User login
Clinical Pearl: Mohs Cantaloupe Analogy for the Dermatology Resident
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
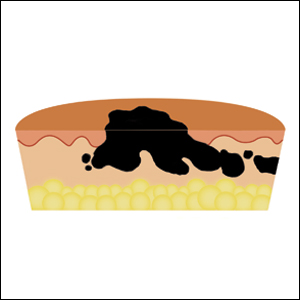
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).

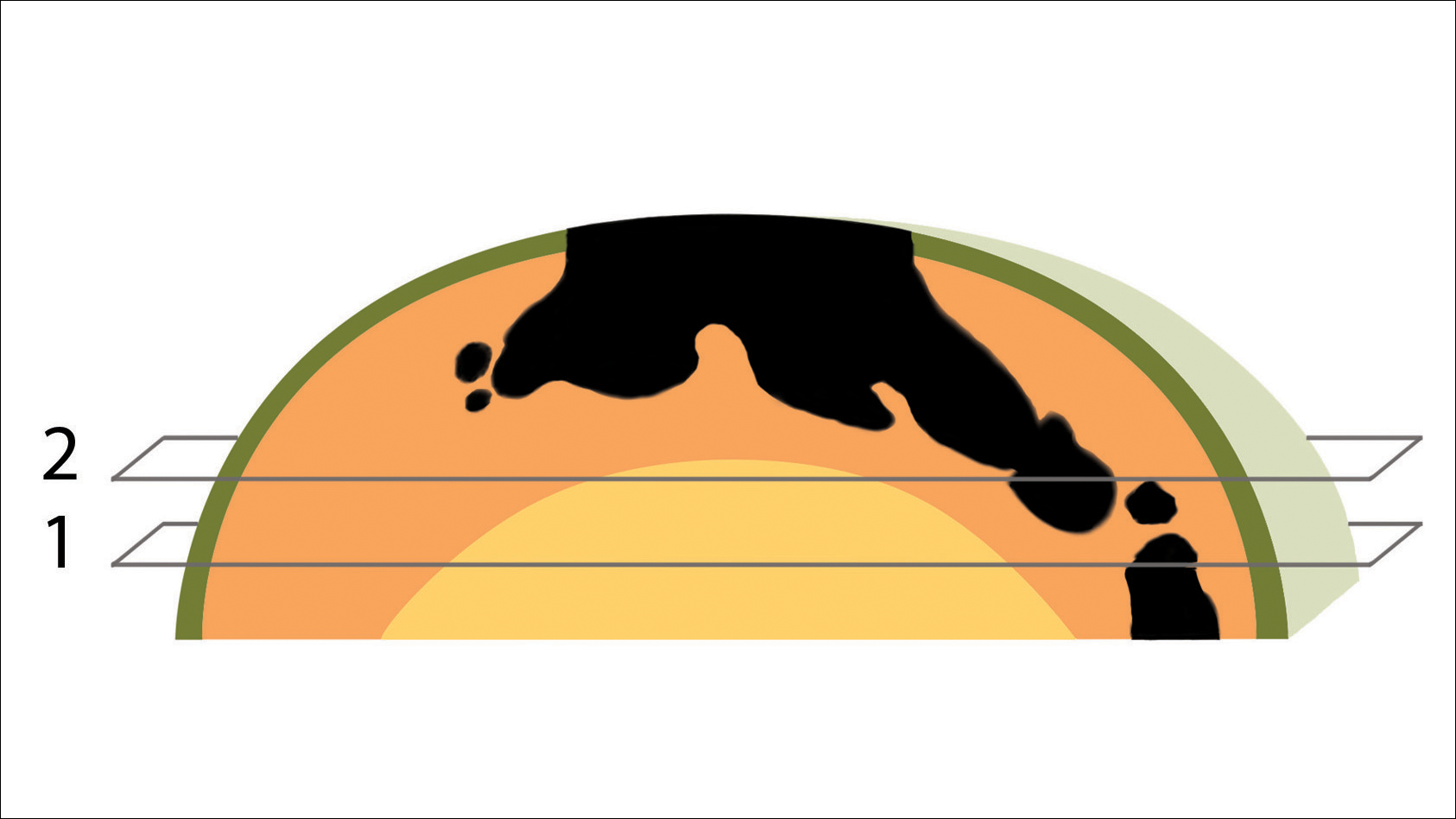
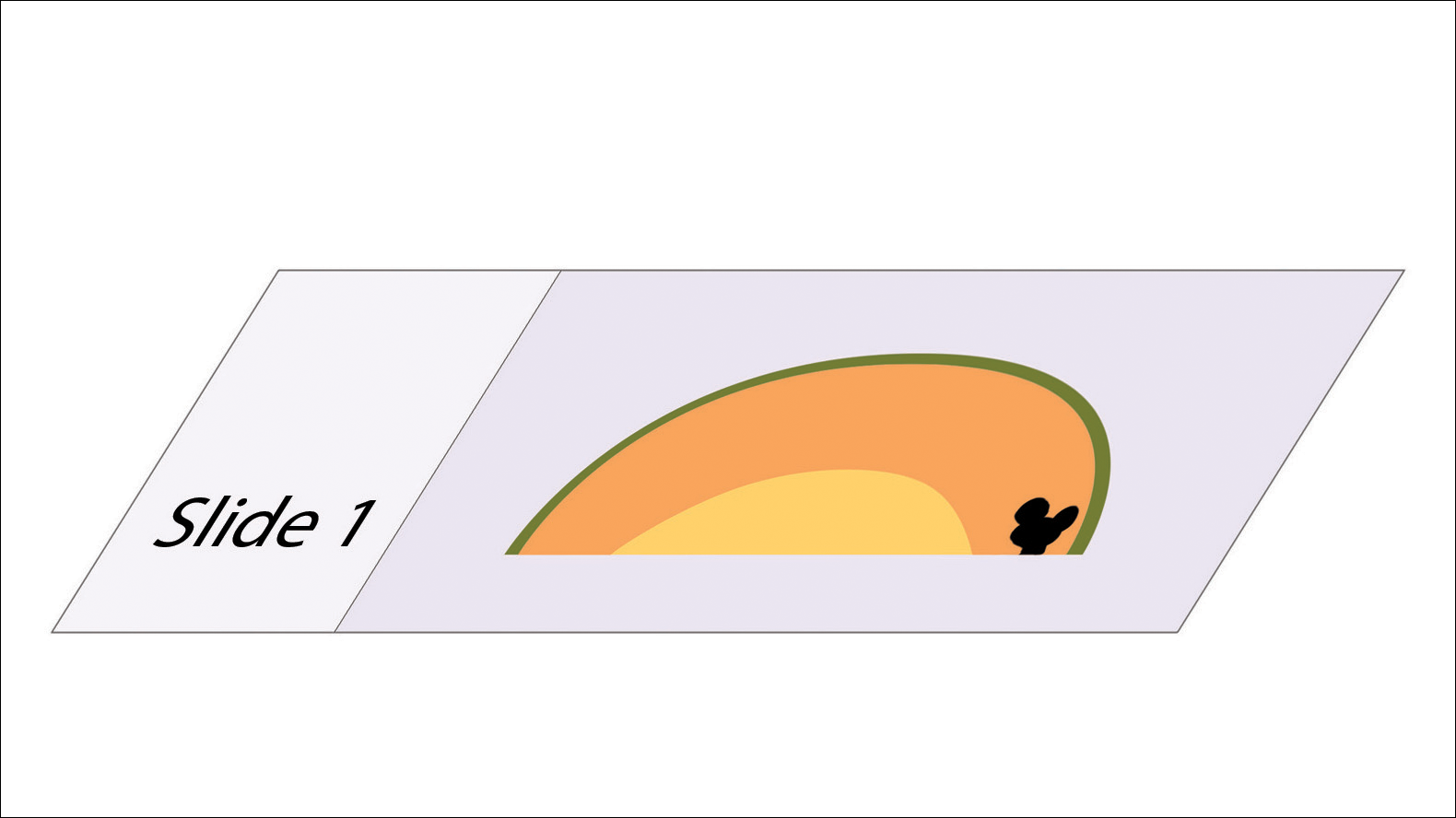
In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.
Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
Practice Gap
Mohs micrographic surgery (MMS) is a highly curative tissue-sparing skin cancer treatment1 and is a required component of dermatology residency training. According to the Accreditation Council for Graduate Medical Education, residents must have exposure “either through direct observation or as an assistant in Mohs micrographic surgery, and reconstruction of these defects, to include flaps and grafts.”2 The MMS technique allows for complete circumferential peripheral and deep margin assessment of excised specimens; however, the conformation of a 3-dimensional gross tissue specimen into a 2-dimensional specimen as represented on a microscope slide is challenging to conceptualize.
Behavioral science research has shown that analogies and metaphors help integrate topics into a memorable format and produce deeper comprehension.3 As such, analogies can aid in the visualization of these complex spatial concepts. The MMS tissue-processing technique has been compared to flattening a pie pan.4 More recently, a peanut butter cup analogy was described as a visualization tool for explaining the various steps of MMS to patients.5 Although these analogies may help elucidate certain aspects of the MMS technique, none adequately account for the multilayered anatomy of the skin.
The Technique
To address this need, we developed the cantaloupe analogy, which provides visual representation of the 3 basic skin layers: (1) the rind represents the epidermis; (2) the flesh represents the dermis, and (3) the seed cavity represents the subcutaneous layer (Figures 1 and 2).


In MMS tissue processing, the peripheral margin of the ovoid excised skin specimen is pressed down into the same plane as the deepest layer through a process called relaxation.4 The cantaloupe represents the dome shape of the relaxed tissue, which is then serially sectioned in horizontal layers from deep to superficial (Figure 2). The first slice represents the deepest subcutaneous layer and most peripheral dermal and epidermal layers of the specimen (Figure 3). Using the cantaloupe analogy, subsequent stages (if warranted) would be guided by the location of the residual skin cancer. If the skin cancer is in the epidermis (rind) or dermis (flesh), then a skin specimen from the perimeter of the defect would be indicated. Residual skin cancer extending into the subcutaneous layer (seed cavity) would require a deeper resection.

Practice Implications
The cantaloupe provides a simple analogy to conceptualize the transition from the multilayered 3-dimensional skin tissue specimen to the 2-dimensional histologic slide specimen. Use of this cantaloupe analogy will aid dermatology residents and others interested in gaining a clearer understanding of MMS.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
- Semkova K, Mallipeddi R, Robson A, et al. Mohs micrographic surgery concordance between Mohs surgeons and dermatopathologists. Dermatol Surg. 2013;39:1648-1652.
- ACGME program requirements for graduate medical education in dermatology. Accreditation Council for Graduate Medical Education website. https://www.acgme.org/Portals/0/PFAssets/ProgramRequirements/080_dermatology_2017-07-01.pdf. Updated July 1, 2017. Accessed June 6, 2018.
- Wolfe CR. Plant a tree in cyberspace: metaphor and analogy as design elements in Web-based learning environments. CyberPsychol Behav. 2001;4:67-76.
- Beck B, Peters SR. Frozen section techniques used in Mohs micrographic surgery. In: Peters SR, ed. A Practical Guide to Frozen Section Technique. New York, NY: Springer; 2010:151-170.
- Lee E, Wolverton JE, Somani AK. A simple, effective analogy to elucidate the Mohs micrographic surgery procedure—the peanut butter cup. JAMA Dermatol. 2017;153:743-744.
Research supports cannabis in MS, but legal, clinical pictures are murky
The medical marijuana landscape is changing so fast that Colorado Neurological Institute neurologist Allen C. Bowling, MD, PhD, already needs to update a presentation he gave about cannabis in multiple sclerosis in late May.
Since then, both those facts became history over a span of 2 days.
First, on June 25, the FDA announced its approval of Epidiolex (cannabidiol) for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. It’s the first time the FDA has approved a drug with a purified ingredient – cannabidiol, a nonpsychoactive substance – that’s derived from marijuana.
Then, on June 26, voters in Oklahoma approved a ballot measure that allows the possession of marijuana for medical use; users must register with the state. Thirty states and the District of Columbia have made medical marijuana legal, according to the procon.org website, although the two newest ones (Oklahoma and West Virginia) are still developing procedures.
The laws vary widely. Some states don’t allow patients to smoke medical marijuana, and some don’t allow visitors to use out-of-state registry ID cards. And certain states limit the use of medical marijuana to specific conditions. Medical marijuana use by patients with MS is specifically allowed in many states, including Alaska, Arizona, Florida, Minnesota, and several others.
