significant improvement in disease activity on the BICLA scale, compared with placebo patients. The BICLA response was 47.8% in patients taking anifrolumab and 31.5% in placebo-treated patients (P = .001).
In the MUSE phase 2 trial, 305 adults with SLE were randomized to a fixed-dose intravenous infusion of 300 mg or 1,000 mg of anifrolumab or a placebo every 4 weeks, plus SOC, for 48 weeks. Patients in this study showed significant improvement on either dose, compared with placebo.
The results from the MUSE trial were published online in Arthritis & Rheumatology Nov. 7, 2016, followed by the TULIP-1 trial in The Lancet Rheumatology Nov. 11, 2019, and the TULIP-2 trial in the New England Journal of Medicine Jan. 16, 2020.
The most common treatment-related adverse events in all three studies were nasopharyngitis, upper respiratory tract infection, bronchitis, infusion-related reactions, herpes zoster, and cough. Infusion-related reactions in the trials were similar in anifrolumab and placebo patients, and included headache, nausea, vomiting, fatigue, and dizziness.
Anifrolumab has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus and is not recommended for these patients, according to the statement.
AstraZeneca said in its statement that anifrolumab is also under regulatory review in Japan and the European Union, and it continues to evaluate anifrolumab in patients with SLE in a long-term extension phase 3 trial and a phase 3 trial assessing subcutaneous delivery. The company said it “is exploring the potential of Saphnelo in a variety of diseases where type I IFN plays a key role, including lupus nephritis, cutaneous lupus erythematosus, and myositis.”
Anifrolumab, an inhibitor of type 1 interferons, received approval from the Food and Drug Administration for the treatment of adults with moderate to severe systemic lupus erythematosus (SLE) who are receiving standard therapy, according to a statement released Aug. 2 from its manufacturer, AstraZeneca.
Anifrolumab will be marketed as Saphnelo. It is a fully human monoclonal antibody against subunit 1 of the type 1 interferon receptor, and its approval represents the only new treatment approved for patients with SLE in a decade. The recommended dosage is 300 mg as an intravenous infusion over a 30-minute period every 4 weeks, according to its prescribing information, and it will be sold in a single-dose vial containing 300 mg/2 mL (150 mg/mL).
Increased type I interferon (IFN) signaling is associated with increased disease activity in patients with SLE, and the option of a type I IFN receptor antagonist may allow physicians to treat patients with fewer corticosteroids, according to the statement.
The approval was based on data from three trials. The TULIP (Treatment of Uncontrolled Lupus via the Interferon Pathway) phase 3 research included two randomized, double-blind, placebo-controlled studies, TULIP-1 and TULIP-2. The TULIP trials each enrolled seropositive patients with moderate to severe active disease despite standard-of-care therapy (SOC), which included oral corticosteroids, antimalarials, and immunosuppressants (methotrexate, azathioprine, or mycophenolate mofetil). All patients met American College of Rheumatology criteria and had an SLE Disease Activity Index (SLEDAI)-2K of 6 or greater, as well as British Isles Lupus Assessment Group (BILAG) index scoring showing one or more organ systems with grade A involvement or two or more with grade B. Both trials required stable SOC therapy throughout the study except for mandatory attempts at oral corticosteroid tapering for patients who were receiving 10 mg/day or more of prednisone or its equivalent at study entry.
TULIP-1 failed to meet its primary endpoint of SLE Responder Index (SRI) at 52 weeks, but investigators determined after the trial that some patients taking anifrolumab had been inappropriately labeled as nonresponders because the trial automatically required any patient who used a restricted drug, including NSAIDs, to be classified as a nonresponder even if they used the medication for something unrelated to SLE. When these rules were amended in a post hoc analysis, differences between the groups treated with anifrolumab and placebo widened in secondary endpoints for oral corticosteroid dose reduction, Cutaneous Lupus Erythematosus Disease Activity Severity Index response, and BILAG-Based Composite Lupus Assessment (BICLA) response.
The TULIP-2 trial included 362 patients who received a fixed dose of 300 mg anifrolumab or a placebo intravenously every 4 weeks for 48 weeks. In this study, anifrolumab patients showed significant improvement in disease activity on the BICLA scale, compared with placebo patients. The BICLA response was 47.8% in patients taking anifrolumab and 31.5% in placebo-treated patients (P = .001).
In the MUSE phase 2 trial, 305 adults with SLE were randomized to a fixed-dose intravenous infusion of 300 mg or 1,000 mg of anifrolumab or a placebo every 4 weeks, plus SOC, for 48 weeks. Patients in this study showed significant improvement on either dose, compared with placebo.
The results from the MUSE trial were published online in Arthritis & Rheumatology Nov. 7, 2016, followed by the TULIP-1 trial in The Lancet Rheumatology Nov. 11, 2019, and the TULIP-2 trial in the New England Journal of Medicine Jan. 16, 2020.
The most common treatment-related adverse events in all three studies were nasopharyngitis, upper respiratory tract infection, bronchitis, infusion-related reactions, herpes zoster, and cough. Infusion-related reactions in the trials were similar in anifrolumab and placebo patients, and included headache, nausea, vomiting, fatigue, and dizziness.
Anifrolumab has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus and is not recommended for these patients, according to the statement.
AstraZeneca said in its statement that anifrolumab is also under regulatory review in Japan and the European Union, and it continues to evaluate anifrolumab in patients with SLE in a long-term extension phase 3 trial and a phase 3 trial assessing subcutaneous delivery. The company said it “is exploring the potential of Saphnelo in a variety of diseases where type I IFN plays a key role, including lupus nephritis, cutaneous lupus erythematosus, and myositis.”
Anifrolumab, an inhibitor of type 1 interferons, received approval from the Food and Drug Administration for the treatment of adults with moderate to severe systemic lupus erythematosus (SLE) who are receiving standard therapy, according to a statement released Aug. 2 from its manufacturer, AstraZeneca.
Anifrolumab will be marketed as Saphnelo. It is a fully human monoclonal antibody against subunit 1 of the type 1 interferon receptor, and its approval represents the only new treatment approved for patients with SLE in a decade. The recommended dosage is 300 mg as an intravenous infusion over a 30-minute period every 4 weeks, according to its prescribing information, and it will be sold in a single-dose vial containing 300 mg/2 mL (150 mg/mL).
Increased type I interferon (IFN) signaling is associated with increased disease activity in patients with SLE, and the option of a type I IFN receptor antagonist may allow physicians to treat patients with fewer corticosteroids, according to the statement.
