User login
Topical PDE4 Inhibitor Now Approved for Atopic Dermatitis in Children, Adults
On July 9, the aged 6 years or older.
Roflumilast cream 0.15%, which has been developed by Arcutis Biotherapeutics and is marketed under the brand name Zoryve, is a steroid-free topical phosphodiesterase-4 inhibitor that was previously approved in a higher concentration to treat seborrheic dermatitis and plaque psoriasis.
According to a press release from Arcutis, approval for AD was supported by positive results from three phase 3 studies, a phase 2 dose-ranging study, and two phase 1 pharmacokinetic trials. In two identical phase 3 studies known as INTEGUMENT-1 and INTEGUMENT-2, about 40% of children and adults treated with roflumilast cream 0.15% achieved a Validated Investigator Global Assessment for Atopic Dermatitis score of clear (0) or almost clear (1) at week 4 (INTEGUMENT-1: 41.5% vs 25.2%; P < .0001; INTEGUMENT-2: 39% vs 16.9%; P < .0001), with significant improvement as early as week 1 (P < .0001).
Among children and adults who participated in the INTEGUMENT studies for 28 and 56 weeks, 61.3% and 65.7% achieved a 75% reduction in their Eczema Area and Severity Index scores, respectively. According to the company, there were no adverse reactions in the combined phase 3 pivotal trials that occurred in more than 2.9% of participants in either arm. The most common adverse reactions included headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
The product is expected to be available commercially at the end of July 2024, according to Arcutis. Roflumilast cream 0.3% is indicated for topical treatment of plaque psoriasis, including intertriginous areas, in adult and pediatric patients aged 6 years or older; roflumilast foam 0.3% is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients aged 9 years or older.
A version of this article first appeared on Medscape.com.
On July 9, the aged 6 years or older.
Roflumilast cream 0.15%, which has been developed by Arcutis Biotherapeutics and is marketed under the brand name Zoryve, is a steroid-free topical phosphodiesterase-4 inhibitor that was previously approved in a higher concentration to treat seborrheic dermatitis and plaque psoriasis.
According to a press release from Arcutis, approval for AD was supported by positive results from three phase 3 studies, a phase 2 dose-ranging study, and two phase 1 pharmacokinetic trials. In two identical phase 3 studies known as INTEGUMENT-1 and INTEGUMENT-2, about 40% of children and adults treated with roflumilast cream 0.15% achieved a Validated Investigator Global Assessment for Atopic Dermatitis score of clear (0) or almost clear (1) at week 4 (INTEGUMENT-1: 41.5% vs 25.2%; P < .0001; INTEGUMENT-2: 39% vs 16.9%; P < .0001), with significant improvement as early as week 1 (P < .0001).
Among children and adults who participated in the INTEGUMENT studies for 28 and 56 weeks, 61.3% and 65.7% achieved a 75% reduction in their Eczema Area and Severity Index scores, respectively. According to the company, there were no adverse reactions in the combined phase 3 pivotal trials that occurred in more than 2.9% of participants in either arm. The most common adverse reactions included headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
The product is expected to be available commercially at the end of July 2024, according to Arcutis. Roflumilast cream 0.3% is indicated for topical treatment of plaque psoriasis, including intertriginous areas, in adult and pediatric patients aged 6 years or older; roflumilast foam 0.3% is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients aged 9 years or older.
A version of this article first appeared on Medscape.com.
On July 9, the aged 6 years or older.
Roflumilast cream 0.15%, which has been developed by Arcutis Biotherapeutics and is marketed under the brand name Zoryve, is a steroid-free topical phosphodiesterase-4 inhibitor that was previously approved in a higher concentration to treat seborrheic dermatitis and plaque psoriasis.
According to a press release from Arcutis, approval for AD was supported by positive results from three phase 3 studies, a phase 2 dose-ranging study, and two phase 1 pharmacokinetic trials. In two identical phase 3 studies known as INTEGUMENT-1 and INTEGUMENT-2, about 40% of children and adults treated with roflumilast cream 0.15% achieved a Validated Investigator Global Assessment for Atopic Dermatitis score of clear (0) or almost clear (1) at week 4 (INTEGUMENT-1: 41.5% vs 25.2%; P < .0001; INTEGUMENT-2: 39% vs 16.9%; P < .0001), with significant improvement as early as week 1 (P < .0001).
Among children and adults who participated in the INTEGUMENT studies for 28 and 56 weeks, 61.3% and 65.7% achieved a 75% reduction in their Eczema Area and Severity Index scores, respectively. According to the company, there were no adverse reactions in the combined phase 3 pivotal trials that occurred in more than 2.9% of participants in either arm. The most common adverse reactions included headache (2.9%), nausea (1.9%), application-site pain (1.5%), diarrhea (1.5%), and vomiting (1.5%).
The product is expected to be available commercially at the end of July 2024, according to Arcutis. Roflumilast cream 0.3% is indicated for topical treatment of plaque psoriasis, including intertriginous areas, in adult and pediatric patients aged 6 years or older; roflumilast foam 0.3% is indicated for the treatment of seborrheic dermatitis in adult and pediatric patients aged 9 years or older.
A version of this article first appeared on Medscape.com.
Strong Sibling Link With Autism Spectrum Disorder
according to a study published in Pediatrics.
When a baby had more than one older sibling with autism, the family recurrence rate rose to 36.9%, the study found.
The researchers, led by Sally Ozonoff, PhD, Department of Psychiatry and Behavioral Sciences at University of California Davis Health in Sacramento, analyzed data from 1,605 infants who had an older sibling with ASD using data from the global Baby Siblings Research Consortium.
They calculated that the rate of autism recurrence is seven times higher in families who already have one autistic child than in the general population, which points to the importance of close developmental observance in infants born in families with autistic children, particularly male infants in those families. This study replicated a 2011 study, also led by Dr. Ozonoff, which found a similar rate of familial recurrence.
Differences by Sex and Race
Dr. Ozonoff’s team found that sex and race played a part in likelihood of recurrence. Younger siblings of females with ASD were much more likely to develop the disorder (34.7%) than siblings of boys (22.5%). And male younger siblings were more likely to have ASD than girls (25.3% vs. 13.1%).
Additionally, ASD recurrence in White families was 17.8% while across other races collectively the recurrence rate was 25%.
Links with Maternal Education
Differences by maternal education were also striking. Recurrence was 32.6% when mothers had a high school or less education; 25.5% with some college; 19.7 with a college degree; and 16.9% with a graduate degree. The parental education revealed a significant effect only for mothers (P < .01); paternal education was not significant (P = .09).
Suzanne Rybczynski, MD, chief medical officer at East Tennessee Children’s Hospital in Knoxville, who was not part of the study, praised the study for following babies over time, “doing serial evaluation using two very standard tools in diagnosing autism and developmental delay.”
The babies were evaluated as early as 6 months of age, for up to seven visits. A final assessment was made at 36 months.
Dr. Rybczynski said it was interesting to see that, although ASD prevalence has increased substantially from the 2011 study (0.9%-2.8%), the findings regarding the sibling link have been consistent (18.7% in the 2011 study to 20.2% now).
Eliminating Biases
Dr. Rybczynski noted the current study also used diagnoses only from autism experts, which strengthened the findings, noting the potential for overdiagnosis when interviews are with the parents. “This really eliminates those biases.”
The authors explained the factors driving the need to update recurrence rate studies, including the growth in the prevalence of ASD in the last decade to 1 in 36. That may be caused partly by “greater awareness and identification of autistic females and cognitively able, verbal children.”
Also, new diagnostic criteria have been published, with different diagnostic thresholds since the last study. This study, they noted, had a sample size twice as large and more diverse than the 2011 sample.
The size and the diversity are particularly important, Dr. Rybczynski said, as it helps support more recent findings that ASD is not as heavily centered in White males as previously thought.
“We need to make sure we’re monitoring all children, especially from groups where there’s at least one older sibling or multiple siblings with autism or a sister with autism,” she said. The findings of this study are important not just for pediatricians but for families and all who have professional interactions with children.
Dr. Ozonoff reports travel reimbursements and honoraria from Autism Speaks and the Autism Science Foundation and book royalties from Guilford Press. One coauthor has served as a paid consultant to F. Hoffmann–La Roche and Servier and has received royalties from Sage Publications and Guilford Publications. Another is supported by the Stollery Children’s Hospital Foundation Chair in Autism. One coauthor reported a consulting agreement with EarliTec Diagnostics and book royalties from Wiley. A fourth coauthor has received funding from the Simons Foundation and consults for the Beasley Law Firm and Linus Technology. Dr. Rybczynski reported no relevant financial relationships.
according to a study published in Pediatrics.
When a baby had more than one older sibling with autism, the family recurrence rate rose to 36.9%, the study found.
The researchers, led by Sally Ozonoff, PhD, Department of Psychiatry and Behavioral Sciences at University of California Davis Health in Sacramento, analyzed data from 1,605 infants who had an older sibling with ASD using data from the global Baby Siblings Research Consortium.
They calculated that the rate of autism recurrence is seven times higher in families who already have one autistic child than in the general population, which points to the importance of close developmental observance in infants born in families with autistic children, particularly male infants in those families. This study replicated a 2011 study, also led by Dr. Ozonoff, which found a similar rate of familial recurrence.
Differences by Sex and Race
Dr. Ozonoff’s team found that sex and race played a part in likelihood of recurrence. Younger siblings of females with ASD were much more likely to develop the disorder (34.7%) than siblings of boys (22.5%). And male younger siblings were more likely to have ASD than girls (25.3% vs. 13.1%).
Additionally, ASD recurrence in White families was 17.8% while across other races collectively the recurrence rate was 25%.
Links with Maternal Education
Differences by maternal education were also striking. Recurrence was 32.6% when mothers had a high school or less education; 25.5% with some college; 19.7 with a college degree; and 16.9% with a graduate degree. The parental education revealed a significant effect only for mothers (P < .01); paternal education was not significant (P = .09).
Suzanne Rybczynski, MD, chief medical officer at East Tennessee Children’s Hospital in Knoxville, who was not part of the study, praised the study for following babies over time, “doing serial evaluation using two very standard tools in diagnosing autism and developmental delay.”
The babies were evaluated as early as 6 months of age, for up to seven visits. A final assessment was made at 36 months.
Dr. Rybczynski said it was interesting to see that, although ASD prevalence has increased substantially from the 2011 study (0.9%-2.8%), the findings regarding the sibling link have been consistent (18.7% in the 2011 study to 20.2% now).
Eliminating Biases
Dr. Rybczynski noted the current study also used diagnoses only from autism experts, which strengthened the findings, noting the potential for overdiagnosis when interviews are with the parents. “This really eliminates those biases.”
The authors explained the factors driving the need to update recurrence rate studies, including the growth in the prevalence of ASD in the last decade to 1 in 36. That may be caused partly by “greater awareness and identification of autistic females and cognitively able, verbal children.”
Also, new diagnostic criteria have been published, with different diagnostic thresholds since the last study. This study, they noted, had a sample size twice as large and more diverse than the 2011 sample.
The size and the diversity are particularly important, Dr. Rybczynski said, as it helps support more recent findings that ASD is not as heavily centered in White males as previously thought.
“We need to make sure we’re monitoring all children, especially from groups where there’s at least one older sibling or multiple siblings with autism or a sister with autism,” she said. The findings of this study are important not just for pediatricians but for families and all who have professional interactions with children.
Dr. Ozonoff reports travel reimbursements and honoraria from Autism Speaks and the Autism Science Foundation and book royalties from Guilford Press. One coauthor has served as a paid consultant to F. Hoffmann–La Roche and Servier and has received royalties from Sage Publications and Guilford Publications. Another is supported by the Stollery Children’s Hospital Foundation Chair in Autism. One coauthor reported a consulting agreement with EarliTec Diagnostics and book royalties from Wiley. A fourth coauthor has received funding from the Simons Foundation and consults for the Beasley Law Firm and Linus Technology. Dr. Rybczynski reported no relevant financial relationships.
according to a study published in Pediatrics.
When a baby had more than one older sibling with autism, the family recurrence rate rose to 36.9%, the study found.
The researchers, led by Sally Ozonoff, PhD, Department of Psychiatry and Behavioral Sciences at University of California Davis Health in Sacramento, analyzed data from 1,605 infants who had an older sibling with ASD using data from the global Baby Siblings Research Consortium.
They calculated that the rate of autism recurrence is seven times higher in families who already have one autistic child than in the general population, which points to the importance of close developmental observance in infants born in families with autistic children, particularly male infants in those families. This study replicated a 2011 study, also led by Dr. Ozonoff, which found a similar rate of familial recurrence.
Differences by Sex and Race
Dr. Ozonoff’s team found that sex and race played a part in likelihood of recurrence. Younger siblings of females with ASD were much more likely to develop the disorder (34.7%) than siblings of boys (22.5%). And male younger siblings were more likely to have ASD than girls (25.3% vs. 13.1%).
Additionally, ASD recurrence in White families was 17.8% while across other races collectively the recurrence rate was 25%.
Links with Maternal Education
Differences by maternal education were also striking. Recurrence was 32.6% when mothers had a high school or less education; 25.5% with some college; 19.7 with a college degree; and 16.9% with a graduate degree. The parental education revealed a significant effect only for mothers (P < .01); paternal education was not significant (P = .09).
Suzanne Rybczynski, MD, chief medical officer at East Tennessee Children’s Hospital in Knoxville, who was not part of the study, praised the study for following babies over time, “doing serial evaluation using two very standard tools in diagnosing autism and developmental delay.”
The babies were evaluated as early as 6 months of age, for up to seven visits. A final assessment was made at 36 months.
Dr. Rybczynski said it was interesting to see that, although ASD prevalence has increased substantially from the 2011 study (0.9%-2.8%), the findings regarding the sibling link have been consistent (18.7% in the 2011 study to 20.2% now).
Eliminating Biases
Dr. Rybczynski noted the current study also used diagnoses only from autism experts, which strengthened the findings, noting the potential for overdiagnosis when interviews are with the parents. “This really eliminates those biases.”
The authors explained the factors driving the need to update recurrence rate studies, including the growth in the prevalence of ASD in the last decade to 1 in 36. That may be caused partly by “greater awareness and identification of autistic females and cognitively able, verbal children.”
Also, new diagnostic criteria have been published, with different diagnostic thresholds since the last study. This study, they noted, had a sample size twice as large and more diverse than the 2011 sample.
The size and the diversity are particularly important, Dr. Rybczynski said, as it helps support more recent findings that ASD is not as heavily centered in White males as previously thought.
“We need to make sure we’re monitoring all children, especially from groups where there’s at least one older sibling or multiple siblings with autism or a sister with autism,” she said. The findings of this study are important not just for pediatricians but for families and all who have professional interactions with children.
