User login
For MD-IQ use only
Hepatocellular Carcinoma: Leading Causes of Mortality Predicted
TOPLINE:
Alcohol-associated liver disease (ALD) will likely become the leading cause of HCC-related mortality by 2026, and metabolic dysfunction–associated steatotic liver disease (MASLD) is projected to become the second leading cause by 2032, a new analysis found.
METHODOLOGY:
- HCC accounts for 75%-85% of primary liver cancers and most liver cancer deaths. Researchers have observed an upward trend in the incidence of and mortality from HCC in the past 2 decades.
- This cross-sectional study analyzed 188,280 HCC-related deaths among adults aged 25 and older to determine trends in mortality rates and project age-standardized mortality rates through 2040. Data came from the National Vital Statistics System database from 2006 to 2022.
- Researchers stratified mortality data by etiology of liver disease (ALD, hepatitis B virus, hepatitis C virus, and MASLD), age groups (25-64 or 65 and older years), sex, and race/ethnicity.
- Demographic data showed that 77.4% of deaths occurred in men, 55.6% in individuals aged 65 years or older, and 62.3% in White individuals.
TAKEAWAY:
- Overall, the age-standardized mortality rate for HCC-related deaths increased from 3.65 per 100,000 persons in 2006 to 5.03 in 2022 and was projected to increase to 6.39 per 100,000 persons by 2040.
- Sex- and age-related disparities were substantial. Men had much higher rates of HCC-related mortality than women (8.15 vs 2.33 per 100,000 persons), with a projected rate among men of 9.78 per 100,000 persons by 2040. HCC-related mortality rates for people aged 65 years or older were 10 times higher than for those aged 25-64 years (18.37 vs 1.79 per 100,000 persons) in 2022 and was projected to reach 32.81 per 100,000 persons by 2040 in the older group.
- Although hepatitis C virus–related deaths were projected to decline from 0.69 to 0.03 per 100,000 persons by 2034, ALD- and MASLD-related deaths showed increasing trends, with both projected to become the two leading causes of HCC-related mortality in the next few years.
- Racial disparities were also evident. By 2040, the American Indian/Alaska Native population showed the highest increase in projected HCC-related mortality rates, which went from 5.46 per 100,000 persons in 2006 to a project increase to 14.71 per 100,000 persons.
IN PRACTICE:
“HCC mortality was projected to continue increasing in the US, primarily due to rising rates of deaths attributable to ALD and MASLD,” the authors wrote.
This “study highlights the importance of addressing these conditions to decrease the burden of liver disease and liver disease mortality in the future,” Emad Qayed, MD, MPH, Emory University School of Medicine, Atlanta, wrote in an accompanying editorial.
SOURCE:
The study was led by Sikai Qiu, MM, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, and was published online in JAMA Network Open.
LIMITATIONS:
The National Vital Statistics System database used in this study captured only mortality data without access to detailed clinical records or individual medical histories. Researchers could not analyze socioeconomic factors or individual-level risk factors owing to data anonymization requirements. Additionally, the inclusion of the COVID-19 pandemic period could have influenced observed trends and reliability of future projections.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China. Several authors reported receiving consulting fees, speaking fees, or research support from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Alcohol-associated liver disease (ALD) will likely become the leading cause of HCC-related mortality by 2026, and metabolic dysfunction–associated steatotic liver disease (MASLD) is projected to become the second leading cause by 2032, a new analysis found.
METHODOLOGY:
- HCC accounts for 75%-85% of primary liver cancers and most liver cancer deaths. Researchers have observed an upward trend in the incidence of and mortality from HCC in the past 2 decades.
- This cross-sectional study analyzed 188,280 HCC-related deaths among adults aged 25 and older to determine trends in mortality rates and project age-standardized mortality rates through 2040. Data came from the National Vital Statistics System database from 2006 to 2022.
- Researchers stratified mortality data by etiology of liver disease (ALD, hepatitis B virus, hepatitis C virus, and MASLD), age groups (25-64 or 65 and older years), sex, and race/ethnicity.
- Demographic data showed that 77.4% of deaths occurred in men, 55.6% in individuals aged 65 years or older, and 62.3% in White individuals.
TAKEAWAY:
- Overall, the age-standardized mortality rate for HCC-related deaths increased from 3.65 per 100,000 persons in 2006 to 5.03 in 2022 and was projected to increase to 6.39 per 100,000 persons by 2040.
- Sex- and age-related disparities were substantial. Men had much higher rates of HCC-related mortality than women (8.15 vs 2.33 per 100,000 persons), with a projected rate among men of 9.78 per 100,000 persons by 2040. HCC-related mortality rates for people aged 65 years or older were 10 times higher than for those aged 25-64 years (18.37 vs 1.79 per 100,000 persons) in 2022 and was projected to reach 32.81 per 100,000 persons by 2040 in the older group.
- Although hepatitis C virus–related deaths were projected to decline from 0.69 to 0.03 per 100,000 persons by 2034, ALD- and MASLD-related deaths showed increasing trends, with both projected to become the two leading causes of HCC-related mortality in the next few years.
- Racial disparities were also evident. By 2040, the American Indian/Alaska Native population showed the highest increase in projected HCC-related mortality rates, which went from 5.46 per 100,000 persons in 2006 to a project increase to 14.71 per 100,000 persons.
IN PRACTICE:
“HCC mortality was projected to continue increasing in the US, primarily due to rising rates of deaths attributable to ALD and MASLD,” the authors wrote.
This “study highlights the importance of addressing these conditions to decrease the burden of liver disease and liver disease mortality in the future,” Emad Qayed, MD, MPH, Emory University School of Medicine, Atlanta, wrote in an accompanying editorial.
SOURCE:
The study was led by Sikai Qiu, MM, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, and was published online in JAMA Network Open.
LIMITATIONS:
The National Vital Statistics System database used in this study captured only mortality data without access to detailed clinical records or individual medical histories. Researchers could not analyze socioeconomic factors or individual-level risk factors owing to data anonymization requirements. Additionally, the inclusion of the COVID-19 pandemic period could have influenced observed trends and reliability of future projections.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China. Several authors reported receiving consulting fees, speaking fees, or research support from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Alcohol-associated liver disease (ALD) will likely become the leading cause of HCC-related mortality by 2026, and metabolic dysfunction–associated steatotic liver disease (MASLD) is projected to become the second leading cause by 2032, a new analysis found.
METHODOLOGY:
- HCC accounts for 75%-85% of primary liver cancers and most liver cancer deaths. Researchers have observed an upward trend in the incidence of and mortality from HCC in the past 2 decades.
- This cross-sectional study analyzed 188,280 HCC-related deaths among adults aged 25 and older to determine trends in mortality rates and project age-standardized mortality rates through 2040. Data came from the National Vital Statistics System database from 2006 to 2022.
- Researchers stratified mortality data by etiology of liver disease (ALD, hepatitis B virus, hepatitis C virus, and MASLD), age groups (25-64 or 65 and older years), sex, and race/ethnicity.
- Demographic data showed that 77.4% of deaths occurred in men, 55.6% in individuals aged 65 years or older, and 62.3% in White individuals.
TAKEAWAY:
- Overall, the age-standardized mortality rate for HCC-related deaths increased from 3.65 per 100,000 persons in 2006 to 5.03 in 2022 and was projected to increase to 6.39 per 100,000 persons by 2040.
- Sex- and age-related disparities were substantial. Men had much higher rates of HCC-related mortality than women (8.15 vs 2.33 per 100,000 persons), with a projected rate among men of 9.78 per 100,000 persons by 2040. HCC-related mortality rates for people aged 65 years or older were 10 times higher than for those aged 25-64 years (18.37 vs 1.79 per 100,000 persons) in 2022 and was projected to reach 32.81 per 100,000 persons by 2040 in the older group.
- Although hepatitis C virus–related deaths were projected to decline from 0.69 to 0.03 per 100,000 persons by 2034, ALD- and MASLD-related deaths showed increasing trends, with both projected to become the two leading causes of HCC-related mortality in the next few years.
- Racial disparities were also evident. By 2040, the American Indian/Alaska Native population showed the highest increase in projected HCC-related mortality rates, which went from 5.46 per 100,000 persons in 2006 to a project increase to 14.71 per 100,000 persons.
IN PRACTICE:
“HCC mortality was projected to continue increasing in the US, primarily due to rising rates of deaths attributable to ALD and MASLD,” the authors wrote.
This “study highlights the importance of addressing these conditions to decrease the burden of liver disease and liver disease mortality in the future,” Emad Qayed, MD, MPH, Emory University School of Medicine, Atlanta, wrote in an accompanying editorial.
SOURCE:
The study was led by Sikai Qiu, MM, The Second Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China, and was published online in JAMA Network Open.
LIMITATIONS:
The National Vital Statistics System database used in this study captured only mortality data without access to detailed clinical records or individual medical histories. Researchers could not analyze socioeconomic factors or individual-level risk factors owing to data anonymization requirements. Additionally, the inclusion of the COVID-19 pandemic period could have influenced observed trends and reliability of future projections.
DISCLOSURES:
This study was supported by grants from the National Natural Science Foundation of China. Several authors reported receiving consulting fees, speaking fees, or research support from various sources.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
VA Launches New Campaign to Attract More Veterans to Health Care
A new US Department of Veterans Affairs (VA) outreach campaign is encouraging all eligible veterans to enroll in VA health care, aiming to connect the roughly 1 million unenrolled veterans to care.
The campaign was prompted following reports of concerns from veterans about health issues—including mental health hurdles and thoughts of suicide—potentially related to repeated low-level artillery blasts, improvised explosive devices, missile launches, heavy fire, and other blast exposures.
Veterans enrolled in VA health care have access to specialty screenings and services to address issues related to blast exposure. Those who served in Vietnam, the Gulf War, Iraq, Afghanistan, and other specific locations are eligible for these benefits based on their deployments. They do not need to have any health conditions specifically associated with their service to be eligible.
“We take veteran concerns about repeated blast exposure very seriously, and we are studying this matter urgently to learn more about potential health impacts,” VA Secretary Denis McDonough said. “While we do that, we don’t want veterans to wait—they should enroll in VA health care today to get full access to primary care, mental health care, regular screenings, specialty care, and more. That’s what this outreach effort is all about: getting veterans in our care, because veterans who come to VA are proven to do better.”
The campaign will consist of text messages and emails sent directly to veterans, in addition to thousands of nationwide events, advertising, and social media campaigns. It is the latest effort to appeal to more veterans and is part of the largest outreach campaign in VA history, which began when President Joseph R. Biden signed the PACT Act into law in 2022. As a result > 835,000 veterans have enrolled in VA health care (a 37% increase), > 900,000 veterans have upgraded their priority groups, making them eligible for health care with fewer copays (a record), and > 4.4 million veterans and survivors have applied for disability compensation benefits (another record).
Increased enrollment benefits not only the individuals enrolled in VA health care, but those who come after.
"[W]e are constantly looking for ways to improve that care as science and research tells us about new concerns," said VA Under Secretary for Health Shereef Elnahal, MD. "The more veterans who enroll, the more we can learn about the impact of blast exposure—and the better care we can ultimately provide those who served."
A new US Department of Veterans Affairs (VA) outreach campaign is encouraging all eligible veterans to enroll in VA health care, aiming to connect the roughly 1 million unenrolled veterans to care.
The campaign was prompted following reports of concerns from veterans about health issues—including mental health hurdles and thoughts of suicide—potentially related to repeated low-level artillery blasts, improvised explosive devices, missile launches, heavy fire, and other blast exposures.
Veterans enrolled in VA health care have access to specialty screenings and services to address issues related to blast exposure. Those who served in Vietnam, the Gulf War, Iraq, Afghanistan, and other specific locations are eligible for these benefits based on their deployments. They do not need to have any health conditions specifically associated with their service to be eligible.
“We take veteran concerns about repeated blast exposure very seriously, and we are studying this matter urgently to learn more about potential health impacts,” VA Secretary Denis McDonough said. “While we do that, we don’t want veterans to wait—they should enroll in VA health care today to get full access to primary care, mental health care, regular screenings, specialty care, and more. That’s what this outreach effort is all about: getting veterans in our care, because veterans who come to VA are proven to do better.”
The campaign will consist of text messages and emails sent directly to veterans, in addition to thousands of nationwide events, advertising, and social media campaigns. It is the latest effort to appeal to more veterans and is part of the largest outreach campaign in VA history, which began when President Joseph R. Biden signed the PACT Act into law in 2022. As a result > 835,000 veterans have enrolled in VA health care (a 37% increase), > 900,000 veterans have upgraded their priority groups, making them eligible for health care with fewer copays (a record), and > 4.4 million veterans and survivors have applied for disability compensation benefits (another record).
Increased enrollment benefits not only the individuals enrolled in VA health care, but those who come after.
"[W]e are constantly looking for ways to improve that care as science and research tells us about new concerns," said VA Under Secretary for Health Shereef Elnahal, MD. "The more veterans who enroll, the more we can learn about the impact of blast exposure—and the better care we can ultimately provide those who served."
A new US Department of Veterans Affairs (VA) outreach campaign is encouraging all eligible veterans to enroll in VA health care, aiming to connect the roughly 1 million unenrolled veterans to care.
The campaign was prompted following reports of concerns from veterans about health issues—including mental health hurdles and thoughts of suicide—potentially related to repeated low-level artillery blasts, improvised explosive devices, missile launches, heavy fire, and other blast exposures.
Veterans enrolled in VA health care have access to specialty screenings and services to address issues related to blast exposure. Those who served in Vietnam, the Gulf War, Iraq, Afghanistan, and other specific locations are eligible for these benefits based on their deployments. They do not need to have any health conditions specifically associated with their service to be eligible.
“We take veteran concerns about repeated blast exposure very seriously, and we are studying this matter urgently to learn more about potential health impacts,” VA Secretary Denis McDonough said. “While we do that, we don’t want veterans to wait—they should enroll in VA health care today to get full access to primary care, mental health care, regular screenings, specialty care, and more. That’s what this outreach effort is all about: getting veterans in our care, because veterans who come to VA are proven to do better.”
The campaign will consist of text messages and emails sent directly to veterans, in addition to thousands of nationwide events, advertising, and social media campaigns. It is the latest effort to appeal to more veterans and is part of the largest outreach campaign in VA history, which began when President Joseph R. Biden signed the PACT Act into law in 2022. As a result > 835,000 veterans have enrolled in VA health care (a 37% increase), > 900,000 veterans have upgraded their priority groups, making them eligible for health care with fewer copays (a record), and > 4.4 million veterans and survivors have applied for disability compensation benefits (another record).
Increased enrollment benefits not only the individuals enrolled in VA health care, but those who come after.
"[W]e are constantly looking for ways to improve that care as science and research tells us about new concerns," said VA Under Secretary for Health Shereef Elnahal, MD. "The more veterans who enroll, the more we can learn about the impact of blast exposure—and the better care we can ultimately provide those who served."