The approval was based on data from three trials. The TULIP (Treatment of Uncontrolled Lupus via the Interferon Pathway) phase 3 research included two randomized, double-blind, placebo-controlled studies, TULIP-1 and TULIP-2. The TULIP trials each enrolled seropositive patients with moderate to severe active disease despite standard-of-care therapy (SOC), which included oral corticosteroids, antimalarials, and immunosuppressants (methotrexate, azathioprine, or mycophenolate mofetil). All patients met American College of Rheumatology criteria and had an SLE Disease Activity Index (SLEDAI)-2K of 6 or greater, as well as British Isles Lupus Assessment Group (BILAG) index scoring showing one or more organ systems with grade A involvement or two or more with grade B. Both trials required stable SOC therapy throughout the study except for mandatory attempts at oral corticosteroid tapering for patients who were receiving 10 mg/day or more of prednisone or its equivalent at study entry.
TULIP-1 failed to meet its primary endpoint of SLE Responder Index (SRI) at 52 weeks, but investigators determined after the trial that some patients taking anifrolumab had been inappropriately labeled as nonresponders because the trial automatically required any patient who used a restricted drug, including NSAIDs, to be classified as a nonresponder even if they used the medication for something unrelated to SLE. When these rules were amended in a post hoc analysis, differences between the groups treated with anifrolumab and placebo widened in secondary endpoints for oral corticosteroid dose reduction, Cutaneous Lupus Erythematosus Disease Activity Severity Index response, and BILAG-Based Composite Lupus Assessment (BICLA) response.
The TULIP-2 trial included 362 patients who received a fixed dose of 300 mg anifrolumab or a placebo intravenously every 4 weeks for 48 weeks. In this study, anifrolumab patients showed significant improvement in disease activity on the BICLA scale, compared with placebo patients. The BICLA response was 47.8% in patients taking anifrolumab and 31.5% in placebo-treated patients (P = .001).
In the MUSE phase 2 trial, 305 adults with SLE were randomized to a fixed-dose intravenous infusion of 300 mg or 1,000 mg of anifrolumab or a placebo every 4 weeks, plus SOC, for 48 weeks. Patients in this study showed significant improvement on either dose, compared with placebo.
The results from the MUSE trial were published online in Arthritis & Rheumatology Nov. 7, 2016, followed by the TULIP-1 trial in The Lancet Rheumatology Nov. 11, 2019, and the TULIP-2 trial in the New England Journal of Medicine Jan. 16, 2020.
The most common treatment-related adverse events in all three studies were nasopharyngitis, upper respiratory tract infection, bronchitis, infusion-related reactions, herpes zoster, and cough. Infusion-related reactions in the trials were similar in anifrolumab and placebo patients, and included headache, nausea, vomiting, fatigue, and dizziness.
Anifrolumab has not been evaluated in patients with severe active lupus nephritis or severe active central nervous system lupus and is not recommended for these patients, according to the statement.
AstraZeneca said in its statement that anifrolumab is also under regulatory review in Japan and the European Union, and it continues to evaluate anifrolumab in patients with SLE in a long-term extension phase 3 trial and a phase 3 trial assessing subcutaneous delivery. The company said it “is exploring the potential of Saphnelo in a variety of diseases where type I IFN plays a key role, including lupus nephritis, cutaneous lupus erythematosus, and myositis.”
FDA’s fast-track approval process exposed as lax, in need of reform
an in-depth investigation published in The BMJ has determined.
“Despite the pathway’s good intentions to accelerate ‘the availability of drugs that treat serious diseases,’ experts are concerned that it is now being exploited – to the detriment of patients, who may be prescribed a drug that offers little benefit and possible harm, and to taxpayers,” writes Elisabeth Mahase, clinical reporter at The BMJ, who carried out the analysis.
The FDA’s accelerated approval pathway is intended to provide earlier access to drugs for serious diseases when there is lingering uncertainty at the time of approval regarding the drug’s ultimate clinical benefit.
Required studies rarely completed
As part of this fast-track pathway, drug manufacturers must conduct postapproval, phase 4 confirmatory trials to verify the anticipated clinical benefit. If these trials indicate no benefit, FDA approval can be withdrawn.
However, the analysis of FDA data shows once they are approved drugs are rarely taken off the market.
The BMJ investigation that analyzed data up to the end of 2020 shows that 112 of the 253 (44%) medications granted accelerated approval have not been confirmed to be effective.
In addition, 24 (21%) of these questionable drugs have been on the market for more than 5 years and some have been on the market for more than 20 years – often with a hefty price tag.
Furthermore, only 16 drugs approved through the accelerated approval process have ever been withdrawn, and most were shown to be ineffective, but in some cases the confirmatory trials were never done, Ms. Mahase reports.
For example, the COX-2 inhibitor celecoxib (Celebrex), which was granted accelerated approval in 1999 for the treatment of familial adenomatous polyposis, was on the market for 12 years before the FDA finally asked Pfizer to voluntarily withdraw it for this indication because efficacy trials were never completed.
As part of The BMJ’s investigation, Ms. Mahase asked manufacturers of the 24 drugs that have remained on the market for more than 5 years whether they had conducted the required phase 4 confirmatory trials. Six of the drugs had been withdrawn, approved, or postponed.
Of the remaining 18 drugs, the manufacturers provided the relevant trial information for only six. Only four drugmakers had started to recruit patients; two said they were still in discussion with the FDA over the final trial design.
“These products routinely have side effects, but the benefit information is a lot less certain. That’s what we’re concerned about – that we may have drugs on the market that don’t have any benefits, but certainly predictably have harms associated with them,” Huseyin Naci, PhD, MHS, with the London School of Economics, comments in the report.
Call for reform
As reported by this news organization, a 2015 report by the General Accountability Office (GAO) concluded that the FDA does not do an effective job of tracking the clinical efficacy or the safety of drugs with expedited approval after they hit the market.
In April of this year, the Institute for Clinical and Economic Review (ICER) cited a lack of “credible threats” to withdraw approval if companies don’t do confirmatory trials – meaning drugmakers have little incentive to do the trials.
“There are some instances where the companies really do seem to be taking advantage of the accelerated approval pathway and are using it in a way that makes it harder to get at the truth about whether these products really are safe and effective,” Rachel Sachs, JD, MPH, Washington University, St. Louis, said in The BMJ article.
In addition, the authors of a recent viewpoint article in JAMA Internal Medicine assert the recent approval of the controversial anti-amyloid drug aducanumab (Aduhelm, Biogen) shows that the accelerated approval pathway needs to be reformed.
Despite the concerns, Ms. Mahase said all experts who spoke to The BMJ believe the accelerated approval pathway is still useful and can be beneficial to patients, although some changes are needed.
One effective reform might be to have confirmatory trials designed, and even started, as part of accelerated approval.
“One important piece of the puzzle is for the FDA itself to be tougher on these companies, to hold them to the bargain that they have agreed to, and to take action when the company has not met their obligations,” Ms. Sachs told the journal.
An FDA spokesperson told the BMJ that the agency is “committed to working with sponsors to ensure that confirmatory studies are completed in a timely manner.”
“We expect sponsors to commit all resources needed to move trials forward as effectively as possible, with the aim of completing trials as soon as is feasible, while assuring the quality of the data and the robustness of the results,” the agency said.
