User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
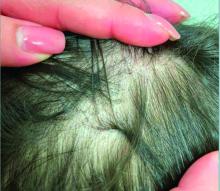
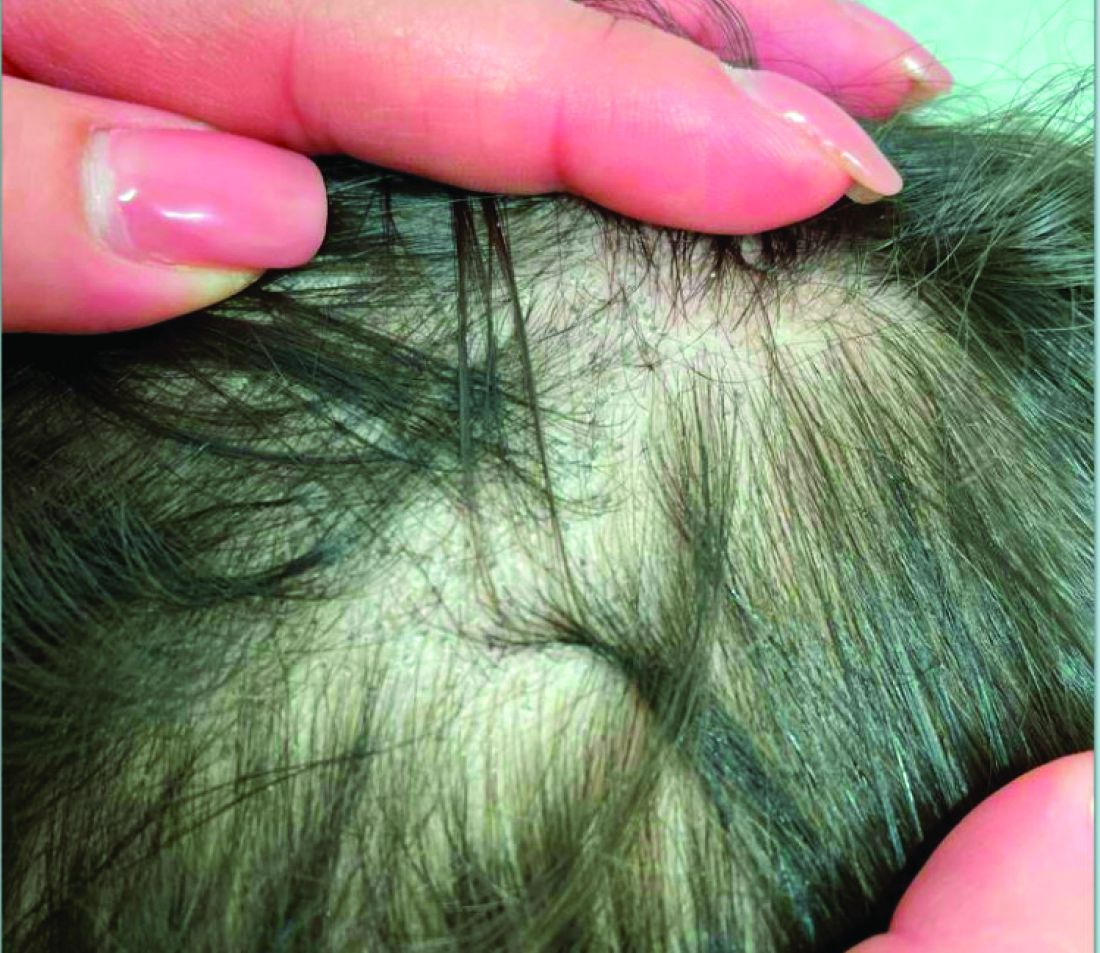
A 7-Year-Old Boy Presents With Dark Spots on His Scalp and Areas of Poor Hair Growth
Given the trichoscopic findings, scrapings from the scaly areas were taken and revealed hyphae, confirming the diagnosis of tinea capitis. A fungal culture identified Trichophyton tonsurans as the causative organism.
Tinea capitis is the most common dermatophyte infection in children. Risk factors include participation in close-contact sports like wrestling or jiu-jitsu, attendance at daycare for younger children, African American hair care practices, pet ownership (particularly cats and rodents), and living in overcrowded conditions.
Diagnosis of tinea capitis requires a thorough clinical history to identify potential risk factors. On physical examination, patchy hair loss with associated scaling should raise suspicion for tinea capitis. Inflammatory signs, such as pustules and swelling, may suggest the presence of a kerion, further supporting the diagnosis. Although some practitioners use Wood’s lamp to help with diagnosis, its utility is limited. It detects fluorescence in Microsporum species (exothrix infections) but not in Trichophyton species (endothrix infections).
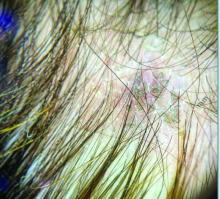
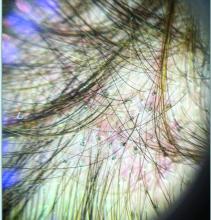
Trichoscopy can be a valuable tool when inflammation is minimal, and only hair loss and scaling are observed. Trichoscopic findings suggestive of tinea capitis include comma hairs, corkscrew hairs (as seen in this patient), Morse code-like hairs, zigzag hairs, bent hairs, block hairs, and i-hairs. Other common, though not characteristic, findings include broken hairs, black dots, perifollicular scaling, and diffuse scaling.
KOH (potassium hydroxide) analysis is another useful method for detecting fungal elements, though it does not identify the specific fungus and may not be available in all clinical settings. Mycologic culture remains the gold standard for diagnosing tinea capitis, though results can take 3-4 weeks. Newer diagnostic techniques, such as PCR analysis and MALDI-TOF/MS, offer more rapid identification of the causative organism.
The differential diagnosis includes:
- Seborrheic dermatitis, which presents with greasy, yellowish scales and itching, with trichoscopy showing twisted, coiled hairs and yellowish scaling.
- Psoriasis, which can mimic tinea capitis but presents with well-demarcated red plaques and silvery-white scales. Trichoscopy shows red dots and uniform scaling.
- Alopecia areata, which causes patchy hair loss without inflammation or scaling, with trichoscopic findings of exclamation mark hairs, black dots, and yellow dots.
- Trichotillomania, a hair-pulling disorder, which results in irregular patches of hair loss. Trichoscopy shows broken hairs of varying lengths, V-sign hairs, and flame-shaped residues at follicular openings.
Treatment of tinea capitis requires systemic antifungals and topical agents to prevent fungal spore spread. Several treatment guidelines are available from different institutions. Griseofulvin (FDA-approved for patients > 2 years of age) has been widely used, particularly for Microsporum canis infections. However, due to limited availability in many countries, terbinafine (FDA-approved for patients > 4 years of age) is now commonly used as first-line therapy, especially for Trichophyton species. Treatment typically lasts 4-6 weeks, and post-treatment cultures may be recommended to confirm mycologic cure.
Concerns about drug resistance have emerged, particularly for terbinafine-resistant dermatophytes linked to mutations in the squalene epoxidase enzyme. Resistance may be driven by limited antifungal availability and poor adherence to prolonged treatment regimens. While fluconazole and itraconazole are used off-label, growing evidence supports their effectiveness, although one large trial showed suboptimal cure rates with fluconazole.
Though systemic antifungals are generally safe, hepatotoxicity remains a concern, especially in patients with hepatic conditions or other comorbidities. Lab monitoring is advised for patients on prolonged or multiple therapies, or for those with coexisting conditions. The decision to conduct lab monitoring should be discussed with parents, balancing the very low risk of hepatotoxicity in healthy children against their comfort level.
An alternative to systemic therapy is photodynamic therapy (PDT), which has been reported as successful in treating tinea capitis infections, particularly in cases of T. mentagrophytes and M. canis. However, large-scale trials are needed to confirm PDT’s efficacy and safety.
In conclusion, children presenting with hair loss, scaling, and associated dark spots on the scalp should be evaluated for fungal infection. While trichoscopy can aid in diagnosis, fungal culture remains the gold standard for confirmation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Rudnicka L et al. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013 Oct;31(4):695-708, x. doi: 10.1016/j.det.2013.06.007.
Gupta AK et al. An update on tinea capitis in children. Pediatr Dermatol. 2024 Aug 7. doi: 10.1111/pde.15708.
Anna Waskiel-Burnat et al. Trichoscopy of tinea capitis: A systematic review. Dermatol Ther (Heidelb). 2020 Feb;10(1):43-52. doi: 10.1007/s13555-019-00350-1.
Given the trichoscopic findings, scrapings from the scaly areas were taken and revealed hyphae, confirming the diagnosis of tinea capitis. A fungal culture identified Trichophyton tonsurans as the causative organism.
Tinea capitis is the most common dermatophyte infection in children. Risk factors include participation in close-contact sports like wrestling or jiu-jitsu, attendance at daycare for younger children, African American hair care practices, pet ownership (particularly cats and rodents), and living in overcrowded conditions.
Diagnosis of tinea capitis requires a thorough clinical history to identify potential risk factors. On physical examination, patchy hair loss with associated scaling should raise suspicion for tinea capitis. Inflammatory signs, such as pustules and swelling, may suggest the presence of a kerion, further supporting the diagnosis. Although some practitioners use Wood’s lamp to help with diagnosis, its utility is limited. It detects fluorescence in Microsporum species (exothrix infections) but not in Trichophyton species (endothrix infections).
Trichoscopy can be a valuable tool when inflammation is minimal, and only hair loss and scaling are observed. Trichoscopic findings suggestive of tinea capitis include comma hairs, corkscrew hairs (as seen in this patient), Morse code-like hairs, zigzag hairs, bent hairs, block hairs, and i-hairs. Other common, though not characteristic, findings include broken hairs, black dots, perifollicular scaling, and diffuse scaling.
KOH (potassium hydroxide) analysis is another useful method for detecting fungal elements, though it does not identify the specific fungus and may not be available in all clinical settings. Mycologic culture remains the gold standard for diagnosing tinea capitis, though results can take 3-4 weeks. Newer diagnostic techniques, such as PCR analysis and MALDI-TOF/MS, offer more rapid identification of the causative organism.
The differential diagnosis includes:
- Seborrheic dermatitis, which presents with greasy, yellowish scales and itching, with trichoscopy showing twisted, coiled hairs and yellowish scaling.
- Psoriasis, which can mimic tinea capitis but presents with well-demarcated red plaques and silvery-white scales. Trichoscopy shows red dots and uniform scaling.
- Alopecia areata, which causes patchy hair loss without inflammation or scaling, with trichoscopic findings of exclamation mark hairs, black dots, and yellow dots.
- Trichotillomania, a hair-pulling disorder, which results in irregular patches of hair loss. Trichoscopy shows broken hairs of varying lengths, V-sign hairs, and flame-shaped residues at follicular openings.
Treatment of tinea capitis requires systemic antifungals and topical agents to prevent fungal spore spread. Several treatment guidelines are available from different institutions. Griseofulvin (FDA-approved for patients > 2 years of age) has been widely used, particularly for Microsporum canis infections. However, due to limited availability in many countries, terbinafine (FDA-approved for patients > 4 years of age) is now commonly used as first-line therapy, especially for Trichophyton species. Treatment typically lasts 4-6 weeks, and post-treatment cultures may be recommended to confirm mycologic cure.
Concerns about drug resistance have emerged, particularly for terbinafine-resistant dermatophytes linked to mutations in the squalene epoxidase enzyme. Resistance may be driven by limited antifungal availability and poor adherence to prolonged treatment regimens. While fluconazole and itraconazole are used off-label, growing evidence supports their effectiveness, although one large trial showed suboptimal cure rates with fluconazole.
Though systemic antifungals are generally safe, hepatotoxicity remains a concern, especially in patients with hepatic conditions or other comorbidities. Lab monitoring is advised for patients on prolonged or multiple therapies, or for those with coexisting conditions. The decision to conduct lab monitoring should be discussed with parents, balancing the very low risk of hepatotoxicity in healthy children against their comfort level.
An alternative to systemic therapy is photodynamic therapy (PDT), which has been reported as successful in treating tinea capitis infections, particularly in cases of T. mentagrophytes and M. canis. However, large-scale trials are needed to confirm PDT’s efficacy and safety.
