User login
Given the choice, T2D patients find their own best meds
Allowing people with type 2 diabetes to try agents from three different classes of antidiabetes drugs showed they usually find a clear preference, often the drug that gives them the best glycemic control and least bothersome adverse effects, according to secondary findings from a randomized study of patients in the United Kingdom.
“This is the first study in which the same patient has tried three different types of glucose-lowering drug, enabling them to directly compare them and then choose which one is best for them,” Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in a written statement. “We’ve shown that going with the patients’ choice results in better glucose control and fewer side effects than any other approach. When it’s not clear which drug is best to use, then patients should try before they choose. Surprisingly, that approach has never been tried before.”
These secondary results from the TriMaster study were recently published in Nature Medicine and presented at the annual meeting of the European Association for the Study of Diabetes (EASD) in September, as reported by this news organization.
TriMaster enrolled adults aged 30-80 years with a clinical diagnosis of type 2 diabetes for at least 12 months. Their glycemia was inadequately controlled despite treatment with metformin alone or two classes of oral glucose-lowering therapy that did not include an agent from any of the three classes tested in the study: dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and thiazolidinediones. The people taking two different drug classes at entry were most often taking metformin and a sulfonylurea.
Do BMI and renal function affect treatment response?
TriMaster tested two hypotheses. Firstly, would people with a body mass index of more than 30 kg/m2 have greater glucose lowering with the thiazolidinedione pioglitazone (Actos) than with the DPP-4 inhibitor sitagliptin (Januvia), compared to people with a lower BMI?
Secondly, would people with an estimated glomerular filtration rate (eGFR) of 60-90 mL/min/1.73 m2 have greater glucose lowering with sitagliptin than with the SGLT2 inhibitor canagliflozin (Invokana), compared with people with higher levels of renal function? The metric for both hypotheses was change in A1c levels from baseline.
The study included 525 adults with type 2 diabetes in a double-blind, three-way crossover trial that assigned each participant a random order of serial 16-week trials of treatment with sitagliptin 100 mg once daily, canagliflozin 100 mg once daily, and pioglitazone 30 mg once daily, with each agent added to the preexisting background regimen.
Analysis showed that for second- or third-line therapy in people with type 2 diabetes “simple predefined stratification using BMI and renal function can determine the choice of the drug most likely to be effective for glucose lowering,” the researchers concluded.
Among those with a BMI of more than 30 kg/m2, patients achieved a lower A1c on pioglitazone, compared with sitagliptin, while those with a lower BMI had their best A1c response on sitagliptin. Patients with impaired renal function (eGFR 60-90 mL/min/1.73 m2) had better A1c lowering with sitagliptin, while those with a higher eGFR had better A1c lowering with canagliflozin.
These results appeared in a second article published in Nature Medicine, and the researchers also presented these findings at the EASD 2021 annual meeting, as reported by this news organization at the time.
Patients identified the agent they liked best
Dr. Hattersley and associates used the TriMaster study to also address the secondary question of which of the three tested agents patients preferred, focusing on the 457 patients who provided information on their treatment preference.
The results showed that patient preference varied: Twenty-four percent liked pioglitazone best, 33% preferred sitagliptin, and 37% said canagliflozin was their favorite, with 6% having no preference. These numbers barely budged when participants learned how well each agent worked for them in terms of reducing their A1c and lowering their BMI.
The findings also showed good correlation between patient preferences and their A1c and adverse-effect responses. The agents that patients identified as their favorites were also the drugs that lowered their A1c the most 53% of the time before they got any feedback on which one gave them their best glycemic control. Once they had this feedback, 70% preferred the most effective agent, with the results likely reflecting that patients feel better when they have improved glucose levels as well as the education patients received that lower A1c levels are better.
Patients also tended to understandably favor the agents that caused the fewest and mildest adverse effects: Sixty-eight percent of the patients who identified a favorite drug picked the one that gave them the best adverse-effect profile.
In an interview at the EASD 2022 annual meeting, Dr. Hattersley promoted the study’s design as a best-practice approach to deciding which drug to next give a person with type 2 diabetes who needs additional glycemic control.
“Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects,” he said. “Patients had overwhelming positivity about being able to choose their drug. Do it when you’re not certain which drug to prescribe,” suggested Dr. Hattersley, a professor and diabetologist at the University of Exeter, England. “We can’t know which drug a patient might prefer.”
But he stressed cautioning patients to return for treatment adjustment sooner than 4 months if they can’t tolerate a new drug they’re trying.
TriMaster received no commercial funding. Dr. Hattersley has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Allowing people with type 2 diabetes to try agents from three different classes of antidiabetes drugs showed they usually find a clear preference, often the drug that gives them the best glycemic control and least bothersome adverse effects, according to secondary findings from a randomized study of patients in the United Kingdom.
“This is the first study in which the same patient has tried three different types of glucose-lowering drug, enabling them to directly compare them and then choose which one is best for them,” Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in a written statement. “We’ve shown that going with the patients’ choice results in better glucose control and fewer side effects than any other approach. When it’s not clear which drug is best to use, then patients should try before they choose. Surprisingly, that approach has never been tried before.”
These secondary results from the TriMaster study were recently published in Nature Medicine and presented at the annual meeting of the European Association for the Study of Diabetes (EASD) in September, as reported by this news organization.
TriMaster enrolled adults aged 30-80 years with a clinical diagnosis of type 2 diabetes for at least 12 months. Their glycemia was inadequately controlled despite treatment with metformin alone or two classes of oral glucose-lowering therapy that did not include an agent from any of the three classes tested in the study: dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and thiazolidinediones. The people taking two different drug classes at entry were most often taking metformin and a sulfonylurea.
Do BMI and renal function affect treatment response?
TriMaster tested two hypotheses. Firstly, would people with a body mass index of more than 30 kg/m2 have greater glucose lowering with the thiazolidinedione pioglitazone (Actos) than with the DPP-4 inhibitor sitagliptin (Januvia), compared to people with a lower BMI?
Secondly, would people with an estimated glomerular filtration rate (eGFR) of 60-90 mL/min/1.73 m2 have greater glucose lowering with sitagliptin than with the SGLT2 inhibitor canagliflozin (Invokana), compared with people with higher levels of renal function? The metric for both hypotheses was change in A1c levels from baseline.
The study included 525 adults with type 2 diabetes in a double-blind, three-way crossover trial that assigned each participant a random order of serial 16-week trials of treatment with sitagliptin 100 mg once daily, canagliflozin 100 mg once daily, and pioglitazone 30 mg once daily, with each agent added to the preexisting background regimen.
Analysis showed that for second- or third-line therapy in people with type 2 diabetes “simple predefined stratification using BMI and renal function can determine the choice of the drug most likely to be effective for glucose lowering,” the researchers concluded.
Among those with a BMI of more than 30 kg/m2, patients achieved a lower A1c on pioglitazone, compared with sitagliptin, while those with a lower BMI had their best A1c response on sitagliptin. Patients with impaired renal function (eGFR 60-90 mL/min/1.73 m2) had better A1c lowering with sitagliptin, while those with a higher eGFR had better A1c lowering with canagliflozin.
These results appeared in a second article published in Nature Medicine, and the researchers also presented these findings at the EASD 2021 annual meeting, as reported by this news organization at the time.
Patients identified the agent they liked best
Dr. Hattersley and associates used the TriMaster study to also address the secondary question of which of the three tested agents patients preferred, focusing on the 457 patients who provided information on their treatment preference.
The results showed that patient preference varied: Twenty-four percent liked pioglitazone best, 33% preferred sitagliptin, and 37% said canagliflozin was their favorite, with 6% having no preference. These numbers barely budged when participants learned how well each agent worked for them in terms of reducing their A1c and lowering their BMI.
The findings also showed good correlation between patient preferences and their A1c and adverse-effect responses. The agents that patients identified as their favorites were also the drugs that lowered their A1c the most 53% of the time before they got any feedback on which one gave them their best glycemic control. Once they had this feedback, 70% preferred the most effective agent, with the results likely reflecting that patients feel better when they have improved glucose levels as well as the education patients received that lower A1c levels are better.
Patients also tended to understandably favor the agents that caused the fewest and mildest adverse effects: Sixty-eight percent of the patients who identified a favorite drug picked the one that gave them the best adverse-effect profile.
In an interview at the EASD 2022 annual meeting, Dr. Hattersley promoted the study’s design as a best-practice approach to deciding which drug to next give a person with type 2 diabetes who needs additional glycemic control.
“Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects,” he said. “Patients had overwhelming positivity about being able to choose their drug. Do it when you’re not certain which drug to prescribe,” suggested Dr. Hattersley, a professor and diabetologist at the University of Exeter, England. “We can’t know which drug a patient might prefer.”
But he stressed cautioning patients to return for treatment adjustment sooner than 4 months if they can’t tolerate a new drug they’re trying.
TriMaster received no commercial funding. Dr. Hattersley has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Allowing people with type 2 diabetes to try agents from three different classes of antidiabetes drugs showed they usually find a clear preference, often the drug that gives them the best glycemic control and least bothersome adverse effects, according to secondary findings from a randomized study of patients in the United Kingdom.
“This is the first study in which the same patient has tried three different types of glucose-lowering drug, enabling them to directly compare them and then choose which one is best for them,” Andrew Hattersley, BMBCh, DM, the study’s principal investigator, said in a written statement. “We’ve shown that going with the patients’ choice results in better glucose control and fewer side effects than any other approach. When it’s not clear which drug is best to use, then patients should try before they choose. Surprisingly, that approach has never been tried before.”
These secondary results from the TriMaster study were recently published in Nature Medicine and presented at the annual meeting of the European Association for the Study of Diabetes (EASD) in September, as reported by this news organization.
TriMaster enrolled adults aged 30-80 years with a clinical diagnosis of type 2 diabetes for at least 12 months. Their glycemia was inadequately controlled despite treatment with metformin alone or two classes of oral glucose-lowering therapy that did not include an agent from any of the three classes tested in the study: dipeptidyl peptidase–4 (DPP-4) inhibitors, sodium-glucose cotransporter 2 (SGLT2) inhibitors, and thiazolidinediones. The people taking two different drug classes at entry were most often taking metformin and a sulfonylurea.
Do BMI and renal function affect treatment response?
TriMaster tested two hypotheses. Firstly, would people with a body mass index of more than 30 kg/m2 have greater glucose lowering with the thiazolidinedione pioglitazone (Actos) than with the DPP-4 inhibitor sitagliptin (Januvia), compared to people with a lower BMI?
Secondly, would people with an estimated glomerular filtration rate (eGFR) of 60-90 mL/min/1.73 m2 have greater glucose lowering with sitagliptin than with the SGLT2 inhibitor canagliflozin (Invokana), compared with people with higher levels of renal function? The metric for both hypotheses was change in A1c levels from baseline.
The study included 525 adults with type 2 diabetes in a double-blind, three-way crossover trial that assigned each participant a random order of serial 16-week trials of treatment with sitagliptin 100 mg once daily, canagliflozin 100 mg once daily, and pioglitazone 30 mg once daily, with each agent added to the preexisting background regimen.
Analysis showed that for second- or third-line therapy in people with type 2 diabetes “simple predefined stratification using BMI and renal function can determine the choice of the drug most likely to be effective for glucose lowering,” the researchers concluded.
Among those with a BMI of more than 30 kg/m2, patients achieved a lower A1c on pioglitazone, compared with sitagliptin, while those with a lower BMI had their best A1c response on sitagliptin. Patients with impaired renal function (eGFR 60-90 mL/min/1.73 m2) had better A1c lowering with sitagliptin, while those with a higher eGFR had better A1c lowering with canagliflozin.
