User login
The Distracted Clinician
The other day, I saw my health care provider for a routine appointment—and indeed, it seemed that I saw him, rather than the other way around. After having my vital signs measured by the medical assistant, I was led into the exam room. To my surprise, the provider (I will not divulge whether he was a physician, PA, or NP) was already there, sitting in front of his computer. He glanced up to say hello, but did not stand up, shake my hand, or maintain any level of eye contact. He did swear under his breath several times—something about his hatred of electronic medical records (EMRs)—while he asked me questions, hammering away on his laptop in time with my responses. After confirming that I was there for a prescription refill, he picked up his laptop and walked out of the room. A few minutes later, he popped back in to say, “Gee, I guess I should listen to your heart.” He placed the stethoscope on my chest over my shirt for a fraction of a second and was gone again. When I got to the pharmacy, I discovered he had called in the wrong prescription.
When Harvard professor Clayton M. Christensen coined the phrase disruptive technology, I’m not sure he imagined quite this level of impact! The time focused on a computer or device, rather than on the patient, has become so disproportionate that Dr. Abraham Verghese coined the term iPatient—a result of what he calls the chart-as-surrogate-for-the-patient approach.1
While I hope my experience is not a regular occurrence in health care today, I’m well aware that the addition of e-this and e-that (computers, tablets, smartphones) at the bedside has clinicians multitasking more and more. Sure, performing more than one task at a time can be time-saving. But it can also lead to preoccupation and medical errors—at a time when medical errors are the third leading cause of death in the United States.2
We, as clinicians and as a larger society, are fascinated by speed. We want information faster than ever: medical information, lab results, etc. Our devices, stimulating and exhilarating as they are, have created a new society. Tell me you have not noticed the zombie-like motions of our colleagues walking in an electronic trance, pecking away at their preferred device! (OK, I am guilty of this, as well.)
Furthermore—and counterintuitively—efficiency in the clinic has been decimated by technology. In the “old days,” we could see patients roughly every 15 minutes, and many were double-booked. No problem; we merely dictated a note while walking from room to room, turned in our tapes at the end of the day, and signed a stack of notes two days later. Now, documentation alone takes at least 15 minutes, because it’s not just the note; it’s also the charges and the visit summary that is supposed to (but never does) go home with the patient.
So, if you want to see patients, if you want to generate revenue, if you want to keep the corporate slave drivers at bay, you either skimp on patient care or you document on your own time. One colleague lamented to me that, by implementing cost-saving measures to eliminate medical transcription ($2-$3/h outsourced to India), administrators and EMRs have reduced clinicians to the role of “Doc-retary.”
The diversion of attention, coupled with pressure to “perform,” is at the heart of the problem. Lately, every clinician I have spoken to seems to feel burdened by an influx of demands to see more patients in abbreviated visits while maintaining detailed records and documenting everything. It is no wonder that more than 75% of respondents in a study on physician distress met the criteria for burnout.3 I worry that NPs and PAs are not far behind. In a 2018 study, more than half (55.6%) of PAs rated “spending too many hours at work” as an important contributor to stress, and about 29% had previously quit a job due to stress.4
Continue to: If my own editorials are anything to judge by...
If my own editorials are anything to judge by, the joys and (welcome) challenges of the job are increasingly rare. I’ve discussed the “lost art” of the physical examination (November 2010); lamented the “death of altruism” (April 2016); and listed the pros and cons (mostly cons) of social media use (December 2017). Is careful listening to the patient the next thing to go?
We know intuitively that careful listening leads to better diagnosis and fewer errors. In fact, Balogh and colleagues identified patient engagement as one of four major cultural movements in health care (the others are patient safety, professionalism, and collaboration) that health care organizations need to foster in order to improve diagnosis and reduce errors.5 To my mind, that means finding ways to bring back the interpersonal relationship between clinician and patient and finding ways to remove the barriers that electronics can build.
I know exam room computing and EMRs are here to stay—and even, I suspect, likely to increase. But it is still possible, in my opinion, to incorporate patients into the interaction between clinician and computer. It is also possible, with the use of scribes, to have a third party transcribe your thoughts and actions as you interact directly with the patient. The last clinic I worked at operated this way, and it was liberating to be able to spend my time doing what I love best: interacting with my patients.
For those of you saying, “Yes, but my practice won’t hire scribes,” there is good advice out there on how to improve your interaction with patients in the Digital Age. In 2016, Frankel introduced the mnemonic POISED to enhance patient encounters while incorporating technologic devices:
Prepare. Review the patient’s medical records before you enter the exam room.
Continue to: Prepare
Orient. Let the patient know what you are doing or plan to do, and explain the use of the computer or scribe.
Information gathering. Although clinician-centric, this process should involve a two-way conversation between the clinician and patient.
Share. Use audiovisual sources (ie, your computer screen) to share information
Educate. Similarly, the computer can be a useful tool for educating the patient, as can low-tech materials like pictures and/or models.
Continue to: Debrief
Debrief. Review what has been said and make sure the patient has a chance to ask questions.6
The use of computers, EMRs, and other gadgets carries many potential consequences—but when used appropriately, these devices can be valuable tools for clinicians to interact with patients, stimulate engagement, and enrich patient-centered relationships. Do you agree? Please share with me your ideas on how we can better use the technology being placed before us at [email protected].
1. Verghese A. Culture shock-patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374(9702):1714-1721.
4. Coplan B, McCall T, Smith N, et al. Burnout, job satisfaction, and stress levels of PAs. JAAPA. 2018;31(9):42-46.
5. Balogh EP, Miller BT, Ball JR; National Academies of Sciences, Engineering, and Medicine. Improving diagnosis in health care. Washington, DC; National Academies Press: 2016.
6. Frankel RM. Computers in the examination room. JAMA Intern Med. 2016;176(1):128-129.
The other day, I saw my health care provider for a routine appointment—and indeed, it seemed that I saw him, rather than the other way around. After having my vital signs measured by the medical assistant, I was led into the exam room. To my surprise, the provider (I will not divulge whether he was a physician, PA, or NP) was already there, sitting in front of his computer. He glanced up to say hello, but did not stand up, shake my hand, or maintain any level of eye contact. He did swear under his breath several times—something about his hatred of electronic medical records (EMRs)—while he asked me questions, hammering away on his laptop in time with my responses. After confirming that I was there for a prescription refill, he picked up his laptop and walked out of the room. A few minutes later, he popped back in to say, “Gee, I guess I should listen to your heart.” He placed the stethoscope on my chest over my shirt for a fraction of a second and was gone again. When I got to the pharmacy, I discovered he had called in the wrong prescription.
When Harvard professor Clayton M. Christensen coined the phrase disruptive technology, I’m not sure he imagined quite this level of impact! The time focused on a computer or device, rather than on the patient, has become so disproportionate that Dr. Abraham Verghese coined the term iPatient—a result of what he calls the chart-as-surrogate-for-the-patient approach.1
While I hope my experience is not a regular occurrence in health care today, I’m well aware that the addition of e-this and e-that (computers, tablets, smartphones) at the bedside has clinicians multitasking more and more. Sure, performing more than one task at a time can be time-saving. But it can also lead to preoccupation and medical errors—at a time when medical errors are the third leading cause of death in the United States.2
We, as clinicians and as a larger society, are fascinated by speed. We want information faster than ever: medical information, lab results, etc. Our devices, stimulating and exhilarating as they are, have created a new society. Tell me you have not noticed the zombie-like motions of our colleagues walking in an electronic trance, pecking away at their preferred device! (OK, I am guilty of this, as well.)
Furthermore—and counterintuitively—efficiency in the clinic has been decimated by technology. In the “old days,” we could see patients roughly every 15 minutes, and many were double-booked. No problem; we merely dictated a note while walking from room to room, turned in our tapes at the end of the day, and signed a stack of notes two days later. Now, documentation alone takes at least 15 minutes, because it’s not just the note; it’s also the charges and the visit summary that is supposed to (but never does) go home with the patient.
So, if you want to see patients, if you want to generate revenue, if you want to keep the corporate slave drivers at bay, you either skimp on patient care or you document on your own time. One colleague lamented to me that, by implementing cost-saving measures to eliminate medical transcription ($2-$3/h outsourced to India), administrators and EMRs have reduced clinicians to the role of “Doc-retary.”
The diversion of attention, coupled with pressure to “perform,” is at the heart of the problem. Lately, every clinician I have spoken to seems to feel burdened by an influx of demands to see more patients in abbreviated visits while maintaining detailed records and documenting everything. It is no wonder that more than 75% of respondents in a study on physician distress met the criteria for burnout.3 I worry that NPs and PAs are not far behind. In a 2018 study, more than half (55.6%) of PAs rated “spending too many hours at work” as an important contributor to stress, and about 29% had previously quit a job due to stress.4
Continue to: If my own editorials are anything to judge by...
If my own editorials are anything to judge by, the joys and (welcome) challenges of the job are increasingly rare. I’ve discussed the “lost art” of the physical examination (November 2010); lamented the “death of altruism” (April 2016); and listed the pros and cons (mostly cons) of social media use (December 2017). Is careful listening to the patient the next thing to go?
We know intuitively that careful listening leads to better diagnosis and fewer errors. In fact, Balogh and colleagues identified patient engagement as one of four major cultural movements in health care (the others are patient safety, professionalism, and collaboration) that health care organizations need to foster in order to improve diagnosis and reduce errors.5 To my mind, that means finding ways to bring back the interpersonal relationship between clinician and patient and finding ways to remove the barriers that electronics can build.
I know exam room computing and EMRs are here to stay—and even, I suspect, likely to increase. But it is still possible, in my opinion, to incorporate patients into the interaction between clinician and computer. It is also possible, with the use of scribes, to have a third party transcribe your thoughts and actions as you interact directly with the patient. The last clinic I worked at operated this way, and it was liberating to be able to spend my time doing what I love best: interacting with my patients.
For those of you saying, “Yes, but my practice won’t hire scribes,” there is good advice out there on how to improve your interaction with patients in the Digital Age. In 2016, Frankel introduced the mnemonic POISED to enhance patient encounters while incorporating technologic devices:
Prepare. Review the patient’s medical records before you enter the exam room.
Continue to: Prepare
Orient. Let the patient know what you are doing or plan to do, and explain the use of the computer or scribe.
