User login
Short bursts of activity may cut cancer risk
, a new study published in JAMA Oncology says.
Researchers at the University of Sydney studied data from wearable fitness devices worn by more than 22,000 “non-exercisers,” then examined their health records for 6 or 7 years.
The scientists found that people who did 4-5 minutes of “vigorous intermittent lifestyle physical activity” (VILPA) had a “substantially” lower cancer risk than people who did no VILPA.
Examples of VILPA are vigorous housework, carrying heavy shopping bags around the grocery store, bursts of power walking, and playing high-energy games with children. The activities could occur in 1-minute bursts, instead of all at once.

The study found that a minimum of around 3.5 minutes of daily VILPA was linked to an 18% reduction in cancer rates, compared with no VILPA. The study said 4.5 minutes of daily VILPA was linked to a 32% reduction in cancers related to physical activity, including lung, kidney, bladder, and stomach cancers.
“We know the majority of middle-aged people don’t regularly exercise, which puts them at increased cancer risk, but it’s only through the advent of wearable technology like activity trackers that we are able to look at the impact of short bursts of incidental physical activity done as part of daily living,” Emmanuel Stamatakis, PhD, the lead author of the study and a professor at the University of Sydney’s Charles Perkins Centre, said in a news release.
Study participants had an average age of 62 and reported that they didn’t exercise in their spare time. VILPA, a concept coined by researchers at the university, was measured by wrist accelerometers that people in the study wore over 7 days at the start of the study, the news release said.
“We are just starting to glimpse the potential of wearable technology to track physical activity and understand how unexplored aspects of our lives affect our long-term health – the potential impact on cancer prevention and a host of other health outcomes is enormous,” Dr. Stamatakis said.
A version of this article first appeared on WebMD.com.
, a new study published in JAMA Oncology says.
Researchers at the University of Sydney studied data from wearable fitness devices worn by more than 22,000 “non-exercisers,” then examined their health records for 6 or 7 years.
The scientists found that people who did 4-5 minutes of “vigorous intermittent lifestyle physical activity” (VILPA) had a “substantially” lower cancer risk than people who did no VILPA.
Examples of VILPA are vigorous housework, carrying heavy shopping bags around the grocery store, bursts of power walking, and playing high-energy games with children. The activities could occur in 1-minute bursts, instead of all at once.

The study found that a minimum of around 3.5 minutes of daily VILPA was linked to an 18% reduction in cancer rates, compared with no VILPA. The study said 4.5 minutes of daily VILPA was linked to a 32% reduction in cancers related to physical activity, including lung, kidney, bladder, and stomach cancers.
“We know the majority of middle-aged people don’t regularly exercise, which puts them at increased cancer risk, but it’s only through the advent of wearable technology like activity trackers that we are able to look at the impact of short bursts of incidental physical activity done as part of daily living,” Emmanuel Stamatakis, PhD, the lead author of the study and a professor at the University of Sydney’s Charles Perkins Centre, said in a news release.
Study participants had an average age of 62 and reported that they didn’t exercise in their spare time. VILPA, a concept coined by researchers at the university, was measured by wrist accelerometers that people in the study wore over 7 days at the start of the study, the news release said.
“We are just starting to glimpse the potential of wearable technology to track physical activity and understand how unexplored aspects of our lives affect our long-term health – the potential impact on cancer prevention and a host of other health outcomes is enormous,” Dr. Stamatakis said.
A version of this article first appeared on WebMD.com.
, a new study published in JAMA Oncology says.
Researchers at the University of Sydney studied data from wearable fitness devices worn by more than 22,000 “non-exercisers,” then examined their health records for 6 or 7 years.
The scientists found that people who did 4-5 minutes of “vigorous intermittent lifestyle physical activity” (VILPA) had a “substantially” lower cancer risk than people who did no VILPA.
Examples of VILPA are vigorous housework, carrying heavy shopping bags around the grocery store, bursts of power walking, and playing high-energy games with children. The activities could occur in 1-minute bursts, instead of all at once.

The study found that a minimum of around 3.5 minutes of daily VILPA was linked to an 18% reduction in cancer rates, compared with no VILPA. The study said 4.5 minutes of daily VILPA was linked to a 32% reduction in cancers related to physical activity, including lung, kidney, bladder, and stomach cancers.
“We know the majority of middle-aged people don’t regularly exercise, which puts them at increased cancer risk, but it’s only through the advent of wearable technology like activity trackers that we are able to look at the impact of short bursts of incidental physical activity done as part of daily living,” Emmanuel Stamatakis, PhD, the lead author of the study and a professor at the University of Sydney’s Charles Perkins Centre, said in a news release.
Study participants had an average age of 62 and reported that they didn’t exercise in their spare time. VILPA, a concept coined by researchers at the university, was measured by wrist accelerometers that people in the study wore over 7 days at the start of the study, the news release said.
“We are just starting to glimpse the potential of wearable technology to track physical activity and understand how unexplored aspects of our lives affect our long-term health – the potential impact on cancer prevention and a host of other health outcomes is enormous,” Dr. Stamatakis said.
A version of this article first appeared on WebMD.com.
FROM JAMA ONCOLOGY
MRI-guided SBRT cuts radiation toxicity in prostate cancer
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
TOPLINE
The use of magnetic resonance–guided daily adaptive stereotactic body radiotherapy for patients with prostate cancer reduces the risk of acute urinary side effects of grade 2 or higher by 44% and the risk of acute bowel side effects of grade 2 or higher by 60%, compared with standard CT-guided SBRT (CT‐SBRT).
METHODOLOGY
- With the use of magnetic resonance–guided daily adaptive SBRT, clinicians can customize radiation dosing to accommodate changes in prostate anatomy during treatment, which may also make SBRT safer and less toxic for patients.
- To determine whether this approach does reduce patient side effects, investigators ran a meta-analysis that included 29 studies with 2547 patients comparing the incidence of short-term, physician-assessed bowel and genitourinary side effects between the MRI-guided approach and standard CT-SBRT.
- The investigators reported no statistically significant differences in age, prescribed radiation doses, planning target volumes, or International Prostatism Symptom Scores between the two groups; the use of rectal spacers and the number of patients who received pelvic lymph node radiation were low in both.
- The average window for collecting acute toxicity data was 70 days in the MRI-guided investigations and 94 days in CT-SBRT investigations.
TAKEAWAY
- (odds ratio, 0.56; P = .04).
- The pooled estimate for grade 2 or higher gastrointestinal toxicity was 4% with the MRI approach versus 9% with CT-SBRT (OR, 0.40; P = .04).
- There were no differences in grade 3 or higher events, which were rare, between the groups.
- There was also no difference in toxicity among CT‐SBRT studies that used fiducial markers and those that did not.
IN PRACTICE
“These findings suggest that the technical advantages in precision of radiotherapy delivery afforded by [MRI-guided] SBRT translate to measurable clinical benefit,” the authors concluded. Potential reasons for the reduced risk of acute toxicity with the MRI-guided approach include “daily online adaptive planning, MRI‐based contouring that results in smaller treatment volumes, and MRI tracking, all of which may facilitate the precision and accuracy of treatment delivery.”
SOURCE
The study was led by Jonathan Leeman, MD, of the Dana-Farber Cancer Institute, Boston, and was published July 24 in Cancer.
LIMITATIONS
- The analysis did not account for differences in dosimetry, radiation planning, and toxicity management and assessment between the studies.
- Late toxicity and cancer control rates were not tracked and may have differed between the two approaches.
DISCLOSURES
- No external funding was reported.
- The investigators reported grants and consulting, personal, and other payments from Novartis, AstraZeneca, Janssen, and other companies.
A version of this article appeared on Medscape.com.
FROM CANCER
‘Treatment holiday’ in prostate cancer with tailored dosing
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
and improve patient outcomes, new research suggests.
The findings indicate that implementing a personalized dosing strategy with the radioligand therapy “allowed for treatment holidays in excellent responders, continuous 6-weekly treatments in moderate responders, and [allowed us] to consider changing or adding treatment in limited responders,” said study author Andrew Nguyen, MBBS, FRACP, AANMS, senior staff specialist in the department of theranostics and nuclear medicine at St. Vincent’s Hospital in Sydney.
The research was presented at the annual meeting of the Society of Nuclear Medicine and Molecular Imaging.
Although clinical trials have demonstrated that 177Lu-PSMA is an effective treatment for metastatic castration-resistant prostate cancer, the question remains: Can patient outcomes be improved through the use of biomarkers and by escalating or deescalating treatment as appropriate? asked Dr. Nguyen, who presented the findings at the meeting.
Clinical trials use standardized dosing intervals. Adjusting treatment intervals through the use of early-biomarker responses could give some patients a break from treatment and improve overall survival outcomes, Dr. Nguyen explained. For example, the 2021 REALITY study showed that overall survival was significantly better for patients who received 177Lu-PSMA plus standard care, compared with patients who received standard care alone (median, 15.3 vs. 11.3 months), and that overall survival was better among patients with early prostate-specific antigen (PSA) responses.
In the current study, Dr. Nguyen and colleagues used composite early biomarkers of PSA, imaging with 177Lu-PSMA SPECT, and diagnostic CT to guide a personalized dosing interval strategy for patients with metastatic castration-resistant prostate cancer receiving 177Lu-PSMA. The team evaluated progression-free survival and overall survival among these patients to determine whether personalizing dosing on the basis of early biomarker levels was associated with survival outcomes.
The cohort included 125 men who received six weekly doses of 177Lu-PSMA and who underwent imaging with 177Lu-SPECT/CT after each dose. After the second dose, investigators used the composite of PSA and 177Lu SPECT/CT response to determine which patients had a partial response, which had stable disease, and which had progressive disease.
The men were divided into three groups on the basis of their level of response. Group 1, which included 35% of participants, achieved a significant reduction in PSA levels and a partial response on 177Lu-SPECT. These patients were advised to discontinue treatment until PSA levels increased. This treatment holiday lasted a median of about 6 months.
Group 2, which represented 34% of the cohort, had stable or reduced PSA levels as well as stable disease on SPECT imaging. For these patients, the treatment regimen continued.
Group 3 demonstrated rising PSA levels and progressive disease on SPECT imaging. These men were offered an alternative therapy.
Overall, median PSA progression-free survival was 12.1 months in group 1, 6.1 months in group 2, and 2.6 months in group 3. Median overall survival was also significantly better among patients who showed early responses to therapy: 19.2 months in group 1, 13.2 months in group 2, and 11. 2 months in group 3.
Dr. Nguyen noted several limitations to the findings, including the study’s retrospective nature and the fact that some patients in group 1 chose not to resume further treatment after their PSA levels rose.
“Personalizing dosing intervals using early-response biomarkers with 177Lu-PSMA has the potential to achieve similar overall treatment responses to that published for continuous dosing, while allowing treatment holidays in responders and early crossover to potentially more effective therapies in nonresponders,” the authors conclude.
Given the effectiveness of this strategy, Dr. Nguyen says his team “now routinely uses these composite biomarkers when treating clinical patients.”
A version of this article appeared on Medscape.com.
FROM SNMMI 2023
Cancer Patients: Who’s at Risk for Venous Thromboembolism?
Patients with cancer are at a high risk of venous thromboembolism (VTE)—in fact, it’s one of the leading causes of death in patients who receive systemic therapy for cancer. But as cancer treatment has evolved, have the incidence and risk of VTE changed too?
