User login
New ESC guidelines for cutting CV risk in noncardiac surgery
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
The European Society of Cardiology guidelines on cardiovascular assessment and management of patients undergoing noncardiac surgery have seen extensive revision since the 2014 version.
They still have the same aim – to prevent surgery-related bleeding complications, perioperative myocardial infarction/injury (PMI), stent thrombosis, acute heart failure, arrhythmias, pulmonary embolism, ischemic stroke, and cardiovascular (CV) death.
Cochairpersons Sigrun Halvorsen, MD, PhD, and Julinda Mehilli, MD, presented highlights from the guidelines at the annual congress of the European Society of Cardiology and the document was simultaneously published online in the European Heart Journal.
The document classifies noncardiac surgery into three levels of 30-day risk of CV death, MI, or stroke. Low (< 1%) risk includes eye or thyroid surgery; intermediate (1%-5%) risk includes knee or hip replacement or renal transplant; and high (> 5%) risk includes aortic aneurysm, lung transplant, or pancreatic or bladder cancer surgery (see more examples below).
It classifies patients as low risk if they are younger than 65 without CV disease or CV risk factors (smoking, hypertension, diabetes, dyslipidemia, family history); intermediate risk if they are 65 or older or have CV risk factors; and high risk if they have CVD.
In an interview, Dr. Halvorsen, professor in cardiology, University of Oslo, zeroed in on three important revisions:
First, recommendations for preoperative ECG and biomarkers are more specific, he noted.
The guidelines advise that before intermediate- or high-risk noncardiac surgery, in patients who have known CVD, CV risk factors (including age 65 or older), or symptoms suggestive of CVD:
- It is recommended to obtain a preoperative 12-lead ECG (class I).
- It is recommended to measure high-sensitivity cardiac troponin T (hs-cTn T) or high-sensitivity cardiac troponin I (hs-cTn I). It is also recommended to measure these biomarkers at 24 hours and 48 hours post surgery (class I).
- It should be considered to measure B-type natriuretic peptide or N-terminal of the prohormone BNP (NT-proBNP).
However, for low-risk patients undergoing low- and intermediate-risk noncardiac surgery, it is not recommended to routinely obtain preoperative ECG, hs-cTn T/I, or BNP/NT-proBNP concentrations (class III).
Troponins have a stronger class I recommendation, compared with the IIA recommendation for BNP, because they are useful for preoperative risk stratification and for diagnosis of PMI, Dr. Halvorsen explained. “Patients receive painkillers after surgery and may have no pain,” she noted, but they may have PMI, which has a bad prognosis.
Second, the guidelines recommend that “all patients should stop smoking 4 weeks before noncardiac surgery [class I],” she noted. Clinicians should also “measure hemoglobin, and if the patient is anemic, treat the anemia.”
Third, the sections on antithrombotic treatment have been significantly revised. “Bridging – stopping an oral antithrombotic drug and switching to a subcutaneous or IV drug – has been common,” Dr. Halvorsen said, “but recently we have new evidence that in most cases that increases the risk of bleeding.”
“We are [now] much more restrictive with respect to bridging” with unfractionated heparin or low-molecular-weight heparin, she said. “We recommend against bridging in patients with low to moderate thrombotic risk,” and bridging should only be considered in patients with mechanical prosthetic heart valves or with very high thrombotic risk.
More preoperative recommendations
In the guideline overview session at the congress, Dr. Halverson highlighted some of the new recommendations for preoperative risk assessment.
If time allows, it is recommended to optimize guideline-recommended treatment of CVD and control of CV risk factors including blood pressure, dyslipidemia, and diabetes, before noncardiac surgery (class I).
Patients commonly have “murmurs, chest pain, dyspnea, and edema that may suggest severe CVD, but may also be caused by noncardiac disease,” she noted. The guidelines state that “for patients with a newly detected murmur and symptoms or signs of CVD, transthoracic echocardiography is recommended before noncardiac surgery (class I).
“Many studies have been performed to try to find out if initiation of specific drugs before surgery could reduce the risk of complications,” Dr. Halvorsen noted. However, few have shown any benefit and “the question of presurgery initiation of beta-blockers has been greatly debated,” she said. “We have again reviewed the literature and concluded ‘Routine initiation of beta-blockers perioperatively is not recommended (class IIIA).’ “
“We adhere to the guidelines on acute and chronic coronary syndrome recommending 6-12 months of dual antiplatelet treatment as a standard before elective surgery,” she said. “However, in case of time-sensitive surgery, the duration of that treatment can be shortened down to a minimum of 1 month after elective PCI and a minimum of 3 months after PCI and ACS.”
Patients with specific types of CVD
Dr. Mehilli, a professor at Landshut-Achdorf (Germany) Hospital, highlighted some new guideline recommendations for patients who have specific types of cardiovascular disease.
Coronary artery disease (CAD). “For chronic coronary syndrome, a cardiac workup is recommended only for patients undergoing intermediate risk or high-risk noncardiac surgery.”
“Stress imaging should be considered before any high risk, noncardiac surgery in asymptomatic patients with poor functional capacity and prior PCI or coronary artery bypass graft (new recommendation, class IIa).”
Mitral valve regurgitation. For patients undergoing scheduled noncardiac surgery, who remain symptomatic despite guideline-directed medical treatment for mitral valve regurgitation (including resynchronization and myocardial revascularization), consider a valve intervention – either transcatheter or surgical – before noncardiac surgery in eligible patients with acceptable procedural risk (new recommendation).
Cardiac implantable electronic devices (CIED). For high-risk patients with CIEDs undergoing noncardiac surgery with high probability of electromagnetic interference, a CIED checkup and necessary reprogramming immediately before the procedure should be considered (new recommendation).
Arrhythmias. “I want only to stress,” Dr. Mehilli said, “in patients with atrial fibrillation with acute or worsening hemodynamic instability undergoing noncardiac surgery, an emergency electrical cardioversion is recommended (class I).”
Peripheral artery disease (PAD) and abdominal aortic aneurysm. For these patients “we do not recommend a routine referral for a cardiac workup. But we recommend it for patients with poor functional capacity or with significant risk factors or symptoms (new recommendations).”
Chronic arterial hypertension. “We have modified the recommendation, recommending avoidance of large perioperative fluctuations in blood pressure, and we do not recommend deferring noncardiac surgery in patients with stage 1 or 2 hypertension,” she said.
Postoperative cardiovascular complications
The most frequent postoperative cardiovascular complication is PMI, Dr. Mehilli noted.
“In the BASEL-PMI registry, the incidence of this complication around intermediate or high-risk noncardiac surgery was up to 15% among patients older than 65 years or with a history of CAD or PAD, which makes this kind of complication really important to prevent, to assess, and to know how to treat.”
“It is recommended to have a high awareness for perioperative cardiovascular complications, combined with surveillance for PMI in patients undergoing intermediate- or high-risk noncardiac surgery” based on serial measurements of high-sensitivity cardiac troponin.