Information gathering. Although clinician-centric, this process should involve a two-way conversation between the clinician and patient.
Share. Use audiovisual sources (ie, your computer screen) to share information
Educate. Similarly, the computer can be a useful tool for educating the patient, as can low-tech materials like pictures and/or models.
Continue to: Debrief
Debrief. Review what has been said and make sure the patient has a chance to ask questions.6
The use of computers, EMRs, and other gadgets carries many potential consequences—but when used appropriately, these devices can be valuable tools for clinicians to interact with patients, stimulate engagement, and enrich patient-centered relationships. Do you agree? Please share with me your ideas on how we can better use the technology being placed before us at [email protected].
The other day, I saw my health care provider for a routine appointment—and indeed, it seemed that I saw him, rather than the other way around. After having my vital signs measured by the medical assistant, I was led into the exam room. To my surprise, the provider (I will not divulge whether he was a physician, PA, or NP) was already there, sitting in front of his computer. He glanced up to say hello, but did not stand up, shake my hand, or maintain any level of eye contact. He did swear under his breath several times—something about his hatred of electronic medical records (EMRs)—while he asked me questions, hammering away on his laptop in time with my responses. After confirming that I was there for a prescription refill, he picked up his laptop and walked out of the room. A few minutes later, he popped back in to say, “Gee, I guess I should listen to your heart.” He placed the stethoscope on my chest over my shirt for a fraction of a second and was gone again. When I got to the pharmacy, I discovered he had called in the wrong prescription.
When Harvard professor Clayton M. Christensen coined the phrase disruptive technology, I’m not sure he imagined quite this level of impact! The time focused on a computer or device, rather than on the patient, has become so disproportionate that Dr. Abraham Verghese coined the term iPatient—a result of what he calls the chart-as-surrogate-for-the-patient approach.1
While I hope my experience is not a regular occurrence in health care today, I’m well aware that the addition of e-this and e-that (computers, tablets, smartphones) at the bedside has clinicians multitasking more and more. Sure, performing more than one task at a time can be time-saving. But it can also lead to preoccupation and medical errors—at a time when medical errors are the third leading cause of death in the United States.2
We, as clinicians and as a larger society, are fascinated by speed. We want information faster than ever: medical information, lab results, etc. Our devices, stimulating and exhilarating as they are, have created a new society. Tell me you have not noticed the zombie-like motions of our colleagues walking in an electronic trance, pecking away at their preferred device! (OK, I am guilty of this, as well.)
Furthermore—and counterintuitively—efficiency in the clinic has been decimated by technology. In the “old days,” we could see patients roughly every 15 minutes, and many were double-booked. No problem; we merely dictated a note while walking from room to room, turned in our tapes at the end of the day, and signed a stack of notes two days later. Now, documentation alone takes at least 15 minutes, because it’s not just the note; it’s also the charges and the visit summary that is supposed to (but never does) go home with the patient.
So, if you want to see patients, if you want to generate revenue, if you want to keep the corporate slave drivers at bay, you either skimp on patient care or you document on your own time. One colleague lamented to me that, by implementing cost-saving measures to eliminate medical transcription ($2-$3/h outsourced to India), administrators and EMRs have reduced clinicians to the role of “Doc-retary.”
The diversion of attention, coupled with pressure to “perform,” is at the heart of the problem. Lately, every clinician I have spoken to seems to feel burdened by an influx of demands to see more patients in abbreviated visits while maintaining detailed records and documenting everything. It is no wonder that more than 75% of respondents in a study on physician distress met the criteria for burnout.3 I worry that NPs and PAs are not far behind. In a 2018 study, more than half (55.6%) of PAs rated “spending too many hours at work” as an important contributor to stress, and about 29% had previously quit a job due to stress.4
Continue to: If my own editorials are anything to judge by...
If my own editorials are anything to judge by, the joys and (welcome) challenges of the job are increasingly rare. I’ve discussed the “lost art” of the physical examination (November 2010); lamented the “death of altruism” (April 2016); and listed the pros and cons (mostly cons) of social media use (December 2017). Is careful listening to the patient the next thing to go?
We know intuitively that careful listening leads to better diagnosis and fewer errors. In fact, Balogh and colleagues identified patient engagement as one of four major cultural movements in health care (the others are patient safety, professionalism, and collaboration) that health care organizations need to foster in order to improve diagnosis and reduce errors.5 To my mind, that means finding ways to bring back the interpersonal relationship between clinician and patient and finding ways to remove the barriers that electronics can build.
I know exam room computing and EMRs are here to stay—and even, I suspect, likely to increase. But it is still possible, in my opinion, to incorporate patients into the interaction between clinician and computer. It is also possible, with the use of scribes, to have a third party transcribe your thoughts and actions as you interact directly with the patient. The last clinic I worked at operated this way, and it was liberating to be able to spend my time doing what I love best: interacting with my patients.
For those of you saying, “Yes, but my practice won’t hire scribes,” there is good advice out there on how to improve your interaction with patients in the Digital Age. In 2016, Frankel introduced the mnemonic POISED to enhance patient encounters while incorporating technologic devices:
Prepare. Review the patient’s medical records before you enter the exam room.
Continue to: Prepare
Orient. Let the patient know what you are doing or plan to do, and explain the use of the computer or scribe.
Information gathering. Although clinician-centric, this process should involve a two-way conversation between the clinician and patient.
Share. Use audiovisual sources (ie, your computer screen) to share information
Educate. Similarly, the computer can be a useful tool for educating the patient, as can low-tech materials like pictures and/or models.
Continue to: Debrief
Debrief. Review what has been said and make sure the patient has a chance to ask questions.6
The use of computers, EMRs, and other gadgets carries many potential consequences—but when used appropriately, these devices can be valuable tools for clinicians to interact with patients, stimulate engagement, and enrich patient-centered relationships. Do you agree? Please share with me your ideas on how we can better use the technology being placed before us at [email protected].
1. Verghese A. Culture shock-patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374(9702):1714-1721.
4. Coplan B, McCall T, Smith N, et al. Burnout, job satisfaction, and stress levels of PAs. JAAPA. 2018;31(9):42-46.
5. Balogh EP, Miller BT, Ball JR; National Academies of Sciences, Engineering, and Medicine. Improving diagnosis in health care. Washington, DC; National Academies Press: 2016.
6. Frankel RM. Computers in the examination room. JAMA Intern Med. 2016;176(1):128-129.
1. Verghese A. Culture shock-patient as icon, icon as patient. N Engl J Med. 2008;359(26):2748-2751.
2. Makary MA, Daniel M. Medical error—the third leading cause of death in the US. BMJ. 2016;353:i2139.
3. Wallace JE, Lemaire JB, Ghali WA. Physician wellness: a missing quality indicator. Lancet. 2009;374(9702):1714-1721.
4. Coplan B, McCall T, Smith N, et al. Burnout, job satisfaction, and stress levels of PAs. JAAPA. 2018;31(9):42-46.
5. Balogh EP, Miller BT, Ball JR; National Academies of Sciences, Engineering, and Medicine. Improving diagnosis in health care. Washington, DC; National Academies Press: 2016.
6. Frankel RM. Computers in the examination room. JAMA Intern Med. 2016;176(1):128-129.
We need to reassess our primitive understanding of the venous system
If one includes the entire spectrum of venous disease, it is a more common pathology than peripheral arterial disease. The financial impact of venous disease is substantial. Why, then, has it taken so long to generate enthusiasm for venous disease of the femorocaval and subclaviocaval segments? For years, the endovascular management of venous disease used technology and techniques borrowed from the arterial space; although results were encouraging, it is clear that they varied widely and continue to do so. Management of these vascular beds is very reminiscent of the barrage of devices we have thrown at the superficial femoral artery.
In peripheral arterial disease, there have been much education and research focused on understanding atherosclerosis and its interaction with arterial devices. However, the paucity of investigation and enlightenment in the venous domain is evident when a literature search is performed. Certainly there are data from Comerota et al. showing an increased amount of collagen in the walls of chronically diseased veins. While this is a reasonable start, it is not sufficient data on which to build an entire treatment paradigm. Just like peripheral arterial disease, venous pathology presents in a continuum. Without an in-depth appreciation of the variability of those presentations, it is difficult to envision targeted therapies.
Although vendors have recently engaged in the development of venous-specific devices, it is in great part grounded in expert opinion rather than in hard data. The Medicare Evidence Development & Coverage Advisory Committee has made it known that we need more evidence on the efficacy of all venous procedures. Peter Gloviczki, MD, a vascular surgeon at Mayo Clinic, in Rochester, Minn., put it succinctly in an issue of Venous News: “We need to focus on venous research and never forget that whoever owns research owns the disease. We must continue innovation and collaboration, with other venous specialties and with industry.” Truth be told, there doesn’t seem to be much fascination with comprehension of the disease, but there appears to be an enormous drive from a variety of specialties to do procedures.
In July 2015, Gerard O’Sullivan, MD, wrote of a multidisciplinary group in Europe established to develop some standardization in venous stenting guidelines. He describes a “need for consistent guidelines for preoperative imaging, follow-up, anticoagulation duration and type, stent diameter, length into the inferior vena cava and lower end in relation to the internal iliac vein/external iliac vein.” I concur, that this would be utopic. I have not come across such guidelines to date.
Current basic science research focuses on pathologic considerations of venous thrombosis, including the consequences related to mechanical behavior of the venous wall in those conditions. In our group’s opinion, these considerations are elemental in determining the next steps in the research paradigm. What determines the remodeling of a vein, with or without intervention? How does a stent influence remodeling? Not surprisingly there are numerous questions that remain unanswered.
Translational investigation has provided insight into innovative ways to use computed tomography and magnetic resonance imaging. The ability to stage venous disease noninvasively could have a profound impact on how and why we manage the pathology. Additionally, knowing what the pathology looks like and potentially behaves like has the potential to promote more appropriate therapies. Intravascular ultrasound is well described by users and essential to the management of venous disease as it allows us to visualize and appreciate the pathology being treated in real time.
IVUS, though, is primarily used in the context of delivering a therapeutic tool as well as being invasive. Until recently, we have not been able to bring the power of cross-sectional imaging into the operative space. Our group has published on the use of multimodal imaging techniques such as magnetic resonance venography and fluoroscopic image fusion, which can potentially guide future interventions and optimize therapeutic decision-making.