Physician Attitudes About Veterans Affairs Video Connect Encounters
Physician Attitudes About Veterans Affairs Video Connect Encounters
Prior to the COVID-19 pandemic, health care systems had been increasingly focused on expanding care delivery through clinical video telehealth (CVT) services.1-3 These modalities offer clinicians and patients opportunities to interact without needing face-to-face visits. CVT services offer significant advantages to patients who encounter challenges accessing traditional face-to-face services, including those living in rural or underserved areas, individuals with mobility limitations, and those with difficulty attending appointments due to work or caregiving commitments.4 The COVID-19 pandemic accelerated the expansion of CVT services to mitigate the spread of the virus.1
Despite its evident advantages, widespread adoption of CVT has encountered resistance.2 Physicians have frequently expressed concerns about the reliability and functionality of CVT platforms for scheduled encounters and frustration with inadequate training.4-6 Additionally, there is a lack trust in the technology, as physicians are unfamiliar with reimbursement or workload capture associated with CVT. Physicians have concerns that telecommunication may diminish the intangible aspects of the “art of medicine.”4 As a result, the implementation of telehealth services has been inconsistent, with successful adoption limited to specific medical and surgical specialties.4 Only recently have entire departments within major health care systems expressed interest in providing comprehensive CVT services in response to the challenges posed by the COVID-19 pandemic.4
The Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) provides an appropriate setting for assessing clinician perceptions of telehealth services. Since 2003, the VHA has significantly expanded CVT services to eligible veterans and has used the VA Video Connect (VVC) platform since 2018.7-10 Through VVC, VA staff and clinicians may schedule video visits with patients, meet with patients through virtual face-to-face interaction, and share relevant laboratory results and imaging through screen sharing. Prior research has shown increased accessibility to care through VVC. For example, a single-site study demonstrated that VVC implementation for delivering psychotherapies significantly increased CVT encounters from 15% to 85% among veterans with anxiety and/or depression.11
The VA New Mexico Healthcare System (VANMHCS) serves a high volume of veterans living in remote and rural regions and significantly increased its use of CVT during the COVID-19 pandemic to reduce in-person visits. Expectedly, this was met with a variety of challenges. Herein, we sought to assess physician perspectives, concerns, and attitudes toward VVC via semistructured interviews. Our hypothesis was that VA physicians may feel uncomfortable with video encounters but recognize the growing importance of such practices providing specialty care to veterans in rural areas.
METHODS
A semistructured interview protocol was created following discussions with physicians from the VANMHCS Medicine Service. Questions were constructed to assess the following domains: overarching views of video telehealth, perceptions of various applications for conducting VVC encounters, and barriers to the broad implementation of video telehealth. A qualitative investigation specialist aided with question development. Two pilot interviews were conducted prior to performing the interviews with the recruited participants to evaluate the quality and delivery of questions.
All VANMHCS physicians who provided outpatient care within the Department of Medicine and had completed ≥ 1 VVC encounter were eligible to participate. Invitations were disseminated via email, and follow-up emails to encourage participation were sent periodically for 2 months following the initial request. Union approval was obtained to interview employees for a research study. In total, 64 physicians were invited and 13 (20%) chose to participate. As the study did not involve assessing medical interventions among patients, a waiver of informed consent was granted by the VANMHCS Institutional Review Board. Physicians who participated in this study were informed that their responses would be used for reporting purposes and could be rescinded at any time.
Data Analysis
Semistructured interviews were conducted by a single interviewer and recorded using Microsoft Teams. The interviews took place between February 2021 and December 2021 and lasted 5 to 15 minutes, with a mean duration of 9 minutes. Verbal informed consent was obtained from all participants before the interviews. Interviewees were encouraged to expand on their responses to structured questions by recounting past experiences with VVC. Recorded audio was additionally transcribed via Microsoft Teams, and the research team reviewed the transcriptions to ensure accuracy.
The tracking and coding of responses to interview questions were conducted using Microsoft Excel. Initially, 5 transcripts were reviewed and responses were assessed by 2 study team members through open coding. All team members examined the 5 coded transcripts to identify differences and reach a consensus for any discrepancies. Based on recommendations from all team members regarding nuanced excerpts of transcripts, 1 study team member coded the remaining interviews. Thematic analysis was subsequently conducted according to the method described by Braun and Clarke.12 Themes were developed both deductively and inductively by reviewing the direct responses to interview questions and identifying emerging patterns of data, respectively. Indicative quotes representing each theme were carefully chosen for reporting.
RESULTS
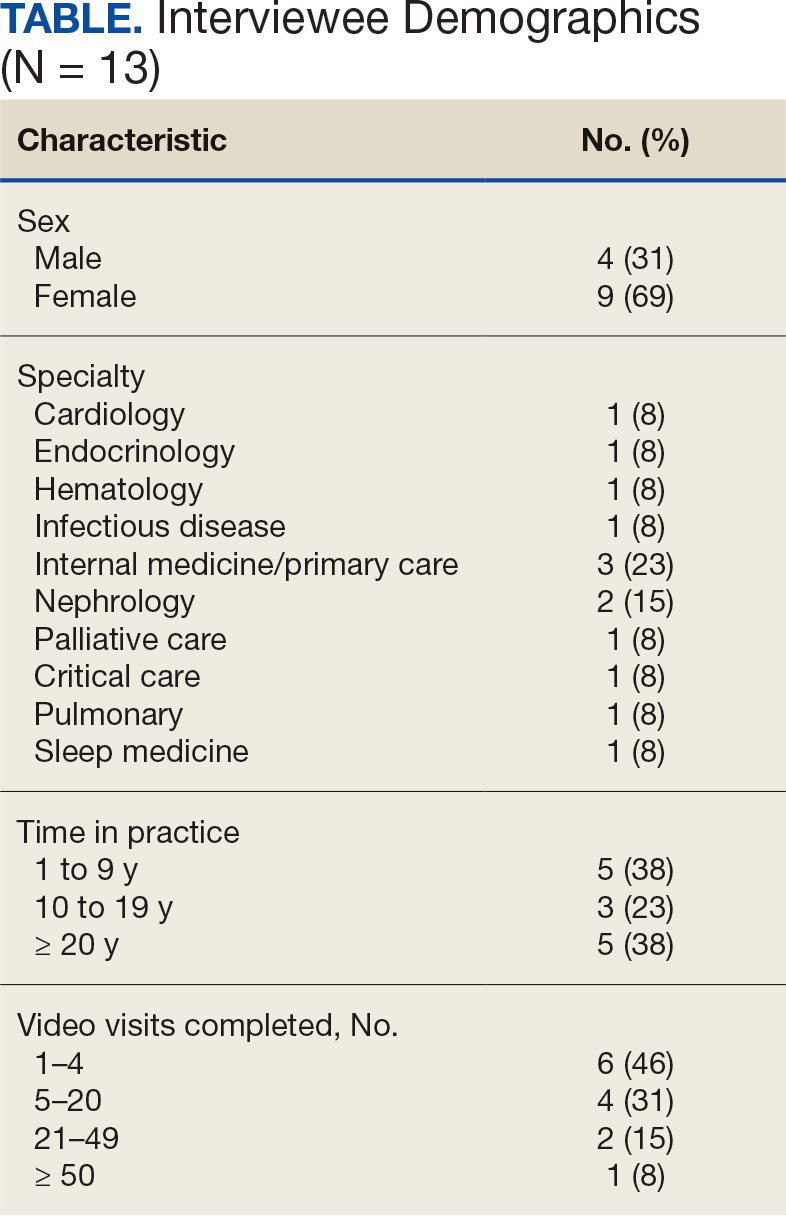
Thirteen interviews were conducted and 9 participants (69%) were female. Participating physicians included 3 internal medicine/primary care physicians (23%), 2 nephrologists (15%), and 1 (8%) from cardiology, endocrinology, hematology, infectious diseases, palliative care, critical care, pulmonology, and sleep medicine. Years of post training experience among physicians ranged from 1 to 9 years (n = 5, 38%), 10 to 19 years (n = 3, 23%), and . 20 years (n = 5, 38%). Seven participants (54%) had conducted ≥ 5 VVC visits, with 1 physician completing > 50 video visits (Table).
Using open coding and a deductive approach to thematic analysis, 5 themes were identified: (1) VVC software and internet connection issues affected implementation; (2) patient technological literacy affected veteran and physician comfort with VVC; (3) integration of supportive measures was desired; (4) CVT services may increasingly be used to enhance access to care; and (5) in-person encounters afforded unique advantages over CVT. Illustrative quotes from physicians that reflect these themes can be found in the Appendix.
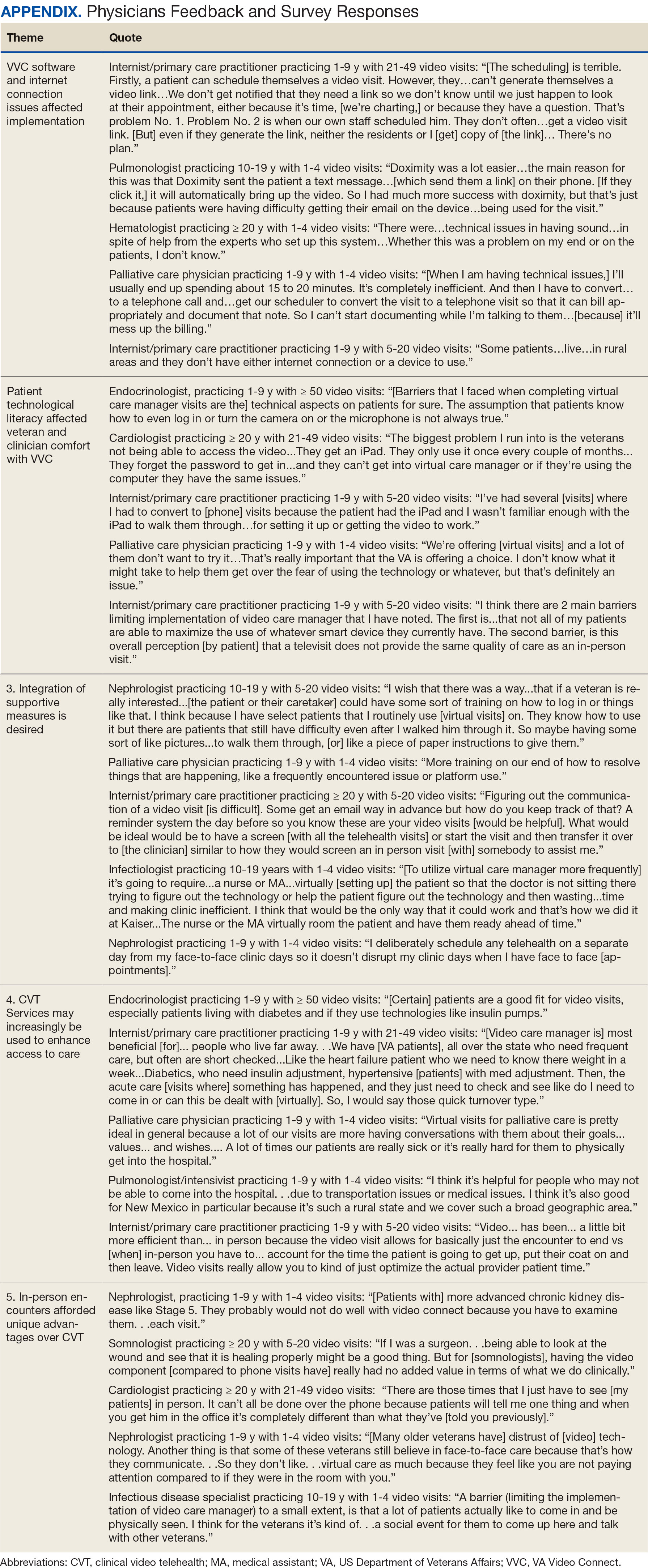
Theme 1: VVC software and internet connection issues affected its implementation. Most participants expressed concern about the technical challenges with VVC. Interviewees cited inconsistencies for both patients and physicians receiving emails with links to join VVC visits, which should be generated when appointments are scheduled. Some physicians were unaware of scheduled VVC visits until the day of the appointment and only received the link via email. Such issues appeared to occur regardless whether the physicians or support staff scheduled the encounter. Poor video and audio quality was also cited as significant barriers to successful VVC visits and were often not resolvable through troubleshooting efforts by physicians, patients, or support personnel. Given the limited time allotted to each patient encounter, such issues could significantly impact the physician’s ability to remain on schedule. Moreover, connectivity problems led to significant lapses, delays in audio and video transmission, and complete disconnections from the VVC encounter. This was a significant concern for participants, given the rural nature of New Mexico and the large geographical gaps in internet service throughout the state.
Theme 2: Patient technological literacy affected veteran and physician comfort with VVC. Successful VVC appointments require high-speed Internet and compatible hardware. Physicians indicated that some patients reported difficulties with critical steps in the process, such as logging into the VVC platform or ensuring their microphones and cameras were active. Physicians also expressed concern about older veterans’ ability to utilize electronic devices, noting they may generally be less technology savvy. Additionally, physicians reported that despite offering the option of a virtual visit, many veterans preferred in-person visits, regardless of the drive time required. This appeared related to a fear of using the technology, which led veterans to believe that virtual visits do not provide the same quality of care as in-person visits.
Theme 3: Integration of supportive measures is desired. Interviewees felt that integrated VVC technical assistance and technology literacy education were imperative. First, training the patient or the patient’s caregiver on how to complete a VVC encounter using the preferred device and the VVC platform would be beneficial. Second, education to inform physicians about common troubleshooting issues could help streamline VVC encounters. Third, managing a VVC encounter similarly to standard in-person visits could allow for better patient and physician experience. For example, physicians suggested that a medical assistant or a nurse triage the patient, take vital signs, and set them up in a room, potentially at a regional VA community based outpatient clinic. Such efforts would also allow patients to receive specialty care in remote areas where only primary care is generally offered. Support staff could assist with technological issues, such as setting up the VVC encounter and addressing potential problems before the physician joins the encounter, thereby preventing delays in patient care. Finally, physicians felt that designating a day solely for CVT visits would help prevent disruption in care with in-person visits.
Theme 4: CVT services may increasingly be used to enhance access to care. Physicians felt that VVC would help patients encountering obstacles in accessing conventional in person services, including patients in rural and underserved areas, with disabilities, or with scheduling challenges.4 Patients with chronic conditions might drive the use of virtual visits, as many of these patients are already accustomed to remote medical monitoring. Data from devices such as scales and continuous glucose monitors can be easily reviewed during VVC visits. Second, video encounters facilitate closer monitoring that some patients might otherwise skip due to significant travel barriers, especially in a rural state like New Mexico. Lastly, VVC may be more efficient than in person visits as they eliminate the need for lengthy parking, checking in, and checking out processes. Thus, if technological issues are resolved, a typical physician’s day in the clinic may be more efficient with virtual visits.
Theme 5: In-person encounters afforded unique advantages over CVT. Some physicians felt in-person visits still offer unique advantages. They opined that the selection of appropriate candidates for CVT is critical. Patients requiring a physical examination should be scheduled for in person visits. For example, patients with advanced chronic kidney disease who require accurate volume status assessment or patients who have recently undergone surgery and need detailed wound inspection should be seen in the clinic. In-person visits may also be preferable for patients with recurrent admissions, or those whose condition is difficult to assess; accurate assessments of such patients may help prevent readmissions. Finally, many patients are more comfortable and satisfied with in-person visits, which are perceived as a more standard or traditional process. Respondents noted that some patients felt physicians may not focus as much attention during a VVC visit as they do during in-person visits. There were also concerns that some patients feel more motivation to come to in-person visits, as they see the VA as a place to interact with other veterans and staff with whom they are familiar and comfortable.