A version of this article first appeared on Medscape.com.
an in-depth investigation published in The BMJ has determined.
“Despite the pathway’s good intentions to accelerate ‘the availability of drugs that treat serious diseases,’ experts are concerned that it is now being exploited – to the detriment of patients, who may be prescribed a drug that offers little benefit and possible harm, and to taxpayers,” writes Elisabeth Mahase, clinical reporter at The BMJ, who carried out the analysis.
The FDA’s accelerated approval pathway is intended to provide earlier access to drugs for serious diseases when there is lingering uncertainty at the time of approval regarding the drug’s ultimate clinical benefit.
Required studies rarely completed
As part of this fast-track pathway, drug manufacturers must conduct postapproval, phase 4 confirmatory trials to verify the anticipated clinical benefit. If these trials indicate no benefit, FDA approval can be withdrawn.
However, the analysis of FDA data shows once they are approved drugs are rarely taken off the market.
The BMJ investigation that analyzed data up to the end of 2020 shows that 112 of the 253 (44%) medications granted accelerated approval have not been confirmed to be effective.
In addition, 24 (21%) of these questionable drugs have been on the market for more than 5 years and some have been on the market for more than 20 years – often with a hefty price tag.
Furthermore, only 16 drugs approved through the accelerated approval process have ever been withdrawn, and most were shown to be ineffective, but in some cases the confirmatory trials were never done, Ms. Mahase reports.
For example, the COX-2 inhibitor celecoxib (Celebrex), which was granted accelerated approval in 1999 for the treatment of familial adenomatous polyposis, was on the market for 12 years before the FDA finally asked Pfizer to voluntarily withdraw it for this indication because efficacy trials were never completed.
As part of The BMJ’s investigation, Ms. Mahase asked manufacturers of the 24 drugs that have remained on the market for more than 5 years whether they had conducted the required phase 4 confirmatory trials. Six of the drugs had been withdrawn, approved, or postponed.
Of the remaining 18 drugs, the manufacturers provided the relevant trial information for only six. Only four drugmakers had started to recruit patients; two said they were still in discussion with the FDA over the final trial design.
“These products routinely have side effects, but the benefit information is a lot less certain. That’s what we’re concerned about – that we may have drugs on the market that don’t have any benefits, but certainly predictably have harms associated with them,” Huseyin Naci, PhD, MHS, with the London School of Economics, comments in the report.
Call for reform
As reported by this news organization, a 2015 report by the General Accountability Office (GAO) concluded that the FDA does not do an effective job of tracking the clinical efficacy or the safety of drugs with expedited approval after they hit the market.
In April of this year, the Institute for Clinical and Economic Review (ICER) cited a lack of “credible threats” to withdraw approval if companies don’t do confirmatory trials – meaning drugmakers have little incentive to do the trials.
“There are some instances where the companies really do seem to be taking advantage of the accelerated approval pathway and are using it in a way that makes it harder to get at the truth about whether these products really are safe and effective,” Rachel Sachs, JD, MPH, Washington University, St. Louis, said in The BMJ article.
In addition, the authors of a recent viewpoint article in JAMA Internal Medicine assert the recent approval of the controversial anti-amyloid drug aducanumab (Aduhelm, Biogen) shows that the accelerated approval pathway needs to be reformed.
Despite the concerns, Ms. Mahase said all experts who spoke to The BMJ believe the accelerated approval pathway is still useful and can be beneficial to patients, although some changes are needed.
One effective reform might be to have confirmatory trials designed, and even started, as part of accelerated approval.
“One important piece of the puzzle is for the FDA itself to be tougher on these companies, to hold them to the bargain that they have agreed to, and to take action when the company has not met their obligations,” Ms. Sachs told the journal.
An FDA spokesperson told the BMJ that the agency is “committed to working with sponsors to ensure that confirmatory studies are completed in a timely manner.”
“We expect sponsors to commit all resources needed to move trials forward as effectively as possible, with the aim of completing trials as soon as is feasible, while assuring the quality of the data and the robustness of the results,” the agency said.
A version of this article first appeared on Medscape.com.
an in-depth investigation published in The BMJ has determined.
“Despite the pathway’s good intentions to accelerate ‘the availability of drugs that treat serious diseases,’ experts are concerned that it is now being exploited – to the detriment of patients, who may be prescribed a drug that offers little benefit and possible harm, and to taxpayers,” writes Elisabeth Mahase, clinical reporter at The BMJ, who carried out the analysis.
The FDA’s accelerated approval pathway is intended to provide earlier access to drugs for serious diseases when there is lingering uncertainty at the time of approval regarding the drug’s ultimate clinical benefit.
Required studies rarely completed
As part of this fast-track pathway, drug manufacturers must conduct postapproval, phase 4 confirmatory trials to verify the anticipated clinical benefit. If these trials indicate no benefit, FDA approval can be withdrawn.
However, the analysis of FDA data shows once they are approved drugs are rarely taken off the market.
The BMJ investigation that analyzed data up to the end of 2020 shows that 112 of the 253 (44%) medications granted accelerated approval have not been confirmed to be effective.
In addition, 24 (21%) of these questionable drugs have been on the market for more than 5 years and some have been on the market for more than 20 years – often with a hefty price tag.
Furthermore, only 16 drugs approved through the accelerated approval process have ever been withdrawn, and most were shown to be ineffective, but in some cases the confirmatory trials were never done, Ms. Mahase reports.
For example, the COX-2 inhibitor celecoxib (Celebrex), which was granted accelerated approval in 1999 for the treatment of familial adenomatous polyposis, was on the market for 12 years before the FDA finally asked Pfizer to voluntarily withdraw it for this indication because efficacy trials were never completed.
As part of The BMJ’s investigation, Ms. Mahase asked manufacturers of the 24 drugs that have remained on the market for more than 5 years whether they had conducted the required phase 4 confirmatory trials. Six of the drugs had been withdrawn, approved, or postponed.
Of the remaining 18 drugs, the manufacturers provided the relevant trial information for only six. Only four drugmakers had started to recruit patients; two said they were still in discussion with the FDA over the final trial design.
“These products routinely have side effects, but the benefit information is a lot less certain. That’s what we’re concerned about – that we may have drugs on the market that don’t have any benefits, but certainly predictably have harms associated with them,” Huseyin Naci, PhD, MHS, with the London School of Economics, comments in the report.
Call for reform
As reported by this news organization, a 2015 report by the General Accountability Office (GAO) concluded that the FDA does not do an effective job of tracking the clinical efficacy or the safety of drugs with expedited approval after they hit the market.
In April of this year, the Institute for Clinical and Economic Review (ICER) cited a lack of “credible threats” to withdraw approval if companies don’t do confirmatory trials – meaning drugmakers have little incentive to do the trials.
