User login
A young woman with enlarged lymph nodes
A previously healthy 25-year-old woman presents to her primary care physician with a “lump in the neck”—a painless, swollen area under the lower part of her left jaw that she noticed several weeks ago and that continues to enlarge. She has also noted a recent increase in fatigue, as well as the onset of generalized headaches and mild sinus congestion. Presumed by another physician to have sinusitis, she had already received a 2-week course of an antibiotic (she could not recall which antibiotic), with no improvement in her symptoms. She has been trying to lose weight and has lost 5 pounds in the last 4 months. She reports no fevers, chills, or night sweats.
She works as a special-education teacher and lives in a rural area. She has not travelled during the past year, inside or outside the United States. When she was an adolescent, she underwent tonsillectomy and had her wisdom teeth extracted. Her family has no history of hematologic dyscrasia or malignancy. She has two dogs, which are indoor pets, and she utilizes a city water supply.
1. Which of the following causes of a lump in the neck is most important to exclude?
- Viral or bacterial infection
- Lymphoma
- Oral cavity abscess
- Infectious mononucleosis
- Congenital anomaly
A lump in the neck can be broadly categorized as congenital, inflammatory, or malignant. Congenital causes include branchial cleft cyst (anterior to the sternocleidomastoid muscle) and thyroglossal duct cyst (usually in the midline between the hyoid bone and the isthmus of the thyroid gland). Other possibilities include lipoma and, less frequently, a salivary gland disorder such as sialadenitis.
A complaint of a neck lump very often correlates with the physical finding of lymphadenopathy, and a standard approach in evaluation should be undertaken, based on the mnemonic “PAAA”—ie, palpation, age, area, and associated symptoms.
Palpation. In palpation of the lymph node group, one should note the size and tactile quality of the lymph nodes and assess for abnormal temperature, tenderness, fluctuance, and mobility. In general, lymph nodes larger than 1.5 cm by 1.5 cm are more likely to be of granulomatous or neoplastic origin.1 Nodes that are tender, warm, or fluctuant are likely reactive to a local infectious process; nodes that are firm, matted, and fixed are most characteristic of malignancy; and rubbery, mobile nodes may represent either granulomatous disease or lymphoma.2
Age helps stratify the risk of malignancy as an underlying cause, which is increased in people over age 50 presenting with lymphadenopathy.1
Area. An assessment of the extent of the lymphadenopathy can guide the search either for a cause of generalized lymphadenopathy or for pathology in the anatomic area drained by the particular lymph node group, including the scalp (occipital or preauricular); external ear (posterior auricular); oral cavity (submandibular, submental); soft tissues of the face and neck (superficial cervical); upper respiratory tract and thyroid (deep cervical); and thoracic cavity and abdominal cavity (supraclavicular).
Asking the patient about occupational, environmental, and behavioral risk factors and associated signs and symptoms such as fever, rash, diaphoresis, unintentional weight loss, and splenomegaly helps to narrow the differential diagnosis. Common diagnoses to consider in the evaluation of peripheral lymphadenopathy are listed in Table 1.
A viral or bacterial upper respiratory infection is one of the most common causes of cervical lymphadenopathy, although this usually does not persist for many weeks. Mononucleosis more commonly involves the posterior cervical chain and is often accompanied by splenomegaly. Because of the prolonged presence of the lump, malignancy, including lymphoma, is the most important of the answer choices to consider and rule out in a timely fashion.
INITIAL PHYSICAL EXAMINATION
The woman appears to be well and is in no acute distress. Her oral temperature is 98.1°F (36.7°C), blood pressure 119/72 mm Hg, heart rate 86 beats per minute, and respiratory rate 18 breaths per minute.
The head and neck appear normal. The nares are patent with normal mucosa and no visible drainage. There is no tenderness during palpation of the facial sinuses. The ear canals, tympanic membranes, oropharynx, and tongue appear normal. Several firm, mobile, nontender lymph nodes about 1 cm in diameter are palpable in the left submandibular and right supraclavicular area. No other occipital, submental, axillary, or inguinal lymphadenopathy is noted. There is no overlying erythema or warmth. The cardiac examination is normal, and the lungs are clear on auscultation. The abdomen is soft, nontender, and nondistended, with no organomegaly. The skin appears normal, and the neurologic examination is normal.
INITIAL LABORATORY TESTS
Results of initial laboratory tests are as follows:
- White blood cell count 5.74 × 109/L (reference range 3.70–11.00)
- Red blood cell count 4.49 × 109/L (3.90–5.20)
- Hemoglobin 13.0 g/dL (11.5–15.5)
- Hematocrit 38.4% (36.0–46.0)
- Platelet count 210 × 109/L (150–400)
- Mean corpuscular volume 85 fL (80–100)
- Absolute neutrophil count 3.26 × 109/L (1.45–7.50)
- Blood urea nitrogen 8 mg/dL (8–25)
- Creatinine 0.65 mg/dL (0.70–1.40)
- Lactate dehydrogenase 146 U/L (100–220)
- Uric acid 4.0 mg/dL (2.0–7.0)
- Thyrotropin (thyroid-stimulating hormone) 1.86 μIU/mL (0.4–5.5).
A recent tuberculin skin test obtained as part of her employment screening was negative, and so was a test for antibody to human immunodeficiency virus (HIV), obtained recently before donating plasma. A urine pregnancy test done in the office was also negative. A peripheral blood smear showed slight toxic granulation with rare reactive lymphocytes.
2. Which test would provide the greatest diagnostic yield at this point?
- Needle aspiration biopsy of lymph node
- Excisional lymph node biopsy
- Polymerase chain reaction (PCR) testing for HIV
- Antistreptolysin-O (ASO) titer
Because of the persistent enlargement of the patient’s lymph nodes despite several weeks of antibiotic treatment, and because submandibular and supraclavicular nodes were involved, excisional lymph node biopsy would be the best of these choices to evaluate for malignancy. Compared with needle aspiration biopsy, it is the gold standard, preserving the nodal architecture and providing ample tissue for immunostaining and additional studies.
Needle aspiration biopsy is safe, inexpensive, and easy to do and can be useful in situations of limited resources, but it does not reliably distinguish between a reactive and a neoplastic process.1 Its collection and interpretation are highly variable and personnel-dependent, and its sensitivity for detecting lymphoma is reported to be as low as 7.1% (95% confidence interval 0.9% to 23.5%).2
An acute retroviral syndrome can cause adenopathy, especially before seroconversion is evident, but it is usually associated with an influenza-like illness and monocytosis. Although this patient had no apparent risk factors for HIV, ordering PCR testing for HIV is also an important step when the clinical situation is suggestive. In the absence of an abnormal-appearing oropharynx, tonsillar exudate, or high fever, the pretest probability of streptococcal pharyngitis is low, and an ASO titer is unlikely to be diagnostic in this case.
CASE CONTINUED: BIOPSY PERFORMED
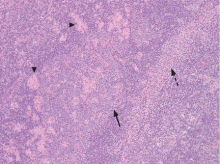
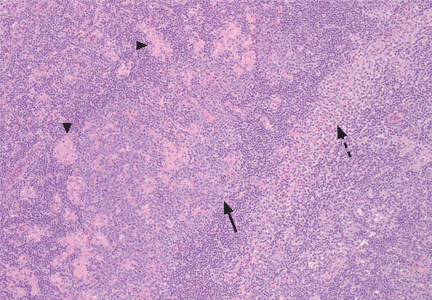
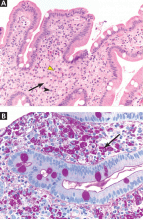
An incisional lymph node biopsy was obtained (Figure 1).
3. Which can confirm the suspected diagnosis?
- Tissue culture
- Test for immunoglobulin (Ig) G and IgM antibodies to Toxoplasma gondii
- Serum PCR testing for T gondii
- T gondii IgG avidity testing
DIAGNOSIS OF TOXOPLASMOSIS
In this patient, acute toxoplasmosis was suspected based on recognition of the morphologic triad seen in Toxoplasma lymphadenitis— ie, follicular hyperplasia, abundance of monocytoid cells, and clusters of epithelioid lymphocytes.3,4
Detection and measurement of IgM antibodies against T gondii is the most widely used serologic test for acute toxoplasmosis and is often considered the reference standard among the most common commercially available agglutination screening assays. It has a sensitivity between 93.7% and 100% and a specificity of 97.1% to 99.2%.5 Confirmation is generally done with enzyme-linked immunoassay or chemiluminescent-based tests, which can detect lower levels of IgG and IgM.5
A positive serum IgG confirms seroconversion but by itself cannot distinguish between acute and chronic infection, although it is commonly obtained in conjunction with IgM levels.6 Since both IgG and IgM can be elevated months after initial infection, serum IgA and IgE levels can more accurately suggest the timing of infection if clarification is needed.6,7 In addition, IgG avidity testing can distinguish acute infection from chronic infection: a high avidity index suggests the acute infection occurred at least 3 to 5 months ago, whereas the avidity index may be low or zero if acute infection occurred within the past 4 weeks.7 The sensitivity of avidity testing is 91.3% to 94.4%, and the specificity 87.8% to 98.5%.7
Serum PCR testing for T gondii is useful when toxoplasmosis is suspected in patients whose immune system may not be able to mount an adequate antibody response or in patients in the hyperacute phase of infection, even before a detectable antibody response can be formed.8,9 However, because of limitations of equipment, expertise, and overall cost, this method is not universally available. Additionally, blood cultures and PCR testing or tissue culture of pathologic specimens cannot routinely be relied on for diagnosis, as often the burden of microorganisms present in these specimens is low. When positive, culture specimens may yield bradyzoites or tachyzoites, but only after considerable latency of many days to weeks.10
How people acquire acute toxoplasmosis
T gondii is an obligate intracellular protozoan parasite. Sexual replication of the organism takes place within the intestines of cats (the definitive host), with subsequent excretion of infective oocysts in feces.11 These hardy oocysts can contaminate soil or water supplies and can survive for months, depending on ambient temperature and humidity. Ingestion of oocysts can lead to infection of a variety of mammals, including sheep, pigs, chicken, and cattle.
Infection in humans can occur with consumption of raw or undercooked foods contaminated with oocysts, and inadequate hand-washing and poor kitchen hygiene substantially increase the risk of infection.12 Activities such as gardening can expose humans to oocysts in contaminated water and soil. In addition, direct contact with cat feces, such as when cleaning the litterbox, is a known exposure risk. Vertical transmission can manifest as congenital toxoplasmosis in a fetus when transmitted from an infected pregnant mother.
Eating raw or undercooked food is considered to be the greatest risk factor for acquired toxoplasmosis and is believed to be responsible for about 50% of all cases.12 However, in pooled data from 14 case-control studies, no clear risk factor for Toxoplasma infection could be identified in up to 60% of affected people, leading many experts to believe contaminated water may play a larger role in acquisition than previously surmised.12
Toxoplasma cysts have a predilection for muscle and neural tissue, resulting in myositis, myocarditis, encephalitis, and chorioretinitis. Severe systemic manifestations are seen in people with impaired T-cell immunity, such as those with HIV infection and acquired immunodeficiency syndrome; or hematologic malignancy; in recipients of solid-organ transplants; or in people taking corticosteroids or cytotoxic drugs. Congenital infection can result in stillbirth, microcephaly, developmental delay, or deafness in the developing fetus and is an important cause of infant morbidity and death worldwide.13
Infection in immunocompetent people is usually asymptomatic.14 However, up to 20% of immunocompetent patients develop symptoms that tend to be nonspecific and include muscle aches and lymphadenopathy, and these are often mistaken for an influenza-like illness.14,15 Other symptoms include malaise, fevers, night sweats, pharyngitis, abdominal pain, hepatosplenomegaly, maculopapular rash, and atypical lymphocytosis (less than 10% of peripheral blood).11 The most common physical manifestation of acute toxoplasmosis is isolated cervical lymphadenopathy, although any lymph node group can be affected.14 Lymph nodes are not fixed or matted and generally are neither tender nor suppurative.16
4. What is the correct treatment strategy for acute toxoplasmosis in this case?
- Symptomatic treatment only
- Trimethoprim 160 mg and sulfamethoxazole 800 mg daily
- Combination of atovaquone and clindamycin
- Combination pyrimethamine, sulfadiazine, and folinic acid
TREATMENT AND PROGNOSIS OF ACUTE TOXOPLASMOSIS
No antimicrobial treatment is required for most immunocompetent patients. Symptoms are self-limited and resolve within 1 to 2 months in 60% of patients.14 A substantial proportion of patients—25%—will have lingering symptoms at 2 to 4 months, and some (10%) can have mild symptoms for 6 months or longer.16
Symptomatic treatment with analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) is appropriate.
Immunocompromised and critically ill patients and those with ocular manifestations require combination therapy with pyrimethamine, sulfadiazine, and folinic acid.17 Trimethoprim-sulfamethoxazole is effective as prophylaxis against T gondii infection in immunocompromised patients at a dosage of 160 mg trimethoprim/800 mg sulfamethoxazole daily, but it is also an alternative for treatment at higher dosages (5 mg/kg trimethoprim and 25 mg/kg sulfamethoxazole twice daily).
Atovaquone and clindamycin can be used in sulfa-sensitive patients17 and also in those with latent toxoplasmosis for better penetration of tissue cysts. Corticosteroids are used as adjuncts in those with ocular involvement.
Spiramycin is the treatment of choice in pregnant women and can be given throughout the pregnancy.17,18 A recent comparative study by Hotop et al18 reported a reduction in the rate of fetal transmission (1.6% vs 4.8%) when spiramycin was given from the time of diagnosis through the 16th week of pregnancy, followed by a minimum of 4 weeks of combination therapy with pyrimethamine, sulfadiazine, and folinic acid.18
CASE CONCLUDED
Serologic testing was positive for IgM and IgG antibodies to T gondii, which suggested subacute infection. The patient received no antimicrobial therapy and her lymphadenopathy eventually resolved. Her generalized fatigue gradually resolved over the next year without antimicrobial treatment.
A thorough re-review of potential exposures was done at subsequent office visits to help elucidate how she may have acquired the infection. She recalled no recent exposure to cats or rodents, nor consumption of raw meat. We could only suppose that there may have been inadvertent exposure to oocyst-containing soil or water or to undercooked meat products. Thus, the diagnosis of acute toxoplasmosis should be kept in mind in the evaluation of lymphadenopathy, even in the absence of a clear history of exposure.
- Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc 2000; 75:723–732.
- Khillan R, Sidhu G, Axiotis C, Braverman AS. Fine needle aspiration (FNA) cytology for diagnosis of cervical lymphadenopathy. Int J Hematol 2012; 95:282–284.
- Dorfman RF, Remington JS. Value of lymph-node biopsy in the diagnosis of acute acquired toxoplasmosis. N Engl J Med 1973; 289:878–881.
- Eapen M, Mathew CF, Aravindan KP. Evidence based criteria for the histopathological diagnosis of toxoplasmic lymphadenopathy. J Clin Pathol 2005; 58:1143–1146.
- Villard O, Cimon B, Franck J, et al; Network from the French National Reference Center for Toxoplasmosis. Evaluation of the usefulness of six commercial agglutination assays for serologic diagnosis of toxoplasmosis. Diagn Microbiol Infect Dis 2012; 73:231–235.
- Suzuki LA, Rocha RJ, Rossi CL. Evaluation of serological markers for the immunodiagnosis of acute acquired toxoplasmosis. J Med Microbiol 2001; 50:62–70.
- Lachaud L, Calas O, Picot MC, Albaba S, Bourgeois N, Pratlong F. Value of 2 IgG avidity commercial tests used alone or in association to date toxoplasmosis contamination. Diagn Microbiol Infect Dis 2009; 64:267–274.
- Rahumatullah A, Khoo BY, Noordin R. Triplex PCR using new primers for the detection of Toxoplasma gondii. Exp Parasitol 2012; 131:231–238.
- Contini C, Giuliodori M, Cultrera R, Seraceni S. Detection of clinical-stage specific molecular Toxoplasma gondii gene patterns in patients with toxoplasmic lymphadenitis. J Med Microbiol 2006; 55:771–774.
- Silveira C, Vallochi AL, Rodrigues da Silva U, et al. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol 2011; 95:396–400.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363:1965–1976.
- Petersen E, Vesco G, Villari S, Buffolano W. What do we know about risk factors for infection in humans with Toxoplasma gondii and how can we prevent infections? Zoonoses Public Health 2010; 57:8–17.
- Feldman DM, Timms D, Borgida AF. Toxoplasmosis, parvovirus, and cytomegalovirus in pregnancy. Clin Lab Med 2010; 30:709–720.
- Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol 2009; 39:895–901.
- Remington JS. Toxoplasmosis in the adult. Bull NY Acad Med 1974; 50:211–227.
- McCabe RE, Brooks RG, Dorfman RF, Remington JS. Clinical spectrum in 107 cases of toxoplasmic lymphadenopathy. Rev Infect Dis 1987; 9:754–774.
- Toxoplasmosis. The Medical Letter, Drugs for Parasitic Infections. New Rochelle, NY: The Medical Letter Inc, June 1, 2010:57–58.
- Hotop A, Hlobil H, Gross U. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2012; 54:1545–1552.
A previously healthy 25-year-old woman presents to her primary care physician with a “lump in the neck”—a painless, swollen area under the lower part of her left jaw that she noticed several weeks ago and that continues to enlarge. She has also noted a recent increase in fatigue, as well as the onset of generalized headaches and mild sinus congestion. Presumed by another physician to have sinusitis, she had already received a 2-week course of an antibiotic (she could not recall which antibiotic), with no improvement in her symptoms. She has been trying to lose weight and has lost 5 pounds in the last 4 months. She reports no fevers, chills, or night sweats.
She works as a special-education teacher and lives in a rural area. She has not travelled during the past year, inside or outside the United States. When she was an adolescent, she underwent tonsillectomy and had her wisdom teeth extracted. Her family has no history of hematologic dyscrasia or malignancy. She has two dogs, which are indoor pets, and she utilizes a city water supply.
1. Which of the following causes of a lump in the neck is most important to exclude?
- Viral or bacterial infection
- Lymphoma
- Oral cavity abscess
- Infectious mononucleosis
- Congenital anomaly
A lump in the neck can be broadly categorized as congenital, inflammatory, or malignant. Congenital causes include branchial cleft cyst (anterior to the sternocleidomastoid muscle) and thyroglossal duct cyst (usually in the midline between the hyoid bone and the isthmus of the thyroid gland). Other possibilities include lipoma and, less frequently, a salivary gland disorder such as sialadenitis.
A complaint of a neck lump very often correlates with the physical finding of lymphadenopathy, and a standard approach in evaluation should be undertaken, based on the mnemonic “PAAA”—ie, palpation, age, area, and associated symptoms.
Palpation. In palpation of the lymph node group, one should note the size and tactile quality of the lymph nodes and assess for abnormal temperature, tenderness, fluctuance, and mobility. In general, lymph nodes larger than 1.5 cm by 1.5 cm are more likely to be of granulomatous or neoplastic origin.1 Nodes that are tender, warm, or fluctuant are likely reactive to a local infectious process; nodes that are firm, matted, and fixed are most characteristic of malignancy; and rubbery, mobile nodes may represent either granulomatous disease or lymphoma.2
Age helps stratify the risk of malignancy as an underlying cause, which is increased in people over age 50 presenting with lymphadenopathy.1
Area. An assessment of the extent of the lymphadenopathy can guide the search either for a cause of generalized lymphadenopathy or for pathology in the anatomic area drained by the particular lymph node group, including the scalp (occipital or preauricular); external ear (posterior auricular); oral cavity (submandibular, submental); soft tissues of the face and neck (superficial cervical); upper respiratory tract and thyroid (deep cervical); and thoracic cavity and abdominal cavity (supraclavicular).
Asking the patient about occupational, environmental, and behavioral risk factors and associated signs and symptoms such as fever, rash, diaphoresis, unintentional weight loss, and splenomegaly helps to narrow the differential diagnosis. Common diagnoses to consider in the evaluation of peripheral lymphadenopathy are listed in Table 1.
A viral or bacterial upper respiratory infection is one of the most common causes of cervical lymphadenopathy, although this usually does not persist for many weeks. Mononucleosis more commonly involves the posterior cervical chain and is often accompanied by splenomegaly. Because of the prolonged presence of the lump, malignancy, including lymphoma, is the most important of the answer choices to consider and rule out in a timely fashion.
INITIAL PHYSICAL EXAMINATION
The woman appears to be well and is in no acute distress. Her oral temperature is 98.1°F (36.7°C), blood pressure 119/72 mm Hg, heart rate 86 beats per minute, and respiratory rate 18 breaths per minute.
The head and neck appear normal. The nares are patent with normal mucosa and no visible drainage. There is no tenderness during palpation of the facial sinuses. The ear canals, tympanic membranes, oropharynx, and tongue appear normal. Several firm, mobile, nontender lymph nodes about 1 cm in diameter are palpable in the left submandibular and right supraclavicular area. No other occipital, submental, axillary, or inguinal lymphadenopathy is noted. There is no overlying erythema or warmth. The cardiac examination is normal, and the lungs are clear on auscultation. The abdomen is soft, nontender, and nondistended, with no organomegaly. The skin appears normal, and the neurologic examination is normal.
INITIAL LABORATORY TESTS
Results of initial laboratory tests are as follows:
- White blood cell count 5.74 × 109/L (reference range 3.70–11.00)
- Red blood cell count 4.49 × 109/L (3.90–5.20)
- Hemoglobin 13.0 g/dL (11.5–15.5)
- Hematocrit 38.4% (36.0–46.0)
- Platelet count 210 × 109/L (150–400)
- Mean corpuscular volume 85 fL (80–100)
- Absolute neutrophil count 3.26 × 109/L (1.45–7.50)
- Blood urea nitrogen 8 mg/dL (8–25)
- Creatinine 0.65 mg/dL (0.70–1.40)
- Lactate dehydrogenase 146 U/L (100–220)
- Uric acid 4.0 mg/dL (2.0–7.0)
- Thyrotropin (thyroid-stimulating hormone) 1.86 μIU/mL (0.4–5.5).
A recent tuberculin skin test obtained as part of her employment screening was negative, and so was a test for antibody to human immunodeficiency virus (HIV), obtained recently before donating plasma. A urine pregnancy test done in the office was also negative. A peripheral blood smear showed slight toxic granulation with rare reactive lymphocytes.
2. Which test would provide the greatest diagnostic yield at this point?
- Needle aspiration biopsy of lymph node
- Excisional lymph node biopsy
- Polymerase chain reaction (PCR) testing for HIV
- Antistreptolysin-O (ASO) titer
Because of the persistent enlargement of the patient’s lymph nodes despite several weeks of antibiotic treatment, and because submandibular and supraclavicular nodes were involved, excisional lymph node biopsy would be the best of these choices to evaluate for malignancy. Compared with needle aspiration biopsy, it is the gold standard, preserving the nodal architecture and providing ample tissue for immunostaining and additional studies.
Needle aspiration biopsy is safe, inexpensive, and easy to do and can be useful in situations of limited resources, but it does not reliably distinguish between a reactive and a neoplastic process.1 Its collection and interpretation are highly variable and personnel-dependent, and its sensitivity for detecting lymphoma is reported to be as low as 7.1% (95% confidence interval 0.9% to 23.5%).2
An acute retroviral syndrome can cause adenopathy, especially before seroconversion is evident, but it is usually associated with an influenza-like illness and monocytosis. Although this patient had no apparent risk factors for HIV, ordering PCR testing for HIV is also an important step when the clinical situation is suggestive. In the absence of an abnormal-appearing oropharynx, tonsillar exudate, or high fever, the pretest probability of streptococcal pharyngitis is low, and an ASO titer is unlikely to be diagnostic in this case.
CASE CONTINUED: BIOPSY PERFORMED
An incisional lymph node biopsy was obtained (Figure 1).
3. Which can confirm the suspected diagnosis?
- Tissue culture
- Test for immunoglobulin (Ig) G and IgM antibodies to Toxoplasma gondii
- Serum PCR testing for T gondii
- T gondii IgG avidity testing
DIAGNOSIS OF TOXOPLASMOSIS
In this patient, acute toxoplasmosis was suspected based on recognition of the morphologic triad seen in Toxoplasma lymphadenitis— ie, follicular hyperplasia, abundance of monocytoid cells, and clusters of epithelioid lymphocytes.3,4
Detection and measurement of IgM antibodies against T gondii is the most widely used serologic test for acute toxoplasmosis and is often considered the reference standard among the most common commercially available agglutination screening assays. It has a sensitivity between 93.7% and 100% and a specificity of 97.1% to 99.2%.5 Confirmation is generally done with enzyme-linked immunoassay or chemiluminescent-based tests, which can detect lower levels of IgG and IgM.5
A positive serum IgG confirms seroconversion but by itself cannot distinguish between acute and chronic infection, although it is commonly obtained in conjunction with IgM levels.6 Since both IgG and IgM can be elevated months after initial infection, serum IgA and IgE levels can more accurately suggest the timing of infection if clarification is needed.6,7 In addition, IgG avidity testing can distinguish acute infection from chronic infection: a high avidity index suggests the acute infection occurred at least 3 to 5 months ago, whereas the avidity index may be low or zero if acute infection occurred within the past 4 weeks.7 The sensitivity of avidity testing is 91.3% to 94.4%, and the specificity 87.8% to 98.5%.7
Serum PCR testing for T gondii is useful when toxoplasmosis is suspected in patients whose immune system may not be able to mount an adequate antibody response or in patients in the hyperacute phase of infection, even before a detectable antibody response can be formed.8,9 However, because of limitations of equipment, expertise, and overall cost, this method is not universally available. Additionally, blood cultures and PCR testing or tissue culture of pathologic specimens cannot routinely be relied on for diagnosis, as often the burden of microorganisms present in these specimens is low. When positive, culture specimens may yield bradyzoites or tachyzoites, but only after considerable latency of many days to weeks.10
How people acquire acute toxoplasmosis
T gondii is an obligate intracellular protozoan parasite. Sexual replication of the organism takes place within the intestines of cats (the definitive host), with subsequent excretion of infective oocysts in feces.11 These hardy oocysts can contaminate soil or water supplies and can survive for months, depending on ambient temperature and humidity. Ingestion of oocysts can lead to infection of a variety of mammals, including sheep, pigs, chicken, and cattle.
Infection in humans can occur with consumption of raw or undercooked foods contaminated with oocysts, and inadequate hand-washing and poor kitchen hygiene substantially increase the risk of infection.12 Activities such as gardening can expose humans to oocysts in contaminated water and soil. In addition, direct contact with cat feces, such as when cleaning the litterbox, is a known exposure risk. Vertical transmission can manifest as congenital toxoplasmosis in a fetus when transmitted from an infected pregnant mother.
Eating raw or undercooked food is considered to be the greatest risk factor for acquired toxoplasmosis and is believed to be responsible for about 50% of all cases.12 However, in pooled data from 14 case-control studies, no clear risk factor for Toxoplasma infection could be identified in up to 60% of affected people, leading many experts to believe contaminated water may play a larger role in acquisition than previously surmised.12
Toxoplasma cysts have a predilection for muscle and neural tissue, resulting in myositis, myocarditis, encephalitis, and chorioretinitis. Severe systemic manifestations are seen in people with impaired T-cell immunity, such as those with HIV infection and acquired immunodeficiency syndrome; or hematologic malignancy; in recipients of solid-organ transplants; or in people taking corticosteroids or cytotoxic drugs. Congenital infection can result in stillbirth, microcephaly, developmental delay, or deafness in the developing fetus and is an important cause of infant morbidity and death worldwide.13
Infection in immunocompetent people is usually asymptomatic.14 However, up to 20% of immunocompetent patients develop symptoms that tend to be nonspecific and include muscle aches and lymphadenopathy, and these are often mistaken for an influenza-like illness.14,15 Other symptoms include malaise, fevers, night sweats, pharyngitis, abdominal pain, hepatosplenomegaly, maculopapular rash, and atypical lymphocytosis (less than 10% of peripheral blood).11 The most common physical manifestation of acute toxoplasmosis is isolated cervical lymphadenopathy, although any lymph node group can be affected.14 Lymph nodes are not fixed or matted and generally are neither tender nor suppurative.16
4. What is the correct treatment strategy for acute toxoplasmosis in this case?
- Symptomatic treatment only
- Trimethoprim 160 mg and sulfamethoxazole 800 mg daily
- Combination of atovaquone and clindamycin
- Combination pyrimethamine, sulfadiazine, and folinic acid
TREATMENT AND PROGNOSIS OF ACUTE TOXOPLASMOSIS
No antimicrobial treatment is required for most immunocompetent patients. Symptoms are self-limited and resolve within 1 to 2 months in 60% of patients.14 A substantial proportion of patients—25%—will have lingering symptoms at 2 to 4 months, and some (10%) can have mild symptoms for 6 months or longer.16
Symptomatic treatment with analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) is appropriate.