DISCUSSION
VANMHCS physicians, which serves veterans across an expansive territory ranging from Southern Colorado to West Texas. About 4.6 million veterans reside in rural regions, constituting roughly 25% of the total veteran population, a pattern mirrored in New Mexico.13 Medicine Service physicians agreed on a number of themes: VVC user-interface issues may affect its use and effectiveness, technological literacy was important for both patients and health care staff, technical support staff roles before and during VVC visits should be standardized, CVT is likely to increase health care delivery, and in-person encounters are preferred for many patients.
This is the first study to qualitatively evaluate a diverse group of physicians at a VA medical center incorporating CVT services across specialties. A few related qualitative studies have been conducted external to VHA, generally evaluating clinicians within a single specialty. Kalicki and colleagues surveyed 16 physicians working at a large home-based primary care program in New York City between April and June 2020 to identify and explore barriers to telehealth among homebound older adults. Similarly to our study, physicians noted that many patients required assistance (family members or caregivers) with the visit, either due to technological literacy issues or medical conditions like dementia.14
Heyer and colleagues surveyed 29 oncologists at an urban academic center prior to the COVID-19 pandemic. Similar to our observations, the oncologists said telemedicine helped eliminate travel as a barrier to health care. Heyer and colleagues noted difficulty for oncologists in performing virtual physical examinations, despite training. This group did note the benefits when being selective as to which clinical issues they would handle virtually vs in person.15
Budhwani and colleagues reported that mental health professionals in an academic setting cited difficulty establishing therapeutic relationships via telehealth and felt that this affected quality of care.16 While this was not a topic during our interviews, it is reasonable to question how potentially missed nonverbal cues may impact patient assessments.
Notably, technological issues were common among all reviewed studies. These ranged from internet connectivity issues to necessary electronic devices. As mentioned, these barriers are more prevalent in rural states like New Mexico.
Limitations
All participants in this study were Medicine Service physicians of a single VA health care system, which may limit generalizability. Many of our respondents were female (69%), compared with 39.2% of active internal medicine physicians and therefore may not be representative.17 Nearly one-half of our participants only completed 1 to 4 VVC encounters, which may have contributed to the emergence of a common theme regarding technological issues. Physicians with more experience with CVT services may be more skilled at troubleshooting technological issues that arise during visits.
CONCLUSIONS
Our study, conducted with VANMHCS physicians, illuminated 5 key themes influencing the use and implementation of video encounters: technological issues, technological literacy, a desire for integrated support measures, perceived future growth of video telehealth, and the unique advantages of in-person visits. Addressing technological barriers and providing more extensive training may streamline CVT use. However, it is vital to recognize the unique benefits of in-person visits and consider the benefits of each modality along with patient preferences when selecting the best care venue. As health care evolves, better understanding and acting upon these themes will optimize telehealth services, particularly in rural areas. Future research should involve patients and other health care team members to further explore strategies for effective CVT service integration.
Appendix
- Monaghesh E, Hajizadeh A. The role of telehealth during covid-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20(1):1193. doi:10.1186/s12889-020-09301-4
- Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018;24(1):4-12. doi:10.1177/1357633X16674087
- Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
- Yellowlees P, Nakagawa K, Pakyurek M, Hanson A, Elder J, Kales HC. Rapid conversion of an outpatient psychiatric clinic to a 100% virtual telepsychiatry clinic in response to covid-19. Pyschiatr Serv. 2020;71(7):749-752. doi:10.1176/appi.ps.202000230
- Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324. doi:10.1258/1357633042602053
- Osuji TA, Macias M, McMullen C, et al. Clinician perspectives on implementing video visits in home-based palliative care. Palliat Med Rep. 2020;1(1):221-226. doi:10.1089/pmr.2020.0074
- Darkins A. The growth of telehealth services in the Veterans Health Administration between 1994 and 2014: a study in the diffusion of innovation. Telemed J E Health. 2014;20(9):761-768. doi:10.1089/tmj.2014.0143
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/nejmra1601705
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA video connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: Baseline assessment. BMC Geriatr. 2021;21(1):502. doi:10.1186/s12877-021-02454-w
- Padala KP, Wilson KB, Gauss CH, Stovall JD, Padala PR. VA video connect for clinical care in older adults in a rural state during the covid-19 pandemic: cross-sectional study. J Med Internet Res. 2020;22(9)e21561. doi:10.2196/21561
- Myers US, Coulon S, Knies K, et al. Lessons learned in implementing VA video connect for evidence-based psychotherapies for anxiety and depression in the veterans healthcare administration. J Technol Behav Sci. 2020;6(2):320-326. doi:10.1007/s41347-020-00161-8
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. doi:10.1191/1478088706qp063oa
- US Department of Veterans Affairs, National Center for Feterans Analysis and Statistics. Accessed September 18, 2024. www.va.gov/vetdata/report.asp
- Kalicki AV, Moody KA, Franzosa E, Gliatto PM, Ornstein KA. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc. 2021;69(9):2404-2411. doi:10.1111/jgs.17163
- Heyer A, Granberg RE, Rising KL, Binder AF, Gentsch AT, Handley NR. Medical oncology professionals’ perceptions of telehealth video visits. JAMA Netw Open. 2021;4(1) e2033967. doi:10.1001/jamanetworkopen.2020.33967
- Budhwani S, Fujioka JK, Chu C, et al. Delivering mental health care virtually during the COVID-19 pandemic: qualitative evaluation of provider experiences in a scaled context. JMIR Form Res. 2021;5(9)e30280. doi:10.2196/30280
- Association of American Medical Colleges. Active physicians by sex and specialty, 2021. AAMC. Accessed September 18, 2024. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-specialty-2021
Prior to the COVID-19 pandemic, health care systems had been increasingly focused on expanding care delivery through clinical video telehealth (CVT) services.1-3 These modalities offer clinicians and patients opportunities to interact without needing face-to-face visits. CVT services offer significant advantages to patients who encounter challenges accessing traditional face-to-face services, including those living in rural or underserved areas, individuals with mobility limitations, and those with difficulty attending appointments due to work or caregiving commitments.4 The COVID-19 pandemic accelerated the expansion of CVT services to mitigate the spread of the virus.1
Despite its evident advantages, widespread adoption of CVT has encountered resistance.2 Physicians have frequently expressed concerns about the reliability and functionality of CVT platforms for scheduled encounters and frustration with inadequate training.4-6 Additionally, there is a lack trust in the technology, as physicians are unfamiliar with reimbursement or workload capture associated with CVT. Physicians have concerns that telecommunication may diminish the intangible aspects of the “art of medicine.”4 As a result, the implementation of telehealth services has been inconsistent, with successful adoption limited to specific medical and surgical specialties.4 Only recently have entire departments within major health care systems expressed interest in providing comprehensive CVT services in response to the challenges posed by the COVID-19 pandemic.4
The Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) provides an appropriate setting for assessing clinician perceptions of telehealth services. Since 2003, the VHA has significantly expanded CVT services to eligible veterans and has used the VA Video Connect (VVC) platform since 2018.7-10 Through VVC, VA staff and clinicians may schedule video visits with patients, meet with patients through virtual face-to-face interaction, and share relevant laboratory results and imaging through screen sharing. Prior research has shown increased accessibility to care through VVC. For example, a single-site study demonstrated that VVC implementation for delivering psychotherapies significantly increased CVT encounters from 15% to 85% among veterans with anxiety and/or depression.11
The VA New Mexico Healthcare System (VANMHCS) serves a high volume of veterans living in remote and rural regions and significantly increased its use of CVT during the COVID-19 pandemic to reduce in-person visits. Expectedly, this was met with a variety of challenges. Herein, we sought to assess physician perspectives, concerns, and attitudes toward VVC via semistructured interviews. Our hypothesis was that VA physicians may feel uncomfortable with video encounters but recognize the growing importance of such practices providing specialty care to veterans in rural areas.
METHODS
A semistructured interview protocol was created following discussions with physicians from the VANMHCS Medicine Service. Questions were constructed to assess the following domains: overarching views of video telehealth, perceptions of various applications for conducting VVC encounters, and barriers to the broad implementation of video telehealth. A qualitative investigation specialist aided with question development. Two pilot interviews were conducted prior to performing the interviews with the recruited participants to evaluate the quality and delivery of questions.
All VANMHCS physicians who provided outpatient care within the Department of Medicine and had completed ≥ 1 VVC encounter were eligible to participate. Invitations were disseminated via email, and follow-up emails to encourage participation were sent periodically for 2 months following the initial request. Union approval was obtained to interview employees for a research study. In total, 64 physicians were invited and 13 (20%) chose to participate. As the study did not involve assessing medical interventions among patients, a waiver of informed consent was granted by the VANMHCS Institutional Review Board. Physicians who participated in this study were informed that their responses would be used for reporting purposes and could be rescinded at any time.
Data Analysis
Semistructured interviews were conducted by a single interviewer and recorded using Microsoft Teams. The interviews took place between February 2021 and December 2021 and lasted 5 to 15 minutes, with a mean duration of 9 minutes. Verbal informed consent was obtained from all participants before the interviews. Interviewees were encouraged to expand on their responses to structured questions by recounting past experiences with VVC. Recorded audio was additionally transcribed via Microsoft Teams, and the research team reviewed the transcriptions to ensure accuracy.
The tracking and coding of responses to interview questions were conducted using Microsoft Excel. Initially, 5 transcripts were reviewed and responses were assessed by 2 study team members through open coding. All team members examined the 5 coded transcripts to identify differences and reach a consensus for any discrepancies. Based on recommendations from all team members regarding nuanced excerpts of transcripts, 1 study team member coded the remaining interviews. Thematic analysis was subsequently conducted according to the method described by Braun and Clarke.12 Themes were developed both deductively and inductively by reviewing the direct responses to interview questions and identifying emerging patterns of data, respectively. Indicative quotes representing each theme were carefully chosen for reporting.
RESULTS
Thirteen interviews were conducted and 9 participants (69%) were female. Participating physicians included 3 internal medicine/primary care physicians (23%), 2 nephrologists (15%), and 1 (8%) from cardiology, endocrinology, hematology, infectious diseases, palliative care, critical care, pulmonology, and sleep medicine. Years of post training experience among physicians ranged from 1 to 9 years (n = 5, 38%), 10 to 19 years (n = 3, 23%), and . 20 years (n = 5, 38%). Seven participants (54%) had conducted ≥ 5 VVC visits, with 1 physician completing > 50 video visits (Table).

Using open coding and a deductive approach to thematic analysis, 5 themes were identified: (1) VVC software and internet connection issues affected implementation; (2) patient technological literacy affected veteran and physician comfort with VVC; (3) integration of supportive measures was desired; (4) CVT services may increasingly be used to enhance access to care; and (5) in-person encounters afforded unique advantages over CVT. Illustrative quotes from physicians that reflect these themes can be found in the Appendix.
Theme 1: VVC software and internet connection issues affected its implementation. Most participants expressed concern about the technical challenges with VVC. Interviewees cited inconsistencies for both patients and physicians receiving emails with links to join VVC visits, which should be generated when appointments are scheduled. Some physicians were unaware of scheduled VVC visits until the day of the appointment and only received the link via email. Such issues appeared to occur regardless whether the physicians or support staff scheduled the encounter. Poor video and audio quality was also cited as significant barriers to successful VVC visits and were often not resolvable through troubleshooting efforts by physicians, patients, or support personnel. Given the limited time allotted to each patient encounter, such issues could significantly impact the physician’s ability to remain on schedule. Moreover, connectivity problems led to significant lapses, delays in audio and video transmission, and complete disconnections from the VVC encounter. This was a significant concern for participants, given the rural nature of New Mexico and the large geographical gaps in internet service throughout the state.
Theme 2: Patient technological literacy affected veteran and physician comfort with VVC. Successful VVC appointments require high-speed Internet and compatible hardware. Physicians indicated that some patients reported difficulties with critical steps in the process, such as logging into the VVC platform or ensuring their microphones and cameras were active. Physicians also expressed concern about older veterans’ ability to utilize electronic devices, noting they may generally be less technology savvy. Additionally, physicians reported that despite offering the option of a virtual visit, many veterans preferred in-person visits, regardless of the drive time required. This appeared related to a fear of using the technology, which led veterans to believe that virtual visits do not provide the same quality of care as in-person visits.
Theme 3: Integration of supportive measures is desired. Interviewees felt that integrated VVC technical assistance and technology literacy education were imperative. First, training the patient or the patient’s caregiver on how to complete a VVC encounter using the preferred device and the VVC platform would be beneficial. Second, education to inform physicians about common troubleshooting issues could help streamline VVC encounters. Third, managing a VVC encounter similarly to standard in-person visits could allow for better patient and physician experience. For example, physicians suggested that a medical assistant or a nurse triage the patient, take vital signs, and set them up in a room, potentially at a regional VA community based outpatient clinic. Such efforts would also allow patients to receive specialty care in remote areas where only primary care is generally offered. Support staff could assist with technological issues, such as setting up the VVC encounter and addressing potential problems before the physician joins the encounter, thereby preventing delays in patient care. Finally, physicians felt that designating a day solely for CVT visits would help prevent disruption in care with in-person visits.
Theme 4: CVT services may increasingly be used to enhance access to care. Physicians felt that VVC would help patients encountering obstacles in accessing conventional in person services, including patients in rural and underserved areas, with disabilities, or with scheduling challenges.4 Patients with chronic conditions might drive the use of virtual visits, as many of these patients are already accustomed to remote medical monitoring. Data from devices such as scales and continuous glucose monitors can be easily reviewed during VVC visits. Second, video encounters facilitate closer monitoring that some patients might otherwise skip due to significant travel barriers, especially in a rural state like New Mexico. Lastly, VVC may be more efficient than in person visits as they eliminate the need for lengthy parking, checking in, and checking out processes. Thus, if technological issues are resolved, a typical physician’s day in the clinic may be more efficient with virtual visits.
Theme 5: In-person encounters afforded unique advantages over CVT. Some physicians felt in-person visits still offer unique advantages. They opined that the selection of appropriate candidates for CVT is critical. Patients requiring a physical examination should be scheduled for in person visits. For example, patients with advanced chronic kidney disease who require accurate volume status assessment or patients who have recently undergone surgery and need detailed wound inspection should be seen in the clinic. In-person visits may also be preferable for patients with recurrent admissions, or those whose condition is difficult to assess; accurate assessments of such patients may help prevent readmissions. Finally, many patients are more comfortable and satisfied with in-person visits, which are perceived as a more standard or traditional process. Respondents noted that some patients felt physicians may not focus as much attention during a VVC visit as they do during in-person visits. There were also concerns that some patients feel more motivation to come to in-person visits, as they see the VA as a place to interact with other veterans and staff with whom they are familiar and comfortable.