There’s another complexity: According to procon.org, 17 states have laws about the use of cannabidiol. In Georgia, for instance, the use of some cannabis oil is allowed for the treatment of MS and other conditions.
In the wake of the FDA ruling, Dr. Bowling spoke in an interview about cannabis, MS, and the questions that neurologists should be asking themselves.
Q: What are studies telling us about cannabis and MS?
A: There are lots of clinical studies – 19 randomized controlled trials. A consistent finding is that there’s benefit in terms of pain and people’s subjective sense of spasticity (Neurology. 2014 Apr 29;82(17):1556-63).
Q: During your CMSC presentation, you talked about how “fidelity” has been a problem in cannabis research. Could you elaborate on what you mean?
A: The products used in these studies are generally standardized, research-grade products that you can’t buy in any U.S. dispensary.
Cannabis is complex and contains more than 100 different potentially pharmacologically active molecules. You can’t conclude that if you see a product in clinical trials, you’ll then be able to walk into a dispensary for recreational or medical cannabis and get a product that produces the same effect.
Q: What have you seen in your own patient population in terms of cannabis use?
A: I find what’s been found with the studies: It helps with pain and people’s sense of muscle stiffness.
It’s especially helpful in people with pain and spasticity that breaks through in the late afternoon or at night when they’re trying to go to sleep. Just a little bit of cannabis can get them through those difficult times and improve their quality of life.
Q: What choices do patients make regarding whether to get high from the cannabis they use?
A: Some have absolutely zero interest in getting high, and they try to avoid the THC-containing products. Other like getting high in addition to getting help with pain and spasticity.
Q: Who should not use medical marijuana in the MS community?
A: Patients who don’t have symptoms that could respond.
I’m also very concerned about patients who are 25 years and younger because of the effects that cannabis can have on brain development out to age 25 and the higher risk of addiction in people who are younger.
Q: What do you think the future will hold on the cannabis front?
A: Now that it’s less of a taboo topic, there’s an ever-growing number of trials each year, including very high-quality studies.
Pharmaceutically produced, cannabis-based medicines will be a growing area. Epidiolex is a perfect example of that.
It’s important for physicians to know that the way cannabis-based medicine is produced by a pharmaceutical company is different in so many levels than the cannabis in states with recreational and medical marijuana.
Q: What are some ways that the pharmaceutical products are different?
A: The rigor of the production process, the standardization, the purity, the correct labeling and expiration dates. Plus, the lack of the use of pesticides and other contaminants. And they’re distributed by pharmacists.
Q: What should neurologists be thinking if they’re considering whether to recommend cannabis to their patients?
A: This is a very complex topic, and it’s not something that most of us have training in. You can’t sit down for 1 or 2 hours, get up to speed, and have your own well-informed opinion on it. You really need to put more time and effort.
Q: What are some issues that neurologists should consider?
A: You really need to find out what your state is doing about it and see how you feel about that.
How is your state administering medical and/or recreational marijuana? The administration of these programs is extremely different from state to state. Do these details satisfy you, and are you content having your patients interface with these programs?
Dr. Bowling reports no relevant disclosures.
The medical marijuana landscape is changing so fast that Colorado Neurological Institute neurologist Allen C. Bowling, MD, PhD, already needs to update a presentation he gave about cannabis in multiple sclerosis in late May.
Since then, both those facts became history over a span of 2 days.
First, on June 25, the FDA announced its approval of Epidiolex (cannabidiol) for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. It’s the first time the FDA has approved a drug with a purified ingredient – cannabidiol, a nonpsychoactive substance – that’s derived from marijuana.
Then, on June 26, voters in Oklahoma approved a ballot measure that allows the possession of marijuana for medical use; users must register with the state. Thirty states and the District of Columbia have made medical marijuana legal, according to the procon.org website, although the two newest ones (Oklahoma and West Virginia) are still developing procedures.
The laws vary widely. Some states don’t allow patients to smoke medical marijuana, and some don’t allow visitors to use out-of-state registry ID cards. And certain states limit the use of medical marijuana to specific conditions. Medical marijuana use by patients with MS is specifically allowed in many states, including Alaska, Arizona, Florida, Minnesota, and several others.
There’s another complexity: According to procon.org, 17 states have laws about the use of cannabidiol. In Georgia, for instance, the use of some cannabis oil is allowed for the treatment of MS and other conditions.
In the wake of the FDA ruling, Dr. Bowling spoke in an interview about cannabis, MS, and the questions that neurologists should be asking themselves.
Q: What are studies telling us about cannabis and MS?
A: There are lots of clinical studies – 19 randomized controlled trials. A consistent finding is that there’s benefit in terms of pain and people’s subjective sense of spasticity (Neurology. 2014 Apr 29;82(17):1556-63).
Q: During your CMSC presentation, you talked about how “fidelity” has been a problem in cannabis research. Could you elaborate on what you mean?
A: The products used in these studies are generally standardized, research-grade products that you can’t buy in any U.S. dispensary.
Cannabis is complex and contains more than 100 different potentially pharmacologically active molecules. You can’t conclude that if you see a product in clinical trials, you’ll then be able to walk into a dispensary for recreational or medical cannabis and get a product that produces the same effect.
Q: What have you seen in your own patient population in terms of cannabis use?
A: I find what’s been found with the studies: It helps with pain and people’s sense of muscle stiffness.
It’s especially helpful in people with pain and spasticity that breaks through in the late afternoon or at night when they’re trying to go to sleep. Just a little bit of cannabis can get them through those difficult times and improve their quality of life.
Q: What choices do patients make regarding whether to get high from the cannabis they use?
A: Some have absolutely zero interest in getting high, and they try to avoid the THC-containing products. Other like getting high in addition to getting help with pain and spasticity.
Q: Who should not use medical marijuana in the MS community?
A: Patients who don’t have symptoms that could respond.
I’m also very concerned about patients who are 25 years and younger because of the effects that cannabis can have on brain development out to age 25 and the higher risk of addiction in people who are younger.
Q: What do you think the future will hold on the cannabis front?
A: Now that it’s less of a taboo topic, there’s an ever-growing number of trials each year, including very high-quality studies.
Pharmaceutically produced, cannabis-based medicines will be a growing area. Epidiolex is a perfect example of that.
It’s important for physicians to know that the way cannabis-based medicine is produced by a pharmaceutical company is different in so many levels than the cannabis in states with recreational and medical marijuana.
Q: What are some ways that the pharmaceutical products are different?
A: The rigor of the production process, the standardization, the purity, the correct labeling and expiration dates. Plus, the lack of the use of pesticides and other contaminants. And they’re distributed by pharmacists.
Q: What should neurologists be thinking if they’re considering whether to recommend cannabis to their patients?
A: This is a very complex topic, and it’s not something that most of us have training in. You can’t sit down for 1 or 2 hours, get up to speed, and have your own well-informed opinion on it. You really need to put more time and effort.
Q: What are some issues that neurologists should consider?
A: You really need to find out what your state is doing about it and see how you feel about that.
How is your state administering medical and/or recreational marijuana? The administration of these programs is extremely different from state to state. Do these details satisfy you, and are you content having your patients interface with these programs?
Dr. Bowling reports no relevant disclosures.
The medical marijuana landscape is changing so fast that Colorado Neurological Institute neurologist Allen C. Bowling, MD, PhD, already needs to update a presentation he gave about cannabis in multiple sclerosis in late May.
Since then, both those facts became history over a span of 2 days.
First, on June 25, the FDA announced its approval of Epidiolex (cannabidiol) for the treatment of seizures in two rare forms of epilepsy, Lennox-Gastaut syndrome and Dravet syndrome. It’s the first time the FDA has approved a drug with a purified ingredient – cannabidiol, a nonpsychoactive substance – that’s derived from marijuana.
Then, on June 26, voters in Oklahoma approved a ballot measure that allows the possession of marijuana for medical use; users must register with the state. Thirty states and the District of Columbia have made medical marijuana legal, according to the procon.org website, although the two newest ones (Oklahoma and West Virginia) are still developing procedures.
The laws vary widely. Some states don’t allow patients to smoke medical marijuana, and some don’t allow visitors to use out-of-state registry ID cards. And certain states limit the use of medical marijuana to specific conditions. Medical marijuana use by patients with MS is specifically allowed in many states, including Alaska, Arizona, Florida, Minnesota, and several others.
There’s another complexity: According to procon.org, 17 states have laws about the use of cannabidiol. In Georgia, for instance, the use of some cannabis oil is allowed for the treatment of MS and other conditions.
In the wake of the FDA ruling, Dr. Bowling spoke in an interview about cannabis, MS, and the questions that neurologists should be asking themselves.
Q: What are studies telling us about cannabis and MS?
A: There are lots of clinical studies – 19 randomized controlled trials. A consistent finding is that there’s benefit in terms of pain and people’s subjective sense of spasticity (Neurology. 2014 Apr 29;82(17):1556-63).
Q: During your CMSC presentation, you talked about how “fidelity” has been a problem in cannabis research. Could you elaborate on what you mean?
A: The products used in these studies are generally standardized, research-grade products that you can’t buy in any U.S. dispensary.
Cannabis is complex and contains more than 100 different potentially pharmacologically active molecules. You can’t conclude that if you see a product in clinical trials, you’ll then be able to walk into a dispensary for recreational or medical cannabis and get a product that produces the same effect.
Q: What have you seen in your own patient population in terms of cannabis use?
A: I find what’s been found with the studies: It helps with pain and people’s sense of muscle stiffness.
It’s especially helpful in people with pain and spasticity that breaks through in the late afternoon or at night when they’re trying to go to sleep. Just a little bit of cannabis can get them through those difficult times and improve their quality of life.
Q: What choices do patients make regarding whether to get high from the cannabis they use?
A: Some have absolutely zero interest in getting high, and they try to avoid the THC-containing products. Other like getting high in addition to getting help with pain and spasticity.
Q: Who should not use medical marijuana in the MS community?
A: Patients who don’t have symptoms that could respond.
I’m also very concerned about patients who are 25 years and younger because of the effects that cannabis can have on brain development out to age 25 and the higher risk of addiction in people who are younger.
Q: What do you think the future will hold on the cannabis front?
A: Now that it’s less of a taboo topic, there’s an ever-growing number of trials each year, including very high-quality studies.
Pharmaceutically produced, cannabis-based medicines will be a growing area. Epidiolex is a perfect example of that.
It’s important for physicians to know that the way cannabis-based medicine is produced by a pharmaceutical company is different in so many levels than the cannabis in states with recreational and medical marijuana.
Q: What are some ways that the pharmaceutical products are different?
A: The rigor of the production process, the standardization, the purity, the correct labeling and expiration dates. Plus, the lack of the use of pesticides and other contaminants. And they’re distributed by pharmacists.
Q: What should neurologists be thinking if they’re considering whether to recommend cannabis to their patients?
A: This is a very complex topic, and it’s not something that most of us have training in. You can’t sit down for 1 or 2 hours, get up to speed, and have your own well-informed opinion on it. You really need to put more time and effort.
Q: What are some issues that neurologists should consider?
A: You really need to find out what your state is doing about it and see how you feel about that.
How is your state administering medical and/or recreational marijuana? The administration of these programs is extremely different from state to state. Do these details satisfy you, and are you content having your patients interface with these programs?
Dr. Bowling reports no relevant disclosures.
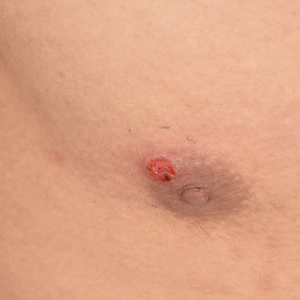
Painless Ulcer on the Areola
The Diagnosis: Primary Syphilitic Chancre of the Nipple
Because laboratory investigation was negative, a primary syphilitic chancre was suspected based on clinical findings, which was confirmed by a positive rapid plasma reagin with a titer of 1:32 and a positive Treponema pallidum particle agglutination assay. Results were negative for human immunodeficiency virus. On further inquiry, the patient acknowledged that the right areola had been traumatized during sexual activity with his regular male partner 1 month prior. In the last year he reported having had 5 different male partners. He was treated with a single dose of 2.4 million IU of intramuscular benzathine penicillin. Screening for other sexually transmitted infections revealed concomitant gonococcal infection of the pharynx and chlamydia proctitis, both of which were subsequently treated. On follow-up 2 weeks after presentation the ulcer had resolved, and he currently is undergoing serial rapid plasma reagin titer monitoring.