Immunocompromised and critically ill patients and those with ocular manifestations require combination therapy with pyrimethamine, sulfadiazine, and folinic acid.17 Trimethoprim-sulfamethoxazole is effective as prophylaxis against T gondii infection in immunocompromised patients at a dosage of 160 mg trimethoprim/800 mg sulfamethoxazole daily, but it is also an alternative for treatment at higher dosages (5 mg/kg trimethoprim and 25 mg/kg sulfamethoxazole twice daily).
Atovaquone and clindamycin can be used in sulfa-sensitive patients17 and also in those with latent toxoplasmosis for better penetration of tissue cysts. Corticosteroids are used as adjuncts in those with ocular involvement.
Spiramycin is the treatment of choice in pregnant women and can be given throughout the pregnancy.17,18 A recent comparative study by Hotop et al18 reported a reduction in the rate of fetal transmission (1.6% vs 4.8%) when spiramycin was given from the time of diagnosis through the 16th week of pregnancy, followed by a minimum of 4 weeks of combination therapy with pyrimethamine, sulfadiazine, and folinic acid.18
CASE CONCLUDED
Serologic testing was positive for IgM and IgG antibodies to T gondii, which suggested subacute infection. The patient received no antimicrobial therapy and her lymphadenopathy eventually resolved. Her generalized fatigue gradually resolved over the next year without antimicrobial treatment.
A thorough re-review of potential exposures was done at subsequent office visits to help elucidate how she may have acquired the infection. She recalled no recent exposure to cats or rodents, nor consumption of raw meat. We could only suppose that there may have been inadvertent exposure to oocyst-containing soil or water or to undercooked meat products. Thus, the diagnosis of acute toxoplasmosis should be kept in mind in the evaluation of lymphadenopathy, even in the absence of a clear history of exposure.
A previously healthy 25-year-old woman presents to her primary care physician with a “lump in the neck”—a painless, swollen area under the lower part of her left jaw that she noticed several weeks ago and that continues to enlarge. She has also noted a recent increase in fatigue, as well as the onset of generalized headaches and mild sinus congestion. Presumed by another physician to have sinusitis, she had already received a 2-week course of an antibiotic (she could not recall which antibiotic), with no improvement in her symptoms. She has been trying to lose weight and has lost 5 pounds in the last 4 months. She reports no fevers, chills, or night sweats.
She works as a special-education teacher and lives in a rural area. She has not travelled during the past year, inside or outside the United States. When she was an adolescent, she underwent tonsillectomy and had her wisdom teeth extracted. Her family has no history of hematologic dyscrasia or malignancy. She has two dogs, which are indoor pets, and she utilizes a city water supply.
1. Which of the following causes of a lump in the neck is most important to exclude?
- Viral or bacterial infection
- Lymphoma
- Oral cavity abscess
- Infectious mononucleosis
- Congenital anomaly
A lump in the neck can be broadly categorized as congenital, inflammatory, or malignant. Congenital causes include branchial cleft cyst (anterior to the sternocleidomastoid muscle) and thyroglossal duct cyst (usually in the midline between the hyoid bone and the isthmus of the thyroid gland). Other possibilities include lipoma and, less frequently, a salivary gland disorder such as sialadenitis.
A complaint of a neck lump very often correlates with the physical finding of lymphadenopathy, and a standard approach in evaluation should be undertaken, based on the mnemonic “PAAA”—ie, palpation, age, area, and associated symptoms.
Palpation. In palpation of the lymph node group, one should note the size and tactile quality of the lymph nodes and assess for abnormal temperature, tenderness, fluctuance, and mobility. In general, lymph nodes larger than 1.5 cm by 1.5 cm are more likely to be of granulomatous or neoplastic origin.1 Nodes that are tender, warm, or fluctuant are likely reactive to a local infectious process; nodes that are firm, matted, and fixed are most characteristic of malignancy; and rubbery, mobile nodes may represent either granulomatous disease or lymphoma.2
Age helps stratify the risk of malignancy as an underlying cause, which is increased in people over age 50 presenting with lymphadenopathy.1
Area. An assessment of the extent of the lymphadenopathy can guide the search either for a cause of generalized lymphadenopathy or for pathology in the anatomic area drained by the particular lymph node group, including the scalp (occipital or preauricular); external ear (posterior auricular); oral cavity (submandibular, submental); soft tissues of the face and neck (superficial cervical); upper respiratory tract and thyroid (deep cervical); and thoracic cavity and abdominal cavity (supraclavicular).
Asking the patient about occupational, environmental, and behavioral risk factors and associated signs and symptoms such as fever, rash, diaphoresis, unintentional weight loss, and splenomegaly helps to narrow the differential diagnosis. Common diagnoses to consider in the evaluation of peripheral lymphadenopathy are listed in Table 1.
A viral or bacterial upper respiratory infection is one of the most common causes of cervical lymphadenopathy, although this usually does not persist for many weeks. Mononucleosis more commonly involves the posterior cervical chain and is often accompanied by splenomegaly. Because of the prolonged presence of the lump, malignancy, including lymphoma, is the most important of the answer choices to consider and rule out in a timely fashion.
INITIAL PHYSICAL EXAMINATION
The woman appears to be well and is in no acute distress. Her oral temperature is 98.1°F (36.7°C), blood pressure 119/72 mm Hg, heart rate 86 beats per minute, and respiratory rate 18 breaths per minute.
The head and neck appear normal. The nares are patent with normal mucosa and no visible drainage. There is no tenderness during palpation of the facial sinuses. The ear canals, tympanic membranes, oropharynx, and tongue appear normal. Several firm, mobile, nontender lymph nodes about 1 cm in diameter are palpable in the left submandibular and right supraclavicular area. No other occipital, submental, axillary, or inguinal lymphadenopathy is noted. There is no overlying erythema or warmth. The cardiac examination is normal, and the lungs are clear on auscultation. The abdomen is soft, nontender, and nondistended, with no organomegaly. The skin appears normal, and the neurologic examination is normal.
INITIAL LABORATORY TESTS
Results of initial laboratory tests are as follows:
- White blood cell count 5.74 × 109/L (reference range 3.70–11.00)
- Red blood cell count 4.49 × 109/L (3.90–5.20)
- Hemoglobin 13.0 g/dL (11.5–15.5)
- Hematocrit 38.4% (36.0–46.0)
- Platelet count 210 × 109/L (150–400)
- Mean corpuscular volume 85 fL (80–100)
- Absolute neutrophil count 3.26 × 109/L (1.45–7.50)
- Blood urea nitrogen 8 mg/dL (8–25)
- Creatinine 0.65 mg/dL (0.70–1.40)
- Lactate dehydrogenase 146 U/L (100–220)
- Uric acid 4.0 mg/dL (2.0–7.0)
- Thyrotropin (thyroid-stimulating hormone) 1.86 μIU/mL (0.4–5.5).
A recent tuberculin skin test obtained as part of her employment screening was negative, and so was a test for antibody to human immunodeficiency virus (HIV), obtained recently before donating plasma. A urine pregnancy test done in the office was also negative. A peripheral blood smear showed slight toxic granulation with rare reactive lymphocytes.
2. Which test would provide the greatest diagnostic yield at this point?
- Needle aspiration biopsy of lymph node
- Excisional lymph node biopsy
- Polymerase chain reaction (PCR) testing for HIV
- Antistreptolysin-O (ASO) titer
Because of the persistent enlargement of the patient’s lymph nodes despite several weeks of antibiotic treatment, and because submandibular and supraclavicular nodes were involved, excisional lymph node biopsy would be the best of these choices to evaluate for malignancy. Compared with needle aspiration biopsy, it is the gold standard, preserving the nodal architecture and providing ample tissue for immunostaining and additional studies.
Needle aspiration biopsy is safe, inexpensive, and easy to do and can be useful in situations of limited resources, but it does not reliably distinguish between a reactive and a neoplastic process.1 Its collection and interpretation are highly variable and personnel-dependent, and its sensitivity for detecting lymphoma is reported to be as low as 7.1% (95% confidence interval 0.9% to 23.5%).2
An acute retroviral syndrome can cause adenopathy, especially before seroconversion is evident, but it is usually associated with an influenza-like illness and monocytosis. Although this patient had no apparent risk factors for HIV, ordering PCR testing for HIV is also an important step when the clinical situation is suggestive. In the absence of an abnormal-appearing oropharynx, tonsillar exudate, or high fever, the pretest probability of streptococcal pharyngitis is low, and an ASO titer is unlikely to be diagnostic in this case.
CASE CONTINUED: BIOPSY PERFORMED
An incisional lymph node biopsy was obtained (Figure 1).
3. Which can confirm the suspected diagnosis?
- Tissue culture
- Test for immunoglobulin (Ig) G and IgM antibodies to Toxoplasma gondii
- Serum PCR testing for T gondii
- T gondii IgG avidity testing
DIAGNOSIS OF TOXOPLASMOSIS
In this patient, acute toxoplasmosis was suspected based on recognition of the morphologic triad seen in Toxoplasma lymphadenitis— ie, follicular hyperplasia, abundance of monocytoid cells, and clusters of epithelioid lymphocytes.3,4
Detection and measurement of IgM antibodies against T gondii is the most widely used serologic test for acute toxoplasmosis and is often considered the reference standard among the most common commercially available agglutination screening assays. It has a sensitivity between 93.7% and 100% and a specificity of 97.1% to 99.2%.5 Confirmation is generally done with enzyme-linked immunoassay or chemiluminescent-based tests, which can detect lower levels of IgG and IgM.5
A positive serum IgG confirms seroconversion but by itself cannot distinguish between acute and chronic infection, although it is commonly obtained in conjunction with IgM levels.6 Since both IgG and IgM can be elevated months after initial infection, serum IgA and IgE levels can more accurately suggest the timing of infection if clarification is needed.6,7 In addition, IgG avidity testing can distinguish acute infection from chronic infection: a high avidity index suggests the acute infection occurred at least 3 to 5 months ago, whereas the avidity index may be low or zero if acute infection occurred within the past 4 weeks.7 The sensitivity of avidity testing is 91.3% to 94.4%, and the specificity 87.8% to 98.5%.7
Serum PCR testing for T gondii is useful when toxoplasmosis is suspected in patients whose immune system may not be able to mount an adequate antibody response or in patients in the hyperacute phase of infection, even before a detectable antibody response can be formed.8,9 However, because of limitations of equipment, expertise, and overall cost, this method is not universally available. Additionally, blood cultures and PCR testing or tissue culture of pathologic specimens cannot routinely be relied on for diagnosis, as often the burden of microorganisms present in these specimens is low. When positive, culture specimens may yield bradyzoites or tachyzoites, but only after considerable latency of many days to weeks.10
How people acquire acute toxoplasmosis
T gondii is an obligate intracellular protozoan parasite. Sexual replication of the organism takes place within the intestines of cats (the definitive host), with subsequent excretion of infective oocysts in feces.11 These hardy oocysts can contaminate soil or water supplies and can survive for months, depending on ambient temperature and humidity. Ingestion of oocysts can lead to infection of a variety of mammals, including sheep, pigs, chicken, and cattle.
Infection in humans can occur with consumption of raw or undercooked foods contaminated with oocysts, and inadequate hand-washing and poor kitchen hygiene substantially increase the risk of infection.12 Activities such as gardening can expose humans to oocysts in contaminated water and soil. In addition, direct contact with cat feces, such as when cleaning the litterbox, is a known exposure risk. Vertical transmission can manifest as congenital toxoplasmosis in a fetus when transmitted from an infected pregnant mother.
Eating raw or undercooked food is considered to be the greatest risk factor for acquired toxoplasmosis and is believed to be responsible for about 50% of all cases.12 However, in pooled data from 14 case-control studies, no clear risk factor for Toxoplasma infection could be identified in up to 60% of affected people, leading many experts to believe contaminated water may play a larger role in acquisition than previously surmised.12
Toxoplasma cysts have a predilection for muscle and neural tissue, resulting in myositis, myocarditis, encephalitis, and chorioretinitis. Severe systemic manifestations are seen in people with impaired T-cell immunity, such as those with HIV infection and acquired immunodeficiency syndrome; or hematologic malignancy; in recipients of solid-organ transplants; or in people taking corticosteroids or cytotoxic drugs. Congenital infection can result in stillbirth, microcephaly, developmental delay, or deafness in the developing fetus and is an important cause of infant morbidity and death worldwide.13
Infection in immunocompetent people is usually asymptomatic.14 However, up to 20% of immunocompetent patients develop symptoms that tend to be nonspecific and include muscle aches and lymphadenopathy, and these are often mistaken for an influenza-like illness.14,15 Other symptoms include malaise, fevers, night sweats, pharyngitis, abdominal pain, hepatosplenomegaly, maculopapular rash, and atypical lymphocytosis (less than 10% of peripheral blood).11 The most common physical manifestation of acute toxoplasmosis is isolated cervical lymphadenopathy, although any lymph node group can be affected.14 Lymph nodes are not fixed or matted and generally are neither tender nor suppurative.16
4. What is the correct treatment strategy for acute toxoplasmosis in this case?
- Symptomatic treatment only
- Trimethoprim 160 mg and sulfamethoxazole 800 mg daily
- Combination of atovaquone and clindamycin
- Combination pyrimethamine, sulfadiazine, and folinic acid
TREATMENT AND PROGNOSIS OF ACUTE TOXOPLASMOSIS
No antimicrobial treatment is required for most immunocompetent patients. Symptoms are self-limited and resolve within 1 to 2 months in 60% of patients.14 A substantial proportion of patients—25%—will have lingering symptoms at 2 to 4 months, and some (10%) can have mild symptoms for 6 months or longer.16
Symptomatic treatment with analgesics such as nonsteroidal anti-inflammatory drugs (NSAIDs) is appropriate.
Immunocompromised and critically ill patients and those with ocular manifestations require combination therapy with pyrimethamine, sulfadiazine, and folinic acid.17 Trimethoprim-sulfamethoxazole is effective as prophylaxis against T gondii infection in immunocompromised patients at a dosage of 160 mg trimethoprim/800 mg sulfamethoxazole daily, but it is also an alternative for treatment at higher dosages (5 mg/kg trimethoprim and 25 mg/kg sulfamethoxazole twice daily).
Atovaquone and clindamycin can be used in sulfa-sensitive patients17 and also in those with latent toxoplasmosis for better penetration of tissue cysts. Corticosteroids are used as adjuncts in those with ocular involvement.
Spiramycin is the treatment of choice in pregnant women and can be given throughout the pregnancy.17,18 A recent comparative study by Hotop et al18 reported a reduction in the rate of fetal transmission (1.6% vs 4.8%) when spiramycin was given from the time of diagnosis through the 16th week of pregnancy, followed by a minimum of 4 weeks of combination therapy with pyrimethamine, sulfadiazine, and folinic acid.18
CASE CONCLUDED
Serologic testing was positive for IgM and IgG antibodies to T gondii, which suggested subacute infection. The patient received no antimicrobial therapy and her lymphadenopathy eventually resolved. Her generalized fatigue gradually resolved over the next year without antimicrobial treatment.
A thorough re-review of potential exposures was done at subsequent office visits to help elucidate how she may have acquired the infection. She recalled no recent exposure to cats or rodents, nor consumption of raw meat. We could only suppose that there may have been inadvertent exposure to oocyst-containing soil or water or to undercooked meat products. Thus, the diagnosis of acute toxoplasmosis should be kept in mind in the evaluation of lymphadenopathy, even in the absence of a clear history of exposure.
- Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc 2000; 75:723–732.
- Khillan R, Sidhu G, Axiotis C, Braverman AS. Fine needle aspiration (FNA) cytology for diagnosis of cervical lymphadenopathy. Int J Hematol 2012; 95:282–284.
- Dorfman RF, Remington JS. Value of lymph-node biopsy in the diagnosis of acute acquired toxoplasmosis. N Engl J Med 1973; 289:878–881.
- Eapen M, Mathew CF, Aravindan KP. Evidence based criteria for the histopathological diagnosis of toxoplasmic lymphadenopathy. J Clin Pathol 2005; 58:1143–1146.
- Villard O, Cimon B, Franck J, et al; Network from the French National Reference Center for Toxoplasmosis. Evaluation of the usefulness of six commercial agglutination assays for serologic diagnosis of toxoplasmosis. Diagn Microbiol Infect Dis 2012; 73:231–235.
- Suzuki LA, Rocha RJ, Rossi CL. Evaluation of serological markers for the immunodiagnosis of acute acquired toxoplasmosis. J Med Microbiol 2001; 50:62–70.
- Lachaud L, Calas O, Picot MC, Albaba S, Bourgeois N, Pratlong F. Value of 2 IgG avidity commercial tests used alone or in association to date toxoplasmosis contamination. Diagn Microbiol Infect Dis 2009; 64:267–274.
- Rahumatullah A, Khoo BY, Noordin R. Triplex PCR using new primers for the detection of Toxoplasma gondii. Exp Parasitol 2012; 131:231–238.
- Contini C, Giuliodori M, Cultrera R, Seraceni S. Detection of clinical-stage specific molecular Toxoplasma gondii gene patterns in patients with toxoplasmic lymphadenitis. J Med Microbiol 2006; 55:771–774.
- Silveira C, Vallochi AL, Rodrigues da Silva U, et al. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol 2011; 95:396–400.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363:1965–1976.
- Petersen E, Vesco G, Villari S, Buffolano W. What do we know about risk factors for infection in humans with Toxoplasma gondii and how can we prevent infections? Zoonoses Public Health 2010; 57:8–17.
- Feldman DM, Timms D, Borgida AF. Toxoplasmosis, parvovirus, and cytomegalovirus in pregnancy. Clin Lab Med 2010; 30:709–720.
- Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol 2009; 39:895–901.
- Remington JS. Toxoplasmosis in the adult. Bull NY Acad Med 1974; 50:211–227.
- McCabe RE, Brooks RG, Dorfman RF, Remington JS. Clinical spectrum in 107 cases of toxoplasmic lymphadenopathy. Rev Infect Dis 1987; 9:754–774.
- Toxoplasmosis. The Medical Letter, Drugs for Parasitic Infections. New Rochelle, NY: The Medical Letter Inc, June 1, 2010:57–58.
- Hotop A, Hlobil H, Gross U. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2012; 54:1545–1552.
- Habermann TM, Steensma DP. Lymphadenopathy. Mayo Clin Proc 2000; 75:723–732.
- Khillan R, Sidhu G, Axiotis C, Braverman AS. Fine needle aspiration (FNA) cytology for diagnosis of cervical lymphadenopathy. Int J Hematol 2012; 95:282–284.
- Dorfman RF, Remington JS. Value of lymph-node biopsy in the diagnosis of acute acquired toxoplasmosis. N Engl J Med 1973; 289:878–881.
- Eapen M, Mathew CF, Aravindan KP. Evidence based criteria for the histopathological diagnosis of toxoplasmic lymphadenopathy. J Clin Pathol 2005; 58:1143–1146.
- Villard O, Cimon B, Franck J, et al; Network from the French National Reference Center for Toxoplasmosis. Evaluation of the usefulness of six commercial agglutination assays for serologic diagnosis of toxoplasmosis. Diagn Microbiol Infect Dis 2012; 73:231–235.
- Suzuki LA, Rocha RJ, Rossi CL. Evaluation of serological markers for the immunodiagnosis of acute acquired toxoplasmosis. J Med Microbiol 2001; 50:62–70.
- Lachaud L, Calas O, Picot MC, Albaba S, Bourgeois N, Pratlong F. Value of 2 IgG avidity commercial tests used alone or in association to date toxoplasmosis contamination. Diagn Microbiol Infect Dis 2009; 64:267–274.
- Rahumatullah A, Khoo BY, Noordin R. Triplex PCR using new primers for the detection of Toxoplasma gondii. Exp Parasitol 2012; 131:231–238.
- Contini C, Giuliodori M, Cultrera R, Seraceni S. Detection of clinical-stage specific molecular Toxoplasma gondii gene patterns in patients with toxoplasmic lymphadenitis. J Med Microbiol 2006; 55:771–774.
- Silveira C, Vallochi AL, Rodrigues da Silva U, et al. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol 2011; 95:396–400.
- Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004; 363:1965–1976.
- Petersen E, Vesco G, Villari S, Buffolano W. What do we know about risk factors for infection in humans with Toxoplasma gondii and how can we prevent infections? Zoonoses Public Health 2010; 57:8–17.
- Feldman DM, Timms D, Borgida AF. Toxoplasmosis, parvovirus, and cytomegalovirus in pregnancy. Clin Lab Med 2010; 30:709–720.
- Weiss LM, Dubey JP. Toxoplasmosis: a history of clinical observations. Int J Parasitol 2009; 39:895–901.
- Remington JS. Toxoplasmosis in the adult. Bull NY Acad Med 1974; 50:211–227.
- McCabe RE, Brooks RG, Dorfman RF, Remington JS. Clinical spectrum in 107 cases of toxoplasmic lymphadenopathy. Rev Infect Dis 1987; 9:754–774.
- Toxoplasmosis. The Medical Letter, Drugs for Parasitic Infections. New Rochelle, NY: The Medical Letter Inc, June 1, 2010:57–58.
- Hotop A, Hlobil H, Gross U. Efficacy of rapid treatment initiation following primary Toxoplasma gondii infection during pregnancy. Clin Infect Dis 2012; 54:1545–1552.
A review of uncommon swelling provides useful reminders
Angioedema associated with insufficient activity of circulating complement component 1 (C1) inhibitor is a rare condition that can be hereditary or acquired. The clinical characteristics of this disorder are nicely reviewed by Drs. Tse and Zuraw in this issue of the Journal. Despite the rarity of this condition, there are good reasons to spend a few minutes with this article.
Angioedema and the urticarias differ in their clinical manifestations and diagnostic and therapeutic implications. Patients who have recurrent urticaria, no matter the severity, need not be evaluated for C1 inhibitor deficiency. Some patients who have recurrent angioedema without urticaria may respond well to antiallergic therapies (eg, antihistamines, corticosteroids), and these patients also are unlikely to have C1 inhibitor deficiency. Other patients who have intermittent localized peripheral swelling (or visceral edema manifest as abdominal pain) without urticaria may not respond, and it is in these patients that a pathophysiologic mechanism other than typical allergy should be considered.
In some patients, bradykinin-mediated angioedema is due to deficient C1 inhibitor activity, in others, to angiotensin-converting enzyme (ACE) inhibition. Although the biochemical site of action is different, both of these conditions result from an imbalance of protease activation and inhibition. If there is not enough C1 inhibition, there is too much kallikrein activity, which leads to excess proteolytic generation of the peptide bradykinin from kininogen. In contrast, ACE inhibitors occasionally cause angioedema by decreasing catabolism of bradykinin. In either case, bradykinin-mediated angioedema is generally clinically resistant to antiallergic therapy.
Thinking about these mechanisms reminds us of the complexity and overlap of many physiologic proteolytic cascades. Although C1 inhibitor is known as an inhibitor of C1 esterase and C4 levels are almost always low when C1 inhibitor activity is depressed, the angioedema in C1-inhibitor-deficiency states is more a result of decreased inhibition of enzymes in the coagulation cascade than in the complement cascade. Similarly, ACE-inhibitor-associated angioedema has little to do with the decreased generation of angiotensin that accounts for these drugs’ antihypertensive effect.
Generalizing these principles should make us vigilant for unpredicted adverse reactions to other newer drugs such as protease and kinase inhibitors, which may variably affect multiple biochemical pathways.
Angioedema associated with insufficient activity of circulating complement component 1 (C1) inhibitor is a rare condition that can be hereditary or acquired. The clinical characteristics of this disorder are nicely reviewed by Drs. Tse and Zuraw in this issue of the Journal. Despite the rarity of this condition, there are good reasons to spend a few minutes with this article.
Angioedema and the urticarias differ in their clinical manifestations and diagnostic and therapeutic implications. Patients who have recurrent urticaria, no matter the severity, need not be evaluated for C1 inhibitor deficiency. Some patients who have recurrent angioedema without urticaria may respond well to antiallergic therapies (eg, antihistamines, corticosteroids), and these patients also are unlikely to have C1 inhibitor deficiency. Other patients who have intermittent localized peripheral swelling (or visceral edema manifest as abdominal pain) without urticaria may not respond, and it is in these patients that a pathophysiologic mechanism other than typical allergy should be considered.
In some patients, bradykinin-mediated angioedema is due to deficient C1 inhibitor activity, in others, to angiotensin-converting enzyme (ACE) inhibition. Although the biochemical site of action is different, both of these conditions result from an imbalance of protease activation and inhibition. If there is not enough C1 inhibition, there is too much kallikrein activity, which leads to excess proteolytic generation of the peptide bradykinin from kininogen. In contrast, ACE inhibitors occasionally cause angioedema by decreasing catabolism of bradykinin. In either case, bradykinin-mediated angioedema is generally clinically resistant to antiallergic therapy.
Thinking about these mechanisms reminds us of the complexity and overlap of many physiologic proteolytic cascades. Although C1 inhibitor is known as an inhibitor of C1 esterase and C4 levels are almost always low when C1 inhibitor activity is depressed, the angioedema in C1-inhibitor-deficiency states is more a result of decreased inhibition of enzymes in the coagulation cascade than in the complement cascade. Similarly, ACE-inhibitor-associated angioedema has little to do with the decreased generation of angiotensin that accounts for these drugs’ antihypertensive effect.
Generalizing these principles should make us vigilant for unpredicted adverse reactions to other newer drugs such as protease and kinase inhibitors, which may variably affect multiple biochemical pathways.
Angioedema associated with insufficient activity of circulating complement component 1 (C1) inhibitor is a rare condition that can be hereditary or acquired. The clinical characteristics of this disorder are nicely reviewed by Drs. Tse and Zuraw in this issue of the Journal. Despite the rarity of this condition, there are good reasons to spend a few minutes with this article.
Angioedema and the urticarias differ in their clinical manifestations and diagnostic and therapeutic implications. Patients who have recurrent urticaria, no matter the severity, need not be evaluated for C1 inhibitor deficiency. Some patients who have recurrent angioedema without urticaria may respond well to antiallergic therapies (eg, antihistamines, corticosteroids), and these patients also are unlikely to have C1 inhibitor deficiency. Other patients who have intermittent localized peripheral swelling (or visceral edema manifest as abdominal pain) without urticaria may not respond, and it is in these patients that a pathophysiologic mechanism other than typical allergy should be considered.
In some patients, bradykinin-mediated angioedema is due to deficient C1 inhibitor activity, in others, to angiotensin-converting enzyme (ACE) inhibition. Although the biochemical site of action is different, both of these conditions result from an imbalance of protease activation and inhibition. If there is not enough C1 inhibition, there is too much kallikrein activity, which leads to excess proteolytic generation of the peptide bradykinin from kininogen. In contrast, ACE inhibitors occasionally cause angioedema by decreasing catabolism of bradykinin. In either case, bradykinin-mediated angioedema is generally clinically resistant to antiallergic therapy.
Thinking about these mechanisms reminds us of the complexity and overlap of many physiologic proteolytic cascades. Although C1 inhibitor is known as an inhibitor of C1 esterase and C4 levels are almost always low when C1 inhibitor activity is depressed, the angioedema in C1-inhibitor-deficiency states is more a result of decreased inhibition of enzymes in the coagulation cascade than in the complement cascade. Similarly, ACE-inhibitor-associated angioedema has little to do with the decreased generation of angiotensin that accounts for these drugs’ antihypertensive effect.
Generalizing these principles should make us vigilant for unpredicted adverse reactions to other newer drugs such as protease and kinase inhibitors, which may variably affect multiple biochemical pathways.
Recognizing and managing hereditary angioedema
Hereditary angioedema due to deficiency of C1 inhibitor is a rare autosomal dominant disease that can be life-threatening. It affects about 1 in 50,000 people,1 or about 6,000 people in the United States. There are no known differences in prevalence by ethnicity or sex. A form of hereditary angioedema with normal C1 inhibitor levels has also recently been identified.
Despite a growing awareness of hereditary angioedema in the medical community, repeated surveys have found an average gap of 10 years between the first appearance of symptoms and the correct diagnosis. In view of the risk of morbidity and death, recognizing this disease sooner is critical.
This article will discuss how to recognize hereditary angioedema and how to differentiate it from other forms of recurring angioedema. We will also review its acute and long-term management, with special attention to new therapies and clinical challenges.
EPISODES OF SWELLING WITHOUT HIVES
Hereditary angioedema involves recurrent episodes of nonpruritic, nonpitting, subcutaneous and submucosal edema that can affect the face, tongue, larynx, trunk, extremities, bowels, or genitals. Attacks typically follow a predictable course: swelling that increases slowly and continuously for 24 hours and then gradually subsides over the next 48 to 72 hours. Attacks that involve the oropharynx, larynx, or abdomen carry the highest risk of morbidity and death.1
The frequency and severity of attacks are highly variable and unpredictable. A few patients have no attacks, a few have two attacks per week, and most fall in between.