DISCUSSION
VANMHCS physicians, which serves veterans across an expansive territory ranging from Southern Colorado to West Texas. About 4.6 million veterans reside in rural regions, constituting roughly 25% of the total veteran population, a pattern mirrored in New Mexico.13 Medicine Service physicians agreed on a number of themes: VVC user-interface issues may affect its use and effectiveness, technological literacy was important for both patients and health care staff, technical support staff roles before and during VVC visits should be standardized, CVT is likely to increase health care delivery, and in-person encounters are preferred for many patients.
This is the first study to qualitatively evaluate a diverse group of physicians at a VA medical center incorporating CVT services across specialties. A few related qualitative studies have been conducted external to VHA, generally evaluating clinicians within a single specialty. Kalicki and colleagues surveyed 16 physicians working at a large home-based primary care program in New York City between April and June 2020 to identify and explore barriers to telehealth among homebound older adults. Similarly to our study, physicians noted that many patients required assistance (family members or caregivers) with the visit, either due to technological literacy issues or medical conditions like dementia.14
Heyer and colleagues surveyed 29 oncologists at an urban academic center prior to the COVID-19 pandemic. Similar to our observations, the oncologists said telemedicine helped eliminate travel as a barrier to health care. Heyer and colleagues noted difficulty for oncologists in performing virtual physical examinations, despite training. This group did note the benefits when being selective as to which clinical issues they would handle virtually vs in person.15
Budhwani and colleagues reported that mental health professionals in an academic setting cited difficulty establishing therapeutic relationships via telehealth and felt that this affected quality of care.16 While this was not a topic during our interviews, it is reasonable to question how potentially missed nonverbal cues may impact patient assessments.
Notably, technological issues were common among all reviewed studies. These ranged from internet connectivity issues to necessary electronic devices. As mentioned, these barriers are more prevalent in rural states like New Mexico.
Limitations
All participants in this study were Medicine Service physicians of a single VA health care system, which may limit generalizability. Many of our respondents were female (69%), compared with 39.2% of active internal medicine physicians and therefore may not be representative.17 Nearly one-half of our participants only completed 1 to 4 VVC encounters, which may have contributed to the emergence of a common theme regarding technological issues. Physicians with more experience with CVT services may be more skilled at troubleshooting technological issues that arise during visits.
CONCLUSIONS
Our study, conducted with VANMHCS physicians, illuminated 5 key themes influencing the use and implementation of video encounters: technological issues, technological literacy, a desire for integrated support measures, perceived future growth of video telehealth, and the unique advantages of in-person visits. Addressing technological barriers and providing more extensive training may streamline CVT use. However, it is vital to recognize the unique benefits of in-person visits and consider the benefits of each modality along with patient preferences when selecting the best care venue. As health care evolves, better understanding and acting upon these themes will optimize telehealth services, particularly in rural areas. Future research should involve patients and other health care team members to further explore strategies for effective CVT service integration.
Appendix

Prior to the COVID-19 pandemic, health care systems had been increasingly focused on expanding care delivery through clinical video telehealth (CVT) services.1-3 These modalities offer clinicians and patients opportunities to interact without needing face-to-face visits. CVT services offer significant advantages to patients who encounter challenges accessing traditional face-to-face services, including those living in rural or underserved areas, individuals with mobility limitations, and those with difficulty attending appointments due to work or caregiving commitments.4 The COVID-19 pandemic accelerated the expansion of CVT services to mitigate the spread of the virus.1
Despite its evident advantages, widespread adoption of CVT has encountered resistance.2 Physicians have frequently expressed concerns about the reliability and functionality of CVT platforms for scheduled encounters and frustration with inadequate training.4-6 Additionally, there is a lack trust in the technology, as physicians are unfamiliar with reimbursement or workload capture associated with CVT. Physicians have concerns that telecommunication may diminish the intangible aspects of the “art of medicine.”4 As a result, the implementation of telehealth services has been inconsistent, with successful adoption limited to specific medical and surgical specialties.4 Only recently have entire departments within major health care systems expressed interest in providing comprehensive CVT services in response to the challenges posed by the COVID-19 pandemic.4
The Veterans Health Administration (VHA) of the US Department of Veterans Affairs (VA) provides an appropriate setting for assessing clinician perceptions of telehealth services. Since 2003, the VHA has significantly expanded CVT services to eligible veterans and has used the VA Video Connect (VVC) platform since 2018.7-10 Through VVC, VA staff and clinicians may schedule video visits with patients, meet with patients through virtual face-to-face interaction, and share relevant laboratory results and imaging through screen sharing. Prior research has shown increased accessibility to care through VVC. For example, a single-site study demonstrated that VVC implementation for delivering psychotherapies significantly increased CVT encounters from 15% to 85% among veterans with anxiety and/or depression.11
The VA New Mexico Healthcare System (VANMHCS) serves a high volume of veterans living in remote and rural regions and significantly increased its use of CVT during the COVID-19 pandemic to reduce in-person visits. Expectedly, this was met with a variety of challenges. Herein, we sought to assess physician perspectives, concerns, and attitudes toward VVC via semistructured interviews. Our hypothesis was that VA physicians may feel uncomfortable with video encounters but recognize the growing importance of such practices providing specialty care to veterans in rural areas.
METHODS
A semistructured interview protocol was created following discussions with physicians from the VANMHCS Medicine Service. Questions were constructed to assess the following domains: overarching views of video telehealth, perceptions of various applications for conducting VVC encounters, and barriers to the broad implementation of video telehealth. A qualitative investigation specialist aided with question development. Two pilot interviews were conducted prior to performing the interviews with the recruited participants to evaluate the quality and delivery of questions.
All VANMHCS physicians who provided outpatient care within the Department of Medicine and had completed ≥ 1 VVC encounter were eligible to participate. Invitations were disseminated via email, and follow-up emails to encourage participation were sent periodically for 2 months following the initial request. Union approval was obtained to interview employees for a research study. In total, 64 physicians were invited and 13 (20%) chose to participate. As the study did not involve assessing medical interventions among patients, a waiver of informed consent was granted by the VANMHCS Institutional Review Board. Physicians who participated in this study were informed that their responses would be used for reporting purposes and could be rescinded at any time.
Data Analysis
Semistructured interviews were conducted by a single interviewer and recorded using Microsoft Teams. The interviews took place between February 2021 and December 2021 and lasted 5 to 15 minutes, with a mean duration of 9 minutes. Verbal informed consent was obtained from all participants before the interviews. Interviewees were encouraged to expand on their responses to structured questions by recounting past experiences with VVC. Recorded audio was additionally transcribed via Microsoft Teams, and the research team reviewed the transcriptions to ensure accuracy.
The tracking and coding of responses to interview questions were conducted using Microsoft Excel. Initially, 5 transcripts were reviewed and responses were assessed by 2 study team members through open coding. All team members examined the 5 coded transcripts to identify differences and reach a consensus for any discrepancies. Based on recommendations from all team members regarding nuanced excerpts of transcripts, 1 study team member coded the remaining interviews. Thematic analysis was subsequently conducted according to the method described by Braun and Clarke.12 Themes were developed both deductively and inductively by reviewing the direct responses to interview questions and identifying emerging patterns of data, respectively. Indicative quotes representing each theme were carefully chosen for reporting.
RESULTS
Thirteen interviews were conducted and 9 participants (69%) were female. Participating physicians included 3 internal medicine/primary care physicians (23%), 2 nephrologists (15%), and 1 (8%) from cardiology, endocrinology, hematology, infectious diseases, palliative care, critical care, pulmonology, and sleep medicine. Years of post training experience among physicians ranged from 1 to 9 years (n = 5, 38%), 10 to 19 years (n = 3, 23%), and . 20 years (n = 5, 38%). Seven participants (54%) had conducted ≥ 5 VVC visits, with 1 physician completing > 50 video visits (Table).

Using open coding and a deductive approach to thematic analysis, 5 themes were identified: (1) VVC software and internet connection issues affected implementation; (2) patient technological literacy affected veteran and physician comfort with VVC; (3) integration of supportive measures was desired; (4) CVT services may increasingly be used to enhance access to care; and (5) in-person encounters afforded unique advantages over CVT. Illustrative quotes from physicians that reflect these themes can be found in the Appendix.
Theme 1: VVC software and internet connection issues affected its implementation. Most participants expressed concern about the technical challenges with VVC. Interviewees cited inconsistencies for both patients and physicians receiving emails with links to join VVC visits, which should be generated when appointments are scheduled. Some physicians were unaware of scheduled VVC visits until the day of the appointment and only received the link via email. Such issues appeared to occur regardless whether the physicians or support staff scheduled the encounter. Poor video and audio quality was also cited as significant barriers to successful VVC visits and were often not resolvable through troubleshooting efforts by physicians, patients, or support personnel. Given the limited time allotted to each patient encounter, such issues could significantly impact the physician’s ability to remain on schedule. Moreover, connectivity problems led to significant lapses, delays in audio and video transmission, and complete disconnections from the VVC encounter. This was a significant concern for participants, given the rural nature of New Mexico and the large geographical gaps in internet service throughout the state.
Theme 2: Patient technological literacy affected veteran and physician comfort with VVC. Successful VVC appointments require high-speed Internet and compatible hardware. Physicians indicated that some patients reported difficulties with critical steps in the process, such as logging into the VVC platform or ensuring their microphones and cameras were active. Physicians also expressed concern about older veterans’ ability to utilize electronic devices, noting they may generally be less technology savvy. Additionally, physicians reported that despite offering the option of a virtual visit, many veterans preferred in-person visits, regardless of the drive time required. This appeared related to a fear of using the technology, which led veterans to believe that virtual visits do not provide the same quality of care as in-person visits.
Theme 3: Integration of supportive measures is desired. Interviewees felt that integrated VVC technical assistance and technology literacy education were imperative. First, training the patient or the patient’s caregiver on how to complete a VVC encounter using the preferred device and the VVC platform would be beneficial. Second, education to inform physicians about common troubleshooting issues could help streamline VVC encounters. Third, managing a VVC encounter similarly to standard in-person visits could allow for better patient and physician experience. For example, physicians suggested that a medical assistant or a nurse triage the patient, take vital signs, and set them up in a room, potentially at a regional VA community based outpatient clinic. Such efforts would also allow patients to receive specialty care in remote areas where only primary care is generally offered. Support staff could assist with technological issues, such as setting up the VVC encounter and addressing potential problems before the physician joins the encounter, thereby preventing delays in patient care. Finally, physicians felt that designating a day solely for CVT visits would help prevent disruption in care with in-person visits.
Theme 4: CVT services may increasingly be used to enhance access to care. Physicians felt that VVC would help patients encountering obstacles in accessing conventional in person services, including patients in rural and underserved areas, with disabilities, or with scheduling challenges.4 Patients with chronic conditions might drive the use of virtual visits, as many of these patients are already accustomed to remote medical monitoring. Data from devices such as scales and continuous glucose monitors can be easily reviewed during VVC visits. Second, video encounters facilitate closer monitoring that some patients might otherwise skip due to significant travel barriers, especially in a rural state like New Mexico. Lastly, VVC may be more efficient than in person visits as they eliminate the need for lengthy parking, checking in, and checking out processes. Thus, if technological issues are resolved, a typical physician’s day in the clinic may be more efficient with virtual visits.
Theme 5: In-person encounters afforded unique advantages over CVT. Some physicians felt in-person visits still offer unique advantages. They opined that the selection of appropriate candidates for CVT is critical. Patients requiring a physical examination should be scheduled for in person visits. For example, patients with advanced chronic kidney disease who require accurate volume status assessment or patients who have recently undergone surgery and need detailed wound inspection should be seen in the clinic. In-person visits may also be preferable for patients with recurrent admissions, or those whose condition is difficult to assess; accurate assessments of such patients may help prevent readmissions. Finally, many patients are more comfortable and satisfied with in-person visits, which are perceived as a more standard or traditional process. Respondents noted that some patients felt physicians may not focus as much attention during a VVC visit as they do during in-person visits. There were also concerns that some patients feel more motivation to come to in-person visits, as they see the VA as a place to interact with other veterans and staff with whom they are familiar and comfortable.
DISCUSSION
VANMHCS physicians, which serves veterans across an expansive territory ranging from Southern Colorado to West Texas. About 4.6 million veterans reside in rural regions, constituting roughly 25% of the total veteran population, a pattern mirrored in New Mexico.13 Medicine Service physicians agreed on a number of themes: VVC user-interface issues may affect its use and effectiveness, technological literacy was important for both patients and health care staff, technical support staff roles before and during VVC visits should be standardized, CVT is likely to increase health care delivery, and in-person encounters are preferred for many patients.
This is the first study to qualitatively evaluate a diverse group of physicians at a VA medical center incorporating CVT services across specialties. A few related qualitative studies have been conducted external to VHA, generally evaluating clinicians within a single specialty. Kalicki and colleagues surveyed 16 physicians working at a large home-based primary care program in New York City between April and June 2020 to identify and explore barriers to telehealth among homebound older adults. Similarly to our study, physicians noted that many patients required assistance (family members or caregivers) with the visit, either due to technological literacy issues or medical conditions like dementia.14
Heyer and colleagues surveyed 29 oncologists at an urban academic center prior to the COVID-19 pandemic. Similar to our observations, the oncologists said telemedicine helped eliminate travel as a barrier to health care. Heyer and colleagues noted difficulty for oncologists in performing virtual physical examinations, despite training. This group did note the benefits when being selective as to which clinical issues they would handle virtually vs in person.15
Budhwani and colleagues reported that mental health professionals in an academic setting cited difficulty establishing therapeutic relationships via telehealth and felt that this affected quality of care.16 While this was not a topic during our interviews, it is reasonable to question how potentially missed nonverbal cues may impact patient assessments.
Notably, technological issues were common among all reviewed studies. These ranged from internet connectivity issues to necessary electronic devices. As mentioned, these barriers are more prevalent in rural states like New Mexico.
Limitations
All participants in this study were Medicine Service physicians of a single VA health care system, which may limit generalizability. Many of our respondents were female (69%), compared with 39.2% of active internal medicine physicians and therefore may not be representative.17 Nearly one-half of our participants only completed 1 to 4 VVC encounters, which may have contributed to the emergence of a common theme regarding technological issues. Physicians with more experience with CVT services may be more skilled at troubleshooting technological issues that arise during visits.
CONCLUSIONS
Our study, conducted with VANMHCS physicians, illuminated 5 key themes influencing the use and implementation of video encounters: technological issues, technological literacy, a desire for integrated support measures, perceived future growth of video telehealth, and the unique advantages of in-person visits. Addressing technological barriers and providing more extensive training may streamline CVT use. However, it is vital to recognize the unique benefits of in-person visits and consider the benefits of each modality along with patient preferences when selecting the best care venue. As health care evolves, better understanding and acting upon these themes will optimize telehealth services, particularly in rural areas. Future research should involve patients and other health care team members to further explore strategies for effective CVT service integration.