Primary syphilitic chancres can occur at any mucocutaneous site of inoculation, most frequently on the genitalia.1 Classically, after an incubation period of 9 to 90 days, a painless indurated ulcer forms2 and heals spontaneously after 3 to 6 weeks if left untreated.3 Chancres at extragenital sites are uncommon, occurring in approximately 2% of patients with primary syphilis.1 Of them, common sites include the lips and mouth (40%-70%),4 with areolar involvement rarely being reported. A PubMed search of articles indexed for MEDLINE using the terms nipple and chancre revealed 9 case reports in the English-language literature, with the first 2 cases being reported by Lee et al5 in 2006. The characteristics of these cases and our patient are summarized in the Table.5-12
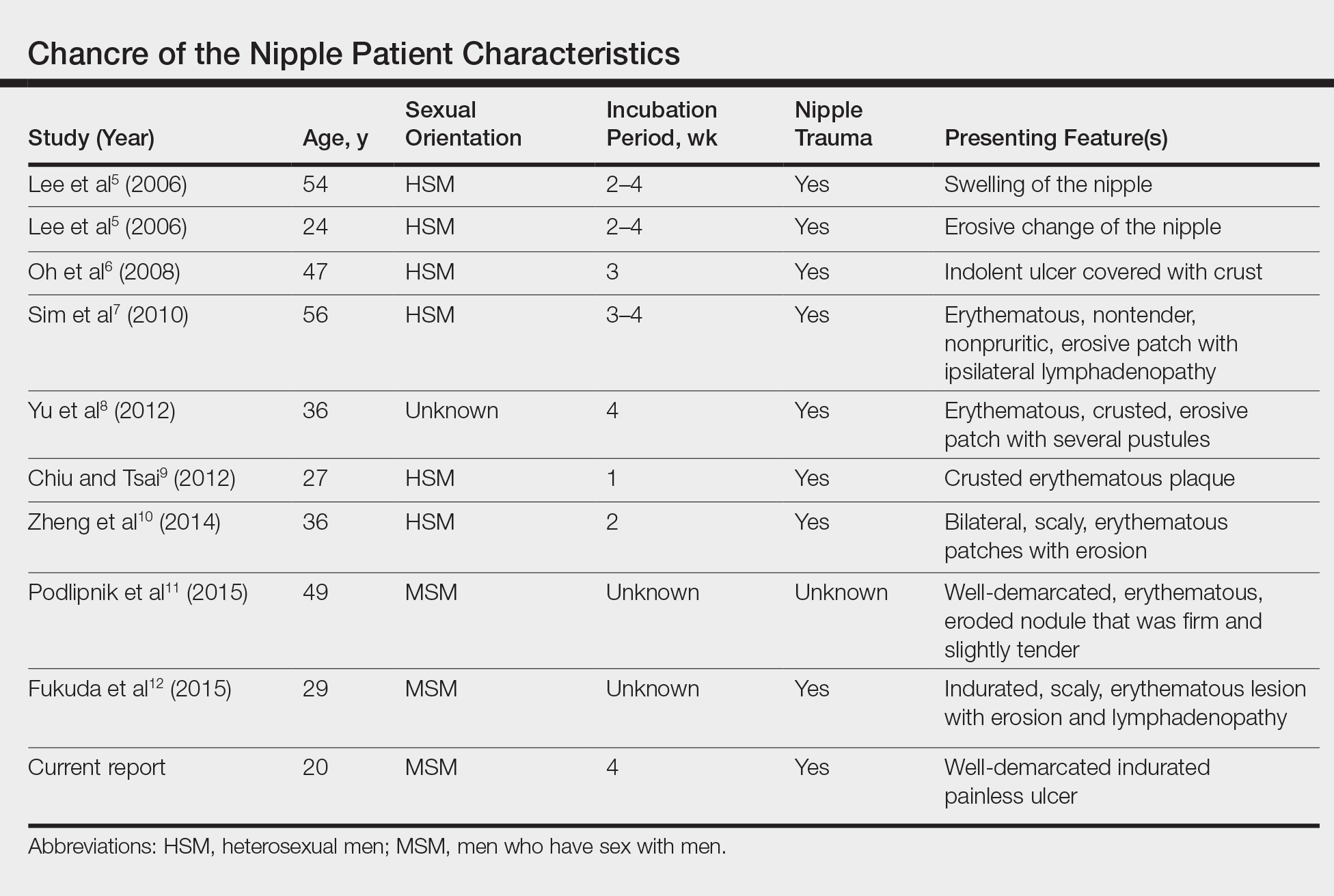
Oral contact or traumatization of the nipple by the patient's sexual partner was reported in all but one of these cases5-10,12; trauma was unknown in one case.11 Our patient reported a similar history of trauma to the nipple. It is known that transmission of syphilis can take place via kissing or oral contact, and it has been asserted that oral syphilitic lesions are highly infectious.13 Syphilis also can be transmitted by an already infected sexual partner sustaining minor trauma at the oral mucosa, allowing Treponema pallidum from the bloodstream to be inoculated onto the nipple. Another explanation for transmission could be the Koebner phenomenon, whereby trauma at the nipple of an already infected patient could lead to the formation of a chancre.6,8
The differential diagnosis includes erosive adenomatosis of the nipple, nipple eczema, Paget disease of the breast, and ulcerated basal cell carcinoma. Erosive adenomatosis of the nipple is a benign tumor of unilateral involvement that presents as an asymptomatic eroded/ulcerated papule. Clinically, it is similar to Paget disease of the breast. Eczema of the nipple usually is associated with pruritus and epidermal changes such as scaling.7,8 Paget disease of the breast arises from the extension of breast ductal carcinoma in situ onto the skin overlying the nipple. It can present as a unilateral nipple plaque with ulceration and bloody discharge. The diagnoses of erosive adenomatosis and Paget disease are confirmed with histologic examination. Basal cell carcinoma is the most common nonmelanoma skin cancer and can present as an ulcerated plaque, often with rolled borders, pearly edges, and overlying telangiectasia. It is known to be locally invasive. A punch biopsy and histopathologic examination would confirm the diagnosis of basal cell carcinoma.14
Extragenital chancres, especially those occurring at unusual sites, are uncommon. Therefore, a high index of suspicion is required to diagnose and initiate appropriate treatment for these patients.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Goh B. Syphilis in adults. Sex Transm Infect. 2005;81:448-452.
- Katz KA. Syphilis. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:2471-2492.
- Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187-209.
- Lee JY, Lin MH, Jung YC. Extragenital syphilitic chancre manifesting as a solitary nodule of the nipple. J Eur Acad Dermatol Venereol. 2006;20:886-887.
- Oh Y, Ahn S, Hong SP, et al. A case of extragenital chancre on a nipple from a human bite during sexual intercourse. Int J Dermatol. 2008;47:978-980.
- Sim JH, Lee MG, In SI, et al. Erythematous erosive patch on the left nipple--quiz case. diagnosis: extragenital syphilitic chancres. Arch Dermatol. 2010;146:81-86.
- Yu M, Lee HR, Han TY, et al. A solitary erosive patch on the left nipple. extragenital syphilitic chancres. Int J Dermatol. 2012;51:27-28.
- Chiu HY, Tsai TF. A crusted plaque on the right nipple. JAMA. 2012;308:403-404.
- Zheng S, Liu J, Xu XG, et al. Primary syphilis presenting as bilateral nipple-areola eczematoid lesions. Acta Derm Venereol. 2014;94:617-618.
- Podlipnik S, Giavedoni P, Alsina M, et al. An erythematous nodule on the nipple: an unusual presentation of primary syphilis. J Cutan Pathol. 2015;42:239-243.
- Fukuda H, Takahashi M, Kato K, et al. Multiple primary syphilis on the lip, nipple-areola and penis: an immunohistochemical examination of Treponema pallidum localization using an anti-T. pallidum antibody. J Dermatol. 2015;42:515-517.
- Yu X, Zheng H. Syphilitic chancre of the lips transmitted by kissing. Medicine (Baltimore). 2016;95:E3303.
- Carucci JA, Leffell DJ, Pettersen JS. Basal cell carcinoma. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:1294-1303.
The Diagnosis: Primary Syphilitic Chancre of the Nipple
Because laboratory investigation was negative, a primary syphilitic chancre was suspected based on clinical findings, which was confirmed by a positive rapid plasma reagin with a titer of 1:32 and a positive Treponema pallidum particle agglutination assay. Results were negative for human immunodeficiency virus. On further inquiry, the patient acknowledged that the right areola had been traumatized during sexual activity with his regular male partner 1 month prior. In the last year he reported having had 5 different male partners. He was treated with a single dose of 2.4 million IU of intramuscular benzathine penicillin. Screening for other sexually transmitted infections revealed concomitant gonococcal infection of the pharynx and chlamydia proctitis, both of which were subsequently treated. On follow-up 2 weeks after presentation the ulcer had resolved, and he currently is undergoing serial rapid plasma reagin titer monitoring.
Primary syphilitic chancres can occur at any mucocutaneous site of inoculation, most frequently on the genitalia.1 Classically, after an incubation period of 9 to 90 days, a painless indurated ulcer forms2 and heals spontaneously after 3 to 6 weeks if left untreated.3 Chancres at extragenital sites are uncommon, occurring in approximately 2% of patients with primary syphilis.1 Of them, common sites include the lips and mouth (40%-70%),4 with areolar involvement rarely being reported. A PubMed search of articles indexed for MEDLINE using the terms nipple and chancre revealed 9 case reports in the English-language literature, with the first 2 cases being reported by Lee et al5 in 2006. The characteristics of these cases and our patient are summarized in the Table.5-12

Oral contact or traumatization of the nipple by the patient's sexual partner was reported in all but one of these cases5-10,12; trauma was unknown in one case.11 Our patient reported a similar history of trauma to the nipple. It is known that transmission of syphilis can take place via kissing or oral contact, and it has been asserted that oral syphilitic lesions are highly infectious.13 Syphilis also can be transmitted by an already infected sexual partner sustaining minor trauma at the oral mucosa, allowing Treponema pallidum from the bloodstream to be inoculated onto the nipple. Another explanation for transmission could be the Koebner phenomenon, whereby trauma at the nipple of an already infected patient could lead to the formation of a chancre.6,8
The differential diagnosis includes erosive adenomatosis of the nipple, nipple eczema, Paget disease of the breast, and ulcerated basal cell carcinoma. Erosive adenomatosis of the nipple is a benign tumor of unilateral involvement that presents as an asymptomatic eroded/ulcerated papule. Clinically, it is similar to Paget disease of the breast. Eczema of the nipple usually is associated with pruritus and epidermal changes such as scaling.7,8 Paget disease of the breast arises from the extension of breast ductal carcinoma in situ onto the skin overlying the nipple. It can present as a unilateral nipple plaque with ulceration and bloody discharge. The diagnoses of erosive adenomatosis and Paget disease are confirmed with histologic examination. Basal cell carcinoma is the most common nonmelanoma skin cancer and can present as an ulcerated plaque, often with rolled borders, pearly edges, and overlying telangiectasia. It is known to be locally invasive. A punch biopsy and histopathologic examination would confirm the diagnosis of basal cell carcinoma.14
Extragenital chancres, especially those occurring at unusual sites, are uncommon. Therefore, a high index of suspicion is required to diagnose and initiate appropriate treatment for these patients.
The Diagnosis: Primary Syphilitic Chancre of the Nipple
Because laboratory investigation was negative, a primary syphilitic chancre was suspected based on clinical findings, which was confirmed by a positive rapid plasma reagin with a titer of 1:32 and a positive Treponema pallidum particle agglutination assay. Results were negative for human immunodeficiency virus. On further inquiry, the patient acknowledged that the right areola had been traumatized during sexual activity with his regular male partner 1 month prior. In the last year he reported having had 5 different male partners. He was treated with a single dose of 2.4 million IU of intramuscular benzathine penicillin. Screening for other sexually transmitted infections revealed concomitant gonococcal infection of the pharynx and chlamydia proctitis, both of which were subsequently treated. On follow-up 2 weeks after presentation the ulcer had resolved, and he currently is undergoing serial rapid plasma reagin titer monitoring.