In conclusion, children presenting with hair loss, scaling, and associated dark spots on the scalp should be evaluated for fungal infection. While trichoscopy can aid in diagnosis, fungal culture remains the gold standard for confirmation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Rudnicka L et al. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013 Oct;31(4):695-708, x. doi: 10.1016/j.det.2013.06.007.
Gupta AK et al. An update on tinea capitis in children. Pediatr Dermatol. 2024 Aug 7. doi: 10.1111/pde.15708.
Anna Waskiel-Burnat et al. Trichoscopy of tinea capitis: A systematic review. Dermatol Ther (Heidelb). 2020 Feb;10(1):43-52. doi: 10.1007/s13555-019-00350-1.
Given the trichoscopic findings, scrapings from the scaly areas were taken and revealed hyphae, confirming the diagnosis of tinea capitis. A fungal culture identified Trichophyton tonsurans as the causative organism.
Tinea capitis is the most common dermatophyte infection in children. Risk factors include participation in close-contact sports like wrestling or jiu-jitsu, attendance at daycare for younger children, African American hair care practices, pet ownership (particularly cats and rodents), and living in overcrowded conditions.
Diagnosis of tinea capitis requires a thorough clinical history to identify potential risk factors. On physical examination, patchy hair loss with associated scaling should raise suspicion for tinea capitis. Inflammatory signs, such as pustules and swelling, may suggest the presence of a kerion, further supporting the diagnosis. Although some practitioners use Wood’s lamp to help with diagnosis, its utility is limited. It detects fluorescence in Microsporum species (exothrix infections) but not in Trichophyton species (endothrix infections).
Trichoscopy can be a valuable tool when inflammation is minimal, and only hair loss and scaling are observed. Trichoscopic findings suggestive of tinea capitis include comma hairs, corkscrew hairs (as seen in this patient), Morse code-like hairs, zigzag hairs, bent hairs, block hairs, and i-hairs. Other common, though not characteristic, findings include broken hairs, black dots, perifollicular scaling, and diffuse scaling.
KOH (potassium hydroxide) analysis is another useful method for detecting fungal elements, though it does not identify the specific fungus and may not be available in all clinical settings. Mycologic culture remains the gold standard for diagnosing tinea capitis, though results can take 3-4 weeks. Newer diagnostic techniques, such as PCR analysis and MALDI-TOF/MS, offer more rapid identification of the causative organism.
The differential diagnosis includes:
- Seborrheic dermatitis, which presents with greasy, yellowish scales and itching, with trichoscopy showing twisted, coiled hairs and yellowish scaling.
- Psoriasis, which can mimic tinea capitis but presents with well-demarcated red plaques and silvery-white scales. Trichoscopy shows red dots and uniform scaling.
- Alopecia areata, which causes patchy hair loss without inflammation or scaling, with trichoscopic findings of exclamation mark hairs, black dots, and yellow dots.
- Trichotillomania, a hair-pulling disorder, which results in irregular patches of hair loss. Trichoscopy shows broken hairs of varying lengths, V-sign hairs, and flame-shaped residues at follicular openings.
Treatment of tinea capitis requires systemic antifungals and topical agents to prevent fungal spore spread. Several treatment guidelines are available from different institutions. Griseofulvin (FDA-approved for patients > 2 years of age) has been widely used, particularly for Microsporum canis infections. However, due to limited availability in many countries, terbinafine (FDA-approved for patients > 4 years of age) is now commonly used as first-line therapy, especially for Trichophyton species. Treatment typically lasts 4-6 weeks, and post-treatment cultures may be recommended to confirm mycologic cure.
Concerns about drug resistance have emerged, particularly for terbinafine-resistant dermatophytes linked to mutations in the squalene epoxidase enzyme. Resistance may be driven by limited antifungal availability and poor adherence to prolonged treatment regimens. While fluconazole and itraconazole are used off-label, growing evidence supports their effectiveness, although one large trial showed suboptimal cure rates with fluconazole.
Though systemic antifungals are generally safe, hepatotoxicity remains a concern, especially in patients with hepatic conditions or other comorbidities. Lab monitoring is advised for patients on prolonged or multiple therapies, or for those with coexisting conditions. The decision to conduct lab monitoring should be discussed with parents, balancing the very low risk of hepatotoxicity in healthy children against their comfort level.
An alternative to systemic therapy is photodynamic therapy (PDT), which has been reported as successful in treating tinea capitis infections, particularly in cases of T. mentagrophytes and M. canis. However, large-scale trials are needed to confirm PDT’s efficacy and safety.
In conclusion, children presenting with hair loss, scaling, and associated dark spots on the scalp should be evaluated for fungal infection. While trichoscopy can aid in diagnosis, fungal culture remains the gold standard for confirmation.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
References
Rudnicka L et al. Hair shafts in trichoscopy: clues for diagnosis of hair and scalp diseases. Dermatol Clin. 2013 Oct;31(4):695-708, x. doi: 10.1016/j.det.2013.06.007.
Gupta AK et al. An update on tinea capitis in children. Pediatr Dermatol. 2024 Aug 7. doi: 10.1111/pde.15708.
Anna Waskiel-Burnat et al. Trichoscopy of tinea capitis: A systematic review. Dermatol Ther (Heidelb). 2020 Feb;10(1):43-52. doi: 10.1007/s13555-019-00350-1.
Pulsed Dye Laser a “Go-To Device” Option for Acne Treatment When Access to 1726-nm Lasers Is Limited
CARLSBAD, CALIF. — Lasers and energy-based treatments alone or in combination with medical therapy may improve outcomes for patients with moderate to severe acne, according to Arielle Kauvar, MD.
At the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium, Kauvar, director of New York Laser & Skin Care, New York City, highlighted several reasons why using lasers for acne is beneficial. “First, we know that topical therapy alone is often ineffective, and antibiotic treatment does not address the cause of acne and can alter the skin and gut microbiome,” she said. “Isotretinoin is highly effective, but there’s an increasing reluctance to use it. Lasers and energy devices are effective in treating acne and may also treat the post-inflammatory hyperpigmentation and scarring associated with it.”
The pathogenesis of acne is multifactorial, she continued, including a disruption of sebaceous gland activity, with overproduction and alteration of sebum and abnormal follicular keratinization. Acne also causes an imbalance of the skin microbiome, local inflammation, and activation of both innate and adaptive immunity.
“Many studies point to the fact that inflammation and immune system activation may actually be the primary event” of acne formation, said Kauvar, who is also a clinical professor of dermatology at New York University, New York City. “This persistent immune activation is also associated with scarring,” she noted. “So, are we off the mark in terms of trying to kill sebaceous glands? Should we be concentrating on anti-inflammatory approaches?”
AviClear became the first 1726-nm laser cleared by the US Food and Drug Administration (FDA) for the treatment of mild to severe acne vulgaris in 2022, followed a few months later with the FDA clearance of another 1726-nm laser, the Accure Acne Laser System in November 2022. These lasers cause selective photothermolysis of sebaceous glands, but according to Kauvar, “access to these devices is somewhat limited at this time.”
What is available includes her go-to device, the pulsed dye laser (PDL), which has been widely studied and shown in a systematic review and meta-analysis of studies to be effective for acne. The PDL “targets dermal blood vessels facilitating inflammation, upregulates TGF-beta, and inhibits CD4+ T cell-mediated inflammation,” she said. “It can also treat PIH [post-inflammatory hyperpigmentation] and may be helpful in scar prevention.”
In an abstract presented at The American Society for Laser Medicine and Surgery (ASLMS) 2024 annual meeting, Kauvar and colleagues conducted a real-world study of PDL therapy in 15 adult women with recalcitrant acne who were maintained on their medical treatment regimen. Their mean age was 27 years, and they had skin types II-IV; they underwent four monthly PDL treatments with follow-up at 1 and 3 months. At each visit, the researchers took digital photographs and counted inflammatory acne lesions, non-inflammatory acne lesions, and post-inflammatory pigment alteration (PIPA) lesions.
The main outcomes of interest were the investigator global assessment (IGA) scores at the 1- and 3-month follow-up visits. Kauvar and colleagues observed a significant improvement in IGA scores at the 1- and 3-month follow-up visits (P < .05), with an average decrease of 1.8 and 1.6 points in the acne severity scale, respectively, from a baseline score of 3.4. By the 3-month follow-up visits, counts of inflammatory and non-inflammatory lesions decreased significantly (P < .05), and 61% of study participants showed a decrease in the PIPA count. No adverse events occurred.
Kauvar disclosed that she has conducted research for Candela, Lumenis, and Sofwave, and is an adviser to Acclaro.
A version of this article first appeared on Medscape.com.
CARLSBAD, CALIF. — Lasers and energy-based treatments alone or in combination with medical therapy may improve outcomes for patients with moderate to severe acne, according to Arielle Kauvar, MD.
At the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium, Kauvar, director of New York Laser & Skin Care, New York City, highlighted several reasons why using lasers for acne is beneficial. “First, we know that topical therapy alone is often ineffective, and antibiotic treatment does not address the cause of acne and can alter the skin and gut microbiome,” she said. “Isotretinoin is highly effective, but there’s an increasing reluctance to use it. Lasers and energy devices are effective in treating acne and may also treat the post-inflammatory hyperpigmentation and scarring associated with it.”
The pathogenesis of acne is multifactorial, she continued, including a disruption of sebaceous gland activity, with overproduction and alteration of sebum and abnormal follicular keratinization. Acne also causes an imbalance of the skin microbiome, local inflammation, and activation of both innate and adaptive immunity.
“Many studies point to the fact that inflammation and immune system activation may actually be the primary event” of acne formation, said Kauvar, who is also a clinical professor of dermatology at New York University, New York City. “This persistent immune activation is also associated with scarring,” she noted. “So, are we off the mark in terms of trying to kill sebaceous glands? Should we be concentrating on anti-inflammatory approaches?”
AviClear became the first 1726-nm laser cleared by the US Food and Drug Administration (FDA) for the treatment of mild to severe acne vulgaris in 2022, followed a few months later with the FDA clearance of another 1726-nm laser, the Accure Acne Laser System in November 2022. These lasers cause selective photothermolysis of sebaceous glands, but according to Kauvar, “access to these devices is somewhat limited at this time.”
What is available includes her go-to device, the pulsed dye laser (PDL), which has been widely studied and shown in a systematic review and meta-analysis of studies to be effective for acne. The PDL “targets dermal blood vessels facilitating inflammation, upregulates TGF-beta, and inhibits CD4+ T cell-mediated inflammation,” she said. “It can also treat PIH [post-inflammatory hyperpigmentation] and may be helpful in scar prevention.”
In an abstract presented at The American Society for Laser Medicine and Surgery (ASLMS) 2024 annual meeting, Kauvar and colleagues conducted a real-world study of PDL therapy in 15 adult women with recalcitrant acne who were maintained on their medical treatment regimen. Their mean age was 27 years, and they had skin types II-IV; they underwent four monthly PDL treatments with follow-up at 1 and 3 months. At each visit, the researchers took digital photographs and counted inflammatory acne lesions, non-inflammatory acne lesions, and post-inflammatory pigment alteration (PIPA) lesions.
The main outcomes of interest were the investigator global assessment (IGA) scores at the 1- and 3-month follow-up visits. Kauvar and colleagues observed a significant improvement in IGA scores at the 1- and 3-month follow-up visits (P < .05), with an average decrease of 1.8 and 1.6 points in the acne severity scale, respectively, from a baseline score of 3.4. By the 3-month follow-up visits, counts of inflammatory and non-inflammatory lesions decreased significantly (P < .05), and 61% of study participants showed a decrease in the PIPA count. No adverse events occurred.
Kauvar disclosed that she has conducted research for Candela, Lumenis, and Sofwave, and is an adviser to Acclaro.
A version of this article first appeared on Medscape.com.
CARLSBAD, CALIF. — Lasers and energy-based treatments alone or in combination with medical therapy may improve outcomes for patients with moderate to severe acne, according to Arielle Kauvar, MD.
At the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium, Kauvar, director of New York Laser & Skin Care, New York City, highlighted several reasons why using lasers for acne is beneficial. “First, we know that topical therapy alone is often ineffective, and antibiotic treatment does not address the cause of acne and can alter the skin and gut microbiome,” she said. “Isotretinoin is highly effective, but there’s an increasing reluctance to use it. Lasers and energy devices are effective in treating acne and may also treat the post-inflammatory hyperpigmentation and scarring associated with it.”