Dr. Ozonoff reports travel reimbursements and honoraria from Autism Speaks and the Autism Science Foundation and book royalties from Guilford Press. One coauthor has served as a paid consultant to F. Hoffmann–La Roche and Servier and has received royalties from Sage Publications and Guilford Publications. Another is supported by the Stollery Children’s Hospital Foundation Chair in Autism. One coauthor reported a consulting agreement with EarliTec Diagnostics and book royalties from Wiley. A fourth coauthor has received funding from the Simons Foundation and consults for the Beasley Law Firm and Linus Technology. Dr. Rybczynski reported no relevant financial relationships.
FROM PEDIATRICS
School Avoidance
The start of the school year is a time that is always full of anticipation and even anxiety. Who will my teachers be? Will I be in classes with friends? Have some of my friends changed over the summer? Will the work be too hard? For some children this anxiety will be so intense that they will resist going back to school. School avoidance is very important to identify and address quickly, as it can intensify and threaten development. Each day of school missed due to accommodating to a child’s anxiety makes a return to school more difficult and less likely. Days can easily become weeks and even months of missed school. A child who misses a substantial amount of school is inevitably going to face developmental delays: academic, social, behavioral and emotional. The pediatrician is often brought into these situations early, as when a child complains of vague physical symptoms that are keeping him or her from school or when a previously calm child becomes inconsolable about going to school in the mornings. With a thoughtful assessment of the potential causes of school avoidance, you can help almost all children return to school successfully.
School Refusal
Sustained school avoidance is now called “school refusal,” a term coined in the late 1990s to describe a school attendance problem driven by emotional distress, as opposed to truancy. It affects up to 15% of children (depending on the operational definition) and seems to peak in the earliest years of elementary school and again in early high school. These are not occasional absences, but missing over 80% of classroom time in a 2-week period. It is also marked by the presence of an anxiety disorder and the absence of conduct disorder. Often in such cases the parents are aware of their child’s whereabouts and motivated to return them to school. Youth with school refusal experience social and academic consequences in the short term and, over the long term, have shown problems with social, family, and professional performance, along with higher rates of major depressive disorder than is seen in the general population. Early identification of these children can make addressing the underlying distress and return to school much easier than attempts to treat after weeks or months out of school.
Identifying the Problem
With younger children, school avoidance is most commonly associated with an anxious temperament or an underlying anxiety disorder, such as separation anxiety disorder or social phobia. A family history of anxiety may contribute or impact a parent’s approach to the issue. Children often present with vague somatic concerns that are genuine symptoms of anxiety (upset stomach, headache). A screening instrument such as the Screen for Child Anxiety Related Disorders (SCARED) can be helpful, but so is inquiring about sleep and other anxiety symptoms. Do the symptoms remit on weekends or in after-school hours? Are there other environmental factors that may be stressing younger children: Are they being teased or bullied at school? Are they struggling to find friends in a new classroom? Might they be having trouble with reading or other new tasks? Perhaps they are afraid of walking to school alone. Has there been a recent change or stress at home, such as a move or parental illness? Younger children may feel more anxious about separating from parents in the face of stress. But when parents accommodate a child’s wish to avoid school, the child’s anxiety, briefly relieved, grows more persistent, gets rewarded by parental attention, and reinforces their reluctance to try new things.
Adolescents may be facing more complex challenges that lead to school avoidance. They may have an undiagnosed anxiety or mood disorder, perhaps complicated by substance abuse that is presenting as an inability to perform at school or to manage the challenge of keeping up with higher workloads. They may be facing complex situations with friends, bullying, or rejection. Those adolescents who are prone to procrastination may avoid school to manage their workload and their distress, which can then become tangled up with symptoms of anxiety and dysphoria. Missing school compounds this problem rather than solving it. Adolescents outside of the structure of school, hungry for socializing and new experiences, often turn to social media for entertainment. Days without exercise and nights without adequate sleep can make mood, attention, and anxiety symptoms worse while overdue work grows. Parents often fear that setting limits or “pushing” their stuck and miserable child may make them more depressed or even suicidal.
Accommodating the Problem Will Likely Make It Worse
It is worth noting that children with a genuine medical illness can also experience school avoidance. Temperamentally anxious children who stay home for several days with a febrile illness may find it overwhelming to return to school as they have become so comfortable at home. Adolescents may have fallen behind with work and find themselves unable to set a schedule and return to more structure. Youth who are managing a known mood or anxiety disorder often have low motivation or high anxiety and want to wait to feel entirely better before returning to school. Youth with a chronic condition such as severe allergies or a sustained viral infection may be anxious about managing symptoms at school. Their parents may have kept them home to be safe or until they feel better, unwittingly making the school avoidance worse.
Formulating a Management Plan
When you suspect school avoidance is present, the critical first step is to engage the parents alongside their child. Without their understanding of the nature of this behavior, it will continue. Start by acknowledging the real physical and emotional symptoms their child is experiencing; it is important that parents and patients not feel that they are being told this is “just” a psychological problem. Children rarely feign illness or manipulate; they genuinely feel bad enough to stay home. It is important that they understand this is a common problem that will get worse unless it is addressed directly. If you believe they are suffering from a mood or anxiety disorder, talk about treatment options and consider getting started with treatment while finding a therapist to participate in their care. Help everyone listen to the child or teenager to understand any realistic basis for anxiety and attempt to address it (e.g. address bullying, provide a tutor, support a parent dependent on the child, etc.)
You can partner with parents and the school to provide the child with structure and support to make the return to school manageable. Frame the challenge of “demagnetizing” home and “remagnetizing” school. When they are at home, there should be no screen time except to catch up or keep up with homework. The child should not be in bed all day unless he or she has a fever. There needs to be close attention paid to maintaining a regular routine, with bedtime and wake time, meals with family, and regular exercise. This may mean turning off the Wi-Fi while a child is at home and parents are at work and providing them with books.
Work with the school to make getting into school and staying there as easy as possible. If a child has very high distress or has been out of school for a long time, he or she may need to return gradually; perhaps aim for the child to spend an hour at school for the first few days and then gradually work up to half and full days. Younger children may benefit from having a “buddy” who meets them outside and enters school with them. This can help avoid intense emotional scenes with parents that heighten distress and lead to accommodation. The child can identify a preferred teacher (or librarian, coach, or school nurse). When they feel overwhelmed, they can have a “break” with that teacher to avoid leaving school altogether. If they enjoy sports, music, or art, emphasize these classes or practices as part of their return to school.
Remind parents and your patients that it is not a matter of making the distress better first and then returning to school. They can be in treatment for an illness and manage returning to school at the same time. Indeed, the distress around school will only get better by getting back to school. This is hard! Ask about previous challenges they have managed or mastered and remind them that this is no different. Providing parents with knowledge and support will help them to be validating of their children without accommodating their wish to avoid discomfort. This support of your patient and the parents is the first step in helping them manage a difficult period and stay on their healthiest developmental trajectory.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
The start of the school year is a time that is always full of anticipation and even anxiety. Who will my teachers be? Will I be in classes with friends? Have some of my friends changed over the summer? Will the work be too hard? For some children this anxiety will be so intense that they will resist going back to school. School avoidance is very important to identify and address quickly, as it can intensify and threaten development. Each day of school missed due to accommodating to a child’s anxiety makes a return to school more difficult and less likely. Days can easily become weeks and even months of missed school. A child who misses a substantial amount of school is inevitably going to face developmental delays: academic, social, behavioral and emotional. The pediatrician is often brought into these situations early, as when a child complains of vague physical symptoms that are keeping him or her from school or when a previously calm child becomes inconsolable about going to school in the mornings. With a thoughtful assessment of the potential causes of school avoidance, you can help almost all children return to school successfully.
School Refusal
Sustained school avoidance is now called “school refusal,” a term coined in the late 1990s to describe a school attendance problem driven by emotional distress, as opposed to truancy. It affects up to 15% of children (depending on the operational definition) and seems to peak in the earliest years of elementary school and again in early high school. These are not occasional absences, but missing over 80% of classroom time in a 2-week period. It is also marked by the presence of an anxiety disorder and the absence of conduct disorder. Often in such cases the parents are aware of their child’s whereabouts and motivated to return them to school. Youth with school refusal experience social and academic consequences in the short term and, over the long term, have shown problems with social, family, and professional performance, along with higher rates of major depressive disorder than is seen in the general population. Early identification of these children can make addressing the underlying distress and return to school much easier than attempts to treat after weeks or months out of school.
Identifying the Problem
With younger children, school avoidance is most commonly associated with an anxious temperament or an underlying anxiety disorder, such as separation anxiety disorder or social phobia. A family history of anxiety may contribute or impact a parent’s approach to the issue. Children often present with vague somatic concerns that are genuine symptoms of anxiety (upset stomach, headache). A screening instrument such as the Screen for Child Anxiety Related Disorders (SCARED) can be helpful, but so is inquiring about sleep and other anxiety symptoms. Do the symptoms remit on weekends or in after-school hours? Are there other environmental factors that may be stressing younger children: Are they being teased or bullied at school? Are they struggling to find friends in a new classroom? Might they be having trouble with reading or other new tasks? Perhaps they are afraid of walking to school alone. Has there been a recent change or stress at home, such as a move or parental illness? Younger children may feel more anxious about separating from parents in the face of stress. But when parents accommodate a child’s wish to avoid school, the child’s anxiety, briefly relieved, grows more persistent, gets rewarded by parental attention, and reinforces their reluctance to try new things.
Adolescents may be facing more complex challenges that lead to school avoidance. They may have an undiagnosed anxiety or mood disorder, perhaps complicated by substance abuse that is presenting as an inability to perform at school or to manage the challenge of keeping up with higher workloads. They may be facing complex situations with friends, bullying, or rejection. Those adolescents who are prone to procrastination may avoid school to manage their workload and their distress, which can then become tangled up with symptoms of anxiety and dysphoria. Missing school compounds this problem rather than solving it. Adolescents outside of the structure of school, hungry for socializing and new experiences, often turn to social media for entertainment. Days without exercise and nights without adequate sleep can make mood, attention, and anxiety symptoms worse while overdue work grows. Parents often fear that setting limits or “pushing” their stuck and miserable child may make them more depressed or even suicidal.
Accommodating the Problem Will Likely Make It Worse
It is worth noting that children with a genuine medical illness can also experience school avoidance. Temperamentally anxious children who stay home for several days with a febrile illness may find it overwhelming to return to school as they have become so comfortable at home. Adolescents may have fallen behind with work and find themselves unable to set a schedule and return to more structure. Youth who are managing a known mood or anxiety disorder often have low motivation or high anxiety and want to wait to feel entirely better before returning to school. Youth with a chronic condition such as severe allergies or a sustained viral infection may be anxious about managing symptoms at school. Their parents may have kept them home to be safe or until they feel better, unwittingly making the school avoidance worse.
Formulating a Management Plan
When you suspect school avoidance is present, the critical first step is to engage the parents alongside their child. Without their understanding of the nature of this behavior, it will continue. Start by acknowledging the real physical and emotional symptoms their child is experiencing; it is important that parents and patients not feel that they are being told this is “just” a psychological problem. Children rarely feign illness or manipulate; they genuinely feel bad enough to stay home. It is important that they understand this is a common problem that will get worse unless it is addressed directly. If you believe they are suffering from a mood or anxiety disorder, talk about treatment options and consider getting started with treatment while finding a therapist to participate in their care. Help everyone listen to the child or teenager to understand any realistic basis for anxiety and attempt to address it (e.g. address bullying, provide a tutor, support a parent dependent on the child, etc.)
You can partner with parents and the school to provide the child with structure and support to make the return to school manageable. Frame the challenge of “demagnetizing” home and “remagnetizing” school. When they are at home, there should be no screen time except to catch up or keep up with homework. The child should not be in bed all day unless he or she has a fever. There needs to be close attention paid to maintaining a regular routine, with bedtime and wake time, meals with family, and regular exercise. This may mean turning off the Wi-Fi while a child is at home and parents are at work and providing them with books.
Work with the school to make getting into school and staying there as easy as possible. If a child has very high distress or has been out of school for a long time, he or she may need to return gradually; perhaps aim for the child to spend an hour at school for the first few days and then gradually work up to half and full days. Younger children may benefit from having a “buddy” who meets them outside and enters school with them. This can help avoid intense emotional scenes with parents that heighten distress and lead to accommodation. The child can identify a preferred teacher (or librarian, coach, or school nurse). When they feel overwhelmed, they can have a “break” with that teacher to avoid leaving school altogether. If they enjoy sports, music, or art, emphasize these classes or practices as part of their return to school.
Remind parents and your patients that it is not a matter of making the distress better first and then returning to school. They can be in treatment for an illness and manage returning to school at the same time. Indeed, the distress around school will only get better by getting back to school. This is hard! Ask about previous challenges they have managed or mastered and remind them that this is no different. Providing parents with knowledge and support will help them to be validating of their children without accommodating their wish to avoid discomfort. This support of your patient and the parents is the first step in helping them manage a difficult period and stay on their healthiest developmental trajectory.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
The start of the school year is a time that is always full of anticipation and even anxiety. Who will my teachers be? Will I be in classes with friends? Have some of my friends changed over the summer? Will the work be too hard? For some children this anxiety will be so intense that they will resist going back to school. School avoidance is very important to identify and address quickly, as it can intensify and threaten development. Each day of school missed due to accommodating to a child’s anxiety makes a return to school more difficult and less likely. Days can easily become weeks and even months of missed school. A child who misses a substantial amount of school is inevitably going to face developmental delays: academic, social, behavioral and emotional. The pediatrician is often brought into these situations early, as when a child complains of vague physical symptoms that are keeping him or her from school or when a previously calm child becomes inconsolable about going to school in the mornings. With a thoughtful assessment of the potential causes of school avoidance, you can help almost all children return to school successfully.
School Refusal
Sustained school avoidance is now called “school refusal,” a term coined in the late 1990s to describe a school attendance problem driven by emotional distress, as opposed to truancy. It affects up to 15% of children (depending on the operational definition) and seems to peak in the earliest years of elementary school and again in early high school. These are not occasional absences, but missing over 80% of classroom time in a 2-week period. It is also marked by the presence of an anxiety disorder and the absence of conduct disorder. Often in such cases the parents are aware of their child’s whereabouts and motivated to return them to school. Youth with school refusal experience social and academic consequences in the short term and, over the long term, have shown problems with social, family, and professional performance, along with higher rates of major depressive disorder than is seen in the general population. Early identification of these children can make addressing the underlying distress and return to school much easier than attempts to treat after weeks or months out of school.