Researchers from Veterans Affairs Boston Healthcare System in Massachusetts conducted a study with 434,203 veterans to evaluate the pattern of VTE incidence over 16 years, focusing on the types of cancer, treatment, race and ethnicity, and other factors related to cancer-associated thrombosis (CAT).
In contrast with other large population studies, this study found the overall incidence of CAT remained largely stable over time. At 12 months, the incidence was 4.5%, with yearly trends ranging between 4.2% and 4.7%. “As expected,” the researchers say, the subset of patients receiving systemic therapy had a higher incidence of VTE at 12 months (7.7%) than did the overall cohort. The pattern was “particularly pronounced” in gynecologic, testicular, and kidney cancers, where the incidence of VTE was 2 to 3 times higher in the treated cohort compared with the overall cohort.
Cancer type and diagnosis were the most statistically and clinically significant associations with CAT, with up to a 6-fold difference between cancer subtypes. The patients at the highest risk of VTE were those with pancreatic cancer and acute lymphoblastic leukemia.
Most studies have focused only on patients with solid tumors, but these researchers observed novel patterns among patients with hematologic neoplasms. Specifically, a higher incidence of VTE among patients with aggressive vs indolent leukemias and lymphomas. This trend, the researchers say, may be associated in part with catheter-related events.
Furthermore, the type of system treatment was associated with the risk of VTE, the researchers say, although to a lesser extent. Chemotherapy- and immunotherapy-based regimens had the highest risk of VTE, relative to no treatment. Targeted and endocrine therapy also carried a higher risk compared with no treatment but to a lesser degree.
The researchers found significant heterogeneity by race and ethnicity across cancer types. Non-Hispanic Black patients had about 20% higher risk of VTE compared with non-Hispanic White patients. Asian and Pacific Islander patients had about 20% lower risk compared with non-Hispanic White patients.
Male sex was also associated with VTE. However, “interestingly,” the researchers note, neighborhood-level socioeconomic factors and patients’ comorbidities were not associated with CAT but were associated with mortality.
Their results suggest that patient- and treatment-specific factors play a critical role in assessing the risk of CAT, and “ongoing efforts to identify these patterns are of utmost importance for risk stratification and prognostic assessment.”
Patients with cancer are at a high risk of venous thromboembolism (VTE)—in fact, it’s one of the leading causes of death in patients who receive systemic therapy for cancer. But as cancer treatment has evolved, have the incidence and risk of VTE changed too?
Researchers from Veterans Affairs Boston Healthcare System in Massachusetts conducted a study with 434,203 veterans to evaluate the pattern of VTE incidence over 16 years, focusing on the types of cancer, treatment, race and ethnicity, and other factors related to cancer-associated thrombosis (CAT).
In contrast with other large population studies, this study found the overall incidence of CAT remained largely stable over time. At 12 months, the incidence was 4.5%, with yearly trends ranging between 4.2% and 4.7%. “As expected,” the researchers say, the subset of patients receiving systemic therapy had a higher incidence of VTE at 12 months (7.7%) than did the overall cohort. The pattern was “particularly pronounced” in gynecologic, testicular, and kidney cancers, where the incidence of VTE was 2 to 3 times higher in the treated cohort compared with the overall cohort.
Cancer type and diagnosis were the most statistically and clinically significant associations with CAT, with up to a 6-fold difference between cancer subtypes. The patients at the highest risk of VTE were those with pancreatic cancer and acute lymphoblastic leukemia.
Most studies have focused only on patients with solid tumors, but these researchers observed novel patterns among patients with hematologic neoplasms. Specifically, a higher incidence of VTE among patients with aggressive vs indolent leukemias and lymphomas. This trend, the researchers say, may be associated in part with catheter-related events.
Furthermore, the type of system treatment was associated with the risk of VTE, the researchers say, although to a lesser extent. Chemotherapy- and immunotherapy-based regimens had the highest risk of VTE, relative to no treatment. Targeted and endocrine therapy also carried a higher risk compared with no treatment but to a lesser degree.
The researchers found significant heterogeneity by race and ethnicity across cancer types. Non-Hispanic Black patients had about 20% higher risk of VTE compared with non-Hispanic White patients. Asian and Pacific Islander patients had about 20% lower risk compared with non-Hispanic White patients.
Male sex was also associated with VTE. However, “interestingly,” the researchers note, neighborhood-level socioeconomic factors and patients’ comorbidities were not associated with CAT but were associated with mortality.
Their results suggest that patient- and treatment-specific factors play a critical role in assessing the risk of CAT, and “ongoing efforts to identify these patterns are of utmost importance for risk stratification and prognostic assessment.”
Patients with cancer are at a high risk of venous thromboembolism (VTE)—in fact, it’s one of the leading causes of death in patients who receive systemic therapy for cancer. But as cancer treatment has evolved, have the incidence and risk of VTE changed too?
Researchers from Veterans Affairs Boston Healthcare System in Massachusetts conducted a study with 434,203 veterans to evaluate the pattern of VTE incidence over 16 years, focusing on the types of cancer, treatment, race and ethnicity, and other factors related to cancer-associated thrombosis (CAT).
In contrast with other large population studies, this study found the overall incidence of CAT remained largely stable over time. At 12 months, the incidence was 4.5%, with yearly trends ranging between 4.2% and 4.7%. “As expected,” the researchers say, the subset of patients receiving systemic therapy had a higher incidence of VTE at 12 months (7.7%) than did the overall cohort. The pattern was “particularly pronounced” in gynecologic, testicular, and kidney cancers, where the incidence of VTE was 2 to 3 times higher in the treated cohort compared with the overall cohort.
Cancer type and diagnosis were the most statistically and clinically significant associations with CAT, with up to a 6-fold difference between cancer subtypes. The patients at the highest risk of VTE were those with pancreatic cancer and acute lymphoblastic leukemia.
Most studies have focused only on patients with solid tumors, but these researchers observed novel patterns among patients with hematologic neoplasms. Specifically, a higher incidence of VTE among patients with aggressive vs indolent leukemias and lymphomas. This trend, the researchers say, may be associated in part with catheter-related events.
Furthermore, the type of system treatment was associated with the risk of VTE, the researchers say, although to a lesser extent. Chemotherapy- and immunotherapy-based regimens had the highest risk of VTE, relative to no treatment. Targeted and endocrine therapy also carried a higher risk compared with no treatment but to a lesser degree.
The researchers found significant heterogeneity by race and ethnicity across cancer types. Non-Hispanic Black patients had about 20% higher risk of VTE compared with non-Hispanic White patients. Asian and Pacific Islander patients had about 20% lower risk compared with non-Hispanic White patients.
Male sex was also associated with VTE. However, “interestingly,” the researchers note, neighborhood-level socioeconomic factors and patients’ comorbidities were not associated with CAT but were associated with mortality.
Their results suggest that patient- and treatment-specific factors play a critical role in assessing the risk of CAT, and “ongoing efforts to identify these patterns are of utmost importance for risk stratification and prognostic assessment.”
No benefit to adding limited radiation in advanced cancer
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
Cancer Data Trends 2023
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
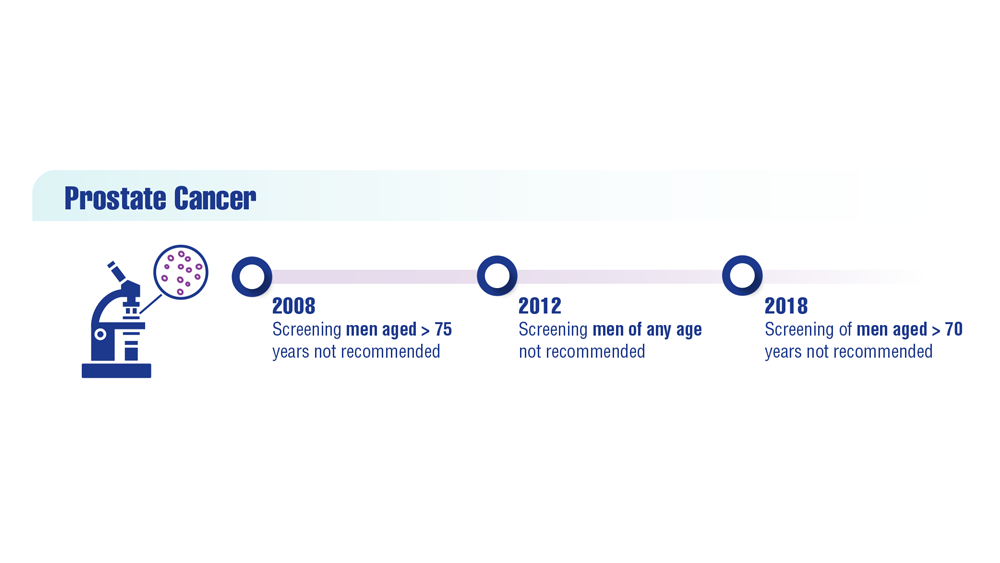
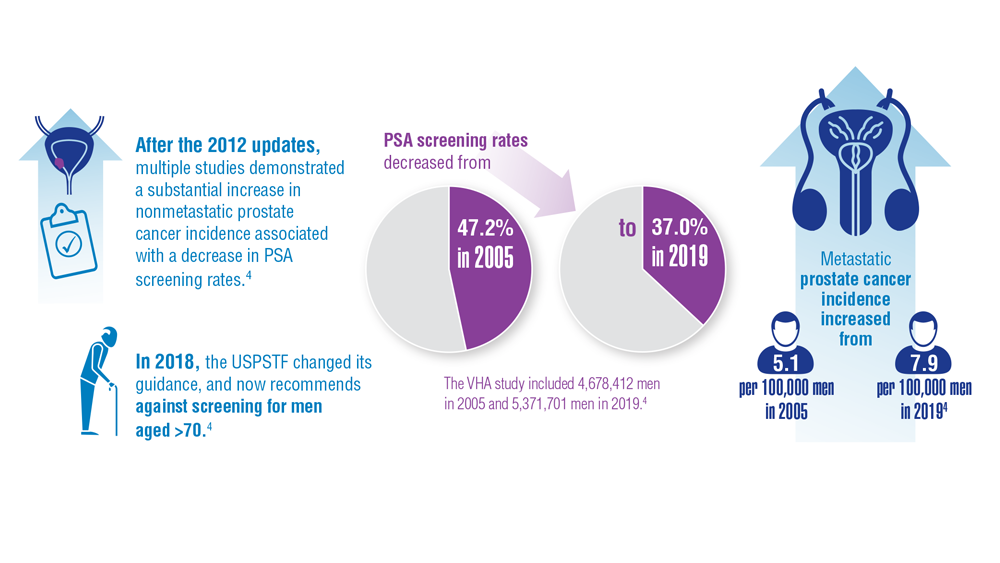
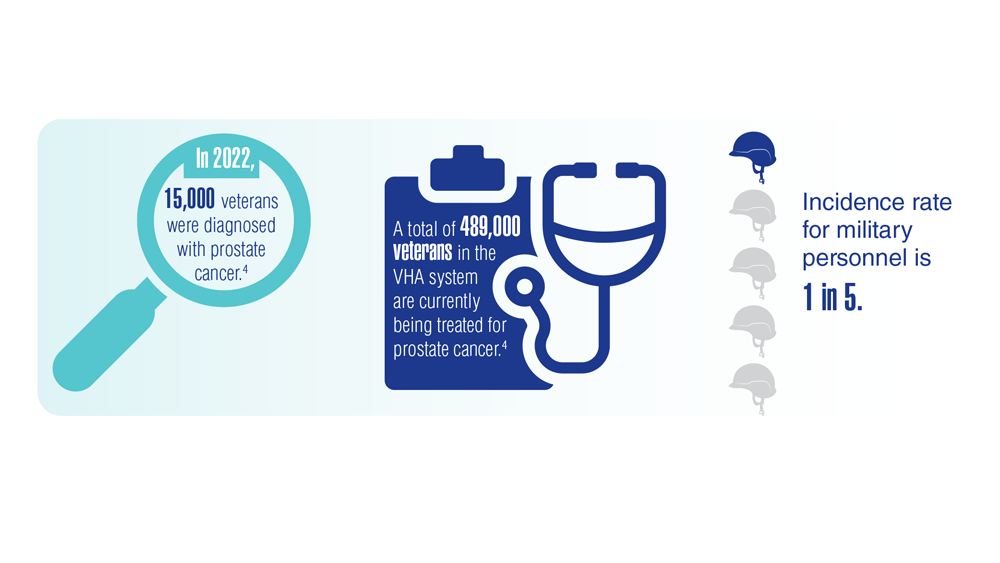