“There are some instances where the companies really do seem to be taking advantage of the accelerated approval pathway and are using it in a way that makes it harder to get at the truth about whether these products really are safe and effective,” Rachel Sachs, JD, MPH, Washington University, St. Louis, said in The BMJ article.
In addition, the authors of a recent viewpoint article in JAMA Internal Medicine assert the recent approval of the controversial anti-amyloid drug aducanumab (Aduhelm, Biogen) shows that the accelerated approval pathway needs to be reformed.
Despite the concerns, Ms. Mahase said all experts who spoke to The BMJ believe the accelerated approval pathway is still useful and can be beneficial to patients, although some changes are needed.
One effective reform might be to have confirmatory trials designed, and even started, as part of accelerated approval.
“One important piece of the puzzle is for the FDA itself to be tougher on these companies, to hold them to the bargain that they have agreed to, and to take action when the company has not met their obligations,” Ms. Sachs told the journal.
An FDA spokesperson told the BMJ that the agency is “committed to working with sponsors to ensure that confirmatory studies are completed in a timely manner.”
“We expect sponsors to commit all resources needed to move trials forward as effectively as possible, with the aim of completing trials as soon as is feasible, while assuring the quality of the data and the robustness of the results,” the agency said.
A version of this article first appeared on Medscape.com.
COVID-19 leaves wake of medical debt among U.S. adults
Despite the passage of four major relief bills in 2020 and 2021 and federal efforts to offset pandemic- and job-related coverage loss, many people continued to face financial challenges, especially those with a low income and those who are Black or Latino.
The survey, which included responses from 5,450 adults, revealed that 10% of adults aged 19-64 were uninsured during the first half of 2021, a rate lower than what was recorded in 2020 and 2019 in both federal and private surveys. However, uninsured rates were highest among those with low income, those younger than 50 years old, and Black and Latino adults.
For most adults who lost employee health insurance, the coverage gap was relatively brief, with 54% saying their coverage gap lasted 3-4 months. Only 16% of adults said coverage gaps lasted a year or longer.
“The good news is that this survey is suggesting that the coverage losses during the pandemic may have been offset by federal efforts to help people get and maintain health insurance coverage,” lead author Sara Collins, PhD, Commonwealth Fund vice president for health care coverage, access, and tracking, said in an interview.
“The bad news is that a third of Americans continue to struggle with medical bills and medical debt, even among those who have health insurance coverage,” Dr. Collins added.
Indeed, the survey found that about one-third of insured adults reported a medical bill problem or that they were paying off medical debt, as did approximately half of those who were uninsured. Medical debt caused 35% of respondents to use up most or all of their savings to pay it off.
Meanwhile, 27% of adults said medical bills left them unable to pay for necessities such as food, heat, or rent. What surprised Dr. Collins was that 43% of adults said they received a lower credit rating as a result of their medical debt, and 35% said they had taken on more credit card debt to pay off these bills.
“The fact that it’s bleeding over into people’s financial security in terms of their credit scores, I think is something that really needs to be looked at by policymakers,” Dr. Collins said.
When analyzed by race/ethnicity, the researchers found that 55% of Black adults and 44% of Latino/Hispanic adults reported medical bills and debt problems, compared with 32% of White adults. In addition, 47% of those living below the poverty line also reported problems with medical bills.
According to the survey, 45% of respondents were directly affected by the pandemic in at least one of three ways – testing positive or getting sick from COVID-19, losing income, or losing employer coverage – with Black and Latinx adults and those with lower incomes at greater risk.
George Abraham, MD, president of the American College of Physicians, said the Commonwealth Fund’s findings were not surprising because it has always been known that underrepresented populations struggle for access to care because of socioeconomic factors. He said these populations were more vulnerable in terms of more severe infections and disease burden during the pandemic.
“[This study] validates what primary care physicians have been saying all along in regard to our patients’ access to care and their ability to cover health care costs,” said Dr. Abraham, who was not involved with the study. “This will hopefully be an eye-opener and wake-up call that reiterates that we still do not have equitable access to care and vulnerable populations are disproportionately affected.”
He believes that, although people are insured, many of them may contend with medical debt when they fall ill because they can’t afford the premiums.
“Even though they may have been registered for health coverage, they may not have active coverage at the time of illness simply because they weren’t able to make their last premium payments because they’ve been down, because they lost their job, or whatever else,” Dr. Abraham explained. “On paper, they appear to have health care coverage. But in reality, clearly, that coverage does not match their needs or it’s not affordable.”
For Dr. Abraham, the study emphasizes the need to continue support for health care reform, including pricing it so that insurance is available for those with fewer socioeconomic resources.
Yalda Jabbarpour, MD, medical director of the Robert Graham Center for Policy Studies, Washington, said high-deductible health plans need to be “reined in” because they can lead to greater debt, particularly among vulnerable populations.
“Hopefully this will encourage policymakers to look more closely at the problem of medical debt as a contributing factor to financial instability,” Dr. Jabbarpour said. “Federal relief is important, so is expanding access to comprehensive, affordable health care coverage.”
Dr. Collins said there should also be a way to raise awareness of the health care marketplace and coverage options so that people have an easier time getting insured.
A version of this article first appeared on Medscape.com.
Despite the passage of four major relief bills in 2020 and 2021 and federal efforts to offset pandemic- and job-related coverage loss, many people continued to face financial challenges, especially those with a low income and those who are Black or Latino.
The survey, which included responses from 5,450 adults, revealed that 10% of adults aged 19-64 were uninsured during the first half of 2021, a rate lower than what was recorded in 2020 and 2019 in both federal and private surveys. However, uninsured rates were highest among those with low income, those younger than 50 years old, and Black and Latino adults.
For most adults who lost employee health insurance, the coverage gap was relatively brief, with 54% saying their coverage gap lasted 3-4 months. Only 16% of adults said coverage gaps lasted a year or longer.
“The good news is that this survey is suggesting that the coverage losses during the pandemic may have been offset by federal efforts to help people get and maintain health insurance coverage,” lead author Sara Collins, PhD, Commonwealth Fund vice president for health care coverage, access, and tracking, said in an interview.
“The bad news is that a third of Americans continue to struggle with medical bills and medical debt, even among those who have health insurance coverage,” Dr. Collins added.
Indeed, the survey found that about one-third of insured adults reported a medical bill problem or that they were paying off medical debt, as did approximately half of those who were uninsured. Medical debt caused 35% of respondents to use up most or all of their savings to pay it off.
Meanwhile, 27% of adults said medical bills left them unable to pay for necessities such as food, heat, or rent. What surprised Dr. Collins was that 43% of adults said they received a lower credit rating as a result of their medical debt, and 35% said they had taken on more credit card debt to pay off these bills.
“The fact that it’s bleeding over into people’s financial security in terms of their credit scores, I think is something that really needs to be looked at by policymakers,” Dr. Collins said.
