User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
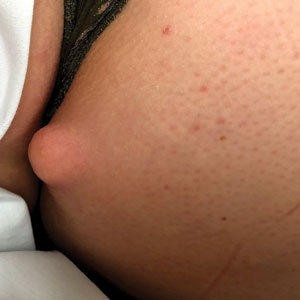
Don’t call me ‘Dr.,’ say some physicians – but most prefer the title
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
When Mark Cucuzzella, MD, meets a new patient at the West Virginia Medical School clinic, he introduces himself as “Mark.” For one thing, says Dr. Cucuzzella, his last name is a mouthful. For another, the 56-year-old general practitioner asserts that getting on a first-name basis with his patients is integral to delivering the best care.
“I’m trying to break down the old paternalistic barriers of the doctor/patient relationship,” he says. “Titles create an environment where the doctors are making all the decisions and not involving the patient in any course of action.”
Aniruddh Setya, MD, has a different take on informality between patients and doctors: It’s not OK. “I am not your friend,” says the 35-year-old pediatrician from Florida-based KIDZ Medical Services. “There has to be a level of respect for the education and accomplishment of being a physician.”
published in JAMA Network Open. But that doesn’t mean most physicians support the practice. In fact, some doctors contend that it can be harmful, particularly to female physicians.
“My concern is that untitling (so termed by Amy Diehl, PhD, and Leanne Dzubinski, PhD) intrudes upon important professional boundaries and might be correlated with diminishing the value of someone’s time,” says Leah Witt, MD, a geriatrician at UCSF Health, San Francisco. Dr. Witt, along with colleague Lekshmi Santhosh, MD, a pulmonologist, offered commentary on the study results. “Studies have shown that women physicians get more patient portal messages, spend more time in the electronic health record, and have longer visits,” Dr. Witt said. “Dr. Santhosh and I wonder if untitling is a signifier of this diminished value of our time, and an assumption of increased ease of access leading to this higher workload.”
To compile the results reported in JAMA Network Open, Mayo Clinic researchers analyzed more than 90,000 emails from patients to doctors over the course of 3 years, beginning in 2018. Of those emails, more than 32% included the physician’s first name in greeting or salutation. For women physicians, the odds were twice as high that their titles would be omitted in the correspondence. The same holds true for doctors of osteopathic medicine (DOs) compared with MDs, and primary care physicians had similar odds for a title drop compared with specialists.
Dr. Witt says the findings are not surprising. “They match my experience as a woman in medicine, as Dr. Santhosh and I write in our commentary,” she says. “We think the findings could easily be replicated at other centers.”
Indeed, research on 321 speaker introductions at a medical rounds found that when female physicians introduced other physicians, they usually applied the doctor title. When the job of introducing colleagues fell to male physicians, however, the stats fell to 72.4% for male peers and only 49.2% when introducing female peers.
The Mayo Clinic study authors identified the pitfalls of patients who informally address their doctors. They wrote, “Untitling may have a negative impact on physicians, demonstrate lack of respect, and can lead to reduction in formality of the physician/patient relationship or workplace.”
Physician preferences vary
Although the results of the Mayo Clinic analysis didn’t and couldn’t address physician sentiments on patient informality, Dr. Setya observes that American culture is becoming less formal. “I’ve been practicing for over 10 years, and the number of people who consider doctors as equals is growing,” he says. “This has been particularly true over the last couple of years.”
This change was documented in 2015. Add in the pandemic and an entire society that is now accustomed to working from home in sweats, and it’s not a stretch to understand why some patients have become less formal in many settings. The 2015 article noted, however, that most physicians prefer to keep titles in the mix.
Perhaps most troublesome, says Dr. Setya, is that patients forgo asking whether it’s OK to use his first name and simply assume it’s acceptable. “It bothers me,” he says. “I became a doctor for more than the money.”
He suspects that his cultural background (Dr. Setya is of Indian descent) plays a role in how strongly he feels about patient-doctor informality. “As a British colony, Indian culture dictates that you pay respect to elders and to accomplishment,” he points out. “America is far looser when it comes to salutations.”
Dr. Cucuzzella largely agrees with Dr. Setya, but has a different view of the role culture plays in how physicians prefer to be addressed. “If your last name is difficult to pronounce, it can put the patient at ease if you give them an option,” he says. “I like my patients to feel comfortable and have a friendly conversation, so I don’t ask them to try to manage my last name.”
When patients revert to using Dr. Cucuzzella’s last name and title, this often breaks down along generational lines, Dr. Cucuzzella has found: Older patients might drop his title, whereas younger patients might keep it as a sign of respect. In some cases, Dr. Cucuzzella tries to bridge this gap, and offers the option of “Dr. Mark.” In his small West Virginia community, this is how people often refer to him.
Dr. Setya says that most of the older physicians he works with still prefer that patients and younger colleagues use their title, but he has witnessed exceptions to this. “My boss in residence hated to be called ‘Sir’ or ‘Doctor,’ ” he says. “In a situation like that, it is reasonable to ask, ‘How can I address you?’ But it has to be mutually agreed upon.”
Dr. Cucuzzella cites informality as the preferred mode for older patients. “If I have a 70-year-old patient, it seems natural they shouldn’t use my title,” he says. “They are worthy of equality in the community. If I’m talking to a retired CEO or state delegate, it’s uncomfortable if they call me doctor.”
Moreover, Dr. Cucuzzella maintains that establishing a less formal environment with patients leads to better outcomes. “Shared decision-making is a basic human right,” he says. “In 2022, doctors shouldn’t make decisions without patient input, unless it’s an emergency situation. Removing the title barriers makes that easier.”
How to handle informality
If you fall more in line with Dr. Setya, there are strategies you can use to try to keep formality in your doctor-patient relationships. Dr. Setya’s approach is indirect. “I don’t correct a patient if they use my first name, because that might seem hostile,” he says. “But I alert them in the way I address them back. A Sir, a Mrs., or a Mr. needs to go both ways.”
This particularly holds true in pediatrics, Dr. Setya has found. He has witnessed many colleagues addressing parents as “Mommy and Daddy,” something he says lacks respect and sets too informal a tone. “It’s almost universal that parents don’t like that, and we need to act accordingly.”
Dr. Witt also avoids directly correcting patients, but struggles when they drop her title. “The standard signature I use to sign every patient portal message I respond to includes my first and last name and credentials,” she says. “I maintain formality in most circumstances with that standard reply.”
Beneath the surface, however, Dr. Witt wishes it were easier. “I have struggled with answering the question, ‘Is it OK if I call you Leah?’ she says. “I want to keep our interaction anchored in professionalism without sacrificing the warmth I think is important to a productive patient-physician relationship. For this reason, I tend to say yes to this request, even though I’d rather patients didn’t make such requests.”
In the Fast Company article by Amy Diehl, PhD, and Leanne Dzubinski, PhD, on the topic of untitling professional women, the authors suggest several actions, beginning with leadership that sets expectations on the topic. They also suggest that physicians use polite corrections if patients untitle them. Supplying positive reinforcement when patients include your title can help, too. If all else fails, you can call out the offensive untitling. More often than not, especially with female physicians, the patient is demonstrating an unconscious bias rather than something deliberate.
Opinions vary on the topic of untitling, and ultimately each physician must make the decision for themselves. But creating informal cultures in an organization can have unintended consequences, especially for female peers.
Says Dr. Witt, “We all want to give our patients the best care we can, but professional boundaries are critical to time management, equitable care, and maintaining work-life balance. I would love to see a study that examines untitling by self-reported race and/or ethnicity of physicians, because we know that women of color experience higher rates of burnout and depression, and I wonder if untitling may be part of this.”
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Stage 3 melanoma attacked with immunotherapy and a virus-like particle
The result led researchers to call for a future study comparing the regimen against a suitable control group.
“We were very excited to see the ability of intratumoral vidutolimod to augment T-cell infiltrate. (Pathologic) response was associated with a dense infiltrate of CD8 T cells. We were also able to demonstrate for what I think may be the first time, that intratumoral CpG resulted in clear evidence of CD303+ plasmacytoid dendritic cells [pDCs],” said Diwakar Davar, MD, assistant professor of medicine at the University of Pittsburgh, during a presentation of the results at the annual meeting of the Society for Immunotherapy of Cancer. He noted that pDCs represent a very rare cell population, less than 0.4% of circulating peripheral blood mononuclear cells, and tend to be found in lymph nodes.
The current standard of care for stage 3 melanoma is up-front surgery followed by adjuvant therapy – anti–PD-1 therapy for patients with wild-type or BRAF-mutant cancers, and targeted therapy with BRAF/MEK inhibitors in patients with BRAF mutations. However, preclinical studies suggest that neoadjuvant immunotherapy could lead to a stronger antitumor T-cell response than adjuvant immunotherapy.
Vidutolimod targets the toll-like receptor 9 (TLR-9) endosomal receptor found in B cells and pDC cells. The formulation is a virus-like particle (VLP) that contains unmethylated cytosine guanine–rich oligonucleotides (CpG ODN). Bacterial and viral genomes tend to be enriched in CpG ODN, and this acts as a TLR-9 agonist. TLR-9 activation in turn triggers an interferon response, and this may help overcome PD-1 blockade resistance in metastatic melanoma.
The researchers conducted a nonrandomized, open-label trial that included 30 patients with stage 3 melanoma (14 women; median age, 61 years). Patients received neoadjuvant nivolumab and vidutolimod for 8 weeks, then were evaluated for surgery. Patients continued both drugs in the adjuvant setting for 48 weeks. 47% experienced complete pathologic response, 10% a major pathologic response, and 10% a partial pathologic response.
Analysis of resected samples revealed clear evidence of an immune response, Dr. Davar said during a press conference held in advance of the meeting. “Pathologic response was associated with compelling evidence of immune activation both peripherally and within the tumor, with clear evidence of pDC infiltrate and pDC activation – something that has not previously been seen in human specimens.”