Primary syphilitic chancres can occur at any mucocutaneous site of inoculation, most frequently on the genitalia.1 Classically, after an incubation period of 9 to 90 days, a painless indurated ulcer forms2 and heals spontaneously after 3 to 6 weeks if left untreated.3 Chancres at extragenital sites are uncommon, occurring in approximately 2% of patients with primary syphilis.1 Of them, common sites include the lips and mouth (40%-70%),4 with areolar involvement rarely being reported. A PubMed search of articles indexed for MEDLINE using the terms nipple and chancre revealed 9 case reports in the English-language literature, with the first 2 cases being reported by Lee et al5 in 2006. The characteristics of these cases and our patient are summarized in the Table.5-12

Oral contact or traumatization of the nipple by the patient's sexual partner was reported in all but one of these cases5-10,12; trauma was unknown in one case.11 Our patient reported a similar history of trauma to the nipple. It is known that transmission of syphilis can take place via kissing or oral contact, and it has been asserted that oral syphilitic lesions are highly infectious.13 Syphilis also can be transmitted by an already infected sexual partner sustaining minor trauma at the oral mucosa, allowing Treponema pallidum from the bloodstream to be inoculated onto the nipple. Another explanation for transmission could be the Koebner phenomenon, whereby trauma at the nipple of an already infected patient could lead to the formation of a chancre.6,8
The differential diagnosis includes erosive adenomatosis of the nipple, nipple eczema, Paget disease of the breast, and ulcerated basal cell carcinoma. Erosive adenomatosis of the nipple is a benign tumor of unilateral involvement that presents as an asymptomatic eroded/ulcerated papule. Clinically, it is similar to Paget disease of the breast. Eczema of the nipple usually is associated with pruritus and epidermal changes such as scaling.7,8 Paget disease of the breast arises from the extension of breast ductal carcinoma in situ onto the skin overlying the nipple. It can present as a unilateral nipple plaque with ulceration and bloody discharge. The diagnoses of erosive adenomatosis and Paget disease are confirmed with histologic examination. Basal cell carcinoma is the most common nonmelanoma skin cancer and can present as an ulcerated plaque, often with rolled borders, pearly edges, and overlying telangiectasia. It is known to be locally invasive. A punch biopsy and histopathologic examination would confirm the diagnosis of basal cell carcinoma.14
Extragenital chancres, especially those occurring at unusual sites, are uncommon. Therefore, a high index of suspicion is required to diagnose and initiate appropriate treatment for these patients.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Goh B. Syphilis in adults. Sex Transm Infect. 2005;81:448-452.
- Katz KA. Syphilis. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:2471-2492.
- Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187-209.
- Lee JY, Lin MH, Jung YC. Extragenital syphilitic chancre manifesting as a solitary nodule of the nipple. J Eur Acad Dermatol Venereol. 2006;20:886-887.
- Oh Y, Ahn S, Hong SP, et al. A case of extragenital chancre on a nipple from a human bite during sexual intercourse. Int J Dermatol. 2008;47:978-980.
- Sim JH, Lee MG, In SI, et al. Erythematous erosive patch on the left nipple--quiz case. diagnosis: extragenital syphilitic chancres. Arch Dermatol. 2010;146:81-86.
- Yu M, Lee HR, Han TY, et al. A solitary erosive patch on the left nipple. extragenital syphilitic chancres. Int J Dermatol. 2012;51:27-28.
- Chiu HY, Tsai TF. A crusted plaque on the right nipple. JAMA. 2012;308:403-404.
- Zheng S, Liu J, Xu XG, et al. Primary syphilis presenting as bilateral nipple-areola eczematoid lesions. Acta Derm Venereol. 2014;94:617-618.
- Podlipnik S, Giavedoni P, Alsina M, et al. An erythematous nodule on the nipple: an unusual presentation of primary syphilis. J Cutan Pathol. 2015;42:239-243.
- Fukuda H, Takahashi M, Kato K, et al. Multiple primary syphilis on the lip, nipple-areola and penis: an immunohistochemical examination of Treponema pallidum localization using an anti-T. pallidum antibody. J Dermatol. 2015;42:515-517.
- Yu X, Zheng H. Syphilitic chancre of the lips transmitted by kissing. Medicine (Baltimore). 2016;95:E3303.
- Carucci JA, Leffell DJ, Pettersen JS. Basal cell carcinoma. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:1294-1303.
- Mindel A, Tovey SJ, Timmins DJ, et al. Primary and secondary syphilis, 20 years' experience. 2. clinical features. Genitourin Med. 1989;65:1-3.
- Goh B. Syphilis in adults. Sex Transm Infect. 2005;81:448-452.
- Katz KA. Syphilis. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:2471-2492.
- Singh AE, Romanowski B. Syphilis: review with emphasis on clinical, epidemiologic, and some biologic features. Clin Microbiol Rev. 1999;12:187-209.
- Lee JY, Lin MH, Jung YC. Extragenital syphilitic chancre manifesting as a solitary nodule of the nipple. J Eur Acad Dermatol Venereol. 2006;20:886-887.
- Oh Y, Ahn S, Hong SP, et al. A case of extragenital chancre on a nipple from a human bite during sexual intercourse. Int J Dermatol. 2008;47:978-980.
- Sim JH, Lee MG, In SI, et al. Erythematous erosive patch on the left nipple--quiz case. diagnosis: extragenital syphilitic chancres. Arch Dermatol. 2010;146:81-86.
- Yu M, Lee HR, Han TY, et al. A solitary erosive patch on the left nipple. extragenital syphilitic chancres. Int J Dermatol. 2012;51:27-28.
- Chiu HY, Tsai TF. A crusted plaque on the right nipple. JAMA. 2012;308:403-404.
- Zheng S, Liu J, Xu XG, et al. Primary syphilis presenting as bilateral nipple-areola eczematoid lesions. Acta Derm Venereol. 2014;94:617-618.
- Podlipnik S, Giavedoni P, Alsina M, et al. An erythematous nodule on the nipple: an unusual presentation of primary syphilis. J Cutan Pathol. 2015;42:239-243.
- Fukuda H, Takahashi M, Kato K, et al. Multiple primary syphilis on the lip, nipple-areola and penis: an immunohistochemical examination of Treponema pallidum localization using an anti-T. pallidum antibody. J Dermatol. 2015;42:515-517.
- Yu X, Zheng H. Syphilitic chancre of the lips transmitted by kissing. Medicine (Baltimore). 2016;95:E3303.
- Carucci JA, Leffell DJ, Pettersen JS. Basal cell carcinoma. In: Goldsmith LA, Katz SI, Gilchrest BA, eds. Fitzpatrick's Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill Medical; 2012:1294-1303.
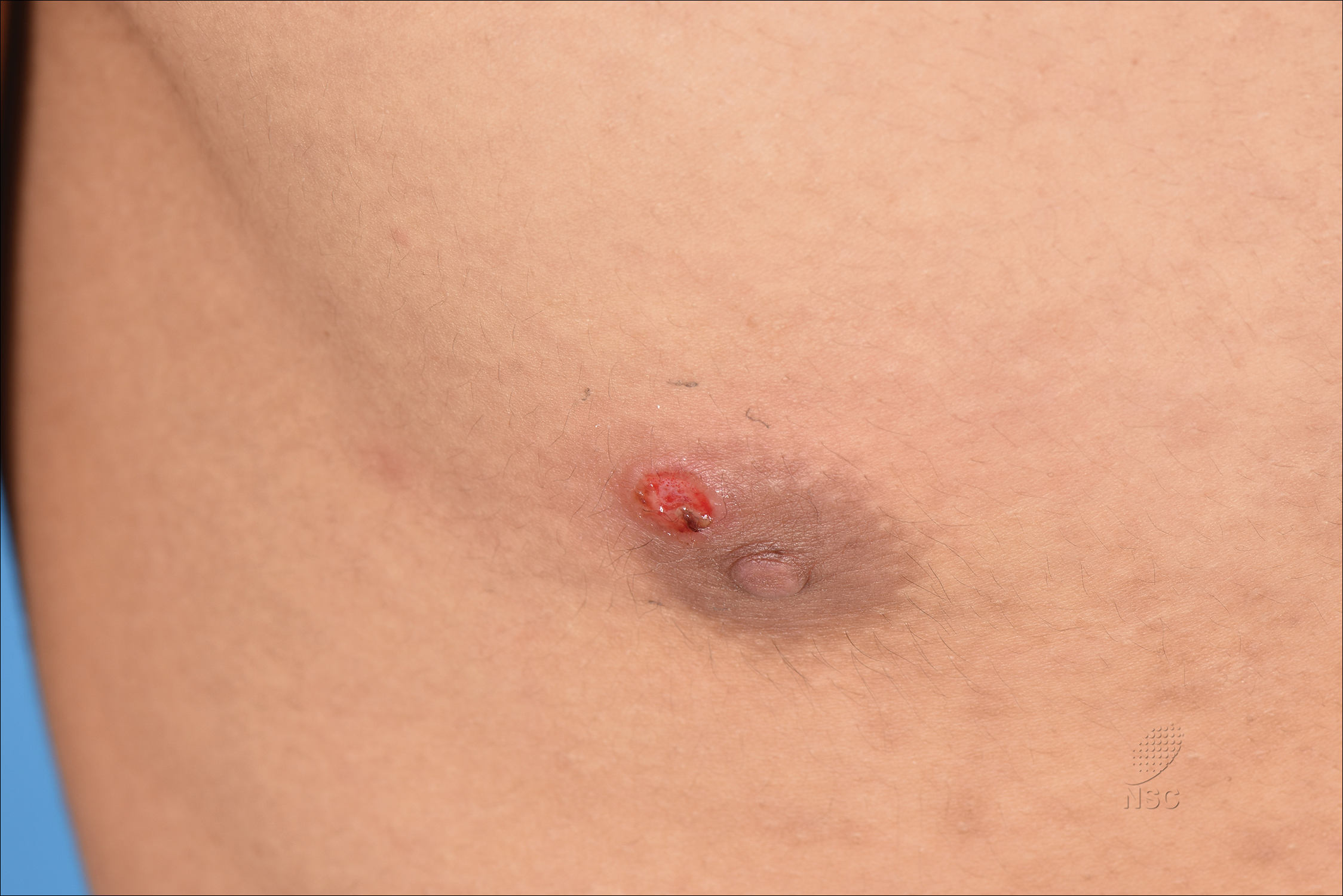
A previously healthy 20-year-old Chinese man presented to our dermatology outpatient clinic with a solitary painless ulcer on the right areola of 1 week's duration. Examination showed a small, slightly indurated ulcer with well-defined borders. No lesions were noted elsewhere. Swabs for pyogenic culture and herpes simplex virus polymerase chain reaction tests were sent, and he was treated empirically with oral cephalexin and tetracycline ointment 3%. At 1-week follow-up the ulcer had dried up and begun to heal, and results from the laboratory investigations were negative.
New insights into sleep, pregnancy weight gain
BALTIMORE – Pregnant women who are overweight and obese are like the general population in that the less they sleep, the more weight they gain, particularly in the first half of pregnancy. However, unlike in the larger adult population, prolonged daily total eating time was not associated with gestational weight gain in these women, particularly early in pregnancy, according to findings from a small study presented at the Associated Professional Sleep Societies annual meeting.
Those findings point to a need to further study the , said Rachel P. Kolko, PhD, a postdoctoral scholar at the University of Pittsburgh, Western Psychiatric Institute and Clinic.
“The association with total sleep time was found to be significant, such that if you had less sleep, you had higher amounts of weight gain; we did not find a significant relation with our eating window variable,” Dr. Kolko said.
She reported on research involving 62 pregnant women, 53% of whom were overweight with a body mass index of 25-29.9 kg/m2 and 47% of whom were obese with BMI greater than 30. Forty-seven percent of the study population was nonwhite.
The research grew out of a need to identify potentially modifiable factors to curtail excessive gestational weight gain during pregnancy, she said. The study hypotheses were that both shorter total sleep time and longer total eating time would lead to higher gestational weight gain, but the study confirmed only the former as a contributing factor.
The women in the study were at 12-20 weeks of pregnancy. Gestational weight gain was calculated as the difference between self-reported prepregnancy weight and current weight. Total sleep time was based on the Pittsburgh Sleep Quality Index, and total eating time was calculated as the time difference between the day’s first meal or snack of more than 50 calories and the last, as self-reported.
Average total sleep time was 7.8 hours, with total eating time spanning 10.8 hours. On average, study participants gained 9.7 pounds through the first half of pregnancy, Dr. Kolko said. She noted that the Institute of Medicine, now known as the National Academy of Medicine, recommends that women who are overweight women gain 15-25 pounds during pregnancy and women who are obese gain 11-20 pounds (JAMA. 2017;317:2207-25). “Already about 20% of our sample has gained that amount of weight within the first half of pregnancy,” she said.