These results appeared in a second article published in Nature Medicine, and the researchers also presented these findings at the EASD 2021 annual meeting, as reported by this news organization at the time.
Patients identified the agent they liked best
Dr. Hattersley and associates used the TriMaster study to also address the secondary question of which of the three tested agents patients preferred, focusing on the 457 patients who provided information on their treatment preference.
The results showed that patient preference varied: Twenty-four percent liked pioglitazone best, 33% preferred sitagliptin, and 37% said canagliflozin was their favorite, with 6% having no preference. These numbers barely budged when participants learned how well each agent worked for them in terms of reducing their A1c and lowering their BMI.
The findings also showed good correlation between patient preferences and their A1c and adverse-effect responses. The agents that patients identified as their favorites were also the drugs that lowered their A1c the most 53% of the time before they got any feedback on which one gave them their best glycemic control. Once they had this feedback, 70% preferred the most effective agent, with the results likely reflecting that patients feel better when they have improved glucose levels as well as the education patients received that lower A1c levels are better.
Patients also tended to understandably favor the agents that caused the fewest and mildest adverse effects: Sixty-eight percent of the patients who identified a favorite drug picked the one that gave them the best adverse-effect profile.
In an interview at the EASD 2022 annual meeting, Dr. Hattersley promoted the study’s design as a best-practice approach to deciding which drug to next give a person with type 2 diabetes who needs additional glycemic control.
“Whenever you’re not sure how to balance adverse effects and positive effects the best person to decide is the one who experiences the effects,” he said. “Patients had overwhelming positivity about being able to choose their drug. Do it when you’re not certain which drug to prescribe,” suggested Dr. Hattersley, a professor and diabetologist at the University of Exeter, England. “We can’t know which drug a patient might prefer.”
But he stressed cautioning patients to return for treatment adjustment sooner than 4 months if they can’t tolerate a new drug they’re trying.
TriMaster received no commercial funding. Dr. Hattersley has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New AHA statement on complementary medicine in heart failure
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
FDA okays Dexcom G7 continuous glucose monitoring system
The U.S. Food and Drug Administration has cleared the Dexcom G7 continuous glucose monitoring (CGM) system for people with all types of diabetes aged 2 years and older and for use during pregnancy.
The G7 has several improvements over the current G6 model, including a 60% smaller size, a 30-minute warm-up period (compared with 2 hours), an all-in-one sensor and transmitter (as opposed to the two separate devices), a mean absolute relative difference (compared with a standard, an assessment of accuracy) of 8.2% (compared with 12.8%), a 12-hour grace period (in contrast to the G6’s hard shutoff), and a redesigned mobile app.
It is indicated for wear on the back of the upper arm for people aged 2 years and older or the upper buttocks for ages 2-17 years old.
As an “integrated” CGM, the G7 has the capacity to work as part of automated insulin delivery systems, but that will require further FDA action. “Dexcom is working closely with its insulin pump partners to integrate Dexcom G7 into current and future automated insulin delivery systems as quickly as possible,” the company said in a statement.
Like the G6, it requires no fingersticks, scanning, or calibration. It provides real-time glucose readings every 5 minutes to a compatible device, including Apple Watch and other digital health apps, and allows for remote monitoring of data by as many as 10 followers.
Dexcom expects to initiate a U.S. launch of Dexcom G7 in early 2023. To facilitate immediate access to G7 for as many users as possible, the company will have accessible cash pay options in place as the company transitions coverage with availability for G7, the statement says.
The Dexcom G7 was granted a CE Mark (Conformité Européenne) in March 2022, which means it is approved for use in people with diabetes aged 2 years and older, including pregnant women, in Europe.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has cleared the Dexcom G7 continuous glucose monitoring (CGM) system for people with all types of diabetes aged 2 years and older and for use during pregnancy.
The G7 has several improvements over the current G6 model, including a 60% smaller size, a 30-minute warm-up period (compared with 2 hours), an all-in-one sensor and transmitter (as opposed to the two separate devices), a mean absolute relative difference (compared with a standard, an assessment of accuracy) of 8.2% (compared with 12.8%), a 12-hour grace period (in contrast to the G6’s hard shutoff), and a redesigned mobile app.
It is indicated for wear on the back of the upper arm for people aged 2 years and older or the upper buttocks for ages 2-17 years old.
As an “integrated” CGM, the G7 has the capacity to work as part of automated insulin delivery systems, but that will require further FDA action. “Dexcom is working closely with its insulin pump partners to integrate Dexcom G7 into current and future automated insulin delivery systems as quickly as possible,” the company said in a statement.
Like the G6, it requires no fingersticks, scanning, or calibration. It provides real-time glucose readings every 5 minutes to a compatible device, including Apple Watch and other digital health apps, and allows for remote monitoring of data by as many as 10 followers.
Dexcom expects to initiate a U.S. launch of Dexcom G7 in early 2023. To facilitate immediate access to G7 for as many users as possible, the company will have accessible cash pay options in place as the company transitions coverage with availability for G7, the statement says.
The Dexcom G7 was granted a CE Mark (Conformité Européenne) in March 2022, which means it is approved for use in people with diabetes aged 2 years and older, including pregnant women, in Europe.
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration has cleared the Dexcom G7 continuous glucose monitoring (CGM) system for people with all types of diabetes aged 2 years and older and for use during pregnancy.
The G7 has several improvements over the current G6 model, including a 60% smaller size, a 30-minute warm-up period (compared with 2 hours), an all-in-one sensor and transmitter (as opposed to the two separate devices), a mean absolute relative difference (compared with a standard, an assessment of accuracy) of 8.2% (compared with 12.8%), a 12-hour grace period (in contrast to the G6’s hard shutoff), and a redesigned mobile app.
It is indicated for wear on the back of the upper arm for people aged 2 years and older or the upper buttocks for ages 2-17 years old.
As an “integrated” CGM, the G7 has the capacity to work as part of automated insulin delivery systems, but that will require further FDA action. “Dexcom is working closely with its insulin pump partners to integrate Dexcom G7 into current and future automated insulin delivery systems as quickly as possible,” the company said in a statement.
Like the G6, it requires no fingersticks, scanning, or calibration. It provides real-time glucose readings every 5 minutes to a compatible device, including Apple Watch and other digital health apps, and allows for remote monitoring of data by as many as 10 followers.
Dexcom expects to initiate a U.S. launch of Dexcom G7 in early 2023. To facilitate immediate access to G7 for as many users as possible, the company will have accessible cash pay options in place as the company transitions coverage with availability for G7, the statement says.
The Dexcom G7 was granted a CE Mark (Conformité Européenne) in March 2022, which means it is approved for use in people with diabetes aged 2 years and older, including pregnant women, in Europe.
A version of this article first appeared on Medscape.com.
Statins tied to lower ICH risk regardless of bleed location
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
‘Clear answer’: ALL study defies conventional wisdom
The same study also addressed two other issues related to standard care for these patients: 1) the dosage of dexamethasone used during the first treatment phase (results of which had already been reported some years ago) and 2) the impact of omitting monthly pulses of dexamethasone and vincristine after initial treatment.
“The trial did not give us the answers we were looking for, but that’s why we do randomized trials, and at least we have one clear answer, which is that high-dose methotrexate does not seem to have benefit in reducing the risk of CNS relapse,” reported study investigator Ajay Vora, MSc, from Great Ormond Street Hospital, London.
Among 1,570 patients randomly assigned in one group of the UKALL2011 trial, 5-year rates of CNS relapse were identical at 5.6% for patients treated with either high-dose methotrexate or standard interim maintenance with oral mercaptopurine and oral and intrathecal methotrexate.
There was a hint, however, that high-dose methotrexate could have a beneficial effect by reducing relapses in bone marrow for some subgroups of patients with B-lineage disease after dexamethasone induction, Dr. Vora commented.
He was speaking at a press briefing at the annual meeting of the American Society of Hematology, prior to the presentation of the data by Amy A. Kirkwood, MSc, from the University College London Cancer Institute.
Reacting to the results, Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., emphasized that “in patients treated with the UKALL regimen, high doses of methotrexate did not reduce the rate of CNS relapse, contrary to our long-standing beliefs.”
“Going forward, patients can be spared the risk of high-dose methotrexate without increasing their risk of recurrence in the central nervous system,” she said.
“As researchers in hematology, we look at it as our duty to question the standard approaches that we use to treat patients, even those that we thought of as tried-and-true,” said briefing moderator Mikkael Sekeres, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami. This is one of the abstracts that “challenge some of those standards and in fact reveal that in many cases, giving less therapy and being less restrictive is actually better for patients or at least no worse.”
Complex design
The UKALL2011 trial had a byzantine design, with the overarching goal of finding out which treatment and maintenance strategy best finds the sweet spot between efficacy and toxicity in children and young adults (up to age 25) with ALL and lymphoblastic lymphoma.
One question that was already answered, as investigators reported at the 2017 ASH annual meeting, came from the first randomization in the study, designed to see whether a shorter course of dexamethasone – 14 days versus the standard 28 days – could reduce induction toxicity. It did not.
Now, at ASH 2022, the investigators reported outcomes from the second phase of the trial, which included two randomizations: one comparing high-dose methotrexate with standard interim maintenance to reduce CNS relapse risk, and one to see whether forgoing pulses of vincristine/dexamethasone could reduce maintenance morbidity.
Patients were stratified by National Cancer Institute minimal residual disease (MRD) risk categories, cytogenetics, and end-of-induction MRD to receive one of three treatment regimens. Patients with MRD high risk, defined as MRD greater than 0.5% at the end of consolidation, were not eligible for second-phase randomization and instead received off-protocol therapies.The second randomization was factorial, stratified by NCI and MRD risk groups, resulting in four arms: high-dose methotrexate with or without pulses and standard interim maintenance with our without pulses.
Standard interim maintenance in this trial was 2 months of oral mercaptopurine/methotrexate monthly pulses and single intrathecal methotrexate in two of the regimens, as well as five doses of escalating intravenous methotrexate plus vincristine and two doses of pegylated asparaginase in the third.
High-dose methotrexate was given at a dose of 5 g/m2 for four doses 2 weeks apart, low dose 6-mercaptopurine, plus two doses of pegylated asparaginase in one regimen only.
Equivocal conclusions
As noted above, CNS relapse, the primary endpoint for the interim maintenance randomization, did not differ between the groups, with identical 5-year relapse rates. Similarly, 5-year event-free survival (EFS) rates were 90.3% in the high-dose group and 89.5% in the standard group, a difference that was not statistically significant (P = .68).
There was, however, an interaction between the first (short- vs. standard-course dexamethasone) and the interim maintenance randomizations, indicating significantly inferior EFS outcomes for patients who had received the short dose of dexamethasone followed by high-dose methotrexate, especially among patients who did not receive pulses (P = .006).
An analysis of patients treated with standard dexamethasone showed that those who received high-dose methotrexate had a lower risk for bone marrow relapse, with a hazard ratio of 0.62 (P = .029), and trends, albeit nonsignificant, toward better EFS and overall survival.
In addition, the overall results suggested that steroid pulses could be safely omitted without leading to an increase in bone marrow relapses: the 5-year rates of bone marrow relapse were 10.2% with pulses and 12.2% without, although omitting pulses was associated with a slight but significant decrease in EFS overall (P = .01). The effect was attenuated among patients who had received standard-course dexamethasone and high-dose methotrexate. Leaving out the pulses also reduced rates of grade 3 or 4 adverse events, including febrile neutropenia, Ms. Kirkwood noted in her presentation.