Ultimately, we believe that diseased veins behave differently than arteries do. Therefore, managing veins with tools meant for another space is likely not ideal. Many venous interventions use arterial devices that are not optimized for venous pathologies and underline the fact that we need to continue to develop tools specifically designed for the venous space. The ATTRACT (Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-Directed Thrombolysis) trial has been extremely impactful in the treatment paradigm of venous thrombosis. Although the results remain heavily debated and, on some level, contested, it is a critical trial and should – in many ways – serve as an example of the good research being executed in venous disease.
A quote many have attributed to Albert Einstein says: “The one who follows the crowd will usually go no further than the crowd. Those who walk alone are likely to find themselves in places no one has ever been before.” We have an opportunity to be more enlightened with respect to central venous therapies; let’s not act like lemmings and follow one another off the cliff.
Dr. Bismuth is an associate professor of surgery and associate program director, Houston Methodist Hospital. He reported that he had no relevant disclosures.
References
Comerota AJ et al. 2015 May. Thromb Res. 135(5):882-7.
Vedantham S et al. 2017 Dec 7. N Engl J Med. 377(23):2240-52.
O’Sullivan G 2015 Jul. Endovascular Today.14;7:60-2.
Gloviczki P 2017 Apr. Venous News.1:8.
If one includes the entire spectrum of venous disease, it is a more common pathology than peripheral arterial disease. The financial impact of venous disease is substantial. Why, then, has it taken so long to generate enthusiasm for venous disease of the femorocaval and subclaviocaval segments? For years, the endovascular management of venous disease used technology and techniques borrowed from the arterial space; although results were encouraging, it is clear that they varied widely and continue to do so. Management of these vascular beds is very reminiscent of the barrage of devices we have thrown at the superficial femoral artery.
In peripheral arterial disease, there have been much education and research focused on understanding atherosclerosis and its interaction with arterial devices. However, the paucity of investigation and enlightenment in the venous domain is evident when a literature search is performed. Certainly there are data from Comerota et al. showing an increased amount of collagen in the walls of chronically diseased veins. While this is a reasonable start, it is not sufficient data on which to build an entire treatment paradigm. Just like peripheral arterial disease, venous pathology presents in a continuum. Without an in-depth appreciation of the variability of those presentations, it is difficult to envision targeted therapies.
Although vendors have recently engaged in the development of venous-specific devices, it is in great part grounded in expert opinion rather than in hard data. The Medicare Evidence Development & Coverage Advisory Committee has made it known that we need more evidence on the efficacy of all venous procedures. Peter Gloviczki, MD, a vascular surgeon at Mayo Clinic, in Rochester, Minn., put it succinctly in an issue of Venous News: “We need to focus on venous research and never forget that whoever owns research owns the disease. We must continue innovation and collaboration, with other venous specialties and with industry.” Truth be told, there doesn’t seem to be much fascination with comprehension of the disease, but there appears to be an enormous drive from a variety of specialties to do procedures.
In July 2015, Gerard O’Sullivan, MD, wrote of a multidisciplinary group in Europe established to develop some standardization in venous stenting guidelines. He describes a “need for consistent guidelines for preoperative imaging, follow-up, anticoagulation duration and type, stent diameter, length into the inferior vena cava and lower end in relation to the internal iliac vein/external iliac vein.” I concur, that this would be utopic. I have not come across such guidelines to date.
Current basic science research focuses on pathologic considerations of venous thrombosis, including the consequences related to mechanical behavior of the venous wall in those conditions. In our group’s opinion, these considerations are elemental in determining the next steps in the research paradigm. What determines the remodeling of a vein, with or without intervention? How does a stent influence remodeling? Not surprisingly there are numerous questions that remain unanswered.
Translational investigation has provided insight into innovative ways to use computed tomography and magnetic resonance imaging. The ability to stage venous disease noninvasively could have a profound impact on how and why we manage the pathology. Additionally, knowing what the pathology looks like and potentially behaves like has the potential to promote more appropriate therapies. Intravascular ultrasound is well described by users and essential to the management of venous disease as it allows us to visualize and appreciate the pathology being treated in real time.
IVUS, though, is primarily used in the context of delivering a therapeutic tool as well as being invasive. Until recently, we have not been able to bring the power of cross-sectional imaging into the operative space. Our group has published on the use of multimodal imaging techniques such as magnetic resonance venography and fluoroscopic image fusion, which can potentially guide future interventions and optimize therapeutic decision-making.
Ultimately, we believe that diseased veins behave differently than arteries do. Therefore, managing veins with tools meant for another space is likely not ideal. Many venous interventions use arterial devices that are not optimized for venous pathologies and underline the fact that we need to continue to develop tools specifically designed for the venous space. The ATTRACT (Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-Directed Thrombolysis) trial has been extremely impactful in the treatment paradigm of venous thrombosis. Although the results remain heavily debated and, on some level, contested, it is a critical trial and should – in many ways – serve as an example of the good research being executed in venous disease.
A quote many have attributed to Albert Einstein says: “The one who follows the crowd will usually go no further than the crowd. Those who walk alone are likely to find themselves in places no one has ever been before.” We have an opportunity to be more enlightened with respect to central venous therapies; let’s not act like lemmings and follow one another off the cliff.
Dr. Bismuth is an associate professor of surgery and associate program director, Houston Methodist Hospital. He reported that he had no relevant disclosures.
References
Comerota AJ et al. 2015 May. Thromb Res. 135(5):882-7.
Vedantham S et al. 2017 Dec 7. N Engl J Med. 377(23):2240-52.
O’Sullivan G 2015 Jul. Endovascular Today.14;7:60-2.
Gloviczki P 2017 Apr. Venous News.1:8.
If one includes the entire spectrum of venous disease, it is a more common pathology than peripheral arterial disease. The financial impact of venous disease is substantial. Why, then, has it taken so long to generate enthusiasm for venous disease of the femorocaval and subclaviocaval segments? For years, the endovascular management of venous disease used technology and techniques borrowed from the arterial space; although results were encouraging, it is clear that they varied widely and continue to do so. Management of these vascular beds is very reminiscent of the barrage of devices we have thrown at the superficial femoral artery.
In peripheral arterial disease, there have been much education and research focused on understanding atherosclerosis and its interaction with arterial devices. However, the paucity of investigation and enlightenment in the venous domain is evident when a literature search is performed. Certainly there are data from Comerota et al. showing an increased amount of collagen in the walls of chronically diseased veins. While this is a reasonable start, it is not sufficient data on which to build an entire treatment paradigm. Just like peripheral arterial disease, venous pathology presents in a continuum. Without an in-depth appreciation of the variability of those presentations, it is difficult to envision targeted therapies.
Although vendors have recently engaged in the development of venous-specific devices, it is in great part grounded in expert opinion rather than in hard data. The Medicare Evidence Development & Coverage Advisory Committee has made it known that we need more evidence on the efficacy of all venous procedures. Peter Gloviczki, MD, a vascular surgeon at Mayo Clinic, in Rochester, Minn., put it succinctly in an issue of Venous News: “We need to focus on venous research and never forget that whoever owns research owns the disease. We must continue innovation and collaboration, with other venous specialties and with industry.” Truth be told, there doesn’t seem to be much fascination with comprehension of the disease, but there appears to be an enormous drive from a variety of specialties to do procedures.
In July 2015, Gerard O’Sullivan, MD, wrote of a multidisciplinary group in Europe established to develop some standardization in venous stenting guidelines. He describes a “need for consistent guidelines for preoperative imaging, follow-up, anticoagulation duration and type, stent diameter, length into the inferior vena cava and lower end in relation to the internal iliac vein/external iliac vein.” I concur, that this would be utopic. I have not come across such guidelines to date.
Current basic science research focuses on pathologic considerations of venous thrombosis, including the consequences related to mechanical behavior of the venous wall in those conditions. In our group’s opinion, these considerations are elemental in determining the next steps in the research paradigm. What determines the remodeling of a vein, with or without intervention? How does a stent influence remodeling? Not surprisingly there are numerous questions that remain unanswered.
Translational investigation has provided insight into innovative ways to use computed tomography and magnetic resonance imaging. The ability to stage venous disease noninvasively could have a profound impact on how and why we manage the pathology. Additionally, knowing what the pathology looks like and potentially behaves like has the potential to promote more appropriate therapies. Intravascular ultrasound is well described by users and essential to the management of venous disease as it allows us to visualize and appreciate the pathology being treated in real time.
IVUS, though, is primarily used in the context of delivering a therapeutic tool as well as being invasive. Until recently, we have not been able to bring the power of cross-sectional imaging into the operative space. Our group has published on the use of multimodal imaging techniques such as magnetic resonance venography and fluoroscopic image fusion, which can potentially guide future interventions and optimize therapeutic decision-making.
Ultimately, we believe that diseased veins behave differently than arteries do. Therefore, managing veins with tools meant for another space is likely not ideal. Many venous interventions use arterial devices that are not optimized for venous pathologies and underline the fact that we need to continue to develop tools specifically designed for the venous space. The ATTRACT (Acute Venous Thrombosis: Thrombus Removal With Adjunctive Catheter-Directed Thrombolysis) trial has been extremely impactful in the treatment paradigm of venous thrombosis. Although the results remain heavily debated and, on some level, contested, it is a critical trial and should – in many ways – serve as an example of the good research being executed in venous disease.
A quote many have attributed to Albert Einstein says: “The one who follows the crowd will usually go no further than the crowd. Those who walk alone are likely to find themselves in places no one has ever been before.” We have an opportunity to be more enlightened with respect to central venous therapies; let’s not act like lemmings and follow one another off the cliff.
Dr. Bismuth is an associate professor of surgery and associate program director, Houston Methodist Hospital. He reported that he had no relevant disclosures.
References
Comerota AJ et al. 2015 May. Thromb Res. 135(5):882-7.
Vedantham S et al. 2017 Dec 7. N Engl J Med. 377(23):2240-52.
O’Sullivan G 2015 Jul. Endovascular Today.14;7:60-2.
Gloviczki P 2017 Apr. Venous News.1:8.