Hives suggests an allergic or idiopathic rather than hereditary cause and will not be discussed here in detail. A history of angioedema that was rapidly aborted by antihistamines, corticosteroids, or epinephrine also suggests an allergic rather than hereditary cause.
UNCHECKED BRADYKININ PRODUCTION
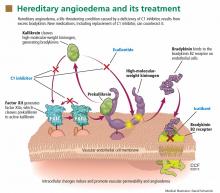
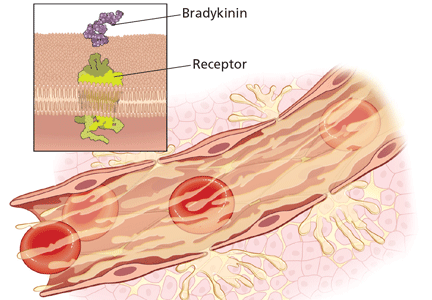
Substantial evidence indicates that hereditary angioedema results from extravasation of plasma into deeper cutaneous or mucosal compartments as a result of overproduction of the vasoactive mediator bradykinin (Figure 1).
Activated factor XII cleaves plasma prekallikrein to generate active plasma kallikrein (which, in turn, activates more factor XII).2 Once generated, plasma kallikrein cleaves high-molecular-weight kininogen, releasing bradykinin. Bradykinin binds to the B2 bradykinin receptor on endothelial cells, increasing the permeability of the endothelium.
Normally, C1 inhibitor helps control bradykinin production by inhibiting plasma kallikrein and activated factor XII. Without enough C1 inhibitor, the contact system is uninhibited and results in bradykinin being inappropriately generated.
Because the attacks of hereditary angioedema involve excessive bradykinin, they do not respond to the usual treatments for anaphylaxis and allergic angioedema (which involve mast cell degranulation), such as antihistamines, corticosteroids, and epinephrine.
TWO TYPES OF HEREDITARY ANGIOEDEMA
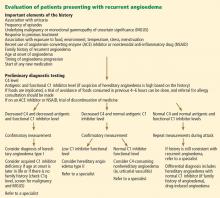
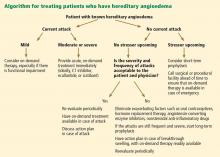
Figure 2 shows the evaluation of patients with suspected hereditary angioedema.
Hereditary angioedema due to C1 inhibitor deficiency
The classic forms of hereditary angioedema (types I and II) involve loss-of-function mutations in SERPING1—the gene that encodes for C1 inhibitor—resulting in low levels of functional C1 inhibitor.3 The mutation is inherited in an autosomal dominant pattern; however, in about 25% of cases, it appears to arise spontaneously,4 so a family history is not required for diagnosis.
Although C1 inhibitor deficiency is present from birth, the clinical disease most commonly presents for the first time when the patient is of school age. Half of patients have their first episode in the first decade of life, and another one-third first develop symptoms over the next 10 years.5
Clinically, types I and II are indistinguishable. Type I, accounting for 85% of cases,1 results from low production of C1 inhibitor. Laboratory studies reveal low antigenic and functional levels of C1 inhibitor.
In type II, the mutant C1 inhibitor protein is present but dysfunctional and unable to inhibit target proteases. On laboratory testing, the functional level of C1 inhibitor is low but its antigenic level is normal (Table 1). Function can be tested by either chromogenic assay or enzyme-linked immunosorbent assay; the former is preferred because it is more sensitive.6
Because C1 inhibitor deficiency results in chronic activation of the complement system, patients with type I or II disease usually have low C4 levels regardless of disease activity, making measuring C4 the most economical screening test. When suspicion for hereditary angioedema is high, based on the presentation and family and clinical history, measuring antigenic and functional C1 inhibitor levels and C4 simultaneously is more efficient.
Hereditary angioedema with normal C1 inhibitor levels
Hereditary angioedema with normal C1 inhibitor levels is also inherited in an autosomal dominant pattern. It is often estrogen-sensitive, making it more severe in women. Symptoms tend to develop slightly later in life than in type I or II disease.7
Angioedema with normal C1 inhibitor levels has been associated with factor XII mutations in a minority of cases, but most patients do not have a specific laboratory abnormality. Because there is no specific laboratory profile, the diagnosis is based on clinical criteria. Hereditary angioedema with normal C1 inhibitor levels should be considered in patients who have recurrent angioedema, normal C4, normal antigenic and functional C1 inhibitor levels, a lack of response to high-dose antihistamines, and either a family history of angioedema without hives or a known factor XII mutation.7 However, other forms of angioedema (allergic, drug-induced, and idiopathic) should also be considered, as C4 and C1 inhibitor levels are normal in these forms as well.
DIFFERENTIAL DIAGNOSIS: OTHER TYPES OF ANGIOEDEMA
Acquired C1 inhibitor deficiency
Symptoms of acquired C1 inhibitor deficiency resemble those of hereditary angioedema but typically do not emerge until the fourth decade of life or later, and patients have no family history of the condition. It is often associated with other diseases, most commonly B-cell lymphoproliferative disorders, which cause uncontrolled complement activation and consumption of C1 inhibitor.
In some patients, autoantibodies to C1 inhibitor develop, greatly reducing its effectiveness and resulting in enhanced consumption. The autoantibody is often associated with a monoclonal gammopathy of unknown significance. The presence of a C1 inhibitor autoantibody does not preclude the presence of an underlying disorder, and vice versa.
Laboratory studies reveal low C4, low C1-inhibitor antigenic and functional levels, and usually a low C1q level owing to consumption of complement. Autoantibodies to C1 inhibitor can be detected by laboratory testing.
Because of the association with autoimmune disease and malignant disorders (especially B-cell malignancy), a patient diagnosed with acquired C1 inhibitor deficiency should be further evaluated for underlying conditions.
Allergic angioedema
Allergic angioedema results from preformed antigen-specific immunoglobulin E (IgE) antibodies that stimulate mast cells to degranulate when patients are exposed to a particular allergen—most commonly food, insect venom, latex, or drugs. IgE-mediated histamine release causes swelling, as histamine is a potent vasodilator.
Symptoms often begin within 2 hours of exposure to the allergen and generally include concurrent urticaria and swelling that last less than 24 hours. Unlike in hereditary angioedema, the swelling responds to antihistamines and corticosteroids. When very severe, these symptoms may also be accompanied by bronchoconstriction and gastrointestinal symptoms, especially if the allergen is ingested.
Histamine-mediated angioedema may also be associated with exercise as part of a syndrome called exercise-induced anaphylaxis or angioedema.
Drug-induced angioedema
Drug-induced angioedema is typically associated with angiotensin-converting enzyme (ACE) inhibitors or nonsteroidal anti-inflammatory drugs (NSAIDs).
Angioedema associated with ACE inhibitors is estimated to affect 0.1% to 6% of patients taking these medications, with African Americans being at significantly higher risk. Although 25% of affected patients develop symptoms of angioedema within the first month of taking the drugs, some tolerate them for as long as 10 years before the first episode.9 The swelling is not allergic or histamine-related. ACE normally degrades bradykinin; therefore, inhibiting ACE leads to accumulation of bradykinin. Because all ACE inhibitors have this effect, this class of drug should be discontinued in any patient who develops isolated angioedema.
NSAID-induced angioedema is often accompanied by other symptoms, including urticaria, rhinitis, cough, hoarseness, or breathlessness.10 The mechanism of NSAID-induced angioedema involves cyclooxygenase (COX) 1 (and to a lesser extent COX-2) inhibition. All NSAIDs (and aspirin) should be avoided in patients with recurrent angioedema. Specific COX-2 inhibitors, while theoretically capable of causing angioedema by the same mechanism, are generally well tolerated in patients who have had COX-1 inhibitor reactions.
Idiopathic angioedema
If no clear cause of recurrent angioedema (at least three episodes in a year) can be found, it is labeled idiopathic.11 Some patients with idiopathic angioedema fail to benefit from high doses of antihistamines, suggesting that the cause is bradykinin-mediated.
CLINICAL MANIFESTATIONS OF HEREDITARY ANGIOEDEMA
Attacks may start at one site and progress to involve additional sites.
Prodromal symptoms may begin up to several days before an attack and include tingling, warmth, burning, or itching at the affected site; increased fatigue or malaise; nausea, abdominal distention, or gassiness; or increased hunger, particularly before an abdominal attack.5 The most characteristic prodromal symptom is erythema marginatum—a raised, serpiginous, nonpruritic rash on the trunk, arms, and legs but often sparing the face.
Abdominal attacks are easily confused with acute abdomen
Almost half of attacks involve the abdomen, and almost all patients with type I or II disease experience at least one such attack.12 Symptoms can include severe abdominal pain, nausea, vomiting, and diarrhea. Abdominal attacks account for many emergency department visits, hospitalizations, and surgical procedures for acute abdomen; about one-third of patients with undiagnosed hereditary angioedema undergo an unnecessary surgery during an abdominal attack. Angioedema of the gastrointestinal tract can result in enough plasma extravasation and vasodilation to cause hypovolemic shock.
Eradicating Helicobacter pylori infection may alleviate abdominal attacks.13
Attacks of the extremities can be painful and disabling
Attacks of the extremities affect 96% of patients12 and can be very disfiguring and disabling. Driving or using the phone is often difficult when the hands are affected. When feet are involved, walking and standing become painful. While these symptoms rarely result in a lengthy hospitalization, they interfere with work and school and require immediate medical attention because they can progress to other parts of the body.
Laryngeal attacks are life-threatening
About half of patients with hereditary angioedema have an attack of laryngeal edema at some point in their lives.12 If not effectively managed, laryngeal angioedema can progress to asphyxiation. A survey of family history in 58 patients with hereditary angioedema suggested a 40% incidence of asphyxiation in untreated laryngeal attacks, and 25% to 30% of patients are estimated to have died of laryngeal edema before effective treatment became available.14
Symptoms of a laryngeal attack include change in voice, hoarseness, trouble swallowing, shortness of breath, and wheezing. Physicians must recognize these symptoms quickly and give effective treatment early in the attack to prevent morbidity and death.
Establishing an airway can be life-saving in the absence of effective therapy, but extensive swelling of the upper airway can make intubation extremely difficult.
Genitourinary attacks also occur
Attacks involving the scrotum and labia have been reported in up to two-thirds of patients with hereditary angioedema at some point in their lives. Attacks involving the bladder and kidneys have also been reported but are less common, affecting about 5% of patients.12 Genitourinary attacks may be triggered by local trauma, such as horseback riding or sexual intercourse, although no trigger may be evident.
MANAGING ACUTE ATTACKS
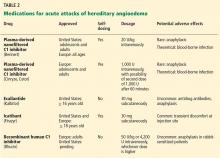
The goals of treatment are to alleviate acute exacerbations with on-demand treatment and to reduce the number of attacks with prophylaxis. Therapy should be individualized to each patient’s needs. Treatments have advanced greatly in the last several years, and new medications for treating acute attacks and preventing attacks have shown great promise (Figure 3, Table 2).
Patients tend to have recurrent symptoms interspersed with periods of health, suggesting that attacks ought to have identifiable triggers, although in most, no trigger is evident. The most commonly identified are local trauma (including medical and dental procedures), emotional stress, and acute infection. Disease severity may be worsened by menstruation, estrogen-containing oral contraceptives, hormone replacement therapy, ACE inhibitors, and NSAIDs.
It is critical that attacks be treated with an effective medication as soon as possible. Consensus guidelines state that all patients with hereditary angioedema due to C1 inhibitor deficiency, even if they are still asymptomatic, should have access to at least one of the drugs approved for on-demand treatment.15 The guidelines further state that whenever possible, “patients should have the on-demand medicine to treat acute attacks at home and should be trained to self-administer these medicines.”15
Plasma-derived C1 inhibitors
Several plasma-derived C1 inhibitors are available (Cinryze, Berinert, Cetor). They are prepared from fractionated plasma obtained from donors, then pasteurized and nanofiltered.
Berinert and Cinryze were each found to be superior to placebo in double-blind, placebo-controlled trials: attacks usually resolved 30 to 60 minutes after intravenous injection.16,17 Berinert 20 U/kg is associated with the onset of symptom relief as early as half an hour after administration, compared with 1.5 hours with placebo. Early use (at the onset of symptoms) of a plasma-derived C1 inhibitor in a low dose (500 U) can also be effective.18,19 Efficacy appears to be consistent at all sites of attack involvement, including laryngeal edema. Safety and efficacy have been demonstrated during pregnancy and lactation and in young children and babies.20
Plasma-derived C1 inhibitors can be self-administered. The safety and efficacy of self-administration (under physician supervision) were demonstrated in a study of Cinryze and Cetor, in which attack duration, pain medication use, and graded attack severity were significantly less with self-administered therapy than with therapy in the clinic.21
A concern about plasma-derived products is the possibility of blood-borne infection, but this has not been confirmed by experience.22
Recombinant human C1 inhibitor
A recombinant human C1 inhibitor (Rhucin) has been studied in two randomized placebo-controlled trials. Although this product has a shorter half-life than the plasma-derived C1 inhibitors (3 vs more than 24 hours), the two are equipotent: 1 U of recombinant human C1 inhibitor is equivalent to 1 U of plasma-derived C1 inhibitor. Because the supply of recombinant human C1 inhibitor is elastic, dosing has been higher, which may provide more efficacy.23 Similar to plasma-derived C1 inhibitor products, the recombinant human C1 inhibitor resulted in more rapid symptom relief than with saline (66 vs 122 minutes) and in a shorter time to minimal symptoms (247−266 vs 1,210 minutes).24
Allergy is of concern: in one study, a healthy volunteer with undisclosed rabbit allergy experienced an allergic reaction. Patients should be screened by a skin-prick test or serum testing for specific IgE to rabbit epithelium before being prescribed recombinant human C1 inhibitor. No data are available for use during pregnancy or breastfeeding.
Ecallantide
Ecallantide (Kalbitor) is a selective inhibitor of plasma kallikrein that is given in three subcutaneous injections. Ecallantide 30 mg was found superior to placebo during acute attacks.25,26
Ecallantide is well tolerated, with the most common adverse effects being headache, nausea, fatigue, diarrhea, and local injection-site reactions. Antibodies to ecallantide can be found in patients with increasing drug exposure but do not appear to correlate with adverse events. Hypersensitivity reactions have been observed in 2% to 3% of patients receiving repeated doses. Because of anaphylaxis risk, ecallantide must be administered by a health care professional.
Icatibant
Icatibant (Firazyr) is a bradykinin receptor-2 antagonist that is given in a single subcutaneous injection. Icatibant 30 mg significantly shortened time to symptom relief and time to almost complete resolution compared with placebo.27,28 Icatibant’s main adverse effect is transient local pain, swelling, and erythema at the injection site. Icatibant can be self-administered by patients.
Fresh-frozen plasma
Fresh-frozen plasma contains C1 inhibitor and was used before the newer products became available. Several noncontrolled studies reported benefit of its use in acute attacks.29 However, its use is controversial because it also contains contact-system proteins that could provide additional substrate for the generation of bradykinin, which could exacerbate attacks in some patients.1 This may be particularly dangerous in patients presenting with laryngeal edema: in such a situation, the physician should be ready to treat a sudden exacerbation with intubation. The risk of acquiring a blood-borne pathogen is also higher than with plasma-derived C1 inhibitor.
PROPHYLACTIC MANAGEMENT
Short-term and long-term prophylaxis have important roles in preventing attacks (Table 3).
Short-term prophylaxis before an anticipated attack
Short-term prophylaxis is used for patients whose disease is generally well controlled but who anticipate exposure to a potentially exacerbating situation, such as an invasive medical, surgical, or dental procedure. (Routine dental cleanings are generally considered safe and do not require prophylaxis.)
Prophylactic treatments include:
- Plasma-derived C1 inhibitor, 500 to 1,500 U 1 hour before the provoking event
- High-dose 17-alpha alkylated (attenuated) androgens (eg, danazol [Danocrine] 200 mg orally 3 times daily) for 5 to 10 days before the provoking event
- Fresh-frozen plasma, 2 U 1 to 12 hours before the event.1
Yet even with short-term prophylaxis, on-demand treatment should be available.
Long-term prophylaxis
While many patients can be managed with on-demand treatment only, other patients (reflecting the severity of their attacks, as well as their individual needs) may benefit from a combination of on-demand treatment plus long-term prophylaxis. Several options are available (Table 3).
17-alpha alkylated androgens. Patients treated with danazol 600 mg/day were attack-free 90% of the time during a 28-day period compared with only 2.2% of the time in placebo-treated patients.30 Use of anabolic androgens, however, is limited by their adverse effects, including weight gain, virilization, menstrual irregularities, headaches, depression, dyslipidemia, liver enzyme elevation, liver adenomas, and hepatocellular carcinoma. Arterial hypertension occurs in about 25% of treated patients.
Because adverse effects are dose-dependent, treatment should be empirically titrated to find the minimal effective dose, generally recommended to be no more than 200 mg per day of danazol or the equivalent.15
Contraindications include use by women during pregnancy or lactation and by children until growth is complete.
Regular follow-up is recommended every 6 months, with monitoring of liver enzymes, lipids, complete blood counts, alpha fetoprotein, and urinalysis. Abdominal ultrasonography (every 6 months if receiving 100 mg/day or more of danazol, every 12 months if less than 100 mg/day) is advisable for early diagnosis of liver tumors.
Antifibrinolytic drugs. Tranexamic acid (Lysteda) and aminocaproic acid (Amicar) have been found to be effective in reducing the number of attacks of hereditary angioedema compared with placebo but are considered to be less reliable than androgens. These drugs have been used in patients who do not tolerate anabolic androgens, and in children and pregnant women. Tranexamic acid is given at a dose of 20 to 50 mg/kg/day divided into two or three doses per day. The therapeutic dose of aminocaproic acid is 1 g orally three to four times per day.31 Patients with a personal or family history of thromboembolic disease may be at greater risk of venous or arterial thrombosis, but this has not occurred in clinical studies.
Plasma-derived C1 inhibitors. In a 24-week crossover study in 22 patients with hereditary angioedema, Cinryze 1,000 U every 3 to 4 days reduced the rate of attacks by 50% while also reducing their severity and duration.17 An open-label extension study in 146 patients for almost 3 years documented a 90% reduction in attack frequency with no evidence of tachyphylaxis.32
New treatments are costlier
The newer on-demand and prophylactic drugs are substantially costlier than the older alternatives (androgens, antifibrinolytics, and fresh-frozen plasma); however, they have a substantially better benefit-to-risk ratio. Furthermore, the costs of care for an attack requiring emergency treatment are also high. Hereditary angioedema patients are often young, otherwise healthy, and capable of leading normal productive lives. While formal pharmacoeconomic studies of the optimal use of these newer drugs have not yet been done, it is important that the use of these drugs be well justified. Ideally, physicians who prescribe these drugs should be knowledgeable in the management of hereditary angioedema.
SPECIAL CHALLENGES IN WOMEN
Women with hereditary angioedema have more frequent attacks and generally a more severe disease course than men.12 Optimizing care for women is challenging because hormonal changes often cause the disease to flare up in menarche, pregnancy, lactation, and menopause. Women also have a higher rate of discontinuing long-term androgen therapy because of side effects, including virilization and menstrual irregularities. Spironolactone (Aldactone) 100 to 200 mg daily can be used to control hirsutism.33
Contraception
Because estrogen can trigger attacks, progesterone-only formulations, intrauterine devices, or barrier methods are recommended for contraception.33 Progesterone-only pills are preferred and improve symptoms in more than 60% of women. Etonogestrel, another alternative, is available as an implant (Implanon) or vaginal ring (Nuvaring). Intrauterine devices are generally well tolerated, and no prophylaxis is needed during placement. The progesterone-eluting intrauterine device (Mirena) could be beneficial.34
Pregnancy and lactation
Pregnancy and lactation pose particular challenges. Anabolic androgens are contraindicated during pregnancy as well as during breastfeeding because they can be passed on in breast milk. Women receiving androgen prophylaxis should understand that they can still ovulate and need contraception if they are sexually active.34 Patients on attenuated androgens who desire pregnancy should discontinue them 2 months before trying to conceive.
Changes in attack patterns can be unpredictable during pregnancy. Attacks tend to be more severe during the first trimester and more frequent during the third. Due to its safety and efficacy, plasma-derived C1 inhibitor has become the treatment of choice for on-demand or prophylactic treatment during pregnancy and lactation. Antifibrinolytics are considered only when plasma-derived C1 inhibitor is not available.31 Ecallantide and icatibant have not been studied in pregnancy. If neither plasma-derived C1 inhibitor nor antifibrinolytics are available, fresh-frozen plasma or solvent-and-detergent-treated plasma can be used.
Short-term prophylaxis should be considered before amniocentesis, chorionic villous sampling, and dilation and curettage. Delivery should take place in a facility with rapid access to plasma-derived C1 inhibitor as well as consultants in obstetrics, anesthesiology, and perinatology. Although plasma-derived C1 inhibitor should be available at all times during labor and delivery, its prophylactic use is not required unless labor and delivery are particularly traumatic, the underlying hereditary angioedema is very severe, or if forceps, vacuum delivery, or cesarian section is performed. Close monitoring is recommended for at least 72 hours after routine vaginal delivery and for 1 week after cesarian section.
CONCLUSION
The goals of hereditary angioedema treatment are to alleviate morbidity and mortality associated with the disease and to improve the patient’s quality of life. Achieving these goals requires timely diagnosis, patient education, and careful selection of therapeutic modalities that are individualized to the needs of that patient. Treatments have advanced greatly in the last 4 years, and new medications for both the acute and chronic symptoms of hereditary angioedema have shown great promise.
Acknowledgment: K.T. is funded by National Institutes of Health grant T32 AI 07469.
- Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med 2008; 359:1027–1036.
- Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol 2010; 126:918–925.
- Davis AE. C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol 1988; 6:595–628.
- Pappalardo E, Cicardi M, Duponchel C, et al. Frequent de novo mutations and exon deletions in the C1inhibitor gene of patients with angioedema. J Allergy Clin Immunol 2000; 106:1147–1154.
- Frigas E, Park M. Idiopathic recurrent angioedema. Immunol Allergy Clin North Am 2006; 26:739–751.
- Wagenaar-Bos IG, Drouet C, Aygören-Pursun E, et al. Functional C1-inhibitor diagnostics in hereditary angioedema: assay evaluation and recommendations. J Immunol Methods 2008; 338:14–20.
- Bork K. Diagnosis and treatment of hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol 2010; 6:15.
- Zuraw BL, Bork K, Binkley KE, et al. Hereditary angioedema with normal C1 inhibitor function: consensus of an international expert panel. Allergy Asthma Proc 2012; 33:S145–S156.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am 2006; 26:725–737.
- Busse PJ. Angioedema: differential diagnosis and treatment. Allergy Asthma Proc 2011; 32(suppl 1):S3–S11.
- Prematta MJ, Kemp JG, Gibbs JG, Mende C, Rhoads C, Craig TJ. Frequency, timing, and type of prodromal symptoms associated with hereditary angioedema attacks. Allergy Asthma Proc 2009; 30:506–511.
- Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 2006; 119:267–274.
- Farkas H, Füst G, Fekete B, Karádi I, Varga L. Eradication of Helicobacter pylori and improvement of hereditary angioneurotic oedema. Lancet 2001; 358:1695–1696.
- Bork K, Barnstedt SE. Treatment of 193 episodes of laryngeal edema with C1 inhibitor concentrate in patients with hereditary angioedema. Arch Intern Med 2001; 161:714–718.
- Cicardi M, Bork K, Caballero T, et al; HAWK (Hereditary Angioedema International Working Group). Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy 2012; 67:147–157.
- Craig TJ, Levy RJ, Wasserman RL, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol 2009; 124:801–808.
- Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med 2010; 363:513–522.
- Bork K, Meng G, Staubach P, Hardt J. Treatment with C1 inhibitor concentrate in abdominal pain attacks of patients with hereditary angioedema. Transfusion 2005; 45:1774–1784.
- Kreuz W, Martinez-Saguer I, Aygören-Pürsün E, Rusicke E, Heller C, Klingebiel T. C1-inhibitor concentrate for individual replacement therapy in patients with severe hereditary angioedema refractory to danazol prophylaxis. Transfusion 2009; 49:1987–1995.
- Farkas H, Varga L, Széplaki G, Visy B, Harmat G, Bowen T. Management of hereditary angioedema in pediatric patients. Pediatrics 2007; 120:e713–e722.
- Tourangeau LM, Castaldo AJ, Davis DK, Koziol J, Christiansen SC, Zuraw BL. Safety and efficacy of physician-supervised self-managed C1 inhibitor replacement therapy. Int Arch Allergy Immunol 2012; 157:417–424.
- De Serres J, Gröner A, Lindner J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apher Sci 2003; 29:247–254.
- Hack CE, Relan A, van Amersfoort ES, Cicardi M. Target levels of functional C1-inhibitor in hereditary angioedema. Allergy 2012; 67:123–130.
- Zuraw B, Cicardi M, Levy RJ, et al. Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol 2010; 126:821–827.e14.
- Cicardi M, Levy RJ, McNeil DL, et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med 2010; 363:523–531.
- Levy RJ, Lumry WR, McNeil DL, et al. EDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol 2010; 104:523–529.
- Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med 2010; 363:532–541.
- Lumry WR, Li HH, Levy RJ, et al. Randomized placebo-controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol 2011; 107:529–537.
- Prematta M, Gibbs JG, Pratt EL, Stoughton TR, Craig TJ. Fresh frozen plasma for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol 2007; 98:383–388.
- Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med 1976; 295:1444–1448.
- Zuraw BL. HAE therapies: past present and future. Allergy Asthma Clin Immunol 2010; 6:23.
- Zuraw BL, Kalfus I. Safety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedema. Am J Med 2012: Epub ahead of print.
- Caballero T, Farkas H, Bouillet L, et al; C-1-INH Deficiency Working Group. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol 2012; 129:308–320.
- Bouillet L, Longhurst H, Boccon-Gibod I, et al. Disease expression in women with hereditary angioedema. Am J Obstet Gynecol 2008; 199:484.e1–e4.
Hereditary angioedema due to deficiency of C1 inhibitor is a rare autosomal dominant disease that can be life-threatening. It affects about 1 in 50,000 people,1 or about 6,000 people in the United States. There are no known differences in prevalence by ethnicity or sex. A form of hereditary angioedema with normal C1 inhibitor levels has also recently been identified.
Despite a growing awareness of hereditary angioedema in the medical community, repeated surveys have found an average gap of 10 years between the first appearance of symptoms and the correct diagnosis. In view of the risk of morbidity and death, recognizing this disease sooner is critical.
This article will discuss how to recognize hereditary angioedema and how to differentiate it from other forms of recurring angioedema. We will also review its acute and long-term management, with special attention to new therapies and clinical challenges.
EPISODES OF SWELLING WITHOUT HIVES
Hereditary angioedema involves recurrent episodes of nonpruritic, nonpitting, subcutaneous and submucosal edema that can affect the face, tongue, larynx, trunk, extremities, bowels, or genitals. Attacks typically follow a predictable course: swelling that increases slowly and continuously for 24 hours and then gradually subsides over the next 48 to 72 hours. Attacks that involve the oropharynx, larynx, or abdomen carry the highest risk of morbidity and death.1
The frequency and severity of attacks are highly variable and unpredictable. A few patients have no attacks, a few have two attacks per week, and most fall in between.
Hives suggests an allergic or idiopathic rather than hereditary cause and will not be discussed here in detail. A history of angioedema that was rapidly aborted by antihistamines, corticosteroids, or epinephrine also suggests an allergic rather than hereditary cause.
UNCHECKED BRADYKININ PRODUCTION
Substantial evidence indicates that hereditary angioedema results from extravasation of plasma into deeper cutaneous or mucosal compartments as a result of overproduction of the vasoactive mediator bradykinin (Figure 1).
Activated factor XII cleaves plasma prekallikrein to generate active plasma kallikrein (which, in turn, activates more factor XII).2 Once generated, plasma kallikrein cleaves high-molecular-weight kininogen, releasing bradykinin. Bradykinin binds to the B2 bradykinin receptor on endothelial cells, increasing the permeability of the endothelium.
Normally, C1 inhibitor helps control bradykinin production by inhibiting plasma kallikrein and activated factor XII. Without enough C1 inhibitor, the contact system is uninhibited and results in bradykinin being inappropriately generated.
Because the attacks of hereditary angioedema involve excessive bradykinin, they do not respond to the usual treatments for anaphylaxis and allergic angioedema (which involve mast cell degranulation), such as antihistamines, corticosteroids, and epinephrine.