The investigators plan to analyze quality-of-life outcomes related to dexamethasone-vincristine pulses to see whether doing so could tip the balance in favor of leaving them out of therapy, and they will continue to follow patients to see whether their findings hold.
UKALL2011 was funded by Children with Cancer UK, Blood Cancer UK, and Cancer Research UK. Ms. Kirkwood disclosed consulting for and receiving honoraria from Kite. Dr. Vora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The same study also addressed two other issues related to standard care for these patients: 1) the dosage of dexamethasone used during the first treatment phase (results of which had already been reported some years ago) and 2) the impact of omitting monthly pulses of dexamethasone and vincristine after initial treatment.
“The trial did not give us the answers we were looking for, but that’s why we do randomized trials, and at least we have one clear answer, which is that high-dose methotrexate does not seem to have benefit in reducing the risk of CNS relapse,” reported study investigator Ajay Vora, MSc, from Great Ormond Street Hospital, London.
Among 1,570 patients randomly assigned in one group of the UKALL2011 trial, 5-year rates of CNS relapse were identical at 5.6% for patients treated with either high-dose methotrexate or standard interim maintenance with oral mercaptopurine and oral and intrathecal methotrexate.
There was a hint, however, that high-dose methotrexate could have a beneficial effect by reducing relapses in bone marrow for some subgroups of patients with B-lineage disease after dexamethasone induction, Dr. Vora commented.
He was speaking at a press briefing at the annual meeting of the American Society of Hematology, prior to the presentation of the data by Amy A. Kirkwood, MSc, from the University College London Cancer Institute.
Reacting to the results, Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., emphasized that “in patients treated with the UKALL regimen, high doses of methotrexate did not reduce the rate of CNS relapse, contrary to our long-standing beliefs.”
“Going forward, patients can be spared the risk of high-dose methotrexate without increasing their risk of recurrence in the central nervous system,” she said.
“As researchers in hematology, we look at it as our duty to question the standard approaches that we use to treat patients, even those that we thought of as tried-and-true,” said briefing moderator Mikkael Sekeres, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami. This is one of the abstracts that “challenge some of those standards and in fact reveal that in many cases, giving less therapy and being less restrictive is actually better for patients or at least no worse.”
Complex design
The UKALL2011 trial had a byzantine design, with the overarching goal of finding out which treatment and maintenance strategy best finds the sweet spot between efficacy and toxicity in children and young adults (up to age 25) with ALL and lymphoblastic lymphoma.
One question that was already answered, as investigators reported at the 2017 ASH annual meeting, came from the first randomization in the study, designed to see whether a shorter course of dexamethasone – 14 days versus the standard 28 days – could reduce induction toxicity. It did not.
Now, at ASH 2022, the investigators reported outcomes from the second phase of the trial, which included two randomizations: one comparing high-dose methotrexate with standard interim maintenance to reduce CNS relapse risk, and one to see whether forgoing pulses of vincristine/dexamethasone could reduce maintenance morbidity.
Patients were stratified by National Cancer Institute minimal residual disease (MRD) risk categories, cytogenetics, and end-of-induction MRD to receive one of three treatment regimens. Patients with MRD high risk, defined as MRD greater than 0.5% at the end of consolidation, were not eligible for second-phase randomization and instead received off-protocol therapies.The second randomization was factorial, stratified by NCI and MRD risk groups, resulting in four arms: high-dose methotrexate with or without pulses and standard interim maintenance with our without pulses.
Standard interim maintenance in this trial was 2 months of oral mercaptopurine/methotrexate monthly pulses and single intrathecal methotrexate in two of the regimens, as well as five doses of escalating intravenous methotrexate plus vincristine and two doses of pegylated asparaginase in the third.
High-dose methotrexate was given at a dose of 5 g/m2 for four doses 2 weeks apart, low dose 6-mercaptopurine, plus two doses of pegylated asparaginase in one regimen only.
Equivocal conclusions
As noted above, CNS relapse, the primary endpoint for the interim maintenance randomization, did not differ between the groups, with identical 5-year relapse rates. Similarly, 5-year event-free survival (EFS) rates were 90.3% in the high-dose group and 89.5% in the standard group, a difference that was not statistically significant (P = .68).
There was, however, an interaction between the first (short- vs. standard-course dexamethasone) and the interim maintenance randomizations, indicating significantly inferior EFS outcomes for patients who had received the short dose of dexamethasone followed by high-dose methotrexate, especially among patients who did not receive pulses (P = .006).
An analysis of patients treated with standard dexamethasone showed that those who received high-dose methotrexate had a lower risk for bone marrow relapse, with a hazard ratio of 0.62 (P = .029), and trends, albeit nonsignificant, toward better EFS and overall survival.
In addition, the overall results suggested that steroid pulses could be safely omitted without leading to an increase in bone marrow relapses: the 5-year rates of bone marrow relapse were 10.2% with pulses and 12.2% without, although omitting pulses was associated with a slight but significant decrease in EFS overall (P = .01). The effect was attenuated among patients who had received standard-course dexamethasone and high-dose methotrexate. Leaving out the pulses also reduced rates of grade 3 or 4 adverse events, including febrile neutropenia, Ms. Kirkwood noted in her presentation.
The investigators plan to analyze quality-of-life outcomes related to dexamethasone-vincristine pulses to see whether doing so could tip the balance in favor of leaving them out of therapy, and they will continue to follow patients to see whether their findings hold.
UKALL2011 was funded by Children with Cancer UK, Blood Cancer UK, and Cancer Research UK. Ms. Kirkwood disclosed consulting for and receiving honoraria from Kite. Dr. Vora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The same study also addressed two other issues related to standard care for these patients: 1) the dosage of dexamethasone used during the first treatment phase (results of which had already been reported some years ago) and 2) the impact of omitting monthly pulses of dexamethasone and vincristine after initial treatment.
“The trial did not give us the answers we were looking for, but that’s why we do randomized trials, and at least we have one clear answer, which is that high-dose methotrexate does not seem to have benefit in reducing the risk of CNS relapse,” reported study investigator Ajay Vora, MSc, from Great Ormond Street Hospital, London.
Among 1,570 patients randomly assigned in one group of the UKALL2011 trial, 5-year rates of CNS relapse were identical at 5.6% for patients treated with either high-dose methotrexate or standard interim maintenance with oral mercaptopurine and oral and intrathecal methotrexate.
There was a hint, however, that high-dose methotrexate could have a beneficial effect by reducing relapses in bone marrow for some subgroups of patients with B-lineage disease after dexamethasone induction, Dr. Vora commented.
He was speaking at a press briefing at the annual meeting of the American Society of Hematology, prior to the presentation of the data by Amy A. Kirkwood, MSc, from the University College London Cancer Institute.
Reacting to the results, Cynthia E. Dunbar, MD, chief of the Translational Stem Cell Biology Branch at the National Heart, Lung, and Blood Institute in Bethesda, Md., emphasized that “in patients treated with the UKALL regimen, high doses of methotrexate did not reduce the rate of CNS relapse, contrary to our long-standing beliefs.”
“Going forward, patients can be spared the risk of high-dose methotrexate without increasing their risk of recurrence in the central nervous system,” she said.
“As researchers in hematology, we look at it as our duty to question the standard approaches that we use to treat patients, even those that we thought of as tried-and-true,” said briefing moderator Mikkael Sekeres, MD, of the Sylvester Comprehensive Cancer Center at the University of Miami. This is one of the abstracts that “challenge some of those standards and in fact reveal that in many cases, giving less therapy and being less restrictive is actually better for patients or at least no worse.”
Complex design
The UKALL2011 trial had a byzantine design, with the overarching goal of finding out which treatment and maintenance strategy best finds the sweet spot between efficacy and toxicity in children and young adults (up to age 25) with ALL and lymphoblastic lymphoma.
One question that was already answered, as investigators reported at the 2017 ASH annual meeting, came from the first randomization in the study, designed to see whether a shorter course of dexamethasone – 14 days versus the standard 28 days – could reduce induction toxicity. It did not.
Now, at ASH 2022, the investigators reported outcomes from the second phase of the trial, which included two randomizations: one comparing high-dose methotrexate with standard interim maintenance to reduce CNS relapse risk, and one to see whether forgoing pulses of vincristine/dexamethasone could reduce maintenance morbidity.
Patients were stratified by National Cancer Institute minimal residual disease (MRD) risk categories, cytogenetics, and end-of-induction MRD to receive one of three treatment regimens. Patients with MRD high risk, defined as MRD greater than 0.5% at the end of consolidation, were not eligible for second-phase randomization and instead received off-protocol therapies.The second randomization was factorial, stratified by NCI and MRD risk groups, resulting in four arms: high-dose methotrexate with or without pulses and standard interim maintenance with our without pulses.
Standard interim maintenance in this trial was 2 months of oral mercaptopurine/methotrexate monthly pulses and single intrathecal methotrexate in two of the regimens, as well as five doses of escalating intravenous methotrexate plus vincristine and two doses of pegylated asparaginase in the third.
High-dose methotrexate was given at a dose of 5 g/m2 for four doses 2 weeks apart, low dose 6-mercaptopurine, plus two doses of pegylated asparaginase in one regimen only.
Equivocal conclusions
As noted above, CNS relapse, the primary endpoint for the interim maintenance randomization, did not differ between the groups, with identical 5-year relapse rates. Similarly, 5-year event-free survival (EFS) rates were 90.3% in the high-dose group and 89.5% in the standard group, a difference that was not statistically significant (P = .68).
There was, however, an interaction between the first (short- vs. standard-course dexamethasone) and the interim maintenance randomizations, indicating significantly inferior EFS outcomes for patients who had received the short dose of dexamethasone followed by high-dose methotrexate, especially among patients who did not receive pulses (P = .006).
An analysis of patients treated with standard dexamethasone showed that those who received high-dose methotrexate had a lower risk for bone marrow relapse, with a hazard ratio of 0.62 (P = .029), and trends, albeit nonsignificant, toward better EFS and overall survival.
In addition, the overall results suggested that steroid pulses could be safely omitted without leading to an increase in bone marrow relapses: the 5-year rates of bone marrow relapse were 10.2% with pulses and 12.2% without, although omitting pulses was associated with a slight but significant decrease in EFS overall (P = .01). The effect was attenuated among patients who had received standard-course dexamethasone and high-dose methotrexate. Leaving out the pulses also reduced rates of grade 3 or 4 adverse events, including febrile neutropenia, Ms. Kirkwood noted in her presentation.
The investigators plan to analyze quality-of-life outcomes related to dexamethasone-vincristine pulses to see whether doing so could tip the balance in favor of leaving them out of therapy, and they will continue to follow patients to see whether their findings hold.
UKALL2011 was funded by Children with Cancer UK, Blood Cancer UK, and Cancer Research UK. Ms. Kirkwood disclosed consulting for and receiving honoraria from Kite. Dr. Vora reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASH 2022
‘Astonishing’ results: Skip salvage chemo, proceed to HSCT
NEW ORLEANS –
The results come from the phase 3 ASAP Trial and were presented at the annual meeting of the American Society of Hematology.
“We selected this to be in the plenary because it completely changes how we’ve traditionally thought about acute myeloid leukemia,” commented press briefing moderator Mikkael A. Sekeres, MD, from the University of Miami, who also serves as chair of the ASH Committee on Communications.