Identifying the Problem
With younger children, school avoidance is most commonly associated with an anxious temperament or an underlying anxiety disorder, such as separation anxiety disorder or social phobia. A family history of anxiety may contribute or impact a parent’s approach to the issue. Children often present with vague somatic concerns that are genuine symptoms of anxiety (upset stomach, headache). A screening instrument such as the Screen for Child Anxiety Related Disorders (SCARED) can be helpful, but so is inquiring about sleep and other anxiety symptoms. Do the symptoms remit on weekends or in after-school hours? Are there other environmental factors that may be stressing younger children: Are they being teased or bullied at school? Are they struggling to find friends in a new classroom? Might they be having trouble with reading or other new tasks? Perhaps they are afraid of walking to school alone. Has there been a recent change or stress at home, such as a move or parental illness? Younger children may feel more anxious about separating from parents in the face of stress. But when parents accommodate a child’s wish to avoid school, the child’s anxiety, briefly relieved, grows more persistent, gets rewarded by parental attention, and reinforces their reluctance to try new things.
Adolescents may be facing more complex challenges that lead to school avoidance. They may have an undiagnosed anxiety or mood disorder, perhaps complicated by substance abuse that is presenting as an inability to perform at school or to manage the challenge of keeping up with higher workloads. They may be facing complex situations with friends, bullying, or rejection. Those adolescents who are prone to procrastination may avoid school to manage their workload and their distress, which can then become tangled up with symptoms of anxiety and dysphoria. Missing school compounds this problem rather than solving it. Adolescents outside of the structure of school, hungry for socializing and new experiences, often turn to social media for entertainment. Days without exercise and nights without adequate sleep can make mood, attention, and anxiety symptoms worse while overdue work grows. Parents often fear that setting limits or “pushing” their stuck and miserable child may make them more depressed or even suicidal.
Accommodating the Problem Will Likely Make It Worse
It is worth noting that children with a genuine medical illness can also experience school avoidance. Temperamentally anxious children who stay home for several days with a febrile illness may find it overwhelming to return to school as they have become so comfortable at home. Adolescents may have fallen behind with work and find themselves unable to set a schedule and return to more structure. Youth who are managing a known mood or anxiety disorder often have low motivation or high anxiety and want to wait to feel entirely better before returning to school. Youth with a chronic condition such as severe allergies or a sustained viral infection may be anxious about managing symptoms at school. Their parents may have kept them home to be safe or until they feel better, unwittingly making the school avoidance worse.
Formulating a Management Plan
When you suspect school avoidance is present, the critical first step is to engage the parents alongside their child. Without their understanding of the nature of this behavior, it will continue. Start by acknowledging the real physical and emotional symptoms their child is experiencing; it is important that parents and patients not feel that they are being told this is “just” a psychological problem. Children rarely feign illness or manipulate; they genuinely feel bad enough to stay home. It is important that they understand this is a common problem that will get worse unless it is addressed directly. If you believe they are suffering from a mood or anxiety disorder, talk about treatment options and consider getting started with treatment while finding a therapist to participate in their care. Help everyone listen to the child or teenager to understand any realistic basis for anxiety and attempt to address it (e.g. address bullying, provide a tutor, support a parent dependent on the child, etc.)
You can partner with parents and the school to provide the child with structure and support to make the return to school manageable. Frame the challenge of “demagnetizing” home and “remagnetizing” school. When they are at home, there should be no screen time except to catch up or keep up with homework. The child should not be in bed all day unless he or she has a fever. There needs to be close attention paid to maintaining a regular routine, with bedtime and wake time, meals with family, and regular exercise. This may mean turning off the Wi-Fi while a child is at home and parents are at work and providing them with books.
Work with the school to make getting into school and staying there as easy as possible. If a child has very high distress or has been out of school for a long time, he or she may need to return gradually; perhaps aim for the child to spend an hour at school for the first few days and then gradually work up to half and full days. Younger children may benefit from having a “buddy” who meets them outside and enters school with them. This can help avoid intense emotional scenes with parents that heighten distress and lead to accommodation. The child can identify a preferred teacher (or librarian, coach, or school nurse). When they feel overwhelmed, they can have a “break” with that teacher to avoid leaving school altogether. If they enjoy sports, music, or art, emphasize these classes or practices as part of their return to school.
Remind parents and your patients that it is not a matter of making the distress better first and then returning to school. They can be in treatment for an illness and manage returning to school at the same time. Indeed, the distress around school will only get better by getting back to school. This is hard! Ask about previous challenges they have managed or mastered and remind them that this is no different. Providing parents with knowledge and support will help them to be validating of their children without accommodating their wish to avoid discomfort. This support of your patient and the parents is the first step in helping them manage a difficult period and stay on their healthiest developmental trajectory.
Dr. Swick is physician in chief at Ohana, Center for Child and Adolescent Behavioral Health, Community Hospital of the Monterey (Calif.) Peninsula. Dr. Jellinek is professor emeritus of psychiatry and pediatrics, Harvard Medical School, Boston. Email them at [email protected].
Summer Is Not Over: Let's Talk About Recreational Water–Associated Illnesses
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Recently I was in Wyoming. As I rode down the Snake River, the guide pointed out tree trunks that had been chewed on by beavers. Days later I joined a local friend for a hike to Taggart Lake. Upon reaching the end of the trail as I began to cast my eyes on the magnificent scenery, I could not help but notice several children, including toddlers, playing in the fresh warm water. The next thing out of my friend’s mouth was “You know there is Giardia in there.” Little did she know, she and the guide had just helped me select a topic for ID Consult.
Giardia, aka ”beaver fever,” was discussed in detail in this column as part of the differential of a diarrheal illness by Christopher J. Harrison, MD. However, it is the perfect time of year to revisit other recreational water–associated illnesses.
Infections acquired during recreational water activity can lead to illnesses involving the gastrointestinal tract, central nervous system, respiratory tract, skin, eyes, and ears. Pathogens, chemicals, and toxins are transmitted by ingestion, contact with contaminated water or a sick individual or animal, and inhalation of aerosols. The National Waterborne Disease and Outbreak Surveillance System (WBDOSS) collects data on waterborne disease and outbreaks associated with recreational water, drinking water, and environmental and undetermined exposures to water. All reporting to the Centers for Disease Control and Prevention (CDC) is voluntary. However, mandatory pathogen reporting requirements can vary by state. Ideally, once an agency has completed the outbreak investigation, the definitive cause and source will be determined, and interventions to prevent future outbreaks implemented.
Treated Versus Untreated Water
One useful way to help narrow the etiology of a patient’s symptoms is to consider those illnesses associated with treated water venues (e.g., pools, hot tubs, water parks) versus untreated water venues (e.g., rivers, lakes, oceans). Parents may forget to offer that information since they may not perceive a connection between water exposure and the illness, especially if they traveled within the US.
In 2021, the CDC reported results of data submitted between 2015 and 2019 from treated recreational water facilities. Of the 208 outbreaks, most (96%) were associated with public pools, hot tubs, or water playgrounds. These outbreaks resulted in at least 3,646 cases of illness, 286 hospitalizations, and 13 deaths. Overall infectious etiologies were the primary cause of illness. Of the 155 outbreaks with a confirmed etiology, Cryptosporidium was the causative pathogen in 49% of the outbreaks and accounted for 84% (2,492) of cases, while Legionella caused 42% of outbreaks, accounted for 13% (354) of cases, and was responsible for all 13 deaths. Slightly more than half (107 of 208) of the outbreaks started between June-August with Cryptosporidium accounting for 63 of the outbreaks during that period. A little more than one-third were associated with a hotel or resort. The majority of hotel recreational water–associated illnesses was associated with hot tubs. Of the 53 outbreaks without a confirmed etiology, 20 were suspected to have a chemical related etiology (excess chlorine, altered pool chemistry).
In contrast, there were 140 untreated recreational water outbreaks reported between 2000 and 2014 from 35 states and Guam involving 4,958 cases and 2 deaths. The etiology was confirmed for 103 (74%) outbreaks including 5 that had multiple etiologies and 8 due to toxins or chemicals; 7 of 8 toxins were from harmful algal blooms. Enteric pathogens were the etiology in 84% of outbreaks including: Norovirus (n = 1459), Shigella (n = 362) Avian schistosomes (n = 345), Cryptosporidium (n = 314) and Escherichia coli (n = 155).There were 24 cases of Giardia. The two deaths were due to Naegleria fowleri. The top 2 settings for these outbreaks were public parks (36%) and beaches (32%) with most outbreaks (n = 117) being associated with a lake /pond venue. Most outbreaks began between June and August.
The major differences between the two types of recreational water–associated illnesses are their most common settings and etiologies. With that in mind, let us briefly review the most common etiology from each venue.
Treated Water Venue: Cryptosporidiosis
Cryptosporidium is an oocyst-forming protozoa that causes a self-limited watery, nonbloody diarrhea which usually resolves within 10-14 days. Most patients have associated abdominal cramps, fever, and vomiting although infected persons can be asymptomatic. Infection in the immunocompromised potentially can lead to profuse and prolonged diarrhea. Oocysts are excreted in the feces of infected hosts and as little as 10 can cause infection. They can survive extreme environmental conditions in water and soil for several months and even survive up to 7 days in a properly chlorinated pool. Transmission occurs between humans via contaminated food and water or from infected animals. Oocysts have been isolated in raw or unpasteurized milk and apple cider. Incidence is highest in children 1 through 4 years of age.
Diagnosis today is usually via molecular methods (nucleic acid amplification tests, aka NAATs), due to their high sensitivity and specificity and is the preferred method. These tests can identify multiple gastrointestinal tract pathogens with a single assay. Diagnosis by microscopy or fecal immunoassay antigens are still available. Treatment is supportive in most cases. If needed, a 3-day course of nitazoxanide can be prescribed. Immunocompromised patients should be managed in consultation with an infectious disease specialist.
Untreated Water Venue: Norovirus
Norovirus is a viral illness characterized by the abrupt onset of vomiting and/or watery diarrhea, usually associated with nausea and abdominal cramps. Symptoms persist 24-72 hours, however they may be prolonged in the immunocompromised and persons at the extremes of the age spectrum. Norovirus has replaced rotavirus as the major cause of medically attended gastroenteritis. While a major cause of recreational water–associated illnesses, high attack rates also occur in semi closed communities including cruise ships, childcare centers, and schools. Transmission is fecal-oral, vomitus oral, person to person, by ingestion of contaminated food and water or touching contaminated surfaces with subsequent touching of the mouth. Asymptomatic viral shedding may occur, especially in children. Prolonged shedding (> 6 mos.) has been reported in immunocompromised hosts.
Molecular diagnosis with stool is utilized most often. Treatment is supportive.
Take Home Message
When evaluating your patients for an acute gastrointestinal illness, consider water-related activities and their potential for being the source. Encourage patients not to ignore posted advisories on beaches, to not swim if they have diarrhea, not to swallow the water they swim in and to minimize water entering their nose while swimming in warm freshwater. If you start seeing several patients with similar symptoms and/or etiology, consider contacting your local or state health department. It could be the beginning of an outbreak.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She has no relevant financial disclosures.
Suggested Readings
Graciaa DS et al. Outbreaks Associated with Untreated Recreational Water — United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2018 Jun 29;67(25):701-706. doi: 10.15585/mmwr.mm6725a1.
Hlavsa MC et al. Outbreaks Associated with Treated Recreational Water — United States, 2015–2019. MMWR Morb Mortal Wkly Rep. 2021;70:733–738. doi: 10.15585/mmwr.mm7020a1.
Kimberlin DW et al., eds. Red Book Report of the Committee on Infectious Diseases. 33rd ed. American Academy of Pediatrics. 2024. Cryptosporidiosis, p 338-40 and Norovirus, p 622-624.Waterborne Outbreaks Summary Reports. CDC. 2024 April 18.
Let ’em Play: In Defense of Youth Football
Over the last couple of decades, I have become increasingly more uncomfortable watching American-style football on television. Lax refereeing coupled with over-juiced players who can generate g-forces previously attainable only on a NASA rocket sled has resulted in a spate of injuries I find unacceptable. The revolving door of transfers from college to college has made the term scholar-athlete a relic that can be applied to only a handful of players at the smallest uncompetitive schools.
Many of you who are regular readers of Letters from Maine have probably tired of my boasting that when I played football in high school we wore leather helmets. I enjoyed playing football and continued playing in college for a couple of years until it became obvious that “bench” was going to be my usual position. But, I would not want my grandson to play college football. Certainly, not at the elite college level. Were he to do so, he would be putting himself at risk for significant injury by participating in what I no longer view as an appealing activity. Let me add that I am not including chronic traumatic encephalopathy among my concerns, because I think its association with football injuries is far from settled. My concern is more about spinal cord injuries, which, although infrequent, are almost always devastating.
I should also make it perfectly clear that my lack of enthusiasm for college and professional football does not place me among the increasingly vocal throng calling for the elimination of youth football. For the 5- to 12-year-olds, putting on pads and a helmet and scrambling around on a grassy field bumping shoulders and heads with their peers is a wonderful way to burn off energy and satisfies a need for roughhousing that comes naturally to most young boys (and many girls). The chance of anyone of those kids playing youth football reaching the elite college or professional level is extremely unlikely. Other activities and the realization that football is not in their future weeds the field during adolescence.
Although there have been some studies suggesting that starting football at an early age is associated with increased injury risk, a recent and well-controlled study published in the journal Sports Medicine has found no such association in professional football players. This finding makes some sense when you consider that most of the children in this age group are not mustering g-forces anywhere close to those a college or professional athlete can generate.
Another recent study published in the Journal of Pediatrics offers more evidence to consider before one passes judgment on youth football. When reviewing the records of nearly 1500 patients in a specialty-care concussion setting at the Children’s Hospital of Philadelphia, investigators found that recreation-related concussions and non–sport- or recreation-related concussions were more prevalent than sports-related concussions. The authors propose that “less supervision at the time of injury and less access to established concussion healthcare following injury” may explain their observations.
Of course as a card-carrying AARP old fogey, I long for the good old days when youth sports were organized by the kids in backyards and playgrounds. There we learned to pick teams and deal with the disappointment of not being a first-round pick and the embarrassment of being a last rounder. We settled out-of-bounds calls and arguments about ball possession without adults’ assistance — or video replays for that matter. But those days are gone and likely never to return, with parental anxiety running at record highs. We must accept youth sports organized for kids by adults is the way it’s going to be for the foreseeable future.