Appendix

- Monaghesh E, Hajizadeh A. The role of telehealth during covid-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20(1):1193. doi:10.1186/s12889-020-09301-4
- Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018;24(1):4-12. doi:10.1177/1357633X16674087
- Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
- Yellowlees P, Nakagawa K, Pakyurek M, Hanson A, Elder J, Kales HC. Rapid conversion of an outpatient psychiatric clinic to a 100% virtual telepsychiatry clinic in response to covid-19. Pyschiatr Serv. 2020;71(7):749-752. doi:10.1176/appi.ps.202000230
- Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324. doi:10.1258/1357633042602053
- Osuji TA, Macias M, McMullen C, et al. Clinician perspectives on implementing video visits in home-based palliative care. Palliat Med Rep. 2020;1(1):221-226. doi:10.1089/pmr.2020.0074
- Darkins A. The growth of telehealth services in the Veterans Health Administration between 1994 and 2014: a study in the diffusion of innovation. Telemed J E Health. 2014;20(9):761-768. doi:10.1089/tmj.2014.0143
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/nejmra1601705
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA video connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: Baseline assessment. BMC Geriatr. 2021;21(1):502. doi:10.1186/s12877-021-02454-w
- Padala KP, Wilson KB, Gauss CH, Stovall JD, Padala PR. VA video connect for clinical care in older adults in a rural state during the covid-19 pandemic: cross-sectional study. J Med Internet Res. 2020;22(9)e21561. doi:10.2196/21561
- Myers US, Coulon S, Knies K, et al. Lessons learned in implementing VA video connect for evidence-based psychotherapies for anxiety and depression in the veterans healthcare administration. J Technol Behav Sci. 2020;6(2):320-326. doi:10.1007/s41347-020-00161-8
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. doi:10.1191/1478088706qp063oa
- US Department of Veterans Affairs, National Center for Feterans Analysis and Statistics. Accessed September 18, 2024. www.va.gov/vetdata/report.asp
- Kalicki AV, Moody KA, Franzosa E, Gliatto PM, Ornstein KA. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc. 2021;69(9):2404-2411. doi:10.1111/jgs.17163
- Heyer A, Granberg RE, Rising KL, Binder AF, Gentsch AT, Handley NR. Medical oncology professionals’ perceptions of telehealth video visits. JAMA Netw Open. 2021;4(1) e2033967. doi:10.1001/jamanetworkopen.2020.33967
- Budhwani S, Fujioka JK, Chu C, et al. Delivering mental health care virtually during the COVID-19 pandemic: qualitative evaluation of provider experiences in a scaled context. JMIR Form Res. 2021;5(9)e30280. doi:10.2196/30280
- Association of American Medical Colleges. Active physicians by sex and specialty, 2021. AAMC. Accessed September 18, 2024. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-specialty-2021
- Monaghesh E, Hajizadeh A. The role of telehealth during covid-19 outbreak: a systematic review based on current evidence. BMC Public Health. 2020;20(1):1193. doi:10.1186/s12889-020-09301-4
- Scott Kruse C, Karem P, Shifflett K, Vegi L, Ravi K, Brooks M. Evaluating barriers to adopting telemedicine worldwide: a systematic review. J Telemed Telecare. 2018;24(1):4-12. doi:10.1177/1357633X16674087
- Bashshur RL, Howell JD, Krupinski EA, Harms KM, Bashshur N, Doarn CR. The empirical foundations of telemedicine interventions in primary care. Telemed J E Health. 2016;22(5):342-375. doi:10.1089/tmj.2016.0045
- Yellowlees P, Nakagawa K, Pakyurek M, Hanson A, Elder J, Kales HC. Rapid conversion of an outpatient psychiatric clinic to a 100% virtual telepsychiatry clinic in response to covid-19. Pyschiatr Serv. 2020;71(7):749-752. doi:10.1176/appi.ps.202000230
- Hailey D, Ohinmaa A, Roine R. Study quality and evidence of benefit in recent assessments of telemedicine. J Telemed Telecare. 2004;10(6):318-324. doi:10.1258/1357633042602053
- Osuji TA, Macias M, McMullen C, et al. Clinician perspectives on implementing video visits in home-based palliative care. Palliat Med Rep. 2020;1(1):221-226. doi:10.1089/pmr.2020.0074
- Darkins A. The growth of telehealth services in the Veterans Health Administration between 1994 and 2014: a study in the diffusion of innovation. Telemed J E Health. 2014;20(9):761-768. doi:10.1089/tmj.2014.0143
- Dorsey ER, Topol EJ. State of telehealth. N Engl J Med. 2016;375(2):154-161. doi:10.1056/nejmra1601705
- Alexander NB, Phillips K, Wagner-Felkey J, et al. Team VA video connect (VVC) to optimize mobility and physical activity in post-hospital discharge older veterans: Baseline assessment. BMC Geriatr. 2021;21(1):502. doi:10.1186/s12877-021-02454-w
- Padala KP, Wilson KB, Gauss CH, Stovall JD, Padala PR. VA video connect for clinical care in older adults in a rural state during the covid-19 pandemic: cross-sectional study. J Med Internet Res. 2020;22(9)e21561. doi:10.2196/21561
- Myers US, Coulon S, Knies K, et al. Lessons learned in implementing VA video connect for evidence-based psychotherapies for anxiety and depression in the veterans healthcare administration. J Technol Behav Sci. 2020;6(2):320-326. doi:10.1007/s41347-020-00161-8
- Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol. 2006;3(2):77-101. doi:10.1191/1478088706qp063oa
- US Department of Veterans Affairs, National Center for Feterans Analysis and Statistics. Accessed September 18, 2024. www.va.gov/vetdata/report.asp
- Kalicki AV, Moody KA, Franzosa E, Gliatto PM, Ornstein KA. Barriers to telehealth access among homebound older adults. J Am Geriatr Soc. 2021;69(9):2404-2411. doi:10.1111/jgs.17163
- Heyer A, Granberg RE, Rising KL, Binder AF, Gentsch AT, Handley NR. Medical oncology professionals’ perceptions of telehealth video visits. JAMA Netw Open. 2021;4(1) e2033967. doi:10.1001/jamanetworkopen.2020.33967
- Budhwani S, Fujioka JK, Chu C, et al. Delivering mental health care virtually during the COVID-19 pandemic: qualitative evaluation of provider experiences in a scaled context. JMIR Form Res. 2021;5(9)e30280. doi:10.2196/30280
- Association of American Medical Colleges. Active physicians by sex and specialty, 2021. AAMC. Accessed September 18, 2024. https://www.aamc.org/data-reports/workforce/data/active-physicians-sex-specialty-2021
Physician Attitudes About Veterans Affairs Video Connect Encounters
Physician Attitudes About Veterans Affairs Video Connect Encounters
Allergic Contact Dermatitis: New Culprits
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
New allergens responsible for contact dermatitis emerge regularly. During the Dermatology Days of Paris 2024 conference, Angèle Soria, MD, PhD, a dermatologist at Tenon Hospital in Paris, France, outlined four major categories driving this trend. Among them are (meth)acrylates found in nail cosmetics used in salons or do-it-yourself false nail kits that can be bought online.
Isothiazolinones
a preservative used in many cosmetics; (meth)acrylates; essential oils; and epoxy resins used in industry and leisure activities.
Around 15 years ago, parabens, commonly used as preservatives in cosmetics, were identified as endocrine disruptors. In response, they were largely replaced by newer preservatives, notably MI. However, this led to a proliferation of allergic contact dermatitis in Europe between 2010 and 2013.
“About 10% of the population that we tested showed allergies to these preservatives, primarily found in cosmetics,” explained Soria. Since 2015, the use of MI in leave-on cosmetics has been prohibited in Europe and its concentration restricted in rinse-off products. However, cosmetics sold online from outside Europe may not comply with these regulations.
MI is also present in water-based paints to prevent mold. “A few years ago, we started seeing patients with facial angioedema, sometimes combined with asthma, caused by these isothiazolinone preservatives, including in patients who are not professional painters,” said Soria. More recently, attention has shifted to MI’s presence in household cleaning products. A 2020 Spanish study found MI in 76% of 34 analyzed cleaning products.
MI-based fungicides are also used to treat leather during transport, which can lead to contact allergies among professionals and consumers alike. Additionally, MI has been identified in children’s toys, including slime gels, and in florists’ gel cubes used to preserve flowers.
“We are therefore surrounded by these preservatives, which are no longer only in cosmetics,” warned the dermatologist.
(Meth)acrylates
Another major allergen category is (meth)acrylates, responsible for many cases of allergic contact dermatitis. Acrylates and their derivatives are widely used in everyday items. They are low–molecular weight monomers, sensitizing on contact with the skin. Their polymerized forms include materials like Plexiglas.
“We are currently witnessing an epidemic of contact dermatitis in the general population, mainly due to nail cosmetics, such as semipermanent nail polishes and at-home false nail kits,” reported Soria. Nail cosmetics account for 97% of new sensitization cases involving (meth)acrylates. These allergens often cause severe dermatitis, prompting the European Union to mandate labeling in 2020, warning that these products are “for professional use only” and can “cause allergic reactions.”
Beyond nail cosmetics, these allergens are also found in dental products (such as trays), ECG electrodes, prosthetics, glucose sensors, surgical adhesives, and some electronic devices like earbuds and phone screens. Notably, patients sensitized to acrylates via nail kits may experience reactions during dental treatments involving acrylates.
Investigating Essential Oil Use
Essential oils, distinct from vegetable oils like almond or argan, are another known allergen. Often considered risk-free due to their “natural” label, these products are widely used topically, orally, or via inhalation for various purposes, such as treating respiratory infections or creating relaxing atmospheres. However, essential oils contain fragrant molecules like terpenes, which can become highly allergenic over time, especially after repeated exposure.
Soria emphasized the importance of asking patients about their use of essential oils, especially tea tree and lavender oils, which are commonly used but rarely mentioned by patients unless prompted.
Epoxy Resins in Recreational Use
Epoxy resins are a growing cause of contact allergies, not just in professional settings such as aeronautics and construction work but also increasingly in recreational activities. Soria highlighted the case of a 12-year-old girl hospitalized for severe facial edema after engaging in resin crafts inspired by TikTok. For 6 months, she had been creating resin objects, such as bowls and cutting boards, using vinyl gloves and a Filtering FacePiece 2 mask under adult supervision.
“The growing popularity and online availability of epoxy resins mean that allergic reactions should now be considered even in nonprofessional contexts,” warned Soria.
Clinical Approach
When dermatologists suspect allergic contact dermatitis, the first step is to treat the condition with corticosteroid creams. This is followed by a detailed patient interview to identify suspected allergens in products they’ve used.
Patch testing is then conducted to confirm the allergen. Small chambers containing potential allergens are applied to the upper back for 48 hours without removal. Results are read 2-5 days later, with some cases requiring a 7-day follow-up.
The patient’s occupation is an important factor, as certain professions, such as hairdressing, healthcare, or beauty therapy, are known to trigger allergic contact dermatitis. Similarly, certain hobbies may also play a role.
A thorough approach ensures accurate diagnosis and targeted prevention strategies.
This story was translated from Medscape’s French edition using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Skin Cancer Risk Elevated Among Blood, Marrow Transplant Survivors
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
with a cumulative incidence of 27.4% over 30 years, according to the results of a cohort study.
METHODOLOGY:
- The retrospective cohort study included 3880 BMT survivors (median age, 44 years; 55.8% men; 4.9% Black, 12.1 Hispanic, and 74.7% non-Hispanic White individuals) who underwent transplant between 1974 to 2014.
- Participants completed the BMT Survivor Study survey and were followed up for a median of 9.5 years.
- The primary outcomes were the development of subsequent cutaneous malignant neoplasms (BCC, SCC, or melanoma).
TAKEAWAY:
- The 30-year cumulative incidence of any cutaneous malignant neoplasm was 27.4% — 18% for BCC, 9.8% for SCC, and 3.7% for melanoma.
- A higher risk for skin cancer was reported for patients aged 50 years or more (subdistribution hazard ratio [SHR], 2.23; 95% CI, 1.83-2.71), and men (SHR, 1.40; 95% CI, 1.18-1.65).
- Allogeneic BMT with chronic graft-vs-host disease (cGVHD) increased the risk for skin cancer (SHR, 1.84; 95% CI, 1.37-2.47), compared with autologous BMT, while post-BMT immunosuppression increased risk for all types (overall SHR, 1.53; 95% CI, 1.26-1.86).
- The risk for any skin cancer was significantly lower in Black individuals (SHR, 0.14; 95% CI, 0.05-0.37), Hispanic individuals (SHR, 0.29; 95%CI, 0.20-0.62), and patients of other races or who were multiracial (SHR, 0.22; 95% CI, 0.13-0.37) than in non-Hispanic White patients.
IN PRACTICE:
In the study, “risk factors for post-BMT cutaneous malignant neoplasms included pretransplant treatment with a monoclonal antibody, cGVHD, and posttransplant immunosuppression,” the authors wrote, adding that the findings “could inform targeted surveillance of BMT survivors.” Most BMT survivors, “do not undergo routine dermatologic surveillance, highlighting the need to understand risk factors and incorporate risk-informed dermatologic surveillance into survivorship care plans.”
SOURCE:
The study was led by Kristy K. Broman, MD, MPH, University of Alabama at Birmingham, and was published online on December 18 in JAMA Dermatology.
LIMITATIONS:
Limitations included self-reported data and possible underreporting of melanoma cases in the SEER database. Additionally, the study did not capture other risk factors for cutaneous malignant neoplasms such as skin phototype, ultraviolet light exposure, or family history. The duration of posttransplant immunosuppression was not collected, and surveys were administered at variable intervals, though all were completed more than 2 years post BMT.
DISCLOSURES:
The study was supported by the National Cancer Institute (NCI) and the Leukemia and Lymphoma Society. Broman received grants from NCI, the National Center for Advancing Translational Sciences, the American Society of Clinical Oncology, and the American College of Surgeons. Another author reported receiving grants outside this work.
This article was created using several editorial tools, including artificial intelligence, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Eye Toxicities Are a Growing Concern With Certain ADCs
Such experts called for greater collaboration between oncologists and ophthalmologists, in interviews with Medscape Medical News.
ADCs combine a monoclonal antibody targeted at an antigen overexpressed on cancer cells with a toxic chemotherapy payload — the aim being to maximize the effectiveness of the drug against the tumor while minimizing the damage to healthy tissues and reducing systemic toxicity.
Yet trastuzumab duocarmazine (T-Duo), a third-generation human epidermal growth factor receptor 2 (HER2)–targeted ADC designed to treat HER2-positive breast cancer, was recently found to have a notable adverse effect in the TULIP trial of 437 patients.
As reported by Medscape Medical News, the drug was associated with a significant increase in progression-free survival over physician’s choice of therapy. However, 78% of patients in the ADC group experienced at least one treatment-emergent ocular toxicity adverse event vs 29.2% of those in the control group.
Moreover, grade 3 or high ocular toxicity events were reported by 21% of patients in the experimental group compared with none of those who received physician’s choice.
Ocular Toxicities Seen on Ocular Surface
Ocular toxicities with these drugs are “not necessarily a new thing,” said Joann J. Kang, MD, director, Cornea and Refractive Surgery, and associate professor of ophthalmology at Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York.
“But what we’re seeing with certain ADCs is a lot of ocular toxicity, especially on the ocular surface,” with the degree toxicity varying depending on the ADC in question. “It’s definitely a real concern.”
Kang noted that separate from T-Duo, certain ADCs already come with black box warnings for ocular toxicity, including:
- Belantamab mafodotin (Blenrep) — approved for relapsed or refractory multiple myeloma and carries a warning specifically for keratopathy.