The guidelines define PMI as “an increase in the delta of high-sensitivity troponin more than the upper level of normal,” Dr. Mehilli said. “It’s different from the one used in a rule-in algorithm for non-STEMI acute coronary syndrome.”
Postoperative atrial fibrillation (AFib) is observed in 2%-30% of noncardiac surgery patients in different registries, particularly in patients undergoing intermediate or high-risk noncardiac surgery, she noted.
“We propose an algorithm on how to prevent and treat this complication. I want to highlight that in patients with hemodynamic unstable postoperative AF[ib], an emergency cardioversion is indicated. For the others, a rate control with the target heart rate of less than 110 beats per minute is indicated.”
In patients with postoperative AFib, long-term oral anticoagulation therapy should be considered in all patients at risk for stroke, considering the anticipated net clinical benefit of oral anticoagulation therapy as well as informed patient preference (new recommendations).
Routine use of beta-blockers to prevent postoperative AFib in patients undergoing noncardiac surgery is not recommended.
The document also covers the management of patients with kidney disease, diabetes, cancer, obesity, and COVID-19. In general, elective noncardiac surgery should be postponed after a patient has COVID-19, until he or she recovers completely, and coexisting conditions are optimized.
The guidelines are available from the ESC website in several formats: pocket guidelines, pocket guidelines smartphone app, guidelines slide set, essential messages, and the European Heart Journal article.
Noncardiac surgery risk categories
The guideline includes a table that classifies noncardiac surgeries into three groups, based on the associated 30-day risk of death, MI, or stroke:
- Low (< 1%): breast, dental, eye, thyroid, and minor gynecologic, orthopedic, and urologic surgery.
- Intermediate (1%-5%): carotid surgery, endovascular aortic aneurysm repair, gallbladder surgery, head or neck surgery, hernia repair, peripheral arterial angioplasty, renal transplant, major gynecologic, orthopedic, or neurologic (hip or spine) surgery, or urologic surgery
- High (> 5%): aortic and major vascular surgery (including aortic aneurysm), bladder removal (usually as a result of cancer), limb amputation, lung or liver transplant, pancreatic surgery, or perforated bowel repair.
The guidelines were endorsed by the European Society of Anaesthesiology and Intensive Care. The guideline authors reported numerous disclosures.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Vitamins or cocoa: Which preserves cognition?
Unexpected results from a phase 3 trial exploring the effect of multivitamins and cognition have now been published.
Originally presented last November at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference, this is the first large-scale, long-term randomized controlled trial to examine the effects of cocoa extract and multivitamins on global cognition. The trial’s primary focus was on cocoa extract, which earlier studies suggest may preserve cognitive function. Analyzing the effect of multivitamins was a secondary outcome.
Showing vitamins, but not cocoa, were beneficial is the exact opposite of what researchers expected. Still, the results offer an interesting new direction for future study, lead investigator Laura D. Baker, PhD, professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C., said in an interview.
“This study made us take notice of a pathway for possible cognitive protection,” Dr. Baker said. “Without this study, we would never have looked down that road.”
The full results were published online in Alzheimer’s and Dementia.
Unexpected effect
The COSMOS-Mind study is a substudy to a larger parent trial called COSMOS. It investigated the effects of cocoa extract and a standard multivitamin-mineral on cardiovascular and cancer outcomes in more than 21,000 older participants.
In COSMOS-Mind, researchers tested whether daily intake of cocoa extract vs. placebo and a multivitamin-mineral vs. placebo improved cognition in older adults.
More than 2,200 participants aged 65 and older were enrolled and followed for 3 years. They completed tests over the telephone at baseline and annually to evaluate memory and other cognitive abilities.
Results showed cocoa extract had no effect on global cognition compared with placebo (mean z-score, 0.03; P = .28). Daily multivitamin use, however, did show significant benefits on global cognition vs. placebo (mean z, 0.07, P = .007).
The beneficial effect was most pronounced in participants with a history of cardiovascular disease (no history 0.06 vs. history 0.14; P = .01).
Researchers found similar protective effects for memory and executive function.
Dr. Baker suggested one possible explanation for the positive effects of multivitamins may be the boost in micronutrients and essential minerals they provided.
“With nutrient-deficient diets plus a high prevalence of cardiovascular disease, diabetes, and other medical comorbidities that we know impact the bioavailability of these nutrients, we are possibly dealing with older adults who are at below optimum in terms of their essential micronutrients and minerals,” she said.
“Even suboptimum levels of micronutrients and essential minerals can have significant consequences for brain health,” she added.
More research needed
Intriguing as the results may be, more work is needed before the findings could affect nutritional guidance, according to Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association.
“While the Alzheimer’s Association is encouraged by these results, we are not ready to recommend widespread use of a multivitamin supplement to reduce risk of cognitive decline in older adults,” Dr. Carrillo said in a statement.
“For now, and until there is more data, people should talk with their health care providers about the benefits and risks of all dietary supplements, including multivitamins,” she added.
Dr. Baker agreed, noting that the study was not designed to measure multivitamin use as a primary outcome. In addition, nearly 90% of the participants were non-Hispanic White, which is not representative of the overall population demographics.
The investigators are now designing another, larger trial that would include a more diverse participant pool. It will be aimed specifically at learning more about how and why multivitamins seem to offer a protective effect on cognition, Dr. Baker noted.
The study was funded by the National Institute on Aging of the National Institutes of Health. Dr. Baker and Dr. Carrillo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unexpected results from a phase 3 trial exploring the effect of multivitamins and cognition have now been published.
Originally presented last November at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference, this is the first large-scale, long-term randomized controlled trial to examine the effects of cocoa extract and multivitamins on global cognition. The trial’s primary focus was on cocoa extract, which earlier studies suggest may preserve cognitive function. Analyzing the effect of multivitamins was a secondary outcome.
Showing vitamins, but not cocoa, were beneficial is the exact opposite of what researchers expected. Still, the results offer an interesting new direction for future study, lead investigator Laura D. Baker, PhD, professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C., said in an interview.
“This study made us take notice of a pathway for possible cognitive protection,” Dr. Baker said. “Without this study, we would never have looked down that road.”
The full results were published online in Alzheimer’s and Dementia.
Unexpected effect
The COSMOS-Mind study is a substudy to a larger parent trial called COSMOS. It investigated the effects of cocoa extract and a standard multivitamin-mineral on cardiovascular and cancer outcomes in more than 21,000 older participants.
In COSMOS-Mind, researchers tested whether daily intake of cocoa extract vs. placebo and a multivitamin-mineral vs. placebo improved cognition in older adults.
More than 2,200 participants aged 65 and older were enrolled and followed for 3 years. They completed tests over the telephone at baseline and annually to evaluate memory and other cognitive abilities.
Results showed cocoa extract had no effect on global cognition compared with placebo (mean z-score, 0.03; P = .28). Daily multivitamin use, however, did show significant benefits on global cognition vs. placebo (mean z, 0.07, P = .007).
