User login
June 2018 Question 2
Correct answer: C
Rationale
This patient has long-standing diabetes with associated complications from prolonged hyperglycemia, with symptoms of delayed gastric emptying. The next best step would be to perform a gastric-emptying study or scintigraphy to confirm the diagnosis of diabetic gastroparesis. A dietitian consult will be necessary once gastroparesis is confirmed, since dietary modifications are the mainstay of treatment. Strict blood glucose control is necessary to prevent worsening gastrointestinal symptoms, and an evaluation by an endocrinologist is reasonable if gastroparesis is confirmed. A trial of metoclopramide may be necessary if gastroparesis symptoms are not controlled with dietary modifications, but it would not be first-line treatment in diabetic gastroparesis.
References
1. Camilleri M. Advances in diabetic gastroparesis. Rev Gastroenterol Disord. 2002;2:47-56.
2. Camilleri M, Vazquez-Roque MI. Gastric dysmotility at the organ level in gastroparesis. In: Parkman H, McCallum R. Gastroparesis: Pathophysiology, presentation, diagnosis, and treatment. New York: Springer; 2011. p. 37-46.
Correct answer: C
Rationale
This patient has long-standing diabetes with associated complications from prolonged hyperglycemia, with symptoms of delayed gastric emptying. The next best step would be to perform a gastric-emptying study or scintigraphy to confirm the diagnosis of diabetic gastroparesis. A dietitian consult will be necessary once gastroparesis is confirmed, since dietary modifications are the mainstay of treatment. Strict blood glucose control is necessary to prevent worsening gastrointestinal symptoms, and an evaluation by an endocrinologist is reasonable if gastroparesis is confirmed. A trial of metoclopramide may be necessary if gastroparesis symptoms are not controlled with dietary modifications, but it would not be first-line treatment in diabetic gastroparesis.
References
1. Camilleri M. Advances in diabetic gastroparesis. Rev Gastroenterol Disord. 2002;2:47-56.
2. Camilleri M, Vazquez-Roque MI. Gastric dysmotility at the organ level in gastroparesis. In: Parkman H, McCallum R. Gastroparesis: Pathophysiology, presentation, diagnosis, and treatment. New York: Springer; 2011. p. 37-46.
Correct answer: C
Rationale
This patient has long-standing diabetes with associated complications from prolonged hyperglycemia, with symptoms of delayed gastric emptying. The next best step would be to perform a gastric-emptying study or scintigraphy to confirm the diagnosis of diabetic gastroparesis. A dietitian consult will be necessary once gastroparesis is confirmed, since dietary modifications are the mainstay of treatment. Strict blood glucose control is necessary to prevent worsening gastrointestinal symptoms, and an evaluation by an endocrinologist is reasonable if gastroparesis is confirmed. A trial of metoclopramide may be necessary if gastroparesis symptoms are not controlled with dietary modifications, but it would not be first-line treatment in diabetic gastroparesis.
References
1. Camilleri M. Advances in diabetic gastroparesis. Rev Gastroenterol Disord. 2002;2:47-56.
2. Camilleri M, Vazquez-Roque MI. Gastric dysmotility at the organ level in gastroparesis. In: Parkman H, McCallum R. Gastroparesis: Pathophysiology, presentation, diagnosis, and treatment. New York: Springer; 2011. p. 37-46.
A 55-year-old obese man with long-standing type 2 diabetes mellitus complains of nausea and early satiety for over a year. His medical history is significant for retinopathy, neuropathy, and nephropathy. His diabetes is treated with subcutaneous insulin and an oral hypoglycemic agent, but his recent glycosylated hemoglobin was 11.2%. Since the onset of symptoms, he has lost approximately 30 pounds. Recent upper endoscopy was normal.
Mild TBI May Increase Risk of Parkinson’s Disease Among Military Veterans
Among military veterans, mild traumatic brain injury (TBI) is associated with a 56% increased risk of developing Parkinson’s disease over 12 years of follow-up, according to data published online ahead of print April 18 in Neurology. Prior TBI also is associated with a diagnosis of Parkinson’s disease two years earlier than among controls.
“Our findings highlight the critical importance of unraveling mechanisms subserving the association between TBI and Parkinson’s disease to inform treatment and prevention of post-TBI Parkinson’s disease,” said Raquel C. Gardner, MD, Assistant Professor of Neurology at the University of California, San Francisco.
A Longitudinal Cohort Study
Every year, mild TBI affects an estimated 42 million people worldwide. It is especially common among athletes and military personnel and is a growing epidemic among the elderly. In 2008, the Institute of Medicine found sufficient evidence to suggest an association between moderate to severe TBI and a clinical diagnosis of Parkinson’s disease, but limited evidence for an association between mild TBI with loss of consciousness and a clinical diagnosis of Parkinson’s disease. One small case–control study assessed the risk of Parkinson’s disease following mild TBI among military veterans, but the results were inconclusive, said the authors.
Dr. Gardner and colleagues conducted a longitudinal cohort study to evaluate the risk of Parkinson’s disease following TBI, including mild TBI, among patients in the Veterans Health Administration (VHA). They analyzed data from three nationwide VHA health care system databases and identified patients with a diagnosis of TBI from October 2002 to September 2014. Participants were age 18 or older without Parkinson’s disease or dementia at baseline and were age-matched 1:1 to a random sample of patients without TBI.
Researchers defined moderate to severe TBI as a loss of consciousness for more than 30 minutes, alteration of consciousness for more than 24 hours, or amnesia for more than 24 hours. They defined mild TBI as loss of consciousness for zero to 30 minutes, alteration of consciousness for a moment to 24 hours, or amnesia for zero to 24 hours.
TBI exposure and severity were determined via detailed clinical assessments or ICD-9 codes using Department of Defense and Defense and Veterans Brain Injury Center criteria. Baseline comorbidities and incident Parkinson’s disease at more than one year post TBI were identified using ICD-9 codes. In addition, investigators used Cox proportional hazard models adjusted for demographics and medical and psychiatric comorbidities to assess risk of Parkinson’s disease after TBI.
Prior TBI Was Associated With Minority Status
A total of 325,870 patients were included in the study with an average age of 47.9 and an average follow-up of 4.6 years. In all, 1,462 patients were diagnosed with Parkinson’s disease during follow-up. After adjusting for age, sex, race, education, and other health conditions, the researchers found that patients with any severity of TBI had a 71% increased risk of Parkinson’s disease; participants with moderate to severe TBI had an 83% increased risk.
Overall, patients with prior TBI were diagnosed with Parkinson’s disease at a significantly younger age, had significantly higher prevalence of non-Hispanic black and Hispanic race or ethnicity, and had significantly higher prevalence of all medical and psychiatric comorbidities, compared with those without prior TBI.
“Given the growing evidence for several potentially modifiable risk factors for Parkinson’s disease, an important area for future research will be to determine whether improved management of specific highly prevalent comorbidities among TBI-exposed veterans may reduce risk of subsequent Parkinson’s disease,” said the researchers.
Strengths of this study include the use of physicians’ diagnosis of TBI and Parkinson’s disease, a longitudinal cohort design, and a large sample size. One of the study’s limitations was the use of ICD-9 codes for the diagnosis of TBI and Parkinson’s disease, which may have overlooked some cases, such as TBI with polytrauma or mild
—Erica Tricarico
Suggested Reading
Gardner RC, Byers AL, Barnes DE, et al. Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology. 2018 Apr 18 [Epub ahead of print].
Among military veterans, mild traumatic brain injury (TBI) is associated with a 56% increased risk of developing Parkinson’s disease over 12 years of follow-up, according to data published online ahead of print April 18 in Neurology. Prior TBI also is associated with a diagnosis of Parkinson’s disease two years earlier than among controls.
“Our findings highlight the critical importance of unraveling mechanisms subserving the association between TBI and Parkinson’s disease to inform treatment and prevention of post-TBI Parkinson’s disease,” said Raquel C. Gardner, MD, Assistant Professor of Neurology at the University of California, San Francisco.
A Longitudinal Cohort Study
Every year, mild TBI affects an estimated 42 million people worldwide. It is especially common among athletes and military personnel and is a growing epidemic among the elderly. In 2008, the Institute of Medicine found sufficient evidence to suggest an association between moderate to severe TBI and a clinical diagnosis of Parkinson’s disease, but limited evidence for an association between mild TBI with loss of consciousness and a clinical diagnosis of Parkinson’s disease. One small case–control study assessed the risk of Parkinson’s disease following mild TBI among military veterans, but the results were inconclusive, said the authors.