Is Vitiligo in Vogue? The Changing Face of Vitiligo
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
Vitiligo is a disfiguring skin condition that is thought to result from autoimmune destruction of melanocytes in the skin, leading to patchy depigmentation. The prevalence of vitiligo is estimated at 1% worldwide.1 Once seen as merely a cosmetic disorder, it is increasingly recognized for its devastating psychological effects. As skin quality, texture, and color are a few of the first things people notice about others, skin plays a major role in our daily interactions with the world. Vitiligo often affects the face and other visible areas of the body; thus, it is associated with impaired quality of life, and affected individuals often experience psychosocial impairment including anxiety, depression, stigmatization, and self-harm ideation.2 Indeed, vitiligo is a condition with not only a visible skin component but a deeper psychological component that also is important to recognize and address. However, due in large part to recent exposure to vitiligo through mainstream media, general understanding about and attitudes toward this condition are changing. As a result, vitiligo has seen a surge in outreach by those affected by the disease.
Perhaps the most well-known current face of vitiligo is Chantelle Brown-Young, a black fashion model, activist, and vitiligo spokesperson known professionally as Winnie Harlow. Diagnosed with vitiligo in childhood, she revealed she was teased and bullied and at one point contemplated suicide. “The continuous harassment and the despair that [vitiligo] brought on my life was so unbearably dehumanizing that I wanted to kill myself,” she disclosed.3 After competing on America’s Next Top Model in 2014, Winnie Harlow became a household name for redefining global standards of beauty and, in her own words, accepting the differences that make us unique and authentic.4 She went on to speak at the Dove Self-Esteem Project panel at the 2015 Women in the World London Summit and was presented with the Role Model award at the Portuguese GQ Men of the Year event that same year.5
More recently, Amy Deanna, a model with vitiligo, was featured in videos for CoverGirl’s 2018 “I Am What I Make Up” campaign in which she is shown enhancing her various skin tones rather than hiding them by applying both light and dark shades of makeup on her face. In a press release she stated, “Vitiligo awareness is something that is very important to me. Being given a platform to [raise awareness] means so much.”6
Additionally, Brock Elbank, a London-based photographer, recently launched a photograph series of men and women with vitiligo on the digital platform Instagram.7 In a recent interview he stated, “I see beauty in what many see as different. Unique individuals who stand out from the crowd are what inspire me to do what I do.”7
Lee Thomas, a television broadcaster and author of the book Turning White: A Memoir of Change is yet another example of a vitiligo patient who recently stopped hiding his condition. He admitted he has had people refuse to shake his hand due to his condition but has used the experience to educate others. He stated, “Because I’m in this position, I think this is where my next thing is supposed to be. It’s supposed to be about sharing and helping, and hopefully leaving the planet a little better for everybody else who comes along with vitiligo.”8 Thomas is dedicated to inspiring others with the condition and started the Clarity Lee Thomas Foundation to provide emotional and mental support to those with vitiligo.
Critics may say this vitiligo movement is merely another example of exploitation of what is unique or different by mainstream media and the fashion industry, similar to prior movements for plus-sized models, natural hairstyles in black women, and transgender identification. Even if partially true, the ultimate effect has been an increase in attention and representation of individuals with vitiligo in mainstream media. At the time this article was being published (September 2018), an Instagram search for #vitiligo yielded approximately 226,000 posts. For comparison with other much more common dermatologic conditions, #eczema returned approximately 958,000 results, #moles returned approximately 65,000 results, and #skincancer returned approximately 104,000 results. Additionally, the Vitiligo Research Foundation currently has more than 5000 followers on Instagram, which is as many as the Melanoma Research Foundation and almost twice as many as the Skin Cancer Foundation, supporting the idea that mainstream representation of individuals with vitiligo is contributing to raising awareness and backing of organizations aimed at making advancements in this area of dermatology.
As more individuals gain an understanding and curiosity about this disease, perhaps more research and investigation will be done to improve treatment options and outcomes for patients with vitiligo. With this movement, perhaps vitiligo patients will feel more comfortable and confident in their skin.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
- Ezzedine K, Eleftheriadou V, Whitton M, et al. Vitiligo. Lancet. 2015;386:74-84.
- Tomas‐Aragones L, Marron SE. Body image and body dysmorphic concerns. Acta Derm Venereol. 2016;96:47-50.
- Rodney D. From suicide thoughts to finalist in America’s Next Top Model. The Gleaner. February 25, 2014. http://jamaica-gleaner.com/gleaner/20140225/news/news1.html. Accessed September 7, 2018.
- Keyes-Bevan B. Winnie Harlow: her emotional story with vitiligo. Personal Health News website. http://www.personalhealthnews.ca/prevention-and-treatment/her-emotional-story-with-vitiligo. Accessed September 7, 2018.
- Giles K, Davidson R. ‘I think I’m beautiful’: model Winnie Harlow, who suffers from rare vitiligo skin condition, gives empowering talk at Women in the World event. Daily Mail. October 9, 2015. http://www.dailymail.co.uk/tvshowbiz/article-3266579/I-think-m-beautiful-Model-Winnie-Harlow-suffers-rare-Vitiligo-skin-condition-gives-empowering-talk-Women-World-event.html. Updated October 13, 2015. Accessed September 7, 2018.
- Ruffo J. CoverGirl’s first model with vitiligo stars in new campaign: ‘w
e have to be more inclusive.’ People. February 20, 2018. https://people.com/style/covergirl-first-model-with-vitiligo-interview/. Accessed September 25, 2018. - Blair O. This vitiligo photo series is absolutely breathtaking. Cosmopolitan. March 23, 2018. https://www.cosmopolitan.com/uk/beauty-hair/a19494259/vitiligo-photo-series-instagram/. Accessed September 7, 2018.
- Broadcaster opens up about living with vitiligo. People. February 20, 2018. http://people.com/health/lee-thomas-tv-reporter-on-his-vitiligo/. Accessed April 1, 2018.
A (former) skeptic’s view of bariatric surgery
Because of the high prevalence of obesity and diabetes, bariatric surgery has become very popular. In the United States alone, there were an estimated 228,000 weight loss surgical procedures performed in 2017.1
But I must confess that for many years, I was skeptical about the value of surgery to treat obesity. Yes, everyone who had a bariatric procedure lost weight, but did the long-term benefits really outweigh the harms? I wondered if most people gradually gained back the weight they lost. And the harms can be significant, including dumping syndrome, hypoglycemia, and malabsorption—in addition to the potential for surgical complications and repeat surgery. And, I must confess that my views were likely affected by the death of a friend from complications of gastric bypass 25 years ago.
My skepticism, however, has changed to cautious optimism for carefully selected patients. I say this because we now have long-term follow-up studies demonstrating the value of bariatric procedures—especially for people with type 2 diabetes.
Most studies have been cohort studies that compare results to similar patients with obesity who did not have surgery, and the outcomes have been consistently better in patients who underwent surgery. Two recent meta-analyses summarized these results; one for all patients with obesity and the other for patients with type 2 diabetes.
Continue to: The first meta-analysis
The first meta-analysis included 11 randomized trials, 4 nonrandomized controlled trials, and 17 cohort studies and showed probable reductions in all-cause mortality and possible reductions in cancer and cardiovascular events.2 The second demonstrated significant improvements in microvascular and macrovascular disease and reduced mortality.3 The data were limited, however, because of the lack of large randomized trials with long-term follow-up.
The Stampede trial is one of a few bariatric surgery randomized trials focusing on patients with diabetes.4 The 5-year follow-up results are impressive. Nearly 30% of patients who had gastric bypass and 23% who had sleeve gastrectomy had an A1C ≤6 at 5 years compared to only 5% of those treated medically. Some patients discontinued all medications for diabetes, hypertension, and hyperlipidemia.
There is now adequate research to show that bariatric surgery provides significant benefits to properly selected patients who understand the risks. I no longer hesitate to refer patients for bariatric surgery who have been unsuccessful with weight loss—despite their best efforts.
Where do you stand?
1. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed September 18, 2018.
2. Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26:2590-2601.
3. Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27:2724-2732.
4. Schauer PR, Bhatt DL, Kirwan JP; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
Because of the high prevalence of obesity and diabetes, bariatric surgery has become very popular. In the United States alone, there were an estimated 228,000 weight loss surgical procedures performed in 2017.1
But I must confess that for many years, I was skeptical about the value of surgery to treat obesity. Yes, everyone who had a bariatric procedure lost weight, but did the long-term benefits really outweigh the harms? I wondered if most people gradually gained back the weight they lost. And the harms can be significant, including dumping syndrome, hypoglycemia, and malabsorption—in addition to the potential for surgical complications and repeat surgery. And, I must confess that my views were likely affected by the death of a friend from complications of gastric bypass 25 years ago.
My skepticism, however, has changed to cautious optimism for carefully selected patients. I say this because we now have long-term follow-up studies demonstrating the value of bariatric procedures—especially for people with type 2 diabetes.
Most studies have been cohort studies that compare results to similar patients with obesity who did not have surgery, and the outcomes have been consistently better in patients who underwent surgery. Two recent meta-analyses summarized these results; one for all patients with obesity and the other for patients with type 2 diabetes.
Continue to: The first meta-analysis
The first meta-analysis included 11 randomized trials, 4 nonrandomized controlled trials, and 17 cohort studies and showed probable reductions in all-cause mortality and possible reductions in cancer and cardiovascular events.2 The second demonstrated significant improvements in microvascular and macrovascular disease and reduced mortality.3 The data were limited, however, because of the lack of large randomized trials with long-term follow-up.
The Stampede trial is one of a few bariatric surgery randomized trials focusing on patients with diabetes.4 The 5-year follow-up results are impressive. Nearly 30% of patients who had gastric bypass and 23% who had sleeve gastrectomy had an A1C ≤6 at 5 years compared to only 5% of those treated medically. Some patients discontinued all medications for diabetes, hypertension, and hyperlipidemia.
There is now adequate research to show that bariatric surgery provides significant benefits to properly selected patients who understand the risks. I no longer hesitate to refer patients for bariatric surgery who have been unsuccessful with weight loss—despite their best efforts.
Where do you stand?