As long as the program is organized with the emphasis on fun nor structured as a fast track to elite play it will be healthier for the kids than sitting on the couch at home watching the carnage on TV.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Over the last couple of decades, I have become increasingly more uncomfortable watching American-style football on television. Lax refereeing coupled with over-juiced players who can generate g-forces previously attainable only on a NASA rocket sled has resulted in a spate of injuries I find unacceptable. The revolving door of transfers from college to college has made the term scholar-athlete a relic that can be applied to only a handful of players at the smallest uncompetitive schools.
Many of you who are regular readers of Letters from Maine have probably tired of my boasting that when I played football in high school we wore leather helmets. I enjoyed playing football and continued playing in college for a couple of years until it became obvious that “bench” was going to be my usual position. But, I would not want my grandson to play college football. Certainly, not at the elite college level. Were he to do so, he would be putting himself at risk for significant injury by participating in what I no longer view as an appealing activity. Let me add that I am not including chronic traumatic encephalopathy among my concerns, because I think its association with football injuries is far from settled. My concern is more about spinal cord injuries, which, although infrequent, are almost always devastating.
I should also make it perfectly clear that my lack of enthusiasm for college and professional football does not place me among the increasingly vocal throng calling for the elimination of youth football. For the 5- to 12-year-olds, putting on pads and a helmet and scrambling around on a grassy field bumping shoulders and heads with their peers is a wonderful way to burn off energy and satisfies a need for roughhousing that comes naturally to most young boys (and many girls). The chance of anyone of those kids playing youth football reaching the elite college or professional level is extremely unlikely. Other activities and the realization that football is not in their future weeds the field during adolescence.
Although there have been some studies suggesting that starting football at an early age is associated with increased injury risk, a recent and well-controlled study published in the journal Sports Medicine has found no such association in professional football players. This finding makes some sense when you consider that most of the children in this age group are not mustering g-forces anywhere close to those a college or professional athlete can generate.
Another recent study published in the Journal of Pediatrics offers more evidence to consider before one passes judgment on youth football. When reviewing the records of nearly 1500 patients in a specialty-care concussion setting at the Children’s Hospital of Philadelphia, investigators found that recreation-related concussions and non–sport- or recreation-related concussions were more prevalent than sports-related concussions. The authors propose that “less supervision at the time of injury and less access to established concussion healthcare following injury” may explain their observations.
Of course as a card-carrying AARP old fogey, I long for the good old days when youth sports were organized by the kids in backyards and playgrounds. There we learned to pick teams and deal with the disappointment of not being a first-round pick and the embarrassment of being a last rounder. We settled out-of-bounds calls and arguments about ball possession without adults’ assistance — or video replays for that matter. But those days are gone and likely never to return, with parental anxiety running at record highs. We must accept youth sports organized for kids by adults is the way it’s going to be for the foreseeable future.
As long as the program is organized with the emphasis on fun nor structured as a fast track to elite play it will be healthier for the kids than sitting on the couch at home watching the carnage on TV.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Over the last couple of decades, I have become increasingly more uncomfortable watching American-style football on television. Lax refereeing coupled with over-juiced players who can generate g-forces previously attainable only on a NASA rocket sled has resulted in a spate of injuries I find unacceptable. The revolving door of transfers from college to college has made the term scholar-athlete a relic that can be applied to only a handful of players at the smallest uncompetitive schools.
Many of you who are regular readers of Letters from Maine have probably tired of my boasting that when I played football in high school we wore leather helmets. I enjoyed playing football and continued playing in college for a couple of years until it became obvious that “bench” was going to be my usual position. But, I would not want my grandson to play college football. Certainly, not at the elite college level. Were he to do so, he would be putting himself at risk for significant injury by participating in what I no longer view as an appealing activity. Let me add that I am not including chronic traumatic encephalopathy among my concerns, because I think its association with football injuries is far from settled. My concern is more about spinal cord injuries, which, although infrequent, are almost always devastating.
I should also make it perfectly clear that my lack of enthusiasm for college and professional football does not place me among the increasingly vocal throng calling for the elimination of youth football. For the 5- to 12-year-olds, putting on pads and a helmet and scrambling around on a grassy field bumping shoulders and heads with their peers is a wonderful way to burn off energy and satisfies a need for roughhousing that comes naturally to most young boys (and many girls). The chance of anyone of those kids playing youth football reaching the elite college or professional level is extremely unlikely. Other activities and the realization that football is not in their future weeds the field during adolescence.
Although there have been some studies suggesting that starting football at an early age is associated with increased injury risk, a recent and well-controlled study published in the journal Sports Medicine has found no such association in professional football players. This finding makes some sense when you consider that most of the children in this age group are not mustering g-forces anywhere close to those a college or professional athlete can generate.
Another recent study published in the Journal of Pediatrics offers more evidence to consider before one passes judgment on youth football. When reviewing the records of nearly 1500 patients in a specialty-care concussion setting at the Children’s Hospital of Philadelphia, investigators found that recreation-related concussions and non–sport- or recreation-related concussions were more prevalent than sports-related concussions. The authors propose that “less supervision at the time of injury and less access to established concussion healthcare following injury” may explain their observations.
Of course as a card-carrying AARP old fogey, I long for the good old days when youth sports were organized by the kids in backyards and playgrounds. There we learned to pick teams and deal with the disappointment of not being a first-round pick and the embarrassment of being a last rounder. We settled out-of-bounds calls and arguments about ball possession without adults’ assistance — or video replays for that matter. But those days are gone and likely never to return, with parental anxiety running at record highs. We must accept youth sports organized for kids by adults is the way it’s going to be for the foreseeable future.
As long as the program is organized with the emphasis on fun nor structured as a fast track to elite play it will be healthier for the kids than sitting on the couch at home watching the carnage on TV.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Tackling Inflammatory and Infectious Nail Disorders in Children
Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.
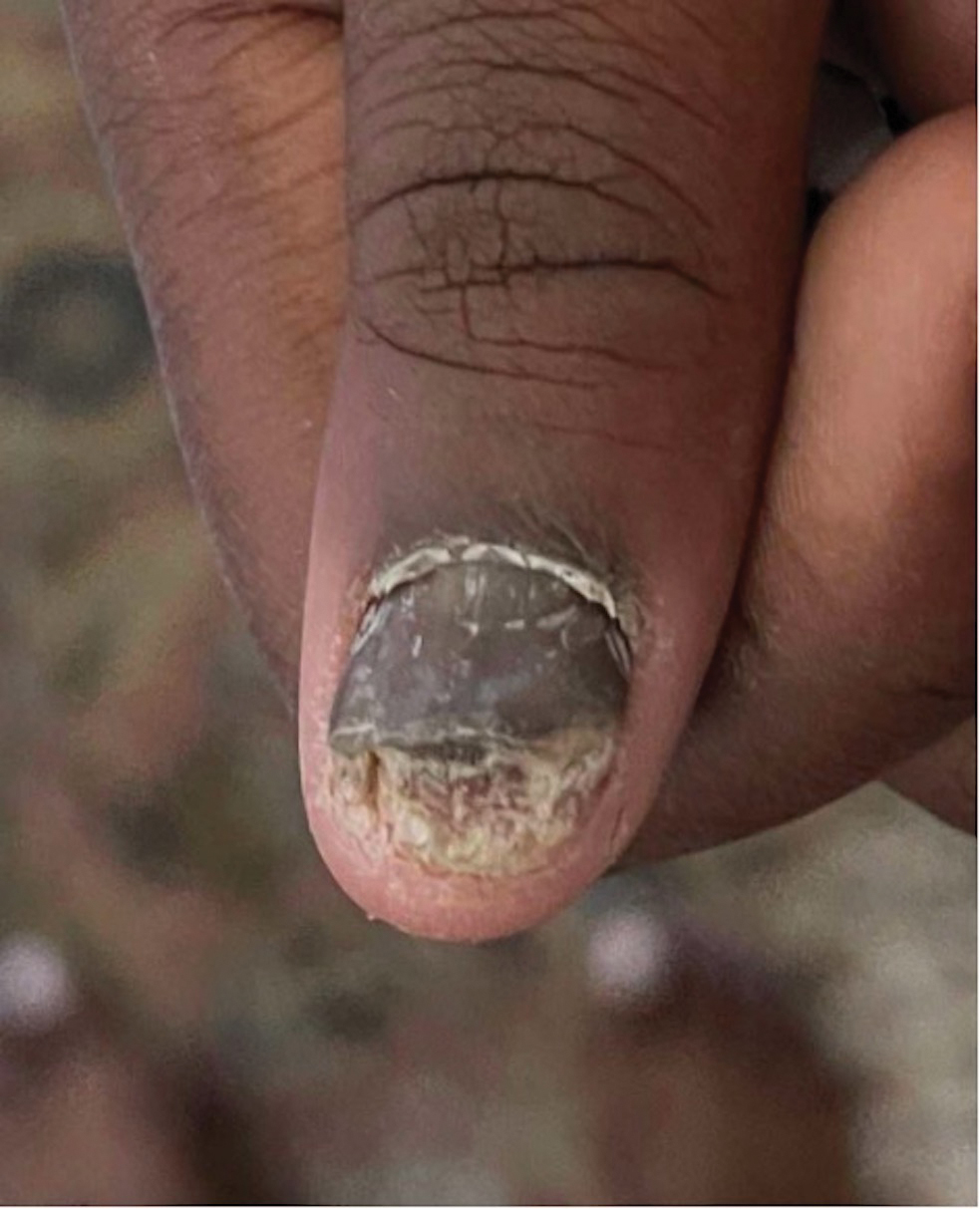
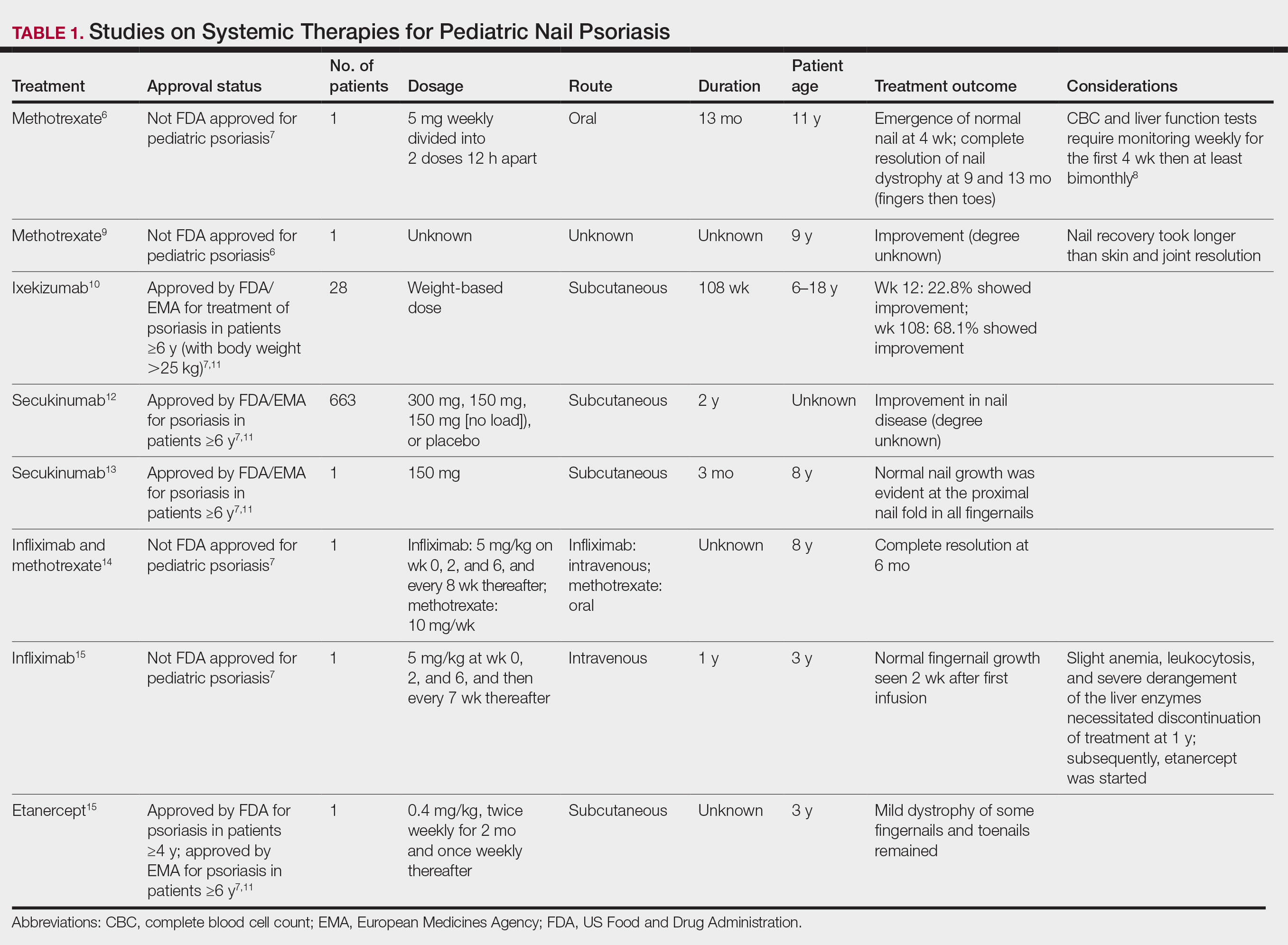
Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30
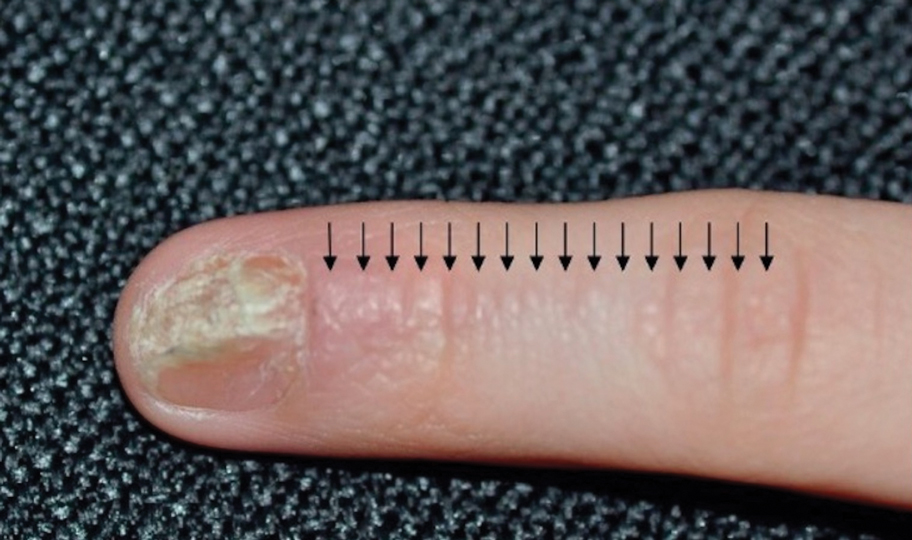
Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.