Dr. Gardner and colleagues conducted a longitudinal cohort study to evaluate the risk of Parkinson’s disease following TBI, including mild TBI, among patients in the Veterans Health Administration (VHA). They analyzed data from three nationwide VHA health care system databases and identified patients with a diagnosis of TBI from October 2002 to September 2014. Participants were age 18 or older without Parkinson’s disease or dementia at baseline and were age-matched 1:1 to a random sample of patients without TBI.
Researchers defined moderate to severe TBI as a loss of consciousness for more than 30 minutes, alteration of consciousness for more than 24 hours, or amnesia for more than 24 hours. They defined mild TBI as loss of consciousness for zero to 30 minutes, alteration of consciousness for a moment to 24 hours, or amnesia for zero to 24 hours.
TBI exposure and severity were determined via detailed clinical assessments or ICD-9 codes using Department of Defense and Defense and Veterans Brain Injury Center criteria. Baseline comorbidities and incident Parkinson’s disease at more than one year post TBI were identified using ICD-9 codes. In addition, investigators used Cox proportional hazard models adjusted for demographics and medical and psychiatric comorbidities to assess risk of Parkinson’s disease after TBI.
Prior TBI Was Associated With Minority Status
A total of 325,870 patients were included in the study with an average age of 47.9 and an average follow-up of 4.6 years. In all, 1,462 patients were diagnosed with Parkinson’s disease during follow-up. After adjusting for age, sex, race, education, and other health conditions, the researchers found that patients with any severity of TBI had a 71% increased risk of Parkinson’s disease; participants with moderate to severe TBI had an 83% increased risk.
Overall, patients with prior TBI were diagnosed with Parkinson’s disease at a significantly younger age, had significantly higher prevalence of non-Hispanic black and Hispanic race or ethnicity, and had significantly higher prevalence of all medical and psychiatric comorbidities, compared with those without prior TBI.
“Given the growing evidence for several potentially modifiable risk factors for Parkinson’s disease, an important area for future research will be to determine whether improved management of specific highly prevalent comorbidities among TBI-exposed veterans may reduce risk of subsequent Parkinson’s disease,” said the researchers.
Strengths of this study include the use of physicians’ diagnosis of TBI and Parkinson’s disease, a longitudinal cohort design, and a large sample size. One of the study’s limitations was the use of ICD-9 codes for the diagnosis of TBI and Parkinson’s disease, which may have overlooked some cases, such as TBI with polytrauma or mild
—Erica Tricarico
Suggested Reading
Gardner RC, Byers AL, Barnes DE, et al. Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology. 2018 Apr 18 [Epub ahead of print].
Among military veterans, mild traumatic brain injury (TBI) is associated with a 56% increased risk of developing Parkinson’s disease over 12 years of follow-up, according to data published online ahead of print April 18 in Neurology. Prior TBI also is associated with a diagnosis of Parkinson’s disease two years earlier than among controls.
“Our findings highlight the critical importance of unraveling mechanisms subserving the association between TBI and Parkinson’s disease to inform treatment and prevention of post-TBI Parkinson’s disease,” said Raquel C. Gardner, MD, Assistant Professor of Neurology at the University of California, San Francisco.
A Longitudinal Cohort Study
Every year, mild TBI affects an estimated 42 million people worldwide. It is especially common among athletes and military personnel and is a growing epidemic among the elderly. In 2008, the Institute of Medicine found sufficient evidence to suggest an association between moderate to severe TBI and a clinical diagnosis of Parkinson’s disease, but limited evidence for an association between mild TBI with loss of consciousness and a clinical diagnosis of Parkinson’s disease. One small case–control study assessed the risk of Parkinson’s disease following mild TBI among military veterans, but the results were inconclusive, said the authors.
Dr. Gardner and colleagues conducted a longitudinal cohort study to evaluate the risk of Parkinson’s disease following TBI, including mild TBI, among patients in the Veterans Health Administration (VHA). They analyzed data from three nationwide VHA health care system databases and identified patients with a diagnosis of TBI from October 2002 to September 2014. Participants were age 18 or older without Parkinson’s disease or dementia at baseline and were age-matched 1:1 to a random sample of patients without TBI.
Researchers defined moderate to severe TBI as a loss of consciousness for more than 30 minutes, alteration of consciousness for more than 24 hours, or amnesia for more than 24 hours. They defined mild TBI as loss of consciousness for zero to 30 minutes, alteration of consciousness for a moment to 24 hours, or amnesia for zero to 24 hours.
TBI exposure and severity were determined via detailed clinical assessments or ICD-9 codes using Department of Defense and Defense and Veterans Brain Injury Center criteria. Baseline comorbidities and incident Parkinson’s disease at more than one year post TBI were identified using ICD-9 codes. In addition, investigators used Cox proportional hazard models adjusted for demographics and medical and psychiatric comorbidities to assess risk of Parkinson’s disease after TBI.
Prior TBI Was Associated With Minority Status
A total of 325,870 patients were included in the study with an average age of 47.9 and an average follow-up of 4.6 years. In all, 1,462 patients were diagnosed with Parkinson’s disease during follow-up. After adjusting for age, sex, race, education, and other health conditions, the researchers found that patients with any severity of TBI had a 71% increased risk of Parkinson’s disease; participants with moderate to severe TBI had an 83% increased risk.
Overall, patients with prior TBI were diagnosed with Parkinson’s disease at a significantly younger age, had significantly higher prevalence of non-Hispanic black and Hispanic race or ethnicity, and had significantly higher prevalence of all medical and psychiatric comorbidities, compared with those without prior TBI.
“Given the growing evidence for several potentially modifiable risk factors for Parkinson’s disease, an important area for future research will be to determine whether improved management of specific highly prevalent comorbidities among TBI-exposed veterans may reduce risk of subsequent Parkinson’s disease,” said the researchers.
Strengths of this study include the use of physicians’ diagnosis of TBI and Parkinson’s disease, a longitudinal cohort design, and a large sample size. One of the study’s limitations was the use of ICD-9 codes for the diagnosis of TBI and Parkinson’s disease, which may have overlooked some cases, such as TBI with polytrauma or mild
—Erica Tricarico
Suggested Reading
Gardner RC, Byers AL, Barnes DE, et al. Mild TBI and risk of Parkinson disease: a chronic effects of neurotrauma consortium study. Neurology. 2018 Apr 18 [Epub ahead of print].
June 2018 Question 1
Correct answer: C
Rationale
This patient is presenting early post-liver transplant with severe hepatic dysfunction. This severity of enzyme elevation is concerning for an underlying hepatic artery thrombosis. The next appropriate diagnostic test for this patient is a hepatic ultrasound with Dopplers to assess hepatic artery patency. CMV infection does not typically occur within the first month post-liver transplant and would not be expected to cause this degree of elevation in the liver enzymes. Performance of liver biopsy, MRCP, or ERCP would not reveal the underlying etiology and may result in delay in diagnosis.
Reference
1. Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transplantation 2003;9:612-20.
Correct answer: C
Rationale
This patient is presenting early post-liver transplant with severe hepatic dysfunction. This severity of enzyme elevation is concerning for an underlying hepatic artery thrombosis. The next appropriate diagnostic test for this patient is a hepatic ultrasound with Dopplers to assess hepatic artery patency. CMV infection does not typically occur within the first month post-liver transplant and would not be expected to cause this degree of elevation in the liver enzymes. Performance of liver biopsy, MRCP, or ERCP would not reveal the underlying etiology and may result in delay in diagnosis.
Reference
1. Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transplantation 2003;9:612-20.
Correct answer: C
Rationale
This patient is presenting early post-liver transplant with severe hepatic dysfunction. This severity of enzyme elevation is concerning for an underlying hepatic artery thrombosis. The next appropriate diagnostic test for this patient is a hepatic ultrasound with Dopplers to assess hepatic artery patency. CMV infection does not typically occur within the first month post-liver transplant and would not be expected to cause this degree of elevation in the liver enzymes. Performance of liver biopsy, MRCP, or ERCP would not reveal the underlying etiology and may result in delay in diagnosis.
Reference
1. Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transplantation 2003;9:612-20.
A 62-year-old man underwent deceased-donor liver transplant 36 hours ago for decompensated chronic hepatitis C cirrhosis. He did well initially posttransplant with a steady decline in his transaminases and improvement in hepatic synthetic function. But he has had a rapidly progressive decline in his clinical status over the past 12 hours. On physical exam, his mental status is notable for new confusion. His temperature is 38.9 ºC. Laboratory data reveal the following:
AST 10,300 U/L
ALT 14,550 U/L
total bilirubin 9.6 mg/dL
alkaline phosphatase 693 IU/L
INR 3.6
creatinine 4.6 mg/dL with oliguria.