The pathogenesis of acne is multifactorial, she continued, including a disruption of sebaceous gland activity, with overproduction and alteration of sebum and abnormal follicular keratinization. Acne also causes an imbalance of the skin microbiome, local inflammation, and activation of both innate and adaptive immunity.
“Many studies point to the fact that inflammation and immune system activation may actually be the primary event” of acne formation, said Kauvar, who is also a clinical professor of dermatology at New York University, New York City. “This persistent immune activation is also associated with scarring,” she noted. “So, are we off the mark in terms of trying to kill sebaceous glands? Should we be concentrating on anti-inflammatory approaches?”
AviClear became the first 1726-nm laser cleared by the US Food and Drug Administration (FDA) for the treatment of mild to severe acne vulgaris in 2022, followed a few months later with the FDA clearance of another 1726-nm laser, the Accure Acne Laser System in November 2022. These lasers cause selective photothermolysis of sebaceous glands, but according to Kauvar, “access to these devices is somewhat limited at this time.”
What is available includes her go-to device, the pulsed dye laser (PDL), which has been widely studied and shown in a systematic review and meta-analysis of studies to be effective for acne. The PDL “targets dermal blood vessels facilitating inflammation, upregulates TGF-beta, and inhibits CD4+ T cell-mediated inflammation,” she said. “It can also treat PIH [post-inflammatory hyperpigmentation] and may be helpful in scar prevention.”
In an abstract presented at The American Society for Laser Medicine and Surgery (ASLMS) 2024 annual meeting, Kauvar and colleagues conducted a real-world study of PDL therapy in 15 adult women with recalcitrant acne who were maintained on their medical treatment regimen. Their mean age was 27 years, and they had skin types II-IV; they underwent four monthly PDL treatments with follow-up at 1 and 3 months. At each visit, the researchers took digital photographs and counted inflammatory acne lesions, non-inflammatory acne lesions, and post-inflammatory pigment alteration (PIPA) lesions.
The main outcomes of interest were the investigator global assessment (IGA) scores at the 1- and 3-month follow-up visits. Kauvar and colleagues observed a significant improvement in IGA scores at the 1- and 3-month follow-up visits (P < .05), with an average decrease of 1.8 and 1.6 points in the acne severity scale, respectively, from a baseline score of 3.4. By the 3-month follow-up visits, counts of inflammatory and non-inflammatory lesions decreased significantly (P < .05), and 61% of study participants showed a decrease in the PIPA count. No adverse events occurred.
Kauvar disclosed that she has conducted research for Candela, Lumenis, and Sofwave, and is an adviser to Acclaro.
A version of this article first appeared on Medscape.com.
A Hard Look at Toxic Workplace Culture in Medicine
While Kellie Lease Stecher, MD, was working as an ob.gyn. in Minneapolis, Minnesota, a patient confided in her a sexual assault allegation about one of Stecher’s male colleagues. Stecher shared the allegation with her supervisor, who told Stecher not to file a report and chose not to address the issue with the patient. Stecher weighed how to do the right thing: Should she speak up? What were the ethical and legal implications of speaking up vs staying silent?
After seeking advice from her mentors, Stecher felt it was her moral and legal duty to report the allegation to the Minnesota Medical Board. Once she did, her supervisor chastised her repeatedly for reporting the allegation. Stecher soon found herself in a hostile work environment where she was regularly singled out and silenced by her supervisor and colleagues.
“I got to a point where I felt like I couldn’t say anything at any meetings without somehow being targeted after the meeting. There was an individual who was even allowed to fat-shame me with no consequences,” Stecher said. “[Being bullied at work is] a struggle because you have no voice, you have no opportunities, and there’s someone who is intentionally making your life uncomfortable.”
Stecher’s experience is not unusual. Mistreatment is a common issue among healthcare workers, ranging from rudeness to bullying and harassment and permeating every level and specialty of the medical profession. A 2019 research review estimated that 26.3% of healthcare workers had experienced bullying and found bullying in healthcare to be associated with mental health problems such as burnout and depression, physical health problems such as insomnia and headaches, and physicians taking more sick leave.
The Medscape Physician Workplace Culture Report 2024 found similarly bleak results:
- 38% said workplace culture is declining.
- 70% don’t see a big commitment from employers for positive culture.
- 48% said staff isn’t committed to positive culture.
The irony, of course, is that most physicians enter the field to care for people. As individuals go from medical school to residency and on with the rest of their careers, they often experience a rude awakening.
It’s Everywhere
Noticing the prevalence of workplace bullying in the medical field, endocrinologist Farah Khan, MD, at UW Medicine in Seattle, Washington, decided to conduct a survey on the issue.
Khan collected 122 responses from colleagues, friends, and acquaintances in the field. When asked if they had ever been bullied in medicine, 68% of respondents said yes. But here’s the fascinating part: She tried to pinpoint one particular area or source of toxicity in the progression of a physician’s career — and couldn’t because it existed at all levels.
More than one third of respondents said their worst bullying experiences occurred in residency, while 30% said mistreatment was worst in medical school, and 24% indicated their worst experience had occurred once they became an attending.
The litany of experiences included being belittled, excluded, yelled at, criticized, shamed, unfairly blamed, threatened, sexually harassed, subjected to bigotry and slurs, and humiliated.
“What surprised me the most was how widespread this problem is and the many different layers of healthcare it permeates through, from operating room staff to medical students to hospital HR to residents and attendings,” Khan said of her findings.
Who Cares for the Caregivers?
When hematologist Mikkael Sekeres, MD, was in medical school, he seriously considered a career as a surgeon. Following success in his surgical rotations, he scrubbed in with a cardiothoracic surgeon who was well known for both his status as a surgeon and his fiery temper. Sekeres witnessed the surgeon yelling at whoever was nearby: Medical students, fellows, residents, operating room nurses.
“At the end of that experience, any passing thoughts I had of going into cardiothoracic surgery were gone,” Sekeres said. “Some of the people I met in surgery were truly wonderful. Some were unhappy people.”
He has clear ideas why. Mental health struggles that are all too common among physicians can be caused or exacerbated by mistreatment and can also lead a physician to mistreat others.
“People bully when they themselves are hurting,” Sekeres said. “It begs the question, why are people hurting? What’s driving them to be bullies? I think part of the reason is that they’re working really hard and they’re tired, and nobody’s caring for them. It’s hard to care for others when you feel as if you’re hurting more than they are.”
Gail Gazelle, MD, experienced something like this. In her case, the pressure to please and to be a perfect professional and mother affected how she interacted with those around her. While working as a hospice medical director and an academician and clinician at Harvard Medical School, Boston, Massachusetts, she found herself feeling exhausted and burnt out but simultaneously guilty for not doing enough at work or at home.
Guess what happened? She became irritable, lashing out at her son and not putting her best foot forward with coworkers or patients.
After trying traditional therapy and self-help through books and podcasts, Gazelle found her solution in life coaching. “I realized just how harsh I was being on myself and found ways to reverse that pattern,” she said. “I learned ways of regulating myself emotionally that I definitely didn’t learn in my training.”
Today, Gazelle works as a life coach herself, guiding physicians through common challenges of the profession — particularly bullying, which she sees often. She remembers one client, an oncologist, who was being targeted by a nurse practitioner she was training. The nurse practitioner began talking back to the oncologist, as well as gossiping and bad-mouthing her to the nurses in the practice. The nurses then began excluding the oncologist from their cafeteria table at lunchtime, which felt blatant in such a small practice.
A core component of Gazelle’s coaching strategy was helping the client reclaim her self-esteem by focusing on her strengths. She instructed the client to write down what went well that day each night rather than lying in bed ruminating. Such self-care strategies can not only help bullied physicians but also prevent some of the challenges that might cause a physician to bully or lash out at another in the first place.
Such strategies, along with the recent influx of wellness programs available in healthcare facilities, can help physicians cope with the mental health impacts of bullying and the job in general. But even life coaches like Gazelle acknowledge that they are often band-aids on the system’s deeper wounds. Bullying in healthcare is not an individual issue; at its core, it’s an institutional one.
Negative Hierarchies in Healthcare
When Stecher’s contract expired, she was fired by the supervisor who had been bullying her. Stecher has since filed a lawsuit, claiming sexual discrimination, defamation, and wrongful termination.
The medical field has a long history of hierarchy, and while this rigidity has softened over time, negative hierarchical dynamics are often perpetuated by leaders. Phenomena like cronyism and cliques and behaviors like petty gossip, lunchroom exclusion (which in the worst cases can mimic high school dynamics), and targeting can be at play in the healthcare workplace.
The classic examples, Stecher said, can usually be spotted: “If you threaten the status quo or offer different ideas, you are seen as a threat. Cronyism ... strict hierarchies ... people who elevate individuals in their social arena into leadership positions. Physicians don’t get the leadership training that they really need; they are often just dumped into roles with no previous experience because they’re someone’s golfing buddy.”
The question is how to get workplace culture momentum moving in a positive direction. When Gazelle’s clients are hesitant to voice concerns, she emphasizes doing so can and should benefit leadership, as well as patients and the wider healthcare system.
“The win-win is that you have a healthy culture of respect and dignity and civility rather than the opposite,” she said. “The leader will actually have more staff retention, which everybody’s concerned about, given the shortage of healthcare workers.”
And that’s a key incentive that may not be discussed as much: Talent drain from toxicity. The Medscape Workplace Culture Report asked about culture as it applies to physicians looking to join up. Notably, 93% of doctors say culture is important when mulling a job offer, 70% said culture is equal to money, and 18% ranked it as more important than money, and 46% say a positive atmosphere is the top priority.
Ultimately, it comes down to who is willing to step in and stand up. Respondents to Khan’s survey counted anonymous reporting systems, more supportive administration teams, and zero-tolerance policies as potential remedies. Gazelle, Sekeres, and Stecher all emphasize the need for zero-tolerance policies for bullying and mistreatment.
“We can’t afford to have things going on like this that just destroy the fabric of the healthcare endeavor,” Gazelle said. “They come out sideways eventually. They come out in terms of poor patient care because there are greater errors. There’s a lack of respect for patients. There’s anger and irritability and so much spillover. We have to have zero-tolerance policies from the top down.”
A version of this article appeared on Medscape.com.
While Kellie Lease Stecher, MD, was working as an ob.gyn. in Minneapolis, Minnesota, a patient confided in her a sexual assault allegation about one of Stecher’s male colleagues. Stecher shared the allegation with her supervisor, who told Stecher not to file a report and chose not to address the issue with the patient. Stecher weighed how to do the right thing: Should she speak up? What were the ethical and legal implications of speaking up vs staying silent?
After seeking advice from her mentors, Stecher felt it was her moral and legal duty to report the allegation to the Minnesota Medical Board. Once she did, her supervisor chastised her repeatedly for reporting the allegation. Stecher soon found herself in a hostile work environment where she was regularly singled out and silenced by her supervisor and colleagues.
“I got to a point where I felt like I couldn’t say anything at any meetings without somehow being targeted after the meeting. There was an individual who was even allowed to fat-shame me with no consequences,” Stecher said. “[Being bullied at work is] a struggle because you have no voice, you have no opportunities, and there’s someone who is intentionally making your life uncomfortable.”
Stecher’s experience is not unusual. Mistreatment is a common issue among healthcare workers, ranging from rudeness to bullying and harassment and permeating every level and specialty of the medical profession. A 2019 research review estimated that 26.3% of healthcare workers had experienced bullying and found bullying in healthcare to be associated with mental health problems such as burnout and depression, physical health problems such as insomnia and headaches, and physicians taking more sick leave.
The Medscape Physician Workplace Culture Report 2024 found similarly bleak results:
- 38% said workplace culture is declining.
- 70% don’t see a big commitment from employers for positive culture.
- 48% said staff isn’t committed to positive culture.
The irony, of course, is that most physicians enter the field to care for people. As individuals go from medical school to residency and on with the rest of their careers, they often experience a rude awakening.
It’s Everywhere
Noticing the prevalence of workplace bullying in the medical field, endocrinologist Farah Khan, MD, at UW Medicine in Seattle, Washington, decided to conduct a survey on the issue.
Khan collected 122 responses from colleagues, friends, and acquaintances in the field. When asked if they had ever been bullied in medicine, 68% of respondents said yes. But here’s the fascinating part: She tried to pinpoint one particular area or source of toxicity in the progression of a physician’s career — and couldn’t because it existed at all levels.