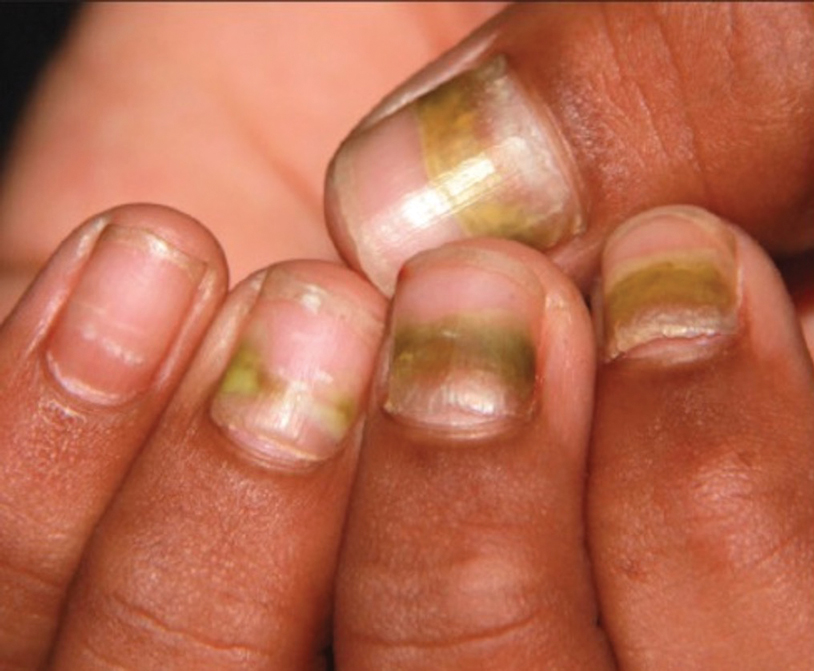
FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.
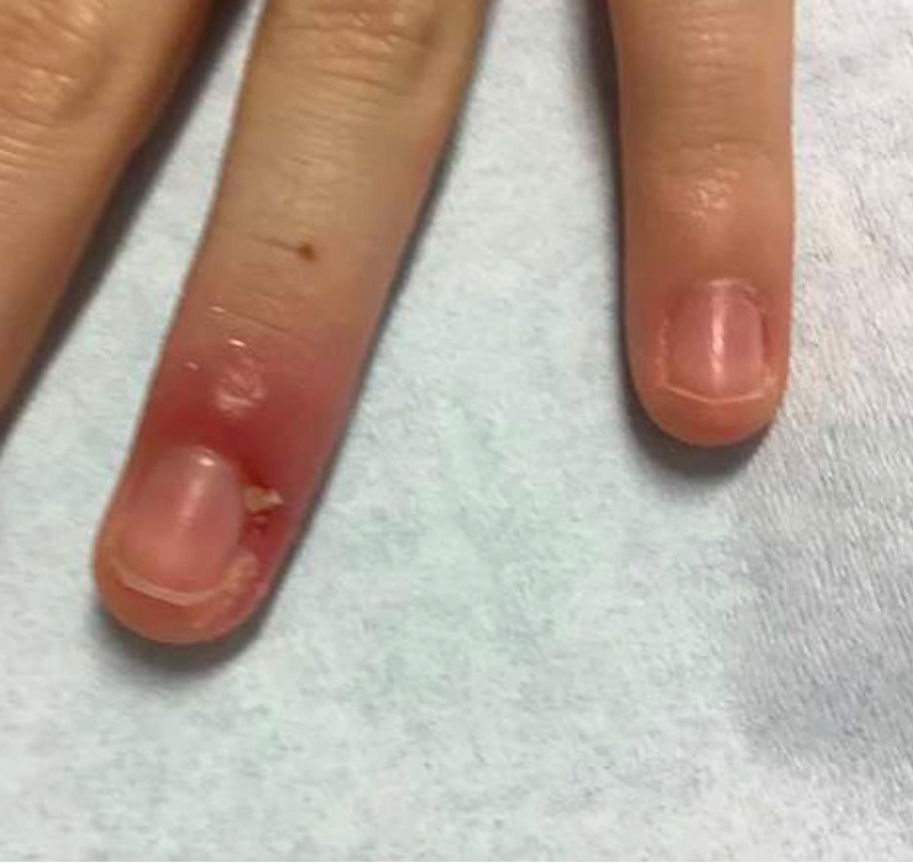
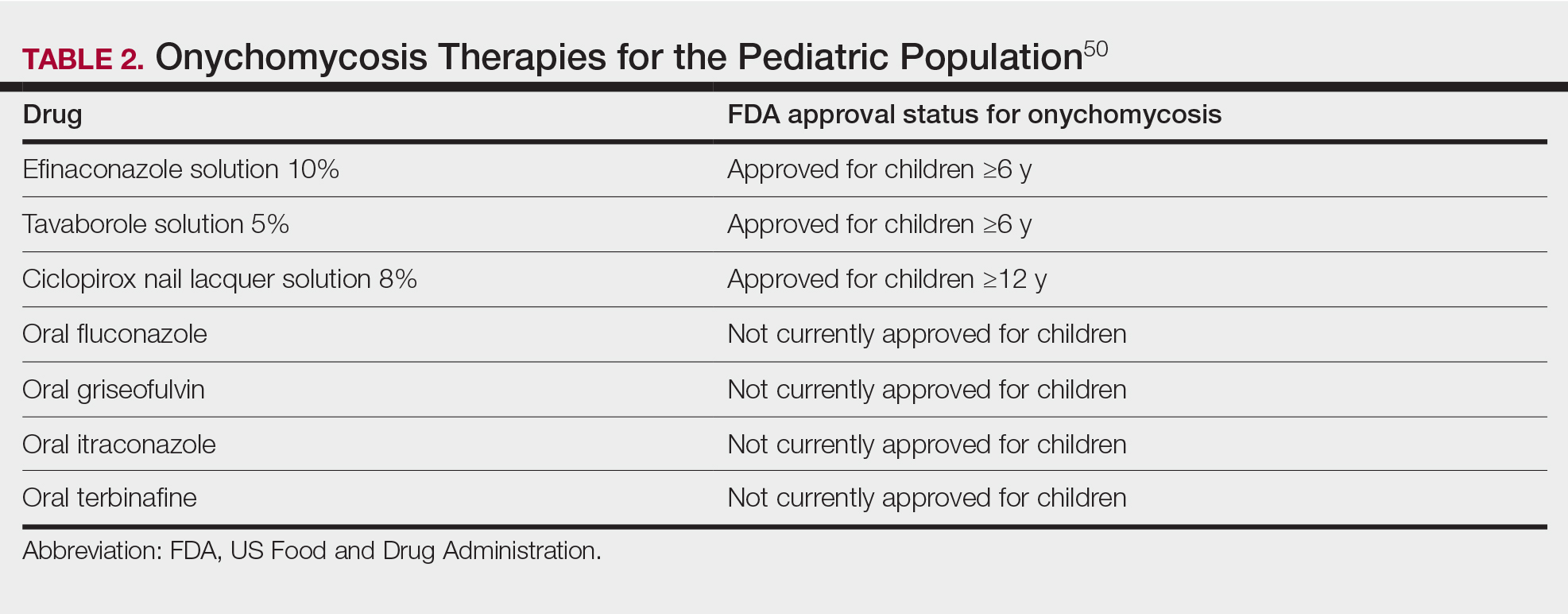
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.


Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30

Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.

FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.


Nail disorders are common among pediatric patients but often are underdiagnosed or misdiagnosed because of their unique disease manifestations. These conditions may severely impact quality of life. There are few nail disease clinical trials that include children. Consequently, most treatment recommendations are based on case series and expert consensus recommendations. We review inflammatory and infectious nail disorders in pediatric patients. By describing characteristics, clinical manifestations, and management approaches for these conditions, we aim to provide guidance to dermatologists in their diagnosis and treatment.
INFLAMMATORY NAIL DISORDERS
Nail Psoriasis
Nail involvement in children with psoriasis is common, with prevalence estimates ranging from 17% to 39.2%.1 Nail matrix psoriasis may manifest with pitting (large irregular pits) and leukonychia as well as chromonychia and nail plate crumbling. Onycholysis, oil drop spots (salmon patches), and subungual hyperkeratosis can be seen in nail bed psoriasis. Nail pitting is the most frequently observed clinical finding (Figure 1).2,3 In a cross-sectional multicenter study of 313 children with cutaneous psoriasis in France, nail findings were present in 101 patients (32.3%). There were associations between nail findings and presence of psoriatic arthritis (P=.03), palmoplantar psoriasis (P<.001), and severity of psoriatic disease, defined as use of systemic treatment with phototherapy (psoralen plus UVA, UVB), traditional systemic treatment (acitretin, methotrexate, cyclosporine), or a biologic (P=.003).4
Topical steroids and vitamin D analogues may be used with or without occlusion and may be efficacious.5 Several case reports describe systemic treatments for psoriasis in children, including methotrexate, acitretin, and apremilast (approved for children 6 years and older for plaque psoriasis by the US Food and Drug Administration [FDA]).2 There are 5 biologic drugs currently approved for the treatment of pediatric psoriasis—adalimumab, etanercept, ustekinumab, secukinumab, ixekizumab—and 6 drugs currently undergoing phase 3 studies—brodalumab, guselkumab, risankizumab, tildrakizumab, certolizumab pegol, and deucravacitinib (Table 1).6-15 Adalimumab is specifically approved for moderate to severe nail psoriasis in adults 18 years and older.


Intralesional steroid injections are sometimes useful in the management of nail matrix psoriasis; however, appropriate patient selection is critical due to the pain associated with the procedure. In a prospective study of 16 children (age range, 9–17 years) with nail psoriasis treated with intralesional triamcinolone (ILTAC) 2.5 to 5 mg/mL every 4 to 8 weeks for a minimum of 3 to 6 months, 9 patients achieved resolution and 6 had improvement of clinical findings.16 Local adverse events were mild, including injection-site pain (66%), subungual hematoma (n=1), Beau lines (n=1), proximal nail fold hypopigmentation (n=2), and proximal nail fold atrophy (n=2). Because the proximal nail fold in children is thinner than in adults, there may be an increased risk for nail fold hypopigmentation and atrophy in children. Therefore, a maximum ILTAC concentration of 2.5 mg/mL with 0.2 mL maximum volume per nail per session is recommended for children younger than 15 years.16
Nail Lichen Planus
Nail lichen planus (NLP) is uncommon in children, with few biopsy-proven cases documented in the literature.17 Common clinical findings are onychorrhexis, nail plate thinning, fissuring, splitting, and atrophy with koilonychia.5 Although pterygium development (irreversible nail matrix scarring) is uncommon in pediatric patients, NLP can be progressive and may cause irreversible destruction of the nail matrix and subsequent nail loss, warranting therapeutic intervention.18
Treatment of NLP may be difficult, as there are no options that work in all patients. Current literature supports the use of systemic corticosteroids or ILTAC for the treatment of NLP; however, recurrence rates can be high. According to an expert consensus paper on NLP treatment, ILTAC may be injected in a concentration of 2.5, 5, or 10 mg/mL according to disease severity.19 In severe or resistant cases, intramuscular (IM) triamcinolone may be considered, especially if more than 3 nails are affected. A dosage of 0.5 to 1 mg/kg/mo for at least 3 to 6 months is recommended for both children and adults, with 1 mg/kg/mo recommended in the active treatment phase (first 2–3 months).19 In a retrospective review of 5 pediatric patients with NLP treated with IM triamcinolone 0.5 mg/kg/mo, 3 patients had resolution and 2 improved with treatment.20 In a prospective study of 10 children with NLP, IM triamcinolone at a dosage of 0.5 to 1 mg/kg every 30 days for 3 to 6 months resulted in resolution of nail findings in 9 patients.17 In a prospective study of 14 pediatric patients with NLP treated with 2.5 to 5 mg/mL of ILTAC, 10 achieved resolution and 3 improved.16
Intralesional triamcinolone injections may be better suited for teenagers compared to younger children who may be more apprehensive of needles. To minimize pain, it is recommended to inject ILTAC slowly at room temperature, with use of “talkesthesia” and vibration devices, 1% lidocaine, or ethyl chloride spray.18
Trachyonychia
Trachyonychia is characterized by the presence of sandpaperlike nails. It manifests with brittle thin nails with longitudinal ridging, onychoschizia, and thickened hyperkeratotic cuticles. Trachyonychia typically involves multiple nails, with a peak age of onset between 3 and 12 years.21,22 There are 2 variants: the opaque type with rough longitudinal ridging, and the shiny variant with opalescent nails and pits that reflect light. The opaque variant is more common and is associated with psoriasis, whereas the shiny variant is less common and is associated with alopecia areata.23 Although most cases are idiopathic, some are associated with psoriasis and alopecia areata, as previously noted, as well as atopic dermatitis (AD) and lichen planus.22,24
Fortunately, trachyonychia does not lead to permanent nail damage or pterygium, making treatment primarily focused on addressing functional and cosmetic concerns.24 Spontaneous resolution occurs in approximately 50% of patients. In a prospective study of 11 patients with idiopathic trachyonychia, there was partial improvement in 5 of 9 patients treated with topical steroids, 1 with only petrolatum, and 1 with vitamin supplements. Complete resolution was reported in 1 patient treated with topical steroids.25 Because trachyonychia often is self-resolving, no treatment is required and a conservative approach is strongly recommended.26 Treatment options include topical corticosteroids, tazarotene, and 5-fluorouracil. Intralesional triamcinolone, systemic cyclosporine, retinoids, systemic corticosteroids, and tofacitinib have been described in case reports, though none of these have been shown to be 100% efficacious.24
Nail Lichen Striatus
Lichen striatus involving the nail is uncommon and is characterized by onycholysis, longitudinal ridging, splitting, and fraying, as well as what appears to be a subungual tumor. It can encompass the entire nail or may be isolated to a portion of the nail (Figure 2). Usually, a Blaschko-linear array of flesh-colored papules on the more proximal digit directly adjacent to the nail dystrophy will be seen, though nail findings can occur in isolation.27-29 The underlying pathophysiology is not clear; however, one hypothesis is that a triggering event, such as trauma, induces the expression of antigens that elicit a self-limiting immune-mediated response by CD8 T lymphocytes.30

Generally, nail lichen striatus spontaneously resolves in 1 to 2 years without treatment. In a prospective study of 5 patients with nail lichen striatus, the median time to resolution was 22.6 months (range, 10–30 months).31 Topical steroids may be used for pruritus. In one case report, a 3-year-old boy with nail lichen striatus of 4 months’ duration was treated with tacrolimus ointment 0.03% daily for 3 months.28
Nail AD
Nail changes with AD may be more common in adults than children or are underreported. In a study of 777 adults with AD, nail dystrophy was present in 124 patients (16%), whereas in a study of 250 pediatric patients with AD (aged 0-2 years), nail dystrophy was present in only 4 patients.32,33
Periungual inflammation from AD causes the nail changes.34 In a cross-sectional study of 24 pediatric patients with nail dystrophy due to AD, transverse grooves (Beau lines) were present in 25% (6/24), nail pitting in 16.7% (4/24), koilonychia in 16.7% (4/24), trachyonychia in 12.5% (3/24), leukonychia in 12.5% (3/24), brachyonychia in 8.3% (2/24), melanonychia in 8.3% (2/24), onychomadesis in 8.3% (2/24), onychoschizia in 8.3% (2/24), and onycholysis in 8.3% (2/24). There was an association between disease severity and presence of toenail dystrophy (P=.03).35
Topical steroids with or without occlusion can be used to treat nail changes. Although there is limited literature describing the treatment of nail AD in children, a 61-year-old man with nail changes associated with AD achieved resolution with 3 months of treatment with dupilumab.36 Anecdotally, most patients will improve with usual cutaneous AD management.
