User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Men Wanted: New Efforts to Attract Male Nurses
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Only 12% of the nurses providing patient care at hospitals and health clinics today are men. Although the percentage of nurses has increased — men made up just 2.7% of nurses in 1970 — nursing is still considered a “pink collar” profession, a female-dominated field.
“We’ve made strides over the last couple of decades, but [the number of men pursuing nursing careers] is leveling out,” said Jason Dunne, DNP, MSN, RN, chief academic officer at the Arizona College of Nursing, Phoenix. “There continues to be persistent gender stereotypes that [have] discouraged men from entering the profession.”
“The nursing shortage is very real,” Dunne said. “We need to be highly focused on the shortage and look at opportunities to bring diversity into the profession, and one big way to solve it is bringing more men into nursing.”
Representation Matters
Colleges recognize the need to diversify their nursing student population and have turned their attention to increasing the number of men attending informational sessions and career days. Dunne believes, “There is a general lack of awareness of nursing as a career choice [for men].”
The Nursing Consortium of Florida hosts a “Day in the Life of a Nurse” program to introduce high school students to nursing careers, and the University of Virginia School of Nursing invites male nursing students to speak at educational events to promote workforce diversity.
“When I was growing up, the males wouldn’t have been included in those sessions,” said Melissa Gilbert Gomes, PhD, APRN, PMHNP-BC, FNAP, FAAN, associate dean for diversity, equity, and inclusion at the University of Virginia School of Nursing, Charlottesville, Virginia. “It was nice to see their interest and to have a male student there for them to ask questions and to help them see that this could be a place for them.”
Nursing schools have also engaged in other efforts to encourage more men to consider nursing careers, from highlighting male nurses in marketing materials and engaging with men at career fairs to updating course curriculum to include content on men’s health and connecting male nursing students with men in nursing faculty or clinical settings.
Focusing on nursing as a lucrative career choice could also attract more men to the profession. On average, male registered nurses (RNs) make $7300 per year more than their female counterparts due to the gender pay gap. The median wage for male RNs in acute care, cardiology, and perioperative specialties is $90,000 annually.
At the University of Virginia School of Nursing, which the American Association for Men in Nursing (AAMN) named “Best School for Men in Nursing” in 2023, 20% of nursing students are men.
The school has a Men Advancing Nursing club and is in the process of chartering a new AAMN chapter. The goal, according to Gomes, is to create an environment where male nursing students feel represented and supported.
“Valuing the perspective that men bring [to nursing] is important,” she said. “Coming together [and] having that camaraderie and intrinsic motivation to specifically speak to areas that impact men ... is important.”
Promoting Patient Care
Highlighting the diversity of career options within the nursing profession is also essential. RNs can pursue careers in specialties ranging from pediatrics, orthopedics, and occupational health to anesthesia, cardiology, and nephrology. The specialty with the highest number of male RNs tends to be acute care, which encompasses emergency/trauma and medical-surgical.
John Schmidt, DNP, MSN, BSN, faculty member and program lead for the acute care nurse practitioner program at Purdue Global School of Nursing, refers to these specialties as having a high excitement factor.
“Men gravitate to nursing to help people,” he said. “In critical care, there is instant gratification. You see patients get better. It’s the same in the [intensive care unit] and the emergency department. We take care of them and can see how we made a difference.”
When hospitals and health systems create environments that support men in nursing, patients also benefit. Research shows that patients often prefer nurses of the same gender, and a more diverse healthcare workforce has been linked to improved patient outcomes. Reducing gender inequities among nursing staff could also improve job satisfaction and retention rates for men in nursing.
“When you’re in a vulnerable space as a patient ... it’s important to know that your care provider understands you [and] having men as nurses is a part of that,” said Gomes. “Even though patients might not be used to having a male nurse at the bedside, once they have the experience, it challenges preconceived notions [and] that connection is important.”
Hospitals must proactively support men in nursing to achieve the benefits of greater gender diversity in the nursing workforce. Male nurses have fewer role models and report higher levels of loneliness, isolation, and role strain.
Groups such as NYC Men in Nursing and mentorship programs such as Men in Nursing at RUSH University College of Nursing and RUSH University Medical Center, and the North Carolina Healthcare Association Diverse Healthcare Leaders Mentorship Program were designed to provide coaching, education, and networking opportunities and connect men in nursing.
Male nurses, Dunne added, must be role models and must take the lead in changing the conversations about gender roles in nursing. Establishing support systems and mentorship opportunities is instrumental in inspiring men to pursue nursing careers and creating visibility into the profession and “would create a level of parity for men in the profession and encourage them to want to stay in nursing as a long-term career.”
He told this news organization that creating scholarships for men enrolled in nursing school, increasing the involvement of male nurse leaders in recruitment efforts, and updating curriculum to ensure men are reflected in the materials is also essential.
“We’ve got to be willing and open to having the conversations to end the stereotypes that have plagued the profession,” said Dunne. “And we’ve got to push men in nursing to be front and center so folks see that there are opportunities for men in nursing.”
A version of this article appeared on Medscape.com.
Lawmakers Rush to Stave Off Doctor Pay Cuts as Medicare Finalizes 2025 Rates
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Federal lawmakers are rushing to soften the blow of Medicare’s 2025 effective pay cut for doctors in 2025, introducing a bill that could limit the cut. But they have little time to act.
In 2025, the conversion factor used to calculate payment to doctors and hospitals caring for Medicare patients will drop to $32.35, a nearly 3% decrease from the current level.
Congress likely will act before the cuts take effect, said Rep. Larry Bucshon, MD (R-IN), who specialized in cardiothoracic surgery before joining Congress. Lawmakers in past years have typically tinkered with the Medicare physician fee schedule at the last minute, tucking in fixes to December legislative packages and spending bills.
“I’m pretty optimistic that a good portion of the fee cuts will be mitigated and they won’t go through,” Bucshon told this news organization in an interview.
Bruce A. Scott, MD, president of the American Medical Association (AMA) said in a statement that CMS’ release of the final fee schedule on November 1 should trigger serious work on a change to the 2025 Medicare physician fee schedule.
“The fee schedule rule released [on November 1] starts the clock — with January 1 looming,” Scott said. “A legislative remedy will require hard work and compromise. The 66 million patients who rely on Medicare are counting on that.”
Both Bucshon and Scott also joined many lawmakers and medical associations in calling on Congress for a larger overhaul of the Medicare physician fee schedule, well beyond whatever temporary adjustment may be made in the months ahead to avoid or soften the 2025 cuts.
The physician fee schedule sets formulas and rules regarding how the largest US buyer of health services pays the almost 1.3 million clinicians who bill Medicare. Of these, 51% are physicians. The physician fee schedule also covers payments for nurse practitioners, physician assistants, physical therapists, and other health professionals.
Last Major Overhaul Unpopular
There’s broad dissatisfaction with Congress’ last major overhaul of the Medicare physician fee schedule. The 2015 Medicare Access and CHIP Reauthorization Act (MACRA) aimed to shift clinicians toward programs tying pay increases to quality measures. But the implementation of that aim through the Merit-based Incentive Payment System is widely considered a disappointment.
MACRA was intended to end the need for annual “doc fixes,” as Congress’ last-minute Medicare adjustments are known. Seventeen such tweaks passed before MACRA took effect.
But MACRA did not include a broad-based inflation adjuster, and some clinicians’ incomes are lagging as inflation rates — and practice costs — have risen. Scott said the Medicare Economic Index, which is a measure used to gauge increases in practice costs for clinicians, is expected to rise by 3.5%.
“To put it bluntly, Medicare plans to pay us less while costs go up. You don’t have to be an economist to know that is an unsustainable trend, though one that has been going on for decades,” Scott said. “For physician practices operating on small margins already, this means it is harder to acquire new equipment, harder to retain staff, harder to take on new Medicare patients, and harder to keep the doors open, particularly in rural and underserved areas.”
In a statement, Jen Brull, MD, president of the American Academy of Family Physicians, noted that this likely will be the fifth year in a row that Congress will need to do a patch to prevent cuts in pay to clinicians.
Bucshon, who will retire from the House in January, said he expects Congress to pass legislation tying Medicare payment rates to inflation — eventually.
“People want to find a way to fix this problem, but also do it in a way that does not cut benefits to anyone, and that’s the key,” Bucshon said. “We’re going to have to find a way to make sure that providers are properly reimbursed.”
A version of this article first appeared on Medscape.com.
Lichenoid Drug Eruption Secondary to Apalutamide Treatment
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
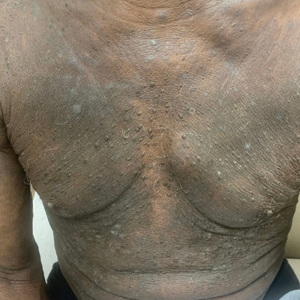
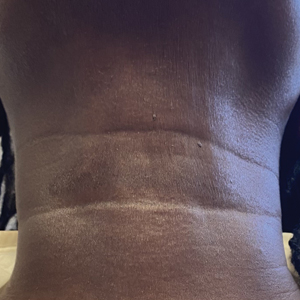
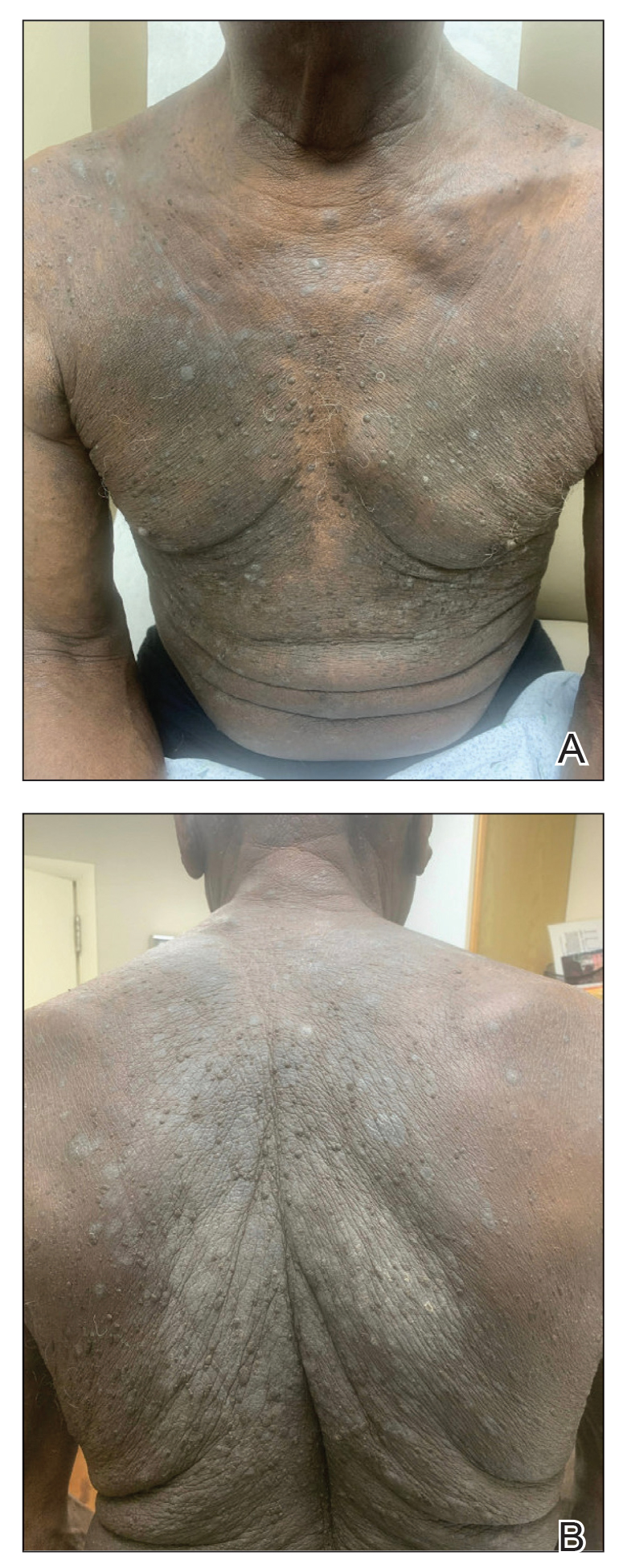
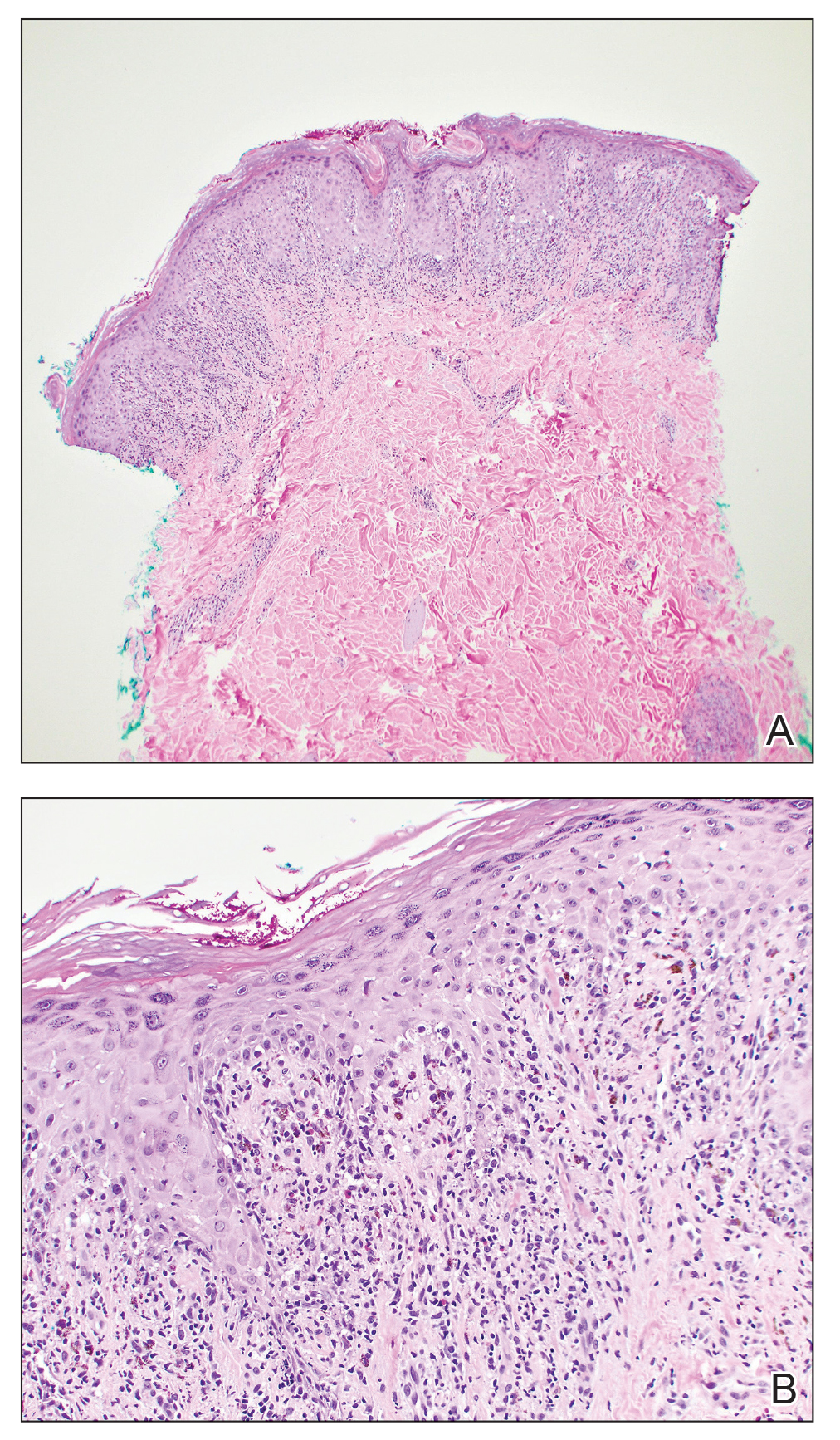
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.
Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.


Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
To the Editor:
Lichenoid drug eruptions are lichen planus–like hypersensitivity reactions induced by medications. These reactions are rare but cause irritation to the skin, as extreme pruritus is common. One review of 300 consecutive cases of drug eruptions submitted to dermatopathology revealed that 12% of cases were classified as lichenoid drug reactions.1 Lichenoid dermatitis is characterized by extremely pruritic, scaly, eczematous or psoriasiform papules, often along the extensor surfaces and trunk.2 The pruritic nature of the rash can negatively impact quality of life. Treatment typically involves discontinuation of the offending medication, although complete resolution can take months, even after the drug is stopped. Although there have been some data suggesting that topical and/or oral corticosteroids can help with resolution, the rash can persist even with steroid treatment.2
The histopathologic findings of lichenoid drug eruptions show lichen planus–like changes such as hyperkeratosis, irregular acanthosis, and lichenoid interface dermatitis. Accordingly, idiopathic lichen planus is an important differential diagnosis for lichenoid drug eruptions; however, compared to idiopathic lichen planus, lichenoid drug eruptions are more likely to be associated with eosinophils and parakeratosis.1,3 In some cases, the histopathologic distinction between the 2 conditions is impossible, and clinical history needs to be considered to make a diagnosis.1 Drugs known to cause lichenoid drug reactions more commonly include angiotensin-converting enzyme inhibitors, beta blockers, thiazides, gold, penicillamine, and antimalarials.2 Lichenoid drug eruptions also have been documented in patients taking the second-generation nonsteroidal androgen receptor antagonist enzalutamide, which is used for the treatment of prostate cancer.4 More recently, the newer second-generation nonsteroidal androgen receptor antagonist apalutamide has been implicated in several cases of lichenoid drug eruptions.5,6
We present a case of an apalutamide-induced lichenoid drug eruption that was resistant to dose reduction and required discontinuation of treatment due to the negative impact on the patient’s quality of life. Once the rash resolved, the patient transitioned to enzalutamide without any adverse events (AEs).
A 72-year-old man with a history of metastatic prostate cancer (stage IVB) presented to the dermatology clinic with a 4-month history of a dry itchy rash on the face, chest, back, and legs that had developed 2 to 3 months after oncology started him on apalutamide. The patient initially received apalutamide 240 mg/d, which was reduced by his oncologist 3 months later to 180 mg/d following the appearance of the rash. Then apalutamide was held as he awaited improvement of the rash.
One week after the apalutamide was held, the patient presented to dermatology. He reported that he had tried over-the-counter ammonium lactate 12% lotion twice daily when the rash first developed without improvement. When the apalutamide was held, oncology prescribed mupirocin ointment 2% 3 times daily which yielded minimal relief. On physical examination, widespread lichenified papules and plaques were noted on the face, chest, back, and legs (Figure 1). Dermatology initially prescribed triamcinolone ointment 0.1% twice daily. A 4-mm punch biopsy specimen of the upper back revealed a lichenoid interface dermatitis with numerous eosinophils compatible with a lichenoid hypersensitivity reaction (Figure 2). Considering the clinical and histologic findings, a diagnosis of lichenoid drug eruption secondary to apalutamide treatment was made.


Two weeks after discontinuation of the medication, the rash improved, and the patient restarted apalutamide at a dosage of 120 mg/d; however, the rash re-emerged within 1 month and was resistant to the triamcinolone ointment 0.1%. Apalutamide was again discontinued, and oncology switched the patient to enzalutamide 160 mg/d in an effort to find a medication the patient could better tolerate. Two months after starting enzalutamide, the patient had resolution of the rash and no further dermatologic complications.
Apalutamide is a second-generation nonsteroidal androgen receptor antagonist used in the treatment of nonmetastatic castration-resistant prostate cancer (CRPC) and metastatic castration-sensitive prostate cancer (CSPC).7 It stops the spread and growth of prostate cancer cells by several different mechanisms, including competitively binding androgen receptors, preventing 5α-dihydrotestosterone from binding to androgen receptors, blocking androgen receptor nuclear translocation, impairing co-activator recruitment, and restraining androgen receptor DNA binding.7 The SPARTAN and TITAN phase 3 clinical trials demonstrated increased overall survival and time to progression with apalutamide in both nonmetastatic CRPC and metastatic CSPC. In both trials, the rash was shown to be an AE more commonly associated with apalutamide than placebo.8,9
Until recently, the characteristics of apalutamide-induced drug rashes have not been well described. One literature review reported 6 cases of cutaneous apalutamide-induced drug eruptions.5 Four (66.7%) of these eruptions were maculopapular rashes, only 2 of which were histologically classified as lichenoid in nature. The other 2 eruptions were classified as toxic epidermal necrosis.5 Another study of 303 patients with prostate cancer who were treated with apalutamide recorded the frequency and time to onset of dermatologic AEs.6 Seventy-one (23.4%) of the patients had dermatologic AEs, and of those, only 20 (28.2%) had AEs that resulted in interruptions in apalutamide therapy (with only 5 [25.0%] requiring medication discontinuation). Thirty-two (45.1%) patients were managed with topical or oral corticosteroids or dose modification. In this study, histopathology was examined in 8 cases (one of which had 2 biopsies for a total of 9 biopsies), 7 of which were consistent with lichenoid interface dermatitis.6
Lichenoid interface dermatitis is a rare manifestation of an apalutamide-induced drug eruption and also has been reported secondary to treatment with enzalutamide, another second-generation nonsteroidal androgen receptor antagonist.4 Enzalutamide was the first second-generation nonsteroidal androgen receptor antagonist approved for the treatment of prostate cancer. It originally was approved only for metastatic CRPC after docetaxel therapy in 2012, then later was expanded to metastatic and nonmetastatic CRPC in 2012 and 2018, respectively, as well as metastatic CSPC in 2019.7 Because enzalutamide is from the same medication class as apalutamide and has been on the market longer for the treatment of nonmetastatic CRPC and metastatic CSPC, it is not surprising that similar drug eruptions now are being reported secondary to apalutamide use as well.
It is important for providers to consider lichenoid drug eruptions in the differential diagnosis of pruritic rashes in patients taking second-generation nonsteroidal androgen receptor antagonists such as apalutamide or enzalutamide. Although dose reduction or treatment discontinuation have been the standard of care for patients with extremely pruritic lichenoid drug eruptions secondary to these medications, these are not ideal because they are important for cancer treatment. Interestingly, after our patient’s apalutamide-induced rash resolved and he was switched to enzalutamide, he did not develop any AEs. Based on our patient’s experience, physicians could consider switching their patients to another drug of the same class, as they may be able tolerate that medication. More research is needed to determine how commonly patients tolerate a different second-generation nonsteroidal androgen receptor antagonist after not tolerating another medication from the same class.
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
- Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1:33-47. doi:10.5826/dpc.0101a09
- Cheraghlou S, Levy LL. Fixed drug eruption, bullous drug eruptions, and lichenoid drug eruptions. Clin Dermatol. 2020;38:679-692. doi:10.1016/j.clindermatol.2020.06.010
- Thompson DF, Skaehill PA. Drug-induced lichen planus. Pharmacotherapy. 1994;14:561-571.
- Khan S, Saizan AL, O’Brien K, et al. Diffuse hyperpigmented lichenoid drug eruption secondary to enzalutamide. Curr Probl Cancer Case Rep. 2022;5:100135. doi:10.1016/j.cpccr.2021.100135
- Katayama H, Saeki H, Osada S-I. Maculopapular drug eruption caused by apalutamide: case report and review of the literature. J Nippon Med Sch. 2022;89:550-554. doi:10.1272/jnms.JNMS.2022_89-503
- Pan A, Reingold RE, Zhao JL, et al. Dermatologic adverse events in prostate cancer patients treated with the androgen receptor inhibitor apalutamide. J Urol. 2022;207:1010-1019. doi:10.1097/JU.0000000000002425
- Rajaram P, Rivera A, Muthima K, et al. Second-generation androgen receptor antagonists as hormonal therapeutics for three forms of prostate cancer. Molecules. 2020;25:2448. doi:10.3390/molecules25102448
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N Engl J Med. 2018;378:1408-1418. doi:10.1056/NEJMoa1715546
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensative prostate cancer. N Engl J Med. 2019;381:13-24. doi:10.1056/NEJMoa1903307
Practice Points
- Although it is rare, patients can develop lichenoid drug eruptions secondary to treatment with second-generation nonsteroidal androgen receptor antagonists such as apalutamide.
- If a patient develops a lichenoid drug eruption while taking a specific second-generation nonsteroidal androgen receptor antagonist, the entire class of medications should not be ruled out, as some patients can tolerate other drugs from that class.
Botulinum Toxin Injection for Treatment of Scleroderma-Related Anterior Neck Sclerosis
To the Editor:
Scleroderma is a chronic autoimmune connective tissue disease that results in excessive collagen deposition in the skin and other organs throughout the body. On its own or in the setting of mixed connective tissue disease, scleroderma can result in systemic or localized symptoms that can limit patients’ functional capabilities, cause pain and discomfort, and reduce self-esteem—all negatively impacting patients’ quality of life.1,2 Neck sclerosis is a common manifestation of scleroderma. There is no curative treatment for scleroderma; thus, therapy is focused on slowing disease progression and improving quality of life. We present a case of neck sclerosis in a 44-year-old woman with scleroderma that was successfully treated with botulinum toxin (BTX) type A injection, resulting in improved skin laxity and appearance with high patient satisfaction. Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region.
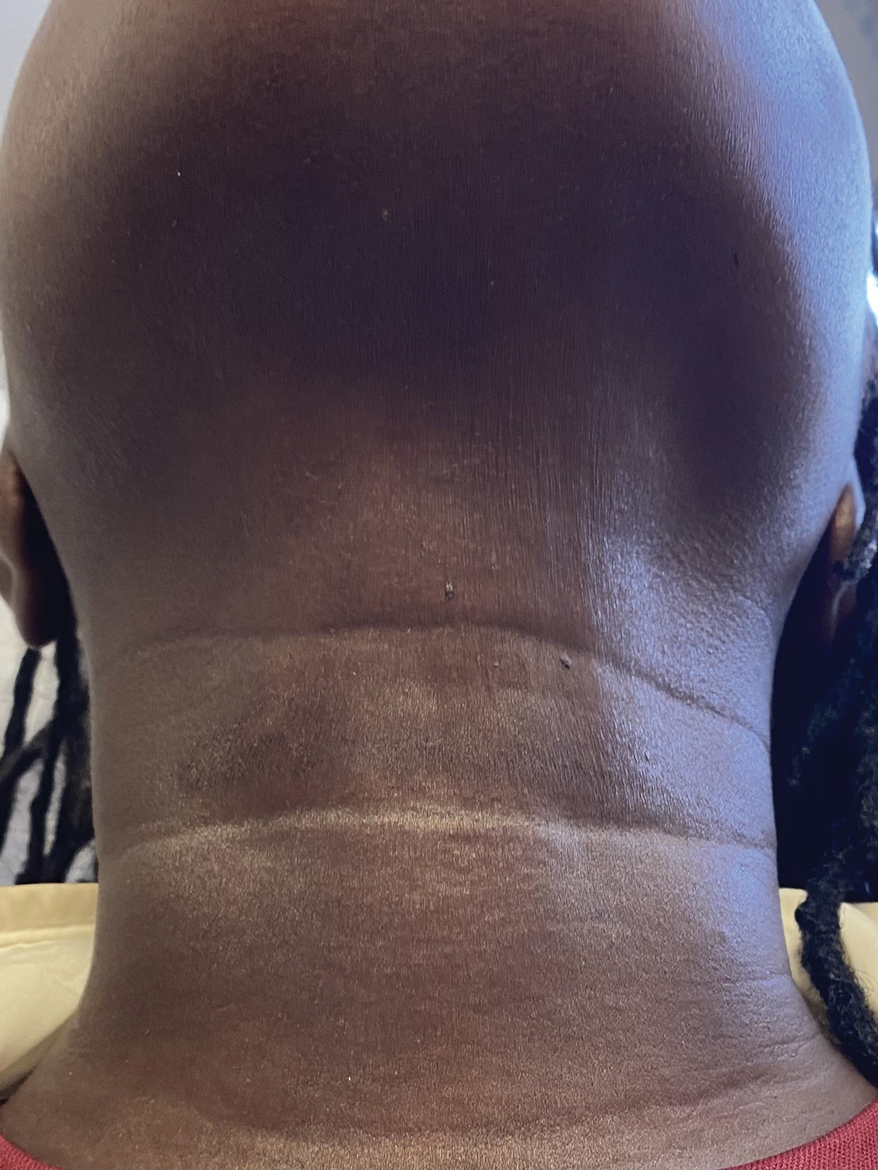
A 44-year-old woman presented to the dermatology clinic for treatment of thickened neck skin with stiffness and tightness that had been present for months to years. She had a history of mixed connective tissue disease (MCTD)(positive anti-ribonucleoprotein, anti–Sjögren syndrome–related antigen, and anti-Smith antibodies) with features of scleroderma and polyarthritis. The patient currently was taking sulfasalazine for the polyarthritis; she previously had taken hydroxychloroquine but discontinued treatment due to ineffectiveness. She was not taking any topical or systemic medications for scleroderma. On physical examination, the skin on the anterior neck appeared thickened with shiny patches (Figure 1). Pinching the skin in the affected area demonstrated sclerosis with high tension.