More than one third of respondents said their worst bullying experiences occurred in residency, while 30% said mistreatment was worst in medical school, and 24% indicated their worst experience had occurred once they became an attending.
The litany of experiences included being belittled, excluded, yelled at, criticized, shamed, unfairly blamed, threatened, sexually harassed, subjected to bigotry and slurs, and humiliated.
“What surprised me the most was how widespread this problem is and the many different layers of healthcare it permeates through, from operating room staff to medical students to hospital HR to residents and attendings,” Khan said of her findings.
Who Cares for the Caregivers?
When hematologist Mikkael Sekeres, MD, was in medical school, he seriously considered a career as a surgeon. Following success in his surgical rotations, he scrubbed in with a cardiothoracic surgeon who was well known for both his status as a surgeon and his fiery temper. Sekeres witnessed the surgeon yelling at whoever was nearby: Medical students, fellows, residents, operating room nurses.
“At the end of that experience, any passing thoughts I had of going into cardiothoracic surgery were gone,” Sekeres said. “Some of the people I met in surgery were truly wonderful. Some were unhappy people.”
He has clear ideas why. Mental health struggles that are all too common among physicians can be caused or exacerbated by mistreatment and can also lead a physician to mistreat others.
“People bully when they themselves are hurting,” Sekeres said. “It begs the question, why are people hurting? What’s driving them to be bullies? I think part of the reason is that they’re working really hard and they’re tired, and nobody’s caring for them. It’s hard to care for others when you feel as if you’re hurting more than they are.”
Gail Gazelle, MD, experienced something like this. In her case, the pressure to please and to be a perfect professional and mother affected how she interacted with those around her. While working as a hospice medical director and an academician and clinician at Harvard Medical School, Boston, Massachusetts, she found herself feeling exhausted and burnt out but simultaneously guilty for not doing enough at work or at home.
Guess what happened? She became irritable, lashing out at her son and not putting her best foot forward with coworkers or patients.
After trying traditional therapy and self-help through books and podcasts, Gazelle found her solution in life coaching. “I realized just how harsh I was being on myself and found ways to reverse that pattern,” she said. “I learned ways of regulating myself emotionally that I definitely didn’t learn in my training.”
Today, Gazelle works as a life coach herself, guiding physicians through common challenges of the profession — particularly bullying, which she sees often. She remembers one client, an oncologist, who was being targeted by a nurse practitioner she was training. The nurse practitioner began talking back to the oncologist, as well as gossiping and bad-mouthing her to the nurses in the practice. The nurses then began excluding the oncologist from their cafeteria table at lunchtime, which felt blatant in such a small practice.
A core component of Gazelle’s coaching strategy was helping the client reclaim her self-esteem by focusing on her strengths. She instructed the client to write down what went well that day each night rather than lying in bed ruminating. Such self-care strategies can not only help bullied physicians but also prevent some of the challenges that might cause a physician to bully or lash out at another in the first place.
Such strategies, along with the recent influx of wellness programs available in healthcare facilities, can help physicians cope with the mental health impacts of bullying and the job in general. But even life coaches like Gazelle acknowledge that they are often band-aids on the system’s deeper wounds. Bullying in healthcare is not an individual issue; at its core, it’s an institutional one.
Negative Hierarchies in Healthcare
When Stecher’s contract expired, she was fired by the supervisor who had been bullying her. Stecher has since filed a lawsuit, claiming sexual discrimination, defamation, and wrongful termination.
The medical field has a long history of hierarchy, and while this rigidity has softened over time, negative hierarchical dynamics are often perpetuated by leaders. Phenomena like cronyism and cliques and behaviors like petty gossip, lunchroom exclusion (which in the worst cases can mimic high school dynamics), and targeting can be at play in the healthcare workplace.
The classic examples, Stecher said, can usually be spotted: “If you threaten the status quo or offer different ideas, you are seen as a threat. Cronyism ... strict hierarchies ... people who elevate individuals in their social arena into leadership positions. Physicians don’t get the leadership training that they really need; they are often just dumped into roles with no previous experience because they’re someone’s golfing buddy.”
The question is how to get workplace culture momentum moving in a positive direction. When Gazelle’s clients are hesitant to voice concerns, she emphasizes doing so can and should benefit leadership, as well as patients and the wider healthcare system.
“The win-win is that you have a healthy culture of respect and dignity and civility rather than the opposite,” she said. “The leader will actually have more staff retention, which everybody’s concerned about, given the shortage of healthcare workers.”
And that’s a key incentive that may not be discussed as much: Talent drain from toxicity. The Medscape Workplace Culture Report asked about culture as it applies to physicians looking to join up. Notably, 93% of doctors say culture is important when mulling a job offer, 70% said culture is equal to money, and 18% ranked it as more important than money, and 46% say a positive atmosphere is the top priority.
Ultimately, it comes down to who is willing to step in and stand up. Respondents to Khan’s survey counted anonymous reporting systems, more supportive administration teams, and zero-tolerance policies as potential remedies. Gazelle, Sekeres, and Stecher all emphasize the need for zero-tolerance policies for bullying and mistreatment.
“We can’t afford to have things going on like this that just destroy the fabric of the healthcare endeavor,” Gazelle said. “They come out sideways eventually. They come out in terms of poor patient care because there are greater errors. There’s a lack of respect for patients. There’s anger and irritability and so much spillover. We have to have zero-tolerance policies from the top down.”
A version of this article appeared on Medscape.com.
While Kellie Lease Stecher, MD, was working as an ob.gyn. in Minneapolis, Minnesota, a patient confided in her a sexual assault allegation about one of Stecher’s male colleagues. Stecher shared the allegation with her supervisor, who told Stecher not to file a report and chose not to address the issue with the patient. Stecher weighed how to do the right thing: Should she speak up? What were the ethical and legal implications of speaking up vs staying silent?
After seeking advice from her mentors, Stecher felt it was her moral and legal duty to report the allegation to the Minnesota Medical Board. Once she did, her supervisor chastised her repeatedly for reporting the allegation. Stecher soon found herself in a hostile work environment where she was regularly singled out and silenced by her supervisor and colleagues.
“I got to a point where I felt like I couldn’t say anything at any meetings without somehow being targeted after the meeting. There was an individual who was even allowed to fat-shame me with no consequences,” Stecher said. “[Being bullied at work is] a struggle because you have no voice, you have no opportunities, and there’s someone who is intentionally making your life uncomfortable.”
Stecher’s experience is not unusual. Mistreatment is a common issue among healthcare workers, ranging from rudeness to bullying and harassment and permeating every level and specialty of the medical profession. A 2019 research review estimated that 26.3% of healthcare workers had experienced bullying and found bullying in healthcare to be associated with mental health problems such as burnout and depression, physical health problems such as insomnia and headaches, and physicians taking more sick leave.
The Medscape Physician Workplace Culture Report 2024 found similarly bleak results:
- 38% said workplace culture is declining.
- 70% don’t see a big commitment from employers for positive culture.
- 48% said staff isn’t committed to positive culture.
The irony, of course, is that most physicians enter the field to care for people. As individuals go from medical school to residency and on with the rest of their careers, they often experience a rude awakening.
It’s Everywhere
Noticing the prevalence of workplace bullying in the medical field, endocrinologist Farah Khan, MD, at UW Medicine in Seattle, Washington, decided to conduct a survey on the issue.
Khan collected 122 responses from colleagues, friends, and acquaintances in the field. When asked if they had ever been bullied in medicine, 68% of respondents said yes. But here’s the fascinating part: She tried to pinpoint one particular area or source of toxicity in the progression of a physician’s career — and couldn’t because it existed at all levels.
More than one third of respondents said their worst bullying experiences occurred in residency, while 30% said mistreatment was worst in medical school, and 24% indicated their worst experience had occurred once they became an attending.
The litany of experiences included being belittled, excluded, yelled at, criticized, shamed, unfairly blamed, threatened, sexually harassed, subjected to bigotry and slurs, and humiliated.
“What surprised me the most was how widespread this problem is and the many different layers of healthcare it permeates through, from operating room staff to medical students to hospital HR to residents and attendings,” Khan said of her findings.
Who Cares for the Caregivers?
When hematologist Mikkael Sekeres, MD, was in medical school, he seriously considered a career as a surgeon. Following success in his surgical rotations, he scrubbed in with a cardiothoracic surgeon who was well known for both his status as a surgeon and his fiery temper. Sekeres witnessed the surgeon yelling at whoever was nearby: Medical students, fellows, residents, operating room nurses.
“At the end of that experience, any passing thoughts I had of going into cardiothoracic surgery were gone,” Sekeres said. “Some of the people I met in surgery were truly wonderful. Some were unhappy people.”
He has clear ideas why. Mental health struggles that are all too common among physicians can be caused or exacerbated by mistreatment and can also lead a physician to mistreat others.
“People bully when they themselves are hurting,” Sekeres said. “It begs the question, why are people hurting? What’s driving them to be bullies? I think part of the reason is that they’re working really hard and they’re tired, and nobody’s caring for them. It’s hard to care for others when you feel as if you’re hurting more than they are.”
Gail Gazelle, MD, experienced something like this. In her case, the pressure to please and to be a perfect professional and mother affected how she interacted with those around her. While working as a hospice medical director and an academician and clinician at Harvard Medical School, Boston, Massachusetts, she found herself feeling exhausted and burnt out but simultaneously guilty for not doing enough at work or at home.
Guess what happened? She became irritable, lashing out at her son and not putting her best foot forward with coworkers or patients.
After trying traditional therapy and self-help through books and podcasts, Gazelle found her solution in life coaching. “I realized just how harsh I was being on myself and found ways to reverse that pattern,” she said. “I learned ways of regulating myself emotionally that I definitely didn’t learn in my training.”
Today, Gazelle works as a life coach herself, guiding physicians through common challenges of the profession — particularly bullying, which she sees often. She remembers one client, an oncologist, who was being targeted by a nurse practitioner she was training. The nurse practitioner began talking back to the oncologist, as well as gossiping and bad-mouthing her to the nurses in the practice. The nurses then began excluding the oncologist from their cafeteria table at lunchtime, which felt blatant in such a small practice.
A core component of Gazelle’s coaching strategy was helping the client reclaim her self-esteem by focusing on her strengths. She instructed the client to write down what went well that day each night rather than lying in bed ruminating. Such self-care strategies can not only help bullied physicians but also prevent some of the challenges that might cause a physician to bully or lash out at another in the first place.
Such strategies, along with the recent influx of wellness programs available in healthcare facilities, can help physicians cope with the mental health impacts of bullying and the job in general. But even life coaches like Gazelle acknowledge that they are often band-aids on the system’s deeper wounds. Bullying in healthcare is not an individual issue; at its core, it’s an institutional one.
Negative Hierarchies in Healthcare
When Stecher’s contract expired, she was fired by the supervisor who had been bullying her. Stecher has since filed a lawsuit, claiming sexual discrimination, defamation, and wrongful termination.
The medical field has a long history of hierarchy, and while this rigidity has softened over time, negative hierarchical dynamics are often perpetuated by leaders. Phenomena like cronyism and cliques and behaviors like petty gossip, lunchroom exclusion (which in the worst cases can mimic high school dynamics), and targeting can be at play in the healthcare workplace.
The classic examples, Stecher said, can usually be spotted: “If you threaten the status quo or offer different ideas, you are seen as a threat. Cronyism ... strict hierarchies ... people who elevate individuals in their social arena into leadership positions. Physicians don’t get the leadership training that they really need; they are often just dumped into roles with no previous experience because they’re someone’s golfing buddy.”
The question is how to get workplace culture momentum moving in a positive direction. When Gazelle’s clients are hesitant to voice concerns, she emphasizes doing so can and should benefit leadership, as well as patients and the wider healthcare system.
“The win-win is that you have a healthy culture of respect and dignity and civility rather than the opposite,” she said. “The leader will actually have more staff retention, which everybody’s concerned about, given the shortage of healthcare workers.”
And that’s a key incentive that may not be discussed as much: Talent drain from toxicity. The Medscape Workplace Culture Report asked about culture as it applies to physicians looking to join up. Notably, 93% of doctors say culture is important when mulling a job offer, 70% said culture is equal to money, and 18% ranked it as more important than money, and 46% say a positive atmosphere is the top priority.