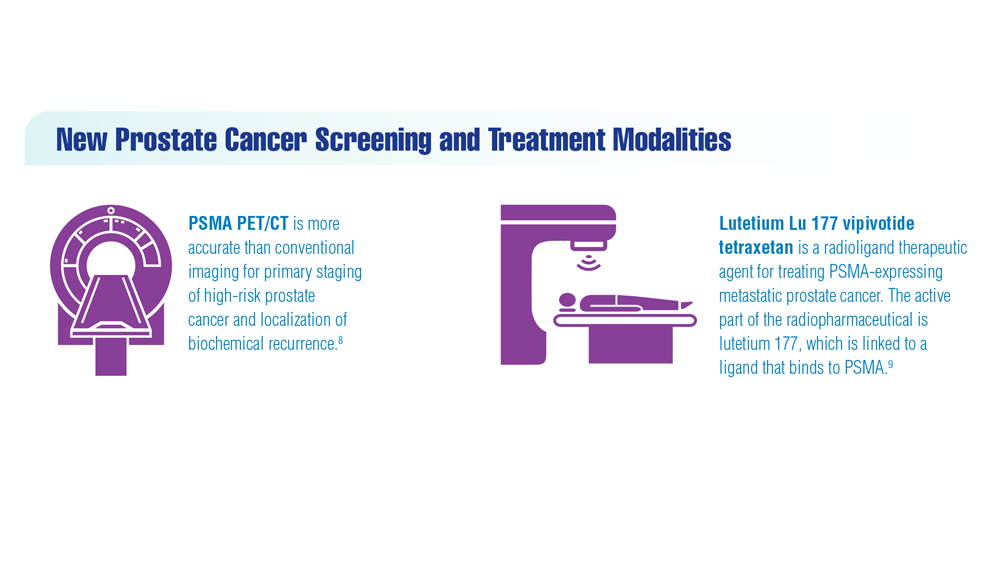
Promising New Approaches for Testicular and Prostate Cancer
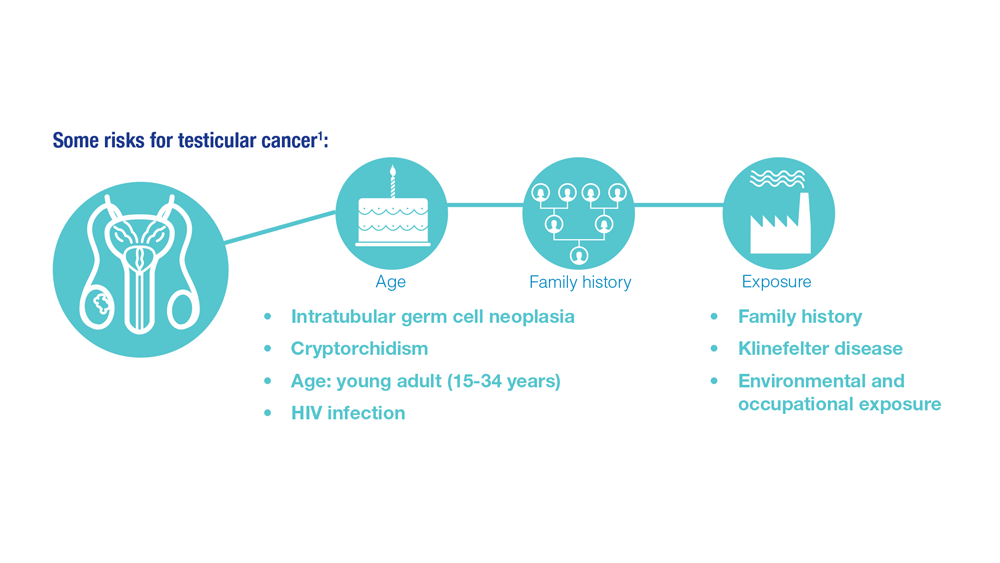
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
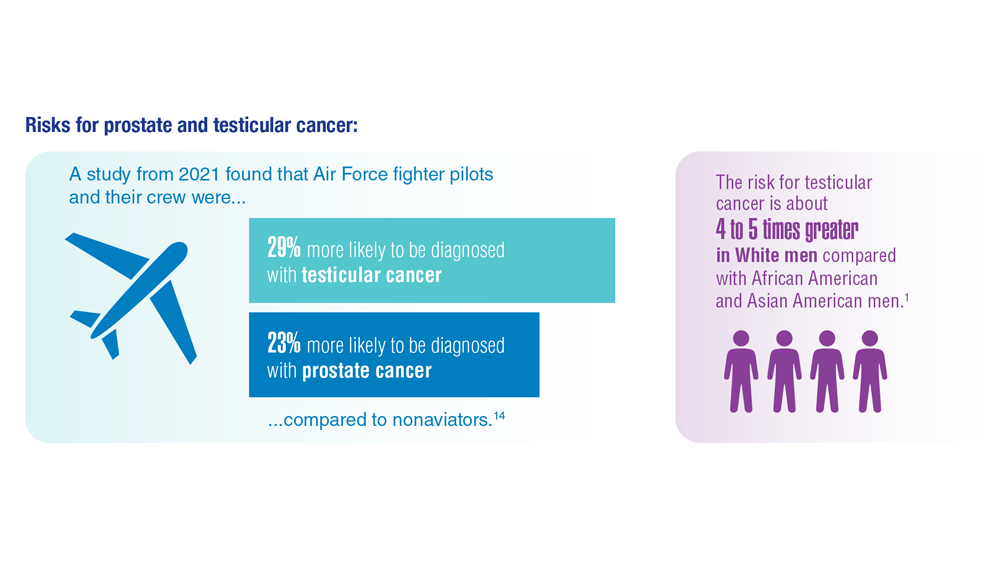
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
- Risk factors for testicular cancer. American Cancer Society. Updated May 17, 2018. Accessed December 15, 2022. https://www.cancer.org/cancer/testicular-cancer/causes-risks-prevention/risk-factors.html
- Chovanec M, Cheng L. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Tavares NT et al. J Pathol. 2022. doi:10.1002/path.6037
- Bryant AK et al. JAMA Oncol. 2022;e224319. doi:10.1001/jamaoncol.2022.4319
- Kabasakal L et al. Nucl Med Commun. 2017;38(2):149-155. doi:10.1097/MNM.0000000000000617
- Sartor O et al; VISION Investigators. N Engl J Med. 2021;385(12):1091-1103. doi:10.1056/NEJMoa2107322
- Rowe SP et al. Annu Rev Med. 2019;70:461-477. doi:10.1146/annurev-med-062117-073027
- Pomykala KL et al. Eur Urol Oncol. 2022;S2588-9311(22)00177-8. doi:10.1016/j.euo.2022.10.007
- Keam SJ. Mol Diagn Ther. 2022;26(4):467-475. doi:10.1007/s40291-022-00594-2
- Lovejoy LA et al. Mil Med. 2022:usac297. doi:10.1093/milmed/usac297
- Smith ZL et al. Med Clin North Am. 2018;102(2):251-264. doi:10.1016/j.mcna.2017.10.003
- Hohnloser JH et al. Eur J Med Res.1996;1(11):509-514.
- Johns Hopkins Medicine website. Testicular Cancer tumor Markers. Accessed December 2022. https://www.hopkinsmedicine.org/health/conditions-and-diseases/testicular-cancer/testicular-cancer-tumor-markers
- Webber BJ et al. J Occup Environ Med. 2022;64(1):71-78. doi:10.1097/JOM.0000000000002353
Progress in Treating Testicular Cancer
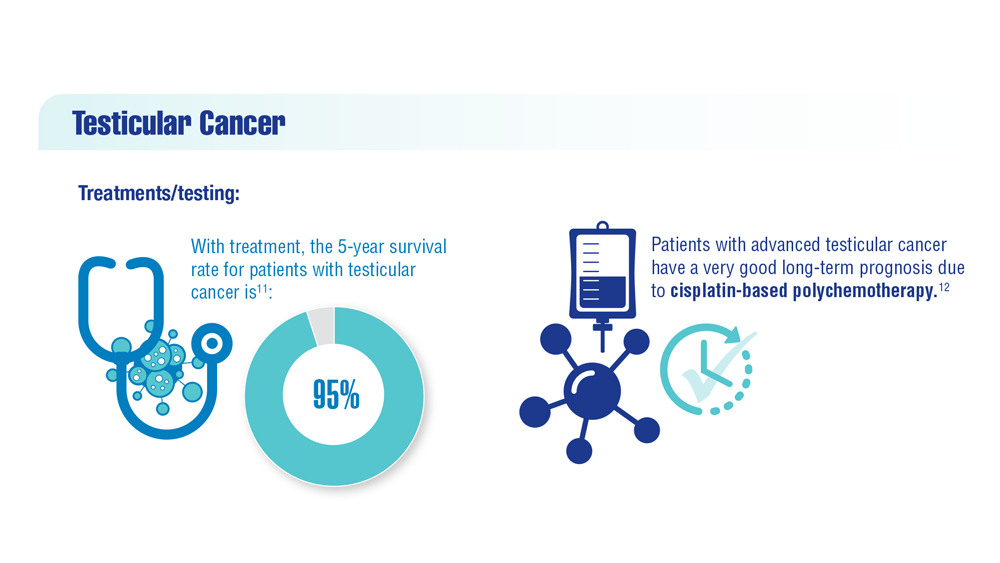
Mortality from TC has been decreasing since the 1970s due to cisplatin-based chemotherapy regimens2,3; TC is among the most curable of solid neoplasms, with a 5-year relative survival rate of 95%.2-4 Thus, the focus of research has shifted from optimizing treatments for improved survival to decreasing treatment-related, long-term adverse events (AEs).5
New Modifications in Risk Assessment and Prognostication
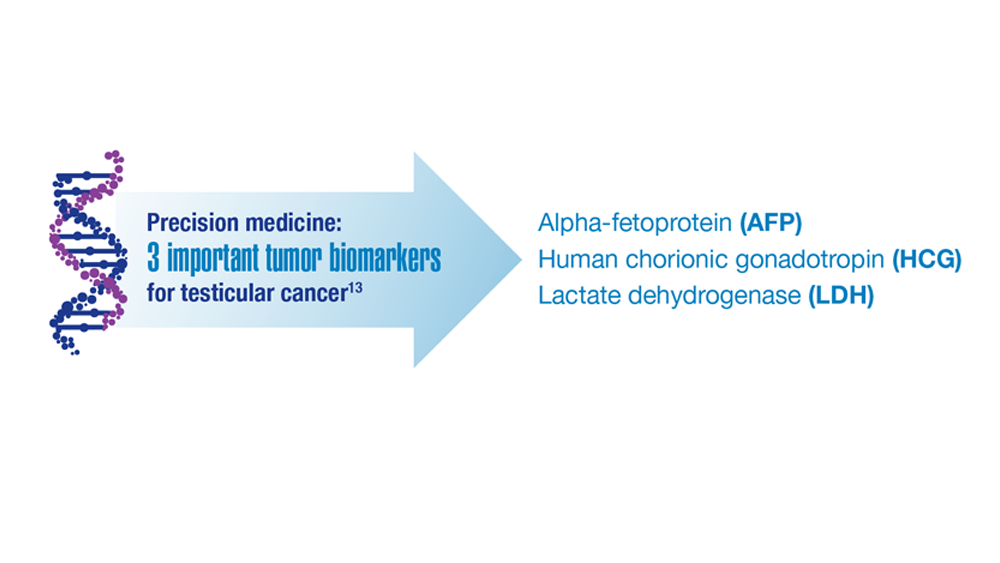
The widely accepted risk stratification model in use today was first developed in 1997 by the International Germ Cell Cancer Collaborative Group (IGCCCG) after studying data on patients with seminoma and NSGCTs.6 The original classification categorized metastatic NSGCTs as having good, intermediate, or poor prognosis based on levels of alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), lactate dehydrogenase (LDH), and the presence of nonpulmonary visceral metastases (NPVM). Primary mediastinal NSGCTs were classified as having poor prognosis regardless of the other factors.6 Metastatic seminoma GCTs were categorized as having good or intermediate prognosis based on the occurrence of brain, liver, or bone metastasis.7
Using contemporary data from more than 12,000 patients with metastatic GCTs who received either cisplatin or etoposide, the IGCCCG model was updated in 2021. For seminoma GCTs, 5-year progression-free survival (PFS) and 5-year overall survival (OS) were extended for both good and intermediate prognostic groups.7 LDH remained the most significant prognostic factor for determining good prognosis however, patients with LDH above 2.5× upper limit of normal (ULN) before chemotherapy had worse survival probabilities than patients with LDH at 2.5× ULN or lower. The survival probabilities for patients with otherwise good prognosis with LDH of more than 2.5× ULN were like those for patients with intermediate prognosis.7 Thus, using LDH of more than 2.5× ULN has revealed a subgroup with significantly worse outcomes within the “good” prognostic group.7,8
For NSGCTs, 5-year PFS rates did not differ from the original IGCCCG for good and intermediate prognostic groups; however, the 2021 update revealed an improved PFS for the poor prognostic group. The 2021 update also demonstrated that 5-year OS rates improved for each group, and further confirmed that the 2 most important prognostic factors for NSGCT were the presence of NPVM and the presence of a mediastinal primary tumor. The update added 2 new adverse prognostic variables: age and metastases. Risk of progression increases 25% with every decade-of-life increase, and 66% with the presence of lung metastases. The LDH groups were reduced to a single cutoff at 2.5× ULN for NSGCTs.8
Primary and Subsequent Treatments for TC
Guideline-directed first-line and subsequent treatments for seminomas and NSGCTs have been developed by several organizations, including the National Comprehensive Cancer Network, IGCCCG, and the American Urological Association (see Figure 1 and 2). An analysis of the most used treatments was performed using the National Cancer Database.2 Most patients underwent orchiectomy without chemotherapy or radiation for both stage I seminomas (78%) and NSGCTs (57%). For stage II and III seminomas, most patients underwent surgery with chemotherapy (66% and 68%, respectively). Nearly half of patients with stage II NSGCTs were treated with surgery and chemotherapy (49%), and a third were treated with retroperitoneal lymph node dissection (RPLND) in addition to surgery and chemotherapy. Surgery with chemotherapy was used for 55% of stage III NSGCTs; other treatments included surgery combined with chemotherapy and RPLND (19%), and chemotherapy with or without radiation (20%).2 However, nearly 30% of patients with TC do not receive guideline-directed therapy, including inappropriate imaging and overtreatment; and nonguideline–directed therapy has been independently associated with risk of relapse.12,13
TC Survivorship
The trend of improved OS after treatment for metastatic GCTs highlights a need to focus on survivorship. The 10-year survival rate for TC post-treatment is 95%.14 Latest estimates suggest there are more than 300,000 TC survivors in the United States,2 accounting for approximately 4% of all US male cancer survivors.14 With longer-term survival, however, comes the risk for long-term complications from cancer treatments. For example, circulating platinum has been detected in the plasma of men up to 28 years after undergoing cisplatin-based chemotherapy for TC.15 Increasing levels of residual serum platinum have also been shown to correlate with severity of neurotoxicity between 5 and 20 years after treatment.16
A significant concern with cancer treatment is the development of second malignant neoplasms (SMNs).14,17 The relative risk of the development of SMNs depends on whetherradiation therapy or chemotherapy, or both, was used as the primary treatment. Patients who received either radiation therapy or chemotherapy are at increased risk for leukemia and solid cancers, including gastrointestinal cancers. For patients treated with cisplatin, a significant dose-response relationship between cumulative dose and leukemic risk has been reported.14
Other concerns are increased non-TC mortality and SMN mortality. Hellesnes et al examined cause-specific, non-TC mortality using a population-based cohort in Norway.18 They determined that the overall 25-year, non-TC mortality risk was 13.7% (95% CI, 12.5-14.9) for patients who previously had TC vs 11.3% for patients who never had TC. The highest mortality rates were reported for patients who had radiation (19%) or platinum-based chemotherapy plus radiation (18.4%); the lowest mortality rate was reported for patients who had received platinum-based chemotherapy only (9.5%). Patients with the highest non-TC mortality risk were fewer than 20 years post-cancer diagnosis. Non-TC mortality excess ranged from 23% to 40% for patients with a prior TC diagnosis, and a significant 1.43- to 3.24-fold increase in SMN mortality emerged after treatment with platinum-based chemotherapy or radiation therapy, or both.19 Awareness of the increased premature mortality risk is crucial for both TC survivors and their care providers.18
Quality of life for TC survivors appears to be affected by the presence of long-term treatment-related AEs.18 The relative risk of developing cardiovascular disease increases after treatment with chemotherapy. Raynaud phenomenon resulting from bleomycin-induced vascular damage developed within 4 to 12 months after chemotherapy for 18.7% to 39% of TC survivors.14,19 Bleomycin may also cause pulmonary toxicity. Pulmonary surgery, tobacco use of ≥ 20 pack-years, and a cumulative cisplatin dose of > 850 mg are risk factors for late bleomycin-associated pulmonary toxicity.14