The dermatologist (J.J.) discussed potential treatment options to help relax the tension in the skin of the anterior neck, including BTX injections. After receiving counsel on adverse effects, alternative treatments, and postprocedural care, the patient decided to proceed with the procedure. The anterior neck was cleansed with an alcohol swab and 37 units (range, 25–50 units) of incobotulinumtoxinA (reconstituted using 2.5-mL bacteriostatic normal saline per 100 units) was injected transdermally using a 9-point injection technique, with each injection placed approximately 1 cm apart. The approximate treatment area included the space between the sternocleidomastoid anterior edges and below the hyoid bone up to the cricothyroid membrane (anatomic zone II).
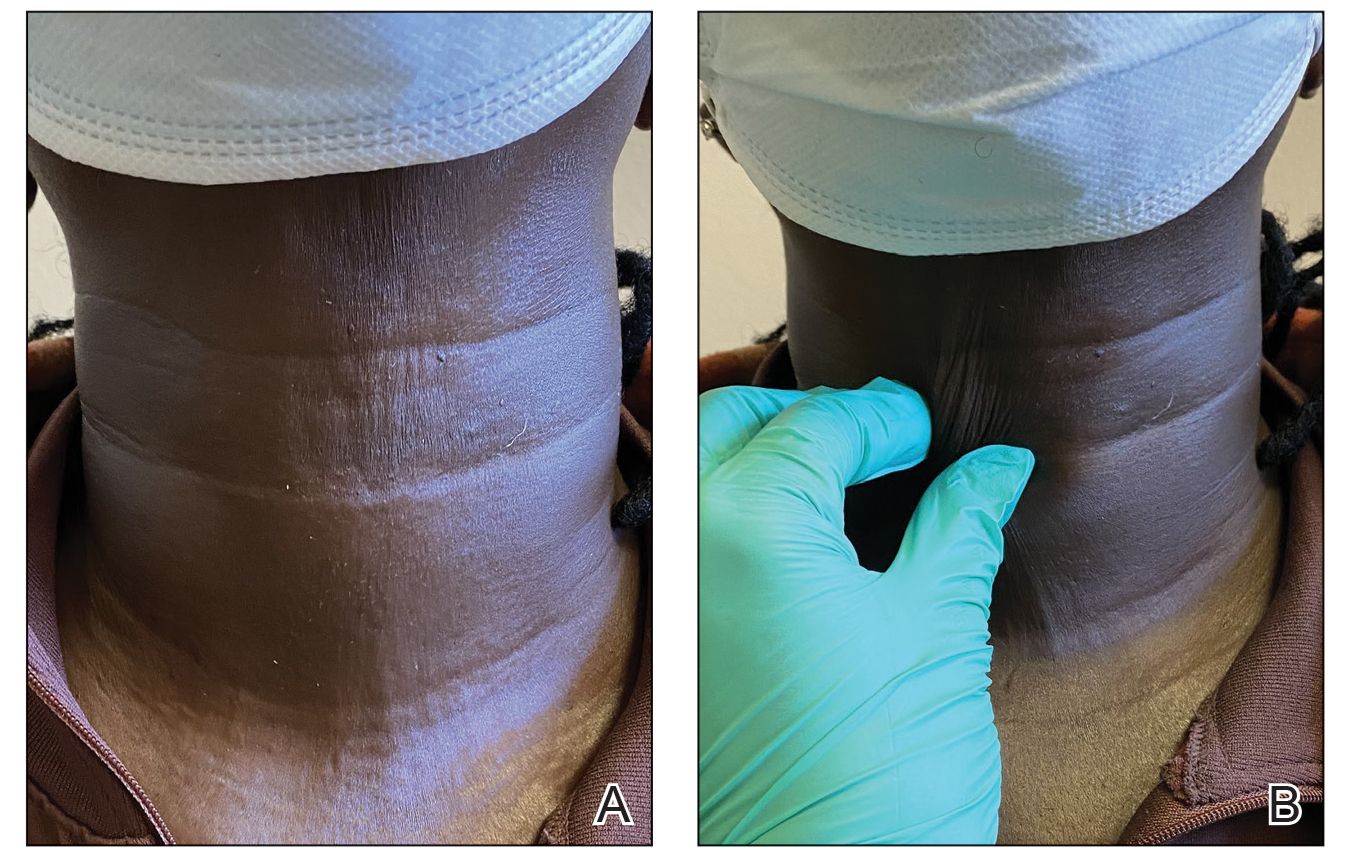
When the patient returned for follow-up 3 weeks later, she reported considerable improvement in the stiffness and appearance of the skin on the anterior neck. On physical examination, the skin of the neck appeared softened, and improved laxity was seen on pinching the skin compared to the initial presentation (Figure 2). The patient expressed satisfaction with the results and denied any adverse events following the procedure.
Mixed connective tissue disease manifests with a combination of features from various disorders—mainly lupus, scleroderma, polymyositis, and rheumatoid arthritis. It is most prevalent in females and often is diagnosed in the third decade of life.3 It is associated with positive antinuclear antibodies and human leukocyte antigen (HLA) II alleles (HLA-DR4, HLA-DR1, and HLA-DR2). Raynaud phenomenon (RP), one of the most common skin manifestations in both scleroderma and MCTD, is present in 75% to 90% of patients with MCTD.3
Scleroderma is a chronic connective tissue disorder that results in excessive collagen deposition in the skin and other organs throughout the body.4 Although the etiology is unknown, scleroderma develops when overactivation of the immune system leads to CD4+ T-lymphocyte infiltration in the skin, along with the release of profibrotic interleukins and growth factors, resulting in fibrosis.4 Subtypes include localized scleroderma (morphea), limited cutaneous systemic sclerosis (formerly known as CREST [calcinosis, RP, esophageal dysmotility, sclerodactyly, and telangiectasia] syndrome), diffuse cutaneous systemic sclerosis, and systemic sclerosis sine scleroderma.5 Scleroderma is associated with positive antinuclear antibodies and HLA II alleles (HLA-DR2 and HLA-DR5).
On its own or in the setting of MCTD, scleroderma can result in systemic or localized symptoms. Overall, the most common symptom is RP.5 Localized scleroderma and limited cutaneous systemic sclerosis manifest with symptoms of the skin and underlying tissues. Diffuse cutaneous systemic sclerosis involves cutaneous and visceral symptoms, including lung, esophageal, and vascular involvement.6 Similar to MCTD, scleroderma is most prevalent in middle-aged females,7 though it occurs at a higher rate and with a more severe disease course in Black patients.8
A highly sensitive and specific test for scleroderma that can aid in diagnosis is the neck sign—tightening of the skin of the neck when the head extends.9,10 In one study, the neck sign was positive in more than 90% of patients with scleroderma and negative for control patients and those with primary RP.9 Thus, neck sclerosis is a common manifestation of scleroderma for which patients may seek treatment.
While there is no curative treatment for scleroderma, skin manifestations can be treated with mycophenolate mofetil or methotrexate.5 Systemic treatments may be recommended if the patient has additional symptoms, such as azathioprine for myositis/arthritis and cyclophosphamide for interstitial lung disease.5 However, it is important to note that these medications are associated with risk for gastrointestinal upset, mouth sores, fatigue, or other complications.
Botulinum toxin is a bacterial protein toxin and neuromodulator that inhibits neurotransmitter release by cleaving SNARE proteins at peripheral nerve terminal junctions.11 It has been used in a variety of dermatologic and nondermatologic conditions, including migraines, hyperhidrosis, contractures, scars, and overactive bladder. It also has been used in aesthetics for facial rejuvenation and minimization of wrinkle appearance. Dermatologists and rheumatologists have successfully used BTX to treat primary and secondary RP—the most common symptom of scleroderma—due to its vasodilatation properties.12 Although our patient did not have RP, use of BTX to treat other features of scleroderma, including en coup de sabre, thoracic outlet syndrome, dyspareunia, gastroparesis, pterygium inversum unguis, and dysphagia has been documented.13-18 An in vivo mouse study that examined the possible mechanism for BTX as a treatment in scleroderma found that BTX injections significantly decreased dermal thickness and inflammation in fibrosis (P<.05). An analysis of oxidative stress and mRNA expression showed that BTX may treat fibrosis by suppressing oxidative stress and inflammatory cells, resulting in decreased apoptosis and oxidant-induced intracellular accumulation of reactive oxygen species.19 Another animal study demonstrated the positive effects of BTX treatment for fibrosis of the bladder in rats.20 In one case report, a female patient with scleroderma and facial fibrosis received perioral BTX injections for cosmetic purposes but also observed improvement in mouth constriction, demonstrating the potential efficacy of BTX for facial fibrosis.21
Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region. We recommend assessing the efficacy of the initial BTX treatment after 2 to 3 weeks, with additional injections as needed to achieve the patient’s desired level of comfort and appearance at approximately 3-month intervals (aligning with the expected duration of efficacy of BTX).22 Our patient experienced considerable relief and high satisfaction with BTX treatment. Given the limitations of sclerosis treatments and the unwanted adverse-effect profile of systemic treatments, BTX injections may be a preferrable treatment option for cutaneous manifestations of scleroderma among patients. Future studies with larger patient populations and a control group are warranted to further explore the use of BTX for the dermatologic treatment of scleroderma.
- Lis-S´wie¸ty A, Skrzypek-Salamon A, Ranosz-Janicka I, et al. Health-related quality of life and its influencing factors in adult patients with localized scleroderma—a cross-sectional study. Health Qual Life Outcomes. 2020;18:133. doi:10.1186/s12955-020-01386-0
- Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev. 2015;14:1087-1096. doi:10.1016/j.autrev.2015.07.012
- Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol. 2012;26:61-72. doi:10.1016/j.berh.2012.01.009
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinico-pathological correlation. G Ital Dermatol Venereol. 2018;153:208-215. doi:10.23736/S0392-0488.18.05922-9
- Fett N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437. doi:10.1016/j.clindermatol.2013.01.010
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Calderon LM, Pope JE. Scleroderma epidemiology update. Curr Opin Rheumatol. 2021;33:122-127. doi:10.1097/BOR.0000000000000785
- Morgan ND, Gelber AC. African Americans and scleroderma: examining the root cause of the association. Arthritis Care Res (Hoboken). 2019;71:1151-1153. doi:10.1002/acr.23860
- Barnett AJ. The “neck sign” in scleroderma. Arthritis Rheum. 1989;32:209-211. doi:10.1002/anr.1780320215
- Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis). Postgrad Med J. 1988;64:121-125. doi:10.1136/pgmj.64.748.121
- Rossetto O, Pirazzini M, Fabris F, et al. Botulinum neurotoxins: mechanism of action. Handb Exp Pharmacol. 2021;263:35-47.doi:10.1007/164_2020_355
- Ennis D, Ahmad Z, Anderson MA, et al. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2021;35:101684. doi:10.1016/j.berh.2021.101684
- Turkmani MG, Alnomair N. Enhancement of the aesthetic outcome of scleroderma en coup de sabre with botulinum toxin injection. JAAD Case Rep. 2018;4:579-581. doi:10.1016/j.jdcr.2018.03.023
- Le EN, Freischlag JA, Christo PJ, et al. Thoracic outlet syndrome secondary to localized scleroderma treated with botulinum toxin injection. Arthritis Care Res (Hoboken). 2010;62:430-433. doi:10.1002/acr.20099
- Mousty E, Rathat G, Rouleau C, et al. Botulinum toxin type A for treatment of dyspareunia caused by localized scleroderma. Acta Obstet Gynecol Scand. 2011;90:926-927. doi:10.1111/j.1600-0412.2011.01183.x
- Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74-101. doi:10.1016/j.disamonth.2010.12.007
- Katschinski M. [Diagnosis and treatment of esophageal motility disorders]. Ther Umsch. 2001;58:128-133. doi:10.1024/0040-5930.58.3.128
- Kim DJ, Odell ID. Improvement of pterygium inversum unguis and Raynaud phenomenon with interdigital botulinum toxin injections. JAAD Case Rep. 2022;26:79-81. doi:10.1016/j.jdcr.2022.06.009
- Baral H, Sekiguchi A, Uchiyama A, et al. Inhibition of skin fibrosis in systemic sclerosis by botulinum toxin B via the suppression of oxidative stress. J Dermatol. 2021;48:1052-1061. doi:10.1111/1346-8138.15888
- Jia C, Xing T, Shang Z, et al. Botulinum toxin A improves neurogenic bladder fibrosis by suppressing transforming growth factor β1 expression in rats. Transl Androl Urol. 2021;10:2000-2007. doi:10.21037/tau-21-62
- Hoverson K, Love T, Lam TK, et al. A novel treatment for limited mouth opening due to facial fibrosis: a case series. J Am Acad Dermatol. 2018;78:190-192. doi:10.1016/j.jaad.2017.07.006
- Kollewe K, Mohammadi B, Köhler S, et al. Blepharospasm: long-term treatment with either Botox®, Xeomin® or Dysport®. J Neural Transm (Vienna). 2015;122:427-431. doi:10.1007/s00702-014-1278-z
To the Editor:
Scleroderma is a chronic autoimmune connective tissue disease that results in excessive collagen deposition in the skin and other organs throughout the body. On its own or in the setting of mixed connective tissue disease, scleroderma can result in systemic or localized symptoms that can limit patients’ functional capabilities, cause pain and discomfort, and reduce self-esteem—all negatively impacting patients’ quality of life.1,2 Neck sclerosis is a common manifestation of scleroderma. There is no curative treatment for scleroderma; thus, therapy is focused on slowing disease progression and improving quality of life. We present a case of neck sclerosis in a 44-year-old woman with scleroderma that was successfully treated with botulinum toxin (BTX) type A injection, resulting in improved skin laxity and appearance with high patient satisfaction. Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region.
