User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Lupus landmark study aims for personalized medicine goals
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
A new prospective, observational study from the Lupus Research Alliance (LRA) aims to enroll 3,500 patients in an effort to accelerate the development of personalized treatments for individuals living with systemic lupus erythematosus (SLE).
The LRA on May 23 announced the launch of the Lupus Landmark Study (LLS). The study will be conducted in partnership with Lupus Therapeutics, the clinical research affiliate of the LRA.
The study will be a key feature of the Lupus Nexus, a unique combination of lupus patient registry, biorepository, and portal for data sharing and analysis.
“The aim of the Lupus Nexus is to transform lupus research and drug development through unprecedented information exchange capabilities,” according to the LRA press release.
“SLE is a debilitating autoimmune disease that disproportionately impacts women and people from minority groups, but the cause of lupus is unknown, and no single laboratory test can definitively identify lupus,” lead investigator S. Sam Lim, MD, of Emory University, Atlanta, told this news organization.
“Nevertheless, early detection and treatment can often lessen the progression and severity of the disease. Although there are numerous contributing factors to the lag in research discoveries and new treatments for patients with lupus, limited access to standardized, high-quality biological samples and natural history data provides a significant roadblock to advancing lupus research,” Dr. Lim said.
“Existing registry and biorepository resources in the lupus field are largely siloed, mostly limited to relatively small or discrete patient populations, and frequently not designed for broad sharing across all stakeholders of the research community,” Dr. Lim said. The LRA and its affiliate Lupus Therapeutics are committed to developing Lupus Nexus, a first-of-its-kind registry and biorepository, to serve as a collaborative research platform for lupus and a leading source of prospective, longitudinal patient data and biological samples for the research community, Dr. Lim added.
“The Lupus Landmark Study will form the foundation of this registry and biorepository and will provide a critical resource to enable the understanding of lupus heterogeneity at the molecular level,” Dr. Lim said. The molecular data can be linked to clinical phenotypes, he explained, “while providing an opportunity to better understand the holistic experience of patients with lupus, thus helping patients address the daily life challenges they face.”
The Lupus Accelerating Breakthroughs Consortium (Lupus ABC) was announced earlier this spring by the LRA. It represents a collaboration between the U.S. Food and Drug Administration and the lupus community to improve and accelerate the development of safer and more effective treatments for people with lupus, Dr. Lim said. “Data and other results from the LLS will inform this collaboration,” he said.
“The LLS will provide greater insight into the pathogenesis and evolution of the condition, providing much needed information and guidance to clinicians so that the disease can be detected and treated earlier and with better precision,” Dr. Lim said. “The partnership with patients will ensure that advances will not only be meaningful to clinicians but their patients and caregivers as well,” he added.
Individuals living with lupus were essential to the development of the Lupus Nexus, and patients will continue to be engaged through participation in the LLS, which will not only generate data to promote patient-centered treatments but will also give participants more insight into their health data, according to the LRA press release.
The clinical coordinating center and biorepository elements of the Lupus Nexus will be managed by Embleema and Azenta Life Sciences, respectively, according the LRA.
Biomarker analysis will be conducted by DxTerity Diagnostics via the company’s proprietary DxCollection MicroCollection Device and Modular Immune Profile platform.
The LLS is scheduled to begin enrolling patients through select academic medical centers in the Lupus Therapeutics Lupus Clinical Investigators Network later in 2023, with an expanded roll-out in 2024, according to the press release. More information about the Lupus Landmark Study is available from Lupus Nexus at [email protected].
A version of this article first appeared on Medscape.com.
Chondrodermatitis Nodularis Helicis After Mohs Micrographic Surgery and Radiation Therapy
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.
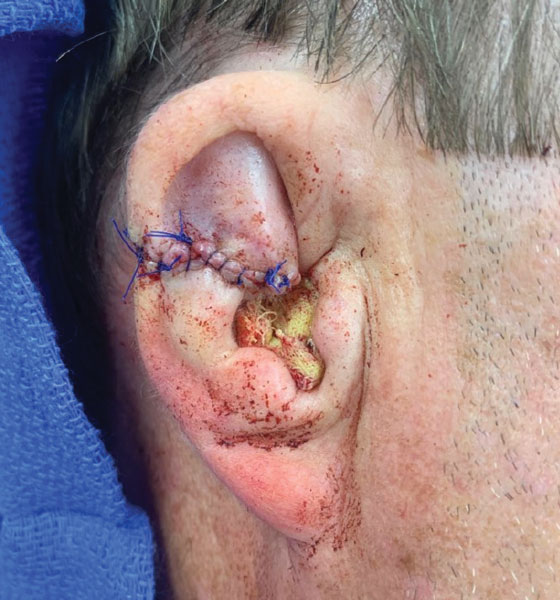
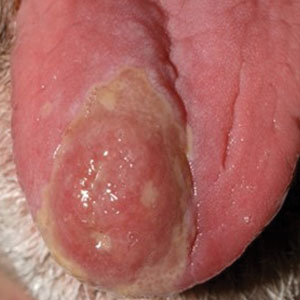
A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.
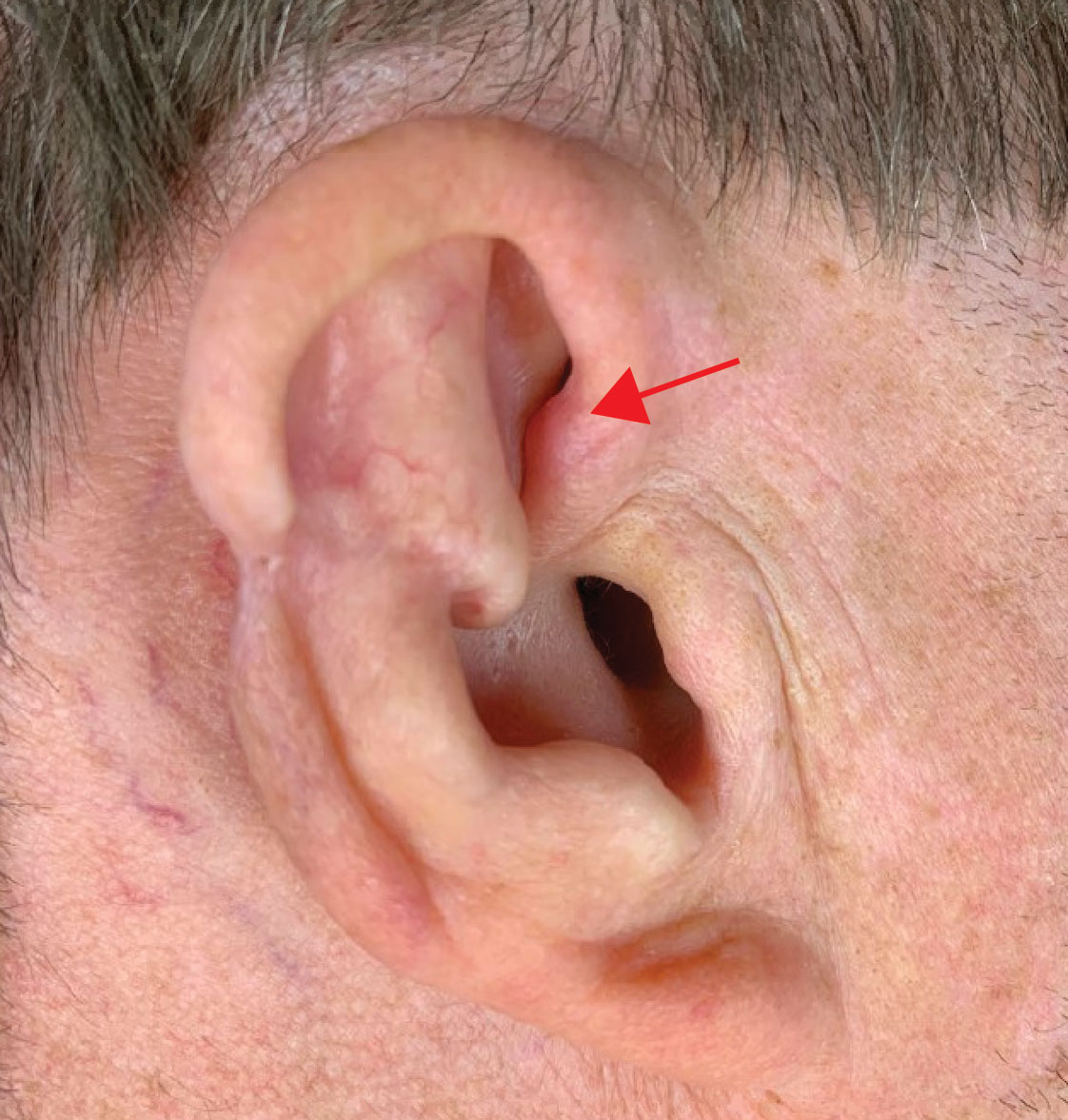
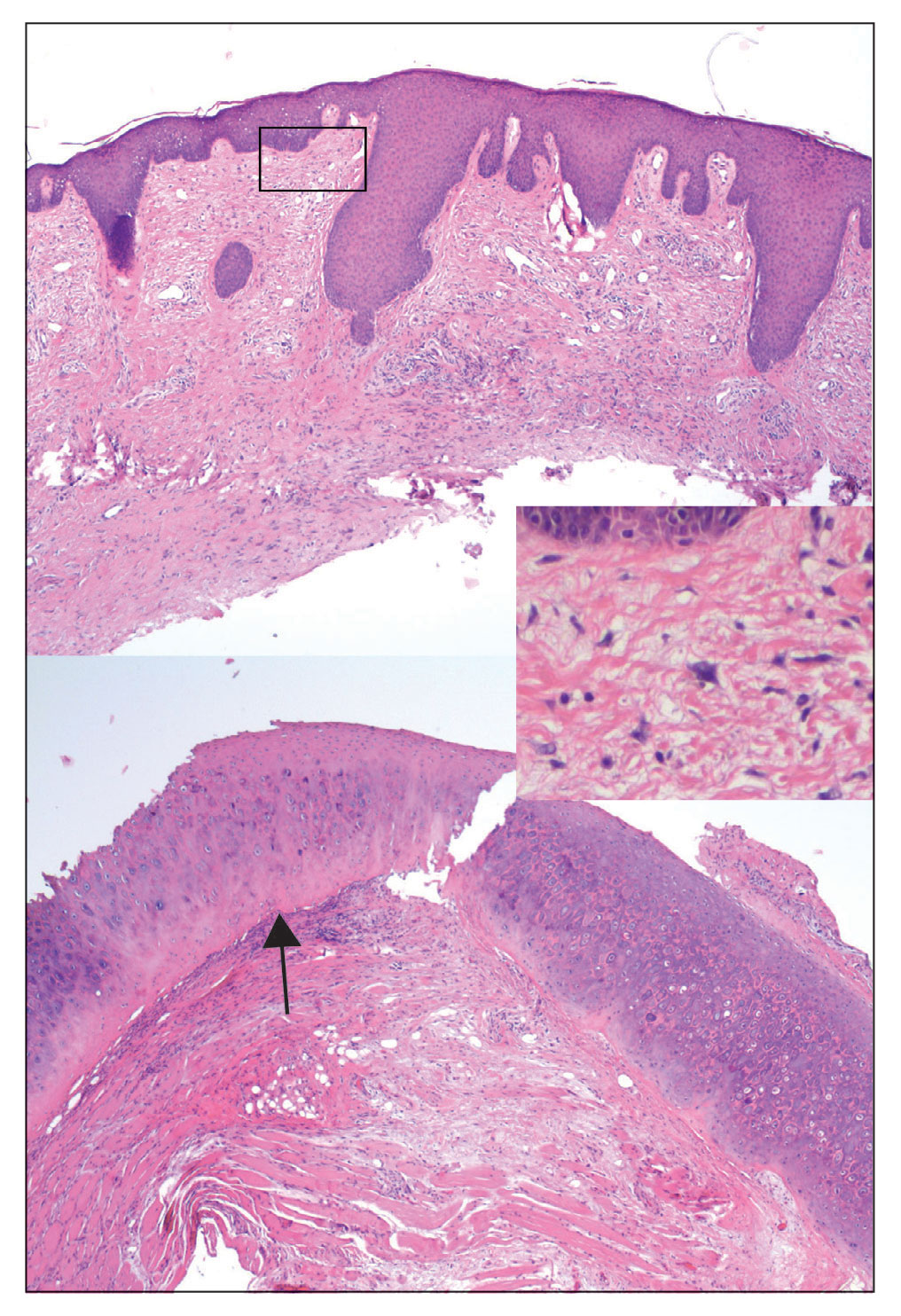
Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).
Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
To the Editor:
Chondrodermatitis nodularis helicis (CNH) is a benign inflammatory condition of the cartilage of the helix or antihelix as well as the overlying skin. Inflammation produces a firm painful nodule that often forms a central crust and enlarges rapidly, mimicking cutaneous malignancy. Chondrodermatitis nodularis helicis is believed to be caused by chronic pressure on the pinna, usually from sleeping, which causes compromised blood supply. However, there is a wide range of additional risk factors,1 including trauma (eg, pressure), environmental insult (eg, sun or cold exposure), and autoimmune processes (eg, systemic lupus erythematosus, scleroderma). Chondrodermatitis nodularis helicis after Mohs micrographic surgery (MMS) is rare. We report a novel case of CNH as a postoperative complication of MMS following adjuvant radiation therapy.

A 61-year-old man presented to the MMS clinic for treatment of a primary squamous cell carcinoma of the right posterior helix. Stage I MMS demonstrated tumor invasion in the deep dermis directly overlying the auricular cartilage, as well as large-nerve (ie, >0.1 mm) perineural invasion. Two additional stages were taken; negative margins were obtained on Stage III. The defect was repaired by primary closure (Figure 1). Considering the presence of perineural invasion around a large nerve, the patient elected to receive adjuvant radiation therapy consisting of 50 Gy in 20 fractions administered to the right ear over 1 month.

Two months after completion of adjuvant radiation therapy, the patient returned to the clinic with a tender pink papule on the right crus within the radiation portal but nonadjacent to the surgical scar (Figure 2). Histopathology from a tangential biopsy revealed acanthosis, dermal sclerosis, and degenerated cartilage, consistent with CNH. Stellate fibroblasts also were seen, suggesting changes related to prior radiation therapy (Figure 3).

Although CNH is a benign condition, it can be concerning in the context of patient follow-up after MMS given its clinical appearance, which is similar to nonmelanoma skin cancer. The differential diagnosis of CNH includes hypertrophic actinic keratosis, basal cell carcinoma, and squamous cell carcinoma. The diagnosis is based on clinical history and confirmed by histopathologic examination.
Chondrodermatitis nodularis helicis in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis. The histopathology of CNH often is characterized by epidermal acanthosis with ulceration, perichondral fibrosis, and a variable degree of cartilage degeneration associated with granulation tissue.2
The scarce subcutaneous tissue and limited blood supply of the pinna offer minimal cushioning and poor circulation to underlying cartilage. These anatomic features predispose the pinna to inflammation and ischemia.1 Mohs micrographic surgery may inadvertently cause damage to surrounding tissue because of excision of cartilage, mechanical manipulation, severance of the extant blood supply, electrocautery, fenestration in preparation for skin grafting, compression from a wound dressing, and other factors related to surgery. In addition, following MMS, scar tissue and swelling with compression of adjacent structures can further inhibit circulation and lead to CNH.
In our case, multiple factors may have contributed to CNH after MMS, including postoperative swelling and compression, prior actinic damage, and other environmental factors. Given that CNH occurred within the radiation portal, we postulated that adjuvant radiation may have played a role in the pathogenesis of the patient’s CNH. Pandya et al3 reported CNH after radiation therapy for a brain tumor.
One prior study showed that CNH treated by surgical excision recurred in 34% of patients.4 In all of these patients, the CNH was completely excised; however, trauma from the surgical procedure itself likely resulted in recurrence of CNH. Darragh et al5 reported a case of CNH after MMS on the right nasal vestibule following wound reconstruction that utilized a cartilage graft from the right ear.
Our patient demonstrated an unusual but concerning complication associated with MMS. The location of CNH also was not in a traditional location but rather near the superior helical crus. Although CNH is benign by nature, it can mimic recurrence of a tumor when it presents close to the site of prior MMS. Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
- Salah H, Urso B, Khachemoune A. Review of the etiopathogenesis and management options of chondrodermatitis nodularis chronica helicis. Cureus. 2018;10:E2367. doi:10.7759/cureus.2367
- Juul Nielsen L, Holkmann Olsen C, Lock-Andersen J. Therapeutic options of chondrodermatitis nodularis helicis. Plast Surg Int. 2016;2016:4340168. doi:10.1155/2016/4340168
- Pandya AG, Kettler AH, Hoffmann TJ, et al. Chondrodermatitis helicis arising after radiation therapy. Arch Dermatol. 1988;124:185-186.
- Moncrieff M, Sassoon EM. Effective treatment of chondrodermatitis nodularis chronica helicis using a conservative approach. Br J Dermatol. 2004;150:892-894. doi:10.1111/j.1365-2133.2004.05961.x
- Darragh CT, Om A, Zwerner JP. Chondrodermatitis nodularis chronica helicis of the right nasal vestibule. Dermatol Surg. 2018;44:1475-1476. doi:10.1097/DSS.0000000000001515
Practice Points
- Although chondrodermatitis nodularis helicis (CNH) is benign by nature, it can mimic tumor recurrence when it presents close to the site of prior Mohs micrographic surgery (MMS). Diagnostic biopsy of CNH should be considered to rule out recurrence of skin cancer.
- Skin lesions in close proximity to a prior MMS site should lower the threshold for biopsy because the area is already known to be affected by actinic damage and cutaneous carcinogenesis.
An Evaluation of Spin in the Abstracts of Systematic Reviews and Meta-analyses on the Treatment of Psoriasis: A Cross-sectional Analysis
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
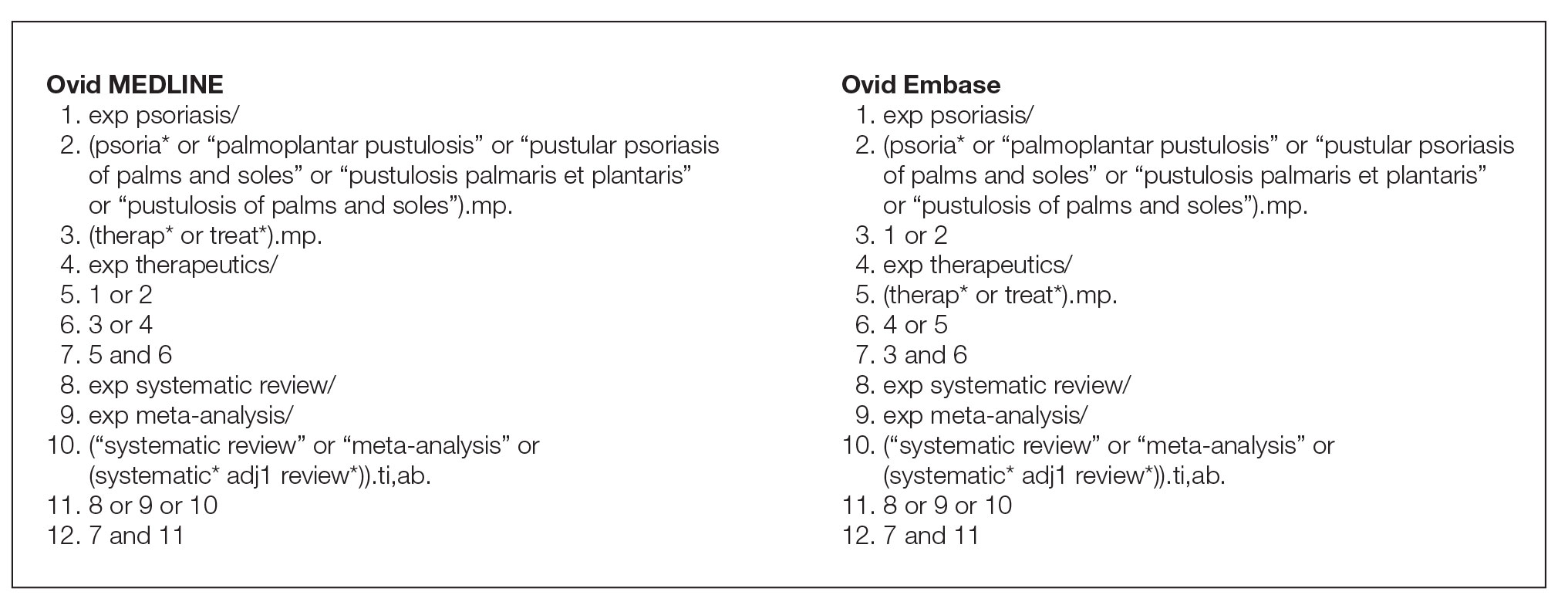
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.
Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
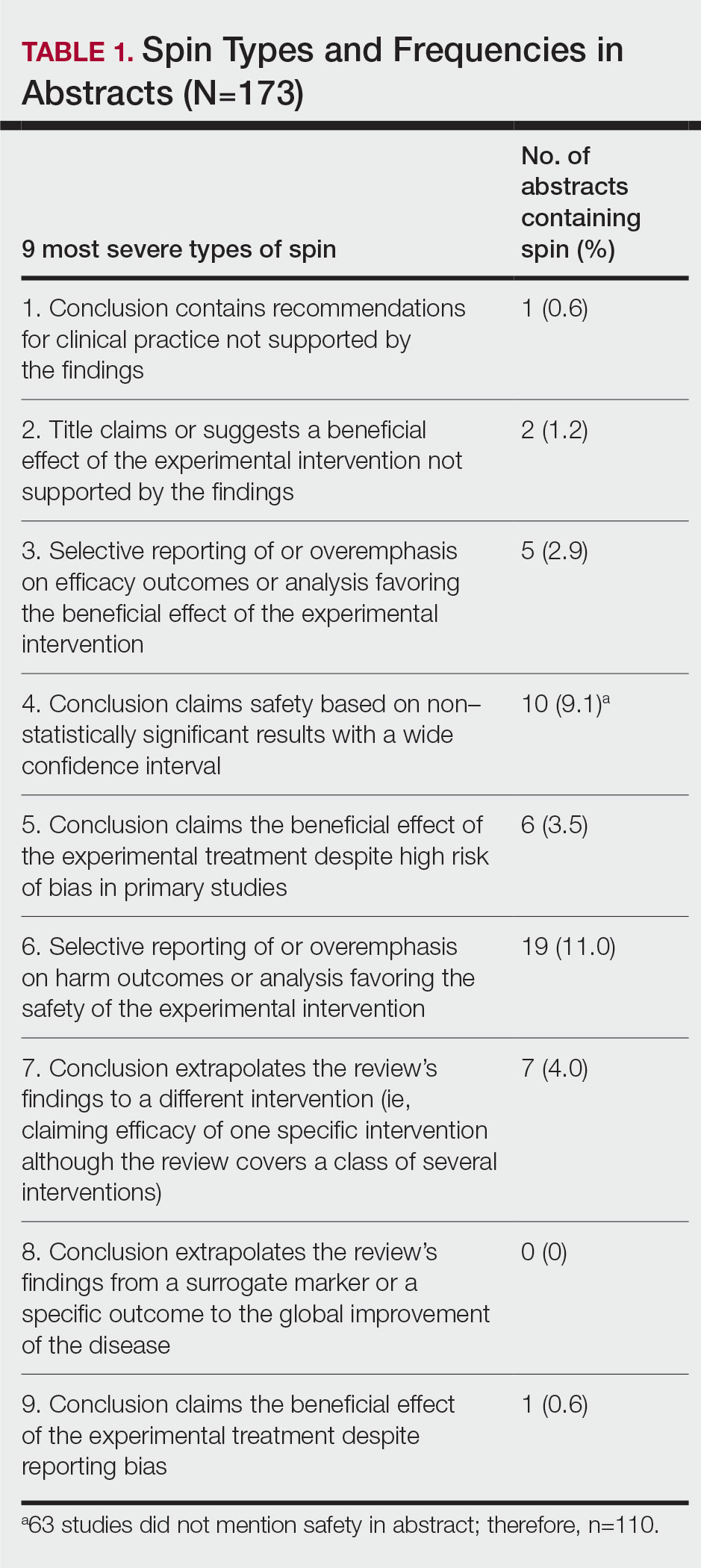
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19
During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
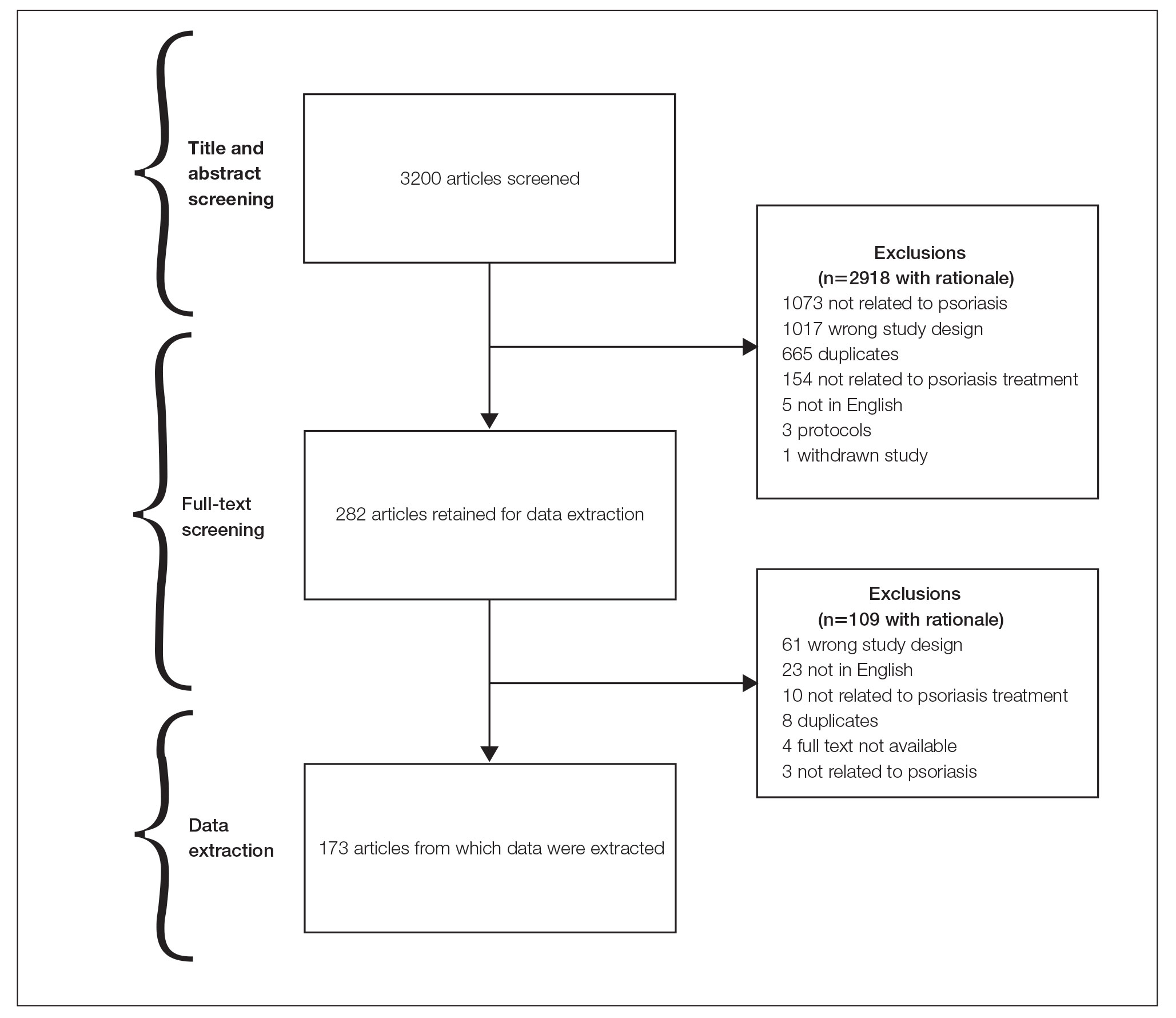
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.
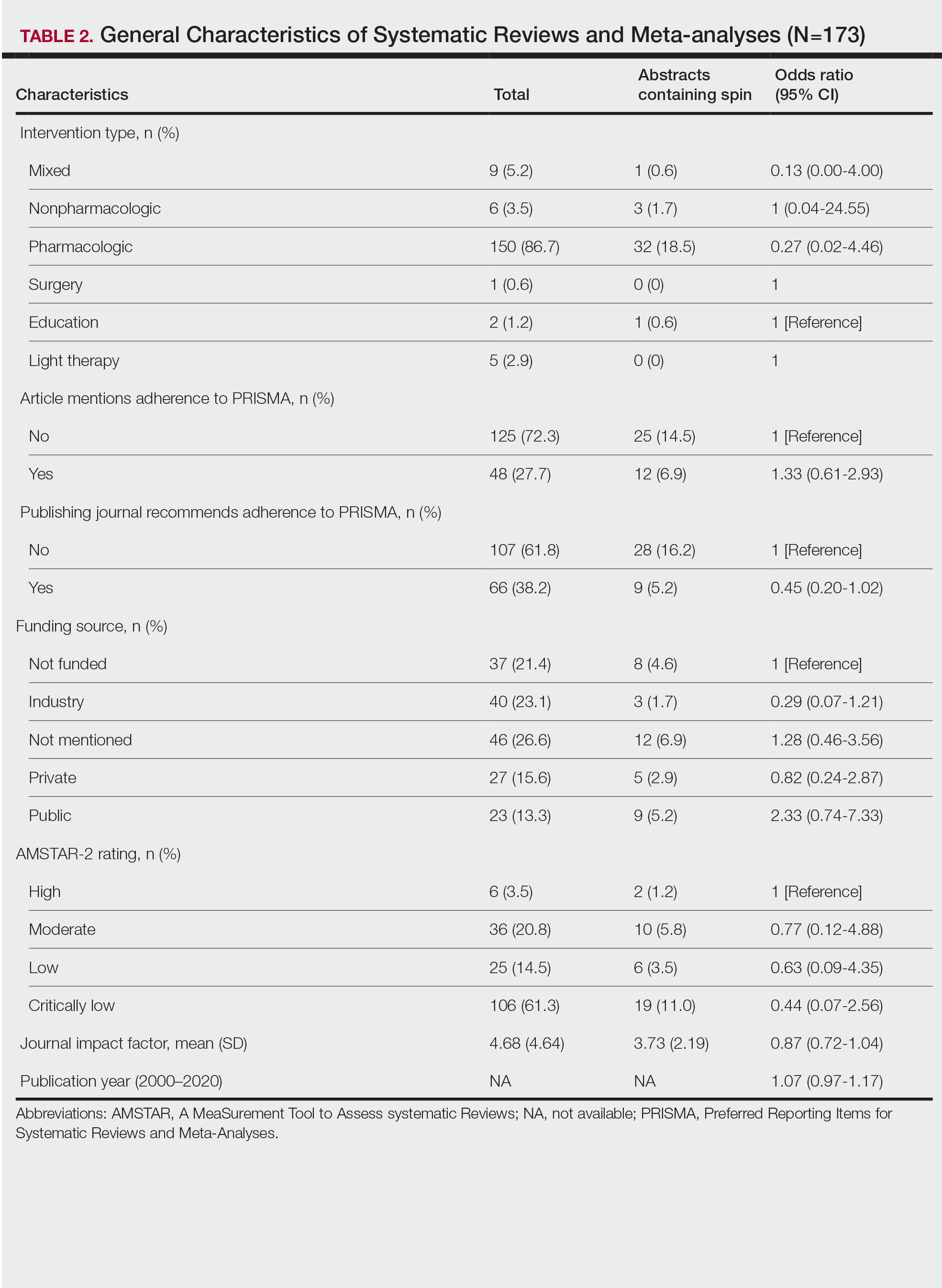
Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).
Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
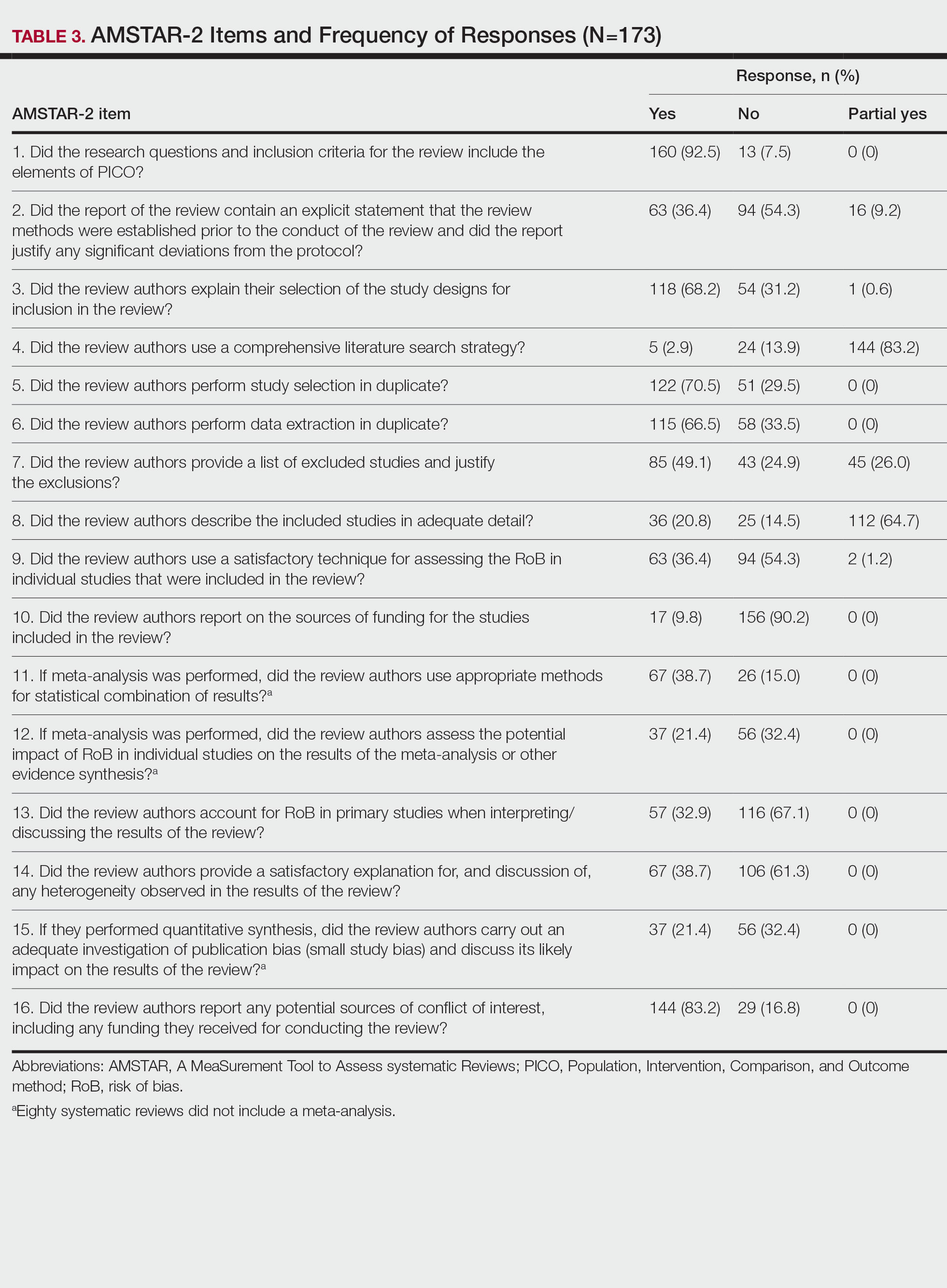
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.
Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.

Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19

During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.

Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).

Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.

Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
Psoriasis is an inflammatory autoimmune skin condition that affects approximately 125 million individuals worldwide, with approximately 8 million patients in the United States.1 Psoriasis not only involves a cosmetic component but also comprises other comorbidities, such as psoriatic arthritis, cardiovascular disease, and psychiatric disorders, that can influence patient quality of life.2-4 In addition, the costs associated with psoriasis are substantial, with an estimated economic burden of $35.2 billion in the United States in 2015.5 Given the prevalence of psoriasis and its many effects on patients, it is important that providers have high-quality evidence regarding efficacious treatment options.
Systematic reviews, which compile all available evidence on a subject to answer a specific question, represent the gold standard of research.6 However, studies have demonstrated that when referencing research literature, physicians tend to read only the abstract of a study rather than the entire article.7,8 A study by Marcelo et al8 showed that residents at a tertiary care center answered clinical questions using only the abstract of a paper 69% of the time. Based on these findings, it is imperative that the results of systematic reviews be accurately reported in their abstracts because they can influence patient care.
Referencing only the abstracts of systematic reviews can be problematic if the abstract contains spin. Spin is a form of reporting that inappropriately highlights the benefits of a treatment with greater emphasis than what is shown by the results.9 Research has identified the presence of spin in the abstracts of randomized controlled trials.10-12 For example, Cooper et al10 found that 70% (33/47) of abstracts in otolaryngology randomized controlled trials contained spin. Additionally, Arthur et al11 and Austin et al12 had similar findings within abstracts of orthopedic and obesity trials, where 44.8% (112/250) and 46.7% (21/45) contained spin, respectively. Ottwell et al13 found that the presence of spin in abstracts is not limited to randomized controlled trials; they demonstrated that the abstracts of nearly one-third (31% [11/36]) of systematic reviews focused on the treatment of acne vulgaris contained spin.
In our study, we aimed to evaluate the presence of spin in the abstracts of systematic reviews focused on the treatment of psoriasis.
Methods
Reproducibility and Reporting—Our study did not meet the regulatory definition for human subjects research per the US Code of Federal Regulations because the study did not involve human research subjects. The study also was not subject to review by the institutional review board. Our protocol, data set, analysis scripts, extraction forms, and other material related to the study have been placed on Open Science Framework to provide transparency and ensure reproducibility. To further allow for analytic reproducibility, our data set was given to an independent laboratory and reanalyzed with a masked approach. Our study was carried out alongside other studies assessing spin in systematic reviews regarding different specialties and disease states. Because these studies were similar in design, this methodology also has been reported elsewhere. The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA)14 and the guidelines for meta-epidemiological studies developed by Murad and Wang15 were used in drafting this article.
Search Strategy—The search strategies for the MEDLINE (Ovid) and Embase (Ovid) databases were created by a systematic review librarian (D.N.W.) to identify systematic reviews and meta-analyses regarding treatments for psoriasis (Figure 1). The searches were performed on June 2, 2020, and uploaded to Rayyan, a systematic review screening platform.16 After duplicates were removed, the records were screened for eligibility by 2 authors (C.H. and A.L.) using the titles and abstracts. Screening was conducted independently while each of these authors was masked to the other’s results; disagreements were resolved through discussion.

Eligibility Criteria—An article had to meet the following criteria for inclusion in our study: (1) be a systematic review with or without a meta-analysis; (2) relate to the treatment of psoriasis; and (3) be written in English and include human patients only. The PRISMA definition of systematic reviews and meta-analyses was applied.17
Training—Various training occurred throughout our study to ensure understanding of each step and mitigate subjectivity. Before beginning screening, 2 investigators (C.H. and A.L.) completed the Introduction to Systematic Review and Meta-Analysis course offered by Johns Hopkins University.18 They also underwent 2 days of online and in-person training on the definition and interpretation of the 9 most severe types of spin found in the abstracts of systematic reviews as defined by Yavchitz et al.9 Finally, they were trained to use A MeaSurement Tool to Assess systematic Reviews (AMSTAR-2) to appraise the methodological quality of each systematic review. Our protocol contained an outline of all training modules used.
Data Extraction—The investigators (C.H. and A.L.) analyzed included abstracts for the 9 most severe types of spin (Table 1). Data were extracted in a masked duplicate fashion using the Google form. AMSTAR-2 was used to assess systematic reviews for methodological quality. AMSTAR-2 is an appraisal tool consisting of a 16-item checklist for systematic reviews or meta-analyses. Scores range from critically low to high based on the methodological quality of the review. Interrater reliability of AMSTAR-2 scores has been moderate to high across studies. Construct validity coefficients have been high with the original AMSTAR instrument (r=0.91) and the Risk of Bias in Systematic Reviews instrument (r=0.84).19

During data extraction from each included systematic review, the following additional items were obtained: (1) the date the review was received; (2) intervention type (ie, pharmacologic, nonpharmacologic, surgery, light therapy, mixed); (3) the funding source(s) for each systematic review (ie, industry, private, public, none, not mentioned, hospital, a combination of funding not including industry, a combination of funding including industry, other); (4) whether the journal submission guidelines suggested adherence to PRISMA guidelines; (5) whether the review discussed adherence to PRISMA14 or PRISMA for Abstracts20 (PRISMA-A); (6) the publishing journal’s 5-year impact factor; and (6) the country of the systematic review’s origin. When data extraction was complete, investigators (C.H. and A.L.) were unmasked and met to resolve any disagreements by discussion. Two authors (R.O. or M.V.) served as arbiters in the case that an agreement between C.H. and A.L. could not be reached.
Statistical Analysis—Frequencies and percentages were calculated to evaluate the most common types of spin found within systematic reviews and meta-analyses. One author (M.H.) prespecified the possibility of a binary logistic regression and calculated a power analysis to determine sample size, as stated in our protocol. Our final sample size of 173 was not powered to perform the multivariable logistic regression; therefore, we calculated unadjusted odds ratios to enable assessing relationships between the presence of spin in abstracts and the various study characteristics. We used Stata 16.1 for all analyses, and all analytic decisions can be found in our protocol.
Results
General Characteristics—Our systematic search of MEDLINE and Embase returned 3200 articles, of which 665 were duplicates that were removed. An additional 2253 articles were excluded during initial abstract and title screening, and full-text screening led to the exclusion of another 109 articles. In total, 173 systematic reviews were included for data extraction. Figure 2 illustrates the screening process with the rationale for all exclusions.

Of the 173 included systematic reviews and meta-analyses, 150 (86.7%) focused on pharmacologic interventions. The majority of studies did not mention adhering to PRISMA guidelines (125/173 [72.3%]), and the publishing journals recommended their authors adhere to PRISMA for only 66 (38.2%) of the included articles. For the articles that received funding (90/173 [52.0%]), industry sources were the most common funding source (40/90 [44.4%]), followed by private (27/90 [30%]) and public funding sources (23/90 [25.6%]). Of the remaining studies, 46 articles did not include a funding statement (46/83 [55.4%]), and 37 studies were not funded (37/83 [44.6%]). The average (SD) 5-year impact factor of our included journals was 4.68 (4.64). Systematic reviews were from 31 different countries. All studies were received by their respective journals between the years 2000 and 2020 (Table 2).

Abstracts Containing Spin—We found that 37 (21.4%) of the abstracts of systematic reviews focused on psoriasis treatments contained at least 1 type of spin. Some abstracts had more than 1 type; thus, a total of 51 different instances of spin were detected. Spin type 6—selective reporting of or overemphasis on harm outcomes or analysis favoring the safety of the experimental intervention—was the most common type ofspin, found in 19 of 173 abstracts (11.0%). The most severe type of spin—type 1 (conclusion contains recommendations for clinical practice not supported by the findings)—occurred in only 1 abstract (0.6%). Spin type 8 did not occur in any of the abstracts (Table 1). There was no statistically significant association between the presence of spin and any of the study characteristics (Table 2).
AMSTAR Ratings—After using AMSTAR-2 to appraise the included systematic reviews, we found that 6 (3.5%) of the 173 studies could be rated as high; 36 (20.8%) as moderate; 25 (14.5%) as low; and 106 (61.3%) as critically low. Of the 37 abstracts containing spin, 2 (5.4%) had an AMSTAR-2 rating of high, 10 (27%) had a rating of moderate, 6 (16.2%) had a rating of low, and 19 (51.4%) had a rating of critically low (Table 2). No statistically significant associations were seen between abstracts found to have spin and the AMSTAR-2 rating of the review.
Nearly all (160/173 [92.5%]) of the included reviews were compliant with the inclusion of Population, Intervention, Comparison, and Outcome (PICO) method. Only 17 of 173 (9.8%) reviews reported funding sources for the studies included. See Table 3 for all AMSTAR-2 items.

Comment
Primary Findings—We evaluated the abstracts of systematic reviews for the treatment of psoriasis and found that more than one-fifth of them contained spin. Our study contributes to the existing literature surrounding spin. Spin in randomized controlled trials is well documented across several fields of medicine, including otolaryngology,10 obesity medicine,12 dermatology,21 anesthesiology,22 psychiatry,23 orthopedics,24 emergency medicine,25 oncology,26 and cardiology.27 More recently, studies have emerged evaluating the presence of spin in systematic reviews. Specific to dermatology, one study found that 74% (84/113) of systematic reviews related to atopic dermatitis treatment contained spin.28 Additionally, Ottwell et al13 identified spin in 31% (11/36) of the systematic reviews related to the treatment of acne vulgaris, which is similar to our results for systematic reviews focused on psoriasis treatments. When comparing the presence of spin in abstracts of systematic reviews from the field of dermatology with other specialties, dermatology-focused systematic reviews appear to contain more spin in the abstract than systematic reviews focused on tinnitus and glaucoma therapies.29,30 However, systematic reviews from the field of dermatology appear to contain less spin than systematic reviews focused on therapies for lower back pain.31 For example, Nascimento et al31 found that 80% (53/66) of systematic reviews focused on low-back pain treatments contained spin.
Examples of Spin—The most common type of spin found in our study was type 6.9 An example of spin type 6 can be found in an article by Bai et al32 that investigated the short-term efficacy and safety of multiple interleukin inhibitors for the treatment of plaque psoriasis. The conclusion of the abstract states, “Risankizumab appeared to have relatively high efficacy and low risk.” However, in the results section, the authors showed that risankizumab had the highest risk of serious adverse events and was ranked highest for discontinuation because of adverse events when compared with other interleukin inhibitors. Here, the presence of spin in the abstract may mislead the reader to accept the “low risk” of risankizumab without understanding the study’s full results.32
Another example of selective reporting of harm outcomes in a systematic review can be found in the article by Wu et al,33 which focused on assessing IL-17 antagonists for the treatment of plaque psoriasis. The conclusion of the abstract indicated that IL-17 antagonists should be accepted as safe; however, in the results section, the authors discussed serious safety concerns with brodalumab, including the death of 4 patients from suicide.33 This example of spin type 6 highlights how the overgeneralization of a drug’s safety profile neglects serious harm outcomes that are critical to patient safety. In fact, against the safety claims of Wu et al,33 brodalumab later received a boxed warning from the US Food and Drug Administration after 6 patients died from suicide while receiving the drug, which led to early discontinuation of the trials.34,35 Although studies suggest this relationship is not causal,34-36 the purpose of our study was not to investigate this association but to highlight the importance of this finding. Thus, with this example of spin in mind, we offer recommendations that we believe will improve reporting in abstracts as well as quality of patient care.
Recommendations for Reporting in Abstracts—Regarding the boxed warning37 for brodalumab because of suicidal ideation and behavior, the US Food and Drug Administration recommends that prior to prescribing brodalumab, clinicians consider the potential benefits and risks in patients with a history of depression and/or suicidal ideation or behavior. However, a clinician would not adequately assess the full risks and benefits when an abstract, such as that for the article by Wu et al,33 contains spin through selectively reporting harm outcomes. Arguably, clinicians could just read the full text; however, research confirms that abstracts often are utilized by clinicians and commonly are used to guide clinical decisions.7,38 It is reasonable that clinicians would use abstracts in this fashion because they provide a quick synopsis of the full article’s findings and are widely available to clinicians who may not have access to article databases. Initiatives are in place to improve the quality of reporting in an abstract, such as PRISMA-A,20 but even this fails to address spin. In fact, it may suggest spin because checklist item 10 of PRISMA-A advises authors of systematic reviews to provide a “general interpretation of the results and important implications.” This item is concerning because it suggests that the authors interpret importance rather than the clinician who prescribes the drug and is ultimately responsible for patient safety. Therefore, we recommend a reform to abstract reporting and an update to PRISMA-A that leads authors to report all benefits and risks encountered instead of reporting what the authors define as important.
Strengths and Limitations—Our study has several strengths as well as limitations. One of these strengths is that our protocol was strictly adhered to; any deviations were noted and added as an amendment. Our protocol, data, and all study artifacts were made freely available online on the Open Science Framework to strengthen reproducibility (https://osf.io/zrxh8/). Investigators underwent training to ensure comprehension of spin and systematic review designs. All data were extracted in masked duplicate fashion per the Cochrane Handbook for Systematic Reviews of Interventions.39
Regarding limitations, only 2 databases were searched—MEDLINE and Embase. Therefore, our screening process may not have included every available systematic review on the treatment of psoriasis. Journal impact factors may be inaccurate for the systematic reviews that were published earlier in our data date range; however, we attempted to negate this limitation by using a 5-year average. Our study characteristic regarding PRISMA adherence did not account for studies published before the PRISMA statement release; we also could not access prior submission guidelines to determine when a journal began recommending PRISMA adherence. Another limitation of our study was the intrinsic subjectivity behind spin. Some may disagree with our classifications. Finally, our cross-sectional design should not be generalized to study types that are not systematic reviews or published in other journals during different periods.
Conclusion
Evidence of spin was present in many of the abstracts of systematic reviews pertaining to the treatment of psoriasis. Future clinical research should investigate any reporting of spin and search for ways to better reduce spin within literature. Continued research is necessary to evaluate the presence of spin within dermatology and other specialties.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
- Psoriasis statistics. National Psoriasis Foundation. Updated December 21, 2022. Accessed March 6, 2023. https://www.psoriasis.org/content/statistics
- Greb JE, Goldminz AM, Elder JT, et al. Psoriasis. Nat Rev Dis Primers. 2016;2:16082.
- Hu SCS, Lan CCE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment. Int J Mol Sci. 2017;18:2211.
- Patel N, Nadkarni A, Cardwell LA, et al. Psoriasis, depression, and inflammatory overlap: a review. Am J Clin Dermatol. 2017;18:613-620.
- Brezinski EA, Dhillon JS, Armstrong AW. Economic burden of psoriasis in the United States: a systematic review. JAMA Dermatol. 2015;151:651-658.
- Gopalakrishnan S, Ganeshkumar P. Systematic reviews and meta‑analysis: understanding the best evidence in primary healthcare. J Fam Med Prim Care. 2013;2:9-14.
- Barry HC, Ebell MH, Shaughnessy AF, et al. Family physicians’ use of medical abstracts to guide decision making: style or substance? J Am Board Fam Pract. 2001;14:437-442.
- Marcelo A, Gavino A, Isip-Tan IT, et al. A comparison of the accuracy of clinical decisions based on full-text articles and on journal abstracts alone: a study among residents in a tertiary care hospital. Evid Based Med. 2013;18:48-53.
- Yavchitz A, Ravaud P, Altman DG, et al. A new classification of spin in systematic reviews and meta-analyses was developed and ranked according to the severity. J Clin Epidemiol. 2016;75:56-65.
- Cooper CM, Gray HM, Ross AE, et al. Evaluation of spin in the abstracts of otolaryngology randomized controlled trials. Laryngoscope. 2019;129:2036-2040.
- Arthur W, Zaaza Z, Checketts JX, et al. Analyzing spin in abstracts of orthopaedic randomized controlled trials with statistically insignificant primary endpoints. Arthroscopy. 2020;36:1443-1450.
- Austin J, Smith C, Natarajan K, et al. Evaluation of spin within abstracts in obesity randomized clinical trials: a cross-sectional review. Clin Obes. 2019;9:E12292.
- Ottwell R, Rogers TC, Michael Anderson J, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on the treatment of acne vulgaris: cross-sectional analysis. JMIR Dermatol. 2020;3:E16978.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:E1000100.
- Murad MH, Wang Z. Guidelines for reporting meta-epidemiological methodology research. Evid Based Med. 2017;22:139-142.
- Rayyan QCRI. Accessed September 10, 2019. https://rayyan.qcri.org/reviews/81224
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647.
- Coursera. Introduction to systematic review and meta-analysis. Accessed May 18, 2023. https://www.coursera.org/learn/systematic-review
- Lorenz RC, Matthias K, Pieper D, et al. A psychometric study found AMSTAR 2 to be a valid and moderately reliable appraisal tool. J Clin Epidemiol. 2019;114:133-140.
- Beller EM, Glasziou PP, Altman DG, et al. PRISMA for abstracts: reporting systematic reviews in journal and conference abstracts. PLoS Med. 2013;10:E1001419.
- Motosko CC, Ault AK, Kimberly LL, et al. Analysis of spin in the reporting of studies of topical treatments of photoaged skin. J Am Acad Dermatol. 2019;80:516-522.e12.
- Kinder NC, Weaver MD, Wayant C, et al. Presence of “spin” in the abstracts and titles of anaesthesiology randomised controlled trials. Br J Anaesth. 2019;122:E13-E14.
- Jellison S, Roberts W, Bowers A, et al. Evaluation of spin in abstracts of papers in psychiatry and psychology journals. BMJ Evid Based Med. 2019;5:178-181.
- Checketts JX, Riddle J, Zaaza Z, et al. An evaluation of spin in lower extremity joint trials. J Arthroplasty. 2019;34:1008-1012.
- Reynolds-Vaughn V, Riddle J, Brown J, et al. Evaluation of spin in the abstracts of emergency medicine randomized controlled trials. Ann Emerg Med. 2019;14:423-431.
- Wayant C, Margalski D, Vaughn K, et al. Evaluation of spin in oncology clinical trials. Crit Rev Oncol Hematol. 2019;144:102821.
- Khan MS, Lateef N, Siddiqi TJ, et al. Level and prevalence of spin in published cardiovascular randomized clinical trial reports with statistically nonsignificant primary outcomes: a systematic review. JAMA Netw Open. 2019;2:E192622.
- Lin V, Patel R, Wirtz A, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of atopic dermatitis treatments and interventions. Dermatology. 2021;237:496-505.
- Rucker B, Umbarger E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses focused on tinnitus. Otol Neurotol. 2021;10:1237-1244.
- Okonya O, Lai E, Ottwell R, et al. Evaluation of spin in the abstracts of systematic reviews and meta-analyses of treatments for glaucoma. J Glaucoma. 2021;30:235-241.
- Nascimento DP, Gonzalez GZ, Araujo AC, et al. Eight out of every ten abstracts of low back pain systematic reviews presented spin and inconsistencies with the full text: an analysis of 66 systematic reviews. J Orthop Sports Phys Ther. 2020;50:17-23.
- Bai F, Li GG, Liu Q, et al. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: a systematic review and network meta-analysis of randomized controlled trials. J Immunol Res. 2019;2019:2546161.
- Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: a meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-1003.
- Rusta-Sallehy S, Gooderham M, Papp K. Brodalumab: a review of safety. Skin Therapy Lett. 2018;23:1-3.
- Rodrigeuz-Bolanos F, Gooderham M, Papp K. A closer look at the data regarding suicidal ideation and behavior in psoriasis patients: the case of brodalumab. Skin Therapy Lett. 2019;24:1-4.
- Danesh MJ, Kimball AB. Brodalumab and suicidal ideation in the context of a recent economic crisis in the United States. J Am Acad Dermatol. 2016;74:190-192.
- Siliq. Prescribing information. Valeant Pharmaceuticals North America LLC; 2017. Accessed May 18, 2023. chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/761032lbl.pdf
- Johnson HL, Fontelo P, Olsen CH, et al. Family nurse practitioner student perception of journal abstract usefulness in clinical decision making: a randomized controlled trial. J Am Assoc Nurse Pract. 2013;25:597-603.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons; 2019.
Practice Points
- Spin is defined as the intentional or unintentional misrepresentation of findings and can inappropriately highlight results and disregard results of equal importance.
- Our findings show that more than 20% of systematic reviews focused on the treatment of psoriasis contained some form of spin within the abstract.
- Because spin has the potential to misrepresent findings and distort a reader’s perception of psoriasis therapies, efforts are needed to prevent its occurrence.
Papular Acneform Eruption With Mucositis
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.
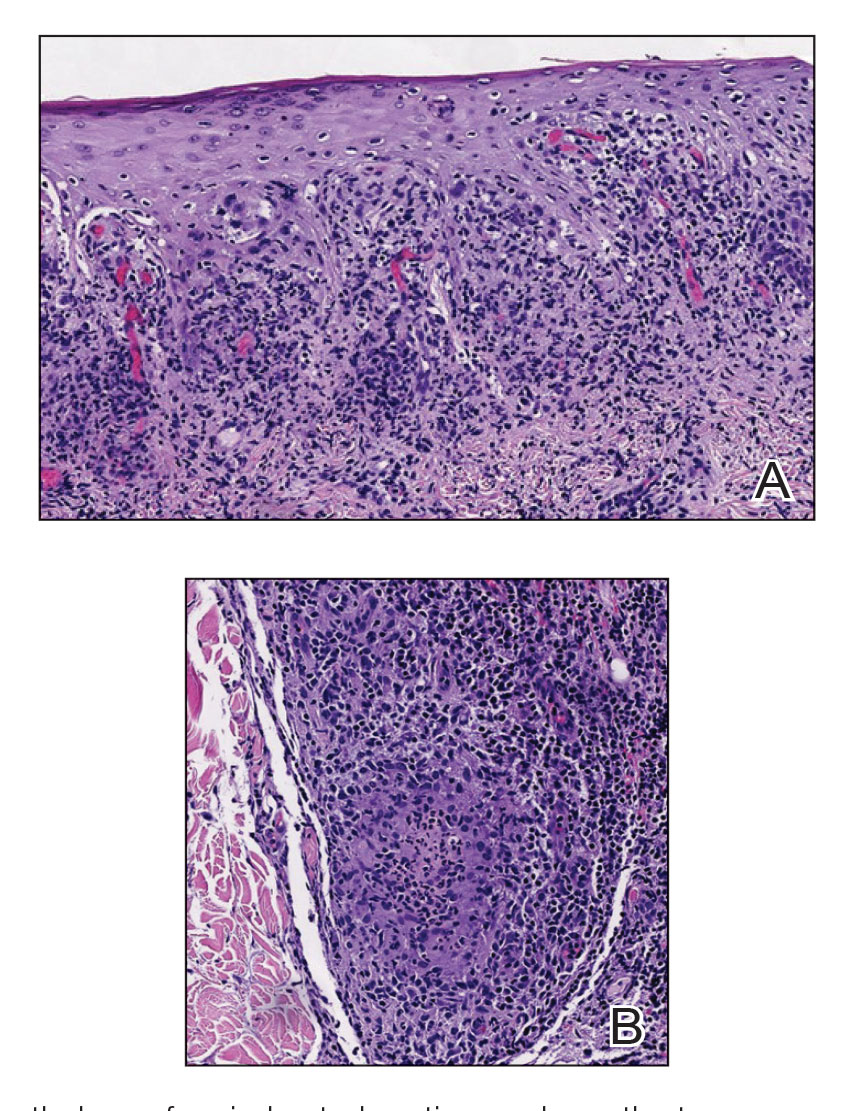
Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
The Diagnosis: Syphilis
Histopathology revealed psoriasiform hyperplasia, endothelial cell swelling, and a brisk lichenoid inflammation with plasma cells (Figure, A). There also was pustular folliculitis in association with well-formed granulomatous inflammation and a prominent number of plasma cells (Figure, B). Treponema pallidum immunostaining showed numerous organisms in the epidermal and follicular epithelium. Rapid plasma reagin was found to be positive with a titer of 1:128. Evaluation for neurosyphilis through lumbar puncture was negative; the patient also was HIV negative. All of our patient’s skin lesions cleared after a 3-week course of weekly intramuscular benzathine G injections. Due to his substantial clinical improvement, the patient was subsequently lost to follow-up.

Syphilis, an infectious disease caused by the spirochete bacterium T pallidum, has a well-known natural history defined by various stages classically categorized as primary, secondary, latent, or late (tertiary).1 The classic lesion in primary syphilis is the chancre, a painless ulcer with raised borders that develops within approximately 3 weeks following the initial inoculation.2 Secondary syphilis manifests with mucocutaneous findings in up to 97% of patients, and untreated patients develop secondary syphilis at a rate of approximately 25%.3 Although mucocutaneous findings in secondary syphilis can vary widely, patients most commonly develop a diffuse maculopapular exanthem, and 40% develop mucosal findings including genital ulcers, mucous patches, and condylomata lata.1 In latent syphilis, there is seroreactivity, but otherwise there are no clinical symptoms. A clear symptomatic history of prior primary or secondary syphilis may be known or unknown. Latent syphilis is divided into early and late phases, and the World Health Organization designates 2 years after the first suspected exposure as the cutoff point for early and late latency.4 During the first 4 years of latent syphilis, patients may exhibit mucocutaneous relapses. Our patient denied any sexual activity for more than 3 years prior to presentation. Because of the start of iatrogenic immunosuppression during this period, this case was classified as late latent syphilis with mucocutaneous reactivation.
Behçet disease was included within the differential diagnosis but is characterized by multiorgan systemic vasculitis that causes various mucocutaneous findings including aphthous ulcers, papulopustular lesions, and genital ulcers.5 Histopathologic features are nonspecific, and the clinical finding of recurrent genital and oral ulceration should be present for diagnosis. This disease predominantly occurs in East Asian or Mediterranean populations and is otherwise rare in White individuals.
SAPHO (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome is a rare disorder consisting of skin, joint, and bone manifestations.6 Severe acne generally is accompanied by palmoplantar pustulosis along with pain and joint tenderness involving the anterior chest and axial skeleton, both of which were absent in our patient.
Pustular psoriasis can be localized or generalized. Localized presentations frequently are acral and may be associated with a variable degree of nail dystrophy and arthritis. Generalized presentations are characterized by hyperemic, well-defined patches with variable numbers of pustules.7 The pustules are the consequence of exuberate neutrophilic exocytosis into the epidermis and are nonfollicular.
Steroid-induced acne may be considered in the proper clinical setting of an acneform eruption with a prior history of systemic steroid treatment. However, additional findings of mucositis would not be expected, and although our patient was prescribed prednisone from his primary care physician prior to presentation to our clinic, this medication was given after the onset of the cutaneous eruption.
Syphilis commonly is referred to as the great mimicker due to its potential diverse morphologic presentations, which can involve acneform eruptions, though rare.8 In the setting of mucositis, generalized acneform eruptions should raise suspicion for the possibility of syphilis, even in the absence of other more classic cutaneous features.
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14.
- Sparling PF. Natural history of syphilis. In: Holmes KK, Mardh PA, Sparling PF, et al, eds. Sexually Transmitted Diseases. McGraw Hill; 1990:213.
- Clark EG, Danbolt N. The Oslo study of the natural course of untreated syphilis: an epidemiologic investigation based on a re-study of the Boeck-Bruusgaard material. Med Clin North Am. 1964;48:613.
- Sule RR, Deshpande SG, Dharmadhikari NJ, et al. Late cutaneous syphilis. Cutis. 1997;59:135-137.
- Wilder EG, Frieder J, Sulhan S, et al. Spectrum of orocutaneous disease associations: genodermatoses and inflammatory conditions. J Am Acad Dermatol. 2017;77:809-830.
- Carneiro S, Sampaio-Barros PD. SAPHO syndrome. Rheum Dis Clin North Am. 2013;39:401-418.
- Bachelez H. Pustular psoriasis and related pustular skin diseases. Br J Dermatol. 2018;178:614-618.
- Domantay-Apostol GP, Handog EB, Gabriel MT. Syphilis: the international challenge of the great imitator. Dermatol Clin. 2008; 26:191-202, v. doi:10.1016/j.det.2007.12.001
A 48-year-old man with a history of ulcerative colitis that was well-controlled with adalimumab presented with a generalized acneform eruption involving the face, chest (top) and back, as well as a well-defined ovoid ulcer on the anterior aspect of the tongue (bottom) of 2 months’ duration. Prior treatment with prednisone 60 mg daily for 14 days resulted in no improvement. He denied unintentional weight loss, cyclic fever, or arthritis. A complete blood cell count with differential showed mild anemia (hemoglobin, 11.6 g/dL [reference range, 13.2–16.6 g/dL]) with a differential cell count that was within reference range for each cell type. The erythrocyte sedimentation rate was elevated at 44 mm/h (reference range, 0–22 mm/h). A 4-mm punch biopsy specimen of an indurated cystic papule on the torso was obtained.
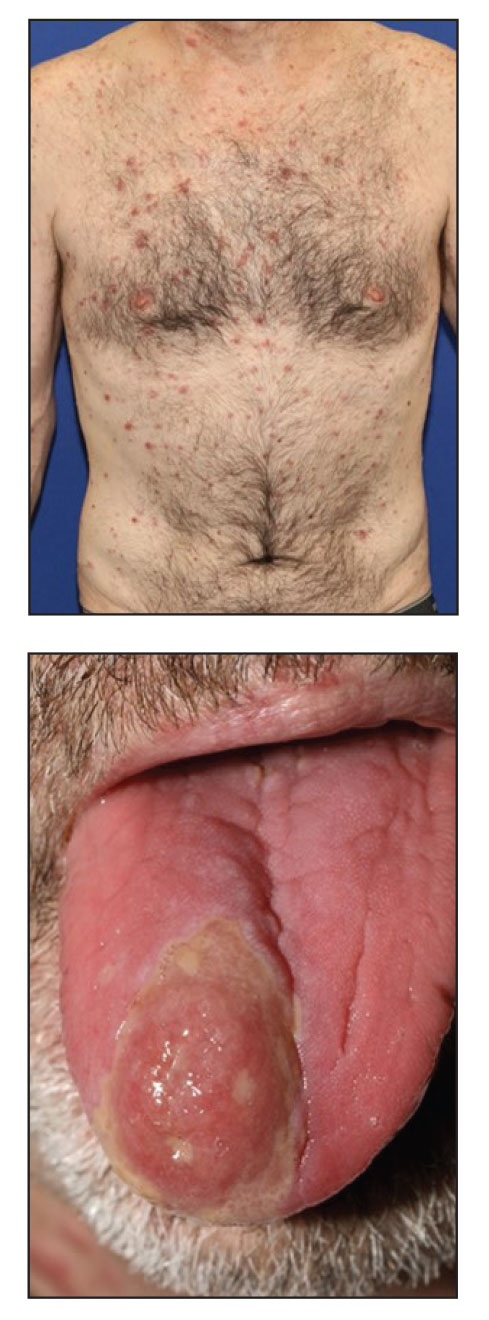
FDA approves autoinjector pen for Humira biosimilar, Cyltezo
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
The U.S. Food and Drug Administration on May 22 approved a new autoinjection option for adalimumab-adbm (Cyltezo), a biosimilar to AbbVie’s adalimumab (Humira), ahead of Cyltezo’s commercial launch on July 1, 2023.
Cyltezo was approved by the FDA in 2017 as a prefilled syringe and was the first biosimilar deemed to be interchangeable with Humira in 2021. It is indicated to treat multiple chronic inflammatory conditions, including rheumatoid arthritis, polyarticular juvenile idiopathic arthritis, plaque psoriasis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, and hidradenitis suppurativa. This new design, which features one-button, three-step activation, has been certified as an “Ease of Use” product by the Arthritis Foundation, Boehringer Ingelheim said in a press release. The 40-mg, prefilled Cyltezo Pen will be available in two-, four-, and six-pack options.
“The FDA approval of the Cyltezo Pen is great news for patients living with chronic inflammatory diseases who may prefer administering the medication needed to manage their conditions via an autoinjector,” said Stephen Pagnotta, the executive director and biosimilar commercial lead at Boehringer Ingelheim in a statement; “we’re excited to be able to offer the Cyltezo Pen as an additional option to patients at Cyltezo’s launch on July 1.”
A version of this article first appeared on Medscape.com.
Beta-blocker gel shows promise for diabetic foot ulcers
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
say Indian researchers.
Esmolol is a short-acting beta-adrenergic receptor blocker that is currently approved by the Food and Drug Administration for cardiac indications such as short-term use for supraventricular tachycardia.
As a gel, esmolol hydrochloride is administered topically to stimulate wound healing via mechanisms such as the migration of keratinocytes, fibroblasts, and endothelial cells into wound tissue.
The current trial enrolled patients with type 1 or 2 diabetes, finding that, among 140 assessed, target ulcer closure within 12 weeks was more than twice as likely in those assigned esmolol gel plus standard of care than those given standard of care alone.
The impact of adding esmolol gel to standard of care was even greater in patients with a body mass index (BMI) over 25 kg/m2 and in those who weighed more than 80 kg (176 lb).
“The use of esmolol in the treatment of diabetic foot ulcers in addition to standard of care may be an important addition to the endeavor of healing diabetic foot ulcers,” wrote Ashu Rastogi, MD, DM, department of endocrinology, Post Graduate Institute of Medical Education and Research, Chandigarh, India, and colleagues, in their article recently published in JAMA Network Open.
Dr. Rastogi first presented the findings at the 2022 annual meeting of the European Association for the Study of Diabetes. The results were well received, with one clinician describing them as “astounding.”
However, Andrew Boulton, MD, PhD, said in an interview that, although the final published data are “interesting,” they “need further confirmation” because “there are one or two unusual features” about the study. Dr. Boulton is a professor of medicine, division of diabetes, endocrinology & gastroenterology, at the University of Manchester (England).
He highlighted that the study was of “basically neuropathic ulcers, many of which were plantar and should be able to heal without any specific additional therapy.”
In addition, the inclusion criteria state that the ulcers could be below the malleoli or 5 cm above them, which Dr. Boulton explained is “very unusual and would therefore include some atypical and not truly diabetic ‘foot’ ulcers.”
And Frances Game, MBBCh, department of diabetes and endocrinology, University Hospitals of Derby (England) and Burton NHS Foundation Trust, added that there are questions about the study methodology.
She said in an interview that although it is a “fascinating study,” the main comparison group did not receive vehicle, or placebo, gel in addition to standard of care. “How were they blinded [to treatment]?”
The “biggest problem” with the study, however, is that the primary outcome was reported as a per-protocol endpoint, not as a standard intention-to-treat analysis, which allowed the researchers to exclude patients whose ulcers increased in size by over 30% on two consecutive visits.
“That kind of makes [esmolol gel] look better than it is because they’ve taken out the ones who got worse,” Dr. Game noted. However, the findings, while not conclusive, do warrant further study of esmolol gel.
The authors noted that diabetic foot ulcers are a severe complication of diabetes, with a prevalence of 1.3%-12.0% across various countries, And the complication contributes to patient morbidity and mortality, with a 5-year mortality that is substantially higher than that of many cancers.
Moreover, “even with the best therapy,” such as advanced moist wound therapy, bioengineered tissue or skin substitutes, peptides, growth factors, electric stimulation, and negative-pressure wound therapy, just 30% of wounds linked to diabetes heal and recurrence is as high as 70%.
Against this backdrop, topical esmolol 14% gel was shown in a phase 1/2 study to be associated with ulcer area reduction and earlier wound closure versus standard of care plus a control vehicle gel.
The current phase 3, randomized, controlled trial involved individuals aged 18-75 years with type 1 or type 2 diabetes and noninfected diabetic foot ulcers classified as grade 1A and 1C on the University of Texas Wound Classification System, which had been open for at least 6 weeks and had an area of 2-25 cm2.
Patients from 27 tertiary care centers across India were enrolled in 2018-2020. They were randomized in a 3:3:1 ratio to one of three groups: esmolol 14% gel plus standard of care, standard of care only, or vehicle plus standard of care.
The study lasted 25 weeks and included a 1-week screening phase, during which all patients received standard of care, a 12-week treatment phase, and a 12-week follow-up phase. The latter included a closure confirmation period of 4 weeks and an observation period of 8 weeks.
Patients were assessed once a week during the treatment phase, and then at weeks 14, 16, 20, and 24.
In all, 176 patients were enrolled. Participants were a mean age of 56.4 years and 69.3% were men. Average hemoglobin A1c was 8.6%. Mean diabetic foot ulcer area was 4.7 cm2 and the average ulcer duration was 49.8 weeks.
The primary outcome was the proportion of patients who achieved target ulcer closure during the 12-week treatment phase and was assessed in 140 patients.
Overall, 60.3% of patients treated with esmolol gel plus standard of care achieved target ulcer closure versus 41.7% of those in the standard of care alone group (odds ratio, 2.13; P = .03).
The secondary outcome was the proportion of patients with target ulcer closure by the study end and was assessed in 120 patients.
In total, 77.2% of patients in the esmolol gel plus standard of care group met the secondary endpoint, compared with 55.6% of those receiving standard of care alone (OR, 1.72; P = .01).
Further analysis suggested the benefit seen with esmolol gel plus standard of care was greater in patients with a weight greater than 80 kg versus standard of care alone (OR, 4.04; P = .04), and in those with a BMI greater than 25 (OR, 2.72; P = .03).
Treatment-emergent adverse events were reported by 33 (18.8%) participants, with 12 events deemed serious. “However, none of the serious adverse events were considered as drug-related by the investigators,” concluded the researchers.
The study was partly funded by NovaLead Pharma and the Biotechnology Industry Research Assistance Council, New Delhi, set up by the Department of Biotechnology, Government of India. Dr. Rastogi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
AD in infancy: Diagnostic advice and treatment tips
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
WASHINGTON – mean it is often “woefully undertreated,” Robert Sidbury, MD, MPH, said at the annual Revolutionizing Atopic Dermatitis conference.
Identifying and mitigating triggers – such as irritation, contact allergy, and infection – is a cornerstone of treatment in infants, but tailored therapy with topical corticosteroids, topical calcineurin inhibitors (TCIs), and topical phosphodiesterase 4 (PDE4) inhibitors also have roles to play, said Dr. Sidbury, chief of dermatology at Seattle Children’s Hospital and professor in the department of pediatrics at the University of Washington, Seattle.
Views on the use of dupilumab as a systemic agent for severe infantile AD, meanwhile, have shifted significantly in the past year with the Food and Drug Administration approval of the biologic for children aged 6 months to 5 years and with extended experience with the biologic in all ages, including children, Lawrence F. Eichenfield, MD, professor of dermatology and pediatrics at the University of California, San Diego, said at the meeting.
The pediatric dermatologists spoke during a session devoted to AD in infants, during which the diagnosis of AD and the role – and risks – of food allergy testing were also discussed. Diagnosis, said Elaine C. Siegfried, MD, who also spoke during the session, requires careful consideration of mimicking conditions and a broader list of differential diagnoses in those infants with poor growth or frequent infections.
Here are some of the experts’ pearls for practice.
Diagnosing AD in infants
Among infants who are growing well and otherwise healthy, the infantile eczema phenotype encompasses AD, seborrheic dermatitis, contact dermatitis, psoriasis – and overlap of more than one of these conditions. “Overlap is common,” said Dr. Siegfried, professor of pediatrics and dermatology at Saint Louis University, and director of the division of pediatric dermatology at Cardinal Glennon Children’s Hospital.
(Initial topical treatment for all these conditions is similar, but optimal treatment may differ for young children with moderate to severe disease that requires systemic treatment, she said in an interview after the meeting.)
Sparing of the diaper area that reflects skin barrier integrity is a classic feature of AD in infants and can be a useful diagnostic sign. In addition, “hypopigmentation is more characteristic of psoriasis” than AD, whereas AD tends to be hyperpigmented, which is most obvious in skin-of-color patients, Dr. Siegfried said.
Disease-specific pigment changes may be related to microbial colonization – such as Malassezia-associated hypopigmentation – or cell turnover, which is faster in psoriasis and slower in AD – with corresponding differences in pigment retention, and may be more obvious in children than adults, she said.
A less common scenario is dermatitis in infants who are not growing well. For these patients, she noted, the differential diagnosis includes metabolic or immune deficiency dermatitis as well as a variety of genodermatoses.
Generalized redness and scaling present on the first day of life is suggestive of non-atopic dermatitis. “If you’re born with red scaly skin, that’s very different than if you develop red skin in the first month or two of life,” Dr. Siegfried said.
When there is diaper area involvement with AD, contact dermatitis, impetigo, and Candida may be complicating factors. And in infants with other morbidities – especially those who are not growing well – diaper area involvement suggests a broader differential diagnosis. “I implore you, if you see children, make sure you weigh and measure them at every appointment,” she said.
Dr. Siegfried has seen infants with Netherton syndrome, and those with cystic fibrosis with zinc deficiency, for instance, presenting with “an eczematous-like picture,” diaper-area involvement, and other morbidities.
For infants with AD, she maintains a high index of suspicion for secondary infections such as molluscum, herpes simplex virus (HSV) with or without streptococci, scabies, tinea, and group A streptococci. “Secondary infections ... may be incognito,” she said. “Look for subtle signs. Even molluscum can be very subtle.”
Secondary allergic contact dermatitis is also common although it’s “technically difficult to confirm the diagnosis,” she said. Patch testing in infants is technically challenging, sensitivity is low, and monosensitization is uncommon. “So I do initial empiric topical allergen avoidance,” she said, keeping in mind ubiquitous and avoidable topical allergens such as Kathon, cocamidopropyl betaine, propylene glycol, disperse blue, and adhesives.
Treating AD in infancy
Irritation “is probably one of the biggest triggers” of AD in infants, and the often “pristine” diaper area compared with inflamed eczema elsewhere can demonstrate the importance of moisturization for healthy skin in atopic infants, Dr. Sidbury said.
Among treatments that “punch above their weight” for AD in infants is an ointment-based barrier applied around the mouth, chin, and chest – where the wet/dry impact of drooling is maximal – before and after meals, he said.
Another is hydrocortisone 2.5% mixed 1:1 with mupirocin for those infants who have secondary infections and “that exudative, weepy-looking appearance on the face,” he said. The topical antibiotic in the combination cream “lessens the potency of the steroid and oftentimes by synergy, makes it more effective” by simultaneously treating inflammation, he said. He cautions against products containing neomycin, which can be an allergen.
A combination antibiotic-steroid-emollient cream (the Aron Regimen) can also “sometimes punch above its weight,” Dr. Sidbury said.
Infections typically involve Staphylococcus aureus, but in up to 16% of cases Streptococcus is involved. And notably, lurking underneath the honey-colored crusting of S. aureus infections may be the grouped vesicles that characterize eczema herpeticum, Dr. Sidbury said.
“Counsel [parents] preemptively to treat cold sores immediately [in order to] decrease HSV shedding and minimize risk to their baby,” Dr. Sidbury said.
For treating AD-associated inflammation in skin not affected by secondary infections, over-the-counter 1% hydrocortisone cream is often sufficient, and “for very young babies and preemies in particular, I generally don’t use anything stronger because their skin barrier isn’t fully complete yet, so they absorb more than an older child does,” he said, referring to ages 2 months corrected as a marker for considering a stronger formulation if needed.
Many parents are “very concerned” about topical corticosteroid (TCS) use and pediatricians are also “often concerned,” Dr. Sidbury said. Addressing this concern, he tries to provide context and promote adherence by pointing out that infants have an easily visible vein at the temple area where the skin is naturally thin. If parents were to see this appearance for the first time in other areas while using topical steroids, he tells them, it may be the first sign of skin thinning, but “it’s entirely reversible at that stage.”
He also stressed the cost of not treating. It’s unknown whether “treating aggressively early on prevents any future development or manifestation of eczema, or future comorbidities, but we don’t know that it doesn’t,” Dr. Sidbury said. “And we certainly know how miserable that baby with eczema can be in the short term. So we need to use these medicines.”
Dr. Sidbury utilizes tacrolimus 0.03% ointment, a topical calcineurin inhibitor (TCI), only if he is worried about overuse of steroids, and uses a regimen that alternates the TCI (used in infants off-label because it is approved for ages 2 years and older) with TCS in periods of similar duration (for example, treatment with TCS for 1 week and TCI for 1 week, or rotations of 2 weeks each or 3 days each). “And these rotations may be dynamic depending on severity of the flare at any given time,” he said after the meeting.
Preapproval data from the pivotal trials of tacrolimus are reassuring and can be shared with parents. “Two-year-olds had 90% of BSA [body surface area] treated for 12 weeks” with no signs of systemic risks, Dr. Sidbury said at the meeting.
Crisaborole, a topical phosphodiesterase 4 (PDE4) inhibitor approved for AD down to age 3 months, does not, like tacrolimus, have a boxed warning about a possible risk for cancer, and may also be alternated with TCSs. It will cause stinging in some children, but TCSs and TCIs can also sting in some children, he said, noting that samples can be helpful to predict what will or won’t sting each infant.
Systemic treatment in infants
The Liberty AD PRESCHOOL phase 3 trial that supported the FDA’s approval of dupilumab down to 6 months of age, published in 2022 in The Lancet, covered ages 6 months to 5 years but included only six children under the age of 2, “leaving us with a very limited dataset in this age group,” Dr. Eichenfield said at the meeting.
Other data and analyses that have provided reassurance, such as a laboratory safety analysis published online in 2022 showing no meaningful changes in laboratory safety parameters in children as young as 6 months, and pediatric data (not including infants) presented at a RAD meeting in 2022 showing that dupilumab, an interleukin-4 receptor alpha antagonist, may have positive effects on bone mineral density.
Data from the Liberty AD PRESCHOOL open-label extension study presented at the American Academy of Dermatology meeting in 2023, meanwhile, show that “the adverse event profile is not looking much different than what we see in older children,” Dr. Eichenfield said. “There are low rates of severe adverse events and a very low rate of discontinuations.”
At Rady Children’s Hospital, where Dr. Eichenfield is chief of pediatric and adolescent dermatology, dupilumab has become a first-line systemic agent for severe infantile AD, supplanting prior traditional but little used systemic agents such as oral corticosteroids, cyclosporine, methotrexate, azathioprine, or mycophenolate, he said after the meeting.
The decision to use systemics in the first 2 years of life is “a comprehensive one,” requiring knowledge of the child’s history, disease course, and assessment of response to prior therapies, comorbidities and severity, he said.
Food allergy in infants with AD
Food allergy is common in children with moderate to severe AD, but true food-triggered AD, with AD being the only symptom of food allergy, is rare, said Anne Marie Singh, MD, associate professor in the division of allergy and immunology and rheumatology at the University of Wisconsin, Madison, who focuses on pediatrics.
Over the years, studies of double-blind placebo-controlled food challenge tests in children with AD have tended to conflate immediate IgE hypersensitivity (and skin symptoms like urticaria) with AD, said Dr. Singh, who directs the university’s Food Allergy Research and Education Center of Excellence. In a recently published study she led involving 374 children with AD referred to allergy and/or dermatology subspecialty clinics at the University of Wisconsin, Madison, 55% had a food allergy but only 2% had food-triggered AD “where eczema is the only symptom and removal of the food cleared up the eczema and its return brought it back,” Dr. Singh said at the meeting. Another 4% had combined IgE-mediated food allergy and food-triggered AD. Almost half of the children with food-triggered AD were under 1 year of age, and egg was the most common trigger, she noted.
Food should be implicated largely by history, Dr. Singh emphasized.
Food allergy testing in the context of AD can be done but is challenging, with the clinical relevance of skin prick testing and food-specific immunoglobulin E (sIgE) difficult to predict. Predictive values of sIgE levels are established for immediate IgE mediated food allergy, but “cut-offs” for food-triggered AD are not established, she explained, noting that “cut-offs are likely higher for our children with AD.”
Elimination diets, moreover, pose significant risks of future oral tolerance and risks of nutritional deficiencies and poor growth, Dr. Singh said. New and immediate reactions to foods that are reintroduced after an elimination diet are common, and research has shown that 20% or more of such reactions involve anaphylaxis. “If an elimination diet is undertaken, you need emergency action plans, injectable epinephrine, and nutrition counseling,” she said.
A recent systematic review and meta-analysis conditionally recommended against elimination diets for the treatment of AD, Dr. Singh noted.
Asked by Dr. Sidbury whether there “is a sweet spot where you can eliminate [foods] without going all the way,” Dr. Singh said she will sometimes do a “diagnostic elimination trial” with food elimination for 2-4 weeks only – a time period after which “I’ll feel really comfortable reintroducing the food.”
Dr. Singh urged dermatologists to “know your allergist” because “patients respond best with a consistent message.”
Dr. Sidbury reported ties with Regeneron, UCB, Pfizer, Leo Pharma, Lilly, and Beiersdorf. Dr. Siegfried reported ties with Pfizer, Regeneron, Sanofi Genzyme, Pfizer, UCB, Novan and Leo Pharma. Dr. Singh reported ties with Incyte and Siolta Therapeutics. Dr. Eichenfield reported ties with Pfizer, Regeneron, Sanofi Genzyme, Incyte, and Pfizer.
AT RAD 2023
FDA OKs first-ever topical gene therapy, for rare skin disease
(DEB), a rare skin disease.
The therapy, Vyjuvek (beremagene geperpavec, formerly known as B-VEC), uses a nonreplicating herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells, restoring the COL7 protein fibrils that stabilize skin structure.
Vyjuvek, developed by Krystal Biotech, is designed to be used repetitively to heal a single wound or on more than one wound. In the pivotal study, the gene therapy, delivered in a topical gel, was administered once a week for 6 months.
The FDA gave Vyjuvek priority review, approving the therapy just 9 months after Krystal filed its application for approval. Vyjuvek is also an orphan drug, giving it potentially 7 years of marketing exclusivity.
“Vyjuvek is the first FDA-approved gene therapy treatment for DEB, a rare and serious genetic skin disorder,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the FDA’s statement announcing the approval.
“With the FDA approval of Vyjuvek, the DEB population has reached a monumental milestone in the treatment of this horrible disorder,” said Brett Kopelan, executive director of debra of America, a national advocacy group for people with DEB, in a statement issued by Krystal Biotech. “Our hopes have now been realized for a safe and effective treatment for one of the most devastating symptoms of the disorder,” said Mr. Kopelan.
“This is a devastating disease,” said M. Peter Marinkovich, MD, primary investigator of Krystal’s pivotal GEM-3 trial and director of the Blistering Disease Clinic at Stanford Health Care, in the statement issued by Krystal. “Until now, doctors and nurses had no way to stop blisters and wounds from developing on dystrophic EB patient skin, and all we could do was to give them bandages and helplessly watch as new blisters formed,” said Dr. Marinkovich, who is also associate professor of dermatology at Stanford (Calif.) University School of Medicine.
“Because it’s safe and easy to apply directly to wounds, it doesn’t require a lot of supporting technology or specialized expertise, making Vyjuvek highly accessible even to patients who live far away from specialized centers,” he said.
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, said that Vyjuvek is an important advance for DEB. “This to me would be a treatment that I would consider for almost all patients,” Dr. Vakharia said in an interview.
Dr. Vakharia, who was not involved with trials of Vyjuvek, said he had concerns about whether patients might develop antibodies to either HSV or C7 but that the greater initial worry is if Vyjuvek will be affordable and accessible. “I envision that it will be a costly medication,” he said.
Mr. Kopelan, the patient advocate, said that his organization “will continue to work closely with Krystal to assure patients have ready access to Vyjuvek.”
Krystal did not respond before press time to a request for comment on pricing.
Dystrophic epidermolysis bullosa affects 1 to 3 people per million in the United States. It is caused by mutations in the COL7A1 gene, which encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue. COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile that it tears with the slightest touch.
DEB usually presents at birth and is divided into two major types depending on the inheritance pattern: recessive dystrophic epidermolysis bullosa and dominant dystrophic epidermolysis bullosa.
People with the dominant form typically have mild cases with blistering primarily on the hands, feet, knees, and elbows. The recessive form can be painful and debilitating, causing widespread blistering. Depending on the severity, it can lead to nonhealing wounds, fusing of fingers and toes, anemia, weak bones, and esophageal and genitourinary strictures. Squamous cell cancers are a frequent cause of death.
Vyjuvek is mixed into an inactive gel and is evenly applied to a wound once a week by a health care professional, according to the FDA.
The agency recommends the following precautions for patients and caregivers:
- Avoid direct contact with treated wounds and dressings of treated wounds for 24 hours following application. Clean any area that is accidentally exposed.
- Wash hands and wear protective gloves when changing wound dressings.
- Disinfect the bandages used for the first dressing with a viricidal agent, such as 70% isopropyl alcohol, 6% hydrogen peroxide, or less than 0.4% ammonium chloride, and dispose of them in a separate, sealed plastic bag in household waste. Subsequent used dressings and cleaning materials should be disposed of in sealed plastic bags in household waste.
FDA approval of Vyjuvek was based on a randomized, double-blinded, placebo-controlled, 31-patient, phase 3 trial published in the New England Journal of Medicine, which showed that 71% of wounds treated with the gene therapy had complete healing at 3 months, compared with 20% of those treated with placebo (95% confidence interval, 29-73; P < .001). At 6 months, 67% of wounds treated with the gene therapy had complete healing, compared with 22% of wounds treated with placebo (95% CI, 24-68; P = .002).
Almost two-thirds of the patients had at least one adverse event. Most were mild or moderate. The most common events were pruritus, chills, and squamous cell carcinoma, reported in three patients each. SCC cases occurred at wound sites that had not been exposed to Vyjuvek or placebo. Serious adverse events, which were unrelated to the treatment, occurred in three patients and included diarrhea, anemia, and cellulitis.
Krystal will also be seeking marketing approval for Vyjuvek in the European Union, likely in 2024, said the company. In September, the European Medicines Agency’s Pediatric Committee issued a positive opinion on the gene therapy and Krystal’s plan to test B-VEC in children.
Dr. Marinkovich and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(DEB), a rare skin disease.
The therapy, Vyjuvek (beremagene geperpavec, formerly known as B-VEC), uses a nonreplicating herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells, restoring the COL7 protein fibrils that stabilize skin structure.
Vyjuvek, developed by Krystal Biotech, is designed to be used repetitively to heal a single wound or on more than one wound. In the pivotal study, the gene therapy, delivered in a topical gel, was administered once a week for 6 months.
The FDA gave Vyjuvek priority review, approving the therapy just 9 months after Krystal filed its application for approval. Vyjuvek is also an orphan drug, giving it potentially 7 years of marketing exclusivity.
“Vyjuvek is the first FDA-approved gene therapy treatment for DEB, a rare and serious genetic skin disorder,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the FDA’s statement announcing the approval.
“With the FDA approval of Vyjuvek, the DEB population has reached a monumental milestone in the treatment of this horrible disorder,” said Brett Kopelan, executive director of debra of America, a national advocacy group for people with DEB, in a statement issued by Krystal Biotech. “Our hopes have now been realized for a safe and effective treatment for one of the most devastating symptoms of the disorder,” said Mr. Kopelan.
“This is a devastating disease,” said M. Peter Marinkovich, MD, primary investigator of Krystal’s pivotal GEM-3 trial and director of the Blistering Disease Clinic at Stanford Health Care, in the statement issued by Krystal. “Until now, doctors and nurses had no way to stop blisters and wounds from developing on dystrophic EB patient skin, and all we could do was to give them bandages and helplessly watch as new blisters formed,” said Dr. Marinkovich, who is also associate professor of dermatology at Stanford (Calif.) University School of Medicine.
“Because it’s safe and easy to apply directly to wounds, it doesn’t require a lot of supporting technology or specialized expertise, making Vyjuvek highly accessible even to patients who live far away from specialized centers,” he said.
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, said that Vyjuvek is an important advance for DEB. “This to me would be a treatment that I would consider for almost all patients,” Dr. Vakharia said in an interview.
Dr. Vakharia, who was not involved with trials of Vyjuvek, said he had concerns about whether patients might develop antibodies to either HSV or C7 but that the greater initial worry is if Vyjuvek will be affordable and accessible. “I envision that it will be a costly medication,” he said.
Mr. Kopelan, the patient advocate, said that his organization “will continue to work closely with Krystal to assure patients have ready access to Vyjuvek.”
Krystal did not respond before press time to a request for comment on pricing.
Dystrophic epidermolysis bullosa affects 1 to 3 people per million in the United States. It is caused by mutations in the COL7A1 gene, which encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue. COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile that it tears with the slightest touch.
DEB usually presents at birth and is divided into two major types depending on the inheritance pattern: recessive dystrophic epidermolysis bullosa and dominant dystrophic epidermolysis bullosa.
People with the dominant form typically have mild cases with blistering primarily on the hands, feet, knees, and elbows. The recessive form can be painful and debilitating, causing widespread blistering. Depending on the severity, it can lead to nonhealing wounds, fusing of fingers and toes, anemia, weak bones, and esophageal and genitourinary strictures. Squamous cell cancers are a frequent cause of death.
Vyjuvek is mixed into an inactive gel and is evenly applied to a wound once a week by a health care professional, according to the FDA.
The agency recommends the following precautions for patients and caregivers:
- Avoid direct contact with treated wounds and dressings of treated wounds for 24 hours following application. Clean any area that is accidentally exposed.
- Wash hands and wear protective gloves when changing wound dressings.
- Disinfect the bandages used for the first dressing with a viricidal agent, such as 70% isopropyl alcohol, 6% hydrogen peroxide, or less than 0.4% ammonium chloride, and dispose of them in a separate, sealed plastic bag in household waste. Subsequent used dressings and cleaning materials should be disposed of in sealed plastic bags in household waste.
FDA approval of Vyjuvek was based on a randomized, double-blinded, placebo-controlled, 31-patient, phase 3 trial published in the New England Journal of Medicine, which showed that 71% of wounds treated with the gene therapy had complete healing at 3 months, compared with 20% of those treated with placebo (95% confidence interval, 29-73; P < .001). At 6 months, 67% of wounds treated with the gene therapy had complete healing, compared with 22% of wounds treated with placebo (95% CI, 24-68; P = .002).
Almost two-thirds of the patients had at least one adverse event. Most were mild or moderate. The most common events were pruritus, chills, and squamous cell carcinoma, reported in three patients each. SCC cases occurred at wound sites that had not been exposed to Vyjuvek or placebo. Serious adverse events, which were unrelated to the treatment, occurred in three patients and included diarrhea, anemia, and cellulitis.
Krystal will also be seeking marketing approval for Vyjuvek in the European Union, likely in 2024, said the company. In September, the European Medicines Agency’s Pediatric Committee issued a positive opinion on the gene therapy and Krystal’s plan to test B-VEC in children.
Dr. Marinkovich and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
(DEB), a rare skin disease.
The therapy, Vyjuvek (beremagene geperpavec, formerly known as B-VEC), uses a nonreplicating herpes simplex virus type 1 (HSV-1) vector to deliver the COL7A1 gene directly to skin cells, restoring the COL7 protein fibrils that stabilize skin structure.
Vyjuvek, developed by Krystal Biotech, is designed to be used repetitively to heal a single wound or on more than one wound. In the pivotal study, the gene therapy, delivered in a topical gel, was administered once a week for 6 months.
The FDA gave Vyjuvek priority review, approving the therapy just 9 months after Krystal filed its application for approval. Vyjuvek is also an orphan drug, giving it potentially 7 years of marketing exclusivity.
“Vyjuvek is the first FDA-approved gene therapy treatment for DEB, a rare and serious genetic skin disorder,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research, in the FDA’s statement announcing the approval.
“With the FDA approval of Vyjuvek, the DEB population has reached a monumental milestone in the treatment of this horrible disorder,” said Brett Kopelan, executive director of debra of America, a national advocacy group for people with DEB, in a statement issued by Krystal Biotech. “Our hopes have now been realized for a safe and effective treatment for one of the most devastating symptoms of the disorder,” said Mr. Kopelan.
“This is a devastating disease,” said M. Peter Marinkovich, MD, primary investigator of Krystal’s pivotal GEM-3 trial and director of the Blistering Disease Clinic at Stanford Health Care, in the statement issued by Krystal. “Until now, doctors and nurses had no way to stop blisters and wounds from developing on dystrophic EB patient skin, and all we could do was to give them bandages and helplessly watch as new blisters formed,” said Dr. Marinkovich, who is also associate professor of dermatology at Stanford (Calif.) University School of Medicine.
“Because it’s safe and easy to apply directly to wounds, it doesn’t require a lot of supporting technology or specialized expertise, making Vyjuvek highly accessible even to patients who live far away from specialized centers,” he said.
Paras P. Vakharia, MD, PharmD, assistant professor of dermatology at Northwestern University, Chicago, said that Vyjuvek is an important advance for DEB. “This to me would be a treatment that I would consider for almost all patients,” Dr. Vakharia said in an interview.
Dr. Vakharia, who was not involved with trials of Vyjuvek, said he had concerns about whether patients might develop antibodies to either HSV or C7 but that the greater initial worry is if Vyjuvek will be affordable and accessible. “I envision that it will be a costly medication,” he said.
Mr. Kopelan, the patient advocate, said that his organization “will continue to work closely with Krystal to assure patients have ready access to Vyjuvek.”
Krystal did not respond before press time to a request for comment on pricing.
Dystrophic epidermolysis bullosa affects 1 to 3 people per million in the United States. It is caused by mutations in the COL7A1 gene, which encodes the alpha-1 chain of collagen type VII (C7) protein. C7 forms the anchoring fibrils that attach the epidermis to the underlying dermal connective tissue. COL71A mutations that lead to defective, decreased, or absent C7 can make the skin so fragile that it tears with the slightest touch.
DEB usually presents at birth and is divided into two major types depending on the inheritance pattern: recessive dystrophic epidermolysis bullosa and dominant dystrophic epidermolysis bullosa.
People with the dominant form typically have mild cases with blistering primarily on the hands, feet, knees, and elbows. The recessive form can be painful and debilitating, causing widespread blistering. Depending on the severity, it can lead to nonhealing wounds, fusing of fingers and toes, anemia, weak bones, and esophageal and genitourinary strictures. Squamous cell cancers are a frequent cause of death.
Vyjuvek is mixed into an inactive gel and is evenly applied to a wound once a week by a health care professional, according to the FDA.
The agency recommends the following precautions for patients and caregivers:
- Avoid direct contact with treated wounds and dressings of treated wounds for 24 hours following application. Clean any area that is accidentally exposed.
- Wash hands and wear protective gloves when changing wound dressings.
- Disinfect the bandages used for the first dressing with a viricidal agent, such as 70% isopropyl alcohol, 6% hydrogen peroxide, or less than 0.4% ammonium chloride, and dispose of them in a separate, sealed plastic bag in household waste. Subsequent used dressings and cleaning materials should be disposed of in sealed plastic bags in household waste.
FDA approval of Vyjuvek was based on a randomized, double-blinded, placebo-controlled, 31-patient, phase 3 trial published in the New England Journal of Medicine, which showed that 71% of wounds treated with the gene therapy had complete healing at 3 months, compared with 20% of those treated with placebo (95% confidence interval, 29-73; P < .001). At 6 months, 67% of wounds treated with the gene therapy had complete healing, compared with 22% of wounds treated with placebo (95% CI, 24-68; P = .002).
Almost two-thirds of the patients had at least one adverse event. Most were mild or moderate. The most common events were pruritus, chills, and squamous cell carcinoma, reported in three patients each. SCC cases occurred at wound sites that had not been exposed to Vyjuvek or placebo. Serious adverse events, which were unrelated to the treatment, occurred in three patients and included diarrhea, anemia, and cellulitis.
Krystal will also be seeking marketing approval for Vyjuvek in the European Union, likely in 2024, said the company. In September, the European Medicines Agency’s Pediatric Committee issued a positive opinion on the gene therapy and Krystal’s plan to test B-VEC in children.
Dr. Marinkovich and Dr. Vakharia have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
MACE, VTE rates compared between TNF and JAK inhibitors for AxSpA and PsA
CLEVELAND – Patients with axial spondyloarthritis or psoriatic arthritis who used Janus kinase (JAK) inhibitors did not have higher risk of myocardial infarction, stroke, or venous thromboembolism (VTE), compared with those who used tumor necrosis factor inhibitors (TNFi), according to new research.
The information was presented in a poster at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) have increased cardiovascular risk compared with the general population. Emerging evidence has suggested that TNFi may protect the cardiovascular system and that there are cardiovascular and thrombotic concerns with JAK inhibitors.
Sali Merjanah, MD, a rheumatology fellow at Boston University, and colleagues, compared how drugs in the two treatment classes affected the likelihood of major adverse cardiovascular events (MACE) or VTE. MACE in this study were myocardial infarction and stroke.
In a search of the Marketscan Database during 2006-2021, the researchers identified 1,621 TNFi and 47 JAK inhibitor users with 273 and 8 cases of MACE, respectively. They identified 2,507 TNFi and 96 JAK users with 452 and 26 cases of VTE, respectively. Patients were aged 18-65 years and had at least one inpatient or two outpatient axSpA or PsA ICD-9 or ICD-10 diagnosis codes separated by at least 7 days.
The likelihood of MACE was 14% lower among JAK inhibitor users than TNFi users (the reference group), whereas the likelihood of VTE was 39% higher for JAK inhibitor users, but neither comparison was statistically significant. JAK/TNFi nonusers had a statistically significant 27% greater likelihood of MACE than did TNFi users. The likelihood for VTE was 12% higher for JAK/TNFi nonusers, compared with TNFi users, but this finding was not statistically significant. The researchers adjusted comparisons for age, medications, and comorbidities.
Small numbers complicate the research
Lianne Gensler, MD, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco, who was not part of the study, said the limitations the authors list are important to note. The researchers said that the study’s small number of JAK inhibitor users, short duration of exposure, and low event rate limit its precision, and there is potential misclassification of TNF/JAK inhibitor exposure, as well as confounding by indication.
Dr. Gensler noted that these same limitations apply to studies of patients with RA as well that try to answer the question of risk for MACE and malignancy when using these drugs,
“MACE is a rare event, malignancy is a rare event. So it’s like finding a needle in a haystack, and the haystack is really big. You either have to enrich the haystack with more needles or you have to make a smaller haystack,” Dr. Gensler said.
Nevertheless, she said, she credits the researchers for bringing the available information to light.
“I think we have to do this many different ways to try to get at the answer in a partial way,” she said.
The data were drawn from 2006 to 2021, but JAK inhibitors have only been approved for axSpA in the last one and a half years and for PsA at the end of 2017.
Additionally, the people taking JAK inhibitors would have likely already failed TNFis, she said, adding that this can make it hard to tell whether an event was linked with the JAK or the TNFi.
Nonusers may have other risk factors
She pointed out that in this study patients who were not using TNF or JAK inhibitors had slightly higher risk numerically for both MACE and VTE than did those using TNFis.
“There, the assumption is always that this is confounding by indication, meaning it is likely that the people who are nonusers have other risk factors for MACE, which is why we’re not giving them these drugs.”
Having heart failure, for instance, is a contraindication for using a TNF inhibitor, she noted. “So it’s not that these are protective compared to nonusers. It’s probably that the nonuser has higher risk and is not getting treated with these drugs to begin with.”
The authors properly concluded from the data that patients using JAK inhibitors did not have higher risk of MACE or VTE, compared with those who used TNFis, she said, but larger studies with more follow-up are needed.
“No evidence doesn’t mean no effect,” she said. “Part of it depends on the [statistical] power and the population you’re studying.”
Dr. Gensler is a consultant for AbbVie, Acceleron, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and has received grant support from Novartis and UCB. The authors’ financial relationships were not available.
CLEVELAND – Patients with axial spondyloarthritis or psoriatic arthritis who used Janus kinase (JAK) inhibitors did not have higher risk of myocardial infarction, stroke, or venous thromboembolism (VTE), compared with those who used tumor necrosis factor inhibitors (TNFi), according to new research.
The information was presented in a poster at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) have increased cardiovascular risk compared with the general population. Emerging evidence has suggested that TNFi may protect the cardiovascular system and that there are cardiovascular and thrombotic concerns with JAK inhibitors.
Sali Merjanah, MD, a rheumatology fellow at Boston University, and colleagues, compared how drugs in the two treatment classes affected the likelihood of major adverse cardiovascular events (MACE) or VTE. MACE in this study were myocardial infarction and stroke.
In a search of the Marketscan Database during 2006-2021, the researchers identified 1,621 TNFi and 47 JAK inhibitor users with 273 and 8 cases of MACE, respectively. They identified 2,507 TNFi and 96 JAK users with 452 and 26 cases of VTE, respectively. Patients were aged 18-65 years and had at least one inpatient or two outpatient axSpA or PsA ICD-9 or ICD-10 diagnosis codes separated by at least 7 days.
The likelihood of MACE was 14% lower among JAK inhibitor users than TNFi users (the reference group), whereas the likelihood of VTE was 39% higher for JAK inhibitor users, but neither comparison was statistically significant. JAK/TNFi nonusers had a statistically significant 27% greater likelihood of MACE than did TNFi users. The likelihood for VTE was 12% higher for JAK/TNFi nonusers, compared with TNFi users, but this finding was not statistically significant. The researchers adjusted comparisons for age, medications, and comorbidities.
Small numbers complicate the research
Lianne Gensler, MD, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco, who was not part of the study, said the limitations the authors list are important to note. The researchers said that the study’s small number of JAK inhibitor users, short duration of exposure, and low event rate limit its precision, and there is potential misclassification of TNF/JAK inhibitor exposure, as well as confounding by indication.
Dr. Gensler noted that these same limitations apply to studies of patients with RA as well that try to answer the question of risk for MACE and malignancy when using these drugs,
“MACE is a rare event, malignancy is a rare event. So it’s like finding a needle in a haystack, and the haystack is really big. You either have to enrich the haystack with more needles or you have to make a smaller haystack,” Dr. Gensler said.
Nevertheless, she said, she credits the researchers for bringing the available information to light.
“I think we have to do this many different ways to try to get at the answer in a partial way,” she said.
The data were drawn from 2006 to 2021, but JAK inhibitors have only been approved for axSpA in the last one and a half years and for PsA at the end of 2017.
Additionally, the people taking JAK inhibitors would have likely already failed TNFis, she said, adding that this can make it hard to tell whether an event was linked with the JAK or the TNFi.
Nonusers may have other risk factors
She pointed out that in this study patients who were not using TNF or JAK inhibitors had slightly higher risk numerically for both MACE and VTE than did those using TNFis.
“There, the assumption is always that this is confounding by indication, meaning it is likely that the people who are nonusers have other risk factors for MACE, which is why we’re not giving them these drugs.”
Having heart failure, for instance, is a contraindication for using a TNF inhibitor, she noted. “So it’s not that these are protective compared to nonusers. It’s probably that the nonuser has higher risk and is not getting treated with these drugs to begin with.”
The authors properly concluded from the data that patients using JAK inhibitors did not have higher risk of MACE or VTE, compared with those who used TNFis, she said, but larger studies with more follow-up are needed.
“No evidence doesn’t mean no effect,” she said. “Part of it depends on the [statistical] power and the population you’re studying.”
Dr. Gensler is a consultant for AbbVie, Acceleron, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and has received grant support from Novartis and UCB. The authors’ financial relationships were not available.
CLEVELAND – Patients with axial spondyloarthritis or psoriatic arthritis who used Janus kinase (JAK) inhibitors did not have higher risk of myocardial infarction, stroke, or venous thromboembolism (VTE), compared with those who used tumor necrosis factor inhibitors (TNFi), according to new research.
The information was presented in a poster at the annual meeting of the Spondyloarthritis Research and Treatment Network (SPARTAN).
Patients with axial spondyloarthritis (axSpA) and psoriatic arthritis (PsA) have increased cardiovascular risk compared with the general population. Emerging evidence has suggested that TNFi may protect the cardiovascular system and that there are cardiovascular and thrombotic concerns with JAK inhibitors.
Sali Merjanah, MD, a rheumatology fellow at Boston University, and colleagues, compared how drugs in the two treatment classes affected the likelihood of major adverse cardiovascular events (MACE) or VTE. MACE in this study were myocardial infarction and stroke.
In a search of the Marketscan Database during 2006-2021, the researchers identified 1,621 TNFi and 47 JAK inhibitor users with 273 and 8 cases of MACE, respectively. They identified 2,507 TNFi and 96 JAK users with 452 and 26 cases of VTE, respectively. Patients were aged 18-65 years and had at least one inpatient or two outpatient axSpA or PsA ICD-9 or ICD-10 diagnosis codes separated by at least 7 days.
The likelihood of MACE was 14% lower among JAK inhibitor users than TNFi users (the reference group), whereas the likelihood of VTE was 39% higher for JAK inhibitor users, but neither comparison was statistically significant. JAK/TNFi nonusers had a statistically significant 27% greater likelihood of MACE than did TNFi users. The likelihood for VTE was 12% higher for JAK/TNFi nonusers, compared with TNFi users, but this finding was not statistically significant. The researchers adjusted comparisons for age, medications, and comorbidities.
Small numbers complicate the research
Lianne Gensler, MD, director of the Ankylosing Spondylitis Clinic at the University of California, San Francisco, who was not part of the study, said the limitations the authors list are important to note. The researchers said that the study’s small number of JAK inhibitor users, short duration of exposure, and low event rate limit its precision, and there is potential misclassification of TNF/JAK inhibitor exposure, as well as confounding by indication.
Dr. Gensler noted that these same limitations apply to studies of patients with RA as well that try to answer the question of risk for MACE and malignancy when using these drugs,
“MACE is a rare event, malignancy is a rare event. So it’s like finding a needle in a haystack, and the haystack is really big. You either have to enrich the haystack with more needles or you have to make a smaller haystack,” Dr. Gensler said.
Nevertheless, she said, she credits the researchers for bringing the available information to light.
“I think we have to do this many different ways to try to get at the answer in a partial way,” she said.
The data were drawn from 2006 to 2021, but JAK inhibitors have only been approved for axSpA in the last one and a half years and for PsA at the end of 2017.
Additionally, the people taking JAK inhibitors would have likely already failed TNFis, she said, adding that this can make it hard to tell whether an event was linked with the JAK or the TNFi.
Nonusers may have other risk factors
She pointed out that in this study patients who were not using TNF or JAK inhibitors had slightly higher risk numerically for both MACE and VTE than did those using TNFis.
“There, the assumption is always that this is confounding by indication, meaning it is likely that the people who are nonusers have other risk factors for MACE, which is why we’re not giving them these drugs.”
Having heart failure, for instance, is a contraindication for using a TNF inhibitor, she noted. “So it’s not that these are protective compared to nonusers. It’s probably that the nonuser has higher risk and is not getting treated with these drugs to begin with.”
The authors properly concluded from the data that patients using JAK inhibitors did not have higher risk of MACE or VTE, compared with those who used TNFis, she said, but larger studies with more follow-up are needed.
“No evidence doesn’t mean no effect,” she said. “Part of it depends on the [statistical] power and the population you’re studying.”
Dr. Gensler is a consultant for AbbVie, Acceleron, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and has received grant support from Novartis and UCB. The authors’ financial relationships were not available.
AT SPARTAN 2023
AI at the office: Are clinicians prepared?
AURORA, COLO. – Artificial Intelligence has arrived at medical offices, whether or not clinicians feel ready for it.
AI might result in more accurate, efficient, and cost-effective care. But it’s possible it could cause harm. That’s according to Benjamin Collins, MD, at Vanderbilt University Medical Center, Nashville, Tenn., who spoke on the subject at the annual meeting of the Society of General Internal Medicine.
Understanding the nuances of AI is even more important because of the quick development of the algorithms.
“When I submitted this workshop, there was no ChatGPT,” said Dr. Collins, referring to Chat Generative Pre-trained Transformer, a recently released natural language processing model. “A lot has already changed.”
Biased data
Biased data are perhaps the biggest pitfall of AI algorithms, Dr. Collins said. If garbage data go in, garbage predictions come out.
If the dataset that trains the algorithm underrepresents a particular gender or ethnic group, for example, the algorithm may not respond accurately to prompts. When an AI tool compounds existing inequalities related to socioeconomic status, ethnicity, or sexual orientation, the algorithm is biased, according to Harvard researchers.
“People often assume that artificial intelligence is free of bias due to the use of scientific processes and its development,” he said. “But whatever flaws exist in data collection and old data can lead to poor representation or underrepresentation in the data used to train the AI tool.”
Racial minorities are underrepresented in studies; therefore, data input into an AI tool might skew results for these patients.
The Framingham Heart Study, for example, which began in 1948, examined heart disease in mainly White participants. The findings from the study resulted in the creation of a sex-specific algorithm that was used to estimate the 10-year cardiovascular risk of a patient. While the cardiovascular risk score was accurate for White persons, it was less accurate for Black patients.
A study published in Science in 2019 revealed bias in an algorithm that used health care costs as a proxy for health needs. Because less money was spent on Black patients who had the same level of need as their White counterparts, the output inaccurately showed that Black patients were healthier and thus did not require extra care.
Developers can also be a source of bias, inasmuch as AI often reflects preexisting human biases, Dr. Collins said.
“Algorithmic bias presents a clear risk of harm that clinicians must play against the benefits of using AI,” Dr. Collins said. “That risk of harm is often disproportionately distributed to marginalized populations.”
As clinicians use AI algorithms to diagnose and detect disease, predict outcomes, and guide treatment, trouble comes when those algorithms perform well for some patients and poorly for others. This gap can exacerbate existing disparities in health care outcomes.
Dr. Collins advised clinicians to push to find out what data were used to train AI algorithms to determine how bias could have influenced the model and whether the developers risk-adjusted for bias. If the training data are not available, clinicians should ask their employers and AI developers to know more about the system.
Clinicians may face the so-called black box phenomenon, which occurs when developers cannot or will not explain what data went into an AI model, Dr. Collins said.
According to Stanford (Calif.) University, AI must be trained on large datasets of images that have been annotated by human experts. Those datasets can cost millions of dollars to create, meaning corporations often fund them and do not always share the data publicly.
Some groups, such as Stanford’s Center for Artificial Intelligence in Medicine and Imaging, are working to acquire annotated datasets so researchers who train AI models can know where the data came from.
Paul Haidet, MD, MPH, an internist at Penn State College of Medicine, Hershey, sees the technology as a tool that requires careful handling.
“It takes a while to learn how to use a stethoscope, and AI is like that,” Dr. Haidet said. “The thing about AI, though, is that it can be just dropped into a system and no one knows how it works.”
Dr. Haidet said he likes knowing how the sausage is made, something AI developers are often reticent to make known.
“If you’re just putting blind faith in a tool, that’s scary,” Dr. Haidet said.
Transparency and ‘explainability’
The ability to explain what goes into tools is essential to maintaining trust in the health care system, Dr. Collins said.
“Part of knowing how much trust to place in the system is the transparency of those systems and the ability to audit how well the algorithm is performing,” Dr. Collins said. “The system should also regularly report to users the level of certainty with which it is providing an output rather than providing a simple binary output.”
Dr. Collins recommends that providers develop an understanding of the limits of AI regulations as well, which might including learning how the system was approved and how it is monitored.
“The FDA has oversight over some applications of AI and health care for software as a medical device, but there’s currently no dedicated process to evaluate the systems for the presence of bias,” Dr. Collins said. “The gaps in regulation leave the door open for the use of AI in clinical care that contain significant biases.”
Dr. Haidet likened AI tools to the Global Positioning System: A good GPS system will let users see alternate routes, opt out of toll roads or highways, and will highlight why routes have changed. But users need to understand how to read the map so they can tell when something seems amiss.
Dr. Collins and Dr. Haidet report no relevant financial relationships
A version of this article first appeared on Medscape.com.
AURORA, COLO. – Artificial Intelligence has arrived at medical offices, whether or not clinicians feel ready for it.
AI might result in more accurate, efficient, and cost-effective care. But it’s possible it could cause harm. That’s according to Benjamin Collins, MD, at Vanderbilt University Medical Center, Nashville, Tenn., who spoke on the subject at the annual meeting of the Society of General Internal Medicine.
Understanding the nuances of AI is even more important because of the quick development of the algorithms.
“When I submitted this workshop, there was no ChatGPT,” said Dr. Collins, referring to Chat Generative Pre-trained Transformer, a recently released natural language processing model. “A lot has already changed.”
Biased data
Biased data are perhaps the biggest pitfall of AI algorithms, Dr. Collins said. If garbage data go in, garbage predictions come out.
If the dataset that trains the algorithm underrepresents a particular gender or ethnic group, for example, the algorithm may not respond accurately to prompts. When an AI tool compounds existing inequalities related to socioeconomic status, ethnicity, or sexual orientation, the algorithm is biased, according to Harvard researchers.
“People often assume that artificial intelligence is free of bias due to the use of scientific processes and its development,” he said. “But whatever flaws exist in data collection and old data can lead to poor representation or underrepresentation in the data used to train the AI tool.”
Racial minorities are underrepresented in studies; therefore, data input into an AI tool might skew results for these patients.
The Framingham Heart Study, for example, which began in 1948, examined heart disease in mainly White participants. The findings from the study resulted in the creation of a sex-specific algorithm that was used to estimate the 10-year cardiovascular risk of a patient. While the cardiovascular risk score was accurate for White persons, it was less accurate for Black patients.
A study published in Science in 2019 revealed bias in an algorithm that used health care costs as a proxy for health needs. Because less money was spent on Black patients who had the same level of need as their White counterparts, the output inaccurately showed that Black patients were healthier and thus did not require extra care.
Developers can also be a source of bias, inasmuch as AI often reflects preexisting human biases, Dr. Collins said.
“Algorithmic bias presents a clear risk of harm that clinicians must play against the benefits of using AI,” Dr. Collins said. “That risk of harm is often disproportionately distributed to marginalized populations.”
As clinicians use AI algorithms to diagnose and detect disease, predict outcomes, and guide treatment, trouble comes when those algorithms perform well for some patients and poorly for others. This gap can exacerbate existing disparities in health care outcomes.
Dr. Collins advised clinicians to push to find out what data were used to train AI algorithms to determine how bias could have influenced the model and whether the developers risk-adjusted for bias. If the training data are not available, clinicians should ask their employers and AI developers to know more about the system.
Clinicians may face the so-called black box phenomenon, which occurs when developers cannot or will not explain what data went into an AI model, Dr. Collins said.
According to Stanford (Calif.) University, AI must be trained on large datasets of images that have been annotated by human experts. Those datasets can cost millions of dollars to create, meaning corporations often fund them and do not always share the data publicly.
Some groups, such as Stanford’s Center for Artificial Intelligence in Medicine and Imaging, are working to acquire annotated datasets so researchers who train AI models can know where the data came from.
Paul Haidet, MD, MPH, an internist at Penn State College of Medicine, Hershey, sees the technology as a tool that requires careful handling.
“It takes a while to learn how to use a stethoscope, and AI is like that,” Dr. Haidet said. “The thing about AI, though, is that it can be just dropped into a system and no one knows how it works.”
Dr. Haidet said he likes knowing how the sausage is made, something AI developers are often reticent to make known.
“If you’re just putting blind faith in a tool, that’s scary,” Dr. Haidet said.
Transparency and ‘explainability’
The ability to explain what goes into tools is essential to maintaining trust in the health care system, Dr. Collins said.
“Part of knowing how much trust to place in the system is the transparency of those systems and the ability to audit how well the algorithm is performing,” Dr. Collins said. “The system should also regularly report to users the level of certainty with which it is providing an output rather than providing a simple binary output.”
Dr. Collins recommends that providers develop an understanding of the limits of AI regulations as well, which might including learning how the system was approved and how it is monitored.
“The FDA has oversight over some applications of AI and health care for software as a medical device, but there’s currently no dedicated process to evaluate the systems for the presence of bias,” Dr. Collins said. “The gaps in regulation leave the door open for the use of AI in clinical care that contain significant biases.”
Dr. Haidet likened AI tools to the Global Positioning System: A good GPS system will let users see alternate routes, opt out of toll roads or highways, and will highlight why routes have changed. But users need to understand how to read the map so they can tell when something seems amiss.
Dr. Collins and Dr. Haidet report no relevant financial relationships
A version of this article first appeared on Medscape.com.
AURORA, COLO. – Artificial Intelligence has arrived at medical offices, whether or not clinicians feel ready for it.
AI might result in more accurate, efficient, and cost-effective care. But it’s possible it could cause harm. That’s according to Benjamin Collins, MD, at Vanderbilt University Medical Center, Nashville, Tenn., who spoke on the subject at the annual meeting of the Society of General Internal Medicine.
Understanding the nuances of AI is even more important because of the quick development of the algorithms.
“When I submitted this workshop, there was no ChatGPT,” said Dr. Collins, referring to Chat Generative Pre-trained Transformer, a recently released natural language processing model. “A lot has already changed.”
Biased data
Biased data are perhaps the biggest pitfall of AI algorithms, Dr. Collins said. If garbage data go in, garbage predictions come out.
If the dataset that trains the algorithm underrepresents a particular gender or ethnic group, for example, the algorithm may not respond accurately to prompts. When an AI tool compounds existing inequalities related to socioeconomic status, ethnicity, or sexual orientation, the algorithm is biased, according to Harvard researchers.
“People often assume that artificial intelligence is free of bias due to the use of scientific processes and its development,” he said. “But whatever flaws exist in data collection and old data can lead to poor representation or underrepresentation in the data used to train the AI tool.”
Racial minorities are underrepresented in studies; therefore, data input into an AI tool might skew results for these patients.
The Framingham Heart Study, for example, which began in 1948, examined heart disease in mainly White participants. The findings from the study resulted in the creation of a sex-specific algorithm that was used to estimate the 10-year cardiovascular risk of a patient. While the cardiovascular risk score was accurate for White persons, it was less accurate for Black patients.
A study published in Science in 2019 revealed bias in an algorithm that used health care costs as a proxy for health needs. Because less money was spent on Black patients who had the same level of need as their White counterparts, the output inaccurately showed that Black patients were healthier and thus did not require extra care.
Developers can also be a source of bias, inasmuch as AI often reflects preexisting human biases, Dr. Collins said.
“Algorithmic bias presents a clear risk of harm that clinicians must play against the benefits of using AI,” Dr. Collins said. “That risk of harm is often disproportionately distributed to marginalized populations.”
As clinicians use AI algorithms to diagnose and detect disease, predict outcomes, and guide treatment, trouble comes when those algorithms perform well for some patients and poorly for others. This gap can exacerbate existing disparities in health care outcomes.
Dr. Collins advised clinicians to push to find out what data were used to train AI algorithms to determine how bias could have influenced the model and whether the developers risk-adjusted for bias. If the training data are not available, clinicians should ask their employers and AI developers to know more about the system.
Clinicians may face the so-called black box phenomenon, which occurs when developers cannot or will not explain what data went into an AI model, Dr. Collins said.
According to Stanford (Calif.) University, AI must be trained on large datasets of images that have been annotated by human experts. Those datasets can cost millions of dollars to create, meaning corporations often fund them and do not always share the data publicly.
Some groups, such as Stanford’s Center for Artificial Intelligence in Medicine and Imaging, are working to acquire annotated datasets so researchers who train AI models can know where the data came from.
Paul Haidet, MD, MPH, an internist at Penn State College of Medicine, Hershey, sees the technology as a tool that requires careful handling.
“It takes a while to learn how to use a stethoscope, and AI is like that,” Dr. Haidet said. “The thing about AI, though, is that it can be just dropped into a system and no one knows how it works.”
Dr. Haidet said he likes knowing how the sausage is made, something AI developers are often reticent to make known.
“If you’re just putting blind faith in a tool, that’s scary,” Dr. Haidet said.
Transparency and ‘explainability’
The ability to explain what goes into tools is essential to maintaining trust in the health care system, Dr. Collins said.
“Part of knowing how much trust to place in the system is the transparency of those systems and the ability to audit how well the algorithm is performing,” Dr. Collins said. “The system should also regularly report to users the level of certainty with which it is providing an output rather than providing a simple binary output.”
Dr. Collins recommends that providers develop an understanding of the limits of AI regulations as well, which might including learning how the system was approved and how it is monitored.
“The FDA has oversight over some applications of AI and health care for software as a medical device, but there’s currently no dedicated process to evaluate the systems for the presence of bias,” Dr. Collins said. “The gaps in regulation leave the door open for the use of AI in clinical care that contain significant biases.”
Dr. Haidet likened AI tools to the Global Positioning System: A good GPS system will let users see alternate routes, opt out of toll roads or highways, and will highlight why routes have changed. But users need to understand how to read the map so they can tell when something seems amiss.
Dr. Collins and Dr. Haidet report no relevant financial relationships
A version of this article first appeared on Medscape.com.
AT SGIM 2023