Because of the high prevalence of obesity and diabetes, bariatric surgery has become very popular. In the United States alone, there were an estimated 228,000 weight loss surgical procedures performed in 2017.1
But I must confess that for many years, I was skeptical about the value of surgery to treat obesity. Yes, everyone who had a bariatric procedure lost weight, but did the long-term benefits really outweigh the harms? I wondered if most people gradually gained back the weight they lost. And the harms can be significant, including dumping syndrome, hypoglycemia, and malabsorption—in addition to the potential for surgical complications and repeat surgery. And, I must confess that my views were likely affected by the death of a friend from complications of gastric bypass 25 years ago.
My skepticism, however, has changed to cautious optimism for carefully selected patients. I say this because we now have long-term follow-up studies demonstrating the value of bariatric procedures—especially for people with type 2 diabetes.
Most studies have been cohort studies that compare results to similar patients with obesity who did not have surgery, and the outcomes have been consistently better in patients who underwent surgery. Two recent meta-analyses summarized these results; one for all patients with obesity and the other for patients with type 2 diabetes.
Continue to: The first meta-analysis
The first meta-analysis included 11 randomized trials, 4 nonrandomized controlled trials, and 17 cohort studies and showed probable reductions in all-cause mortality and possible reductions in cancer and cardiovascular events.2 The second demonstrated significant improvements in microvascular and macrovascular disease and reduced mortality.3 The data were limited, however, because of the lack of large randomized trials with long-term follow-up.
The Stampede trial is one of a few bariatric surgery randomized trials focusing on patients with diabetes.4 The 5-year follow-up results are impressive. Nearly 30% of patients who had gastric bypass and 23% who had sleeve gastrectomy had an A1C ≤6 at 5 years compared to only 5% of those treated medically. Some patients discontinued all medications for diabetes, hypertension, and hyperlipidemia.
There is now adequate research to show that bariatric surgery provides significant benefits to properly selected patients who understand the risks. I no longer hesitate to refer patients for bariatric surgery who have been unsuccessful with weight loss—despite their best efforts.
Where do you stand?
1. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed September 18, 2018.
2. Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26:2590-2601.
3. Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27:2724-2732.
4. Schauer PR, Bhatt DL, Kirwan JP; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
1. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2017. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. Accessed September 18, 2018.
2. Zhou X, Yu J, Li L, et al. Effects of bariatric surgery on mortality, cardiovascular events, and cancer outcomes in obese patients: systematic review and meta-analysis. Obes Surg. 2016;26:2590-2601.
3. Sheng B, Truong K, Spitler H, et al. The long-term effects of bariatric surgery on type 2 diabetes remission, microvascular and macrovascular complications, and mortality: a systematic review and meta-analysis. Obes Surg. 2017;27:2724-2732.
4. Schauer PR, Bhatt DL, Kirwan JP; STAMPEDE Investigators. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med. 2017;376:641-651.
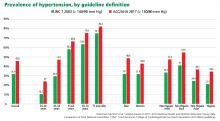
2017 ACC/AHA hypertension guidelines: Toward tighter control
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
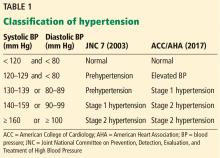
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
In 2017, the American College of Cardiology (ACC), American Heart Association (AHA), and 9 other professional associations published a new guideline on high blood pressure in adults.1 Their document addresses a range of topics relevant to preventing, diagnosing, and managing hypertension. It incorporates evidence from randomized controlled trials, including the Systolic Blood Pressure Intervention Trial (SPRINT),2 systematic reviews, and expert opinion.
The new guidelines contain many noteworthy changes, some of which are generating intense debate and discussion. Here, we provide our opinions to help practicing clinicians broaden their perspective and make informed decisions about management.
ACC AND AHA ARE NOW RESPONSIBLE FOR HYPERTENSION GUIDELINES
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC), organized by the National Heart, Lung, and Blood Institute, began issuing hypertension guidelines in 1977. Based on observational and clinical trial data, succeeding JNC reports recommended ever-lower blood pressure goals, with emphasis shifting to treatment of systolic hypertension.
The last official JNC report—JNC 7—was published in 2003.3 In 2013, the Institute transferred the responsibility for cardiovascular prevention guidelines to the ACC and AHA.4
A report from the panel members appointed to JNC 8 was published independently in 2014.5 It focused on a few key questions and used evidence limited to randomized controlled trials. In this report, the panel relaxed the goals for many subgroups, leading to criticism from many professional societies and from some members of the panel writing group.6
WHAT'S NEW IN THE 2017 GUIDELINES?
The new ACC/AHA guidelines contain a number of changes from previous documents that have been the topic of debate.
New definition and classification of hypertension
Strong recommendation, based on moderate-quality evidence.
Our opinion. While this new classification is intended to promote closer monitoring and earlier intervention to lower cardiovascular event rates, creating a new level of disease may lead to more pharmacologic treatment for those with lower risk, without emphasis on lifestyle modifications.
Emphasis on measurement technique and out-of-office measurements
Strong recommendation, based on expert opinion, for accurate measurement of blood pressure in the office, high-quality evidence from systematic review for out-of-office measurement.
Appropriate management of hypertension entails accurate blood pressure measurement. While office-based measurement remains the most commonly used method, this “snapshot” may not reflect a patient’s true baseline blood pressure.
Out-of-office measurements. Based on the results of a systematic review commissioned by the guideline committee, out-of-office measurements are now recommended to confirm the diagnosis of hypertension and to assess response to therapy.
Ambulatory blood pressure monitoring should be strongly considered as the preferred method for out-of-office monitoring; home blood pressure monitoring can be done if ambulatory monitoring is not feasible. Ambulatory monitoring provides additional information on nighttime blood pressure, including the dipping status (normal defined as a nighttime blood pressure decrease of 10% to 20%). Ambulatory monitoring predicts long-term cardiovascular outcomes independent of office blood pressure, and elevated nighttime pressure and non-dipping have been shown to be independently associated with increased cardiovascular mortality rates.8,9 Unfortunately, despite evidence supporting its use, ambulatory blood pressure monitoring is not widely available for a variety of reasons, including high cost (roughly $2,000–$4,000) and minimal reimbursement.
Out-of-office measurements can also detect white coat hypertension and masked hypertension. White coat hypertension is defined as blood pressure that is elevated in the office but normal in an out-of-office setting, and masked hypertension is blood pressure that is normal in the office and elevated in an out-of-office setting. Currently, pharmacologic therapy is not recommended to treat white coat hypertension, and treatment for masked hypertension should be the same as for sustained hypertension.
While the guidelines do not comment specifically on manual office measurement vs automated office measurements using devices that take multiple measurements with the patient alone in the room to reduce the white coat effect, they acknowledge “increasing evidence” favoring the use of automated office measurement.
Proper technique for measuring blood pressure is appropriately emphasized; correct patient positioning, allowing a period of rest, and using the appropriate cuff size are all important. Unfortunately, many busy clinical practices may not follow correct technique when measuring blood pressure in the office, leading to misdiagnosis and unnecessary pharmacologic therapy that may result in adverse events.
Of note, the SPRINT trial, which informed many of the new guideline recommendations, followed a strict protocol of blood pressure measurement with an automated device, checking sitting blood pressure 3 times at 1-minute intervals, with the patient alone in the room and without an observer present at many of the sites.10
Most guidelines11,12 agree on an average of at least 135/85 mm Hg as the threshold for diagnosing hypertension by home monitoring, or an average daytime pressure of at least 135/85 mm Hg by ambulatory monitoring, corresponding with office-based blood pressure of 140/90 mm Hg. However, the new guidelines recommend a lower threshold of 130/80 mm Hg for both home monitoring and average daytime ambulatory monitoring, corresponding with an office blood pressure of 130/80 mm Hg. They do not specify whether the office-based measurement is manual or automated.
Our opinion. Since office-based measurement will likely remain the principal method for managing hypertension due to constraints with ambulatory or home monitoring, the use of automated devices for office measurement should be strongly considered. Studies have shown that, compared with routine office measurements, automated measurements more closely approximate those obtained by ambulatory and home blood pressure monitoring.13
Risk-based approach to hypertension management
The algorithm for hypertension management now incorporates objective assessment of cardiovascular risk. Specifically, it calls for estimation of the 10-year risk of atherosclerotic cardiovascular disease, defined as coronary heart disease death, nonfatal myocardial infarction, or fatal or nonfatal stroke.
The information required to estimate risk includes age, sex, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure, use of blood pressure-lowering medication, diabetes status, and smoking status. The guideline recommends an easy-to-use online risk calculator (http://tools.acc.org/ASCVD-Risk-Estimator).
A 10-year risk of 10% or more is designated as the cutoff between high risk and low risk. However, this is not based on trial evidence, and the risk calculator has not been verified in prospective trials to show that its use reduces cardiovascular events. The SPRINT trial,2 which was a study of blood pressure-lowering in high-risk patients, used a 10-year risk of 15% or more based on the Framingham risk score to delineate high risk.
Additionally, the 10-year risk calculator is valid only in patients ages 40 through 79, and some studies indicate that it may overestimate risk in older adults.14,15 This overestimation may lead to patients being started on pharmacologic therapy when it may not truly be indicated. The risk calculator controversy has been discussed in a previous issue of this journal.16
Blood pressure goals
Strong recommendation for known cardiovascular disease or atherosclerotic cardiovascular disease risk 10% or greater, weak recommendation for risk less than 10%, based on moderate-quality evidence for systolic blood pressure, expert opinion for diastolic.
The guidelines recommend a blood pressure goal of less than 130/80 mm Hg for all patients, including the elderly and patients with chronic kidney disease or diabetes.
The SPRINT trial,2 which showed better cardiovascular outcomes in the intensive treatment group (aiming for systolic pressure < 120 mm Hg) compared with a standard treatment group (aiming for systolic pressure < 140 mm Hg), excluded participants with diabetes and severe chronic kidney disease (estimated glomerular filtration rate < 20 mL/min/m2 and proteinuria > 1 g/day), and those who were in nursing homes or had dementia.
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) blood pressure trial showed that intensive blood pressure control did not have cardiovascular benefits compared with standard therapy.17 However, many now believe that the study may have been underpowered due to its design, and a meta-analysis of the results from SPRINT and ACCORD suggested that findings from both trials were consistent, favoring intensive blood pressure control in a high-risk population.18
While the totality of evidence favors a lower achieved blood pressure for many patients, this lower goal may be difficult to achieve in many, particularly those with vascular stiffness, which is common in the elderly. These patients also tend to have low diastolic pressure, and lowering diastolic pressure below 60 mm Hg in those with documented coronary artery disease could increase the risk of adverse cardiovascular outcomes.19,20 The guidelines do not address the potential issues with lowering diastolic blood pressure.