When analyzed by race/ethnicity, the researchers found that 55% of Black adults and 44% of Latino/Hispanic adults reported medical bills and debt problems, compared with 32% of White adults. In addition, 47% of those living below the poverty line also reported problems with medical bills.
According to the survey, 45% of respondents were directly affected by the pandemic in at least one of three ways – testing positive or getting sick from COVID-19, losing income, or losing employer coverage – with Black and Latinx adults and those with lower incomes at greater risk.
George Abraham, MD, president of the American College of Physicians, said the Commonwealth Fund’s findings were not surprising because it has always been known that underrepresented populations struggle for access to care because of socioeconomic factors. He said these populations were more vulnerable in terms of more severe infections and disease burden during the pandemic.
“[This study] validates what primary care physicians have been saying all along in regard to our patients’ access to care and their ability to cover health care costs,” said Dr. Abraham, who was not involved with the study. “This will hopefully be an eye-opener and wake-up call that reiterates that we still do not have equitable access to care and vulnerable populations are disproportionately affected.”
He believes that, although people are insured, many of them may contend with medical debt when they fall ill because they can’t afford the premiums.
“Even though they may have been registered for health coverage, they may not have active coverage at the time of illness simply because they weren’t able to make their last premium payments because they’ve been down, because they lost their job, or whatever else,” Dr. Abraham explained. “On paper, they appear to have health care coverage. But in reality, clearly, that coverage does not match their needs or it’s not affordable.”
For Dr. Abraham, the study emphasizes the need to continue support for health care reform, including pricing it so that insurance is available for those with fewer socioeconomic resources.
Yalda Jabbarpour, MD, medical director of the Robert Graham Center for Policy Studies, Washington, said high-deductible health plans need to be “reined in” because they can lead to greater debt, particularly among vulnerable populations.
“Hopefully this will encourage policymakers to look more closely at the problem of medical debt as a contributing factor to financial instability,” Dr. Jabbarpour said. “Federal relief is important, so is expanding access to comprehensive, affordable health care coverage.”
Dr. Collins said there should also be a way to raise awareness of the health care marketplace and coverage options so that people have an easier time getting insured.
A version of this article first appeared on Medscape.com.
Despite the passage of four major relief bills in 2020 and 2021 and federal efforts to offset pandemic- and job-related coverage loss, many people continued to face financial challenges, especially those with a low income and those who are Black or Latino.
The survey, which included responses from 5,450 adults, revealed that 10% of adults aged 19-64 were uninsured during the first half of 2021, a rate lower than what was recorded in 2020 and 2019 in both federal and private surveys. However, uninsured rates were highest among those with low income, those younger than 50 years old, and Black and Latino adults.
For most adults who lost employee health insurance, the coverage gap was relatively brief, with 54% saying their coverage gap lasted 3-4 months. Only 16% of adults said coverage gaps lasted a year or longer.
“The good news is that this survey is suggesting that the coverage losses during the pandemic may have been offset by federal efforts to help people get and maintain health insurance coverage,” lead author Sara Collins, PhD, Commonwealth Fund vice president for health care coverage, access, and tracking, said in an interview.
“The bad news is that a third of Americans continue to struggle with medical bills and medical debt, even among those who have health insurance coverage,” Dr. Collins added.
Indeed, the survey found that about one-third of insured adults reported a medical bill problem or that they were paying off medical debt, as did approximately half of those who were uninsured. Medical debt caused 35% of respondents to use up most or all of their savings to pay it off.
Meanwhile, 27% of adults said medical bills left them unable to pay for necessities such as food, heat, or rent. What surprised Dr. Collins was that 43% of adults said they received a lower credit rating as a result of their medical debt, and 35% said they had taken on more credit card debt to pay off these bills.
“The fact that it’s bleeding over into people’s financial security in terms of their credit scores, I think is something that really needs to be looked at by policymakers,” Dr. Collins said.
When analyzed by race/ethnicity, the researchers found that 55% of Black adults and 44% of Latino/Hispanic adults reported medical bills and debt problems, compared with 32% of White adults. In addition, 47% of those living below the poverty line also reported problems with medical bills.
According to the survey, 45% of respondents were directly affected by the pandemic in at least one of three ways – testing positive or getting sick from COVID-19, losing income, or losing employer coverage – with Black and Latinx adults and those with lower incomes at greater risk.
George Abraham, MD, president of the American College of Physicians, said the Commonwealth Fund’s findings were not surprising because it has always been known that underrepresented populations struggle for access to care because of socioeconomic factors. He said these populations were more vulnerable in terms of more severe infections and disease burden during the pandemic.
“[This study] validates what primary care physicians have been saying all along in regard to our patients’ access to care and their ability to cover health care costs,” said Dr. Abraham, who was not involved with the study. “This will hopefully be an eye-opener and wake-up call that reiterates that we still do not have equitable access to care and vulnerable populations are disproportionately affected.”
He believes that, although people are insured, many of them may contend with medical debt when they fall ill because they can’t afford the premiums.
“Even though they may have been registered for health coverage, they may not have active coverage at the time of illness simply because they weren’t able to make their last premium payments because they’ve been down, because they lost their job, or whatever else,” Dr. Abraham explained. “On paper, they appear to have health care coverage. But in reality, clearly, that coverage does not match their needs or it’s not affordable.”
For Dr. Abraham, the study emphasizes the need to continue support for health care reform, including pricing it so that insurance is available for those with fewer socioeconomic resources.
Yalda Jabbarpour, MD, medical director of the Robert Graham Center for Policy Studies, Washington, said high-deductible health plans need to be “reined in” because they can lead to greater debt, particularly among vulnerable populations.
“Hopefully this will encourage policymakers to look more closely at the problem of medical debt as a contributing factor to financial instability,” Dr. Jabbarpour said. “Federal relief is important, so is expanding access to comprehensive, affordable health care coverage.”
Dr. Collins said there should also be a way to raise awareness of the health care marketplace and coverage options so that people have an easier time getting insured.
A version of this article first appeared on Medscape.com.
Study highlights impact of acne in adult women on quality of life, mental health
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
results from a qualitative study demonstrated.
“Nearly 50% of women experience acne in their 20s, and 35% experience acne in their 30s,” the study’s corresponding author, John S. Barbieri, MD, MBA, formerly of the department of dermatology at the University of Pennsylvania, Philadelphia, told this news organization. “While several qualitative studies have examined acne in adolescence, the lived experience of adult female acne has not been explored in detail and prior studies have included relatively few patients. As a result, we conducted a series of semistructured interviews among adult women with acne to examine the lived experience of adult acne and its treatment.”
For the study, published online July 28, 2021, in JAMA Dermatology, Dr. Barbieri and colleagues conducted voluntary, confidential phone interviews with 50 women aged between 18 and 40 years with moderate to severe acne who were recruited from the University of Pennsylvania Health System and from a private dermatology clinic in Cincinnati. They used free listing and open-ended, semistructured interviews to elicit opinions from the women on how acne affected their lives; their experience with acne treatments, dermatologists, and health care systems; as well as their views on treatment success.