INFECTIOUS NAIL DISORDERS
Viral Infections
Hand, Foot, and Mouth Disease—Hand, foot, and mouth disease (HFMD) is a common childhood viral infection caused by various enteroviruses, most commonly coxsackievirus A16, with the A6 variant causing more severe disease. Fever and painful vesicles involving the oral mucosa as well as palms and soles give the disease its name. Nail changes are common. In a prospective study involving 130 patients with laboratory-confirmed coxsackievirus CA6 serotype infection, 37% developed onychomadesis vs only 5% of 145 cases with non-CA6 enterovirus infection who developed nail findings. There was an association between CA6 infection and presence of nail changes (P<.001).37
Findings ranging from transverse grooves (Beau lines) to complete nail shedding (onychomadesis)(Figure 3) may be seen.38,39 Nail findings in HFMD are due to transient inhibition of nail growth and present approximately 3 to 6 weeks after infection.40 Onychomadesis is seen in 30% to 68% of patients with HFMD.37,41,42 Nail findings in HFMD spontaneously resolve with nail growth (2–3 mm per month for fingernails and 1 mm per month for toenails) and do not require specific treatment. Although the appearance of nail changes associated with HFMD can be disturbing, dermatologists can reassure children and their parents that the nails will resolve with the next cycle of growth.
Kawasaki Disease—Kawasaki disease (KD) is a vasculitis primarily affecting children and infants. Although the specific pathogen and pathophysiology is not entirely clear, clinical observations have suggested an infectious cause, most likely a virus.43 In Japan, more than 15,000 cases of KD are documented annually, while approximately 4200 cases are seen in the United States.44 In a prospective study from 1984 to 1990, 4 of 26 (15.4%) patients with KD presented with nail manifestations during the late acute phase or early convalescent phase of disease. There were no significant associations between nail dystrophy and severity of KD, such as coronary artery aneurysm.45
Nail changes reported in children with KD include onychomadesis, onycholysis, orange-brown chromonychia, splinter hemorrhages, Beau lines, and pincer nails. In a review of nail changes associated with KD from 1980 to 2021, orange-brown transverse chromonychia, which may evolve into transverse leukonychia, was the most common nail finding reported, occurring in 17 of 31 (54.8%) patients.44 It has been hypothesized that nail changes may result from blood flow disturbance due to the underlying vasculitis.46 Nail changes appear several weeks after the onset of fever and are self-limited. Resolution occurs with nail growth, with no treatment required.

FUNGAL INFECTIONS
Onychomycosis
Onychomycosis is a fungal infection of the nails that occurs in 0.2% to 5.5% of pediatric patients, and its prevalence may be increasing, which may be due to environmental factors or increased rates of diabetes mellitus and obesity in the pediatric population.47 Onychomycosis represents 15.5% of nail dystrophies in pediatric patients.48 Some dermatologists treat presumptive onychomycosis without confirmation; however, we do not recommend that approach. Because the differential is broad and the duration of treatment is long, mycologic examination (potassium hydroxide preparation, fungal culture, polymerase chain reaction, and/or histopathology) should be obtained to confirm onychomycosis prior to initiation of antifungal management. Family members of affected individuals should be evaluated and treated, if indicated, for onychomycosis and tinea pedis, as household transmission is common.
Currently, there are 2 topical FDA-approved treatments for pediatric onychomycosis in children 6 years and older (Table 2).49,50 There is a discussion of the need for confirmatory testing for onychomycosis in children, particularly when systemic treatment is prescribed. In a retrospective review of 269 pediatric patients with onychomycosis prescribed terbinafine, 53.5% (n=144) underwent laboratory monitoring of liver function and complete blood cell counts, and 12.5% had grade 1 laboratory abnormalities either prior to (12/144 [8.3%]) or during (6/144 [4.2%]) therapy.51 Baseline transaminase monitoring is recommended, though subsequent routine laboratory monitoring in healthy children may have limited utility with associated increased costs, incidental findings, and patient discomfort and likely is not needed.51
Pediatric onychomycosis responds better to topical therapy than adult disease, and pediatric patients do not always require systemic treatment.52 Ciclopirox is not FDA approved for the treatment of pediatric onychomycosis, but in a 32-week clinical trial of ciclopirox lacquer 8% use in 40 patients, 77% (27/35) of treated patients achieved mycologic cure. Overall, 71% of treated patients (25/35) vs 22% (2/9) of controls achieved efficacy (defined as investigator global assessment score of 2 or lower).52 In an open-label, single-arm clinical trial assessing tavaborole solution 5% applied once daily for 48 weeks for the treatment of toenail onychomycosis in pediatric patients (aged 6–17 years), 36.2% (20/55) of patients achieved mycologic cure, and 8.5% (5/55) achieved complete cure at week 52 with mild or minimal adverse effects.53 In an open-label, phase 4 study of the safety and efficacy of efinaconazole solution 10% applied once daily for 48 weeks in pediatric patients (aged 6 to 16 years) (n=60), 65% (35/60) achieved mycologic cure, 42% (25/60) achieved clinical cure, and 40% (24/60) achieved complete cure at 52 weeks. The most common adverse effects of efinaconazole were local and included ingrown toenail (1/60), application-site dermatitis (1/60), application-site vesicles (1/60), and application-site pain (1/60).54
In a systematic review of systemic antifungals for onychomycosis in 151 pediatric patients, itraconazole, fluconazole, griseofulvin, and terbinafine resulted in complete cure rates similar to those of the adult population, with excellent safety profiles.55 Depending on the situation, initiation of treatment with topical medications followed by addition of systemic antifungal agents only if needed may be an appropriate course of action.
BACTERIAL INFECTIONS
Acute Paronychia
Acute paronychia is a nail-fold infection that develops after the protective nail barrier has been compromised.56 In children, thumb-sucking, nail-biting, frequent oral manipulation of the digits, and poor skin hygiene are risk factors. Acute paronychia also may develop in association with congenital malalignment of the great toenails.57
Clinical manifestations include localized pain, erythema, and nail fold edema (Figure 4). Purulent material and abscess formation may ensue. Staphylococcus aureus as well as methicillin-resistant S aureus and Streptococcus pyogenes are classically the most common causes of acute paronychia. Treatment of paronychia is based on severity. In mild cases, warm soaks with topical antibiotics are indicated. Oral antibiotics should be prescribed for more severe presentations. If there is no improvement after 48 hours, surgical drainage is required to facilitate healing.56
FINAL THOUGHTS
Inflammatory and infectious nail disorders in children are relatively common and may impact the physical and emotional well-being of young patients. By understanding the distinctive features of these nail disorders in pediatric patients, dermatologists can provide anticipatory guidance and informed treatment options to children and their parents. Further research is needed to expand our understanding of pediatric nail disorders and create targeted therapeutic interventions, particularly for NLP and psoriasis.


- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
- Uber M, Carvalho VO, Abagge KT, et al. Clinical features and nail clippings in 52 children with psoriasis. Pediatr Dermatol. 2018;35:202-207. doi:10.1111/pde.13402
- Plachouri KM, Mulita F, Georgiou S. Management of pediatric nail psoriasis. Cutis. 2021;108:292-294. doi:10.12788/cutis.0386
- Smith RJ, Rubin AI. Pediatric nail disorders: a review. Curr Opin Pediatr. 2020;32:506-515. doi:10.1097/mop.0000000000000921
- Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34:58-63. doi:10.1111/pde.13028
- Richert B, André J. Nail disorders in children: diagnosis and management. Am J Clin Dermatol. 2011;12:101-112. doi:10.2165/11537110-000000000-00000
- Lee JYY. Severe 20-nail psoriasis successfully treated by low dose methotrexate. Dermatol Online J. 2009;15:8.
- Nogueira M, Paller AS, Torres T. Targeted therapy for pediatric psoriasis. Paediatr Drugs. May 2021;23:203-212. doi:10.1007/s40272-021-00443-5
- Hanoodi M, Mittal M. Methotrexate. StatPearls [Internet]. Updated August 16, 2023. Accessed July 1, 2024. https://www.ncbi.nlm.nih.gov/books/NBK556114/
- Teran CG, Teran-Escalera CN, Balderrama C. A severe case of erythrodermic psoriasis associated with advanced nail and joint manifestations: a case report. J Med Case Rep. 2010;4:179. doi:10.1186/1752-1947-4-179
- Paller AS, Seyger MMB, Magariños GA, et al. Long-term efficacy and safety of up to 108 weeks of ixekizumab in pediatric patients with moderate to severe plaque psoriasis: the IXORA-PEDS randomized clinical trial. JAMA Dermatol. 2022;158:533-541. doi:10.1001/jamadermatol.2022.0655
- Diotallevi F, Simonetti O, Rizzetto G, et al. Biological treatments for pediatric psoriasis: state of the art and future perspectives. Int J Mol Sci. 2022;23:11128. doi:10.3390/ijms231911128
- Nash P, Mease PJ, Kirkham B, et al. Secukinumab provides sustained improvement in nail psoriasis, signs and symptoms of psoriatic arthritis and low rate of radiographic progression in patients with concomitant nail involvement: 2-year results from the Phase III FUTURE 5 study. Clin Exp Rheumatol. 2022;40:952-959. doi:10.55563/clinexprheumatol/3nuz51
- Wells LE, Evans T, Hilton R, et al. Use of secukinumab in a pediatric patient leads to significant improvement in nail psoriasis and psoriatic arthritis. Pediatr Dermatol. 2019;36:384-385. doi:10.1111/pde.13767
- Watabe D, Endoh K, Maeda F, et al. Childhood-onset psoriaticonycho-pachydermo-periostitis treated successfully with infliximab. Eur J Dermatol. 2015;25:506-508. doi:10.1684/ejd.2015.2616
- Pereira TM, Vieira AP, Fernandes JC, et al. Anti-TNF-alpha therapy in childhood pustular psoriasis. Dermatology. 2006;213:350-352. doi:10.1159/000096202
- Iorizzo M, Gioia Di Chiacchio N, Di Chiacchio N, et al. Intralesional steroid injections for inflammatory nail dystrophies in the pediatric population. Pediatr Dermatol. 2023;40:759-761. doi:10.1111/pde.15295
- Tosti A, Piraccini BM, Cambiaghi S, et al. Nail lichen planus in children: clinical features, response to treatment, and long-term follow-up. Arch Dermatol. 2001;137:1027-1032.