Our opinion. While a “universal” blood pressure goal may simplify decision-making, we believe it is important to individualize goals, taking into account patient characteristics, lifestyle factors, medication side effects, patient preferences, cost issues, and adherence to therapy.
The goal blood pressure should also consider the method of measurement. Systolic blood pressure readings have been reported to be 5 to 10 mm Hg lower with automated office measurement than with routine office measurement.21
It is also not clear that the magnitude of absolute benefit from pursuing more intensive blood pressure control with antihypertensive therapy in patients with high cardiovascular risk (as in SPRINT) would translate to similar benefits in a lower-risk population. Thus, we believe that in patients with lower cardiovascular risk, a goal blood pressure of less than 140/90 mm Hg (if routine office measurement is done) and less than 135/85 mm Hg (if automated office measurement is done) would be reasonable.
We also believe that it is reasonable to relax these goals in the very elderly (age ≥ 80), especially those who are frail and at risk of falls, with low diastolic pressures. In these patients, we recommend individualizing blood pressure goals that can be achieved without significant side effects from antihypertensive therapy.
Nonpharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials
Nonpharmacologic therapy and lifestyle modification are appropriately emphasized in the new guidelines. Most of the lifestyle changes that are recommended are in concordance with prior JNC 7 recommendations.3
Recognizing the roles of sodium and potassium in the pathogenesis of hypertension, the guidelines emphasize a diet that is higher in potassium, the DASH (Dietary Approaches to Stop Hypertension) diet, and a low-sodium diet. The recommended optimal goal of sodium intake of less than 1,500 mg/day may be difficult to achieve with a Western diet, and there is debate about the potential adverse effects of a very-low sodium diet.22 The general recommendation for sodium intake of less than 2,300 mg/day is supported in the literature, and it is unclear if further reduction has additional beneficial effects on blood pressure.23
The guidelines recommend a 3- to 6-month reassessment of patients who are prescribed risk-factor modification, but are unclear about initiation of pharmacologic therapy or other steps if these low-risk patients have not responded to lifestyle modifications alone at the time of reassessment.
Pharmacologic therapy
Strong recommendation, based on high-quality evidence from randomized controlled trials for systolic blood pressure, expert opinion for diastolic blood pressure for those with atherosclerotic cardiovascular disease risk 10% or greater, and limited data for those with risk less than 10%.
Pharmacologic therapy is recommended in patients with stage 1 hypertension and pre-existing cardiovascular disease or 10-year risk of atherosclerotic cardiovascular disease of 10% or more, and in those with stage 2 hypertension even if their 10-year risk is less than 10%.
In the absence of compelling indications, the primary drugs recommended for initial therapy are:
- Thiazide or thiazide-type diuretics (preferably chlorthalidone)
- Angiotensin-converting enzyme (ACE) inhibitors
- Angiotensin II receptor blockers (ARBs)
- Calcium channel blockers (CCBs).
In black adults, thiazide diuretics or CCBs are recommended for initial therapy. Beta-blockers are not recommended as first-line agents in the absence of a compelling indication, although meta-analyses that suggested beta-blockers are less effective than other classes of agents included trials that used beta-blockers in doses now considered suboptimal. ACE inhibitors or ARBs are recommended as initial therapy in proteinuric patients with chronic kidney disease or diabetes. Combining an ACE inhibitor and an ARB or renin inhibitor is potentially harmful and is not recommended. The guidelines provide a helpful table describing important characteristics and available dosage forms of the commonly used antihypertensive agents.
These recommendations are concordant with the JNC 8 panel recommendations,5 and differ from JNC 7, which recommended thiazide-type diuretics as first-line therapy.3 The European guidelines recommend that all major classes of antihypertensive agents, including beta-blockers, are suitable for initiation of therapy.24 The UK National Institute for Clinical Excellence guidelines adopt an age-based approach to deciding initial therapy—with ACE inhibitors or ARBs favored in those below the age of 55 and CCBs in those who are 55 and older.25
Starting with a single antihypertensive agent is recommended for stage 1 hypertension with increased cardiovascular risk, and starting with 2 agents (either separately or in fixed-dose combination) is recommended for stage 2 hypertension. The guidelines emphasize a team-based approach to improve hypertension care, using adjunctive interventions such as telehealth strategies and leveraging electronic medical records to guide quality improvement initiatives.
Our opinion. We agree with Bakris and Sorrentino26 that general patient profiles should be considered to decide on efficient pharmacologic management in clinical practice—thiazide diuretics would be best in those who are volume-expanded; ACE inhibitors, ARBs, or CCBs in those who are obese or have metabolic syndrome; and beta-blockers or nondihydropyridine CCBs in those who are hyperadrenergic. More patients will likely be classified as having resistant hypertension based on the blood pressure goal of less than 130/80 mm Hg, which may require greater use of mineralocorticoid receptor antagonists such as spironolactone.
COMPARISONS WITH OTHER GUIDELINES
STRENGTHS AND LIMITATIONS
The new guidelines stress correct technique of blood pressure measurement, out-of-office and self-monitoring of blood pressure, and lifestyle modifications. In addition, they comprehensively review topics relevant to hypertension management of practical use for healthcare providers, including resistant hypertension, secondary hypertension, hypertensive crises, and special populations. The guidelines also incorporate multiple lines of evidence rather than just randomized controlled trials (which may not be available for every scenario).
There will be ongoing debate and discussion about the new definition and classification of hypertension, and the “conversion” of previously healthy adults to a new disease category. The blood pressure goals will also be debated: Should the goal for a young patient be applied to an elderly patient? The pathophysiology of the disease process should be considered rather than a one-size-fits-all approach. For example, older patients with stiff arteries and low diastolic blood pressure will have more difficulty achieving a lower systolic pressure, are more likely to experience medication side effects, and may have adherence issues due to polypharmacy.
A clinical trial, with strict adherence to protocols and rigorous follow-up procedures, is different from real-world clinical practice. Busy clinical practices with time and space constraints may forgo the steps needed for accurate blood pressure measurement in the office and may not reinforce lifestyle modifications, instead opting for more pharmacologic therapy to achieve a blood pressure goal that may become mandated by healthcare payment models without consideration for clinical judgment and individual patient characteristics.
The ACC/AHA guidelines have not been universally endorsed. The American College of Physicians and the American Academy of Family Physicians released their own guidelines for older adults earlier in 2017, echoing the recommendations from the panel appointed to JNC 8.27 Contrasting recommendations can unfortunately lead to confusion among healthcare providers and patients and can undermine confidence and trust in the healthcare system.
In the background of ongoing debate, where battle lines have been drawn by key stakeholders with regard to their contrasting positions, it is even more important for the practicing clinician who is in the front lines of hypertension management to be knowledgeable about the pros and cons of different recommendations as they apply to individual patients, and to be able to clearly communicate this with patients when deciding on a treatment plan.
FINAL THOUGHTS
- Accurate measurement of blood pressure in the office is imperative—position the patient properly, use an appropriately sized cuff, and allow for a period of rest. Consider using automated office measurement to minimize potential white coat effect.
- Out-of-office blood pressure monitoring is recommended to confirm the diagnosis of hypertension and for monitoring response to therapy. Ambulatory monitoring is preferred, but home blood pressure monitoring can be done if ambulatory monitoring is unavailable or unfeasible.
- Nonpharmacologic therapy should be emphasized for everyone, regardless of blood pressure level.
- Guidelines should be used as a framework for management. Individualize decisions about blood pressure goals and pharmacologic therapy based on patient characteristics and clinical judgment.
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
- Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2017. doi:10.1016/j.jacc.2017.11.006
- SPRINT Research Group. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med 2015; 373(22):2103-2116. doi:10.1056/NEJMoa1511939
- Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003; 289(19):2560–2571. doi:10.1001/jama.289.19.2560
- Gibbons GH, Shurin SB, Mensah GA, Lauer MS. Refocusing the agenda on cardiovascular guidelines: an announcement from the National Heart, Lung, and Blood Institute. Circulation 2013; 128(15)1713–1715. doi:10.1161/CIRCULATIONAHA.113.004587
- James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311(5):507–520. doi:10.1001/jama.2013.284427
- Wright JT, Fine LJ, Lackland DT, Ogedegbe G, Himmelfarb CR. Evidence supporting a systolic blood pressure goal of less than 150 mm Hg in patients aged 60 years or older: the minority view. Ann Intern Med 2014; 160(7):499–503. doi:10.7326/M13-2981
- Muntner P, Carey RM, Gidding S, et al. Potential US population impact of the 2017 ACC/AHA high blood pressure guideline. Circulation 2018; 137(2):109–118. doi:10.1161/CIRCULATIONAHA.117.032582
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E, Whitlock EP. Diagnostic and predictive accuracy of blood pressure screening methods with consideration of rescreening intervals: a systematic review for the US Preventive Services Task Force. Ann Intern Med 2015; 162(3):192–204. doi:10.7326/M14-1539
- Boggia J, Li Y, Thijs L, et al. Prognostic accuracy of day versus night ambulatory blood pressure: a cohort study. Lancet 2007; 370(9594): 1219–1229. doi:10.1016/S0140-6736(07)61538-4
- Drawz PE, Ix JH. BP measurement in clinical practice: time to SPRINT to guideline-recommended protocols. J Am Soc Nephrol 2017: 29(2):383–388. doi:10.1681/ASN.2017070753
- O’Brien E, Parati G, Stergiou G, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens 2013; 31(9):1731–1768. doi:10.1097/HJH.0b013e328363e964
- Nerenberg KA, Zarnke KB, Leung AA, et al. Hypertension Canada’s 2018 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34(5):506–525. doi:10.1016/j.cjca.2018.02.022
- Myers MG, Godwin M, Dawes M, et al. Conventional versus automated measurement of blood pressure in primary care patients with systolic hypertension: randomised parallel design controlled trial. BMJ 2011; 342:d286. doi:10.1136/bmj.d286
- Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet 2013; 382(9907):1762–1765. doi:10.1016/S0140-6736(13)62388-0
- DeFilippis AP, Young R, McEvoy JW, et al. Risk score overestimation: the impact of individual cardiovascular risk factors and preventive therapies on the performance of the American Heart Association-American College of Cardiology-Atherosclerotic Cardiovascular Disease risk score in a modern multi-ethnic cohort. Eur Heart J 2017; 38(8):598–608. doi:10.1093/eurheartj/ehw301
- Raymond C, Cho L, Rocco M, Hazen SL. New cholesterol guidelines: worth the wait? Cleve Clin J Med 2014; 81(1):11–19. doi:10.3949/ccjm.81a.13161
- ACCORD Study Group, Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362(17):1575–1585. doi:10.1056/NEJMoa1001286
- Perkovic V, Rodgers A. Redefining blood-pressure targets – SPRINT starts the marathon. N Engl J Med 2015; 373(22):2175–2178. doi:10.1056/NEJMe1513301
- Vidal-Petiot E, Ford I, Greenlaw N, et al. Cardiovascular event rates and mortality according to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet 2016; 388(10056):2142–2152. doi:10.1016/S0140-6736(16)31326-5
- McEvoy JW, Chen Y, Rawlings A, et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events: implications for blood pressure control. J Am Coll Cardiol 2016; 68(16):1713–1722. doi:10.1016/j.jacc.2016.07.754
- Bakris GL. The implications of blood pressure measurement methods on treatment targets for blood pressure. Circulation 2016; 134(13):904–905. doi:10.1161/CIRCULATIONAHA.116.022536
- O’Donnell M, Mente A, Rangarajan S, et al. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371(7):612–623. doi:10.1056/NEJMoa1311889
- Sacks FM, Svetkey LP, Vollmer WM, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 2001; 344(1):3–10. doi:10.1056/NEJM200101043440101
- Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2013; 34(28):2159–2219. doi:10.1093/eurheartj/eht151
- National Institute for Health and Care Excellence (NICE). Hypertension in adults: diagnosis and management. Clinical guideline CG127. http://www.nice.org.uk/guidance/CG127. Accessed August 6, 2018.