Ultimately, it comes down to who is willing to step in and stand up. Respondents to Khan’s survey counted anonymous reporting systems, more supportive administration teams, and zero-tolerance policies as potential remedies. Gazelle, Sekeres, and Stecher all emphasize the need for zero-tolerance policies for bullying and mistreatment.
“We can’t afford to have things going on like this that just destroy the fabric of the healthcare endeavor,” Gazelle said. “They come out sideways eventually. They come out in terms of poor patient care because there are greater errors. There’s a lack of respect for patients. There’s anger and irritability and so much spillover. We have to have zero-tolerance policies from the top down.”
A version of this article appeared on Medscape.com.
NY Nurse Practitioners Sue State Over Pay Equity, Alleged Gender Inequality
A
The New York State Civil Service Commission understates the job function of NPs, overstates their dependence on physicians, and inadequately pays them for their work, according to the complaint filed in the US District Court for the Northern District of New York.
The nurses claim the mistreatment is a consequence of the fact that “at least 80% of the state’s employed NPs are women.”
Michael H. Sussman, a Goshen, New York–based attorney for the nurses, said in an interview that New York NPs are increasingly being used essentially as doctors at state-run facilities, including prisons, yet the state has failed to adequately pay them.
The lawsuit comes after a decade-long attempt by NPs to attain equitable pay and the ability to advance their civil service careers, he said.
“New York state has not addressed the heart of the issue, which is that the classification of this position is much lower than other positions in the state which are not so female-dominated and which engage in very similar activities,” Sussman said.
The lawsuit claims that “the work of NPs is complex, equaling that of a medical specialist, psychiatrist, or clinical physician.”
A spokesman for the New York State Civil Service Commission declined comment, saying the department does not comment on pending litigation.
Novel Gender Discrimination Argument
Gender discrimination is a relatively new argument avenue in the larger equal work, equal pay debate, said Joanne Spetz, PhD, director of the Institute for Health Policy Studies at the University of California, San Francisco.
“This is the first time I’ve heard of [such] a case being really gender discrimination focused,” she said in an interview. “On one level, I think it’s groundbreaking as a legal approach, but it’s also limited because it’s focused on public, state employees.”
Spetz noted that New York has significantly expanded NPs’ scope of practice, enacting in 2022 legislation that granted NPs full practice authority. The law means NPs can evaluate, order, diagnose, manage treatments, and prescribe medications for patients without physician supervision.
“They are in a role where they are stepping back and saying, ‘Wait, why are [we] not receiving equal pay for equal work?’ ” Spetz said. “It’s a totally fair area for debate, especially because they are now authorized to do essentially equal work with a high degree of autonomy.”
Debate Over Pay Grade
The nurses’ complaint centers on the New York State Civil Service Commission’s classification for NPs, which hasn’t changed since 2006. NPs are classified at grade 24, and they have no possibility of internal advancement associated with their title, according to the legal complaint filed on September 17.
To comply with a state legislative directive, the commission in 2018 conducted a study of the NP classification but recommended against reclassification or implementing a career ladder. The study noted the subordinate role of NPs to physicians and the substantial difference between physician classification (entry at grade 34) and that of NPs, psychologists (grade 25), and pharmacists (grade 25).
The study concluded that higher classified positions have higher levels of educational attainment and licensure requirements and no supervision or collaboration requirements, according to the complaint.
At the time, groups such as the Nurse Practitioner Association and the Public Employees Federation (PEF) criticized the findings, but the commission stuck to its classification.
Following the NP Modernization Act that allowed NPs to practice independently, PEF sought an increase for NPs to grade 28 with a progression to grade 34 depending on experience.
“But to this date, despite altering the starting salaries of NPs, defendants have failed and refused to alter the compensation offered to the substantial majority of NPs, and each plaintiff remains cabined in a grade 24 with a discriminatorily low salary when compared with males in other job classifications doing highly similar functions,” the lawsuit contended.
Six plaintiffs are named in the lawsuit, all of whom are women and work for state agencies. Plaintiff Rachel Burns, for instance, works as a psychiatric mental health NP in West Seneca and is responsible for performing psychiatric evaluations for patients, diagnosis, prescribing medication, ordering labs, and determining risks. The evaluations are identical for a psychiatrist and require her to complete the same forms, according to the suit.
Another plaintiff, Amber Hawthorne Lashway, works at a correctional facility in Altona, where for many years she was the sole medical provider, according to the lawsuit. Lashway’s duties, which include diagnoses and treatment of inmates’ medical conditions, mirror those performed by clinical physicians, the suit stated.
The plaintiffs are requesting the court accept jurisdiction of the matter and certify the class they seek to represent. They are also demanding prospective pay equity and compensatory damages for the distress caused by “the long-standing discriminatory” treatment by the state.
The Civil Service Commission and state of New York have not yet responded to the complaint. Their responses are due on November 12.
Attorney: Case Impact Limited
Benjamin McMichael, PhD, JD, said the New York case is not surprising as more states across the country are granting nurses more practice autonomy. The current landscape tends to favor the nurses, he said, with about half of states now allowing NPs full practice authority.
“I think the [New York] NPs are correct that they are underpaid,” said McMichael, an associate professor of law and director of the Interdisciplinary Legal Studies Initiative at The University of Alabama in Tuscaloosa. “With that said, the nature of the case does not clearly lend itself to national change.”
The fact that the NP plaintiffs are employed by the state means they are using a specific set of laws to advance their cause, he said. Other NPs in other employment situations may not have access to the same laws.
A version of this article first appeared on Medscape.com.
A
The New York State Civil Service Commission understates the job function of NPs, overstates their dependence on physicians, and inadequately pays them for their work, according to the complaint filed in the US District Court for the Northern District of New York.
The nurses claim the mistreatment is a consequence of the fact that “at least 80% of the state’s employed NPs are women.”
Michael H. Sussman, a Goshen, New York–based attorney for the nurses, said in an interview that New York NPs are increasingly being used essentially as doctors at state-run facilities, including prisons, yet the state has failed to adequately pay them.
The lawsuit comes after a decade-long attempt by NPs to attain equitable pay and the ability to advance their civil service careers, he said.
“New York state has not addressed the heart of the issue, which is that the classification of this position is much lower than other positions in the state which are not so female-dominated and which engage in very similar activities,” Sussman said.
The lawsuit claims that “the work of NPs is complex, equaling that of a medical specialist, psychiatrist, or clinical physician.”
A spokesman for the New York State Civil Service Commission declined comment, saying the department does not comment on pending litigation.
Novel Gender Discrimination Argument
Gender discrimination is a relatively new argument avenue in the larger equal work, equal pay debate, said Joanne Spetz, PhD, director of the Institute for Health Policy Studies at the University of California, San Francisco.
“This is the first time I’ve heard of [such] a case being really gender discrimination focused,” she said in an interview. “On one level, I think it’s groundbreaking as a legal approach, but it’s also limited because it’s focused on public, state employees.”
Spetz noted that New York has significantly expanded NPs’ scope of practice, enacting in 2022 legislation that granted NPs full practice authority. The law means NPs can evaluate, order, diagnose, manage treatments, and prescribe medications for patients without physician supervision.
“They are in a role where they are stepping back and saying, ‘Wait, why are [we] not receiving equal pay for equal work?’ ” Spetz said. “It’s a totally fair area for debate, especially because they are now authorized to do essentially equal work with a high degree of autonomy.”
Debate Over Pay Grade
The nurses’ complaint centers on the New York State Civil Service Commission’s classification for NPs, which hasn’t changed since 2006. NPs are classified at grade 24, and they have no possibility of internal advancement associated with their title, according to the legal complaint filed on September 17.
To comply with a state legislative directive, the commission in 2018 conducted a study of the NP classification but recommended against reclassification or implementing a career ladder. The study noted the subordinate role of NPs to physicians and the substantial difference between physician classification (entry at grade 34) and that of NPs, psychologists (grade 25), and pharmacists (grade 25).
The study concluded that higher classified positions have higher levels of educational attainment and licensure requirements and no supervision or collaboration requirements, according to the complaint.
At the time, groups such as the Nurse Practitioner Association and the Public Employees Federation (PEF) criticized the findings, but the commission stuck to its classification.
Following the NP Modernization Act that allowed NPs to practice independently, PEF sought an increase for NPs to grade 28 with a progression to grade 34 depending on experience.
“But to this date, despite altering the starting salaries of NPs, defendants have failed and refused to alter the compensation offered to the substantial majority of NPs, and each plaintiff remains cabined in a grade 24 with a discriminatorily low salary when compared with males in other job classifications doing highly similar functions,” the lawsuit contended.
Six plaintiffs are named in the lawsuit, all of whom are women and work for state agencies. Plaintiff Rachel Burns, for instance, works as a psychiatric mental health NP in West Seneca and is responsible for performing psychiatric evaluations for patients, diagnosis, prescribing medication, ordering labs, and determining risks. The evaluations are identical for a psychiatrist and require her to complete the same forms, according to the suit.
Another plaintiff, Amber Hawthorne Lashway, works at a correctional facility in Altona, where for many years she was the sole medical provider, according to the lawsuit. Lashway’s duties, which include diagnoses and treatment of inmates’ medical conditions, mirror those performed by clinical physicians, the suit stated.
The plaintiffs are requesting the court accept jurisdiction of the matter and certify the class they seek to represent. They are also demanding prospective pay equity and compensatory damages for the distress caused by “the long-standing discriminatory” treatment by the state.
The Civil Service Commission and state of New York have not yet responded to the complaint. Their responses are due on November 12.
Attorney: Case Impact Limited
Benjamin McMichael, PhD, JD, said the New York case is not surprising as more states across the country are granting nurses more practice autonomy. The current landscape tends to favor the nurses, he said, with about half of states now allowing NPs full practice authority.
“I think the [New York] NPs are correct that they are underpaid,” said McMichael, an associate professor of law and director of the Interdisciplinary Legal Studies Initiative at The University of Alabama in Tuscaloosa. “With that said, the nature of the case does not clearly lend itself to national change.”
The fact that the NP plaintiffs are employed by the state means they are using a specific set of laws to advance their cause, he said. Other NPs in other employment situations may not have access to the same laws.
A version of this article first appeared on Medscape.com.
A
The New York State Civil Service Commission understates the job function of NPs, overstates their dependence on physicians, and inadequately pays them for their work, according to the complaint filed in the US District Court for the Northern District of New York.
The nurses claim the mistreatment is a consequence of the fact that “at least 80% of the state’s employed NPs are women.”
Michael H. Sussman, a Goshen, New York–based attorney for the nurses, said in an interview that New York NPs are increasingly being used essentially as doctors at state-run facilities, including prisons, yet the state has failed to adequately pay them.
The lawsuit comes after a decade-long attempt by NPs to attain equitable pay and the ability to advance their civil service careers, he said.
“New York state has not addressed the heart of the issue, which is that the classification of this position is much lower than other positions in the state which are not so female-dominated and which engage in very similar activities,” Sussman said.
The lawsuit claims that “the work of NPs is complex, equaling that of a medical specialist, psychiatrist, or clinical physician.”
A spokesman for the New York State Civil Service Commission declined comment, saying the department does not comment on pending litigation.
Novel Gender Discrimination Argument
Gender discrimination is a relatively new argument avenue in the larger equal work, equal pay debate, said Joanne Spetz, PhD, director of the Institute for Health Policy Studies at the University of California, San Francisco.
“This is the first time I’ve heard of [such] a case being really gender discrimination focused,” she said in an interview. “On one level, I think it’s groundbreaking as a legal approach, but it’s also limited because it’s focused on public, state employees.”
Spetz noted that New York has significantly expanded NPs’ scope of practice, enacting in 2022 legislation that granted NPs full practice authority. The law means NPs can evaluate, order, diagnose, manage treatments, and prescribe medications for patients without physician supervision.
“They are in a role where they are stepping back and saying, ‘Wait, why are [we] not receiving equal pay for equal work?’ ” Spetz said. “It’s a totally fair area for debate, especially because they are now authorized to do essentially equal work with a high degree of autonomy.”
Debate Over Pay Grade
The nurses’ complaint centers on the New York State Civil Service Commission’s classification for NPs, which hasn’t changed since 2006. NPs are classified at grade 24, and they have no possibility of internal advancement associated with their title, according to the legal complaint filed on September 17.