Other late-developing toxicities resulting from cisplatin treatment include ototoxicity, neurotoxicity, nephrotoxicity, chronic fatigue, and hypogonadism.14,19 Nearly 1 in 5 North American survivors treated with cisplatin reported severe-to-profound hearing loss within a median of 4.3 years. The extent of hearing loss has been directly associated with the increase in cumulative cisplatin dose. Peripheral neurotoxicity after cisplatin-based chemotherapy is reported to be as high as 40%.14 Chronic cancer-related fatigue can range from 15% to 27%, and has been associated with peripheral neuropathy, low testosterone levels, low physical activity, anxiety, and depression. Post-treatment hypogonadism ranges from 11% to 16%.14,17,20,21
Psychosocial issues are also of concern. Mild-to-moderate psychological distress with diagnosis and survivorship has been reported.17 Anxiety and depression are higher in TC survivors than in the general population. Variables associated with clinically significant anxiety include younger age and shorter time from diagnosis; whereas feeling helpless/hopeless, having less social support, having a higher number of physical symptoms, and having children are factors associated with higher levels of depression. A moderate-to-high level of fear of recurrence has also been reported.17
Recent Clinical Trials in Stage II Disease
Stage II disease has been the focus of current research to reduce treatment-related toxicities and limit longer-term complications. While few phase 3 clinical trials are ongoing (see Table), the results of several phase 2 trials have been reported recently.22-24
PRIMETEST was a single-arm, single-center, phase 2 study examining the efficacy and surgical safety of primary RPLND for stage II disease.22 Participants underwent either open or robot-assisted unilateral RPLND for stage IIA or B seminoma. No adjuvant treatment was permitted. Of the 33 participants, 9 presented initially with clinical stage II disease (27%) and 24 (73%) had recurrence during active surveillance. Five of the 24 had 1 cycle of carboplatin prior to progressing to stage II. With a median follow-up of 32 months, the study did not meet its primary endpoint of PFS at 36 months. After 32 months, 10 recurrences (30%) were detected, yielding a PFS rate of 70%. All 10 patients with recurrence received chemotherapy and were alive without evidence of disease at the time of publication. This study demonstrates that RPLND may be appropriate for select patients; however, criteria for selecting patients to receive only RPLND need to be clearly defined.22
The SEMS (surgery in early metastatic seminoma) trial was a single-arm, international, phase 2 study of RPLND as first-line treatment for early metastatic seminoma with isolated retroperitoneal lymphadenopathy between 1 and 3 cm (stage II).23 With a median follow-up of 24 months, OS was 100% and 2-year recurrence-free survival was 87%. Recurrence rate was 18% (10 recurrences) with a median time to recurrence of 8 months. Short-term complications occurred in 7 patients (13%), and no patients reported long-term complications. The authors suggested that RPLND is a therapeutic option for first-line treatment in early metastatic seminoma.23
SAKK 01/10 was a single-arm, international, phase 2 study examining the de-escalation of treatment to potentially avoid toxic effects for patients with either stage IIA or stage IIB seminoma.24 Treatment included carboplatin (area under the curve [AUC] 7 mg/mL/min) followed 3 weeks later with involvednode radiotherapy (30 Gy in 15 fractions for stage IIA and 36 Gy in 18 fractions for stage IIB). The study did not meet its primary endpoint of PFS of 95% at 3 years. Grade ≥ 3 treatment-related AEs (TRAEs) included neutropenia (4%), thrombocytopenia (3%), and vomiting (1%). No treatment-related deaths and no late TRAEs were reported. One case of transient creatinine increase was reported as a serious AE, and second primary tumors were reported in 4 participants. Although the primary endpoint was not met, long-term AEs continue to be recorded for potentially up to 20 years. The favorable efficacy and toxicity profile observed in the deescalation combination treatment warrants further study.24
Emerging Trends and Future Directions for TC Treatment
Although the outlook for most newly diagnosed patients with TC is promising, especially for those diagnosed with early-stage disease and good prognosis advanced disease, treatment challenges remain. Between 10% and 20% of patients will have a relapse of TC after initially achieving a complete remission. Most patients will have a relapse within 2 years of initial treatment, but a small subgroup will have a relapse more than 5 years after therapy. Most recurrences occur in the retroperitoneum and lungs and require definitive therapy using chemotherapy and surgical resection.21
Patients with platinum-refractory disease may still achiev long-term remission with salvage therapy of surgery, conventional-dose chemotherapy, or high-dose chemotherapy with autologous stem cell transplantation; however, these treatments will fail for some patients, resulting in poor prognosis. Targeted therapy for TC has not produced meaningful benefits for this population with refractory disease, and the optimal treatment for this group of patients with TC remains to be determined.21
Although current guidelines recommend determining the levels of AFP, hCG, and LDH for clinical staging, treatment monitoring, and follow-up, limitations exist with their usage.9 The assays for these markers have low sensitivity and lack specificity; about half of all GCTs express only 1 of the 3 biomarkers, and seminomas lack AFP expression.7,25,26 Further research is needed on LDH. An emerging group of patients with LDH below 2.5× ULN may be candidates for de-escalatio strategies to reduce treatment burden, while inferior outcomes remain for patients with either good prognosis seminoma and elevated LDH, or intermediate prognosis seminoma.7
Other biomarkers, such as miRNA371a-3p and PD-L1, are being investigated; miRNA371a-3p has been shown to have prognostic significance. The results of this assay can be informative for both seminomas and NSGCTs.26 However, the protocol for quantification and implementation still needs to be determined.27
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4(1):29. doi:10.1038/s41572-018-0029-03
- Chovanec M, Cheng L. Advances in diagnosis and treatment of testicular cancer. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Gaddam SJ, Chesnut GT. Testicle cancer. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated October 16, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK563159/
- Yang H, Obiora D, Tomaszewski JJ. Outcomes and expanding indications for robotic retroperitoneal lymph node dissection for testicular cancer. Transl Androl Urol. 2021;10(5):2188-2194. doi:10.21037/tau.2020.03.14
- International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15(2):594-603. doi:10.1200/JCO.1997.15.2.594
- Beyer J, Collette L, Sauvé N, et al. Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-Update Consortium. J Clin Oncol. 2021;39(14):1553-1562. doi:10.1200/JCO.20.03292
- Gillessen S, Sauvé N, Collette L, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG Update Consortium. J Clin Oncol. 2021;39(14):1563-1574. doi:10.1200/JCO.20.03296
- Gilligan T, Lin DW, Aggarwal R, et al. Testicular cancer, version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(12):1529-1554. doi:10.6004/jnccn.2019.0058
- Heinzelbecker J, Schmidt S, Lackner J, et al. Therapy of clinical stage IIa and IIb seminoma: a systematic review. World J Urol. 2022;40(12):2829-2841. doi:10.1007/s00345-021-03873-5
- Oldenburg J, Berney DM, Bokemeyer C, et al. Testicular seminoma and nonseminoma: ESMA-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(4):362-375. doi:10.1016/j.annonc.2022.01.002
- Wymer KM, Pearce SM, Harris KT, Pierorazio PM, Daneshmand S, Eggener SE. Adherence to National Comprehensive Cancer Network® guidelines for testicular cancer. J Urol. 2017;197(3 pt 1):684-689. doi:10.1016/j.juro.2016.09.073
- Saoud RM, Andolfi C, Aizen J, et al. Impact of non-guideline-directed care on quality of life in testicular cancer survivors. Eur Urol Focus. 2021;7(5):1137-1142. doi:10.1016/j.euf.2020.10.005
- Fung C, Dinh PC, Fossa SD, Travis LB. Testicular cancer survivorship. J Natl Compr Canc Netw. 2019;17(12):1557-1568. doi:10.6004/jnccn.2019.7369
- Guo CC, Czerniak B. Somatic-type malignancies in testicular germ cell tumors. Hum Pathol. 2022;127:123-135.