The study regimen appeared safe, with no dose-limiting toxicities or grade 4 or 5 adverse events. He noted that the regimen is now being tested in the phase 2 ECOG-ACRIN trial.
The results are “very exciting,” said Pamela Ohashi, PhD, who commented on the study during the press conference. The virus-like nature of vidutolimod may be an important element of the therapy. “I think scientifically we would have predicted that the VLP carrying the CPG would be very good at activating the CD8 cells, which in fact is what you’re seeing. So I think it’s very exciting and has lots of potential for future combinations,” said Dr. Ohashi, who is director of the tumor immunotherapy program at the Princess Margaret Cancer Centre, Toronto.
The study was funded by Checkmate Pharmaceuticals. Dr. Davar has financial relationships with Checkmate Pharmaceuticals and Regeneron, which has acquired Checkmate Pharmaceuticals.
The result led researchers to call for a future study comparing the regimen against a suitable control group.
“We were very excited to see the ability of intratumoral vidutolimod to augment T-cell infiltrate. (Pathologic) response was associated with a dense infiltrate of CD8 T cells. We were also able to demonstrate for what I think may be the first time, that intratumoral CpG resulted in clear evidence of CD303+ plasmacytoid dendritic cells [pDCs],” said Diwakar Davar, MD, assistant professor of medicine at the University of Pittsburgh, during a presentation of the results at the annual meeting of the Society for Immunotherapy of Cancer. He noted that pDCs represent a very rare cell population, less than 0.4% of circulating peripheral blood mononuclear cells, and tend to be found in lymph nodes.
The current standard of care for stage 3 melanoma is up-front surgery followed by adjuvant therapy – anti–PD-1 therapy for patients with wild-type or BRAF-mutant cancers, and targeted therapy with BRAF/MEK inhibitors in patients with BRAF mutations. However, preclinical studies suggest that neoadjuvant immunotherapy could lead to a stronger antitumor T-cell response than adjuvant immunotherapy.
Vidutolimod targets the toll-like receptor 9 (TLR-9) endosomal receptor found in B cells and pDC cells. The formulation is a virus-like particle (VLP) that contains unmethylated cytosine guanine–rich oligonucleotides (CpG ODN). Bacterial and viral genomes tend to be enriched in CpG ODN, and this acts as a TLR-9 agonist. TLR-9 activation in turn triggers an interferon response, and this may help overcome PD-1 blockade resistance in metastatic melanoma.
The researchers conducted a nonrandomized, open-label trial that included 30 patients with stage 3 melanoma (14 women; median age, 61 years). Patients received neoadjuvant nivolumab and vidutolimod for 8 weeks, then were evaluated for surgery. Patients continued both drugs in the adjuvant setting for 48 weeks. 47% experienced complete pathologic response, 10% a major pathologic response, and 10% a partial pathologic response.
Analysis of resected samples revealed clear evidence of an immune response, Dr. Davar said during a press conference held in advance of the meeting. “Pathologic response was associated with compelling evidence of immune activation both peripherally and within the tumor, with clear evidence of pDC infiltrate and pDC activation – something that has not previously been seen in human specimens.”
The study regimen appeared safe, with no dose-limiting toxicities or grade 4 or 5 adverse events. He noted that the regimen is now being tested in the phase 2 ECOG-ACRIN trial.
The results are “very exciting,” said Pamela Ohashi, PhD, who commented on the study during the press conference. The virus-like nature of vidutolimod may be an important element of the therapy. “I think scientifically we would have predicted that the VLP carrying the CPG would be very good at activating the CD8 cells, which in fact is what you’re seeing. So I think it’s very exciting and has lots of potential for future combinations,” said Dr. Ohashi, who is director of the tumor immunotherapy program at the Princess Margaret Cancer Centre, Toronto.
The study was funded by Checkmate Pharmaceuticals. Dr. Davar has financial relationships with Checkmate Pharmaceuticals and Regeneron, which has acquired Checkmate Pharmaceuticals.
The result led researchers to call for a future study comparing the regimen against a suitable control group.
“We were very excited to see the ability of intratumoral vidutolimod to augment T-cell infiltrate. (Pathologic) response was associated with a dense infiltrate of CD8 T cells. We were also able to demonstrate for what I think may be the first time, that intratumoral CpG resulted in clear evidence of CD303+ plasmacytoid dendritic cells [pDCs],” said Diwakar Davar, MD, assistant professor of medicine at the University of Pittsburgh, during a presentation of the results at the annual meeting of the Society for Immunotherapy of Cancer. He noted that pDCs represent a very rare cell population, less than 0.4% of circulating peripheral blood mononuclear cells, and tend to be found in lymph nodes.
The current standard of care for stage 3 melanoma is up-front surgery followed by adjuvant therapy – anti–PD-1 therapy for patients with wild-type or BRAF-mutant cancers, and targeted therapy with BRAF/MEK inhibitors in patients with BRAF mutations. However, preclinical studies suggest that neoadjuvant immunotherapy could lead to a stronger antitumor T-cell response than adjuvant immunotherapy.
Vidutolimod targets the toll-like receptor 9 (TLR-9) endosomal receptor found in B cells and pDC cells. The formulation is a virus-like particle (VLP) that contains unmethylated cytosine guanine–rich oligonucleotides (CpG ODN). Bacterial and viral genomes tend to be enriched in CpG ODN, and this acts as a TLR-9 agonist. TLR-9 activation in turn triggers an interferon response, and this may help overcome PD-1 blockade resistance in metastatic melanoma.
The researchers conducted a nonrandomized, open-label trial that included 30 patients with stage 3 melanoma (14 women; median age, 61 years). Patients received neoadjuvant nivolumab and vidutolimod for 8 weeks, then were evaluated for surgery. Patients continued both drugs in the adjuvant setting for 48 weeks. 47% experienced complete pathologic response, 10% a major pathologic response, and 10% a partial pathologic response.
Analysis of resected samples revealed clear evidence of an immune response, Dr. Davar said during a press conference held in advance of the meeting. “Pathologic response was associated with compelling evidence of immune activation both peripherally and within the tumor, with clear evidence of pDC infiltrate and pDC activation – something that has not previously been seen in human specimens.”
The study regimen appeared safe, with no dose-limiting toxicities or grade 4 or 5 adverse events. He noted that the regimen is now being tested in the phase 2 ECOG-ACRIN trial.
The results are “very exciting,” said Pamela Ohashi, PhD, who commented on the study during the press conference. The virus-like nature of vidutolimod may be an important element of the therapy. “I think scientifically we would have predicted that the VLP carrying the CPG would be very good at activating the CD8 cells, which in fact is what you’re seeing. So I think it’s very exciting and has lots of potential for future combinations,” said Dr. Ohashi, who is director of the tumor immunotherapy program at the Princess Margaret Cancer Centre, Toronto.
The study was funded by Checkmate Pharmaceuticals. Dr. Davar has financial relationships with Checkmate Pharmaceuticals and Regeneron, which has acquired Checkmate Pharmaceuticals.
FROM SITC 2022
Stool transplants may boost immunotherapy success in melanoma
, defined as complete response, partial response, or stable disease that lasted 6 months or longer. The results come from a small, single arm phase 1 study whose primary endpoint was safety.
“We know that the gut microbiome has shown the ability to affect the systemic antitumor immunity by affecting the CD8+ T cells and CD4+ T cells, and these are the cells that are ultimately important for the function of checkpoint inhibitors. There is now clinical evidence that has shown that changing patient microbiota via fecal microbiota transplantation using stool from previous responder patients has the capacity to sensitize immunotherapy refractory melanomas to anti–PD-1 therapy, (with) about 30% response in this setting,” said Saman Maleki, PhD, during his presentation of the results at the Society for Immunotherapy of Cancer’s 37th Annual Meeting. He also noted that broad-spectrum antibiotics have been shown to negatively influence responses to immunotherapy.
Rather than using stool from donors who responded to immunotherapy, the researchers chose instead to use stool from healthy donors.
The study included 20 patients with advanced melanoma who had not been treated with anti–PD-1 therapy. The median age was 75.5 years, 40% were female, and 75% had wild type BRAF. All patients underwent bowel prep and then received fecal transplants from healthy donors, followed by a 7-day engraftment period before initiating anti–PD-1 therapy in the form of nivolumab or pembrolizumab.
The primary endpoint of the study was safety, and no grade 3 or 4 toxicities were observed during the FMT, and safety signals associated with anti–PD-1 therapies were in line with previous experience.
Fifteen percent of patients had a complete response, 50% had a partial response, 15% had stable disease, and 20% had progressive disease. Seventy-five percent of patients had a complete response, partial response, or stable disease that lasted at least 6 months.
Analysis of the microbiomes showed much higher diversity in the donor microbiomes than in patients. “What was really interesting was that the success of engraftment and retention of the donor microbiome was really key in determining between responders and nonresponders. Responders had successful engraftment that lasted over time, and in nonresponders we did not see that,” said Dr. Maleki, who is a cancer immunology researcher at the University of Western Ontario, London.
They also saw differences between responders and nonresponders in how their microbiome evolved over time. Responders had enrichment in Ruminococcus callidus and other bacteria, while nonresponders had enrichment in different bacteria, among them Catabacter hongkongensis, which has previously been implicated as negatively impacting anti–PD-1 responses, according to Dr. Maleki.
Microbiomes from healthy donors had greater diversity than the patients. Following FMT, patients’ microbiomes increased regardless of clinical response to immunotherapy. However, the tendency for patients to trend toward and retain greater diversity over time was associated with treatment success. “What we saw that was key in patients’ response to immunotherapy was the ability of the patients to retain the donor microbiome. All patients’ microbiomes changed and shifted toward the donors’ post FMT. However, only the responders were able to keep the donor microbiome over time, and the nonresponders’ microbiomes reverted to the previous microbiome,” Dr. Maleki said.
The researchers also conducted a mouse version of the clinical trial. They transplanted mice with the baseline fecal samples of a human responder and then exposed the animals to tumors. They then conducted a second FMT with stool from the human donor, and the animals then responded to anti–PD-1 therapy. The results further confirm “that the donor still has the capacity to drive response in this setting,” Dr. Maleki said.