“When we have a patient who has relapsed or refractory AML, that person is in a very, very difficult situation, and the mortality among those sort of patients is incredibly high,” Dr. Sekeres commented. “So traditionally we’ve given them very high doses of chemotherapy to try to reduce the tumor burden – at least that’s been the theory – to then get them successfully to a transplant.”
This new finding “completely upends that, if these results hold,” he said. The clinical implication is that “we no longer have to hospitalize these patients and give them very aggressive chemotherapy ... [and] we don’t introduce all the morbidity from giving them very high dose chemotherapy, which can actually prevent a transplant from happening if they get sick enough, and we can get them to a transplant quicker.”
The ASAP trial was conducted in patients with an unfavorable risk AML who either had a poor response to first induction therapy or a relapse after first induction therapy.
They were randomly assigned to either a remission-induction strategy aiming for a better response prior to an allogeneic hematopoietic stem cell transplant (alloHCT), or a disease-control strategy consisting primarily of watchful waiting with low-dose cytarabine and single doses of mitoxantrone as needed, followed by sequential conditioning and alloHCT.
The results after 4 years of follow-up showed no differences in either leukemia-free survival or overall survival between patients who underwent additional chemotherapy with the remission-induction strategy and those who went straight to transplant, reported Johannes Schetelig, MD, MSc, from the Clinical Trials Unit at DKMS, Dresden, Germany.
“We expected non-inferiority – this was what we tested, and of course this was based on an assumption that we could get close or even somewhat better with respect to the primary endpoint, disease-free survival, after transplantation,” he said.
“What we did not expect is that the early success, [complete response] on day 56 after transplantation, also translates into equal long-term benefit, so this is what I was really astonished about,” Dr. Schetelig said at a press briefing prior to his presentation.
Less intensive approach
Dr. Schetelig explained that the rationale for the study was previous work by his group and others showing that alloHCT in patients with residual aplasia after first induction is feasible, with favorable outcomes, compared with standard of care. Additionally, the impetus for the research was evidence that sequential conditioning based on high-dose cytarabine or melphalan plus reduced-intensity conditioning and alloHCT resulted in long-term control for relapsed or refractory AML.
Dr. Schetelig also gave details of the two treatment arms of the ASAP trial. The remission-inducing arm consisted of cytarabine (3 g/m2 for younger patients or 1 g/m2 for patients over age 60) twice daily on days 1-3 plus 10 mitoxantrone mg/m2 on days 3-5 and subsequent alloHCT. In the other group – disease control prior to sequential conditioning and alloHCT – watchful waiting was recommended, but low-dose cytarabine (LDAC) and single doses of mitoxantrone were permitted for disease control.
Although, as Dr. Schetelig noted, the statistical goal of the study was to show non-inferiority of the disease control arm, this less intensive strategy exceeded expectations for meeting the primary endpoint of disease-free survival (DFS; a maintained complete response) by day 56 after alloHCT.
In an intention-to-treat and per-protocol analysis, the respective rates of DFS at 56 days in the disease control group were 83.5% and 84.1%. In comparison, the respective rates in the remission-induction group were 81% and 81.3%.
Further, after a median follow-up from randomization of 37 months, there were no differences in either leukemia-free survival or overall survival out to 4 years after DFS at day 56.
The disease-control strategy was also associated with significantly fewer adverse events grade 3 or greater (23% vs. 64%, P < .001), and fewer days in hospital prior to transplant (mean 19 vs. 42, P < .001). There were no significant differences between the trial arms in either deaths within 28 days of randomization or time to discharge from hospital (28 days in each arm).
“These data support sequential conditioning and alloHCT without prior remission-induction chemotherapy whenever a stem cell donor is readily available,” the researchers concluded.
“These results underline the importance of facilitating alloHCT as [the] most effective anti-leukemic therapy in patients with [relapsed or refractory] AML and stress the need for starting donor search at diagnosis,” they added.
The study was sponsored by DKMS gemeinnützige GmbH. Dr. Schetelig disclosed honoraria from BeiGene, BMS, Janssen, AstraZeneca, AbbVie, and DKMS. Dr. Sekkeres reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
The results come from the phase 3 ASAP Trial and were presented at the annual meeting of the American Society of Hematology.
“We selected this to be in the plenary because it completely changes how we’ve traditionally thought about acute myeloid leukemia,” commented press briefing moderator Mikkael A. Sekeres, MD, from the University of Miami, who also serves as chair of the ASH Committee on Communications.
“When we have a patient who has relapsed or refractory AML, that person is in a very, very difficult situation, and the mortality among those sort of patients is incredibly high,” Dr. Sekeres commented. “So traditionally we’ve given them very high doses of chemotherapy to try to reduce the tumor burden – at least that’s been the theory – to then get them successfully to a transplant.”
This new finding “completely upends that, if these results hold,” he said. The clinical implication is that “we no longer have to hospitalize these patients and give them very aggressive chemotherapy ... [and] we don’t introduce all the morbidity from giving them very high dose chemotherapy, which can actually prevent a transplant from happening if they get sick enough, and we can get them to a transplant quicker.”
The ASAP trial was conducted in patients with an unfavorable risk AML who either had a poor response to first induction therapy or a relapse after first induction therapy.
They were randomly assigned to either a remission-induction strategy aiming for a better response prior to an allogeneic hematopoietic stem cell transplant (alloHCT), or a disease-control strategy consisting primarily of watchful waiting with low-dose cytarabine and single doses of mitoxantrone as needed, followed by sequential conditioning and alloHCT.
The results after 4 years of follow-up showed no differences in either leukemia-free survival or overall survival between patients who underwent additional chemotherapy with the remission-induction strategy and those who went straight to transplant, reported Johannes Schetelig, MD, MSc, from the Clinical Trials Unit at DKMS, Dresden, Germany.
“We expected non-inferiority – this was what we tested, and of course this was based on an assumption that we could get close or even somewhat better with respect to the primary endpoint, disease-free survival, after transplantation,” he said.
“What we did not expect is that the early success, [complete response] on day 56 after transplantation, also translates into equal long-term benefit, so this is what I was really astonished about,” Dr. Schetelig said at a press briefing prior to his presentation.
Less intensive approach
Dr. Schetelig explained that the rationale for the study was previous work by his group and others showing that alloHCT in patients with residual aplasia after first induction is feasible, with favorable outcomes, compared with standard of care. Additionally, the impetus for the research was evidence that sequential conditioning based on high-dose cytarabine or melphalan plus reduced-intensity conditioning and alloHCT resulted in long-term control for relapsed or refractory AML.
Dr. Schetelig also gave details of the two treatment arms of the ASAP trial. The remission-inducing arm consisted of cytarabine (3 g/m2 for younger patients or 1 g/m2 for patients over age 60) twice daily on days 1-3 plus 10 mitoxantrone mg/m2 on days 3-5 and subsequent alloHCT. In the other group – disease control prior to sequential conditioning and alloHCT – watchful waiting was recommended, but low-dose cytarabine (LDAC) and single doses of mitoxantrone were permitted for disease control.
Although, as Dr. Schetelig noted, the statistical goal of the study was to show non-inferiority of the disease control arm, this less intensive strategy exceeded expectations for meeting the primary endpoint of disease-free survival (DFS; a maintained complete response) by day 56 after alloHCT.
In an intention-to-treat and per-protocol analysis, the respective rates of DFS at 56 days in the disease control group were 83.5% and 84.1%. In comparison, the respective rates in the remission-induction group were 81% and 81.3%.
Further, after a median follow-up from randomization of 37 months, there were no differences in either leukemia-free survival or overall survival out to 4 years after DFS at day 56.
The disease-control strategy was also associated with significantly fewer adverse events grade 3 or greater (23% vs. 64%, P < .001), and fewer days in hospital prior to transplant (mean 19 vs. 42, P < .001). There were no significant differences between the trial arms in either deaths within 28 days of randomization or time to discharge from hospital (28 days in each arm).
“These data support sequential conditioning and alloHCT without prior remission-induction chemotherapy whenever a stem cell donor is readily available,” the researchers concluded.
“These results underline the importance of facilitating alloHCT as [the] most effective anti-leukemic therapy in patients with [relapsed or refractory] AML and stress the need for starting donor search at diagnosis,” they added.
The study was sponsored by DKMS gemeinnützige GmbH. Dr. Schetelig disclosed honoraria from BeiGene, BMS, Janssen, AstraZeneca, AbbVie, and DKMS. Dr. Sekkeres reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NEW ORLEANS –
The results come from the phase 3 ASAP Trial and were presented at the annual meeting of the American Society of Hematology.
“We selected this to be in the plenary because it completely changes how we’ve traditionally thought about acute myeloid leukemia,” commented press briefing moderator Mikkael A. Sekeres, MD, from the University of Miami, who also serves as chair of the ASH Committee on Communications.
“When we have a patient who has relapsed or refractory AML, that person is in a very, very difficult situation, and the mortality among those sort of patients is incredibly high,” Dr. Sekeres commented. “So traditionally we’ve given them very high doses of chemotherapy to try to reduce the tumor burden – at least that’s been the theory – to then get them successfully to a transplant.”
This new finding “completely upends that, if these results hold,” he said. The clinical implication is that “we no longer have to hospitalize these patients and give them very aggressive chemotherapy ... [and] we don’t introduce all the morbidity from giving them very high dose chemotherapy, which can actually prevent a transplant from happening if they get sick enough, and we can get them to a transplant quicker.”
The ASAP trial was conducted in patients with an unfavorable risk AML who either had a poor response to first induction therapy or a relapse after first induction therapy.
They were randomly assigned to either a remission-induction strategy aiming for a better response prior to an allogeneic hematopoietic stem cell transplant (alloHCT), or a disease-control strategy consisting primarily of watchful waiting with low-dose cytarabine and single doses of mitoxantrone as needed, followed by sequential conditioning and alloHCT.
The results after 4 years of follow-up showed no differences in either leukemia-free survival or overall survival between patients who underwent additional chemotherapy with the remission-induction strategy and those who went straight to transplant, reported Johannes Schetelig, MD, MSc, from the Clinical Trials Unit at DKMS, Dresden, Germany.
“We expected non-inferiority – this was what we tested, and of course this was based on an assumption that we could get close or even somewhat better with respect to the primary endpoint, disease-free survival, after transplantation,” he said.
“What we did not expect is that the early success, [complete response] on day 56 after transplantation, also translates into equal long-term benefit, so this is what I was really astonished about,” Dr. Schetelig said at a press briefing prior to his presentation.
Less intensive approach
Dr. Schetelig explained that the rationale for the study was previous work by his group and others showing that alloHCT in patients with residual aplasia after first induction is feasible, with favorable outcomes, compared with standard of care. Additionally, the impetus for the research was evidence that sequential conditioning based on high-dose cytarabine or melphalan plus reduced-intensity conditioning and alloHCT resulted in long-term control for relapsed or refractory AML.
Dr. Schetelig also gave details of the two treatment arms of the ASAP trial. The remission-inducing arm consisted of cytarabine (3 g/m2 for younger patients or 1 g/m2 for patients over age 60) twice daily on days 1-3 plus 10 mitoxantrone mg/m2 on days 3-5 and subsequent alloHCT. In the other group – disease control prior to sequential conditioning and alloHCT – watchful waiting was recommended, but low-dose cytarabine (LDAC) and single doses of mitoxantrone were permitted for disease control.