- Tisotumab vedotin (Tivdak) — indicated for recurrent or metastatic cervical cancer and can cause changes in the corneal epithelium and conjunctiva.
- Mirvetuximab soravtansine (Elahere) — used to treat folate receptor (FR) alpha–positive ovarian, fallopian tube, and peritoneal cancers and can lead to keratopathy, blurred vision, and dry eyes.
Indeed, the American Academy of Ophthalmology 2024 annual meeting saw research presented indicating that mirvetuximab was associated with moderate or severe corneal toxicity in 47% of patients treated for primary gynecologic malignancies.
As reported by Medscape Medical News, the study, by researchers at Byers Eye Institute of Stanford University in Stanford, California, was a retrospective analysis of 36 eyes of 18 women who received mirvetuximab for FR alpha–positive, platinum-resistant primary ovarian cancer.
What Are the Causes?
But why would a drug that is targeted specifically to a cancer tumor, thanks to the presence of a monoclonal antibody, cause off-target effects such as ocular toxicity?
Kathy D. Miller, MD, professor of oncology and medicine at Indiana University School of Medicine in Indianapolis, pointed out that they are targeted in a relative and not absolute sense, meaning that the antigen target may not be truly limited to the tumor cells.
There can also be “a lot of ways that you could get systemic toxicities,” she said.
For example, if the linker connecting the antibody and the chemotherapy payload breaks prematurely or is not stable, or if the drug leaches out into the tumor microenvironment and then is “picked up into the circulation, that can give you systemic toxicity,” she said.
In addition, the drug may, once it is in the tumor cells, be metabolized to an active metabolite that could, again, result in systemic exposure.
Side Effects Are Underappreciated and Distressing
Ocular toxicity remains underappreciated among oncologists prescribing these drugs. One reason is that it “did not get enough attention” in the initial clinical trial reports, Miller said she suspects.
Another potential reason for this is that “we’re not used to thinking about it because it’s not particularly common among the drugs that oncologists use frequently,” she added. Additionally, it tends to come up later during treatment, “so people have to be on therapy for some time before you start to see it.”
Nevertheless, Miller underlined that ocular toxicity “can be particularly distressing for patients, as it’s uncomfortable [and] can lead to scarring, so some of the vision issues can be permanent.”
“We often see in these situations that there are different types of ocular toxicities that present in different patients,” said Jane L. Meisel, MD, co-director, Breast Medical Oncology, Department of Hematology and Medical Oncology at Emory University School of Medicine in Atlanta.
“Corneal damage is pretty common, and patients can present with blurry vision, or dry eyes, or light sensitivity. And unlike some side effects, these are things that really impact people at every waking moment of their day.”
“So they’re pretty clinically significant side effects, even if they’re not life-threatening,” Meisel emphasized.
Miller suspects that more heavily pretreated patients may be more likely to experience ocular toxicity, as “there’s a much higher incidence of dry eyes in our patients than we recognize.”
She added: “We don’t usually ask about it, and we certainly don’t routinely do Schirmer’s tests,” which determine whether the eye produces enough tears to keep it moist.
Preventive Measures
For patients receiving tisotumab or mirvetuximab who experience ocular toxicity, Kang said the recommendation is to use steroid eye drops before, during, and after treatment with the ADC.
However, she noted that steroids have not been found to be useful in patients given belantamab, so clinicians have tried vasoconstrictor eye drops immediately prior to the infusion, as well as ocular cooling masks, which “are thought to help by reducing blood supply to the ocular areas.”
Other approaches to minimize ocular toxicity have included longer infusion times, so it’s “not so much of a hefty dose at one time,” Kang added.
She underlined that grade 2 and 3 ocular toxicities can lead to dose delays or dose modifications, and “usually by the time you get a grade 4 event, then you may need to discontinue the medication.”
This can have consequences for the patients because they are often “very sick, and this may be their third agent that they’re trying,” or it may be that their tumor is responding to a new treatment, but it has to be withheld because of an ocular toxicity.
“It can be incredibly frustrating for patients, and also for oncologists, and then for ophthalmologists,” Kang said.
Closer Collaboration Between Specialists Needed
What’s known about ocular side effects in patients taking ADCs underlines that there is a need for closer collaboration between oncologists and ophthalmologists.
“In oncology, especially as immunotherapies came to the forefront, our relationships with our endocrinology colleagues have become stronger because we’ve needed them to help us manage things like thyroid toxicity and pituitary issues related to immunotherapy,” Meisel said.
With toxicities that may be “very impactful for patient quality of life, like ocular toxicity, we will need to learn more about them and develop protocols for management, along with our ophthalmology colleagues, so that we can keep patients as comfortable as possible, while maximizing the efficacy of these drugs.”
Miller agreed, saying oncologists need to have “a conversation with a local ophthalmologist,” although she conceded that, in many areas, such specialists “are in short supply.”
The oncologist “not only needs to be aware” of and looking for ocular toxicity when using these ADCs but also needs to be thinking: “If I run into trouble here, who’s my ophthalmology backup? Are they familiar with this drug? And do we have a plan for the multispecialty management of patients who run into this toxicity?”
Setting Counts When Assessing Toxicities
But do all these considerations mean that ADCs’ potential ocular toxicity should give clinicians pause when considering whether to use these drugs?
“What my patients most want are drugs that work; that are effective in controlling their tumors,” Miller said.
“Every drug we use has potential toxicities, and which toxicities are most physically troublesome [or] are the greatest concern may vary from patient to patient, and it may vary a lot from patients with metastatic disease to those in the curative setting.”
She explained that “toxicities that might not be prohibitive at all in the metastatic setting [may] have to be a much bigger part of our considerations” when moving drugs into the adjuvant or neoadjuvant setting.
This, Miller underlined, is where the ocular toxicity with these ADCs “may be much more prohibitive.”
TULIP was funded by Byondis BV.
Turner declared relationships with Novartis, AstraZeneca, Pfizer, Merck Sharp & Dohme, Lilly, Repare Therapeutics, Roche, GlaxoSmithKline, Gilead Sciences, Inivata, Guardant Health, Exact Sciences, and Relay Therapeutics.
Meisel declared relationships with Novartis, AstraZeneca, Genentech, Seagen, Olema Oncology, GE Healthcare, Pfizer, Stemline, and Sermonix Pharmaceuticals.
A version of this article appeared on Medscape.com.
Such experts called for greater collaboration between oncologists and ophthalmologists, in interviews with Medscape Medical News.
ADCs combine a monoclonal antibody targeted at an antigen overexpressed on cancer cells with a toxic chemotherapy payload — the aim being to maximize the effectiveness of the drug against the tumor while minimizing the damage to healthy tissues and reducing systemic toxicity.
Yet trastuzumab duocarmazine (T-Duo), a third-generation human epidermal growth factor receptor 2 (HER2)–targeted ADC designed to treat HER2-positive breast cancer, was recently found to have a notable adverse effect in the TULIP trial of 437 patients.
As reported by Medscape Medical News, the drug was associated with a significant increase in progression-free survival over physician’s choice of therapy. However, 78% of patients in the ADC group experienced at least one treatment-emergent ocular toxicity adverse event vs 29.2% of those in the control group.
Moreover, grade 3 or high ocular toxicity events were reported by 21% of patients in the experimental group compared with none of those who received physician’s choice.
Ocular Toxicities Seen on Ocular Surface
Ocular toxicities with these drugs are “not necessarily a new thing,” said Joann J. Kang, MD, director, Cornea and Refractive Surgery, and associate professor of ophthalmology at Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York.
“But what we’re seeing with certain ADCs is a lot of ocular toxicity, especially on the ocular surface,” with the degree toxicity varying depending on the ADC in question. “It’s definitely a real concern.”
Kang noted that separate from T-Duo, certain ADCs already come with black box warnings for ocular toxicity, including:
- Belantamab mafodotin (Blenrep) — approved for relapsed or refractory multiple myeloma and carries a warning specifically for keratopathy.
- Tisotumab vedotin (Tivdak) — indicated for recurrent or metastatic cervical cancer and can cause changes in the corneal epithelium and conjunctiva.
- Mirvetuximab soravtansine (Elahere) — used to treat folate receptor (FR) alpha–positive ovarian, fallopian tube, and peritoneal cancers and can lead to keratopathy, blurred vision, and dry eyes.
Indeed, the American Academy of Ophthalmology 2024 annual meeting saw research presented indicating that mirvetuximab was associated with moderate or severe corneal toxicity in 47% of patients treated for primary gynecologic malignancies.
As reported by Medscape Medical News, the study, by researchers at Byers Eye Institute of Stanford University in Stanford, California, was a retrospective analysis of 36 eyes of 18 women who received mirvetuximab for FR alpha–positive, platinum-resistant primary ovarian cancer.
What Are the Causes?
But why would a drug that is targeted specifically to a cancer tumor, thanks to the presence of a monoclonal antibody, cause off-target effects such as ocular toxicity?
Kathy D. Miller, MD, professor of oncology and medicine at Indiana University School of Medicine in Indianapolis, pointed out that they are targeted in a relative and not absolute sense, meaning that the antigen target may not be truly limited to the tumor cells.
There can also be “a lot of ways that you could get systemic toxicities,” she said.
For example, if the linker connecting the antibody and the chemotherapy payload breaks prematurely or is not stable, or if the drug leaches out into the tumor microenvironment and then is “picked up into the circulation, that can give you systemic toxicity,” she said.
In addition, the drug may, once it is in the tumor cells, be metabolized to an active metabolite that could, again, result in systemic exposure.
Side Effects Are Underappreciated and Distressing
Ocular toxicity remains underappreciated among oncologists prescribing these drugs. One reason is that it “did not get enough attention” in the initial clinical trial reports, Miller said she suspects.
Another potential reason for this is that “we’re not used to thinking about it because it’s not particularly common among the drugs that oncologists use frequently,” she added. Additionally, it tends to come up later during treatment, “so people have to be on therapy for some time before you start to see it.”
Nevertheless, Miller underlined that ocular toxicity “can be particularly distressing for patients, as it’s uncomfortable [and] can lead to scarring, so some of the vision issues can be permanent.”
“We often see in these situations that there are different types of ocular toxicities that present in different patients,” said Jane L. Meisel, MD, co-director, Breast Medical Oncology, Department of Hematology and Medical Oncology at Emory University School of Medicine in Atlanta.
“Corneal damage is pretty common, and patients can present with blurry vision, or dry eyes, or light sensitivity. And unlike some side effects, these are things that really impact people at every waking moment of their day.”
“So they’re pretty clinically significant side effects, even if they’re not life-threatening,” Meisel emphasized.
Miller suspects that more heavily pretreated patients may be more likely to experience ocular toxicity, as “there’s a much higher incidence of dry eyes in our patients than we recognize.”
She added: “We don’t usually ask about it, and we certainly don’t routinely do Schirmer’s tests,” which determine whether the eye produces enough tears to keep it moist.
Preventive Measures
For patients receiving tisotumab or mirvetuximab who experience ocular toxicity, Kang said the recommendation is to use steroid eye drops before, during, and after treatment with the ADC.
However, she noted that steroids have not been found to be useful in patients given belantamab, so clinicians have tried vasoconstrictor eye drops immediately prior to the infusion, as well as ocular cooling masks, which “are thought to help by reducing blood supply to the ocular areas.”
Other approaches to minimize ocular toxicity have included longer infusion times, so it’s “not so much of a hefty dose at one time,” Kang added.
She underlined that grade 2 and 3 ocular toxicities can lead to dose delays or dose modifications, and “usually by the time you get a grade 4 event, then you may need to discontinue the medication.”
This can have consequences for the patients because they are often “very sick, and this may be their third agent that they’re trying,” or it may be that their tumor is responding to a new treatment, but it has to be withheld because of an ocular toxicity.
“It can be incredibly frustrating for patients, and also for oncologists, and then for ophthalmologists,” Kang said.
Closer Collaboration Between Specialists Needed
What’s known about ocular side effects in patients taking ADCs underlines that there is a need for closer collaboration between oncologists and ophthalmologists.
“In oncology, especially as immunotherapies came to the forefront, our relationships with our endocrinology colleagues have become stronger because we’ve needed them to help us manage things like thyroid toxicity and pituitary issues related to immunotherapy,” Meisel said.
With toxicities that may be “very impactful for patient quality of life, like ocular toxicity, we will need to learn more about them and develop protocols for management, along with our ophthalmology colleagues, so that we can keep patients as comfortable as possible, while maximizing the efficacy of these drugs.”
Miller agreed, saying oncologists need to have “a conversation with a local ophthalmologist,” although she conceded that, in many areas, such specialists “are in short supply.”
The oncologist “not only needs to be aware” of and looking for ocular toxicity when using these ADCs but also needs to be thinking: “If I run into trouble here, who’s my ophthalmology backup? Are they familiar with this drug? And do we have a plan for the multispecialty management of patients who run into this toxicity?”
Setting Counts When Assessing Toxicities
But do all these considerations mean that ADCs’ potential ocular toxicity should give clinicians pause when considering whether to use these drugs?
“What my patients most want are drugs that work; that are effective in controlling their tumors,” Miller said.
“Every drug we use has potential toxicities, and which toxicities are most physically troublesome [or] are the greatest concern may vary from patient to patient, and it may vary a lot from patients with metastatic disease to those in the curative setting.”
She explained that “toxicities that might not be prohibitive at all in the metastatic setting [may] have to be a much bigger part of our considerations” when moving drugs into the adjuvant or neoadjuvant setting.
This, Miller underlined, is where the ocular toxicity with these ADCs “may be much more prohibitive.”
TULIP was funded by Byondis BV.
Turner declared relationships with Novartis, AstraZeneca, Pfizer, Merck Sharp & Dohme, Lilly, Repare Therapeutics, Roche, GlaxoSmithKline, Gilead Sciences, Inivata, Guardant Health, Exact Sciences, and Relay Therapeutics.
Meisel declared relationships with Novartis, AstraZeneca, Genentech, Seagen, Olema Oncology, GE Healthcare, Pfizer, Stemline, and Sermonix Pharmaceuticals.
A version of this article appeared on Medscape.com.
Such experts called for greater collaboration between oncologists and ophthalmologists, in interviews with Medscape Medical News.
ADCs combine a monoclonal antibody targeted at an antigen overexpressed on cancer cells with a toxic chemotherapy payload — the aim being to maximize the effectiveness of the drug against the tumor while minimizing the damage to healthy tissues and reducing systemic toxicity.
Yet trastuzumab duocarmazine (T-Duo), a third-generation human epidermal growth factor receptor 2 (HER2)–targeted ADC designed to treat HER2-positive breast cancer, was recently found to have a notable adverse effect in the TULIP trial of 437 patients.
As reported by Medscape Medical News, the drug was associated with a significant increase in progression-free survival over physician’s choice of therapy. However, 78% of patients in the ADC group experienced at least one treatment-emergent ocular toxicity adverse event vs 29.2% of those in the control group.
Moreover, grade 3 or high ocular toxicity events were reported by 21% of patients in the experimental group compared with none of those who received physician’s choice.
Ocular Toxicities Seen on Ocular Surface
Ocular toxicities with these drugs are “not necessarily a new thing,” said Joann J. Kang, MD, director, Cornea and Refractive Surgery, and associate professor of ophthalmology at Albert Einstein College of Medicine, Montefiore Medical Center, Bronx, New York.
“But what we’re seeing with certain ADCs is a lot of ocular toxicity, especially on the ocular surface,” with the degree toxicity varying depending on the ADC in question. “It’s definitely a real concern.”
Kang noted that separate from T-Duo, certain ADCs already come with black box warnings for ocular toxicity, including:
- Belantamab mafodotin (Blenrep) — approved for relapsed or refractory multiple myeloma and carries a warning specifically for keratopathy.
- Tisotumab vedotin (Tivdak) — indicated for recurrent or metastatic cervical cancer and can cause changes in the corneal epithelium and conjunctiva.
- Mirvetuximab soravtansine (Elahere) — used to treat folate receptor (FR) alpha–positive ovarian, fallopian tube, and peritoneal cancers and can lead to keratopathy, blurred vision, and dry eyes.
Indeed, the American Academy of Ophthalmology 2024 annual meeting saw research presented indicating that mirvetuximab was associated with moderate or severe corneal toxicity in 47% of patients treated for primary gynecologic malignancies.
As reported by Medscape Medical News, the study, by researchers at Byers Eye Institute of Stanford University in Stanford, California, was a retrospective analysis of 36 eyes of 18 women who received mirvetuximab for FR alpha–positive, platinum-resistant primary ovarian cancer.
What Are the Causes?
But why would a drug that is targeted specifically to a cancer tumor, thanks to the presence of a monoclonal antibody, cause off-target effects such as ocular toxicity?
Kathy D. Miller, MD, professor of oncology and medicine at Indiana University School of Medicine in Indianapolis, pointed out that they are targeted in a relative and not absolute sense, meaning that the antigen target may not be truly limited to the tumor cells.
There can also be “a lot of ways that you could get systemic toxicities,” she said.
For example, if the linker connecting the antibody and the chemotherapy payload breaks prematurely or is not stable, or if the drug leaches out into the tumor microenvironment and then is “picked up into the circulation, that can give you systemic toxicity,” she said.
In addition, the drug may, once it is in the tumor cells, be metabolized to an active metabolite that could, again, result in systemic exposure.
Side Effects Are Underappreciated and Distressing
Ocular toxicity remains underappreciated among oncologists prescribing these drugs. One reason is that it “did not get enough attention” in the initial clinical trial reports, Miller said she suspects.
Another potential reason for this is that “we’re not used to thinking about it because it’s not particularly common among the drugs that oncologists use frequently,” she added. Additionally, it tends to come up later during treatment, “so people have to be on therapy for some time before you start to see it.”
Nevertheless, Miller underlined that ocular toxicity “can be particularly distressing for patients, as it’s uncomfortable [and] can lead to scarring, so some of the vision issues can be permanent.”
“We often see in these situations that there are different types of ocular toxicities that present in different patients,” said Jane L. Meisel, MD, co-director, Breast Medical Oncology, Department of Hematology and Medical Oncology at Emory University School of Medicine in Atlanta.
“Corneal damage is pretty common, and patients can present with blurry vision, or dry eyes, or light sensitivity. And unlike some side effects, these are things that really impact people at every waking moment of their day.”
“So they’re pretty clinically significant side effects, even if they’re not life-threatening,” Meisel emphasized.
Miller suspects that more heavily pretreated patients may be more likely to experience ocular toxicity, as “there’s a much higher incidence of dry eyes in our patients than we recognize.”
She added: “We don’t usually ask about it, and we certainly don’t routinely do Schirmer’s tests,” which determine whether the eye produces enough tears to keep it moist.
Preventive Measures
For patients receiving tisotumab or mirvetuximab who experience ocular toxicity, Kang said the recommendation is to use steroid eye drops before, during, and after treatment with the ADC.
However, she noted that steroids have not been found to be useful in patients given belantamab, so clinicians have tried vasoconstrictor eye drops immediately prior to the infusion, as well as ocular cooling masks, which “are thought to help by reducing blood supply to the ocular areas.”
Other approaches to minimize ocular toxicity have included longer infusion times, so it’s “not so much of a hefty dose at one time,” Kang added.
She underlined that grade 2 and 3 ocular toxicities can lead to dose delays or dose modifications, and “usually by the time you get a grade 4 event, then you may need to discontinue the medication.”
This can have consequences for the patients because they are often “very sick, and this may be their third agent that they’re trying,” or it may be that their tumor is responding to a new treatment, but it has to be withheld because of an ocular toxicity.
“It can be incredibly frustrating for patients, and also for oncologists, and then for ophthalmologists,” Kang said.
Closer Collaboration Between Specialists Needed
What’s known about ocular side effects in patients taking ADCs underlines that there is a need for closer collaboration between oncologists and ophthalmologists.
“In oncology, especially as immunotherapies came to the forefront, our relationships with our endocrinology colleagues have become stronger because we’ve needed them to help us manage things like thyroid toxicity and pituitary issues related to immunotherapy,” Meisel said.
With toxicities that may be “very impactful for patient quality of life, like ocular toxicity, we will need to learn more about them and develop protocols for management, along with our ophthalmology colleagues, so that we can keep patients as comfortable as possible, while maximizing the efficacy of these drugs.”
Miller agreed, saying oncologists need to have “a conversation with a local ophthalmologist,” although she conceded that, in many areas, such specialists “are in short supply.”
The oncologist “not only needs to be aware” of and looking for ocular toxicity when using these ADCs but also needs to be thinking: “If I run into trouble here, who’s my ophthalmology backup? Are they familiar with this drug? And do we have a plan for the multispecialty management of patients who run into this toxicity?”
Setting Counts When Assessing Toxicities
But do all these considerations mean that ADCs’ potential ocular toxicity should give clinicians pause when considering whether to use these drugs?
“What my patients most want are drugs that work; that are effective in controlling their tumors,” Miller said.
“Every drug we use has potential toxicities, and which toxicities are most physically troublesome [or] are the greatest concern may vary from patient to patient, and it may vary a lot from patients with metastatic disease to those in the curative setting.”
She explained that “toxicities that might not be prohibitive at all in the metastatic setting [may] have to be a much bigger part of our considerations” when moving drugs into the adjuvant or neoadjuvant setting.
This, Miller underlined, is where the ocular toxicity with these ADCs “may be much more prohibitive.”
TULIP was funded by Byondis BV.
Turner declared relationships with Novartis, AstraZeneca, Pfizer, Merck Sharp & Dohme, Lilly, Repare Therapeutics, Roche, GlaxoSmithKline, Gilead Sciences, Inivata, Guardant Health, Exact Sciences, and Relay Therapeutics.
Meisel declared relationships with Novartis, AstraZeneca, Genentech, Seagen, Olema Oncology, GE Healthcare, Pfizer, Stemline, and Sermonix Pharmaceuticals.
A version of this article appeared on Medscape.com.
Daratumumab Confirmed as SOC for AL Amyloidosis
Adding DARA to VCd (D-VCd; Darzalex Faspro; Janssen Biotech) provided deeper and more rapid hematologic response and clinically meaningful and statistically significant improvement in overall survival (OS) and major organ deterioration progression-free survival (MOD-PFS), combined with 40.7% cardiac complete response (CR), first author Efstathios Kastritis, MD, said during presentation of an oral abstract at the American Society of Hematology (ASH) 2024 Annual Meeting.
“The Andromeda study is the first comparing two contemporary regimens that shows a significant survival improvement for patients with AL amyloidosis,” said Kastritis, an associate professor at the National and Kapodistrian University of Athens in Greece. “These findings reaffirm frontline D-VCd as the standard of care in this difficult-to-treat disease.”
The regimen was approved for this indication in 2021 based on prior earlier findings from the Andromeda trial. The current results are from a preplanned analysis for MOD-PFS and OS.
At a median follow-up of 61.4 months, the overall hematologic CR rates were 59.5% and 19.2% among 388 patients randomized to receive D-VCd or VCd, respectively (odds ratio, 6.03), which showed continued improvement with additional DARA vs the 53.3% and 18.1% rates observed at the primary analysis, Kastritis reported.
Time to hematologic CR was 67.5 days and 85.0 days in the treatment groups, respectively, and median duration of hematologic CR was not reached in either group.
A significant 56% improvement was also observed in MOD-PFS (hazard ratio [HR], 0.44). Median MOD-PFS was not reached in the D-VCd group and was 30.3 months in the VCd group.
A significant 38% improvement was observed in OS (HR, 0.62), 5-year OS was 76.1% vs 64.7% in the D-VCd and VCd groups, respectively, he said.
“[The OS] benefit occurred even though more than 70% of the patients in the VCd arm who received a subsequent therapy were treated with a DARA-based regimen,” he stressed. “This further emphasizes the importance of using DSTS in the frontline setting.”
Trial participants had newly diagnosed AL amyloidosis with measurable hematologic disease, one or more involved organs, cardiac stage I-IIIA, estimated glomerular filtration rate of at least 20 mL/min, and absence of symptomatic multiple myeloma. They were randomized 1:1 to the two treatment groups. All patients received 1.3 mg/m2 of bortezomib by weekly injection, 300 mg/m2 of cyclophosphamide either by weekly oral or intravenous administration, and 20-40 mg of dexamethasone by weekly oral or intravenous administration for six 28-day cycles.
Those in the D-VCd group also received 1800 mg of DARA coformulated with rHuPH20 as a weekly injection in cycles 1-2, as a biweekly injection in cycles 3-6, and by injection every 4 weeks thereafter for up to 24 28-day cycles.
The median duration of treatment was 21.3 months for D-VCd and 5.3 months for VCd, and of 122 patients who received subsequent therapy, 82 (67%) received subsequent DARA.
Patients who achieved hematologic CR had better MOD-PFS and OS (HR, 0.30 and 0.41, respectively), regardless of the treatment received, he noted, adding that “this further supports that complete hematologic response is a valid early endpoint for the evaluation of anti-monoclonal therapies in AL amyloidosis.”
“I think this is very important for the further development of new treatments in this disease,” he said.
Of note, cardiac and renal response rates in the D-VCd group were about two to three times greater than those in the VCd group at 6, 12, 24, 36, and 48 months, Kastritis said.
Among 235 patients with an evaluable cardiac response, 113 achieved a very good partial response or better, including 76 of 118 (64.4%) in the D-VCd group and 37 of 117 (31.6%) in the VCd group. Of these, 48 (40.7%) and 16 (13.7%) achieved a cardiac CR.
Grade 3 or 4 treatment-emergent adverse events (TEAEs) occurring in at least 5% of patients in the D-VCd and VCd groups, respectively, were lymphopenia (13% and 10%), pneumonia (8% and 4%), hypokalemia (2% and 5%), and peripheral edema (3% and 6%), he noted, adding that systemic administration-related reactions occurred in 14 (7%) of patients receiving D-VCd; all were grade 1 or 2 and most (86%) occurred after the first injection. TEAEs led to treatment discontinuation in 5% and 4% of patients in the groups, respectively.
No new safety signals were observed during the extended follow-up, he said.
Kastritis reported relationships with Pfizer, Genesis Pharma, Sanofi, AbbVie, GSK, Prothena, Janssen, and Amgen.
A version of this article first appeared on Medscape.com.
Adding DARA to VCd (D-VCd; Darzalex Faspro; Janssen Biotech) provided deeper and more rapid hematologic response and clinically meaningful and statistically significant improvement in overall survival (OS) and major organ deterioration progression-free survival (MOD-PFS), combined with 40.7% cardiac complete response (CR), first author Efstathios Kastritis, MD, said during presentation of an oral abstract at the American Society of Hematology (ASH) 2024 Annual Meeting.
“The Andromeda study is the first comparing two contemporary regimens that shows a significant survival improvement for patients with AL amyloidosis,” said Kastritis, an associate professor at the National and Kapodistrian University of Athens in Greece. “These findings reaffirm frontline D-VCd as the standard of care in this difficult-to-treat disease.”
The regimen was approved for this indication in 2021 based on prior earlier findings from the Andromeda trial. The current results are from a preplanned analysis for MOD-PFS and OS.
At a median follow-up of 61.4 months, the overall hematologic CR rates were 59.5% and 19.2% among 388 patients randomized to receive D-VCd or VCd, respectively (odds ratio, 6.03), which showed continued improvement with additional DARA vs the 53.3% and 18.1% rates observed at the primary analysis, Kastritis reported.
Time to hematologic CR was 67.5 days and 85.0 days in the treatment groups, respectively, and median duration of hematologic CR was not reached in either group.
A significant 56% improvement was also observed in MOD-PFS (hazard ratio [HR], 0.44). Median MOD-PFS was not reached in the D-VCd group and was 30.3 months in the VCd group.
A significant 38% improvement was observed in OS (HR, 0.62), 5-year OS was 76.1% vs 64.7% in the D-VCd and VCd groups, respectively, he said.
“[The OS] benefit occurred even though more than 70% of the patients in the VCd arm who received a subsequent therapy were treated with a DARA-based regimen,” he stressed. “This further emphasizes the importance of using DSTS in the frontline setting.”
Trial participants had newly diagnosed AL amyloidosis with measurable hematologic disease, one or more involved organs, cardiac stage I-IIIA, estimated glomerular filtration rate of at least 20 mL/min, and absence of symptomatic multiple myeloma. They were randomized 1:1 to the two treatment groups. All patients received 1.3 mg/m2 of bortezomib by weekly injection, 300 mg/m2 of cyclophosphamide either by weekly oral or intravenous administration, and 20-40 mg of dexamethasone by weekly oral or intravenous administration for six 28-day cycles.
Those in the D-VCd group also received 1800 mg of DARA coformulated with rHuPH20 as a weekly injection in cycles 1-2, as a biweekly injection in cycles 3-6, and by injection every 4 weeks thereafter for up to 24 28-day cycles.
The median duration of treatment was 21.3 months for D-VCd and 5.3 months for VCd, and of 122 patients who received subsequent therapy, 82 (67%) received subsequent DARA.
Patients who achieved hematologic CR had better MOD-PFS and OS (HR, 0.30 and 0.41, respectively), regardless of the treatment received, he noted, adding that “this further supports that complete hematologic response is a valid early endpoint for the evaluation of anti-monoclonal therapies in AL amyloidosis.”
“I think this is very important for the further development of new treatments in this disease,” he said.
Of note, cardiac and renal response rates in the D-VCd group were about two to three times greater than those in the VCd group at 6, 12, 24, 36, and 48 months, Kastritis said.
Among 235 patients with an evaluable cardiac response, 113 achieved a very good partial response or better, including 76 of 118 (64.4%) in the D-VCd group and 37 of 117 (31.6%) in the VCd group. Of these, 48 (40.7%) and 16 (13.7%) achieved a cardiac CR.
Grade 3 or 4 treatment-emergent adverse events (TEAEs) occurring in at least 5% of patients in the D-VCd and VCd groups, respectively, were lymphopenia (13% and 10%), pneumonia (8% and 4%), hypokalemia (2% and 5%), and peripheral edema (3% and 6%), he noted, adding that systemic administration-related reactions occurred in 14 (7%) of patients receiving D-VCd; all were grade 1 or 2 and most (86%) occurred after the first injection. TEAEs led to treatment discontinuation in 5% and 4% of patients in the groups, respectively.