Dr. Maleki is a board member of IMV Inc.
, defined as complete response, partial response, or stable disease that lasted 6 months or longer. The results come from a small, single arm phase 1 study whose primary endpoint was safety.
“We know that the gut microbiome has shown the ability to affect the systemic antitumor immunity by affecting the CD8+ T cells and CD4+ T cells, and these are the cells that are ultimately important for the function of checkpoint inhibitors. There is now clinical evidence that has shown that changing patient microbiota via fecal microbiota transplantation using stool from previous responder patients has the capacity to sensitize immunotherapy refractory melanomas to anti–PD-1 therapy, (with) about 30% response in this setting,” said Saman Maleki, PhD, during his presentation of the results at the Society for Immunotherapy of Cancer’s 37th Annual Meeting. He also noted that broad-spectrum antibiotics have been shown to negatively influence responses to immunotherapy.
Rather than using stool from donors who responded to immunotherapy, the researchers chose instead to use stool from healthy donors.
The study included 20 patients with advanced melanoma who had not been treated with anti–PD-1 therapy. The median age was 75.5 years, 40% were female, and 75% had wild type BRAF. All patients underwent bowel prep and then received fecal transplants from healthy donors, followed by a 7-day engraftment period before initiating anti–PD-1 therapy in the form of nivolumab or pembrolizumab.
The primary endpoint of the study was safety, and no grade 3 or 4 toxicities were observed during the FMT, and safety signals associated with anti–PD-1 therapies were in line with previous experience.
Fifteen percent of patients had a complete response, 50% had a partial response, 15% had stable disease, and 20% had progressive disease. Seventy-five percent of patients had a complete response, partial response, or stable disease that lasted at least 6 months.
Analysis of the microbiomes showed much higher diversity in the donor microbiomes than in patients. “What was really interesting was that the success of engraftment and retention of the donor microbiome was really key in determining between responders and nonresponders. Responders had successful engraftment that lasted over time, and in nonresponders we did not see that,” said Dr. Maleki, who is a cancer immunology researcher at the University of Western Ontario, London.
They also saw differences between responders and nonresponders in how their microbiome evolved over time. Responders had enrichment in Ruminococcus callidus and other bacteria, while nonresponders had enrichment in different bacteria, among them Catabacter hongkongensis, which has previously been implicated as negatively impacting anti–PD-1 responses, according to Dr. Maleki.
Microbiomes from healthy donors had greater diversity than the patients. Following FMT, patients’ microbiomes increased regardless of clinical response to immunotherapy. However, the tendency for patients to trend toward and retain greater diversity over time was associated with treatment success. “What we saw that was key in patients’ response to immunotherapy was the ability of the patients to retain the donor microbiome. All patients’ microbiomes changed and shifted toward the donors’ post FMT. However, only the responders were able to keep the donor microbiome over time, and the nonresponders’ microbiomes reverted to the previous microbiome,” Dr. Maleki said.
The researchers also conducted a mouse version of the clinical trial. They transplanted mice with the baseline fecal samples of a human responder and then exposed the animals to tumors. They then conducted a second FMT with stool from the human donor, and the animals then responded to anti–PD-1 therapy. The results further confirm “that the donor still has the capacity to drive response in this setting,” Dr. Maleki said.
Dr. Maleki is a board member of IMV Inc.
, defined as complete response, partial response, or stable disease that lasted 6 months or longer. The results come from a small, single arm phase 1 study whose primary endpoint was safety.
“We know that the gut microbiome has shown the ability to affect the systemic antitumor immunity by affecting the CD8+ T cells and CD4+ T cells, and these are the cells that are ultimately important for the function of checkpoint inhibitors. There is now clinical evidence that has shown that changing patient microbiota via fecal microbiota transplantation using stool from previous responder patients has the capacity to sensitize immunotherapy refractory melanomas to anti–PD-1 therapy, (with) about 30% response in this setting,” said Saman Maleki, PhD, during his presentation of the results at the Society for Immunotherapy of Cancer’s 37th Annual Meeting. He also noted that broad-spectrum antibiotics have been shown to negatively influence responses to immunotherapy.
Rather than using stool from donors who responded to immunotherapy, the researchers chose instead to use stool from healthy donors.
The study included 20 patients with advanced melanoma who had not been treated with anti–PD-1 therapy. The median age was 75.5 years, 40% were female, and 75% had wild type BRAF. All patients underwent bowel prep and then received fecal transplants from healthy donors, followed by a 7-day engraftment period before initiating anti–PD-1 therapy in the form of nivolumab or pembrolizumab.
The primary endpoint of the study was safety, and no grade 3 or 4 toxicities were observed during the FMT, and safety signals associated with anti–PD-1 therapies were in line with previous experience.
Fifteen percent of patients had a complete response, 50% had a partial response, 15% had stable disease, and 20% had progressive disease. Seventy-five percent of patients had a complete response, partial response, or stable disease that lasted at least 6 months.
Analysis of the microbiomes showed much higher diversity in the donor microbiomes than in patients. “What was really interesting was that the success of engraftment and retention of the donor microbiome was really key in determining between responders and nonresponders. Responders had successful engraftment that lasted over time, and in nonresponders we did not see that,” said Dr. Maleki, who is a cancer immunology researcher at the University of Western Ontario, London.
They also saw differences between responders and nonresponders in how their microbiome evolved over time. Responders had enrichment in Ruminococcus callidus and other bacteria, while nonresponders had enrichment in different bacteria, among them Catabacter hongkongensis, which has previously been implicated as negatively impacting anti–PD-1 responses, according to Dr. Maleki.
Microbiomes from healthy donors had greater diversity than the patients. Following FMT, patients’ microbiomes increased regardless of clinical response to immunotherapy. However, the tendency for patients to trend toward and retain greater diversity over time was associated with treatment success. “What we saw that was key in patients’ response to immunotherapy was the ability of the patients to retain the donor microbiome. All patients’ microbiomes changed and shifted toward the donors’ post FMT. However, only the responders were able to keep the donor microbiome over time, and the nonresponders’ microbiomes reverted to the previous microbiome,” Dr. Maleki said.
The researchers also conducted a mouse version of the clinical trial. They transplanted mice with the baseline fecal samples of a human responder and then exposed the animals to tumors. They then conducted a second FMT with stool from the human donor, and the animals then responded to anti–PD-1 therapy. The results further confirm “that the donor still has the capacity to drive response in this setting,” Dr. Maleki said.
Dr. Maleki is a board member of IMV Inc.
FROM SITC 2022
Consider gaps in access and knowledge in diagnosis and treatment in skin of color
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
LAS VEGAS – and patients, Susan C. Taylor, MD, said in a presentation at MedscapeLive’s annual Las Vegas Dermatology Seminar.
Additionally, some disparities occur because of gaps in access to health care, said Dr. Taylor, vice chair, diversity, equity and inclusion, in the department of dermatology at the University of Pennsylvania, Philadelphia, who moderated an expert panel discussion of treatment tips for several common dermatologic conditions in skin of color patients.
Atopic dermatitis angles
Atopic dermatitis (AD) is the fourth most common dermatologic complaint in Black patients, based on data from the United States National Ambulatory Medical Care Survey. Also, data from the National Health and Nutrition Examination Survey show that Black children are nearly twice as likely as White children to develop AD after controlling for socioeconomic factors, Dr. Taylor said.
When Black patients present with AD, “you may not see the erythema,” said Valerie D. Callender, MD, of Howard University, Washington, who presented on AD. Instead, “you may see more follicular and papular presentations.” Erythema and erythroderma can present as shades of violet, gray, or dark brown in patients with rich skin tones, added Dr. Callender, who practices in Glenn Dale, Md.
Consequently, disease severity can be misinterpreted, she said, noting that data suggest that scoring systems such as the Eczema Area and Severity Index and Scoring Atopic Dermatitis underestimate AD severity in dark skin.
As for treatment, skin of color patients with AD are often as bothered by postinflammatory hyperpigmentation (PIH) as by active lesions, so treatment should take these concerns into account, Dr. Callender said. Studies evaluating the effectiveness of AD treatments in diverse populations are limited by lack of representation of racial groups in clinical trials and lack of subset analyses by race.
Acne awareness
An important consideration of acne in skin of color patients is that the acne “might not be red, it might just be darker,” said Andrew F. Alexis, MD, vice-chair for diversity and inclusion in the department of dermatology, and professor of clinical dermatology at Weill Cornell Medicine, New York. A study published in JAMA Dermatology of nearly 30,000 patients with acne from 2007 to 2017 found that non-Hispanic Black patients were more likely than non-Hispanic White patients to see a dermatologist for acne, but Black patients received fewer prescriptions for acne medications than White patients.
The study also showed that Black patients who received prescriptions for acne were more likely to receive topical retinoids and topical antibiotics, and less likely to receive oral antibiotics, spironolactone, or isotretinoin, compared with White patients. Similarly, Asian patients were more likely to receive topical antibiotics and less likely to receive oral antibiotics, compared with White patients.
Other panelists shared some of their best practices for acne in patients with skin of color, including treatment with topical retinoids (for inflammation) and spironolactone, and therapies that address both inflammation and pigmentation, such as salicylic acid and azelaic acid. Dr. Callender also advised asking patients about makeup, as they may not know that many types of makeup used to cover acne are in fact comedogenic.
Melanoma misconceptions
One of the most common misperceptions about melanoma among skin of color patients is that they don’t think they can get it, Dr. Taylor said. Many health care providers don’t think about melanoma in skin of color patients because of the dramatically lower incidence in this population, but as a result, cases may go undiagnosed, and as studies have shown, the mortality rate from melanoma is higher in Black patients.
Consider the palms, soles, nails, and web spaces as possible melanoma sites, Dr. Taylor added.
