User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
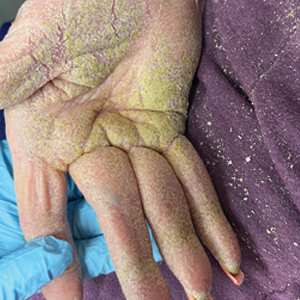
Second Treatment for Prurigo Nodularis Approved by FDA
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
On August 13, 2024, the
A first-in-class monoclonal antibody specifically designed to inhibit interleukin (IL)–31 signaling, nemolizumab, will be available in a prefilled pen for subcutaneous injection and will be marketed as Nemluvio. It is currently under FDA review for treating atopic dermatitis in adolescents and adults.
Approval for PN is based on data from the phase 3 OLYMPIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in 560 patients with PN, according to a press release from Galderma, the manufacturer.
According to the press release, in OLYMPIA 1 and OLYMPIA 2, 58% and 56% of patients, respectively, achieved at least a 4-point reduction in itch intensity at week 16 as measured by the Peak Pruritus Numerical Rating Scale, compared with 16% in both placebo groups (P < .0001). At the same time, 26% and 38% of nemolizumab-treated patients reached clearance or almost-clearance of skin lesions on the Investigator Global Assessment score at week 16, compared with 7% and 11% in the placebo groups (P < .0001).
According to the company press release, the most common side effects of nemolizumab are headache and rashes in the form of eczema, atopic dermatitis, and nummular eczema.
“By inhibiting the signaling of IL-31, Nemluvio addresses a key driver of prurigo nodularis, safely and effectively improving itch as well as skin nodules,” Shawn G. Kwatra, MD, PhD, professor and chair of dermatology at the University of Maryland School of Medicine, Baltimore, and lead investigator of the OLYMPIA program, stated in the press release.
The regulatory submission of nemolizumab in atopic dermatitis is based on data from the phase 3 ARCADIA clinical trial program, which evaluated the efficacy and safety of nemolizumab administered subcutaneously every 4 weeks in adolescents and adults with moderate to severe atopic dermatitis. A decision on approval for this indication from the FDA is expected in December 2024.
In September 2022, dupilumab became the first FDA-approved treatment for PN in the United States.
A version of this article first appeared on Medscape.com.
PrEP Prescription Pickups Vary With Prescriber Specialty
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
Preexposure prophylaxis prescription reversals and abandonments were lower for patients seen by primary care clinicians than by other non–infectious disease clinicians, based on data from approximately 37,000 individuals.
Although preexposure prophylaxis (PrEP) has been associated with a reduced risk of HIV (human immunodeficiency virus) infection when used as prescribed, the association between PrEP prescription pickup and specialty of the prescribing clinician has not been examined, wrote Lorraine T. Dean, ScD, an epidemiologist at Johns Hopkins University, Baltimore, Maryland, and colleagues.
“HIV PrEP is highly effective at preventing new HIV cases, and while use is on the rise, is still used much less than it should be by people who are at risk of HIV,” Dr. Dean said in an interview. “This study is helpful in pinpointing who is at risk for not picking up PrEP and in helping us think through how to reach them so that they can be better positioned to get PrEP,” she said.
In a study published in JAMA Internal Medicine, the researchers reviewed data for PrEP care. The study population included 37,003 patients aged 18 years and older who received new insurer-approved PrEP prescriptions between 2015 and 2019. Most of the patients (77%) ranged in age from 25 to 64 years; 88% were male.
Pharmacy claims data were matched with clinician data from the US National Plan and Provider Enumeration System.
Clinicians were divided into three groups: primary care providers (PCPs), infectious disease specialists (IDs), and other specialists (defined as any clinician prescribing PrEP but not classified as a PCP or an ID specialist). The main binary outcomes were prescription reversal (defined as when a patient failed to retrieve a prescription) and abandonment (defined as when a patient neglected to pick up a prescription for 1 year).
Overall, of 24,604 patients 67% received prescriptions from PCPs, 3,571 (10%) received prescriptions from ID specialists, and 8828 (24%) received prescriptions from other specialty clinicians.
The prevalence of reversals for patients seen by PCPs, ID specialists, and other specialty clinicians was 18%, 18%, and 25%, respectively. The prevalence of abandonments by clinician group was 12%, 12%, and 20%, respectively.
In a regression analysis, patients prescribed PrEP by ID specialists had 10% lower odds of reversals and 12% lower odds of abandonments compared to those seen by PCPs (odds ratio 0.90 and 0.88, respectively). However, patients seen by other clinicians (not primary care or ID) were 33% and 54% more likely to have reversals and abandonments, respectively, compared with those seen by PCPs.
Many patients at risk for HIV first see a PCP and then are referred to a specialist, such as an ID physician, Dr. Dean said. “The patients who take the time to then follow up with a specialist may be most motivated and able to follow through with the specialist’s request, in this case, accessing their PrEP prescription,” she said. In the current study, the researchers were most surprised by how many other specialty providers are involved in PrEP care, which is very positive given the effectiveness of the medication, she noted.
“Our results suggest that a wide range of prescribers, regardless of specialty, should be equipped to prescribe PrEP as well as offer PrEP counseling,” Dr. Dean said. A key takeaway for clinicians is that PrEP should have no cost for the majority of patients in the United States, she emphasized. The absence of cost expands the population who should be interested and able to access PrEP, she said. Therefore, providers should be prepared to recommend PrEP to eligible patients, and seek training or continuing medical education for themselves so they feel equipped to prescribe and counsel patients on PrEP, she said.
“One limitation of this work is that, while it can point to what is happening, it cannot tell us why the reversals are happening; what is the reason patients prescribed by certain providers are more or less likely to get their PrEP,” Dr. Dean explained. “We have tried to do interviews with patients to understand why this might be happening, but it’s hard to find people who aren’t showing up to do something, compared to finding people who are showing up to do something,” she said. Alternatively, researchers could interview providers to understand their perspective on why differences in prescription pickups occur across specialties, she said.
Looking ahead, “a national PrEP program that includes elements of required clinician training could be beneficial, and research on how a national PrEP program could be implemented and impact HIV rates would be helpful in considering this strategy of prevention,” said Dr. Dean.
Support All Prescribers to Increase PrEP Adherence
Differences in uptake of PrEP prescriptions may be explained by the different populations seen by various specialties, Meredith Green, MD, of Indiana University School of Medicine, Indianapolis, and Lona Mody, MD, of the University of Michigan, Ann Arbor, wrote in an accompanying editorial. However, the key question is how to support all prescribers and promote initiation and adherence to PrEP, they said.
Considerations include whether people at risk for HIV prefer to discuss PrEP with a clinician they already know, vs. a new specialist, but many PCPs are not familiar with the latest PrEP guidelines, they said.
“Interventions that support PrEP provision by PCPs, especially since they prescribed the largest proportion of PrEP prescriptions, can accelerate the uptake of PrEP,” the editorialists wrote.
“Supporting a diverse clinician workforce reflective of communities most impacted by HIV will remain critical, as will acknowledging and addressing HIV stigma,” they said. Educational interventions, including online programs and specialist access for complex cases, would help as well, they said. The approval of additional PrEP agents since the current study was conducted make it even more important to support PrEP prescribers and promote treatment adherence for those at risk for HIV, Dr. Green and Dr. Mody emphasized.
The study was funded by the National Institutes of Health. Dr. Dean had no financial conflicts to disclose. Dr. Green disclosed grants from Gilead and royalties from Wolters Kluwer unrelated to the current study; she also disclosed serving on the Centers for Disease Control and Prevention/Health Resources and Services Administration advisory committee on HIV, viral hepatitis, and sexually transmitted infection prevention and treatment. Dr. Mody disclosed grants from the US National Institute on Aging, Veterans Affairs, Centers for Disease Control and Prevention, NanoVibronix, and UpToDate unrelated to the current study.
FROM JAMA INTERNAL MEDICINE
Low HPV Vaccination in the United States Is a Public Health ‘Failure’
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
I would like to briefly discuss what I consider to be a very discouraging report and one that I believe we as an oncology society and, quite frankly, as a medical community need to deal with.
The manuscript I’m referring to is from the United States Department of Health and Human Services, titled, “Human Papillomavirus Vaccination Coverage in Children Ages 9-17 Years: United States, 2022.” This particular analysis looked at the coverage of both men and women — young boys and young girls, I would say — receiving at least one dose of the recommended human papillomavirus (HPV) vaccination.
Since 2006, girls have been recommended to receive HPV vaccination; for boys, it’s been since 2011. Certainly, the time period that we’re considering falls within the recommendations based on overwhelmingly positive data. Now, today, still, the recommendation is for more than one vaccine. Obviously, there may be evidence in the future that a single vaccination may be acceptable or appropriate. But today, it’s more than one.
In this particular analysis, they were looking at just a single vaccination. The vaccines have targeted young individuals, both male and female children aged 11-12 years, but it’s certainly acceptable to look starting at age 9.
What is the bottom line? At least one dose of the HPV vaccination was given to 38.6% of children aged 9-17 years in 2022. We are talking about a cancer-preventive vaccine, which on the basis of population-based data in the United States, but also in other countries, is incredibly effective in preventing HPV-associated cancers. This not only includes cervical cancer, but also a large percentage of head and neck cancers.
For this vaccine, which is incredibly safe and incredibly effective, in this country, only 38.6% have received even a single dose. It is noted that the individuals with private insurance had a higher rate, at 41.5%, than individuals with no insurance, at only 20.7%.
In my opinion, this is clearly a failure of our public health establishment at all levels. My own focus has been in gynecologic cancers. I’ve seen young women with advanced cervical cancer, and this is a disease we can prevent. Yet, this is where we are.
For those of you who are interested in cancer prevention or public health, I think this is a very sobering statistic. It’s my plea and my hope that we can, as a society, somehow do something about it.
I thank you for listening. I would encourage you to think about this question if you’re in this area.
Dr. Markman, professor, Department of Medical Oncology and Therapeutics Research, City of Hope, Duarte, California, and president of Medicine & Science, City of Hope Atlanta, Chicago, and Phoenix, disclosed ties with GlaxoSmithKline and AstraZeneca.
A version of this article appeared on Medscape.com.
Applications for the CUTIS 2025 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2025 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2025.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Alicia Sonners ([email protected]) by November 1. The residents who are selected to write the column for the upcoming year will be notified by November 15.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
Storybooks Can Help Children Deal with Skin Conditions
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
TORONTO —
So far, “the study demonstrates that these books have value to patients and families,” one of the study authors, Sonia Havele, MD, a pediatrician and dermatology resident at Children’s Mercy Hospital Kansas City, Kansas City, Missouri, said in an interview.
“There are tools to help kids cope with their skin conditions, but we’re underutilizing them,” she added. “And part of the reason we’re underutilizing storybooks is that we just don’t know what’s out there.” For the study, the researchers received funding to purchase 18 “creative and thoughtful” storybooks related to pediatric skin conditions, reviewed by at least two pediatric dermatologists before being selected, which are just a sample of related books that are available.
The study results were presented as a poster at the annual meeting of the Society for Pediatric Dermatology.
Children with visible skin conditions, which can include port-wine stains, capillary malformations, and congenital moles, may be subjected to teasing or bullying at school, and the conditions can also affect their quality of life.
Beauty and the Birthmark
The books include one titled “Beauty with a Birthmark” and another, “My Hair Went on Vacation.” An illustrated book, “Just Ask: Be Different, Be Brave, Be You,” by US Supreme Court Justice Sonia Sotomayor, offers tips on how to answer common questions about someone’s appearance.
Dr. Havele said that Justice Sotomayor’s book “empowers kids, their siblings, their classmates ... to ask questions, and it teaches patients not to be afraid of those questions, and to really lean into educating their peers, and their family members.”
“Kids are really just curious,” she added. “They’ll make comments like: ‘Hey, what’s that spot on your face?’ Or, they’ll ask about vitiligo because they’ve never seen somebody with it before.”
To evaluate the psychosocial impact of these types of books for children with visible skin conditions, Dr. Havele and colleagues designed a study that includes patients aged 2-12 years dealing with issues related to self-esteem, acceptance, coping, or bullying. Parents are provided with a relevant storybook to read at home with their child in a “safe and comfortable space” and “at their own pace and their own time,” said Dr. Havele.
Inside the book is a QR code to access the validated Children’s Dermatology Life Quality Index (CDLQI). Families complete the survey at baseline and provide feedback after reading the book. Researchers collect information about demographics, age, gender, and skin conditions, which included atopic dermatitis, alopecia areata, vitiligo, hemangioma, and port-wine stain.
The response rate so far is 34%, and close to 80 parents have completed the survey with their child, Dr. Havele said.
At baseline, many of the children were either moderately or severely affected in terms of their quality of life (45% scored ≥ 6 on the CDLQI).
After reading the book, about 80% of parents reported it had a positive impact, and about 20% said it had a somewhat positive impact on their child’s self-image or confidence. Almost 80% agreed, and the remainder somewhat agreed it encouraged their child to embrace differences.
Most respondents also said the book helped the parent and child cope with the child’s condition. “So really, it was overall a positive response,” said Dr. Havele. “We are able to demonstrate that these books have value in a more scientific or objective way.”
This may not be surprising. Dr. Havele referred to more formal bibliotherapy (book therapy), which has been studied in other pediatric populations, including patients with cancer and those who have experienced trauma.
Awesome Space
Pediatric dermatologists are perfectly positioned to play a role in improving the lives of their patients with skin issues. “We see the impact of visible skin disease on children all the time,” said Dr. Havele. “The dermatology visit is an awesome space and opportunity to introduce these books to families and potentially help them talk about the skin condition with their child.”
In addition to prescribing therapies, “we’re also with these kids through an emotional journey, and I think giving them tools for that emotional journey is very helpful,” she added.
Such books would have been a great help to Dr. Havele herself. Growing up, she had severe atopic dermatitis covering much of her body. “Having such a resource would have helped me better cope with my reality of being different than everyone else.”
She hopes a database will be established to house these resources so other providers can refer patients to the list of books. Other books include “The Itchy-saurus: The Dino with an itch that can’t be scratched,” “Hair in My Brush,” and “I am Unique!”
Dr. Havele had no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM SPD 2024
Experts Highlight Challenges That Remain for AI Devices in Triaging Skin Cancer
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.
Emerging according to researchers and dermatologists investigating AI.
While some AI-integrated devices designed to triage skin lesions have emerged, including one that received Food and Drug Administration (FDA) clearance earlier in 2024, it may be some time before AI has a meaningful clinical impact in dermatology and, more specifically, the diagnosis of skin cancer, Ivy Lee, MD, a dermatologist in Pasadena, California, and chair of the American Academy of Dermatology’s augmented intelligence committee, told this news organization.
“It hasn’t really translated into clinical practice yet,” Dr. Lee said of AI in dermatology. “There have been significant advances in terms of the technical possibility and feasibility of these tools, but the translation and integration of AI into actual clinical work flows to benefit patients beyond academic research studies has been limited.” More studies and more “easily accessible and digestible information” are needed to evaluate AI tools in dermatologic practice.
“In dermatology, we’re on a cusp with AI,” said Rebecca Hartman, MD, MPH, chief of dermatology at the VA Boston Healthcare System and director of melanoma epidemiology at Brigham and Women’s Hospital, Boston, Massachusetts. “I think it’s going to come and change what we do,” which is especially true for any image-based specialty,” including radiology and pathology, in addition to dermatology.
Dr. Hartman led a study of one of these emerging technologies, the handheld elastic scattering spectroscopy device DermaSensor, which was cleared by the FDA in January for evaluating skin lesions suggestive of skin cancer.
Early AI Devices for Skin Cancer Detection
At the American Society for Laser Medicine and Surgery (ASLMS) meeting in April, a panel explored a number of algorithms with dermatologic applications that use AI to triage skin lesions, including DermaSensor.
Raman spectroscopy, which contains a handheld Raman probe, a diode laser, and a detecting spectrograph. A laser beam — which at 1.56 W/cm2 is below the maximum permissible exposure — focuses on the skin target with a 3.5-mm spot, gathers data on the target, and feeds it back into the unit that houses the algorithm that evaluates the spot analysis. It’s still in the investigative phase. A clinical trial, published almost 5 years ago, demonstrated a sensitivity of 90%-99% and a specificity of 24%-66% for skin cancer.
A dermatoscope called Sklip clips onto a smartphone and performs what company cofounder Alexander Witkowski, MD, PhD, described as an “optical painless virtual biopsy” for at-home use. The device uploads the captured image to an AI platform for analysis. It received FDA breakthrough device designation in 2022. At the ASLMS meeting, Dr. Witkowski said that clinical performance showed the device had a 97% sensitivity and 30% specificity for skin cancer.
DermaSensor, described in the study conducted by Dr. Hartman and others as a noninvasive, point-and-click spectrometer, is a wireless handheld piece that weighs about 10 ounces. The unit captures five recordings to generate a spectral reading, which an algorithm in the software unit analyzes. The study found a sensitivity of 95.5% and specificity of 32.5% for melanoma detection with the device.
The target market for DermaSensor is primary care physicians, and, according to the FDA announcement in January, it is indicated for evaluating skin lesions “suggestive” of melanoma, basal cell carcinoma (BCC), and/or squamous cell carcinoma (SCC) in patients aged 40 and older to “assist healthcare providers in determining whether to refer a patient to a dermatologist.”
So Many Cases, So Few Dermatologists
In dermatology, AI devices have the potential to streamline the crushing burden of diagnosing skin cancer, said Yun Liu, PhD, a senior staff scientist at Google Research, Mountain View, California, who’s worked on developing machine-learning tools in dermatology among other medical fields. “Many people cannot access dermatology expertise when they most need it, ie, without waiting a long time. This causes substantial morbidity for patients,” Dr. Liu said in an interview.
His own research of an AI-based tool to help primary care physicians and nurse practitioners in teledermatology practices diagnose skin conditions documented the shortage of dermatologists to triage lesions, including a finding that only about one quarter of skin conditions are seen by a specialist and that nonspecialists play a pivotal role in the management of skin lesions.
The Centers for Disease Control and Prevention reports that about 6.1 million adults are treated for BCC and SCCs each year. The American Medical Association estimates that 13,200 active dermatologists practice in the United States.
Overcoming Barriers to AI in Dermatology
Before AI makes significant inroads in dermatology, clinicians need to see more verifiable data, said Roxana Daneshjou, MD, PhD, assistant professor of biomedical data science and dermatology at Stanford University, Stanford, California. “One of the challenges is having the availability of models that actually improve clinical care because we have some very early prospective trials on different devices, but we don’t have large-scale randomized clinical trials of AI devices showing definitive behaviors such as improved patient outcomes, that it helps curb skin cancer, or it catches it like dermatologists but helps reduce the biopsy load,” she said. “You need good data.”
Another challenge she noted was overcoming biases built into medicine. “A lot of the image-based models are built on datasets depicting skin disease on White skin, and those models don’t work so well on people with brown and black skin, who have historically had worse outcomes and also have been underrepresented in dermatology,” said Dr. Daneshjou, an associate editor of NEJM AI.
There’s also the challenge of getting verified AI models into the clinic. “Similar to many medical AI endeavors, developing a proof-of-concept or research prototype is far easier and faster than bringing the development to real users,” Dr. Liu said. “In particular, it is important to conduct thorough validation studies on various patient populations and settings and understand how these AI tools can best fit into the workflow or patient journey.”
A study published in 2023 documented progress Google made in deploying AI models in retina specialty clinics in India and Thailand, Dr. Liu noted.
Another challenge is to avoid overdiagnosis with these new technologies, Dr. Hartman said. Her group’s study showed the DermaSensor had a positive predictive value of 16% and a negative predictive value of 98.5%. “I think there’s some question about how this will factor into overdiagnosis. Could this actually bombard dermatologists more if the positive predictive value’s only 16%?”
One key to dermatologists accepting AI tools is having a transparent process for validating them, Dr. Lee said. “Even with FDA clearance, we don’t have the transparency we need as clinicians, researchers, and advocates of machine learning and AI in healthcare.”
But, Dr. Lee noted, the FDA in June took a step toward illuminating its validation process when it adopted guiding principles for transparency for machine learning–enabled devices. “Once we can get more access to this information and have more transparency, that’s where we can think about actually about making the decision to implement or not implement into local healthcare settings,” she said. The process was further enabled by a White House executive order in October 2023 on the safe, secure, and trustworthy development and use of AI.
The experience with telehealth during the COVID-19 pandemic, when patients and providers quickly embraced the technology to stay connected, serves as a potential template for AI, Dr. Lee noted. “As we’d seen with telehealth through the pandemic, you also need the cultural evolution and the development of the infrastructure around it to actually make sure this is a sustainable implementation and a scalable implementation in healthcare.”
Dr. Lee had no relevant relationships to disclose. Dr. Hartman received funding from DermaSensor for a study. Dr. Witkowski is a cofounder of Sklip. Dr. Liu is an employee of Google Research. Dr. Daneshjou reported financial relationships with MD Algorithms, Revea, and L’Oreal.
A version of this article first appeared on Medscape.com.
Who’s Behind Cosmetic Procedures at MedSpas?
CARLSBAD, CALIFORNIA — according to Sara Hogan, MD.
“I’m not anti-MedSpa; I’m pro-patient safety,” Dr. Hogan, clinical assistant professor of dermatology at George Washington University, Washington, DC, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “The MedSpa industry is booming; it brought in $17 billion in 2023. There are as many MedSpas in the United States as there are practicing dermatologists, and that number is set to exceed the number of dermatologists.”
According to industry data from the American Med Spa Association, 63% of member MedSpas have non-MD ownership. Among MedSpas owned by physicians, 80% are of a non–core specialty, meaning a specialty other than dermatology, plastic surgery, otorhinolaryngology, or ophthalmology. Of MedSpa medical directors, 69% are from non–core physician specialties. “There’s an increasing amount of data that shows a relatively higher incidence of complications from cosmetic procedures that are delivered at MedSpas,” Dr. Hogan said. “A 2020 study suggested that this is likely due to improper training, improper technique, and/or improper device settings.”
Dr. Hogan also cited adverse effects linked to counterfeit or mishandled botulinum toxin injections that prompted the Centers for Disease Control and Prevention to issue an alert to clinicians in April 2024. Clusters of 22 people in 11 states reported adverse effects after receiving injections with counterfeit botulinum toxin or injections administered by unlicensed or untrained individuals or in non-healthcare settings, such as homes or spas.
To better understand who performs cosmetic procedures, provides medical supervision, and follows safety protocols at MedSpas, Dr. Hogan and colleagues conducted a “truth in advertising” study of 127 MedSpas in the greater Chicago area. They chose this geographic location because an analysis published in 2021 identified Chicago as having the third highest number of aesthetic physicians and the fifth highest number of MedSpas in the United States. The researchers enlisted help from “secret shoppers” who contacted the MedSpas by telephone to ask about the level of training, if patients underwent a review of medical history, the level of on-site physician supervision, and the protocol for complications.
The top five cosmetic procedures offered by the 127 surveyed MedSpas were facials (85.0%), hair removal (85.0%), botulinum toxin injections (83.5%), dermal fillers (82.7%), and chemical peels (76.4%). About two thirds of cosmetic procedures were performed by aestheticians (66.9%), followed by registered nurses or licensed practical nurses (52.8%), board-certified physicians (48.8%, mostly plastic and reconstructive surgeons), nurse practitioners (27.6%), and physician assistants (9.4%).
In the realm of supervision, 16.5% of MedSpas surveyed reported that a medical director or supervising physician is always on site. “If not located on site, when asked where the physicians are, the majority of the time they were at the physician’s primary practice, clinic, or hospital,” Dr. Hogan said. “Only 65% of the MedSpas surveyed stated that they informed the patient that the supervising physician is not on site. In addition, a patient’s medical history is reviewed at only 40% of the MedSpas. To give context, in Illinois, a physician can only deliver care after a physician-patient relationship has been established, meaning that a good faith exam has been performed. And if they are to delegate any type of service, they must always be on site to provide assistance.”
Dr. Hogan noted that there are no federal statutes or agencies that regulate or oversee MedSpas. “Regulation and oversight are often delegated to state licensing agencies that are overwhelmed and often stretched thin regarding personnel and budgets,” she said. To raise awareness of this issue, the American Society for Dermatologic Surgery Association (ASDSA) launched the Medical Spa Safety Coalition, which aims to promote model legislation for states known as the Medical Spa Safety Act. Highlights of the bill include clear definitions of medical spa and medical director, as well as the requirement of an on-site medical director who must be a physician trained in all procedures performed at the MedSpa. Coalition members include 16 state dermatology boards as well as the ASDSA, the American Academy of Dermatology Association, the American Society for Laser Medicine & Surgery, and the American Society of Plastic Surgeons.
The ASDSA provided funding to support the published study. Dr. Hogan reported having no financial disclosures.
A version of this article appeared on Medscape.com.
CARLSBAD, CALIFORNIA — according to Sara Hogan, MD.
“I’m not anti-MedSpa; I’m pro-patient safety,” Dr. Hogan, clinical assistant professor of dermatology at George Washington University, Washington, DC, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “The MedSpa industry is booming; it brought in $17 billion in 2023. There are as many MedSpas in the United States as there are practicing dermatologists, and that number is set to exceed the number of dermatologists.”
According to industry data from the American Med Spa Association, 63% of member MedSpas have non-MD ownership. Among MedSpas owned by physicians, 80% are of a non–core specialty, meaning a specialty other than dermatology, plastic surgery, otorhinolaryngology, or ophthalmology. Of MedSpa medical directors, 69% are from non–core physician specialties. “There’s an increasing amount of data that shows a relatively higher incidence of complications from cosmetic procedures that are delivered at MedSpas,” Dr. Hogan said. “A 2020 study suggested that this is likely due to improper training, improper technique, and/or improper device settings.”
Dr. Hogan also cited adverse effects linked to counterfeit or mishandled botulinum toxin injections that prompted the Centers for Disease Control and Prevention to issue an alert to clinicians in April 2024. Clusters of 22 people in 11 states reported adverse effects after receiving injections with counterfeit botulinum toxin or injections administered by unlicensed or untrained individuals or in non-healthcare settings, such as homes or spas.
To better understand who performs cosmetic procedures, provides medical supervision, and follows safety protocols at MedSpas, Dr. Hogan and colleagues conducted a “truth in advertising” study of 127 MedSpas in the greater Chicago area. They chose this geographic location because an analysis published in 2021 identified Chicago as having the third highest number of aesthetic physicians and the fifth highest number of MedSpas in the United States. The researchers enlisted help from “secret shoppers” who contacted the MedSpas by telephone to ask about the level of training, if patients underwent a review of medical history, the level of on-site physician supervision, and the protocol for complications.
The top five cosmetic procedures offered by the 127 surveyed MedSpas were facials (85.0%), hair removal (85.0%), botulinum toxin injections (83.5%), dermal fillers (82.7%), and chemical peels (76.4%). About two thirds of cosmetic procedures were performed by aestheticians (66.9%), followed by registered nurses or licensed practical nurses (52.8%), board-certified physicians (48.8%, mostly plastic and reconstructive surgeons), nurse practitioners (27.6%), and physician assistants (9.4%).
In the realm of supervision, 16.5% of MedSpas surveyed reported that a medical director or supervising physician is always on site. “If not located on site, when asked where the physicians are, the majority of the time they were at the physician’s primary practice, clinic, or hospital,” Dr. Hogan said. “Only 65% of the MedSpas surveyed stated that they informed the patient that the supervising physician is not on site. In addition, a patient’s medical history is reviewed at only 40% of the MedSpas. To give context, in Illinois, a physician can only deliver care after a physician-patient relationship has been established, meaning that a good faith exam has been performed. And if they are to delegate any type of service, they must always be on site to provide assistance.”
Dr. Hogan noted that there are no federal statutes or agencies that regulate or oversee MedSpas. “Regulation and oversight are often delegated to state licensing agencies that are overwhelmed and often stretched thin regarding personnel and budgets,” she said. To raise awareness of this issue, the American Society for Dermatologic Surgery Association (ASDSA) launched the Medical Spa Safety Coalition, which aims to promote model legislation for states known as the Medical Spa Safety Act. Highlights of the bill include clear definitions of medical spa and medical director, as well as the requirement of an on-site medical director who must be a physician trained in all procedures performed at the MedSpa. Coalition members include 16 state dermatology boards as well as the ASDSA, the American Academy of Dermatology Association, the American Society for Laser Medicine & Surgery, and the American Society of Plastic Surgeons.
The ASDSA provided funding to support the published study. Dr. Hogan reported having no financial disclosures.
A version of this article appeared on Medscape.com.
CARLSBAD, CALIFORNIA — according to Sara Hogan, MD.
“I’m not anti-MedSpa; I’m pro-patient safety,” Dr. Hogan, clinical assistant professor of dermatology at George Washington University, Washington, DC, said at the Controversies & Conversations in Laser & Cosmetic Surgery symposium. “The MedSpa industry is booming; it brought in $17 billion in 2023. There are as many MedSpas in the United States as there are practicing dermatologists, and that number is set to exceed the number of dermatologists.”
According to industry data from the American Med Spa Association, 63% of member MedSpas have non-MD ownership. Among MedSpas owned by physicians, 80% are of a non–core specialty, meaning a specialty other than dermatology, plastic surgery, otorhinolaryngology, or ophthalmology. Of MedSpa medical directors, 69% are from non–core physician specialties. “There’s an increasing amount of data that shows a relatively higher incidence of complications from cosmetic procedures that are delivered at MedSpas,” Dr. Hogan said. “A 2020 study suggested that this is likely due to improper training, improper technique, and/or improper device settings.”
Dr. Hogan also cited adverse effects linked to counterfeit or mishandled botulinum toxin injections that prompted the Centers for Disease Control and Prevention to issue an alert to clinicians in April 2024. Clusters of 22 people in 11 states reported adverse effects after receiving injections with counterfeit botulinum toxin or injections administered by unlicensed or untrained individuals or in non-healthcare settings, such as homes or spas.
To better understand who performs cosmetic procedures, provides medical supervision, and follows safety protocols at MedSpas, Dr. Hogan and colleagues conducted a “truth in advertising” study of 127 MedSpas in the greater Chicago area. They chose this geographic location because an analysis published in 2021 identified Chicago as having the third highest number of aesthetic physicians and the fifth highest number of MedSpas in the United States. The researchers enlisted help from “secret shoppers” who contacted the MedSpas by telephone to ask about the level of training, if patients underwent a review of medical history, the level of on-site physician supervision, and the protocol for complications.
The top five cosmetic procedures offered by the 127 surveyed MedSpas were facials (85.0%), hair removal (85.0%), botulinum toxin injections (83.5%), dermal fillers (82.7%), and chemical peels (76.4%). About two thirds of cosmetic procedures were performed by aestheticians (66.9%), followed by registered nurses or licensed practical nurses (52.8%), board-certified physicians (48.8%, mostly plastic and reconstructive surgeons), nurse practitioners (27.6%), and physician assistants (9.4%).
In the realm of supervision, 16.5% of MedSpas surveyed reported that a medical director or supervising physician is always on site. “If not located on site, when asked where the physicians are, the majority of the time they were at the physician’s primary practice, clinic, or hospital,” Dr. Hogan said. “Only 65% of the MedSpas surveyed stated that they informed the patient that the supervising physician is not on site. In addition, a patient’s medical history is reviewed at only 40% of the MedSpas. To give context, in Illinois, a physician can only deliver care after a physician-patient relationship has been established, meaning that a good faith exam has been performed. And if they are to delegate any type of service, they must always be on site to provide assistance.”
Dr. Hogan noted that there are no federal statutes or agencies that regulate or oversee MedSpas. “Regulation and oversight are often delegated to state licensing agencies that are overwhelmed and often stretched thin regarding personnel and budgets,” she said. To raise awareness of this issue, the American Society for Dermatologic Surgery Association (ASDSA) launched the Medical Spa Safety Coalition, which aims to promote model legislation for states known as the Medical Spa Safety Act. Highlights of the bill include clear definitions of medical spa and medical director, as well as the requirement of an on-site medical director who must be a physician trained in all procedures performed at the MedSpa. Coalition members include 16 state dermatology boards as well as the ASDSA, the American Academy of Dermatology Association, the American Society for Laser Medicine & Surgery, and the American Society of Plastic Surgeons.
The ASDSA provided funding to support the published study. Dr. Hogan reported having no financial disclosures.
A version of this article appeared on Medscape.com.
Misdiagnosis of Crusted Scabies: Skin Excoriations Resembling Brown Sugar Are Characteristic
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.
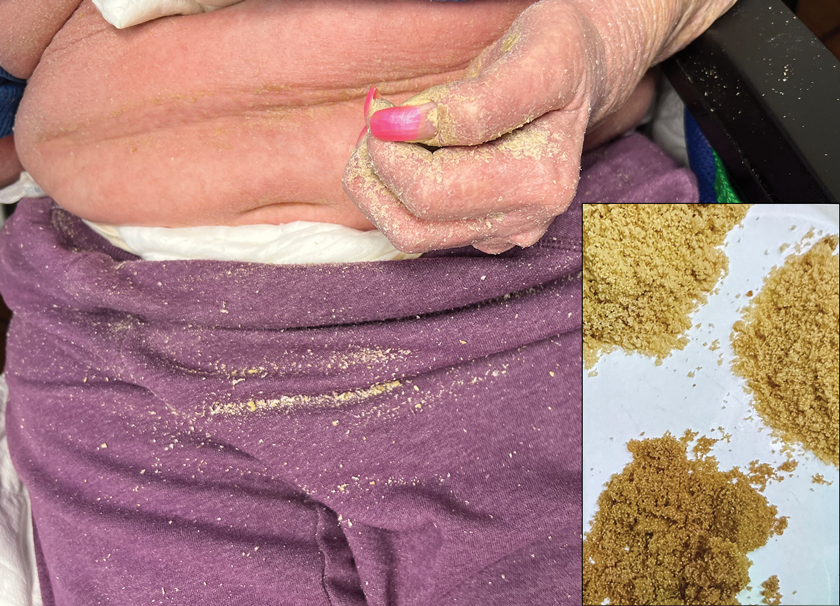
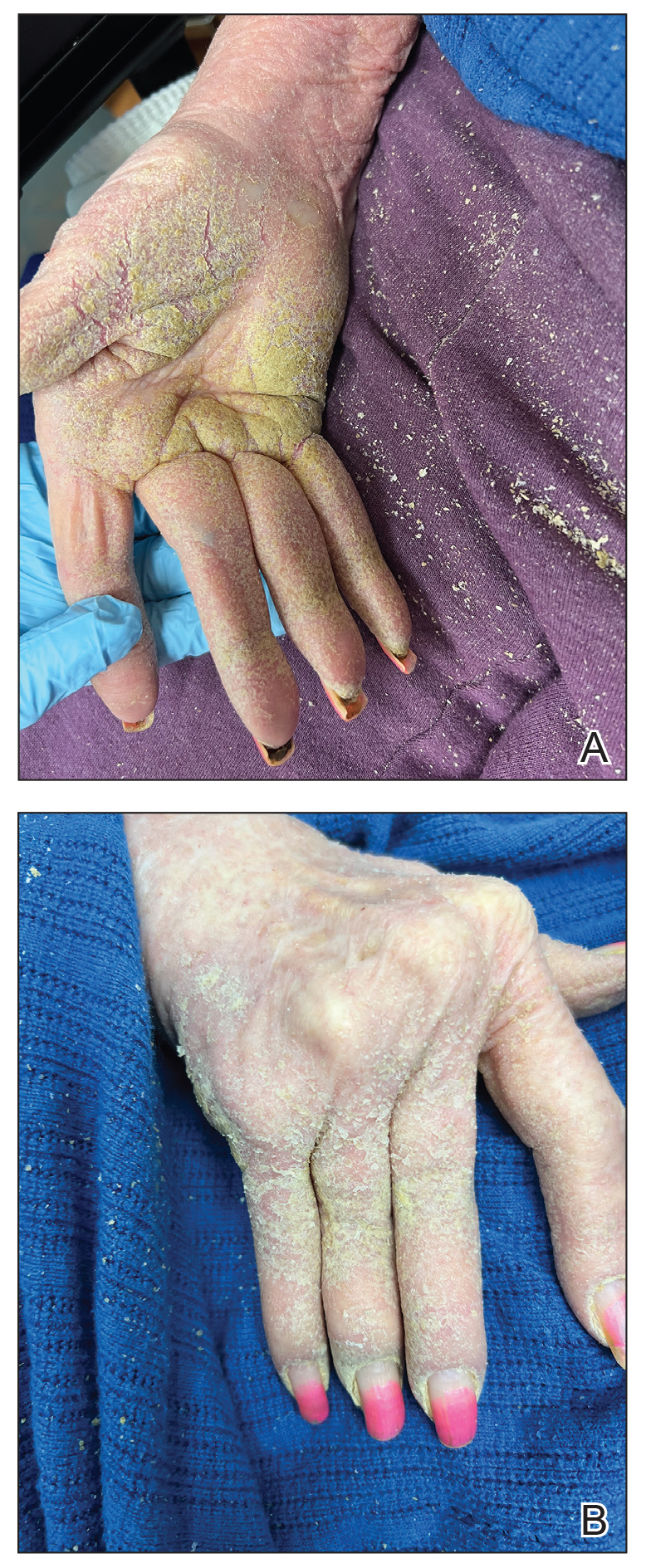
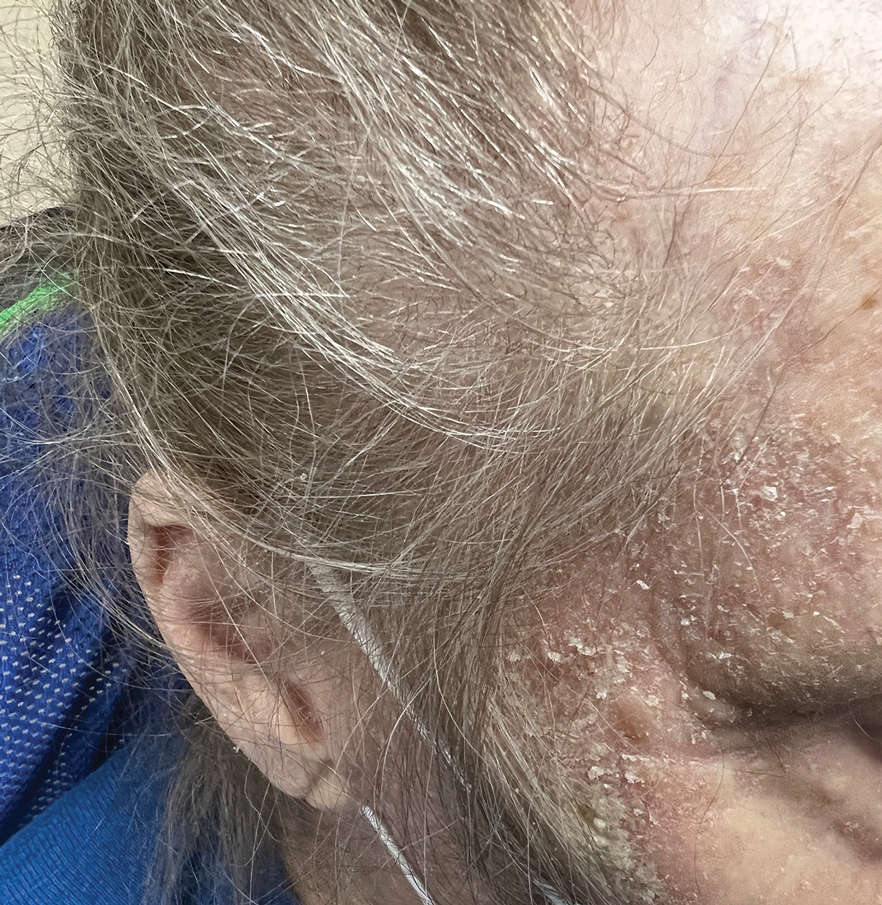
The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.
Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.



The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.

Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
To the Editor:
Crusted scabies (formerly known as Norwegian scabies) is a rare and highly contagious variant of scabies, in which the skin is infested with thousands to millions of Sarcoptes scabiei var hominis mites. We present a case of skin changes that were misdiagnosed as atopic dermatitis, seborrhea, xerosis, and drug eruption on initial presentation, which prompted treatment with a corticosteroid that inadvertently caused progression to crusted scabies.
A 79-year-old woman who uses a wheelchair presented to the clinic with skin changes that consisted of diffuse, severely pruritic, erythematous plaques on the head, neck, trunk, face, and extremities of 2 years’ duration. She had a medical history of hyperlipidemia, hypertension, and hyperglycemia, as well as a stroke that required hospitalization 2 years prior to the onset of the skin changes. She had no history of allergies.
Prior clinical diagnoses by primary care and dermatology included xerosis, atopic dermatitis, seborrhea, and drug eruption. She was treated with a mid-potency topical corticosteroid (triamcinolone acetonide cream 0.1%) twice daily and prednisone 40 mg once daily for 2- to 4-week courses over an 8-month period without reduction in symptoms.
Physical examination at the current presentation revealed golden, crusted, fine, powdery but slightly sticky flakes that spread diffusely across the entire body and came off in crumbles with a simple touch. These widespread crusts were easily visible on clothing. There was underlying diffuse erythema beneath the flaking skin on the trunk and proximal extremities. The scale and shedding skin laid in piles on the patient’s lap and resembled brown sugar (Figure 1). The patient also reported decreased hand function and dexterity due to the yellowbrown, thick, crusty plaques that had developed on both the palmar and dorsal sides of the hands (Figure 2). Erythematous plaques on the scalp, forehead, and inner ears resembled seborrhea (Figure 3). Pruritus severity was rated by the patient as 10 of 10, and she scratched her skin the entire time she was in the clinic. The patient was emotional and stated that she had not been able to sleep due to the discomfort. We suspected scabies, and the patient was reassured to learn that it could be confirmed with a simple skin scrape test.



The crusted lesions on the patient's hands were scraped with a #15-blade scalpel, and a routine potassium hydroxide mount was performed. The skin scrapings were placed on a slide with a drop of 10% potassium hydroxide and observed under low-power (×10) and high-power (×40) microscopy, which revealed thousands of mites and eggs (along with previously hatched eggs) (Figure 4) and quickly confirmed a diagnosis of crusted scabies.an extremely contagious form of scabies seen in older patients with compromised immune systems, malnutrition, or disabilities. The patient was prescribed oral ivermectin (3 mg dosed at 200 μg/kg of body weight) and topical permethrin 5%, neither of which she took, as she died of a COVID-19 infection complication 3 days after this diagnostic clinic visit.

Classic and crusted scabies are both caused by infestation of the Sarcoptes scabiei var hominis mite. Classic scabies is a result of an infestation of a small number of mites (commonly 5–15 mites), while crusted scabies is due to hyperinfestation by as many as millions of mites, the latter often requiring more aggressive treatment. The mites are first transmitted to humans by either skin-toskin contact or fomites on bedding and clothing. The scabies mite undergoes 4 life cycle stages: egg, larvae, nymph, and adult. Once female mites are transmitted, they burrow under the skin and lay 2 to 3 eggs per day. The eggs hatch within 3 to 4 days, after which the larvae migrate to the skin surface. The larval stage lasts for 3 to 4 days, during which the larvae burrow into the stratum corneum to create molting pouches, until they molt into slightly larger nymphs. Nymphs can be found in hair follicles or molting pouches until they further molt within 3 to 4 days into adults, which are round, saclike mites. The adult male and female mites then mate, leaving the female fertile for the rest of her 1- to 2-month lifespan. Impregnated female mites traverse the skin surface in search of a burrow site, using the pulvilli on the anterior aspect of 2 legs to hold onto the skin. Once burrowed, the female mite continues to lay eggs for the rest of her life, with approximately 10% of her eggs resulting in adult mites. Male mites feed in shallow pits of the skin until they find a female burrow site for mating.1 This continuous life cycle of the scabies mite gives rise to highly transmissible, pruritic skin excoriations, as demonstrated in our patient.
The skin has a relatively late inflammatory and adaptive immune response to scabies, typically occurring 4 to 6 weeks after the initial infestation.2 This delayed inflammatory response and onset of symptoms may be due to the scabies mite’s ability to alter aspects of the host’s immune response, which differs in classic vs crusted scabies. In classic scabies, there is a predominance of CD4+ T cells in the dermis and minimal CD8+ T cells. The opposite is true in crusted scabies— there is an overwhelming infiltration of CD8+ T cells and minimal CD4+ T cells.3 The CD8+ T-cell predominance in crusted scabies is hypothesized to be the cause of keratinocyte apoptosis, resulting in epidermal hyperproliferation. Keratinocyte apoptosis also secretes cytokines, which may lead to the immunologic targeting of healthy skin cells. The damage of healthy dermal cells contributes to the inability of the skin’s immune system to mount an effective response, allowing the parasite to grow uncontrollably in patients with crusted scabies.4
This ineffective immune response is further exacerbated by corticosteroids, which are commonly prescribed for pruritus experienced by patients with scabies infestations. The mechanism of action of corticosteroids is the production of anti-inflammatory, antimitotic, and immunosuppressive effects.5 Because the integumentary immune system is imbalanced during crusted scabies infestation, the immunosuppressive mechanism of oral and topical corticosteroids further reduces the cellular immune response to scabies. The flourishing of the scabies mites along with keratinocyte apoptosis4 results in the development of hyperkeratotic skin crusting, most frequently on the palms, soles, arms, and legs. Risk factors for crusted scabies include immunosuppression, hospitalization, crowded living conditions, and poor hygiene, though no known risk factors were documented in up to 42% (33/78) of patients with crusted scabies in one study.6
Patients with crusted scabies typically present with generalized, poorly defined, erythematous, fissured plaques covered by scaling and crusts. Plaques on bony prominences such as finger articulations and elbows may have a thick verrucous aspect.1 Skin flaking that resembles brown sugar—a mixture of white sugar and molasses—is a clue to the diagnosis of crusted scabies. Brown sugar has a slightly sandy and sticky texture that ranges in color from very light brown to very dark brown. When present, flakes always appears slightly lighter than the patient’s skin tone. Although skin burrows are pathognomonic and clinically recognizable features of scabies, these burrows can be disguised by lesions, such as the hyperkeratotic plaques seen in our patient. The lesions may or may not be associated with pruritus, which may occur only at night, and bacterial superinfection has been reported in severe cases of crusted scabies,7 as scratching can cause sores, which may lead to infection. In severe cases, the constant scratching could lead to sepsis if the infection enters the bloodstream.8 Another symptom of scabies is a rash that causes small bumps that tend to form in a line, resembling small bites, hives, or pimples, and scaly plaques can lead to misdiagnosis as atopic dermatitis.
Treatment often is delayed due to misdiagnosis, as seen in our patient. Common misdiagnoses include atopic dermatitis, pityriasis rosea, systemic lupus erythematosus, bullous pemphigoid, lichen planus, pediculosis corporis, seborrheic scalp dermatitis, and adverse drug reactions.9 Patients with extensive infestations of crusted scabies should be treated with a 4-week course of permethrin cream 5% daily for 1 week, then twice per week until resolved, and oral ivermectin 200 μg/kg dosed 1 week apart for up to 4 weeks, if needed.1 Topical permethrin works by producing a selective neurotoxic effect on invertebrates such as scabies mites, which disrupts the function of voltage-gated sodium channels, thereby paralyzing the adult mites to halt the spread of infestation. However, treatment with topical medications can be difficult due to the thick crusts that have formed, which make it more challenging for the skin to properly absorb the treatment. Additionally, surgical debridement as an adjunct procedure has been done to improve the effectiveness of topical medications by removing all the mites in skin.10 On the other hand, the mechanism in which ivermectin treats scabies infestations is poorly understood. Current research suggests that ivermectin works by causing persistent opening of pH-gated chloride channels in scabies mites.11 There is emerging concern for drug resistance to these scabicides,12 revealing a need for further research of treatment options.
Patients with crusted scabies can have an extremely large number of mites (up to 2 million), making them more infectious than patients with classic scabies.13 As a result, it is imperative to reduce environmental transmission and risk for reinfection with mites during treatment. Because crusted scabies is transmitted by prolonged skinto- skin contact or by contact with personal items of an infected person (eg, bedding, clothing), treatment guidelines require all clothing, bedding, and towels of a patient with scabies to be machine-washed and dried with hot water and hot dryer cycles. If an item cannot be washed, it should be stored in a sealed plastic bag for 1 week, as scabies mites cannot survive more than 2 to 3 days away from their host of human skin.13 Treatment of close contacts of patients with scabies is recommended, as well as for those in endemic areas or closed communities, such as nursing homes or jails.
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
- Salavastru CM, Chosidow O, Boffa MJ, et al. European guideline for the management of scabies. J Eur Acad Dermatol Venereol. 2017;31:1248-1253. doi:10.1111/jdv.14351
- Morgan MS, Arlian LG, Markey MP. Sarcoptes scabiei mites modulate gene expression in human skin equivalents. PLoS One. 2013;8:e71143. doi:10.1371/journal.pone.0071143
- Walton SF, Beroukas D, Roberts-Thomson P, et al. New insights into disease pathogenesis in crusted (Norwegian) scabies: the skin immune response in crusted scabies. Br J Dermatol. 2008;158:1247-1255. doi:10.1111/j.1365-2133.2008.08541.x
- Bhat SA, Mounsey KE, Liu X, et al. Host immune responses to the itch mite, Sarcoptes scabiei, in humans. Parasit Vectors. 2017;10:385. doi:10.1186/s13071-017-2320-4
- Binic´ I, Jankovic´ A, Jovanovic´ D, et al. Crusted (Norwegian) scabies following systemic and topical corticosteroid therapy. J Korean Med Sci. 2009;25:188-191. doi:10.3346/jkms.2010.25.1.188
- Roberts LJ, Huffam SE, Walton SF, et al. Crusted scabies: clinical and immunological findings in seventy-eight patients and a review of the literature. J Infect. 2005;50:375-381. doi:10.1016/j.jinf.2004.08.033
- Yari N, Malone CH, Rivas A. Misdiagnosed crusted scabies in an AIDS patient leads to hyperinfestation. Cutis. 2017;99:202-204.
- American Academy of Dermatology Association. Scabies: signs and symptoms. Accessed July 12, 2024. https://www.aad.org/public/diseases/a-z/scabies-symptoms
- Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884-917. doi:10.3390/jcm4050884
- Maghrabi MM, Lum S, Joba AT, et al. Norwegian crusted scabies: an unusual case presentation. J Foot Ankle Surg. 2014;53:62-66. doi:10.1053/j.jfas.2013.09.002
- Currie BJ, McCarthy JS. Permethrin and ivermectin for scabies. N Engl J Med. 2010;362:717-725. doi:10.1056/NEJMct0910329
- Andriantsoanirina V, Izri A, Botterel F, et al. Molecular survey of knockdown resistance to pyrethroids in human scabies mites. Clin Microbiol Infect. 2014;20:O139-O141. doi:10.1111/1469-0691.12334
- Centers for Disease Control and Prevention. Preventing scabies. Published December 18, 2023. Accessed August 9, 2024. https://www.cdc.gov/scabies/prevention/index.html
PRACTICE POINTS
- Crusted scabies often is misdiagnosed because it mimics common dermatologic conditions, such as atopic dermatitis, psoriasis, drug eruption, and seborrhea. A unique feature of crusted scabies is fine or coarse scaling that resembles brown sugar.
- Immunosuppressants, such as topical corticosteroids, worsen the skin’s immune response to classic scabies infestations, which leads to parasitic overgrowth and the development of crusted scabies.
- Treatment of crusted scabies requires topical and oral scabicide; in addition, all clothing, bedding, and towels should be machine-washed and dried with hot water and hot dryer cycles to prevent environmental transmission and reinfection.
Doctors Are Seeking Professional Coaches More Often. Here’s Why
When Andrea Austin, MD, an emergency medicine specialist, left the military in 2020, she knew the adjustment to civilian life and practice might be difficult. To help smooth the transition, she reached out to a physician mentor who also had a professional coaching certificate. After a conversation, Dr. Austin signed up for 6 months of career coaching.
It was time well spent, according to Dr. Austin, who today is a coach herself. “It was really the first time I had the ability to choose what I wanted to do, and that required a mindset shift,” she explains. “A big part of coaching is helping physicians discover their agency so that they can make the best career choices.”
Physicians have long lacked the coaching resources typically made available to corporate executives. But that’s changing. In today’s high-pressure environment, where doctors are burning out at a rapid pace, coaching can sometimes be an avenue to staying in the field, especially if that coach is a fellow physician who understands what you’re facing.
With a physician shortage that the Association of American Medical Colleges expects to hit 86,000 in the next decade or so, coaching could be a stone worth turning over. A 2024 report in JAMA Network Open found that coaching provided by physician peers led to a significant reduction in interpersonal disengagement and burnout.
“What I think is exciting about coaching is that it allows you to better understand yourself and know your strengths and weaknesses,” said Dr. Austin. “It might seem simple, but many ‘soft skills’ aren’t considered mainstream in medicine. Coaching allows us to understand them and ourselves better.”
Why Are Doctors Using Coaches?
Although it’s hard to put a number on how many physicians are turning to coaches, the number of coaches available for doctors is growing rapidly. The American Medical Women’s Association maintains a database of physician coaches. According to deputy director Jodi Godfrey, MS, RDN, the number of members who have added coaching to their skill set has tripled in the past 4 years. “Many cite burnout as the reason they sought coaching support, and then they decided to go on to get certified in coaching.”
The pandemic is one reason physician coaching has grown, said Elizabeth Esparaz, MD, an ophthalmologist and physician coach. “Since the pandemic, the word ‘burnout’ is thrown around a good deal.” And the causes are clear. “Doctors are facing longer hours, they must make split-second decisions, they’re multitasking, and they have less support staff.”
Among her coaching clients, Dr. Austin has noticed other common struggles: fears of litigation, time scarcity with patients, declining reimbursement that hasn’t kept up with inflation, and loss of autonomy because of the corporatization of healthcare.
Coaching, Dr. Esparaz believes, can be an antidote to many of these issues. “Coaches help doctors see their strengths and find better ways of applying them,” she said. “We help them move forward, and also see their blind spots.”
Clarity, Goals, and Making the Right Choices
Physician coaching comes in a variety of flavors — some one on one, and others in the form of group sessions. All, however, serve the purpose of helping physicians gain career clarity. “Sometimes clients realize their job may not be working for them, but that there are things they can do to change that without having to leave the field,” said Jattu Senesie, MD, a former ob.gyn. who is now a physician coach.
Dr. Esparaz works with doctors to establish SMART goals: specific, measurable, attainable, realistic, and time based. She gave the example of learning how to set boundaries. “If a physician is asked to create a presentation for work, I encourage them to ask for compensation or administrative time before committing to unpaid tasks.”
Another big issue: charting. It’s increasingly burdensome, and many doctors find it encroaching on their home lives. “If we can identify a problem like that, we can come up with a strategy for mitigating it,” Dr. Esparaz said. This might include setting a goal of getting 80% of charting completed immediately after the patient encounter on the busiest clinic day of the week. The client tests the experiment and then revisits it with the coach to discuss what worked and what didn’t, refining the process until it has freed up the physician’s home life.
The younger generation of doctors often struggles with career choices, too, because it’s the first time they are without structure, said Dr. Senesie. There’s med school and residency, which puts a framework around every move a doctor makes. But once they become attending physicians, the choices are endless. “Coaching can help them find a new structure and systems that will allow them to thrive.”
Although mentoring has been a well-embraced concept for decades, it “hits a wall,” at some point in terms of what it can offer, Dr. Austin said. That’s where coaching can take over. “There’s a point where a mentor cannot help someone self-actualize. As a coach, you don’t need to know everything about a doctor’s life, but you can help them learn to ask themselves the right questions to solve problems.”
Should You Stay or Should You Go?
Dr. Austin’s approach begins with the premise that healthcare today is challenging and dysfunctional — but doctors still have agency. She has worked with clients on the verge of leaving the field and helped them find their way back.
“They have a light bulb moment and open up to the idea that they have much to give still,” she said. “We take an inventory to help them better communicate their needs and make changes, and I help them connect to their values. Sometimes that exercise allows them to reframe their current work environment.”
Not every doctor who goes through coaching remains in the field. But “that’s the exception, not the rule,” Dr. Austin said. And that’s okay. “If that’s the outcome, coaching probably helped them get to that point faster, and with an informed decision.”
Dr. Senesie has been coaching for about a decade, and in that time, she’s seen a shift that goes beyond figuring out career goals. “Doctors are more aware of the need for well-being today. The pandemic made it impossible to ignore what doesn’t work for us. When I work with clients, we look for ways to make the job more tenable.”
According to Dr. Senesie, younger doctors are looking for that balance at the outset. “They want to be physicians, but they also want a life,” she said. “It’s a challenge for them because in addition to that mindset, they’re also coming out with more debt than older generations. They want out from underneath that.”
When It’s Time to Find a Physician Coach
Wondering whether coaching is right for you? Consider these symptoms:
- You need help setting boundaries at work.
- You feel like you’re sacrificing your own well-being for your job.
- You’re using maladaptive strategies to cope with the stress at work.
- You’ve reached a point where you are considering leaving the field.
If you’re interested in finding a physician coach, there are several places to begin your search, word of mouth being one of them. “Conferences and social media can also expose you to coaches,” suggested Dr. Esparaz. There are different methods and approaches to coaching. So, as you research, “make sure the coach you choose has techniques and a framework that fit what you’re after.”
Dr. Austin warned that it is an unregulated industry, so buyer beware. To ensure you’re getting an accredited physician coach, look for people who have obtained an International Coach Federation (ICF) accreditation. These coaches will hold an associate certified coach credential, which requires at least 60 hours of coaching-specific training approved by the ICF, in addition to other assessments and education.
Ensure that the coach you choose is within your budget. “There are some people charging astronomical rates out there,” Dr. Austin said. “If you’re burned out or struggling, it can be easy to reach for your credit card.”
Dr. Austin also cautioned doctors seeking a coach to avoid promises that sound too good to be true. Some coaching can have a gaslighting quality to it, she warned, “suggesting it can allow you to endure any environment.” But positive self-talk alone won’t cure an abusive or discriminatory situation. “If a client describes a toxic work environment,” the coach has an “ethical imperative” to help that person protect themselves.
A Side Gig or a New Career Path
After Dr. Austin’s experience with her coach, she made the choice to continue as an emergency physician part-time while starting her own coaching business. “It’s important for me personally to keep in touch with what’s happening on the ground, but I have no judgment for anyone who chooses to leave clinical practice to become a coach.”
When Dr. Senesie looks back on her own struggles as a clinician, she recognizes the state of burnout she was in 10 years ago. “I knew there was an issue, but I didn’t have the mindset to find a way to make it work,” she said. “I left the field when I was at my depths of burnout, which is generally not the best way to go about it.”
Guidance might have allowed her to take into account other avenues and helped her remain in the field, said Dr. Senesie. She has since learned that “there are many ways to practice medicine, and the way we’ve gone about it traditionally has worked for some, but not necessarily for everyone.”
There may be more possibilities than you think. By helping you assess your path and make meaningful changes, a physician coach might be the key to remaining in the field you love.
A version of this article first appeared on Medscape.com.
When Andrea Austin, MD, an emergency medicine specialist, left the military in 2020, she knew the adjustment to civilian life and practice might be difficult. To help smooth the transition, she reached out to a physician mentor who also had a professional coaching certificate. After a conversation, Dr. Austin signed up for 6 months of career coaching.
It was time well spent, according to Dr. Austin, who today is a coach herself. “It was really the first time I had the ability to choose what I wanted to do, and that required a mindset shift,” she explains. “A big part of coaching is helping physicians discover their agency so that they can make the best career choices.”
Physicians have long lacked the coaching resources typically made available to corporate executives. But that’s changing. In today’s high-pressure environment, where doctors are burning out at a rapid pace, coaching can sometimes be an avenue to staying in the field, especially if that coach is a fellow physician who understands what you’re facing.
With a physician shortage that the Association of American Medical Colleges expects to hit 86,000 in the next decade or so, coaching could be a stone worth turning over. A 2024 report in JAMA Network Open found that coaching provided by physician peers led to a significant reduction in interpersonal disengagement and burnout.
“What I think is exciting about coaching is that it allows you to better understand yourself and know your strengths and weaknesses,” said Dr. Austin. “It might seem simple, but many ‘soft skills’ aren’t considered mainstream in medicine. Coaching allows us to understand them and ourselves better.”
Why Are Doctors Using Coaches?
Although it’s hard to put a number on how many physicians are turning to coaches, the number of coaches available for doctors is growing rapidly. The American Medical Women’s Association maintains a database of physician coaches. According to deputy director Jodi Godfrey, MS, RDN, the number of members who have added coaching to their skill set has tripled in the past 4 years. “Many cite burnout as the reason they sought coaching support, and then they decided to go on to get certified in coaching.”
The pandemic is one reason physician coaching has grown, said Elizabeth Esparaz, MD, an ophthalmologist and physician coach. “Since the pandemic, the word ‘burnout’ is thrown around a good deal.” And the causes are clear. “Doctors are facing longer hours, they must make split-second decisions, they’re multitasking, and they have less support staff.”
Among her coaching clients, Dr. Austin has noticed other common struggles: fears of litigation, time scarcity with patients, declining reimbursement that hasn’t kept up with inflation, and loss of autonomy because of the corporatization of healthcare.
Coaching, Dr. Esparaz believes, can be an antidote to many of these issues. “Coaches help doctors see their strengths and find better ways of applying them,” she said. “We help them move forward, and also see their blind spots.”
Clarity, Goals, and Making the Right Choices
Physician coaching comes in a variety of flavors — some one on one, and others in the form of group sessions. All, however, serve the purpose of helping physicians gain career clarity. “Sometimes clients realize their job may not be working for them, but that there are things they can do to change that without having to leave the field,” said Jattu Senesie, MD, a former ob.gyn. who is now a physician coach.
Dr. Esparaz works with doctors to establish SMART goals: specific, measurable, attainable, realistic, and time based. She gave the example of learning how to set boundaries. “If a physician is asked to create a presentation for work, I encourage them to ask for compensation or administrative time before committing to unpaid tasks.”
Another big issue: charting. It’s increasingly burdensome, and many doctors find it encroaching on their home lives. “If we can identify a problem like that, we can come up with a strategy for mitigating it,” Dr. Esparaz said. This might include setting a goal of getting 80% of charting completed immediately after the patient encounter on the busiest clinic day of the week. The client tests the experiment and then revisits it with the coach to discuss what worked and what didn’t, refining the process until it has freed up the physician’s home life.
The younger generation of doctors often struggles with career choices, too, because it’s the first time they are without structure, said Dr. Senesie. There’s med school and residency, which puts a framework around every move a doctor makes. But once they become attending physicians, the choices are endless. “Coaching can help them find a new structure and systems that will allow them to thrive.”
Although mentoring has been a well-embraced concept for decades, it “hits a wall,” at some point in terms of what it can offer, Dr. Austin said. That’s where coaching can take over. “There’s a point where a mentor cannot help someone self-actualize. As a coach, you don’t need to know everything about a doctor’s life, but you can help them learn to ask themselves the right questions to solve problems.”
Should You Stay or Should You Go?
Dr. Austin’s approach begins with the premise that healthcare today is challenging and dysfunctional — but doctors still have agency. She has worked with clients on the verge of leaving the field and helped them find their way back.
“They have a light bulb moment and open up to the idea that they have much to give still,” she said. “We take an inventory to help them better communicate their needs and make changes, and I help them connect to their values. Sometimes that exercise allows them to reframe their current work environment.”
Not every doctor who goes through coaching remains in the field. But “that’s the exception, not the rule,” Dr. Austin said. And that’s okay. “If that’s the outcome, coaching probably helped them get to that point faster, and with an informed decision.”
Dr. Senesie has been coaching for about a decade, and in that time, she’s seen a shift that goes beyond figuring out career goals. “Doctors are more aware of the need for well-being today. The pandemic made it impossible to ignore what doesn’t work for us. When I work with clients, we look for ways to make the job more tenable.”
According to Dr. Senesie, younger doctors are looking for that balance at the outset. “They want to be physicians, but they also want a life,” she said. “It’s a challenge for them because in addition to that mindset, they’re also coming out with more debt than older generations. They want out from underneath that.”
When It’s Time to Find a Physician Coach
Wondering whether coaching is right for you? Consider these symptoms:
- You need help setting boundaries at work.
- You feel like you’re sacrificing your own well-being for your job.
- You’re using maladaptive strategies to cope with the stress at work.
- You’ve reached a point where you are considering leaving the field.
If you’re interested in finding a physician coach, there are several places to begin your search, word of mouth being one of them. “Conferences and social media can also expose you to coaches,” suggested Dr. Esparaz. There are different methods and approaches to coaching. So, as you research, “make sure the coach you choose has techniques and a framework that fit what you’re after.”
Dr. Austin warned that it is an unregulated industry, so buyer beware. To ensure you’re getting an accredited physician coach, look for people who have obtained an International Coach Federation (ICF) accreditation. These coaches will hold an associate certified coach credential, which requires at least 60 hours of coaching-specific training approved by the ICF, in addition to other assessments and education.
Ensure that the coach you choose is within your budget. “There are some people charging astronomical rates out there,” Dr. Austin said. “If you’re burned out or struggling, it can be easy to reach for your credit card.”
Dr. Austin also cautioned doctors seeking a coach to avoid promises that sound too good to be true. Some coaching can have a gaslighting quality to it, she warned, “suggesting it can allow you to endure any environment.” But positive self-talk alone won’t cure an abusive or discriminatory situation. “If a client describes a toxic work environment,” the coach has an “ethical imperative” to help that person protect themselves.
A Side Gig or a New Career Path
After Dr. Austin’s experience with her coach, she made the choice to continue as an emergency physician part-time while starting her own coaching business. “It’s important for me personally to keep in touch with what’s happening on the ground, but I have no judgment for anyone who chooses to leave clinical practice to become a coach.”
When Dr. Senesie looks back on her own struggles as a clinician, she recognizes the state of burnout she was in 10 years ago. “I knew there was an issue, but I didn’t have the mindset to find a way to make it work,” she said. “I left the field when I was at my depths of burnout, which is generally not the best way to go about it.”
Guidance might have allowed her to take into account other avenues and helped her remain in the field, said Dr. Senesie. She has since learned that “there are many ways to practice medicine, and the way we’ve gone about it traditionally has worked for some, but not necessarily for everyone.”
There may be more possibilities than you think. By helping you assess your path and make meaningful changes, a physician coach might be the key to remaining in the field you love.
A version of this article first appeared on Medscape.com.
When Andrea Austin, MD, an emergency medicine specialist, left the military in 2020, she knew the adjustment to civilian life and practice might be difficult. To help smooth the transition, she reached out to a physician mentor who also had a professional coaching certificate. After a conversation, Dr. Austin signed up for 6 months of career coaching.
It was time well spent, according to Dr. Austin, who today is a coach herself. “It was really the first time I had the ability to choose what I wanted to do, and that required a mindset shift,” she explains. “A big part of coaching is helping physicians discover their agency so that they can make the best career choices.”
Physicians have long lacked the coaching resources typically made available to corporate executives. But that’s changing. In today’s high-pressure environment, where doctors are burning out at a rapid pace, coaching can sometimes be an avenue to staying in the field, especially if that coach is a fellow physician who understands what you’re facing.
With a physician shortage that the Association of American Medical Colleges expects to hit 86,000 in the next decade or so, coaching could be a stone worth turning over. A 2024 report in JAMA Network Open found that coaching provided by physician peers led to a significant reduction in interpersonal disengagement and burnout.
“What I think is exciting about coaching is that it allows you to better understand yourself and know your strengths and weaknesses,” said Dr. Austin. “It might seem simple, but many ‘soft skills’ aren’t considered mainstream in medicine. Coaching allows us to understand them and ourselves better.”
Why Are Doctors Using Coaches?
Although it’s hard to put a number on how many physicians are turning to coaches, the number of coaches available for doctors is growing rapidly. The American Medical Women’s Association maintains a database of physician coaches. According to deputy director Jodi Godfrey, MS, RDN, the number of members who have added coaching to their skill set has tripled in the past 4 years. “Many cite burnout as the reason they sought coaching support, and then they decided to go on to get certified in coaching.”
The pandemic is one reason physician coaching has grown, said Elizabeth Esparaz, MD, an ophthalmologist and physician coach. “Since the pandemic, the word ‘burnout’ is thrown around a good deal.” And the causes are clear. “Doctors are facing longer hours, they must make split-second decisions, they’re multitasking, and they have less support staff.”
Among her coaching clients, Dr. Austin has noticed other common struggles: fears of litigation, time scarcity with patients, declining reimbursement that hasn’t kept up with inflation, and loss of autonomy because of the corporatization of healthcare.
Coaching, Dr. Esparaz believes, can be an antidote to many of these issues. “Coaches help doctors see their strengths and find better ways of applying them,” she said. “We help them move forward, and also see their blind spots.”
Clarity, Goals, and Making the Right Choices
Physician coaching comes in a variety of flavors — some one on one, and others in the form of group sessions. All, however, serve the purpose of helping physicians gain career clarity. “Sometimes clients realize their job may not be working for them, but that there are things they can do to change that without having to leave the field,” said Jattu Senesie, MD, a former ob.gyn. who is now a physician coach.
Dr. Esparaz works with doctors to establish SMART goals: specific, measurable, attainable, realistic, and time based. She gave the example of learning how to set boundaries. “If a physician is asked to create a presentation for work, I encourage them to ask for compensation or administrative time before committing to unpaid tasks.”
Another big issue: charting. It’s increasingly burdensome, and many doctors find it encroaching on their home lives. “If we can identify a problem like that, we can come up with a strategy for mitigating it,” Dr. Esparaz said. This might include setting a goal of getting 80% of charting completed immediately after the patient encounter on the busiest clinic day of the week. The client tests the experiment and then revisits it with the coach to discuss what worked and what didn’t, refining the process until it has freed up the physician’s home life.
The younger generation of doctors often struggles with career choices, too, because it’s the first time they are without structure, said Dr. Senesie. There’s med school and residency, which puts a framework around every move a doctor makes. But once they become attending physicians, the choices are endless. “Coaching can help them find a new structure and systems that will allow them to thrive.”
Although mentoring has been a well-embraced concept for decades, it “hits a wall,” at some point in terms of what it can offer, Dr. Austin said. That’s where coaching can take over. “There’s a point where a mentor cannot help someone self-actualize. As a coach, you don’t need to know everything about a doctor’s life, but you can help them learn to ask themselves the right questions to solve problems.”
Should You Stay or Should You Go?
Dr. Austin’s approach begins with the premise that healthcare today is challenging and dysfunctional — but doctors still have agency. She has worked with clients on the verge of leaving the field and helped them find their way back.
“They have a light bulb moment and open up to the idea that they have much to give still,” she said. “We take an inventory to help them better communicate their needs and make changes, and I help them connect to their values. Sometimes that exercise allows them to reframe their current work environment.”
Not every doctor who goes through coaching remains in the field. But “that’s the exception, not the rule,” Dr. Austin said. And that’s okay. “If that’s the outcome, coaching probably helped them get to that point faster, and with an informed decision.”
Dr. Senesie has been coaching for about a decade, and in that time, she’s seen a shift that goes beyond figuring out career goals. “Doctors are more aware of the need for well-being today. The pandemic made it impossible to ignore what doesn’t work for us. When I work with clients, we look for ways to make the job more tenable.”
According to Dr. Senesie, younger doctors are looking for that balance at the outset. “They want to be physicians, but they also want a life,” she said. “It’s a challenge for them because in addition to that mindset, they’re also coming out with more debt than older generations. They want out from underneath that.”
When It’s Time to Find a Physician Coach
Wondering whether coaching is right for you? Consider these symptoms:
- You need help setting boundaries at work.
- You feel like you’re sacrificing your own well-being for your job.
- You’re using maladaptive strategies to cope with the stress at work.
- You’ve reached a point where you are considering leaving the field.
If you’re interested in finding a physician coach, there are several places to begin your search, word of mouth being one of them. “Conferences and social media can also expose you to coaches,” suggested Dr. Esparaz. There are different methods and approaches to coaching. So, as you research, “make sure the coach you choose has techniques and a framework that fit what you’re after.”
Dr. Austin warned that it is an unregulated industry, so buyer beware. To ensure you’re getting an accredited physician coach, look for people who have obtained an International Coach Federation (ICF) accreditation. These coaches will hold an associate certified coach credential, which requires at least 60 hours of coaching-specific training approved by the ICF, in addition to other assessments and education.
Ensure that the coach you choose is within your budget. “There are some people charging astronomical rates out there,” Dr. Austin said. “If you’re burned out or struggling, it can be easy to reach for your credit card.”
Dr. Austin also cautioned doctors seeking a coach to avoid promises that sound too good to be true. Some coaching can have a gaslighting quality to it, she warned, “suggesting it can allow you to endure any environment.” But positive self-talk alone won’t cure an abusive or discriminatory situation. “If a client describes a toxic work environment,” the coach has an “ethical imperative” to help that person protect themselves.
A Side Gig or a New Career Path
After Dr. Austin’s experience with her coach, she made the choice to continue as an emergency physician part-time while starting her own coaching business. “It’s important for me personally to keep in touch with what’s happening on the ground, but I have no judgment for anyone who chooses to leave clinical practice to become a coach.”
When Dr. Senesie looks back on her own struggles as a clinician, she recognizes the state of burnout she was in 10 years ago. “I knew there was an issue, but I didn’t have the mindset to find a way to make it work,” she said. “I left the field when I was at my depths of burnout, which is generally not the best way to go about it.”
Guidance might have allowed her to take into account other avenues and helped her remain in the field, said Dr. Senesie. She has since learned that “there are many ways to practice medicine, and the way we’ve gone about it traditionally has worked for some, but not necessarily for everyone.”
There may be more possibilities than you think. By helping you assess your path and make meaningful changes, a physician coach might be the key to remaining in the field you love.
A version of this article first appeared on Medscape.com.
Fillers, Hyaluronidase Relieve Orofacial Changes in Patients with Scleroderma
CARLSBAD, CALIFORNIA — In 2003, researchers asked 303 patients with systemic sclerosis (scleroderma) what bothered them most about their disease from an aesthetic standpoint: Orofacial features, such as thin lips and mouth furrows, or non-facial features, such as fingertip ulceration and waxy changes to the skin.
Respondents expressed significant concern about specific orofacial features, including thin lips (73%), mouth furrows (80%), loss of facial lines (68%), and a smaller, tighter mouth (77%).
“Patients with systemic sclerosis may have loss of vermilion lip, microstomia, and perioral rhytids,” Kathleen Cook Suozzi, MD, who directs the Aesthetic Dermatology Program at Yale University School of Medicine, New Haven, Connecticut, said at the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium. “How can we address these changes for our patients?”
Recent research has shown that hyaluronidase injections can help improve orofacial changes commonly experienced by patients with scleroderma. In 2019, researchers in Alabama reported the case of a 53-year-old woman treated with hyaluronidase for scleroderma-induced microstomia. After four visits over 7 months and a total hyaluronidase dose of 470 IU, the patient reported an improved Mouth Handicap in Systemic Sclerosis (MHISS) score (38 of 48); subjective improvement of symptoms, including greater ease in eating and undergoing dental treatment; and improved mouth closure.
In 2023, researchers published a cohort study of four women between the ages of 43 and 61 with autoimmune sclerosing conditions that resulted in oral microstomia. Following hyaluronidase injections, all improved in mouth opening capacity and MHISS, with change stabilizing between three and five treatments. More recently, in a study pending publication in JAAD Case Reports, Dr. Suozzi and colleagues retrospectively evaluated 12 women with scleroderma who received between 150 and 300 units of hyaluronic acid (HA) filler for microstomia between 2020 and 2023. Of the 12 women, 58% had diffuse disease, and 42% had limited disease. Overall, oral aperture width increased by 0.65 cm (P = .0027) and oral aperture height increased by 0.88 cm (P < .0001). “In general, patients needed three to four treatments to reach peak effect, and then they reached a plateau,” Dr. Suozzi said. “It wasn’t that the treatment wasn’t working anymore, but it was because their oral aperture had gotten to a size of around 5 cm, which is clinically normal. Interestingly, we found that if the patient’s disease flared and their microstomia started to return, when you rechallenged them, they continued to respond. So, patients can continue to use this treatment over time.”
In a separate case series of seven patients, Dr. Suozzi and colleagues prospectively evaluated the effect of HA soft tissue filler with Restylane Silk for lip augmentation. Study participants experienced statistically significant increases in the difference between pre- and postinjection fullness in both upper and lower lips. Also, the mean posttreatment score fell between “much improved” (2) and “improved” (3) on both the Investigator Global Aesthetic Improvement Scale and the Subject Global Aesthetic Improvement Scale.
Dr. Suozzi recommends using nerve blocks for injecting HA filler or hyaluronidase in patients with scleroderma and minimizing the injection points. “Initially, we were using 30% lidocaine preparations around the mouth for an hour before the procedure, and patients were still having pain, so now we use nerve blocks,” she said. “For hyaluronidase, we do perform a test dose of 75-100 units, usually in the commissure. It’s amazing how well it works; people will usually come back after their test dose and have improvements in their measurements. This is a really easy treatment to perform, and I think it can be done in the office of a general dermatologist. There is concern about cross-reactivity with bee venom, so you want to ask patients about that.”
Dr. Suozzi reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CARLSBAD, CALIFORNIA — In 2003, researchers asked 303 patients with systemic sclerosis (scleroderma) what bothered them most about their disease from an aesthetic standpoint: Orofacial features, such as thin lips and mouth furrows, or non-facial features, such as fingertip ulceration and waxy changes to the skin.
Respondents expressed significant concern about specific orofacial features, including thin lips (73%), mouth furrows (80%), loss of facial lines (68%), and a smaller, tighter mouth (77%).
“Patients with systemic sclerosis may have loss of vermilion lip, microstomia, and perioral rhytids,” Kathleen Cook Suozzi, MD, who directs the Aesthetic Dermatology Program at Yale University School of Medicine, New Haven, Connecticut, said at the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium. “How can we address these changes for our patients?”
Recent research has shown that hyaluronidase injections can help improve orofacial changes commonly experienced by patients with scleroderma. In 2019, researchers in Alabama reported the case of a 53-year-old woman treated with hyaluronidase for scleroderma-induced microstomia. After four visits over 7 months and a total hyaluronidase dose of 470 IU, the patient reported an improved Mouth Handicap in Systemic Sclerosis (MHISS) score (38 of 48); subjective improvement of symptoms, including greater ease in eating and undergoing dental treatment; and improved mouth closure.
In 2023, researchers published a cohort study of four women between the ages of 43 and 61 with autoimmune sclerosing conditions that resulted in oral microstomia. Following hyaluronidase injections, all improved in mouth opening capacity and MHISS, with change stabilizing between three and five treatments. More recently, in a study pending publication in JAAD Case Reports, Dr. Suozzi and colleagues retrospectively evaluated 12 women with scleroderma who received between 150 and 300 units of hyaluronic acid (HA) filler for microstomia between 2020 and 2023. Of the 12 women, 58% had diffuse disease, and 42% had limited disease. Overall, oral aperture width increased by 0.65 cm (P = .0027) and oral aperture height increased by 0.88 cm (P < .0001). “In general, patients needed three to four treatments to reach peak effect, and then they reached a plateau,” Dr. Suozzi said. “It wasn’t that the treatment wasn’t working anymore, but it was because their oral aperture had gotten to a size of around 5 cm, which is clinically normal. Interestingly, we found that if the patient’s disease flared and their microstomia started to return, when you rechallenged them, they continued to respond. So, patients can continue to use this treatment over time.”
In a separate case series of seven patients, Dr. Suozzi and colleagues prospectively evaluated the effect of HA soft tissue filler with Restylane Silk for lip augmentation. Study participants experienced statistically significant increases in the difference between pre- and postinjection fullness in both upper and lower lips. Also, the mean posttreatment score fell between “much improved” (2) and “improved” (3) on both the Investigator Global Aesthetic Improvement Scale and the Subject Global Aesthetic Improvement Scale.
Dr. Suozzi recommends using nerve blocks for injecting HA filler or hyaluronidase in patients with scleroderma and minimizing the injection points. “Initially, we were using 30% lidocaine preparations around the mouth for an hour before the procedure, and patients were still having pain, so now we use nerve blocks,” she said. “For hyaluronidase, we do perform a test dose of 75-100 units, usually in the commissure. It’s amazing how well it works; people will usually come back after their test dose and have improvements in their measurements. This is a really easy treatment to perform, and I think it can be done in the office of a general dermatologist. There is concern about cross-reactivity with bee venom, so you want to ask patients about that.”
Dr. Suozzi reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
CARLSBAD, CALIFORNIA — In 2003, researchers asked 303 patients with systemic sclerosis (scleroderma) what bothered them most about their disease from an aesthetic standpoint: Orofacial features, such as thin lips and mouth furrows, or non-facial features, such as fingertip ulceration and waxy changes to the skin.
Respondents expressed significant concern about specific orofacial features, including thin lips (73%), mouth furrows (80%), loss of facial lines (68%), and a smaller, tighter mouth (77%).
“Patients with systemic sclerosis may have loss of vermilion lip, microstomia, and perioral rhytids,” Kathleen Cook Suozzi, MD, who directs the Aesthetic Dermatology Program at Yale University School of Medicine, New Haven, Connecticut, said at the Controversies and Conversations in Laser and Cosmetic Surgery annual symposium. “How can we address these changes for our patients?”
Recent research has shown that hyaluronidase injections can help improve orofacial changes commonly experienced by patients with scleroderma. In 2019, researchers in Alabama reported the case of a 53-year-old woman treated with hyaluronidase for scleroderma-induced microstomia. After four visits over 7 months and a total hyaluronidase dose of 470 IU, the patient reported an improved Mouth Handicap in Systemic Sclerosis (MHISS) score (38 of 48); subjective improvement of symptoms, including greater ease in eating and undergoing dental treatment; and improved mouth closure.
In 2023, researchers published a cohort study of four women between the ages of 43 and 61 with autoimmune sclerosing conditions that resulted in oral microstomia. Following hyaluronidase injections, all improved in mouth opening capacity and MHISS, with change stabilizing between three and five treatments. More recently, in a study pending publication in JAAD Case Reports, Dr. Suozzi and colleagues retrospectively evaluated 12 women with scleroderma who received between 150 and 300 units of hyaluronic acid (HA) filler for microstomia between 2020 and 2023. Of the 12 women, 58% had diffuse disease, and 42% had limited disease. Overall, oral aperture width increased by 0.65 cm (P = .0027) and oral aperture height increased by 0.88 cm (P < .0001). “In general, patients needed three to four treatments to reach peak effect, and then they reached a plateau,” Dr. Suozzi said. “It wasn’t that the treatment wasn’t working anymore, but it was because their oral aperture had gotten to a size of around 5 cm, which is clinically normal. Interestingly, we found that if the patient’s disease flared and their microstomia started to return, when you rechallenged them, they continued to respond. So, patients can continue to use this treatment over time.”
In a separate case series of seven patients, Dr. Suozzi and colleagues prospectively evaluated the effect of HA soft tissue filler with Restylane Silk for lip augmentation. Study participants experienced statistically significant increases in the difference between pre- and postinjection fullness in both upper and lower lips. Also, the mean posttreatment score fell between “much improved” (2) and “improved” (3) on both the Investigator Global Aesthetic Improvement Scale and the Subject Global Aesthetic Improvement Scale.
Dr. Suozzi recommends using nerve blocks for injecting HA filler or hyaluronidase in patients with scleroderma and minimizing the injection points. “Initially, we were using 30% lidocaine preparations around the mouth for an hour before the procedure, and patients were still having pain, so now we use nerve blocks,” she said. “For hyaluronidase, we do perform a test dose of 75-100 units, usually in the commissure. It’s amazing how well it works; people will usually come back after their test dose and have improvements in their measurements. This is a really easy treatment to perform, and I think it can be done in the office of a general dermatologist. There is concern about cross-reactivity with bee venom, so you want to ask patients about that.”
Dr. Suozzi reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.