DMTs Are Associated With Reduced Stroke Risk in Patients With MS
LOS ANGELES—Among patients with multiple sclerosis (MS), treatment with disease-modifying therapy is associated with a significantly decreased likelihood of ischemic stroke, compared with no disease-modifying therapy, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology.
Stroke is a major cause of morbidity and mortality. One-third to one-half of stroke survivors have post-stoke disability. Autoimmune diseases are associated with an increased risk of ischemic stroke. To examine the role of disease-modifying therapy in the prevention of ischemic stroke in MS, Asad Ikram, MD, a clinical research fellow in the Department of Neurology at the University of New Mexico School of Medicine in Albuquerque, and colleagues conducted a nationwide, case–control, population-based study.
Investigators extracted data from the Cerner’s Health Facts database and used ICD-9/10 codes to identify all patients diagnosed with MS between 2000 and 2016. The researchers identified patients with MS who had an ischemic stroke and categorized them as having received disease-modifying therapy or not. They excluded patients younger than 18 and patients for whom they did not have information about sex.
The investigators used multiple logistic regression to evaluate the likelihood of ischemic stroke in patients treated with disease-modifying therapy. In the final model, they also adjusted for patients’ baseline characteristics and medications.
In all, Dr. Ikram and colleagues identified 57,769 patients with a diagnosis of MS, 1,349 of whom had a CT-confirmed ischemic stroke (2.34%).
The study group (ie, patients with ischemic stroke who were receiving disease-modifying therapy) included 126 patients (89 females), and the control group (ie, patients with ischemic stroke who had not received disease-modifying therapy) included 1,002 patients.
Patients not treated with disease-modifying therapy were approximately twice as likely to have an ischemic stroke, compared with patients treated with disease-modifying therapy (odds ratio = 1.91).
After controlling for covariates (eg, smoking, hypertension, diabetes, heart failure, atrial fibrillation, age, and sex), the likelihood of ischemic stroke remained higher in the untreated group.
“This study suggests that the early treatment of MS with disease-modifying therapies may reduce the risk of ischemic stroke in the MS population,” said Dr. Ikram and colleagues.
—Jake Remaly
LOS ANGELES—Among patients with multiple sclerosis (MS), treatment with disease-modifying therapy is associated with a significantly decreased likelihood of ischemic stroke, compared with no disease-modifying therapy, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology.
Stroke is a major cause of morbidity and mortality. One-third to one-half of stroke survivors have post-stoke disability. Autoimmune diseases are associated with an increased risk of ischemic stroke. To examine the role of disease-modifying therapy in the prevention of ischemic stroke in MS, Asad Ikram, MD, a clinical research fellow in the Department of Neurology at the University of New Mexico School of Medicine in Albuquerque, and colleagues conducted a nationwide, case–control, population-based study.
Investigators extracted data from the Cerner’s Health Facts database and used ICD-9/10 codes to identify all patients diagnosed with MS between 2000 and 2016. The researchers identified patients with MS who had an ischemic stroke and categorized them as having received disease-modifying therapy or not. They excluded patients younger than 18 and patients for whom they did not have information about sex.
The investigators used multiple logistic regression to evaluate the likelihood of ischemic stroke in patients treated with disease-modifying therapy. In the final model, they also adjusted for patients’ baseline characteristics and medications.
In all, Dr. Ikram and colleagues identified 57,769 patients with a diagnosis of MS, 1,349 of whom had a CT-confirmed ischemic stroke (2.34%).
The study group (ie, patients with ischemic stroke who were receiving disease-modifying therapy) included 126 patients (89 females), and the control group (ie, patients with ischemic stroke who had not received disease-modifying therapy) included 1,002 patients.
Patients not treated with disease-modifying therapy were approximately twice as likely to have an ischemic stroke, compared with patients treated with disease-modifying therapy (odds ratio = 1.91).
After controlling for covariates (eg, smoking, hypertension, diabetes, heart failure, atrial fibrillation, age, and sex), the likelihood of ischemic stroke remained higher in the untreated group.
“This study suggests that the early treatment of MS with disease-modifying therapies may reduce the risk of ischemic stroke in the MS population,” said Dr. Ikram and colleagues.
—Jake Remaly
LOS ANGELES—Among patients with multiple sclerosis (MS), treatment with disease-modifying therapy is associated with a significantly decreased likelihood of ischemic stroke, compared with no disease-modifying therapy, according to a study presented at the 70th Annual Meeting of the American Academy of Neurology.
Stroke is a major cause of morbidity and mortality. One-third to one-half of stroke survivors have post-stoke disability. Autoimmune diseases are associated with an increased risk of ischemic stroke. To examine the role of disease-modifying therapy in the prevention of ischemic stroke in MS, Asad Ikram, MD, a clinical research fellow in the Department of Neurology at the University of New Mexico School of Medicine in Albuquerque, and colleagues conducted a nationwide, case–control, population-based study.
Investigators extracted data from the Cerner’s Health Facts database and used ICD-9/10 codes to identify all patients diagnosed with MS between 2000 and 2016. The researchers identified patients with MS who had an ischemic stroke and categorized them as having received disease-modifying therapy or not. They excluded patients younger than 18 and patients for whom they did not have information about sex.
The investigators used multiple logistic regression to evaluate the likelihood of ischemic stroke in patients treated with disease-modifying therapy. In the final model, they also adjusted for patients’ baseline characteristics and medications.
In all, Dr. Ikram and colleagues identified 57,769 patients with a diagnosis of MS, 1,349 of whom had a CT-confirmed ischemic stroke (2.34%).
The study group (ie, patients with ischemic stroke who were receiving disease-modifying therapy) included 126 patients (89 females), and the control group (ie, patients with ischemic stroke who had not received disease-modifying therapy) included 1,002 patients.
Patients not treated with disease-modifying therapy were approximately twice as likely to have an ischemic stroke, compared with patients treated with disease-modifying therapy (odds ratio = 1.91).
After controlling for covariates (eg, smoking, hypertension, diabetes, heart failure, atrial fibrillation, age, and sex), the likelihood of ischemic stroke remained higher in the untreated group.
“This study suggests that the early treatment of MS with disease-modifying therapies may reduce the risk of ischemic stroke in the MS population,” said Dr. Ikram and colleagues.
—Jake Remaly
Safety of MRI in patients with implantable cardiac devices
Clinical question: Is MRI safe for patients who have implanted ICD or pacemakers that have not been deemed to be “MRI conditional” by the Food and Drug Administration?
Background: The majority of patients with implantable cardiac devices have a clinical indication for MRI within 10 years. Devices that meet certain criteria specified by the Food and Drug Administration are not felt to pose any safety hazards and are deemed “MRI conditional.” Those that do not meet these criteria are referred to as “legacy” devices and are considered to be a contraindication to MRI by the FDA and device manufacturers. The majority of ICDs and pacemakers currently in use are legacy devices and access to MRI for patients who have these devices has been very limited. This study is the first large prospective study to evaluate the safety of an MRI protocol in patients with legacy ICDs and pacemakers.
Study design: Prospective nonrandomized study.
Setting: Single academic medical center.
Synopsis: During 2003-2015, 1,509 patients with ICDs (629 patients) and pacemakers (880 patients) were enrolled and underwent 2,103 MRI examinations supervised by either an electrophysiologist or a registered nurse with cardiac device programming experience.
Study outcomes included safety and device function immediately after MRI and change in device parameters both immediately after MRI and at long-term follow-up. The most important clinical adverse event that occurred was a reset of device to backup settings referred to as “power on reset” that occurred in nine examinations. Of these nine events, one was associated with mild physical discomfort, one led to device replacement, and one was associated with transient inhibition of pacing. Small changes in P- or R-wave amplitude and atrial or ventricular capture were noted at long-term follow-up. However, none of these were large enough to result in lead revision or device reprogramming. Notable limitations of this study include that it is a single-center study limiting its ability to be generalized and that nearly 20% of patients were lost to long term follow up.
Bottom line: When performed at an institution with an established safety protocol, MRI examinations in patients with legacy devices are not associated with clinically significant adverse safety events or changes in device function that require reprogramming. Multicenter studies are necessary to determine if these results can be generalizable.
Citation: Nazarian S et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017 Dec 28;377(26):2555-64.
Dr. Scaletta is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Clinical question: Is MRI safe for patients who have implanted ICD or pacemakers that have not been deemed to be “MRI conditional” by the Food and Drug Administration?