TWO TYPES OF HEREDITARY ANGIOEDEMA
Figure 2 shows the evaluation of patients with suspected hereditary angioedema.
Hereditary angioedema due to C1 inhibitor deficiency
The classic forms of hereditary angioedema (types I and II) involve loss-of-function mutations in SERPING1—the gene that encodes for C1 inhibitor—resulting in low levels of functional C1 inhibitor.3 The mutation is inherited in an autosomal dominant pattern; however, in about 25% of cases, it appears to arise spontaneously,4 so a family history is not required for diagnosis.
Although C1 inhibitor deficiency is present from birth, the clinical disease most commonly presents for the first time when the patient is of school age. Half of patients have their first episode in the first decade of life, and another one-third first develop symptoms over the next 10 years.5
Clinically, types I and II are indistinguishable. Type I, accounting for 85% of cases,1 results from low production of C1 inhibitor. Laboratory studies reveal low antigenic and functional levels of C1 inhibitor.
In type II, the mutant C1 inhibitor protein is present but dysfunctional and unable to inhibit target proteases. On laboratory testing, the functional level of C1 inhibitor is low but its antigenic level is normal (Table 1). Function can be tested by either chromogenic assay or enzyme-linked immunosorbent assay; the former is preferred because it is more sensitive.6
Because C1 inhibitor deficiency results in chronic activation of the complement system, patients with type I or II disease usually have low C4 levels regardless of disease activity, making measuring C4 the most economical screening test. When suspicion for hereditary angioedema is high, based on the presentation and family and clinical history, measuring antigenic and functional C1 inhibitor levels and C4 simultaneously is more efficient.
Hereditary angioedema with normal C1 inhibitor levels
Hereditary angioedema with normal C1 inhibitor levels is also inherited in an autosomal dominant pattern. It is often estrogen-sensitive, making it more severe in women. Symptoms tend to develop slightly later in life than in type I or II disease.7
Angioedema with normal C1 inhibitor levels has been associated with factor XII mutations in a minority of cases, but most patients do not have a specific laboratory abnormality. Because there is no specific laboratory profile, the diagnosis is based on clinical criteria. Hereditary angioedema with normal C1 inhibitor levels should be considered in patients who have recurrent angioedema, normal C4, normal antigenic and functional C1 inhibitor levels, a lack of response to high-dose antihistamines, and either a family history of angioedema without hives or a known factor XII mutation.7 However, other forms of angioedema (allergic, drug-induced, and idiopathic) should also be considered, as C4 and C1 inhibitor levels are normal in these forms as well.
DIFFERENTIAL DIAGNOSIS: OTHER TYPES OF ANGIOEDEMA
Acquired C1 inhibitor deficiency
Symptoms of acquired C1 inhibitor deficiency resemble those of hereditary angioedema but typically do not emerge until the fourth decade of life or later, and patients have no family history of the condition. It is often associated with other diseases, most commonly B-cell lymphoproliferative disorders, which cause uncontrolled complement activation and consumption of C1 inhibitor.
In some patients, autoantibodies to C1 inhibitor develop, greatly reducing its effectiveness and resulting in enhanced consumption. The autoantibody is often associated with a monoclonal gammopathy of unknown significance. The presence of a C1 inhibitor autoantibody does not preclude the presence of an underlying disorder, and vice versa.
Laboratory studies reveal low C4, low C1-inhibitor antigenic and functional levels, and usually a low C1q level owing to consumption of complement. Autoantibodies to C1 inhibitor can be detected by laboratory testing.
Because of the association with autoimmune disease and malignant disorders (especially B-cell malignancy), a patient diagnosed with acquired C1 inhibitor deficiency should be further evaluated for underlying conditions.
Allergic angioedema
Allergic angioedema results from preformed antigen-specific immunoglobulin E (IgE) antibodies that stimulate mast cells to degranulate when patients are exposed to a particular allergen—most commonly food, insect venom, latex, or drugs. IgE-mediated histamine release causes swelling, as histamine is a potent vasodilator.
Symptoms often begin within 2 hours of exposure to the allergen and generally include concurrent urticaria and swelling that last less than 24 hours. Unlike in hereditary angioedema, the swelling responds to antihistamines and corticosteroids. When very severe, these symptoms may also be accompanied by bronchoconstriction and gastrointestinal symptoms, especially if the allergen is ingested.
Histamine-mediated angioedema may also be associated with exercise as part of a syndrome called exercise-induced anaphylaxis or angioedema.
Drug-induced angioedema
Drug-induced angioedema is typically associated with angiotensin-converting enzyme (ACE) inhibitors or nonsteroidal anti-inflammatory drugs (NSAIDs).
Angioedema associated with ACE inhibitors is estimated to affect 0.1% to 6% of patients taking these medications, with African Americans being at significantly higher risk. Although 25% of affected patients develop symptoms of angioedema within the first month of taking the drugs, some tolerate them for as long as 10 years before the first episode.9 The swelling is not allergic or histamine-related. ACE normally degrades bradykinin; therefore, inhibiting ACE leads to accumulation of bradykinin. Because all ACE inhibitors have this effect, this class of drug should be discontinued in any patient who develops isolated angioedema.
NSAID-induced angioedema is often accompanied by other symptoms, including urticaria, rhinitis, cough, hoarseness, or breathlessness.10 The mechanism of NSAID-induced angioedema involves cyclooxygenase (COX) 1 (and to a lesser extent COX-2) inhibition. All NSAIDs (and aspirin) should be avoided in patients with recurrent angioedema. Specific COX-2 inhibitors, while theoretically capable of causing angioedema by the same mechanism, are generally well tolerated in patients who have had COX-1 inhibitor reactions.
Idiopathic angioedema
If no clear cause of recurrent angioedema (at least three episodes in a year) can be found, it is labeled idiopathic.11 Some patients with idiopathic angioedema fail to benefit from high doses of antihistamines, suggesting that the cause is bradykinin-mediated.
CLINICAL MANIFESTATIONS OF HEREDITARY ANGIOEDEMA
Attacks may start at one site and progress to involve additional sites.
Prodromal symptoms may begin up to several days before an attack and include tingling, warmth, burning, or itching at the affected site; increased fatigue or malaise; nausea, abdominal distention, or gassiness; or increased hunger, particularly before an abdominal attack.5 The most characteristic prodromal symptom is erythema marginatum—a raised, serpiginous, nonpruritic rash on the trunk, arms, and legs but often sparing the face.
Abdominal attacks are easily confused with acute abdomen
Almost half of attacks involve the abdomen, and almost all patients with type I or II disease experience at least one such attack.12 Symptoms can include severe abdominal pain, nausea, vomiting, and diarrhea. Abdominal attacks account for many emergency department visits, hospitalizations, and surgical procedures for acute abdomen; about one-third of patients with undiagnosed hereditary angioedema undergo an unnecessary surgery during an abdominal attack. Angioedema of the gastrointestinal tract can result in enough plasma extravasation and vasodilation to cause hypovolemic shock.
Eradicating Helicobacter pylori infection may alleviate abdominal attacks.13
Attacks of the extremities can be painful and disabling
Attacks of the extremities affect 96% of patients12 and can be very disfiguring and disabling. Driving or using the phone is often difficult when the hands are affected. When feet are involved, walking and standing become painful. While these symptoms rarely result in a lengthy hospitalization, they interfere with work and school and require immediate medical attention because they can progress to other parts of the body.
Laryngeal attacks are life-threatening
About half of patients with hereditary angioedema have an attack of laryngeal edema at some point in their lives.12 If not effectively managed, laryngeal angioedema can progress to asphyxiation. A survey of family history in 58 patients with hereditary angioedema suggested a 40% incidence of asphyxiation in untreated laryngeal attacks, and 25% to 30% of patients are estimated to have died of laryngeal edema before effective treatment became available.14
Symptoms of a laryngeal attack include change in voice, hoarseness, trouble swallowing, shortness of breath, and wheezing. Physicians must recognize these symptoms quickly and give effective treatment early in the attack to prevent morbidity and death.
Establishing an airway can be life-saving in the absence of effective therapy, but extensive swelling of the upper airway can make intubation extremely difficult.
Genitourinary attacks also occur
Attacks involving the scrotum and labia have been reported in up to two-thirds of patients with hereditary angioedema at some point in their lives. Attacks involving the bladder and kidneys have also been reported but are less common, affecting about 5% of patients.12 Genitourinary attacks may be triggered by local trauma, such as horseback riding or sexual intercourse, although no trigger may be evident.
MANAGING ACUTE ATTACKS
The goals of treatment are to alleviate acute exacerbations with on-demand treatment and to reduce the number of attacks with prophylaxis. Therapy should be individualized to each patient’s needs. Treatments have advanced greatly in the last several years, and new medications for treating acute attacks and preventing attacks have shown great promise (Figure 3, Table 2).
Patients tend to have recurrent symptoms interspersed with periods of health, suggesting that attacks ought to have identifiable triggers, although in most, no trigger is evident. The most commonly identified are local trauma (including medical and dental procedures), emotional stress, and acute infection. Disease severity may be worsened by menstruation, estrogen-containing oral contraceptives, hormone replacement therapy, ACE inhibitors, and NSAIDs.
It is critical that attacks be treated with an effective medication as soon as possible. Consensus guidelines state that all patients with hereditary angioedema due to C1 inhibitor deficiency, even if they are still asymptomatic, should have access to at least one of the drugs approved for on-demand treatment.15 The guidelines further state that whenever possible, “patients should have the on-demand medicine to treat acute attacks at home and should be trained to self-administer these medicines.”15
Plasma-derived C1 inhibitors
Several plasma-derived C1 inhibitors are available (Cinryze, Berinert, Cetor). They are prepared from fractionated plasma obtained from donors, then pasteurized and nanofiltered.
Berinert and Cinryze were each found to be superior to placebo in double-blind, placebo-controlled trials: attacks usually resolved 30 to 60 minutes after intravenous injection.16,17 Berinert 20 U/kg is associated with the onset of symptom relief as early as half an hour after administration, compared with 1.5 hours with placebo. Early use (at the onset of symptoms) of a plasma-derived C1 inhibitor in a low dose (500 U) can also be effective.18,19 Efficacy appears to be consistent at all sites of attack involvement, including laryngeal edema. Safety and efficacy have been demonstrated during pregnancy and lactation and in young children and babies.20
Plasma-derived C1 inhibitors can be self-administered. The safety and efficacy of self-administration (under physician supervision) were demonstrated in a study of Cinryze and Cetor, in which attack duration, pain medication use, and graded attack severity were significantly less with self-administered therapy than with therapy in the clinic.21
A concern about plasma-derived products is the possibility of blood-borne infection, but this has not been confirmed by experience.22
Recombinant human C1 inhibitor
A recombinant human C1 inhibitor (Rhucin) has been studied in two randomized placebo-controlled trials. Although this product has a shorter half-life than the plasma-derived C1 inhibitors (3 vs more than 24 hours), the two are equipotent: 1 U of recombinant human C1 inhibitor is equivalent to 1 U of plasma-derived C1 inhibitor. Because the supply of recombinant human C1 inhibitor is elastic, dosing has been higher, which may provide more efficacy.23 Similar to plasma-derived C1 inhibitor products, the recombinant human C1 inhibitor resulted in more rapid symptom relief than with saline (66 vs 122 minutes) and in a shorter time to minimal symptoms (247−266 vs 1,210 minutes).24
Allergy is of concern: in one study, a healthy volunteer with undisclosed rabbit allergy experienced an allergic reaction. Patients should be screened by a skin-prick test or serum testing for specific IgE to rabbit epithelium before being prescribed recombinant human C1 inhibitor. No data are available for use during pregnancy or breastfeeding.
Ecallantide
Ecallantide (Kalbitor) is a selective inhibitor of plasma kallikrein that is given in three subcutaneous injections. Ecallantide 30 mg was found superior to placebo during acute attacks.25,26
Ecallantide is well tolerated, with the most common adverse effects being headache, nausea, fatigue, diarrhea, and local injection-site reactions. Antibodies to ecallantide can be found in patients with increasing drug exposure but do not appear to correlate with adverse events. Hypersensitivity reactions have been observed in 2% to 3% of patients receiving repeated doses. Because of anaphylaxis risk, ecallantide must be administered by a health care professional.
Icatibant
Icatibant (Firazyr) is a bradykinin receptor-2 antagonist that is given in a single subcutaneous injection. Icatibant 30 mg significantly shortened time to symptom relief and time to almost complete resolution compared with placebo.27,28 Icatibant’s main adverse effect is transient local pain, swelling, and erythema at the injection site. Icatibant can be self-administered by patients.
Fresh-frozen plasma
Fresh-frozen plasma contains C1 inhibitor and was used before the newer products became available. Several noncontrolled studies reported benefit of its use in acute attacks.29 However, its use is controversial because it also contains contact-system proteins that could provide additional substrate for the generation of bradykinin, which could exacerbate attacks in some patients.1 This may be particularly dangerous in patients presenting with laryngeal edema: in such a situation, the physician should be ready to treat a sudden exacerbation with intubation. The risk of acquiring a blood-borne pathogen is also higher than with plasma-derived C1 inhibitor.
PROPHYLACTIC MANAGEMENT
Short-term and long-term prophylaxis have important roles in preventing attacks (Table 3).
Short-term prophylaxis before an anticipated attack
Short-term prophylaxis is used for patients whose disease is generally well controlled but who anticipate exposure to a potentially exacerbating situation, such as an invasive medical, surgical, or dental procedure. (Routine dental cleanings are generally considered safe and do not require prophylaxis.)
Prophylactic treatments include:
- Plasma-derived C1 inhibitor, 500 to 1,500 U 1 hour before the provoking event
- High-dose 17-alpha alkylated (attenuated) androgens (eg, danazol [Danocrine] 200 mg orally 3 times daily) for 5 to 10 days before the provoking event
- Fresh-frozen plasma, 2 U 1 to 12 hours before the event.1
Yet even with short-term prophylaxis, on-demand treatment should be available.
Long-term prophylaxis
While many patients can be managed with on-demand treatment only, other patients (reflecting the severity of their attacks, as well as their individual needs) may benefit from a combination of on-demand treatment plus long-term prophylaxis. Several options are available (Table 3).
17-alpha alkylated androgens. Patients treated with danazol 600 mg/day were attack-free 90% of the time during a 28-day period compared with only 2.2% of the time in placebo-treated patients.30 Use of anabolic androgens, however, is limited by their adverse effects, including weight gain, virilization, menstrual irregularities, headaches, depression, dyslipidemia, liver enzyme elevation, liver adenomas, and hepatocellular carcinoma. Arterial hypertension occurs in about 25% of treated patients.
Because adverse effects are dose-dependent, treatment should be empirically titrated to find the minimal effective dose, generally recommended to be no more than 200 mg per day of danazol or the equivalent.15
Contraindications include use by women during pregnancy or lactation and by children until growth is complete.
Regular follow-up is recommended every 6 months, with monitoring of liver enzymes, lipids, complete blood counts, alpha fetoprotein, and urinalysis. Abdominal ultrasonography (every 6 months if receiving 100 mg/day or more of danazol, every 12 months if less than 100 mg/day) is advisable for early diagnosis of liver tumors.
Antifibrinolytic drugs. Tranexamic acid (Lysteda) and aminocaproic acid (Amicar) have been found to be effective in reducing the number of attacks of hereditary angioedema compared with placebo but are considered to be less reliable than androgens. These drugs have been used in patients who do not tolerate anabolic androgens, and in children and pregnant women. Tranexamic acid is given at a dose of 20 to 50 mg/kg/day divided into two or three doses per day. The therapeutic dose of aminocaproic acid is 1 g orally three to four times per day.31 Patients with a personal or family history of thromboembolic disease may be at greater risk of venous or arterial thrombosis, but this has not occurred in clinical studies.
Plasma-derived C1 inhibitors. In a 24-week crossover study in 22 patients with hereditary angioedema, Cinryze 1,000 U every 3 to 4 days reduced the rate of attacks by 50% while also reducing their severity and duration.17 An open-label extension study in 146 patients for almost 3 years documented a 90% reduction in attack frequency with no evidence of tachyphylaxis.32
New treatments are costlier
The newer on-demand and prophylactic drugs are substantially costlier than the older alternatives (androgens, antifibrinolytics, and fresh-frozen plasma); however, they have a substantially better benefit-to-risk ratio. Furthermore, the costs of care for an attack requiring emergency treatment are also high. Hereditary angioedema patients are often young, otherwise healthy, and capable of leading normal productive lives. While formal pharmacoeconomic studies of the optimal use of these newer drugs have not yet been done, it is important that the use of these drugs be well justified. Ideally, physicians who prescribe these drugs should be knowledgeable in the management of hereditary angioedema.
SPECIAL CHALLENGES IN WOMEN
Women with hereditary angioedema have more frequent attacks and generally a more severe disease course than men.12 Optimizing care for women is challenging because hormonal changes often cause the disease to flare up in menarche, pregnancy, lactation, and menopause. Women also have a higher rate of discontinuing long-term androgen therapy because of side effects, including virilization and menstrual irregularities. Spironolactone (Aldactone) 100 to 200 mg daily can be used to control hirsutism.33
Contraception
Because estrogen can trigger attacks, progesterone-only formulations, intrauterine devices, or barrier methods are recommended for contraception.33 Progesterone-only pills are preferred and improve symptoms in more than 60% of women. Etonogestrel, another alternative, is available as an implant (Implanon) or vaginal ring (Nuvaring). Intrauterine devices are generally well tolerated, and no prophylaxis is needed during placement. The progesterone-eluting intrauterine device (Mirena) could be beneficial.34
Pregnancy and lactation
Pregnancy and lactation pose particular challenges. Anabolic androgens are contraindicated during pregnancy as well as during breastfeeding because they can be passed on in breast milk. Women receiving androgen prophylaxis should understand that they can still ovulate and need contraception if they are sexually active.34 Patients on attenuated androgens who desire pregnancy should discontinue them 2 months before trying to conceive.
Changes in attack patterns can be unpredictable during pregnancy. Attacks tend to be more severe during the first trimester and more frequent during the third. Due to its safety and efficacy, plasma-derived C1 inhibitor has become the treatment of choice for on-demand or prophylactic treatment during pregnancy and lactation. Antifibrinolytics are considered only when plasma-derived C1 inhibitor is not available.31 Ecallantide and icatibant have not been studied in pregnancy. If neither plasma-derived C1 inhibitor nor antifibrinolytics are available, fresh-frozen plasma or solvent-and-detergent-treated plasma can be used.
Short-term prophylaxis should be considered before amniocentesis, chorionic villous sampling, and dilation and curettage. Delivery should take place in a facility with rapid access to plasma-derived C1 inhibitor as well as consultants in obstetrics, anesthesiology, and perinatology. Although plasma-derived C1 inhibitor should be available at all times during labor and delivery, its prophylactic use is not required unless labor and delivery are particularly traumatic, the underlying hereditary angioedema is very severe, or if forceps, vacuum delivery, or cesarian section is performed. Close monitoring is recommended for at least 72 hours after routine vaginal delivery and for 1 week after cesarian section.
CONCLUSION
The goals of hereditary angioedema treatment are to alleviate morbidity and mortality associated with the disease and to improve the patient’s quality of life. Achieving these goals requires timely diagnosis, patient education, and careful selection of therapeutic modalities that are individualized to the needs of that patient. Treatments have advanced greatly in the last 4 years, and new medications for both the acute and chronic symptoms of hereditary angioedema have shown great promise.
Acknowledgment: K.T. is funded by National Institutes of Health grant T32 AI 07469.
Hereditary angioedema due to deficiency of C1 inhibitor is a rare autosomal dominant disease that can be life-threatening. It affects about 1 in 50,000 people,1 or about 6,000 people in the United States. There are no known differences in prevalence by ethnicity or sex. A form of hereditary angioedema with normal C1 inhibitor levels has also recently been identified.
Despite a growing awareness of hereditary angioedema in the medical community, repeated surveys have found an average gap of 10 years between the first appearance of symptoms and the correct diagnosis. In view of the risk of morbidity and death, recognizing this disease sooner is critical.
This article will discuss how to recognize hereditary angioedema and how to differentiate it from other forms of recurring angioedema. We will also review its acute and long-term management, with special attention to new therapies and clinical challenges.
EPISODES OF SWELLING WITHOUT HIVES
Hereditary angioedema involves recurrent episodes of nonpruritic, nonpitting, subcutaneous and submucosal edema that can affect the face, tongue, larynx, trunk, extremities, bowels, or genitals. Attacks typically follow a predictable course: swelling that increases slowly and continuously for 24 hours and then gradually subsides over the next 48 to 72 hours. Attacks that involve the oropharynx, larynx, or abdomen carry the highest risk of morbidity and death.1
The frequency and severity of attacks are highly variable and unpredictable. A few patients have no attacks, a few have two attacks per week, and most fall in between.
Hives suggests an allergic or idiopathic rather than hereditary cause and will not be discussed here in detail. A history of angioedema that was rapidly aborted by antihistamines, corticosteroids, or epinephrine also suggests an allergic rather than hereditary cause.
UNCHECKED BRADYKININ PRODUCTION
Substantial evidence indicates that hereditary angioedema results from extravasation of plasma into deeper cutaneous or mucosal compartments as a result of overproduction of the vasoactive mediator bradykinin (Figure 1).
Activated factor XII cleaves plasma prekallikrein to generate active plasma kallikrein (which, in turn, activates more factor XII).2 Once generated, plasma kallikrein cleaves high-molecular-weight kininogen, releasing bradykinin. Bradykinin binds to the B2 bradykinin receptor on endothelial cells, increasing the permeability of the endothelium.
Normally, C1 inhibitor helps control bradykinin production by inhibiting plasma kallikrein and activated factor XII. Without enough C1 inhibitor, the contact system is uninhibited and results in bradykinin being inappropriately generated.
Because the attacks of hereditary angioedema involve excessive bradykinin, they do not respond to the usual treatments for anaphylaxis and allergic angioedema (which involve mast cell degranulation), such as antihistamines, corticosteroids, and epinephrine.
TWO TYPES OF HEREDITARY ANGIOEDEMA
Figure 2 shows the evaluation of patients with suspected hereditary angioedema.
Hereditary angioedema due to C1 inhibitor deficiency
The classic forms of hereditary angioedema (types I and II) involve loss-of-function mutations in SERPING1—the gene that encodes for C1 inhibitor—resulting in low levels of functional C1 inhibitor.3 The mutation is inherited in an autosomal dominant pattern; however, in about 25% of cases, it appears to arise spontaneously,4 so a family history is not required for diagnosis.
Although C1 inhibitor deficiency is present from birth, the clinical disease most commonly presents for the first time when the patient is of school age. Half of patients have their first episode in the first decade of life, and another one-third first develop symptoms over the next 10 years.5
Clinically, types I and II are indistinguishable. Type I, accounting for 85% of cases,1 results from low production of C1 inhibitor. Laboratory studies reveal low antigenic and functional levels of C1 inhibitor.
In type II, the mutant C1 inhibitor protein is present but dysfunctional and unable to inhibit target proteases. On laboratory testing, the functional level of C1 inhibitor is low but its antigenic level is normal (Table 1). Function can be tested by either chromogenic assay or enzyme-linked immunosorbent assay; the former is preferred because it is more sensitive.6
Because C1 inhibitor deficiency results in chronic activation of the complement system, patients with type I or II disease usually have low C4 levels regardless of disease activity, making measuring C4 the most economical screening test. When suspicion for hereditary angioedema is high, based on the presentation and family and clinical history, measuring antigenic and functional C1 inhibitor levels and C4 simultaneously is more efficient.
Hereditary angioedema with normal C1 inhibitor levels
Hereditary angioedema with normal C1 inhibitor levels is also inherited in an autosomal dominant pattern. It is often estrogen-sensitive, making it more severe in women. Symptoms tend to develop slightly later in life than in type I or II disease.7
Angioedema with normal C1 inhibitor levels has been associated with factor XII mutations in a minority of cases, but most patients do not have a specific laboratory abnormality. Because there is no specific laboratory profile, the diagnosis is based on clinical criteria. Hereditary angioedema with normal C1 inhibitor levels should be considered in patients who have recurrent angioedema, normal C4, normal antigenic and functional C1 inhibitor levels, a lack of response to high-dose antihistamines, and either a family history of angioedema without hives or a known factor XII mutation.7 However, other forms of angioedema (allergic, drug-induced, and idiopathic) should also be considered, as C4 and C1 inhibitor levels are normal in these forms as well.
DIFFERENTIAL DIAGNOSIS: OTHER TYPES OF ANGIOEDEMA
Acquired C1 inhibitor deficiency
Symptoms of acquired C1 inhibitor deficiency resemble those of hereditary angioedema but typically do not emerge until the fourth decade of life or later, and patients have no family history of the condition. It is often associated with other diseases, most commonly B-cell lymphoproliferative disorders, which cause uncontrolled complement activation and consumption of C1 inhibitor.
In some patients, autoantibodies to C1 inhibitor develop, greatly reducing its effectiveness and resulting in enhanced consumption. The autoantibody is often associated with a monoclonal gammopathy of unknown significance. The presence of a C1 inhibitor autoantibody does not preclude the presence of an underlying disorder, and vice versa.
Laboratory studies reveal low C4, low C1-inhibitor antigenic and functional levels, and usually a low C1q level owing to consumption of complement. Autoantibodies to C1 inhibitor can be detected by laboratory testing.
Because of the association with autoimmune disease and malignant disorders (especially B-cell malignancy), a patient diagnosed with acquired C1 inhibitor deficiency should be further evaluated for underlying conditions.
Allergic angioedema
Allergic angioedema results from preformed antigen-specific immunoglobulin E (IgE) antibodies that stimulate mast cells to degranulate when patients are exposed to a particular allergen—most commonly food, insect venom, latex, or drugs. IgE-mediated histamine release causes swelling, as histamine is a potent vasodilator.
Symptoms often begin within 2 hours of exposure to the allergen and generally include concurrent urticaria and swelling that last less than 24 hours. Unlike in hereditary angioedema, the swelling responds to antihistamines and corticosteroids. When very severe, these symptoms may also be accompanied by bronchoconstriction and gastrointestinal symptoms, especially if the allergen is ingested.
Histamine-mediated angioedema may also be associated with exercise as part of a syndrome called exercise-induced anaphylaxis or angioedema.
Drug-induced angioedema
Drug-induced angioedema is typically associated with angiotensin-converting enzyme (ACE) inhibitors or nonsteroidal anti-inflammatory drugs (NSAIDs).
Angioedema associated with ACE inhibitors is estimated to affect 0.1% to 6% of patients taking these medications, with African Americans being at significantly higher risk. Although 25% of affected patients develop symptoms of angioedema within the first month of taking the drugs, some tolerate them for as long as 10 years before the first episode.9 The swelling is not allergic or histamine-related. ACE normally degrades bradykinin; therefore, inhibiting ACE leads to accumulation of bradykinin. Because all ACE inhibitors have this effect, this class of drug should be discontinued in any patient who develops isolated angioedema.
NSAID-induced angioedema is often accompanied by other symptoms, including urticaria, rhinitis, cough, hoarseness, or breathlessness.10 The mechanism of NSAID-induced angioedema involves cyclooxygenase (COX) 1 (and to a lesser extent COX-2) inhibition. All NSAIDs (and aspirin) should be avoided in patients with recurrent angioedema. Specific COX-2 inhibitors, while theoretically capable of causing angioedema by the same mechanism, are generally well tolerated in patients who have had COX-1 inhibitor reactions.
Idiopathic angioedema
If no clear cause of recurrent angioedema (at least three episodes in a year) can be found, it is labeled idiopathic.11 Some patients with idiopathic angioedema fail to benefit from high doses of antihistamines, suggesting that the cause is bradykinin-mediated.
CLINICAL MANIFESTATIONS OF HEREDITARY ANGIOEDEMA
Attacks may start at one site and progress to involve additional sites.
Prodromal symptoms may begin up to several days before an attack and include tingling, warmth, burning, or itching at the affected site; increased fatigue or malaise; nausea, abdominal distention, or gassiness; or increased hunger, particularly before an abdominal attack.5 The most characteristic prodromal symptom is erythema marginatum—a raised, serpiginous, nonpruritic rash on the trunk, arms, and legs but often sparing the face.
Abdominal attacks are easily confused with acute abdomen
Almost half of attacks involve the abdomen, and almost all patients with type I or II disease experience at least one such attack.12 Symptoms can include severe abdominal pain, nausea, vomiting, and diarrhea. Abdominal attacks account for many emergency department visits, hospitalizations, and surgical procedures for acute abdomen; about one-third of patients with undiagnosed hereditary angioedema undergo an unnecessary surgery during an abdominal attack. Angioedema of the gastrointestinal tract can result in enough plasma extravasation and vasodilation to cause hypovolemic shock.