- Sprauten M, Darrah TH, Peterson DR, et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in cisplatin-treated survivors of testicular cancer. J Clin Oncol. 2012;30(3):300-307. doi:10.1200/JCO.2011.37.4025
- Shrem NS, Wood L, Hamilton RJ, et al. Testicular cancer survivorship: long-term toxicity and management. Can Urol Assoc J. 2022;16(8):257-272. doi:10.5489/cuaj.8009
- Hellesnes R, Myklebust TA, Fosså SD, et al. Testicular cancer in the cisplatin era: causes of death and mortality rates in a population-based cohort. J Clin Oncol. 2021;39(32):3561-3573. doi:10.1200/JCO.21.00637
- Mercieca-Bebber R, Naher SK, Rincones O, Smith AB, Stockler MR. Patient-reported outcomes associated with treatments for testicular cancer: a systematic review. Patient Relat Outcome Meas. 2021;12:129-171. doi:10.2147/PROM.S242754
- Sprauten M, Haugnes HS, Brydøy M, et al. Chronic fatigue in 812 testicular cancer survivors during long-term follow-up: increasing prevalence and risk factors. Ann Oncol. 2015;26(10):2133-2140. doi:10.1093/annonc/mdv328
- King J, Adra N, Einhorn LH. Testicular cancer: biology to bedside. Cancer Res. 2021;81(21):5369-5376. doi:10.1158/0008-5472.CAN-21-1452
- Hiester A, Che Y, Lusch A, et al. Phase 2 single-arm trial of primary retroperitoneal lymph node dissection in patients with seminomatous testicular germ cell tumors with clinical stage IIA/B (PRIMETEST). Eur Urol. 2022;S0302-2838(22)02775-0. doi:10.1016/j.eururo.2022.10.021
- Daneshmand S, Cary C, Masterson TA, et al. SEMS trial: result of a prospective, multi-institutional phase II clinical trial of surgery in early metastatic seminoma. J Clin Oncol. 2021;39(6 suppl):Abstract 375. doi:10.1200JCO.2021.39.6_suppl.375
- Papachristofilou A, Bedke J, Hayoz S, et al. Single-dose carboplatin followed by involved-node radiotherapy for stage IIA and stage IIB seminoma (SAKK 01/10): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(11):1441-1450. doi:10.1016/S1470-2045(22)00564-2
- Dieckmann KP, Richter-Simonsen H, Kulejewski M, et al. Testicular germ-cell tumours: a descriptive analysis of clinical characteristics at first presentation. Urol Int. 2018;100(4):409-419. doi:10.1159/000488284
- Murray MJ, Huddart RA, Coleman N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat Rev Urol. 2016;13(12):715-725. doi:10.1038/nrurol.2016.170
Mortality from TC has been decreasing since the 1970s due to cisplatin-based chemotherapy regimens2,3; TC is among the most curable of solid neoplasms, with a 5-year relative survival rate of 95%.2-4 Thus, the focus of research has shifted from optimizing treatments for improved survival to decreasing treatment-related, long-term adverse events (AEs).5
New Modifications in Risk Assessment and Prognostication
The widely accepted risk stratification model in use today was first developed in 1997 by the International Germ Cell Cancer Collaborative Group (IGCCCG) after studying data on patients with seminoma and NSGCTs.6 The original classification categorized metastatic NSGCTs as having good, intermediate, or poor prognosis based on levels of alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), lactate dehydrogenase (LDH), and the presence of nonpulmonary visceral metastases (NPVM). Primary mediastinal NSGCTs were classified as having poor prognosis regardless of the other factors.6 Metastatic seminoma GCTs were categorized as having good or intermediate prognosis based on the occurrence of brain, liver, or bone metastasis.7
Using contemporary data from more than 12,000 patients with metastatic GCTs who received either cisplatin or etoposide, the IGCCCG model was updated in 2021. For seminoma GCTs, 5-year progression-free survival (PFS) and 5-year overall survival (OS) were extended for both good and intermediate prognostic groups.7 LDH remained the most significant prognostic factor for determining good prognosis however, patients with LDH above 2.5× upper limit of normal (ULN) before chemotherapy had worse survival probabilities than patients with LDH at 2.5× ULN or lower. The survival probabilities for patients with otherwise good prognosis with LDH of more than 2.5× ULN were like those for patients with intermediate prognosis.7 Thus, using LDH of more than 2.5× ULN has revealed a subgroup with significantly worse outcomes within the “good” prognostic group.7,8
For NSGCTs, 5-year PFS rates did not differ from the original IGCCCG for good and intermediate prognostic groups; however, the 2021 update revealed an improved PFS for the poor prognostic group. The 2021 update also demonstrated that 5-year OS rates improved for each group, and further confirmed that the 2 most important prognostic factors for NSGCT were the presence of NPVM and the presence of a mediastinal primary tumor. The update added 2 new adverse prognostic variables: age and metastases. Risk of progression increases 25% with every decade-of-life increase, and 66% with the presence of lung metastases. The LDH groups were reduced to a single cutoff at 2.5× ULN for NSGCTs.8
Primary and Subsequent Treatments for TC
Guideline-directed first-line and subsequent treatments for seminomas and NSGCTs have been developed by several organizations, including the National Comprehensive Cancer Network, IGCCCG, and the American Urological Association (see Figure 1 and 2). An analysis of the most used treatments was performed using the National Cancer Database.2 Most patients underwent orchiectomy without chemotherapy or radiation for both stage I seminomas (78%) and NSGCTs (57%). For stage II and III seminomas, most patients underwent surgery with chemotherapy (66% and 68%, respectively). Nearly half of patients with stage II NSGCTs were treated with surgery and chemotherapy (49%), and a third were treated with retroperitoneal lymph node dissection (RPLND) in addition to surgery and chemotherapy. Surgery with chemotherapy was used for 55% of stage III NSGCTs; other treatments included surgery combined with chemotherapy and RPLND (19%), and chemotherapy with or without radiation (20%).2 However, nearly 30% of patients with TC do not receive guideline-directed therapy, including inappropriate imaging and overtreatment; and nonguideline–directed therapy has been independently associated with risk of relapse.12,13
TC Survivorship
The trend of improved OS after treatment for metastatic GCTs highlights a need to focus on survivorship. The 10-year survival rate for TC post-treatment is 95%.14 Latest estimates suggest there are more than 300,000 TC survivors in the United States,2 accounting for approximately 4% of all US male cancer survivors.14 With longer-term survival, however, comes the risk for long-term complications from cancer treatments. For example, circulating platinum has been detected in the plasma of men up to 28 years after undergoing cisplatin-based chemotherapy for TC.15 Increasing levels of residual serum platinum have also been shown to correlate with severity of neurotoxicity between 5 and 20 years after treatment.16
A significant concern with cancer treatment is the development of second malignant neoplasms (SMNs).14,17 The relative risk of the development of SMNs depends on whetherradiation therapy or chemotherapy, or both, was used as the primary treatment. Patients who received either radiation therapy or chemotherapy are at increased risk for leukemia and solid cancers, including gastrointestinal cancers. For patients treated with cisplatin, a significant dose-response relationship between cumulative dose and leukemic risk has been reported.14
Other concerns are increased non-TC mortality and SMN mortality. Hellesnes et al examined cause-specific, non-TC mortality using a population-based cohort in Norway.18 They determined that the overall 25-year, non-TC mortality risk was 13.7% (95% CI, 12.5-14.9) for patients who previously had TC vs 11.3% for patients who never had TC. The highest mortality rates were reported for patients who had radiation (19%) or platinum-based chemotherapy plus radiation (18.4%); the lowest mortality rate was reported for patients who had received platinum-based chemotherapy only (9.5%). Patients with the highest non-TC mortality risk were fewer than 20 years post-cancer diagnosis. Non-TC mortality excess ranged from 23% to 40% for patients with a prior TC diagnosis, and a significant 1.43- to 3.24-fold increase in SMN mortality emerged after treatment with platinum-based chemotherapy or radiation therapy, or both.19 Awareness of the increased premature mortality risk is crucial for both TC survivors and their care providers.18
Quality of life for TC survivors appears to be affected by the presence of long-term treatment-related AEs.18 The relative risk of developing cardiovascular disease increases after treatment with chemotherapy. Raynaud phenomenon resulting from bleomycin-induced vascular damage developed within 4 to 12 months after chemotherapy for 18.7% to 39% of TC survivors.14,19 Bleomycin may also cause pulmonary toxicity. Pulmonary surgery, tobacco use of ≥ 20 pack-years, and a cumulative cisplatin dose of > 850 mg are risk factors for late bleomycin-associated pulmonary toxicity.14
Other late-developing toxicities resulting from cisplatin treatment include ototoxicity, neurotoxicity, nephrotoxicity, chronic fatigue, and hypogonadism.14,19 Nearly 1 in 5 North American survivors treated with cisplatin reported severe-to-profound hearing loss within a median of 4.3 years. The extent of hearing loss has been directly associated with the increase in cumulative cisplatin dose. Peripheral neurotoxicity after cisplatin-based chemotherapy is reported to be as high as 40%.14 Chronic cancer-related fatigue can range from 15% to 27%, and has been associated with peripheral neuropathy, low testosterone levels, low physical activity, anxiety, and depression. Post-treatment hypogonadism ranges from 11% to 16%.14,17,20,21
Psychosocial issues are also of concern. Mild-to-moderate psychological distress with diagnosis and survivorship has been reported.17 Anxiety and depression are higher in TC survivors than in the general population. Variables associated with clinically significant anxiety include younger age and shorter time from diagnosis; whereas feeling helpless/hopeless, having less social support, having a higher number of physical symptoms, and having children are factors associated with higher levels of depression. A moderate-to-high level of fear of recurrence has also been reported.17
Recent Clinical Trials in Stage II Disease
Stage II disease has been the focus of current research to reduce treatment-related toxicities and limit longer-term complications. While few phase 3 clinical trials are ongoing (see Table), the results of several phase 2 trials have been reported recently.22-24
PRIMETEST was a single-arm, single-center, phase 2 study examining the efficacy and surgical safety of primary RPLND for stage II disease.22 Participants underwent either open or robot-assisted unilateral RPLND for stage IIA or B seminoma. No adjuvant treatment was permitted. Of the 33 participants, 9 presented initially with clinical stage II disease (27%) and 24 (73%) had recurrence during active surveillance. Five of the 24 had 1 cycle of carboplatin prior to progressing to stage II. With a median follow-up of 32 months, the study did not meet its primary endpoint of PFS at 36 months. After 32 months, 10 recurrences (30%) were detected, yielding a PFS rate of 70%. All 10 patients with recurrence received chemotherapy and were alive without evidence of disease at the time of publication. This study demonstrates that RPLND may be appropriate for select patients; however, criteria for selecting patients to receive only RPLND need to be clearly defined.22