Background: The majority of patients with implantable cardiac devices have a clinical indication for MRI within 10 years. Devices that meet certain criteria specified by the Food and Drug Administration are not felt to pose any safety hazards and are deemed “MRI conditional.” Those that do not meet these criteria are referred to as “legacy” devices and are considered to be a contraindication to MRI by the FDA and device manufacturers. The majority of ICDs and pacemakers currently in use are legacy devices and access to MRI for patients who have these devices has been very limited. This study is the first large prospective study to evaluate the safety of an MRI protocol in patients with legacy ICDs and pacemakers.
Study design: Prospective nonrandomized study.
Setting: Single academic medical center.
Synopsis: During 2003-2015, 1,509 patients with ICDs (629 patients) and pacemakers (880 patients) were enrolled and underwent 2,103 MRI examinations supervised by either an electrophysiologist or a registered nurse with cardiac device programming experience.
Study outcomes included safety and device function immediately after MRI and change in device parameters both immediately after MRI and at long-term follow-up. The most important clinical adverse event that occurred was a reset of device to backup settings referred to as “power on reset” that occurred in nine examinations. Of these nine events, one was associated with mild physical discomfort, one led to device replacement, and one was associated with transient inhibition of pacing. Small changes in P- or R-wave amplitude and atrial or ventricular capture were noted at long-term follow-up. However, none of these were large enough to result in lead revision or device reprogramming. Notable limitations of this study include that it is a single-center study limiting its ability to be generalized and that nearly 20% of patients were lost to long term follow up.
Bottom line: When performed at an institution with an established safety protocol, MRI examinations in patients with legacy devices are not associated with clinically significant adverse safety events or changes in device function that require reprogramming. Multicenter studies are necessary to determine if these results can be generalizable.
Citation: Nazarian S et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017 Dec 28;377(26):2555-64.
Dr. Scaletta is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Clinical question: Is MRI safe for patients who have implanted ICD or pacemakers that have not been deemed to be “MRI conditional” by the Food and Drug Administration?
Background: The majority of patients with implantable cardiac devices have a clinical indication for MRI within 10 years. Devices that meet certain criteria specified by the Food and Drug Administration are not felt to pose any safety hazards and are deemed “MRI conditional.” Those that do not meet these criteria are referred to as “legacy” devices and are considered to be a contraindication to MRI by the FDA and device manufacturers. The majority of ICDs and pacemakers currently in use are legacy devices and access to MRI for patients who have these devices has been very limited. This study is the first large prospective study to evaluate the safety of an MRI protocol in patients with legacy ICDs and pacemakers.
Study design: Prospective nonrandomized study.
Setting: Single academic medical center.
Synopsis: During 2003-2015, 1,509 patients with ICDs (629 patients) and pacemakers (880 patients) were enrolled and underwent 2,103 MRI examinations supervised by either an electrophysiologist or a registered nurse with cardiac device programming experience.
Study outcomes included safety and device function immediately after MRI and change in device parameters both immediately after MRI and at long-term follow-up. The most important clinical adverse event that occurred was a reset of device to backup settings referred to as “power on reset” that occurred in nine examinations. Of these nine events, one was associated with mild physical discomfort, one led to device replacement, and one was associated with transient inhibition of pacing. Small changes in P- or R-wave amplitude and atrial or ventricular capture were noted at long-term follow-up. However, none of these were large enough to result in lead revision or device reprogramming. Notable limitations of this study include that it is a single-center study limiting its ability to be generalized and that nearly 20% of patients were lost to long term follow up.
Bottom line: When performed at an institution with an established safety protocol, MRI examinations in patients with legacy devices are not associated with clinically significant adverse safety events or changes in device function that require reprogramming. Multicenter studies are necessary to determine if these results can be generalizable.
Citation: Nazarian S et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017 Dec 28;377(26):2555-64.
Dr. Scaletta is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Coding considerations in investigating chronic pelvic pain
Nonsurgical interventions for chronic pelvic pain may include evaluation and management visits for managing medications, trigger point injections, or pelvic floor physical therapy. However, while these management options can be coded, some of them may have limitations imposed by payers on the frequency of care and by whom the care may be rendered.
For encounters that involve the management of pain medications, it is important that documentation for each of these visits clearly spells out the progress the patient is making in setting goals for pain management. Frequent office visits may send a flag to the payer for overutilization; complete documentation will go a long way to support the medical necessity of each visit at the level billed.
Who renders treatment?
Sometimes, chronic pelvic pain management involves pelvic floor physical therapy, such as teaching pelvic floor exercises or using biofeedback to control certain aspects of the pain. The majority of payers have strict guidelines dictating who can render these services by way of licensure and training if performed by someone other than the physician, and at what frequency. Typically, the person performing the therapy must be, at minimum, a licensed physical therapist.
Frequency
Frequency is often limited to 1 to 2 times a week in increments of 4 weeks before additional authorization is granted. Again, careful and detailed documentation of the patient’s progress will be crucial to continued therapy.
The TABLE shows typical Current Procedural Terminology (CPT) codes that might be authorized by the payer for this type of management.
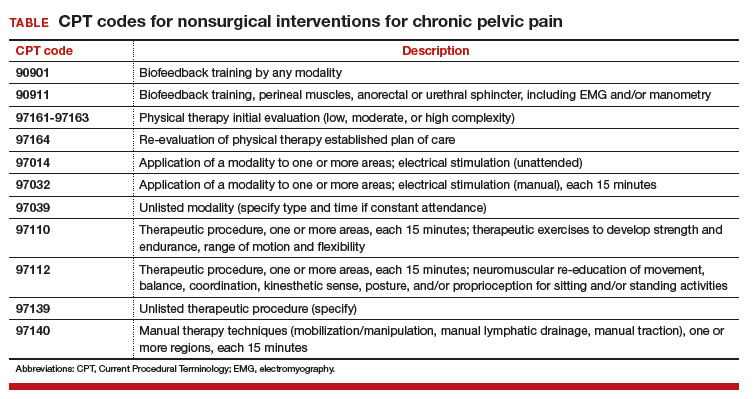
Timed codes
Keep in mind that the “timed” codes listed above are based on the provider’s time spent one-on-one in direct contact with the patient. The time must have been used to provide skilled services and includes pre-, intra-, and posttreatment. CPT also has clarified that if “less than 15 minutes of service is provided, then the reduced services modifier -52 should be appended to the code to identify the reduction of service.” It will therefore be important that the provider accurately document the time involved in the therapy session for these codes.
Trigger point injections
Another treatment option is the use of trigger point injections to control pelvic pain. CPT provides 2 codes to report these:
- 20552, Injection(s); single or multiple trigger point(s), 1 or 2 muscle(s)
- 20553, Injection(s); single or multiple trigger point(s), 3 or more muscles.
Notice that the choice of code depends on the number of muscles the anesthetic is injected into, not the number of injections given at that muscle site.
The relative values units assigned to these codes are based on the injection procedure alone. Normally, the anesthetic used (lidocaine or bupivacaine) can be billed in addition; however, there are no specific Healthcare Common Procedure Coding System (HCPCS) “J” codes for either. Code J2001, Injection, lidocaine HCl, can only be reported for an intravenous infusion, not intramuscular, and the only current code for bupivacaine is an “S” code that is only recognized by some Blue Cross/Blue Shield payers (S0020, Injection, bupivacaine HCl, 30 ml).
Some physicians also inject sodium bicarbonate, but this, too, has no specific “J” code. Because of this, the only correct J code to report these drugs will be J3490, Unclassified drugs. Be sure to include the National Drug Code (NDC) number (usually found on the package insert) for each drug, and an invoice showing your cost with the claim.
ICD-10-CM codes needed for support
Billing for services will not be complete without a supporting diagnostic code. For pelvic pain in particular, 1 or more of the following ICD-10-CM codes may provide the medical justification for the provided nonsurgical services so long as there are no identified psychological factors:
- G89.0, Central pain syndrome
- G89.29, Other chronic pain
- N94.10, Unspecified dyspareunia
- N94.11, Superficial (introital) dyspareunia
- N94.12, Deep dyspareunia
- N94.19, Other specified dyspareunia
- N94.2, Vaginismus
- N94.4, Primary dysmenorrhea
- N94.5, Secondary dysmenorrhea
- N94.6, Dysmenorrhea, unspecified
- N94.810, Vulvar vestibulitis
- N94.818, Other vulvodynia
- N94.819, Vulvodynia, unspecified
- R10.2, Pelvic and perineal pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Nonsurgical interventions for chronic pelvic pain may include evaluation and management visits for managing medications, trigger point injections, or pelvic floor physical therapy. However, while these management options can be coded, some of them may have limitations imposed by payers on the frequency of care and by whom the care may be rendered.