Educating skin of color patients about melanoma is important, although the incidence is 20 to 30 times lower than in non-Hispanic Whites, said Nada Elbuluk, MD, the founder and director of the University of Southern California Skin of Color Center and Pigmentary Disorders Clinic, Los Angeles. A 2020 editorial published in Cancer Cytopathology pointed out that 1 in 3 Black men or women with a melanoma diagnosis in the United States dies of the disease, compared with 1 in 7 non-Hispanic White men and 1 in 11 non-Hispanic White women with melanoma.
Don’t skip the total body skin exam in these patients, Dr. Elbuluk emphasized. Many patients will only partially undress, and areas such as toes can be missed.
Rosacea review
For patients with skin of color, clinicians need to look for different signs of rosacea than those typically seen in White patients, Dr. Elbuluk said. “The most common presentation of rosacea in skin of color is papulopustular,” and the granulomatous variant.
“These patients will often give you a history of sensitivity to products,” Dr. Elbuluk noted. They may not always have the flushing, but they may report warmth or itching, in addition to product sensitivity.
When considering rosacea in skin of color patients, be sure to have good lighting for close examination, as skin thickening is another subtle sign of rosacea in these patients, she said. Skin thickening “is a very early sign that will present in skin of color with no erythema, so keep that in mind.”
Stinging and burning sensations may be reported by skin of color patients with rosacea. Use patient history to confirm the diagnosis of rosacea, which is often delayed in skin of color patients because of a low index of suspicion, she said.
Psoriasis pointers
Psoriasis in skin of color patients used to be considered rare, “but that is far from true,” Dr. Alexis said. In fact, many cases of psoriasis are undiagnosed or the diagnosis is delayed in these patients.
The panelists noted that current guidelines for psoriasis treatment are based on clinical trials composed mainly of White patients, and do not contain specific recommendations for skin of color patients.
Notably, the morphology, location, and color of psoriasis lesions may be different for patients with darker skin, such as thicker plaques and more scaling over larger areas, they said. Also, skin of color patients may experience long-lasting dyspigmentation from psoriasis lesions that have resolved.
When developing a strategy for psoriasis in skin of color patients, consider not only disease severity, but also comorbidities and medications, response (if any) to prior therapies, patient preferences, and quality of life, the panelists said.
Dr. Callender, Dr. Elbuluk, Dr. Taylor, and Dr. Alexis reported conflicts of interest from numerous sources in industry. MedscapeLive and this news organization are owned by the same parent company.
AT INNOVATIONS IN DERMATOLOGY
Skinny-label biosimilars provide substantial savings to Medicare
Recent court rulings could put such saving under threat
Competition between five biologic drugs and their skinny-label biosimilars saved Medicare an estimated $1.5 billion during 2015-2020. But these savings accruing to Medicare and the availability of those and other biosimilars through skinny labeling is under threat from recent court rulings, according to a research letter published online in JAMA Internal Medicine.
The authors highlighted the need for such savings by noting that, while biologics comprise less than 5% of prescription drug use, their price tag amounts to about 40% of U.S. drug spending, Biologic manufacturers often delay the availability of biosimilars for additional years beyond the original patent expiration through further patents for supplemental indications. To provide a counterbalance, federal law allows the Food and Drug Administration to approve “skinny-label” generics and biosimilars that carve out patent-protected indications or regulatory exclusivities. But once a generic drug reaches the market through this process with a skinny label, it may often be substituted for indications that go beyond the ones listed on the skinny label. In fact, some state laws mandate that pharmacists substitute interchangeable generics for brand-name drugs, helping to decrease drug prices. In response to legal threats to the skinny-label pathway, Alexander C. Egilman and colleagues at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, assessed the frequency of approval and marketing of skinny-label biosimilars from 2015 to 2021 and the resultant savings to Medicare.
The authors estimated annual Part B (clinician-administered) savings from skinny-label biosimilars through 2020 by comparing actual biologic and skinny-label biosimilar spending with estimated biologic spending without competition using the Medicare Dashboard. They assumed that the unit price of the biologic would increase at its 5-year compound annual growth rate prior to competition.
In that period, the FDA approved 33 biosimilars linked to 11 biologics. Among them, 22 (66.7%) had a skinny label. Of 21 biosimilars marketed before 2022, 13 (61.9%) were launched with a skinny label. Of the 8 biologics linked to these 21 biosimilars, 5 of the first-to-market biosimilars had skinny labels (bevacizumab, filgrastim, infliximab, pegfilgrastim, and rituximab), leading to earlier competition through 2021.
The estimated $1.5 billion in savings to Medicare from these skinny-label biosimilars over the 2015-2020 span represents 4.9% of the $30.2 billion that Medicare spent on the five biologics during this period. The researchers pointed out that once adalimumab (Humira) faces skinny-label biosimilar competition in 2023, savings will likely grow substantially.
In response to the research letter, an editor’s note by JAMA Internal Medicine Editorial Fellow Eric Ward, MD, and JAMA Internal Medicine Editor at Large and Online Editor Robert Steinbrook, MD, stated that, between 2015 and 2019, 24 (43%) of 56 brand-name drugs had competition from skinny-labeled generic formulations after first becoming available as generics.
The editors also referenced a JAMA Viewpoints article from 2021 that reviewed the most recent case challenging the skinny-label pathway in which GlaxoSmithKline sued Teva for its marketing of a skinny-label generic of the brand-name beta-blocker carvedilol (Coreg) that the plaintive claimed “induced physicians to prescribe carvedilol for indications that had been carved out by Teva’s skinny label, thus infringing GlaxoSmithKline’s patents.” A $235 million judgment against Teva was overturned by a district court and then reversed again by a Federal Circuit court that, after receiving criticism, reconsidered the case, and a panel affirmed the judgment against Teva.
“The Federal Circuit panel’s decision has the potential to put generic drugs that fail to adequately carve out indications from the brand name labeling at risk for damages related to infringement,” the authors wrote. Similar claims of infringement are being heard in other courts, they wrote, and they urged careful targeting of skinny-label carveouts, and suggest also that challenges to the arguments used against Teva focus on preservation of First Amendment rights as protection for lawful and accurate speech in drug labels.
“The legal uncertainties are likely to continue, as manufacturers pursue novel and complex strategies to protect the patents and regulatory exclusivities of brand-name drugs and biologics,” Dr. Ward and Dr. Steinbrook wrote, adding that “the path forward is for Congress to enact additional legislation that reaffirms and strengthens the permissibility of skinny labeling.”
The research letter’s corresponding author, Ameet Sarpatwari, PhD, JD, assistant professor at Harvard Medical School, and assistant director for the Harvard Program On Regulation, Therapeutics, And Law, echoed concerns over the Teva case in an interview. “There has certainly been concern that should the appellate decision stand, there will be a chilling effect. As the lone dissenter in that case noted, ‘no skinny-label generic is safe.’ I think many generic and biosimilar manufacturers are awaiting to see whether the Supreme Court will take the case.”
He added: “I do not believe the likelihood of skinny-label-supportive legislation making it through Congress will be greatly diminished in a divided Congress. Democrats and Republicans alike should seek to promote competition in the marketplace, which is what the skinny-labeling pathway accomplishes.”
The authors reported no relevant conflicts of interest. The research was funded by a grant from Arnold Ventures.
Recent court rulings could put such saving under threat
Recent court rulings could put such saving under threat
Competition between five biologic drugs and their skinny-label biosimilars saved Medicare an estimated $1.5 billion during 2015-2020. But these savings accruing to Medicare and the availability of those and other biosimilars through skinny labeling is under threat from recent court rulings, according to a research letter published online in JAMA Internal Medicine.
The authors highlighted the need for such savings by noting that, while biologics comprise less than 5% of prescription drug use, their price tag amounts to about 40% of U.S. drug spending, Biologic manufacturers often delay the availability of biosimilars for additional years beyond the original patent expiration through further patents for supplemental indications. To provide a counterbalance, federal law allows the Food and Drug Administration to approve “skinny-label” generics and biosimilars that carve out patent-protected indications or regulatory exclusivities. But once a generic drug reaches the market through this process with a skinny label, it may often be substituted for indications that go beyond the ones listed on the skinny label. In fact, some state laws mandate that pharmacists substitute interchangeable generics for brand-name drugs, helping to decrease drug prices. In response to legal threats to the skinny-label pathway, Alexander C. Egilman and colleagues at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, assessed the frequency of approval and marketing of skinny-label biosimilars from 2015 to 2021 and the resultant savings to Medicare.
The authors estimated annual Part B (clinician-administered) savings from skinny-label biosimilars through 2020 by comparing actual biologic and skinny-label biosimilar spending with estimated biologic spending without competition using the Medicare Dashboard. They assumed that the unit price of the biologic would increase at its 5-year compound annual growth rate prior to competition.
In that period, the FDA approved 33 biosimilars linked to 11 biologics. Among them, 22 (66.7%) had a skinny label. Of 21 biosimilars marketed before 2022, 13 (61.9%) were launched with a skinny label. Of the 8 biologics linked to these 21 biosimilars, 5 of the first-to-market biosimilars had skinny labels (bevacizumab, filgrastim, infliximab, pegfilgrastim, and rituximab), leading to earlier competition through 2021.
The estimated $1.5 billion in savings to Medicare from these skinny-label biosimilars over the 2015-2020 span represents 4.9% of the $30.2 billion that Medicare spent on the five biologics during this period. The researchers pointed out that once adalimumab (Humira) faces skinny-label biosimilar competition in 2023, savings will likely grow substantially.
In response to the research letter, an editor’s note by JAMA Internal Medicine Editorial Fellow Eric Ward, MD, and JAMA Internal Medicine Editor at Large and Online Editor Robert Steinbrook, MD, stated that, between 2015 and 2019, 24 (43%) of 56 brand-name drugs had competition from skinny-labeled generic formulations after first becoming available as generics.