The mean age of the participants was 28 years and 48% were white (10% were Black, 8% were Asian, 4% were more than one race, and the rest abstained from answering this question; 10% said they were Hispanic).
More than three-quarters (78%) reported prior treatment with topical retinoids, followed by spironolactone (70%), topical antibiotics (43%), combined oral contraceptives (43%), and isotretinoin (41%). During the free-listing part of interviews, where the women reported the first words that came to their mind when asked about success of treatment and adverse effects, the most important terms expressed related to treatment success were clear skin, no scarring, and no acne. The most important terms related to treatment adverse effects were dryness, redness, and burning.
In the semistructured interview portion of the study, the main themes expressed were acne-related concerns about appearance, including feeling less confident at work; mental and emotional health, including feelings of depression, anxiety, depression, and low self-worth during acne breakouts; and everyday life impact, including the notion that acne affected how other people perceived them. The other main themes included successful treatment, with clear skin and having a manageable number of lesions being desirable outcomes; and interactions with health care, including varied experiences with dermatologists. The researchers observed that most participants did not think oral antibiotics were appropriate treatments for their acne, specifically because of limited long-term effectiveness.
“Many patients described frustration with finding a dermatologist with whom they were comfortable and with identifying effective treatments for their acne,” the authors wrote. “In contrast, those who thought their dermatologist listened to their concerns and individualized their treatment plan reported higher levels of satisfaction.”
In an interview, Dr. Barbieri, who is now with the department of dermatology at Brigham and Women’s Hospital, Boston, said that he was surprised by how many patients expressed interest in nonantibiotic treatments for acne, “given that oral antibiotics are by far the most commonly prescribed systemic treatment for acne.”
Moreover, he added, “although I have experienced many patients being hesitant about isotretinoin, I was surprised by how strong patients’ concerns were about isotretinoin side effects. Unfortunately, there are many misconceptions about isotretinoin that limit use of this treatment that can be highly effective and safe for the appropriate patient.”
In an accompanying editorial, dermatologists Diane M. Thiboutot, MD and Andrea L. Zaenglein, MD, with Penn State University, Hershey, and Alison M. Layton, MB, ChB, with the Harrogate Foundation Trust, Harrogate, North Yorkshire, England, wrote that the findings from the study “resonate with those recently reported in several international studies that examine the impacts of acne, how patients assess treatment success, and what is important to measure from a patient and health care professional perspective in a clinical trial for acne.”
A large systematic review on the impact of acne on patients, conducted by the Acne Core Outcomes Research Network (ACORN), found that “appearance-related concerns and negative psychosocial effects were found to be a major impact of acne,” they noted. “Surprisingly, only 22 of the 473 studies identified in this review included qualitative data gathered from patient interviews. It is encouraging to see the concordance between the concerns voiced by the participants in the current study and those identified from the literature review, wherein a variety of methods were used to assess acne impacts.”
For his part, Dr. Barbieri said that the study findings “justify the importance of having a discussion with patients about their unique lived experience of acne and individualizing treatment to their specific needs. Patient reported outcome measures could be a useful adjunctive tool to capture these impacts on quality of life.”
This study was funded by grant from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Barbieri disclosed that he received partial salary support through a Pfizer Fellowship in Dermatology Patient Oriented Research grant to the Trustees of the University of Pennsylvania. Dr. Thiboutot reported receiving consultant fees from Galderma and Novartis outside the submitted work. Dr. Layton reported receiving unrestricted educational presentation, advisory board, and consultancy fees from Galderma Honoraria; unrestricted educational presentation and advisory board honoraria from Leo; advisory board honoraria from Novartis and Mylan; consultancy honoraria from Procter and Gamble and Meda; grants from Galderma; and consultancy and advisory board honoraria from Origimm outside the submitted work.
FROM JAMA DERMATOLOGY
Clinical Edge Journal Scan Commentary: Atopic Dermatitis August 2021
Atopic Dermatitis (AD) is complex with heterogeneous symptoms (e.g. skin-pain, sleep disturbance), signs (e.g. lichenification, prurigo nodules, follicular accentuation), and longitudinal course (intermittent, persistent). These disparate signs and symptoms should be addressed in optimize disease control.
Multiple extracellular cytokines are upregulated in skin of AD patients, including interleukins 4, 5, 13, 22, 31 and thymic stromal lymphopoietin, all of which signal intracellularly through Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathways. Differential cytokine expression is proposed to underlie clinical variability. It may be necessary to inhibit signaling of multiple cytokines to achieve adequate control of AD.
Dupilumab is currently the only biologic treatment approved in the United States for moderate-severe AD. Dupilumab revolutionized AD management. However, there remain unmet needs, including the need for faster and more potent efficacy, and oral treatment options. Recently, oral JAK-inhibitors were investigated as treatments for moderate-severe AD. Multiple JAK-inhibitors demonstrated strong and rapid efficacy across multiple clinician-reported and patient-reported outcomes.
- Miao et al. recently conducted a meta-analysis of 10 randomized controlled trials and found that patients receiving JAK inhibitors showed significantly higher efficacy for eczema area and severity index (EASI) and Numeric Rating Scale (NRS)-itch scores and similar rates of adverse-events.
- Kim et al. pooled data from 3 randomized controlled trials of abrocitinib and found significantly higher proportions of clinically meaningful responses for itch in patients receiving abrocitinib 200 mg and 100 mg vs placebo as early as week 2 which continued through week 12.
- Lio et al. performed a post-hoc analysis of a phase 3 study of conducted in North America and found significant improvements for itch severity and sleep disturbance in patients treated with baricitinib 1 mg and 2 mg vs placebo. In particular, patients who achieved improvement of itch or sleep disturbance compared to those who did not were more likely to report having no impact on quality of life impact and improved work productivity.
This new therapeutic class will be an important addition to our therapeutic armamentarium and has potential to transform the AD treatment landscape.
- Many patients prefer taking pills over injections.
- Rapid-onset of efficacy for JAK-inhibitors will certainly be appreciated by patients, especially when trying to control tough flares. It may even guide clinical decision-making. Patients who have a good clinical response to JAK-inhibitors tend to do so within 4-8 weeks. By 8 weeks, if patients have no clinical response, they are likely not going to respond and may benefit from switching to alternative therapies.
- JAK-inhibitors can have robust efficacy, with higher doses of upadacitinib and abrocritinib showing greater efficacy than dupilumab at 12-16 weeks. This makes them attractive options to consider in patients who previously failed dupilumab.
- On the other hand, JAK-inhibitors have laboratory monitoring requirements, including complete blood count, comprehensive metabolic panel, lipid panel, etc.