- Lipner SR. Nail lichen planus: a true nail emergency. J Am Acad Dermatol. 2019;80:e177-e178. doi:10.1016/j.jaad.2018.11.065
- Iorizzo M, Tosti A, Starace M, et al. Isolated nail lichen planus: an expert consensus on treatment of the classical form. J Am Acad Dermatol. 2020;83:1717-1723. doi:10.1016/j.jaad.2020.02.056
- Piraccini BM, Saccani E, Starace M, et al. Nail lichen planus: response to treatment and long term follow-up. Eur J Dermatol. 2010;20:489-496. doi:10.1684/ejd.2010.0952
- Mahajan R, Kaushik A, De D, et al. Pediatric trachyonychia- a retrospective study of 17 cases. Indian J Dermatol. 2021;66:689-690. doi:10.4103/ijd.ijd_42_21
- Leung AKC, Leong KF, Barankin B. Trachyonychia. J Pediatr. 2020;216:239-239.e1. doi:10.1016/j.jpeds.2019.08.034
- Haber JS, Chairatchaneeboon M, Rubin AI. Trachyonychia: review and update on clinical aspects, histology, and therapy. Skin Appendage Disord. 2017;2:109-115. doi:10.1159/000449063
- Jacobsen AA, Tosti A. Trachyonychia and twenty-nail dystrophy: a comprehensive review and discussion of diagnostic accuracy. Skin Appendage Disord. 2016;2:7-13. doi:10.1159/000445544
- Kumar MG, Ciliberto H, Bayliss SJ. Long-term follow-up of pediatric trachyonychia. Pediatr Dermatol. 2015;32:198-200. doi:10.1111/pde.12427
- Tosti A, Piraccini BM, Iorizzo M. Trachyonychia and related disorders: evaluation and treatment plans. Dermatolog Ther. 2002;15:121-125. doi:10.1046/j.1529-8019.2002.01511.x
- Leung AKC, Leong KF, Barankin B. Lichen striatus with nail involvement in a 6-year-old boy. Case Rep Pediatr. 2020;2020:1494760. doi:10.1155/2020/1494760
- Kim GW, Kim SH, Seo SH, et al. Lichen striatus with nail abnormality successfully treated with tacrolimus ointment. J Dermatol. 2009;36:616-617. doi:10.1111/j.1346-8138.2009.00720.x
- Iorizzo M, Rubin AI, Starace M. Nail lichen striatus: is dermoscopy useful for the diagnosis? Pediatr Dermatol. 2019;36:859-863. doi:10.1111/pde.13916
- Karp DL, Cohen BA. Onychodystrophy in lichen striatus. Pediatr Dermatol. 1993;10:359-361. doi:10.1111/j.1525-1470.1993.tb00399.x
- Tosti A, Peluso AM, Misciali C, et al. Nail lichen striatus: clinical features and long-term follow-up of five patients. J Am Acad Dermatol. 1997;36(6, pt 1):908-913. doi:10.1016/s0190-9622(97)80270-8
- Simpson EL, Thompson MM, Hanifin JM. Prevalence and morphology of hand eczema in patients with atopic dermatitis. Dermatitis. 2006;17:123-127. doi:10.2310/6620.2006.06005
- Sarifakioglu E, Yilmaz AE, Gorpelioglu C. Nail alterations in 250 infant patients: a clinical study. J Eur Acad Dermatol Venereol. 2008;22:741-744. doi:10.1111/j.1468-3083.2008.02592.x
- Milanesi N, D’Erme AM, Gola M. Nail improvement during alitretinoin treatment: three case reports and review of the literature. Clin Exp Dermatol. 2015;40:533-536. doi:10.1111/ced.12584
- Chung BY, Choi YW, Kim HO, et al. Nail dystrophy in patients with atopic dermatitis and its association with disease severity. Ann Dermatol. 2019;31:121-126. doi:10.5021/ad.2019.31.2.121
- Navarro-Triviño FJ, Vega-Castillo JJ, Ruiz-Villaverde R. Nail changes successfully treated with dupilumab in a patient with severe atopic dermatitis. Australas J Dermatol. 2021;62:e468-e469. doi:10.1111/ajd.13633
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346. doi:10.1186/1471-2334-11-346
- Shin JY, Cho BK, Park HJ. A clinical study of nail changes occurring secondary to hand-foot-mouth disease: onychomadesis and Beau’s lines. Ann Dermatol. 2014;26:280-283. doi:10.5021/ad.2014.26.2.280
- Verma S, Singal A. Nail changes in hand-foot-and-mouth disease (HFMD). Indian Dermatol Online J. 2021;12:656-657. doi:10.4103 /idoj.IDOJ_271_20
- Giordano LMC, de la Fuente LA, Lorca JMB, et al. Onychomadesis secondary to hand-foot-mouth disease: a frequent manifestation and cause of concern for parents. Article in Spanish. Rev Chil Pediatr. 2018;89:380-383. doi:10.4067/s0370-41062018005000203
- Justino MCA, da SMD, Souza MF, et al. Atypical hand-foot-mouth disease in Belém, Amazon region, northern Brazil, with detection of coxsackievirus A6. J Clin Virol. 2020;126:104307. doi:10.1016/j.jcv.2020.104307
- Cheng FF, Zhang BB, Cao ML, et al. Clinical characteristics of 68 children with atypical hand, foot, and mouth disease caused by coxsackievirus A6: a single-center retrospective analysis. Transl Pediatr. 2022;11:1502-1509. doi:10.21037/tp-22-352
- Nagata S. Causes of Kawasaki disease-from past to present. Front Pediatr. 2019;7:18. doi:10.3389/fped.2019.00018
- Mitsuishi T, Miyata K, Ando A, et al. Characteristic nail lesions in Kawasaki disease: case series and literature review. J Dermatol. 2022;49:232-238. doi:10.1111/1346-8138.16276
- Lindsley CB. Nail-bed lines in Kawasaki disease. Am J Dis Child. 1992;146:659-660. doi:10.1001/archpedi.1992.02160180017005
- Matsumura O, Nakagishi Y. Pincer nails upon convalescence from Kawasaki disease. J Pediatr. 2022;246:279. doi:10.1016/j.jpeds.2022.03.002
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Gupta AK, Mays RR, Versteeg SG, et al. Onychomycosis in children: safety and efficacy of antifungal agents. Pediatr Dermatol. 2018;35:552-559. doi:10.1111/pde.13561
- 49. Gupta AK, Venkataraman M, Shear NH, et al. Labeled use of efinaconazole topical solution 10% in treating onychomycosis in children and a review of the management of pediatric onychomycosis. Dermatol Ther. 2020;33:e13613. doi:10.1111/dth.13613
- Falotico JM, Lipner SR. Updated perspectives on the diagnosis and management of onychomycosis. Clin Cosmet Investig Dermatol. 2022;15:1933-1957. doi:10.2147/ccid.S362635
- Patel D, Castelo-Soccio LA, Rubin AI, et al. Laboratory monitoring during systemic terbinafine therapy for pediatric onychomycosis. JAMA Dermatol. 2017;153:1326-1327. doi:10.1001/jamadermatol.2017.4483
- Friedlander SF, Chan YC, Chan YH, et al. Onychomycosis does not always require systemic treatment for cure: a trial using topical therapy. Pediatr Dermatol. 2013;30:316-322. doi:10.1111/pde.12064
- Rich P, Spellman M, Purohit V, et al. Tavaborole 5% topical solution for the treatment of toenail onychomycosis in pediatric patients: results from a phase 4 open-label study. J Drugs Dermatol. 2019;18:190-195.
- Gupta AK, Venkataraman M, Abramovits W, et al. JUBLIA (efinaconazole 10% solution) in the treatment of pediatric onychomycosis. Skinmed. 2021;19:206-210.
- Gupta AK, Paquet M. Systemic antifungals to treat onychomycosis in children: a systematic review. Pediatr Dermatol. 2013;30:294-302. doi:10.1111/pde.12048
- Leggit JC. Acute and chronic paronychia. Am Fam Physician. 2017;96:44-51.
- Lipner SR, Scher RK. Congenital malalignment of the great toenails with acute paronychia. Pediatr Dermatol. 2016;33:e288-e289.doi:10.1111/pde.12924
Practice Points
- Nail plate pitting is the most common clinical sign of nail psoriasis in children.
- Nail changes are common in hand, foot, and mouth disease, with the most frequent being onychomadesis.
- Because onychomycosis may resemble other nail disorders, mycologic confirmation is recommended to avoid misdiagnosis.
- Many nail conditions in children self-resolve but recognizing these manifestations is important in providing anticipatory guidance to patients and caregivers.
Gut Biomarkers Accurately Flag Autism Spectrum Disorder
, new research shows.
The findings could form the basis for development of a noninvasive diagnostic test for ASD and also provide novel therapeutic targets, wrote investigators, led by Siew C. Ng, MBBS, PhD, with the Microbiota I-Center (MagIC), the Chinese University of Hong Kong.
Their study was published online in Nature Microbiology.
Beyond Bacteria
The gut microbiome has been shown to play a central role in modulating the gut-brain axis, potentially influencing the development of ASD.
However, most studies in ASD have focused on the bacterial component of the microbiome. Whether nonbacterial microorganisms (such as gut archaea, fungi, and viruses) or function of the gut microbiome are altered in ASD remains unclear.
To investigate, the researchers performed metagenomic sequencing on fecal samples from 1627 boys and girls aged 1-13 years with and without ASD from five cohorts in China.
After controlling for diet, medication, and comorbidity, they identified 14 archaea, 51 bacteria, 7 fungi, 18 viruses, 27 microbial genes, and 12 metabolic pathways that were altered in children with ASD.
Machine-learning models using single-kingdom panels (archaea, bacteria, fungi, viruses) achieved area under the curve (AUC) values ranging from 0.68 to 0.87 in differentiating children with ASD from neurotypical control children.
A model based on a panel of 31 multikingdom and functional markers achieved “high predictive value” for ASD, achieving an AUC of 0.91, with comparable performance among boys and girls.
“The reproducible performance of the models across ages, sexes, and cohorts highlights their potential as promising diagnostic tools for ASD,” the investigators wrote.
They also noted that the accuracy of the model was largely driven by the biosynthesis pathways of ubiquinol-7 and thiamine diphosphate, which were less abundant in children with ASD, and may serve as therapeutic targets.
‘Exciting’ Possibilities
“This study broadens our understanding by including fungi, archaea, and viruses, where previous studies have largely focused on the role of gut bacteria in autism,” Bhismadev Chakrabarti, PhD, research director of the Centre for Autism at the University of Reading, United Kingdom, said in a statement from the nonprofit UK Science Media Centre.
“The results are broadly in line with previous studies that show reduced microbial diversity in autistic individuals. It also examines one of the largest samples seen in a study like this, which further strengthens the results,” Dr. Chakrabarti added.
He said this research may provide “new ways of detecting autism, if microbial markers turn out to strengthen the ability of genetic and behavioral tests to detect autism. A future platform that can combine genetic, microbial, and simple behavioral assessments could help address the detection gap.
“One limitation of this data is that it cannot assess any causal role for the microbiota in the development of autism,” Dr. Chakrabarti noted.
This study was supported by InnoHK, the Government of Hong Kong, Special Administrative Region of the People’s Republic of China, The D. H. Chen Foundation, and the New Cornerstone Science Foundation through the New Cornerstone Investigator Program. Dr. Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and AbbVie; has received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, AbbVie, and Takeda; is a scientific cofounder and shareholder of GenieBiome; receives patent royalties through her affiliated institutions; and is named as a co-inventor of patent applications that cover the therapeutic and diagnostic use of microbiome. Dr. Chakrabarti has no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, new research shows.
The findings could form the basis for development of a noninvasive diagnostic test for ASD and also provide novel therapeutic targets, wrote investigators, led by Siew C. Ng, MBBS, PhD, with the Microbiota I-Center (MagIC), the Chinese University of Hong Kong.
Their study was published online in Nature Microbiology.
Beyond Bacteria
The gut microbiome has been shown to play a central role in modulating the gut-brain axis, potentially influencing the development of ASD.
However, most studies in ASD have focused on the bacterial component of the microbiome. Whether nonbacterial microorganisms (such as gut archaea, fungi, and viruses) or function of the gut microbiome are altered in ASD remains unclear.
To investigate, the researchers performed metagenomic sequencing on fecal samples from 1627 boys and girls aged 1-13 years with and without ASD from five cohorts in China.
After controlling for diet, medication, and comorbidity, they identified 14 archaea, 51 bacteria, 7 fungi, 18 viruses, 27 microbial genes, and 12 metabolic pathways that were altered in children with ASD.
Machine-learning models using single-kingdom panels (archaea, bacteria, fungi, viruses) achieved area under the curve (AUC) values ranging from 0.68 to 0.87 in differentiating children with ASD from neurotypical control children.
A model based on a panel of 31 multikingdom and functional markers achieved “high predictive value” for ASD, achieving an AUC of 0.91, with comparable performance among boys and girls.
“The reproducible performance of the models across ages, sexes, and cohorts highlights their potential as promising diagnostic tools for ASD,” the investigators wrote.
They also noted that the accuracy of the model was largely driven by the biosynthesis pathways of ubiquinol-7 and thiamine diphosphate, which were less abundant in children with ASD, and may serve as therapeutic targets.
‘Exciting’ Possibilities
“This study broadens our understanding by including fungi, archaea, and viruses, where previous studies have largely focused on the role of gut bacteria in autism,” Bhismadev Chakrabarti, PhD, research director of the Centre for Autism at the University of Reading, United Kingdom, said in a statement from the nonprofit UK Science Media Centre.
“The results are broadly in line with previous studies that show reduced microbial diversity in autistic individuals. It also examines one of the largest samples seen in a study like this, which further strengthens the results,” Dr. Chakrabarti added.
He said this research may provide “new ways of detecting autism, if microbial markers turn out to strengthen the ability of genetic and behavioral tests to detect autism. A future platform that can combine genetic, microbial, and simple behavioral assessments could help address the detection gap.
“One limitation of this data is that it cannot assess any causal role for the microbiota in the development of autism,” Dr. Chakrabarti noted.
This study was supported by InnoHK, the Government of Hong Kong, Special Administrative Region of the People’s Republic of China, The D. H. Chen Foundation, and the New Cornerstone Science Foundation through the New Cornerstone Investigator Program. Dr. Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and AbbVie; has received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, AbbVie, and Takeda; is a scientific cofounder and shareholder of GenieBiome; receives patent royalties through her affiliated institutions; and is named as a co-inventor of patent applications that cover the therapeutic and diagnostic use of microbiome. Dr. Chakrabarti has no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, new research shows.
The findings could form the basis for development of a noninvasive diagnostic test for ASD and also provide novel therapeutic targets, wrote investigators, led by Siew C. Ng, MBBS, PhD, with the Microbiota I-Center (MagIC), the Chinese University of Hong Kong.
Their study was published online in Nature Microbiology.
Beyond Bacteria
The gut microbiome has been shown to play a central role in modulating the gut-brain axis, potentially influencing the development of ASD.
However, most studies in ASD have focused on the bacterial component of the microbiome. Whether nonbacterial microorganisms (such as gut archaea, fungi, and viruses) or function of the gut microbiome are altered in ASD remains unclear.
To investigate, the researchers performed metagenomic sequencing on fecal samples from 1627 boys and girls aged 1-13 years with and without ASD from five cohorts in China.
After controlling for diet, medication, and comorbidity, they identified 14 archaea, 51 bacteria, 7 fungi, 18 viruses, 27 microbial genes, and 12 metabolic pathways that were altered in children with ASD.
Machine-learning models using single-kingdom panels (archaea, bacteria, fungi, viruses) achieved area under the curve (AUC) values ranging from 0.68 to 0.87 in differentiating children with ASD from neurotypical control children.
A model based on a panel of 31 multikingdom and functional markers achieved “high predictive value” for ASD, achieving an AUC of 0.91, with comparable performance among boys and girls.
“The reproducible performance of the models across ages, sexes, and cohorts highlights their potential as promising diagnostic tools for ASD,” the investigators wrote.
They also noted that the accuracy of the model was largely driven by the biosynthesis pathways of ubiquinol-7 and thiamine diphosphate, which were less abundant in children with ASD, and may serve as therapeutic targets.
‘Exciting’ Possibilities
“This study broadens our understanding by including fungi, archaea, and viruses, where previous studies have largely focused on the role of gut bacteria in autism,” Bhismadev Chakrabarti, PhD, research director of the Centre for Autism at the University of Reading, United Kingdom, said in a statement from the nonprofit UK Science Media Centre.
“The results are broadly in line with previous studies that show reduced microbial diversity in autistic individuals. It also examines one of the largest samples seen in a study like this, which further strengthens the results,” Dr. Chakrabarti added.
He said this research may provide “new ways of detecting autism, if microbial markers turn out to strengthen the ability of genetic and behavioral tests to detect autism. A future platform that can combine genetic, microbial, and simple behavioral assessments could help address the detection gap.
“One limitation of this data is that it cannot assess any causal role for the microbiota in the development of autism,” Dr. Chakrabarti noted.
This study was supported by InnoHK, the Government of Hong Kong, Special Administrative Region of the People’s Republic of China, The D. H. Chen Foundation, and the New Cornerstone Science Foundation through the New Cornerstone Investigator Program. Dr. Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and AbbVie; has received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, AbbVie, and Takeda; is a scientific cofounder and shareholder of GenieBiome; receives patent royalties through her affiliated institutions; and is named as a co-inventor of patent applications that cover the therapeutic and diagnostic use of microbiome. Dr. Chakrabarti has no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE MICROBIOLOGY
Should Cancer Trial Eligibility Become More Inclusive?