The editors also referenced a JAMA Viewpoints article from 2021 that reviewed the most recent case challenging the skinny-label pathway in which GlaxoSmithKline sued Teva for its marketing of a skinny-label generic of the brand-name beta-blocker carvedilol (Coreg) that the plaintive claimed “induced physicians to prescribe carvedilol for indications that had been carved out by Teva’s skinny label, thus infringing GlaxoSmithKline’s patents.” A $235 million judgment against Teva was overturned by a district court and then reversed again by a Federal Circuit court that, after receiving criticism, reconsidered the case, and a panel affirmed the judgment against Teva.
“The Federal Circuit panel’s decision has the potential to put generic drugs that fail to adequately carve out indications from the brand name labeling at risk for damages related to infringement,” the authors wrote. Similar claims of infringement are being heard in other courts, they wrote, and they urged careful targeting of skinny-label carveouts, and suggest also that challenges to the arguments used against Teva focus on preservation of First Amendment rights as protection for lawful and accurate speech in drug labels.
“The legal uncertainties are likely to continue, as manufacturers pursue novel and complex strategies to protect the patents and regulatory exclusivities of brand-name drugs and biologics,” Dr. Ward and Dr. Steinbrook wrote, adding that “the path forward is for Congress to enact additional legislation that reaffirms and strengthens the permissibility of skinny labeling.”
The research letter’s corresponding author, Ameet Sarpatwari, PhD, JD, assistant professor at Harvard Medical School, and assistant director for the Harvard Program On Regulation, Therapeutics, And Law, echoed concerns over the Teva case in an interview. “There has certainly been concern that should the appellate decision stand, there will be a chilling effect. As the lone dissenter in that case noted, ‘no skinny-label generic is safe.’ I think many generic and biosimilar manufacturers are awaiting to see whether the Supreme Court will take the case.”
He added: “I do not believe the likelihood of skinny-label-supportive legislation making it through Congress will be greatly diminished in a divided Congress. Democrats and Republicans alike should seek to promote competition in the marketplace, which is what the skinny-labeling pathway accomplishes.”
The authors reported no relevant conflicts of interest. The research was funded by a grant from Arnold Ventures.
Competition between five biologic drugs and their skinny-label biosimilars saved Medicare an estimated $1.5 billion during 2015-2020. But these savings accruing to Medicare and the availability of those and other biosimilars through skinny labeling is under threat from recent court rulings, according to a research letter published online in JAMA Internal Medicine.
The authors highlighted the need for such savings by noting that, while biologics comprise less than 5% of prescription drug use, their price tag amounts to about 40% of U.S. drug spending, Biologic manufacturers often delay the availability of biosimilars for additional years beyond the original patent expiration through further patents for supplemental indications. To provide a counterbalance, federal law allows the Food and Drug Administration to approve “skinny-label” generics and biosimilars that carve out patent-protected indications or regulatory exclusivities. But once a generic drug reaches the market through this process with a skinny label, it may often be substituted for indications that go beyond the ones listed on the skinny label. In fact, some state laws mandate that pharmacists substitute interchangeable generics for brand-name drugs, helping to decrease drug prices. In response to legal threats to the skinny-label pathway, Alexander C. Egilman and colleagues at Brigham and Women’s Hospital and Harvard Medical School, both in Boston, assessed the frequency of approval and marketing of skinny-label biosimilars from 2015 to 2021 and the resultant savings to Medicare.
The authors estimated annual Part B (clinician-administered) savings from skinny-label biosimilars through 2020 by comparing actual biologic and skinny-label biosimilar spending with estimated biologic spending without competition using the Medicare Dashboard. They assumed that the unit price of the biologic would increase at its 5-year compound annual growth rate prior to competition.
In that period, the FDA approved 33 biosimilars linked to 11 biologics. Among them, 22 (66.7%) had a skinny label. Of 21 biosimilars marketed before 2022, 13 (61.9%) were launched with a skinny label. Of the 8 biologics linked to these 21 biosimilars, 5 of the first-to-market biosimilars had skinny labels (bevacizumab, filgrastim, infliximab, pegfilgrastim, and rituximab), leading to earlier competition through 2021.
The estimated $1.5 billion in savings to Medicare from these skinny-label biosimilars over the 2015-2020 span represents 4.9% of the $30.2 billion that Medicare spent on the five biologics during this period. The researchers pointed out that once adalimumab (Humira) faces skinny-label biosimilar competition in 2023, savings will likely grow substantially.
In response to the research letter, an editor’s note by JAMA Internal Medicine Editorial Fellow Eric Ward, MD, and JAMA Internal Medicine Editor at Large and Online Editor Robert Steinbrook, MD, stated that, between 2015 and 2019, 24 (43%) of 56 brand-name drugs had competition from skinny-labeled generic formulations after first becoming available as generics.
The editors also referenced a JAMA Viewpoints article from 2021 that reviewed the most recent case challenging the skinny-label pathway in which GlaxoSmithKline sued Teva for its marketing of a skinny-label generic of the brand-name beta-blocker carvedilol (Coreg) that the plaintive claimed “induced physicians to prescribe carvedilol for indications that had been carved out by Teva’s skinny label, thus infringing GlaxoSmithKline’s patents.” A $235 million judgment against Teva was overturned by a district court and then reversed again by a Federal Circuit court that, after receiving criticism, reconsidered the case, and a panel affirmed the judgment against Teva.
“The Federal Circuit panel’s decision has the potential to put generic drugs that fail to adequately carve out indications from the brand name labeling at risk for damages related to infringement,” the authors wrote. Similar claims of infringement are being heard in other courts, they wrote, and they urged careful targeting of skinny-label carveouts, and suggest also that challenges to the arguments used against Teva focus on preservation of First Amendment rights as protection for lawful and accurate speech in drug labels.
“The legal uncertainties are likely to continue, as manufacturers pursue novel and complex strategies to protect the patents and regulatory exclusivities of brand-name drugs and biologics,” Dr. Ward and Dr. Steinbrook wrote, adding that “the path forward is for Congress to enact additional legislation that reaffirms and strengthens the permissibility of skinny labeling.”
The research letter’s corresponding author, Ameet Sarpatwari, PhD, JD, assistant professor at Harvard Medical School, and assistant director for the Harvard Program On Regulation, Therapeutics, And Law, echoed concerns over the Teva case in an interview. “There has certainly been concern that should the appellate decision stand, there will be a chilling effect. As the lone dissenter in that case noted, ‘no skinny-label generic is safe.’ I think many generic and biosimilar manufacturers are awaiting to see whether the Supreme Court will take the case.”
He added: “I do not believe the likelihood of skinny-label-supportive legislation making it through Congress will be greatly diminished in a divided Congress. Democrats and Republicans alike should seek to promote competition in the marketplace, which is what the skinny-labeling pathway accomplishes.”
The authors reported no relevant conflicts of interest. The research was funded by a grant from Arnold Ventures.
FROM JAMA INTERNAL MEDICINE
Therapeutic Considerations in Adults With Hidradenitis Suppurativa
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by recurrent boils, abscesses, and nodules that can progress to narrow channels that form under the skin. An estimated 1%-4% of the US population has the condition, and women are affected more commonly than men.
Treatment of HS is challenging and the pathogenesis is still under investigation. Many believe that the disease involves follicular occlusion that leads to perifollicular cyst development followed by ruptures of the cyst contents. Many drug classes, including antibiotics and topical therapies, as well as lifestyle modifications, have been used to successfully treat mild to moderate HS. Management of moderate to severe HS has been less successful, however.
Dr Jennifer Hsiao, from the University of Southern California, highlights the various approaches to HS treatment, including medical, procedural, and emerging options.
--
Jennifer Hsiao, MD, Associate Professor, Physician, Department of Dermatology, University of Southern California, Los Angeles, California
Jennifer Hsiao, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Novartis; UCB
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by recurrent boils, abscesses, and nodules that can progress to narrow channels that form under the skin. An estimated 1%-4% of the US population has the condition, and women are affected more commonly than men.
Treatment of HS is challenging and the pathogenesis is still under investigation. Many believe that the disease involves follicular occlusion that leads to perifollicular cyst development followed by ruptures of the cyst contents. Many drug classes, including antibiotics and topical therapies, as well as lifestyle modifications, have been used to successfully treat mild to moderate HS. Management of moderate to severe HS has been less successful, however.
Dr Jennifer Hsiao, from the University of Southern California, highlights the various approaches to HS treatment, including medical, procedural, and emerging options.
--
Jennifer Hsiao, MD, Associate Professor, Physician, Department of Dermatology, University of Southern California, Los Angeles, California
Jennifer Hsiao, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Novartis; UCB
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition characterized by recurrent boils, abscesses, and nodules that can progress to narrow channels that form under the skin. An estimated 1%-4% of the US population has the condition, and women are affected more commonly than men.
Treatment of HS is challenging and the pathogenesis is still under investigation. Many believe that the disease involves follicular occlusion that leads to perifollicular cyst development followed by ruptures of the cyst contents. Many drug classes, including antibiotics and topical therapies, as well as lifestyle modifications, have been used to successfully treat mild to moderate HS. Management of moderate to severe HS has been less successful, however.
Dr Jennifer Hsiao, from the University of Southern California, highlights the various approaches to HS treatment, including medical, procedural, and emerging options.
--
Jennifer Hsiao, MD, Associate Professor, Physician, Department of Dermatology, University of Southern California, Los Angeles, California
Jennifer Hsiao, MD, has disclosed the following relevant financial relationships:
Serve(d) as a director, officer, partner, employee, advisor, consultant, or trustee for: AbbVie; Novartis; UCB
Serve(d) as a speaker or a member of a speakers bureau for: AbbVie
The right indoor relative humidity could ward off COVID
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
The “sweet spot” associated with reduced COVID-19 cases and deaths is 40%-60% indoor relative humidity, an MIT news release said. People who maintained indoor relative humidity outside those parameters had higher rates of catching COVID-19.
Most people are comfortable with 30%-50% relative humidity, researchers said. An airplane cabin has about 20% relative humidity.
Relative humidity is the amount of moisture in the air, compared with the total moisture the air can hold at a given temperature before saturating and forming condensation.
The study was published in The Journal of the Royal Society Interface. Researchers examined COVID-19 data and meteorological measurements from 121 countries from January 2020 through August 2020, before vaccines became available to the public.