For encounters that involve the management of pain medications, it is important that documentation for each of these visits clearly spells out the progress the patient is making in setting goals for pain management. Frequent office visits may send a flag to the payer for overutilization; complete documentation will go a long way to support the medical necessity of each visit at the level billed.
Who renders treatment?
Sometimes, chronic pelvic pain management involves pelvic floor physical therapy, such as teaching pelvic floor exercises or using biofeedback to control certain aspects of the pain. The majority of payers have strict guidelines dictating who can render these services by way of licensure and training if performed by someone other than the physician, and at what frequency. Typically, the person performing the therapy must be, at minimum, a licensed physical therapist.
Frequency
Frequency is often limited to 1 to 2 times a week in increments of 4 weeks before additional authorization is granted. Again, careful and detailed documentation of the patient’s progress will be crucial to continued therapy.
The TABLE shows typical Current Procedural Terminology (CPT) codes that might be authorized by the payer for this type of management.

Timed codes
Keep in mind that the “timed” codes listed above are based on the provider’s time spent one-on-one in direct contact with the patient. The time must have been used to provide skilled services and includes pre-, intra-, and posttreatment. CPT also has clarified that if “less than 15 minutes of service is provided, then the reduced services modifier -52 should be appended to the code to identify the reduction of service.” It will therefore be important that the provider accurately document the time involved in the therapy session for these codes.
Trigger point injections
Another treatment option is the use of trigger point injections to control pelvic pain. CPT provides 2 codes to report these:
- 20552, Injection(s); single or multiple trigger point(s), 1 or 2 muscle(s)
- 20553, Injection(s); single or multiple trigger point(s), 3 or more muscles.
Notice that the choice of code depends on the number of muscles the anesthetic is injected into, not the number of injections given at that muscle site.
The relative values units assigned to these codes are based on the injection procedure alone. Normally, the anesthetic used (lidocaine or bupivacaine) can be billed in addition; however, there are no specific Healthcare Common Procedure Coding System (HCPCS) “J” codes for either. Code J2001, Injection, lidocaine HCl, can only be reported for an intravenous infusion, not intramuscular, and the only current code for bupivacaine is an “S” code that is only recognized by some Blue Cross/Blue Shield payers (S0020, Injection, bupivacaine HCl, 30 ml).
Some physicians also inject sodium bicarbonate, but this, too, has no specific “J” code. Because of this, the only correct J code to report these drugs will be J3490, Unclassified drugs. Be sure to include the National Drug Code (NDC) number (usually found on the package insert) for each drug, and an invoice showing your cost with the claim.
ICD-10-CM codes needed for support
Billing for services will not be complete without a supporting diagnostic code. For pelvic pain in particular, 1 or more of the following ICD-10-CM codes may provide the medical justification for the provided nonsurgical services so long as there are no identified psychological factors:
- G89.0, Central pain syndrome
- G89.29, Other chronic pain
- N94.10, Unspecified dyspareunia
- N94.11, Superficial (introital) dyspareunia
- N94.12, Deep dyspareunia
- N94.19, Other specified dyspareunia
- N94.2, Vaginismus
- N94.4, Primary dysmenorrhea
- N94.5, Secondary dysmenorrhea
- N94.6, Dysmenorrhea, unspecified
- N94.810, Vulvar vestibulitis
- N94.818, Other vulvodynia
- N94.819, Vulvodynia, unspecified
- R10.2, Pelvic and perineal pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Nonsurgical interventions for chronic pelvic pain may include evaluation and management visits for managing medications, trigger point injections, or pelvic floor physical therapy. However, while these management options can be coded, some of them may have limitations imposed by payers on the frequency of care and by whom the care may be rendered.
For encounters that involve the management of pain medications, it is important that documentation for each of these visits clearly spells out the progress the patient is making in setting goals for pain management. Frequent office visits may send a flag to the payer for overutilization; complete documentation will go a long way to support the medical necessity of each visit at the level billed.
Who renders treatment?
Sometimes, chronic pelvic pain management involves pelvic floor physical therapy, such as teaching pelvic floor exercises or using biofeedback to control certain aspects of the pain. The majority of payers have strict guidelines dictating who can render these services by way of licensure and training if performed by someone other than the physician, and at what frequency. Typically, the person performing the therapy must be, at minimum, a licensed physical therapist.
Frequency
Frequency is often limited to 1 to 2 times a week in increments of 4 weeks before additional authorization is granted. Again, careful and detailed documentation of the patient’s progress will be crucial to continued therapy.
The TABLE shows typical Current Procedural Terminology (CPT) codes that might be authorized by the payer for this type of management.

Timed codes
Keep in mind that the “timed” codes listed above are based on the provider’s time spent one-on-one in direct contact with the patient. The time must have been used to provide skilled services and includes pre-, intra-, and posttreatment. CPT also has clarified that if “less than 15 minutes of service is provided, then the reduced services modifier -52 should be appended to the code to identify the reduction of service.” It will therefore be important that the provider accurately document the time involved in the therapy session for these codes.
Trigger point injections
Another treatment option is the use of trigger point injections to control pelvic pain. CPT provides 2 codes to report these:
- 20552, Injection(s); single or multiple trigger point(s), 1 or 2 muscle(s)
- 20553, Injection(s); single or multiple trigger point(s), 3 or more muscles.
Notice that the choice of code depends on the number of muscles the anesthetic is injected into, not the number of injections given at that muscle site.
The relative values units assigned to these codes are based on the injection procedure alone. Normally, the anesthetic used (lidocaine or bupivacaine) can be billed in addition; however, there are no specific Healthcare Common Procedure Coding System (HCPCS) “J” codes for either. Code J2001, Injection, lidocaine HCl, can only be reported for an intravenous infusion, not intramuscular, and the only current code for bupivacaine is an “S” code that is only recognized by some Blue Cross/Blue Shield payers (S0020, Injection, bupivacaine HCl, 30 ml).
Some physicians also inject sodium bicarbonate, but this, too, has no specific “J” code. Because of this, the only correct J code to report these drugs will be J3490, Unclassified drugs. Be sure to include the National Drug Code (NDC) number (usually found on the package insert) for each drug, and an invoice showing your cost with the claim.
ICD-10-CM codes needed for support
Billing for services will not be complete without a supporting diagnostic code. For pelvic pain in particular, 1 or more of the following ICD-10-CM codes may provide the medical justification for the provided nonsurgical services so long as there are no identified psychological factors:
- G89.0, Central pain syndrome
- G89.29, Other chronic pain
- N94.10, Unspecified dyspareunia
- N94.11, Superficial (introital) dyspareunia
- N94.12, Deep dyspareunia
- N94.19, Other specified dyspareunia
- N94.2, Vaginismus
- N94.4, Primary dysmenorrhea
- N94.5, Secondary dysmenorrhea
- N94.6, Dysmenorrhea, unspecified
- N94.810, Vulvar vestibulitis
- N94.818, Other vulvodynia
- N94.819, Vulvodynia, unspecified
- R10.2, Pelvic and perineal pain.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Ixekizumab improves psoriatic arthritis patient-reported outcomes
LIVERPOOL, ENGLAND – In biologic-experienced patients with psoriatic arthritis, the interleukin-17 inhibitor ixekizumab not only met the primary efficacy endpoint of a pivotal phase 3 trial, but also improved multiple patient-reported outcomes in doing so.
Newly-released results from the Study of Ixekizumab in Participants With Active Psoriatic Arthritis (SPIRIT-P2) showed that patients who received active treatment exhibited significantly better changes in physical function, quality of life, itch score, and work productivity compared with those given placebo.
Patients treated with ixekizumab 80 mg every 2 or 4 weeks more often achieved the MCID by week 24, reaching 40% for 80 mg every 2 weeks and 43% for every 4 weeks, compared with 17% for placebo.
A total of 363 patients who met CASPAR (Classification Criteria for Psoriatic Arthritis) criteria were randomized into the SPIRIT-P2 trial. Patients could be included only if they had at least three tender and three swollen joints, active skin lesions, or a documented history of skin psoriasis, and had received prior treatment with a tumor necrosis factor inhibitor (TNFi).
“The population of patients studied is representative of the patients we see in our clinics,” said Dr. Marzo-Ortega, a consultant rheumatologist at Leeds Teaching Hospitals NHS Trust, England. The mean age was 52 years, a similar percentage of men and women were seen, and the majority (53%-58%) were inadequate responders to one TNFi. One-third had not responded to two TNFis, and 8%-10% had an intolerance.