A 44-year-old woman presented to the dermatology clinic for treatment of thickened neck skin with stiffness and tightness that had been present for months to years. She had a history of mixed connective tissue disease (MCTD)(positive anti-ribonucleoprotein, anti–Sjögren syndrome–related antigen, and anti-Smith antibodies) with features of scleroderma and polyarthritis. The patient currently was taking sulfasalazine for the polyarthritis; she previously had taken hydroxychloroquine but discontinued treatment due to ineffectiveness. She was not taking any topical or systemic medications for scleroderma. On physical examination, the skin on the anterior neck appeared thickened with shiny patches (Figure 1). Pinching the skin in the affected area demonstrated sclerosis with high tension.

The dermatologist (J.J.) discussed potential treatment options to help relax the tension in the skin of the anterior neck, including BTX injections. After receiving counsel on adverse effects, alternative treatments, and postprocedural care, the patient decided to proceed with the procedure. The anterior neck was cleansed with an alcohol swab and 37 units (range, 25–50 units) of incobotulinumtoxinA (reconstituted using 2.5-mL bacteriostatic normal saline per 100 units) was injected transdermally using a 9-point injection technique, with each injection placed approximately 1 cm apart. The approximate treatment area included the space between the sternocleidomastoid anterior edges and below the hyoid bone up to the cricothyroid membrane (anatomic zone II).
When the patient returned for follow-up 3 weeks later, she reported considerable improvement in the stiffness and appearance of the skin on the anterior neck. On physical examination, the skin of the neck appeared softened, and improved laxity was seen on pinching the skin compared to the initial presentation (Figure 2). The patient expressed satisfaction with the results and denied any adverse events following the procedure.

Mixed connective tissue disease manifests with a combination of features from various disorders—mainly lupus, scleroderma, polymyositis, and rheumatoid arthritis. It is most prevalent in females and often is diagnosed in the third decade of life.3 It is associated with positive antinuclear antibodies and human leukocyte antigen (HLA) II alleles (HLA-DR4, HLA-DR1, and HLA-DR2). Raynaud phenomenon (RP), one of the most common skin manifestations in both scleroderma and MCTD, is present in 75% to 90% of patients with MCTD.3
Scleroderma is a chronic connective tissue disorder that results in excessive collagen deposition in the skin and other organs throughout the body.4 Although the etiology is unknown, scleroderma develops when overactivation of the immune system leads to CD4+ T-lymphocyte infiltration in the skin, along with the release of profibrotic interleukins and growth factors, resulting in fibrosis.4 Subtypes include localized scleroderma (morphea), limited cutaneous systemic sclerosis (formerly known as CREST [calcinosis, RP, esophageal dysmotility, sclerodactyly, and telangiectasia] syndrome), diffuse cutaneous systemic sclerosis, and systemic sclerosis sine scleroderma.5 Scleroderma is associated with positive antinuclear antibodies and HLA II alleles (HLA-DR2 and HLA-DR5).
On its own or in the setting of MCTD, scleroderma can result in systemic or localized symptoms. Overall, the most common symptom is RP.5 Localized scleroderma and limited cutaneous systemic sclerosis manifest with symptoms of the skin and underlying tissues. Diffuse cutaneous systemic sclerosis involves cutaneous and visceral symptoms, including lung, esophageal, and vascular involvement.6 Similar to MCTD, scleroderma is most prevalent in middle-aged females,7 though it occurs at a higher rate and with a more severe disease course in Black patients.8
A highly sensitive and specific test for scleroderma that can aid in diagnosis is the neck sign—tightening of the skin of the neck when the head extends.9,10 In one study, the neck sign was positive in more than 90% of patients with scleroderma and negative for control patients and those with primary RP.9 Thus, neck sclerosis is a common manifestation of scleroderma for which patients may seek treatment.
While there is no curative treatment for scleroderma, skin manifestations can be treated with mycophenolate mofetil or methotrexate.5 Systemic treatments may be recommended if the patient has additional symptoms, such as azathioprine for myositis/arthritis and cyclophosphamide for interstitial lung disease.5 However, it is important to note that these medications are associated with risk for gastrointestinal upset, mouth sores, fatigue, or other complications.
Botulinum toxin is a bacterial protein toxin and neuromodulator that inhibits neurotransmitter release by cleaving SNARE proteins at peripheral nerve terminal junctions.11 It has been used in a variety of dermatologic and nondermatologic conditions, including migraines, hyperhidrosis, contractures, scars, and overactive bladder. It also has been used in aesthetics for facial rejuvenation and minimization of wrinkle appearance. Dermatologists and rheumatologists have successfully used BTX to treat primary and secondary RP—the most common symptom of scleroderma—due to its vasodilatation properties.12 Although our patient did not have RP, use of BTX to treat other features of scleroderma, including en coup de sabre, thoracic outlet syndrome, dyspareunia, gastroparesis, pterygium inversum unguis, and dysphagia has been documented.13-18 An in vivo mouse study that examined the possible mechanism for BTX as a treatment in scleroderma found that BTX injections significantly decreased dermal thickness and inflammation in fibrosis (P<.05). An analysis of oxidative stress and mRNA expression showed that BTX may treat fibrosis by suppressing oxidative stress and inflammatory cells, resulting in decreased apoptosis and oxidant-induced intracellular accumulation of reactive oxygen species.19 Another animal study demonstrated the positive effects of BTX treatment for fibrosis of the bladder in rats.20 In one case report, a female patient with scleroderma and facial fibrosis received perioral BTX injections for cosmetic purposes but also observed improvement in mouth constriction, demonstrating the potential efficacy of BTX for facial fibrosis.21
Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region. We recommend assessing the efficacy of the initial BTX treatment after 2 to 3 weeks, with additional injections as needed to achieve the patient’s desired level of comfort and appearance at approximately 3-month intervals (aligning with the expected duration of efficacy of BTX).22 Our patient experienced considerable relief and high satisfaction with BTX treatment. Given the limitations of sclerosis treatments and the unwanted adverse-effect profile of systemic treatments, BTX injections may be a preferrable treatment option for cutaneous manifestations of scleroderma among patients. Future studies with larger patient populations and a control group are warranted to further explore the use of BTX for the dermatologic treatment of scleroderma.
To the Editor:
Scleroderma is a chronic autoimmune connective tissue disease that results in excessive collagen deposition in the skin and other organs throughout the body. On its own or in the setting of mixed connective tissue disease, scleroderma can result in systemic or localized symptoms that can limit patients’ functional capabilities, cause pain and discomfort, and reduce self-esteem—all negatively impacting patients’ quality of life.1,2 Neck sclerosis is a common manifestation of scleroderma. There is no curative treatment for scleroderma; thus, therapy is focused on slowing disease progression and improving quality of life. We present a case of neck sclerosis in a 44-year-old woman with scleroderma that was successfully treated with botulinum toxin (BTX) type A injection, resulting in improved skin laxity and appearance with high patient satisfaction. Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region.
A 44-year-old woman presented to the dermatology clinic for treatment of thickened neck skin with stiffness and tightness that had been present for months to years. She had a history of mixed connective tissue disease (MCTD)(positive anti-ribonucleoprotein, anti–Sjögren syndrome–related antigen, and anti-Smith antibodies) with features of scleroderma and polyarthritis. The patient currently was taking sulfasalazine for the polyarthritis; she previously had taken hydroxychloroquine but discontinued treatment due to ineffectiveness. She was not taking any topical or systemic medications for scleroderma. On physical examination, the skin on the anterior neck appeared thickened with shiny patches (Figure 1). Pinching the skin in the affected area demonstrated sclerosis with high tension.

The dermatologist (J.J.) discussed potential treatment options to help relax the tension in the skin of the anterior neck, including BTX injections. After receiving counsel on adverse effects, alternative treatments, and postprocedural care, the patient decided to proceed with the procedure. The anterior neck was cleansed with an alcohol swab and 37 units (range, 25–50 units) of incobotulinumtoxinA (reconstituted using 2.5-mL bacteriostatic normal saline per 100 units) was injected transdermally using a 9-point injection technique, with each injection placed approximately 1 cm apart. The approximate treatment area included the space between the sternocleidomastoid anterior edges and below the hyoid bone up to the cricothyroid membrane (anatomic zone II).
When the patient returned for follow-up 3 weeks later, she reported considerable improvement in the stiffness and appearance of the skin on the anterior neck. On physical examination, the skin of the neck appeared softened, and improved laxity was seen on pinching the skin compared to the initial presentation (Figure 2). The patient expressed satisfaction with the results and denied any adverse events following the procedure.

Mixed connective tissue disease manifests with a combination of features from various disorders—mainly lupus, scleroderma, polymyositis, and rheumatoid arthritis. It is most prevalent in females and often is diagnosed in the third decade of life.3 It is associated with positive antinuclear antibodies and human leukocyte antigen (HLA) II alleles (HLA-DR4, HLA-DR1, and HLA-DR2). Raynaud phenomenon (RP), one of the most common skin manifestations in both scleroderma and MCTD, is present in 75% to 90% of patients with MCTD.3
Scleroderma is a chronic connective tissue disorder that results in excessive collagen deposition in the skin and other organs throughout the body.4 Although the etiology is unknown, scleroderma develops when overactivation of the immune system leads to CD4+ T-lymphocyte infiltration in the skin, along with the release of profibrotic interleukins and growth factors, resulting in fibrosis.4 Subtypes include localized scleroderma (morphea), limited cutaneous systemic sclerosis (formerly known as CREST [calcinosis, RP, esophageal dysmotility, sclerodactyly, and telangiectasia] syndrome), diffuse cutaneous systemic sclerosis, and systemic sclerosis sine scleroderma.5 Scleroderma is associated with positive antinuclear antibodies and HLA II alleles (HLA-DR2 and HLA-DR5).
On its own or in the setting of MCTD, scleroderma can result in systemic or localized symptoms. Overall, the most common symptom is RP.5 Localized scleroderma and limited cutaneous systemic sclerosis manifest with symptoms of the skin and underlying tissues. Diffuse cutaneous systemic sclerosis involves cutaneous and visceral symptoms, including lung, esophageal, and vascular involvement.6 Similar to MCTD, scleroderma is most prevalent in middle-aged females,7 though it occurs at a higher rate and with a more severe disease course in Black patients.8
A highly sensitive and specific test for scleroderma that can aid in diagnosis is the neck sign—tightening of the skin of the neck when the head extends.9,10 In one study, the neck sign was positive in more than 90% of patients with scleroderma and negative for control patients and those with primary RP.9 Thus, neck sclerosis is a common manifestation of scleroderma for which patients may seek treatment.
While there is no curative treatment for scleroderma, skin manifestations can be treated with mycophenolate mofetil or methotrexate.5 Systemic treatments may be recommended if the patient has additional symptoms, such as azathioprine for myositis/arthritis and cyclophosphamide for interstitial lung disease.5 However, it is important to note that these medications are associated with risk for gastrointestinal upset, mouth sores, fatigue, or other complications.
Botulinum toxin is a bacterial protein toxin and neuromodulator that inhibits neurotransmitter release by cleaving SNARE proteins at peripheral nerve terminal junctions.11 It has been used in a variety of dermatologic and nondermatologic conditions, including migraines, hyperhidrosis, contractures, scars, and overactive bladder. It also has been used in aesthetics for facial rejuvenation and minimization of wrinkle appearance. Dermatologists and rheumatologists have successfully used BTX to treat primary and secondary RP—the most common symptom of scleroderma—due to its vasodilatation properties.12 Although our patient did not have RP, use of BTX to treat other features of scleroderma, including en coup de sabre, thoracic outlet syndrome, dyspareunia, gastroparesis, pterygium inversum unguis, and dysphagia has been documented.13-18 An in vivo mouse study that examined the possible mechanism for BTX as a treatment in scleroderma found that BTX injections significantly decreased dermal thickness and inflammation in fibrosis (P<.05). An analysis of oxidative stress and mRNA expression showed that BTX may treat fibrosis by suppressing oxidative stress and inflammatory cells, resulting in decreased apoptosis and oxidant-induced intracellular accumulation of reactive oxygen species.19 Another animal study demonstrated the positive effects of BTX treatment for fibrosis of the bladder in rats.20 In one case report, a female patient with scleroderma and facial fibrosis received perioral BTX injections for cosmetic purposes but also observed improvement in mouth constriction, demonstrating the potential efficacy of BTX for facial fibrosis.21
Our case demonstrates the potential positive effects of BTX treatment in patients with features of sclerosis or fibrosis, particularly in the neck region. We recommend assessing the efficacy of the initial BTX treatment after 2 to 3 weeks, with additional injections as needed to achieve the patient’s desired level of comfort and appearance at approximately 3-month intervals (aligning with the expected duration of efficacy of BTX).22 Our patient experienced considerable relief and high satisfaction with BTX treatment. Given the limitations of sclerosis treatments and the unwanted adverse-effect profile of systemic treatments, BTX injections may be a preferrable treatment option for cutaneous manifestations of scleroderma among patients. Future studies with larger patient populations and a control group are warranted to further explore the use of BTX for the dermatologic treatment of scleroderma.