The SEMS (surgery in early metastatic seminoma) trial was a single-arm, international, phase 2 study of RPLND as first-line treatment for early metastatic seminoma with isolated retroperitoneal lymphadenopathy between 1 and 3 cm (stage II).23 With a median follow-up of 24 months, OS was 100% and 2-year recurrence-free survival was 87%. Recurrence rate was 18% (10 recurrences) with a median time to recurrence of 8 months. Short-term complications occurred in 7 patients (13%), and no patients reported long-term complications. The authors suggested that RPLND is a therapeutic option for first-line treatment in early metastatic seminoma.23
SAKK 01/10 was a single-arm, international, phase 2 study examining the de-escalation of treatment to potentially avoid toxic effects for patients with either stage IIA or stage IIB seminoma.24 Treatment included carboplatin (area under the curve [AUC] 7 mg/mL/min) followed 3 weeks later with involvednode radiotherapy (30 Gy in 15 fractions for stage IIA and 36 Gy in 18 fractions for stage IIB). The study did not meet its primary endpoint of PFS of 95% at 3 years. Grade ≥ 3 treatment-related AEs (TRAEs) included neutropenia (4%), thrombocytopenia (3%), and vomiting (1%). No treatment-related deaths and no late TRAEs were reported. One case of transient creatinine increase was reported as a serious AE, and second primary tumors were reported in 4 participants. Although the primary endpoint was not met, long-term AEs continue to be recorded for potentially up to 20 years. The favorable efficacy and toxicity profile observed in the deescalation combination treatment warrants further study.24
Emerging Trends and Future Directions for TC Treatment
Although the outlook for most newly diagnosed patients with TC is promising, especially for those diagnosed with early-stage disease and good prognosis advanced disease, treatment challenges remain. Between 10% and 20% of patients will have a relapse of TC after initially achieving a complete remission. Most patients will have a relapse within 2 years of initial treatment, but a small subgroup will have a relapse more than 5 years after therapy. Most recurrences occur in the retroperitoneum and lungs and require definitive therapy using chemotherapy and surgical resection.21
Patients with platinum-refractory disease may still achiev long-term remission with salvage therapy of surgery, conventional-dose chemotherapy, or high-dose chemotherapy with autologous stem cell transplantation; however, these treatments will fail for some patients, resulting in poor prognosis. Targeted therapy for TC has not produced meaningful benefits for this population with refractory disease, and the optimal treatment for this group of patients with TC remains to be determined.21
Although current guidelines recommend determining the levels of AFP, hCG, and LDH for clinical staging, treatment monitoring, and follow-up, limitations exist with their usage.9 The assays for these markers have low sensitivity and lack specificity; about half of all GCTs express only 1 of the 3 biomarkers, and seminomas lack AFP expression.7,25,26 Further research is needed on LDH. An emerging group of patients with LDH below 2.5× ULN may be candidates for de-escalatio strategies to reduce treatment burden, while inferior outcomes remain for patients with either good prognosis seminoma and elevated LDH, or intermediate prognosis seminoma.7
Other biomarkers, such as miRNA371a-3p and PD-L1, are being investigated; miRNA371a-3p has been shown to have prognostic significance. The results of this assay can be informative for both seminomas and NSGCTs.26 However, the protocol for quantification and implementation still needs to be determined.27
Mortality from TC has been decreasing since the 1970s due to cisplatin-based chemotherapy regimens2,3; TC is among the most curable of solid neoplasms, with a 5-year relative survival rate of 95%.2-4 Thus, the focus of research has shifted from optimizing treatments for improved survival to decreasing treatment-related, long-term adverse events (AEs).5
New Modifications in Risk Assessment and Prognostication
The widely accepted risk stratification model in use today was first developed in 1997 by the International Germ Cell Cancer Collaborative Group (IGCCCG) after studying data on patients with seminoma and NSGCTs.6 The original classification categorized metastatic NSGCTs as having good, intermediate, or poor prognosis based on levels of alpha-fetoprotein (AFP), human chorionic gonadotropin (hCG), lactate dehydrogenase (LDH), and the presence of nonpulmonary visceral metastases (NPVM). Primary mediastinal NSGCTs were classified as having poor prognosis regardless of the other factors.6 Metastatic seminoma GCTs were categorized as having good or intermediate prognosis based on the occurrence of brain, liver, or bone metastasis.7
Using contemporary data from more than 12,000 patients with metastatic GCTs who received either cisplatin or etoposide, the IGCCCG model was updated in 2021. For seminoma GCTs, 5-year progression-free survival (PFS) and 5-year overall survival (OS) were extended for both good and intermediate prognostic groups.7 LDH remained the most significant prognostic factor for determining good prognosis however, patients with LDH above 2.5× upper limit of normal (ULN) before chemotherapy had worse survival probabilities than patients with LDH at 2.5× ULN or lower. The survival probabilities for patients with otherwise good prognosis with LDH of more than 2.5× ULN were like those for patients with intermediate prognosis.7 Thus, using LDH of more than 2.5× ULN has revealed a subgroup with significantly worse outcomes within the “good” prognostic group.7,8
For NSGCTs, 5-year PFS rates did not differ from the original IGCCCG for good and intermediate prognostic groups; however, the 2021 update revealed an improved PFS for the poor prognostic group. The 2021 update also demonstrated that 5-year OS rates improved for each group, and further confirmed that the 2 most important prognostic factors for NSGCT were the presence of NPVM and the presence of a mediastinal primary tumor. The update added 2 new adverse prognostic variables: age and metastases. Risk of progression increases 25% with every decade-of-life increase, and 66% with the presence of lung metastases. The LDH groups were reduced to a single cutoff at 2.5× ULN for NSGCTs.8
Primary and Subsequent Treatments for TC
Guideline-directed first-line and subsequent treatments for seminomas and NSGCTs have been developed by several organizations, including the National Comprehensive Cancer Network, IGCCCG, and the American Urological Association (see Figure 1 and 2). An analysis of the most used treatments was performed using the National Cancer Database.2 Most patients underwent orchiectomy without chemotherapy or radiation for both stage I seminomas (78%) and NSGCTs (57%). For stage II and III seminomas, most patients underwent surgery with chemotherapy (66% and 68%, respectively). Nearly half of patients with stage II NSGCTs were treated with surgery and chemotherapy (49%), and a third were treated with retroperitoneal lymph node dissection (RPLND) in addition to surgery and chemotherapy. Surgery with chemotherapy was used for 55% of stage III NSGCTs; other treatments included surgery combined with chemotherapy and RPLND (19%), and chemotherapy with or without radiation (20%).2 However, nearly 30% of patients with TC do not receive guideline-directed therapy, including inappropriate imaging and overtreatment; and nonguideline–directed therapy has been independently associated with risk of relapse.12,13
TC Survivorship
The trend of improved OS after treatment for metastatic GCTs highlights a need to focus on survivorship. The 10-year survival rate for TC post-treatment is 95%.14 Latest estimates suggest there are more than 300,000 TC survivors in the United States,2 accounting for approximately 4% of all US male cancer survivors.14 With longer-term survival, however, comes the risk for long-term complications from cancer treatments. For example, circulating platinum has been detected in the plasma of men up to 28 years after undergoing cisplatin-based chemotherapy for TC.15 Increasing levels of residual serum platinum have also been shown to correlate with severity of neurotoxicity between 5 and 20 years after treatment.16
A significant concern with cancer treatment is the development of second malignant neoplasms (SMNs).14,17 The relative risk of the development of SMNs depends on whetherradiation therapy or chemotherapy, or both, was used as the primary treatment. Patients who received either radiation therapy or chemotherapy are at increased risk for leukemia and solid cancers, including gastrointestinal cancers. For patients treated with cisplatin, a significant dose-response relationship between cumulative dose and leukemic risk has been reported.14
Other concerns are increased non-TC mortality and SMN mortality. Hellesnes et al examined cause-specific, non-TC mortality using a population-based cohort in Norway.18 They determined that the overall 25-year, non-TC mortality risk was 13.7% (95% CI, 12.5-14.9) for patients who previously had TC vs 11.3% for patients who never had TC. The highest mortality rates were reported for patients who had radiation (19%) or platinum-based chemotherapy plus radiation (18.4%); the lowest mortality rate was reported for patients who had received platinum-based chemotherapy only (9.5%). Patients with the highest non-TC mortality risk were fewer than 20 years post-cancer diagnosis. Non-TC mortality excess ranged from 23% to 40% for patients with a prior TC diagnosis, and a significant 1.43- to 3.24-fold increase in SMN mortality emerged after treatment with platinum-based chemotherapy or radiation therapy, or both.19 Awareness of the increased premature mortality risk is crucial for both TC survivors and their care providers.18
Quality of life for TC survivors appears to be affected by the presence of long-term treatment-related AEs.18 The relative risk of developing cardiovascular disease increases after treatment with chemotherapy. Raynaud phenomenon resulting from bleomycin-induced vascular damage developed within 4 to 12 months after chemotherapy for 18.7% to 39% of TC survivors.14,19 Bleomycin may also cause pulmonary toxicity. Pulmonary surgery, tobacco use of ≥ 20 pack-years, and a cumulative cisplatin dose of > 850 mg are risk factors for late bleomycin-associated pulmonary toxicity.14
Other late-developing toxicities resulting from cisplatin treatment include ototoxicity, neurotoxicity, nephrotoxicity, chronic fatigue, and hypogonadism.14,19 Nearly 1 in 5 North American survivors treated with cisplatin reported severe-to-profound hearing loss within a median of 4.3 years. The extent of hearing loss has been directly associated with the increase in cumulative cisplatin dose. Peripheral neurotoxicity after cisplatin-based chemotherapy is reported to be as high as 40%.14 Chronic cancer-related fatigue can range from 15% to 27%, and has been associated with peripheral neuropathy, low testosterone levels, low physical activity, anxiety, and depression. Post-treatment hypogonadism ranges from 11% to 16%.14,17,20,21
Psychosocial issues are also of concern. Mild-to-moderate psychological distress with diagnosis and survivorship has been reported.17 Anxiety and depression are higher in TC survivors than in the general population. Variables associated with clinically significant anxiety include younger age and shorter time from diagnosis; whereas feeling helpless/hopeless, having less social support, having a higher number of physical symptoms, and having children are factors associated with higher levels of depression. A moderate-to-high level of fear of recurrence has also been reported.17
Recent Clinical Trials in Stage II Disease
Stage II disease has been the focus of current research to reduce treatment-related toxicities and limit longer-term complications. While few phase 3 clinical trials are ongoing (see Table), the results of several phase 2 trials have been reported recently.22-24