The primary endpoint results, which have been previously presented and published (Lancet. 2017;389[10086]:2317-27), showed that a significantly (P less than .0001) higher percentage of patients treated with either of the two regimens of ixekizumab achieved a 20% response level on American College of Rheumatology criteria (ACR20) at 24 weeks. Indeed, 48% of 123 patients given 80 mg of ixekizumab every 2 weeks and 53% of 122 given 80 mg every 4 weeks achieved an ACR20 versus 20% of 118 placebo-treated patients. Also, on two key secondary endpoints at 24 weeks, an ACR50 response was achieved by a respective 33%, 35%, and 5% of patients, and an ACR70 by 12%, 22%, and 0%, she said.
Dr. Marzo-Ortega noted that the study had only been powered to show a difference between the active treatment and placebo, and not between the two doses, and that, looking at the speed of response, a difference from placebo was already being seen by week 2 “and certainly by week 4,” indicating an early effect. Data from the trial at 52 weeks are being analyzed and should be available soon, she said.
Another efficacy measure used was the percentage of patients achieving minimum disease activity at week 24. “These are stringent criteria to achieve: Five of seven criteria need to be met,” Dr. Marzo-Ortega said. “This was achieved by almost 30% of patients on the 4-weekly dose,” and by 24% on the 2-weekly dose, but by just 3% of those given placebo.
“One of the remarkable things is that nearly a third [of patients] achieved PASI [Psoriasis Area Severity Index] 100 by week 24, which is complete resolution of skin psoriasis,” she said. This is one of the first times this type of skin response has been seen in a psoriasis trial, she noted.
However, there was little difference between the active treatment and placebo arms in terms of the percentage of patients seeing a response on enthesitis, and only the dose taken every 4 weeks had a significant benefit over placebo in terms of improving dactylitis.
It is not clear why these modest results were seen in the joints, perhaps there were too few patients. While this is surprising, Dr. Marzo-Ortega noted that she “wouldn’t put too much weight on” the lack of an enthesis response; these are “fantastic drugs for the skin,” she said. “There is no doubt about it.”
Other findings from the trial included a significant improvement in itch with both ixekizumab regimens versus placebo, as shown by a greater reduction in numerical rating scale scores from baseline to week 24 (–3.4 and –3.5 vs. –1.2; P less than .001).
Significant improvements with ixekizumab versus placebo in patients’ mental and physical health were also seen when data from the Short Form–36 and EQ-5D instruments were analyzed.
There was also evidence that treatment with ixekizumab significantly improved patients’ presenteeism, work productivity, and activity impairment at work when compared against placebo. There was no difference in absenteeism, as measured by changes in Work Productivity and Activity Impairment Questionnaire–Specific Health Problem scores from baseline to week 24.
SPIRIT-P2 is one of two pivotal trials conducted with ixekizumab in patients with psoriatic arthritis; the other is SPIRIT-P1, which was conducted in biologic-naive patients (Ann Rheum Dis. 2017;76[1]:79-87). Investigators recently reported 1-year data from it (J Rheumatol. 2018;45[3]:367-77.).
Eli Lilly, which markets ixekizumab as Taltz, sponsored the study. Dr. Marzo-Ortega disclosed receiving honoraria from AbbVie, Celgene, Eli Lilly, Novartis, and UCB, and honoraria and research funding from Janssen and Pfizer. Several other authors reported disclosures with many manufacturers of biologics for psoriatic arthritis, including Eli Lilly.
SOURCE: Marzo-Ortega H et al. Rheumatology. 2018;57[Suppl. 3]:key075.185.
LIVERPOOL, ENGLAND – In biologic-experienced patients with psoriatic arthritis, the interleukin-17 inhibitor ixekizumab not only met the primary efficacy endpoint of a pivotal phase 3 trial, but also improved multiple patient-reported outcomes in doing so.
Newly-released results from the Study of Ixekizumab in Participants With Active Psoriatic Arthritis (SPIRIT-P2) showed that patients who received active treatment exhibited significantly better changes in physical function, quality of life, itch score, and work productivity compared with those given placebo.
Patients treated with ixekizumab 80 mg every 2 or 4 weeks more often achieved the MCID by week 24, reaching 40% for 80 mg every 2 weeks and 43% for every 4 weeks, compared with 17% for placebo.
A total of 363 patients who met CASPAR (Classification Criteria for Psoriatic Arthritis) criteria were randomized into the SPIRIT-P2 trial. Patients could be included only if they had at least three tender and three swollen joints, active skin lesions, or a documented history of skin psoriasis, and had received prior treatment with a tumor necrosis factor inhibitor (TNFi).
“The population of patients studied is representative of the patients we see in our clinics,” said Dr. Marzo-Ortega, a consultant rheumatologist at Leeds Teaching Hospitals NHS Trust, England. The mean age was 52 years, a similar percentage of men and women were seen, and the majority (53%-58%) were inadequate responders to one TNFi. One-third had not responded to two TNFis, and 8%-10% had an intolerance.
The primary endpoint results, which have been previously presented and published (Lancet. 2017;389[10086]:2317-27), showed that a significantly (P less than .0001) higher percentage of patients treated with either of the two regimens of ixekizumab achieved a 20% response level on American College of Rheumatology criteria (ACR20) at 24 weeks. Indeed, 48% of 123 patients given 80 mg of ixekizumab every 2 weeks and 53% of 122 given 80 mg every 4 weeks achieved an ACR20 versus 20% of 118 placebo-treated patients. Also, on two key secondary endpoints at 24 weeks, an ACR50 response was achieved by a respective 33%, 35%, and 5% of patients, and an ACR70 by 12%, 22%, and 0%, she said.
Dr. Marzo-Ortega noted that the study had only been powered to show a difference between the active treatment and placebo, and not between the two doses, and that, looking at the speed of response, a difference from placebo was already being seen by week 2 “and certainly by week 4,” indicating an early effect. Data from the trial at 52 weeks are being analyzed and should be available soon, she said.
Another efficacy measure used was the percentage of patients achieving minimum disease activity at week 24. “These are stringent criteria to achieve: Five of seven criteria need to be met,” Dr. Marzo-Ortega said. “This was achieved by almost 30% of patients on the 4-weekly dose,” and by 24% on the 2-weekly dose, but by just 3% of those given placebo.
“One of the remarkable things is that nearly a third [of patients] achieved PASI [Psoriasis Area Severity Index] 100 by week 24, which is complete resolution of skin psoriasis,” she said. This is one of the first times this type of skin response has been seen in a psoriasis trial, she noted.
However, there was little difference between the active treatment and placebo arms in terms of the percentage of patients seeing a response on enthesitis, and only the dose taken every 4 weeks had a significant benefit over placebo in terms of improving dactylitis.
It is not clear why these modest results were seen in the joints, perhaps there were too few patients. While this is surprising, Dr. Marzo-Ortega noted that she “wouldn’t put too much weight on” the lack of an enthesis response; these are “fantastic drugs for the skin,” she said. “There is no doubt about it.”
Other findings from the trial included a significant improvement in itch with both ixekizumab regimens versus placebo, as shown by a greater reduction in numerical rating scale scores from baseline to week 24 (–3.4 and –3.5 vs. –1.2; P less than .001).
Significant improvements with ixekizumab versus placebo in patients’ mental and physical health were also seen when data from the Short Form–36 and EQ-5D instruments were analyzed.
There was also evidence that treatment with ixekizumab significantly improved patients’ presenteeism, work productivity, and activity impairment at work when compared against placebo. There was no difference in absenteeism, as measured by changes in Work Productivity and Activity Impairment Questionnaire–Specific Health Problem scores from baseline to week 24.
SPIRIT-P2 is one of two pivotal trials conducted with ixekizumab in patients with psoriatic arthritis; the other is SPIRIT-P1, which was conducted in biologic-naive patients (Ann Rheum Dis. 2017;76[1]:79-87). Investigators recently reported 1-year data from it (J Rheumatol. 2018;45[3]:367-77.).
Eli Lilly, which markets ixekizumab as Taltz, sponsored the study. Dr. Marzo-Ortega disclosed receiving honoraria from AbbVie, Celgene, Eli Lilly, Novartis, and UCB, and honoraria and research funding from Janssen and Pfizer. Several other authors reported disclosures with many manufacturers of biologics for psoriatic arthritis, including Eli Lilly.
SOURCE: Marzo-Ortega H et al. Rheumatology. 2018;57[Suppl. 3]:key075.185.
LIVERPOOL, ENGLAND – In biologic-experienced patients with psoriatic arthritis, the interleukin-17 inhibitor ixekizumab not only met the primary efficacy endpoint of a pivotal phase 3 trial, but also improved multiple patient-reported outcomes in doing so.