The beneficial effect was most pronounced in participants with a history of cardiovascular disease (no history 0.06 vs. history 0.14; P = .01).
Researchers found similar protective effects for memory and executive function.
Dr. Baker suggested one possible explanation for the positive effects of multivitamins may be the boost in micronutrients and essential minerals they provided.
“With nutrient-deficient diets plus a high prevalence of cardiovascular disease, diabetes, and other medical comorbidities that we know impact the bioavailability of these nutrients, we are possibly dealing with older adults who are at below optimum in terms of their essential micronutrients and minerals,” she said.
“Even suboptimum levels of micronutrients and essential minerals can have significant consequences for brain health,” she added.
More research needed
Intriguing as the results may be, more work is needed before the findings could affect nutritional guidance, according to Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association.
“While the Alzheimer’s Association is encouraged by these results, we are not ready to recommend widespread use of a multivitamin supplement to reduce risk of cognitive decline in older adults,” Dr. Carrillo said in a statement.
“For now, and until there is more data, people should talk with their health care providers about the benefits and risks of all dietary supplements, including multivitamins,” she added.
Dr. Baker agreed, noting that the study was not designed to measure multivitamin use as a primary outcome. In addition, nearly 90% of the participants were non-Hispanic White, which is not representative of the overall population demographics.
The investigators are now designing another, larger trial that would include a more diverse participant pool. It will be aimed specifically at learning more about how and why multivitamins seem to offer a protective effect on cognition, Dr. Baker noted.
The study was funded by the National Institute on Aging of the National Institutes of Health. Dr. Baker and Dr. Carrillo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unexpected results from a phase 3 trial exploring the effect of multivitamins and cognition have now been published.
Originally presented last November at the 14th Clinical Trials on Alzheimer’s Disease (CTAD) conference, this is the first large-scale, long-term randomized controlled trial to examine the effects of cocoa extract and multivitamins on global cognition. The trial’s primary focus was on cocoa extract, which earlier studies suggest may preserve cognitive function. Analyzing the effect of multivitamins was a secondary outcome.
Showing vitamins, but not cocoa, were beneficial is the exact opposite of what researchers expected. Still, the results offer an interesting new direction for future study, lead investigator Laura D. Baker, PhD, professor of gerontology and geriatric medicine at Wake Forest University, Winston-Salem, N.C., said in an interview.
“This study made us take notice of a pathway for possible cognitive protection,” Dr. Baker said. “Without this study, we would never have looked down that road.”
The full results were published online in Alzheimer’s and Dementia.
Unexpected effect
The COSMOS-Mind study is a substudy to a larger parent trial called COSMOS. It investigated the effects of cocoa extract and a standard multivitamin-mineral on cardiovascular and cancer outcomes in more than 21,000 older participants.
In COSMOS-Mind, researchers tested whether daily intake of cocoa extract vs. placebo and a multivitamin-mineral vs. placebo improved cognition in older adults.
More than 2,200 participants aged 65 and older were enrolled and followed for 3 years. They completed tests over the telephone at baseline and annually to evaluate memory and other cognitive abilities.
Results showed cocoa extract had no effect on global cognition compared with placebo (mean z-score, 0.03; P = .28). Daily multivitamin use, however, did show significant benefits on global cognition vs. placebo (mean z, 0.07, P = .007).
The beneficial effect was most pronounced in participants with a history of cardiovascular disease (no history 0.06 vs. history 0.14; P = .01).
Researchers found similar protective effects for memory and executive function.
Dr. Baker suggested one possible explanation for the positive effects of multivitamins may be the boost in micronutrients and essential minerals they provided.
“With nutrient-deficient diets plus a high prevalence of cardiovascular disease, diabetes, and other medical comorbidities that we know impact the bioavailability of these nutrients, we are possibly dealing with older adults who are at below optimum in terms of their essential micronutrients and minerals,” she said.
“Even suboptimum levels of micronutrients and essential minerals can have significant consequences for brain health,” she added.
More research needed
Intriguing as the results may be, more work is needed before the findings could affect nutritional guidance, according to Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association.
“While the Alzheimer’s Association is encouraged by these results, we are not ready to recommend widespread use of a multivitamin supplement to reduce risk of cognitive decline in older adults,” Dr. Carrillo said in a statement.
“For now, and until there is more data, people should talk with their health care providers about the benefits and risks of all dietary supplements, including multivitamins,” she added.
Dr. Baker agreed, noting that the study was not designed to measure multivitamin use as a primary outcome. In addition, nearly 90% of the participants were non-Hispanic White, which is not representative of the overall population demographics.
The investigators are now designing another, larger trial that would include a more diverse participant pool. It will be aimed specifically at learning more about how and why multivitamins seem to offer a protective effect on cognition, Dr. Baker noted.
The study was funded by the National Institute on Aging of the National Institutes of Health. Dr. Baker and Dr. Carrillo report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA
Cumulative blood pressure load: A better predictor of CV events?
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
Cumulative systolic blood pressure load, which can be calculated from serial blood pressure measurements, may provide better prediction of major cardiovascular events, compared with traditional blood pressure measures, a new study suggests.
“Our results suggest that cumulative blood pressure load is an independent predictor of cardiovascular events and should be used in future cardiovascular risk prediction algorithms,” the authors, led by Nelson Wang, MD, George Institute for Global Health, Sydney, conclude.
The study was published online in the Journal of the American College of Cardiology.
The researchers explain that the management of hypertension has traditionally centered around blood pressure measurements taken at a single timepoint, with adequate control defined as those measurements being below a predefined target threshold.
However, this approach fails to recognize blood pressure as a continuous measure that fluctuates over time and does not acknowledge that the most recently recorded measurement may not reflect previous blood pressure control.
More recently, studies have defined the time a patient spends below blood pressure target, or TIme at TaRgEt (TITRE), as a novel marker of cardiovascular risk that is independent of mean blood pressure.
Although TITRE has the added advantage of incorporating duration of control, it is unable to characterize the magnitude of blood pressure elevation, the researchers note.
They point out that an optimal measure as a risk factor for cardiovascular disease would account for both the magnitude and duration of elevated blood pressure.
Such a measure is cumulative blood pressure load, defined as the area under the curve (AUC) expressed in units of mm Hg by time.
The only prior study of this measure was small and retrospective and calculated cumulative blood pressure load from ambulatory blood pressure monitoring estimated over a short (24-hour) period.
Therefore, the aim of the current study was to estimate the association between cumulative systolic blood pressure load over a longer period (24 months) and subsequent major cardiovascular events.
To do this, the researchers conducted a post-hoc analysis of 9,338 patients with type 2 diabetes in the ADVANCE-ON study.
Cumulative systolic blood pressure load was defined as the AUC for systolic blood pressure values above 130 mm Hg divided by the AUC for all measured systolic blood pressure values over a 24-month exposure period.