“When outdoor temperatures were below the typical human comfort range, they assumed indoor spaces were heated to reach that comfort range. Based on the added heating, they calculated the associated drop in indoor relative humidity,” the MIT news release said.
The research teams found that when a region reported a rise in COVID-19 cases and deaths, the region’s estimated indoor relative humidity was either lower than 40% or higher than 60%, the release said.
“There’s potentially a protective effect of this intermediate indoor relative humidity,” said Connor Verheyen, the lead author and a PhD student in medical engineering and medical physics in the Harvard-MIT Program in Health Sciences and Technology.
Widespread use of the 40%-60% indoor humidity range could reduce the need for lockdowns and other widespread restrictions, the study concluded.
“Unlike measures that depend on individual compliance (for example, masking or hand-washing), indoor RH optimization would achieve high compliance because all occupants of a common indoor space would be exposed to similar ambient conditions,” the study said. “Compared to the long timelines and high costs of vaccine production and distribution, humidity control systems could potentially be implemented more quickly and cheaply in certain indoor settings.”
A version of this article first appeared on WebMD.com.
FROM THE JOURNAL OF THE ROYAL SOCIETY INTERFACE
Laser and light devices for acne treatment continue to advance
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
The calendar year
This was preceded by the FDA clearance of AviClear, marketed by Cutera, in March, and the commercial launch of TheraClearX, marketed by StrataSkin, in July.
“It’s an exciting time to be working with acne,” Fernanda H. Sakamoto, MD, PhD, a dermatologist at the Wellman Center for Photomedicine at Massachusetts General Hospital, Boston. “We’ll see a lot of people using new devices. I’m looking forward to seeing results in the long term.”
AviClear and the Accure Laser System, marketed by Accure, are both powered by a 1,726-nm laser, but they work differently. AviClear, which was cleared for the treatment of mild, moderate, and severe acne, has a maximum fluence of 30 J/cm2 in single-pulse mode and a maximum fluence of 20 J/cm2 in double-pulse mode. The treatment handpiece has an integrated scanner for delivering treatment spot(s) in an operator-selected pattern. “It’s a little bit lower powered than the Accure and has a maximum pulse energy of 5 joules and a pulse duration of up to 50 milliseconds,” Dr. Sakamoto said. In the treatment of acne, laser and light treatments target the sebaceous gland.
In pivotal data submitted to the FDA, 104 patients with acne who were enrolled at 7 U.S. sites received 304 treatments with AviClear spaced 2-5 weeks apart. Each treatment took about 30 minutes. Treatment success was defined as having at least 50% fewer inflammatory acne lesions 12 weeks after the final treatment visit, compared with baseline. At the week 4 follow-up visit, there were median and mean reductions of 42% and 37%, respectively, in the inflammatory lesion counts from baseline (P < .001). The researchers found that, at the week 4 follow-up visit, 36% of patients had achieved treatment success, which increased to 78% at the 12-week follow-up visit. Treatment was considered safe and tolerable, according to the manufacturer.
The other newcomer device with a 1,726-nm wavelength is the Accure Laser System, which features a smart laser handpiece for real-time thermal monitoring and precise delivery of laser emissions. The device received CE Mark approval in 2020 for the treatment of moderate acne, and on Nov. 22, 2022, the manufacturer announced that it had been cleared by the FDA for the treatment of mild to severe inflammatory acne vulgaris.
Dr. Sakamoto and her Wellman colleagues have been working with five dermatologists to conduct clinical trials of the device: Emil Tanghetti, MD, and Mitchel Goldman, MD, in California; Roy Geronemus, MD, in New York; Joel Cohen, MD, in Colorado; and Daniel Friedmann, MD, in Texas. As of Oct. 2, 2022, more than 50 patients with mild to severe acne were enrolled in four studies and an additional 30 were enrolled in a pilot facial acne trial, Dr. Sakamoto said. In the trials, patients are followed at 4, 8, 12, and 24 weeks post treatment.
Among patients enrolled in the facial acne trial, researchers have observed a 100% responder rate for patients with more than five acne lesions at 4, 8, 12, and 24 weeks post treatment after four monthly treatment sessions. The average lesion reduction at week 12 was 82% and the mean visual analog scale score immediately after treatment was 2.09 out of 10. Each patient received more than 12,000 trigger pulls of energy from the device overall with no adverse events reported. At 12 months, they observed a 90% inflammatory lesion count reduction from baseline and a rapid response to treatment: a 73% reduction achieved after the first two treatment sessions. Histologic studies revealed selective sebaceous gland destruction with no damage to the epidermis, surrounding dermis, or other skin structures.
Dr. Sakamoto emphasized that to date no direct clinical comparisons have been made between the AviClear and Accure devices. “Are all 1,726-nm lasers made equal? That is a question that we have to keep in our mind,” she said during the meeting, which was sponsored by Harvard Medical School, Massachusetts General Hospital, and the Wellman Center for Photomedicine. “They are using the same wavelength, but they are different types of lasers.”
For example, the Accure Laser treats to temperature, relies on air cooling, and is targeted to dermatologists and plastic surgeons, while the AviClear treats to fluence, relies on contact cooling, and includes med spas and other nonphysician providers as the target users. “Mathematically, the difference between the two devices is that the Accure can achieve deeper penetration in a single pulse, while the AviClear is a little more superficial,” she said. “Whether that is translated clinically is unknown at this point.”
Dr. Sakamoto also discussed the TheraClearX, which is FDA cleared for the treatment of mild, moderate, and severe acne, including comedonal, pustular, and inflammatory acne vulgaris. The device, which is a new version of the Palomar Acleara, uses a vacuum technique with up to 3 psi pressure in conjunction with broadband light with a wavelength spectrum of 500 nm–1,200 nm delivered through a liquid-cooled, handheld delivery system. The predicate device was the Aesthera Isolaz System. The vacuum extracts buildup of sebaceous material. “At the same time, it takes the blood out of the competing chromophore,” she said. “By doing so, it potentially damages the sebaceous glands and reduces the inflammatory lesions.”
Dr. Sakamoto disclosed that she is the founder of and science advisor for Lightwater Bioscience. She is also a science advisor for Accure Acne and has received portions of patent royalties from Massachusetts General Hospital.
FROM A LASER & AESTHETIC SKIN THERAPY COURSE
Primary Malignant Melanoma of the Middle Ear
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
To the Editor:
An 82-year-old man presented to our dermatology clinic for a total-body skin examination due to a recently diagnosed primary melanoma of the left middle ear. He reported pain of the left ear and water behind the left eardrum of 1 year’s duration. An otorhinolaryngologist performed surgery due to the severe mastoiditis. A biopsy of the contents of the left middle ear revealed malignant melanoma. Positron emission tomography–computed tomography revealed the mass was mainly located in the anterior aspect of the left middle ear with suspicion of tumor extension into the bony portion of the eustachian tube. No other disease was present. Prior to presentation to dermatology, gross excision of the left middle ear with removal of additional melanoma was confirmed by biopsy, and further analysis revealed v-Raf murine sarcoma viral oncogene (BRAF) was not detected while cellular proto-oncogene receptor kinase (KIT) mutation was detected on exon 13p (K642E).
The patient had no family history of melanoma. He never smoked and did not have contact with hazardous material. Initial examination at our clinic revealed no other suspicious pigmented lesions. After additional negative workup by the oncologist, the patient was presented to the tumor board, and postoperative radiotherapy was recommended to improve local control. Eight months after the patient’s initial diagnosis of the primary middle ear melanoma, a computed tomography–guided right lung biopsy showed metastatic melanoma. After various treatment modalities were discussed with the patient and his family, he was started on pembrolizumab. After 6 months on pembrolizumab, the patient developed autoimmune pneumonitis and pembrolizumab was discontinued. The patient elected to discontinue treatment and died 6 months later.