Newly-released results from the Study of Ixekizumab in Participants With Active Psoriatic Arthritis (SPIRIT-P2) showed that patients who received active treatment exhibited significantly better changes in physical function, quality of life, itch score, and work productivity compared with those given placebo.
Patients treated with ixekizumab 80 mg every 2 or 4 weeks more often achieved the MCID by week 24, reaching 40% for 80 mg every 2 weeks and 43% for every 4 weeks, compared with 17% for placebo.
A total of 363 patients who met CASPAR (Classification Criteria for Psoriatic Arthritis) criteria were randomized into the SPIRIT-P2 trial. Patients could be included only if they had at least three tender and three swollen joints, active skin lesions, or a documented history of skin psoriasis, and had received prior treatment with a tumor necrosis factor inhibitor (TNFi).
“The population of patients studied is representative of the patients we see in our clinics,” said Dr. Marzo-Ortega, a consultant rheumatologist at Leeds Teaching Hospitals NHS Trust, England. The mean age was 52 years, a similar percentage of men and women were seen, and the majority (53%-58%) were inadequate responders to one TNFi. One-third had not responded to two TNFis, and 8%-10% had an intolerance.
The primary endpoint results, which have been previously presented and published (Lancet. 2017;389[10086]:2317-27), showed that a significantly (P less than .0001) higher percentage of patients treated with either of the two regimens of ixekizumab achieved a 20% response level on American College of Rheumatology criteria (ACR20) at 24 weeks. Indeed, 48% of 123 patients given 80 mg of ixekizumab every 2 weeks and 53% of 122 given 80 mg every 4 weeks achieved an ACR20 versus 20% of 118 placebo-treated patients. Also, on two key secondary endpoints at 24 weeks, an ACR50 response was achieved by a respective 33%, 35%, and 5% of patients, and an ACR70 by 12%, 22%, and 0%, she said.
Dr. Marzo-Ortega noted that the study had only been powered to show a difference between the active treatment and placebo, and not between the two doses, and that, looking at the speed of response, a difference from placebo was already being seen by week 2 “and certainly by week 4,” indicating an early effect. Data from the trial at 52 weeks are being analyzed and should be available soon, she said.
Another efficacy measure used was the percentage of patients achieving minimum disease activity at week 24. “These are stringent criteria to achieve: Five of seven criteria need to be met,” Dr. Marzo-Ortega said. “This was achieved by almost 30% of patients on the 4-weekly dose,” and by 24% on the 2-weekly dose, but by just 3% of those given placebo.
“One of the remarkable things is that nearly a third [of patients] achieved PASI [Psoriasis Area Severity Index] 100 by week 24, which is complete resolution of skin psoriasis,” she said. This is one of the first times this type of skin response has been seen in a psoriasis trial, she noted.
However, there was little difference between the active treatment and placebo arms in terms of the percentage of patients seeing a response on enthesitis, and only the dose taken every 4 weeks had a significant benefit over placebo in terms of improving dactylitis.
It is not clear why these modest results were seen in the joints, perhaps there were too few patients. While this is surprising, Dr. Marzo-Ortega noted that she “wouldn’t put too much weight on” the lack of an enthesis response; these are “fantastic drugs for the skin,” she said. “There is no doubt about it.”
Other findings from the trial included a significant improvement in itch with both ixekizumab regimens versus placebo, as shown by a greater reduction in numerical rating scale scores from baseline to week 24 (–3.4 and –3.5 vs. –1.2; P less than .001).
Significant improvements with ixekizumab versus placebo in patients’ mental and physical health were also seen when data from the Short Form–36 and EQ-5D instruments were analyzed.
There was also evidence that treatment with ixekizumab significantly improved patients’ presenteeism, work productivity, and activity impairment at work when compared against placebo. There was no difference in absenteeism, as measured by changes in Work Productivity and Activity Impairment Questionnaire–Specific Health Problem scores from baseline to week 24.
SPIRIT-P2 is one of two pivotal trials conducted with ixekizumab in patients with psoriatic arthritis; the other is SPIRIT-P1, which was conducted in biologic-naive patients (Ann Rheum Dis. 2017;76[1]:79-87). Investigators recently reported 1-year data from it (J Rheumatol. 2018;45[3]:367-77.).
Eli Lilly, which markets ixekizumab as Taltz, sponsored the study. Dr. Marzo-Ortega disclosed receiving honoraria from AbbVie, Celgene, Eli Lilly, Novartis, and UCB, and honoraria and research funding from Janssen and Pfizer. Several other authors reported disclosures with many manufacturers of biologics for psoriatic arthritis, including Eli Lilly.
SOURCE: Marzo-Ortega H et al. Rheumatology. 2018;57[Suppl. 3]:key075.185.
REPORTING FROM RHEUMATOLOGY 2018
Key clinical point: Ixekizumab has multiple beneficial effects in patients with psoriatic arthritis previously treated with biologics.
Major finding: Mean HAQ-DI score changes (baseline to week 24) were –0.4 and –0.6 with ixekizumab (80 mg every 2 or 4 weeks) and –0.2 for placebo (P less than or equal to .001).
Study details: SPIRIT-P2: A randomized, double-blind, placebo-controlled phase 3 trial of ixekizumab in 363 biologic-experienced patients.
Disclosures: Eli Lilly, which markets ixekizumab as Taltz, sponsored the study. Dr. Marzo-Ortega disclosed receiving honoraria from AbbVie, Celgene, Eli Lilly, Novartis, and UCB, and honoraria and research funding from Janssen and Pfizer. Several other authors reported disclosures with many manufacturers of biologics for psoriatic arthritis, including Eli Lilly.
Source: Marzo-Ortega H et al. Rheumatology. 2018;57[Suppl. 3]:key075.185.
Peer mentorship, groups help combat burnout in female physicians
NEW YORK – Female physicians are at higher risk for burnout compared with their male counterparts, and the reasons and potential solutions for the problem were addressed at a symposium during the annual meeting of the American Psychiatric Association.
The work environment for women has improved over time, but lingering implicit and unconscious biases are part of the reason for the high burnout rate among women who are physicians, as are some inherent biological differences, according to Cynthia M. Stonnington, MD, of the Mayo Clinic, Phoenix.
In this video interview, Dr. Stonnington, symposium chair, discussed potential solutions, including facilitated peer mentorship and group support. She also reviewed recent data on how group support can be of benefit, and noted that “there is power in numbers.
“,” she said.
Dr. Stonnington reported having no disclosures.
NEW YORK – Female physicians are at higher risk for burnout compared with their male counterparts, and the reasons and potential solutions for the problem were addressed at a symposium during the annual meeting of the American Psychiatric Association.
The work environment for women has improved over time, but lingering implicit and unconscious biases are part of the reason for the high burnout rate among women who are physicians, as are some inherent biological differences, according to Cynthia M. Stonnington, MD, of the Mayo Clinic, Phoenix.
In this video interview, Dr. Stonnington, symposium chair, discussed potential solutions, including facilitated peer mentorship and group support. She also reviewed recent data on how group support can be of benefit, and noted that “there is power in numbers.
“,” she said.
Dr. Stonnington reported having no disclosures.
NEW YORK – Female physicians are at higher risk for burnout compared with their male counterparts, and the reasons and potential solutions for the problem were addressed at a symposium during the annual meeting of the American Psychiatric Association.
The work environment for women has improved over time, but lingering implicit and unconscious biases are part of the reason for the high burnout rate among women who are physicians, as are some inherent biological differences, according to Cynthia M. Stonnington, MD, of the Mayo Clinic, Phoenix.
In this video interview, Dr. Stonnington, symposium chair, discussed potential solutions, including facilitated peer mentorship and group support. She also reviewed recent data on how group support can be of benefit, and noted that “there is power in numbers.
“,” she said.
Dr. Stonnington reported having no disclosures.
REPORTING FROM APA
New agents may bring hope for SLE patients
SANDESTIN, FLA. – Several drugs approved for other conditions may also have good effect in patients with systemic lupus erythematosus, Michelle Petri, MD, said in an interview at the annual Congress of Clinical Rheumatology.
The molecules target several different disease pathways, said Dr. Petri, director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
Ustekinumab (Stelara) has accumulated the most data so far. A phase 2 study presented last fall at the annual meeting of the American College of Rheumatology found that it conferred significant benefits relative to placebo, including a 60% responder rate (29% better than placebo), a significantly lower flare rate, and improvements in musculoskeletal and mucocutaneous disease features. The rate of serious adverse events was acceptable (8.3% vs. 9.5% for placebo).