Eradicating Helicobacter pylori infection may alleviate abdominal attacks.13
Attacks of the extremities can be painful and disabling
Attacks of the extremities affect 96% of patients12 and can be very disfiguring and disabling. Driving or using the phone is often difficult when the hands are affected. When feet are involved, walking and standing become painful. While these symptoms rarely result in a lengthy hospitalization, they interfere with work and school and require immediate medical attention because they can progress to other parts of the body.
Laryngeal attacks are life-threatening
About half of patients with hereditary angioedema have an attack of laryngeal edema at some point in their lives.12 If not effectively managed, laryngeal angioedema can progress to asphyxiation. A survey of family history in 58 patients with hereditary angioedema suggested a 40% incidence of asphyxiation in untreated laryngeal attacks, and 25% to 30% of patients are estimated to have died of laryngeal edema before effective treatment became available.14
Symptoms of a laryngeal attack include change in voice, hoarseness, trouble swallowing, shortness of breath, and wheezing. Physicians must recognize these symptoms quickly and give effective treatment early in the attack to prevent morbidity and death.
Establishing an airway can be life-saving in the absence of effective therapy, but extensive swelling of the upper airway can make intubation extremely difficult.
Genitourinary attacks also occur
Attacks involving the scrotum and labia have been reported in up to two-thirds of patients with hereditary angioedema at some point in their lives. Attacks involving the bladder and kidneys have also been reported but are less common, affecting about 5% of patients.12 Genitourinary attacks may be triggered by local trauma, such as horseback riding or sexual intercourse, although no trigger may be evident.
MANAGING ACUTE ATTACKS
The goals of treatment are to alleviate acute exacerbations with on-demand treatment and to reduce the number of attacks with prophylaxis. Therapy should be individualized to each patient’s needs. Treatments have advanced greatly in the last several years, and new medications for treating acute attacks and preventing attacks have shown great promise (Figure 3, Table 2).
Patients tend to have recurrent symptoms interspersed with periods of health, suggesting that attacks ought to have identifiable triggers, although in most, no trigger is evident. The most commonly identified are local trauma (including medical and dental procedures), emotional stress, and acute infection. Disease severity may be worsened by menstruation, estrogen-containing oral contraceptives, hormone replacement therapy, ACE inhibitors, and NSAIDs.
It is critical that attacks be treated with an effective medication as soon as possible. Consensus guidelines state that all patients with hereditary angioedema due to C1 inhibitor deficiency, even if they are still asymptomatic, should have access to at least one of the drugs approved for on-demand treatment.15 The guidelines further state that whenever possible, “patients should have the on-demand medicine to treat acute attacks at home and should be trained to self-administer these medicines.”15
Plasma-derived C1 inhibitors
Several plasma-derived C1 inhibitors are available (Cinryze, Berinert, Cetor). They are prepared from fractionated plasma obtained from donors, then pasteurized and nanofiltered.
Berinert and Cinryze were each found to be superior to placebo in double-blind, placebo-controlled trials: attacks usually resolved 30 to 60 minutes after intravenous injection.16,17 Berinert 20 U/kg is associated with the onset of symptom relief as early as half an hour after administration, compared with 1.5 hours with placebo. Early use (at the onset of symptoms) of a plasma-derived C1 inhibitor in a low dose (500 U) can also be effective.18,19 Efficacy appears to be consistent at all sites of attack involvement, including laryngeal edema. Safety and efficacy have been demonstrated during pregnancy and lactation and in young children and babies.20
Plasma-derived C1 inhibitors can be self-administered. The safety and efficacy of self-administration (under physician supervision) were demonstrated in a study of Cinryze and Cetor, in which attack duration, pain medication use, and graded attack severity were significantly less with self-administered therapy than with therapy in the clinic.21
A concern about plasma-derived products is the possibility of blood-borne infection, but this has not been confirmed by experience.22
Recombinant human C1 inhibitor
A recombinant human C1 inhibitor (Rhucin) has been studied in two randomized placebo-controlled trials. Although this product has a shorter half-life than the plasma-derived C1 inhibitors (3 vs more than 24 hours), the two are equipotent: 1 U of recombinant human C1 inhibitor is equivalent to 1 U of plasma-derived C1 inhibitor. Because the supply of recombinant human C1 inhibitor is elastic, dosing has been higher, which may provide more efficacy.23 Similar to plasma-derived C1 inhibitor products, the recombinant human C1 inhibitor resulted in more rapid symptom relief than with saline (66 vs 122 minutes) and in a shorter time to minimal symptoms (247−266 vs 1,210 minutes).24
Allergy is of concern: in one study, a healthy volunteer with undisclosed rabbit allergy experienced an allergic reaction. Patients should be screened by a skin-prick test or serum testing for specific IgE to rabbit epithelium before being prescribed recombinant human C1 inhibitor. No data are available for use during pregnancy or breastfeeding.
Ecallantide
Ecallantide (Kalbitor) is a selective inhibitor of plasma kallikrein that is given in three subcutaneous injections. Ecallantide 30 mg was found superior to placebo during acute attacks.25,26
Ecallantide is well tolerated, with the most common adverse effects being headache, nausea, fatigue, diarrhea, and local injection-site reactions. Antibodies to ecallantide can be found in patients with increasing drug exposure but do not appear to correlate with adverse events. Hypersensitivity reactions have been observed in 2% to 3% of patients receiving repeated doses. Because of anaphylaxis risk, ecallantide must be administered by a health care professional.
Icatibant
Icatibant (Firazyr) is a bradykinin receptor-2 antagonist that is given in a single subcutaneous injection. Icatibant 30 mg significantly shortened time to symptom relief and time to almost complete resolution compared with placebo.27,28 Icatibant’s main adverse effect is transient local pain, swelling, and erythema at the injection site. Icatibant can be self-administered by patients.
Fresh-frozen plasma
Fresh-frozen plasma contains C1 inhibitor and was used before the newer products became available. Several noncontrolled studies reported benefit of its use in acute attacks.29 However, its use is controversial because it also contains contact-system proteins that could provide additional substrate for the generation of bradykinin, which could exacerbate attacks in some patients.1 This may be particularly dangerous in patients presenting with laryngeal edema: in such a situation, the physician should be ready to treat a sudden exacerbation with intubation. The risk of acquiring a blood-borne pathogen is also higher than with plasma-derived C1 inhibitor.
PROPHYLACTIC MANAGEMENT
Short-term and long-term prophylaxis have important roles in preventing attacks (Table 3).
Short-term prophylaxis before an anticipated attack
Short-term prophylaxis is used for patients whose disease is generally well controlled but who anticipate exposure to a potentially exacerbating situation, such as an invasive medical, surgical, or dental procedure. (Routine dental cleanings are generally considered safe and do not require prophylaxis.)
Prophylactic treatments include:
- Plasma-derived C1 inhibitor, 500 to 1,500 U 1 hour before the provoking event
- High-dose 17-alpha alkylated (attenuated) androgens (eg, danazol [Danocrine] 200 mg orally 3 times daily) for 5 to 10 days before the provoking event
- Fresh-frozen plasma, 2 U 1 to 12 hours before the event.1
Yet even with short-term prophylaxis, on-demand treatment should be available.
Long-term prophylaxis
While many patients can be managed with on-demand treatment only, other patients (reflecting the severity of their attacks, as well as their individual needs) may benefit from a combination of on-demand treatment plus long-term prophylaxis. Several options are available (Table 3).
17-alpha alkylated androgens. Patients treated with danazol 600 mg/day were attack-free 90% of the time during a 28-day period compared with only 2.2% of the time in placebo-treated patients.30 Use of anabolic androgens, however, is limited by their adverse effects, including weight gain, virilization, menstrual irregularities, headaches, depression, dyslipidemia, liver enzyme elevation, liver adenomas, and hepatocellular carcinoma. Arterial hypertension occurs in about 25% of treated patients.
Because adverse effects are dose-dependent, treatment should be empirically titrated to find the minimal effective dose, generally recommended to be no more than 200 mg per day of danazol or the equivalent.15
Contraindications include use by women during pregnancy or lactation and by children until growth is complete.
Regular follow-up is recommended every 6 months, with monitoring of liver enzymes, lipids, complete blood counts, alpha fetoprotein, and urinalysis. Abdominal ultrasonography (every 6 months if receiving 100 mg/day or more of danazol, every 12 months if less than 100 mg/day) is advisable for early diagnosis of liver tumors.
Antifibrinolytic drugs. Tranexamic acid (Lysteda) and aminocaproic acid (Amicar) have been found to be effective in reducing the number of attacks of hereditary angioedema compared with placebo but are considered to be less reliable than androgens. These drugs have been used in patients who do not tolerate anabolic androgens, and in children and pregnant women. Tranexamic acid is given at a dose of 20 to 50 mg/kg/day divided into two or three doses per day. The therapeutic dose of aminocaproic acid is 1 g orally three to four times per day.31 Patients with a personal or family history of thromboembolic disease may be at greater risk of venous or arterial thrombosis, but this has not occurred in clinical studies.
Plasma-derived C1 inhibitors. In a 24-week crossover study in 22 patients with hereditary angioedema, Cinryze 1,000 U every 3 to 4 days reduced the rate of attacks by 50% while also reducing their severity and duration.17 An open-label extension study in 146 patients for almost 3 years documented a 90% reduction in attack frequency with no evidence of tachyphylaxis.32
New treatments are costlier
The newer on-demand and prophylactic drugs are substantially costlier than the older alternatives (androgens, antifibrinolytics, and fresh-frozen plasma); however, they have a substantially better benefit-to-risk ratio. Furthermore, the costs of care for an attack requiring emergency treatment are also high. Hereditary angioedema patients are often young, otherwise healthy, and capable of leading normal productive lives. While formal pharmacoeconomic studies of the optimal use of these newer drugs have not yet been done, it is important that the use of these drugs be well justified. Ideally, physicians who prescribe these drugs should be knowledgeable in the management of hereditary angioedema.
SPECIAL CHALLENGES IN WOMEN
Women with hereditary angioedema have more frequent attacks and generally a more severe disease course than men.12 Optimizing care for women is challenging because hormonal changes often cause the disease to flare up in menarche, pregnancy, lactation, and menopause. Women also have a higher rate of discontinuing long-term androgen therapy because of side effects, including virilization and menstrual irregularities. Spironolactone (Aldactone) 100 to 200 mg daily can be used to control hirsutism.33
Contraception
Because estrogen can trigger attacks, progesterone-only formulations, intrauterine devices, or barrier methods are recommended for contraception.33 Progesterone-only pills are preferred and improve symptoms in more than 60% of women. Etonogestrel, another alternative, is available as an implant (Implanon) or vaginal ring (Nuvaring). Intrauterine devices are generally well tolerated, and no prophylaxis is needed during placement. The progesterone-eluting intrauterine device (Mirena) could be beneficial.34
Pregnancy and lactation
Pregnancy and lactation pose particular challenges. Anabolic androgens are contraindicated during pregnancy as well as during breastfeeding because they can be passed on in breast milk. Women receiving androgen prophylaxis should understand that they can still ovulate and need contraception if they are sexually active.34 Patients on attenuated androgens who desire pregnancy should discontinue them 2 months before trying to conceive.
Changes in attack patterns can be unpredictable during pregnancy. Attacks tend to be more severe during the first trimester and more frequent during the third. Due to its safety and efficacy, plasma-derived C1 inhibitor has become the treatment of choice for on-demand or prophylactic treatment during pregnancy and lactation. Antifibrinolytics are considered only when plasma-derived C1 inhibitor is not available.31 Ecallantide and icatibant have not been studied in pregnancy. If neither plasma-derived C1 inhibitor nor antifibrinolytics are available, fresh-frozen plasma or solvent-and-detergent-treated plasma can be used.
Short-term prophylaxis should be considered before amniocentesis, chorionic villous sampling, and dilation and curettage. Delivery should take place in a facility with rapid access to plasma-derived C1 inhibitor as well as consultants in obstetrics, anesthesiology, and perinatology. Although plasma-derived C1 inhibitor should be available at all times during labor and delivery, its prophylactic use is not required unless labor and delivery are particularly traumatic, the underlying hereditary angioedema is very severe, or if forceps, vacuum delivery, or cesarian section is performed. Close monitoring is recommended for at least 72 hours after routine vaginal delivery and for 1 week after cesarian section.
CONCLUSION
The goals of hereditary angioedema treatment are to alleviate morbidity and mortality associated with the disease and to improve the patient’s quality of life. Achieving these goals requires timely diagnosis, patient education, and careful selection of therapeutic modalities that are individualized to the needs of that patient. Treatments have advanced greatly in the last 4 years, and new medications for both the acute and chronic symptoms of hereditary angioedema have shown great promise.
Acknowledgment: K.T. is funded by National Institutes of Health grant T32 AI 07469.
- Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med 2008; 359:1027–1036.
- Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol 2010; 126:918–925.
- Davis AE. C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol 1988; 6:595–628.
- Pappalardo E, Cicardi M, Duponchel C, et al. Frequent de novo mutations and exon deletions in the C1inhibitor gene of patients with angioedema. J Allergy Clin Immunol 2000; 106:1147–1154.
- Frigas E, Park M. Idiopathic recurrent angioedema. Immunol Allergy Clin North Am 2006; 26:739–751.
- Wagenaar-Bos IG, Drouet C, Aygören-Pursun E, et al. Functional C1-inhibitor diagnostics in hereditary angioedema: assay evaluation and recommendations. J Immunol Methods 2008; 338:14–20.
- Bork K. Diagnosis and treatment of hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol 2010; 6:15.
- Zuraw BL, Bork K, Binkley KE, et al. Hereditary angioedema with normal C1 inhibitor function: consensus of an international expert panel. Allergy Asthma Proc 2012; 33:S145–S156.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am 2006; 26:725–737.
- Busse PJ. Angioedema: differential diagnosis and treatment. Allergy Asthma Proc 2011; 32(suppl 1):S3–S11.
- Prematta MJ, Kemp JG, Gibbs JG, Mende C, Rhoads C, Craig TJ. Frequency, timing, and type of prodromal symptoms associated with hereditary angioedema attacks. Allergy Asthma Proc 2009; 30:506–511.
- Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 2006; 119:267–274.
- Farkas H, Füst G, Fekete B, Karádi I, Varga L. Eradication of Helicobacter pylori and improvement of hereditary angioneurotic oedema. Lancet 2001; 358:1695–1696.
- Bork K, Barnstedt SE. Treatment of 193 episodes of laryngeal edema with C1 inhibitor concentrate in patients with hereditary angioedema. Arch Intern Med 2001; 161:714–718.
- Cicardi M, Bork K, Caballero T, et al; HAWK (Hereditary Angioedema International Working Group). Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy 2012; 67:147–157.
- Craig TJ, Levy RJ, Wasserman RL, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol 2009; 124:801–808.
- Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med 2010; 363:513–522.
- Bork K, Meng G, Staubach P, Hardt J. Treatment with C1 inhibitor concentrate in abdominal pain attacks of patients with hereditary angioedema. Transfusion 2005; 45:1774–1784.
- Kreuz W, Martinez-Saguer I, Aygören-Pürsün E, Rusicke E, Heller C, Klingebiel T. C1-inhibitor concentrate for individual replacement therapy in patients with severe hereditary angioedema refractory to danazol prophylaxis. Transfusion 2009; 49:1987–1995.
- Farkas H, Varga L, Széplaki G, Visy B, Harmat G, Bowen T. Management of hereditary angioedema in pediatric patients. Pediatrics 2007; 120:e713–e722.
- Tourangeau LM, Castaldo AJ, Davis DK, Koziol J, Christiansen SC, Zuraw BL. Safety and efficacy of physician-supervised self-managed C1 inhibitor replacement therapy. Int Arch Allergy Immunol 2012; 157:417–424.
- De Serres J, Gröner A, Lindner J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apher Sci 2003; 29:247–254.
- Hack CE, Relan A, van Amersfoort ES, Cicardi M. Target levels of functional C1-inhibitor in hereditary angioedema. Allergy 2012; 67:123–130.
- Zuraw B, Cicardi M, Levy RJ, et al. Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol 2010; 126:821–827.e14.
- Cicardi M, Levy RJ, McNeil DL, et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med 2010; 363:523–531.
- Levy RJ, Lumry WR, McNeil DL, et al. EDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol 2010; 104:523–529.
- Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med 2010; 363:532–541.
- Lumry WR, Li HH, Levy RJ, et al. Randomized placebo-controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol 2011; 107:529–537.
- Prematta M, Gibbs JG, Pratt EL, Stoughton TR, Craig TJ. Fresh frozen plasma for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol 2007; 98:383–388.
- Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med 1976; 295:1444–1448.
- Zuraw BL. HAE therapies: past present and future. Allergy Asthma Clin Immunol 2010; 6:23.
- Zuraw BL, Kalfus I. Safety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedema. Am J Med 2012: Epub ahead of print.
- Caballero T, Farkas H, Bouillet L, et al; C-1-INH Deficiency Working Group. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol 2012; 129:308–320.
- Bouillet L, Longhurst H, Boccon-Gibod I, et al. Disease expression in women with hereditary angioedema. Am J Obstet Gynecol 2008; 199:484.e1–e4.
- Zuraw BL. Clinical practice. Hereditary angioedema. N Engl J Med 2008; 359:1027–1036.
- Kaplan AP. Enzymatic pathways in the pathogenesis of hereditary angioedema: the role of C1 inhibitor therapy. J Allergy Clin Immunol 2010; 126:918–925.
- Davis AE. C1 inhibitor and hereditary angioneurotic edema. Annu Rev Immunol 1988; 6:595–628.
- Pappalardo E, Cicardi M, Duponchel C, et al. Frequent de novo mutations and exon deletions in the C1inhibitor gene of patients with angioedema. J Allergy Clin Immunol 2000; 106:1147–1154.
- Frigas E, Park M. Idiopathic recurrent angioedema. Immunol Allergy Clin North Am 2006; 26:739–751.
- Wagenaar-Bos IG, Drouet C, Aygören-Pursun E, et al. Functional C1-inhibitor diagnostics in hereditary angioedema: assay evaluation and recommendations. J Immunol Methods 2008; 338:14–20.
- Bork K. Diagnosis and treatment of hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol 2010; 6:15.
- Zuraw BL, Bork K, Binkley KE, et al. Hereditary angioedema with normal C1 inhibitor function: consensus of an international expert panel. Allergy Asthma Proc 2012; 33:S145–S156.
- Byrd JB, Adam A, Brown NJ. Angiotensin-converting enzyme inhibitor-associated angioedema. Immunol Allergy Clin North Am 2006; 26:725–737.
- Busse PJ. Angioedema: differential diagnosis and treatment. Allergy Asthma Proc 2011; 32(suppl 1):S3–S11.
- Prematta MJ, Kemp JG, Gibbs JG, Mende C, Rhoads C, Craig TJ. Frequency, timing, and type of prodromal symptoms associated with hereditary angioedema attacks. Allergy Asthma Proc 2009; 30:506–511.
- Bork K, Meng G, Staubach P, Hardt J. Hereditary angioedema: new findings concerning symptoms, affected organs, and course. Am J Med 2006; 119:267–274.
- Farkas H, Füst G, Fekete B, Karádi I, Varga L. Eradication of Helicobacter pylori and improvement of hereditary angioneurotic oedema. Lancet 2001; 358:1695–1696.
- Bork K, Barnstedt SE. Treatment of 193 episodes of laryngeal edema with C1 inhibitor concentrate in patients with hereditary angioedema. Arch Intern Med 2001; 161:714–718.
- Cicardi M, Bork K, Caballero T, et al; HAWK (Hereditary Angioedema International Working Group). Evidence-based recommendations for the therapeutic management of angioedema owing to hereditary C1 inhibitor deficiency: consensus report of an International Working Group. Allergy 2012; 67:147–157.
- Craig TJ, Levy RJ, Wasserman RL, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol 2009; 124:801–808.
- Zuraw BL, Busse PJ, White M, et al. Nanofiltered C1 inhibitor concentrate for treatment of hereditary angioedema. N Engl J Med 2010; 363:513–522.
- Bork K, Meng G, Staubach P, Hardt J. Treatment with C1 inhibitor concentrate in abdominal pain attacks of patients with hereditary angioedema. Transfusion 2005; 45:1774–1784.
- Kreuz W, Martinez-Saguer I, Aygören-Pürsün E, Rusicke E, Heller C, Klingebiel T. C1-inhibitor concentrate for individual replacement therapy in patients with severe hereditary angioedema refractory to danazol prophylaxis. Transfusion 2009; 49:1987–1995.
- Farkas H, Varga L, Széplaki G, Visy B, Harmat G, Bowen T. Management of hereditary angioedema in pediatric patients. Pediatrics 2007; 120:e713–e722.
- Tourangeau LM, Castaldo AJ, Davis DK, Koziol J, Christiansen SC, Zuraw BL. Safety and efficacy of physician-supervised self-managed C1 inhibitor replacement therapy. Int Arch Allergy Immunol 2012; 157:417–424.
- De Serres J, Gröner A, Lindner J. Safety and efficacy of pasteurized C1 inhibitor concentrate (Berinert P) in hereditary angioedema: a review. Transfus Apher Sci 2003; 29:247–254.
- Hack CE, Relan A, van Amersfoort ES, Cicardi M. Target levels of functional C1-inhibitor in hereditary angioedema. Allergy 2012; 67:123–130.
- Zuraw B, Cicardi M, Levy RJ, et al. Recombinant human C1-inhibitor for the treatment of acute angioedema attacks in patients with hereditary angioedema. J Allergy Clin Immunol 2010; 126:821–827.e14.
- Cicardi M, Levy RJ, McNeil DL, et al. Ecallantide for the treatment of acute attacks in hereditary angioedema. N Engl J Med 2010; 363:523–531.
- Levy RJ, Lumry WR, McNeil DL, et al. EDEMA4: a phase 3, double-blind study of subcutaneous ecallantide treatment for acute attacks of hereditary angioedema. Ann Allergy Asthma Immunol 2010; 104:523–529.
- Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med 2010; 363:532–541.
- Lumry WR, Li HH, Levy RJ, et al. Randomized placebo-controlled trial of the bradykinin B2 receptor antagonist icatibant for the treatment of acute attacks of hereditary angioedema: the FAST-3 trial. Ann Allergy Asthma Immunol 2011; 107:529–537.
- Prematta M, Gibbs JG, Pratt EL, Stoughton TR, Craig TJ. Fresh frozen plasma for the treatment of hereditary angioedema. Ann Allergy Asthma Immunol 2007; 98:383–388.
- Gelfand JA, Sherins RJ, Alling DW, Frank MM. Treatment of hereditary angioedema with danazol. Reversal of clinical and biochemical abnormalities. N Engl J Med 1976; 295:1444–1448.
- Zuraw BL. HAE therapies: past present and future. Allergy Asthma Clin Immunol 2010; 6:23.
- Zuraw BL, Kalfus I. Safety and efficacy of prophylactic nanofiltered C1-inhibitor in hereditary angioedema. Am J Med 2012: Epub ahead of print.
- Caballero T, Farkas H, Bouillet L, et al; C-1-INH Deficiency Working Group. International consensus and practical guidelines on the gynecologic and obstetric management of female patients with hereditary angioedema caused by C1 inhibitor deficiency. J Allergy Clin Immunol 2012; 129:308–320.
- Bouillet L, Longhurst H, Boccon-Gibod I, et al. Disease expression in women with hereditary angioedema. Am J Obstet Gynecol 2008; 199:484.e1–e4.
KEY POINTS
- Swelling in the airways is life-threatening and requires rapid treatment.
- Almost half of attacks involve the abdomen, and abdominal attacks account for many emergency department visits, hospitalizations, and unnecessary surgical procedures for acute abdomen.
- Acute attacks can be managed with plasma-derived or recombinant human preparations of C1 inhibitor (which is the deficient factor in this condition), ecallantide (a specific plasma kallikrein inhibitor), or icatibant (a B2 bradykinin receptor antagonist).
- Short-term prophylaxis may be used before events that could provoke attacks (eg, dental work or surgery). Long-term prophylaxis may be used in patients who have frequent or severe attacks or require more stringent control of their disease. Plasma-derived C1 inhibitor is both safe and effective when used as prophylaxis. Attenuated androgens are effective but associated with many adverse effects.
Not all joint pain is arthritis
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
A 47-year-old man who had been diagnosed with rheumatoid arthritis 5 years previously was referred to us for management of bilateral pleural effusions.
At the time of his diagnosis, his symptoms included pain and swelling of both wrists and the metacarpal joints of both hands. His serum C-reactive protein level had been elevated at that time, but he had no detectable rheumatoid factor. Findings on magnetic resonance imaging of the hand were very suggestive of rheumatoid arthritis (Figure 1).
He had been started on the anti-tumor necrosis factor agent etanercept but his symptoms improved only slightly, and therefore a glucocorticoid had been added.
Two years later, he developed abdominal pain, for which he underwent cholecystectomy. However, he continued to have chronic, generalized abdominal pain, and over the next 4 years he lost 25 lb. Upper endoscopy showed no mucosal changes, and multiple random biopsy samples were obtained for histologic evaluation (FIGURE 2) as part of his workup for chronic abdominal pain.
Q: What is the diagnosis?
A: As shown in Figure 2, staining of duodenal specimens showed intact villous architecture, with focal expansion of the lamina propria by “foamy” macrophages, rare plasma cells, and eosinophils, a key feature of Whipple disease. Periodic acid-Schiff staining showed numerous bacilli within the macrophages, thus confirming the diagnosis of Whipple disease. The diagnosis was also confirmed by polymerase chain reaction testing. Staining for acid-fast bacilli was negative.
WHEN TO CONSIDER WHIPPLE DISEASE
Whipple disease is a rare systemic disease with a very low incidence rate worldwide. Thus, its prevalence is difficult to estimate accurately. It is caused by a gram-positive bacterium, Tropheryma whippelii.1,2 The typical clinical manifestations are diarrhea, abdominal pain, weight loss, and fever. In most patients, these are often preceded by articular symptoms,3 as in our patient, who had articular symptoms for 5 years before he was diagnosed with Whipple disease.
Interestingly, our patient also had pleural effusion, which is uncommon in Whipple disease.4
The pathogenesis of Whipple disease is thought to be related to bacterial replication within macrophages, which leads to a systemic immune response and tissue infiltration by the organism.5 Histologic evaluation is the most common way to confirm the diagnosis.
As our patient’s disease course illustrates, Whipple disease should be part of the differential diagnosis of arthritis, as antibiotic therapy alone leads to a dramatic clinical response.
Our patient was started on a 2-week course of intravenous ceftriaxone followed by oral sulfamethoxazole and trimethoprim, and his abdominal and articular symptoms completely resolved within 4 weeks.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
- Dutly F, Altwegg M. Whipple’s disease and ‘Tropheryma whippelii.’ Clin Microbiol Rev 2001; 14:561–583.
- Raoult D, Birg ML, La Scola B, et al. Cultivation of the bacillus of Whipple’s disease. N Engl J Med 2000; 342:620–625.
- Relman DA, Schmidt TM, MacDermott RP, Falkow S. Identification of the uncultured bacillus of Whipple’s disease. N Engl J Med 1992; 327:293–301.
- Durand DV, Lecomte C, Cathébras P, Rousset H, Godeau P. Whipple disease. Clinical review of 52 cases. The SNFMI Research Group on Whipple disease. Société Nationale Française de Médecine Interne. Medicine (Baltimore) 1997; 76:170–184.
- Dobbins WO, Ruffin JM. A light- and electron-microscopic study of bacterial invasion in Whipple’s disease. Am J Pathol 1967; 51:225–242.
Child’s brain damage blamed on late cesarean … and more
A MOTHER WANTED A HOME BIRTH with a midwife. When complications arose and labor stopped progressing, the midwife called an ambulance. The emergency department (ED) physician ordered an urgent cesarean delivery, but the procedure did not begin for another 2 hours. The child was born with brain damage, multiple physical and mental disabilities, complex seizure disorder, and cerebral palsy.
PARENTS’ CLAIM The child’s injuries occurred because cesarean delivery was delayed for 2 hours. Based on fetal heart-rate monitoring, the injuries most likely occurred in the last 18 minutes before birth, and were probably caused by compression of the umbilical cord. An earlier cesarean delivery would have avoided the injuries.
DEFENDANTS’ DEFENSE All of the injuries occurred prior to the mother’s arrival at the hospital, while she was under the care of the midwife. Fetal distress was present for an hour before the ambulance was called. When the mother arrived at the ED, she was an unknown patient, as the midwife did not have a collaborating physician. While the ED physician determined that a cesarean delivery was required, it was not considered an emergency. The mother was taken to the OR as soon as possible. Fetal monitoring strips at the hospital were reassuring.