PRIMETEST was a single-arm, single-center, phase 2 study examining the efficacy and surgical safety of primary RPLND for stage II disease.22 Participants underwent either open or robot-assisted unilateral RPLND for stage IIA or B seminoma. No adjuvant treatment was permitted. Of the 33 participants, 9 presented initially with clinical stage II disease (27%) and 24 (73%) had recurrence during active surveillance. Five of the 24 had 1 cycle of carboplatin prior to progressing to stage II. With a median follow-up of 32 months, the study did not meet its primary endpoint of PFS at 36 months. After 32 months, 10 recurrences (30%) were detected, yielding a PFS rate of 70%. All 10 patients with recurrence received chemotherapy and were alive without evidence of disease at the time of publication. This study demonstrates that RPLND may be appropriate for select patients; however, criteria for selecting patients to receive only RPLND need to be clearly defined.22
The SEMS (surgery in early metastatic seminoma) trial was a single-arm, international, phase 2 study of RPLND as first-line treatment for early metastatic seminoma with isolated retroperitoneal lymphadenopathy between 1 and 3 cm (stage II).23 With a median follow-up of 24 months, OS was 100% and 2-year recurrence-free survival was 87%. Recurrence rate was 18% (10 recurrences) with a median time to recurrence of 8 months. Short-term complications occurred in 7 patients (13%), and no patients reported long-term complications. The authors suggested that RPLND is a therapeutic option for first-line treatment in early metastatic seminoma.23
SAKK 01/10 was a single-arm, international, phase 2 study examining the de-escalation of treatment to potentially avoid toxic effects for patients with either stage IIA or stage IIB seminoma.24 Treatment included carboplatin (area under the curve [AUC] 7 mg/mL/min) followed 3 weeks later with involvednode radiotherapy (30 Gy in 15 fractions for stage IIA and 36 Gy in 18 fractions for stage IIB). The study did not meet its primary endpoint of PFS of 95% at 3 years. Grade ≥ 3 treatment-related AEs (TRAEs) included neutropenia (4%), thrombocytopenia (3%), and vomiting (1%). No treatment-related deaths and no late TRAEs were reported. One case of transient creatinine increase was reported as a serious AE, and second primary tumors were reported in 4 participants. Although the primary endpoint was not met, long-term AEs continue to be recorded for potentially up to 20 years. The favorable efficacy and toxicity profile observed in the deescalation combination treatment warrants further study.24
Emerging Trends and Future Directions for TC Treatment
Although the outlook for most newly diagnosed patients with TC is promising, especially for those diagnosed with early-stage disease and good prognosis advanced disease, treatment challenges remain. Between 10% and 20% of patients will have a relapse of TC after initially achieving a complete remission. Most patients will have a relapse within 2 years of initial treatment, but a small subgroup will have a relapse more than 5 years after therapy. Most recurrences occur in the retroperitoneum and lungs and require definitive therapy using chemotherapy and surgical resection.21
Patients with platinum-refractory disease may still achiev long-term remission with salvage therapy of surgery, conventional-dose chemotherapy, or high-dose chemotherapy with autologous stem cell transplantation; however, these treatments will fail for some patients, resulting in poor prognosis. Targeted therapy for TC has not produced meaningful benefits for this population with refractory disease, and the optimal treatment for this group of patients with TC remains to be determined.21
Although current guidelines recommend determining the levels of AFP, hCG, and LDH for clinical staging, treatment monitoring, and follow-up, limitations exist with their usage.9 The assays for these markers have low sensitivity and lack specificity; about half of all GCTs express only 1 of the 3 biomarkers, and seminomas lack AFP expression.7,25,26 Further research is needed on LDH. An emerging group of patients with LDH below 2.5× ULN may be candidates for de-escalatio strategies to reduce treatment burden, while inferior outcomes remain for patients with either good prognosis seminoma and elevated LDH, or intermediate prognosis seminoma.7
Other biomarkers, such as miRNA371a-3p and PD-L1, are being investigated; miRNA371a-3p has been shown to have prognostic significance. The results of this assay can be informative for both seminomas and NSGCTs.26 However, the protocol for quantification and implementation still needs to be determined.27
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4(1):29. doi:10.1038/s41572-018-0029-03
- Chovanec M, Cheng L. Advances in diagnosis and treatment of testicular cancer. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Gaddam SJ, Chesnut GT. Testicle cancer. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated October 16, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK563159/
- Yang H, Obiora D, Tomaszewski JJ. Outcomes and expanding indications for robotic retroperitoneal lymph node dissection for testicular cancer. Transl Androl Urol. 2021;10(5):2188-2194. doi:10.21037/tau.2020.03.14
- International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15(2):594-603. doi:10.1200/JCO.1997.15.2.594
- Beyer J, Collette L, Sauvé N, et al. Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-Update Consortium. J Clin Oncol. 2021;39(14):1553-1562. doi:10.1200/JCO.20.03292
- Gillessen S, Sauvé N, Collette L, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG Update Consortium. J Clin Oncol. 2021;39(14):1563-1574. doi:10.1200/JCO.20.03296
- Gilligan T, Lin DW, Aggarwal R, et al. Testicular cancer, version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(12):1529-1554. doi:10.6004/jnccn.2019.0058
- Heinzelbecker J, Schmidt S, Lackner J, et al. Therapy of clinical stage IIa and IIb seminoma: a systematic review. World J Urol. 2022;40(12):2829-2841. doi:10.1007/s00345-021-03873-5
- Oldenburg J, Berney DM, Bokemeyer C, et al. Testicular seminoma and nonseminoma: ESMA-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(4):362-375. doi:10.1016/j.annonc.2022.01.002
- Wymer KM, Pearce SM, Harris KT, Pierorazio PM, Daneshmand S, Eggener SE. Adherence to National Comprehensive Cancer Network® guidelines for testicular cancer. J Urol. 2017;197(3 pt 1):684-689. doi:10.1016/j.juro.2016.09.073
- Saoud RM, Andolfi C, Aizen J, et al. Impact of non-guideline-directed care on quality of life in testicular cancer survivors. Eur Urol Focus. 2021;7(5):1137-1142. doi:10.1016/j.euf.2020.10.005
- Fung C, Dinh PC, Fossa SD, Travis LB. Testicular cancer survivorship. J Natl Compr Canc Netw. 2019;17(12):1557-1568. doi:10.6004/jnccn.2019.7369
- Guo CC, Czerniak B. Somatic-type malignancies in testicular germ cell tumors. Hum Pathol. 2022;127:123-135.
- Sprauten M, Darrah TH, Peterson DR, et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in cisplatin-treated survivors of testicular cancer. J Clin Oncol. 2012;30(3):300-307. doi:10.1200/JCO.2011.37.4025
- Shrem NS, Wood L, Hamilton RJ, et al. Testicular cancer survivorship: long-term toxicity and management. Can Urol Assoc J. 2022;16(8):257-272. doi:10.5489/cuaj.8009
- Hellesnes R, Myklebust TA, Fosså SD, et al. Testicular cancer in the cisplatin era: causes of death and mortality rates in a population-based cohort. J Clin Oncol. 2021;39(32):3561-3573. doi:10.1200/JCO.21.00637
- Mercieca-Bebber R, Naher SK, Rincones O, Smith AB, Stockler MR. Patient-reported outcomes associated with treatments for testicular cancer: a systematic review. Patient Relat Outcome Meas. 2021;12:129-171. doi:10.2147/PROM.S242754
- Sprauten M, Haugnes HS, Brydøy M, et al. Chronic fatigue in 812 testicular cancer survivors during long-term follow-up: increasing prevalence and risk factors. Ann Oncol. 2015;26(10):2133-2140. doi:10.1093/annonc/mdv328
- King J, Adra N, Einhorn LH. Testicular cancer: biology to bedside. Cancer Res. 2021;81(21):5369-5376. doi:10.1158/0008-5472.CAN-21-1452
- Hiester A, Che Y, Lusch A, et al. Phase 2 single-arm trial of primary retroperitoneal lymph node dissection in patients with seminomatous testicular germ cell tumors with clinical stage IIA/B (PRIMETEST). Eur Urol. 2022;S0302-2838(22)02775-0. doi:10.1016/j.eururo.2022.10.021
- Daneshmand S, Cary C, Masterson TA, et al. SEMS trial: result of a prospective, multi-institutional phase II clinical trial of surgery in early metastatic seminoma. J Clin Oncol. 2021;39(6 suppl):Abstract 375. doi:10.1200JCO.2021.39.6_suppl.375
- Papachristofilou A, Bedke J, Hayoz S, et al. Single-dose carboplatin followed by involved-node radiotherapy for stage IIA and stage IIB seminoma (SAKK 01/10): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(11):1441-1450. doi:10.1016/S1470-2045(22)00564-2
- Dieckmann KP, Richter-Simonsen H, Kulejewski M, et al. Testicular germ-cell tumours: a descriptive analysis of clinical characteristics at first presentation. Urol Int. 2018;100(4):409-419. doi:10.1159/000488284
- Murray MJ, Huddart RA, Coleman N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat Rev Urol. 2016;13(12):715-725. doi:10.1038/nrurol.2016.170
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73(1):17-48. doi:10.3322/caac.21763
- Cheng L, Albers P, Berney DM, et al. Testicular cancer. Nat Rev Dis Primers. 2018;4(1):29. doi:10.1038/s41572-018-0029-03
- Chovanec M, Cheng L. Advances in diagnosis and treatment of testicular cancer. BMJ. 2022;379:e070499. doi:10.1136/bmj-2022-070499
- Gaddam SJ, Chesnut GT. Testicle cancer. StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022. Updated October 16, 2022. Accessed March 13, 2023. https://www.ncbi.nlm.nih.gov/books/NBK563159/
- Yang H, Obiora D, Tomaszewski JJ. Outcomes and expanding indications for robotic retroperitoneal lymph node dissection for testicular cancer. Transl Androl Urol. 2021;10(5):2188-2194. doi:10.21037/tau.2020.03.14
- International Germ Cell Cancer Collaborative Group. International germ cell consensus classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15(2):594-603. doi:10.1200/JCO.1997.15.2.594
- Beyer J, Collette L, Sauvé N, et al. Survival and new prognosticators in metastatic seminoma: results from the IGCCCG-Update Consortium. J Clin Oncol. 2021;39(14):1553-1562. doi:10.1200/JCO.20.03292
- Gillessen S, Sauvé N, Collette L, et al. Predicting outcomes in men with metastatic nonseminomatous germ cell tumors (NSGCT): results from the IGCCCG Update Consortium. J Clin Oncol. 2021;39(14):1563-1574. doi:10.1200/JCO.20.03296
- Gilligan T, Lin DW, Aggarwal R, et al. Testicular cancer, version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17(12):1529-1554. doi:10.6004/jnccn.2019.0058
- Heinzelbecker J, Schmidt S, Lackner J, et al. Therapy of clinical stage IIa and IIb seminoma: a systematic review. World J Urol. 2022;40(12):2829-2841. doi:10.1007/s00345-021-03873-5
- Oldenburg J, Berney DM, Bokemeyer C, et al. Testicular seminoma and nonseminoma: ESMA-EURACAN Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(4):362-375. doi:10.1016/j.annonc.2022.01.002
- Wymer KM, Pearce SM, Harris KT, Pierorazio PM, Daneshmand S, Eggener SE. Adherence to National Comprehensive Cancer Network® guidelines for testicular cancer. J Urol. 2017;197(3 pt 1):684-689. doi:10.1016/j.juro.2016.09.073
- Saoud RM, Andolfi C, Aizen J, et al. Impact of non-guideline-directed care on quality of life in testicular cancer survivors. Eur Urol Focus. 2021;7(5):1137-1142. doi:10.1016/j.euf.2020.10.005
- Fung C, Dinh PC, Fossa SD, Travis LB. Testicular cancer survivorship. J Natl Compr Canc Netw. 2019;17(12):1557-1568. doi:10.6004/jnccn.2019.7369
- Guo CC, Czerniak B. Somatic-type malignancies in testicular germ cell tumors. Hum Pathol. 2022;127:123-135.