- Bakris G, Sorrentino M. Redefining hypertension—assessing the new blood-pressure guidelines. N Engl Med 2018; 378(6):497–499. doi:10.1056/NEJMp1716193
- Qaseem A, Wilt TJ, Rich R, Humphrey LL, Frost J, Forciea MA. Pharmacologic treatment of hypertension in adults aged 60 years or older to higher versus lower blood pressure targets: a clinical practice guideline from the American College of Physicians and the American Academy of Family Physicians. Ann Intern Med 2017; 166(6): 430-437. doi:10.7326/M16-1785
- Weber MA, Schiffrin EL, White WB, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hyperten 2014; 16(1):14–26. doi:10.1111/jch.12237
- KDIGO Blood Pressure Work Group. KDIGO clinical practice guideline for the management of blood pressure in chronic kidney disease. Kidney Int Suppl 2012; 2(5):337–414.
- De Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care 2017; 40(9):1273–1284. doi:10.2337/dci17-0026
Clove
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Cloves (Syzygium aromaticum, also known as Eugenia caryophyllata) are the aromatic flower buds of a tree in the Myrtaceae family native to Indonesia. The essential oil of clove is known to exhibit antioxidant, anti-inflammatory, antimicrobial, antifungal, antiviral, anticancer, cytotoxic, insect repellent, and anesthetic activities.1,2 It is used topically in herbal medicine to alleviate pain and facilitate healing,3 and has been used in traditional medicine to confer analgesic, anti-inflammatory, antimicrobial, antiviral, and antiseptic activity.4 Cloves also are used in fragrances and for food flavoring.2
The two main constituents of clove oil are eugenol (78%) and beta-caryophyllene (13%). Although clove oil and its primary components are generally recognized as safe, a 2006 in vitro study by Prashar et al. found that clove oil and eugenol displayed cytotoxicity toward human fibroblasts and endothelial cells. Clove oil, in concentrations as low as 0.03%, was noted for being exceedingly cytotoxic, with up to 73% of this effect ascribed to eugenol, with beta-caryophyllene displaying no toxicity.3 In addition to beta-caryophyllene and the phenylpropanoid eugenol, other important constituents of clove essential oil are the phenylpropanoids carvacrol, thymol, and cinnamaldehyde.2
Topical applications and human studies
constituent, eugenol.5 It also has been used as a penetration enhancer in various forms of topical products, including creams, ointments, gels, and patches.6
Palmar hyperhidrosis
In 2017, Ibrahim et al. treated 45 patients with palmar hyperhidrosis with clove oil 45% in liposome, with 20 patients in a control group treated with 0.9% saline solution. Subjects were assessed by gravimetry testing and hyperhidrosis disease severity scale to determine the impact of clove oil on decreasing the sweating rate in patients with idiopathic palmar hyperhidrosis. Gravimetry testing revealed that the sweating rate decreased significantly in the clove oil group but that there was no significant improvement in the placebo group. The investigators concluded that twice-daily topical application of 45% clove oil in liposome for 2 weeks showed promise in significantly reducing palmar sweating.5
Pruritus
That same year Ibrahim et al. evaluated the effects of topically applied clove oil in treating 50 patients with chronic pruritus due to hepatic, renal, or diabetic origin. The investigators divided the subjects into two groups of 25, with the first directed to hydrate their skin before applying topical clove oil twice daily for 2 weeks. The second group was instructed to apply topical petrolatum by hand on the same schedule. Using the 5-D itch scale, researchers noted a significant improvement in all parameters in the patients using clove oil and no such improvements in the petrolatum group. They concluded that particularly for patients whose topical or systemic treatments are not well tolerated or are contraindicated.7
Anal fissure
In 2007, Elwakeel et al. evaluated the use of a clove oil 1% cream for the treatment of chronic anal fissure as opposed to the traditional treatment of stool softeners and lignocaine cream 5% in a single-blind randomized comparative trial over 6 weeks. Healing was observed in 60% of the 30 patients in the clove oil group and in 12% of the 25 patients in the control group at the 3-month follow-up visit. The researchers concluded that topically applied clove oil cream yielded significant benefits in the treatment of chronic anal fissures.8
More recently, Nelson et al. conducted a literature survey to evaluate the efficacy and morbidity of nonsurgical treatments for anal fissures from 1966 to August 2010. Clove oil was among 17 agents used in the 77 cited studies. While no medical therapies were found to display the efficacy of surgical sphincterotomy (or, fortunately, linked to the risk of incontinence), clove oil was identified as one of the “newer” agents demonstrating promise.9
Musculoskeletal pain
Clove oil is included among several herbal ingredients (i.e., eucalyptus oil, gaultheria oil, turpentine oil, menthol, and camphor) associated with analgesic and anti-inflammatory properties that are used in the topical spray Eezpain. Nawaz et al. showed in a prospective pilot study with 20 male and female subjects that the polyherbal formulation was efficacious in relieving mild to moderate knee and wrist joint pain.10
Laboratory studies
Just over a decade ago, Chaieb et al. assessed the antioxidant characteristics of the essential oil of clove, finding that it displayed a robust radical scavenging capacity against 2,2-diphenyl-1-picrylhydrazyl in comparison to the synthetic antioxidant tert-butylated hydroxytoluene. It also showed potent antifungal activity against 53 test strains of human pathogenic yeasts. The authors noted that clove oil is a readily available source of natural antioxidants and is a worthy ingredient in pharmaceutical products.11
Anti-inflammatory activity
In 2017, Han and Parker studied the biological activity of four concentrations of a commercially available clove essential oil product on 17 protein biomarkers important in inflammation in a model of human skin disease. They found that the 0.011% concentration of the oil enacted strong antiproliferative effects on human dermal skin fibroblasts, and significantly suppressed multiple proinflammatory biomarkers as well as tissue remodeling protein molecules. The investigators also observed that essential clove oil significantly influenced global gene expression and signaling pathways involved in inflammation, tissue remodeling, and cancer processes. They concluded that their results indicate anti-inflammatory, anticancer, and tissue-remodeling properties of clove essential oil, and its main active ingredient eugenol, in human dermal fibroblasts.1
UVB protection
Recently, Patwardhan and Bhatt assessed the capacity of flavonoids from clove buds to protect human dermal fibroblasts from UVB exposure. They found that the flavonoid-enriched fraction of clove demonstrated significant potential, as it mitigated the effects of UVB radiation, and delivered protection via the nuclear factor E2-related factor 2-antioxidant response pathway. The flavonoid-enriched clove fraction, they concluded, warrants consideration as a topically applied cutaneous protectant against the effects of UVB exposure.4
Antiviral and immunomodulatory activity
Based on their earlier work showing the antiviral activity of clove bud oil against Pseudomonas aeruginosa PAO1, Haripriyan et al. reported this year that clove bud oil affects pseudomonal proteases (elastase A, elastase B, protease IV, and alkaline protease), attenuating significant viral mechanisms of this noted human disease agent while bolstering host immunomodulatory functions. They concluded that their results suggest the viability of clove bud oil as a topical treatment for infections resistant to antibiotics.12
Acne
In 2017, Owen et al. developed a topical preparation incorporating clove bud, rosewood, and litsea essential oils that compared favorably with the topical antibiotics Dalacin T and Stiemycin in controlling acne vulgaris-linked bacteria. Specifically, the herbal formulation exhibited synergistic activity against Propionibacterium acnes, although not to Staphylococcus epidermidis, and its antimicrobial activity exceeded or equated to that of the tested antibiotics. The investigators suggested that the polyherbal preparation may serve as an option for treating acne-linked bacteria.13
Scabies
In a study 2 years ago to ascertain the efficacy of 10 essential oils against Sarcoptes scabiei, Fang et al. conducted contact bioassays and fumigation bioassays using clove, palmarosa, geranium, tea tree, lavender, Manuka, bitter orange, eucalyptus, Japanese cedar, and cade oil. In the contact bioassays, clove oil 1%, the most effective of the oils, eliminated the mites within 20 minutes. In the fumigation bioassay, clove was second to tea tree oil in efficacy. The investigators concluded that clove, tea tree, palmarosa, and eucalyptus oils demonstrate potential in pest control and for treating scabies infections in humans or animals.14
Conclusion
Clove oil is an active ingredient in various topical treatments. While not typically a first-line therapy, it shows promise for a wider range of applications. Research continues to determine the extent to which this botanical agent can reach into the dermatologic armamentarium and, more importantly, how effective it can be in treating cutaneous disorders.