VERDICT A $55 million Maryland verdict was returned against the hospital, including $26 million in noneconomic damages. After the court reduced noneconomic damages and future lost wages awards, the net verdict was $28 million.
ARDS after hysterectomy
A MORBIDLY OBESE WOMAN underwent a hysterectomy. The asthmatic, 38-year-old patient vomited after surgery. A pulmonologist undertook her care and determined that she had acute respiratory distress syndrome (ARDS). He prescribed the administration of oxygen. When she vomited again during the early morning hours of the second postsurgical day, he ordered intubation and went to the hospital immediately, but the patient quickly deteriorated. She died from cardiac arrest.
ESTATE’S CLAIM The patient’s death was due to failure to diagnose and treat ARDS in a timely manner. A bronchoscopy and frequent radiographs should have been performed. If the patient had been intubated earlier and steps had been taken to reduce the risk of vomiting, she would have had a better chance of survival. She should have been transferred to another facility when ARDS was diagnosed.
DEFENDANTS’ DEFENSE A bronchoscopy was not necessary. ARDS was diagnosed and treated in a timely manner. She was too unstable to transfer to another hospital.
VERDICT The hospital reached a confidential settlement, and the claim against the anesthesiologist was dismissed. The trial proceeded against the pulmonologist and his group. A New York defense verdict was returned.
Mother’s HELLP syndrome missed; fetus dies
DURING HER PREGNANCY, a 23-year-old woman was monitored for hypertension by her ObGyn and nurse midwife. At her 36-week prenatal visit, she was found to have preeclampsia, including proteinuria. She was sent directly to the ED, where the baby was monitored and laboratory tests were ordered by a nurse and nurse midwife. After 2 hours, she was told she had a urinary tract infection and discharged. Three days later, she returned to the ED in critical condition; she had suffered an intrauterine fetal demise.
PARENTS’ CLAIM Lab results showed critical values and confirmed that the patient had developed HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. The ED nurse and nurse midwife were negligent in their treatment: They never read the lab results or reported the results to the patient or an ObGyn.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $950,000 Virginia settlement was reached.
A PREGNANT WOMAN WAS AWAITING TRIAL in County jail when she went into preterm labor. She was taken to the ED but released 2 hours later, although she was dilated 2–3 cm and having contractions. She was returned to her locked cell and not monitored—no deputy or nurse was within sight or sound of the patient. Her water broke and contractions increased. Despite her screams, and those of other inmates, a nurse didn’t arrive for 2 hours, when the baby’s head was crowning. EMS services were called and the baby was delivered in the jail cell. The child had no heartbeat or respiration. Mother and baby were transported to the hospital, where the child was resuscitated. She has severe mental impairment and cerebral palsy.
There is no documentation that the mother received any prenatal or postpartum care in jail. The mother is now serving a life sentence after a conviction for felony murder, kidnapping, and conspiracy.
CHILD’S CLAIM The case was brought on behalf of the child, and claimed that deliberate indifference and the failure to provide medical attention caused the child’s impairments.
DEFENDANTS’ DEFENSE The County claimed qualified immunity as a government entity and argued that, when the child was injured, she was still a fetus, and therefore not protected by the Constitution and civil rights laws.
VERDICT The US Circuit Court of Appeals rejected the County’s argument that the child was not protected by the Constitution. An $8 million Michigan settlement was reached.
Dermoid cyst still present after wrong-site surgery
A DERMOID CYST WAS DETECTED on the left ovary of a 28-year-old woman during prenatal ultrasonography (US). A year later, US confirmed the dermoid cyst, and the patient underwent outpatient cystectomy.
At the first postsurgical visit, the patient reported right pelvic pain. When she called the ObGyn’s office a few days later to again report right pelvic pain, her call was not returned.
She then went to the ED, where testing determined that the ObGyn had performed a right salpingo-oophorectomy and that her left ovary and cyst were still intact. She again attempted to contact the ObGyn, without response.
PATIENT’S CLAIM The ObGyn performed wrong-site surgery. The patient was not informed of the error during a postsurgical visit, nor were her attempts at contacting the physician returned. Still at risk for malignancy, she is facing a second surgical procedure to remove the cyst. Her fertility is diminished due to the surgical error, and she suffers anxiety and mental stress as a result of the situation.
At first, the ObGyn refused to provide medical records to the patient’s lawyer. When the records were obtained and compared with records obtained from another physician who treated the patient, it was evident that the ObGyn had altered the records to state that the patient had complained of right-side pain.
PHYSICIAN’S DEFENSE There was no negligence. The patient was properly treated for right-sided pain. The records were not altered.
VERDICT A $1.42 million Maryland verdict was returned. The state cap on noneconomic damages will reduce the verdict to $680,000.
Sponge left behind after vacuum-assisted closure
A WOMAN WENT TO THE ED with abdominal pain. It was determined that she had an abdominal abscess, and a surgeon assumed her care. After surgically draining the abdominal abscess, the surgeon placed a large black sponge into the abdominal cavity and then used vacuum-assisted closure. The patient was discharged 6 days later. She continued to receive treatment for a surgical-site infection that failed to heal. Two weeks later, the patient was readmitted to the hospital for exploratory surgery. The surgeon found and removed the sponge.
PATIENT’S CLAIM The surgeon was negligent for leaving the surgical sponge in the patient’s abdomen. She claimed pain, scarring, wound necrosis, infection, and the need for additional hospitalizations due to retention of the sponge.
PHYSICIAN’S DEFENSE A settlement was reached during the trial.
VERDICT A confidential Florida settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
A MOTHER WANTED A HOME BIRTH with a midwife. When complications arose and labor stopped progressing, the midwife called an ambulance. The emergency department (ED) physician ordered an urgent cesarean delivery, but the procedure did not begin for another 2 hours. The child was born with brain damage, multiple physical and mental disabilities, complex seizure disorder, and cerebral palsy.
PARENTS’ CLAIM The child’s injuries occurred because cesarean delivery was delayed for 2 hours. Based on fetal heart-rate monitoring, the injuries most likely occurred in the last 18 minutes before birth, and were probably caused by compression of the umbilical cord. An earlier cesarean delivery would have avoided the injuries.
DEFENDANTS’ DEFENSE All of the injuries occurred prior to the mother’s arrival at the hospital, while she was under the care of the midwife. Fetal distress was present for an hour before the ambulance was called. When the mother arrived at the ED, she was an unknown patient, as the midwife did not have a collaborating physician. While the ED physician determined that a cesarean delivery was required, it was not considered an emergency. The mother was taken to the OR as soon as possible. Fetal monitoring strips at the hospital were reassuring.
VERDICT A $55 million Maryland verdict was returned against the hospital, including $26 million in noneconomic damages. After the court reduced noneconomic damages and future lost wages awards, the net verdict was $28 million.
ARDS after hysterectomy
A MORBIDLY OBESE WOMAN underwent a hysterectomy. The asthmatic, 38-year-old patient vomited after surgery. A pulmonologist undertook her care and determined that she had acute respiratory distress syndrome (ARDS). He prescribed the administration of oxygen. When she vomited again during the early morning hours of the second postsurgical day, he ordered intubation and went to the hospital immediately, but the patient quickly deteriorated. She died from cardiac arrest.
ESTATE’S CLAIM The patient’s death was due to failure to diagnose and treat ARDS in a timely manner. A bronchoscopy and frequent radiographs should have been performed. If the patient had been intubated earlier and steps had been taken to reduce the risk of vomiting, she would have had a better chance of survival. She should have been transferred to another facility when ARDS was diagnosed.
DEFENDANTS’ DEFENSE A bronchoscopy was not necessary. ARDS was diagnosed and treated in a timely manner. She was too unstable to transfer to another hospital.
VERDICT The hospital reached a confidential settlement, and the claim against the anesthesiologist was dismissed. The trial proceeded against the pulmonologist and his group. A New York defense verdict was returned.
Mother’s HELLP syndrome missed; fetus dies
DURING HER PREGNANCY, a 23-year-old woman was monitored for hypertension by her ObGyn and nurse midwife. At her 36-week prenatal visit, she was found to have preeclampsia, including proteinuria. She was sent directly to the ED, where the baby was monitored and laboratory tests were ordered by a nurse and nurse midwife. After 2 hours, she was told she had a urinary tract infection and discharged. Three days later, she returned to the ED in critical condition; she had suffered an intrauterine fetal demise.
PARENTS’ CLAIM Lab results showed critical values and confirmed that the patient had developed HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. The ED nurse and nurse midwife were negligent in their treatment: They never read the lab results or reported the results to the patient or an ObGyn.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $950,000 Virginia settlement was reached.
A PREGNANT WOMAN WAS AWAITING TRIAL in County jail when she went into preterm labor. She was taken to the ED but released 2 hours later, although she was dilated 2–3 cm and having contractions. She was returned to her locked cell and not monitored—no deputy or nurse was within sight or sound of the patient. Her water broke and contractions increased. Despite her screams, and those of other inmates, a nurse didn’t arrive for 2 hours, when the baby’s head was crowning. EMS services were called and the baby was delivered in the jail cell. The child had no heartbeat or respiration. Mother and baby were transported to the hospital, where the child was resuscitated. She has severe mental impairment and cerebral palsy.
There is no documentation that the mother received any prenatal or postpartum care in jail. The mother is now serving a life sentence after a conviction for felony murder, kidnapping, and conspiracy.
CHILD’S CLAIM The case was brought on behalf of the child, and claimed that deliberate indifference and the failure to provide medical attention caused the child’s impairments.
DEFENDANTS’ DEFENSE The County claimed qualified immunity as a government entity and argued that, when the child was injured, she was still a fetus, and therefore not protected by the Constitution and civil rights laws.
VERDICT The US Circuit Court of Appeals rejected the County’s argument that the child was not protected by the Constitution. An $8 million Michigan settlement was reached.
Dermoid cyst still present after wrong-site surgery
A DERMOID CYST WAS DETECTED on the left ovary of a 28-year-old woman during prenatal ultrasonography (US). A year later, US confirmed the dermoid cyst, and the patient underwent outpatient cystectomy.
At the first postsurgical visit, the patient reported right pelvic pain. When she called the ObGyn’s office a few days later to again report right pelvic pain, her call was not returned.
She then went to the ED, where testing determined that the ObGyn had performed a right salpingo-oophorectomy and that her left ovary and cyst were still intact. She again attempted to contact the ObGyn, without response.
PATIENT’S CLAIM The ObGyn performed wrong-site surgery. The patient was not informed of the error during a postsurgical visit, nor were her attempts at contacting the physician returned. Still at risk for malignancy, she is facing a second surgical procedure to remove the cyst. Her fertility is diminished due to the surgical error, and she suffers anxiety and mental stress as a result of the situation.
At first, the ObGyn refused to provide medical records to the patient’s lawyer. When the records were obtained and compared with records obtained from another physician who treated the patient, it was evident that the ObGyn had altered the records to state that the patient had complained of right-side pain.
PHYSICIAN’S DEFENSE There was no negligence. The patient was properly treated for right-sided pain. The records were not altered.
VERDICT A $1.42 million Maryland verdict was returned. The state cap on noneconomic damages will reduce the verdict to $680,000.
Sponge left behind after vacuum-assisted closure
A WOMAN WENT TO THE ED with abdominal pain. It was determined that she had an abdominal abscess, and a surgeon assumed her care. After surgically draining the abdominal abscess, the surgeon placed a large black sponge into the abdominal cavity and then used vacuum-assisted closure. The patient was discharged 6 days later. She continued to receive treatment for a surgical-site infection that failed to heal. Two weeks later, the patient was readmitted to the hospital for exploratory surgery. The surgeon found and removed the sponge.
PATIENT’S CLAIM The surgeon was negligent for leaving the surgical sponge in the patient’s abdomen. She claimed pain, scarring, wound necrosis, infection, and the need for additional hospitalizations due to retention of the sponge.
PHYSICIAN’S DEFENSE A settlement was reached during the trial.
VERDICT A confidential Florida settlement was reached.
A MOTHER WANTED A HOME BIRTH with a midwife. When complications arose and labor stopped progressing, the midwife called an ambulance. The emergency department (ED) physician ordered an urgent cesarean delivery, but the procedure did not begin for another 2 hours. The child was born with brain damage, multiple physical and mental disabilities, complex seizure disorder, and cerebral palsy.
PARENTS’ CLAIM The child’s injuries occurred because cesarean delivery was delayed for 2 hours. Based on fetal heart-rate monitoring, the injuries most likely occurred in the last 18 minutes before birth, and were probably caused by compression of the umbilical cord. An earlier cesarean delivery would have avoided the injuries.
DEFENDANTS’ DEFENSE All of the injuries occurred prior to the mother’s arrival at the hospital, while she was under the care of the midwife. Fetal distress was present for an hour before the ambulance was called. When the mother arrived at the ED, she was an unknown patient, as the midwife did not have a collaborating physician. While the ED physician determined that a cesarean delivery was required, it was not considered an emergency. The mother was taken to the OR as soon as possible. Fetal monitoring strips at the hospital were reassuring.
VERDICT A $55 million Maryland verdict was returned against the hospital, including $26 million in noneconomic damages. After the court reduced noneconomic damages and future lost wages awards, the net verdict was $28 million.
ARDS after hysterectomy
A MORBIDLY OBESE WOMAN underwent a hysterectomy. The asthmatic, 38-year-old patient vomited after surgery. A pulmonologist undertook her care and determined that she had acute respiratory distress syndrome (ARDS). He prescribed the administration of oxygen. When she vomited again during the early morning hours of the second postsurgical day, he ordered intubation and went to the hospital immediately, but the patient quickly deteriorated. She died from cardiac arrest.
ESTATE’S CLAIM The patient’s death was due to failure to diagnose and treat ARDS in a timely manner. A bronchoscopy and frequent radiographs should have been performed. If the patient had been intubated earlier and steps had been taken to reduce the risk of vomiting, she would have had a better chance of survival. She should have been transferred to another facility when ARDS was diagnosed.
DEFENDANTS’ DEFENSE A bronchoscopy was not necessary. ARDS was diagnosed and treated in a timely manner. She was too unstable to transfer to another hospital.
VERDICT The hospital reached a confidential settlement, and the claim against the anesthesiologist was dismissed. The trial proceeded against the pulmonologist and his group. A New York defense verdict was returned.
Mother’s HELLP syndrome missed; fetus dies
DURING HER PREGNANCY, a 23-year-old woman was monitored for hypertension by her ObGyn and nurse midwife. At her 36-week prenatal visit, she was found to have preeclampsia, including proteinuria. She was sent directly to the ED, where the baby was monitored and laboratory tests were ordered by a nurse and nurse midwife. After 2 hours, she was told she had a urinary tract infection and discharged. Three days later, she returned to the ED in critical condition; she had suffered an intrauterine fetal demise.
PARENTS’ CLAIM Lab results showed critical values and confirmed that the patient had developed HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome. The ED nurse and nurse midwife were negligent in their treatment: They never read the lab results or reported the results to the patient or an ObGyn.
DEFENDANTS’ DEFENSE The case was settled before trial.
VERDICT A $950,000 Virginia settlement was reached.
A PREGNANT WOMAN WAS AWAITING TRIAL in County jail when she went into preterm labor. She was taken to the ED but released 2 hours later, although she was dilated 2–3 cm and having contractions. She was returned to her locked cell and not monitored—no deputy or nurse was within sight or sound of the patient. Her water broke and contractions increased. Despite her screams, and those of other inmates, a nurse didn’t arrive for 2 hours, when the baby’s head was crowning. EMS services were called and the baby was delivered in the jail cell. The child had no heartbeat or respiration. Mother and baby were transported to the hospital, where the child was resuscitated. She has severe mental impairment and cerebral palsy.
There is no documentation that the mother received any prenatal or postpartum care in jail. The mother is now serving a life sentence after a conviction for felony murder, kidnapping, and conspiracy.
CHILD’S CLAIM The case was brought on behalf of the child, and claimed that deliberate indifference and the failure to provide medical attention caused the child’s impairments.
DEFENDANTS’ DEFENSE The County claimed qualified immunity as a government entity and argued that, when the child was injured, she was still a fetus, and therefore not protected by the Constitution and civil rights laws.
VERDICT The US Circuit Court of Appeals rejected the County’s argument that the child was not protected by the Constitution. An $8 million Michigan settlement was reached.
Dermoid cyst still present after wrong-site surgery
A DERMOID CYST WAS DETECTED on the left ovary of a 28-year-old woman during prenatal ultrasonography (US). A year later, US confirmed the dermoid cyst, and the patient underwent outpatient cystectomy.
At the first postsurgical visit, the patient reported right pelvic pain. When she called the ObGyn’s office a few days later to again report right pelvic pain, her call was not returned.
She then went to the ED, where testing determined that the ObGyn had performed a right salpingo-oophorectomy and that her left ovary and cyst were still intact. She again attempted to contact the ObGyn, without response.
PATIENT’S CLAIM The ObGyn performed wrong-site surgery. The patient was not informed of the error during a postsurgical visit, nor were her attempts at contacting the physician returned. Still at risk for malignancy, she is facing a second surgical procedure to remove the cyst. Her fertility is diminished due to the surgical error, and she suffers anxiety and mental stress as a result of the situation.
At first, the ObGyn refused to provide medical records to the patient’s lawyer. When the records were obtained and compared with records obtained from another physician who treated the patient, it was evident that the ObGyn had altered the records to state that the patient had complained of right-side pain.
PHYSICIAN’S DEFENSE There was no negligence. The patient was properly treated for right-sided pain. The records were not altered.
VERDICT A $1.42 million Maryland verdict was returned. The state cap on noneconomic damages will reduce the verdict to $680,000.
Sponge left behind after vacuum-assisted closure
A WOMAN WENT TO THE ED with abdominal pain. It was determined that she had an abdominal abscess, and a surgeon assumed her care. After surgically draining the abdominal abscess, the surgeon placed a large black sponge into the abdominal cavity and then used vacuum-assisted closure. The patient was discharged 6 days later. She continued to receive treatment for a surgical-site infection that failed to heal. Two weeks later, the patient was readmitted to the hospital for exploratory surgery. The surgeon found and removed the sponge.
PATIENT’S CLAIM The surgeon was negligent for leaving the surgical sponge in the patient’s abdomen. She claimed pain, scarring, wound necrosis, infection, and the need for additional hospitalizations due to retention of the sponge.
PHYSICIAN’S DEFENSE A settlement was reached during the trial.
VERDICT A confidential Florida settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
We want to hear from you! Tell us what you think.
Failure to spot postpartum danger leads to permanent disability
Failure to spot postpartum danger leads to permanent disability
AFTER 2 HOSPITALIZATIONS FOR HYPERTENSION ordered by her physician, a pregnant 41-year-old woman gave birth to a daughter by cesarean section on December 17. She was discharged 2 days later with a blood pressure of 130/90 mm Hg.
On December 21, the woman went to her doctor’s office, complaining that she didn’t feel well and had severe swelling. A nurse took her blood pressure twice, obtaining readings of 170/88 and 168/90 mm Hg. She sent the patient home without an examination by the doctor. On her way out of the office, the patient passed the doctor in the hallway and, she claimed, told him she wasn’t feeling well and that her blood pressure was high. She said he told her to double her blood pressure medication.
That evening the patient had trouble breathing and was taken by paramedics to a hospital, where she was intubated. She didn’t have a pulse for 15 minutes, leading to permanent brain damage.
The patient can’t walk without help and can’t feed herself because her hands are contorted. She’s legally blind, suffers from short-term memory loss, and has difficulty speaking.
PLAINTIFF’S CLAIM The patient had classic signs of postpartum cardiomyopathy. If the doctor had looked at her blood pressure readings and examined her while she was at the office, she would have received appropriate treatment and avoided injury.
THE DEFENSE The patient went to the doctor’s office to show the staff her baby and have her blood pressure checked, not because she was feeling ill. The doctor would have examined the patient if he had been told of the blood pressure readings.
VERDICT $5 million Georgia verdict.
COMMENT For the vast majority of patients, a blood pressure of 170/88 mm Hg is not a medical emergency or even urgent. But for a woman 4 days postpartum with significant edema, it is. This case illustrates the ultimate challenge of family medicine: identifying and treating the dangerous situations among the many mundane ones.
Persistent pain requires more than medication
PAIN IN HER CHEST AND SHOULDERS prompted a 27-year-old woman to seek medical attention. Her physician attributed the pain to muscle strain and prescribed medication. Six months later the patient returned to the doctor complaining of continuing pain. The doctor concluded that the position in which the patient slept was causing the pain and prescribed painkillers.
After 9 months, the pain still had not resolved. The patient was given a diagnosis of stage II Hodgkin’s lymphoma, which went into remission after aggressive treatment.
PLAINTIFF’S CLAIM The pain was caused by the cancer, which had been present at all of the patient’s visits with her doctor. The doctor was negligent in failing to diagnose the cancer promptly, necessitating more aggressive treatment than would otherwise have been required.
THE DEFENSE The patient’s pain was episodic and varied; it didn’t warrant diagnostic testing. The patient failed to follow through on physical therapy that the physician had prescribed. The patient denied that the doctor had prescribed physical therapy.
VERDICT $800,000 New York verdict.
COMMENT Persistence of symptoms dictates persistence of work-up. After 6 months of pain, the patient should have had a more detailed evaluation. On a personal note, I had a patient just like this one several years ago; a chest radiograph revealed her lymphoma.
Failure to spot postpartum danger leads to permanent disability
AFTER 2 HOSPITALIZATIONS FOR HYPERTENSION ordered by her physician, a pregnant 41-year-old woman gave birth to a daughter by cesarean section on December 17. She was discharged 2 days later with a blood pressure of 130/90 mm Hg.
On December 21, the woman went to her doctor’s office, complaining that she didn’t feel well and had severe swelling. A nurse took her blood pressure twice, obtaining readings of 170/88 and 168/90 mm Hg. She sent the patient home without an examination by the doctor. On her way out of the office, the patient passed the doctor in the hallway and, she claimed, told him she wasn’t feeling well and that her blood pressure was high. She said he told her to double her blood pressure medication.
That evening the patient had trouble breathing and was taken by paramedics to a hospital, where she was intubated. She didn’t have a pulse for 15 minutes, leading to permanent brain damage.
The patient can’t walk without help and can’t feed herself because her hands are contorted. She’s legally blind, suffers from short-term memory loss, and has difficulty speaking.
PLAINTIFF’S CLAIM The patient had classic signs of postpartum cardiomyopathy. If the doctor had looked at her blood pressure readings and examined her while she was at the office, she would have received appropriate treatment and avoided injury.
THE DEFENSE The patient went to the doctor’s office to show the staff her baby and have her blood pressure checked, not because she was feeling ill. The doctor would have examined the patient if he had been told of the blood pressure readings.
VERDICT $5 million Georgia verdict.
COMMENT For the vast majority of patients, a blood pressure of 170/88 mm Hg is not a medical emergency or even urgent. But for a woman 4 days postpartum with significant edema, it is. This case illustrates the ultimate challenge of family medicine: identifying and treating the dangerous situations among the many mundane ones.
Persistent pain requires more than medication
PAIN IN HER CHEST AND SHOULDERS prompted a 27-year-old woman to seek medical attention. Her physician attributed the pain to muscle strain and prescribed medication. Six months later the patient returned to the doctor complaining of continuing pain. The doctor concluded that the position in which the patient slept was causing the pain and prescribed painkillers.
After 9 months, the pain still had not resolved. The patient was given a diagnosis of stage II Hodgkin’s lymphoma, which went into remission after aggressive treatment.
PLAINTIFF’S CLAIM The pain was caused by the cancer, which had been present at all of the patient’s visits with her doctor. The doctor was negligent in failing to diagnose the cancer promptly, necessitating more aggressive treatment than would otherwise have been required.
THE DEFENSE The patient’s pain was episodic and varied; it didn’t warrant diagnostic testing. The patient failed to follow through on physical therapy that the physician had prescribed. The patient denied that the doctor had prescribed physical therapy.
VERDICT $800,000 New York verdict.
COMMENT Persistence of symptoms dictates persistence of work-up. After 6 months of pain, the patient should have had a more detailed evaluation. On a personal note, I had a patient just like this one several years ago; a chest radiograph revealed her lymphoma.
Failure to spot postpartum danger leads to permanent disability
AFTER 2 HOSPITALIZATIONS FOR HYPERTENSION ordered by her physician, a pregnant 41-year-old woman gave birth to a daughter by cesarean section on December 17. She was discharged 2 days later with a blood pressure of 130/90 mm Hg.
On December 21, the woman went to her doctor’s office, complaining that she didn’t feel well and had severe swelling. A nurse took her blood pressure twice, obtaining readings of 170/88 and 168/90 mm Hg. She sent the patient home without an examination by the doctor. On her way out of the office, the patient passed the doctor in the hallway and, she claimed, told him she wasn’t feeling well and that her blood pressure was high. She said he told her to double her blood pressure medication.
That evening the patient had trouble breathing and was taken by paramedics to a hospital, where she was intubated. She didn’t have a pulse for 15 minutes, leading to permanent brain damage.
The patient can’t walk without help and can’t feed herself because her hands are contorted. She’s legally blind, suffers from short-term memory loss, and has difficulty speaking.
PLAINTIFF’S CLAIM The patient had classic signs of postpartum cardiomyopathy. If the doctor had looked at her blood pressure readings and examined her while she was at the office, she would have received appropriate treatment and avoided injury.
THE DEFENSE The patient went to the doctor’s office to show the staff her baby and have her blood pressure checked, not because she was feeling ill. The doctor would have examined the patient if he had been told of the blood pressure readings.
VERDICT $5 million Georgia verdict.
COMMENT For the vast majority of patients, a blood pressure of 170/88 mm Hg is not a medical emergency or even urgent. But for a woman 4 days postpartum with significant edema, it is. This case illustrates the ultimate challenge of family medicine: identifying and treating the dangerous situations among the many mundane ones.
Persistent pain requires more than medication
PAIN IN HER CHEST AND SHOULDERS prompted a 27-year-old woman to seek medical attention. Her physician attributed the pain to muscle strain and prescribed medication. Six months later the patient returned to the doctor complaining of continuing pain. The doctor concluded that the position in which the patient slept was causing the pain and prescribed painkillers.
After 9 months, the pain still had not resolved. The patient was given a diagnosis of stage II Hodgkin’s lymphoma, which went into remission after aggressive treatment.
PLAINTIFF’S CLAIM The pain was caused by the cancer, which had been present at all of the patient’s visits with her doctor. The doctor was negligent in failing to diagnose the cancer promptly, necessitating more aggressive treatment than would otherwise have been required.
THE DEFENSE The patient’s pain was episodic and varied; it didn’t warrant diagnostic testing. The patient failed to follow through on physical therapy that the physician had prescribed. The patient denied that the doctor had prescribed physical therapy.
VERDICT $800,000 New York verdict.
COMMENT Persistence of symptoms dictates persistence of work-up. After 6 months of pain, the patient should have had a more detailed evaluation. On a personal note, I had a patient just like this one several years ago; a chest radiograph revealed her lymphoma.
Suspect carpal tunnel? Try this
For best results, use the modified Phalen’s test (MPT) rather than the traditional Phalen’s when you suspect carpal tunnel syndrome (CTS).1
1. Bilkis S, Loveman DM, Eldridge JA, et al. Modified Phalen’s test as an aid in diagnosing carpal tunnel syndrome. Arthritis Care Res. 2012;64:287-289.
STRENGTH OF RECOMMENDATION
B: Based on a single diagnostic cohort study.
ILLUSTRATIVE CASE
A 60-year-old assembly line worker reports bilateral hand numbness and tingling that frequently awaken her at night. What is the best office test to determine if she has CTS?
CTS is one of the most common causes of disability in the United States.2 Among patients with hand paresthesias, one in 5 has CTS.2 Factory workers whose jobs involve repetitive hand movements, females, and the elderly are at increased risk.3 If left untreated, the symptoms are likely to become constant, with thenar muscle wasting and weakness.
Traditional diagnostic test has only 50% sensitivity
In the traditional Phalen’s test (TPT)—commonly used in an office setting—the patient holds his or her wrists in a position of fixed flexion for one minute. The onset of paresthesias is considered a positive result.
The TPT was found in the study reported here to be 100% specific;1 however, other studies have found a wider range of specificity (33%-86%).4 The TPT has a sensitivity of only 50%, which increases the risk that cases of CTS will be missed. This is an important consideration because establishing a diagnosis early in the course of CTS has been shown to minimize disability.5
STUDY SUMMARY: Modified Phalen’s has higher sensitivity
Bilkis et al developed a modified Phalen’s test (MPT) and compared it with the TPT, as well as with electrodiagnostic studies (EDS)—the gold standard for CTS diagnosis. The MPT begins with the TPT position and adds sensory testing with a Semmes-Weinstein 2.83-unit monofilament.
