User login
Are AI-powered skin-check tools on the horizon for dermatologists, PCPs?
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
.
Given that about 6.3 billion smartphones would soon be in use, this AI approach could provide a gateway for “low-cost universal access to vital diagnostic care,” wrote Justin M. Ko, MD, MBA, a dermatologist, and colleagues from Stanford (Calif.) University that included other dermatologists and engineers.
Dr. Ko and his coauthors described how they trained a computer system to identify both benign and cancerous skin lesions. They used an approach known as a convolutional neural network, often deployed for projects seeking to train computers to “see” through image analysis. They said that their test of this system found it to be on par with the performance of 21 board-certified dermatologists.
“This fast, scalable method is deployable on mobile devices and holds the potential for substantial clinical impact, including broadening the scope of primary care practice and augmenting clinical decision-making for dermatology specialists,” they wrote in their paper.
More than 6 years later, there are signs that companies are making progress toward moving skin checks using this technology into U.S. primary care settings – but only with devices that employ special tools.
It may prove tougher for companies to eventually secure the sign-off of the U.S. Food and Drug Administration for mobile apps intended to let consumers handle this task with smartphones.
Such tools would need to be proven highly accurate before release, because too many false positives mean that people would be needlessly exposed to biopsies, said Sancy A. Leachman, MD, PhD, director of the melanoma research program and chair of the department of dermatology at Oregon Health & Science University, Portland.
And false-negative readings would allow melanoma to advance and even be fatal, Dr. Leachman told this news organization.
Roxana Daneshjou, MD, PhD, a dermatologist at Stanford who has studied the promise and the pitfalls of AI in medicine, said that developers of a consumer skin-check app would need to know how people would react to their readings. That includes a good sense of how often they would appropriately seek medical care for a concerning reading. (She was not an author of the previously cited Nature paper but has published widely on AI.)
“The direct-to-consumer diagnostic space makes me nervous,” Dr. Daneshjou said in an interview. “In order to do it, you really need to have good studies in consumer populations prior to release. You need to show how effective it is with follow up.”
FDA shows interest – and reservations
As of July, the FDA had not yet given its okay for marketing of any consumer apps intended to help people detect signs of skin cancer, an agency spokesperson told this news organization.
To date, the agency has only cleared two AI-based products for this task, both meant to be used by dermatologists. And only one of these two products, Scibase’s Nevisense, remains in use in the United States. The other, MelaFind, has been discontinued. In 2017, Strata Skin Sciences said that the product did not win “a significant enough level of acceptance by dermatologists to justify the continued investment” in it. And the company said it notified the 90 owners of MelaFind devices in the United States that it would no longer support the device.
But another company, DermaSensor, said in a 2021 press release that it expects its AI-powered tool, also named DermaSensor, to be the “first ever FDA cleared or approved skin cancer detection device for primary care providers.”
The Miami-based firm said that the FDA had granted its product a “breakthrough” device designation. A breakthrough designation means that agency staff will offer extra help and guidance to companies in developing a product, because of its expected benefit for patients.
In a 2020 press release, 3Derm Systems, now owned by Digital Diagnostics, made a similar announcement about winning FDA breakthrough designation for an AI-powered tool intended to allow skin checks in primary care settings.
(The FDA generally does not comment on its reviews of experimental drugs and devices, but companies can do so. Several other companies have announced FDA breakthrough designations for AI-driven products intended to check for skin lesions, but these might be used in settings other than primary care.)
Both DermaSensor and Digital Diagnostics have chairs with notable track records for winning FDA approvals of other devices. DermaSensor’s Maurice Ferre, MD, also is the chairman of Insightec, which in 2016 won the first FDA approval for a device with a breakthrough designation device that uses ultrasound to treat tremors.
In 2018, the FDA allowed Digital Diagnostics, then called IDx, to introduce in the United States the first medical device using AI in primary care offices to check for signs of diabetic retinopathy. This product also had an FDA breakthrough designation. The executive chairman and founder of Digital Diagnostics is Michael Abramoff, MD, PhD, professor of engineering and ophthalmology at the University of Iowa, Iowa City. Dr. Abramoff and the team behind the AI tool for retinopathy, now called the LumineticsCore system, also scored a notable win with Medicare, which agreed to cover use of the product through a dedicated CPT code.
FDA draft guidance
The FDA has acknowledged the interest in broadening access to skin checks via AI.
This was a topic of discussion at a 2-day advisory committee meeting the FDA held last year. In April 2023, the FDA outlined some of its expectations for future regulation of skin-analyzing tools as part of a wide-ranging draft guidance document intended to aid companies in their efforts to develop products using a form of AI known as machine learning.
In the document, the FDA described how it might approach applications for “hypothetical” devices using this kind of AI, such as a special tool to help primary care clinicians identify lesions in need of further investigation. Such a product would use a specific camera for gathering data for its initial clearance, in the FDA’s hypothetical scenario.
The FDA staff offered technical suggestions about what the developer of this hypothetical device would have to do to extend its use to smartphones and tablets while keeping clinicians as the intended users.
Some of these expanded uses could fall within the bounds of the FDA’s initial clearance and thus not trigger a need for a new marketing submission, the agency said. But seeking to shift this hypothetical product to “patient-facing” use would require a new marketing submission to the FDA, the agency said.
In this scenario, a company would expect people to follow up with a dermatologist after receiving a report suggesting cancer. Thus, this kind of a change could expose patients to “many new, unconsidered risks,” the FDA said.
Reality check?
The state of current efforts to develop consumer apps for checking for skin cancer seems to be summarized well on the website for the MoleMapper. The app was developed by researchers at OHSU to help people track how their moles change over time.
“Mole Mapper is NOT designed to provide medical advice, professional diagnosis, opinion, or treatment. Currently, there is not enough data to develop an app that can diagnose melanoma, but if enough data is collected through Mole Mapper and shared with researchers, it may be possible in the future,” the app’s website says.
OHSU released MoleMapper as an iPhone app in 2015. The aim of this project was to help people track the moles on their skin while also fostering an experiment in “citizen science,” OHSU’s Dr. Leachman told this news organization.
OHSU researchers hoped that the digital images taken by members of the public on cell phones could one day be used to develop diagnostic algorithms for melanoma.
But around 2017, the MoleMapper team realized that they would not be able to create a diagnostic app at this time, Dr. Leachman explained. They could not collect enough data of adequate quality.
And by 2021, it was clear that they could not even develop a successful app to triage patients to assess who needs to be seen quickly. The amount of data required was, at this point, beyond what the team could collect, Dr. Leachman said in an interview.
That was a disappointment because the team had successfully completed the difficult task of creating a confidential pathway for collecting these images via both iPhones and smartphones run on Android.
“We thought if we built it, people would come, but that’s not what happened,” Dr. Leachman said. Many patients didn’t want their images used for research or would fail to follow up with details of biopsy reports. Sometimes images were not captured well enough to be of use.
“You need at least hundreds of thousands, if not millions, of data points that have been verified with pathologies, and nobody was giving us back that data. That was the reality,” Dr. Leachman said.
There were valuable lessons in that setback. The OHSU team now has a better grasp of the challenges of trying to build a data-collection system that could prove helpful in assessing skin lesions.
“If you don’t build it, you don’t know” what can go wrong, she said.
Dr. Leachman said other scientists who have worked on similar projects to build skin-analyzing apps have probably encountered the same difficulties, although they may not reveal these issues. “I think that a lot of people build these things and then they try to make it into something that it’s not,” she said.
In addition to the challenges with gathering images, dermatologists frequently need to rely on touch and other clues from in-person visits when diagnosing a suspicious lesion. “There’s something about seeing and feeling the skin in person that can’t be captured completely with an image,” Dr. Leachman said.
Public demand
Still, regulators must face the strong and immediate interest consumers have in using AI to check on moles and skin conditions, despite continuing questions about how well this approach might work.
In June, Google announced in a blog post that its Google Lens tool can help people research skin conditions.
“Just take a picture or upload a photo through Lens, and you’ll find visual matches to inform your search,” Google said in a blog post. “This feature also works if you’re not sure how to describe something else on your body, like a bump on your lip, a line on your nails or hair loss on your head. This feature is currently available in the U.S.”
Google also continues work on DermAssist, an app that’s intended to help people get personalized information about skin concerns using three photos. It is not currently publicly available, a Google spokesperson told this news organization.
Several skin-analyzing apps are already available in the Apple and Google Play stores. The British Association of Dermatologists last year issued a press release warning consumers that these apps may not be safe or effective and thus may put patients at risk for misdiagnosis.
“Unfortunately, AI-based apps which do not appear to meet regulatory requirements crop up more often than we would like,” the association said. “Additionally, the evidence to support the use of AI to diagnose skin conditions is weak which means that when it is used, it may not be safe or effective and it is possible that AI is putting patients at risk of misdiagnosis.”
Delicate and difficult balancing act
At this time, regulators, entrepreneurs, and the medical community face a delicate balancing act in considering how best to deploy AI in skin care, Dr. Ko said in an interview. (In addition to being one of the authors on the widely cited 2017 Nature paper mentioned above, Dr. Ko served until March as the initial chair of the American Academy of Dermatology’s Augmented Intelligence Committee.)
There are many solid reasons why there hasn’t been speedy progress to deploy AI in dermatology, as many envisioned a few years ago, Dr. Ko said.
Some of those reasons are specific to dermatology; this field doesn’t have a ready set of robust data from which to build AI-driven tools. In this aspect, dermatology is decades behind specialties like radiology, pathology, and ophthalmology, where clinicians have long been accumulating and storing images and other data in more standardized ways, Dr. Ko said.
“If you went to most dermatology practices and said, ‘Hey, let me learn from the data accumulated over the course of your 30-year practice to help us develop new tools,’” there may not be a whole lot there,” Dr. Ko said.
Beyond the start-up hurdles is the larger concern Dr. Ko shares with other dermatologists who work in this field, such as Dr. Daneshjou and Dr. Leachman. What would clinicians without much dermatology training and patients do with the readings from AI-driven tools and apps?
There would need to be significant research to show that such products actually help get people treated for skin diseases, including skin cancer.
Dr. Ko praised Google for being open about the stumbles with its efforts to use its AI tool for identifying diabetic retinopathy in a test in Thailand. Real-world hitches included poor Internet connections and poor image quality.
Developing reliable systems, processes, and workflows will be paramount for eventual widespread use of AI-driven tools, Dr. Ko said.
“It’s all those hidden things that are not sexy,” as are announcements about algorithms working about as well as clinicians in diagnosis, Dr. Ko said. “They don’t get the media attention, but they’re going to be make or break for AI, not just in our field but [for] AI in general.”
But he added that there also needs to be a recognition that AI-driven tools and products, even if somewhat imperfect, can help people get access to care.
In many cases, shortages of specialists prevent people from getting screened for treatable conditions such as skin cancer and retinopathy. The challenge is setting an appropriate standard to make sure that AI-driven products would help most patients in practice, without raising it so high that no such products emerge.
“There’s a risk of holding too high of a bar,” Dr. Ko said. “There is harm in not moving forward as well.”
A version of this article first appeared on Medscape.com.
RFS failed as endpoint in adjuvant immunotherapy trials
TOPLINE:
METHODOLOGY:
- FDA approvals in the adjuvant setting for cancer immunotherapy are increasingly based on trials that use RFS as a surrogate endpoint for overall survival, largely because such a design allows for smaller, speedier trials.
- To test the validity of using RFS as a surrogate for overall survival in this setting, investigators conducted a meta-analysis of 15 phase 2 and 3 randomized controlled trials (RCTs) of adjuvant CTLA4 and anti–PD-1/PD-L1 blockers for melanoma, non–small cell lung cancer, renal cell cancer, and other tumors.
- The team used weighted regression at the arm and trial levels to assess the efficacy of RFS as a surrogate for overall survival.
- The strength of the association was quantified by weighted coefficients of determination (R2)12Dante MT Stdplz make sure all mentions of R’2’ are superscript, with a strong correlation considered to be R2 of 0.7 or higher.
- If there were strong correlations at both the arm and trial levels, RFS would be considered a robust surrogate endpoint for overall survival; however, if one of the correlations at the arm or trial level was not strong, RFS would not be considered a surrogate endpoint for overall survival.
TAKEAWAY:
- At the arm level, moderate and strong associations were observed between 2-year RFS and 3-year overall survival (R2, 0.58) and between 3-year RFS and 5-year overall survival (R2, 0.72; 95% confidence interval, 0.38-.00).
- At the trial level, a moderate association was observed between effect of treatment on RFS and overall survival (R2, 0.63).
- The findings were confirmed in several sensitivity analyses that were based on different trial phases, experimental arms, cancer types, and treatment strategies.
IN PRACTICE:
“Our meta-analysis failed to find a significantly strong association between RFS and OS in RCTs of adjuvant immunotherapy,” the authors concluded. “RFS should not be used as a surrogate endpoint for OS in this clinical context.” Instead, the finding indicates that overall survival is “the ideal primary endpoint” in this setting.
SOURCE:
The study, led by Yuanfang Li, PhD, of Sun Yat-sen University Cancer Center in Guangzhou, China, was published in the Journal of the National Cancer Institute.
LIMITATIONS:
- Correlations were calculated from a relatively limited number of RCTs that involved different types of cancer, and overall survival data were not fully mature in some of the trials.
- The analysis did not include patient-level data.
DISCLOSURES:
- The work was funded by the National Natural Science Foundation of China and others.
- The investigators had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- FDA approvals in the adjuvant setting for cancer immunotherapy are increasingly based on trials that use RFS as a surrogate endpoint for overall survival, largely because such a design allows for smaller, speedier trials.
- To test the validity of using RFS as a surrogate for overall survival in this setting, investigators conducted a meta-analysis of 15 phase 2 and 3 randomized controlled trials (RCTs) of adjuvant CTLA4 and anti–PD-1/PD-L1 blockers for melanoma, non–small cell lung cancer, renal cell cancer, and other tumors.
- The team used weighted regression at the arm and trial levels to assess the efficacy of RFS as a surrogate for overall survival.
- The strength of the association was quantified by weighted coefficients of determination (R2)12Dante MT Stdplz make sure all mentions of R’2’ are superscript, with a strong correlation considered to be R2 of 0.7 or higher.
- If there were strong correlations at both the arm and trial levels, RFS would be considered a robust surrogate endpoint for overall survival; however, if one of the correlations at the arm or trial level was not strong, RFS would not be considered a surrogate endpoint for overall survival.
TAKEAWAY:
- At the arm level, moderate and strong associations were observed between 2-year RFS and 3-year overall survival (R2, 0.58) and between 3-year RFS and 5-year overall survival (R2, 0.72; 95% confidence interval, 0.38-.00).
- At the trial level, a moderate association was observed between effect of treatment on RFS and overall survival (R2, 0.63).
- The findings were confirmed in several sensitivity analyses that were based on different trial phases, experimental arms, cancer types, and treatment strategies.
IN PRACTICE:
“Our meta-analysis failed to find a significantly strong association between RFS and OS in RCTs of adjuvant immunotherapy,” the authors concluded. “RFS should not be used as a surrogate endpoint for OS in this clinical context.” Instead, the finding indicates that overall survival is “the ideal primary endpoint” in this setting.
SOURCE:
The study, led by Yuanfang Li, PhD, of Sun Yat-sen University Cancer Center in Guangzhou, China, was published in the Journal of the National Cancer Institute.
LIMITATIONS:
- Correlations were calculated from a relatively limited number of RCTs that involved different types of cancer, and overall survival data were not fully mature in some of the trials.
- The analysis did not include patient-level data.
DISCLOSURES:
- The work was funded by the National Natural Science Foundation of China and others.
- The investigators had no disclosures.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- FDA approvals in the adjuvant setting for cancer immunotherapy are increasingly based on trials that use RFS as a surrogate endpoint for overall survival, largely because such a design allows for smaller, speedier trials.
- To test the validity of using RFS as a surrogate for overall survival in this setting, investigators conducted a meta-analysis of 15 phase 2 and 3 randomized controlled trials (RCTs) of adjuvant CTLA4 and anti–PD-1/PD-L1 blockers for melanoma, non–small cell lung cancer, renal cell cancer, and other tumors.
- The team used weighted regression at the arm and trial levels to assess the efficacy of RFS as a surrogate for overall survival.
- The strength of the association was quantified by weighted coefficients of determination (R2)12Dante MT Stdplz make sure all mentions of R’2’ are superscript, with a strong correlation considered to be R2 of 0.7 or higher.
- If there were strong correlations at both the arm and trial levels, RFS would be considered a robust surrogate endpoint for overall survival; however, if one of the correlations at the arm or trial level was not strong, RFS would not be considered a surrogate endpoint for overall survival.
TAKEAWAY:
- At the arm level, moderate and strong associations were observed between 2-year RFS and 3-year overall survival (R2, 0.58) and between 3-year RFS and 5-year overall survival (R2, 0.72; 95% confidence interval, 0.38-.00).
- At the trial level, a moderate association was observed between effect of treatment on RFS and overall survival (R2, 0.63).
- The findings were confirmed in several sensitivity analyses that were based on different trial phases, experimental arms, cancer types, and treatment strategies.
IN PRACTICE:
“Our meta-analysis failed to find a significantly strong association between RFS and OS in RCTs of adjuvant immunotherapy,” the authors concluded. “RFS should not be used as a surrogate endpoint for OS in this clinical context.” Instead, the finding indicates that overall survival is “the ideal primary endpoint” in this setting.
SOURCE:
The study, led by Yuanfang Li, PhD, of Sun Yat-sen University Cancer Center in Guangzhou, China, was published in the Journal of the National Cancer Institute.
LIMITATIONS:
- Correlations were calculated from a relatively limited number of RCTs that involved different types of cancer, and overall survival data were not fully mature in some of the trials.
- The analysis did not include patient-level data.
DISCLOSURES:
- The work was funded by the National Natural Science Foundation of China and others.
- The investigators had no disclosures.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF THE NATIONAL CANCER INSTITUTE
What makes teens choose to use sunscreen?
a cornerstone of skin cancer prevention, according to results from a systematic review.
“We know that skin cancer is one of the most common malignancies in the world, and sun protection methods such as sunscreen make it highly preventable,” first author Carly R. Stevens, a student at Tulane University, New Orleans, said in an interview. “This study demonstrates the adolescent populations that are most vulnerable to sun damage and how we can help mitigate their risk of developing skin cancer through education methods, such as Sun Protection Outreach Teaching by Students.”
Ms. Stevens and coauthors presented the findings during a poster session at the annual meeting of the Society for Pediatric Dermatology.
To investigate predictors of sunscreen use among high school students, they searched PubMed, Embase, and Web of Science using the terms (“sunscreen” or “SPF” or “sun protection”) and (“high school” or “teen” or “teenager” or “adolescent”) and limited the analysis to English studies reporting data on sunscreen use in U.S. high school students up to November 2021.
A total of 20 studies were included in the final review. The study populations ranged in number from 208 to 24,645. Of 11 studies that examined gender, all showed increased sunscreen use in females compared with males. Of five studies that examined age, all showed increased sunscreen use in younger adolescents, compared with their older counterparts.
Of four studies that examined the role of ethnicity on sunscreen use, White students were more likely to use sunscreen, compared with their peers of other ethnicities. “This may be due to perceived sun sensitivity, as [these four studies] also showed increased sunscreen use in populations that believed were more susceptible to sun damage,” the researchers wrote in their abstract.
In other findings, two studies that examined perceived self-efficacy concluded that higher levels of sunscreen use correlated with higher self-efficacy, while four studies concluded that high school students were more likely to use sunscreen if their parents encouraged them the wear it or if the parent used it themselves.
“With 40%-50% of ultraviolet damage being done before the age of 20, it’s crucial that we find ways to educate adolescents on the importance of sunscreen use and target those populations who were found to rarely use sunscreen in our study,” Ms. Stevens said.
In one outreach program, Sun Protection Outreach Teaching by Students (SPOTS), medical students visit middle and high schools to educate them about the importance of practicing sun protection. The program began as a collaboration between Saint Louis University and Washington University in St. Louis, but has expanded nationwide. Ms. Stevens described SPOTS as “a great way for medical students to present the information to middle and high school students in a way that is engaging and interactive.”
The researchers reported having no disclosures.
a cornerstone of skin cancer prevention, according to results from a systematic review.
“We know that skin cancer is one of the most common malignancies in the world, and sun protection methods such as sunscreen make it highly preventable,” first author Carly R. Stevens, a student at Tulane University, New Orleans, said in an interview. “This study demonstrates the adolescent populations that are most vulnerable to sun damage and how we can help mitigate their risk of developing skin cancer through education methods, such as Sun Protection Outreach Teaching by Students.”
Ms. Stevens and coauthors presented the findings during a poster session at the annual meeting of the Society for Pediatric Dermatology.
To investigate predictors of sunscreen use among high school students, they searched PubMed, Embase, and Web of Science using the terms (“sunscreen” or “SPF” or “sun protection”) and (“high school” or “teen” or “teenager” or “adolescent”) and limited the analysis to English studies reporting data on sunscreen use in U.S. high school students up to November 2021.
A total of 20 studies were included in the final review. The study populations ranged in number from 208 to 24,645. Of 11 studies that examined gender, all showed increased sunscreen use in females compared with males. Of five studies that examined age, all showed increased sunscreen use in younger adolescents, compared with their older counterparts.
Of four studies that examined the role of ethnicity on sunscreen use, White students were more likely to use sunscreen, compared with their peers of other ethnicities. “This may be due to perceived sun sensitivity, as [these four studies] also showed increased sunscreen use in populations that believed were more susceptible to sun damage,” the researchers wrote in their abstract.
In other findings, two studies that examined perceived self-efficacy concluded that higher levels of sunscreen use correlated with higher self-efficacy, while four studies concluded that high school students were more likely to use sunscreen if their parents encouraged them the wear it or if the parent used it themselves.
“With 40%-50% of ultraviolet damage being done before the age of 20, it’s crucial that we find ways to educate adolescents on the importance of sunscreen use and target those populations who were found to rarely use sunscreen in our study,” Ms. Stevens said.
In one outreach program, Sun Protection Outreach Teaching by Students (SPOTS), medical students visit middle and high schools to educate them about the importance of practicing sun protection. The program began as a collaboration between Saint Louis University and Washington University in St. Louis, but has expanded nationwide. Ms. Stevens described SPOTS as “a great way for medical students to present the information to middle and high school students in a way that is engaging and interactive.”
The researchers reported having no disclosures.
a cornerstone of skin cancer prevention, according to results from a systematic review.
“We know that skin cancer is one of the most common malignancies in the world, and sun protection methods such as sunscreen make it highly preventable,” first author Carly R. Stevens, a student at Tulane University, New Orleans, said in an interview. “This study demonstrates the adolescent populations that are most vulnerable to sun damage and how we can help mitigate their risk of developing skin cancer through education methods, such as Sun Protection Outreach Teaching by Students.”
Ms. Stevens and coauthors presented the findings during a poster session at the annual meeting of the Society for Pediatric Dermatology.
To investigate predictors of sunscreen use among high school students, they searched PubMed, Embase, and Web of Science using the terms (“sunscreen” or “SPF” or “sun protection”) and (“high school” or “teen” or “teenager” or “adolescent”) and limited the analysis to English studies reporting data on sunscreen use in U.S. high school students up to November 2021.
A total of 20 studies were included in the final review. The study populations ranged in number from 208 to 24,645. Of 11 studies that examined gender, all showed increased sunscreen use in females compared with males. Of five studies that examined age, all showed increased sunscreen use in younger adolescents, compared with their older counterparts.
Of four studies that examined the role of ethnicity on sunscreen use, White students were more likely to use sunscreen, compared with their peers of other ethnicities. “This may be due to perceived sun sensitivity, as [these four studies] also showed increased sunscreen use in populations that believed were more susceptible to sun damage,” the researchers wrote in their abstract.
In other findings, two studies that examined perceived self-efficacy concluded that higher levels of sunscreen use correlated with higher self-efficacy, while four studies concluded that high school students were more likely to use sunscreen if their parents encouraged them the wear it or if the parent used it themselves.
“With 40%-50% of ultraviolet damage being done before the age of 20, it’s crucial that we find ways to educate adolescents on the importance of sunscreen use and target those populations who were found to rarely use sunscreen in our study,” Ms. Stevens said.
In one outreach program, Sun Protection Outreach Teaching by Students (SPOTS), medical students visit middle and high schools to educate them about the importance of practicing sun protection. The program began as a collaboration between Saint Louis University and Washington University in St. Louis, but has expanded nationwide. Ms. Stevens described SPOTS as “a great way for medical students to present the information to middle and high school students in a way that is engaging and interactive.”
The researchers reported having no disclosures.
FROM SPD 2023
No benefit to adding limited radiation in advanced cancer
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
TOPLINE:
METHODOLOGY:
- In the phase 2 CHEERS trial, 52 patients with advanced solid tumors were randomized to anti-PD-1/PD-L1 monotherapy and 47 patients to the same treatment plus stereotactic body radiotherapy (3 x 8 Gy) to a maximum of three lesions before the second or third cycle of an immune checkpoint inhibitor.
- Patients had locally advanced or metastatic melanoma, renal cell carcinoma, urothelial carcinoma, non-small cell lung carcinoma, or head and neck squamous cell carcinoma and were treated at five Belgian hospitals.
- Most patients had more than three lesions.
- Seven patients in the experimental group did not complete radiotherapy because of early progression or intercurrent illness.
TAKEAWAY:
- Over a median follow-up of 12.5 months, median progression-free survival was 4.4 months in the radiotherapy group versus 2.8 months in the control group (hazard ratio, 0.95; P = .82).
- Median overall survival was not significantly better with radiotherapy, compared with the control group (14.3 vs. 11 months; HR, 0.82; P = .47), nor was the objective response rate (27% vs. 22%; P = .56).
- However, a post hoc analysis demonstrated a significant association between the number of irradiated lesions and overall survival among patients receiving radiotherapy (HR, 0.31; P = .002).
- The incidence of grade 3 or worse treatment-related adverse events was 18% in both groups.
IN PRACTICE:
Although the study was negative overall, the post hoc analysis coupled with “recent evidence suggests that treating all active disease sites with higher radiation doses ... may be a more promising strategy to optimize systemic disease control,” the authors concluded.
SOURCE:
The study was led by Mathieu Spaas, MD, department of radiation oncology, Ghent (Bellgium) University, and published online in JAMA Oncology.
LIMITATIONS:
- There was insufficient power to detect if certain cancers benefited more from add-on radiation because of the small sample size.
- More than half of patients in the control group had already received some form of radiotherapy before study inclusion, which may mean the study underestimated the benefit of radiotherapy.
DISCLOSURES:
The work was funded by Kom Op Tegen Kanker and Varian Medical Systems.
Investigators disclosed numerous industry ties, including Merck, Novartis, and Bristol Myers Squibb.
A version of this article first appeared on Medscape.com.
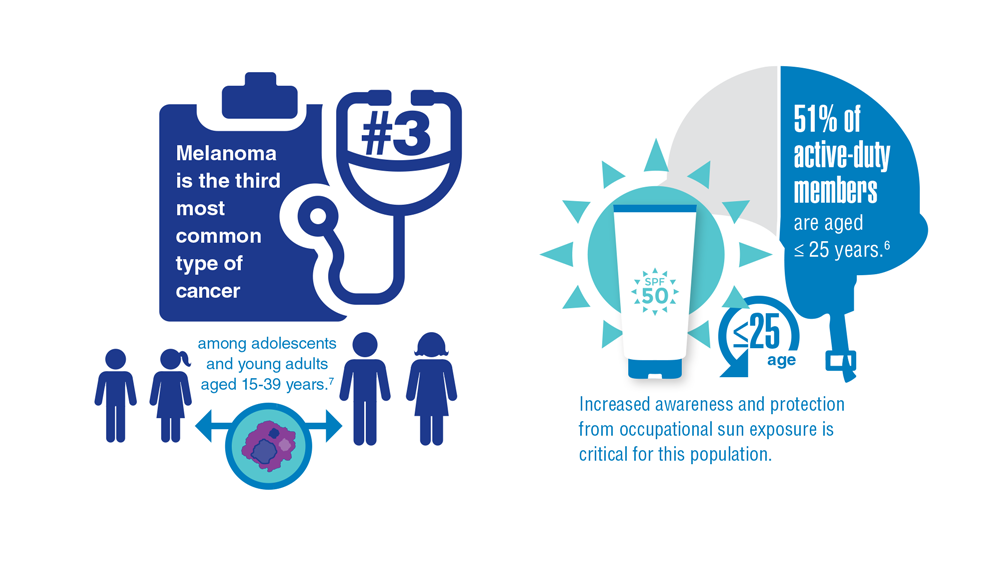
Necessary Updates to Skin Cancer Risk Stratification
1. Powers JG, Patel NA, Powers EA, Mayer JE, Stricklin GP, Geller AC. Skin cancer
risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
2. Balci S, Ayaz L, Gorur A, Yildirim Yaroglu H, Akbayir S, Dogruer Unal N, Bulut B,
Tursen U, Tamer L. microRNA profiling for early detection of nonmelanoma skin cancer. Clin Exp Dermatol. 2016;41(4):346-51. doi:10.1111/ced.12736
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
4. Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748-62.
5. Chou SE, Gaysynsky A, Trivedi N, Vanderpool R. Using social media for health: national data from HINTS 2019. Journ of Health Comm. 2019;26(3):184-193. doi:10.1080/10810730.2021.
6. Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279-82.
7. Dennis LK, et al. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Annals of Epidem. 2008;18(8):614-627. doi:10.1016/j.annepidem.2008.
8. Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomar Prev. 2014;23(6):1080-1089.
9. 2020 Demographics Profile of the military community. US Department of Defense. 2020:iv. Accessed November 15, 2022. 2020 Demographics Profile of the Military Community (militaryonesource.mil)
10. Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1-6.
11. Basch CH, Hillyer GC. Skin cancer on Instagram: implications for adolescents and young adults. Int J Adolesc Med Health. 2022;34(3). doi:10.1515/ijamh-2019-0218
1. Powers JG, Patel NA, Powers EA, Mayer JE, Stricklin GP, Geller AC. Skin cancer
risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
2. Balci S, Ayaz L, Gorur A, Yildirim Yaroglu H, Akbayir S, Dogruer Unal N, Bulut B,
Tursen U, Tamer L. microRNA profiling for early detection of nonmelanoma skin cancer. Clin Exp Dermatol. 2016;41(4):346-51. doi:10.1111/ced.12736
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
4. Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748-62.
5. Chou SE, Gaysynsky A, Trivedi N, Vanderpool R. Using social media for health: national data from HINTS 2019. Journ of Health Comm. 2019;26(3):184-193. doi:10.1080/10810730.2021.
6. Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279-82.
7. Dennis LK, et al. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Annals of Epidem. 2008;18(8):614-627. doi:10.1016/j.annepidem.2008.
8. Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomar Prev. 2014;23(6):1080-1089.
9. 2020 Demographics Profile of the military community. US Department of Defense. 2020:iv. Accessed November 15, 2022. 2020 Demographics Profile of the Military Community (militaryonesource.mil)
10. Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1-6.
11. Basch CH, Hillyer GC. Skin cancer on Instagram: implications for adolescents and young adults. Int J Adolesc Med Health. 2022;34(3). doi:10.1515/ijamh-2019-0218
1. Powers JG, Patel NA, Powers EA, Mayer JE, Stricklin GP, Geller AC. Skin cancer
risk factors and preventative behaviors among United States military veterans deployed to Iraq and Afghanistan. J Invest Dermatol. 2015;135:2871-2873.
2. Balci S, Ayaz L, Gorur A, Yildirim Yaroglu H, Akbayir S, Dogruer Unal N, Bulut B,
Tursen U, Tamer L. microRNA profiling for early detection of nonmelanoma skin cancer. Clin Exp Dermatol. 2016;41(4):346-51. doi:10.1111/ced.12736
3. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7-33. doi:10.3322/caac.21708
4. Agbai ON, Buster K, Sanchez M, Hernandez C, Kundu RV, Chiu M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70(4):748-62.
5. Chou SE, Gaysynsky A, Trivedi N, Vanderpool R. Using social media for health: national data from HINTS 2019. Journ of Health Comm. 2019;26(3):184-193. doi:10.1080/10810730.2021.
6. Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279-82.
7. Dennis LK, et al. Sunburns and risk of cutaneous melanoma: does age matter? A comprehensive meta-analysis. Annals of Epidem. 2008;18(8):614-627. doi:10.1016/j.annepidem.2008.
8. Wu S, Han J, Laden F, Qureshi AA. Long-term ultraviolet flux, other potential risk factors, and skin cancer risk: a cohort study. Cancer Epidemiol Biomar Prev. 2014;23(6):1080-1089.
9. 2020 Demographics Profile of the military community. US Department of Defense. 2020:iv. Accessed November 15, 2022. 2020 Demographics Profile of the Military Community (militaryonesource.mil)
10. Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Ioannides D. Epidemiological trends in skin cancer. Dermatol Pract Concept. 2017;7:1-6.
11. Basch CH, Hillyer GC. Skin cancer on Instagram: implications for adolescents and young adults. Int J Adolesc Med Health. 2022;34(3). doi:10.1515/ijamh-2019-0218
Cancer Data Trends 2023
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Federal Practitioner and the Association of VA Hematology/Oncology (AVAHO) present the 2023 edition of Cancer Data Trends (click to view the digital edition). This special issue provides updates on some of the top cancers and related concerns affecting veterans through original infographics and visual storytelling.
In this issue:
- COVID-19 Outcomes in Veterans With Hematologic Cancers
- Promising New Approaches for Testicular and Prostate Cancer
- Screening Guideline Updates and New Treatments in Colon Cancer
- Exposure-Related Cancers: A Look at the PACT Act
- New Classifications and Emerging Treatments in Brain Cancer
- Gender Disparity in Breast Cancer Among US Veterans
- Lung Cancer Screening in Veterans
- Necessary Updates to Skin Cancer Risk Stratification
- Innovation in Cancer Treatment
Palliative Care: Utilization Patterns in Inpatient Dermatology
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2
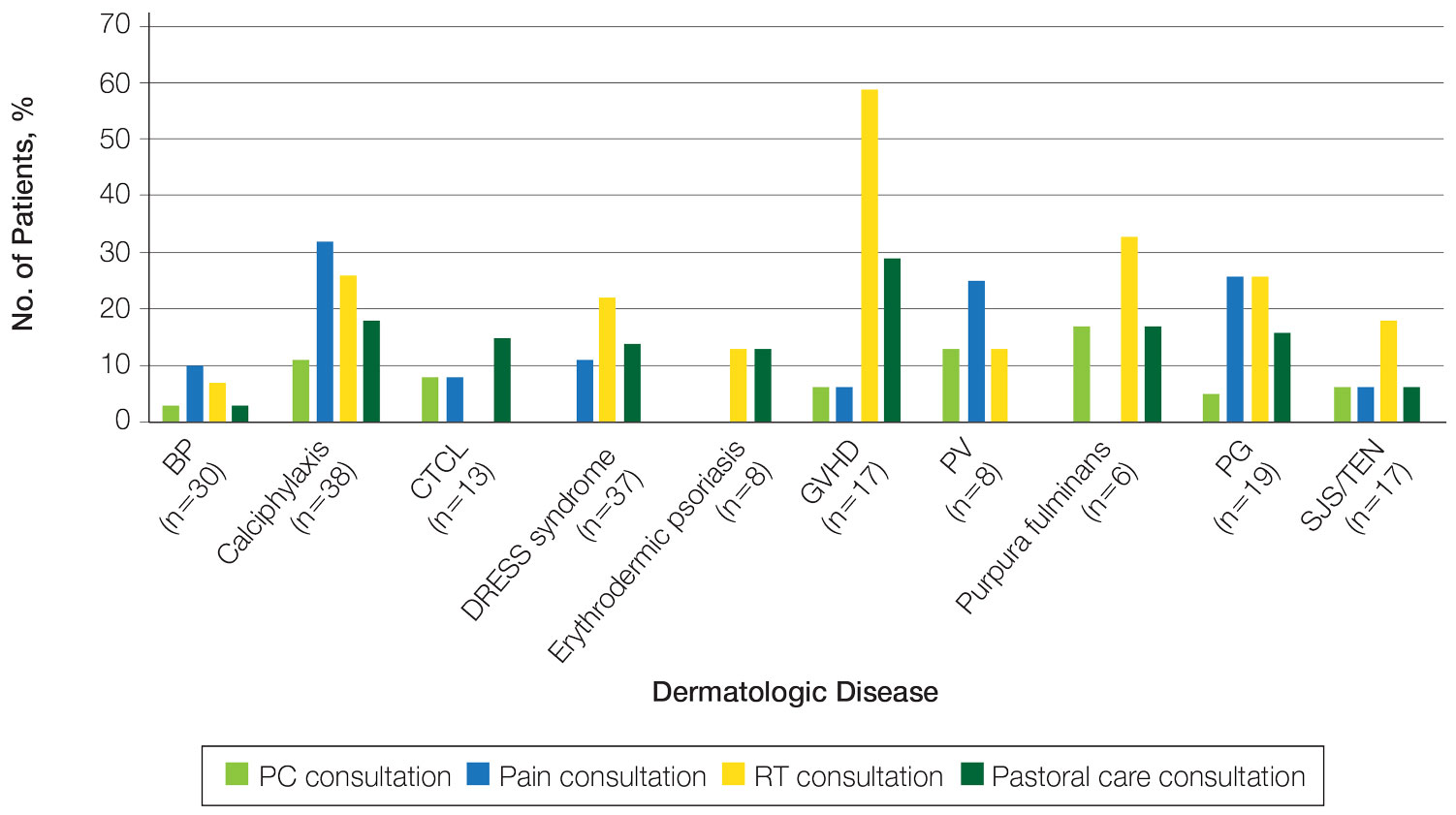
Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
Palliative care (PC) is a field of medicine that focuses on improving quality of life by managing physical symptoms as well as mental and spiritual well-being in patients with severe illnesses.1,2 Despite cases of severe dermatologic disease, the use of PC in the field of dermatology is limited, often leaving patients with a range of unmet needs.2,3 In one study that explored PC in patients with melanoma, only one-third of patients with advanced melanoma had a PC consultation.4 Reasons behind the lack of utilization of PC in dermatology include time constraints and limited training in addressing the complex psychosocial needs of patients with severe dermatologic illnesses.1 We conducted a retrospective, cross-sectional, single-institution study of specific inpatient dermatology consultations over a 5-year period to describe PC utilization among patients who were hospitalized with select severe dermatologic diseases.
Methods
A retrospective, cross-sectional study of inpatient dermatology consultations over a 5-year period (October 2016 to October 2021) was performed at Atrium Health Wake Forest Baptist Medical Center (Winston-Salem, North Carolina). Patients’ medical records were reviewed if they had one of the following diseases: bullous pemphigoid, calciphylaxis, cutaneous T-cell lymphoma (CTCL), drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, erythrodermic psoriasis, graft-vs-host disease, pemphigus vulgaris (PV), purpura fulminans, pyoderma gangrenosum, and Stevens-Johnson syndrome/toxic epidermal necrolysis. These diseases were selected for inclusion because they have been associated with a documented increase in inpatient mortality and have been described in the published literature on PC in dermatology.2 This study was reviewed and approved by the Wake Forest University institutional review board.
Use of PC consultative services along with other associated consultative care (ie, recreation therapy [RT], acute pain management, pastoral care) was assessed for each patient. Recreation therapy included specific interventions such as music therapy, arts/craft therapy, pet therapy, and other services with the goal of improving patient cognitive, emotional, and social function. For patients with a completed PC consultation, goals for PC intervention were recorded.
Results
The total study sample included 193 inpatient dermatology consultations. The mean age of the patients was 58.9 years (range, 2–100 years); 66.8% (129/193) were White and 28.5% (55/193) were Black (Table). Palliative care was consulted in 5.7% of cases, with consultations being requested by the primary care team. Reasons for PC consultation included assessment of the patient’s goals of care (4.1% [8/193]), pain management (3.6% [7/193]), non–pain symptom management (2.6% [5/193]), psychosocial support (1.6% [3/193]), and transitions of care (1.0% [2/193]). The average length of patients’ hospital stay prior to PC consultation was 11.5 days(range, 1–32 days). Acute pain management was the reason for consultation in 15.0% of cases (29/193), RT in 21.8% (42/193), and pastoral care in 13.5% (26/193) of cases. Patients with calciphylaxis received the most PC and pain consultations, but fewer than half received these services. Patients with calciphylaxis, PV, purpura fulminans, and CTCL received a higher percentage of PC consultations than the overall cohort, while patients with calciphylaxis, DRESS syndrome, PV, and pyoderma gangrenosum received relatively more pain consultations than the overall cohort (Figure).

Comment
Clinical practice guidelines for quality PC stress the importance of specialists being familiar with these services and the ability to involve PC as part of the treatment plan to achieve better care for patients with serious illnesses.5 Our results demonstrated low rates of PC consultation services for dermatology patients, which supports the existing literature and suggests that PC may be highly underutilized in inpatient settings for patients with serious skin diseases. Use of PC was infrequent and was initiated relatively late in the course of hospital admission, which can negatively impact a patient’s well-being and care experience and can increase the care burden on their caregivers and families.2

Our results suggest a discrepancy in the frequency of formal PC and other palliative consultative services used for dermatologic diseases, with non-PC services including RT, acute pain management, and pastoral care more likely to be utilized. Impacting this finding may be that RT, pastoral care, and acute pain management are provided by nonphysician providers at our institution, not attending faculty staffing PC services. Patients with calciphylaxis were more likely to have PC consultations, potentially due to medicine providers’ familiarity with its morbidity and mortality, as it is commonly associated with end-stage renal disease. Similarly, internal medicine providers may be more familiar with pain classically associated with PG and PV and may be more likely to engage pain experts. Some diseases with notable morbidity and potential mortality were underrepresented including SJS/TEN, erythrodermic psoriasis, CTCL, and GVHD.
Limitations of our study included examination of data from a single institution, as well as the small sample sizes in specific subgroups, which prevented us from making comparisons between diseases. The cross-sectional design also limited our ability to control for confounding variables.
Conclusion
We urge dermatology consultation services to advocate for patients with serious skin diseases andinclude PC consultation as part of their recommendations to primary care teams. Further research should characterize the specific needs of patients that may be addressed by PC services and explore ways dermatologists and others can identify and provide specialty care to hospitalized patients.
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
- Kelley AS, Morrison RS. Palliative care for the seriously ill. N Engl J Med. 2015;373:747-755.
- Thompson LL, Chen ST, Lawton A, et al. Palliative care in dermatology: a clinical primer, review of the literature, and needs assessment. J Am Acad Dermatol. 2021;85:708-717. doi:10.1016/j.jaad.2020.08.029
- Yang CS, Quan VL, Charrow A. The power of a palliative perspective in dermatology. JAMA Dermatol. 2022;158:609-610. doi:10.1001/jamadermatol.2022.1298
- Osagiede O, Colibaseanu DT, Spaulding AC, et al. Palliative care use among patients with solid cancer tumors. J Palliat Care. 2018;33:149-158.
- Clinical Practice Guidelines for Quality Palliative Care. 4th ed. National Coalition for Hospice and Palliative Care; 2018. Accessed June 21, 2023. https://www.nationalcoalitionhpc.org/wp-content/uploads/2018/10/NCHPC-NCPGuidelines_4thED_web_FINAL.pdf
Practice Points
- Although severe dermatologic disease negatively impacts patients’ quality of life, palliative care may be underutilized in this population.
- Palliative care should be an integral part of caring for patients who are admitted to the hospital with serious dermatologic illnesses.
Imaging techniques will revolutionize cancer detection, expert predicts
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
PHOENIX –
In a lecture during a multispecialty roundup of cutting-edge energy-based device applications at the annual conference of the American Society for Laser Medicine and Surgery, Dr. Barton, a biomedical engineer who directs the BIO5 Institute at the University of Arizona, Tucson, said that while no current modality exists to enable physicians in dermatology and other specialties to view internal structures throughout the entire body with cellular resolution, refining existing technologies is a good way to start.
In 2011, renowned cancer researchers Douglas Hanahan, PhD, and Robert A. Weinberg, PhD, proposed six hallmarks of cancer, which include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Each hallmark poses unique imaging challenges. For example, enabling replicative immortality “means that the cell nuclei change size and shape; they change their position,” said Dr. Barton, who is also professor of biomedical engineering and optical sciences at the university. “If we want to see that, we’re going to need an imaging modality that’s subcellular in resolution.”
Similarly, if clinicians want to view how proliferative signaling is changing, “that means being able to visualize the cell surface receptors; those are even smaller to actually visualize,” she said. “But we have technologies where we can target those receptors with fluorophores. And then we can look at large areas very quickly.” Meanwhile, the ability of cancer cells to resist cell death and evade growth suppressors often results in thickening of epithelium throughout the body. “So, if we can measure the thickness of the epithelium, we can see that there’s something wrong with that tissue,” she said.
As for cancer’s propensity for invasion and metastasis, “here, we’re looking at how the collagen structure [between the cells] has changed and whether there’s layer breakdown or not. Optical imaging can detect cancer. However, high resolution optical techniques can only image about 1 mm deep, so unless you’re looking at the skin or the eye, you’re going to have to develop an endoscope to be able to view these hallmarks.”
OCT images the tissue microstructure, generally in a resolution of 2-20 microns, at a depth of 1-2 mm, and it measures reflected light. When possible, Dr. Barton combines OCT with laser-induced fluorescence for enhanced accuracy of detection of cancer. Induced fluorescence senses molecular information with the natural fluorophores in the body or with targeted exogenous agents. Then there’s multiphoton microscopy, an advanced imaging technique that enables clinicians to view cellular and subcellular events within living tissue. Early models of this technology “took up entire benches” in physics labs, Dr. Barton said, but she and other investigators are designing smaller devices for use in clinics. “This is exciting, because not only do we [view] subcellular structure with this modality, but it can also be highly sensitive to collagen structure,” she said.
Ovarian cancer model
In a model of ovarian cancer, she and colleagues externalized the ovaries of a mouse, imaged the organs, put them back in, and reassessed them at 8 weeks. “This model develops cancer very quickly,” said Dr. Barton, who once worked for McDonnell Douglas on the Space Station program. At 8 weeks, using fluorescence and targeted agents with a tabletop multiphoton microscopy system, they observed that the proliferation signals of cancer had begun. “So, with an agent targeted to the folate receptor or to other receptors that are implicated in cancer development, we can see that ovaries and fallopian tubes are lighting up,” she said.
With proof of concept established with the mouse study, she and other researchers are drawing from technological advances to create tiny laser systems for use in the clinic to image a variety of structures in the human body. Optics advances include bulk optics and all-fiber designs where engineers can create an imaging probe that’s only 125 microns in diameter, “or maybe even as small as 70 microns in diameter,” she said. “We can do fabrications on the tips of endoscopes to redirect the light and focus it. We can also do 3-D printing and spiral scanning to create miniature devices to make new advances. That means that instead of just white light imaging of the colon or the lung like we have had in the past, we can start moving into smaller structures, such as the eustachian tube, the fallopian tube, the bile ducts, or making miniature devices for brain biopsies, lung biopsies, and maybe being able to get into bronchioles and arterioles.”
According to Dr. Barton, prior research has demonstrated that cerebral vasculature can be imaged with a catheter 400 microns in diameter, the spaces in the lungs can be imaged with a needle that is 310 microns in diameter, and the inner structures of the eustachian tube can be viewed with an endoscope 1 mm in diameter.
She and her colleagues are developing an OCT/fluorescence imaging falloposcope that is 0.8 mm in diameter, flexible, and steerable, as a tool for early detection of ovarian cancer in humans. “It’s now known that most ovarian cancer starts in the fallopian tubes,” Dr. Barton said. “It’s metastatic disease when those cells break off from the fallopian tubes and go to the ovaries. We wanted to create an imaging system where we created a fiber bundle that we could navigate with white light and with fluorescence so that we can see these early stages of cancer [and] how they fluoresce differently. We also wanted to have an OCT system so that we could image through the wall of the fallopian tube and look for that layer thickening and other precursors to ovarian cancer.”
To date, in vivo testing in healthy women has demonstrated that the miniature endoscope is able to reach the fallopian tubes through the natural orifice of the vagina and uterus. “That is pretty exciting,” she said. “The images may not be of the highest quality, but we are advancing.”
Dr. Barton reported having no relevant financial disclosures.
AT ASLMS 2023
Multiprong strategy makes clinical trials less White
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
CHICAGO – Clinical trials are so White. Only a small percentage of eligible patients participate in clinical trials in the first place, and very few come from racial and ethnic minority groups.
For example, according to the Food and Drug Administration, in trials that resulted in drug approvals from 2017 to 2020, only 2%-5% of participants were Black patients.
When clinical trials lack diverse patient populations, those who are left out have fewer opportunities to get new therapies. Moreover, the scope of the research is limited by smaller phenotypic and genotypic samples, and the trial results are applicable only to more homogeneous patient groups.
There has been a push to include more underrepresented patients in clinical trials. One group reported its success in doing so here at the annual meeting of the American Society of Clinical Oncology.
a period that included a pandemic-induced hiatus in clinical trials in general.
Alliance member Electra D. Paskett, PhD, from the College of Public Health at the Ohio State University in Columbus, presented accrual data from 117 trials led by the Alliance from 2014 to 2022.
During this period, accrual of racial and ethnic minority patients increased from 13.6% to 25.3% for cancer treatment trials and from 13% to 21.5% for cancer control trials.
Overall, the recruitment program resulted in an absolute increase from 13.5 % to 23.6% of underrepresented populations, which translated into a relative 74.8% improvement.
“We’re focusing now on monitoring accrual of women, rural populations, younger AYAs [adolescents and young adults] and older patients, and we’ll see what strategies we need to implement,” Dr. Packett told this news organization.
The Alliance has implemented a real-time accrual dashboard on its website that allows individual sites to review accrual by trial and overall for all of the identified underrepresented populations, she noted.
Program to increase underrepresented patient accrual
The impetus for the program to increase enrollment of underrepresented patients came from the goal set by Monica M. Bertagnolli, MD, group chair of the Alliance from 2011 to 2022 and currently the director of the U.S. National Cancer Institute.
“Our leader, Dr. Bertagnolli, set out a group-wide goal for accrual of underrepresented minorities to our trials of 20%, and that gave us permission to implement a whole host of new strategies,” Dr. Paskett said in an interview.
“These strategies follow the Accrual of Clinical Trials framework, which essentially says that the interaction between the patient and the provider for going on a clinical trial is not just an interaction between the patient and provider but recognizes, for example, that the provider has coworkers and they have norms and beliefs and attitudes, and the patient comes from a family with their own values. And then there are system-level barriers, and there are community barriers that all relate to this interaction about going on a trial,” Dr. Packett said.
What works?
The study was presented as a poster at the meeting. During the poster discussion session, comoderator Victoria S. Blinder, MD, from Memorial Sloan Kettering Cancer Center in New York, asked Dr. Paskett, “If you had a certain amount of money and you really wanted to use that resource to focus on one area, where would you put that resource?”
“I’m going to violate the rules of your question,” Dr. Paskett replied.
“You cannot change this problem by focusing on one thing, and that’s what we showed in our Alliance poster, and what I’ve said is based on over 30 years of work in this area,” she said.
She cited what she considered as the two most important components for improving accrual of underrepresented populations: a commitment by leadership to a recruitment goal, and the development of protocols with specific accrual goals for minority populations.
Still, those are only two components of a comprehensive program that includes the aforementioned accrual goal set by Dr. Bertagnolli, as well as the following:
- Funding of minority junior investigators and research that focuses on issues of concern to underrepresented populations.
- Establishment of work groups that focus on specific populations with the Alliance health disparities committee.
- Translation of informational materials for patients.
- Opening studies at National Cancer Institute Community. Oncology Research Program–designated minority underserved sites.
- Real-time monitoring of accrual demographics by the Alliance and at the trial site.
- Closing protocol enrollment to majority populations.
- Increasing the study sample sizes to enroll additional minority participants and to allow for subgroup analyses.
The study was funded by the National Institutes of Health. Dr. Packett and Dr. Blinder reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
AT ASCO 2023
CBSM phone app eases anxiety, depression in cancer patients
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
CHICAGO – One-third of patients with cancer also experience anxiety or depression, and an estimated 70% of the 18 million patients with cancer and cancer survivors in the US experience emotional symptoms, including fear of recurrence.
Despite many having these symptoms, few patients with cancer have access to psycho-oncologic support.
A digital cognitive-behavioral stress management (CBSM) application may help to ease some of the burden, reported Allison Ramiller, MPH, of Blue Note Therapeutics in San Francisco, which developed the app version of the program.
In addition, patients assigned to the CBSM app were twice as likely as control persons to report that their symptoms were “much” or “very much” improved after using the app for 12 weeks, Ms. Ramiller reported at an oral abstract session at the annual meeting of the American Society of Clinical Oncology (ASCO).
However, the investigators did not report baseline characteristics of patients in each of the study arms, which might have helped to clarify the depth of the effects they saw.
The CBSM program was developed by Michael H. Antoni, PhD, and colleagues in the University of Miami Health System. It is based on cognitive-behavioral therapy but also includes stress management and relaxation techniques to help patients cope with cancer-specific stress.
“”It has been clinically validated and shown to benefit patients with cancer,” Ms. Ramiller said. “However, access is a problem,” she said.
“There aren’t enough qualified, trained providers for the need, and patients with cancer encounter barriers to in-person participation, including things like transportation or financial barriers. So to overcome this, we developed a digitized version of CBSM,” she explained.
Impressive and elegant
“Everything about [the study] I thought was very impressive, very elegant, very nicely done,” said invited discussant Raymond U. Osarogiagbon, MBBS, FACP, chief scientist at Baptist Memorial Health Care Corp in Memphis, Tenn.
“They showed efficacy, they showed safety – very nice – user friendliness – very good. Certainly they look like they’re trying to address a highly important, unmet need in a very elegant way. Certainly, they pointed out it needs longer follow-up to see sustainability. We need to see will this work in other settings. Will this be cost-effective? You’ve gotta believe it probably will be,” he said.
CBSM has previously been shown to help patients with cancer reduce stress, improve general and cancer-specific quality of life at various stages of treatment, reduce symptom burden, and improve coping skills, Ms. Ramiller said.
To see whether these benefits could be conveyed digitally rather than in face-to-face encounters, Ms. Ramiller and colleagues worked with Dr. Antoni to develop the CBSM app.
Patients using the app received therapeutic content over 10 sessions with audio, video, and interactive tools that mimicked the sessions they would have received during in-person interventions.
They then compared the app against the control educational app in the randomized, decentralized RESTORE study.
High-quality control
Ms. Ramiller said that the control app set “a high bar.”
“The control also offered 10 interactive self-guided sessions. Both treatment apps were professionally designed and visually similar in styling, and they were presented as digital therapeutic-specific for cancer patients. And they were also in a match condition, meaning they received the same attention from study staff and cadence of reminders, but importantly, only the intervention app was based on CBSM,” she explained.
A total of 449 patients with cancers of stage I–III who were undergoing active systemic treatment or were planning to undergo such treatment within 6 months were randomly assigned to the CBSM app or the control app.
The CBSM app was superior to the control app for the primary outcome of anxiety reduction over baseline, as measured at 4, 8 and 12 weeks by the Patient-Reported Outcomes Measurement Information System Anxiety Scale (PROMIS-A) (beta = -.03; P = .019).
CBSM was also significantly better than the control app for the secondary endpoints of reducing symptoms of depression, as measured by the PROMIS-D scale (beta = -.02, P = .042), and also at increasing the percentage of patients who reported improvement in anxiety and depression symptoms on the Patient Global Impression of Change instrument (P < .001)
An extension study of the durability of the effects at 3 and 6 months is underway.
The investigators noted that the incremental cost of management of anxiety or depression is greater than $17,000 per patient per year.
“One of the big promises of a digital therapeutic like this is that it could potentially reduce costs,” Ms. Ramiller told the audience, but she acknowledged, “More work is really needed, however, to directly test the potential savings.”
The RESTORE study is funded by Blue Note Therapeutics. Dr. Osarogiagbon owns stock in Gilead, Lilly, and Pfizer, has received honoraria from Biodesix and Medscape, and has a consulting or advisory role for the American Cancer Society AstraZeneca, Genentech/Roche, LUNGevity, National Cancer Institute, and Triptych Health Partners.
A version of this article originally appeared on Medscape.com.
AT ASCO 2023