- JAK-inhibitors warrant adverse-event monitoring for headache, nausea, acne, herpesvirus infections, risk of venous thromboembolism, etc.
Future research is needed to identify patient subsets who will benefit most from JAK-inhibitor therapy and where to position these agents in treatment guidelines.
Atopic Dermatitis (AD) is complex with heterogeneous symptoms (e.g. skin-pain, sleep disturbance), signs (e.g. lichenification, prurigo nodules, follicular accentuation), and longitudinal course (intermittent, persistent). These disparate signs and symptoms should be addressed in optimize disease control.
Multiple extracellular cytokines are upregulated in skin of AD patients, including interleukins 4, 5, 13, 22, 31 and thymic stromal lymphopoietin, all of which signal intracellularly through Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathways. Differential cytokine expression is proposed to underlie clinical variability. It may be necessary to inhibit signaling of multiple cytokines to achieve adequate control of AD.
Dupilumab is currently the only biologic treatment approved in the United States for moderate-severe AD. Dupilumab revolutionized AD management. However, there remain unmet needs, including the need for faster and more potent efficacy, and oral treatment options. Recently, oral JAK-inhibitors were investigated as treatments for moderate-severe AD. Multiple JAK-inhibitors demonstrated strong and rapid efficacy across multiple clinician-reported and patient-reported outcomes.
- Miao et al. recently conducted a meta-analysis of 10 randomized controlled trials and found that patients receiving JAK inhibitors showed significantly higher efficacy for eczema area and severity index (EASI) and Numeric Rating Scale (NRS)-itch scores and similar rates of adverse-events.
- Kim et al. pooled data from 3 randomized controlled trials of abrocitinib and found significantly higher proportions of clinically meaningful responses for itch in patients receiving abrocitinib 200 mg and 100 mg vs placebo as early as week 2 which continued through week 12.
- Lio et al. performed a post-hoc analysis of a phase 3 study of conducted in North America and found significant improvements for itch severity and sleep disturbance in patients treated with baricitinib 1 mg and 2 mg vs placebo. In particular, patients who achieved improvement of itch or sleep disturbance compared to those who did not were more likely to report having no impact on quality of life impact and improved work productivity.
This new therapeutic class will be an important addition to our therapeutic armamentarium and has potential to transform the AD treatment landscape.
- Many patients prefer taking pills over injections.
- Rapid-onset of efficacy for JAK-inhibitors will certainly be appreciated by patients, especially when trying to control tough flares. It may even guide clinical decision-making. Patients who have a good clinical response to JAK-inhibitors tend to do so within 4-8 weeks. By 8 weeks, if patients have no clinical response, they are likely not going to respond and may benefit from switching to alternative therapies.
- JAK-inhibitors can have robust efficacy, with higher doses of upadacitinib and abrocritinib showing greater efficacy than dupilumab at 12-16 weeks. This makes them attractive options to consider in patients who previously failed dupilumab.
- On the other hand, JAK-inhibitors have laboratory monitoring requirements, including complete blood count, comprehensive metabolic panel, lipid panel, etc.
- JAK-inhibitors warrant adverse-event monitoring for headache, nausea, acne, herpesvirus infections, risk of venous thromboembolism, etc.
Future research is needed to identify patient subsets who will benefit most from JAK-inhibitor therapy and where to position these agents in treatment guidelines.
Atopic Dermatitis (AD) is complex with heterogeneous symptoms (e.g. skin-pain, sleep disturbance), signs (e.g. lichenification, prurigo nodules, follicular accentuation), and longitudinal course (intermittent, persistent). These disparate signs and symptoms should be addressed in optimize disease control.
Multiple extracellular cytokines are upregulated in skin of AD patients, including interleukins 4, 5, 13, 22, 31 and thymic stromal lymphopoietin, all of which signal intracellularly through Janus Kinase (JAK)-Signal Transducer and Activator of Transcription (STAT) pathways. Differential cytokine expression is proposed to underlie clinical variability. It may be necessary to inhibit signaling of multiple cytokines to achieve adequate control of AD.
Dupilumab is currently the only biologic treatment approved in the United States for moderate-severe AD. Dupilumab revolutionized AD management. However, there remain unmet needs, including the need for faster and more potent efficacy, and oral treatment options. Recently, oral JAK-inhibitors were investigated as treatments for moderate-severe AD. Multiple JAK-inhibitors demonstrated strong and rapid efficacy across multiple clinician-reported and patient-reported outcomes.
- Miao et al. recently conducted a meta-analysis of 10 randomized controlled trials and found that patients receiving JAK inhibitors showed significantly higher efficacy for eczema area and severity index (EASI) and Numeric Rating Scale (NRS)-itch scores and similar rates of adverse-events.
- Kim et al. pooled data from 3 randomized controlled trials of abrocitinib and found significantly higher proportions of clinically meaningful responses for itch in patients receiving abrocitinib 200 mg and 100 mg vs placebo as early as week 2 which continued through week 12.
- Lio et al. performed a post-hoc analysis of a phase 3 study of conducted in North America and found significant improvements for itch severity and sleep disturbance in patients treated with baricitinib 1 mg and 2 mg vs placebo. In particular, patients who achieved improvement of itch or sleep disturbance compared to those who did not were more likely to report having no impact on quality of life impact and improved work productivity.
This new therapeutic class will be an important addition to our therapeutic armamentarium and has potential to transform the AD treatment landscape.
- Many patients prefer taking pills over injections.
- Rapid-onset of efficacy for JAK-inhibitors will certainly be appreciated by patients, especially when trying to control tough flares. It may even guide clinical decision-making. Patients who have a good clinical response to JAK-inhibitors tend to do so within 4-8 weeks. By 8 weeks, if patients have no clinical response, they are likely not going to respond and may benefit from switching to alternative therapies.
- JAK-inhibitors can have robust efficacy, with higher doses of upadacitinib and abrocritinib showing greater efficacy than dupilumab at 12-16 weeks. This makes them attractive options to consider in patients who previously failed dupilumab.
- On the other hand, JAK-inhibitors have laboratory monitoring requirements, including complete blood count, comprehensive metabolic panel, lipid panel, etc.
- JAK-inhibitors warrant adverse-event monitoring for headache, nausea, acne, herpesvirus infections, risk of venous thromboembolism, etc.
Future research is needed to identify patient subsets who will benefit most from JAK-inhibitor therapy and where to position these agents in treatment guidelines.
JAK inhibitors could be a promising alternative treatment option for atopic dermatitis
Key clinical point: Janus kinase (JAK) inhibitors were safe and effective in reducing the intensity of signs and symptoms of atopic dermatitis (AD) with significant improvements observed for Eczema Area and Severity Index (EASI) and pruritus numerical rating scale (NRS) scores.
Major finding: Patients receiving JAK inhibitors showed significant improvements in both total EASI score (mean difference [MD], −0.31; 95% confidence interval [CI], −0.46 to −0.17) and pruritus NRS score (MD, −1.15; 95% CI, −1.48 to −0.83). The risk of total adverse events was not significantly different between JAK inhibitor and control groups (risk ratio, 1.02; P = .745).