Although, as Dr. Schetelig noted, the statistical goal of the study was to show non-inferiority of the disease control arm, this less intensive strategy exceeded expectations for meeting the primary endpoint of disease-free survival (DFS; a maintained complete response) by day 56 after alloHCT.
In an intention-to-treat and per-protocol analysis, the respective rates of DFS at 56 days in the disease control group were 83.5% and 84.1%. In comparison, the respective rates in the remission-induction group were 81% and 81.3%.
Further, after a median follow-up from randomization of 37 months, there were no differences in either leukemia-free survival or overall survival out to 4 years after DFS at day 56.
The disease-control strategy was also associated with significantly fewer adverse events grade 3 or greater (23% vs. 64%, P < .001), and fewer days in hospital prior to transplant (mean 19 vs. 42, P < .001). There were no significant differences between the trial arms in either deaths within 28 days of randomization or time to discharge from hospital (28 days in each arm).
“These data support sequential conditioning and alloHCT without prior remission-induction chemotherapy whenever a stem cell donor is readily available,” the researchers concluded.
“These results underline the importance of facilitating alloHCT as [the] most effective anti-leukemic therapy in patients with [relapsed or refractory] AML and stress the need for starting donor search at diagnosis,” they added.
The study was sponsored by DKMS gemeinnützige GmbH. Dr. Schetelig disclosed honoraria from BeiGene, BMS, Janssen, AstraZeneca, AbbVie, and DKMS. Dr. Sekkeres reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT ASH 2022
Post-transplant diet: Gruel no longer rules
NEW ORLEANS – A new Italian study yields more evidence that stem-cell transplant patients need not lose their appetites along with their immune systems. Low-bacterial, gruel-like diets, once the mainstay of immunity-lowering surgeries, don’t actually provide any protection against infections, researchers reported.
University of Miami hematologist Mikkael A. Sekeres, MD, MS, expressed delight to hear these findings.
“Around the world, we should eliminate these silly neutropenic diets, let people eat what they want, and give them a much better quality of life while they’re in the hospital,” said Dr. Sekeres, moderator of the news briefing where these research results were discussed at the annual meeting of the American Society of Hematology.
In recent decades, physicians and nutritionists have questioned the value of low-bacterial/neutropenic menus, designed to protect people with compromised immune systems from germs in food. These diets can be quite strict, outlawing food such as deli, processed, and cured meats; yogurt; hummus; strawberries and raspberries; lettuce; raw nuts; certain kinds of seafood; and herbs and spices such as pepper, unless they were cooked. Patients may be urged to avoid salad bars, buffets, and potlucks.
MD Anderson Cancer Center pediatrician Karen Moody, MD, MS, who has studied the diet, said in an interview that the diet has been around since the 1970s, despite a lack of evidence supporting it. “Cancer patients often suffer from treatment-related side effects that affect taste, appetite, and tolerance of food,” she said. “Further restricting food options in this population can be burdensome and reduce diet-related satisfaction.”
For the new multi-center, phase 3 study, researchers led by hematology resident Federico Stella, MD, of the University of Milan, randomly assigned consecutive adult patients undergoing hematopoietic stem cells transplantation or high-dose induction chemotherapy to either a low-bacterial diet (n = 224) or a non-restrictive diet (n = 224).
The low-bacterial diet emphasized food cooked to at least 176 degrees Fahrenheit and thick-skinned fruit. Raw fruits/vegetables, yogurt, honey, cold cuts and sausages, and raw fish and meat were forbidden.
The two groups were similar in age (median = 56 years), gender (about 57% male), and reason for admission (97% stem-cell transplants and 3% high-dose chemotherapy). The plan was to follow the patients for 100 days (stem-cell transplant recipients) or 30 days (high-dose chemotherapy patients).
No statistically significant differences between the group were found in the rates of infections of higher than Grade 2 (per CTCAE 4.0): These infections occurred in 38 (34%) of those on the low-bacterial diet and 44 (39%) of those on the non-restrictive diet (P = 0.5).
There were also no statistically significant differences in rates of fever of unknown origin (P = 0.2), sepsis (P = 0.5), and gastrointestinal infection (P = 0.7).
The findings show that the “use of a restrictive diet is an unnecessary burden for our patients’ quality of life,” said study lead author Dr. Stella at the news briefing.
Dr. Sekeres, the news briefing moderator, noted that the findings reflect his own suspicions about the worthlessness of the low-bacterial diet. “I’ve never seen a patient die of an infection that was foodborne. So years ago, when I was still in Cleveland, I eliminated the neutropenic diet on the leukemia floor. That did face a lot of resistance, as you can imagine. There were decades of people saying we should do this.”
Now, Dr. Sekeres said, he feels validated. “I love this study because it formalizes what I thought was true,” he said.
Dr. Moody said it’s difficult to evaluate the study since it’s in abstract form, and details are limited. “However,” she said, “the sample size, study design, and outcomes appear very appropriate, and I think most likely the full-length study will provide additional evidence to support abandonment of the low-microbial diet in transplant patients.”
Also, Dr. Moody said, the study “replicates the same findings of other prospective randomized trials of this diet that say it confers no protection from infection and has no identified health benefit whatsoever. Bottom line? This diet has burden without benefit.”
Moving forward, she said, “we need a lot more research on diet in general for cancer patients. Recently, there is a lot more interest in this topic. I think we are going to learn a lot in the next few decades about the relationship between diet, epigenetics, the microbiome, and various cancer-related health issues.”
No study funding was reported. Dr. Stella reports no disclosures, and other authors report various relationships with industry. Dr. Moody reports no disclosures, and disclosures for Dr. Sekeres were not available.
NEW ORLEANS – A new Italian study yields more evidence that stem-cell transplant patients need not lose their appetites along with their immune systems. Low-bacterial, gruel-like diets, once the mainstay of immunity-lowering surgeries, don’t actually provide any protection against infections, researchers reported.
University of Miami hematologist Mikkael A. Sekeres, MD, MS, expressed delight to hear these findings.
“Around the world, we should eliminate these silly neutropenic diets, let people eat what they want, and give them a much better quality of life while they’re in the hospital,” said Dr. Sekeres, moderator of the news briefing where these research results were discussed at the annual meeting of the American Society of Hematology.
In recent decades, physicians and nutritionists have questioned the value of low-bacterial/neutropenic menus, designed to protect people with compromised immune systems from germs in food. These diets can be quite strict, outlawing food such as deli, processed, and cured meats; yogurt; hummus; strawberries and raspberries; lettuce; raw nuts; certain kinds of seafood; and herbs and spices such as pepper, unless they were cooked. Patients may be urged to avoid salad bars, buffets, and potlucks.
MD Anderson Cancer Center pediatrician Karen Moody, MD, MS, who has studied the diet, said in an interview that the diet has been around since the 1970s, despite a lack of evidence supporting it. “Cancer patients often suffer from treatment-related side effects that affect taste, appetite, and tolerance of food,” she said. “Further restricting food options in this population can be burdensome and reduce diet-related satisfaction.”
For the new multi-center, phase 3 study, researchers led by hematology resident Federico Stella, MD, of the University of Milan, randomly assigned consecutive adult patients undergoing hematopoietic stem cells transplantation or high-dose induction chemotherapy to either a low-bacterial diet (n = 224) or a non-restrictive diet (n = 224).
The low-bacterial diet emphasized food cooked to at least 176 degrees Fahrenheit and thick-skinned fruit. Raw fruits/vegetables, yogurt, honey, cold cuts and sausages, and raw fish and meat were forbidden.
The two groups were similar in age (median = 56 years), gender (about 57% male), and reason for admission (97% stem-cell transplants and 3% high-dose chemotherapy). The plan was to follow the patients for 100 days (stem-cell transplant recipients) or 30 days (high-dose chemotherapy patients).
No statistically significant differences between the group were found in the rates of infections of higher than Grade 2 (per CTCAE 4.0): These infections occurred in 38 (34%) of those on the low-bacterial diet and 44 (39%) of those on the non-restrictive diet (P = 0.5).
There were also no statistically significant differences in rates of fever of unknown origin (P = 0.2), sepsis (P = 0.5), and gastrointestinal infection (P = 0.7).
The findings show that the “use of a restrictive diet is an unnecessary burden for our patients’ quality of life,” said study lead author Dr. Stella at the news briefing.
Dr. Sekeres, the news briefing moderator, noted that the findings reflect his own suspicions about the worthlessness of the low-bacterial diet. “I’ve never seen a patient die of an infection that was foodborne. So years ago, when I was still in Cleveland, I eliminated the neutropenic diet on the leukemia floor. That did face a lot of resistance, as you can imagine. There were decades of people saying we should do this.”
Now, Dr. Sekeres said, he feels validated. “I love this study because it formalizes what I thought was true,” he said.
Dr. Moody said it’s difficult to evaluate the study since it’s in abstract form, and details are limited. “However,” she said, “the sample size, study design, and outcomes appear very appropriate, and I think most likely the full-length study will provide additional evidence to support abandonment of the low-microbial diet in transplant patients.”
Also, Dr. Moody said, the study “replicates the same findings of other prospective randomized trials of this diet that say it confers no protection from infection and has no identified health benefit whatsoever. Bottom line? This diet has burden without benefit.”
Moving forward, she said, “we need a lot more research on diet in general for cancer patients. Recently, there is a lot more interest in this topic. I think we are going to learn a lot in the next few decades about the relationship between diet, epigenetics, the microbiome, and various cancer-related health issues.”
No study funding was reported. Dr. Stella reports no disclosures, and other authors report various relationships with industry. Dr. Moody reports no disclosures, and disclosures for Dr. Sekeres were not available.
NEW ORLEANS – A new Italian study yields more evidence that stem-cell transplant patients need not lose their appetites along with their immune systems. Low-bacterial, gruel-like diets, once the mainstay of immunity-lowering surgeries, don’t actually provide any protection against infections, researchers reported.
University of Miami hematologist Mikkael A. Sekeres, MD, MS, expressed delight to hear these findings.
“Around the world, we should eliminate these silly neutropenic diets, let people eat what they want, and give them a much better quality of life while they’re in the hospital,” said Dr. Sekeres, moderator of the news briefing where these research results were discussed at the annual meeting of the American Society of Hematology.
In recent decades, physicians and nutritionists have questioned the value of low-bacterial/neutropenic menus, designed to protect people with compromised immune systems from germs in food. These diets can be quite strict, outlawing food such as deli, processed, and cured meats; yogurt; hummus; strawberries and raspberries; lettuce; raw nuts; certain kinds of seafood; and herbs and spices such as pepper, unless they were cooked. Patients may be urged to avoid salad bars, buffets, and potlucks.
MD Anderson Cancer Center pediatrician Karen Moody, MD, MS, who has studied the diet, said in an interview that the diet has been around since the 1970s, despite a lack of evidence supporting it. “Cancer patients often suffer from treatment-related side effects that affect taste, appetite, and tolerance of food,” she said. “Further restricting food options in this population can be burdensome and reduce diet-related satisfaction.”
For the new multi-center, phase 3 study, researchers led by hematology resident Federico Stella, MD, of the University of Milan, randomly assigned consecutive adult patients undergoing hematopoietic stem cells transplantation or high-dose induction chemotherapy to either a low-bacterial diet (n = 224) or a non-restrictive diet (n = 224).
The low-bacterial diet emphasized food cooked to at least 176 degrees Fahrenheit and thick-skinned fruit. Raw fruits/vegetables, yogurt, honey, cold cuts and sausages, and raw fish and meat were forbidden.