Over a median 7.6 years of follow-up, 1,469 major cardiovascular events, 1,615 deaths, and 660 cardiovascular deaths occurred.
Results showed that each one standard deviation increase in cumulative systolic blood pressure load was associated with a 14% increase in major cardiovascular events, a 13% increase in all-cause mortality, and a 21% increase in cardiovascular death.
Cumulative systolic blood pressure load outperformed mean systolic blood pressure, time-below-target, and visit-to-visit systolic blood pressure variability for the prediction of cardiovascular events and death and also discriminated risk and reclassified more patients’ risk correctly than the other measures.
“Small improvements in risk prediction can have a major impact when scaled up across large high-risk populations. Furthermore, cumulative systolic pressure load may also prove useful to inform the design of future clinical trials,” the researchers say.
Although the present study only assessed cumulative systolic blood pressure load over 24 months, clinicians should recognize the importance of this measure over a lifetime, they note.
“This approach emphasizes the importance of early blood pressure–lowering interventions to reduce the cumulative systolic blood pressure load that each individual experiences over their lifetime,” they conclude.
The researchers suggest that, based on these results, cumulative systolic blood pressure load and visit-to-visit systolic blood pressure variability “should be used in conjunction in future cardiovascular risk prediction algorithms.”
In an accompanying editorial, Donald Lloyd-Jones, MD, Northwestern Feinberg School of Medicine, Chicago, says that before routinely adopting these new measures, several additional questions need to be addressed.
He notes that many patients in the current study already had cardiovascular disease, and it is not known whether the benefit was consistent among those with and without cardiovascular disease. In addition, longer term data using blood pressure measurements in the real-world clinical setting would be desirable, as well as information on whether these new measures add incremental value to existing risk prediction equations.
“Certainly, the next guidelines should reconsider all types of blood pressure measures, and other potential predictors, to optimize risk estimation and identification of patients with greatest net benefit from risk-reducing therapies,” Dr. Lloyd-Jones comments.
“Ultimately, clinicians should leverage as much information on their patients as possible to understand their blood pressure–related cardiovascular risk, to identify those who may be more likely have occult or emerging subclinical target organ damage, and to identify those who may have particular net benefit from earlier or more intensive treatment,” he concludes.
“These opportunities are more readily available with integration of data that allow for visualization of longer-term blood pressure patterns and incorporation of home monitoring and ambulatory monitoring data to monitor out-of-office blood pressure levels and control.”
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Should patients stand for office BP readings?
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
a new study suggests.
Combining three standing and three seated BP measurements in the same visit may lead to a “quicker diagnosis and save people a trip back to the office,” Wanpen Vongpatanasin, MD, professor of internal medicine and director of the hypertension section, cardiology division, UT Southwestern Medical Center, Dallas, said in an interview.
The study was presented at the Hypertension Scientific Sessions, sponsored by the American Heart Association.
Practice changing?
Clinical guidelines recommend office BP be taken in a seated position for most patients.
However, research has suggested that the sensitivity of seated office BP in diagnosing hypertension is about 50%, with high specificity around 90% during a single visit, Dr. Vongpatanasin explained.
At the follow-up visit, however, the second office BP yielded higher sensitivity to 80% but specificity fell to 55%. Nevertheless, the accuracy of standing BP measurements for diagnosing hypertension has not been investigated.
In a cross-sectional study, Dr. Vongpatanasin and colleagues determined the accuracy of both seated and standing BP for diagnosing hypertension in a single visit in 125 healthy adults who had not had a previous diagnosis of hypertension and were not taking any BP medications. The cohort had a mean age of 49 years, 62% were women, and 24% were Black.
During each office visit, seated BP was measured three times, then standing BP was measured three times using an automated and validated device.
Average seated BP was 123/76 mm Hg and average standing BP was 126/80 mm Hg.
Of the 125 participants, 42 (34%) had hypertension, defined as 24-hour ambulatory systolic/diastolic BP (SBP/DBP) of ≥ 125/75 mm Hg.
The sensitivity and specificity of seated SBP for hypertension was 43% and 92%, respectively.
“Interestingly, with standing SBP, sensitivity was improved to 74% and specificity dropped to 65% – which is okay; you will have to confirm a positive test anyway and when screening for a common disease you’d rather have a high sensitivity rather than low sensitivity to pick it up in this case,” Dr. Vongpatanasin said.
The area under receiver operating characteristic curve (AUROC) of standing SBP was significantly higher than seated SBP (Bayes factor [BF] = 11.8) when hypertension was defined as 24-hour SBP ≥ 125 mm Hg.
Similarly, when hypertension was defined as 24-hour DBP ≥ 75 mm Hg or daytime DBP ≥ 80 mm Hg, the AUROC of standing DBP was higher than seated DBP (all BF > 3).
The addition of standing to seated BP improved detection of hypertension compared with seated BP alone based on 24-hour SBP/DBP ≥ 125/75 mm Hg or daytime SBP/DBP ≥ 130/80 mm Hg (all BF > 3).
“In our hypertension clinic, we always measure both seated and standing BP in all of our patients,” John Giacona, PA-C, a PhD candidate at UT Southwestern Medical Center and coauthor of the study, told this news organization,
Multiple readings most important
Reached for comment, Johanna Contreras, MD, a cardiologist at Mount Sinai Hospital in New York, noted that diagnosing hypertension is “difficult” and she agrees that multiple readings are important.
“I usually take at least two readings in two different visits before I tell the patient they have high blood pressure,” Dr. Contreras said in an interview.
Dr. Contreras said she takes blood pressure both seated and standing.
“I’m not sure standing versus seated makes a big difference. However, when the patient first comes into the office, it is really important to let them rest and calm down before taking the blood pressure,” she said.
The study had no commercial funding. The authors and Dr. Contreras have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM HYPERTENSION 2022
Schizophrenia and postmodernism: A philosophical exercise in treatment
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.
Schizophrenia is defined as having episodes of psychosis: periods of time when one suffers from delusions, hallucinations, disorganized behaviors, disorganized speech, and negative symptoms. The concept of schizophrenia can be simplified as a detachment from reality. Patients who struggle with this illness frame their perceptions with a different set of rules and beliefs than the rest of society. These altered perceptions frequently become the basis of delusions, one of the most recognized symptoms of schizophrenia.
A patient with schizophrenia doesn’t have delusions, as much as having a belief system, which is not recognized by any other. It is not the mismatch between “objective reality” and the held belief, which qualifies the belief as delusional, so much as the mismatch with the beliefs of those around you. Heliocentrism denial, denying the knowledge that the earth rotates around the sun, is incorrect because it is not factual. However, heliocentrism denial is not a delusion because it is incorrect, but because society chooses it to be incorrect.
We’d like to invite the reader to a thought experiment. “Objective reality” can be referred to as “anything that exists as it is independent of any conscious awareness of it.”1 “Consciousness awareness” entails an observer. If we remove the concept of consciousness or observer from existence, how would we then define “objective reality,” as the very definition of “objective reality” points to the existence of an observer. One deduces that there is no way to define “objective reality” without invoking the notion of an observer or of consciousness.