Malignant melanoma with primary involvement of the middle ear and mastoid mucosa rarely has been reported.1-3 Primary malignant melanoma of the middle ear mucosa is difficult to diagnose clinically. Difficulty and delay in diagnosis occur because of the location and frequent lack of pathognomonic symptoms of the disease.2 A comprehensive literature review by Maxwell et al3 in 2018 of the 10 reported primary middle ear mucosal melanomas found that patients most commonly presented with otorrhea, aural fullness, and hearing loss. Less common symptoms included otalgia, tinnitus, and facial weakness. Clinical examination revealed patients presented with serous otitis and/or a visible mass within the middle ear or external auditory canal. These melanomas demonstrated particularly poor outcomes, with 70% mortality, 20% local recurrence, and 50% distant metastasis. Distant metastases that occurred with primary middle ear mucosal melanoma include lung, liver, intraparotid, abdomen, and cutaneous metastasis.3
The specific pathophysiologic factors underlying the development of primary malignant melanoma of the middle ear mucosa are not known.2 The middle ear and its components develop from the first and second pharyngeal arches.4 Melanocyte precursors from the neural crest migrate during the seventh or eighth week of embryogenesis. These precursors migrate to the epidermis, various mucosal epithelial, hair follicles, dermis, retina, uveal tract, leptomeninges, inner ear, and other tissues.5 The ossicles of the middle ear develop from the neural crest6 and remain in the mesenchyme until the eighth month, when the surrounding tissue dissolves.4 Cutaneous melanomas arise from the malignant transformation of melanocytes in the skin of neural crest lineage. Noncutaneous melanomas are hypothesized to arise from melanoblasts migrating to noncutaneous organs after neural crest cells undergo an epithelial-mesenchymal translation.7
Melanoma 5-year survival rates vary based on the melanoma disease stage: 98% for stage 1, 90% for stage 2, 70% for stage 3, and 10% for stage 4. Although early-stage disease mainly is treated with surgery, advanced and unresectable disease is managed with different therapeutic options, including BRAF inhibitors such as vemurafenib, dabrafenib mesylate, and encorafenib; immune checkpoint inhibitors such as ipilimumab, nivolumab, and pembrolizumab; and oncolytic virus such as talimogene laherparepvec.8,9
Ninety percent of melanomas are of cutaneous origin. Extracutaneous melanomas may be derived from the uvea, leptomeninges, mucous membranes, and gastrointestinal tract.10 Mucosal melanomas are rare and represent only approximately 1% of all melanomas.11 In order of frequency, primary mucosal melanomas include the head and neck, anorectal region, vulvovaginal region, and urinary tract. UV radiation exposure is an important risk factor for cutaneous melanoma but has not been associated with the development of mucosal melanoma.7 In 2019, Altieri et al11 analyzed 1824 cases of mucosal melanoma and found that anatomic site influences survival because mucosal melanomas in the most occult anatomic sites—spinal/central nervous system, lung and pleura, liver, and pancreas—have the worst prognosis, likely because they have already metastasized by the time they are diagnosed. Due to their occult anatomic location and lack of early presenting signs and symptoms, mucosal melanomas are difficult to diagnose at an early stage, resulting in a poorer prognosis compared with cutaneous melanomas. The most important prognostic indicator for cutaneous melanomas of tumor thickness (ie, Breslow depth) provides less prognostic value for patients with mucosal melanoma. Limitations also include the lack of a standardized staging system for mucosal melanoma, but Altieri et al11 found that poorer survival in patients with mucosal melanoma was observed in relation to stage based on the clinical and pathologic tumor-node-metastasis staging system of the Surveillance, Epidemiology, and End Results program. An aggregate 5-year survival estimate of patients diagnosed with mucosal melanoma is 28%, underscoring that mucosal melanoma is an aggressive melanoma that carries a poor prognosis and warrants a more aggressive treatment approach at the time of diagnosis.11
Common treatment of primary middle ear mucosal melanoma involves a multimodality therapy including surgical oncological resection for most patients. Currently, radiation is in use for adjuvant treatment and definitive therapy in unresectable tumors or patients who are poor surgical candidates. Malignant melanoma traditionally was considered radioresistant, yet considerable variability in responsiveness has been observed both within and between tumors. Although there are no defined indications for adjuvant therapy, it is often administered in advanced or recurrent cases and those with positive or close margins. Chemotherapy generally is reserved for patients with systemic disease. The chemotherapeutic agents that have been used in the treatment of patients with melanoma of the middle ear include the alkylating agents dacarbazine, cisplatin, nimustine, paclitaxel, and temozolomide. Also, chemotherapeutic agents that have been reported in the treatment of melanoma of the middle ear include tamoxifen, the selective estrogen receptor inhibitor, and interferon. Most recently, programed cell death protein 1 inhibitors pembrolizumab and nivolumab have been used in the treatment of middle ear melanoma. Outcomes remain poor with a high rate of mortality. Novel immunotherapeutic agents combined with adjuvant radiotherapy have been proposed to improve disease control and survival rates.3
Data on systemic therapies for mucosal melanomas are limited due to the rarity of the disease. Even with the development of novel therapies, outcomes remain poor for mucosal melanomas, and additional treatment strategies are needed. Although proto-oncogene BRAF mutations occur in 50% to 70% of cutaneous melanomas, these mutations are rare in mucosal melanomas.3 In mucosal melanomas, activating mutations of the cell receptor KIT are identified more frequently.7 Alterations in proto-oncogene KIT have been found in acral, mucosal, and cutaneous melanoma. KIT mutations were found on exons 11 and 13.12 Variability in the biology of KIT is suggested. Treatment of melanomas with the KIT mutations with tyrosine inhibitors imatinib and nilotinib have shown variable benefits.10 In a 2019 study of 44 patients with mucosal melanoma, Moya-Plana et al13 found that in cases of unresectable and/or metastatic disease, immunotherapy with pembrolizumab had a better benefit-risk ratio than immune treatment with ipilimumab, a cytotoxic T-cell lymphocyte-associated protein 4 inhibitor.
Primary malignant melanoma of the middle ear is unusual and difficult to diagnose clinically. These melanomas have a poor prognosis and can have distant metastasis including cutaneous metastasis. We present this case to emphasize the need to be aware that melanoma can arise in the middle ear.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
- Ozturk O, Baglam T, Uneri C, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Kulak Burun Bogaz Ihtis Derg. 2006;16:83-86.
- Idris IA, Daud KM, Yusof Z, et al. Primary malignant melanoma of the middle ear mucosa: a case report. Egypt J ENT Allied Sci. 2017;18:307-309.
- Maxwell AK, Takeda H, Gubbels SP. Primary middle ear mucosal melanoma: case report and comprehensive literature review of 21 cases of primary middle ear and eustachian tube melanoma. Ann Otol Rhinol Laryngol. 2018;127:856-863.
- Sadler TW. Ear. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:324-325.
- Jakubovic HR, Akerman AB. Structure and function of skin: development, morphology and physiology. In: Moschella SL, Hurley HJ, eds. Dermatology. Vol 1. WB Saunders Co; 1985:22-23.
- Sadler TW. The axial skeleton. In: Sadler TW, ed. Langman’s Medical Embryology. Lippincott Williams & Wilkins; 2012:133-137.
- Tacastacas JD, Bray J, Cohen YK, et al. Update on primary mucosal melanoma. J Am Acad Dermatol. 2014;71:366-375.
- Abdutaali R, Alkhattib NS, Oh M, et al. Economic evaluation of talimogene laherparepvec plus ipilimumab combination therapy vs ipilimumab monotherapy in patients with advanced unresectable melanoma. JAMA Dermatol. 2019;155:22-28.
- Skudalski L, Waldeman R, Kerr PE, et al. Melanoma: an update on systemic therapies. J Am Acad Dermatol. 2022;86:515-524.
- Heymann WR. A step toward demystifying melanomas of unknown primary sites. J Am Acad Dermatol. 2018;79:208-209.
- Altieri L, Eguchi M, Peng DH, et al. Predictors of mucosal melanoma survival in a population-based setting. J Am Acad Dermatol. 2019;81:136-142.