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
Opioids Post T&A
I recently encountered a study that reviewed return visits of pediatric patients after undergoing adenotonsillectomy. The investigators discovered that pain-related visits were higher for patients who had received prescriptions for opioids. After the Food and Drug Administration (FDA) issued a boxed warning about the use of codeine in postoperative pediatric tonsillectomy with adenoidectomy (T&A), patients pain-related return visits declined and steroid prescriptions increased.
On the surface, this inverse relationship between opioid prescriptions and pain-related visits seems counterintuitive. This is particularly true if you believe that opioids are effective pain medications. The relationship between pain-related visits, steroid use, and the boxed warning is a bit easier to understand and most likely points to the effectiveness of the steroids.
Keeping in mind this was a single-institution study that included more than 5000 patients and more than 700 return visits, we should be careful in reading too much into these results. However, I can’t resist the temptation to use it as a springboard from which to launch a short dissertation on pain management.
First, let’s consider whether there was something about the opioids that was causing more pain for the patients. I’m not aware of any studies that suggest pain as a side effect of codeine. Nausea and vomiting, yes. And, although the investigators were focusing on pain, it may have been that the general discomfort associated with the gastrointestinal effects of the drug were lowering the patients’ pain threshold. I certainly know of many adults who have said that they now avoid opioids postoperatively because of the general sense of unwellness they have experienced during previous surgical adventures.
However, my bias leads me to focus on this question: If the patients didn’t receive opioids postoperatively, were they receiving something else that was making them less likely to arrive at the hospital or clinic complaining of pain? I assume the researchers would have told us about some new alternative miracle painkiller that was being prescribed.
As a card-carrying nihilist in good standing, I am tempted to claim that this is another example of nothing is better than most well-intentioned somethings. However, I am going to posit that these patients were receiving something that lessened their need to seek help with their pain.
Most likely that something was a thoughtful preemptive dialogue postoperatively about what they (and in most cases their parents) might expect in the way of symptoms. And ... an easy-to-reach contact point preferably with a person with whom they were familiar. And ... were scheduled to receive follow up phone calls at intervals relevant to the details of their surgery.
I know many of you are going to say, “We are already doing those things.” And, if so, you are to be commended. And, I’m sure that every outpatient postoperative manual includes all of those common-sense ingredients of good follow-up care. However, you know as well as I do that not all postoperative instructions are delivered with same degree of thoroughness nor with sufficient pauses thoughtfully delivered to make it a real dialogue. Nor is the follow-up contact person as easy to reach as promised.
I’m not sure how much we can thank the FDA boxed warning about codeine for the decrease in postoperative pain-generated visits. However, it could be that when physicians were discouraged from prescribing postoperative opioids, they may have felt the need to lean more heavily on good old-fashioned postoperative follow-up care. Instructions presented more as a dialogue and preemptive follow-up calls made with an aura of caring are well known deterrents of middle-of-the-night calls for help.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I recently encountered a study that reviewed return visits of pediatric patients after undergoing adenotonsillectomy. The investigators discovered that pain-related visits were higher for patients who had received prescriptions for opioids. After the Food and Drug Administration (FDA) issued a boxed warning about the use of codeine in postoperative pediatric tonsillectomy with adenoidectomy (T&A), patients pain-related return visits declined and steroid prescriptions increased.
On the surface, this inverse relationship between opioid prescriptions and pain-related visits seems counterintuitive. This is particularly true if you believe that opioids are effective pain medications. The relationship between pain-related visits, steroid use, and the boxed warning is a bit easier to understand and most likely points to the effectiveness of the steroids.
Keeping in mind this was a single-institution study that included more than 5000 patients and more than 700 return visits, we should be careful in reading too much into these results. However, I can’t resist the temptation to use it as a springboard from which to launch a short dissertation on pain management.
First, let’s consider whether there was something about the opioids that was causing more pain for the patients. I’m not aware of any studies that suggest pain as a side effect of codeine. Nausea and vomiting, yes. And, although the investigators were focusing on pain, it may have been that the general discomfort associated with the gastrointestinal effects of the drug were lowering the patients’ pain threshold. I certainly know of many adults who have said that they now avoid opioids postoperatively because of the general sense of unwellness they have experienced during previous surgical adventures.
However, my bias leads me to focus on this question: If the patients didn’t receive opioids postoperatively, were they receiving something else that was making them less likely to arrive at the hospital or clinic complaining of pain? I assume the researchers would have told us about some new alternative miracle painkiller that was being prescribed.
As a card-carrying nihilist in good standing, I am tempted to claim that this is another example of nothing is better than most well-intentioned somethings. However, I am going to posit that these patients were receiving something that lessened their need to seek help with their pain.
Most likely that something was a thoughtful preemptive dialogue postoperatively about what they (and in most cases their parents) might expect in the way of symptoms. And ... an easy-to-reach contact point preferably with a person with whom they were familiar. And ... were scheduled to receive follow up phone calls at intervals relevant to the details of their surgery.
I know many of you are going to say, “We are already doing those things.” And, if so, you are to be commended. And, I’m sure that every outpatient postoperative manual includes all of those common-sense ingredients of good follow-up care. However, you know as well as I do that not all postoperative instructions are delivered with same degree of thoroughness nor with sufficient pauses thoughtfully delivered to make it a real dialogue. Nor is the follow-up contact person as easy to reach as promised.
I’m not sure how much we can thank the FDA boxed warning about codeine for the decrease in postoperative pain-generated visits. However, it could be that when physicians were discouraged from prescribing postoperative opioids, they may have felt the need to lean more heavily on good old-fashioned postoperative follow-up care. Instructions presented more as a dialogue and preemptive follow-up calls made with an aura of caring are well known deterrents of middle-of-the-night calls for help.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
I recently encountered a study that reviewed return visits of pediatric patients after undergoing adenotonsillectomy. The investigators discovered that pain-related visits were higher for patients who had received prescriptions for opioids. After the Food and Drug Administration (FDA) issued a boxed warning about the use of codeine in postoperative pediatric tonsillectomy with adenoidectomy (T&A), patients pain-related return visits declined and steroid prescriptions increased.
On the surface, this inverse relationship between opioid prescriptions and pain-related visits seems counterintuitive. This is particularly true if you believe that opioids are effective pain medications. The relationship between pain-related visits, steroid use, and the boxed warning is a bit easier to understand and most likely points to the effectiveness of the steroids.
Keeping in mind this was a single-institution study that included more than 5000 patients and more than 700 return visits, we should be careful in reading too much into these results. However, I can’t resist the temptation to use it as a springboard from which to launch a short dissertation on pain management.
First, let’s consider whether there was something about the opioids that was causing more pain for the patients. I’m not aware of any studies that suggest pain as a side effect of codeine. Nausea and vomiting, yes. And, although the investigators were focusing on pain, it may have been that the general discomfort associated with the gastrointestinal effects of the drug were lowering the patients’ pain threshold. I certainly know of many adults who have said that they now avoid opioids postoperatively because of the general sense of unwellness they have experienced during previous surgical adventures.
However, my bias leads me to focus on this question: If the patients didn’t receive opioids postoperatively, were they receiving something else that was making them less likely to arrive at the hospital or clinic complaining of pain? I assume the researchers would have told us about some new alternative miracle painkiller that was being prescribed.
As a card-carrying nihilist in good standing, I am tempted to claim that this is another example of nothing is better than most well-intentioned somethings. However, I am going to posit that these patients were receiving something that lessened their need to seek help with their pain.
Most likely that something was a thoughtful preemptive dialogue postoperatively about what they (and in most cases their parents) might expect in the way of symptoms. And ... an easy-to-reach contact point preferably with a person with whom they were familiar. And ... were scheduled to receive follow up phone calls at intervals relevant to the details of their surgery.
I know many of you are going to say, “We are already doing those things.” And, if so, you are to be commended. And, I’m sure that every outpatient postoperative manual includes all of those common-sense ingredients of good follow-up care. However, you know as well as I do that not all postoperative instructions are delivered with same degree of thoroughness nor with sufficient pauses thoughtfully delivered to make it a real dialogue. Nor is the follow-up contact person as easy to reach as promised.
I’m not sure how much we can thank the FDA boxed warning about codeine for the decrease in postoperative pain-generated visits. However, it could be that when physicians were discouraged from prescribing postoperative opioids, they may have felt the need to lean more heavily on good old-fashioned postoperative follow-up care. Instructions presented more as a dialogue and preemptive follow-up calls made with an aura of caring are well known deterrents of middle-of-the-night calls for help.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Other than a Littman stethoscope he accepted as a first-year medical student in 1966, Dr. Wilkoff reports having nothing to disclose. Email him at [email protected].
Dupilumab Effective in PPI-Refractory Pediatric EoE
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
Good news for younger children suffering from the uncommon but debilitating gastrointestinal condition eosinophilic esophagitis (EoE): Data from this trial led to a January US Food and Drug Administration (FDA) approval of the anti-inflammatory biologic for patients aged 1-11 years weighing at least 15 kg.
In addition, the trial, published in The New England Journal of Medicine, found that a higher-exposure dupilumab regimen (approximating the trough concentration of a 300-mg dose administered once weekly versus every 2 weeks) improved key secondary end points, according to gastroenterologist Mirna Chehade, MD, MPH, AGAF, a professor of pediatrics at Icahn School of Medicine at Mount Sinai and Mount Sinai Kravis Children’s Hospital in New York City, and colleagues.
In 2022, the FDA approved the drug for those aged 12 or older weighing at least 40 kg.
“Left untreated or inadequately treated, EoE can progress to esophageal narrowing and strictures, leading to increased risk of food impactions and the need for esophageal dilations,” Dr. Chehade said in an interview. “Therefore, it’s important that children with EoE have the FDA-approved treatment option based on our study that can address their underlying disease starting at a young age.”
She added that dupilumab has the exciting potential to transform the standard of care for many young children living with EoE. “There are, however, factors to consider before switching a child to dupilumab — all related to the child’s specific medical history and therefore the perceived potential benefits from the drug.”
Commenting on the study but not involved in it, Toni Webster, DO, a pediatric gastroenterologist at Cohen Children’s Medical Center in Queens, New York, and an assistant professor at the Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York, said, “Like many allergic diseases, EoE is on the rise and, unfortunately, is affecting our children at alarming rates and at earlier ages. Given its efficacy and side-effect profile, dupilumab will vastly change our ability to treat EoE, especially for families who find diet and daily medication to be a challenge.”
Dr. Webster noted that an elimination diet is a rigorous choice that is often difficult to navigate. And the oral administration of off-label choices, proton pump inhibitors, and swallowed topical steroids, as well as the newly FDA-approved oral budesonide therapy (Eohilia), may also be challenging because many children have precluding aversions to oral therapy. “Regardless of age, treatment choice for EoE should be a good fit that is a plausible addition to a family’s lifestyle,” she said.
Blocking interleukin 4 and interleukin 13 inflammatory pathways, dupilumab has shown efficacy in other atopic diseases such as eczema. It broadly inhibits most aspects of type 2 inflammation and that action is reflected in its histologic and transcriptomic effects in affected tissues, Dr. Chehade and associates explained.
The Trial
Conducted at one Canadian and 26 US sites, the two-part phase 3 study randomly assigned 102 EoE patients aged 1-11 years who were refractory to proton pump inhibition in a 2:2:1:1 ratio.
Part A enrolled 102 patients and evaluated dupilumab at a weight-tiered higher-dose or lower-dose regimen vs placebo (two groups) for 16 weeks.
Part B was a 36-week extended active treatment period in which eligible dupilumab recipients from part A maintained their weight-tiered higher- or lower-dose regimen, whereas those in the placebo groups switched to weight-tiered higher- or lower-dose dupilumab.
The primary end point was histologic remission (peak esophageal intraepithelial eosinophil count, ≤ 6 per high-power field) at week 16. Continued dupilumab treatment appeared to maintain its effect through week 52.
During part A, histologic remission occurred in 25 of the 37 higher-exposure patients (68%), 18 of the 31 lower-exposure patients (58%), and one of the 34 placebo patients (3%).
The difference between the higher-exposure regimen and placebo was 65 percentage points (95% confidence interval [CI], 48-81; P < .001), whereas that between the lower-exposure regimen and placebo was 55 percentage points (95% CI, 37-73; P < .001).
Higher exposure led to significant improvements in histologic, endoscopic, and transcriptomic measures over placebo. Improvements between baseline and week 52 in all patients were generally similar to those between baseline and week 16 in patients who received dupilumab in part A.
As for adverse events, in part A, the incidence of coronavirus disease, nausea, injection-site pain, and headache was at least 10 percentage points higher among dupilumab recipients at either dose than among placebo recipients. Serious adverse events were reported in three dupilumab patients during part A and in six patients overall during part B.
A Balanced Approach
On a cautionary note, Eric H. Chiou, MD, an assistant professor of pediatrics at Baylor College of Medicine and a pediatric gastroenterologist at Texas Children’s Hospital in Houston, said that while dupilumab shows great promise, further research is needed on its cost-effectiveness in EoE.
“The cost of treatment will need to be compared relative to potential long-term savings from reduced hospitalizations, fewer complications, and improved quality of life,” said Dr. Chiou, who was not involved in the study. “A balanced approach that considers clinical efficacy, patient well-being, cost-effectiveness, and equity is essential.”
He added that despite the study’s encouraging results, long-term safety and efficacy data are needed to fully understand the impact of dupilumab on pediatric patients with EoE. “Dupilumab will need to be compared with existing treatments for EoE such as dietary management and swallowed topical corticosteroids in terms of efficacy, safety, and quality of life improvements.”
Additionally, further research is required to identify which patients are most likely to benefit from this therapy and to explore any potential complications associated with its long-term use. “Understanding the optimal dosing and duration of treatment will also be crucial for maximizing benefits while minimizing risks,” Dr. Chiou said.
Dr. Chehade agreed. “While it’s that great that young children finally have an FDA-approved drug to treat their EoE, more research is needed to learn which patient subsets would derive maximum benefit from dupilumab and at which specific steps in their medical management journey should dupilumab be used.”
This study was supported by Sanofi and Regeneron Pharmaceuticals. Dr. Chehade disclosed research funding from and consulting for numerous private sector companies, among others, Sanofi and Regeneron Pharmaceuticals, AstraZeneca, Shire-Takeda, and Bristol-Myers Squibb. Multiple study coauthors disclosed various relationships with private-sector companies, including Sanofi and Regeneron Pharmaceuticals, for research funding, consulting, travel, employment, and stock or intellectual ownership. Dr. Webster and Dr. Chiou disclosed no competing interests relevant to their comments.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE