User login
For MD-IQ use only
For Richer, for Poorer: Low-Carb Diets Work for All Incomes
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
For 3 years, Ajala Efem’s type 2 diabetes was so poorly controlled that her blood sugar often soared northward of 500 mg/dL despite insulin shots three to five times a day. She would experience dizziness, vomiting, severe headaches, and the neuropathy in her feet made walking painful. She was also — literally — frothing at the mouth. The 47-year-old single mother of two adult children with mental disabilities feared that she would die.
Ms. Efem lives in the South Bronx, which is among the poorest areas of New York City, where the combined rate of prediabetes and diabetes is close to 30%, the highest rate of any borough in the city.
She had to wait 8 months for an appointment with an endocrinologist, but that visit proved to be life-changing. She lost 28 pounds and got off 15 medications in a single month. She did not join a gym or count calories; she simply changed the food she ate and adopted a low-carb diet.
“I went from being sick to feeling so great,” she told her endocrinologist recently: “My feet aren’t hurting; I’m not in pain; I’m eating as much as I want, and I really enjoy my food so much.”
Ms. Efem’s life-changing visit was with Mariela Glandt, MD, at the offices of Essen Health Care. One month earlier, Dr. Glandt’s company, OwnaHealth, was contracted by Essen to conduct a 100-person pilot program for endocrinology patients. Essen is the largest Medicaid provider in New York City, and “they were desperate for an endocrinologist,” said Dr. Glandt, who trained at Columbia University in New York. So she came — all the way from Madrid, Spain. She commutes monthly, staying for a week each visit.
Dr. Glandt keeps up this punishing schedule because, as she explains, “it’s such a high for me to see these incredible transformations.” Her mostly Black and Hispanic patients are poor and lack resources, yet they lose significant amounts of weight, and their health issues resolve.
“Food is medicine” is an idea very much in vogue. The concept was central to the landmark White House Conference on Hunger, Nutrition, and Health in 2022 and is now the focus of a number of a wide range of government programs. Recently, the Senate held a hearing aimed at further expanding food as medicine programs.
Still, only a single randomized controlled clinical trial has been conducted on this nutritional approach, with unexpectedly disappointing results. In the mid-Atlantic region, 456 food-insecure adults with type 2 diabetes were randomly assigned to usual care or the provision of weekly groceries for their entire families for about 1 year. Provisions for a Mediterranean-style diet included whole grains, fruits and vegetables, lean protein, low-fat dairy products, cereal, brown rice, and bread. In addition, participants received dietary consultations. Yet, those who got free food and coaching did not see improvements in their average blood sugar (the study’s primary outcome), and their low-density lipoprotein (LDL) cholesterol and high-density lipoprotein (HDL) cholesterol levels appeared to have worsened.
“To be honest, I was surprised,” the study’s lead author, Joseph Doyle, PhD, professor at the Sloan School of Management at MIT in Cambridge, Massachusetts, told me. “I was hoping we would show improved outcomes, but the way to make progress is to do well-randomized trials to find out what works.”
I was not surprised by these results because a recent rigorous systematic review and meta-analysis in The BMJ did not show a Mediterranean-style diet to be the most effective for glycemic control. And Ms. Efem was not in fact following a Mediterranean-style diet.
Ms. Efem’s low-carb success story is anecdotal, but Dr. Glandt has an established track record from her 9 years’ experience as the medical director of the eponymous diabetes center she founded in Tel Aviv. A recent audit of 344 patients from the center found that after 6 months of following a very low–carbohydrate diet, 96.3% of those with diabetes saw their A1c fall from a median 7.6% to 6.3%. Weight loss was significant, with a median drop of 6.5 kg (14 pounds) for patients with diabetes and 5.7 kg for those with prediabetes. The diet comprises 5%-10% of calories from carbs, but Dr. Glandt does not use numeric targets with her patients.
Blood pressure, triglycerides, and liver enzymes also improved. And though LDL cholesterol went up by 8%, this result may have been offset by an accompanying 13% rise in HDL cholesterol. Of the 78 patients initially on insulin, 62 were able to stop this medication entirely.
Although these results aren’t from a clinical trial, they’re still highly meaningful because the current dietary standard of care for type 2 diabetes can only slow the progression of the disease, not cause remission. Indeed, the idea that type 2 diabetes could be put into remission was not seriously considered by the American Diabetes Association (ADA) until 2009. By 2019, an ADA report concluded that “[r]educing overall carbohydrate intake for individuals with diabetes has demonstrated the most evidence for improving glycemia.” In other words, the best way to improve the key factor in diabetes is to reduce total carbohydrates. Yet, the ADA still advocates filling one quarter of one’s plate with carbohydrate-based foods, an amount that will prevent remission. Given that the ADA’s vision statement is “a life free of diabetes,” it seems negligent not to tell people with a deadly condition that they can reverse this diagnosis.
A 2023 meta-analysis of 42 controlled clinical trials on 4809 patients showed that a very low–carbohydrate ketogenic diet (keto) was “superior” to alternatives for glycemic control. A more recent review of 11 clinical trials found that this diet was equal but not superior to other nutritional approaches in terms of blood sugar control, but this review also concluded that keto led to greater increases in HDL cholesterol and lower triglycerides.
Dr. Glandt’s patients in the Bronx might not seem like obvious low-carb candidates. The diet is considered expensive and difficult to sustain. My interviews with a half dozen patients revealed some of these difficulties, but even for a woman living in a homeless shelter, the obstacles are not insurmountable.
Jerrilyn, who preferred that I use only her first name, lives in a shelter in Queens. While we strolled through a nearby park, she told me about her desire to lose weight and recover from polycystic ovary syndrome, which terrified her because it had caused dramatic hair loss. When she landed in Dr. Glandt’s office at age 28, she weighed 180 pounds.
Less than 5 months later, Jerrilyn had lost 25 pounds, and her period had returned with some regularity. She said she used “food stamps,” known as the Supplemental Nutrition Assistance Program (SNAP), to buy most of her food at local delis because the meals served at the shelter were too heavy in starches. She starts her day with eggs, turkey bacon, and avocado.
“It was hard to give up carbohydrates because in my culture [Latina], we have nothing but carbs: rice, potatoes, yuca,” Jerrilyn shared. She noticed that carbs make her hungrier, but after 3 days of going low-carb, her cravings diminished. “It was like getting over an addiction,” she said.
Jerrilyn told me she’d seen many doctors but none as involved as Dr. Glandt. “It feels awesome to know that I have a lot of really useful information coming from her all the time.” The OwnaHealth app tracks weight, blood pressure, blood sugar, ketones, meals, mood, and cravings. Patients wear continuous glucose monitors and enter other information manually. Ketone bodies are used to measure dietary adherence and are obtained through finger pricks and test strips provided by OwnaHealth. Dr. Glandt gives patients her own food plan, along with free visual guides to low-carbohydrate foods by dietdoctor.com.
Dr. Glandt also sends her patients for regular blood work. She says she does not frequently see a rise in LDL cholesterol, which can sometimes occur on a low-carbohydrate diet. This effect is most common among people who are lean and fit. She says she doesn’t discontinue statins unless cholesterol levels improve significantly.
Samuel Gonzalez, age 56, weighed 275 pounds when he walked into Dr. Glandt’s office this past November. His A1c was 9.2%, but none of his previous doctors had diagnosed him with diabetes. “I was like a walking bag of sugar!” he joked.
A low-carbohydrate diet seemed absurd to a Puerto Rican like himself: “Having coffee without sugar? That’s like sacrilegious in my culture!” exclaimed Mr. Gonzalez. Still, he managed, with SNAP, to cook eggs and bacon for breakfast and some kind of protein for dinner. He keeps lunch light, “like tuna fish,” and finds checking in with the OwnaHealth app to be very helpful. “Every day, I’m on it,” he said. In the past 7 months, he’s lost 50 pounds, normalized his cholesterol and blood pressure levels, and lowered his A1c to 5.5%.
Mr. Gonzalez gets disability payments due to a back injury, and Ms. Efem receives government payments because her husband died serving in the military. Ms. Efem says her new diet challenges her budget, but Mr. Gonzalez says he manages easily.
Mélissa Cruz, a 28-year-old studying to be a nail technician while also doing back office work at a physical therapy practice, says she’s stretched thin. “I end up sad because I can’t put energy into looking up recipes and cooking for me and my boyfriend,” she told me. She’ll often cook rice and plantains for him and meat for herself, but “it’s frustrating when I’m low on funds and can’t figure out what to eat.”
Low-carbohydrate diets have a reputation for being expensive because people often start eating pricier foods, like meat and cheese, to replace cheaper starchy foods such as pasta and rice.
A 2019 cost analysis published in Nutrition & Dietetics compared a low-carbohydrate dietary pattern with the New Zealand government’s recommended guidelines (which are almost identical to those in the United States) and found that it cost only an extra $1.27 in US dollars per person per day. One explanation is that protein and fat are more satiating than carbohydrates, so people who mostly consume these macronutrients often cut back on snacks like packaged chips, crackers, and even fruits. Also, those on a ketogenic diet usually cut down on medications, so the additional $1.27 daily is likely offset by reduced spending at the pharmacy.
It’s not just Bronx residents with low socioeconomic status (SES) who adapt well to low-carbohydrate diets. Among Alabama state employees with diabetes enrolled in a low-carbohydrate dietary program provided by a company called Virta, the low SES population had the best outcomes. Virta also published survey data in 2023 showing that participants in a program with the Veteran’s Administration did not find additional costs to be an obstacle to dietary adherence. In fact, some participants saw cost reductions due to decreased spending on processed snacks and fast foods.
Ms. Cruz told me she struggles financially, yet she’s still lost nearly 30 pounds in 5 months, and her A1c went from 7.1% down to 5.9%, putting her diabetes into remission. Equally motivating for her are the improvements she’s seen in other hormonal issues. Since childhood, she’s had acanthosis, a condition that causes the skin to darken in velvety patches, and more recently, she developed severe hirsutism to the point of growing sideburns. “I had tried going vegan and fasting, but these just weren’t sustainable for me, and I was so overwhelmed with counting calories all the time.” Now, on a low-carbohydrate diet, which doesn’t require calorie counting, she’s finally seeing both these conditions improve significantly.
When I last checked in with Ms. Cruz, she said she had “kind of ghosted” Dr. Glandt due to her work and school constraints, but she hadn’t abandoned the diet. She appreciated, too, that Dr. Glandt had not given up on her and kept calling and messaging. “She’s not at all like a typical doctor who would just tell me to lose weight and shake their head at me,” Ms. Cruz said.
Because Dr. Glandt’s approach is time-intensive and high-touch, it might seem impractical to scale up, but Dr. Glandt’s app uses artificial intelligence to help with communications thus allowing her, with help from part-time health coaches, to care for patients.
This early success in one of the United States’ poorest and sickest neighborhoods should give us hope that type 2 diabetes need not to be a progressive irreversible disease, even among the disadvantaged.
OwnaHealth’s track record, along with that of Virta and other similar low-carbohydrate medical practices also give hope to the food-is-medicine idea. Diabetes can go into remission, and people can be healed, provided that health practitioners prescribe the right foods. And in truth, it’s not a diet. It’s a way of eating that must be maintained. The sustainability of low-carbohydrate diets has been a point of contention, but the Virta trial, with 38% of patients sustaining remission at 2 years, showed that it’s possible. (OwnaHealth, for its part, offers long-term maintenance plans to help patients stay very low-carb permanently.)
Given the tremendous costs and health burden of diabetes, this approach should no doubt be the first line of treatment for doctors and the ADA. The past two decades of clinical trial research have demonstrated that remission of type 2 diabetes is possible through diet alone. It turns out that for metabolic diseases, only certain foods are truly medicine.
Tools and Tips for Clinicians:
- Free two-page keto starter’s guide by OwnaHealth; Dr. Glandt uses this guide with her patients.
- Illustrated low-carb guides by dietdoctor.com
- Free low-carbohydrate starter guide by the Michigan Collaborative for Type 2 Diabetes
- Low-Carb for Any Budget, a free digital booklet by Mark Cucuzzella, MD, and Kristie Sullivan, PhD
- Recipe and meal ideas from Ruled.me, Keto-Mojo.com, and
Dr. Teicholz is the founder of Nutrition Coalition, an independent nonprofit dedicated to ensuring that US dietary guidelines align with current science. She disclosed receiving book royalties from The Big Fat Surprise, and received honorarium not exceeding $2000 for speeches from various sources.
A version of this article appeared on Medscape.com.
In Some Patients, Antiseizure Medications Can Cause Severe Skin Reactions
according to authors of a recent review. And if putting higher-risk patients on drugs most associated with human leukocyte antigen (HLA)–related reaction risk before test results are available, authors advised starting at low doses and titrating slowly.
“When someone is having a seizure drug prescribed,” said senior author Ram Mani, MD, MSCE, chief of epilepsy at Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey, “it’s often a tense clinical situation because the patient has either had the first few seizures of their life, or they’ve had a worsening in their seizures.”
To help physicians optimize choices, Dr. Mani and colleagues reviewed literature regarding 31 ASMs. Their study was published in Current Treatment Options in Neurology.
Overall, said Dr. Mani, incidence of benign skin reactions such as morbilliform exanthematous eruptions, which account for 95% of cutaneous adverse drug reactions (CADRs), ranges from a few percent up to 15%. “It’s a somewhat common occurrence. Fortunately, the reactions that can lead to morbidity and mortality are fairly rare.”
Severe Cutaneous Adverse Reactions
Among the five ASMs approved by the Food and Drug Administration since 2018, cenobamate has sparked the greatest concern. In early clinical development for epilepsy, a fast titration schedule (starting at 50 mg/day and increasing by 50 mg every 2 weeks to at least 200 mg/day) resulted in three cases of drug reaction with eosinophilia and systemic symptoms (DRESS, also called drug-induced hypersensitivity reaction/DIHS), including one fatal case. Based on a phase 3 trial, the drug’s manufacturer now recommends starting at 12.5 mg and titrating more slowly.
DRESS/DIHS appears within 2-6 weeks of drug exposure. Along with malaise, fever, and conjunctivitis, symptoms can include skin eruptions ranging from morbilliform to hemorrhagic and bullous. “Facial edema and early facial rash are classic findings,” the authors added. DRESS also can involve painful lymphadenopathy and potentially life-threatening damage to the liver, heart, and other organs.
Stevens-Johnson syndrome (SJS), which is characterized by detached skin measuring less than 10% of the entire body surface area, typically happens within the first month of drug exposure. Flu-like symptoms can appear 1-3 days before erythematous to dusky macules, commonly on the chest, as well as cutaneous and mucosal erosions. Along with the skin and conjunctiva, SJS can affect the eyes, lungs, liver, bone marrow, and gastrointestinal tract.
When patients present with possible DRESS or SJS, the authors recommended inpatient multidisciplinary care. Having ready access to blood tests can help assess severity and prognosis, Dr. Mani explained. Inpatient evaluation and treatment also may allow faster access to other specialists as needed, and monitoring of potential seizure exacerbation in patients with uncontrolled seizures for whom the drug provided benefit but required abrupt discontinuation.
Often, he added, all hope is not lost for future use of the medication after a minor skin reaction. A case series and literature review of mild lamotrigine-associated CADRs showed that most patients could reintroduce and titrate lamotrigine by waiting at least 4 weeks, beginning at 5 mg/day, and gradually increasing to 25 mg/day.
Identifying Those at Risk
With millions of patients being newly prescribed ASMs annually, accurately screening out all people at risk of severe cutaneous adverse reactions based on available genetic information is impossible. The complexity of evolving recommendations for HLA testing makes them hard to remember, Dr. Mani said. “Development and better use of clinical decision support systems can help.”
Accordingly, he starts with a thorough history and physical examination, inquiring about prior skin reactions or hypersensitivity, which are risk factors for future reactions to drugs such as carbamazepine, phenytoin, phenobarbital, oxcarbazepine, lamotrigine, rufinamide, and zonisamide. “Most of the medicines that the HLA tests are being done for are not the initial medicines I typically prescribe for a patient with newly diagnosed epilepsy,” said Dr. Mani. For ASM-naive patients with moderate or high risk of skin hypersensitivity reactions, he usually starts with lacosamide, levetiracetam, or brivaracetam. Additional low-risk drugs he considers in more complex cases include valproate, topiramate, and clobazam.
Only if a patient’s initial ASM causes problems will Dr. Mani consider higher-risk options and order HLA tests for patients belonging to indicated groups — such as testing for HLA-B*15:02 in Asian patients being considered for carbamazepine. About once weekly, he must put a patient on a potentially higher-risk drug before test results are available. If after a thorough risk-benefit discussion, he and the patient agree that the higher-risk drug is warranted, Dr. Mani starts at a lower-than-labeled dose, with a slower titration schedule that typically extends the ramp-up period by 1 week.
Fortunately, Dr. Mani said that, in 20 years of practice, he has seen more misdiagnoses — involving rashes from poison ivy, viral infections, or allergies — than actual ASM-induced reactions. “That’s why the patient, family, and practitioner need to be open-minded about what could be causing the rash.”