See how the modified Phalen’s test is done
Courtesy of Clinically Relevant Technologies
The filament is applied perpendicular to the palmar and lateral surface of each distal finger 3 times, with enough pressure to bend the monofilament. In this study, the test was considered positive if the patient did not feel the monofilament in any finger along the distribution of the median nerve. The MPT was negative if the patient correctly reported being touched along this distribution. The fifth, or “pinkie,” finger, which is less likely to be affected by CTS, was used as a control.
Participants in the study were adult patients—mostly women between the ages of 27 and 88 years—at a neurology clinic. Exclusion criteria included cervical radiculopathy, a history of stroke, diabetes mellitus, and concomitant neck injury. A total of 66 hands (and 37 participants) underwent TPT and MPT testing by trained examiners, followed by EDS to confirm the findings.
EDS found evidence of CTS in 46 of the 66 hands studied. The MPT correctly identified 39 of the 46, while the TPT correctly identified 23. Both the traditional and the modified Phalen’s were found to be 100% specific, but the sensitivity of the MPT was 85% (95% confidence interval [CI], 71%-93%), compared with 50% (95% CI, 35%-65%) for the TPT.
WHAT’S NEW: Better results can be achieved in seconds
The addition of monofilament testing to the TPT increases the sensitivity in identifying CTS. The MPT is simple to learn (watch the video on jfponline.com) and, based on our observations, adds only about 10 to 15 seconds to the clinical exam.
CAVEATS: Modification is untested in primary care
A diagnosis of CTS is rarely made on the basis of one test, but rather on a set of signs, symptoms, and physical exam maneuvers. The added value of the MPT needs to be evaluated in the larger context of the comprehensive clinical examination for CTS.6
Notably, the study participants were seen in a neurology clinic, which suggests that they may have had more advanced CTS than typical primary care patients. That would help explain the 100% specificity of both the traditional and modified tests reported by the researchers. The sensitivity of the MPT may therefore be lower in a family physician’s office because the spectrum of disease may be wider. Another study is needed to evaluate the performance of the MPT in a primary care setting.
The monofilament used (Semmes-Weinstein 2.83) is not the same as the typical 5.07 (10-g) monofilament used in diabetic foot screenings. Using this heavier monofilament with a stronger pressure point would likely decrease the sensitivity of the MPT.
CHALLENGES TO IMPLEMENTATION: Taking the time, obtaining the monofilament
Additional time to obtain the correct monofilament and administer the MPT are the key challenges to implementation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Bilkis S, Loveman DM, Eldridge JA, et al. Modified Phalen’s test as an aid in diagnosing carpal tunnel syndrome. Arthritis Care Res. 2012;64:287-289.
2. Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153-158.
3. National Institute of Neurological Disorders and Stroke. Carpal tunnel syndrome fact sheet. National Institutes of Health. July 2012. Available at http://www.ninds.nih.gov/disorders/carpal_tunnel/detail_carpal_tunnel.htm. Accessed April 15, 2013.
4. McGee SR. Evidence-Based Physical Diagnosis. 3rd ed. Philadelphia, Pa: Saunders; 2012:chap 62.
5. Daniell WE, Fulton-Kehoe D, Franklin GM. Work-related carpal tunnel syndrome in Washington State workers’ compensation: utilization of surgery and the duration of lost work. Am J Ind Med. 2009;52:931-942.
6. D’Arcy CA, McGee S. Does this patient have carpal tunnel syndrome? JAMA. 2000;282:3110-3117.
For best results, use the modified Phalen’s test (MPT) rather than the traditional Phalen’s when you suspect carpal tunnel syndrome (CTS).1
1. Bilkis S, Loveman DM, Eldridge JA, et al. Modified Phalen’s test as an aid in diagnosing carpal tunnel syndrome. Arthritis Care Res. 2012;64:287-289.
STRENGTH OF RECOMMENDATION
B: Based on a single diagnostic cohort study.
ILLUSTRATIVE CASE
A 60-year-old assembly line worker reports bilateral hand numbness and tingling that frequently awaken her at night. What is the best office test to determine if she has CTS?
CTS is one of the most common causes of disability in the United States.2 Among patients with hand paresthesias, one in 5 has CTS.2 Factory workers whose jobs involve repetitive hand movements, females, and the elderly are at increased risk.3 If left untreated, the symptoms are likely to become constant, with thenar muscle wasting and weakness.
Traditional diagnostic test has only 50% sensitivity
In the traditional Phalen’s test (TPT)—commonly used in an office setting—the patient holds his or her wrists in a position of fixed flexion for one minute. The onset of paresthesias is considered a positive result.
The TPT was found in the study reported here to be 100% specific;1 however, other studies have found a wider range of specificity (33%-86%).4 The TPT has a sensitivity of only 50%, which increases the risk that cases of CTS will be missed. This is an important consideration because establishing a diagnosis early in the course of CTS has been shown to minimize disability.5
STUDY SUMMARY: Modified Phalen’s has higher sensitivity
Bilkis et al developed a modified Phalen’s test (MPT) and compared it with the TPT, as well as with electrodiagnostic studies (EDS)—the gold standard for CTS diagnosis. The MPT begins with the TPT position and adds sensory testing with a Semmes-Weinstein 2.83-unit monofilament.
See how the modified Phalen’s test is done
Courtesy of Clinically Relevant Technologies
The filament is applied perpendicular to the palmar and lateral surface of each distal finger 3 times, with enough pressure to bend the monofilament. In this study, the test was considered positive if the patient did not feel the monofilament in any finger along the distribution of the median nerve. The MPT was negative if the patient correctly reported being touched along this distribution. The fifth, or “pinkie,” finger, which is less likely to be affected by CTS, was used as a control.
Participants in the study were adult patients—mostly women between the ages of 27 and 88 years—at a neurology clinic. Exclusion criteria included cervical radiculopathy, a history of stroke, diabetes mellitus, and concomitant neck injury. A total of 66 hands (and 37 participants) underwent TPT and MPT testing by trained examiners, followed by EDS to confirm the findings.
EDS found evidence of CTS in 46 of the 66 hands studied. The MPT correctly identified 39 of the 46, while the TPT correctly identified 23. Both the traditional and the modified Phalen’s were found to be 100% specific, but the sensitivity of the MPT was 85% (95% confidence interval [CI], 71%-93%), compared with 50% (95% CI, 35%-65%) for the TPT.
WHAT’S NEW: Better results can be achieved in seconds
The addition of monofilament testing to the TPT increases the sensitivity in identifying CTS. The MPT is simple to learn (watch the video on jfponline.com) and, based on our observations, adds only about 10 to 15 seconds to the clinical exam.
CAVEATS: Modification is untested in primary care
A diagnosis of CTS is rarely made on the basis of one test, but rather on a set of signs, symptoms, and physical exam maneuvers. The added value of the MPT needs to be evaluated in the larger context of the comprehensive clinical examination for CTS.6
Notably, the study participants were seen in a neurology clinic, which suggests that they may have had more advanced CTS than typical primary care patients. That would help explain the 100% specificity of both the traditional and modified tests reported by the researchers. The sensitivity of the MPT may therefore be lower in a family physician’s office because the spectrum of disease may be wider. Another study is needed to evaluate the performance of the MPT in a primary care setting.
The monofilament used (Semmes-Weinstein 2.83) is not the same as the typical 5.07 (10-g) monofilament used in diabetic foot screenings. Using this heavier monofilament with a stronger pressure point would likely decrease the sensitivity of the MPT.
CHALLENGES TO IMPLEMENTATION: Taking the time, obtaining the monofilament
Additional time to obtain the correct monofilament and administer the MPT are the key challenges to implementation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
For best results, use the modified Phalen’s test (MPT) rather than the traditional Phalen’s when you suspect carpal tunnel syndrome (CTS).1
1. Bilkis S, Loveman DM, Eldridge JA, et al. Modified Phalen’s test as an aid in diagnosing carpal tunnel syndrome. Arthritis Care Res. 2012;64:287-289.
STRENGTH OF RECOMMENDATION
B: Based on a single diagnostic cohort study.
ILLUSTRATIVE CASE
A 60-year-old assembly line worker reports bilateral hand numbness and tingling that frequently awaken her at night. What is the best office test to determine if she has CTS?
CTS is one of the most common causes of disability in the United States.2 Among patients with hand paresthesias, one in 5 has CTS.2 Factory workers whose jobs involve repetitive hand movements, females, and the elderly are at increased risk.3 If left untreated, the symptoms are likely to become constant, with thenar muscle wasting and weakness.
Traditional diagnostic test has only 50% sensitivity
In the traditional Phalen’s test (TPT)—commonly used in an office setting—the patient holds his or her wrists in a position of fixed flexion for one minute. The onset of paresthesias is considered a positive result.
The TPT was found in the study reported here to be 100% specific;1 however, other studies have found a wider range of specificity (33%-86%).4 The TPT has a sensitivity of only 50%, which increases the risk that cases of CTS will be missed. This is an important consideration because establishing a diagnosis early in the course of CTS has been shown to minimize disability.5
STUDY SUMMARY: Modified Phalen’s has higher sensitivity
Bilkis et al developed a modified Phalen’s test (MPT) and compared it with the TPT, as well as with electrodiagnostic studies (EDS)—the gold standard for CTS diagnosis. The MPT begins with the TPT position and adds sensory testing with a Semmes-Weinstein 2.83-unit monofilament.
See how the modified Phalen’s test is done
Courtesy of Clinically Relevant Technologies
The filament is applied perpendicular to the palmar and lateral surface of each distal finger 3 times, with enough pressure to bend the monofilament. In this study, the test was considered positive if the patient did not feel the monofilament in any finger along the distribution of the median nerve. The MPT was negative if the patient correctly reported being touched along this distribution. The fifth, or “pinkie,” finger, which is less likely to be affected by CTS, was used as a control.
Participants in the study were adult patients—mostly women between the ages of 27 and 88 years—at a neurology clinic. Exclusion criteria included cervical radiculopathy, a history of stroke, diabetes mellitus, and concomitant neck injury. A total of 66 hands (and 37 participants) underwent TPT and MPT testing by trained examiners, followed by EDS to confirm the findings.
EDS found evidence of CTS in 46 of the 66 hands studied. The MPT correctly identified 39 of the 46, while the TPT correctly identified 23. Both the traditional and the modified Phalen’s were found to be 100% specific, but the sensitivity of the MPT was 85% (95% confidence interval [CI], 71%-93%), compared with 50% (95% CI, 35%-65%) for the TPT.
WHAT’S NEW: Better results can be achieved in seconds
The addition of monofilament testing to the TPT increases the sensitivity in identifying CTS. The MPT is simple to learn (watch the video on jfponline.com) and, based on our observations, adds only about 10 to 15 seconds to the clinical exam.
CAVEATS: Modification is untested in primary care
A diagnosis of CTS is rarely made on the basis of one test, but rather on a set of signs, symptoms, and physical exam maneuvers. The added value of the MPT needs to be evaluated in the larger context of the comprehensive clinical examination for CTS.6
Notably, the study participants were seen in a neurology clinic, which suggests that they may have had more advanced CTS than typical primary care patients. That would help explain the 100% specificity of both the traditional and modified tests reported by the researchers. The sensitivity of the MPT may therefore be lower in a family physician’s office because the spectrum of disease may be wider. Another study is needed to evaluate the performance of the MPT in a primary care setting.
The monofilament used (Semmes-Weinstein 2.83) is not the same as the typical 5.07 (10-g) monofilament used in diabetic foot screenings. Using this heavier monofilament with a stronger pressure point would likely decrease the sensitivity of the MPT.
CHALLENGES TO IMPLEMENTATION: Taking the time, obtaining the monofilament
Additional time to obtain the correct monofilament and administer the MPT are the key challenges to implementation.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center for Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
1. Bilkis S, Loveman DM, Eldridge JA, et al. Modified Phalen’s test as an aid in diagnosing carpal tunnel syndrome. Arthritis Care Res. 2012;64:287-289.
2. Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153-158.
3. National Institute of Neurological Disorders and Stroke. Carpal tunnel syndrome fact sheet. National Institutes of Health. July 2012. Available at http://www.ninds.nih.gov/disorders/carpal_tunnel/detail_carpal_tunnel.htm. Accessed April 15, 2013.
4. McGee SR. Evidence-Based Physical Diagnosis. 3rd ed. Philadelphia, Pa: Saunders; 2012:chap 62.
5. Daniell WE, Fulton-Kehoe D, Franklin GM. Work-related carpal tunnel syndrome in Washington State workers’ compensation: utilization of surgery and the duration of lost work. Am J Ind Med. 2009;52:931-942.
6. D’Arcy CA, McGee S. Does this patient have carpal tunnel syndrome? JAMA. 2000;282:3110-3117.
1. Bilkis S, Loveman DM, Eldridge JA, et al. Modified Phalen’s test as an aid in diagnosing carpal tunnel syndrome. Arthritis Care Res. 2012;64:287-289.
2. Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153-158.
3. National Institute of Neurological Disorders and Stroke. Carpal tunnel syndrome fact sheet. National Institutes of Health. July 2012. Available at http://www.ninds.nih.gov/disorders/carpal_tunnel/detail_carpal_tunnel.htm. Accessed April 15, 2013.
4. McGee SR. Evidence-Based Physical Diagnosis. 3rd ed. Philadelphia, Pa: Saunders; 2012:chap 62.
5. Daniell WE, Fulton-Kehoe D, Franklin GM. Work-related carpal tunnel syndrome in Washington State workers’ compensation: utilization of surgery and the duration of lost work. Am J Ind Med. 2009;52:931-942.
6. D’Arcy CA, McGee S. Does this patient have carpal tunnel syndrome? JAMA. 2000;282:3110-3117.
Copyright © 2013 The Family Physicians Inquiries Network. All rights reserved.
The latest recommendations from the USPSTF
Since the last Practice Alert update on the US Preventive Services Task Force (USPSTF) recommendations,1 the Task Force released 16 final recommendations, through January of this year (TABLE).2 However, none of these were level A recommendations and only 4 were level B. This is significant in that USPSTF level A and B recommendations must now be covered by health insurance plans without patient cost sharing as a result of a clause in the Affordable Care Act. There were 5 D recommendations (recommend against), and some of the tests that fell into this category are in common use. I discuss the B and D recommendations below.
TABLE
Recent recommendations from the USPSTF2
| B recommendations |
The USPSTF recommends:
|
| C recommendations |
The USPSTF recommends against automatically:
|
| D recommendations |
The USPSTF recommends against:
|
| I statements |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of:
|
| For more on the USPSTF’s grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. |
B recommendations
Encourage vitamin D supplementation and regular exercise to prevent falls in elderly
Falls in the elderly are a significant cause of morbidity and mortality. The Task Force found that between 30% and 40% of community-dwelling adults ≥65 years fall each year, and 5% to 10% of those who fall will sustain a fracture, head injury, or laceration.3 Those at highest risk have a history of falls, report mobility problems, have chronic diseases, use psychotropic medications, or have difficulty on a “get up and go” test, which involves rising from a sitting position in an arm chair, walking 10 feet, turning, walking back, and sitting down. If this activity takes more than 10 seconds, the risk of a fall is increased.3
Two interventions were found to be effective in preventing falls: vitamin D supplementation and regular exercise or physical therapy. Vitamin D enhances muscular strength and balance, and supplementation of 800 IU daily for 12 months can decrease the risk of a fall by 17%, with a number needed to treat (NNT) of 10 to prevent one fall.3 Exercise or physical therapy that focuses on gait and balance, strength or resistance training, or general fitness can reduce the risk of falls with an NNT of 16. Individuals who benefit the most are those at higher risk.3
As for multifactorial risk assessment and comprehensive management of risks to prevent falls, a pooled analysis of studies showed that these interventions do little to reduce falls and do not warrant routine use. The Task Force evaluated other interventions—vision correction, medication discontinuation, protein supplementation, education or counseling, and home hazard modification—but could not find sufficient evidence to recommend for or against them.
Screen for obesity in adults
The Task Force reaffirmed its recommendation to screen all adults for obesity and to offer intensive behavioral interventions to those with a body mass index of ≥30 kg/m2. Helpful interventions include multiple behavioral management activities in group or individual sessions; setting weight-loss goals; improving diet or nutrition; physical activity sessions; addressing barriers to change; active use of self-monitoring; and strategizing ways to maintain lifestyle changes. High-intensity programs involve 12 to 26 sessions a year and result, on average, in a reduction of 6% of body weight.4
Counsel fair-skinned patients to minimize sun exposure
The Task Force now recommends counseling fair-skinned children, adolescents, and young adults (10-24 years of age) about reducing their exposure to ultraviolet (UV) radiation. UV radiation exposure occurs when outdoors in the sun, especially in the middle of the day; and when using artificial sources of UV light, such as an indoor tanning bed. Unprotected UV light exposure is a cause of skin cancer, especially when this exposure occurs in childhood or young adulthood.
Behaviors that protect from UV radiation exposure include using broad-spectrum sunscreen with a sun-protection factor of at least 15, wearing hats and protective clothing, avoiding the outdoors during midday hours (10 am-3 pm), and avoiding indoor tanning. Brief counseling offered in a primary care setting can increase protective behaviors in the targeted age group.
UV light exposure in adults is also linked to skin cancer, but the effectiveness of counseling in this population is less certain and the benefit from protective behaviors is less. In addition, almost all studies of skin cancer prevention have been conducted with fair-skinned subjects, so the Task Force limited this recommendation to those who have fair skin and are between the ages of 10 and 24.5
Screen for intimate partner violence
The USPSTF has changed its recommendation on screening women for intimate partner violence (IPV). Previously it said that the evidence was insufficient to make a recommendation. New evidence has since been published and the Task Force recommends that women of childbearing age (14-46 years, with most evidence for those over age 18) be screened using one of 6 screening tools found to have satisfactory performance characteristics.6 IPV means physical, sexual, or psychological abuse by a current or former partner or spouse, among heterosexual or same-sex couples. To learn more, see “Time to routinely screen for intimate partner violence?” (J Fam Pract. 2013;62:90-92).
Services found to be effective in preventing IPV include counseling, home visits, information cards, referrals to community services, and mentoring support provided by physicians or other health professionals.6
The evidence on screening for the prevention of elder abuse and abuse of vulnerable adults still remains insufficient for a recommendation.
D recommendations
No need for prostate cancer screening, or these other interventions
The list of new D recommendations (interventions that have no benefit or that cause more harm than benefit) includes:
- screening for ovarian and prostate cancer
- using estrogen or estrogen combined with progestin in postmenopausal women for the prevention of chronic conditions
- screening with resting or exercise electrocardiography for the prediction of coronary heart disease events in asymptomatic adults at low risk for such events.
The most controversial D recommendation is to avoid measuring prostate-specific antigen (PSA) to screen for prostate cancer. The Task Force has never endorsed use of the PSA test, previously stating that evidence was not of sufficient strength to recommend for or against it in men <75 years and recommending against it for older men. The evidence report conducted for the reconsideration of this topic provided sufficient evidence that the PSA test results in far more harm than benefit.
In February, the USPSTF finalized a recommendation on “Vitamin D and Calcium Supplementation to Prevent Fractures in Adults.” For more information, go to:
http://www.uspreventiveservicestaskforce.org/announcements.htm
The troublesome C recommendation
Proceed with caution with these 2 interventions
The wording of level C recommendations has undergone revision once again. In recognition that some preventive services may benefit select patients—although the overall benefit in the population is small—the USPSTF now states that a C recommendation means that the Task Force “recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences.” This past year, 2 interventions fell into this category: multifactorial risk assessment and management to prevent falls in community dwelling elders, and counseling adults about a healthy diet and exercise to prevent cardiovascular disease (TABLE).2
1. Campos-Outcalt D. The latest recommendations from the USPSTF. J Fam Pract. 2012;61:278-282.
2. USPSTF. Announcements. Available at: http://www.uspreventiveservicestaskforce.org/announcements.htm. Accessed March 6, 2013.
3. USPSTF. Prevention of falls in community dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed March 6, 2013.
4. USPSTF. Screening for and management of obesity in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed March 6, 2013.
5. USPSTF. Behavioral counseling to prevent skin cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/skincancouns/skincancounsrs.htm. Accessed March 6, 2013.
6. USPSTF. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf12/ipvelder/ipvelderfinalrs.htm. Accessed March 6, 2013.
Since the last Practice Alert update on the US Preventive Services Task Force (USPSTF) recommendations,1 the Task Force released 16 final recommendations, through January of this year (TABLE).2 However, none of these were level A recommendations and only 4 were level B. This is significant in that USPSTF level A and B recommendations must now be covered by health insurance plans without patient cost sharing as a result of a clause in the Affordable Care Act. There were 5 D recommendations (recommend against), and some of the tests that fell into this category are in common use. I discuss the B and D recommendations below.
TABLE
Recent recommendations from the USPSTF2
| B recommendations |
The USPSTF recommends:
|
| C recommendations |
The USPSTF recommends against automatically:
|
| D recommendations |
The USPSTF recommends against:
|
| I statements |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of:
|
| For more on the USPSTF’s grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. |
B recommendations
Encourage vitamin D supplementation and regular exercise to prevent falls in elderly
Falls in the elderly are a significant cause of morbidity and mortality. The Task Force found that between 30% and 40% of community-dwelling adults ≥65 years fall each year, and 5% to 10% of those who fall will sustain a fracture, head injury, or laceration.3 Those at highest risk have a history of falls, report mobility problems, have chronic diseases, use psychotropic medications, or have difficulty on a “get up and go” test, which involves rising from a sitting position in an arm chair, walking 10 feet, turning, walking back, and sitting down. If this activity takes more than 10 seconds, the risk of a fall is increased.3
Two interventions were found to be effective in preventing falls: vitamin D supplementation and regular exercise or physical therapy. Vitamin D enhances muscular strength and balance, and supplementation of 800 IU daily for 12 months can decrease the risk of a fall by 17%, with a number needed to treat (NNT) of 10 to prevent one fall.3 Exercise or physical therapy that focuses on gait and balance, strength or resistance training, or general fitness can reduce the risk of falls with an NNT of 16. Individuals who benefit the most are those at higher risk.3
As for multifactorial risk assessment and comprehensive management of risks to prevent falls, a pooled analysis of studies showed that these interventions do little to reduce falls and do not warrant routine use. The Task Force evaluated other interventions—vision correction, medication discontinuation, protein supplementation, education or counseling, and home hazard modification—but could not find sufficient evidence to recommend for or against them.
Screen for obesity in adults
The Task Force reaffirmed its recommendation to screen all adults for obesity and to offer intensive behavioral interventions to those with a body mass index of ≥30 kg/m2. Helpful interventions include multiple behavioral management activities in group or individual sessions; setting weight-loss goals; improving diet or nutrition; physical activity sessions; addressing barriers to change; active use of self-monitoring; and strategizing ways to maintain lifestyle changes. High-intensity programs involve 12 to 26 sessions a year and result, on average, in a reduction of 6% of body weight.4
Counsel fair-skinned patients to minimize sun exposure
The Task Force now recommends counseling fair-skinned children, adolescents, and young adults (10-24 years of age) about reducing their exposure to ultraviolet (UV) radiation. UV radiation exposure occurs when outdoors in the sun, especially in the middle of the day; and when using artificial sources of UV light, such as an indoor tanning bed. Unprotected UV light exposure is a cause of skin cancer, especially when this exposure occurs in childhood or young adulthood.
Behaviors that protect from UV radiation exposure include using broad-spectrum sunscreen with a sun-protection factor of at least 15, wearing hats and protective clothing, avoiding the outdoors during midday hours (10 am-3 pm), and avoiding indoor tanning. Brief counseling offered in a primary care setting can increase protective behaviors in the targeted age group.
UV light exposure in adults is also linked to skin cancer, but the effectiveness of counseling in this population is less certain and the benefit from protective behaviors is less. In addition, almost all studies of skin cancer prevention have been conducted with fair-skinned subjects, so the Task Force limited this recommendation to those who have fair skin and are between the ages of 10 and 24.5
Screen for intimate partner violence
The USPSTF has changed its recommendation on screening women for intimate partner violence (IPV). Previously it said that the evidence was insufficient to make a recommendation. New evidence has since been published and the Task Force recommends that women of childbearing age (14-46 years, with most evidence for those over age 18) be screened using one of 6 screening tools found to have satisfactory performance characteristics.6 IPV means physical, sexual, or psychological abuse by a current or former partner or spouse, among heterosexual or same-sex couples. To learn more, see “Time to routinely screen for intimate partner violence?” (J Fam Pract. 2013;62:90-92).
Services found to be effective in preventing IPV include counseling, home visits, information cards, referrals to community services, and mentoring support provided by physicians or other health professionals.6
The evidence on screening for the prevention of elder abuse and abuse of vulnerable adults still remains insufficient for a recommendation.
D recommendations
No need for prostate cancer screening, or these other interventions
The list of new D recommendations (interventions that have no benefit or that cause more harm than benefit) includes:
- screening for ovarian and prostate cancer
- using estrogen or estrogen combined with progestin in postmenopausal women for the prevention of chronic conditions
- screening with resting or exercise electrocardiography for the prediction of coronary heart disease events in asymptomatic adults at low risk for such events.
The most controversial D recommendation is to avoid measuring prostate-specific antigen (PSA) to screen for prostate cancer. The Task Force has never endorsed use of the PSA test, previously stating that evidence was not of sufficient strength to recommend for or against it in men <75 years and recommending against it for older men. The evidence report conducted for the reconsideration of this topic provided sufficient evidence that the PSA test results in far more harm than benefit.
In February, the USPSTF finalized a recommendation on “Vitamin D and Calcium Supplementation to Prevent Fractures in Adults.” For more information, go to:
http://www.uspreventiveservicestaskforce.org/announcements.htm
The troublesome C recommendation
Proceed with caution with these 2 interventions
The wording of level C recommendations has undergone revision once again. In recognition that some preventive services may benefit select patients—although the overall benefit in the population is small—the USPSTF now states that a C recommendation means that the Task Force “recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences.” This past year, 2 interventions fell into this category: multifactorial risk assessment and management to prevent falls in community dwelling elders, and counseling adults about a healthy diet and exercise to prevent cardiovascular disease (TABLE).2
Since the last Practice Alert update on the US Preventive Services Task Force (USPSTF) recommendations,1 the Task Force released 16 final recommendations, through January of this year (TABLE).2 However, none of these were level A recommendations and only 4 were level B. This is significant in that USPSTF level A and B recommendations must now be covered by health insurance plans without patient cost sharing as a result of a clause in the Affordable Care Act. There were 5 D recommendations (recommend against), and some of the tests that fell into this category are in common use. I discuss the B and D recommendations below.
TABLE
Recent recommendations from the USPSTF2
| B recommendations |
The USPSTF recommends:
|
| C recommendations |
The USPSTF recommends against automatically:
|
| D recommendations |
The USPSTF recommends against:
|
| I statements |
The USPSTF concludes that the current evidence is insufficient to assess the balance of benefits and harms of:
|
| For more on the USPSTF’s grade definitions, see http://www.uspreventiveservicestaskforce.org/uspstf/grades.htm. |
B recommendations
Encourage vitamin D supplementation and regular exercise to prevent falls in elderly
Falls in the elderly are a significant cause of morbidity and mortality. The Task Force found that between 30% and 40% of community-dwelling adults ≥65 years fall each year, and 5% to 10% of those who fall will sustain a fracture, head injury, or laceration.3 Those at highest risk have a history of falls, report mobility problems, have chronic diseases, use psychotropic medications, or have difficulty on a “get up and go” test, which involves rising from a sitting position in an arm chair, walking 10 feet, turning, walking back, and sitting down. If this activity takes more than 10 seconds, the risk of a fall is increased.3
Two interventions were found to be effective in preventing falls: vitamin D supplementation and regular exercise or physical therapy. Vitamin D enhances muscular strength and balance, and supplementation of 800 IU daily for 12 months can decrease the risk of a fall by 17%, with a number needed to treat (NNT) of 10 to prevent one fall.3 Exercise or physical therapy that focuses on gait and balance, strength or resistance training, or general fitness can reduce the risk of falls with an NNT of 16. Individuals who benefit the most are those at higher risk.3
As for multifactorial risk assessment and comprehensive management of risks to prevent falls, a pooled analysis of studies showed that these interventions do little to reduce falls and do not warrant routine use. The Task Force evaluated other interventions—vision correction, medication discontinuation, protein supplementation, education or counseling, and home hazard modification—but could not find sufficient evidence to recommend for or against them.