It is our contention that the concept of an “objective reality” is tautological – it answers itself. This philosophical quandary helps explain why a person with schizophrenia may feel alienated by others who do not appreciate their perceived “objective reality.”
Schizophrenia and ‘objective reality’
A patient with schizophrenia enters a psychiatrist’s office and may realize that their belief is not shared by others and society. The schizophrenic patient may understand the concept of delusions as fixed and false beliefs. However, to them, it is everyone else who is delusional. They may attempt to convince you, as their provider, to switch to their side. They may provide you with evidence for their belief system. One could argue that believing them, in response, would be curative. If not only one’s psychiatrist, but society accepted the schizophrenic patient’s belief system, it would no longer be delusional, whether real or not. Objective reality requires the presence of an object, an observer, to grant its value of truth.
In a simplistic way, those were the arguments of postmodernist philosophers. Reality is tainted by its observer, in a similar way that the Heisenberg uncertainty principle teaches that there is a limit to our simultaneous understanding of position and momentum of particles. This perspective may explain why Michel Foucault, PhD, the famous French postmodernist philosopher, was so interested in psychiatry and in particular schizophrenia. Dr. Foucault was deeply concerned with society imposing its beliefs and value system on patients, and positioning itself as the ultimate arbiter of reality. He went on to postulate that the bigger difference between schizophrenic patients and psychiatrists was not who was in the correct plane of reality but who was granted by society to arbitrate the answer. If reality is a subjective construct enforced by a ruling class, who has the power to rule becomes of the utmost importance.
Intersubjectivity theory in psychoanalysis has many of its sensibilities rooted in such thought. It argues against the myth of the isolated mind. Truth, in the context of psychoanalysis, is seen as an emergent product of dialogue between the therapist/patient dyad. It is in line with the ontological shift from a logical-positivist model to the more modern, constructivist framework. In terms of its view of psychosis, “delusional ideas were understood as a form of absolution – a radical decontextualization serving vital and restorative defensive functions.”2
It is an interesting proposition to advance this theory further in contending that it is not the independent consciousness of two entities that create the intersubjective space; but rather that it is the intersubjective space that literally creates the conscious entities. Could it not be said that the subjective relationship is more fundamental than consciousness itself? As Chris Jaenicke, Dipl.-Psych., wrote, “infant research has opened our eyes to the fact that there is no unilateral action.”3
Postmodernism and psychiatry
Postmodernism and its precursor skepticism have significant histories within the field of philosophy. This article will not summarize centuries of philosophical thought. In brief, skepticism is a powerful philosophical tool that can powerfully point out the limitations of human knowledge and certainty.
As a pedagogic jest to trainees, we will often point out that none of us “really knows” our date of birth with absolute certainty. None of us were conscious enough to remember our birth, conscious enough to understand the concept of date or time, and conscious enough to know who participated in it. At a fundamental level, we chose to believe our date of birth. Similarly, while the world could be a fictionalized simulation,4 we chose to believe that it is real because it behaves in a consistent way that permits scientific study. Postmodernism and skepticism are philosophical tools that permit one to question everything but are themselves limited by the real and empiric lives we live.
Psychiatrists are empiricists. We treat real people, who suffer in a very perceptible way, and live in a very tangible world. We frown on the postmodernist perspective and do not spend much or any time studying it as trainees. However, postmodernism, despite its philosophical and practical flaws, and adjacency to antipsychiatry,5 is an essential tool for the psychiatrist. In addition to the standard treatments for schizophrenia, the psychiatrist should attempt to create a bond with someone who is disconnected from the world. Postmodernism provides us with a way of doing so.
A psychiatrist who understands and appreciates postmodernism can show a patient why at some level we cannot refute all delusions. This psychiatrist can subsequently have empathy that some of the core beliefs of a patient may always be left unanswered. The psychiatrist can appreciate that to some degree the reason why the patient’s beliefs are not true is because society has chosen for them not to be true. Additionally, the psychiatrist can acknowledge to the patient that in some ways the correctness of a delusion is less relevant than the power of society to enforce its reality on the patient. This connection in itself is partially curative as it restores the patient’s attachment to society; we now have some plane of reality, the relationship, which is the same.
Psychiatry and philosophy
However, tempting it may be to be satisfied with this approach as an end in itself; this would be dangerous. While gratifying to the patient to be seen and heard, they will over time only become further entrenched in that compromise formation of delusional beliefs. The role of the psychiatrist, once deep and meaningful rapport has been established and solidified, is to point out to the patient the limitations of the delusions’ belief system.
“I empathize that not all your delusions can be disproved. An extension of that thought is that many beliefs can’t be disproved. Society chooses to believe that aliens do not live on earth but at the same time we can’t disprove with absolute certainty that they don’t. We live in a world where attachment to others enriches our lives. If you continue to believe that aliens affect all existence around you, you will disconnect yourself from all of us. I hope that our therapy has shown you the importance of human connection and the sacrifice of your belief system.”
In the modern day, psychiatry has chosen to believe that schizophrenia is a biological disorder that requires treatment with antipsychotics. We choose to believe that this is likely true, and we think that our empirical experience has been consistent with this belief. However, we also think that patients with this illness are salient beings that deserve to have their thoughts examined and addressed in a therapeutic framework that seeks to understand and acknowledge them as worthy and intelligent individuals. Philosophy provides psychiatry with tools on how to do so.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Khalafian practices full time as a general outpatient psychiatrist. He trained at the University of California, San Diego, for his psychiatric residency and currently works as a telepsychiatrist, serving an outpatient clinic population in northern California. Dr. Badre and Dr. Khalafian have no conflicts of interest.
References
1. https://iep.utm.edu/objectiv/.
2. Stolorow, RD. The phenomenology of trauma and the absolutisms of everyday life: A personal journey. Psychoanal Psychol. 1999;16(3):464-8. doi: 10.1037/0736-9735.16.3.464.
3. Jaenicke C. “The Risk of Relatedness: Intersubjectivity Theory in Clinical Practice” Lanham, Md.: Jason Aronson, 2007.
4. Cuthbertson A. “Elon Musk cites Pong as evidence that we are already living in a simulation” The Independent. 2021 Dec 1. https://www.independent.co.uk/space/elon-musk-simulation-pong-video-game-b1972369.html.
5. Foucault M (Howard R, translator). “Madness and Civilization: A History of Insanity in the Age of Reason” New York: Vintage, 1965.
Hidradenitis Suppurativa Overview
Opioids after lung cancer surgery may up all-cause mortality risk
Patients who undergo lung cancer surgery and who receive long-term opioids for pain relief have an elevated risk of all-cause mortality at 2 years, a new study suggests. That risk was 40% higher than among patients who did not receive opioids.