- Sprauten M, Darrah TH, Peterson DR, et al. Impact of long-term serum platinum concentrations on neuro- and ototoxicity in cisplatin-treated survivors of testicular cancer. J Clin Oncol. 2012;30(3):300-307. doi:10.1200/JCO.2011.37.4025
- Shrem NS, Wood L, Hamilton RJ, et al. Testicular cancer survivorship: long-term toxicity and management. Can Urol Assoc J. 2022;16(8):257-272. doi:10.5489/cuaj.8009
- Hellesnes R, Myklebust TA, Fosså SD, et al. Testicular cancer in the cisplatin era: causes of death and mortality rates in a population-based cohort. J Clin Oncol. 2021;39(32):3561-3573. doi:10.1200/JCO.21.00637
- Mercieca-Bebber R, Naher SK, Rincones O, Smith AB, Stockler MR. Patient-reported outcomes associated with treatments for testicular cancer: a systematic review. Patient Relat Outcome Meas. 2021;12:129-171. doi:10.2147/PROM.S242754
- Sprauten M, Haugnes HS, Brydøy M, et al. Chronic fatigue in 812 testicular cancer survivors during long-term follow-up: increasing prevalence and risk factors. Ann Oncol. 2015;26(10):2133-2140. doi:10.1093/annonc/mdv328
- King J, Adra N, Einhorn LH. Testicular cancer: biology to bedside. Cancer Res. 2021;81(21):5369-5376. doi:10.1158/0008-5472.CAN-21-1452
- Hiester A, Che Y, Lusch A, et al. Phase 2 single-arm trial of primary retroperitoneal lymph node dissection in patients with seminomatous testicular germ cell tumors with clinical stage IIA/B (PRIMETEST). Eur Urol. 2022;S0302-2838(22)02775-0. doi:10.1016/j.eururo.2022.10.021
- Daneshmand S, Cary C, Masterson TA, et al. SEMS trial: result of a prospective, multi-institutional phase II clinical trial of surgery in early metastatic seminoma. J Clin Oncol. 2021;39(6 suppl):Abstract 375. doi:10.1200JCO.2021.39.6_suppl.375
- Papachristofilou A, Bedke J, Hayoz S, et al. Single-dose carboplatin followed by involved-node radiotherapy for stage IIA and stage IIB seminoma (SAKK 01/10): a single-arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(11):1441-1450. doi:10.1016/S1470-2045(22)00564-2
- Dieckmann KP, Richter-Simonsen H, Kulejewski M, et al. Testicular germ-cell tumours: a descriptive analysis of clinical characteristics at first presentation. Urol Int. 2018;100(4):409-419. doi:10.1159/000488284
- Murray MJ, Huddart RA, Coleman N. The present and future of serum diagnostic tests for testicular germ cell tumours. Nat Rev Urol. 2016;13(12):715-725. doi:10.1038/nrurol.2016.170
NORD: Making Progress Through Collaboration
While people living with rare cancers continue to face daunting obstacles, progress is being made, and there are reasons to hope for a better future. Advances in genomic testing and precision medicine provide increasing evidence that rare cancers can be more efficiently and effectively diagnosed and treated. Genomic tests examine tumor DNA to identify mutations that are unique to an individual’s cancer. This genetic information enables a more precise diagnosis and targeted treatment approach. Jim Palma, Co-Lead of the NORD Rare Cancer Coalition, said “There is promise for rare cancer patients due to increased legislative efforts to cover the costs of genomic testing coupled by an increase in FDA approvals for targeted and tissue agnostic therapies.”
In 2019, the National Cancer Institute established MyPART, a vast pediatric and adult rare tumor network that aims to bolster patient involvement in research and develop effective therapies through tumor sample collection, shared data, shared samples, new methods to test treatments, and new trial designs. In 2022, MyPART welcomed NORD’s Rare Cancer Coalition as an advocacy partner.
Meanwhile, advocacy organizations are giving rare cancer a rising voice. NORD’s Rare Cancer Coalition unites rare cancer patient advocacy organizations and helps them drive progress together. The coalition promotes research and awareness through its annual Rare Cancer Day (September 30) campaign. Additionally, NORD has produced over 22 continuing medical education modules on rare cancers in collaboration with PlatformQ Health, providing updates on new therapies and treatment approaches. NORD also offers rare disease reports and educational videos on rare cancers, sessions inclusive of rare cancer topics at the annual NORD Summit, and a quarterly e-newsletter, “Caring for Rare” for healthcare professionals. Please visit us at rarediseases.org to access these resources.
Much work on rare cancers remains to be done, but the progress over recent years points to better outcomes moving forward. We are grateful for the work you do and your dedication to your patients, including those with rare cancers and other rare conditions. We hope you will find the information in this special issue useful for your clinical practice.
– Katie Kowalski, MPH
Associate Director of Education
National Organization for Rare Disorders
- About Rare Cancers. National Cancer Institute. Posted February 27, 2019. Accessed April 28, 2023. http://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/about-rare-cancers
- Gatta G, Capocaccia R, Botta L, et al. Burden and centralized treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017,18(8):1022–1039. doi:10.1016/S1470-2045(17)30445-X
While people living with rare cancers continue to face daunting obstacles, progress is being made, and there are reasons to hope for a better future. Advances in genomic testing and precision medicine provide increasing evidence that rare cancers can be more efficiently and effectively diagnosed and treated. Genomic tests examine tumor DNA to identify mutations that are unique to an individual’s cancer. This genetic information enables a more precise diagnosis and targeted treatment approach. Jim Palma, Co-Lead of the NORD Rare Cancer Coalition, said “There is promise for rare cancer patients due to increased legislative efforts to cover the costs of genomic testing coupled by an increase in FDA approvals for targeted and tissue agnostic therapies.”
In 2019, the National Cancer Institute established MyPART, a vast pediatric and adult rare tumor network that aims to bolster patient involvement in research and develop effective therapies through tumor sample collection, shared data, shared samples, new methods to test treatments, and new trial designs. In 2022, MyPART welcomed NORD’s Rare Cancer Coalition as an advocacy partner.
Meanwhile, advocacy organizations are giving rare cancer a rising voice. NORD’s Rare Cancer Coalition unites rare cancer patient advocacy organizations and helps them drive progress together. The coalition promotes research and awareness through its annual Rare Cancer Day (September 30) campaign. Additionally, NORD has produced over 22 continuing medical education modules on rare cancers in collaboration with PlatformQ Health, providing updates on new therapies and treatment approaches. NORD also offers rare disease reports and educational videos on rare cancers, sessions inclusive of rare cancer topics at the annual NORD Summit, and a quarterly e-newsletter, “Caring for Rare” for healthcare professionals. Please visit us at rarediseases.org to access these resources.
Much work on rare cancers remains to be done, but the progress over recent years points to better outcomes moving forward. We are grateful for the work you do and your dedication to your patients, including those with rare cancers and other rare conditions. We hope you will find the information in this special issue useful for your clinical practice.
– Katie Kowalski, MPH
Associate Director of Education
National Organization for Rare Disorders
While people living with rare cancers continue to face daunting obstacles, progress is being made, and there are reasons to hope for a better future. Advances in genomic testing and precision medicine provide increasing evidence that rare cancers can be more efficiently and effectively diagnosed and treated. Genomic tests examine tumor DNA to identify mutations that are unique to an individual’s cancer. This genetic information enables a more precise diagnosis and targeted treatment approach. Jim Palma, Co-Lead of the NORD Rare Cancer Coalition, said “There is promise for rare cancer patients due to increased legislative efforts to cover the costs of genomic testing coupled by an increase in FDA approvals for targeted and tissue agnostic therapies.”
In 2019, the National Cancer Institute established MyPART, a vast pediatric and adult rare tumor network that aims to bolster patient involvement in research and develop effective therapies through tumor sample collection, shared data, shared samples, new methods to test treatments, and new trial designs. In 2022, MyPART welcomed NORD’s Rare Cancer Coalition as an advocacy partner.
Meanwhile, advocacy organizations are giving rare cancer a rising voice. NORD’s Rare Cancer Coalition unites rare cancer patient advocacy organizations and helps them drive progress together. The coalition promotes research and awareness through its annual Rare Cancer Day (September 30) campaign. Additionally, NORD has produced over 22 continuing medical education modules on rare cancers in collaboration with PlatformQ Health, providing updates on new therapies and treatment approaches. NORD also offers rare disease reports and educational videos on rare cancers, sessions inclusive of rare cancer topics at the annual NORD Summit, and a quarterly e-newsletter, “Caring for Rare” for healthcare professionals. Please visit us at rarediseases.org to access these resources.
Much work on rare cancers remains to be done, but the progress over recent years points to better outcomes moving forward. We are grateful for the work you do and your dedication to your patients, including those with rare cancers and other rare conditions. We hope you will find the information in this special issue useful for your clinical practice.
– Katie Kowalski, MPH
Associate Director of Education
National Organization for Rare Disorders
- About Rare Cancers. National Cancer Institute. Posted February 27, 2019. Accessed April 28, 2023. http://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/about-rare-cancers
- Gatta G, Capocaccia R, Botta L, et al. Burden and centralized treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017,18(8):1022–1039. doi:10.1016/S1470-2045(17)30445-X
- About Rare Cancers. National Cancer Institute. Posted February 27, 2019. Accessed April 28, 2023. http://www.cancer.gov/pediatric-adult-rare-tumor/rare-tumors/about-rare-cancers
- Gatta G, Capocaccia R, Botta L, et al. Burden and centralized treatment in Europe of rare tumours: Results of RARECAREnet-a population-based study. Lancet Oncol. 2017,18(8):1022–1039. doi:10.1016/S1470-2045(17)30445-X
Multiprong strategy makes clinical trials less White
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ASCO 2023