- Volpe VO, Klufas DM, Hegde U, et al. The new paradigm of systemic therapies for metastatic melanoma. J Am Acad Dermatol. 2017;77:356-368.
- Moya-Plana A, Herrera Gomez RG, Rossoni C, et al. Evaluation of the efficacy of immunotherapy for non-resectable mucosal melanoma. Cancer Immunol Immunother. 2019;68:1171-1178.
PRACTICE POINTS
- Primary malignant melanoma of the middle ear is rare and has poor prognosis.
- Distant metastasis, including cutaneous metastasis, results from primary middle ear melanoma.
A Trauma-Induced Fatty Mass: The Facts About Posttraumatic Pseudolipomas
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.
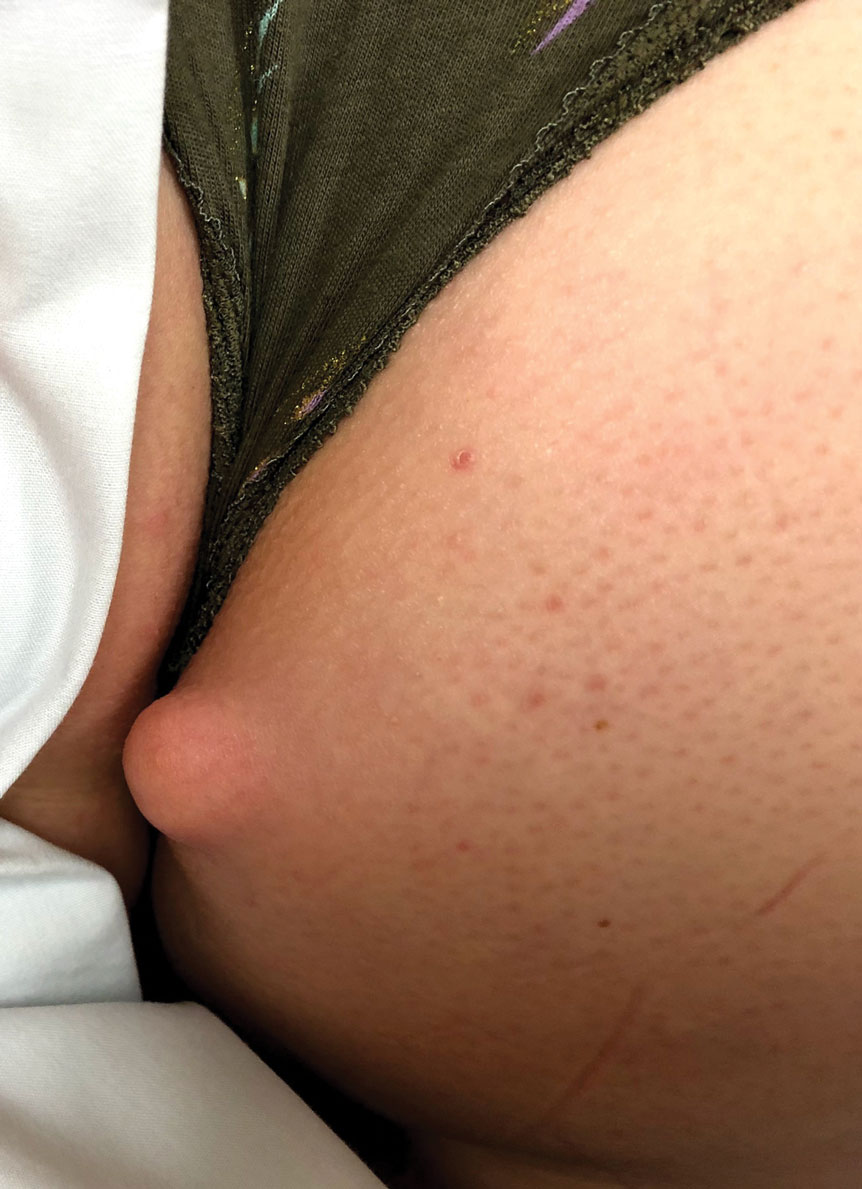
In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5
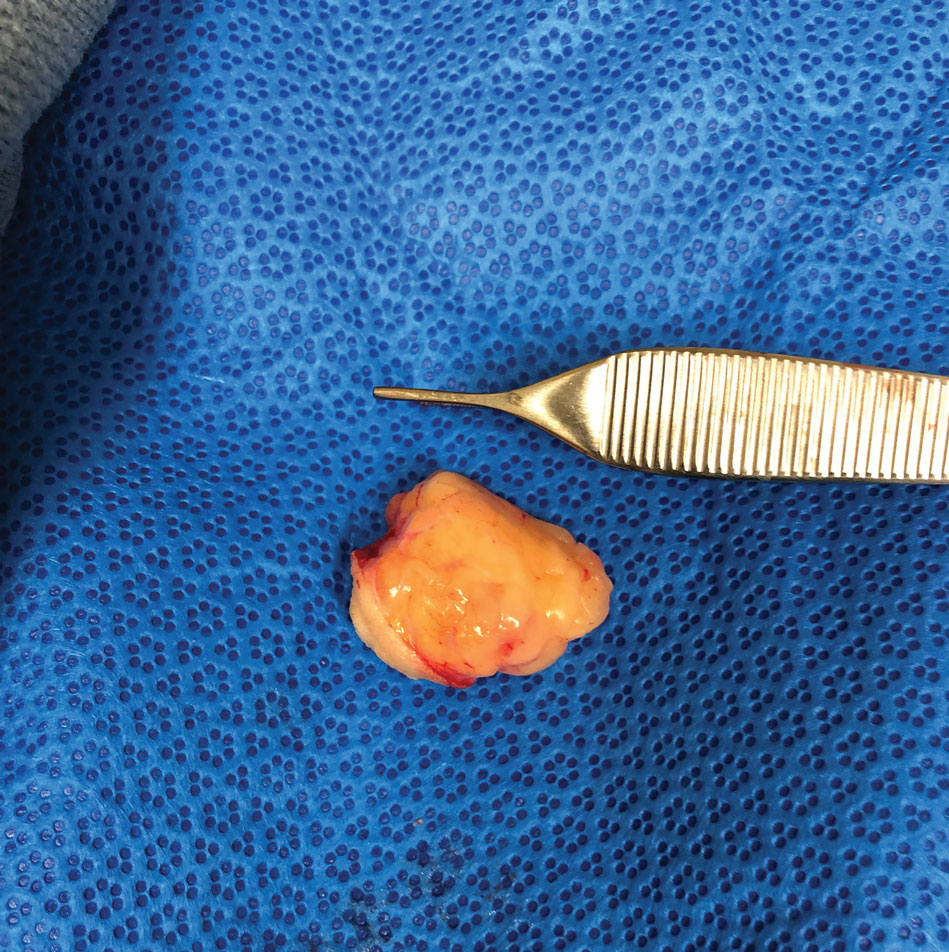
Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
To the Editor:
The posttraumatic pseudolipoma (PTL) is a painless localized mass comprised of unencapsulated adipose tissue that develops at the site of acute or prolonged blunt soft tissue trauma. It may be round or fusiform in shape and has areas of saponification leading to fat necrosis.1 Posttraumatic pseudolipomas are 12 times more likely to occur in females, which may be attributed to sex-determined adipose tissue distribution or cosmetic concerns.2 Most PTLs are found in areas of the body with high adiposity, including the hip, thigh, and gluteal regions.3 A patient history of a traumatic event resulting in a hematoma and a subsequent latent period of several months to years before the pseudolipoma formation occurs is common.1,2,4-6
A 27-year-old woman presented to the family medicine clinic for examination of a deformity on the right buttock. She noticed a soft protruding mass months after landing on the buttocks and on top of a stick during routine physical training. Prior ultrasonography of the deformity proved unhelpful in determining the etiology. Physical examination revealed a protruding, 2-cm, flesh-colored mass on the right buttock intergluteal fold that was soft, compressible, and nontender (Figure 1). There was no capsule, nodule, loculation, or sinus tract. The patient underwent excisional resection with findings of benign-appearing unencapsulated adipose tissue (Figure 2). The wound was closed without difficulty. After several weeks, she had a well-healing scar without contour deficits of the buttocks. Two to 3 months after the initial repair, the patient presented to the family medicine clinic with recurrence of the fatty protrusion. She was referred for consultation and definitive management to a plastic surgeon but was lost to follow up.

In a systematic review of the literature to research pathogenesis theories, a PubMed search of articles indexed for MEDLINE using the terms trauma and pseudolipoma, lipoma, fat, or adipose yielded 45 citations, with only 10 publications addressing the pathology specific to pseudolipomas. Two leading theories of the pathogenesis of PTLs include the adipose herniation pathway and the inflammatory proliferation pathway.4,5

Adipose tissue comprises fat lobules that are organized underneath the supportive elastic fascial layers. Injury from forces exceeding the fascial strength is the basis for the oldest pathogenesis theory. The adipose herniation theory suggests that fat lobules are displaced through the damaged septae, allowing for the development of an epidermal pseudolipoma at the site of blunt trauma.7 This theory has been supported by many case reports; however, more recent reports have identified a larger number of PTL cases that showed no identifiable disruptions in the fascia.1,4,8
In 1997, the inflammatory proliferation theory began to gain attention. The theory describes how local tissue trauma leads to the release of inflammatory cytokines, which successively signals the development of preadipocytes or adipose tissue–derived stem cells (ASCs) into mature adipocytes.4 Most patients report a history of a hematoma in the area of pseudolipoma development, which strongly supports this newer theory. Studies exploring hematomas have found elevated levels of growth factors and inflammatory markers.2,9 In particular, tumor necrosis factor α, peroxisome proliferator–activated receptor γ, vascular endothelial growth factor, and IL-6 and IL-8 may foster an environment in which adipogenic cells are both chemotaxed to the area of trauma and differentiated to white adipose tissue.2,10
Despite addressing the role of the preadipocyte, the available research fails to address the general development of mesenchymal cells into the preadipocyte. White adipose tissue develops at sites of neovascularization and frequently has been observed spreading into the nearby tissue toward other blood vessels. Furthermore, these white adipose tissue expansions remain reliant on multiple growth factors and cell-signaling molecules.10 Numerous investigations into stem cell grafting have found that implantation of ASCs in vivo within animal models does not result in the proliferation and differentiation of ASCs unless specific conditions have been met such as prior tissue injury or immunodeficiency.10-12 These investigations support and expand on the inflammatory proliferation pathway. Thus, most of the true PTLs in the available research appear as de novo tumors and are more congruent with the inflammatory proliferation model.1,2,4-6,8
Typical treatment of a PTL is surgical excision or liposuction depending on the pathology and size of the pseudolipoma. Biopsy examination prior to liposuction is critical for evaluation of liposarcoma and may help identify damage to Scarpa fascia. Recurrence of a PTL is rare regardless of treatment method; however, in a study of 31 PTL cases, only 6 were pathologically identified as PTLs without fibrous material.1
Our patient experienced a blunt trauma to the buttocks and subsequently developed a PTL that was surgically excised and recurred within 3 months. Research surrounding the pathogenesis of the PTL has evolved from the theory of physical herniation of adipose tissue to an inflammatory differentiation of preadipocytes, but there is still much to learn about how and why it occurs and the mesenchymal differentiation following tissue injury.
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
- Aust MC, Spies M, Kall S, et al. Lipomas after blunt soft tissue trauma: are they real? analysis of 31 cases. Br J Dermatol. 2007;157:92-99. doi:10.1111/j.1365-2133.2007.07970.x
- Galea LA, Penington AJ, Morrison WA. Post-traumatic pseudolipomas—a review and postulated mechanisms of their development. J Plast Reconstr Aesthet Surg. 2009;62:737-741. doi:10.1016/j.bjps.2008.12.021
- Zajac JC, Mandelbaum M, Economides JM, et al. Immediate massive posttraumatic pseudolipoma of the buttocks: a case of a heterotopic “love handle.” Plast Reconstr Surg Glob Open. 2018;6:E1887. doi:10.1097/GOX.0000000000001887
- Signorini M, Campiglio GL. Posttraumatic lipomas: where do they really come from? Plast Reconstr Surg. 1998;101:699-705. doi:10.1097/00006534-199803000-00017
- Khadilkar AS, Goyal A, Gauba K. The enigma of “traumatic pseudolipoma” and “traumatic herniation of buccal fat pad”: a systematic review and new classification system of post-traumatic craniofacial fatty masses. J Oral Maxillofac Surg. 2018;76:1267-1278. doi:10.1016/j.joms.2017.01.024
- Copcu E, Sivrioglu NS. Posttraumatic lipoma: analysis of 10 cases and explanation of possible mechanisms. Dermatol Surg. 2003;29:215-220. doi:10.1046/j.1524-4725.2003.29052.x
- Penoff JH. Traumatic lipomas/pseudolipomas. J Trauma. 1982;22:63-65. doi:10.1097/00005373-198201000-00013
- Theumann N, Abdelmoumene A, Wintermark M, et al. Posttraumatic pseudolipoma: MRI appearances. Eur Radiol. 2005;15:1876-1880. doi:10.1007/s00330-005-2757-2
- David LR, DeFranzo A, Marks M, et al. Posttraumatic pseudolipoma. J Trauma. 1996;40:396-400. doi:10.1097/00005373-199603000-00012
- Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227-246. doi:10.1194/jlr.R021089
- Joe AW, Yi L, Natarajan A, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153-163. doi:10.1038/ncb2015
- Miranville A, Heeschen C, Sengenès C, et al. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation. 2004;110:349-355. doi:10.1161/01.Cir.0000135466.16823.D0
Practice Points
- Physicians should include pseudolipoma in the differential diagnosis when evaluating masses that develop in patients at sites of blunt or prolonged trauma.
- A pseudolipoma is an unencapsulated, round, or fusiform fatty mass that differs from a traditional lipoma by the absence of a capsule.
- Further research may elucidate the pathogenesis of these adiposities.