Baricitinib is also being investigated in SLE, Dr. Petri said. A phase 2 study conducted by Eli Lilly closed late last year and will be reported on June 13 at the European League Against Rheumatism’s opening plenary session (Wallace et al. EULAR 2018 abstract OP0019).
The three-armed, placebo-controlled study comprised 314 patients who were randomized to placebo or one of two baricitinib doses, given orally for 24 weeks. The primary outcome was remission of arthritis and/or rash as measured by the SLE Disease Activity Index 2000 (SLEDAI-2K). Secondary endpoints included responder rate, change from baseline in the SLEDAI-2K, change in the Global Assessment of Disease Activity score, and pharmacokinetic measures.
Dr. Petri disclosed relationships with Amgen, Boston Pharmaceuticals, Bristol-Myers Squibb, EMD Serono, Janssen, Novartis, and GlaxoSmithKline.
SANDESTIN, FLA. – Several drugs approved for other conditions may also have good effect in patients with systemic lupus erythematosus, Michelle Petri, MD, said in an interview at the annual Congress of Clinical Rheumatology.
The molecules target several different disease pathways, said Dr. Petri, director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
Ustekinumab (Stelara) has accumulated the most data so far. A phase 2 study presented last fall at the annual meeting of the American College of Rheumatology found that it conferred significant benefits relative to placebo, including a 60% responder rate (29% better than placebo), a significantly lower flare rate, and improvements in musculoskeletal and mucocutaneous disease features. The rate of serious adverse events was acceptable (8.3% vs. 9.5% for placebo).
Baricitinib is also being investigated in SLE, Dr. Petri said. A phase 2 study conducted by Eli Lilly closed late last year and will be reported on June 13 at the European League Against Rheumatism’s opening plenary session (Wallace et al. EULAR 2018 abstract OP0019).
The three-armed, placebo-controlled study comprised 314 patients who were randomized to placebo or one of two baricitinib doses, given orally for 24 weeks. The primary outcome was remission of arthritis and/or rash as measured by the SLE Disease Activity Index 2000 (SLEDAI-2K). Secondary endpoints included responder rate, change from baseline in the SLEDAI-2K, change in the Global Assessment of Disease Activity score, and pharmacokinetic measures.
Dr. Petri disclosed relationships with Amgen, Boston Pharmaceuticals, Bristol-Myers Squibb, EMD Serono, Janssen, Novartis, and GlaxoSmithKline.
SANDESTIN, FLA. – Several drugs approved for other conditions may also have good effect in patients with systemic lupus erythematosus, Michelle Petri, MD, said in an interview at the annual Congress of Clinical Rheumatology.
The molecules target several different disease pathways, said Dr. Petri, director of the Hopkins Lupus Center at Johns Hopkins University, Baltimore.
Ustekinumab (Stelara) has accumulated the most data so far. A phase 2 study presented last fall at the annual meeting of the American College of Rheumatology found that it conferred significant benefits relative to placebo, including a 60% responder rate (29% better than placebo), a significantly lower flare rate, and improvements in musculoskeletal and mucocutaneous disease features. The rate of serious adverse events was acceptable (8.3% vs. 9.5% for placebo).
Baricitinib is also being investigated in SLE, Dr. Petri said. A phase 2 study conducted by Eli Lilly closed late last year and will be reported on June 13 at the European League Against Rheumatism’s opening plenary session (Wallace et al. EULAR 2018 abstract OP0019).
The three-armed, placebo-controlled study comprised 314 patients who were randomized to placebo or one of two baricitinib doses, given orally for 24 weeks. The primary outcome was remission of arthritis and/or rash as measured by the SLE Disease Activity Index 2000 (SLEDAI-2K). Secondary endpoints included responder rate, change from baseline in the SLEDAI-2K, change in the Global Assessment of Disease Activity score, and pharmacokinetic measures.
Dr. Petri disclosed relationships with Amgen, Boston Pharmaceuticals, Bristol-Myers Squibb, EMD Serono, Janssen, Novartis, and GlaxoSmithKline.
EXPERT ANALYSIS FROM CCR 18
CMSC looks to increase advocacy efforts to address looming concerns
As patients with multiple sclerosis and their caregivers face various challenges in the American health care system, the Consortium of Multiple Sclerosis Centers (CMSC) is re-energizing its focus on advocacy. The 2018 annual meeting in Nashville, Tenn., will feature programming designed to teach MS professionals and researchers how to speak up and let their voices be heard.
“CMSC has re-engaged in advocacy, in a more focused and energized way, because of mounting concerns that there is inadequate advocacy pressure on some of the critical issues negatively impacting the quality and comprehensive nature of MS care required of people living with MS,” said Lisa Taylor Skutnik, a physical therapist and chief operating officer of the CMSC. “Both people living with MS and the clinicians dedicated to treating them are increasingly frustrated and powerless in attempts to facilitate the right care and resources for people living with MS. Our members are facing significant barriers and obstacles to ensure timely and seamless comprehensive care.”
The development of quality measures in MS is also a “huge concern,” she said, referring to the federal government’s efforts to develop new ways to measure whether health professionals are providing high-quality care. Physicians have expressed widespread concern about the measures, which are being linked to reimbursement from Medicare and Medicaid.
Among the highlights of the CMSC meeting will be an educational session that “will focus on the available resources within the MS community for MS professionals and researchers to access advocacy efforts and get involved,” Ms. Skutnik said.
Another educational session “will focus on helping MS clinicians and researchers to understand all of the various stakeholders and their respective perspectives related to access to comprehensive care services for those living with MS,” such as pharmacy benefit managers and insurers, she said.
As patients with multiple sclerosis and their caregivers face various challenges in the American health care system, the Consortium of Multiple Sclerosis Centers (CMSC) is re-energizing its focus on advocacy. The 2018 annual meeting in Nashville, Tenn., will feature programming designed to teach MS professionals and researchers how to speak up and let their voices be heard.
“CMSC has re-engaged in advocacy, in a more focused and energized way, because of mounting concerns that there is inadequate advocacy pressure on some of the critical issues negatively impacting the quality and comprehensive nature of MS care required of people living with MS,” said Lisa Taylor Skutnik, a physical therapist and chief operating officer of the CMSC. “Both people living with MS and the clinicians dedicated to treating them are increasingly frustrated and powerless in attempts to facilitate the right care and resources for people living with MS. Our members are facing significant barriers and obstacles to ensure timely and seamless comprehensive care.”
The development of quality measures in MS is also a “huge concern,” she said, referring to the federal government’s efforts to develop new ways to measure whether health professionals are providing high-quality care. Physicians have expressed widespread concern about the measures, which are being linked to reimbursement from Medicare and Medicaid.
Among the highlights of the CMSC meeting will be an educational session that “will focus on the available resources within the MS community for MS professionals and researchers to access advocacy efforts and get involved,” Ms. Skutnik said.
Another educational session “will focus on helping MS clinicians and researchers to understand all of the various stakeholders and their respective perspectives related to access to comprehensive care services for those living with MS,” such as pharmacy benefit managers and insurers, she said.
As patients with multiple sclerosis and their caregivers face various challenges in the American health care system, the Consortium of Multiple Sclerosis Centers (CMSC) is re-energizing its focus on advocacy. The 2018 annual meeting in Nashville, Tenn., will feature programming designed to teach MS professionals and researchers how to speak up and let their voices be heard.
“CMSC has re-engaged in advocacy, in a more focused and energized way, because of mounting concerns that there is inadequate advocacy pressure on some of the critical issues negatively impacting the quality and comprehensive nature of MS care required of people living with MS,” said Lisa Taylor Skutnik, a physical therapist and chief operating officer of the CMSC. “Both people living with MS and the clinicians dedicated to treating them are increasingly frustrated and powerless in attempts to facilitate the right care and resources for people living with MS. Our members are facing significant barriers and obstacles to ensure timely and seamless comprehensive care.”
The development of quality measures in MS is also a “huge concern,” she said, referring to the federal government’s efforts to develop new ways to measure whether health professionals are providing high-quality care. Physicians have expressed widespread concern about the measures, which are being linked to reimbursement from Medicare and Medicaid.
Among the highlights of the CMSC meeting will be an educational session that “will focus on the available resources within the MS community for MS professionals and researchers to access advocacy efforts and get involved,” Ms. Skutnik said.
Another educational session “will focus on helping MS clinicians and researchers to understand all of the various stakeholders and their respective perspectives related to access to comprehensive care services for those living with MS,” such as pharmacy benefit managers and insurers, she said.