Dr. Mani reported no relevant conflicts. The study authors reported no funding sources.
according to authors of a recent review. And if putting higher-risk patients on drugs most associated with human leukocyte antigen (HLA)–related reaction risk before test results are available, authors advised starting at low doses and titrating slowly.
“When someone is having a seizure drug prescribed,” said senior author Ram Mani, MD, MSCE, chief of epilepsy at Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey, “it’s often a tense clinical situation because the patient has either had the first few seizures of their life, or they’ve had a worsening in their seizures.”
To help physicians optimize choices, Dr. Mani and colleagues reviewed literature regarding 31 ASMs. Their study was published in Current Treatment Options in Neurology.
Overall, said Dr. Mani, incidence of benign skin reactions such as morbilliform exanthematous eruptions, which account for 95% of cutaneous adverse drug reactions (CADRs), ranges from a few percent up to 15%. “It’s a somewhat common occurrence. Fortunately, the reactions that can lead to morbidity and mortality are fairly rare.”
Severe Cutaneous Adverse Reactions
Among the five ASMs approved by the Food and Drug Administration since 2018, cenobamate has sparked the greatest concern. In early clinical development for epilepsy, a fast titration schedule (starting at 50 mg/day and increasing by 50 mg every 2 weeks to at least 200 mg/day) resulted in three cases of drug reaction with eosinophilia and systemic symptoms (DRESS, also called drug-induced hypersensitivity reaction/DIHS), including one fatal case. Based on a phase 3 trial, the drug’s manufacturer now recommends starting at 12.5 mg and titrating more slowly.
DRESS/DIHS appears within 2-6 weeks of drug exposure. Along with malaise, fever, and conjunctivitis, symptoms can include skin eruptions ranging from morbilliform to hemorrhagic and bullous. “Facial edema and early facial rash are classic findings,” the authors added. DRESS also can involve painful lymphadenopathy and potentially life-threatening damage to the liver, heart, and other organs.
Stevens-Johnson syndrome (SJS), which is characterized by detached skin measuring less than 10% of the entire body surface area, typically happens within the first month of drug exposure. Flu-like symptoms can appear 1-3 days before erythematous to dusky macules, commonly on the chest, as well as cutaneous and mucosal erosions. Along with the skin and conjunctiva, SJS can affect the eyes, lungs, liver, bone marrow, and gastrointestinal tract.
When patients present with possible DRESS or SJS, the authors recommended inpatient multidisciplinary care. Having ready access to blood tests can help assess severity and prognosis, Dr. Mani explained. Inpatient evaluation and treatment also may allow faster access to other specialists as needed, and monitoring of potential seizure exacerbation in patients with uncontrolled seizures for whom the drug provided benefit but required abrupt discontinuation.
Often, he added, all hope is not lost for future use of the medication after a minor skin reaction. A case series and literature review of mild lamotrigine-associated CADRs showed that most patients could reintroduce and titrate lamotrigine by waiting at least 4 weeks, beginning at 5 mg/day, and gradually increasing to 25 mg/day.
Identifying Those at Risk
With millions of patients being newly prescribed ASMs annually, accurately screening out all people at risk of severe cutaneous adverse reactions based on available genetic information is impossible. The complexity of evolving recommendations for HLA testing makes them hard to remember, Dr. Mani said. “Development and better use of clinical decision support systems can help.”
Accordingly, he starts with a thorough history and physical examination, inquiring about prior skin reactions or hypersensitivity, which are risk factors for future reactions to drugs such as carbamazepine, phenytoin, phenobarbital, oxcarbazepine, lamotrigine, rufinamide, and zonisamide. “Most of the medicines that the HLA tests are being done for are not the initial medicines I typically prescribe for a patient with newly diagnosed epilepsy,” said Dr. Mani. For ASM-naive patients with moderate or high risk of skin hypersensitivity reactions, he usually starts with lacosamide, levetiracetam, or brivaracetam. Additional low-risk drugs he considers in more complex cases include valproate, topiramate, and clobazam.
Only if a patient’s initial ASM causes problems will Dr. Mani consider higher-risk options and order HLA tests for patients belonging to indicated groups — such as testing for HLA-B*15:02 in Asian patients being considered for carbamazepine. About once weekly, he must put a patient on a potentially higher-risk drug before test results are available. If after a thorough risk-benefit discussion, he and the patient agree that the higher-risk drug is warranted, Dr. Mani starts at a lower-than-labeled dose, with a slower titration schedule that typically extends the ramp-up period by 1 week.
Fortunately, Dr. Mani said that, in 20 years of practice, he has seen more misdiagnoses — involving rashes from poison ivy, viral infections, or allergies — than actual ASM-induced reactions. “That’s why the patient, family, and practitioner need to be open-minded about what could be causing the rash.”
Dr. Mani reported no relevant conflicts. The study authors reported no funding sources.
according to authors of a recent review. And if putting higher-risk patients on drugs most associated with human leukocyte antigen (HLA)–related reaction risk before test results are available, authors advised starting at low doses and titrating slowly.
“When someone is having a seizure drug prescribed,” said senior author Ram Mani, MD, MSCE, chief of epilepsy at Rutgers Robert Wood Johnson Medical School in New Brunswick, New Jersey, “it’s often a tense clinical situation because the patient has either had the first few seizures of their life, or they’ve had a worsening in their seizures.”
To help physicians optimize choices, Dr. Mani and colleagues reviewed literature regarding 31 ASMs. Their study was published in Current Treatment Options in Neurology.
Overall, said Dr. Mani, incidence of benign skin reactions such as morbilliform exanthematous eruptions, which account for 95% of cutaneous adverse drug reactions (CADRs), ranges from a few percent up to 15%. “It’s a somewhat common occurrence. Fortunately, the reactions that can lead to morbidity and mortality are fairly rare.”
Severe Cutaneous Adverse Reactions
Among the five ASMs approved by the Food and Drug Administration since 2018, cenobamate has sparked the greatest concern. In early clinical development for epilepsy, a fast titration schedule (starting at 50 mg/day and increasing by 50 mg every 2 weeks to at least 200 mg/day) resulted in three cases of drug reaction with eosinophilia and systemic symptoms (DRESS, also called drug-induced hypersensitivity reaction/DIHS), including one fatal case. Based on a phase 3 trial, the drug’s manufacturer now recommends starting at 12.5 mg and titrating more slowly.
DRESS/DIHS appears within 2-6 weeks of drug exposure. Along with malaise, fever, and conjunctivitis, symptoms can include skin eruptions ranging from morbilliform to hemorrhagic and bullous. “Facial edema and early facial rash are classic findings,” the authors added. DRESS also can involve painful lymphadenopathy and potentially life-threatening damage to the liver, heart, and other organs.
Stevens-Johnson syndrome (SJS), which is characterized by detached skin measuring less than 10% of the entire body surface area, typically happens within the first month of drug exposure. Flu-like symptoms can appear 1-3 days before erythematous to dusky macules, commonly on the chest, as well as cutaneous and mucosal erosions. Along with the skin and conjunctiva, SJS can affect the eyes, lungs, liver, bone marrow, and gastrointestinal tract.
When patients present with possible DRESS or SJS, the authors recommended inpatient multidisciplinary care. Having ready access to blood tests can help assess severity and prognosis, Dr. Mani explained. Inpatient evaluation and treatment also may allow faster access to other specialists as needed, and monitoring of potential seizure exacerbation in patients with uncontrolled seizures for whom the drug provided benefit but required abrupt discontinuation.
Often, he added, all hope is not lost for future use of the medication after a minor skin reaction. A case series and literature review of mild lamotrigine-associated CADRs showed that most patients could reintroduce and titrate lamotrigine by waiting at least 4 weeks, beginning at 5 mg/day, and gradually increasing to 25 mg/day.
Identifying Those at Risk
With millions of patients being newly prescribed ASMs annually, accurately screening out all people at risk of severe cutaneous adverse reactions based on available genetic information is impossible. The complexity of evolving recommendations for HLA testing makes them hard to remember, Dr. Mani said. “Development and better use of clinical decision support systems can help.”
Accordingly, he starts with a thorough history and physical examination, inquiring about prior skin reactions or hypersensitivity, which are risk factors for future reactions to drugs such as carbamazepine, phenytoin, phenobarbital, oxcarbazepine, lamotrigine, rufinamide, and zonisamide. “Most of the medicines that the HLA tests are being done for are not the initial medicines I typically prescribe for a patient with newly diagnosed epilepsy,” said Dr. Mani. For ASM-naive patients with moderate or high risk of skin hypersensitivity reactions, he usually starts with lacosamide, levetiracetam, or brivaracetam. Additional low-risk drugs he considers in more complex cases include valproate, topiramate, and clobazam.
Only if a patient’s initial ASM causes problems will Dr. Mani consider higher-risk options and order HLA tests for patients belonging to indicated groups — such as testing for HLA-B*15:02 in Asian patients being considered for carbamazepine. About once weekly, he must put a patient on a potentially higher-risk drug before test results are available. If after a thorough risk-benefit discussion, he and the patient agree that the higher-risk drug is warranted, Dr. Mani starts at a lower-than-labeled dose, with a slower titration schedule that typically extends the ramp-up period by 1 week.
Fortunately, Dr. Mani said that, in 20 years of practice, he has seen more misdiagnoses — involving rashes from poison ivy, viral infections, or allergies — than actual ASM-induced reactions. “That’s why the patient, family, and practitioner need to be open-minded about what could be causing the rash.”
Dr. Mani reported no relevant conflicts. The study authors reported no funding sources.
FROM CURRENT TREATMENT OPTIONS IN NEUROLOGY
Study Estimates Global Prevalence of Seborrheic Dermatitis
TOPLINE:
, according to a meta-analysis that also found a higher prevalence in adults than in children.
METHODOLOGY:
- Researchers conducted a meta-analysis of 121 studies, which included 1,260,163 people with clinician-diagnosed seborrheic dermatitis.
- The included studies represented nine countries; most were from India (n = 18), Turkey (n = 13), and the United States (n = 8).
- The primary outcome was the pooled prevalence of seborrheic dermatitis.
TAKEAWAY:
- The overall pooled prevalence of seborrheic dermatitis was 4.38%, 4.08% in clinical settings, and 4.71% in the studies conducted in the general population.
- The prevalence of seborrheic dermatitis was higher among adults (5.64%) than in children (3.7%) and neonates (0.23%).
- A significant variation was observed across countries, with South Africa having the highest prevalence at 8.82%, followed by the United States at 5.86% and Turkey at 3.74%, while India had the lowest prevalence at 2.62%.
IN PRACTICE:
The global prevalence in this meta-analysis was “higher than previous large-scale global estimates, with notable geographic and sociodemographic variability, highlighting the potential impact of environmental factors and cultural practices,” the authors wrote.
SOURCE:
The study was led by Meredith Tyree Polaskey, MS, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, and was published online on July 3, 2024, in the JAMA Dermatology.
LIMITATIONS:
Interpretation of the findings is limited by research gaps in Central Asia, much of Sub-Saharan Africa, Eastern Europe, Southeast Asia, Latin America (excluding Brazil), and the Caribbean, along with potential underreporting in regions with restricted healthcare access and significant heterogeneity across studies.
DISCLOSURES:
Funding information was not available. One author reported serving as an advisor, consultant, speaker, and/or investigator for multiple pharmaceutical companies, including AbbVie, Amgen, and Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a meta-analysis that also found a higher prevalence in adults than in children.
METHODOLOGY:
- Researchers conducted a meta-analysis of 121 studies, which included 1,260,163 people with clinician-diagnosed seborrheic dermatitis.
- The included studies represented nine countries; most were from India (n = 18), Turkey (n = 13), and the United States (n = 8).
- The primary outcome was the pooled prevalence of seborrheic dermatitis.
TAKEAWAY:
- The overall pooled prevalence of seborrheic dermatitis was 4.38%, 4.08% in clinical settings, and 4.71% in the studies conducted in the general population.
- The prevalence of seborrheic dermatitis was higher among adults (5.64%) than in children (3.7%) and neonates (0.23%).
- A significant variation was observed across countries, with South Africa having the highest prevalence at 8.82%, followed by the United States at 5.86% and Turkey at 3.74%, while India had the lowest prevalence at 2.62%.
IN PRACTICE:
The global prevalence in this meta-analysis was “higher than previous large-scale global estimates, with notable geographic and sociodemographic variability, highlighting the potential impact of environmental factors and cultural practices,” the authors wrote.
SOURCE:
The study was led by Meredith Tyree Polaskey, MS, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, and was published online on July 3, 2024, in the JAMA Dermatology.
LIMITATIONS:
Interpretation of the findings is limited by research gaps in Central Asia, much of Sub-Saharan Africa, Eastern Europe, Southeast Asia, Latin America (excluding Brazil), and the Caribbean, along with potential underreporting in regions with restricted healthcare access and significant heterogeneity across studies.
DISCLOSURES:
Funding information was not available. One author reported serving as an advisor, consultant, speaker, and/or investigator for multiple pharmaceutical companies, including AbbVie, Amgen, and Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a meta-analysis that also found a higher prevalence in adults than in children.
METHODOLOGY:
- Researchers conducted a meta-analysis of 121 studies, which included 1,260,163 people with clinician-diagnosed seborrheic dermatitis.
- The included studies represented nine countries; most were from India (n = 18), Turkey (n = 13), and the United States (n = 8).
- The primary outcome was the pooled prevalence of seborrheic dermatitis.
TAKEAWAY:
- The overall pooled prevalence of seborrheic dermatitis was 4.38%, 4.08% in clinical settings, and 4.71% in the studies conducted in the general population.
- The prevalence of seborrheic dermatitis was higher among adults (5.64%) than in children (3.7%) and neonates (0.23%).
- A significant variation was observed across countries, with South Africa having the highest prevalence at 8.82%, followed by the United States at 5.86% and Turkey at 3.74%, while India had the lowest prevalence at 2.62%.
IN PRACTICE:
The global prevalence in this meta-analysis was “higher than previous large-scale global estimates, with notable geographic and sociodemographic variability, highlighting the potential impact of environmental factors and cultural practices,” the authors wrote.
SOURCE:
The study was led by Meredith Tyree Polaskey, MS, Chicago Medical School, Rosalind Franklin University of Medicine and Science, North Chicago, Illinois, and was published online on July 3, 2024, in the JAMA Dermatology.
LIMITATIONS:
Interpretation of the findings is limited by research gaps in Central Asia, much of Sub-Saharan Africa, Eastern Europe, Southeast Asia, Latin America (excluding Brazil), and the Caribbean, along with potential underreporting in regions with restricted healthcare access and significant heterogeneity across studies.
DISCLOSURES:
Funding information was not available. One author reported serving as an advisor, consultant, speaker, and/or investigator for multiple pharmaceutical companies, including AbbVie, Amgen, and Pfizer.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Should Cancer Trial Eligibility Become More Inclusive?
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
The study, published online in Clinical Cancer Research, highlighted the potential benefits of broadening eligibility criteria for clinical trials.
“It is well known that results in an ‘ideal’ population do not always translate to the real-world population,” senior author Hans Gelderblom, MD, chair of the Department of Medical Oncology at the Leiden University Medical Center, Leiden, the Netherlands, said in a press release. “Eligibility criteria are often too strict, and educated exemptions by experienced investigators can help individual patients, especially in a last-resort trial.”
Although experts have expressed interest in improving trial inclusivity, it’s unclear how doing so might impact treatment safety and efficacy.
In the Drug Rediscovery Protocol (DRUP), Dr. Gelderblom and colleagues examined the impact of broadening trial eligibility on patient outcomes. DRUP is an ongoing Dutch national, multicenter, pan-cancer, nonrandomized clinical trial in which patients are treated off-label with approved molecularly targeted or immunotherapies.
In the trial, 1019 patients with treatment-refractory disease were matched to one of the available study drugs based on their tumor molecular profile and enrolled in parallel cohorts. Cohorts were defined by tumor type, molecular profile, and study drug.
Among these patients, 82 patients — 8% of the cohort — were granted waivers to participate. Most waivers (45%) were granted as exceptions to general- or drug-related eligibility criteria, often because of out-of-range lab results. Other categories included treatment and testing exceptions, as well as out-of-window testing.
The researchers then compared safety and efficacy outcomes between the 82 participants granted waivers and the 937 who did not receive waivers.
Overall, Dr. Gelderblom’s team found that the rate of serious adverse events was similar between patients who received a waiver and those who did not: 39% vs 41%, respectively.
A relationship between waivers and serious adverse events was deemed “unlikely” for 86% of patients and “possible” for 14%. In two cases concerning a direct relationship, for instance, patients who received waivers for decreased hemoglobin levels developed anemia.
The rate of clinical benefit — defined as an objective response or stable disease for at least 16 weeks — was similar between the groups. Overall, 40% of patients who received a waiver (33 of 82) had a clinical benefit vs 33% of patients without a waiver (P = .43). Median overall survival for patients that received a waiver was also similar — 11 months in the waiver group and 8 months in the nonwaiver group (hazard ratio, 0.87; P = .33).
“Safety and clinical benefit were preserved in patients for whom a waiver was granted,” the authors concluded.
The study had several limitations. The diversity of cancer types, treatments, and reasons for protocol exemptions precluded subgroup analyses. In addition, because the decision to grant waivers depended in large part on the likelihood of clinical benefit, “it is possible that patients who received waivers were positively selected for clinical benefit compared with the general study population,” the authors wrote.
So, “although the clinical benefit rate of the patient group for whom a waiver was granted appears to be slightly higher, this difference might be explained by the selection process of the central study team, in which each waiver request was carefully considered, weighing the risks and potential benefits for the patient in question,” the authors explained.
Overall, “these findings advocate for a broader and more inclusive design when establishing novel trials, paving the way for a more effective and tailored application of cancer therapies in patients with advanced or refractory disease,” Dr. Gelderblom said.
Commenting on the study, Bishal Gyawali, MD, PhD, said that “relaxing eligibility criteria is important, and I support this. Trials should include patients that are more representative of the real-world, so that results are generalizable.”
However, “the paper overemphasized efficacy,” said Dr. Gyawali, from Queen’s University, Kingston, Ontario, Canada. The sample size of waiver-granted patients was small, plus “the clinical benefit rate is not a marker of efficacy.
“The response rate is somewhat better, but for a heterogeneous study with multiple targets and drugs, it is difficult to say much about treatment effects here,” Dr. Gyawali added. Overall, “we shouldn’t read too much into treatment benefits based on these numbers.”
Funding for the study was provided by the Stelvio for Life Foundation, the Dutch Cancer Society, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, pharma&, Eisai Co., Ipsen, Merck Sharp & Dohme, Novartis, Pfizer, and Roche. Dr. Gelderblom declared no conflicts of interest, and Dr. Gyawali declared no conflicts of interest related to his comment.
A version of this article appeared on Medscape.com.
Suspected Orbital Compartment Syndrome Leading to Visual Loss After Pterional Craniotomy
Perioperative visual loss (POVL) is a well-documented yet uncommon complication of nonocular surgery. Patients undergoing cardiac and spinal surgery are at the greatest risk, though POVL may occur during other neurosurgical and vascular procedures as well. The most common causes of POVL are central retinal artery occlusion (CRAO) and ischemic optic neuropathy (ION),1-3 though cases of orbital compartment syndrome (OCS) have also been reported.4-7
We describe a case of POVL during a temporal meningioma excision using the pterional approach. Though the etiology is not fully understood, the patient’s clinical course was complicated by a third cranial nerve (CN III) palsy and CRAO, which, together with the patient’s presentation, were consistent with previously documented cases of OCS. The goals of this case report are to increase awareness of this surgical outcome, identify practices that may have contributed to its development, and delineate methods to minimize its occurrence.
Informed consent regarding this research was obtained from the patient and an institutional Health Insurance Portability and Accountability Act authorization form was completed. This manuscript adheres to the applicable Enhancing the Quality and Transparency of Health Research guideline.8
Case Presentation
A 47-year-old woman underwent a left temporal craniotomy for resection of a sphenoid wing meningioma discovered during a workup for persistent headaches. She had no medical history of diabetes, hypertension, coronary artery disease, or ophthalmic disease. Two months before her scheduled surgery, the patient reported bilateral blurry vision and underwent ophthalmologic evaluation. Her intraocular pressure (IOP) was normal, and she had no pupillary or retinal disease. She showed evidence of decreased vision in her left eye, suggesting a possible mass effect from her meningioma. Subsequent imaging of the optic nerve and retina had unremarkable physiology (Figure 1). Preoperative magnetic resonance imaging (MRI) demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus(Figure 2). There was a superior mass effect on the left middle cerebral artery, but all vessels were patent without evidence of thrombosis.
The patient underwent general anesthesia with invasive hemodynamic monitoring used throughout the procedure. She was induced with fentanyl, propofol, and rocuronium; anesthesia was maintained with isoflurane and a remifentanil infusion. Hypotension was treated with phenylephrine and intravenous fluids. Intraoperative neuromonitoring with electroencephalogram (EEG) and somatosensory evoked potentials was performed. During the surgery, the patient was positioned supine in a Mayfield 3-point head fixation system. All pressure points were padded appropriately and continually checked. A standard left pterional craniotomy was performed, and the scalp was reflected anteriorly and secured using fish hooks with rubber bands. The operation did not violate the cavernous sinus or orbital compartment. There was no evidence of active bleeding upon inspection nor with the Valsalva maneuver. No changes were noted in EEG or somatosensory evoked potentials; blood pressure remained within 20 mm Hg of the patient’s baseline. She was extubated at the end of the 10-hour case and was hemodynamically stable upon transport to the surgical intensive care unit. Postoperative imaging confirmed the successful removal of the left sphenoid wing meningioma.
The patient’s postoperative examination demonstrated a 5 mm dilated, nonresponsive left pupil, though the patient did not report visual loss at that time. Defects were noted in the inferior oblique, superior, inferior, and medial rectus muscles, consistent with CN III palsy. The surgery included manipulation of CN III, which made this a possible outcome, but an alternate causative pathology like OCS was not immediately suspected. Postoperative computed tomography (CT) showed an expected pneumocephalus and left scalp swelling without evidence of mass effect or midline shift.
On the morning of postoperative Day 1, the patient reported vision loss in her left eye, while her clinical examination revealed erythema and conjunctival chemosis with left eyelid swelling. The ophthalmologic evaluation was notable for a continued leftCN III palsy with intact lateral rectus and superior oblique function, a nonreactive and dilated left eye with 3+ afferent pupillary defect by reverse (light perception), pallor throughout, a flat cherry red macula with blurred disc margins, left upper eyelid edema, and 18 mm Hg intraocular pressure bilaterally (reference range, 8 to 21 mm Hg). Fundoscopic examination showed a clear vitreous without plaques or occlusions, no perivascular sheathing, and no retinal hemorrhages. CT angiography revealed small outpouchings at the superolateral aspect of the left and right cavernous carotid, consistent with atherosclerotic calcifications. An echocardiogram revealed a Valsalva-dependent patent foramen ovale, but a venous Doppler ultrasound yielded negative results.
Repeat MRI showed denervation of the left medial rectus and minimal left-sided proptosis. A 3-month ophthalmologic follow-up revealed a persistent CN III palsy, including an afferent pupillary defect, absence of light perception in her left eye, and continued ophthalmoplegia. Repeat examination showed a left-sided 4+ afferent pupillary defect unreactive to light, 4+ pallor surrounding the optic nerve, macular atrophy, sclerotic vessels, and 17 mm Hg intraocular pressure bilaterally. The eye had diffuse atrophy of the inner retina and significant patchy atrophy of the outer retinal components without neovascularization of the iris. Postoperative retinal imaging can be seen in Figure 3. Her vision loss persisted at this encounter and has continued through subsequent follow-up examinations.
Discussion
Perioperative visual loss is a rare surgical complication, with an estimated incidence of once in every 60,000 to 125,000 cases.9 The mechanism of injury is variable and dependent upon the type of surgical intervention, with cardiac and spine surgeries carrying the greatest risk.10,11 The injury often results in either CRAO or ION, which may result in visual loss.1-3 POVL can also occur in the aftermath of rapid changes in intracranial pressure during decompressive craniotomies, though the pathophysiology in such cases is not well understood.5
Among the myriad ways in which POVL can occur, neurosurgical cases carry the unique risk of direct cranial nerve injury. Such an insult can lead to vision loss via optic nerve damage or ophthalmoplegia if damage occurs to CN III, IV, or VI. This can occur during manipulation or resection, especially if the surgical approach involves the orbital cavity or the cavernous sinus. Though neither space was entered in this patient, direct injury cannot be ruled out as the etiology for either her vision loss or persistent ophthalmoplegia. An alternate causative scenario for both symptoms involve an impaired blood supply, with the vision loss potentially occurring secondary to CRAO and the ophthalmoplegia to an alternate cause of decreased blood flow. It is unclear which of these 2 conditions occurred first or if they occurred due to the same insult, but OCS could lead to both. Though it is a less common etiology for POVL, this patient’s presentation was similar to those in previously reported cases, and OCS was identified as the likely diagnosis.
OCS is precipitated by an elevated orbital pressure, which leads to ischemia of the retina and damage to orbital contents. Though associated with retrobulbar hemorrhage and orbital trauma, another proposed mechanism for OCS is extrinsic orbital compression, resulting in increased IOP and subsequent CRAO.10 A cherry red spot is visible on fundoscopy, as only the macula with its thin retinal layer will permit the choroidal vessels to be visualized. In a separate process, the relative increase in orbital pressure may lead to impaired perfusion or damage of CN III. However, a causative relationship between the 2 may be difficult to establish. Such an injury to the oculomotor nerve is demonstrated by impaired function of the inferior oblique, superior rectus, inferior rectus, and medial rectus muscles, which may persist even after the compressive symptoms of OCS have resolved.12 Other reported symptoms of OCS include erythema, ophthalmoplegia, conjunctival chemosis, ptosis, corneal abrasion, and eyelid edema.12-15
Alternate Diagnoses
OCS is a diagnosis of exclusion, and several alternate mechanisms were considered before identifying it as the likely etiology. The patient’s preoperative imaging demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus, with displacement of the left middle cerebral artery, left cavernous internal carotid artery, and left optic canal. Dissection and removal of this tumor could have compromised the arterial or venous blood supply to the orbit, thus causing ischemia to the retina and other ocular structures. CN III was manipulated during surgery, and it may have been inadvertently damaged during exposure or resection of the tumor.
The patient’s Valsalva-dependent patent foramen ovale put her at risk of a paroxysmal embolus as an alternate explanation, particularly as a Valsalva maneuver was utilized to confirm hemostasis. The patient did not, however, demonstrate any evidence of venous thromboembolism (VTE) on ultrasound, nor did she have the common risk factors of hypertension, diabetes, or smoking history that would increase VTE risk.16 Her cancer diagnosis and surgical status may have put her at risk of VTE, but she did not have any clinical or laboratory values suggestive of hypercoagulability. Had an embolism occurred, it may have compromised the orbital blood supply and led to the CRAO. A similar scenario may have occurred from an atherosclerotic plaque in either of her carotid arteries, as she did have evidence of atherosclerosis on postoperative CT angiography. However, atherosclerosis as a risk factor for POVL appears to be related more to its impact upon impaired blood supply rather than as an embolic source. The patient did not have any significant intraoperative hypotensive episodes, making ION in the setting of atherosclerosis and hypotension a less likely etiology.17
This patient differed from other reported OCS cases. She was never placed in a prone or jackknife position, nor was she agitated or straining for a sustained period. These factors, along with the fact that the orbital compartment was not entered, decreased the likelihood of intraorbital hemorrhage and other intrinsic causes of elevated IOP.12 Additionally, the presentation of our patient’s vision loss was delayed compared with other cases, despite clinicians observing a dilated left pupil and CN III palsy on examination immediately after surgery.14 It is significant to note that OCS may not demonstrate a significant increase in IOP once the source of compression is removed, which may explain the absence of proptosis on her postoperative examination.
The diagnosis of OCS was primarily implicated by the positioning of the myocutaneous flap during the pterional approach to craniotomy. It was retracted anteriorly and superiorly, ultimately resting over her left orbit for most of the 10-hour surgery. Kim and colleagues found that myocutaneous flaps may increase IOP as much as 17.5 mm Hg if improperly positioned, providing an unrecognized source of compression and increasing the risk of damage to orbital contents. According to their review, elevated IOP > 40 mm Hg, particularly over several hours, can compromise blood flow to the optic nerve and increase the risk for POVL.18 The flap was secured using fish hooks and rubber bands. However, it is suspected that the orbital rim did not fully support its pressure, thereby resting to some degree directly on the globe for an extended period and compromising the orbital blood supply. There are no current methods for measuring intraoperative IOP, though surrogate markers are under investigation and may yield clinical utility.18 The myocutaneous flap was created and positioned by the surgeons, but it may be that increased vigilance and communication from the anesthesia and nursing teams could have prevented it from remaining in an improper position.
Conclusions
Despite having few reported cases, OCS must be considered in neurosurgical patients with ophthalmoplegia and CRAO on postoperative examinations. Myocutaneous flaps that are retracted across the orbit can lead to significant elevations in IOP, leading to vision loss, which likely occurred with the patient in this case. Though protecting neurovascular structures is within the purview of the surgeon, all members of the intraoperative team should assist with ensuring proper flap positioning. These measures can help ensure adequate blood flow to the ophthalmic artery, decrease the likelihood of elevated IOP due to extrinsic compression, and help prevent the development of POVL and OCS in these patients.
1. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125(10):1597-1607. doi:10.1016/j.ophtha.2018.03.054
2. Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;372(25):2428-2436. doi:10.1056/NEJMra1413352
3. Shah SH, Chen YF, Moss HE, Rubin DS, Joslin CE, Roth S. Predicting risk of perioperative ischemic optic neuropathy in spine fusion surgery: a cohort study using the national inpatient sample. Anesth Analg. 2020;130(4):967-974. doi:10.1213/ANE.0000000000004383
4. Habets JGV, Haeren RHL, Lie SAN, Bauer NJC, Dings JTA. Acute monocular blindness due to orbital compartment syndrome following pterional craniotomy. World Neurosurg. 2018;114:72-75. doi:10.1016/j.wneu.2018.03.013
5. Vahedi P, Meshkini A, Mohajernezhadfard Z, Tubbs RS. Post-craniotomy blindness in the supine position: Unlikely or ignored? Asian J Neurosurg. 2013;8(1):36-41. doi:10.4103/1793-5482.110278
6. Kang S, Yang Y, Kim T, Kim J. Sudden unilateral blindness after intracranial aneurysm surgery. Acta Neurochir (Wien). 1997;139(3):221-226. doi:10.1007/BF01844755
7. Zimmerman CF, Van Patten PD, Golnik KC, Kopitnik TA Jr, Anand R. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology. 1995;102(4):594-598. doi:10.1016/s0161-6420(95)30979-7
8. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 23;2013:bcr2013201554. doi:10.1136/bcr-2013-201554
9. Raphael J, Moss HE, Roth S. Perioperative visual loss in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(5):1420-429. doi:10.1053/j.jvca.2018.11.035
10. Kansakar P, Sundar G. Vision loss associated with orbital surgery - a major review. Orbit. 2020;39(3):197-208. doi:10.1080/01676830.2019.1658790
11. Dohlman JC, Yoon MK. Principles of protection of the eye and vision in orbital surgery. J Neurol Surg B Skull Base. 2020;81(4):381-384. doi:10.1055/s-0040-1714077
12. Pahl FH, de Oliveira MF, Dal Col Lúcio JE, Souza E Castro EF. Orbital compartment syndrome after frontotemporal craniotomy: case report and review of literature. World Neurosurg. 2018;109:218-221. doi:10.1016/j.wneu.2017.09.167
13. Grossman W, Ward WT. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine (Phila Pa 1976). 1993;18(9):1226-1228. doi:10.1097/00007632-199307000-00017
14. Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145(4):604-610. doi:10.1016/j.ajo.2007.09.016
15. Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104(5):1185-1187. doi:10.1213/01.ane.0000264319.57758.55
16. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85. doi:10.1097/01.brs.0000152169.48117.c7
17. Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop. 2014;5(2):100-106. Published 2014 April 18. doi:10.5312/wjo.v5.i2.100
18. Kim TS, Hur JW, Park DH, et al. Extraocular ressure measurements to avoid orbital compartment syndrome in aneurysm surgery. World Neurosurg. 2018;118:e601-e609. doi:10.1016/j.wneu.2018.06.248
Perioperative visual loss (POVL) is a well-documented yet uncommon complication of nonocular surgery. Patients undergoing cardiac and spinal surgery are at the greatest risk, though POVL may occur during other neurosurgical and vascular procedures as well. The most common causes of POVL are central retinal artery occlusion (CRAO) and ischemic optic neuropathy (ION),1-3 though cases of orbital compartment syndrome (OCS) have also been reported.4-7
We describe a case of POVL during a temporal meningioma excision using the pterional approach. Though the etiology is not fully understood, the patient’s clinical course was complicated by a third cranial nerve (CN III) palsy and CRAO, which, together with the patient’s presentation, were consistent with previously documented cases of OCS. The goals of this case report are to increase awareness of this surgical outcome, identify practices that may have contributed to its development, and delineate methods to minimize its occurrence.
Informed consent regarding this research was obtained from the patient and an institutional Health Insurance Portability and Accountability Act authorization form was completed. This manuscript adheres to the applicable Enhancing the Quality and Transparency of Health Research guideline.8
Case Presentation
A 47-year-old woman underwent a left temporal craniotomy for resection of a sphenoid wing meningioma discovered during a workup for persistent headaches. She had no medical history of diabetes, hypertension, coronary artery disease, or ophthalmic disease. Two months before her scheduled surgery, the patient reported bilateral blurry vision and underwent ophthalmologic evaluation. Her intraocular pressure (IOP) was normal, and she had no pupillary or retinal disease. She showed evidence of decreased vision in her left eye, suggesting a possible mass effect from her meningioma. Subsequent imaging of the optic nerve and retina had unremarkable physiology (Figure 1). Preoperative magnetic resonance imaging (MRI) demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus(Figure 2). There was a superior mass effect on the left middle cerebral artery, but all vessels were patent without evidence of thrombosis.
The patient underwent general anesthesia with invasive hemodynamic monitoring used throughout the procedure. She was induced with fentanyl, propofol, and rocuronium; anesthesia was maintained with isoflurane and a remifentanil infusion. Hypotension was treated with phenylephrine and intravenous fluids. Intraoperative neuromonitoring with electroencephalogram (EEG) and somatosensory evoked potentials was performed. During the surgery, the patient was positioned supine in a Mayfield 3-point head fixation system. All pressure points were padded appropriately and continually checked. A standard left pterional craniotomy was performed, and the scalp was reflected anteriorly and secured using fish hooks with rubber bands. The operation did not violate the cavernous sinus or orbital compartment. There was no evidence of active bleeding upon inspection nor with the Valsalva maneuver. No changes were noted in EEG or somatosensory evoked potentials; blood pressure remained within 20 mm Hg of the patient’s baseline. She was extubated at the end of the 10-hour case and was hemodynamically stable upon transport to the surgical intensive care unit. Postoperative imaging confirmed the successful removal of the left sphenoid wing meningioma.
The patient’s postoperative examination demonstrated a 5 mm dilated, nonresponsive left pupil, though the patient did not report visual loss at that time. Defects were noted in the inferior oblique, superior, inferior, and medial rectus muscles, consistent with CN III palsy. The surgery included manipulation of CN III, which made this a possible outcome, but an alternate causative pathology like OCS was not immediately suspected. Postoperative computed tomography (CT) showed an expected pneumocephalus and left scalp swelling without evidence of mass effect or midline shift.
On the morning of postoperative Day 1, the patient reported vision loss in her left eye, while her clinical examination revealed erythema and conjunctival chemosis with left eyelid swelling. The ophthalmologic evaluation was notable for a continued leftCN III palsy with intact lateral rectus and superior oblique function, a nonreactive and dilated left eye with 3+ afferent pupillary defect by reverse (light perception), pallor throughout, a flat cherry red macula with blurred disc margins, left upper eyelid edema, and 18 mm Hg intraocular pressure bilaterally (reference range, 8 to 21 mm Hg). Fundoscopic examination showed a clear vitreous without plaques or occlusions, no perivascular sheathing, and no retinal hemorrhages. CT angiography revealed small outpouchings at the superolateral aspect of the left and right cavernous carotid, consistent with atherosclerotic calcifications. An echocardiogram revealed a Valsalva-dependent patent foramen ovale, but a venous Doppler ultrasound yielded negative results.
Repeat MRI showed denervation of the left medial rectus and minimal left-sided proptosis. A 3-month ophthalmologic follow-up revealed a persistent CN III palsy, including an afferent pupillary defect, absence of light perception in her left eye, and continued ophthalmoplegia. Repeat examination showed a left-sided 4+ afferent pupillary defect unreactive to light, 4+ pallor surrounding the optic nerve, macular atrophy, sclerotic vessels, and 17 mm Hg intraocular pressure bilaterally. The eye had diffuse atrophy of the inner retina and significant patchy atrophy of the outer retinal components without neovascularization of the iris. Postoperative retinal imaging can be seen in Figure 3. Her vision loss persisted at this encounter and has continued through subsequent follow-up examinations.
Discussion
Perioperative visual loss is a rare surgical complication, with an estimated incidence of once in every 60,000 to 125,000 cases.9 The mechanism of injury is variable and dependent upon the type of surgical intervention, with cardiac and spine surgeries carrying the greatest risk.10,11 The injury often results in either CRAO or ION, which may result in visual loss.1-3 POVL can also occur in the aftermath of rapid changes in intracranial pressure during decompressive craniotomies, though the pathophysiology in such cases is not well understood.5
Among the myriad ways in which POVL can occur, neurosurgical cases carry the unique risk of direct cranial nerve injury. Such an insult can lead to vision loss via optic nerve damage or ophthalmoplegia if damage occurs to CN III, IV, or VI. This can occur during manipulation or resection, especially if the surgical approach involves the orbital cavity or the cavernous sinus. Though neither space was entered in this patient, direct injury cannot be ruled out as the etiology for either her vision loss or persistent ophthalmoplegia. An alternate causative scenario for both symptoms involve an impaired blood supply, with the vision loss potentially occurring secondary to CRAO and the ophthalmoplegia to an alternate cause of decreased blood flow. It is unclear which of these 2 conditions occurred first or if they occurred due to the same insult, but OCS could lead to both. Though it is a less common etiology for POVL, this patient’s presentation was similar to those in previously reported cases, and OCS was identified as the likely diagnosis.
OCS is precipitated by an elevated orbital pressure, which leads to ischemia of the retina and damage to orbital contents. Though associated with retrobulbar hemorrhage and orbital trauma, another proposed mechanism for OCS is extrinsic orbital compression, resulting in increased IOP and subsequent CRAO.10 A cherry red spot is visible on fundoscopy, as only the macula with its thin retinal layer will permit the choroidal vessels to be visualized. In a separate process, the relative increase in orbital pressure may lead to impaired perfusion or damage of CN III. However, a causative relationship between the 2 may be difficult to establish. Such an injury to the oculomotor nerve is demonstrated by impaired function of the inferior oblique, superior rectus, inferior rectus, and medial rectus muscles, which may persist even after the compressive symptoms of OCS have resolved.12 Other reported symptoms of OCS include erythema, ophthalmoplegia, conjunctival chemosis, ptosis, corneal abrasion, and eyelid edema.12-15
Alternate Diagnoses
OCS is a diagnosis of exclusion, and several alternate mechanisms were considered before identifying it as the likely etiology. The patient’s preoperative imaging demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus, with displacement of the left middle cerebral artery, left cavernous internal carotid artery, and left optic canal. Dissection and removal of this tumor could have compromised the arterial or venous blood supply to the orbit, thus causing ischemia to the retina and other ocular structures. CN III was manipulated during surgery, and it may have been inadvertently damaged during exposure or resection of the tumor.
The patient’s Valsalva-dependent patent foramen ovale put her at risk of a paroxysmal embolus as an alternate explanation, particularly as a Valsalva maneuver was utilized to confirm hemostasis. The patient did not, however, demonstrate any evidence of venous thromboembolism (VTE) on ultrasound, nor did she have the common risk factors of hypertension, diabetes, or smoking history that would increase VTE risk.16 Her cancer diagnosis and surgical status may have put her at risk of VTE, but she did not have any clinical or laboratory values suggestive of hypercoagulability. Had an embolism occurred, it may have compromised the orbital blood supply and led to the CRAO. A similar scenario may have occurred from an atherosclerotic plaque in either of her carotid arteries, as she did have evidence of atherosclerosis on postoperative CT angiography. However, atherosclerosis as a risk factor for POVL appears to be related more to its impact upon impaired blood supply rather than as an embolic source. The patient did not have any significant intraoperative hypotensive episodes, making ION in the setting of atherosclerosis and hypotension a less likely etiology.17
This patient differed from other reported OCS cases. She was never placed in a prone or jackknife position, nor was she agitated or straining for a sustained period. These factors, along with the fact that the orbital compartment was not entered, decreased the likelihood of intraorbital hemorrhage and other intrinsic causes of elevated IOP.12 Additionally, the presentation of our patient’s vision loss was delayed compared with other cases, despite clinicians observing a dilated left pupil and CN III palsy on examination immediately after surgery.14 It is significant to note that OCS may not demonstrate a significant increase in IOP once the source of compression is removed, which may explain the absence of proptosis on her postoperative examination.
The diagnosis of OCS was primarily implicated by the positioning of the myocutaneous flap during the pterional approach to craniotomy. It was retracted anteriorly and superiorly, ultimately resting over her left orbit for most of the 10-hour surgery. Kim and colleagues found that myocutaneous flaps may increase IOP as much as 17.5 mm Hg if improperly positioned, providing an unrecognized source of compression and increasing the risk of damage to orbital contents. According to their review, elevated IOP > 40 mm Hg, particularly over several hours, can compromise blood flow to the optic nerve and increase the risk for POVL.18 The flap was secured using fish hooks and rubber bands. However, it is suspected that the orbital rim did not fully support its pressure, thereby resting to some degree directly on the globe for an extended period and compromising the orbital blood supply. There are no current methods for measuring intraoperative IOP, though surrogate markers are under investigation and may yield clinical utility.18 The myocutaneous flap was created and positioned by the surgeons, but it may be that increased vigilance and communication from the anesthesia and nursing teams could have prevented it from remaining in an improper position.
Conclusions
Despite having few reported cases, OCS must be considered in neurosurgical patients with ophthalmoplegia and CRAO on postoperative examinations. Myocutaneous flaps that are retracted across the orbit can lead to significant elevations in IOP, leading to vision loss, which likely occurred with the patient in this case. Though protecting neurovascular structures is within the purview of the surgeon, all members of the intraoperative team should assist with ensuring proper flap positioning. These measures can help ensure adequate blood flow to the ophthalmic artery, decrease the likelihood of elevated IOP due to extrinsic compression, and help prevent the development of POVL and OCS in these patients.
Perioperative visual loss (POVL) is a well-documented yet uncommon complication of nonocular surgery. Patients undergoing cardiac and spinal surgery are at the greatest risk, though POVL may occur during other neurosurgical and vascular procedures as well. The most common causes of POVL are central retinal artery occlusion (CRAO) and ischemic optic neuropathy (ION),1-3 though cases of orbital compartment syndrome (OCS) have also been reported.4-7
We describe a case of POVL during a temporal meningioma excision using the pterional approach. Though the etiology is not fully understood, the patient’s clinical course was complicated by a third cranial nerve (CN III) palsy and CRAO, which, together with the patient’s presentation, were consistent with previously documented cases of OCS. The goals of this case report are to increase awareness of this surgical outcome, identify practices that may have contributed to its development, and delineate methods to minimize its occurrence.
Informed consent regarding this research was obtained from the patient and an institutional Health Insurance Portability and Accountability Act authorization form was completed. This manuscript adheres to the applicable Enhancing the Quality and Transparency of Health Research guideline.8
Case Presentation
A 47-year-old woman underwent a left temporal craniotomy for resection of a sphenoid wing meningioma discovered during a workup for persistent headaches. She had no medical history of diabetes, hypertension, coronary artery disease, or ophthalmic disease. Two months before her scheduled surgery, the patient reported bilateral blurry vision and underwent ophthalmologic evaluation. Her intraocular pressure (IOP) was normal, and she had no pupillary or retinal disease. She showed evidence of decreased vision in her left eye, suggesting a possible mass effect from her meningioma. Subsequent imaging of the optic nerve and retina had unremarkable physiology (Figure 1). Preoperative magnetic resonance imaging (MRI) demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus(Figure 2). There was a superior mass effect on the left middle cerebral artery, but all vessels were patent without evidence of thrombosis.
The patient underwent general anesthesia with invasive hemodynamic monitoring used throughout the procedure. She was induced with fentanyl, propofol, and rocuronium; anesthesia was maintained with isoflurane and a remifentanil infusion. Hypotension was treated with phenylephrine and intravenous fluids. Intraoperative neuromonitoring with electroencephalogram (EEG) and somatosensory evoked potentials was performed. During the surgery, the patient was positioned supine in a Mayfield 3-point head fixation system. All pressure points were padded appropriately and continually checked. A standard left pterional craniotomy was performed, and the scalp was reflected anteriorly and secured using fish hooks with rubber bands. The operation did not violate the cavernous sinus or orbital compartment. There was no evidence of active bleeding upon inspection nor with the Valsalva maneuver. No changes were noted in EEG or somatosensory evoked potentials; blood pressure remained within 20 mm Hg of the patient’s baseline. She was extubated at the end of the 10-hour case and was hemodynamically stable upon transport to the surgical intensive care unit. Postoperative imaging confirmed the successful removal of the left sphenoid wing meningioma.
The patient’s postoperative examination demonstrated a 5 mm dilated, nonresponsive left pupil, though the patient did not report visual loss at that time. Defects were noted in the inferior oblique, superior, inferior, and medial rectus muscles, consistent with CN III palsy. The surgery included manipulation of CN III, which made this a possible outcome, but an alternate causative pathology like OCS was not immediately suspected. Postoperative computed tomography (CT) showed an expected pneumocephalus and left scalp swelling without evidence of mass effect or midline shift.
On the morning of postoperative Day 1, the patient reported vision loss in her left eye, while her clinical examination revealed erythema and conjunctival chemosis with left eyelid swelling. The ophthalmologic evaluation was notable for a continued leftCN III palsy with intact lateral rectus and superior oblique function, a nonreactive and dilated left eye with 3+ afferent pupillary defect by reverse (light perception), pallor throughout, a flat cherry red macula with blurred disc margins, left upper eyelid edema, and 18 mm Hg intraocular pressure bilaterally (reference range, 8 to 21 mm Hg). Fundoscopic examination showed a clear vitreous without plaques or occlusions, no perivascular sheathing, and no retinal hemorrhages. CT angiography revealed small outpouchings at the superolateral aspect of the left and right cavernous carotid, consistent with atherosclerotic calcifications. An echocardiogram revealed a Valsalva-dependent patent foramen ovale, but a venous Doppler ultrasound yielded negative results.
Repeat MRI showed denervation of the left medial rectus and minimal left-sided proptosis. A 3-month ophthalmologic follow-up revealed a persistent CN III palsy, including an afferent pupillary defect, absence of light perception in her left eye, and continued ophthalmoplegia. Repeat examination showed a left-sided 4+ afferent pupillary defect unreactive to light, 4+ pallor surrounding the optic nerve, macular atrophy, sclerotic vessels, and 17 mm Hg intraocular pressure bilaterally. The eye had diffuse atrophy of the inner retina and significant patchy atrophy of the outer retinal components without neovascularization of the iris. Postoperative retinal imaging can be seen in Figure 3. Her vision loss persisted at this encounter and has continued through subsequent follow-up examinations.
Discussion
Perioperative visual loss is a rare surgical complication, with an estimated incidence of once in every 60,000 to 125,000 cases.9 The mechanism of injury is variable and dependent upon the type of surgical intervention, with cardiac and spine surgeries carrying the greatest risk.10,11 The injury often results in either CRAO or ION, which may result in visual loss.1-3 POVL can also occur in the aftermath of rapid changes in intracranial pressure during decompressive craniotomies, though the pathophysiology in such cases is not well understood.5
Among the myriad ways in which POVL can occur, neurosurgical cases carry the unique risk of direct cranial nerve injury. Such an insult can lead to vision loss via optic nerve damage or ophthalmoplegia if damage occurs to CN III, IV, or VI. This can occur during manipulation or resection, especially if the surgical approach involves the orbital cavity or the cavernous sinus. Though neither space was entered in this patient, direct injury cannot be ruled out as the etiology for either her vision loss or persistent ophthalmoplegia. An alternate causative scenario for both symptoms involve an impaired blood supply, with the vision loss potentially occurring secondary to CRAO and the ophthalmoplegia to an alternate cause of decreased blood flow. It is unclear which of these 2 conditions occurred first or if they occurred due to the same insult, but OCS could lead to both. Though it is a less common etiology for POVL, this patient’s presentation was similar to those in previously reported cases, and OCS was identified as the likely diagnosis.
OCS is precipitated by an elevated orbital pressure, which leads to ischemia of the retina and damage to orbital contents. Though associated with retrobulbar hemorrhage and orbital trauma, another proposed mechanism for OCS is extrinsic orbital compression, resulting in increased IOP and subsequent CRAO.10 A cherry red spot is visible on fundoscopy, as only the macula with its thin retinal layer will permit the choroidal vessels to be visualized. In a separate process, the relative increase in orbital pressure may lead to impaired perfusion or damage of CN III. However, a causative relationship between the 2 may be difficult to establish. Such an injury to the oculomotor nerve is demonstrated by impaired function of the inferior oblique, superior rectus, inferior rectus, and medial rectus muscles, which may persist even after the compressive symptoms of OCS have resolved.12 Other reported symptoms of OCS include erythema, ophthalmoplegia, conjunctival chemosis, ptosis, corneal abrasion, and eyelid edema.12-15
Alternate Diagnoses
OCS is a diagnosis of exclusion, and several alternate mechanisms were considered before identifying it as the likely etiology. The patient’s preoperative imaging demonstrated a stable enhancing mass involving the left great sphenoid wing and left cavernous sinus, with displacement of the left middle cerebral artery, left cavernous internal carotid artery, and left optic canal. Dissection and removal of this tumor could have compromised the arterial or venous blood supply to the orbit, thus causing ischemia to the retina and other ocular structures. CN III was manipulated during surgery, and it may have been inadvertently damaged during exposure or resection of the tumor.
The patient’s Valsalva-dependent patent foramen ovale put her at risk of a paroxysmal embolus as an alternate explanation, particularly as a Valsalva maneuver was utilized to confirm hemostasis. The patient did not, however, demonstrate any evidence of venous thromboembolism (VTE) on ultrasound, nor did she have the common risk factors of hypertension, diabetes, or smoking history that would increase VTE risk.16 Her cancer diagnosis and surgical status may have put her at risk of VTE, but she did not have any clinical or laboratory values suggestive of hypercoagulability. Had an embolism occurred, it may have compromised the orbital blood supply and led to the CRAO. A similar scenario may have occurred from an atherosclerotic plaque in either of her carotid arteries, as she did have evidence of atherosclerosis on postoperative CT angiography. However, atherosclerosis as a risk factor for POVL appears to be related more to its impact upon impaired blood supply rather than as an embolic source. The patient did not have any significant intraoperative hypotensive episodes, making ION in the setting of atherosclerosis and hypotension a less likely etiology.17
This patient differed from other reported OCS cases. She was never placed in a prone or jackknife position, nor was she agitated or straining for a sustained period. These factors, along with the fact that the orbital compartment was not entered, decreased the likelihood of intraorbital hemorrhage and other intrinsic causes of elevated IOP.12 Additionally, the presentation of our patient’s vision loss was delayed compared with other cases, despite clinicians observing a dilated left pupil and CN III palsy on examination immediately after surgery.14 It is significant to note that OCS may not demonstrate a significant increase in IOP once the source of compression is removed, which may explain the absence of proptosis on her postoperative examination.
The diagnosis of OCS was primarily implicated by the positioning of the myocutaneous flap during the pterional approach to craniotomy. It was retracted anteriorly and superiorly, ultimately resting over her left orbit for most of the 10-hour surgery. Kim and colleagues found that myocutaneous flaps may increase IOP as much as 17.5 mm Hg if improperly positioned, providing an unrecognized source of compression and increasing the risk of damage to orbital contents. According to their review, elevated IOP > 40 mm Hg, particularly over several hours, can compromise blood flow to the optic nerve and increase the risk for POVL.18 The flap was secured using fish hooks and rubber bands. However, it is suspected that the orbital rim did not fully support its pressure, thereby resting to some degree directly on the globe for an extended period and compromising the orbital blood supply. There are no current methods for measuring intraoperative IOP, though surrogate markers are under investigation and may yield clinical utility.18 The myocutaneous flap was created and positioned by the surgeons, but it may be that increased vigilance and communication from the anesthesia and nursing teams could have prevented it from remaining in an improper position.
Conclusions
Despite having few reported cases, OCS must be considered in neurosurgical patients with ophthalmoplegia and CRAO on postoperative examinations. Myocutaneous flaps that are retracted across the orbit can lead to significant elevations in IOP, leading to vision loss, which likely occurred with the patient in this case. Though protecting neurovascular structures is within the purview of the surgeon, all members of the intraoperative team should assist with ensuring proper flap positioning. These measures can help ensure adequate blood flow to the ophthalmic artery, decrease the likelihood of elevated IOP due to extrinsic compression, and help prevent the development of POVL and OCS in these patients.
1. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125(10):1597-1607. doi:10.1016/j.ophtha.2018.03.054
2. Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;372(25):2428-2436. doi:10.1056/NEJMra1413352
3. Shah SH, Chen YF, Moss HE, Rubin DS, Joslin CE, Roth S. Predicting risk of perioperative ischemic optic neuropathy in spine fusion surgery: a cohort study using the national inpatient sample. Anesth Analg. 2020;130(4):967-974. doi:10.1213/ANE.0000000000004383
4. Habets JGV, Haeren RHL, Lie SAN, Bauer NJC, Dings JTA. Acute monocular blindness due to orbital compartment syndrome following pterional craniotomy. World Neurosurg. 2018;114:72-75. doi:10.1016/j.wneu.2018.03.013
5. Vahedi P, Meshkini A, Mohajernezhadfard Z, Tubbs RS. Post-craniotomy blindness in the supine position: Unlikely or ignored? Asian J Neurosurg. 2013;8(1):36-41. doi:10.4103/1793-5482.110278
6. Kang S, Yang Y, Kim T, Kim J. Sudden unilateral blindness after intracranial aneurysm surgery. Acta Neurochir (Wien). 1997;139(3):221-226. doi:10.1007/BF01844755
7. Zimmerman CF, Van Patten PD, Golnik KC, Kopitnik TA Jr, Anand R. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology. 1995;102(4):594-598. doi:10.1016/s0161-6420(95)30979-7
8. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 23;2013:bcr2013201554. doi:10.1136/bcr-2013-201554
9. Raphael J, Moss HE, Roth S. Perioperative visual loss in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(5):1420-429. doi:10.1053/j.jvca.2018.11.035
10. Kansakar P, Sundar G. Vision loss associated with orbital surgery - a major review. Orbit. 2020;39(3):197-208. doi:10.1080/01676830.2019.1658790
11. Dohlman JC, Yoon MK. Principles of protection of the eye and vision in orbital surgery. J Neurol Surg B Skull Base. 2020;81(4):381-384. doi:10.1055/s-0040-1714077
12. Pahl FH, de Oliveira MF, Dal Col Lúcio JE, Souza E Castro EF. Orbital compartment syndrome after frontotemporal craniotomy: case report and review of literature. World Neurosurg. 2018;109:218-221. doi:10.1016/j.wneu.2017.09.167
13. Grossman W, Ward WT. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine (Phila Pa 1976). 1993;18(9):1226-1228. doi:10.1097/00007632-199307000-00017
14. Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145(4):604-610. doi:10.1016/j.ajo.2007.09.016
15. Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104(5):1185-1187. doi:10.1213/01.ane.0000264319.57758.55
16. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85. doi:10.1097/01.brs.0000152169.48117.c7
17. Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop. 2014;5(2):100-106. Published 2014 April 18. doi:10.5312/wjo.v5.i2.100
18. Kim TS, Hur JW, Park DH, et al. Extraocular ressure measurements to avoid orbital compartment syndrome in aneurysm surgery. World Neurosurg. 2018;118:e601-e609. doi:10.1016/j.wneu.2018.06.248
1. Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125(10):1597-1607. doi:10.1016/j.ophtha.2018.03.054
2. Biousse V, Newman NJ. Ischemic optic neuropathies. N Engl J Med. 2015;372(25):2428-2436. doi:10.1056/NEJMra1413352
3. Shah SH, Chen YF, Moss HE, Rubin DS, Joslin CE, Roth S. Predicting risk of perioperative ischemic optic neuropathy in spine fusion surgery: a cohort study using the national inpatient sample. Anesth Analg. 2020;130(4):967-974. doi:10.1213/ANE.0000000000004383
4. Habets JGV, Haeren RHL, Lie SAN, Bauer NJC, Dings JTA. Acute monocular blindness due to orbital compartment syndrome following pterional craniotomy. World Neurosurg. 2018;114:72-75. doi:10.1016/j.wneu.2018.03.013
5. Vahedi P, Meshkini A, Mohajernezhadfard Z, Tubbs RS. Post-craniotomy blindness in the supine position: Unlikely or ignored? Asian J Neurosurg. 2013;8(1):36-41. doi:10.4103/1793-5482.110278
6. Kang S, Yang Y, Kim T, Kim J. Sudden unilateral blindness after intracranial aneurysm surgery. Acta Neurochir (Wien). 1997;139(3):221-226. doi:10.1007/BF01844755
7. Zimmerman CF, Van Patten PD, Golnik KC, Kopitnik TA Jr, Anand R. Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology. 1995;102(4):594-598. doi:10.1016/s0161-6420(95)30979-7
8. Gagnier JJ, Kienle G, Altman DG, et al. The CARE guidelines: consensus-based clinical case reporting guideline development. BMJ Case Rep. 23;2013:bcr2013201554. doi:10.1136/bcr-2013-201554
9. Raphael J, Moss HE, Roth S. Perioperative visual loss in cardiac surgery. J Cardiothorac Vasc Anesth. 2019;33(5):1420-429. doi:10.1053/j.jvca.2018.11.035
10. Kansakar P, Sundar G. Vision loss associated with orbital surgery - a major review. Orbit. 2020;39(3):197-208. doi:10.1080/01676830.2019.1658790
11. Dohlman JC, Yoon MK. Principles of protection of the eye and vision in orbital surgery. J Neurol Surg B Skull Base. 2020;81(4):381-384. doi:10.1055/s-0040-1714077
12. Pahl FH, de Oliveira MF, Dal Col Lúcio JE, Souza E Castro EF. Orbital compartment syndrome after frontotemporal craniotomy: case report and review of literature. World Neurosurg. 2018;109:218-221. doi:10.1016/j.wneu.2017.09.167
13. Grossman W, Ward WT. Central retinal artery occlusion after scoliosis surgery with a horseshoe headrest. Case report and literature review. Spine (Phila Pa 1976). 1993;18(9):1226-1228. doi:10.1097/00007632-199307000-00017
14. Newman NJ. Perioperative visual loss after nonocular surgeries. Am J Ophthalmol. 2008;145(4):604-610. doi:10.1016/j.ajo.2007.09.016
15. Roth S, Tung A, Ksiazek S. Visual loss in a prone-positioned spine surgery patient with the head on a foam headrest and goggles covering the eyes: an old complication with a new mechanism. Anesth Analg. 2007;104(5):1185-1187. doi:10.1213/01.ane.0000264319.57758.55
16. Katz DA, Karlin LI. Visual field defect after posterior spine fusion. Spine (Phila Pa 1976). 2005;30(3):E83-E85. doi:10.1097/01.brs.0000152169.48117.c7
17. Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop. 2014;5(2):100-106. Published 2014 April 18. doi:10.5312/wjo.v5.i2.100
18. Kim TS, Hur JW, Park DH, et al. Extraocular ressure measurements to avoid orbital compartment syndrome in aneurysm surgery. World Neurosurg. 2018;118:e601-e609. doi:10.1016/j.wneu.2018.06.248
Fit for Promotion: Navy Changes the Policy
Time was—recent time, that is—sailors had two chances to pass a physical fitness assessment (PFA). Failing the first meant no promotion. Failing the second: No career. They could neither be promoted nor reenlist.
That’s changed; as of this month, the Navy now allows the sailor’s commanding officer to decide whether the sailor gets to go on, even after failing a second test.
In an administrative letter, Vice Adm. Rick Cheeseman, chief of naval personnel, said, "Commanding officers can now evaluate a sailor's physical readiness progress or lack of progress in performance evaluations, giving them the ability to manage risk, recognize earnest effort, and best take care of their people.”
According to the new policy, sailors who fail any PFA no longer need to have it noted on their annual evaluation (although they still may not advance until they pass another test). Enlisted sailors who fail a second consecutive PFA are no longer required to receive the lowest possible score in the "Military Bearing/Professionalism" category and are not denied the ability to reenlist.
In assessing eligibility for enlisted members, the memo states that commanders should consider a sailor’s ability to perform the functions of their rate without physical or medical limitation at sea, shore or isolated duty; their overall ability to contribute to Navy missions; and the likelihood of improvement in meeting PFA standards within the next 12 months.
“Building the bodies of great people,” Cheeseman wrote, “is more than annual (or biannual) testing and includes ensuring healthy food, adequate sleep, opportunities to exercise (especially outside), and medical readiness.”
According to a report by Military.com, “critics have argued that many of the changes were the Navy relaxing its standards in the face of a challenging recruiting environment and an increasingly overweight population of Americans.” However, Navy data provided in November indicate that the number of sailors failing PFAs has remained very low. In 2017, nearly 98% of sailors passed the PFA, and 95.1% passed the first post-pandemic PFA in 2022.
The PFA policy changes are part of the Navy’s Culture of Excellence 2.0, initiated earlier this year, Cheeseman says. This initiative “charges our leaders to build great people, great leaders, and great teams: their minds, bodies, and spirits, eliminating barriers wherever possible. In response, we are modernizing our PFA policy to acknowledge our diverse population, increase sailor trust, and enhance quality of service.”
Time was—recent time, that is—sailors had two chances to pass a physical fitness assessment (PFA). Failing the first meant no promotion. Failing the second: No career. They could neither be promoted nor reenlist.
That’s changed; as of this month, the Navy now allows the sailor’s commanding officer to decide whether the sailor gets to go on, even after failing a second test.
In an administrative letter, Vice Adm. Rick Cheeseman, chief of naval personnel, said, "Commanding officers can now evaluate a sailor's physical readiness progress or lack of progress in performance evaluations, giving them the ability to manage risk, recognize earnest effort, and best take care of their people.”
According to the new policy, sailors who fail any PFA no longer need to have it noted on their annual evaluation (although they still may not advance until they pass another test). Enlisted sailors who fail a second consecutive PFA are no longer required to receive the lowest possible score in the "Military Bearing/Professionalism" category and are not denied the ability to reenlist.
In assessing eligibility for enlisted members, the memo states that commanders should consider a sailor’s ability to perform the functions of their rate without physical or medical limitation at sea, shore or isolated duty; their overall ability to contribute to Navy missions; and the likelihood of improvement in meeting PFA standards within the next 12 months.
“Building the bodies of great people,” Cheeseman wrote, “is more than annual (or biannual) testing and includes ensuring healthy food, adequate sleep, opportunities to exercise (especially outside), and medical readiness.”
According to a report by Military.com, “critics have argued that many of the changes were the Navy relaxing its standards in the face of a challenging recruiting environment and an increasingly overweight population of Americans.” However, Navy data provided in November indicate that the number of sailors failing PFAs has remained very low. In 2017, nearly 98% of sailors passed the PFA, and 95.1% passed the first post-pandemic PFA in 2022.
The PFA policy changes are part of the Navy’s Culture of Excellence 2.0, initiated earlier this year, Cheeseman says. This initiative “charges our leaders to build great people, great leaders, and great teams: their minds, bodies, and spirits, eliminating barriers wherever possible. In response, we are modernizing our PFA policy to acknowledge our diverse population, increase sailor trust, and enhance quality of service.”
Time was—recent time, that is—sailors had two chances to pass a physical fitness assessment (PFA). Failing the first meant no promotion. Failing the second: No career. They could neither be promoted nor reenlist.
That’s changed; as of this month, the Navy now allows the sailor’s commanding officer to decide whether the sailor gets to go on, even after failing a second test.
In an administrative letter, Vice Adm. Rick Cheeseman, chief of naval personnel, said, "Commanding officers can now evaluate a sailor's physical readiness progress or lack of progress in performance evaluations, giving them the ability to manage risk, recognize earnest effort, and best take care of their people.”
According to the new policy, sailors who fail any PFA no longer need to have it noted on their annual evaluation (although they still may not advance until they pass another test). Enlisted sailors who fail a second consecutive PFA are no longer required to receive the lowest possible score in the "Military Bearing/Professionalism" category and are not denied the ability to reenlist.
In assessing eligibility for enlisted members, the memo states that commanders should consider a sailor’s ability to perform the functions of their rate without physical or medical limitation at sea, shore or isolated duty; their overall ability to contribute to Navy missions; and the likelihood of improvement in meeting PFA standards within the next 12 months.
“Building the bodies of great people,” Cheeseman wrote, “is more than annual (or biannual) testing and includes ensuring healthy food, adequate sleep, opportunities to exercise (especially outside), and medical readiness.”
According to a report by Military.com, “critics have argued that many of the changes were the Navy relaxing its standards in the face of a challenging recruiting environment and an increasingly overweight population of Americans.” However, Navy data provided in November indicate that the number of sailors failing PFAs has remained very low. In 2017, nearly 98% of sailors passed the PFA, and 95.1% passed the first post-pandemic PFA in 2022.
The PFA policy changes are part of the Navy’s Culture of Excellence 2.0, initiated earlier this year, Cheeseman says. This initiative “charges our leaders to build great people, great leaders, and great teams: their minds, bodies, and spirits, eliminating barriers wherever possible. In response, we are modernizing our PFA policy to acknowledge our diverse population, increase sailor trust, and enhance quality of service.”
Weight Loss Drugs Cut Cancer Risk in Diabetes Patients
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Recent research on popular weight loss drugs has uncovered surprising benefits beyond their intended use, like lowering the risk of fatal heart attacks. And now there may be another unforeseen advantage:
That’s according to a study published July 5 in JAMA Network Open where researchers studied glucagon-like peptide receptor agonists (known as GLP-1RAs), a class of drugs used to treat diabetes and obesity. Ozempic, Wegovy, Mounjaro, and Zepbound, which have become well-known recently because they are linked to rapid weight loss, contain GLP-1RAs.
For the study, they looked at electronic health records of 1.7 million patients who had type 2 diabetes, no prior diagnosis of obesity-related cancers, and had been prescribed GLP-1RAs, insulins, or metformin from March 2005 to November 2018.
The scientists found that compared to patients who took insulin, people who took GLP-1RAs had a “significant risk reduction” in 10 of 13 obesity-related cancers. Those 10 cancers were esophageal, colorectal, endometrial, gallbladder, kidney, liver, ovarian, and pancreatic cancers, as well as meningioma and multiple myeloma.
Compared with patients taking insulin, patients taking GLP-1RAs showed no statistically significant reduction in stomach cancer and no reduced risk of breast and thyroid cancers, the study said.
But the study found no decrease in cancer risk with GLP-1RAs compared with metformin.
While the study results suggest that these drugs may reduce the risk of certain obesity-related cancers better than insulins, more research is needed, they said.
A version of this article appeared on WebMD.com.
Histiocytoid Pyoderma Gangrenosum: A Challenging Case With Features of Sweet Syndrome
To the Editor:
Neutrophilic dermatoses—a group of inflammatory cutaneous conditions—include acute febrile neutrophilic dermatosis (Sweet syndrome), pyoderma gangrenosum, and neutrophilic dermatosis of the dorsal hands. Histopathology shows a dense dermal infiltrate of mature neutrophils. In 2005, the histiocytoid subtype of Sweet syndrome was introduced with histopathologic findings of a dermal infiltrate composed of immature myeloid cells that resemble histiocytes in appearance but stain strongly with neutrophil markers on immunohistochemistry.1 We present a case of histiocytoid pyoderma gangrenosum with histopathology that showed a dense dermal histiocytoid infiltrate with strong positivity for neutrophil markers on immunohistochemistry.
An 85-year-old man was seen by dermatology in the inpatient setting for a new-onset painful abdominal wound. He had a medical history of myelodysplastic syndrome (MDS), high-grade invasive papillary urothelial carcinoma of the bladder, and a recent diagnosis of low-grade invasive ascending colon adenocarcinoma. Ten days prior he underwent a right colectomy without intraoperative complications that was followed by septic shock. Workup with urinalysis and urine culture showed minimal pyuria with Pseudomonas aeruginosa. Additional studies, including blood cultures, abdominal wound cultures, computed tomography of the abdomen and pelvis, renal ultrasound, and chest radiographs, were unremarkable and showed no signs of surgical site infection, intra-abdominal or pelvic abscess formation, or pulmonary embolism. Broad-spectrum antibiotics—vancomycin and piperacillin-tazobactam—were started. Persistent fever (Tmax of 102.3 °F [39.1 °C]) and leukocytosis (45.3×109/L [4.2–10×109/L]) despite antibiotic therapy, increasing pressor requirements, and progressive painful erythema and purulence at the abdominal surgical site led to debridement of the wound by the general surgery team on day 9 following the initial surgery due to suspected necrotizing infection. Within 24 hours, dermatology was consulted for continued rapid expansion of the wound. Physical examination of the abdomen revealed a large, well-demarcated, pink-red, indurated, ulcerated plaque with clear to purulent exudate and superficial erosions with violaceous undermined borders extending centrifugally from the abdominal surgical incision line (Figure 1A). Two punch biopsies sent for histopathologic evaluation and tissue culture showed dermal edema with a dense histiocytic infiltrate with nodular foci and admixed mature neutrophils to a lesser degree (Figure 2). Special staining was negative for bacteria, fungi, and mycobacteria. Immunohistochemistry revealed positive staining of the dermal inflammatory infiltrate with CD68, myeloperoxidase, and lysozyme, as well as negative staining with CD34 (Figure 3). These findings were suggestive of a histiocytoid neutrophilic dermatosis such as Sweet syndrome or pyoderma gangrenosum. Due to the morphology of the solitary lesion and the abrupt exacerbation shortly after surgical intervention, the patient was diagnosed with histiocytoid pyoderma gangrenosum. At the same time, the patient’s septic shock was treated with intravenous hydrocortisone (100 mg 3 times daily) for 2 days and also achieved a prompt response in the cutaneous symptoms (Figure 1B).
Sweet syndrome and pyoderma gangrenosum are considered distinct neutrophilic dermatoses that rarely coexist but share several clinical and histopathologic features, which can become a diagnostic challenge.2 Both conditions can manifest clinically as abrupt-onset, tender, erythematous papules; vesiculopustular lesions; or bullae with ulcerative changes. They also exhibit pathergy; present with systemic symptoms such as pyrexia, malaise, and joint pain; are associated with underlying systemic conditions such as infections and/or malignancy; demonstrate a dense neutrophilic infiltrate in the dermis on histopathology; and respond promptly to systemic corticosteroids.2-6 Bullous Sweet syndrome, which can present as vesicles, pustules, or bullae that progress to superficial ulcerations, may represent a variant of neutrophilic dermatosis characterized by features seen in both Sweet syndrome and pyoderma gangrenosum, suggesting that these 2 conditions may be on a spectrum.5Clinical features such as erythema with a blue, gray, or purple hue; undermined and ragged borders; and healing of skin lesions with atrophic or cribriform scarring may favor pyoderma gangrenosum, whereas a dull red or plum color and resolution of lesions without scarring may support the diagnosis of Sweet syndrome.7 Although both conditions can exhibit pathergy secondary to minor skin trauma such as venipuncture and biopsies,2,3,5,8 Sweet syndrome rarely has been described to develop after surgery in a patient without a known history of the condition.9 In contrast, postsurgical pyoderma gangrenosum has been well described as secondary to the pathergy phenomenon.5
Our patient was favored to have pyoderma gangrenosum given the solitary lesion, its abrupt development after surgery, and the morphology of the lesion that exhibited a large violaceous to red ulcerative and exudative plaque with undermined borders with atrophic scarring. In patients with skin disease that cannot be distinguished with certainty as either Sweet syndrome or pyoderma gangrenosum, it is essential to recognize that, as neutrophilic dermatoses, both conditions can be managed with either the first-line treatment option of high-dose systemic steroids or one of the shared alternative first-line or second-line steroid-sparing treatments, such as dapsone and cyclosporine.2
Although the exact pathogenesis of pyoderma gangrenosum remains to be fully understood, paraneoplastic pyoderma gangrenosum is a frequently described phenomenon.10,11 Our patient’s history of multiple malignancies, both solid and hematologic, supports the likelihood of malignancy-induced pyoderma gangrenosum; however, given his history of MDS, several other conditions were ruled out prior to making the diagnosis of pyoderma gangrenosum.
Classically, neutrophilic dermatoses such as pyoderma gangrenosum have a dense dermal neutrophilic infiltrate. Concurrent myeloproliferative disorders can alter the maturation of leukocytes, subsequently leading to an atypical appearance of the inflammatory cells on histopathology. Further, in the setting of myeloproliferative disorders, conditions such as leukemia cutis, in which there can be a cutaneous infiltrate of immature or mature myeloid or lymphocytic cells, must be considered. To ensure our patient’s abdominal skin changes were not a cutaneous manifestation of hematologic malignancy, immunohistochemical staining with CD20 and CD3 was performed and showed only the rare presence of B and T lymphocytes, respectively. Staining with CD34 for lymphocytic and myeloid progenitor cells was negative in the dermal infiltrate and further reduced the likelihood of leukemia cutis. Alternatively, patients can have aleukemic cutaneous myeloid sarcoma or leukemia cutis without an underlying hematologic condition or with latent peripheral blood or bone marrow myeloproliferative disorder, but our patient’s history of MDS eliminated this possibility.12 After exclusion of cutaneous infiltration by malignant leukocytes, our patient was diagnosed with histiocytoid neutrophilic dermatosis.
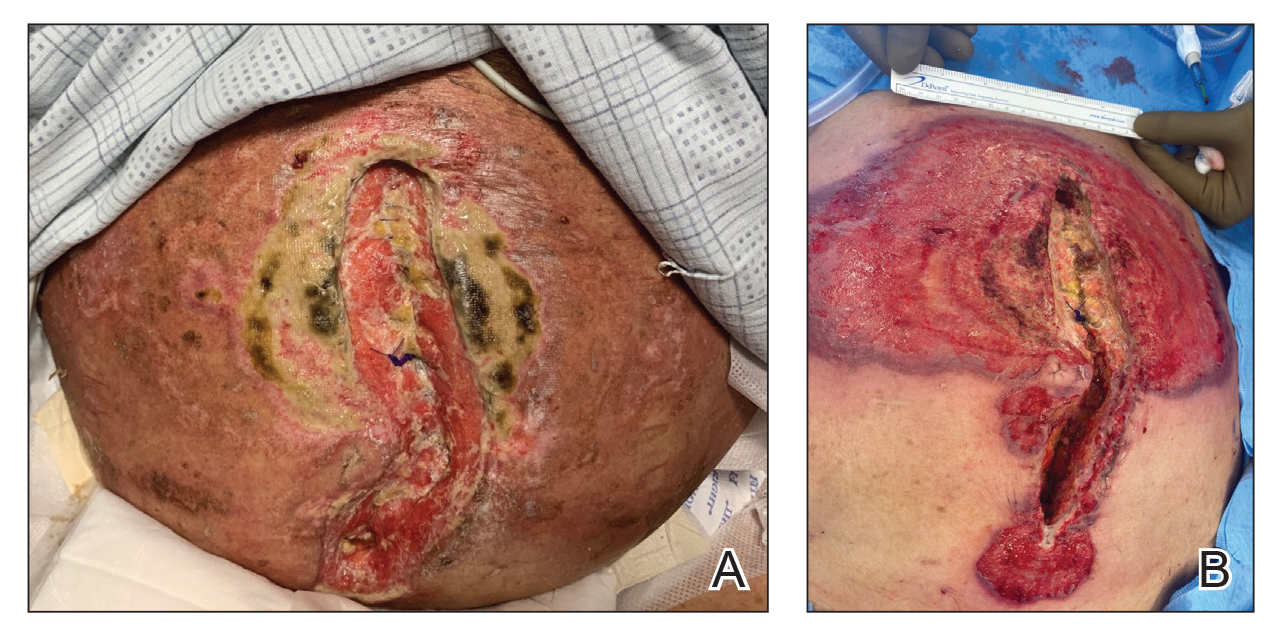
Multiple reports have described histiocytoid Sweet syndrome, in which there is a dense dermal histiocytoid infiltrate on histopathology that demonstrates myeloid lineage with immunologic staining.1,13 The typical pattern of histiocytoid Sweet syndrome includes a predominantly unaffected epidermis with papillary dermal edema, an absence of vasculitis, and a dense dermal infiltrate primarily composed of immature histiocytelike mononuclear cells with a basophilic elongated, twisted, or kidney-shaped nucleus and pale eosinophilic cytoplasm.1,13 In an analogous manner, Morin et al12 described a patient with congenital hypogammaglobulinemia who presented with lesions that clinically resembled pyoderma gangrenosum but revealed a dense dermal infiltrate mostly made of large immature histiocytoid mononuclear cells on histopathology, consistent with the histopathologic features observed in histiocytoid Sweet syndrome. The patient ultimately was diagnosed with histiocytoid pyoderma gangrenosum. Similarly, we believe that our patient also developed histiocytoid pyoderma gangrenosum. As with histiocytoid Sweet syndrome, this diagnosis is based on histopathologic and immunohistochemical findings of a dense dermal infiltrate composed of histiocyte-resembling immature neutrophils.
Typically, pyoderma gangrenosum responds promptly to treatment with systemic corticosteroids.4 Steroid-sparing agents such as cyclosporine, azathioprine, dapsone, and tumor necrosis factor α inhibitors also may be used.4,10 In the setting of MDS, clearance of pyoderma gangrenosum has been reported upon treatment of the underlying malignancy,14 high-dose systemic corticosteroids,11,15 cyclosporine with systemic steroids,16 thalidomide,17 combination therapy with thalidomide and interferon alfa-2a,18 and ustekinumab with vacuum-assisted closure therapy.19 Our patient’s histiocytoid pyoderma gangrenosum in the setting of solid and hematologic malignancy cleared rapidly with high-dose systemic hydrocortisone.
In the setting of malignancy, as in our patient, neutrophilic dermatoses may develop from an aberrant immune system or tumor-induced cytokine dysregulation that leads to increased neutrophil production or dysfunction.4,10,11 Although our patient’s MDS may have contributed to the atypical appearance of the dermal inflammatory infiltrate, it is unclear whether the hematologic disorder increased his risk for the histiocytoid variant of neutrophilic dermatoses. Alegría-Landa et al13 reported that histiocytoid Sweet syndrome is associated with hematologic malignancy at a similar frequency as classic Sweet syndrome. It is unknown if histiocytoid pyoderma gangrenosum would have a strong association with hematologic malignancy. Future reports may elucidate a better understanding of the histiocytoid subtype of pyoderma gangrenosum and its clinical implications.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10:301-312.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Wallach D, Vignon-Pennamen MD. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Lear JT, Atherton MT, Byrne JP. Neutrophilic dermatoses: pyoderma gangrenosum and Sweet’s syndrome. Postgrad Med. 1997;73:65-68.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:E74-E76.
- Shah M, Sachdeva M, Gefri A, et al. Paraneoplastic pyoderma gangrenosum in solid organ malignancy: a literature review. Int J Dermatol. 2020;59:154-158.
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359.
- Morin CB, Côté B, Belisle A. An interesting case of pyoderma gangrenosum with immature histiocytoid neutrophils. J Cutan Pathol. 2018;45:63-66.
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Saleh MFM, Saunthararajah Y. Severe pyoderma gangrenosum caused by myelodysplastic syndrome successfully treated with decitabine administered by a noncytotoxic regimen. Clin Case Rep. 2017;5:2025-2027.
- Yamauchi R, Ishida K, Iwashima Y, et al. Successful treatment of pyoderma gangrenosum that developed in a patient with myelodysplastic syndrome. J Infect Chemother. 2003;9:268-271.
- Ha JW, Hahm JE, Kim KS, et al. A case of pyoderma gangrenosum with myelodysplastic syndrome. Ann Dermatol. 2018;30:392-393.
- Malkan UY, Gunes G, Eliacik E, et al. Treatment of pyoderma gangrenosum with thalidomide in a myelodysplastic syndrome case. Int J Med Case Rep. 2016;9:61-64.
- Koca E, Duman AE, Cetiner D, et al. Successful treatment of myelodysplastic syndrome-induced pyoderma gangrenosum. Neth J Med. 2006;64:422-424.
- Nieto D, Sendagorta E, Rueda JM, et al. Successful treatment with ustekinumab and vacuum-assisted closure therapy in recalcitrant myelodysplastic syndrome-associated pyoderma gangrenosum: case report and literature review. Clin Exp Dermatol. 2019;44:116-119.
To the Editor:
Neutrophilic dermatoses—a group of inflammatory cutaneous conditions—include acute febrile neutrophilic dermatosis (Sweet syndrome), pyoderma gangrenosum, and neutrophilic dermatosis of the dorsal hands. Histopathology shows a dense dermal infiltrate of mature neutrophils. In 2005, the histiocytoid subtype of Sweet syndrome was introduced with histopathologic findings of a dermal infiltrate composed of immature myeloid cells that resemble histiocytes in appearance but stain strongly with neutrophil markers on immunohistochemistry.1 We present a case of histiocytoid pyoderma gangrenosum with histopathology that showed a dense dermal histiocytoid infiltrate with strong positivity for neutrophil markers on immunohistochemistry.
An 85-year-old man was seen by dermatology in the inpatient setting for a new-onset painful abdominal wound. He had a medical history of myelodysplastic syndrome (MDS), high-grade invasive papillary urothelial carcinoma of the bladder, and a recent diagnosis of low-grade invasive ascending colon adenocarcinoma. Ten days prior he underwent a right colectomy without intraoperative complications that was followed by septic shock. Workup with urinalysis and urine culture showed minimal pyuria with Pseudomonas aeruginosa. Additional studies, including blood cultures, abdominal wound cultures, computed tomography of the abdomen and pelvis, renal ultrasound, and chest radiographs, were unremarkable and showed no signs of surgical site infection, intra-abdominal or pelvic abscess formation, or pulmonary embolism. Broad-spectrum antibiotics—vancomycin and piperacillin-tazobactam—were started. Persistent fever (Tmax of 102.3 °F [39.1 °C]) and leukocytosis (45.3×109/L [4.2–10×109/L]) despite antibiotic therapy, increasing pressor requirements, and progressive painful erythema and purulence at the abdominal surgical site led to debridement of the wound by the general surgery team on day 9 following the initial surgery due to suspected necrotizing infection. Within 24 hours, dermatology was consulted for continued rapid expansion of the wound. Physical examination of the abdomen revealed a large, well-demarcated, pink-red, indurated, ulcerated plaque with clear to purulent exudate and superficial erosions with violaceous undermined borders extending centrifugally from the abdominal surgical incision line (Figure 1A). Two punch biopsies sent for histopathologic evaluation and tissue culture showed dermal edema with a dense histiocytic infiltrate with nodular foci and admixed mature neutrophils to a lesser degree (Figure 2). Special staining was negative for bacteria, fungi, and mycobacteria. Immunohistochemistry revealed positive staining of the dermal inflammatory infiltrate with CD68, myeloperoxidase, and lysozyme, as well as negative staining with CD34 (Figure 3). These findings were suggestive of a histiocytoid neutrophilic dermatosis such as Sweet syndrome or pyoderma gangrenosum. Due to the morphology of the solitary lesion and the abrupt exacerbation shortly after surgical intervention, the patient was diagnosed with histiocytoid pyoderma gangrenosum. At the same time, the patient’s septic shock was treated with intravenous hydrocortisone (100 mg 3 times daily) for 2 days and also achieved a prompt response in the cutaneous symptoms (Figure 1B).