To comply with a state legislative directive, the commission in 2018 conducted a study of the NP classification but recommended against reclassification or implementing a career ladder. The study noted the subordinate role of NPs to physicians and the substantial difference between physician classification (entry at grade 34) and that of NPs, psychologists (grade 25), and pharmacists (grade 25).
The study concluded that higher classified positions have higher levels of educational attainment and licensure requirements and no supervision or collaboration requirements, according to the complaint.
At the time, groups such as the Nurse Practitioner Association and the Public Employees Federation (PEF) criticized the findings, but the commission stuck to its classification.
Following the NP Modernization Act that allowed NPs to practice independently, PEF sought an increase for NPs to grade 28 with a progression to grade 34 depending on experience.
“But to this date, despite altering the starting salaries of NPs, defendants have failed and refused to alter the compensation offered to the substantial majority of NPs, and each plaintiff remains cabined in a grade 24 with a discriminatorily low salary when compared with males in other job classifications doing highly similar functions,” the lawsuit contended.
Six plaintiffs are named in the lawsuit, all of whom are women and work for state agencies. Plaintiff Rachel Burns, for instance, works as a psychiatric mental health NP in West Seneca and is responsible for performing psychiatric evaluations for patients, diagnosis, prescribing medication, ordering labs, and determining risks. The evaluations are identical for a psychiatrist and require her to complete the same forms, according to the suit.
Another plaintiff, Amber Hawthorne Lashway, works at a correctional facility in Altona, where for many years she was the sole medical provider, according to the lawsuit. Lashway’s duties, which include diagnoses and treatment of inmates’ medical conditions, mirror those performed by clinical physicians, the suit stated.
The plaintiffs are requesting the court accept jurisdiction of the matter and certify the class they seek to represent. They are also demanding prospective pay equity and compensatory damages for the distress caused by “the long-standing discriminatory” treatment by the state.
The Civil Service Commission and state of New York have not yet responded to the complaint. Their responses are due on November 12.
Attorney: Case Impact Limited
Benjamin McMichael, PhD, JD, said the New York case is not surprising as more states across the country are granting nurses more practice autonomy. The current landscape tends to favor the nurses, he said, with about half of states now allowing NPs full practice authority.
“I think the [New York] NPs are correct that they are underpaid,” said McMichael, an associate professor of law and director of the Interdisciplinary Legal Studies Initiative at The University of Alabama in Tuscaloosa. “With that said, the nature of the case does not clearly lend itself to national change.”
The fact that the NP plaintiffs are employed by the state means they are using a specific set of laws to advance their cause, he said. Other NPs in other employment situations may not have access to the same laws.
A version of this article first appeared on Medscape.com.
Beyond Scope Creep: Why Physicians and PAs Should Come Together for Patients
Over the past few years, many states have attempted to address the ongoing shortage of healthcare workers by introducing new bills to increase the scope of practice for nurse practitioners (NPs) and physician assistants (PAs). The goal of each bill was to improve access to care, particularly for patients who may live in areas where it’s difficult to find a doctor.
In response, the American Medical Association (AMA) launched a targeted campaign to fight “scope creep.” Their goal was to gain the momentum necessary to block proposed legislation to modify or expand the practice authority of nonphysicians, including PAs. A spokesperson for the organization told this news organization that the AMA “greatly values and respects the contributions of PAs as important members of the healthcare team” but emphasized that they do not have the same “skill set or breadth of experience of physicians.”
As such, the AMA argued that expanded practice authority would not only dismantle physician-led care teams but also ultimately lead to higher costs and lower-quality patient care.
The AMA has since launched a large-scale advocacy effort to fight practice expansion legislation — and has a specific page on its website to highlight those efforts. In addition, they have authored model legislation, talking points for AMA members, and a widely read article in AMA News to help them in what they call a “fight for physicians.”
These resources have also been disseminated to the greater healthcare stakeholder community.
Marilyn Suri, PA-C, chief operating officer and senior executive for Advanced Practice Professional Affairs at Vincenzo Novara MDPA and Associates, a critical care pulmonary medicine practice in Miami, Florida, said she found the AMA’s campaign to be “very misleading.”
“PAs are created in the image of physicians to help manage the physician shortage. We are trained very rigorously — to diagnose illness, develop treatment plans, and prescribe medications,” she said. “We’re not trying to expand our scope. We are trying to eliminate or lessen barriers that prevent patients from getting access to care.”
Suri is not alone. Last summer, the American Academy of Physician Associates (AAPA) requested a meeting with the AMA to find ways for the two organizations to collaborate to improve care delivery — as well as find common ground to address issues regarding patient access to care. When the AMA did not respond, the AAPA sent a second letter in September 2024, reiterating their request for a meeting.
That correspondence also included a letter, signed by more than 8000 PAs from across the country, calling for an end to what the AAPA refers to as “damaging rhetoric,” as well as data from a recent survey of PAs regarding the fallout of AMA’s scope creep messaging.
Those survey results highlighted that the vast majority of PAs surveyed feel that the AMA is doing more than just attacking proposed legislation: They believe the association is negatively influencing patients’ understanding of PA qualifications, ultimately affecting their ability to provide care.
“The campaign is unintentionally harming patients by suggesting we are doing more than what we are trained to do,” said Elisa Hock, PA-C, a behavioral health PA in Texas. “And when you work in a place with limited resources, medically speaking — including limited access to providers — this kind of campaign is really detrimental to helping patients.”
Lisa M. Gables, CEO of the AAPA, said the organization is “deeply disappointed” in the AMA’s lack of response to their letters thus far — but remains committed to working with the organization to bring forward new solutions to address healthcare’s most pressing challenges.
“AAPA remains committed to pushing for modernization of practice laws to ensure all providers can practice medicine to the fullest extent of their training, education, and experience,” she said. “That is what patients deserve and want.”
Hock agreed. She told this news organization that the public is not always aware of what PAs can offer in terms of patient care. That said, she believes newer generations of physicians understand the value of PAs and the many skills they bring to the table.
“I’ve been doing this for 17 years, and it’s been an uphill battle, at times, to educate the public about what PAs can and can’t do,” she explained. “To throw more mud in the mix that will confuse patients more about what we do doesn’t help. Healthcare works best with a team-based approach. And that team has been and always will be led by the physician. We are aware of our role and our limitations. But we also know what we can offer patients, especially in areas like El Paso, where there is a real shortage of providers.”
With a growing aging population — and the physician shortage expected to increase in the coming decade — Suri hopes that the AMA will accept AAPA’s invitation to meet — because no one wins with this kind of healthcare infighting. In fact, she said patients will suffer because of it. She hopes that future discussions and collaborations can show providers and patients what team-based healthcare can offer.
“I think it’s important for those in healthcare to be aware that none of us work alone. Even physicians collaborate with other subspecialties, as well as nurses and other healthcare professionals,” said Suri.
A version of this article appeared on Medscape.com.
Over the past few years, many states have attempted to address the ongoing shortage of healthcare workers by introducing new bills to increase the scope of practice for nurse practitioners (NPs) and physician assistants (PAs). The goal of each bill was to improve access to care, particularly for patients who may live in areas where it’s difficult to find a doctor.
In response, the American Medical Association (AMA) launched a targeted campaign to fight “scope creep.” Their goal was to gain the momentum necessary to block proposed legislation to modify or expand the practice authority of nonphysicians, including PAs. A spokesperson for the organization told this news organization that the AMA “greatly values and respects the contributions of PAs as important members of the healthcare team” but emphasized that they do not have the same “skill set or breadth of experience of physicians.”
As such, the AMA argued that expanded practice authority would not only dismantle physician-led care teams but also ultimately lead to higher costs and lower-quality patient care.
The AMA has since launched a large-scale advocacy effort to fight practice expansion legislation — and has a specific page on its website to highlight those efforts. In addition, they have authored model legislation, talking points for AMA members, and a widely read article in AMA News to help them in what they call a “fight for physicians.”
These resources have also been disseminated to the greater healthcare stakeholder community.
Marilyn Suri, PA-C, chief operating officer and senior executive for Advanced Practice Professional Affairs at Vincenzo Novara MDPA and Associates, a critical care pulmonary medicine practice in Miami, Florida, said she found the AMA’s campaign to be “very misleading.”
“PAs are created in the image of physicians to help manage the physician shortage. We are trained very rigorously — to diagnose illness, develop treatment plans, and prescribe medications,” she said. “We’re not trying to expand our scope. We are trying to eliminate or lessen barriers that prevent patients from getting access to care.”
Suri is not alone. Last summer, the American Academy of Physician Associates (AAPA) requested a meeting with the AMA to find ways for the two organizations to collaborate to improve care delivery — as well as find common ground to address issues regarding patient access to care. When the AMA did not respond, the AAPA sent a second letter in September 2024, reiterating their request for a meeting.
That correspondence also included a letter, signed by more than 8000 PAs from across the country, calling for an end to what the AAPA refers to as “damaging rhetoric,” as well as data from a recent survey of PAs regarding the fallout of AMA’s scope creep messaging.
Those survey results highlighted that the vast majority of PAs surveyed feel that the AMA is doing more than just attacking proposed legislation: They believe the association is negatively influencing patients’ understanding of PA qualifications, ultimately affecting their ability to provide care.
“The campaign is unintentionally harming patients by suggesting we are doing more than what we are trained to do,” said Elisa Hock, PA-C, a behavioral health PA in Texas. “And when you work in a place with limited resources, medically speaking — including limited access to providers — this kind of campaign is really detrimental to helping patients.”
Lisa M. Gables, CEO of the AAPA, said the organization is “deeply disappointed” in the AMA’s lack of response to their letters thus far — but remains committed to working with the organization to bring forward new solutions to address healthcare’s most pressing challenges.
“AAPA remains committed to pushing for modernization of practice laws to ensure all providers can practice medicine to the fullest extent of their training, education, and experience,” she said. “That is what patients deserve and want.”
Hock agreed. She told this news organization that the public is not always aware of what PAs can offer in terms of patient care. That said, she believes newer generations of physicians understand the value of PAs and the many skills they bring to the table.
“I’ve been doing this for 17 years, and it’s been an uphill battle, at times, to educate the public about what PAs can and can’t do,” she explained. “To throw more mud in the mix that will confuse patients more about what we do doesn’t help. Healthcare works best with a team-based approach. And that team has been and always will be led by the physician. We are aware of our role and our limitations. But we also know what we can offer patients, especially in areas like El Paso, where there is a real shortage of providers.”
With a growing aging population — and the physician shortage expected to increase in the coming decade — Suri hopes that the AMA will accept AAPA’s invitation to meet — because no one wins with this kind of healthcare infighting. In fact, she said patients will suffer because of it. She hopes that future discussions and collaborations can show providers and patients what team-based healthcare can offer.
“I think it’s important for those in healthcare to be aware that none of us work alone. Even physicians collaborate with other subspecialties, as well as nurses and other healthcare professionals,” said Suri.
A version of this article appeared on Medscape.com.
Over the past few years, many states have attempted to address the ongoing shortage of healthcare workers by introducing new bills to increase the scope of practice for nurse practitioners (NPs) and physician assistants (PAs). The goal of each bill was to improve access to care, particularly for patients who may live in areas where it’s difficult to find a doctor.
In response, the American Medical Association (AMA) launched a targeted campaign to fight “scope creep.” Their goal was to gain the momentum necessary to block proposed legislation to modify or expand the practice authority of nonphysicians, including PAs. A spokesperson for the organization told this news organization that the AMA “greatly values and respects the contributions of PAs as important members of the healthcare team” but emphasized that they do not have the same “skill set or breadth of experience of physicians.”
As such, the AMA argued that expanded practice authority would not only dismantle physician-led care teams but also ultimately lead to higher costs and lower-quality patient care.
The AMA has since launched a large-scale advocacy effort to fight practice expansion legislation — and has a specific page on its website to highlight those efforts. In addition, they have authored model legislation, talking points for AMA members, and a widely read article in AMA News to help them in what they call a “fight for physicians.”
These resources have also been disseminated to the greater healthcare stakeholder community.
Marilyn Suri, PA-C, chief operating officer and senior executive for Advanced Practice Professional Affairs at Vincenzo Novara MDPA and Associates, a critical care pulmonary medicine practice in Miami, Florida, said she found the AMA’s campaign to be “very misleading.”