“This is the first study to identify the association of new long-term opioid use with poorer long-term survival outcomes after lung cancer surgery using real-world data based on a national registration database,” said the authors, led by In-Ae Song, MD, Seoul National University Bundang Hospital, Seongnam, South Korea.
“New long-term opioid use may be associated with poor long-term survival outcomes, especially in potent opioid users,” they concluded.
Long-term opioid use might promote protumor activity secondary to immunosuppression along with migration of tumor cells and angiogenesis, the authors suggested.
The study was published online in Regional Anesthesia and Pain.
The finding comes from a study that used the South Korean National Health Insurance database as a nationwide registration data source. “All patients undergoing lung cancer surgery between 2011 and 2018 were included,” the authors noted.
In total, 54,509 patients were included in the final analysis. Six months after undergoing the procedure, 3,325 patients (6.1%) had been prescribed opioids continuously and regularly. These patients constituted the new long-term opioid user group.
This finding fits in with those from past studies that have suggested that new long-term postoperative pain is reported in 4%-12% of patients who undergo lung cancer surgeries, the authors commented.
The new study found that all-cause mortality at 2 years was significantly higher in the new long-term opioid user group than it was in the non–opioid user group (17.3% vs. 9.3%; P < .001).
Moreover, the new long-term opioid user group were at 43% higher risk of 2-year lung cancer mortality and 29% higher risk of 2-year non–lung cancer mortality.
The investigators divided the patients who had received long-term opioids into two subgroups – those who received more potent opioids (1.6%), and those who received less potent opioids (4.5%).
There was a big difference in the results for all-cause mortality.
Compared with nonopioid users, long-term use of less potent opioids was associated with a 2-year mortality risk of only 22% (P < .001), whereas the patients who used potent opioids were at a 92% increased risk of all-cause mortality.
A number of risk factors were associated with an increased rate of new long-term opioid use. These included older age, being male, length of stay in hospital, and comorbidities.
In addition, patients who were more likely to receive long-term opioids included those who had received neoadjuvant and adjuvant chemotherapy and those who had experienced preoperative anxiety disorder or insomnia disorder.
In contrast, patients who underwent video-assisted thoracoscopic surgery were less likely to receive long-term opioids, the authors noted.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who undergo lung cancer surgery and who receive long-term opioids for pain relief have an elevated risk of all-cause mortality at 2 years, a new study suggests. That risk was 40% higher than among patients who did not receive opioids.
“This is the first study to identify the association of new long-term opioid use with poorer long-term survival outcomes after lung cancer surgery using real-world data based on a national registration database,” said the authors, led by In-Ae Song, MD, Seoul National University Bundang Hospital, Seongnam, South Korea.
“New long-term opioid use may be associated with poor long-term survival outcomes, especially in potent opioid users,” they concluded.
Long-term opioid use might promote protumor activity secondary to immunosuppression along with migration of tumor cells and angiogenesis, the authors suggested.
The study was published online in Regional Anesthesia and Pain.
The finding comes from a study that used the South Korean National Health Insurance database as a nationwide registration data source. “All patients undergoing lung cancer surgery between 2011 and 2018 were included,” the authors noted.
In total, 54,509 patients were included in the final analysis. Six months after undergoing the procedure, 3,325 patients (6.1%) had been prescribed opioids continuously and regularly. These patients constituted the new long-term opioid user group.
This finding fits in with those from past studies that have suggested that new long-term postoperative pain is reported in 4%-12% of patients who undergo lung cancer surgeries, the authors commented.
The new study found that all-cause mortality at 2 years was significantly higher in the new long-term opioid user group than it was in the non–opioid user group (17.3% vs. 9.3%; P < .001).
Moreover, the new long-term opioid user group were at 43% higher risk of 2-year lung cancer mortality and 29% higher risk of 2-year non–lung cancer mortality.
The investigators divided the patients who had received long-term opioids into two subgroups – those who received more potent opioids (1.6%), and those who received less potent opioids (4.5%).
There was a big difference in the results for all-cause mortality.
Compared with nonopioid users, long-term use of less potent opioids was associated with a 2-year mortality risk of only 22% (P < .001), whereas the patients who used potent opioids were at a 92% increased risk of all-cause mortality.
A number of risk factors were associated with an increased rate of new long-term opioid use. These included older age, being male, length of stay in hospital, and comorbidities.
In addition, patients who were more likely to receive long-term opioids included those who had received neoadjuvant and adjuvant chemotherapy and those who had experienced preoperative anxiety disorder or insomnia disorder.
In contrast, patients who underwent video-assisted thoracoscopic surgery were less likely to receive long-term opioids, the authors noted.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Patients who undergo lung cancer surgery and who receive long-term opioids for pain relief have an elevated risk of all-cause mortality at 2 years, a new study suggests. That risk was 40% higher than among patients who did not receive opioids.
“This is the first study to identify the association of new long-term opioid use with poorer long-term survival outcomes after lung cancer surgery using real-world data based on a national registration database,” said the authors, led by In-Ae Song, MD, Seoul National University Bundang Hospital, Seongnam, South Korea.
“New long-term opioid use may be associated with poor long-term survival outcomes, especially in potent opioid users,” they concluded.
Long-term opioid use might promote protumor activity secondary to immunosuppression along with migration of tumor cells and angiogenesis, the authors suggested.
The study was published online in Regional Anesthesia and Pain.
The finding comes from a study that used the South Korean National Health Insurance database as a nationwide registration data source. “All patients undergoing lung cancer surgery between 2011 and 2018 were included,” the authors noted.
In total, 54,509 patients were included in the final analysis. Six months after undergoing the procedure, 3,325 patients (6.1%) had been prescribed opioids continuously and regularly. These patients constituted the new long-term opioid user group.
This finding fits in with those from past studies that have suggested that new long-term postoperative pain is reported in 4%-12% of patients who undergo lung cancer surgeries, the authors commented.
The new study found that all-cause mortality at 2 years was significantly higher in the new long-term opioid user group than it was in the non–opioid user group (17.3% vs. 9.3%; P < .001).
Moreover, the new long-term opioid user group were at 43% higher risk of 2-year lung cancer mortality and 29% higher risk of 2-year non–lung cancer mortality.
The investigators divided the patients who had received long-term opioids into two subgroups – those who received more potent opioids (1.6%), and those who received less potent opioids (4.5%).
There was a big difference in the results for all-cause mortality.
Compared with nonopioid users, long-term use of less potent opioids was associated with a 2-year mortality risk of only 22% (P < .001), whereas the patients who used potent opioids were at a 92% increased risk of all-cause mortality.
A number of risk factors were associated with an increased rate of new long-term opioid use. These included older age, being male, length of stay in hospital, and comorbidities.
In addition, patients who were more likely to receive long-term opioids included those who had received neoadjuvant and adjuvant chemotherapy and those who had experienced preoperative anxiety disorder or insomnia disorder.
In contrast, patients who underwent video-assisted thoracoscopic surgery were less likely to receive long-term opioids, the authors noted.