Sweet syndrome and pyoderma gangrenosum are considered distinct neutrophilic dermatoses that rarely coexist but share several clinical and histopathologic features, which can become a diagnostic challenge.2 Both conditions can manifest clinically as abrupt-onset, tender, erythematous papules; vesiculopustular lesions; or bullae with ulcerative changes. They also exhibit pathergy; present with systemic symptoms such as pyrexia, malaise, and joint pain; are associated with underlying systemic conditions such as infections and/or malignancy; demonstrate a dense neutrophilic infiltrate in the dermis on histopathology; and respond promptly to systemic corticosteroids.2-6 Bullous Sweet syndrome, which can present as vesicles, pustules, or bullae that progress to superficial ulcerations, may represent a variant of neutrophilic dermatosis characterized by features seen in both Sweet syndrome and pyoderma gangrenosum, suggesting that these 2 conditions may be on a spectrum.5Clinical features such as erythema with a blue, gray, or purple hue; undermined and ragged borders; and healing of skin lesions with atrophic or cribriform scarring may favor pyoderma gangrenosum, whereas a dull red or plum color and resolution of lesions without scarring may support the diagnosis of Sweet syndrome.7 Although both conditions can exhibit pathergy secondary to minor skin trauma such as venipuncture and biopsies,2,3,5,8 Sweet syndrome rarely has been described to develop after surgery in a patient without a known history of the condition.9 In contrast, postsurgical pyoderma gangrenosum has been well described as secondary to the pathergy phenomenon.5
Our patient was favored to have pyoderma gangrenosum given the solitary lesion, its abrupt development after surgery, and the morphology of the lesion that exhibited a large violaceous to red ulcerative and exudative plaque with undermined borders with atrophic scarring. In patients with skin disease that cannot be distinguished with certainty as either Sweet syndrome or pyoderma gangrenosum, it is essential to recognize that, as neutrophilic dermatoses, both conditions can be managed with either the first-line treatment option of high-dose systemic steroids or one of the shared alternative first-line or second-line steroid-sparing treatments, such as dapsone and cyclosporine.2
Although the exact pathogenesis of pyoderma gangrenosum remains to be fully understood, paraneoplastic pyoderma gangrenosum is a frequently described phenomenon.10,11 Our patient’s history of multiple malignancies, both solid and hematologic, supports the likelihood of malignancy-induced pyoderma gangrenosum; however, given his history of MDS, several other conditions were ruled out prior to making the diagnosis of pyoderma gangrenosum.
Classically, neutrophilic dermatoses such as pyoderma gangrenosum have a dense dermal neutrophilic infiltrate. Concurrent myeloproliferative disorders can alter the maturation of leukocytes, subsequently leading to an atypical appearance of the inflammatory cells on histopathology. Further, in the setting of myeloproliferative disorders, conditions such as leukemia cutis, in which there can be a cutaneous infiltrate of immature or mature myeloid or lymphocytic cells, must be considered. To ensure our patient’s abdominal skin changes were not a cutaneous manifestation of hematologic malignancy, immunohistochemical staining with CD20 and CD3 was performed and showed only the rare presence of B and T lymphocytes, respectively. Staining with CD34 for lymphocytic and myeloid progenitor cells was negative in the dermal infiltrate and further reduced the likelihood of leukemia cutis. Alternatively, patients can have aleukemic cutaneous myeloid sarcoma or leukemia cutis without an underlying hematologic condition or with latent peripheral blood or bone marrow myeloproliferative disorder, but our patient’s history of MDS eliminated this possibility.12 After exclusion of cutaneous infiltration by malignant leukocytes, our patient was diagnosed with histiocytoid neutrophilic dermatosis.


Multiple reports have described histiocytoid Sweet syndrome, in which there is a dense dermal histiocytoid infiltrate on histopathology that demonstrates myeloid lineage with immunologic staining.1,13 The typical pattern of histiocytoid Sweet syndrome includes a predominantly unaffected epidermis with papillary dermal edema, an absence of vasculitis, and a dense dermal infiltrate primarily composed of immature histiocytelike mononuclear cells with a basophilic elongated, twisted, or kidney-shaped nucleus and pale eosinophilic cytoplasm.1,13 In an analogous manner, Morin et al12 described a patient with congenital hypogammaglobulinemia who presented with lesions that clinically resembled pyoderma gangrenosum but revealed a dense dermal infiltrate mostly made of large immature histiocytoid mononuclear cells on histopathology, consistent with the histopathologic features observed in histiocytoid Sweet syndrome. The patient ultimately was diagnosed with histiocytoid pyoderma gangrenosum. Similarly, we believe that our patient also developed histiocytoid pyoderma gangrenosum. As with histiocytoid Sweet syndrome, this diagnosis is based on histopathologic and immunohistochemical findings of a dense dermal infiltrate composed of histiocyte-resembling immature neutrophils.

Typically, pyoderma gangrenosum responds promptly to treatment with systemic corticosteroids.4 Steroid-sparing agents such as cyclosporine, azathioprine, dapsone, and tumor necrosis factor α inhibitors also may be used.4,10 In the setting of MDS, clearance of pyoderma gangrenosum has been reported upon treatment of the underlying malignancy,14 high-dose systemic corticosteroids,11,15 cyclosporine with systemic steroids,16 thalidomide,17 combination therapy with thalidomide and interferon alfa-2a,18 and ustekinumab with vacuum-assisted closure therapy.19 Our patient’s histiocytoid pyoderma gangrenosum in the setting of solid and hematologic malignancy cleared rapidly with high-dose systemic hydrocortisone.
In the setting of malignancy, as in our patient, neutrophilic dermatoses may develop from an aberrant immune system or tumor-induced cytokine dysregulation that leads to increased neutrophil production or dysfunction.4,10,11 Although our patient’s MDS may have contributed to the atypical appearance of the dermal inflammatory infiltrate, it is unclear whether the hematologic disorder increased his risk for the histiocytoid variant of neutrophilic dermatoses. Alegría-Landa et al13 reported that histiocytoid Sweet syndrome is associated with hematologic malignancy at a similar frequency as classic Sweet syndrome. It is unknown if histiocytoid pyoderma gangrenosum would have a strong association with hematologic malignancy. Future reports may elucidate a better understanding of the histiocytoid subtype of pyoderma gangrenosum and its clinical implications.
To the Editor:
Neutrophilic dermatoses—a group of inflammatory cutaneous conditions—include acute febrile neutrophilic dermatosis (Sweet syndrome), pyoderma gangrenosum, and neutrophilic dermatosis of the dorsal hands. Histopathology shows a dense dermal infiltrate of mature neutrophils. In 2005, the histiocytoid subtype of Sweet syndrome was introduced with histopathologic findings of a dermal infiltrate composed of immature myeloid cells that resemble histiocytes in appearance but stain strongly with neutrophil markers on immunohistochemistry.1 We present a case of histiocytoid pyoderma gangrenosum with histopathology that showed a dense dermal histiocytoid infiltrate with strong positivity for neutrophil markers on immunohistochemistry.
An 85-year-old man was seen by dermatology in the inpatient setting for a new-onset painful abdominal wound. He had a medical history of myelodysplastic syndrome (MDS), high-grade invasive papillary urothelial carcinoma of the bladder, and a recent diagnosis of low-grade invasive ascending colon adenocarcinoma. Ten days prior he underwent a right colectomy without intraoperative complications that was followed by septic shock. Workup with urinalysis and urine culture showed minimal pyuria with Pseudomonas aeruginosa. Additional studies, including blood cultures, abdominal wound cultures, computed tomography of the abdomen and pelvis, renal ultrasound, and chest radiographs, were unremarkable and showed no signs of surgical site infection, intra-abdominal or pelvic abscess formation, or pulmonary embolism. Broad-spectrum antibiotics—vancomycin and piperacillin-tazobactam—were started. Persistent fever (Tmax of 102.3 °F [39.1 °C]) and leukocytosis (45.3×109/L [4.2–10×109/L]) despite antibiotic therapy, increasing pressor requirements, and progressive painful erythema and purulence at the abdominal surgical site led to debridement of the wound by the general surgery team on day 9 following the initial surgery due to suspected necrotizing infection. Within 24 hours, dermatology was consulted for continued rapid expansion of the wound. Physical examination of the abdomen revealed a large, well-demarcated, pink-red, indurated, ulcerated plaque with clear to purulent exudate and superficial erosions with violaceous undermined borders extending centrifugally from the abdominal surgical incision line (Figure 1A). Two punch biopsies sent for histopathologic evaluation and tissue culture showed dermal edema with a dense histiocytic infiltrate with nodular foci and admixed mature neutrophils to a lesser degree (Figure 2). Special staining was negative for bacteria, fungi, and mycobacteria. Immunohistochemistry revealed positive staining of the dermal inflammatory infiltrate with CD68, myeloperoxidase, and lysozyme, as well as negative staining with CD34 (Figure 3). These findings were suggestive of a histiocytoid neutrophilic dermatosis such as Sweet syndrome or pyoderma gangrenosum. Due to the morphology of the solitary lesion and the abrupt exacerbation shortly after surgical intervention, the patient was diagnosed with histiocytoid pyoderma gangrenosum. At the same time, the patient’s septic shock was treated with intravenous hydrocortisone (100 mg 3 times daily) for 2 days and also achieved a prompt response in the cutaneous symptoms (Figure 1B).
Sweet syndrome and pyoderma gangrenosum are considered distinct neutrophilic dermatoses that rarely coexist but share several clinical and histopathologic features, which can become a diagnostic challenge.2 Both conditions can manifest clinically as abrupt-onset, tender, erythematous papules; vesiculopustular lesions; or bullae with ulcerative changes. They also exhibit pathergy; present with systemic symptoms such as pyrexia, malaise, and joint pain; are associated with underlying systemic conditions such as infections and/or malignancy; demonstrate a dense neutrophilic infiltrate in the dermis on histopathology; and respond promptly to systemic corticosteroids.2-6 Bullous Sweet syndrome, which can present as vesicles, pustules, or bullae that progress to superficial ulcerations, may represent a variant of neutrophilic dermatosis characterized by features seen in both Sweet syndrome and pyoderma gangrenosum, suggesting that these 2 conditions may be on a spectrum.5Clinical features such as erythema with a blue, gray, or purple hue; undermined and ragged borders; and healing of skin lesions with atrophic or cribriform scarring may favor pyoderma gangrenosum, whereas a dull red or plum color and resolution of lesions without scarring may support the diagnosis of Sweet syndrome.7 Although both conditions can exhibit pathergy secondary to minor skin trauma such as venipuncture and biopsies,2,3,5,8 Sweet syndrome rarely has been described to develop after surgery in a patient without a known history of the condition.9 In contrast, postsurgical pyoderma gangrenosum has been well described as secondary to the pathergy phenomenon.5
Our patient was favored to have pyoderma gangrenosum given the solitary lesion, its abrupt development after surgery, and the morphology of the lesion that exhibited a large violaceous to red ulcerative and exudative plaque with undermined borders with atrophic scarring. In patients with skin disease that cannot be distinguished with certainty as either Sweet syndrome or pyoderma gangrenosum, it is essential to recognize that, as neutrophilic dermatoses, both conditions can be managed with either the first-line treatment option of high-dose systemic steroids or one of the shared alternative first-line or second-line steroid-sparing treatments, such as dapsone and cyclosporine.2
Although the exact pathogenesis of pyoderma gangrenosum remains to be fully understood, paraneoplastic pyoderma gangrenosum is a frequently described phenomenon.10,11 Our patient’s history of multiple malignancies, both solid and hematologic, supports the likelihood of malignancy-induced pyoderma gangrenosum; however, given his history of MDS, several other conditions were ruled out prior to making the diagnosis of pyoderma gangrenosum.
Classically, neutrophilic dermatoses such as pyoderma gangrenosum have a dense dermal neutrophilic infiltrate. Concurrent myeloproliferative disorders can alter the maturation of leukocytes, subsequently leading to an atypical appearance of the inflammatory cells on histopathology. Further, in the setting of myeloproliferative disorders, conditions such as leukemia cutis, in which there can be a cutaneous infiltrate of immature or mature myeloid or lymphocytic cells, must be considered. To ensure our patient’s abdominal skin changes were not a cutaneous manifestation of hematologic malignancy, immunohistochemical staining with CD20 and CD3 was performed and showed only the rare presence of B and T lymphocytes, respectively. Staining with CD34 for lymphocytic and myeloid progenitor cells was negative in the dermal infiltrate and further reduced the likelihood of leukemia cutis. Alternatively, patients can have aleukemic cutaneous myeloid sarcoma or leukemia cutis without an underlying hematologic condition or with latent peripheral blood or bone marrow myeloproliferative disorder, but our patient’s history of MDS eliminated this possibility.12 After exclusion of cutaneous infiltration by malignant leukocytes, our patient was diagnosed with histiocytoid neutrophilic dermatosis.


Multiple reports have described histiocytoid Sweet syndrome, in which there is a dense dermal histiocytoid infiltrate on histopathology that demonstrates myeloid lineage with immunologic staining.1,13 The typical pattern of histiocytoid Sweet syndrome includes a predominantly unaffected epidermis with papillary dermal edema, an absence of vasculitis, and a dense dermal infiltrate primarily composed of immature histiocytelike mononuclear cells with a basophilic elongated, twisted, or kidney-shaped nucleus and pale eosinophilic cytoplasm.1,13 In an analogous manner, Morin et al12 described a patient with congenital hypogammaglobulinemia who presented with lesions that clinically resembled pyoderma gangrenosum but revealed a dense dermal infiltrate mostly made of large immature histiocytoid mononuclear cells on histopathology, consistent with the histopathologic features observed in histiocytoid Sweet syndrome. The patient ultimately was diagnosed with histiocytoid pyoderma gangrenosum. Similarly, we believe that our patient also developed histiocytoid pyoderma gangrenosum. As with histiocytoid Sweet syndrome, this diagnosis is based on histopathologic and immunohistochemical findings of a dense dermal infiltrate composed of histiocyte-resembling immature neutrophils.

Typically, pyoderma gangrenosum responds promptly to treatment with systemic corticosteroids.4 Steroid-sparing agents such as cyclosporine, azathioprine, dapsone, and tumor necrosis factor α inhibitors also may be used.4,10 In the setting of MDS, clearance of pyoderma gangrenosum has been reported upon treatment of the underlying malignancy,14 high-dose systemic corticosteroids,11,15 cyclosporine with systemic steroids,16 thalidomide,17 combination therapy with thalidomide and interferon alfa-2a,18 and ustekinumab with vacuum-assisted closure therapy.19 Our patient’s histiocytoid pyoderma gangrenosum in the setting of solid and hematologic malignancy cleared rapidly with high-dose systemic hydrocortisone.
In the setting of malignancy, as in our patient, neutrophilic dermatoses may develop from an aberrant immune system or tumor-induced cytokine dysregulation that leads to increased neutrophil production or dysfunction.4,10,11 Although our patient’s MDS may have contributed to the atypical appearance of the dermal inflammatory infiltrate, it is unclear whether the hematologic disorder increased his risk for the histiocytoid variant of neutrophilic dermatoses. Alegría-Landa et al13 reported that histiocytoid Sweet syndrome is associated with hematologic malignancy at a similar frequency as classic Sweet syndrome. It is unknown if histiocytoid pyoderma gangrenosum would have a strong association with hematologic malignancy. Future reports may elucidate a better understanding of the histiocytoid subtype of pyoderma gangrenosum and its clinical implications.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10:301-312.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Wallach D, Vignon-Pennamen MD. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Lear JT, Atherton MT, Byrne JP. Neutrophilic dermatoses: pyoderma gangrenosum and Sweet’s syndrome. Postgrad Med. 1997;73:65-68.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:E74-E76.
- Shah M, Sachdeva M, Gefri A, et al. Paraneoplastic pyoderma gangrenosum in solid organ malignancy: a literature review. Int J Dermatol. 2020;59:154-158.
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359.
- Morin CB, Côté B, Belisle A. An interesting case of pyoderma gangrenosum with immature histiocytoid neutrophils. J Cutan Pathol. 2018;45:63-66.
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Saleh MFM, Saunthararajah Y. Severe pyoderma gangrenosum caused by myelodysplastic syndrome successfully treated with decitabine administered by a noncytotoxic regimen. Clin Case Rep. 2017;5:2025-2027.
- Yamauchi R, Ishida K, Iwashima Y, et al. Successful treatment of pyoderma gangrenosum that developed in a patient with myelodysplastic syndrome. J Infect Chemother. 2003;9:268-271.
- Ha JW, Hahm JE, Kim KS, et al. A case of pyoderma gangrenosum with myelodysplastic syndrome. Ann Dermatol. 2018;30:392-393.
- Malkan UY, Gunes G, Eliacik E, et al. Treatment of pyoderma gangrenosum with thalidomide in a myelodysplastic syndrome case. Int J Med Case Rep. 2016;9:61-64.
- Koca E, Duman AE, Cetiner D, et al. Successful treatment of myelodysplastic syndrome-induced pyoderma gangrenosum. Neth J Med. 2006;64:422-424.
- Nieto D, Sendagorta E, Rueda JM, et al. Successful treatment with ustekinumab and vacuum-assisted closure therapy in recalcitrant myelodysplastic syndrome-associated pyoderma gangrenosum: case report and literature review. Clin Exp Dermatol. 2019;44:116-119.
- Requena L, Kutzner H, Palmedo G, et al. Histiocytoid Sweet syndrome: a dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol. 2005;141:834-842.
- Cohen PR. Neutrophilic dermatoses: a review of current treatment options. Am J Clin Dermatol. 2009;10:301-312.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Braswell SF, Kostopoulos TC, Ortega-Loayza AG. Pathophysiology of pyoderma gangrenosum (PG): an updated review. J Am Acad Dermatol. 2015;73:691-698.
- Wallach D, Vignon-Pennamen MD. Pyoderma gangrenosum and Sweet syndrome: the prototypic neutrophilic dermatoses. Br J Dermatol. 2018;178:595-602.
- Walling HW, Snipes CJ, Gerami P, et al. The relationship between neutrophilic dermatosis of the dorsal hands and Sweet syndrome: report of 9 cases and comparison to atypical pyoderma gangrenosum. Arch Dermatol. 2006;142:57-63.
- Lear JT, Atherton MT, Byrne JP. Neutrophilic dermatoses: pyoderma gangrenosum and Sweet’s syndrome. Postgrad Med. 1997;73:65-68.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006.
- Minocha R, Sebaratnam DF, Choi JY. Sweet’s syndrome following surgery: cutaneous trauma as a possible aetiological co-factor in neutrophilic dermatoses. Australas J Dermatol. 2015;56:E74-E76.
- Shah M, Sachdeva M, Gefri A, et al. Paraneoplastic pyoderma gangrenosum in solid organ malignancy: a literature review. Int J Dermatol. 2020;59:154-158.
- Montagnon CM, Fracica EA, Patel AA, et al. Pyoderma gangrenosum in hematologic malignancies: a systematic review. J Am Acad Dermatol. 2020;82:1346-1359.
- Morin CB, Côté B, Belisle A. An interesting case of pyoderma gangrenosum with immature histiocytoid neutrophils. J Cutan Pathol. 2018;45:63-66.
- Alegría-Landa V, Rodríguez-Pinilla SM, Santos-Briz A, et al. Clinicopathologic, immunohistochemical, and molecular features of histiocytoid Sweet syndrome. JAMA Dermatol. 2017;153:651-659.
- Saleh MFM, Saunthararajah Y. Severe pyoderma gangrenosum caused by myelodysplastic syndrome successfully treated with decitabine administered by a noncytotoxic regimen. Clin Case Rep. 2017;5:2025-2027.
- Yamauchi R, Ishida K, Iwashima Y, et al. Successful treatment of pyoderma gangrenosum that developed in a patient with myelodysplastic syndrome. J Infect Chemother. 2003;9:268-271.
- Ha JW, Hahm JE, Kim KS, et al. A case of pyoderma gangrenosum with myelodysplastic syndrome. Ann Dermatol. 2018;30:392-393.
- Malkan UY, Gunes G, Eliacik E, et al. Treatment of pyoderma gangrenosum with thalidomide in a myelodysplastic syndrome case. Int J Med Case Rep. 2016;9:61-64.
- Koca E, Duman AE, Cetiner D, et al. Successful treatment of myelodysplastic syndrome-induced pyoderma gangrenosum. Neth J Med. 2006;64:422-424.
- Nieto D, Sendagorta E, Rueda JM, et al. Successful treatment with ustekinumab and vacuum-assisted closure therapy in recalcitrant myelodysplastic syndrome-associated pyoderma gangrenosum: case report and literature review. Clin Exp Dermatol. 2019;44:116-119.
Practice Points:
- Dermatologists and dermatopathologists should be aware of the histiocytoid variant of pyoderma gangrenosum, which can clinical and histologic features that overlap with histiocytoid Sweet syndrome.
- When considering a diagnosis of histiocytoid neutrophilic dermatoses, leukemia cutis or aleukemic cutaneous myeloid sarcoma should be ruled out.
- Similar to histiocytoid Sweet syndrome and neutrophilic dermatoses in the setting of hematologic or solid organ malignancy, histiocytoid pyoderma gangrenosum may respond well to high-dose systemic corticosteroids.
Urticaria Linked to Higher Cancer Risk, Study Finds
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
which decreased to 6% in subsequent years, in a cohort study using Danish healthcare databases.
METHODOLOGY:
- Researchers conducted a retrospective cohort study using data from Danish healthcare registries and compared the incident cancer risk between patients with urticaria and the risk in the general population.
- They identified 87,507 patients (58% women) with a primary or secondary first-time hospital outpatient clinic, emergency room, or inpatient diagnosis of urticaria between 1980 and 2022, who were followed for a median of 10.1 years.
- Incident cancers, including nonmelanoma skin cancer, were identified using the Danish Cancer Registry and classified by the extent of spread at the time of diagnosis.
- This study computed the absolute cancer risk within the first year of an urticaria diagnosis and standardized incidence ratios (SIRs), with 95% CIs standardized to Danish national cancer rates.
TAKEAWAY:
- For the first year of follow-up, the absolute risk for all cancer types was 0.7%, and it was 29.5% for subsequent years. The overall SIR for all types of cancer was 1.09 (95% CI, 1.06-1.11), which was based on 7788 observed cancer cases compared with 7161 cases expected over the entire follow-up period.
- Within the first year of follow-up, 588 patients with urticaria were diagnosed with cancer, for an SIR of 1.49 (95% CI, 1.38-1.62) for all cancer types.
- After the first year, the SIR for all cancer sites decreased and stabilized at 1.06 (95% CI, 1.04-1.09), with 7200 observed cancer cases.
- The risk was highest for hematological cancers in the first year, particularly Hodgkin lymphoma (SIR, 5.35; 95% CI, 2.56-9.85).
IN PRACTICE:
“Our study suggests that urticaria may be a marker of occult cancer and that it is associated with a slightly increased long-term cancer risk,” the authors wrote.
SOURCE:
The study was led by Sissel B.T. Sørensen, departments of dermatology and rheumatology, Aarhus University Hospital, Aarhus, Denmark. It was published online on June 27, 2024, in the British Journal of Dermatology.
LIMITATIONS:
The study is limited by its observational design and reliance on registry data, which may be subject to misclassification or incomplete information. In addition, the study could not assess individual patient factors such as lifestyle or genetic predispositions that may influence cancer risk, and the results may not be generalizable to other populations. Finally, the exact biologic mechanisms linking urticaria and cancer remain unclear, warranting further investigation.
DISCLOSURES:
The study did not receive any funding. The authors reported that they had no relevant conflicts of interest.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Time Warp: Fax Machines Still Common in Oncology Practice. Why?
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.
One minute, he’s working on sequencing a tumor genome. The next, he’s sifting through pages of disorganized data from a device that has been around for decades: the fax machine.
“If two doctors’ offices aren’t on the same electronic medical record, one of the main ways to transfer records is still by fax,” said Dr. Lewis, director of gastrointestinal oncology at Intermountain Healthcare in Murray, Utah. “I can go from cutting-edge innovation to relying on, at best, 1980s information technology. It just boggles my mind.”
Dr. Lewis, who has posted about his frustration with fax machines, is far from alone. Oncologists are among the many specialists across the country at the mercy of telecopiers.
According to a 2021 report by the Office of the National Coordinator for Health Information Technology, fax and mail continue to be the most common methods for hospitals and health systems to exchange care record summaries. In 2019, nearly 8 in 10 hospitals used mail or fax to send and receive health information, the report found.
Fax machines are still commonplace across the healthcare spectrum, said Robert Havasy, MS, senior director for informatics strategy at the Healthcare Information and Management Systems Society (HIMSS). Inertia, cost, and more pressing priorities for hospitals and medical institutions contribute to the technology sticking around, he explained.
“Post-COVID, my guess is we’re still at over 50% of healthcare practices using fax for some reason, on a daily basis,” Mr. Havasy said in an interview. “A lot of hospitals just don’t have the time, the money, or the staff to fix that problem because there’s always something a little higher up the priority chain they need to focus on.”
If, for instance, “you’re going to do a process redesign to reduce hospital total acquired infections, your fax machine replacement might be 10th or 12th on the list. It just never gets up to 1 or 2 because it’s ‘not that much of a problem,’ ” he added.
Or is it?
Administrators may not view fax machines as a top concern, but clinicians who deal with the machines daily see it differently.
“What worries me is we’re taking records out of an electronic storehouse [and] converting them to a paper medium,” Dr. Lewis said. “And then we are scanning into another electronic storehouse. The more steps, the more can be lost.”
And when information is lost, patient care can be compromised.
Slower Workflows, Care Concerns
Although there are no published data on fax machine use in oncology specifically, this outdated technology does come into play in a variety of ways along the cancer care continuum.
Radiation oncologist David R. Penberthy, MD, said patients often seek his cancer center’s expertise for second opinions, and that requires collecting patient records from many different practices.
“Ideally, it would come electronically, but sometimes it does come by fax,” said Dr. Penberthy, program director of radiation oncology at the University of Virginia School of Medicine in Charlottesville. “The quality of the fax is not always the best. Sometimes it’s literally a fax of a fax. You’re reading something that’s very difficult to read.”
Orders for new tests are also typically sent and received via fax temporarily while IT teams work to integrate them into the electronic health record (EHR), Dr. Penberthy said.
Insurers and third-party laboratories often send test results back by fax as well.
“Even if I haven’t actually sent my patient out of our institution, this crucial result may only be entered back into the record as a scanned document from a fax, which is not great because it can get lost in the other results that are reported electronically,” Dr. Lewis said. The risk here is that an ordering physician won’t see these results, which can lead to delayed or overlooked care for patients, he explained.
“To me, it’s like a blind spot,” Dr. Lewis said. “Every time we use a fax, I see it actually as an opportunity for oversight and missed opportunity to collect data.”
Dr. Penberthy said faxing can slow things down at his practice, particularly if he faxes a document to another office but receives no confirmation and has to track down what happened.
As for cybersecurity, data that are in transit during faxing are generally considered secure and compliant with the Health Insurance Portability and Accountability Act (HIPAA), said Mr. Havasy of HIMSS. However, the Privacy Rule also requires that data remain secure while at rest, which isn’t always possible, he added.
“That’s where faxes fall down, because generally fax machines are in public, if you will, or open areas in a hospital,” he said. “They just sit on a desk. I don’t know that the next nurse who comes up and looks through that stack was the nurse who was treating the patient.”
Important decisions or results can also be missed when sent by fax, creating headaches for physicians and care problems for patients.
Dr. Lewis recently experienced an insurance-related fax mishap over Memorial Day weekend. He believed his patient had access to the antinausea medication he had prescribed. When Dr. Lewis happened to check the fax machine over the weekend, he found a coverage denial for the medication from the insurer but, at that point, had no recourse to appeal because it was a long holiday weekend.
“Had the denial been sent by an electronic means that was quicker and more readily available, it would have been possible to appeal before the holiday weekend,” he said.
Hematologist Aaron Goodman, MD, encountered a similar problem after an insurer denied coverage of an expensive cancer drug for a patient and faxed over its reason for the denial. Dr. Goodman was not directly notified that the information arrived and didn’t learn about the denial for a week, he said.
“There’s no ‘ding’ in my inbox if something is faxed over and scanned,” said Dr. Goodman, associate professor of medicine at UC San Diego Health. “Once I realized it was denied, I was able to rectify it, but it wasted a week of a patient not getting a drug that I felt would be beneficial for them.”
Broader Health Policy Impacts
The use of outdated technology, such as fax machines, also creates ripple effects that burden the health system, health policy experts say.
Duplicate testing and unnecessary care are top impacts, said Julia Adler-Milstein, PhD, professor of medicine and chief of the division of clinical informatics and digital transformation at the University of California, San Francisco.
Studies show that 20%-30% of the $65 billion spent annually on lab tests is used on unnecessary duplicate tests, and another estimated $30 billion is spent each year on unnecessary duplicate medical imaging. These duplicate tests may be mitigated if hospitals adopt certified EHR technology, research shows.
Still, without EHR interoperability between institutions, new providers may be unaware that tests or past labs for patients exist, leading to repeat tests, said Dr. Adler-Milstein, who researches health IT policy with a focus on EHRs. Patients can sometimes fill in the gaps, but not always.
“Fax machines only help close information gaps if the clinician is aware of where to seek out the information and there is someone at the other organization to locate and transmit the information in a timely manner,” Dr. Adler-Milstein said.
Old technology and poor interoperability also greatly affect data collection for disease surveillance and monitoring, said Janet Hamilton, MPH, executive director for the Council of State and Territorial Epidemiologists. This issue was keenly demonstrated during the pandemic, Ms. Hamilton said.
“It was tragic, quite honestly,” she said. “There was such an immense amount of data that needed to be moved quickly, and that’s when computers are at their best.”
But, she said, “we didn’t have the level of systems in place to do it well.”
Specifically, the lack of electronic case reporting in place during the pandemic — where diagnoses are documented in the record and then immediately sent to the public health system — led to reports that were delayed, not made, or had missing or incomplete information, such as patients’ race and ethnicity or other health conditions, Ms. Hamilton said.
Incomplete or missing data hampered the ability of public health officials and researchers to understand how the virus might affect different patients.
“If you had a chronic condition like cancer, you were less likely to have a positive outcome with COVID,” Ms. Hamilton said. “But because electronic case reporting was not in place, we didn’t get some of those additional pieces of information. We didn’t have people’s underlying oncology status to then say, ‘Here are individuals with these types of characteristics, and these are the things that happen if they also have a cancer.’”
Slow, but Steady, Improvements
Efforts at the state and federal levels have targeted improved health information exchange, but progress takes time, Dr. Adler-Milstein said.
Most states have some form of health information exchange, such as statewide exchanges, regional health information organizations, or clinical data registries. Maryland is often held up as a notable example for its health information exchange, Dr. Adler-Milstein noted.
According to Maryland law, all hospitals under the jurisdiction of the Maryland Health Care Commission are required to electronically connect to the state-designated health information exchange. In 2012, Maryland became the first state to connect all its 46 acute care hospitals in the sharing of real-time data.
The Health Information Technology for Economic and Clinical Health (HITECH) Act provided federal-enhanced Medicaid matching funds to states through 2021 to support efforts to advance electronic exchange. Nearly all states used these funds, and most have identified other sources to sustain the efforts, according to a recent US Government Accountability Office (GAO) report. However, GAO found that small and rural providers are less likely to have the financial and technological resources to participate in or maintain electronic exchange capabilities.
Nationally, several recent initiatives have targeted health data interoperability, including for cancer care. The Centers for Disease Control and Prevention’s Data Modernization Initiative is a multiyear, multi–billion-dollar effort to improve data sharing across the federal and state public health landscape.
Meanwhile, in March 2024, the Biden-Harris administration launched United States Core Data for Interoperability Plus Cancer. The program will define a recommended minimum set of cancer-related data to be included in a patient’s EHR to enhance data exchange for research and clinical care.
EHR vendors are also key to improving the landscape, said Dr. Adler-Milstein. Vendors such as Epic have developed strong sharing capabilities for transmitting health information from site to site, but of course, that only helps if providers have Epic, she said.
“That’s where these national frameworks should help, because we don’t want it to break down by what EHR vendor you have,” she said. “It’s a patchwork. You can go to some places and hear success stories because they have Epic or a state health information exchange, but it’s very heterogeneous. In some places, they have nothing and are using a fax machine.”
Mr. Havasy believes fax machines will ultimately go extinct, particularly as a younger, more digitally savvy generation enters the healthcare workforce. He also foresees that the growing use of artificial intelligence will help eradicate the outdated technology.
But, Ms. Hamilton noted, “unless we have consistent, ongoing, sustained funding, it is very hard to move off [an older] technology that can work. That’s one of the biggest barriers.”
“Public health is about protecting the lives of every single person everywhere,” Ms. Hamilton said, “but when we don’t have the data that comes into the system, we can’t achieve our mission.”
A version of this article appeared on Medscape.com.