“PAs are created in the image of physicians to help manage the physician shortage. We are trained very rigorously — to diagnose illness, develop treatment plans, and prescribe medications,” she said. “We’re not trying to expand our scope. We are trying to eliminate or lessen barriers that prevent patients from getting access to care.”
Suri is not alone. Last summer, the American Academy of Physician Associates (AAPA) requested a meeting with the AMA to find ways for the two organizations to collaborate to improve care delivery — as well as find common ground to address issues regarding patient access to care. When the AMA did not respond, the AAPA sent a second letter in September 2024, reiterating their request for a meeting.
That correspondence also included a letter, signed by more than 8000 PAs from across the country, calling for an end to what the AAPA refers to as “damaging rhetoric,” as well as data from a recent survey of PAs regarding the fallout of AMA’s scope creep messaging.
Those survey results highlighted that the vast majority of PAs surveyed feel that the AMA is doing more than just attacking proposed legislation: They believe the association is negatively influencing patients’ understanding of PA qualifications, ultimately affecting their ability to provide care.
“The campaign is unintentionally harming patients by suggesting we are doing more than what we are trained to do,” said Elisa Hock, PA-C, a behavioral health PA in Texas. “And when you work in a place with limited resources, medically speaking — including limited access to providers — this kind of campaign is really detrimental to helping patients.”
Lisa M. Gables, CEO of the AAPA, said the organization is “deeply disappointed” in the AMA’s lack of response to their letters thus far — but remains committed to working with the organization to bring forward new solutions to address healthcare’s most pressing challenges.
“AAPA remains committed to pushing for modernization of practice laws to ensure all providers can practice medicine to the fullest extent of their training, education, and experience,” she said. “That is what patients deserve and want.”
Hock agreed. She told this news organization that the public is not always aware of what PAs can offer in terms of patient care. That said, she believes newer generations of physicians understand the value of PAs and the many skills they bring to the table.
“I’ve been doing this for 17 years, and it’s been an uphill battle, at times, to educate the public about what PAs can and can’t do,” she explained. “To throw more mud in the mix that will confuse patients more about what we do doesn’t help. Healthcare works best with a team-based approach. And that team has been and always will be led by the physician. We are aware of our role and our limitations. But we also know what we can offer patients, especially in areas like El Paso, where there is a real shortage of providers.”
With a growing aging population — and the physician shortage expected to increase in the coming decade — Suri hopes that the AMA will accept AAPA’s invitation to meet — because no one wins with this kind of healthcare infighting. In fact, she said patients will suffer because of it. She hopes that future discussions and collaborations can show providers and patients what team-based healthcare can offer.
“I think it’s important for those in healthcare to be aware that none of us work alone. Even physicians collaborate with other subspecialties, as well as nurses and other healthcare professionals,” said Suri.
A version of this article appeared on Medscape.com.
Lawsuit Targets Publishers: Is Peer Review Flawed?
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
The peer-review process, which is used by scientific journals to validate legitimate research, is now under legal scrutiny. The US District Court for the Southern District of New York will soon rule on whether scientific publishers have compromised this system for profit. In mid-September, University of California, Los Angeles neuroscientist Lucina Uddin filed a class action lawsuit against six leading academic publishers — Elsevier, Wolters Kluwer, Wiley, Sage Publications, Taylor & Francis, and Springer Nature — accusing them of violating antitrust laws and obstructing academic research.
The lawsuit targets several long-standing practices in scientific publishing, including the lack of compensation for peer reviewers, restrictions that require submitting to only one journal at a time, and bans on sharing manuscripts under review. Uddin’s complaint argues that these practices contribute to inefficiencies in the review process, thus delaying the publication of critical discoveries, which could hinder research, clinical advancements, and the development of new medical treatments.
The suit also noted that these publishers generated $10 billion in revenue in 2023 in peer-reviewed journals. However, the complaint seemingly overlooks the widespread practice of preprint repositories, where many manuscripts are shared while awaiting peer review.
Flawed Reviews
A growing number of studies have highlighted subpar or unethical behaviors among reviewers, who are supposed to adhere to the highest standards of methodological rigor, both in conducting research and reviewing work for journals. One recent study published in Scientometrics in August examined 263 reviews from 37 journals across various disciplines and found alarming patterns of duplication. Many of the reviews contained identical or highly similar language. Some reviewers were found to be suggesting that the authors expand their bibliographies to include the reviewers’ own work, thus inflating their citation counts.
As María Ángeles Oviedo-García from the University of Seville in Spain, pointed out: “The analysis of 263 review reports shows a pattern of vague, repetitive statements — often identical or very similar — along with coercive citations, ultimately resulting in misleading reviews.”
Experts in research integrity and ethics argue that while issues persist, the integrity of scientific research is improving. Increasing research and public disclosure reflect a heightened awareness of problems long overlooked.
Speaking to this news organization, Fanelli, who has been studying scientific misconduct for about 20 years, noted that while his early work left him disillusioned, further research has replaced his cynicism with what he describes as healthy skepticism and a more optimistic outlook. Fanelli also collaborates with the Luxembourg Agency for Research Integrity and the Advisory Committee on Research Ethics and Bioethics at the Italian National Research Council (CNR), where he helped develop the first research integrity guidelines.
Lack of Awareness
A recurring challenge is the difficulty in distinguishing between honest mistakes and intentional misconduct. “This is why greater investment in education is essential,” said Daniel Pizzolato, European Network of Research Ethics Committees, Bonn, Germany, and the Centre for Biomedical Ethics and Law, KU Leuven in Belgium.
While Pizzolato acknowledged that institutions such as the CNR in Italy provide a positive example, awareness of research integrity is generally still lacking across much of Europe, and there are few offices dedicated to promoting research integrity. However, he pointed to promising developments in other countries. “In France and Denmark, researchers are required to be familiar with integrity norms because codes of conduct have legal standing. Some major international funding bodies like the European Molecular Biology Organization are making participation in research integrity courses a condition for receiving grants.”
Pizzolato remains optimistic. “There is a growing willingness to move past this impasse,” he said.
A recent study published in The Journal of Clinical Epidemiology reveals troubling gaps in how retracted biomedical articles are flagged and cited. Led by Caitlin Bakkera, Department of Epidemiology, Maastricht University, Maastricht, the Netherlands, the research sought to determine whether articles retracted because of errors or fraud were properly flagged across various databases.
The results were concerning: Less than 5% of retracted articles had consistent retraction notices across all databases that hosted them, and less than 50% of citations referenced the retraction. None of the 414 retraction notices analyzed met best-practice guidelines for completeness. Bakkera and colleagues warned that these shortcomings threaten the integrity of public health research.
Fanelli’s Perspective
Despite the concerns, Fanelli remains calm. “Science is based on debate and a perspective called organized skepticism, which helps reveal the truth,” he explained. “While there is often excessive skepticism today, the overall quality of clinical trials is improving.
“It’s important to remember that reliable results take time and shouldn’t depend on the outcome of a single study. It’s essential to consider the broader context, the history of the research field, and potential conflicts of interest, both financial and otherwise. Biomedical research requires constant updates,” he concluded.
This story was translated from Univadis Italy using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
How AI Is Revolutionizing Drug Repurposing for Faster, Broader Impact
Summary:
In this segment, the speaker discusses how AI is revolutionizing the drug repurposing process. Previously, drug repurposing was limited by manual research on individual diseases and drugs. With AI, scientists can now analyze a vast array of drugs and diseases simultaneously, generating a ranking system based on the likelihood of success. The Center for Cytokine Storm Treatment and Laboratory, along with the platform Every Cure, uses AI to score 3000 drugs against 18,000 diseases. This platform dramatically reduces the time and resources required for drug repurposing, enabling predictions that can be tested in a fraction of the time.
Key Takeaways:
AI is accelerating the drug repurposing process, offering faster and more comprehensive analysis of possible drug-disease matches.
The AI-based platform assigns a likelihood score to each potential match, streamlining the process for testing and validation.
Our Editors Also Recommend:
AI’s Drug Revolution, Part 1: Faster Trials and Approvals
From AI to Obesity Drugs to Soaring Costs: Medscape Hot Topics in the Medical Profession Report 2024
AI Voice Analysis for Diabetes Screening Shows Promise
To see the full event recording, click here.
A version of this article appeared on Medscape.com.
Summary:
In this segment, the speaker discusses how AI is revolutionizing the drug repurposing process. Previously, drug repurposing was limited by manual research on individual diseases and drugs. With AI, scientists can now analyze a vast array of drugs and diseases simultaneously, generating a ranking system based on the likelihood of success. The Center for Cytokine Storm Treatment and Laboratory, along with the platform Every Cure, uses AI to score 3000 drugs against 18,000 diseases. This platform dramatically reduces the time and resources required for drug repurposing, enabling predictions that can be tested in a fraction of the time.
Key Takeaways:
AI is accelerating the drug repurposing process, offering faster and more comprehensive analysis of possible drug-disease matches.
The AI-based platform assigns a likelihood score to each potential match, streamlining the process for testing and validation.
Our Editors Also Recommend:
AI’s Drug Revolution, Part 1: Faster Trials and Approvals
From AI to Obesity Drugs to Soaring Costs: Medscape Hot Topics in the Medical Profession Report 2024
AI Voice Analysis for Diabetes Screening Shows Promise
To see the full event recording, click here.
A version of this article appeared on Medscape.com.
Summary:
In this segment, the speaker discusses how AI is revolutionizing the drug repurposing process. Previously, drug repurposing was limited by manual research on individual diseases and drugs. With AI, scientists can now analyze a vast array of drugs and diseases simultaneously, generating a ranking system based on the likelihood of success. The Center for Cytokine Storm Treatment and Laboratory, along with the platform Every Cure, uses AI to score 3000 drugs against 18,000 diseases. This platform dramatically reduces the time and resources required for drug repurposing, enabling predictions that can be tested in a fraction of the time.
Key Takeaways:
AI is accelerating the drug repurposing process, offering faster and more comprehensive analysis of possible drug-disease matches.
The AI-based platform assigns a likelihood score to each potential match, streamlining the process for testing and validation.
Our Editors Also Recommend:
AI’s Drug Revolution, Part 1: Faster Trials and Approvals
From AI to Obesity Drugs to Soaring Costs: Medscape Hot Topics in the Medical Profession Report 2024
AI Voice Analysis for Diabetes Screening Shows Promise
To see the full event recording, click here.
A version of this article appeared on Medscape.com.
Mycosis Fungoides: Measured Approach Key to Treatment
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
HUNTINGTON BEACH, CALIFORNIA — When patients of Aaron Mangold, MD, first learn they have mycosis fungoides (MF), the most common form of primary cutaneous T-cell lymphoma (CTCL), some are concerned about whether the diagnosis means a shortened life expectancy.
Dr. Mangold, codirector of the multidisciplinary cutaneous lymphoma clinic at Mayo Clinic, Scottsdale, Arizona, said at the annual meeting of the Pacific Dermatologic Association. “For early-stage disease, I think of it more like diabetes; this is really a chronic disease” that unlikely will be fatal but may be associated with increased morbidity as the disease progresses, and “the overall goal of therapy should be disease control to increase quality of life.”
Patient- and lymphoma-specific factors drive the choice of therapy. The focus for patients with early-stage disease, Dr. Mangold said, is to treat comorbidities and symptoms, such as itch or skin pain, maximize their quality of life, and consider the potential for associated toxicities of therapy as the disease progresses. Start with the least toxic, targeted, nonimmunosuppressive therapy, “then work toward more toxic immunosuppressive therapies,” he advised. “Use toxic agents just long enough to control the disease, then transition to a maintenance regimen with less toxic immunosuppressive agents.”
When Close Follow-Up Is Advised
According to unpublished data from PROCLIPI (the Prospective Cutaneous Lymphoma International Prognostic Index) study presented at the fifth World Congress of Cutaneous Lymphomas earlier in 2024, the following factors warrant consideration for close follow-up and more aggressive treatment: Nodal enlargement greater than 15 mm, age over 60 years, presence of plaques, and large-cell transformation in skin. “These are some of the stigmata in early disease that might guide you toward referring” a patient to a CTCL expert, Dr. Mangold said. (Consensus-based recommendations on the management of MF in children were published in August of 2024.)