The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM REGIONAL ANESTHESIA AND PAIN
Hidradenitis suppurativa
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.
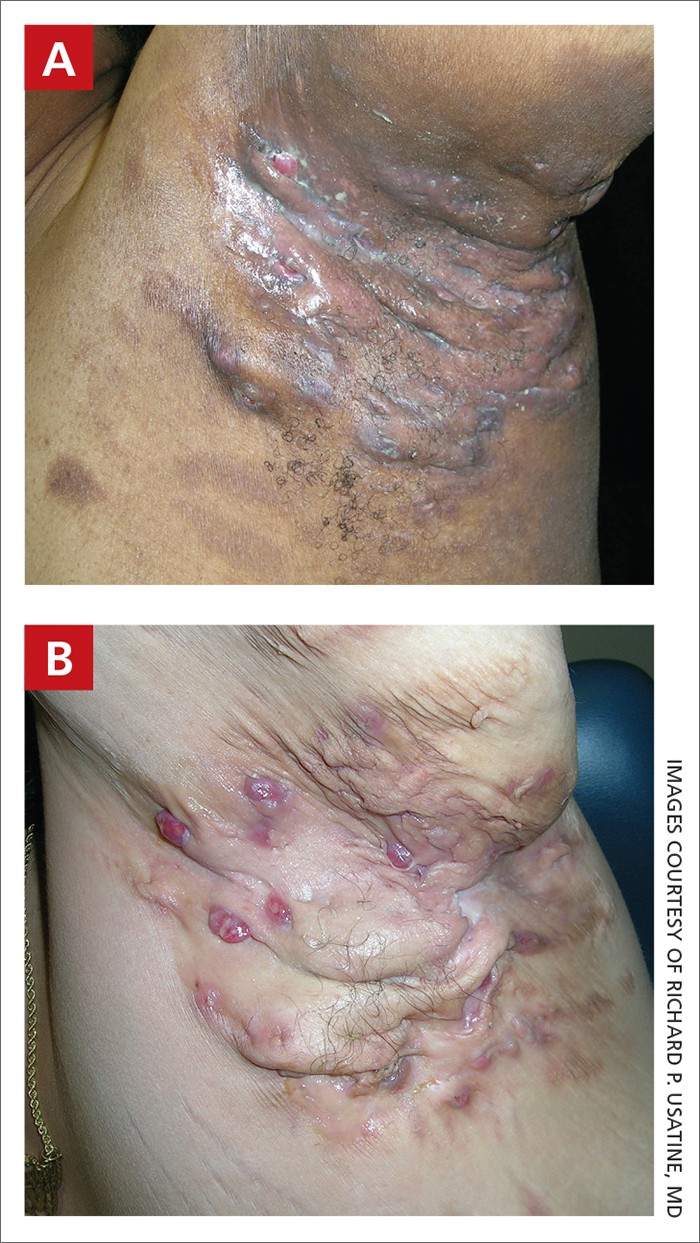
Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
THE COMPARISON
Severe longstanding hidradenitis suppurativa (Hurley stage III) with architectural changes, ropy scarring, granulation tissue, and purulent discharge in the axilla of
A A 35-year-old Black man.
B A 42-year-old Hispanic woman with a light skin tone.

Hidradenitis suppurativa (HS) is a chronic inflammatory condition of the follicular epithelium that most commonly is found in the axillae and buttocks, as well as the inguinal, perianal, and submammary areas. It is characterized by firm and tender chronic nodules, abscesses complicated by sinus tracts, fistulae, and scarring thought to be related to follicular occlusion. Double-open comedones also may be seen.
The Hurley staging system is widely used to characterize the extent of disease in patients with HS:
- Stage I (mild): nodule(s) and abscess(es) without sinus tracts (tunnels) or scarring;
- Stage II (moderate): recurrent nodule(s) and abscess(es) with a limited number of sinus tracts and/or scarring; and
- Stage III (severe): multiple or extensive sinus tracts, abscesses, and/or scarring across the entire area.
Epidemiology
HS is most common in adults and African American patients. It has a prevalence of 1.3% in African Americans.1 When it occurs in children, it generally develops after the onset of puberty. The incidence is higher in females as well as individuals with a history of smoking and obesity (a higher body mass index).2-5
Key clinical features in people with darker skin tones
The erythema associated with HS may be difficult to see in darker skin tones, but violaceous, dark brown, and gray lesions may be present. When active HS lesions subside, intense hyperpigmentation may be left behind, and in some skin tones a pink or violaceous lesion may be apparent.
Worth noting
HS is disfiguring and has a negative impact on quality of life, including social relationships. Mental health support and screening tools are useful. Pain also is a common concern and may warrant referral to a pain specialist.6 In early disease, HS lesions can be misdiagnosed as an infection that recurs in the same location.
Treatments for HS include oral antibiotics (ie, tetracyclines, rifampin, clindamycin), topical antibiotics, immunosuppressing biologics, metformin, and spironolactone.7 Surgical interventions may be considered earlier in HS management and vary based on the location and severity of the lesions.8
Patients with HS are at risk for developing squamous cell carcinoma in scars, even many years later9; therefore, patients should perform skin checks and be referred to a dermatologist. Squamous cell carcinoma is most commonly found on the buttocks of men with HS and has a poor prognosis.
Health disparity highlight
Although those of African American and African descent have the highest rates of HS,1 the clinical trials for adalimumab (the only biologic approved for HS) enrolled a low number of Black patients.
Thirty HS comorbidities have been identified. Garg et al10 recommended that dermatologists perform examinations for comorbid conditions involving the skin and conduct a simple review of systems for extracutaneous comorbidities. Access to medical care is essential, and health care system barriers affect the ability of some patients to receive adequate continuity of care.
The diagnosis of HS often is delayed due to a lack of knowledge about the condition in the medical community at large and delayed presentation to a dermatologist.
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
1. Sachdeva M, Shah M, Alavi A. Race-specific prevalence of hidradenitis suppurativa. J Cutan Med Surg. 2021;25:177-187. doi:10.1177/1203475420972348
2. Zouboulis CC, Goyal M, Byrd AS. Hidradenitis suppurativa in skin of colour. Exp Dermatol. 2021;30(suppl 1):27-30. doi:10.1111 /exd.14341
3. Shalom G, Cohen AD. The epidemiology of hidradenitis suppurativa: what do we know? Br J Dermatol. 2019;180:712-713.
4. Theut Riis P, Pedersen OB, Sigsgaard V, et al. Prevalence of patients with self-reported hidradenitis suppurativa in a cohort of Danish blood donors: a cross-sectional study. Br J Dermatol. 2019;180:774-781.
5. Jemec GB, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7.
6. Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
7. Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: a publication from the United States and Canadian Hidradenitis Suppurativa Foundations: part II: topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81:91-101.