The two groups were similar in age (median = 56 years), gender (about 57% male), and reason for admission (97% stem-cell transplants and 3% high-dose chemotherapy). The plan was to follow the patients for 100 days (stem-cell transplant recipients) or 30 days (high-dose chemotherapy patients).
No statistically significant differences between the group were found in the rates of infections of higher than Grade 2 (per CTCAE 4.0): These infections occurred in 38 (34%) of those on the low-bacterial diet and 44 (39%) of those on the non-restrictive diet (P = 0.5).
There were also no statistically significant differences in rates of fever of unknown origin (P = 0.2), sepsis (P = 0.5), and gastrointestinal infection (P = 0.7).
The findings show that the “use of a restrictive diet is an unnecessary burden for our patients’ quality of life,” said study lead author Dr. Stella at the news briefing.
Dr. Sekeres, the news briefing moderator, noted that the findings reflect his own suspicions about the worthlessness of the low-bacterial diet. “I’ve never seen a patient die of an infection that was foodborne. So years ago, when I was still in Cleveland, I eliminated the neutropenic diet on the leukemia floor. That did face a lot of resistance, as you can imagine. There were decades of people saying we should do this.”
Now, Dr. Sekeres said, he feels validated. “I love this study because it formalizes what I thought was true,” he said.
Dr. Moody said it’s difficult to evaluate the study since it’s in abstract form, and details are limited. “However,” she said, “the sample size, study design, and outcomes appear very appropriate, and I think most likely the full-length study will provide additional evidence to support abandonment of the low-microbial diet in transplant patients.”
Also, Dr. Moody said, the study “replicates the same findings of other prospective randomized trials of this diet that say it confers no protection from infection and has no identified health benefit whatsoever. Bottom line? This diet has burden without benefit.”
Moving forward, she said, “we need a lot more research on diet in general for cancer patients. Recently, there is a lot more interest in this topic. I think we are going to learn a lot in the next few decades about the relationship between diet, epigenetics, the microbiome, and various cancer-related health issues.”
No study funding was reported. Dr. Stella reports no disclosures, and other authors report various relationships with industry. Dr. Moody reports no disclosures, and disclosures for Dr. Sekeres were not available.
AT ASH 2022
Incorporating medication abortion into your ObGyn practice: Why and how
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.
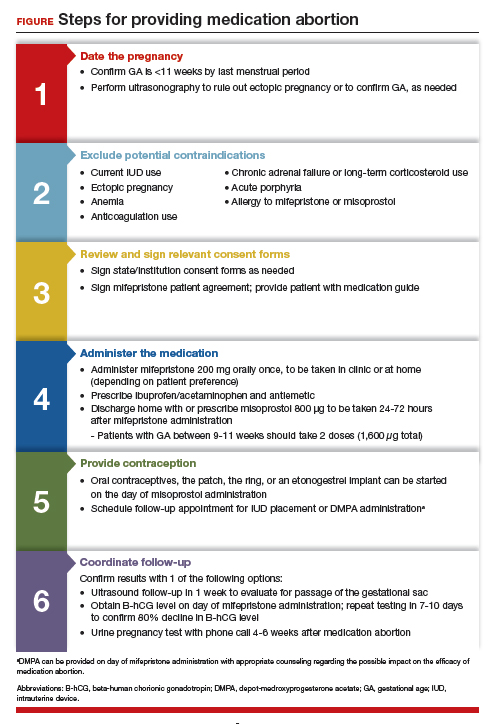
Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
The Supreme Court’s Dobbs decision on June 24, 2022, which nullified the federal protections of Roe v Wade, resulted in the swift and devastating dissolution of access to abortion care for hundreds of thousands of patients in the United States.1 Within days of the decision, 11 states in the South and Midwest implemented complete or 6-week abortion bans that, in part, led to the closure of over half the abortion clinics in these states.2 Abortion bans, severe restrictions, and clinic closures affect all patients and magnify existing health care inequities.
Medication abortion is becoming increasingly popular; as of 2020, approximately 50% of US abortions were performed using this method.3 Through a combination of mifepristone and misoprostol, medication abortion induces the physiologic process and symptoms similar to those of a miscarriage. Notably, this regimen is also the most effective medical management method for a missed abortion in the first trimester, and therefore, should already be incorporated into any general ObGyn practice.4
Although a recent study found that 97% of ObGyn physicians report encountering patients who seek an abortion, only 15% to 25% of them reported providing abortion services.5,6 Given our expertise, ObGyns are well-positioned to incorporate medication abortion into our practices. For those ObGyn providers who practice in states without extreme abortion bans, this article provides guidance on how to incorporate medication abortion into your practice (FIGURE). Several states now have early gestational limits on abortion, and the abortion-dedicated clinics that remain open are over capacity. Therefore, by incorporating medication abortion into your practice you can contribute to timely abortion access for your patients.

Medication abortion: The process
Determine your ability and patient’s eligibility
Abortion-specific laws for your state have now become the first determinant of your ability to provide medication abortion to your patients. The Guttmacher Institute is one reliable source of specific state laws that your practice can reference and is updated regularly.7
From a practice perspective, most ObGyn physicians already have the technical capabilities in place to provide medication abortion. First, you must be able to accurately determine the patient’s gestational age by their last menstrual period, which is often confirmed through ultrasonography.
Medication abortion is safe and routinely used in many practices up to 77 days, or 11 weeks, of gestation. Authors of a recent retrospective cohort study found that medication abortion also may be initiated for a pregnancy of unknown location in patients who are asymptomatic and determined to have low risk for an ectopic pregnancy. In this study, initiation of medication abortion on the day of presentation, with concurrent evaluation for ectopic pregnancy, was associated with a shorter time to a completed abortion, but a lower rate of successful medication abortion when compared with patients who delayed the initiation of medication abortion until a clear intrauterine pregnancy was diagnosed.8
Few medical contraindications exist for patients who seek a medication abortion. These contraindications include allergy to or medication interaction with mifepristone or misoprostol, chronic adrenal failure or long-term corticosteroid therapy, acute porphyria, anemia or the use of anticoagulation therapy, or current intrauterine device (IUD) use.
Continue to: Gather consents and administer treatment...
Gather consents and administer treatment
Historically, mifepristone has been dispensed directly at an ObGyn physician’s office. However, the US Food and Drug Administration (FDA) regulations requiring this were lifted during the COVID-19 pandemic, and as of December 2021, the inperson dispensing requirement was permanently removed.9 To provide mifepristone in a medical practice under current guidelines, a confidential prescriber agreement must be completed once by one person on behalf of the practice. Then each patient must read the manufacturer’s medication guide and sign the patient agreement form as part of the consent process (available on the FDA’s website).10 These agreement forms must be filled out by a physician and each patient if your practice uses mifepristone for any pregnancy indication, including induction of labor or medical management of miscarriage. Given the multiple evidence-based indications for mifepristone in pregnancy, it is hoped that these agreement forms will become a routine part of most ObGyn practices. Other consent requirements vary by state.
After signing consent forms, patients receive and often immediately take mifepristone 200 mg orally. Mifepristone is a progesterone receptor antagonist that sensitizes the uterine myometrium to the effects of prostaglandin.11 Rarely, patients may experience symptoms of bleeding or cramping after mifepristone administration alone.
Patients are discharged home with ibuprofen and an antiemetic for symptom relief to be taken around the time of administration of misoprostol. Misoprostol is a synthetic prostaglandin that causes uterine cramping and expulsion of the pregnancy typically within 4 hours of administration. Patients leave with the pills of misoprostol 800 μg (4 tablets, 200 µg each), which they self-administer buccally 24-48 hours after mifepristone administration. A prescription for misoprostol can be given instead of the actual pills, but geographic distance to the pharmacy and other potential barriers should be considered when evaluating the feasibility and convenience of providing pharmacy-dispensed misoprostol.
We instruct patients to place 2 tablets buccally between each gum and cheek, dosing all 4 tablets at the same time. Patients are instructed to let the tablets dissolve buccally and, after 30 minutes, to swallow the tablets with water. Administration of an automatic second dose of misoprostol 3-6 hours after the first dose for pregnancies between 9-11 weeks of gestation is recommended to increase success rate at these later gestational ages.12,13 Several different routes of administration, including buccal, vaginal, and sublingual, have been used for first trimester medication abortion with misoprostol.
Follow up and confirm the results
Patients can safely follow up after their medication abortion in several ways. In our practice, patients are offered 3 possible options.
- The first is ultrasound follow-up, whereby the patient returns to the clinic 1 week after their medication abortion for a pelvic ultrasound to confirm the gestational sac has passed.
- The second method is to test beta-human chorionic gonadotropin (B-hCG) levels. Patients interested in this option have a baseline B-hCG drawn on the day of presentation and follow up 7-10 days later for a repeat B-hCG test. An 80% drop in B-hCG level is consistent with a successful medication abortion.
- The third option, a phone checklist that is usually combined with a urine pregnancy test 4-6 weeks after a medication abortion, is an effective patient-centered approach. The COVID-19 pandemic and the subsequent compulsory shift to providing medical care via telemedicine highlighted the safety, acceptability, and patient preference for the provision of medication abortion using telehealth platforms.14
Outcomes and complications
Medication abortion using a combined regimen of mifepristone followed by misoprostol is approximately 95% effective at complete expulsion of the pregnancy.15,16 Complications after a first trimester medication abortion are rare. In a retrospective cohort study of 54,911 abortions, the most common complication was incomplete abortion.17 Symptoms concerning for incomplete abortion included persistent heavy vaginal bleeding and pelvic cramping. An incomplete or failed abortion should be managed with an additional dose of misoprostol or dilation and evacuation. Other possible complications such as infection are also rare, and prophylactic antibiotics are not encouraged.18
Future fertility and pregnancy implications
Patients should be counseled that a medication abortion is not associated with infertility or increased risk for adverse outcomes in future pregnancies.19 Contraceptive counseling should be provided to all interested patients at the time of a medication abortion and ideally provided to the patient on the day of their visit. Oral contraceptives, the patch, and the ring can be started on the day of misoprostol administration.20 The optimal timing of IUD insertion has been examined in 2 randomized control trials. Results indicated a higher uptake in the group of patients who received their IUD approximately 1 week after medication abortion versus delaying placement for several weeks, with no difference in IUD expulsion rates.21,22 Patients interested in depot-medroxyprogesterone acetate (DMPA) injection should be counseled on the theoretical decreased efficacy of medication abortion in the setting of concurrent DMPA administration. If possible, a follow-up plan should be made so that the patient can receive DMPA, if desired, at a later date.23 The etonogestrel implant (Nexplanon), however, can be placed on the day of mifepristone administration and does not affect the efficacy of a medication abortion.24,25
Summary
During this critical time for reproductive health care, it is essential that ObGyns consider how their professional position and expertise can assist with the provision of medication abortions. Most ObGyn practices already have the resources in place to effectively care for patients before, during, and after a medication abortion. Integrating abortion health care into your practice promotes patient-centered care, continuity, and patient satisfaction. Furthermore, by improving abortion referrals or offering information on safe, self-procured abortion, you can contribute to destigmatizing abortion care, while playing an integral role in connecting your patients with the care they need and desire. ●
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
- Jones RK, Philbin J, Kirstein M, et al. Long-term decline in US abortions reverses, showing rising need for abortion as Supreme Court is poised to overturn Roe v. Wade. Guttmacher Institute. August 30, 2022. https://www.gut. Accessed November 2, 2022. tmacher.org/article/2022/06 /long-term-decline-us-abortions-reverses-showing-rising -need-abortion-supreme-court.