“Total sleep time was related to a higher early gestational weight in women with overweight and obesity, and it’s possible that addressing this may affect and hopefully improve women’s weight gain during early pregnancy to fit within those guidelines,” she said.
Future research should look at the entire gestational period – possibly targeting sleep patterns during pregnancy – and should expand to include women who are not overweight or obese, Dr. Kolko noted.
Dr. Kolko reported having no financial relationships to disclose.
SOURCE: Kolko RP, et al., SLEEP 2018, Abstract 0692.
BALTIMORE – Pregnant women who are overweight and obese are like the general population in that the less they sleep, the more weight they gain, particularly in the first half of pregnancy. However, unlike in the larger adult population, prolonged daily total eating time was not associated with gestational weight gain in these women, particularly early in pregnancy, according to findings from a small study presented at the Associated Professional Sleep Societies annual meeting.
Those findings point to a need to further study the , said Rachel P. Kolko, PhD, a postdoctoral scholar at the University of Pittsburgh, Western Psychiatric Institute and Clinic.
“The association with total sleep time was found to be significant, such that if you had less sleep, you had higher amounts of weight gain; we did not find a significant relation with our eating window variable,” Dr. Kolko said.
She reported on research involving 62 pregnant women, 53% of whom were overweight with a body mass index of 25-29.9 kg/m2 and 47% of whom were obese with BMI greater than 30. Forty-seven percent of the study population was nonwhite.
The research grew out of a need to identify potentially modifiable factors to curtail excessive gestational weight gain during pregnancy, she said. The study hypotheses were that both shorter total sleep time and longer total eating time would lead to higher gestational weight gain, but the study confirmed only the former as a contributing factor.
The women in the study were at 12-20 weeks of pregnancy. Gestational weight gain was calculated as the difference between self-reported prepregnancy weight and current weight. Total sleep time was based on the Pittsburgh Sleep Quality Index, and total eating time was calculated as the time difference between the day’s first meal or snack of more than 50 calories and the last, as self-reported.
Average total sleep time was 7.8 hours, with total eating time spanning 10.8 hours. On average, study participants gained 9.7 pounds through the first half of pregnancy, Dr. Kolko said. She noted that the Institute of Medicine, now known as the National Academy of Medicine, recommends that women who are overweight women gain 15-25 pounds during pregnancy and women who are obese gain 11-20 pounds (JAMA. 2017;317:2207-25). “Already about 20% of our sample has gained that amount of weight within the first half of pregnancy,” she said.
“Total sleep time was related to a higher early gestational weight in women with overweight and obesity, and it’s possible that addressing this may affect and hopefully improve women’s weight gain during early pregnancy to fit within those guidelines,” she said.
Future research should look at the entire gestational period – possibly targeting sleep patterns during pregnancy – and should expand to include women who are not overweight or obese, Dr. Kolko noted.
Dr. Kolko reported having no financial relationships to disclose.
SOURCE: Kolko RP, et al., SLEEP 2018, Abstract 0692.
BALTIMORE – Pregnant women who are overweight and obese are like the general population in that the less they sleep, the more weight they gain, particularly in the first half of pregnancy. However, unlike in the larger adult population, prolonged daily total eating time was not associated with gestational weight gain in these women, particularly early in pregnancy, according to findings from a small study presented at the Associated Professional Sleep Societies annual meeting.
Those findings point to a need to further study the , said Rachel P. Kolko, PhD, a postdoctoral scholar at the University of Pittsburgh, Western Psychiatric Institute and Clinic.
“The association with total sleep time was found to be significant, such that if you had less sleep, you had higher amounts of weight gain; we did not find a significant relation with our eating window variable,” Dr. Kolko said.
She reported on research involving 62 pregnant women, 53% of whom were overweight with a body mass index of 25-29.9 kg/m2 and 47% of whom were obese with BMI greater than 30. Forty-seven percent of the study population was nonwhite.
The research grew out of a need to identify potentially modifiable factors to curtail excessive gestational weight gain during pregnancy, she said. The study hypotheses were that both shorter total sleep time and longer total eating time would lead to higher gestational weight gain, but the study confirmed only the former as a contributing factor.
The women in the study were at 12-20 weeks of pregnancy. Gestational weight gain was calculated as the difference between self-reported prepregnancy weight and current weight. Total sleep time was based on the Pittsburgh Sleep Quality Index, and total eating time was calculated as the time difference between the day’s first meal or snack of more than 50 calories and the last, as self-reported.
Average total sleep time was 7.8 hours, with total eating time spanning 10.8 hours. On average, study participants gained 9.7 pounds through the first half of pregnancy, Dr. Kolko said. She noted that the Institute of Medicine, now known as the National Academy of Medicine, recommends that women who are overweight women gain 15-25 pounds during pregnancy and women who are obese gain 11-20 pounds (JAMA. 2017;317:2207-25). “Already about 20% of our sample has gained that amount of weight within the first half of pregnancy,” she said.
“Total sleep time was related to a higher early gestational weight in women with overweight and obesity, and it’s possible that addressing this may affect and hopefully improve women’s weight gain during early pregnancy to fit within those guidelines,” she said.
Future research should look at the entire gestational period – possibly targeting sleep patterns during pregnancy – and should expand to include women who are not overweight or obese, Dr. Kolko noted.
Dr. Kolko reported having no financial relationships to disclose.
SOURCE: Kolko RP, et al., SLEEP 2018, Abstract 0692.
REPORTING FROM SLEEP 2018
Key clinical point: In overweight/obese women, shorter sleep times are linked to early gestational weight gain.
Major finding: Overweight/obese women slept 30% less and had higher gestational weight gain in early pregnancy.
Study details: Study of 62 women between 12 and 20 weeks’ gestation with prepregnancy BMI greater than 25 kg/m2.
Disclosures: Dr. Kolko reported having no financial relationships.
Source: Kolko RP et al. SLEEP 2018, Abstract 0692.
Pediatric dermatology admissions: Crunching the numbers
according to data from the Agency for Healthcare Research and Quality.
There were 74,229 such admissions in the United States that year – all others totaled 1.77 million – and the children at the highest risk for dermatology hospitalization were those living in communities with the lowest household incomes, the uninsured and those on Medicaid, and those living in the South, Justin D. Arnold of George Washington University, Washington, and his associates said in Pediatric Dermatology.
“Individuals from communities of low socioeconomic status may be more likely to be hospitalized because of gaps in insurance coverage, difficulty with transportation, or inconsistent access to preventative medical care, which for skin disease, would include access to an outpatient pediatric dermatologist,” they wrote.
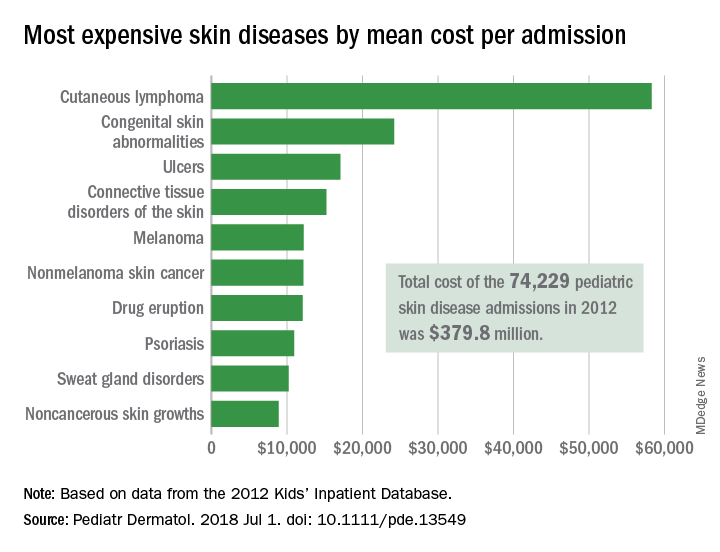
All those admissions for skin diseases cost the health care system $379.8 million in 2012, or 1.9% of the $20.3 billion spent on all pediatric hospitalizations, excluding those related to pregnancy or childbirth. The mean cost of a skin disease admission was $5,211 for a child aged less than 18 years, compared with $11,409 for nondermatology admissions, according to data from the 2012 Kids’ Inpatient Database, which includes records of pediatric discharges from 44 states.
Cutaneous lymphoma was the most expensive skin disease per admission at a mean cost of $58,294, with congenital skin abnormalities second at $24,186, and ulcers third at $17,064. Bacterial skin infections and infestations were only the 19th most expensive admission at $4,135, but it was by far the most common (59,115 admissions) and the most expensive overall, with a total cost of $240 million. The second most common condition was viral diseases with 3,812 admissions and the next most expensive total was $33.5 million for connective tissue disorders, Mr. Arnold and his associates said.
Multivariate models that adjusted for such factors as age, sex, and race revealed that “the risk of hospitalization for skin disease increased as the median income of one’s zip code declined,” the investigators noted. The adjusted odds ratio for hospitalization in the lowest-income quartile (less than $39,000) was 1.22, compared with the highest-income quartile.
Insurance status also affected hospitalization, putting children from families with no insurance (aOR, 1.35) and those on Medicaid (aOR, 1.17) at a disadvantage, compared with those who had private insurance. “Policy makers should consider increasing Medicaid reimbursement rates to outpatient dermatologists, which might encourage more clinicians to accept this form of insurance and thereby expand access to preventative skin care,” they said.
Regional differences also were observed, which put children from the southern states at the highest risk (aOR, 1.32), compared with those in the West, which could be related to access issues. “In 2016, 4 of the 10 communities in the United States with the lowest density of dermatologists were in the South, suggesting that the high rate of hospitalizations there may also be partially attributed to lack of access to dermatologists,” Mr. Arnold and his associates wrote.
The investigators did not report funding or disclose conflicts of interest.
SOURCE: Arnold JD et al. Pediatr Dermatol. 2018 Jul 1. doi: 10.1111/pde.13549.
according to data from the Agency for Healthcare Research and Quality.

There were 74,229 such admissions in the United States that year – all others totaled 1.77 million – and the children at the highest risk for dermatology hospitalization were those living in communities with the lowest household incomes, the uninsured and those on Medicaid, and those living in the South, Justin D. Arnold of George Washington University, Washington, and his associates said in Pediatric Dermatology.
“Individuals from communities of low socioeconomic status may be more likely to be hospitalized because of gaps in insurance coverage, difficulty with transportation, or inconsistent access to preventative medical care, which for skin disease, would include access to an outpatient pediatric dermatologist,” they wrote.
All those admissions for skin diseases cost the health care system $379.8 million in 2012, or 1.9% of the $20.3 billion spent on all pediatric hospitalizations, excluding those related to pregnancy or childbirth. The mean cost of a skin disease admission was $5,211 for a child aged less than 18 years, compared with $11,409 for nondermatology admissions, according to data from the 2012 Kids’ Inpatient Database, which includes records of pediatric discharges from 44 states.
Cutaneous lymphoma was the most expensive skin disease per admission at a mean cost of $58,294, with congenital skin abnormalities second at $24,186, and ulcers third at $17,064. Bacterial skin infections and infestations were only the 19th most expensive admission at $4,135, but it was by far the most common (59,115 admissions) and the most expensive overall, with a total cost of $240 million. The second most common condition was viral diseases with 3,812 admissions and the next most expensive total was $33.5 million for connective tissue disorders, Mr. Arnold and his associates said.
Multivariate models that adjusted for such factors as age, sex, and race revealed that “the risk of hospitalization for skin disease increased as the median income of one’s zip code declined,” the investigators noted. The adjusted odds ratio for hospitalization in the lowest-income quartile (less than $39,000) was 1.22, compared with the highest-income quartile.
Insurance status also affected hospitalization, putting children from families with no insurance (aOR, 1.35) and those on Medicaid (aOR, 1.17) at a disadvantage, compared with those who had private insurance. “Policy makers should consider increasing Medicaid reimbursement rates to outpatient dermatologists, which might encourage more clinicians to accept this form of insurance and thereby expand access to preventative skin care,” they said.
Regional differences also were observed, which put children from the southern states at the highest risk (aOR, 1.32), compared with those in the West, which could be related to access issues. “In 2016, 4 of the 10 communities in the United States with the lowest density of dermatologists were in the South, suggesting that the high rate of hospitalizations there may also be partially attributed to lack of access to dermatologists,” Mr. Arnold and his associates wrote.