- Lis-S´wie¸ty A, Skrzypek-Salamon A, Ranosz-Janicka I, et al. Health-related quality of life and its influencing factors in adult patients with localized scleroderma—a cross-sectional study. Health Qual Life Outcomes. 2020;18:133. doi:10.1186/s12955-020-01386-0
- Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev. 2015;14:1087-1096. doi:10.1016/j.autrev.2015.07.012
- Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol. 2012;26:61-72. doi:10.1016/j.berh.2012.01.009
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinico-pathological correlation. G Ital Dermatol Venereol. 2018;153:208-215. doi:10.23736/S0392-0488.18.05922-9
- Fett N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437. doi:10.1016/j.clindermatol.2013.01.010
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Calderon LM, Pope JE. Scleroderma epidemiology update. Curr Opin Rheumatol. 2021;33:122-127. doi:10.1097/BOR.0000000000000785
- Morgan ND, Gelber AC. African Americans and scleroderma: examining the root cause of the association. Arthritis Care Res (Hoboken). 2019;71:1151-1153. doi:10.1002/acr.23860
- Barnett AJ. The “neck sign” in scleroderma. Arthritis Rheum. 1989;32:209-211. doi:10.1002/anr.1780320215
- Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis). Postgrad Med J. 1988;64:121-125. doi:10.1136/pgmj.64.748.121
- Rossetto O, Pirazzini M, Fabris F, et al. Botulinum neurotoxins: mechanism of action. Handb Exp Pharmacol. 2021;263:35-47.doi:10.1007/164_2020_355
- Ennis D, Ahmad Z, Anderson MA, et al. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2021;35:101684. doi:10.1016/j.berh.2021.101684
- Turkmani MG, Alnomair N. Enhancement of the aesthetic outcome of scleroderma en coup de sabre with botulinum toxin injection. JAAD Case Rep. 2018;4:579-581. doi:10.1016/j.jdcr.2018.03.023
- Le EN, Freischlag JA, Christo PJ, et al. Thoracic outlet syndrome secondary to localized scleroderma treated with botulinum toxin injection. Arthritis Care Res (Hoboken). 2010;62:430-433. doi:10.1002/acr.20099
- Mousty E, Rathat G, Rouleau C, et al. Botulinum toxin type A for treatment of dyspareunia caused by localized scleroderma. Acta Obstet Gynecol Scand. 2011;90:926-927. doi:10.1111/j.1600-0412.2011.01183.x
- Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74-101. doi:10.1016/j.disamonth.2010.12.007
- Katschinski M. [Diagnosis and treatment of esophageal motility disorders]. Ther Umsch. 2001;58:128-133. doi:10.1024/0040-5930.58.3.128
- Kim DJ, Odell ID. Improvement of pterygium inversum unguis and Raynaud phenomenon with interdigital botulinum toxin injections. JAAD Case Rep. 2022;26:79-81. doi:10.1016/j.jdcr.2022.06.009
- Baral H, Sekiguchi A, Uchiyama A, et al. Inhibition of skin fibrosis in systemic sclerosis by botulinum toxin B via the suppression of oxidative stress. J Dermatol. 2021;48:1052-1061. doi:10.1111/1346-8138.15888
- Jia C, Xing T, Shang Z, et al. Botulinum toxin A improves neurogenic bladder fibrosis by suppressing transforming growth factor β1 expression in rats. Transl Androl Urol. 2021;10:2000-2007. doi:10.21037/tau-21-62
- Hoverson K, Love T, Lam TK, et al. A novel treatment for limited mouth opening due to facial fibrosis: a case series. J Am Acad Dermatol. 2018;78:190-192. doi:10.1016/j.jaad.2017.07.006
- Kollewe K, Mohammadi B, Köhler S, et al. Blepharospasm: long-term treatment with either Botox®, Xeomin® or Dysport®. J Neural Transm (Vienna). 2015;122:427-431. doi:10.1007/s00702-014-1278-z
- Lis-S´wie¸ty A, Skrzypek-Salamon A, Ranosz-Janicka I, et al. Health-related quality of life and its influencing factors in adult patients with localized scleroderma—a cross-sectional study. Health Qual Life Outcomes. 2020;18:133. doi:10.1186/s12955-020-01386-0
- Almeida C, Almeida I, Vasconcelos C. Quality of life in systemic sclerosis. Autoimmun Rev. 2015;14:1087-1096. doi:10.1016/j.autrev.2015.07.012
- Ortega-Hernandez OD, Shoenfeld Y. Mixed connective tissue disease: an overview of clinical manifestations, diagnosis and treatment. Best Pract Res Clin Rheumatol. 2012;26:61-72. doi:10.1016/j.berh.2012.01.009
- Rongioletti F, Ferreli C, Atzori L, et al. Scleroderma with an update about clinico-pathological correlation. G Ital Dermatol Venereol. 2018;153:208-215. doi:10.23736/S0392-0488.18.05922-9
- Fett N. Scleroderma: nomenclature, etiology, pathogenesis, prognosis, and treatments: facts and controversies. Clin Dermatol. 2013;31:432-437. doi:10.1016/j.clindermatol.2013.01.010
- Careta MF, Romiti R. Localized scleroderma: clinical spectrum and therapeutic update. An Bras Dermatol. 2015;90:62-73. doi:10.1590/abd1806-4841.20152890
- Calderon LM, Pope JE. Scleroderma epidemiology update. Curr Opin Rheumatol. 2021;33:122-127. doi:10.1097/BOR.0000000000000785
- Morgan ND, Gelber AC. African Americans and scleroderma: examining the root cause of the association. Arthritis Care Res (Hoboken). 2019;71:1151-1153. doi:10.1002/acr.23860
- Barnett AJ. The “neck sign” in scleroderma. Arthritis Rheum. 1989;32:209-211. doi:10.1002/anr.1780320215
- Barnett AJ, Miller M, Littlejohn GO. The diagnosis and classification of scleroderma (systemic sclerosis). Postgrad Med J. 1988;64:121-125. doi:10.1136/pgmj.64.748.121
- Rossetto O, Pirazzini M, Fabris F, et al. Botulinum neurotoxins: mechanism of action. Handb Exp Pharmacol. 2021;263:35-47.doi:10.1007/164_2020_355
- Ennis D, Ahmad Z, Anderson MA, et al. Botulinum toxin in the management of primary and secondary Raynaud’s phenomenon. Best Pract Res Clin Rheumatol. 2021;35:101684. doi:10.1016/j.berh.2021.101684
- Turkmani MG, Alnomair N. Enhancement of the aesthetic outcome of scleroderma en coup de sabre with botulinum toxin injection. JAAD Case Rep. 2018;4:579-581. doi:10.1016/j.jdcr.2018.03.023
- Le EN, Freischlag JA, Christo PJ, et al. Thoracic outlet syndrome secondary to localized scleroderma treated with botulinum toxin injection. Arthritis Care Res (Hoboken). 2010;62:430-433. doi:10.1002/acr.20099
- Mousty E, Rathat G, Rouleau C, et al. Botulinum toxin type A for treatment of dyspareunia caused by localized scleroderma. Acta Obstet Gynecol Scand. 2011;90:926-927. doi:10.1111/j.1600-0412.2011.01183.x
- Tang DM, Friedenberg FK. Gastroparesis: approach, diagnostic evaluation, and management. Dis Mon. 2011;57:74-101. doi:10.1016/j.disamonth.2010.12.007
- Katschinski M. [Diagnosis and treatment of esophageal motility disorders]. Ther Umsch. 2001;58:128-133. doi:10.1024/0040-5930.58.3.128
- Kim DJ, Odell ID. Improvement of pterygium inversum unguis and Raynaud phenomenon with interdigital botulinum toxin injections. JAAD Case Rep. 2022;26:79-81. doi:10.1016/j.jdcr.2022.06.009
- Baral H, Sekiguchi A, Uchiyama A, et al. Inhibition of skin fibrosis in systemic sclerosis by botulinum toxin B via the suppression of oxidative stress. J Dermatol. 2021;48:1052-1061. doi:10.1111/1346-8138.15888
- Jia C, Xing T, Shang Z, et al. Botulinum toxin A improves neurogenic bladder fibrosis by suppressing transforming growth factor β1 expression in rats. Transl Androl Urol. 2021;10:2000-2007. doi:10.21037/tau-21-62
- Hoverson K, Love T, Lam TK, et al. A novel treatment for limited mouth opening due to facial fibrosis: a case series. J Am Acad Dermatol. 2018;78:190-192. doi:10.1016/j.jaad.2017.07.006
- Kollewe K, Mohammadi B, Köhler S, et al. Blepharospasm: long-term treatment with either Botox®, Xeomin® or Dysport®. J Neural Transm (Vienna). 2015;122:427-431. doi:10.1007/s00702-014-1278-z
Practice Points
- Scleroderma is a chronic autoimmune connective tissue disease that results in excessive collagen deposition in the skin and other organs throughout the body.
- Although there is no curative treatment for scleroderma, there are options to slow disease progression and improve quality of life.
- Botulinum toxin injection may be a preferred treatment option in patients with features of sclerosis or fibrosis related to scleroderma, particularly in the neck region.
Anaphylaxis Treatment Uncertainty Persists for Patients and Professionals
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Misinformation and outdated protocols contribute to the suboptimal management of anaphylaxis by patients and healthcare professionals, based on data from two new studies presented at the American College of Allergy, Asthma and Immunology Annual Scientific Meeting.
Anaphylaxis can strike suddenly, and many patients and caregivers at risk do not know which symptoms to treat with epinephrine, said Joni Chow, DO, of Baylor College of Medicine, San Antonio, Texas, in her presentation at the meeting.
“Early identification of anaphylaxis and early intervention with epinephrine are critical for improving patient outcomes,” Chow said in an interview.
“Many allergic reactions occur in community settings, where written action plans serve to instruct patients and caregivers on how to recognize and respond to these emergencies,” she said. “Currently, anaphylaxis action plans are developed based on the consensus of healthcare professionals, with limited information available on the preferences of patients and caregivers,” she noted. However, even with action plans, many patients and families struggle to recognize and manage severe allergic reactions effectively, she added.
In response to this issue, Chow and colleagues created a survey designed to assess the understanding of anaphylaxis recognition and management by patients and caregivers and to identify their preferences regarding the elements included in the action plans.
In the study, Chow and colleagues surveyed 96 patients and caregivers in an allergy clinic waiting room. The majority (95%) of the patients were prescribed epinephrine. Although 73% said they were comfortable identifying signs of anaphylaxis, only 14% said they were likely to use epinephrine as a first-line treatment.
The most common reason given for avoiding epinephrine was uncertainty over which symptoms to treat (40.6%), followed by hesitancy to visit an emergency department (24%), hesitancy to call 911 (17.7%), uncertainty about how to use epinephrine auto-injectors (11.5%), and fear of needles (5.2%).
Although 85% of the respondents understood that antihistamine use does not prevent the need for epinephrine in cases of anaphylactic reactions, 23.7% said they would use an antihistamine as the first treatment in these cases.
For patients with rash and wheezing after a suspected allergen exposure, approximately two thirds (64.5%) of the respondents said they would inject epinephrine and 10.8% would drive to the emergency room before taking any action, Chow said in her presentation.
The relatively low impact of fear of needles was unexpected, as fear of needles is considered a significant deterrent to epinephrine use, Chow told this news organization. “However, our respondents were more inclined to acknowledge a reluctance to escalate to emergency response as the major barrier to treatment,” she said.
The survey also asked patients what features of an anaphylaxis action plan would be most helpful. A majority of respondents (93%) rated a section for the management of mild (non-anaphylactic) allergic reaction symptoms as somewhat or very important. Visual aids for injection of epinephrine and visuals of anaphylaxis symptoms also ranked as somewhat or very important for 87.6% and 81% of respondents, respectively.
The study highlights the importance of educating allergy patients on recognizing and treating anaphylaxis and demonstrates that visuals were preferred in this survey population, Chow said. “Most patients and caregivers from our surveyed population report knowing how to treat anaphylaxis, but many would not use epinephrine as the first treatment,” she noted.
“The study focused on a single community clinic, and it would be beneficial to gather feedback from patients and caregivers representing a wider variety of educational, cultural, social, and socioeconomic backgrounds,” Chow told this news organization. “Additionally, input from other stakeholders, such as school nurses, would enhance knowledge,” she said.
Clinical Anaphylaxis Protocols Fall Short
A second study presented at the meeting showed the need to improve anaphylaxis education for clinicians.
Discrepancies in anaphylaxis management include variations in the definition and treatment of the condition, according to Carly Gunderson, DO, of Memorial Healthcare System, Pembroke Pines, Florida, who presented the study at the meeting.
“So often, we see patients in our office with a history of symptoms that meet criteria for anaphylaxis, yet when they call 911 and emergency medical services (EMS) arrive, they never receive epinephrine,” Gunderson said in an interview. “They receive antihistamines, steroids, everything except epinephrine, which is incredibly concerning given that epinephrine is always the first-line treatment for anaphylaxis,” she said.
“Because EMS providers are often the first healthcare professionals to assess patients experiencing anaphylaxis, their ability to recognize and appropriately treat anaphylaxis is essential,” Gunderson emphasized.
Gunderson and colleagues analyzed data from 30 states with mandatory Advanced Cardiac Life Support protocols to identify gaps in recognizing anaphylaxis and areas for improvement in prehospital management.
Only 15 states (50%) included gastrointestinal symptoms in the definition of anaphylaxis, 40% included neurologic manifestations, and 47% used a two-organ system definition, Gunderson noted in her presentation.
All 30 state protocols recommended diphenhydramine and epinephrine for anaphylactic reactions, 90% recommended albuterol if respiratory symptoms were present, 73% recommended intravenous fluids, and 60% recommended steroids. All but one of the state protocols listed epinephrine as the first-line recommendation for anaphylaxis; 25 states allowed epinephrine autoinjectors and 17 provided autoinjectors.
“We were shocked by how many protocols didn’t include gastrointestinal (abdominal pain, vomiting) or neurologic (lethargy, altered mental status) manifestations, when these are common presenting symptoms of anaphylaxis,” Gunderson told this news organization.
“We were also disappointed by how many protocols continue to recommend outdated interventions such as first-generation antihistamines and corticosteroids in the treatment of anaphylaxis,” she said.
Although anaphylaxis management has come a long way, the current study suggests that there is clearly room for improvement in the education of healthcare providers on how to identify and treat anaphylaxis, said Gunderson. “Most people think of anaphylaxis as the typical ‘face swelling up, throat closing’ type of reaction, which it can be, but in reality, there are so many other ways that it can present,” she said. “Healthcare providers must be aware of all of these possible manifestations so that we can treat in a timely manner to improve outcomes,” she added.
Limitations of the study included the focus only on states with mandatory or model EMS protocols, Gunderson told this news organization. As for additional research, the most important next steps are practical ones, namely, identifying ways to realistically implement necessary protocol changes, she said.
Real-World Data Support Need for Education
Real-world studies are important to identify current practice and opportunities for improvement, S. Shahzad Mustafa, MD, lead physician in allergy, immunology, and rheumatology at Rochester Regional Health and clinical associate professor of medicine at the University of Rochester School of Medicine and Dentistry, Rochester, New York, said in an interview.
“Management of anaphylaxis continues to evolve, and studies like these can help standardize evidence-based care across different medical settings, such as emergency medical services, urgent care, and emergency departments,” said Mustafa, who was not involved in either study.
The findings of the two studies were not unexpected, Mustafa said. “Heterogeneity in medical care is well recognized in numerous conditions, and anaphylaxis is no different. Patients and healthcare providers continue to have hesitation to use epinephrine and continue to overly rely on antihistamines and/or systemic steroids,” he noted.
For both studies, the takeaway message is that education is paramount to optimize anaphylaxis management, Mustafa told this news organization. “Education needs to focus on timely recognition of anaphylaxis, including atypical features such as gastrointestinal symptoms, and appropriate therapy with epinephrine,” he said.
Looking ahead, “research demonstrating differences in clinical outcomes with differing approaches to anaphylaxis may highlight the importance of early recognition and treatment with epinephrine,” said Mustafa. Management of anaphylaxis also lends itself to quality improvement studies, he added.
Neither of the studies received any outside funding. The researchers had no financial conflicts to disclose. Mustafa had no disclosures related to anaphylaxis but disclosed serving on the speakers’ bureau for Genentech, GSK, AstraZeneca, Regeneron/Sanofi, and CSL Behring and received grants from Takeda.
A version of this article first appeared on Medscape.com.
Hyperkeratotic Papules and Black Macules on the Hands
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3
Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3

Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
THE DIAGNOSIS: Acral Hemorrhagic Darier Disease
Darier disease (DD), also known as keratosis follicularis, is a rare autosomal-dominant genodermatosis caused by mutations in the ATPase sarcoplasmic/endoplasmic reticulum Ca2+ transporting 2 gene (ATP2A2). This gene encodes the enzyme sarcoplasmic/endoplasmic reticulum calcium ATPase 2, which results in abnormal calcium signaling in keratinocytes and leads to dyskeratosis.1 Darier disease commonly manifests in the second decade of life with hyperkeratotic papules coalescing into plaques, often accompanied by erosions and fissures that cause discomfort and pruritus. Darier disease also is associated with characteristic nail findings such as the classic candy cane nails and V-shaped nicking.
Acral hemorrhagic lesions are a rare manifestation of DD. Clinically, these lesions can manifest as hemorrhagic macules, papules, and/or vesicles, most commonly occurring following local trauma or retinoid use. Patients with these lesions are believed to have either specific mutations in the ATP2A2 gene that impair sarcoplasmic/endoplasmic reticulum calcium ATPase 2 function in the vascular endothelium or a mutation in the sarcoplasmic/endoplasmic reticulum calcium ATPase protein itself, leading to dysregulation of mitochondrial homeostasis from within the cell, provoking oxidative stress and causing detrimental effects on blood vessels.2 Patients with this variant can present with all the features of classic DD concomitantly, with varying symptom severity or distinct clinical features during separate episodic flares, or as the sole manifestation. Other nonclassical lesions of DD include acral keratoderma, giant comedones, keloidlike vegetations, and leucodermic macules (Figure).3

Acral hemorrhagic DD may appear either in isolation or in tandem with more traditional symptoms, necessitating consideration of other possible differential diagnoses such as acrokeratosis verruciformis of Hopf (AKV), porphyria cutanea tarda, bullous lichen planus (BLP), and hemorrhagic lichen sclerosus.
Sometimes regarded as a variant of DD, AKV is an autosomal- dominant genodermatosis characterized by flat or verrucous hyperkeratotic papules on the hands and feet. In AKV, the nails also may be affected, with changes including striations, subungual hyperkeratosis, and V-shaped nicking of the distal nails. Although our patient displayed features of AKV, it has not been associated with acral hemorrhagic macules, making this diagnosis less likely than DD.4
Porphyria cutanea tarda, a condition caused by decreased levels of uroporphyrinogen decarboxylase, also can cause skin manifestations such as blistering as well as increased skin fragility, predominantly in sun-exposed areas.5 Our patient’s lack of photosensitivity and absence of other common symptoms of this disorder, such as hypertrichosis and hyperpigmentation, made porphyria cutanea tarda less likely.
Bullous lichen planus is a rare subtype of lichen planus characterized by tense bullae arising from preexisting lichen planus lesions or appearing de novo, most commonly manifesting on the oral mucosa or the legs.6 The bullae associated with BLP can rupture and form ulcers—a symptom that could potentially be mistaken for hemorrhagic macules like the ones observed in our patient. However, BLP typically is characterized by erythematous, violaceous, polygonal papules commonly appearing on the oral mucosa and the legs with blisters developing near or on pre-existing lichen planus lesions. These are different from the hyperkeratotic papules and leucodermic macules seen in our patient, which aligned more closely with the clinical presentation of DD.
Hemorrhagic lichen sclerosus presents with white atrophic patches and plaques and hemorrhagic bullae, which may resemble the leucodermic macules and hemorrhagic macules of DD. However, hemorrhagic lichen sclerosus most commonly involves the genital area in postmenopausal women. Extragenital manifestations of lichen sclerosus, although less common, can occur and typically manifest on the thighs, buttocks, breasts, back, chest, axillae, shoulders, and wrists.7 Notably, these hemorrhagic lesions typically are surrounded by hypopigmented skin and display an atrophic appearance.
Management of DD can be challenging. General measures include sun protection, heat avoidance, and friction reduction. Retinoids are considered the first-line therapy for severe DD, as they help normalize keratinocyte differentiation and reduce keratotic scaling.8 Topical corticosteroids can help manage inflammation and reduce the risk for secondary infections. Our patient responded well to this treatment approach, with a notable reduction in the number and severity of the hyperkeratotic plaques and resolution of the acral hemorrhagic lesions.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
- Savignac M, Edir A, Simon M, et al. Darier disease: a disease model of impaired calcium homeostasis in the skin. Biochim Biophys Acta. 2011;1813:1111-1117. doi:10.1016/j.bbamcr.2010.12.006
- Hong E, Hu R, Posligua A, et al. Acral hemorrhagic Darier disease: a case report of a rare presentation and literature review. JAAD Case Rep. 2023;31:93-96. doi:10.1016/j.jdcr.2022.05.030
- Yeshurun A, Ziv M, Cohen-Barak E, et al. An update on the cutaneous manifestations of Darier disease. J Cutan Med Surg. 2021;25:498-503. doi:10.1177/1203475421999331
- Williams GM, Lincoln M. Acrokeratosis verruciformis of Hopf. In: StatPearls. StatPearls Publishing; May 1, 2023.
- Shah A, Bhatt H. Cutanea tarda porphyria. In: StatPearls. StatPearls Publishing; April 17, 2023.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4. doi:10.3315/jdcr.2017.1239
- Arnold N, Manway M, Stephenson S, et al. Extragenital bullous lichen sclerosus on the anterior lower extremities: report of a case and literature review. Dermatol Online J. 2017;23:13030
- Haber RN, Dib NG. Management of Darier disease: a review of the literature and update. Indian J Dermatol Venereol Leprol. 2021;87:14-21. doi:10.25259/IJDVL_963_19 /qt8dn3p7kv.
An elderly woman with a long history of hyperkeratotic papules on the abdomen, forearms, dorsal hands, and skinfolds presented with new lesions on the dorsal hands that had developed over the preceding few months after a lapse in treatment with her previous dermatologist. Her medical history was otherwise unremarkable. Physical examination revealed hyperkeratotic papules, black hemorrhagic macules with jagged borders, and a thin hemorrhagic plaque on the dorsal hands. Nail findings were notable for alternating white and red longitudinal bands with nicking of the distal nail plates. She also had scattered leucodermic macules over the trunk, feet, arms, and legs, as well as numerous hyperkeratotic papules coalescing into plaques over the mons pubis and in the inguinal folds.
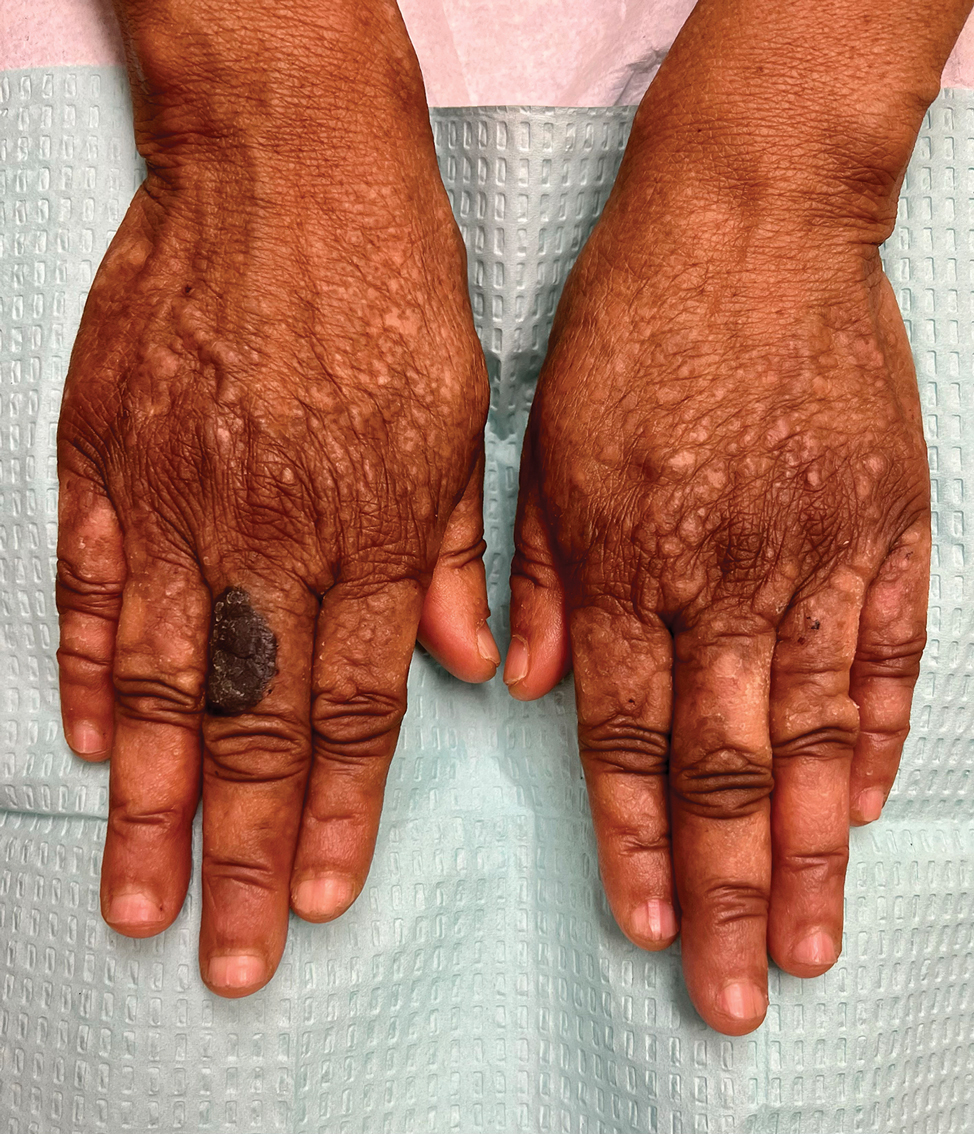
Gardasil 9 at 10 Years: Vaccine Protects Against Multiple Cancers
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Vaccination against human papilloma virus (HPV), a group of more than 200 viruses infecting at least 50% of sexually active people over their lifetimes, has proved more than 90% effective for preventing several diseases caused by high-risk HPV types.
Gardasil 4: 2006
It started in 2006 with the approval of Human Papillomavirus Quadrivalent, types 6, 11, 16, and 18 (Gardasil 4). Merck’s vaccine began to lower rates of cervical cancer, a major global killer of women.
“It’s fair to say the vaccine has been an American and a global public health success story in reducing rates of cervical cancer,” Paula M. Cuccaro, PhD, assistant professor of health promotion and behavioral sciences at University of Texas School of Public Health, Houston, said in an interview.
How does a common virus trigger such a lethal gynecologic malignancy? “It knocks out two important cancer suppressor genes in cells,” explained Christina Annunziata,MD, PhD, a medical oncologist and senior vice president of extramural discovery science for the American Cancer Society. HPV oncoproteins are encoded by the E6 and E7 genes. As in other DNA tumor viruses, the E6 and E7 proteins functionally inactivate the tumor suppressor proteins p53 and pRB, respectively.
US Prevalence
Despite screening and vaccination, cervical cancer is still very much around. This year, 13,820 new cases of invasive cervical cancer will be diagnosed in the United States, and approximately 4360 women will die of it, according to the American Cancer Society. Even before the advent of Gardasil 4, incidence rates had already decreased by more than half from the mid-1970s to the mid-2000s, thanks largely to Pap smear screening programs for treatable premalignant lesions. “The US rate had dropped to about 20 per 100,000 women even before Gardasil 4,” said Annunziata. “After the introduction of the first vaccine, it decreased to 7 per 100,000, a decrease of about 30%, but it remains plateaued now at about the same level.”
Although the past decade has seen rates generally stabilize, there have been some changes in different age groups. In women ages 30-44, rates increased 1.7% each year from 2012 to 2019, while rates declined 11% each year for women ages 20-24— probably reflecting the impact of the first wave of prevention from Gardasil 4.
In one 2021 population-based study of US cancer registry data from 1999 to 2017, rates of both cervical squamous cell carcinoma and adenocarcinoma dropped. The largest declines occurred in females 15-20 years old, the age group most likely to be vaccinated against HPV but not typically screened, suggesting a vaccine-related effect.
Gardasil 9: 2014
With the 2014 approval of the vaccine’s second iteration, Gardasil 9, which replaced Gardasil 4 and targeted 9 HPV strains, immunization has taken broader aim. The strains covered by Gardasil 9 protect against oropharyngeal and other head and neck cancers — as well as penile, anal, vulvar, and vaginal malignancies and premalignancies, and genital warts in both sexes ages 9-45.