- Kirstein M, Jones RK, Philbin J. One month post-roe: at least 43 abortion clinics across 11 states have stopped offering abortion care. Guttmacher Institute. September 5, 2022. https://www.guttmacher.org/article/2022/07/one-month -post-roe-least-43-abortion-clinics-across-11-states-have -stopped-offering. Accessed November 2, 2022.
- Jones RK, Nash E, Cross L, et al. Medication abortion now accounts for more than half of all US abortions. Guttmacher Institute. September 12, 2022. https://www.guttmacher.org /article/2022/02/medication-abortion-now-accounts-more-half-all-us-abortions. Accessed November 2, 2022.
- Schreiber CA, Creinin MD, Atrio J, et al. Mifepristone pretreatment for the medical management of early pregnancy loss. N Engl J Med. 2018;378:2161-2170. doi:10.1056/ nejmoa1715726.
- Stulberg DB, Dude AM, Dahlquist I, Curlin, FA. Abortion provision among practicing obstetrician-gynecologists. Obstet Gynecol. 2011;118:609-614. doi:10.1097/aog.0b013e31822ad973.
- Daniel S, Schulkin J, Grossman D. Obstetrician-gynecologist willingness to provide medication abortion with removal of the in-person dispensing requirement for mifepristone. Contraception. 2021;104:73-76. doi:10.1016/j. contraception.2021.03.026.
- Guttmacher Institute. State legislation tracker. Updated October 31, 2022. https://www.guttmacher.org/state-policy. Accessed November 2, 2022.
- Goldberg AB, Fulcher IR, Fortin J, et al. Mifepristone and misoprostol for undesired pregnancy of unknown location. Obstet Gynecol. 2022;139:771-780. doi:10.1097/ aog.0000000000004756.
- The American College of Obstetricians and Gynecologists. Understanding the practical implications of the FDA’s December 2021 mifepristone REMS decision: a Q&A with Dr. Nisha Verma and Vanessa Wellbery. March 28, 2022. https:// www.acog.org/news/news-articles/2022/03/understanding -the-practical-implications-of-the-fdas-december-2021 -mifepristone-rems-decision. Accessed November 2, 2022.
- US Food and Drug Administration. Mifeprex (mifepristone) information. December 16, 2021. https://www.fda.gov/ drugs/postmarket-drug-safety-information-patients-and-providers/ifeprex-mifepristone-information. Accessed November 2, 2022.
- Cadepond F, Ulmann A, Baulieu EE. Ru486 (mifepristone): mechanisms of action and clinical uses. Annu Rev Med. 1997;48:129-156. doi:10.1146/annurev.med.48.1.129.
- Ashok PW, Templeton A, Wagaarachchi PT, Flett GMM. Factors affecting the outcome of early medical abortion: a review of 4132 consecutive cases. BJOG. 2002;109:1281-1289. doi:10.1046/j.1471-0528.2002.02156.x.
- Coyaji K, Krishna U, Ambardekar S, et al. Are two doses of misoprostol after mifepristone for early abortion better than one? BJOG. 2007;114:271-278. doi:10.1111/j.14710528.2006.01208.x.
- Aiken A, Lohr PA, Lord J, et al. Effectiveness, safety and acceptability of no‐test medical abortion (termination of pregnancy) provided via telemedicine: a national cohort study. BJOG. 2021;128:1464-1474. doi:10.1111/14710528.16668.
- Schaff EA, Eisinger SH, Stadalius LS, et al. Low-dose mifepristone 200 mg and vaginal misoprostol for abortion. Contraception. 1999;59:1-6. doi:10.1016/s00107824(98)00150-4.
- Schaff EA, Fielding SL, Westhoff C. Randomized trial of oral versus vaginal misoprostol at one day after mifepristone for early medical abortion. Contraception. 2001;64:81-85. doi:10.1016/s0010-7824(01)00229-3.
- Upadhyay UD, Desai S, Zlidar V, et al. Incidence of emergency department visits and complications after abortion. Obstet Gynecol. 2015;125:175-183. doi:10.1097/ aog.0000000000000603.
- Shannon C, Brothers LP, Philip NM, Winikoff B. Infection after medical abortion: a review of the literature. Contraception. 2004;70:183-190. doi:10.1016/j.contraception.2004.04.009.
- Virk J, Zhang J, Olsen J. Medical abortion and the risk of subsequent adverse pregnancy outcomes. N Engl J Med. 2007;357:648-653. doi:10.1056/nejmoa070445.
- Mittal S. Contraception after medical abortion. Contraception. 2006;74:56-60. doi:10.1016/j.contraception.2006.03.006.
- Shimoni N, Davis A, Ramos ME, et al. Timing of copper intrauterine device insertion after medical abortion. Obstet Gynecol. 2011;118:623-628. doi:10.1097/aog.0b013e31822ade67.
- Sääv I, Stephansson O, Gemzell-Danielsson K. Early versus delayed insertion of intrauterine contraception after medical abortion—a randomized controlled trial. PloS ONE. 2012;7:e48948. doi:10.1371/journal.pone.0048948.
- Raymond EG, Weaver MA, Louie KS, et al. Effects of depot medroxyprogesterone acetate injection timing on medical abortion efficacy and repeat pregnancy: a randomized controlled trial. Obstet Gynecol. 2016;128:739-745. doi:10.1097/aog.0000000000001627.
- Hognert H, Kopp Kallner H, Cameron S, et al. Immediate versus delayed insertion of an etonogestrel releasing implant at medical abortion—a randomized controlled equivalence trial. Hum Reprod. 2016;31:2484-2490. doi:10.1093/humrep/ dew238.
- Raymond EG, Weaver MA, Tan Y-L, et al. Effect of immediate compared with delayed insertion of etonogestrel implants on medical abortion efficacy and repeat pregnancy. Obstet Gynecol. 2016;127:306-312. doi:10.1097/ aog.0000000000001274.
Global effort needed to widen access to HSCT
The use of HSCT, the main curative option for AML, “remains unacceptably low,” commented Molly Tokaz, MD, a hematology/oncology fellow at the Fred Hutchinson Cancer Center, Seattle.
She was presenting the findings from a study of worldwide HSCT utilization at the annual meeting of the American Society of Hematology.
Globally, the incidence of AML has increased 16.2% – from 101,867 cases in 2009 to 118,404 in 2016, she noted. This in turn has led to a 54.9% increase in the worldwide use of HSCT for AML, from 9,659 to 14,965 transplants per year over the same period.
North America and Europe have the highest utilization rates of allogeneic HSCT for AML, but even so, fewer than 40% of patients have the procedure, raising a “question of how [well] we are prioritizing the use of HSCT, even in these resource-abundant health systems,” Dr. Tokaz said.
Meanwhile, in Africa, South America, and the Eastern Mediterranean, fewer than 5% of AML patients undergo transplant. Although “resource-constrained regions have the largest growth in HSCT use” in recent years, utilization rates remain abysmally low, “which has profound effects on the expected outcomes for patients in these regions,” she said.
Overall, “patients from lower- and middle-income countries face substantial barriers to accessing stem cell transplantation for AML,” commented Chancellor Donald, MD, a hematologist/oncologist at Tulane University, New Orleans, who moderated the session.
The “stark regional differences” illustrate “inequities in the delivery of stem cell transplants” but also opportunities “to improve access to this potentially curative treatment,” he said.
The goal of the study was to establish a global baseline of HSCT utilization to help focus future expansion efforts aimed at closing regional access gaps. It shows there is much work to be done, Dr. Tokaz said.
An international effort is needed to address the issue, including better data collection, implementation of regional HSCT programs, increased representation of ethnic and racial minorities in international donor registries, and other measures. In many cases, telemedicine can help with sharing cross-border expertise.
In short, what’s needed is a “comprehensive global effort to improve outcomes for patients with AML” worldwide, Dr. Tokaz said.
Timing of transplant is similar across regions, generally coming during the first complete remission, and there’s also been a global shift toward collecting stem cells from peripheral blood.
There has also been a marked shift away from autologous procedures and toward allogeneic transplants, she said.
A key difference between regions, however, is that while more than half of transplants are from unrelated donors in Europe and North America, almost all are from related donors in Africa and the Eastern Mediterranean, with an increasing proportion of haploidentical donors. In addition, the majority of transplants in Asia, the western Pacific, and South America are from related donors.
The use of related donors has implications for HSCT treatment algorithms and outcomes, Dr. Tokaz said.
The estimates of AML incidence were obtained from the 2019 Global Burden of Disease study. Data on HSCT utilization came from the Worldwide Network for Blood and Marrow Transplantation. No funding source was reported. Dr. Tokaz reports no relevant financial relationships, but some co-authors had numerous industry ties. Dr. Donald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of HSCT, the main curative option for AML, “remains unacceptably low,” commented Molly Tokaz, MD, a hematology/oncology fellow at the Fred Hutchinson Cancer Center, Seattle.
She was presenting the findings from a study of worldwide HSCT utilization at the annual meeting of the American Society of Hematology.
Globally, the incidence of AML has increased 16.2% – from 101,867 cases in 2009 to 118,404 in 2016, she noted. This in turn has led to a 54.9% increase in the worldwide use of HSCT for AML, from 9,659 to 14,965 transplants per year over the same period.
North America and Europe have the highest utilization rates of allogeneic HSCT for AML, but even so, fewer than 40% of patients have the procedure, raising a “question of how [well] we are prioritizing the use of HSCT, even in these resource-abundant health systems,” Dr. Tokaz said.
Meanwhile, in Africa, South America, and the Eastern Mediterranean, fewer than 5% of AML patients undergo transplant. Although “resource-constrained regions have the largest growth in HSCT use” in recent years, utilization rates remain abysmally low, “which has profound effects on the expected outcomes for patients in these regions,” she said.
Overall, “patients from lower- and middle-income countries face substantial barriers to accessing stem cell transplantation for AML,” commented Chancellor Donald, MD, a hematologist/oncologist at Tulane University, New Orleans, who moderated the session.
The “stark regional differences” illustrate “inequities in the delivery of stem cell transplants” but also opportunities “to improve access to this potentially curative treatment,” he said.
The goal of the study was to establish a global baseline of HSCT utilization to help focus future expansion efforts aimed at closing regional access gaps. It shows there is much work to be done, Dr. Tokaz said.
An international effort is needed to address the issue, including better data collection, implementation of regional HSCT programs, increased representation of ethnic and racial minorities in international donor registries, and other measures. In many cases, telemedicine can help with sharing cross-border expertise.
In short, what’s needed is a “comprehensive global effort to improve outcomes for patients with AML” worldwide, Dr. Tokaz said.
Timing of transplant is similar across regions, generally coming during the first complete remission, and there’s also been a global shift toward collecting stem cells from peripheral blood.
There has also been a marked shift away from autologous procedures and toward allogeneic transplants, she said.
A key difference between regions, however, is that while more than half of transplants are from unrelated donors in Europe and North America, almost all are from related donors in Africa and the Eastern Mediterranean, with an increasing proportion of haploidentical donors. In addition, the majority of transplants in Asia, the western Pacific, and South America are from related donors.
The use of related donors has implications for HSCT treatment algorithms and outcomes, Dr. Tokaz said.
The estimates of AML incidence were obtained from the 2019 Global Burden of Disease study. Data on HSCT utilization came from the Worldwide Network for Blood and Marrow Transplantation. No funding source was reported. Dr. Tokaz reports no relevant financial relationships, but some co-authors had numerous industry ties. Dr. Donald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The use of HSCT, the main curative option for AML, “remains unacceptably low,” commented Molly Tokaz, MD, a hematology/oncology fellow at the Fred Hutchinson Cancer Center, Seattle.