Screen for obesity in adults
The Task Force reaffirmed its recommendation to screen all adults for obesity and to offer intensive behavioral interventions to those with a body mass index of ≥30 kg/m2. Helpful interventions include multiple behavioral management activities in group or individual sessions; setting weight-loss goals; improving diet or nutrition; physical activity sessions; addressing barriers to change; active use of self-monitoring; and strategizing ways to maintain lifestyle changes. High-intensity programs involve 12 to 26 sessions a year and result, on average, in a reduction of 6% of body weight.4
Counsel fair-skinned patients to minimize sun exposure
The Task Force now recommends counseling fair-skinned children, adolescents, and young adults (10-24 years of age) about reducing their exposure to ultraviolet (UV) radiation. UV radiation exposure occurs when outdoors in the sun, especially in the middle of the day; and when using artificial sources of UV light, such as an indoor tanning bed. Unprotected UV light exposure is a cause of skin cancer, especially when this exposure occurs in childhood or young adulthood.
Behaviors that protect from UV radiation exposure include using broad-spectrum sunscreen with a sun-protection factor of at least 15, wearing hats and protective clothing, avoiding the outdoors during midday hours (10 am-3 pm), and avoiding indoor tanning. Brief counseling offered in a primary care setting can increase protective behaviors in the targeted age group.
UV light exposure in adults is also linked to skin cancer, but the effectiveness of counseling in this population is less certain and the benefit from protective behaviors is less. In addition, almost all studies of skin cancer prevention have been conducted with fair-skinned subjects, so the Task Force limited this recommendation to those who have fair skin and are between the ages of 10 and 24.5
Screen for intimate partner violence
The USPSTF has changed its recommendation on screening women for intimate partner violence (IPV). Previously it said that the evidence was insufficient to make a recommendation. New evidence has since been published and the Task Force recommends that women of childbearing age (14-46 years, with most evidence for those over age 18) be screened using one of 6 screening tools found to have satisfactory performance characteristics.6 IPV means physical, sexual, or psychological abuse by a current or former partner or spouse, among heterosexual or same-sex couples. To learn more, see “Time to routinely screen for intimate partner violence?” (J Fam Pract. 2013;62:90-92).
Services found to be effective in preventing IPV include counseling, home visits, information cards, referrals to community services, and mentoring support provided by physicians or other health professionals.6
The evidence on screening for the prevention of elder abuse and abuse of vulnerable adults still remains insufficient for a recommendation.
D recommendations
No need for prostate cancer screening, or these other interventions
The list of new D recommendations (interventions that have no benefit or that cause more harm than benefit) includes:
- screening for ovarian and prostate cancer
- using estrogen or estrogen combined with progestin in postmenopausal women for the prevention of chronic conditions
- screening with resting or exercise electrocardiography for the prediction of coronary heart disease events in asymptomatic adults at low risk for such events.
The most controversial D recommendation is to avoid measuring prostate-specific antigen (PSA) to screen for prostate cancer. The Task Force has never endorsed use of the PSA test, previously stating that evidence was not of sufficient strength to recommend for or against it in men <75 years and recommending against it for older men. The evidence report conducted for the reconsideration of this topic provided sufficient evidence that the PSA test results in far more harm than benefit.
In February, the USPSTF finalized a recommendation on “Vitamin D and Calcium Supplementation to Prevent Fractures in Adults.” For more information, go to:
http://www.uspreventiveservicestaskforce.org/announcements.htm
The troublesome C recommendation
Proceed with caution with these 2 interventions
The wording of level C recommendations has undergone revision once again. In recognition that some preventive services may benefit select patients—although the overall benefit in the population is small—the USPSTF now states that a C recommendation means that the Task Force “recommends selectively offering or providing this service to individual patients based on professional judgment and patient preferences.” This past year, 2 interventions fell into this category: multifactorial risk assessment and management to prevent falls in community dwelling elders, and counseling adults about a healthy diet and exercise to prevent cardiovascular disease (TABLE).2
1. Campos-Outcalt D. The latest recommendations from the USPSTF. J Fam Pract. 2012;61:278-282.
2. USPSTF. Announcements. Available at: http://www.uspreventiveservicestaskforce.org/announcements.htm. Accessed March 6, 2013.
3. USPSTF. Prevention of falls in community dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed March 6, 2013.
4. USPSTF. Screening for and management of obesity in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed March 6, 2013.
5. USPSTF. Behavioral counseling to prevent skin cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/skincancouns/skincancounsrs.htm. Accessed March 6, 2013.
6. USPSTF. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf12/ipvelder/ipvelderfinalrs.htm. Accessed March 6, 2013.
1. Campos-Outcalt D. The latest recommendations from the USPSTF. J Fam Pract. 2012;61:278-282.
2. USPSTF. Announcements. Available at: http://www.uspreventiveservicestaskforce.org/announcements.htm. Accessed March 6, 2013.
3. USPSTF. Prevention of falls in community dwelling older adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/fallsprevention/fallsprevrs.htm. Accessed March 6, 2013.
4. USPSTF. Screening for and management of obesity in adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/obeseadult/obesers.htm. Accessed March 6, 2013.
5. USPSTF. Behavioral counseling to prevent skin cancer. Available at: http://www.uspreventiveservicestaskforce.org/uspstf11/skincancouns/skincancounsrs.htm. Accessed March 6, 2013.
6. USPSTF. Screening for intimate partner violence and abuse of elderly and vulnerable adults. Available at: http://www.uspreventiveservicestaskforce.org/uspstf12/ipvelder/ipvelderfinalrs.htm. Accessed March 6, 2013.
Unusual shoulder injury from a motorcycle crash
A 38-YEAR-OLD MAN was brought into our emergency department (ED) after driving his motorcycle at high speed into a tree. The patient, who hadn’t been wearing a helmet, was thrown 30 feet. When EMS arrived, the patient was unresponsive, with his right arm in the air. En route, the patient regained consciousness; he appeared intoxicated and became combative.
The patient was evaluated in the ED and his vital signs were normal. His right arm was abducted and over his head (FIGURE 1). He reported significant pain with palpation and attempts at range of motion. We were unable to place the patient’s arm at his side. Other than some minor abrasions, the patient appeared to have no other injuries.
FIGURE 1Right upper extremity on presentation
Routine laboratory tests showed an alcohol level of 0.175 g/dL and urine toxicology was positive for benzodiazepines and tetrahydrocannabinol. A focused assessment with sonography in trauma (FAST) exam was negative. We ordered a right shoulder x-ray and a chest x-ray.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Inferior dislocation of the shoulder
The right shoulder x-ray (FIGURE 2) revealed luxatio erecta—an inferior dislocation of the shoulder. The humeral head was displaced inferiorly with respect to the glenoid fossa and there was an associated greater tuberosity fracture. The chest x-ray demonstrated mild pulmonary contusions.
FIGURE 2
Right shoulder radiograph reveals luxatio erecta with greater tuberosity fracture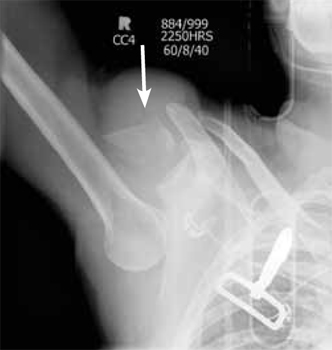
An uncommon dislocation
Inferior shoulder dislocation or luxatio erecta is the least common type of glenohumeral dislocation, comprising only about 0.5% of all shoulder dislocations.1 The 2 other types of shoulder dislocations—anterior and posterior—account for 95% to 97% and 2% to 4% of dislocations, respectively.2
Injury occurs in one of 2 ways, either by a direct or indirect mechanism. A direct dislocation occurs when there is axial loading on an arm that is fully abducted at the shoulder.3 The indirect mechanism, which is more common, is caused by a hyperabduction stress that directs the humeral neck superiorly against the acromion process, forcing the humeral head out of the glenoid fossa inferiorly.2 The indirect mechanism usually occurs when a patient falls and reacts by grasping an object above his or her head, resulting in hyperabduction.
Sometimes, there is no trauma. True inferior dislocations have also been reported in patients with stroke, septic arthritis, and other neuromuscular diseases.4
The presentation is distinctive
Patients with this type of dislocation present with their arm elevated, elbow flexed, and hand behind their head. Due to mechanical entrapment of the humeral head, patients can’t move their arm. The abducted position of the arm may hinder further assessment with computed tomography (CT) for life-threatening injuries, as was the case with our patient.
While an immobile, abducted arm is virtually pathognomonic, radiographs are useful for confirming the diagnosis and assessing for associated fractures. It is essential to obtain anteroposterior, axillary, and Y views.5 Radiographs typically show the shaft of the humerus directed superiorly and parallel to the scapular spine, with the humeral head below the coracoid process or glenoid fossa.3,5
Rotator cuff tears are a common complication
There are a number of complications associated with luxatio erecta. Eighty percent of patients with this injury have either an associated rotator cuff tear or a fracture of the greater tuberosity (which we’ll get to in a bit).3 Magnetic resonance imaging studies have shown rotator cuff injuries to involve the supraspinatus, infraspinatus, and, less frequently, the subscapularis tendon.6 It’s believed that rotator cuff tears may be even more prevalent than reported in the literature since they are often underrecognized at the time of presentation with the dislocation.6
Other complications. Sixty percent of patients report some degree of neurologic dysfunction after the dislocation.5 The most common nerve affected is the axillary, followed by the radial, ulnar, and median nerves.3 These injuries are more likely to occur with associated fractures of the greater tuberosity or axillary artery injuries.7 Symptoms generally resolve after reduction, although there have been cases that have taken up to 6 weeks to resolve.8
Vascular compromise, most commonly occurring as a result of axillary artery injury, has been reported in 3.3% of cases.5 This injury is most common in elderly patients, with 75% of cases occurring in patients older than 60 years.7 It’s been hypothesized that this is due to the loss of arterial elasticity as an individual ages. The most common presenting signs and symptoms include absent radial and/or brachial pulses, severe pain, axillary swelling, axillary masses due to hematoma formation, and neurologic deficits.7 Complications are minimal if diagnosed and treated early.
The most expeditious way to diagnose this complication is to obtain a Doppler ultrasound of the injured extremity. If surgery is indicated, saphenous vein graft has been reported as a successful treatment.3
Fractures are another complication to watch for. The most common fractures are of the greater tuberosity, although fractures to the glenoid, humeral head, acromion, and scapular body have also been reported.8 Fracture management depends on the characteristics of the fracture, including displacement, size of the fragment, and joint stability.
Treatment involves traction and countertraction
Luxatio erecta is normally treated by closed reduction using the traction-countertraction technique. In this maneuver, the shoulder is reduced with direct traction, while countertraction is applied with a sheet wrapped over the clavicle on the affected side and pulled down and across the chest toward the unaffected side. The affected arm is pulled in a cephalad direction and further abducted until the humeral head is reduced within the glenoid fossa. After reduction, the arm is gradually moved downwards toward the patient’s side and splinted in the adducted position.8
Special care should be taken with patients who are at risk of cervical spine injuries. Postreduction radiographs should be obtained to verify proper humeral placement and to assess for any associated fractures. While closed reduction is the definitive treatment, patients run the risk of recurrent instability that may necessitate capsular reconstruction.1
Our patient recovered well
Our patient was sedated with fentanyl and midazolam, and his shoulder was reduced with the traction-countertraction technique described earlier. Postreduction radiographs revealed satisfactory alignment of the right glenohumeral joint and that the greater tuberosity was reduced to within a centimeter of its normal position. No additional fractures were identified.
After the reduction, a head CT scan was done; it revealed a small intracerebral hemorrhage. The patient was admitted overnight and discharged the following day with a sling and swathe and instructions to follow up with orthopedics.
CORRESPONDENCE
Casey Z. MacVane, MD, MPH, Department of Emergency Medicine, Maine Medical Center, 47 Bramhall Street, Portland, ME 04102; [email protected]
1. Groh GI, Wirth MA, Rockwood CA, Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg 2010;19:423-426.
2. Goldstein JR, Eilbert WP. Locked anterior-inferior shoulder subluxation presenting as luxatio erecta. J Emerg Med 2004;27:245-248.
3. Yamamoto T, Yoshiya S, Kurosaka M, et al. Luxatio erecta: a report of 5 cases and a review of the literature. Am J Orthop 2003;32:601-603.
4. Sonanis SV, Das S, Deshmukh N, et al. A true traumatic inferior dislocation of shoulder. Injury 2002;33:842-844.
5. Yanturali S, Aksay E, Holliman CJ, et al. Luxatio erecta: clinical presentation and management in the emergency department. J Emerg Med. 2005;29:85-89.
6. Krug DK, Vinson EN, Helms CA. MRI findings associated with luxatio erecta humeri. Skeletal Radiol. 2010;39:27-33.
7. Plaga BR, Looby P, Feldhaus SJ, et al. Axillary artery injury secondary to inferior shoulder dislocation. J Emerg Med. 2010;39:599-601.
8. Sewecke JJ, Varitimidis SE. Bilateral luxatio erecta: a case report and review of the literature. Am J Orthop. 2006;35:578-580.
A 38-YEAR-OLD MAN was brought into our emergency department (ED) after driving his motorcycle at high speed into a tree. The patient, who hadn’t been wearing a helmet, was thrown 30 feet. When EMS arrived, the patient was unresponsive, with his right arm in the air. En route, the patient regained consciousness; he appeared intoxicated and became combative.
The patient was evaluated in the ED and his vital signs were normal. His right arm was abducted and over his head (FIGURE 1). He reported significant pain with palpation and attempts at range of motion. We were unable to place the patient’s arm at his side. Other than some minor abrasions, the patient appeared to have no other injuries.
FIGURE 1Right upper extremity on presentation
Routine laboratory tests showed an alcohol level of 0.175 g/dL and urine toxicology was positive for benzodiazepines and tetrahydrocannabinol. A focused assessment with sonography in trauma (FAST) exam was negative. We ordered a right shoulder x-ray and a chest x-ray.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Inferior dislocation of the shoulder
The right shoulder x-ray (FIGURE 2) revealed luxatio erecta—an inferior dislocation of the shoulder. The humeral head was displaced inferiorly with respect to the glenoid fossa and there was an associated greater tuberosity fracture. The chest x-ray demonstrated mild pulmonary contusions.
FIGURE 2
Right shoulder radiograph reveals luxatio erecta with greater tuberosity fracture
An uncommon dislocation
Inferior shoulder dislocation or luxatio erecta is the least common type of glenohumeral dislocation, comprising only about 0.5% of all shoulder dislocations.1 The 2 other types of shoulder dislocations—anterior and posterior—account for 95% to 97% and 2% to 4% of dislocations, respectively.2
Injury occurs in one of 2 ways, either by a direct or indirect mechanism. A direct dislocation occurs when there is axial loading on an arm that is fully abducted at the shoulder.3 The indirect mechanism, which is more common, is caused by a hyperabduction stress that directs the humeral neck superiorly against the acromion process, forcing the humeral head out of the glenoid fossa inferiorly.2 The indirect mechanism usually occurs when a patient falls and reacts by grasping an object above his or her head, resulting in hyperabduction.
Sometimes, there is no trauma. True inferior dislocations have also been reported in patients with stroke, septic arthritis, and other neuromuscular diseases.4
The presentation is distinctive
Patients with this type of dislocation present with their arm elevated, elbow flexed, and hand behind their head. Due to mechanical entrapment of the humeral head, patients can’t move their arm. The abducted position of the arm may hinder further assessment with computed tomography (CT) for life-threatening injuries, as was the case with our patient.
While an immobile, abducted arm is virtually pathognomonic, radiographs are useful for confirming the diagnosis and assessing for associated fractures. It is essential to obtain anteroposterior, axillary, and Y views.5 Radiographs typically show the shaft of the humerus directed superiorly and parallel to the scapular spine, with the humeral head below the coracoid process or glenoid fossa.3,5
Rotator cuff tears are a common complication
There are a number of complications associated with luxatio erecta. Eighty percent of patients with this injury have either an associated rotator cuff tear or a fracture of the greater tuberosity (which we’ll get to in a bit).3 Magnetic resonance imaging studies have shown rotator cuff injuries to involve the supraspinatus, infraspinatus, and, less frequently, the subscapularis tendon.6 It’s believed that rotator cuff tears may be even more prevalent than reported in the literature since they are often underrecognized at the time of presentation with the dislocation.6
Other complications. Sixty percent of patients report some degree of neurologic dysfunction after the dislocation.5 The most common nerve affected is the axillary, followed by the radial, ulnar, and median nerves.3 These injuries are more likely to occur with associated fractures of the greater tuberosity or axillary artery injuries.7 Symptoms generally resolve after reduction, although there have been cases that have taken up to 6 weeks to resolve.8
Vascular compromise, most commonly occurring as a result of axillary artery injury, has been reported in 3.3% of cases.5 This injury is most common in elderly patients, with 75% of cases occurring in patients older than 60 years.7 It’s been hypothesized that this is due to the loss of arterial elasticity as an individual ages. The most common presenting signs and symptoms include absent radial and/or brachial pulses, severe pain, axillary swelling, axillary masses due to hematoma formation, and neurologic deficits.7 Complications are minimal if diagnosed and treated early.
The most expeditious way to diagnose this complication is to obtain a Doppler ultrasound of the injured extremity. If surgery is indicated, saphenous vein graft has been reported as a successful treatment.3
Fractures are another complication to watch for. The most common fractures are of the greater tuberosity, although fractures to the glenoid, humeral head, acromion, and scapular body have also been reported.8 Fracture management depends on the characteristics of the fracture, including displacement, size of the fragment, and joint stability.
Treatment involves traction and countertraction
Luxatio erecta is normally treated by closed reduction using the traction-countertraction technique. In this maneuver, the shoulder is reduced with direct traction, while countertraction is applied with a sheet wrapped over the clavicle on the affected side and pulled down and across the chest toward the unaffected side. The affected arm is pulled in a cephalad direction and further abducted until the humeral head is reduced within the glenoid fossa. After reduction, the arm is gradually moved downwards toward the patient’s side and splinted in the adducted position.8
Special care should be taken with patients who are at risk of cervical spine injuries. Postreduction radiographs should be obtained to verify proper humeral placement and to assess for any associated fractures. While closed reduction is the definitive treatment, patients run the risk of recurrent instability that may necessitate capsular reconstruction.1
Our patient recovered well
Our patient was sedated with fentanyl and midazolam, and his shoulder was reduced with the traction-countertraction technique described earlier. Postreduction radiographs revealed satisfactory alignment of the right glenohumeral joint and that the greater tuberosity was reduced to within a centimeter of its normal position. No additional fractures were identified.
After the reduction, a head CT scan was done; it revealed a small intracerebral hemorrhage. The patient was admitted overnight and discharged the following day with a sling and swathe and instructions to follow up with orthopedics.
CORRESPONDENCE
Casey Z. MacVane, MD, MPH, Department of Emergency Medicine, Maine Medical Center, 47 Bramhall Street, Portland, ME 04102; [email protected]
A 38-YEAR-OLD MAN was brought into our emergency department (ED) after driving his motorcycle at high speed into a tree. The patient, who hadn’t been wearing a helmet, was thrown 30 feet. When EMS arrived, the patient was unresponsive, with his right arm in the air. En route, the patient regained consciousness; he appeared intoxicated and became combative.
The patient was evaluated in the ED and his vital signs were normal. His right arm was abducted and over his head (FIGURE 1). He reported significant pain with palpation and attempts at range of motion. We were unable to place the patient’s arm at his side. Other than some minor abrasions, the patient appeared to have no other injuries.
FIGURE 1Right upper extremity on presentation
Routine laboratory tests showed an alcohol level of 0.175 g/dL and urine toxicology was positive for benzodiazepines and tetrahydrocannabinol. A focused assessment with sonography in trauma (FAST) exam was negative. We ordered a right shoulder x-ray and a chest x-ray.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Inferior dislocation of the shoulder
The right shoulder x-ray (FIGURE 2) revealed luxatio erecta—an inferior dislocation of the shoulder. The humeral head was displaced inferiorly with respect to the glenoid fossa and there was an associated greater tuberosity fracture. The chest x-ray demonstrated mild pulmonary contusions.
FIGURE 2
Right shoulder radiograph reveals luxatio erecta with greater tuberosity fracture
An uncommon dislocation
Inferior shoulder dislocation or luxatio erecta is the least common type of glenohumeral dislocation, comprising only about 0.5% of all shoulder dislocations.1 The 2 other types of shoulder dislocations—anterior and posterior—account for 95% to 97% and 2% to 4% of dislocations, respectively.2
Injury occurs in one of 2 ways, either by a direct or indirect mechanism. A direct dislocation occurs when there is axial loading on an arm that is fully abducted at the shoulder.3 The indirect mechanism, which is more common, is caused by a hyperabduction stress that directs the humeral neck superiorly against the acromion process, forcing the humeral head out of the glenoid fossa inferiorly.2 The indirect mechanism usually occurs when a patient falls and reacts by grasping an object above his or her head, resulting in hyperabduction.
Sometimes, there is no trauma. True inferior dislocations have also been reported in patients with stroke, septic arthritis, and other neuromuscular diseases.4
The presentation is distinctive
Patients with this type of dislocation present with their arm elevated, elbow flexed, and hand behind their head. Due to mechanical entrapment of the humeral head, patients can’t move their arm. The abducted position of the arm may hinder further assessment with computed tomography (CT) for life-threatening injuries, as was the case with our patient.
While an immobile, abducted arm is virtually pathognomonic, radiographs are useful for confirming the diagnosis and assessing for associated fractures. It is essential to obtain anteroposterior, axillary, and Y views.5 Radiographs typically show the shaft of the humerus directed superiorly and parallel to the scapular spine, with the humeral head below the coracoid process or glenoid fossa.3,5
Rotator cuff tears are a common complication
There are a number of complications associated with luxatio erecta. Eighty percent of patients with this injury have either an associated rotator cuff tear or a fracture of the greater tuberosity (which we’ll get to in a bit).3 Magnetic resonance imaging studies have shown rotator cuff injuries to involve the supraspinatus, infraspinatus, and, less frequently, the subscapularis tendon.6 It’s believed that rotator cuff tears may be even more prevalent than reported in the literature since they are often underrecognized at the time of presentation with the dislocation.6
Other complications. Sixty percent of patients report some degree of neurologic dysfunction after the dislocation.5 The most common nerve affected is the axillary, followed by the radial, ulnar, and median nerves.3 These injuries are more likely to occur with associated fractures of the greater tuberosity or axillary artery injuries.7 Symptoms generally resolve after reduction, although there have been cases that have taken up to 6 weeks to resolve.8
Vascular compromise, most commonly occurring as a result of axillary artery injury, has been reported in 3.3% of cases.5 This injury is most common in elderly patients, with 75% of cases occurring in patients older than 60 years.7 It’s been hypothesized that this is due to the loss of arterial elasticity as an individual ages. The most common presenting signs and symptoms include absent radial and/or brachial pulses, severe pain, axillary swelling, axillary masses due to hematoma formation, and neurologic deficits.7 Complications are minimal if diagnosed and treated early.
The most expeditious way to diagnose this complication is to obtain a Doppler ultrasound of the injured extremity. If surgery is indicated, saphenous vein graft has been reported as a successful treatment.3
Fractures are another complication to watch for. The most common fractures are of the greater tuberosity, although fractures to the glenoid, humeral head, acromion, and scapular body have also been reported.8 Fracture management depends on the characteristics of the fracture, including displacement, size of the fragment, and joint stability.
Treatment involves traction and countertraction
Luxatio erecta is normally treated by closed reduction using the traction-countertraction technique. In this maneuver, the shoulder is reduced with direct traction, while countertraction is applied with a sheet wrapped over the clavicle on the affected side and pulled down and across the chest toward the unaffected side. The affected arm is pulled in a cephalad direction and further abducted until the humeral head is reduced within the glenoid fossa. After reduction, the arm is gradually moved downwards toward the patient’s side and splinted in the adducted position.8
Special care should be taken with patients who are at risk of cervical spine injuries. Postreduction radiographs should be obtained to verify proper humeral placement and to assess for any associated fractures. While closed reduction is the definitive treatment, patients run the risk of recurrent instability that may necessitate capsular reconstruction.1
Our patient recovered well
Our patient was sedated with fentanyl and midazolam, and his shoulder was reduced with the traction-countertraction technique described earlier. Postreduction radiographs revealed satisfactory alignment of the right glenohumeral joint and that the greater tuberosity was reduced to within a centimeter of its normal position. No additional fractures were identified.
After the reduction, a head CT scan was done; it revealed a small intracerebral hemorrhage. The patient was admitted overnight and discharged the following day with a sling and swathe and instructions to follow up with orthopedics.
CORRESPONDENCE
Casey Z. MacVane, MD, MPH, Department of Emergency Medicine, Maine Medical Center, 47 Bramhall Street, Portland, ME 04102; [email protected]
1. Groh GI, Wirth MA, Rockwood CA, Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg 2010;19:423-426.
2. Goldstein JR, Eilbert WP. Locked anterior-inferior shoulder subluxation presenting as luxatio erecta. J Emerg Med 2004;27:245-248.
3. Yamamoto T, Yoshiya S, Kurosaka M, et al. Luxatio erecta: a report of 5 cases and a review of the literature. Am J Orthop 2003;32:601-603.
4. Sonanis SV, Das S, Deshmukh N, et al. A true traumatic inferior dislocation of shoulder. Injury 2002;33:842-844.
5. Yanturali S, Aksay E, Holliman CJ, et al. Luxatio erecta: clinical presentation and management in the emergency department. J Emerg Med. 2005;29:85-89.
6. Krug DK, Vinson EN, Helms CA. MRI findings associated with luxatio erecta humeri. Skeletal Radiol. 2010;39:27-33.
7. Plaga BR, Looby P, Feldhaus SJ, et al. Axillary artery injury secondary to inferior shoulder dislocation. J Emerg Med. 2010;39:599-601.
8. Sewecke JJ, Varitimidis SE. Bilateral luxatio erecta: a case report and review of the literature. Am J Orthop. 2006;35:578-580.
1. Groh GI, Wirth MA, Rockwood CA, Jr. Results of treatment of luxatio erecta (inferior shoulder dislocation). J Shoulder Elbow Surg 2010;19:423-426.
2. Goldstein JR, Eilbert WP. Locked anterior-inferior shoulder subluxation presenting as luxatio erecta. J Emerg Med 2004;27:245-248.
3. Yamamoto T, Yoshiya S, Kurosaka M, et al. Luxatio erecta: a report of 5 cases and a review of the literature. Am J Orthop 2003;32:601-603.
4. Sonanis SV, Das S, Deshmukh N, et al. A true traumatic inferior dislocation of shoulder. Injury 2002;33:842-844.
5. Yanturali S, Aksay E, Holliman CJ, et al. Luxatio erecta: clinical presentation and management in the emergency department. J Emerg Med. 2005;29:85-89.
6. Krug DK, Vinson EN, Helms CA. MRI findings associated with luxatio erecta humeri. Skeletal Radiol. 2010;39:27-33.
7. Plaga BR, Looby P, Feldhaus SJ, et al. Axillary artery injury secondary to inferior shoulder dislocation. J Emerg Med. 2010;39:599-601.
8. Sewecke JJ, Varitimidis SE. Bilateral luxatio erecta: a case report and review of the literature. Am J Orthop. 2006;35:578-580.
Alcoholism? Ask this
I read with interest Dr. Vinson’s approach to alcohol abuse (Patient abusing alcohol or drugs? Help starts with a single question. J Fam Pract. 2013;62:63-69).
For the past 3 years I have often used my residency director’s approach to patients with substance abuse issues. It, too, involves a single question.
Once I’ve established a rapport with a patient who I suspect has a chronic alcohol problem, I simply ask: “How long have you been an alcoholic?” This establishes that we’re both aware of the patient’s alcoholism and that I now want to establish the duration of the abuse.
Israel Wojnowich, MD
St. Petersburg, Fla
I read with interest Dr. Vinson’s approach to alcohol abuse (Patient abusing alcohol or drugs? Help starts with a single question. J Fam Pract. 2013;62:63-69).
For the past 3 years I have often used my residency director’s approach to patients with substance abuse issues. It, too, involves a single question.
Once I’ve established a rapport with a patient who I suspect has a chronic alcohol problem, I simply ask: “How long have you been an alcoholic?” This establishes that we’re both aware of the patient’s alcoholism and that I now want to establish the duration of the abuse.
Israel Wojnowich, MD
St. Petersburg, Fla
I read with interest Dr. Vinson’s approach to alcohol abuse (Patient abusing alcohol or drugs? Help starts with a single question. J Fam Pract. 2013;62:63-69).
For the past 3 years I have often used my residency director’s approach to patients with substance abuse issues. It, too, involves a single question.
Once I’ve established a rapport with a patient who I suspect has a chronic alcohol problem, I simply ask: “How long have you been an alcoholic?” This establishes that we’re both aware of the patient’s alcoholism and that I now want to establish the duration of the abuse.
Israel Wojnowich, MD
St. Petersburg, Fla