The investigators did not report funding or disclose conflicts of interest.
SOURCE: Arnold JD et al. Pediatr Dermatol. 2018 Jul 1. doi: 10.1111/pde.13549.
according to data from the Agency for Healthcare Research and Quality.

There were 74,229 such admissions in the United States that year – all others totaled 1.77 million – and the children at the highest risk for dermatology hospitalization were those living in communities with the lowest household incomes, the uninsured and those on Medicaid, and those living in the South, Justin D. Arnold of George Washington University, Washington, and his associates said in Pediatric Dermatology.
“Individuals from communities of low socioeconomic status may be more likely to be hospitalized because of gaps in insurance coverage, difficulty with transportation, or inconsistent access to preventative medical care, which for skin disease, would include access to an outpatient pediatric dermatologist,” they wrote.
All those admissions for skin diseases cost the health care system $379.8 million in 2012, or 1.9% of the $20.3 billion spent on all pediatric hospitalizations, excluding those related to pregnancy or childbirth. The mean cost of a skin disease admission was $5,211 for a child aged less than 18 years, compared with $11,409 for nondermatology admissions, according to data from the 2012 Kids’ Inpatient Database, which includes records of pediatric discharges from 44 states.
Cutaneous lymphoma was the most expensive skin disease per admission at a mean cost of $58,294, with congenital skin abnormalities second at $24,186, and ulcers third at $17,064. Bacterial skin infections and infestations were only the 19th most expensive admission at $4,135, but it was by far the most common (59,115 admissions) and the most expensive overall, with a total cost of $240 million. The second most common condition was viral diseases with 3,812 admissions and the next most expensive total was $33.5 million for connective tissue disorders, Mr. Arnold and his associates said.
Multivariate models that adjusted for such factors as age, sex, and race revealed that “the risk of hospitalization for skin disease increased as the median income of one’s zip code declined,” the investigators noted. The adjusted odds ratio for hospitalization in the lowest-income quartile (less than $39,000) was 1.22, compared with the highest-income quartile.
Insurance status also affected hospitalization, putting children from families with no insurance (aOR, 1.35) and those on Medicaid (aOR, 1.17) at a disadvantage, compared with those who had private insurance. “Policy makers should consider increasing Medicaid reimbursement rates to outpatient dermatologists, which might encourage more clinicians to accept this form of insurance and thereby expand access to preventative skin care,” they said.
Regional differences also were observed, which put children from the southern states at the highest risk (aOR, 1.32), compared with those in the West, which could be related to access issues. “In 2016, 4 of the 10 communities in the United States with the lowest density of dermatologists were in the South, suggesting that the high rate of hospitalizations there may also be partially attributed to lack of access to dermatologists,” Mr. Arnold and his associates wrote.
The investigators did not report funding or disclose conflicts of interest.
SOURCE: Arnold JD et al. Pediatr Dermatol. 2018 Jul 1. doi: 10.1111/pde.13549.
FROM PEDIATRIC DERMATOLOGY
Key clinical point: Children at the highest risk for dermatology hospitalization lived in communities with the lowest household incomes, were uninsured or on Medicaid, or lived in the South.
Major finding: Admissions for skin diseases cost $379.8 million in 2012, or 1.9% of all spending on pediatric hospitalizations.
Study details: A statistical analysis of the 2012 Kids’ Inpatient Database.
Disclosures: The investigators did not report funding or disclose conflicts of interest.
Source: Arnold JD et al. Pediatr Dermatol. 2018 Jul 1. doi: 10.1111/pde.13549.
Family separations and the intergenerational transmission of trauma
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
Growing up, I was always intrigued by the strong emotions that even the mildest separation evoked within me. As a psychiatrist, I now believe that these emotions were related to my family’s difficulty with separation, a concept likely transmitted from my grandmother’s sudden separation as a child.
The political circumstances of the “Kindertransport” and the recent family separation at the southern U.S. border differ, but the Kindertransport is a model for studying the effects of forced parent-child separation and its intergenerational transmission. As a rescue operation that took place immediately before World War II, the Kindertransport was the emigration of approximately 10,000 German Jewish children from Germany and Nazi-occupied countries to England. Even though they were not literally forced, the parents involved were compelled to separate from their children to give their children a chance to survive.
In a recent letter1 published in The New York Times, Eva Yachnes, herself a Kindertransport participant, reflected on the current situation at the southern U.S. border. She alluded to the lifelong effects of her own separation at the age of 6 when emigrating from Vienna to Germany. Personally, as a granddaughter of a Kindertransport participant, I am particularly concerned about the intergenerational transmission of the trauma of family separation.
Since early May 2018, more than 2,000 children have been forcibly separated from their parents after illegally crossing the border. As part of a “zero tolerance policy,” the separation was characterized by the Trump administration as a deterrent to illegal border crossings. Although many Americans are horrified by the news reports about family separation, psychiatrists in particular have expressed concern about this trauma. The American Psychiatric Association issued a statement2 warning that “any forced separation is highly stressful for children and can cause lifelong trauma.” Several weeks later, the APA joined several other mental health organizations in a letter3 calling for the immediate end of enforcement of those policies. In that letter, the organizations said forced separations can cause “an increased risk of ... mental illnesses such as depression, anxiety, and posttraumatic stress disorder.”
What must psychiatrists understand about the impact of childhood trauma? How can psychiatrists approach treatment of children separated from their parents? Are there ways to minimize the risk for intergenerational impact of this trauma?
What do we know?
Childhood trauma is influenced by multiple factors and can be expressed in several ways. According to research,4 the age of the child at the time of traumatic event, the frequency of traumatic experiences, and the degree to which the child’s caretakers were involved in the trauma are factors that influence the extent of psychological damage. This research also suggests that childhood trauma is associated with emotional dysregulation, aggression against self and others, difficulties in attention and dissociation, medical problems, and difficulty with navigating adult interpersonal relationships.
When viewed through the lens of attachment theory, the forced separation of a child from its caretakers is a potent form of childhood trauma. Joanna E. Chambers, MD, summarizes and explains John Bowlby’s attachment theory as a neurobiological system originating from an infant’s connection to the primary caretaker. This connection becomes a lifelong model for all subsequent relationships.
Any traumatic disruptions in the development of this system puts the child at risk of developing “insecure attachment.” This insecure attachment can lead to lifelong emotional problems for the child, affecting the quality of subsequent marital relationships, relationships to children, and the development of personality disorders.
In addition, it correlates to the development of psychiatric illnesses, specifically depression and anxiety. There is also a plausible biological basis for attachment theory. Both oxytocin, a hormone released in human bonding, and social interaction itself have been shown to decrease cortisol levels. Elevated cortisol has been found to negatively affect infant brain development, Dr. Chambers argued.5
Given those significant effects of childhood trauma, it is understandable that there exists a concept of “intergenerational transmission of trauma.” Originating from studies of Holocaust survivors and their descendants, researchers Amy Lehrner, PhD, and Rachel Yehuda, PhD, conceptualize intergenerational transmission of trauma as the intergenerational impact of prenatal PTSD.6 This impact is expressed as a predisposition in the offspring of Holocaust survivors to developing PTSD, difficulties in individuation and separation, higher rates of mood and anxiety disorders, and higher rates of physical health issues.
Although they are complex and clearly multidetermined, Dr. Yehuda and Dr. Lehrner also summarize plausible biological theories for the intergenerational transmission of trauma. Epigenetic differences in the hypothalamic-pituitary-adrenal axis, circadian rhythm, urinary and plasma cortisol levels, glucocorticoid sensitivity, and regulation of the glucocorticoid receptor gene all have been found in Holocaust offspring with parental PTSD, in contrast to offspring without parental PTSD.6
What can we as psychiatrists do?
We are uniquely equipped to take several concrete steps to help mitigate the effects of these traumatic events. Among them, we can:
- Provide opportunities for the child and the family to process their experience. This can be profoundly healing and can help minimize the devastating psychological effects of this separation.
- Become acquainted with the concept of intergenerational transmission of resilience.
- Work with trauma survivors to develop their own personal narratives and cultural rituals surrounding the trauma.6
- Encourage second- and third-generation descendants to engage in artistic expression of the trauma, visit places of importance to their parents, and engage in social and political activism. These are all expressions of resilience in the offspring of trauma victims.6
In summary, recent U.S. political events have caused thousands of children to be forcibly separated from their parents. Those separations are traumatic and can have lifelong psychological implications for the children and their offspring. It is important to provide quality mental health treatment to these children with a specific focus on treating PTSD and processing the traumatic experience. Psychological treatment can help mitigate the effects of the traumatic separation and create a sense of resiliency.
References
1. New York Times. June 20, 2018. “My separation trauma.”
2. American Psychiatric Association statement, May 30, 2018.
3. Letter to the departments of Justice, Health & Human Services, and Homeland Security, June 20, 2018.
4. Child Adolesc Psychiatric Clin N Am. 2003;(12.2):293-318.
5. Psychodynamic Psychiatry. 2017 Dec;45(4):542-63.
6. Psychological Trauma: Theory, Research, Practice, and Policy. 2018 Jan;10(1):22-9.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital of the Northwell Health System, Glen Oaks, New York. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine after graduating from the Albert Einstein College of Medicine and Yeshiva University with a B.A. in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
Growing up, I was always intrigued by the strong emotions that even the mildest separation evoked within me. As a psychiatrist, I now believe that these emotions were related to my family’s difficulty with separation, a concept likely transmitted from my grandmother’s sudden separation as a child.
The political circumstances of the “Kindertransport” and the recent family separation at the southern U.S. border differ, but the Kindertransport is a model for studying the effects of forced parent-child separation and its intergenerational transmission. As a rescue operation that took place immediately before World War II, the Kindertransport was the emigration of approximately 10,000 German Jewish children from Germany and Nazi-occupied countries to England. Even though they were not literally forced, the parents involved were compelled to separate from their children to give their children a chance to survive.
In a recent letter1 published in The New York Times, Eva Yachnes, herself a Kindertransport participant, reflected on the current situation at the southern U.S. border. She alluded to the lifelong effects of her own separation at the age of 6 when emigrating from Vienna to Germany. Personally, as a granddaughter of a Kindertransport participant, I am particularly concerned about the intergenerational transmission of the trauma of family separation.
Since early May 2018, more than 2,000 children have been forcibly separated from their parents after illegally crossing the border. As part of a “zero tolerance policy,” the separation was characterized by the Trump administration as a deterrent to illegal border crossings. Although many Americans are horrified by the news reports about family separation, psychiatrists in particular have expressed concern about this trauma. The American Psychiatric Association issued a statement2 warning that “any forced separation is highly stressful for children and can cause lifelong trauma.” Several weeks later, the APA joined several other mental health organizations in a letter3 calling for the immediate end of enforcement of those policies. In that letter, the organizations said forced separations can cause “an increased risk of ... mental illnesses such as depression, anxiety, and posttraumatic stress disorder.”
What must psychiatrists understand about the impact of childhood trauma? How can psychiatrists approach treatment of children separated from their parents? Are there ways to minimize the risk for intergenerational impact of this trauma?
What do we know?
Childhood trauma is influenced by multiple factors and can be expressed in several ways. According to research,4 the age of the child at the time of traumatic event, the frequency of traumatic experiences, and the degree to which the child’s caretakers were involved in the trauma are factors that influence the extent of psychological damage. This research also suggests that childhood trauma is associated with emotional dysregulation, aggression against self and others, difficulties in attention and dissociation, medical problems, and difficulty with navigating adult interpersonal relationships.
When viewed through the lens of attachment theory, the forced separation of a child from its caretakers is a potent form of childhood trauma. Joanna E. Chambers, MD, summarizes and explains John Bowlby’s attachment theory as a neurobiological system originating from an infant’s connection to the primary caretaker. This connection becomes a lifelong model for all subsequent relationships.
Any traumatic disruptions in the development of this system puts the child at risk of developing “insecure attachment.” This insecure attachment can lead to lifelong emotional problems for the child, affecting the quality of subsequent marital relationships, relationships to children, and the development of personality disorders.
In addition, it correlates to the development of psychiatric illnesses, specifically depression and anxiety. There is also a plausible biological basis for attachment theory. Both oxytocin, a hormone released in human bonding, and social interaction itself have been shown to decrease cortisol levels. Elevated cortisol has been found to negatively affect infant brain development, Dr. Chambers argued.5
Given those significant effects of childhood trauma, it is understandable that there exists a concept of “intergenerational transmission of trauma.” Originating from studies of Holocaust survivors and their descendants, researchers Amy Lehrner, PhD, and Rachel Yehuda, PhD, conceptualize intergenerational transmission of trauma as the intergenerational impact of prenatal PTSD.6 This impact is expressed as a predisposition in the offspring of Holocaust survivors to developing PTSD, difficulties in individuation and separation, higher rates of mood and anxiety disorders, and higher rates of physical health issues.