It may be years, however, before the impact of the newer polyvalent formulation is felt. “While the first vaccine has been successful against the prevalent strains of HPV linked to cervical cancer, it’s a little early to call it for the newer vaccine since oropharyngeal cancers tend to develop later in older men,” Cuccaro said. “But the types of HPV linked to mouth and throat cancers and covered by the newer vaccines are much less prevalent in those who are vaccinated. The strains not covered in the vaccine you see are equally present in the vaccinated and non-vaccinated.”
Angela L. Myers, MD, MPH, division director of infectious diseases and medical director of the Center for Wellbeing at Children’s Mercy in Kansas City, Missouri, added, “Unlike for cervical cancer, there are no screening programs for oropharyngeal lesions, so you have to wait to see rates until actual cancer develops.”
A 2023 review reported that HPV vaccination reduced levels of oropharyngeal HPV positivity in men, strengthening the case for pangender immunization.
And in a recent phase 3 doubled-blind trial, GARDASIL 9 reduced the incidence of anogenital persistent infection caused by nine types of HPV compared with a placebo.
Increasing Uptake
The current public health aim is to have 80% of young people in the targeted age group vaccinated with two doses. Today, uptake among those 9-26 years old stands at about 78% of girls and 75% of boys for the first dose, said Annunziata. “But it’s only about 61% for the two doses in the current series, and we want to improve that.”
Some parents may still harbor fears that immunizing teens and tweens — both the American Academy of Pediatrics and the American Cancer Society recommend immunization at age 9 — will open the door to precocious sexual activity.
“But overall, uptake in tweens and young teens has increased because the messaging has changed,” said Myers, with the rationale now focusing on cancer prevention not sexual-infection prophylaxis. “This is similar to the hepatitis B vaccine, which used to be given to young adults and is now given to newborns to prevent cancer.”
Cuccaro added that a proactive presentation by healthcare professionals has a significant effect on vaccine uptake and increases the odds of vaccination ninefold. “Providers should take a presumptive approach and avoid just offering the vaccine as an option. It should be included with regular childhood vaccinations,” she said. “And the advantage of starting early at age 9 is that you can spread the doses out across other regular childhood vaccinations, whereas if you start at age 11, you need to add the HPV vaccine to three other vaccines that are given at that time.”
After age 15, three doses are necessary. “Providers should stress to parents that it’s most effective when given before young people become sexually active and exposed to HPV,” Cuccaro said. And Myers stressed that despite the vaccine’s effectiveness, routine screening for cervical premalignancies is still important.
Despite increasing coverage, vaccination rates have some distance to go before the public health target of at least 80% uptake of the series in the targeted age group, Cuccaro cautioned.
On the global stage, barriers to immunization remain, but the World Health Organization has endorsed a campaign to eradicate cervical cancer through HPV vaccination. It has predicted that the 21st century may be the last to experience HPV-associated cancers, currently responsible for more than 300,000 annual deaths worldwide.
A Brief History of HPV Vaccines
- 1951. Cervical cancer patient Henrietta Lacks’ rapidly dividing cervical cells are collected by George Otto Gey at Johns Hopkins Hospital. They create the first immortal cell line (HeLa) used to study cancers and vaccines worldwide.
- 1976. Harald zur Hausen suggests that genital wart-associated HPV, not herpes simplex, is the probable cause of cervical cancer.
- 1983. HPV is confirmed as a cause of cancer.
- 1991. The first HPV vaccine is developed.
- 2002. Proof of principle and protective efficacy for the monovalent HPV 16 are shown.
- 2006. Merck’s Gardasil 4 (HPV 4) is FDA approved in girls ages 9-26 for protection against strains 6, 11, 16, and 18 — the cause of more than 70% of cervical cancer cases.
- 2009. Approval of Gardasil 4 is expanded to boys ages 9-26 for the prevention of genital warts.
- 2009. The FDA approves GlaxoSmithKline’s Cervarix (HPV 16 and 18) for girls and young women. The vaccine was withdrawn from the US market in 2016 following the success of Gardasil 9 but is used abroad for HPV cancer prevention.
- 2014. The 9-valent recombinant vaccine Gardasil 9 is FDA approved for protection against several low-risk, wart-causing HPV strains as well as the high-risk cancer strains targeted by HPV 4.
- 2018. The FDA expands approval to include females and males 27-45 years old.
- 2020. The FDA extends approval of Gardasil 9 to include prevention not only of cervical cancer but also, vaginal, vulvar, anal, oropharyngeal, and other head and neck cancers.
Annunziata, Cuccaro, and Myers had no competing interests to declare.
A version of this article appeared on Medscape.com.
Cosmetic Dermatology Product Recalls Still Common, Analysis Finds
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Between 2011 and 2023, the US Food and Drug Administration (FDA) reported recalls of 334 cosmetic dermatology products in the United States, affecting over 77 million units, predominantly due to bacterial contamination.
METHODOLOGY:
- Researchers conducted a cross-sectional analysis of the FDA Enforcement Report database for cosmetic dermatology products from 2011 to 2023.
- Cosmetic products are any article “intended for body cleaning or beauty enhancement,” as defined by the FDA.
- Recalls were categorized by product type, reason for the recall, microbial contaminant, inorganic contaminant, distribution, and risk classification.
TAKEAWAY:
- During the study period, 334 voluntary and manufacturer-initiated recalls of cosmetic products were reported, affecting 77,135,700 units.
- A total of 297 recalls (88.9%) were categorized as Class II, indicating that they caused “medically reversible health consequences.” The median recall duration was 307 days.
- Hygiene and cleaning products accounted for most of the recalls (51.5%). Makeup gels, soaps, shampoos, tattoo ink, wipes, and lotions were the most recalled product categories. Nearly 51% of the products were distributed internationally.
- Microbial and inorganic contamination accounted for 76.8% and 10.2% of the recalls (the two most common reasons for the recall), respectively, with bacteria (80%) the most common contaminating pathogen (primarily Pseudomonas and Burkholderia species).
IN PRACTICE:
With 77 million units recalled by the FDA over 12 years, cosmetic recalls have remained common, the authors concluded, adding that “dermatologists should be key voices in pharmacovigilance given scientific expertise and frontline experience managing products and associated concerns.” Dermatologists, they added, “should also be aware of FDA enforcement reports for recall updates given that average recall termination took approximately 1 year.”
SOURCE:
The study was led by Kaushik P. Venkatesh, MBA, MPH, Harvard Medical School, Boston, and was published online on October 29 in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study’s limitations include the potential underreporting of Class III recalls (products that are unlikely to cause any adverse health reaction but violate FDA labeling or manufacturing laws) and lack of complete information on contaminants.
DISCLOSURES:
No information on funding was provided in the study. No conflicts of interest were reported.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Novel Treatment Promising for Cutaneous Lupus in Phase 2 Trial
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
particularly in subacute and chronic cases.
METHODOLOGY:
- Researchers conducted a randomized phase 2 trial to evaluate the efficacy and safety of iberdomide in 288 patients with CLE (mean age, 45 years; 97% women). Iberdomide is a cereblon modulator, which results in degradation of two transcription factors of immune cell development and homeostasis — Ikaros and Aiolos — that have been implicated in the genetic predisposition of systemic lupus.
- CLE Disease Area and Severity Index Activity (CLASI-A) endpoints included mean percent change from baseline and ≥ 50% reduction from baseline (CLASI-50), which were evaluated in all patients with baseline CLASI-A scores ≥ 8 and by CLE subtypes (acute, subacute, and chronic).
- At baseline, 56% of patients had acute CLE, 29% had chronic CLE, and 16% had subacute CLE; 28% of patients had a baseline CLASI-A score ≥ 8.
- Patients were randomly assigned to receive oral iberdomide (0.45 mg, 0.30 mg, 0.15 mg, or placebo daily) for 24 weeks while continuing standard lupus medications. At week 24, patients on placebo were rerandomized to iberdomide 0.45 mg or 0.30 mg once a day, while those on iberdomide continued their assigned dose through week 52.
TAKEAWAY:
- Among patients with baseline CLASI-A ≥ 8, the mean change in CLASI-A score from baseline at week 24 was −66.7% for those on iberdomide 0.45 mg vs −54.2% for placebo (P = .295).
- At week 24, patients with subacute CLE showed a significantly greater mean percent change from baseline in CLASI-A with iberdomide 0.45 mg vs placebo (−90.5% vs −51.2%; P = .007), while no significant differences were observed with the 0.45-mg dose vs placebo in patients with chronic or acute CLE.
- Overall, CLASI-50 responses were not significantly different among those on 0.45 mg vs placebo (55.6% vs 44.6%). The proportions of patients achieving CLASI-50 at week 24 were significantly greater for iberdomide 0.45 mg vs placebo for those with subacute CLE (91.7% vs 52.9%; P = .035) and chronic CLE (62.1% vs 27.8%; P = .029), but not for those with baseline CLASI-A ≥ 8 (66.7% vs 50%).
- More than 80% of patients had treatment-emergent adverse events (TEAEs), which were mostly mild to moderate. Over 2 years, the most common were urinary tract infections, upper respiratory tract infections, neutropenia, and nasopharyngitis. TEAEs leading to iberdomide discontinuation in one or more patients were neutropenia (n = 7), rash (n = 7), increased hepatic enzymes (n = 4), and deep vein thrombosis (n = 3).
IN PRACTICE:
“Data from this phase 2 trial of iberdomide in patients with SLE suggest that a greater proportion of patients with subacute or chronic CLE who received the higher dose of 0.45 mg iberdomide achieved CLASI-50 vs placebo. For the overall population, CLASI-50 response was not significantly different between treatment groups at week 24, partly due to a high placebo response that may have been driven by patients with acute CLE,” the authors wrote.
SOURCE:
The study was led by Victoria P. Werth, MD, of the University of Pennsylvania and the Veteran’s Administration Medical Center, both in Philadelphia, and was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
The study included small patient subgroups for different CLE subtypes, which may affect the generalizability of the findings. CLE subtype was determined by the investigator without additional photographic adjudication. Additionally, the use of background lupus medications could have influenced the placebo group’s response, limiting the ability to observe the treatment effect of iberdomide monotherapy.
DISCLOSURES:
The study was funded by Bristol-Myers Squibb. Six authors reported being employed by Bristol-Myers Squibb, and several others reported consultancy and research support from various sources including Bristol-Myers Squibb.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Parent Perceptions Drive Diet Changes for Children With Atopic Dermatitis
based on survey data from nearly 300 parents.
Although atopic dermatitis can be associated with an increased risk for food allergies, major allergy organizations do not currently recommend elimination diets as a treatment for atopic dermatitis, said Nadia Makkoukdji, MD, a pediatrician at Jackson Memorial Hospital, Miami, in a presentation at the American College of Allergy, Asthma, and Immunology (ACAAI) Annual Scientific Meeting.
“A fear of drastic dietary changes often prevents families from seeking the care their children need,” Makkoukdji said in an interview. In the clinical setting, Makkoukdji noted that she has seen many patients who have started food elimination diets on their own or as recommended by other doctors, and that these diets can lead to dangers such as the development of immunoglobulin E–mediated food allergies on reintroduction of eliminated foods and malnutrition. They can also produce “emotional stress in children and anxiety or depression, while also adding stress to parents and the entire family.”
Makkoukdji conducted the study to explore parents’ perceptions of these diets in management of their children’s atopic dermatitis, she said.
In the study, Makkoukdji and colleagues sought to understand parents’ perceptions of the role of diet in atopic dermatitis in their children. The researchers reviewed surveys from 298 parents of children with atopic dermatitis who were seen at a single academic center. Parents completed the surveys in the emergency department or in an allergy, dermatology, and general pediatrics clinic.
Overall, 42% of parents identified food triggers for their child’s atopic dermatitis. The most commonly identified triggers were milk (32%), tree nuts/seeds/peanuts (16%), and eggs (11%).
Of the parents who reported food triggers, 23% removed the suspected trigger food from the child’s diet completely, 20% removed suspected trigger foods from their own diets while breastfeeding, and 19% changed their infant’s formula.
In the wake of the elimination diets, 38% of the parents reported no improvement in their child’s atopic dermatitis, 35% reported a 25% improvement, and 9% reported complete resolution. The majority (79%) reintroduced eliminated foods and reported no recurrence of atopic dermatitis symptoms.
The researchers were surprised by how many parents changed their child’s diet in the belief that certain foods exacerbated their child’s atopic dermatitis, “although this perception aligns with the common concern that food allergens can trigger or worsen atopic dermatitis flares,” Makkoukdji said.
The current study highlights the need for more awareness of the limited impact of dietary modifications on atopic dermatitis in the absence of confirmed food allergies, Makkoukdji said. “Our study shows that food elimination diets are still commonly being used by parents in the local Miami population.”
The findings were limited by several factors, including the use of data from a single center and the focus only on pediatric patients, but the primary goal was to assess parental perceptions of AD flares in relation to dietary choices, said Makkoukdji. “Future studies that include larger and more diverse populations would be valuable for the field.”
Dietary Modifications Don’t Live Up to Hype
“Food continues to be one of the most discussed aspects of atopic dermatitis,” Peter Lio, MD, clinical assistant professor of dermatology and pediatrics at Northwestern University Feinberg School of Medicine, Chicago, Illinois, said in an interview.
“Almost all of my patients and families ask about dietary modifications, even though almost all of them have experimented with it to some degree,” said Lio. In his experience, diet plays a small role, if any, in the day-to-day management of atopic dermatitis.
This lack of effect of dietary changes is often frustrating to patients because of the persistent “common wisdom” that points to diet as a root cause of atopic dermatitis, Lio said. “Many practitioners continue to recommend excluding foods such as gluten or dairy from the diet, but generally these are only of modest help,” and although patients wish that dietary changes would fix the problem, most are left wondering why these changes didn’t help them.
The current study findings “reflect my own experience after nearly 20 years of being deeply immersed in the world of atopic dermatitis,” Lio said. Although the takeaway message does not argue against eating healthy foods, some foods do seem to make AD worse in some patients and may have nonallergic pro-inflammatory effects.