She was presenting the findings from a study of worldwide HSCT utilization at the annual meeting of the American Society of Hematology.
Globally, the incidence of AML has increased 16.2% – from 101,867 cases in 2009 to 118,404 in 2016, she noted. This in turn has led to a 54.9% increase in the worldwide use of HSCT for AML, from 9,659 to 14,965 transplants per year over the same period.
North America and Europe have the highest utilization rates of allogeneic HSCT for AML, but even so, fewer than 40% of patients have the procedure, raising a “question of how [well] we are prioritizing the use of HSCT, even in these resource-abundant health systems,” Dr. Tokaz said.
Meanwhile, in Africa, South America, and the Eastern Mediterranean, fewer than 5% of AML patients undergo transplant. Although “resource-constrained regions have the largest growth in HSCT use” in recent years, utilization rates remain abysmally low, “which has profound effects on the expected outcomes for patients in these regions,” she said.
Overall, “patients from lower- and middle-income countries face substantial barriers to accessing stem cell transplantation for AML,” commented Chancellor Donald, MD, a hematologist/oncologist at Tulane University, New Orleans, who moderated the session.
The “stark regional differences” illustrate “inequities in the delivery of stem cell transplants” but also opportunities “to improve access to this potentially curative treatment,” he said.
The goal of the study was to establish a global baseline of HSCT utilization to help focus future expansion efforts aimed at closing regional access gaps. It shows there is much work to be done, Dr. Tokaz said.
An international effort is needed to address the issue, including better data collection, implementation of regional HSCT programs, increased representation of ethnic and racial minorities in international donor registries, and other measures. In many cases, telemedicine can help with sharing cross-border expertise.
In short, what’s needed is a “comprehensive global effort to improve outcomes for patients with AML” worldwide, Dr. Tokaz said.
Timing of transplant is similar across regions, generally coming during the first complete remission, and there’s also been a global shift toward collecting stem cells from peripheral blood.
There has also been a marked shift away from autologous procedures and toward allogeneic transplants, she said.
A key difference between regions, however, is that while more than half of transplants are from unrelated donors in Europe and North America, almost all are from related donors in Africa and the Eastern Mediterranean, with an increasing proportion of haploidentical donors. In addition, the majority of transplants in Asia, the western Pacific, and South America are from related donors.
The use of related donors has implications for HSCT treatment algorithms and outcomes, Dr. Tokaz said.
The estimates of AML incidence were obtained from the 2019 Global Burden of Disease study. Data on HSCT utilization came from the Worldwide Network for Blood and Marrow Transplantation. No funding source was reported. Dr. Tokaz reports no relevant financial relationships, but some co-authors had numerous industry ties. Dr. Donald reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM ASH 2022
Poorly matched stem cell transplants linked to ancestry
There is “an intersectionality between ancestry and socioeconomic status and an association with donor type, with the most vulnerable patients” – those of non-European ancestry with low socioeconomic status (SES), especially people of African ancestry – “receiving the most complex [i.e., human leukocyte antigen (HLA)–disparate] transplants,” said lead investigator Warren Fingrut, MD, a research fellow in the Adult Bone Marrow Transplantation Service at Memorial Sloan Kettering Cancer Center, New York.
“Successful extension of transplant access to minority patients will be contingent on addressing [their] financial hardship,” said Dr. Fingrut, who presented the findings at the meeting.
To better channel support services and ensure that resources are available, he also noted that centers will have to do a better job of identifying patients with financial struggles.
“Household income data is not collected at our center, and neither is it collected at most centers,” hence assessments of SES are based on imperfect surrogates, such as neighborhood poverty by zip code. “Interventions to advance equity will require better SES classifications or detailed recording of household income,” Dr. Fingrut said.
Overall, the study highlights “inequities in the delivery of stem cell transplants,” pointing to opportunities “to improve access to this potentially curative treatment,” said hematologist/oncologist Chancellor Donald, MD, of Tulane University, New Orleans, who moderated the study presentation.
Dr. Donald said that the new research shows “how interactions between racial backgrounds and socioeconomic status relate to the type of allogenic stem cell transplant patients receive.” The team “identified that [people] of non-European ancestry and especially those of low SES, are more likely to receive the most specialized type of allogeneic stem cell transplantation, which notably require the highest level of care,” Dr. Donald said.
The investigators reviewed 372 consecutive adults transplanted at MSKCC from March 2020 to February 2022, mostly for myeloid malignancies.
Thirty-one percent of patients had non-European ancestry, including 11% of African, 9% of Asian, and 8% of White Hispanic descent.
With no information about household income, the team used neighborhood poverty (which affected 5% of patients); Medicaid as the primary insurance (6% of patients), and financial support for living and medical expenses (19%) as surrogates of lower SES. Classification depended largely on what criteria were used, with only 20 patients meeting two criteria and only one patient meeting all three.
Overall, more than half (58%) of non-European ancestry patients received HLA-disparate grafts, compared with 24% of people with European ancestry, including 48% of White Hispanic patients, 58% of Asian patients, and 78% of patients of African decent.
Markers of lower SES were more common among non-European patients. For instance, among people of European ancestry, 4% were on Medicaid and 15% were on financial aid, versus 10% on Medicaid and 29% on financial support among people of other ancestries. Medicaid use (12.5%) and financial aid (42.5%) were highest among patients of African descent.
Among patients who received HLA-disparate grafts, patients of non-European descent were three times more likely to be on Medicaid (12% versus 4%) and more than twice as likely to be on financial support (33% versus 15%).
People of African ancestry who received HLA-disparate grafts had the highest proportions of Medicaid reliance (16%) and financial support (45%).
There is “an intersectionality between ancestry and socioeconomic status and an association with donor type, with the most vulnerable patients” – those of non-European ancestry with low socioeconomic status (SES), especially people of African ancestry – “receiving the most complex [i.e., human leukocyte antigen (HLA)–disparate] transplants,” said lead investigator Warren Fingrut, MD, a research fellow in the Adult Bone Marrow Transplantation Service at Memorial Sloan Kettering Cancer Center, New York.
“Successful extension of transplant access to minority patients will be contingent on addressing [their] financial hardship,” said Dr. Fingrut, who presented the findings at the meeting.
To better channel support services and ensure that resources are available, he also noted that centers will have to do a better job of identifying patients with financial struggles.
“Household income data is not collected at our center, and neither is it collected at most centers,” hence assessments of SES are based on imperfect surrogates, such as neighborhood poverty by zip code. “Interventions to advance equity will require better SES classifications or detailed recording of household income,” Dr. Fingrut said.
Overall, the study highlights “inequities in the delivery of stem cell transplants,” pointing to opportunities “to improve access to this potentially curative treatment,” said hematologist/oncologist Chancellor Donald, MD, of Tulane University, New Orleans, who moderated the study presentation.
Dr. Donald said that the new research shows “how interactions between racial backgrounds and socioeconomic status relate to the type of allogenic stem cell transplant patients receive.” The team “identified that [people] of non-European ancestry and especially those of low SES, are more likely to receive the most specialized type of allogeneic stem cell transplantation, which notably require the highest level of care,” Dr. Donald said.
The investigators reviewed 372 consecutive adults transplanted at MSKCC from March 2020 to February 2022, mostly for myeloid malignancies.
Thirty-one percent of patients had non-European ancestry, including 11% of African, 9% of Asian, and 8% of White Hispanic descent.
With no information about household income, the team used neighborhood poverty (which affected 5% of patients); Medicaid as the primary insurance (6% of patients), and financial support for living and medical expenses (19%) as surrogates of lower SES. Classification depended largely on what criteria were used, with only 20 patients meeting two criteria and only one patient meeting all three.
Overall, more than half (58%) of non-European ancestry patients received HLA-disparate grafts, compared with 24% of people with European ancestry, including 48% of White Hispanic patients, 58% of Asian patients, and 78% of patients of African decent.
Markers of lower SES were more common among non-European patients. For instance, among people of European ancestry, 4% were on Medicaid and 15% were on financial aid, versus 10% on Medicaid and 29% on financial support among people of other ancestries. Medicaid use (12.5%) and financial aid (42.5%) were highest among patients of African descent.
Among patients who received HLA-disparate grafts, patients of non-European descent were three times more likely to be on Medicaid (12% versus 4%) and more than twice as likely to be on financial support (33% versus 15%).
People of African ancestry who received HLA-disparate grafts had the highest proportions of Medicaid reliance (16%) and financial support (45%).
There is “an intersectionality between ancestry and socioeconomic status and an association with donor type, with the most vulnerable patients” – those of non-European ancestry with low socioeconomic status (SES), especially people of African ancestry – “receiving the most complex [i.e., human leukocyte antigen (HLA)–disparate] transplants,” said lead investigator Warren Fingrut, MD, a research fellow in the Adult Bone Marrow Transplantation Service at Memorial Sloan Kettering Cancer Center, New York.
“Successful extension of transplant access to minority patients will be contingent on addressing [their] financial hardship,” said Dr. Fingrut, who presented the findings at the meeting.
To better channel support services and ensure that resources are available, he also noted that centers will have to do a better job of identifying patients with financial struggles.
“Household income data is not collected at our center, and neither is it collected at most centers,” hence assessments of SES are based on imperfect surrogates, such as neighborhood poverty by zip code. “Interventions to advance equity will require better SES classifications or detailed recording of household income,” Dr. Fingrut said.
Overall, the study highlights “inequities in the delivery of stem cell transplants,” pointing to opportunities “to improve access to this potentially curative treatment,” said hematologist/oncologist Chancellor Donald, MD, of Tulane University, New Orleans, who moderated the study presentation.
Dr. Donald said that the new research shows “how interactions between racial backgrounds and socioeconomic status relate to the type of allogenic stem cell transplant patients receive.” The team “identified that [people] of non-European ancestry and especially those of low SES, are more likely to receive the most specialized type of allogeneic stem cell transplantation, which notably require the highest level of care,” Dr. Donald said.
The investigators reviewed 372 consecutive adults transplanted at MSKCC from March 2020 to February 2022, mostly for myeloid malignancies.
Thirty-one percent of patients had non-European ancestry, including 11% of African, 9% of Asian, and 8% of White Hispanic descent.
With no information about household income, the team used neighborhood poverty (which affected 5% of patients); Medicaid as the primary insurance (6% of patients), and financial support for living and medical expenses (19%) as surrogates of lower SES. Classification depended largely on what criteria were used, with only 20 patients meeting two criteria and only one patient meeting all three.
Overall, more than half (58%) of non-European ancestry patients received HLA-disparate grafts, compared with 24% of people with European ancestry, including 48% of White Hispanic patients, 58% of Asian patients, and 78% of patients of African decent.
Markers of lower SES were more common among non-European patients. For instance, among people of European ancestry, 4% were on Medicaid and 15% were on financial aid, versus 10% on Medicaid and 29% on financial support among people of other ancestries. Medicaid use (12.5%) and financial aid (42.5%) were highest among patients of African descent.
Among patients who received HLA-disparate grafts, patients of non-European descent were three times more likely to be on Medicaid (12% versus 4%) and more than twice as likely to be on financial support (33% versus 15%).
People of African ancestry who received HLA-disparate grafts had the highest proportions of Medicaid reliance (16%) and financial support (45%).
FROM ASH 2022