Although they are complex and clearly multidetermined, Dr. Yehuda and Dr. Lehrner also summarize plausible biological theories for the intergenerational transmission of trauma. Epigenetic differences in the hypothalamic-pituitary-adrenal axis, circadian rhythm, urinary and plasma cortisol levels, glucocorticoid sensitivity, and regulation of the glucocorticoid receptor gene all have been found in Holocaust offspring with parental PTSD, in contrast to offspring without parental PTSD.6
What can we as psychiatrists do?
We are uniquely equipped to take several concrete steps to help mitigate the effects of these traumatic events. Among them, we can:
- Provide opportunities for the child and the family to process their experience. This can be profoundly healing and can help minimize the devastating psychological effects of this separation.
- Become acquainted with the concept of intergenerational transmission of resilience.
- Work with trauma survivors to develop their own personal narratives and cultural rituals surrounding the trauma.6
- Encourage second- and third-generation descendants to engage in artistic expression of the trauma, visit places of importance to their parents, and engage in social and political activism. These are all expressions of resilience in the offspring of trauma victims.6
In summary, recent U.S. political events have caused thousands of children to be forcibly separated from their parents. Those separations are traumatic and can have lifelong psychological implications for the children and their offspring. It is important to provide quality mental health treatment to these children with a specific focus on treating PTSD and processing the traumatic experience. Psychological treatment can help mitigate the effects of the traumatic separation and create a sense of resiliency.
References
1. New York Times. June 20, 2018. “My separation trauma.”
2. American Psychiatric Association statement, May 30, 2018.
3. Letter to the departments of Justice, Health & Human Services, and Homeland Security, June 20, 2018.
4. Child Adolesc Psychiatric Clin N Am. 2003;(12.2):293-318.
5. Psychodynamic Psychiatry. 2017 Dec;45(4):542-63.
6. Psychological Trauma: Theory, Research, Practice, and Policy. 2018 Jan;10(1):22-9.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital of the Northwell Health System, Glen Oaks, New York. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine after graduating from the Albert Einstein College of Medicine and Yeshiva University with a B.A. in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.
Editor’s Note: Alison M. Heru, MD, the Families in Psychiatry columnist, invited Dr. Reinstein to address this topic.
Growing up, I was always intrigued by the strong emotions that even the mildest separation evoked within me. As a psychiatrist, I now believe that these emotions were related to my family’s difficulty with separation, a concept likely transmitted from my grandmother’s sudden separation as a child.
The political circumstances of the “Kindertransport” and the recent family separation at the southern U.S. border differ, but the Kindertransport is a model for studying the effects of forced parent-child separation and its intergenerational transmission. As a rescue operation that took place immediately before World War II, the Kindertransport was the emigration of approximately 10,000 German Jewish children from Germany and Nazi-occupied countries to England. Even though they were not literally forced, the parents involved were compelled to separate from their children to give their children a chance to survive.
In a recent letter1 published in The New York Times, Eva Yachnes, herself a Kindertransport participant, reflected on the current situation at the southern U.S. border. She alluded to the lifelong effects of her own separation at the age of 6 when emigrating from Vienna to Germany. Personally, as a granddaughter of a Kindertransport participant, I am particularly concerned about the intergenerational transmission of the trauma of family separation.
Since early May 2018, more than 2,000 children have been forcibly separated from their parents after illegally crossing the border. As part of a “zero tolerance policy,” the separation was characterized by the Trump administration as a deterrent to illegal border crossings. Although many Americans are horrified by the news reports about family separation, psychiatrists in particular have expressed concern about this trauma. The American Psychiatric Association issued a statement2 warning that “any forced separation is highly stressful for children and can cause lifelong trauma.” Several weeks later, the APA joined several other mental health organizations in a letter3 calling for the immediate end of enforcement of those policies. In that letter, the organizations said forced separations can cause “an increased risk of ... mental illnesses such as depression, anxiety, and posttraumatic stress disorder.”
What must psychiatrists understand about the impact of childhood trauma? How can psychiatrists approach treatment of children separated from their parents? Are there ways to minimize the risk for intergenerational impact of this trauma?
What do we know?
Childhood trauma is influenced by multiple factors and can be expressed in several ways. According to research,4 the age of the child at the time of traumatic event, the frequency of traumatic experiences, and the degree to which the child’s caretakers were involved in the trauma are factors that influence the extent of psychological damage. This research also suggests that childhood trauma is associated with emotional dysregulation, aggression against self and others, difficulties in attention and dissociation, medical problems, and difficulty with navigating adult interpersonal relationships.
When viewed through the lens of attachment theory, the forced separation of a child from its caretakers is a potent form of childhood trauma. Joanna E. Chambers, MD, summarizes and explains John Bowlby’s attachment theory as a neurobiological system originating from an infant’s connection to the primary caretaker. This connection becomes a lifelong model for all subsequent relationships.
Any traumatic disruptions in the development of this system puts the child at risk of developing “insecure attachment.” This insecure attachment can lead to lifelong emotional problems for the child, affecting the quality of subsequent marital relationships, relationships to children, and the development of personality disorders.
In addition, it correlates to the development of psychiatric illnesses, specifically depression and anxiety. There is also a plausible biological basis for attachment theory. Both oxytocin, a hormone released in human bonding, and social interaction itself have been shown to decrease cortisol levels. Elevated cortisol has been found to negatively affect infant brain development, Dr. Chambers argued.5
Given those significant effects of childhood trauma, it is understandable that there exists a concept of “intergenerational transmission of trauma.” Originating from studies of Holocaust survivors and their descendants, researchers Amy Lehrner, PhD, and Rachel Yehuda, PhD, conceptualize intergenerational transmission of trauma as the intergenerational impact of prenatal PTSD.6 This impact is expressed as a predisposition in the offspring of Holocaust survivors to developing PTSD, difficulties in individuation and separation, higher rates of mood and anxiety disorders, and higher rates of physical health issues.
Although they are complex and clearly multidetermined, Dr. Yehuda and Dr. Lehrner also summarize plausible biological theories for the intergenerational transmission of trauma. Epigenetic differences in the hypothalamic-pituitary-adrenal axis, circadian rhythm, urinary and plasma cortisol levels, glucocorticoid sensitivity, and regulation of the glucocorticoid receptor gene all have been found in Holocaust offspring with parental PTSD, in contrast to offspring without parental PTSD.6
What can we as psychiatrists do?
We are uniquely equipped to take several concrete steps to help mitigate the effects of these traumatic events. Among them, we can:
- Provide opportunities for the child and the family to process their experience. This can be profoundly healing and can help minimize the devastating psychological effects of this separation.
- Become acquainted with the concept of intergenerational transmission of resilience.
- Work with trauma survivors to develop their own personal narratives and cultural rituals surrounding the trauma.6
- Encourage second- and third-generation descendants to engage in artistic expression of the trauma, visit places of importance to their parents, and engage in social and political activism. These are all expressions of resilience in the offspring of trauma victims.6
In summary, recent U.S. political events have caused thousands of children to be forcibly separated from their parents. Those separations are traumatic and can have lifelong psychological implications for the children and their offspring. It is important to provide quality mental health treatment to these children with a specific focus on treating PTSD and processing the traumatic experience. Psychological treatment can help mitigate the effects of the traumatic separation and create a sense of resiliency.
References
1. New York Times. June 20, 2018. “My separation trauma.”
2. American Psychiatric Association statement, May 30, 2018.
3. Letter to the departments of Justice, Health & Human Services, and Homeland Security, June 20, 2018.
4. Child Adolesc Psychiatric Clin N Am. 2003;(12.2):293-318.
5. Psychodynamic Psychiatry. 2017 Dec;45(4):542-63.
6. Psychological Trauma: Theory, Research, Practice, and Policy. 2018 Jan;10(1):22-9.
Dr. Reinstein is a psychiatry attending at Zucker Hillside Hospital of the Northwell Health System, Glen Oaks, New York. Her clinical interests include reproductive psychiatry and family therapy, with a specific focus on maternal mental health. Dr. Reinstein completed her adult psychiatry residency training at Montefiore Hospital/Albert Einstein College of Medicine after graduating from the Albert Einstein College of Medicine and Yeshiva University with a B.A. in biology. She is one of the recipients of the 4th Annual Resident Recognition Award for Excellence in Family Oriented Care.
ICH: Recent NOAC use associated with lower risk of in-hospital mortality, compared with warfarin
Background: Previous studies comparing NOACs with warfarin have demonstrated a lower incidence of ICH in patients receiving NOACs. Data have been limited, though, regarding ICH with recent anticoagulant use and in-hospital mortality.
Study design: Retrospective cohort study.
Setting: More than 1,600 U.S. hospitals that participate in the Get With The Guidelines–Stroke national registry.
Synopsis: Of 141,311 patients admitted with ICH, 10.6% were receiving warfarin and 3.5% were receiving NOACs prior to hospitalization. Prior use of warfarin or NOACs, compared with no anticoagulant use, was associated with higher in-hospital mortality. However, use of NOACs, compared with use of warfarin, was associated with lower in-hospital mortality risk (adjusted risk difference, –5.7%; adjusted odds ratio, 0.75). Among patients with prior NOAC use, 54% of them were using rivaroxaban.
A limitation to this study is that reversal strategies, such as the use of vitamin K, fresh frozen plasma, or intravenous factor concentrates, were not available in the database. In addition, since rivaroxaban accounted for more than half the NOACs used, it may be difficult to apply the overall findings to all other available NOACs.
Bottom line: In patients admitted for ICH, prior use of NOACs, compared with warfarin, was associated with lower risk of in-hospital mortality.
Citation: Inohara T et al. Association of intracerebral hemorrhage among patients taking non–vitamin K antagonist vs. vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA. 2018 Feb 6;319(5):463-73.
Dr. Farkhondehpour is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Background: Previous studies comparing NOACs with warfarin have demonstrated a lower incidence of ICH in patients receiving NOACs. Data have been limited, though, regarding ICH with recent anticoagulant use and in-hospital mortality.
Study design: Retrospective cohort study.
Setting: More than 1,600 U.S. hospitals that participate in the Get With The Guidelines–Stroke national registry.
Synopsis: Of 141,311 patients admitted with ICH, 10.6% were receiving warfarin and 3.5% were receiving NOACs prior to hospitalization. Prior use of warfarin or NOACs, compared with no anticoagulant use, was associated with higher in-hospital mortality. However, use of NOACs, compared with use of warfarin, was associated with lower in-hospital mortality risk (adjusted risk difference, –5.7%; adjusted odds ratio, 0.75). Among patients with prior NOAC use, 54% of them were using rivaroxaban.
A limitation to this study is that reversal strategies, such as the use of vitamin K, fresh frozen plasma, or intravenous factor concentrates, were not available in the database. In addition, since rivaroxaban accounted for more than half the NOACs used, it may be difficult to apply the overall findings to all other available NOACs.
Bottom line: In patients admitted for ICH, prior use of NOACs, compared with warfarin, was associated with lower risk of in-hospital mortality.
Citation: Inohara T et al. Association of intracerebral hemorrhage among patients taking non–vitamin K antagonist vs. vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA. 2018 Feb 6;319(5):463-73.
Dr. Farkhondehpour is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Background: Previous studies comparing NOACs with warfarin have demonstrated a lower incidence of ICH in patients receiving NOACs. Data have been limited, though, regarding ICH with recent anticoagulant use and in-hospital mortality.
Study design: Retrospective cohort study.
Setting: More than 1,600 U.S. hospitals that participate in the Get With The Guidelines–Stroke national registry.
Synopsis: Of 141,311 patients admitted with ICH, 10.6% were receiving warfarin and 3.5% were receiving NOACs prior to hospitalization. Prior use of warfarin or NOACs, compared with no anticoagulant use, was associated with higher in-hospital mortality. However, use of NOACs, compared with use of warfarin, was associated with lower in-hospital mortality risk (adjusted risk difference, –5.7%; adjusted odds ratio, 0.75). Among patients with prior NOAC use, 54% of them were using rivaroxaban.