Study details: Findings are from a meta-analysis of 10 randomized controlled trials including 2583 patients with AD, of which 1,761 were in JAK inhibitor and 822 in control groups.
Disclosures: The study did not report any source of funding. No conflicts of interest were reported.
Source: Miao M et al. J Dermatolog Treat. 2021 Jun 16. doi: 10.1080/09546634.2021.1942422.
Key clinical point: Janus kinase (JAK) inhibitors were safe and effective in reducing the intensity of signs and symptoms of atopic dermatitis (AD) with significant improvements observed for Eczema Area and Severity Index (EASI) and pruritus numerical rating scale (NRS) scores.
Major finding: Patients receiving JAK inhibitors showed significant improvements in both total EASI score (mean difference [MD], −0.31; 95% confidence interval [CI], −0.46 to −0.17) and pruritus NRS score (MD, −1.15; 95% CI, −1.48 to −0.83). The risk of total adverse events was not significantly different between JAK inhibitor and control groups (risk ratio, 1.02; P = .745).
Study details: Findings are from a meta-analysis of 10 randomized controlled trials including 2583 patients with AD, of which 1,761 were in JAK inhibitor and 822 in control groups.
Disclosures: The study did not report any source of funding. No conflicts of interest were reported.
Source: Miao M et al. J Dermatolog Treat. 2021 Jun 16. doi: 10.1080/09546634.2021.1942422.
Key clinical point: Janus kinase (JAK) inhibitors were safe and effective in reducing the intensity of signs and symptoms of atopic dermatitis (AD) with significant improvements observed for Eczema Area and Severity Index (EASI) and pruritus numerical rating scale (NRS) scores.
Major finding: Patients receiving JAK inhibitors showed significant improvements in both total EASI score (mean difference [MD], −0.31; 95% confidence interval [CI], −0.46 to −0.17) and pruritus NRS score (MD, −1.15; 95% CI, −1.48 to −0.83). The risk of total adverse events was not significantly different between JAK inhibitor and control groups (risk ratio, 1.02; P = .745).
Study details: Findings are from a meta-analysis of 10 randomized controlled trials including 2583 patients with AD, of which 1,761 were in JAK inhibitor and 822 in control groups.
Disclosures: The study did not report any source of funding. No conflicts of interest were reported.
Source: Miao M et al. J Dermatolog Treat. 2021 Jun 16. doi: 10.1080/09546634.2021.1942422.
Serum biomarker-based patient clusters identify heterogeneity in pediatric atopic dermatitis
Key clinical point: Analysis of serum biomarker profiles in pediatric patients with atopic dermatitis (AD) indicates the existence of unique endotypes that could predict patients at risk of persistent disease and guide personalized, endotype-driven therapeutic approaches.
Major finding: Distinct biomarker profiles identified Th2/retinol dominant (mean Eczema Area Severity Index [EASI] score: 9.2), skin-homing dominant (mean EASI score: 27.8), Th1/Th2/Th17/IL-1 dominant (mean EASI score: 10.5), and Th1/IL-1/eosinophil inferior (mean EASI score: 12.3) as the 4 distinct pediatric clusters. The clusters were influenced by disease severity and not age, with the skin-homing dominant cluster having more severe AD than other clusters (P less than .001).
Study details: Findings are from an analysis of biomarker profiles of 240 pediatric patients with AD aged 0-17 years compared with previously found profiles in adult patients with AD.
Disclosures: This study was funded by Regeneron and Sanofi-Genzyme pharmaceuticals, Inc. Dr. M de Graaf, Dr. MS de Bruin-Weller, Dr. DS Bakker, Dr. E Knol, and Dr. JL Thijs declared being speaker, principal investigator, consultant, and/or advisory board member for various sources including Sanofi-Genzyme and Regeneron.
Source: Bakker DS et al. J Allergy Clin Immun. 2021 Jul 6. doi: 10.1016/j.jaci.2021.06.029.
Key clinical point: Analysis of serum biomarker profiles in pediatric patients with atopic dermatitis (AD) indicates the existence of unique endotypes that could predict patients at risk of persistent disease and guide personalized, endotype-driven therapeutic approaches.
Major finding: Distinct biomarker profiles identified Th2/retinol dominant (mean Eczema Area Severity Index [EASI] score: 9.2), skin-homing dominant (mean EASI score: 27.8), Th1/Th2/Th17/IL-1 dominant (mean EASI score: 10.5), and Th1/IL-1/eosinophil inferior (mean EASI score: 12.3) as the 4 distinct pediatric clusters. The clusters were influenced by disease severity and not age, with the skin-homing dominant cluster having more severe AD than other clusters (P less than .001).
Study details: Findings are from an analysis of biomarker profiles of 240 pediatric patients with AD aged 0-17 years compared with previously found profiles in adult patients with AD.
Disclosures: This study was funded by Regeneron and Sanofi-Genzyme pharmaceuticals, Inc. Dr. M de Graaf, Dr. MS de Bruin-Weller, Dr. DS Bakker, Dr. E Knol, and Dr. JL Thijs declared being speaker, principal investigator, consultant, and/or advisory board member for various sources including Sanofi-Genzyme and Regeneron.
Source: Bakker DS et al. J Allergy Clin Immun. 2021 Jul 6. doi: 10.1016/j.jaci.2021.06.029.
Key clinical point: Analysis of serum biomarker profiles in pediatric patients with atopic dermatitis (AD) indicates the existence of unique endotypes that could predict patients at risk of persistent disease and guide personalized, endotype-driven therapeutic approaches.
Major finding: Distinct biomarker profiles identified Th2/retinol dominant (mean Eczema Area Severity Index [EASI] score: 9.2), skin-homing dominant (mean EASI score: 27.8), Th1/Th2/Th17/IL-1 dominant (mean EASI score: 10.5), and Th1/IL-1/eosinophil inferior (mean EASI score: 12.3) as the 4 distinct pediatric clusters. The clusters were influenced by disease severity and not age, with the skin-homing dominant cluster having more severe AD than other clusters (P less than .001).
Study details: Findings are from an analysis of biomarker profiles of 240 pediatric patients with AD aged 0-17 years compared with previously found profiles in adult patients with AD.
Disclosures: This study was funded by Regeneron and Sanofi-Genzyme pharmaceuticals, Inc. Dr. M de Graaf, Dr. MS de Bruin-Weller, Dr. DS Bakker, Dr. E Knol, and Dr. JL Thijs declared being speaker, principal investigator, consultant, and/or advisory board member for various sources including Sanofi-Genzyme and Regeneron.
Source: Bakker DS et al. J Allergy Clin Immun. 2021 Jul 6. doi: 10.1016/j.jaci.2021.06.029.