According to Dr. Mangold, topical/skin-directed therapies are best for early-stage disease or in combination with systemic therapies in advanced disease. For early-stage disease, one of his preferred options is daily application of a skin moisturizer plus a topical corticosteroid such as clobetasol, halobetasol, or augmented betamethasone, then evaluating the response at 3 months. “This is a cheap option, and we see response rates as high as 90%,” he said. “I don’t often see steroid atrophy when treating patients with active MF. There’s a tendency to think, ‘I don’t want to overtreat.’ I think you can be aggressive. If you look in the literature, people typically pulse twice daily for a couple of weeks with a 1-week break.”
Mechlorethamine, a topical alkylating gel approved in 2013 for the treatment of early-stage MF, is an option when patients fail to respond to topical steroids, prefer to avoid steroids, or have thick, plaque-like disease. With mechlorethamine, it is important to “start slow and be patient,” Dr. Mangold said. “Real-world data shows that it takes 12-18 months to get a good response. Counsel patients that they are likely to get a rash, and that the risk of rash is dose dependent.”
Other treatment options to consider include imiquimod, which can be used for single refractory spots. He typically recommends application 5 days per week with titration up to daily if tolerated for up to 3 months. “Treat until you get a brisk immune response,” he said. “We’ve seen patients with durable, long-term responses.”
UVB Phototherapy Effective
For patients with stage IB disease, topical therapies are less practical and may be focused on refractory areas of disease. Narrow-band UVB phototherapy is the most practical and cost-effective treatment, Dr. Mangold said. Earlier-stage patch disease responds to phototherapy in up to 80% of cases, while plaque-stage disease responds in up to half of cases. “More frequent use of phototherapy may decrease time to clearance, but overall response is similar.”
Dr. Mangold recommends phototherapy 2-3 days per week, titrating up to a maximal response dose, and maintaining that dose for about 3 months. Maintenance involves tapering the phototherapy dose to a minimal dose with continued response. “The goal is to prevent relapse,” he said.
For patients with MF of stage IIB and higher, he considers total skin electron beam therapy, an oral retinoid with phototherapy, systemic agents, and focal radiation with systemic treatment. One of his go-to systemic options is bexarotene, which he uses for early-stage disease refractory to treatment or for less aggressive advanced disease. “We typically use a low dose ... and about half of patients respond,” Dr. Mangold said. The time to response is about 6 months. Bexarotene causes elevated lipids and low thyroid function, so he initiates patients on fenofibrate and levothyroxine at baseline.
Another systemic option is brentuximab vedotin, a monoclonal antibody that targets cells with CD30 expression, which is typically administered in a specialty center every 3 weeks for up to 16 cycles. “In practice, we often use six to eight cycles to avoid neuropathy,” he said. “It’s a good debulking agent, the time to response is 6-9 weeks, and it has a sustained response of 60%.” Neuropathy can occur with treatment, but improves over time.
Other systemic options for MF include romidepsin, mogamulizumab, and extracorporeal photopheresis used in erythrodermic disease.
Radiation An Option in Some Cases
Dr. Mangold noted that low doses of radiation therapy can effectively treat MF lesions in as little as one dose. “We can use it as a cure for a single spot or to temporarily treat the disease while other therapies are being started,” he said. Long-term side effects need to be considered when using radiation. “The more radiation, the more side effects.”
Dr. Mangold disclosed that he is an investigator for Sun Pharmaceutical, Solagenix, Elorac, miRagen, Kyowa Kirin, the National Clinical Trials Network, and CRISPR Therapeutics. He has also received consulting fees/honoraria from Kirin and Solagenix.
A version of this article first appeared on Medscape.com.
FROM PDA 2024
Why Residents Are Joining Unions in Droves
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Before the 350 residents finalized their union contract at the University of Vermont (UVM) Medical Center, Burlington, in 2022, Jesse Mostoller, DO, now a third-year pathology resident, recalls hearing about another resident at the hospital who resorted to moonlighting as an Uber driver to make ends meet.
“In Vermont, rent and childcare are expensive,” said Dr. Mostoller, adding that, thanks to union bargaining, first-year residents at UVM are now paid $71,000 per year instead of $61,000. In addition, residents now receive $1800 per year for food (up from $200-$300 annually) and a $1800 annual fund to help pay for board exams that can be carried over for 2 years. “When we were negotiating, the biggest item on our list of demands was to help alleviate the financial pressure residents have been facing for years.”
The UVM residents’ collective bargaining also includes a cap on working hours so that residents don’t work 80 hours a week, paid parental leave, affordable housing, and funds for education and wellness.
These are some of the most common challenges that are faced by residents all over the country, said A. Taylor Walker, MD, MPH, family medicine chief physician at Tufts University School of Medicine/Cambridge Health Alliance in Boston, Massachusetts, and national president of the Committee of Interns and Residents (CIR), which is part of the Service Employees International Union.
For these reasons, residents at Montefiore Medical Center, Stanford Health Care, George Washington University, and the University of Pennsylvania have recently voted to unionize, according to Dr. Walker.
And while there are several small local unions that have picked up residents at local hospitals, CIR is the largest union of physicians in the United States, with a total of 33,000 residents and fellows across the country (15% of the staff at more than 60 hospitals nationwide).
“We’ve doubled in size in the last 4 years,” said Dr. Walker. “The reason is that we’re in a national reckoning on the corporatization of American medicine and the way in which graduate medical education is rooted in a cycle of exploitation that doesn’t center on the health, well-being, or safety of our doctors and ultimately negatively affects our patients.”
Here’s what residents are fighting for — right now.
Adequate Parental Leave
Christopher Domanski, MD, a first-year resident in psychiatry at California Pacific Medical Center (CPMC) in San Francisco, is also a new dad to a 5-month-old son and is currently in the sixth week of parental leave. One goal of CPMC’s union, started a year and a half ago, is to expand parental leave to 8 weeks.
“I started as a resident here in mid-June, but the fight with CPMC leaders has been going on for a year and a half,” Dr. Domanski said. “It can feel very frustrating because many times there’s no budge in the conversations we want to have.”
Contract negotiations here continue to be slow — and arduous.
“It goes back and forth,” said Dr. Domanski, who makes about $75,000 a year. “Sometimes they listen to our proposals, but they deny the vast majority or make a paltry increase in salary or time off. It goes like this: We’ll have a negotiation; we’ll talk about it, and then they say, ‘we’re not comfortable doing this’ and it stalls again.”
If a resident hasn’t started a family yet, access to fertility benefits and reproductive healthcare is paramount because most residents are in their 20s and 30s, Dr. Walker said.
“Our reproductive futures are really hindered by what care we have access to and what care is covered,” she added. “We don’t make enough money to pay for reproductive care out of pocket.”
Fair Pay
In Boston, the residents at Mass General Brigham certified their union in June 2023, but they still don’t have a contract.
“When I applied for a residency in September 2023, I spoke to the folks here, and I was basically under the impression that we would have a contract by the time I matched,” said Madison Masters, MD, a resident in internal medicine. “We are not there.”
This timeline isn’t unusual — the 1400 Penn Medicine residents who unionized in 2023 only recently secured a tentative union contract at the end of September, and at Stanford, the process to ratify their first contract took 13 months.
Still, the salary issue remains frustrating as resident compensation doesn’t line up with the cost of living or the amount of work residents do, said Dr. Masters, who says starting salaries at Mass General Brigham are $78,500 plus a $10,000 stipend for housing.
“There’s been a long tradition of underpaying residents — we’re treated like trainees, but we’re also a primary labor force,” Dr. Masters said, adding that nurse practitioners and physician assistants are paid almost twice as much as residents — some make $120,000 per year or more, while the salary range for residents nationwide is $49,000-$65,000 per year.
“Every time we discuss the contract and talk about a financial package, they offer a 1.5% raise for the next 3 years while we had asked for closer to 8%,” Dr. Masters said. “Then, when they come back for the next bargaining session, they go up a quarter of a percent each time. Recently, they said we will need to go to a mediator to try and resolve this.”
Adequate Healthcare
The biggest — and perhaps the most shocking — ask is for robust health insurance coverage.
“At my hospital, they’re telling us to get Amazon One Medical for health insurance,” Dr. Masters said. “They’re saying it’s hard for anyone to get primary care coverage here.”
Inadequate health insurance is a big issue, as burnout among residents and fellows remains a problem. At UVM, a $10,000 annual wellness stipend has helped address some of these issues. Even so, union members at UVM are planning to return to the table within 18 months to continue their collective bargaining.
The ability to access mental health services anywhere you want is also critical for residents, Dr. Walker said.
“If you can only go to a therapist at your own institution, there is a hesitation to utilize that specialist if that’s even offered,” Dr. Walker said. “Do you want to go to therapy with a colleague? Probably not.”
Ultimately, the residents we spoke to are committed to fighting for their workplace rights — no matter how time-consuming or difficult this has been.
“No administration wants us to have to have a union, but it’s necessary,” Dr. Mostoller said. “As an individual, you don’t have leverage to get a seat at the table, but now we have a seat at the table. We have a wonderful contract, but we’re going to keep fighting to make it even better.”
Paving the way for future residents is a key motivator, too.
“There’s this idea of leaving the campsite cleaner than you found it,” Dr. Mostoller told this news organization. “It’s the same thing here — we’re trying to fix this so that the next generation of residents won’t have to.”
A version of this article first appeared on Medscape.com.
Crisugabalin Alleviates Postherpetic Neuralgia Symptoms in Phase 3 Study
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- Researchers conducted a phase 3 multicenter, double-blind study involving 366 patients in China (median age, 63 years; 52.7% men) with PHN with an average daily pain score (ADPS) of 4 or greater on the numeric pain rating scale who were randomly assigned to receive either crisugabalin 40 mg/d (n = 121), 80 mg/d (n = 121), or placebo (n = 124) for 12 weeks.
- Patients who did not experience any serious toxic effects in these 12 weeks entered a 14-week open-label extension phase and received crisugabalin 40 mg twice daily.
- The primary efficacy endpoint was the change in ADPS from baseline at week 12.
- Secondary efficacy endpoints included the proportion of patients achieving at least 30% and 50% reduction in ADPS at week 12; changes in the Short-Form McGill Pain Questionnaire (SF-MPQ), Visual Analog Scale, and Average Daily Sleep Interference Scale scores at week 12; and change in the SF-MPQ Present Pain Intensity scores at weeks 12 and 26.
TAKEAWAY:
- At week 12, among those on crisugabalin 40 mg/d and 80 mg/d, there were significant reductions in ADPS compared with placebo (least squares mean [LSM] change from baseline, −2.2 and −2.6 vs −1.1, respectively; P < .001).
- A greater proportion of patients on crisugabalin 40 mg/d (61.2%) and 80 mg/d (54.5%) achieved 30% or greater reduction in ADPS (P < .001) than patients who received placebo (35.5%). Similarly, a 50% or greater reduction in ADPS was achieved by 37.2% of patients on crisugabalin 40 mg/d (P = .002) and 38% on 80 mg/d (P < .001), compared with 20.2% for placebo.
- Crisugabalin 40 mg/d and crisugabalin 80 mg/d were associated with greater reductions in the pain intensity at week 12 than placebo (LSM, −1.0 and −1.2 vs −0.5, respectively; P < .001). Similar patterns were noted for other pain-related measures at weeks 12 and 26.
- Serious treatment-emergent adverse events occurred in four patients in each group; only 2.4% of those on 40 mg/d and 1.6% on 80 mg/d discontinued treatment because of side effects.
IN PRACTICE:
“Crisugabalin 40 mg/d or crisugabalin 80 mg/d was well-tolerated and significantly improved ADPS compared to placebo,” the authors wrote, adding that “crisugabalin can be flexibly selected depending on individual patient response and tolerability at 40 mg/d or 80 mg/d.”
SOURCE:
The study was led by Daying Zhang, PhD, of the Department of Pain Medicine at The First Affiliated Hospital of Nanchang University, Nanchang, China. It was published online in JAMA Dermatology.
LIMITATIONS:
The findings may not be generalizable to the global population as the study population was limited to Chinese patients. The study only provided short-term efficacy and safety data on crisugabalin, lacked an active comparator, and did not reflect the standard of care observed in the United States or Europe, where oral tricyclic antidepressants, pregabalin, and the lidocaine patch are recommended as first-line therapies.
DISCLOSURES:
The study was sponsored and funded by Haisco Pharmaceutical. Dr. Zhang and another author reported receiving support from Haisco. Two authors are company employees.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.