A limitation to this study is that reversal strategies, such as the use of vitamin K, fresh frozen plasma, or intravenous factor concentrates, were not available in the database. In addition, since rivaroxaban accounted for more than half the NOACs used, it may be difficult to apply the overall findings to all other available NOACs.
Bottom line: In patients admitted for ICH, prior use of NOACs, compared with warfarin, was associated with lower risk of in-hospital mortality.
Citation: Inohara T et al. Association of intracerebral hemorrhage among patients taking non–vitamin K antagonist vs. vitamin K antagonist oral anticoagulants with in-hospital mortality. JAMA. 2018 Feb 6;319(5):463-73.
Dr. Farkhondehpour is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Trial finds link between OSA, aortic diameter
BALTIMORE – Individuals with moderate to severe and thus may be at a heightened risk of cardiovascular events, according to an analysis of a national, multisite research study presented at the annual meeting of the Associated Professional Sleep Societies.
The study evaluated a subgroup of 708 patients with no history of cardiovascular disease (CVD) from the Multi-Ethnic Study of Atherosclerosis (MESA).
Dr. Kwon noted that previous studies have shown that patients with thoracic aortopathy have a high rate of obstructive sleep apnea (OSA) (Am J Respir Crit Care Med. 2003;168:1528-31), and those with OSA tend to have higher thoracic aortic size (J Am Coll Cardiol. 2008;52:885-6). “There’s also a degree of evidence suggesting that OSA is associated with high arterial stiffness, which is a marker of primary organ damage and a major cardiovascular risk that is predictive of cardiovascular disease,” Dr. Kwon said (J Intl Med Res. 2011;39:228-38). However, he also noted that some studies have found no relationship between OSA and aortic disease (Respiration. 2006;73:741-50). “The question can be raised as to whether sleep apnea may have implications” in thoracic aortic disease, he said.
Dr. Kwon’s study evaluated three groups: patients with no OSA (apnea hypopnea index [AHI] less than 5, n = 87); mild OSA (AHI 5-15, n = 215); and severe OSA (AHI greater than 15, n = 406). All patients had polysomnography as part of an ancillary study. Cardiac MRI measured these three features of aortic function and physiology (unadjusted results):
Diameter at the pulmonary artery bifurcation, which ranged from 3.13 cm in patients with no OSA to 3.37 cm in those with severe OSA (P = .0017).
Pulse wave velocity, which averaged 8.07 m/s in the no-OSA group and 9.11 m/s in the severe group (P less than .0001).
Distensibility, or aortic stiffness, which was 1.73%/mm Hg in the no-OSA group, 1.54%/mm Hg in the mild group and 1.68%/mm Hg in the severe group (P = .0141).
“There was maybe some higher pulse wave velocity across the significant OSA group,” Dr. Kwon said. “However, with aortic distensibility, there did not seem to be any significant trend.”
In the adjusted analysis of aortic diameter, “there did appear to be a small but significant difference in the significant OSA group, compared with the reference group,” Dr. Kwon said. He also noted that women with OSA typically had significantly larger aortic diameters than did non-OSA counterparts, whereas that trend was not as pronounced in men.
“Thoracic aorta size does seem to increase with OSA severity, but this has a sex-interaction component; it’s more pronounced in women,” Dr. Kwon said. He also noted a discrepancy in the results: “The functional properties of the aorta did not seem to bear a significant association with OSA severity.”
In explaining why these results differed from previous studies, Dr. Kwon said the study populations or their characteristics may be the cause, or that MRI-based measures of aortic properties have not been extensively studied before. “This is probably the first study to look at an unselected population, use a large sample size that was ethnically diverse, and use cardiac MRI technology,” he said.
Limitations he noted were the study’s cross-sectional nature and its small population of patients with enlarged thoracic aorta size, which left it underpowered to evaluate that population.
Dr. Kwon reported having no financial relationships.
SOURCE: Kwon Y et al. SLEEP 2018, Abstract #0465.
BALTIMORE – Individuals with moderate to severe and thus may be at a heightened risk of cardiovascular events, according to an analysis of a national, multisite research study presented at the annual meeting of the Associated Professional Sleep Societies.
The study evaluated a subgroup of 708 patients with no history of cardiovascular disease (CVD) from the Multi-Ethnic Study of Atherosclerosis (MESA).
Dr. Kwon noted that previous studies have shown that patients with thoracic aortopathy have a high rate of obstructive sleep apnea (OSA) (Am J Respir Crit Care Med. 2003;168:1528-31), and those with OSA tend to have higher thoracic aortic size (J Am Coll Cardiol. 2008;52:885-6). “There’s also a degree of evidence suggesting that OSA is associated with high arterial stiffness, which is a marker of primary organ damage and a major cardiovascular risk that is predictive of cardiovascular disease,” Dr. Kwon said (J Intl Med Res. 2011;39:228-38). However, he also noted that some studies have found no relationship between OSA and aortic disease (Respiration. 2006;73:741-50). “The question can be raised as to whether sleep apnea may have implications” in thoracic aortic disease, he said.
Dr. Kwon’s study evaluated three groups: patients with no OSA (apnea hypopnea index [AHI] less than 5, n = 87); mild OSA (AHI 5-15, n = 215); and severe OSA (AHI greater than 15, n = 406). All patients had polysomnography as part of an ancillary study. Cardiac MRI measured these three features of aortic function and physiology (unadjusted results):
Diameter at the pulmonary artery bifurcation, which ranged from 3.13 cm in patients with no OSA to 3.37 cm in those with severe OSA (P = .0017).
Pulse wave velocity, which averaged 8.07 m/s in the no-OSA group and 9.11 m/s in the severe group (P less than .0001).
Distensibility, or aortic stiffness, which was 1.73%/mm Hg in the no-OSA group, 1.54%/mm Hg in the mild group and 1.68%/mm Hg in the severe group (P = .0141).
“There was maybe some higher pulse wave velocity across the significant OSA group,” Dr. Kwon said. “However, with aortic distensibility, there did not seem to be any significant trend.”
In the adjusted analysis of aortic diameter, “there did appear to be a small but significant difference in the significant OSA group, compared with the reference group,” Dr. Kwon said. He also noted that women with OSA typically had significantly larger aortic diameters than did non-OSA counterparts, whereas that trend was not as pronounced in men.
“Thoracic aorta size does seem to increase with OSA severity, but this has a sex-interaction component; it’s more pronounced in women,” Dr. Kwon said. He also noted a discrepancy in the results: “The functional properties of the aorta did not seem to bear a significant association with OSA severity.”
In explaining why these results differed from previous studies, Dr. Kwon said the study populations or their characteristics may be the cause, or that MRI-based measures of aortic properties have not been extensively studied before. “This is probably the first study to look at an unselected population, use a large sample size that was ethnically diverse, and use cardiac MRI technology,” he said.
Limitations he noted were the study’s cross-sectional nature and its small population of patients with enlarged thoracic aorta size, which left it underpowered to evaluate that population.
Dr. Kwon reported having no financial relationships.
SOURCE: Kwon Y et al. SLEEP 2018, Abstract #0465.
BALTIMORE – Individuals with moderate to severe and thus may be at a heightened risk of cardiovascular events, according to an analysis of a national, multisite research study presented at the annual meeting of the Associated Professional Sleep Societies.
The study evaluated a subgroup of 708 patients with no history of cardiovascular disease (CVD) from the Multi-Ethnic Study of Atherosclerosis (MESA).
Dr. Kwon noted that previous studies have shown that patients with thoracic aortopathy have a high rate of obstructive sleep apnea (OSA) (Am J Respir Crit Care Med. 2003;168:1528-31), and those with OSA tend to have higher thoracic aortic size (J Am Coll Cardiol. 2008;52:885-6). “There’s also a degree of evidence suggesting that OSA is associated with high arterial stiffness, which is a marker of primary organ damage and a major cardiovascular risk that is predictive of cardiovascular disease,” Dr. Kwon said (J Intl Med Res. 2011;39:228-38). However, he also noted that some studies have found no relationship between OSA and aortic disease (Respiration. 2006;73:741-50). “The question can be raised as to whether sleep apnea may have implications” in thoracic aortic disease, he said.
Dr. Kwon’s study evaluated three groups: patients with no OSA (apnea hypopnea index [AHI] less than 5, n = 87); mild OSA (AHI 5-15, n = 215); and severe OSA (AHI greater than 15, n = 406). All patients had polysomnography as part of an ancillary study. Cardiac MRI measured these three features of aortic function and physiology (unadjusted results):
Diameter at the pulmonary artery bifurcation, which ranged from 3.13 cm in patients with no OSA to 3.37 cm in those with severe OSA (P = .0017).
Pulse wave velocity, which averaged 8.07 m/s in the no-OSA group and 9.11 m/s in the severe group (P less than .0001).
Distensibility, or aortic stiffness, which was 1.73%/mm Hg in the no-OSA group, 1.54%/mm Hg in the mild group and 1.68%/mm Hg in the severe group (P = .0141).
“There was maybe some higher pulse wave velocity across the significant OSA group,” Dr. Kwon said. “However, with aortic distensibility, there did not seem to be any significant trend.”
In the adjusted analysis of aortic diameter, “there did appear to be a small but significant difference in the significant OSA group, compared with the reference group,” Dr. Kwon said. He also noted that women with OSA typically had significantly larger aortic diameters than did non-OSA counterparts, whereas that trend was not as pronounced in men.
“Thoracic aorta size does seem to increase with OSA severity, but this has a sex-interaction component; it’s more pronounced in women,” Dr. Kwon said. He also noted a discrepancy in the results: “The functional properties of the aorta did not seem to bear a significant association with OSA severity.”
In explaining why these results differed from previous studies, Dr. Kwon said the study populations or their characteristics may be the cause, or that MRI-based measures of aortic properties have not been extensively studied before. “This is probably the first study to look at an unselected population, use a large sample size that was ethnically diverse, and use cardiac MRI technology,” he said.
Limitations he noted were the study’s cross-sectional nature and its small population of patients with enlarged thoracic aorta size, which left it underpowered to evaluate that population.
Dr. Kwon reported having no financial relationships.
SOURCE: Kwon Y et al. SLEEP 2018, Abstract #0465.
REPORTING FROM SLEEP 2018
Key clinical point: Obstructive sleep apnea severity is related to increased aortic artery size.
Major finding: Ascending aortic diameter measured 3.37 cm in patients with severe OSA vs. 3.13 cm with no OSA.
Study details: Subgroup of 708 patients with OSA but no history of cardiovascular disease from the Multi-Ethnic Study of Atherosclerosis.
Disclosures: Dr. Kwon reported having no financial relationships.
Source: Kwon Y et al. SLEEP 2018, Abstract #0465.
Register Today for UCLA / SVS Review Course
The Third Annual UCLA / SVS Symposium on “What’s New in Vascular and Endovascular Surgery" is less than seven weeks away. Register today for this comprehensive course that includes a survey of basic science, pathogenesis, diagnosis and management of the broad spectrum of vascular disorders, plus important new developments. The course will be Aug. 25 to 27 at the Beverly Hilton, Beverly Hills, Calif.
The course registration includes the new (9th) edition of the textbook, “Vascular and Endovascular Surgery: A Comprehensive Review,” edited by Drs. Wesley S. Moore, Peter F. Lawrence and Gustavo S. Oderich. Learn more and register here.
The Third Annual UCLA / SVS Symposium on “What’s New in Vascular and Endovascular Surgery" is less than seven weeks away. Register today for this comprehensive course that includes a survey of basic science, pathogenesis, diagnosis and management of the broad spectrum of vascular disorders, plus important new developments. The course will be Aug. 25 to 27 at the Beverly Hilton, Beverly Hills, Calif.
The course registration includes the new (9th) edition of the textbook, “Vascular and Endovascular Surgery: A Comprehensive Review,” edited by Drs. Wesley S. Moore, Peter F. Lawrence and Gustavo S. Oderich. Learn more and register here.
The Third Annual UCLA / SVS Symposium on “What’s New in Vascular and Endovascular Surgery" is less than seven weeks away. Register today for this comprehensive course that includes a survey of basic science, pathogenesis, diagnosis and management of the broad spectrum of vascular disorders, plus important new developments. The course will be Aug. 25 to 27 at the Beverly Hilton, Beverly Hills, Calif.
The course registration includes the new (9th) edition of the textbook, “Vascular and Endovascular Surgery: A Comprehensive Review,” edited by Drs. Wesley S. Moore, Peter F. Lawrence and Gustavo S. Oderich. Learn more and register here.