8. Vellaichamy G, Braunberger TL, Nahhas AF, et al. Surgical procedures for hidradenitis suppurativa. Cutis. 2018;102:13-16.
9. Jung JM, Lee KH, Kim Y-J, et al. Assessment of overall and specific cancer risks in patients with hidradenitis suppurativa. JAMA Dermatol. 2020;156:844-853.
10. Garg A, Malviya N, Strunk A, et al. Comorbidity screening in hidradenitis suppurativa: evidence-based recommendations from the US and Canadian Hidradenitis Suppurativa Foundations. J Am Acad Dermatol. 2022;86:1092-1101. doi:10.1016/ j.jaad.2021.01.059
Extravascular ICD surpasses goals in pivotal trial
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
BARCELONA – A novel “extravascular” implantable cardioverter defibrillator (ICD) that uses substernally placed electrodes surpassed its prespecified efficacy and safety targets in the device’s pivotal trial with 299 patients who received an implant.
The results showed that the extravascular ICD “provides antitachycardia pacing and low energy defibrillation while avoiding the vascular space” for lead placement, Ian Crozier, MD, said at the annual congress of the European Society of Cardiology.
“The results are fantastic; they exceeded our expectations,” said Dr. Crozier in an interview, adding that he expects the new device to receive marketing approval from regulatory agencies based on the findings. “This will be the next generation of ICD going forward,” predicted Dr. Crozier, an electrophysiologist cardiologist at Christchurch (New Zealand) Hospital.
Moving beyond transvenous and subcutaneous ICDs
Traditional ICDs use transvenous leads, which can cause vascular injury, are prone to lead fracture over time, and can produce serious infections as well as other potential complications. The U.S. Food and Drug Administration first approved an alternative-design, subcutaneous ICD in 2012 that avoids the need for transvenous leads and the risks they pose. But subcutaneous ICDs have their own limitations: an inability to provide antitachycardia pacing or chronic pacing; a limited ability to provide bradycardia pacing; and an increased device size with shorter battery life, because of the high shock power needed for effective performance. These drawbacks have collectively hindered uptake, Dr. Crozier said.
This led to development of the extravascular ICD – 10 years in the making – which uses substernally placed leads that allow antitachycardia pacing and backup pacing in a device with the size of and the anticipated battery longevity of a transvenous ICD device, noted Dr. Crozier.
A 98.7% rate of arrhythmia termination at implant
The pivotal trial’s primary efficacy endpoint was successful defibrillation based on terminating an induced, sustained, shockable ventricular arrhythmia at the time of implantation. The rate was 98.7%, compared with a prespecified target of 88%. All patients had a class I or IIa indication for an ICD.
The primary safety endpoint was freedom from major system- or procedure-related complications at 6 months, which occurred at a rate of 92.6%, compared with the study’s prespecified target rate of 79%. Both targets were derived from the historical rates of ICDs with transvenous leads.
Simultaneously with Dr. Crozier’s report at the congress, the results also appeared online in the New England Journal of Medicine.
Although the pivotal study met both prespecified endpoints, the evidence has limitations that make it likely that regulatory bodies will seek additional data, commented Fred Kusumoto, MD, director of heart rhythm services for the Mayo Clinic in Jacksonville, Fla.
Short follow-up; questions remain
“Follow-up was relatively short, less than a year,” and “questions remain” about the extravascular ICD’s performance, Dr. Kusumoto said in an interview. “Inappropriate shocks occurred in nearly 10% of patients after 11 month follow-up,” he noted, and also cited the 29 patients who needed revisions including two cases with lead fractures.
“The extravascular lead strategy has an advantage over transvenous systems because of the lower risk for extraction or explant,” and it also provides the antitachycardia pacing that’s not available with subcutaneous ICDs, he granted. But in the new study, antitachycardia termination was delivered to only 10 patients and had “reasonable” effectiveness by resolving 70% of these episodes. “Wide adoption by clinicians will depend on results from larger studies with longer follow-up,” Dr. Kusumoto maintained. He also wanted to see confirmation of the ease of lead removal after longer periods of implantation.
Implantation ‘is not difficult’
The trial ran at 46 sites in 17 countries during September 2019 to October 2021. It enrolled patients with a class I or IIa indication for an ICD, excluding patients with a prior sternotomy or need for chronic pacing, and those unable to undergo defibrillation testing.
Clinicians attempted an implantation in 316 patients and had successful placement in 299 (314 had successful placement of their substernal leads), with 292 having a functional device after 6 months, and 284 completing their planned 6-month follow-up. The median procedure time was 66 minutes, including the time for defibrillation testing.
All of the cardiologists who did the implants had received a full day of training prior to performing the procedure. “This is not a difficult procedure, but it is not a region [the substernal space] that cardiologists are familiar working in,” noted Dr. Crozier, explaining the rationale behind a policy of required implantation training.
Twenty-five adverse events occurred in 23 patients. Eighteen of these events required a system revision, including nine lead dislodgments and five infections. The seven adverse events that did not require a revision included three wound-related episodes and three hospitalizations for inappropriate shock. No patients died, nor were there any cardiac injuries as result of the implant.
During average follow-up of 10.6 months, the implanted devices delivered antitachycardia pacing to 10 patients, successfully terminating 32 of 46 episodes (70%), a rate that Dr. Crozier called “very good, and very comparable to transvenous devices.” The devices also delivered 18 appropriate shocks that successfully converted all 18 episodes.
A 10% rate of inappropriate shocks
However, 29 patients (10% of the study cohort) received inappropriate shocks in 81 episodes, with a total of 118 inappropriate shocks delivered, including 34 episodes (42%) triggered by oversensing of a P wave.
“We fully acknowledge that the inappropriate shock rate is higher than what’s seen with transvenous ICDs, but the rate is comparable to what was seen in the early trials with subcutaneous ICDs,” said Dr. Crozier. “We have a number of strategies to reduce the inappropriate shock rate to what we’d expect with conventional devices,” such as making sure that P waves are not detected by the device at the time of implantation, using new algorithms to mitigate P wave sensing, and other programming changes, he added.
Two patients had lead fractures that Dr. Crozier attributed to atypical lead locations and that are likely avoidable in the future. He expressed optimism that the extravascular ICD will avoid the high lead fracture rate over time that remains a problem for ICDs with transvenous leads.
The study also followed a subgroup of 36 patients who underwent a prespecified protocol of chronic defibrillation testing that was successful in all 36.
Dr. Crozier conceded that the extravascular ICD cannot currently deliver chronic pacing, but he expressed optimism that this capability will be available in the future.
“This innovative [extravascular] ICD system would be particularly beneficial for patients with ventricular arrhythmias that can be reliably pace terminated and avoid a transvenous endocardial lead, but more information is required,” concluded Dr. Kusumoto.
The study was sponsored by Medtronic, the company that is developing the extravascular ICD. Dr. Crozier is a consultant to and has received research funding from Medtronic. Dr. Kusumoto had no disclosures.
AT ESC CONGRESS 2022