Dr. Baumann is a private practice dermatologist, researcher, author and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients,” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Pharm Biol. 2017 Dec;55(1):1619-22.
2. Phytother Res. 2007 Jun;21(6):501-6.
3. Cell Prolif. 2006 Aug;39(4):241-8.
4. Pharmacogn Mag. 2015 Oct;11(Suppl 3):S397-406.
5. J Cosmet Dermatol. 2017 Dec 28. doi: 10.1111/jocd.12471.
6. Curr Drug Deliv. 2012 Mar;9(2):219-30.
7. J Cosmet Dermatol. 2017 Dec;16(4):508-11.
8. Colorectal Dis. 2007 Jul;9(6):549-52
9. Cochrane Database Syst Rev. 2012 Feb 15;(2):CD003431.
10. Pak J Pharm Sci. 2015 Jan;28(1):43-7.
11. Mycoses. 2007 Sep;50(5):403-6.
12. Sci Rep. 2018 Feb 21;8(1):3437.
13. Phytother Res. 2017 Mar;31(3):410-7.
14. Parasit Vectors. 2016 Nov 22;9(1):594.
Preventing brain damage in psychosis
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.
I read with great interest Dr. Nasrallah’s editorial, “FAST and RAPID: Acronyms to prevent brain damage in stroke and psychosis” (From the Editor,
Mitchell L. Glaser, MD
Board-Certified Child/Adolescent and General Psychiatrist
Assistant Professor of Psychiatry
Rush University Medical Center
Chairman
Department of Psychiatry
Medical Director of Child/Adolescent Psychiatry
St. Mary/Elizabeth Medical Center
Clinical Assistant Professor of Psychiatry
Rosalind Franklin University
Chicago, Illinois
Thank you, Dr. Nasrallah, for your incisive thinking and for bringing our attention as psychiatrists to the crucial issues of our clinical practice. I’d like to offer some nuance on the RAPID acronym. First, I’d like to counterpropose DASH: Delusions, Auditory hallucinations, Strange behavior, Hospital now. This is more in line with getting physicians to tune in to the symptoms that should alarm them and bring them to action. I agree that neurodegeneration and illness recurrence are the problems to address. One unsettled issue remains: With early intervention, can we eventually taper patients off antipsychotics to spare them the metabolic and immune morbidity associated with these medications? There is some evidence that this is possible, but it is difficult to collect data. One of the factors delaying treatment, other than lack of recognition, is the general public’s belief that the treatment is sometimes worse than the disease. If we can address this issue in a nuanced fashion, we may get more “early adopters” of these neuron-sparing treatments.
Michael S. Diamond, MD
Private psychiatric practice
Chevy Chase, Maryland
Dr. Nasrallah is right to focus on brain injury patterns, including inflammation and de-myelination, during psychotic episodes. He and Dr. Roque note that starting a patient on a long-acting injectable antipsychotic as soon as possible may prevent subsequent relapse and further brain damage. However, their editorial omits 2 treatments—minocycline and clemastine—that can help stop CNS inflammation, reduce brain damage, and promote remyelination.
Minocycline has been shown to reduce stroke infarct penumbra size and improve outcomes in functional recovery from stroke.1,2 Minocycline’s effects as a potent CNS anti-inflammatory and antiapoptotic agent are well established.
Clemastine has been shown to improve function in multiple sclerosis by activating oligodendrocyte precursor cells into active agents of myelination and fiber bundle stabilization.3 Clemastine reverses acute leukoencephalopathy.4
If we are to treat acute psychosis as a neurologic emergency, we cannot rely on long-acting injectable antipsychotics as the sole treatment. Psychiatric medication alone is not sufficient across every neuropsychiatric condition in which inflammation and white matter damage are part of the etiology, destruction, and pattern of relapse.
The adverse effects risk of adjunctive minocycline and clemastine is low compared with the potential benefits of stopping inflammation, reducing apoptosis, and jump-starting white matter repair. Doses of oral minocycline in the 50- to 100-mg/d range and oral clemastine in the 1.34- to 2.68-mg/d range together can lead to reduced cranial heat, improved cranial suture mobility, and improved elasticity of white matter bundle tracts palpable on physical examination. Both medications show clinical results in improved emotional self-regulation, according to family reports and clinical observations in the outpatient setting. There is no reason to delay neurologic-based adjunctive treatment when our goal is to prevent and reverse brain damage.
Daniel Kerlinsky, MD
Child Psychiatrist
Clinical Assistant Professor
Burrell College of Osteopathic Medicine
Albuquerque, New Mexico
References
1. Hess DH, Fagan SC. Repurposing an old drug to improve the use and safety of tissue plasminogen activator for acute ischemic stroke: minocycline. Rev Neurol Dis. 2010;30(7 pt 2):55S-61S.
2. Vedantam S, Moller AR. Minocycline: a novel stroke therapy. J Neurol Stroke.
3. Green AJ, Gelfand JM, Cree BA, et al. Clemastine fumarate as a remyelinating therapy for multiple sclerosis (ReBUILD): a randomised, controlled, double-blind, crossover trial. Lancet. 2017;390(10111):2481-2489.
4. Cree BAC, Niu J, Hoi KK, et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 2018;141(1):85-98.
Continue to: Dr. Nasrallah responds
Dr. Nasrallah responds
Thanks to my colleagues, Drs. Diamond, Glaser, and Kerlinsky, for their cogent letters about my editorial.
To Dr. Glaser: The “ethics” of conducting placebo-controlled studies when developing a new antipsychotic has been raging for some time. For decades, the FDA has insisted on using a placebo group because around 25% to 30% of research participants respond to placebo, and because participants receiving placebo also complain of many adverse effects. So a new drug has to demonstrate a statistically higher efficacy than a placebo, and the adverse effect profile of the placebo group will put the safety and tolerability profile of a new drug in proper perspective. However, in Europe, they do not conduct placebo-controlled studies; instead, they conduct what is called a “non-inferiority” trial of a new antipsychotic compared with a well-established antipsychotic.
Interestingly, even though the discovery of the neurodegenerative effects of untreated psychosis was only 20 years ago (in 1997 after serial MRI scans revealed progressive atrophy), in the 1960s, the first antipsychotic, chlorpromazine, was compared with placebo in a large national study for 6 months. This study showed without a doubt that chlorpromazine has a higher efficacy than placebo. After the study was done, Dr. Philip May at University of California, Los Angeles looked at what happened to the psychotic patients who received placebo for 6 months and found that they became less responsive to treatment, were re-hospitalized more often, and had more negative symptoms and a poorer overall outcome. That was a clue that untreated psychosis can be harmful, and it supports your point about the ethics of using placebo. In contemporary studies, a trial of oral antipsychotics is 6 weeks, not 6 months. In the year-long, placebo-controlled studies of injectable antipsychotics in stable patients, those who show the slightest increase in delusions, hallucinations, or suicidal/homicidal behavior were promptly taken out of the study and treated. This reduced the “harm,” although not completely. Perhaps the FDA will change its policies and adopt the non-inferiority model. That’s what is done with nonpsychiatric disorders such as pneumonia, stroke, or diabetes. But one last fact has to be stated: The placebo response in anxiety, depression, or psychosis is much higher (25% to 35%) than the 1% placebo response in pneumonia.
To Dr. Diamond: I really like DASH, and it is an acronym for quick symptomatic diagnosis. Speedy treatment then follows with the acronym RAPID to prevent brain damage that gets worse with delay.
As for the second issue of tapering off the antipsychotic medication, the evidence is overwhelming in favor of continuous pharmacotherapy. Just as hypertension and diabetes will return if medications are tapered or stopped, so will psychosis, and vengefully so because treatment resistance increases with each relapse.1 This is also true for bipolar disorder recurrences.2 A recent 20-year follow-up study showed that stopping antipsychotic treatment is associated with a much higher mortality rate than continuation therapy.3 Another 7-year study showed the same thing.4 It is literally deadly, and not just neurodegenerative, for persons with schizophrenia to stop their medications.
To Dr. Kerlinsky: I agree with you about using certain adjunctive pharmacotherapies for acute psychosis, which is associated with neuroinflammation, oxidative stress, and neuropil and myelin damage. I support using agents with anti-inflammatory effects (such as minocycline and omega-3 fatty acid), antioxidant effects (such as N-acetylcysteine), and neuroprotective effects (such as minocycline, clemastine, lithium, vitamin D, erythropoietin, etc.). I refer you to my past editorial, “Are you neuroprotecting your patients? 10 Adjunctive therapies to consider,”5 in which I mentioned all the above. I also pointed out the many neuroprotective effects of atypical antipsychotics in another editorial.6 Although off-label, those supplements can be useful interventions that can ameliorate the gray and white matter damage associated with acute psychotic relapses in patients with schizophrenia.
Henry A. Nasrallah, MD
Editor-in-Chief
The Sydney W. Souers Endowed Chair
Professor and Chairman
Department of Psychiatry and Behavioral Neuroscience
Saint Louis University School of Medicine
St. Louis, Missouri
References
1. Emsley R, Oosthuizen P, Koen L, et al. Comparison of treatment response in second-episode versus first-episode schizophrenia. J Clin Psychopharmacol. 2013;33(1):80-83.
2. Post RM. Preventing the maligna
3. Tiihonen J, Tanskanen A, Taipale H. 20-year nationwide follow-up study on discontinuation of antipsychotic treatment in first-episode schizophrenia. Am J Psychiatry. 2018;175(8):765-773.
4. Taipale H, Mittendorfer-Rutz E, Alexanderson K, et al. Antipsychotics and mortality in a nationwide cohort of 29,823 patients with schizophrenia. Schizophr Res. 2018;197:274-280.
5. Nasrallah HA. Are you neuroprotecting your patients? 10 Adjunctive therapies to consider. Current Psychiatry. 2016;15(12):12-14.
6. Nasrallah HA. A decade after the CATIE study, the focus has shifted from effectiveness to neuroprotection. Current Psychiatry. 2015;14(2):19-21.