“In those cases, it is reasonable to limit or avoid those foods. However, it is extremely difficult to tell what food or foods are driving flare-ups when things are out of control, so dietary modification is generally not the best place to start,” he said.
True food allergies are much more common in patients with atopic dermatitis compared with individuals without atopic dermatitis, but the current study is not addressing these types of allergies, Lio emphasized. “If someone has true allergy to peanuts, for example, they should not be eating them; we also know that they are not ‘cheating’ because these patients would not merely have an eczema flare; they would have urticaria, angioedema, or anaphylaxis. There is tremendous confusion around this point and lots of confusion around allergy testing and its limitations.”
In addition, patients with atopic dermatitis are more likely than those without atopic dermatitis to have abnormalities in the gut microbiome and gut barrier, Lio said.
Abnormalities in the gut microbiome are different from the concept of allergy and may fall into the more complex category of barrier and microbiome disruptors, he said. Therefore, “the food category may not be nearly as important as the specific preparation of the food along with the additives (such as preservatives and emulsifiers) that may actually be driving the problem.”
Although in the past many clinicians advised patients to try cutting out certain foods to see whether atopic dermatitis symptoms improved, this strategy is not without risk, said Lio. “There have been incredible advancements in understanding the role of the gut in tolerization to foods.” Recent research has shown that by eating foods regularly, particularly those such as peanuts that seem to have more allergic potential, the body becomes tolerant, and this prevents the development of true food allergies.
As for additional research, many questions remain about the effects of types of foods, processing methods, and timing of introduction of foods on atopic dermatitis, Lio noted.
“Atopic dermatitis is a systemic condition with the immune system, with the skin/gut/respiratory barriers and microbiome involved; I think we now have a broader view of how big and complex the landscape really is,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Lio had no disclosures relevant to elimination diets but disclosed serving on the speakers bureau for AbbVie, Arcutis Biotherapeutics, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche–Posay/L’Oréal, Pfizer, Pierre Fabre Dermatologie, Regeneron/Sanofi Genzyme, and Verrica Pharmaceuticals; serving on consulting/advisory boards; or having stock options for many pharmaceutical companies. Lio also disclosed a patent pending for a Theraplex product with royalties paid and is a board member and Scientific Advisory Committee member emeritus of the National Eczema Association.
A version of this article first appeared on Medscape.com.
based on survey data from nearly 300 parents.
Although atopic dermatitis can be associated with an increased risk for food allergies, major allergy organizations do not currently recommend elimination diets as a treatment for atopic dermatitis, said Nadia Makkoukdji, MD, a pediatrician at Jackson Memorial Hospital, Miami, in a presentation at the American College of Allergy, Asthma, and Immunology (ACAAI) Annual Scientific Meeting.
“A fear of drastic dietary changes often prevents families from seeking the care their children need,” Makkoukdji said in an interview. In the clinical setting, Makkoukdji noted that she has seen many patients who have started food elimination diets on their own or as recommended by other doctors, and that these diets can lead to dangers such as the development of immunoglobulin E–mediated food allergies on reintroduction of eliminated foods and malnutrition. They can also produce “emotional stress in children and anxiety or depression, while also adding stress to parents and the entire family.”
Makkoukdji conducted the study to explore parents’ perceptions of these diets in management of their children’s atopic dermatitis, she said.
In the study, Makkoukdji and colleagues sought to understand parents’ perceptions of the role of diet in atopic dermatitis in their children. The researchers reviewed surveys from 298 parents of children with atopic dermatitis who were seen at a single academic center. Parents completed the surveys in the emergency department or in an allergy, dermatology, and general pediatrics clinic.
Overall, 42% of parents identified food triggers for their child’s atopic dermatitis. The most commonly identified triggers were milk (32%), tree nuts/seeds/peanuts (16%), and eggs (11%).
Of the parents who reported food triggers, 23% removed the suspected trigger food from the child’s diet completely, 20% removed suspected trigger foods from their own diets while breastfeeding, and 19% changed their infant’s formula.
In the wake of the elimination diets, 38% of the parents reported no improvement in their child’s atopic dermatitis, 35% reported a 25% improvement, and 9% reported complete resolution. The majority (79%) reintroduced eliminated foods and reported no recurrence of atopic dermatitis symptoms.
The researchers were surprised by how many parents changed their child’s diet in the belief that certain foods exacerbated their child’s atopic dermatitis, “although this perception aligns with the common concern that food allergens can trigger or worsen atopic dermatitis flares,” Makkoukdji said.
The current study highlights the need for more awareness of the limited impact of dietary modifications on atopic dermatitis in the absence of confirmed food allergies, Makkoukdji said. “Our study shows that food elimination diets are still commonly being used by parents in the local Miami population.”
The findings were limited by several factors, including the use of data from a single center and the focus only on pediatric patients, but the primary goal was to assess parental perceptions of AD flares in relation to dietary choices, said Makkoukdji. “Future studies that include larger and more diverse populations would be valuable for the field.”
Dietary Modifications Don’t Live Up to Hype
“Food continues to be one of the most discussed aspects of atopic dermatitis,” Peter Lio, MD, clinical assistant professor of dermatology and pediatrics at Northwestern University Feinberg School of Medicine, Chicago, Illinois, said in an interview.
“Almost all of my patients and families ask about dietary modifications, even though almost all of them have experimented with it to some degree,” said Lio. In his experience, diet plays a small role, if any, in the day-to-day management of atopic dermatitis.
This lack of effect of dietary changes is often frustrating to patients because of the persistent “common wisdom” that points to diet as a root cause of atopic dermatitis, Lio said. “Many practitioners continue to recommend excluding foods such as gluten or dairy from the diet, but generally these are only of modest help,” and although patients wish that dietary changes would fix the problem, most are left wondering why these changes didn’t help them.
The current study findings “reflect my own experience after nearly 20 years of being deeply immersed in the world of atopic dermatitis,” Lio said. Although the takeaway message does not argue against eating healthy foods, some foods do seem to make AD worse in some patients and may have nonallergic pro-inflammatory effects.
“In those cases, it is reasonable to limit or avoid those foods. However, it is extremely difficult to tell what food or foods are driving flare-ups when things are out of control, so dietary modification is generally not the best place to start,” he said.
True food allergies are much more common in patients with atopic dermatitis compared with individuals without atopic dermatitis, but the current study is not addressing these types of allergies, Lio emphasized. “If someone has true allergy to peanuts, for example, they should not be eating them; we also know that they are not ‘cheating’ because these patients would not merely have an eczema flare; they would have urticaria, angioedema, or anaphylaxis. There is tremendous confusion around this point and lots of confusion around allergy testing and its limitations.”
In addition, patients with atopic dermatitis are more likely than those without atopic dermatitis to have abnormalities in the gut microbiome and gut barrier, Lio said.
Abnormalities in the gut microbiome are different from the concept of allergy and may fall into the more complex category of barrier and microbiome disruptors, he said. Therefore, “the food category may not be nearly as important as the specific preparation of the food along with the additives (such as preservatives and emulsifiers) that may actually be driving the problem.”
Although in the past many clinicians advised patients to try cutting out certain foods to see whether atopic dermatitis symptoms improved, this strategy is not without risk, said Lio. “There have been incredible advancements in understanding the role of the gut in tolerization to foods.” Recent research has shown that by eating foods regularly, particularly those such as peanuts that seem to have more allergic potential, the body becomes tolerant, and this prevents the development of true food allergies.
As for additional research, many questions remain about the effects of types of foods, processing methods, and timing of introduction of foods on atopic dermatitis, Lio noted.
“Atopic dermatitis is a systemic condition with the immune system, with the skin/gut/respiratory barriers and microbiome involved; I think we now have a broader view of how big and complex the landscape really is,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Lio had no disclosures relevant to elimination diets but disclosed serving on the speakers bureau for AbbVie, Arcutis Biotherapeutics, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche–Posay/L’Oréal, Pfizer, Pierre Fabre Dermatologie, Regeneron/Sanofi Genzyme, and Verrica Pharmaceuticals; serving on consulting/advisory boards; or having stock options for many pharmaceutical companies. Lio also disclosed a patent pending for a Theraplex product with royalties paid and is a board member and Scientific Advisory Committee member emeritus of the National Eczema Association.
A version of this article first appeared on Medscape.com.
based on survey data from nearly 300 parents.
Although atopic dermatitis can be associated with an increased risk for food allergies, major allergy organizations do not currently recommend elimination diets as a treatment for atopic dermatitis, said Nadia Makkoukdji, MD, a pediatrician at Jackson Memorial Hospital, Miami, in a presentation at the American College of Allergy, Asthma, and Immunology (ACAAI) Annual Scientific Meeting.
“A fear of drastic dietary changes often prevents families from seeking the care their children need,” Makkoukdji said in an interview. In the clinical setting, Makkoukdji noted that she has seen many patients who have started food elimination diets on their own or as recommended by other doctors, and that these diets can lead to dangers such as the development of immunoglobulin E–mediated food allergies on reintroduction of eliminated foods and malnutrition. They can also produce “emotional stress in children and anxiety or depression, while also adding stress to parents and the entire family.”
Makkoukdji conducted the study to explore parents’ perceptions of these diets in management of their children’s atopic dermatitis, she said.
In the study, Makkoukdji and colleagues sought to understand parents’ perceptions of the role of diet in atopic dermatitis in their children. The researchers reviewed surveys from 298 parents of children with atopic dermatitis who were seen at a single academic center. Parents completed the surveys in the emergency department or in an allergy, dermatology, and general pediatrics clinic.
Overall, 42% of parents identified food triggers for their child’s atopic dermatitis. The most commonly identified triggers were milk (32%), tree nuts/seeds/peanuts (16%), and eggs (11%).
Of the parents who reported food triggers, 23% removed the suspected trigger food from the child’s diet completely, 20% removed suspected trigger foods from their own diets while breastfeeding, and 19% changed their infant’s formula.
In the wake of the elimination diets, 38% of the parents reported no improvement in their child’s atopic dermatitis, 35% reported a 25% improvement, and 9% reported complete resolution. The majority (79%) reintroduced eliminated foods and reported no recurrence of atopic dermatitis symptoms.
The researchers were surprised by how many parents changed their child’s diet in the belief that certain foods exacerbated their child’s atopic dermatitis, “although this perception aligns with the common concern that food allergens can trigger or worsen atopic dermatitis flares,” Makkoukdji said.
The current study highlights the need for more awareness of the limited impact of dietary modifications on atopic dermatitis in the absence of confirmed food allergies, Makkoukdji said. “Our study shows that food elimination diets are still commonly being used by parents in the local Miami population.”
The findings were limited by several factors, including the use of data from a single center and the focus only on pediatric patients, but the primary goal was to assess parental perceptions of AD flares in relation to dietary choices, said Makkoukdji. “Future studies that include larger and more diverse populations would be valuable for the field.”
Dietary Modifications Don’t Live Up to Hype
“Food continues to be one of the most discussed aspects of atopic dermatitis,” Peter Lio, MD, clinical assistant professor of dermatology and pediatrics at Northwestern University Feinberg School of Medicine, Chicago, Illinois, said in an interview.
“Almost all of my patients and families ask about dietary modifications, even though almost all of them have experimented with it to some degree,” said Lio. In his experience, diet plays a small role, if any, in the day-to-day management of atopic dermatitis.
This lack of effect of dietary changes is often frustrating to patients because of the persistent “common wisdom” that points to diet as a root cause of atopic dermatitis, Lio said. “Many practitioners continue to recommend excluding foods such as gluten or dairy from the diet, but generally these are only of modest help,” and although patients wish that dietary changes would fix the problem, most are left wondering why these changes didn’t help them.
The current study findings “reflect my own experience after nearly 20 years of being deeply immersed in the world of atopic dermatitis,” Lio said. Although the takeaway message does not argue against eating healthy foods, some foods do seem to make AD worse in some patients and may have nonallergic pro-inflammatory effects.
“In those cases, it is reasonable to limit or avoid those foods. However, it is extremely difficult to tell what food or foods are driving flare-ups when things are out of control, so dietary modification is generally not the best place to start,” he said.
True food allergies are much more common in patients with atopic dermatitis compared with individuals without atopic dermatitis, but the current study is not addressing these types of allergies, Lio emphasized. “If someone has true allergy to peanuts, for example, they should not be eating them; we also know that they are not ‘cheating’ because these patients would not merely have an eczema flare; they would have urticaria, angioedema, or anaphylaxis. There is tremendous confusion around this point and lots of confusion around allergy testing and its limitations.”
In addition, patients with atopic dermatitis are more likely than those without atopic dermatitis to have abnormalities in the gut microbiome and gut barrier, Lio said.
Abnormalities in the gut microbiome are different from the concept of allergy and may fall into the more complex category of barrier and microbiome disruptors, he said. Therefore, “the food category may not be nearly as important as the specific preparation of the food along with the additives (such as preservatives and emulsifiers) that may actually be driving the problem.”
Although in the past many clinicians advised patients to try cutting out certain foods to see whether atopic dermatitis symptoms improved, this strategy is not without risk, said Lio. “There have been incredible advancements in understanding the role of the gut in tolerization to foods.” Recent research has shown that by eating foods regularly, particularly those such as peanuts that seem to have more allergic potential, the body becomes tolerant, and this prevents the development of true food allergies.
As for additional research, many questions remain about the effects of types of foods, processing methods, and timing of introduction of foods on atopic dermatitis, Lio noted.
“Atopic dermatitis is a systemic condition with the immune system, with the skin/gut/respiratory barriers and microbiome involved; I think we now have a broader view of how big and complex the landscape really is,” he said.
The study received no outside funding. The researchers had no financial conflicts to disclose. Lio had no disclosures relevant to elimination diets but disclosed serving on the speakers bureau for AbbVie, Arcutis Biotherapeutics, Eli Lilly, Galderma, Hyphens Pharma, Incyte, La Roche–Posay/L’Oréal, Pfizer, Pierre Fabre Dermatologie, Regeneron/Sanofi Genzyme, and Verrica Pharmaceuticals; serving on consulting/advisory boards; or having stock options for many pharmaceutical companies. Lio also disclosed a patent pending for a Theraplex product with royalties paid and is a board member and Scientific Advisory Committee member emeritus of the National Eczema Association.
A version of this article first appeared on Medscape.com.
FROM ACAAI 2024