No new safety signals were observed during the extended follow-up, he said.
Kastritis reported relationships with Pfizer, Genesis Pharma, Sanofi, AbbVie, GSK, Prothena, Janssen, and Amgen.
A version of this article first appeared on Medscape.com.
Adding DARA to VCd (D-VCd; Darzalex Faspro; Janssen Biotech) provided deeper and more rapid hematologic response and clinically meaningful and statistically significant improvement in overall survival (OS) and major organ deterioration progression-free survival (MOD-PFS), combined with 40.7% cardiac complete response (CR), first author Efstathios Kastritis, MD, said during presentation of an oral abstract at the American Society of Hematology (ASH) 2024 Annual Meeting.
“The Andromeda study is the first comparing two contemporary regimens that shows a significant survival improvement for patients with AL amyloidosis,” said Kastritis, an associate professor at the National and Kapodistrian University of Athens in Greece. “These findings reaffirm frontline D-VCd as the standard of care in this difficult-to-treat disease.”
The regimen was approved for this indication in 2021 based on prior earlier findings from the Andromeda trial. The current results are from a preplanned analysis for MOD-PFS and OS.
At a median follow-up of 61.4 months, the overall hematologic CR rates were 59.5% and 19.2% among 388 patients randomized to receive D-VCd or VCd, respectively (odds ratio, 6.03), which showed continued improvement with additional DARA vs the 53.3% and 18.1% rates observed at the primary analysis, Kastritis reported.
Time to hematologic CR was 67.5 days and 85.0 days in the treatment groups, respectively, and median duration of hematologic CR was not reached in either group.
A significant 56% improvement was also observed in MOD-PFS (hazard ratio [HR], 0.44). Median MOD-PFS was not reached in the D-VCd group and was 30.3 months in the VCd group.
A significant 38% improvement was observed in OS (HR, 0.62), 5-year OS was 76.1% vs 64.7% in the D-VCd and VCd groups, respectively, he said.
“[The OS] benefit occurred even though more than 70% of the patients in the VCd arm who received a subsequent therapy were treated with a DARA-based regimen,” he stressed. “This further emphasizes the importance of using DSTS in the frontline setting.”
Trial participants had newly diagnosed AL amyloidosis with measurable hematologic disease, one or more involved organs, cardiac stage I-IIIA, estimated glomerular filtration rate of at least 20 mL/min, and absence of symptomatic multiple myeloma. They were randomized 1:1 to the two treatment groups. All patients received 1.3 mg/m2 of bortezomib by weekly injection, 300 mg/m2 of cyclophosphamide either by weekly oral or intravenous administration, and 20-40 mg of dexamethasone by weekly oral or intravenous administration for six 28-day cycles.
Those in the D-VCd group also received 1800 mg of DARA coformulated with rHuPH20 as a weekly injection in cycles 1-2, as a biweekly injection in cycles 3-6, and by injection every 4 weeks thereafter for up to 24 28-day cycles.
The median duration of treatment was 21.3 months for D-VCd and 5.3 months for VCd, and of 122 patients who received subsequent therapy, 82 (67%) received subsequent DARA.
Patients who achieved hematologic CR had better MOD-PFS and OS (HR, 0.30 and 0.41, respectively), regardless of the treatment received, he noted, adding that “this further supports that complete hematologic response is a valid early endpoint for the evaluation of anti-monoclonal therapies in AL amyloidosis.”
“I think this is very important for the further development of new treatments in this disease,” he said.
Of note, cardiac and renal response rates in the D-VCd group were about two to three times greater than those in the VCd group at 6, 12, 24, 36, and 48 months, Kastritis said.
Among 235 patients with an evaluable cardiac response, 113 achieved a very good partial response or better, including 76 of 118 (64.4%) in the D-VCd group and 37 of 117 (31.6%) in the VCd group. Of these, 48 (40.7%) and 16 (13.7%) achieved a cardiac CR.
Grade 3 or 4 treatment-emergent adverse events (TEAEs) occurring in at least 5% of patients in the D-VCd and VCd groups, respectively, were lymphopenia (13% and 10%), pneumonia (8% and 4%), hypokalemia (2% and 5%), and peripheral edema (3% and 6%), he noted, adding that systemic administration-related reactions occurred in 14 (7%) of patients receiving D-VCd; all were grade 1 or 2 and most (86%) occurred after the first injection. TEAEs led to treatment discontinuation in 5% and 4% of patients in the groups, respectively.
No new safety signals were observed during the extended follow-up, he said.
Kastritis reported relationships with Pfizer, Genesis Pharma, Sanofi, AbbVie, GSK, Prothena, Janssen, and Amgen.
A version of this article first appeared on Medscape.com.
FROM ASH 2024
Lights, Action, Bodycams in the ED
, and one third of those assaults resulted in an injury.
The attacks included verbal assaults with threats of violence (64%), hits/slaps (40%), being spit on (31%), kicked (26%), and punched (25%). Nearly one in four physicians said they were assaulted multiple times.
The same survey found that 85% of emergency physicians believe that violence in the emergency department (ED) has increased over the past 5 years; nearly half (45%) say it has greatly increased.
To offset this disturbing trend, healthcare systems are trying new measures to reduce or prevent violence in the ED and protect staff and patients. EDs have security cameras, metal detectors, panic alarms, and security guards. And now more security guards are using body-worn cameras (bodycams) like police wear, to protect ED doctors and staff.
Bodycams in the ED
Scott Hill, EdD, CPP, CHPA, a board member of the International Association for Healthcare Security and Safety Foundation, recently published a study on the impact of hospital security officers using bodycams. The study surveyed more than 100 hospitals. Fifty-three had security guards wearing bodycams; 57 had security guards without them.
The study supported the benefits of implementing body-worn cameras in a healthcare environment, said Hill, the former director of safety and security for King’s Daughters Health System in Ashland, Kentucky. “We were a little surprised by the data, but basically there was a positive impact…on the safety of hospital staff. There was higher officer confidence, better record-keeping, improved customer service, better training ... and better protection from false allegations.”
Hill told Medscape Medical News that bodycams can make for a safer ED. “The body-worn camera group believed that the cameras would have a positive impact that would make patients and staff feel safer,” said Hill. The idea is that security guards will use force more appropriately because the bodycam provides protection from false accusations.
Appropriate, timely intervention with disruptive or violent patients (or their family members) creates a safer environment for doctors, nurses, and other staff. (While the study found that hospital personnel felt safer with security guards wearing bodycams, physicians and healthcare staff were not surveyed in the study.)
Should Doctors Wear Bodycams?
While the idea of ED physicians or nurses wearing bodycams has been suggested, it’s a novel one to Jeffrey Goodloe, MD, an emergency physician in Tulsa, Oklahoma, and member of the American College of Emergency Physicians’ board of directors. “Even with a very vast network of colleagues, both professional and personal friends…I am personally not aware of an ER physician who wears a bodycam,” said Goodloe.
However, there’s no consensus on whether they should, he added. “If you ask 10 emergency room physicians [about wearing bodycams], some will say, ‘That would help’; some would say, ‘from an academic perspective, we don’t have the data,’ some would say, ‘what about privacy concerns?’ You’ll get all those responses,” said Goodloe.
“If a doctor is wearing a bodycam, one of the risks is that patients may not want to be upfront because they see a camera and they see it’s being recorded,” said Edward Wright, MD, a board-certified emergency medicine physician who owns freestanding emergency rooms (ERs) in San Antonio. “It could cause a lot of damage to the relationship between the patient and the physician.”
Other Potential Drawbacks of Bodycams
When it comes to patient care, “the presence of a body camera [on a security guard] in and of itself is not intrusive or detrimental to the professional actions of an emergency physician,” said Goodloe, who often sees police officers and security guards with bodycams in the ED. “However, when we are in the process of treating patients, there is a certain amount of privacy that patients and their families and loved ones have a reasonable right to expect in an ED.”
Wright has seen police officers wearing body cameras as well as patients using their phones to record in the ED. “That happens a lot…in Texas, anyone in a public place can record audio and video,” said Wright. “I would say the concern with anyone recording things is patient’s privacy and HIPPA concerns.” While some areas, like the waiting room and hallways, are considered public, the patient care areas are typically private — and a bodycam could violate that privacy.
“The biggest concern we have with bodycams is the unintended violation of someone else’s privacy,” said Goodloe. In an ER setting, he explains that it’s difficult for someone wearing a bodycam to prevent unintentionally recording multiple other patients in the footage. “How do we provide care and protect patient privacy?”
And while a camera makes a record, it may not always be an accurate reflection of what happened. “You might have a video record of what I said and what the patient said, but the camera doesn’t tell the whole story,” said Wright. “There are nuances, and there may be family members who are not on camera…you have to consider that something may be missing.”
Most EDs have wall-mounted security cameras; a 2023 study found that 94.7% had security cameras in key locations. Signage alerting patients of cameras is also common; more than half of EDs have signage warning patients and visitors that they are being recorded, so there may be little expectation of privacy in public areas. But unfortunately, sometimes, patients must be triaged, and treated, in the hallways of busy EDs, where they are subject to being recorded either on stationary or body-worn cameras.
Keeping the ED Safe
Putting aside privacy concerns, security measures meant to protect staff and patients can only do so much. “Some security measures are more of a feel-good measure,” said Wright. “We have automatic door locks for our doors, but the whole side of the building is glass…my personal feeling is that cameras can be a deterrent, but if someone has an intention to hurt someone else or is psychotic, you aren’t going to be able to stop them from what they’re going to do.”
Regardless, we’re likely to see more security measures like body-worn cameras in the ED. “Workplace safety is very much on the minds of ER physicians and ER nurses,” said Goodloe. “EDs have become sites of workplace violence with unacceptable increasing frequency…how do we solve this, [while] simultaneously not discouraging or preventing access to emergency care when and where people need it most?”
“We truly care about patient safety and our colleagues’ safety, and we want to be able to come in and make a positive difference,” said Goodloe. But ultimately, doctors want to go home safely to their families, and they want their patients to be able to do that, too.
A version of this article appeared on Medscape.com.
, and one third of those assaults resulted in an injury.
The attacks included verbal assaults with threats of violence (64%), hits/slaps (40%), being spit on (31%), kicked (26%), and punched (25%). Nearly one in four physicians said they were assaulted multiple times.
The same survey found that 85% of emergency physicians believe that violence in the emergency department (ED) has increased over the past 5 years; nearly half (45%) say it has greatly increased.
To offset this disturbing trend, healthcare systems are trying new measures to reduce or prevent violence in the ED and protect staff and patients. EDs have security cameras, metal detectors, panic alarms, and security guards. And now more security guards are using body-worn cameras (bodycams) like police wear, to protect ED doctors and staff.
Bodycams in the ED
Scott Hill, EdD, CPP, CHPA, a board member of the International Association for Healthcare Security and Safety Foundation, recently published a study on the impact of hospital security officers using bodycams. The study surveyed more than 100 hospitals. Fifty-three had security guards wearing bodycams; 57 had security guards without them.
The study supported the benefits of implementing body-worn cameras in a healthcare environment, said Hill, the former director of safety and security for King’s Daughters Health System in Ashland, Kentucky. “We were a little surprised by the data, but basically there was a positive impact…on the safety of hospital staff. There was higher officer confidence, better record-keeping, improved customer service, better training ... and better protection from false allegations.”
Hill told Medscape Medical News that bodycams can make for a safer ED. “The body-worn camera group believed that the cameras would have a positive impact that would make patients and staff feel safer,” said Hill. The idea is that security guards will use force more appropriately because the bodycam provides protection from false accusations.
Appropriate, timely intervention with disruptive or violent patients (or their family members) creates a safer environment for doctors, nurses, and other staff. (While the study found that hospital personnel felt safer with security guards wearing bodycams, physicians and healthcare staff were not surveyed in the study.)
Should Doctors Wear Bodycams?
While the idea of ED physicians or nurses wearing bodycams has been suggested, it’s a novel one to Jeffrey Goodloe, MD, an emergency physician in Tulsa, Oklahoma, and member of the American College of Emergency Physicians’ board of directors. “Even with a very vast network of colleagues, both professional and personal friends…I am personally not aware of an ER physician who wears a bodycam,” said Goodloe.
However, there’s no consensus on whether they should, he added. “If you ask 10 emergency room physicians [about wearing bodycams], some will say, ‘That would help’; some would say, ‘from an academic perspective, we don’t have the data,’ some would say, ‘what about privacy concerns?’ You’ll get all those responses,” said Goodloe.
“If a doctor is wearing a bodycam, one of the risks is that patients may not want to be upfront because they see a camera and they see it’s being recorded,” said Edward Wright, MD, a board-certified emergency medicine physician who owns freestanding emergency rooms (ERs) in San Antonio. “It could cause a lot of damage to the relationship between the patient and the physician.”
Other Potential Drawbacks of Bodycams
When it comes to patient care, “the presence of a body camera [on a security guard] in and of itself is not intrusive or detrimental to the professional actions of an emergency physician,” said Goodloe, who often sees police officers and security guards with bodycams in the ED. “However, when we are in the process of treating patients, there is a certain amount of privacy that patients and their families and loved ones have a reasonable right to expect in an ED.”
Wright has seen police officers wearing body cameras as well as patients using their phones to record in the ED. “That happens a lot…in Texas, anyone in a public place can record audio and video,” said Wright. “I would say the concern with anyone recording things is patient’s privacy and HIPPA concerns.” While some areas, like the waiting room and hallways, are considered public, the patient care areas are typically private — and a bodycam could violate that privacy.
“The biggest concern we have with bodycams is the unintended violation of someone else’s privacy,” said Goodloe. In an ER setting, he explains that it’s difficult for someone wearing a bodycam to prevent unintentionally recording multiple other patients in the footage. “How do we provide care and protect patient privacy?”
And while a camera makes a record, it may not always be an accurate reflection of what happened. “You might have a video record of what I said and what the patient said, but the camera doesn’t tell the whole story,” said Wright. “There are nuances, and there may be family members who are not on camera…you have to consider that something may be missing.”
Most EDs have wall-mounted security cameras; a 2023 study found that 94.7% had security cameras in key locations. Signage alerting patients of cameras is also common; more than half of EDs have signage warning patients and visitors that they are being recorded, so there may be little expectation of privacy in public areas. But unfortunately, sometimes, patients must be triaged, and treated, in the hallways of busy EDs, where they are subject to being recorded either on stationary or body-worn cameras.
Keeping the ED Safe
Putting aside privacy concerns, security measures meant to protect staff and patients can only do so much. “Some security measures are more of a feel-good measure,” said Wright. “We have automatic door locks for our doors, but the whole side of the building is glass…my personal feeling is that cameras can be a deterrent, but if someone has an intention to hurt someone else or is psychotic, you aren’t going to be able to stop them from what they’re going to do.”
Regardless, we’re likely to see more security measures like body-worn cameras in the ED. “Workplace safety is very much on the minds of ER physicians and ER nurses,” said Goodloe. “EDs have become sites of workplace violence with unacceptable increasing frequency…how do we solve this, [while] simultaneously not discouraging or preventing access to emergency care when and where people need it most?”
“We truly care about patient safety and our colleagues’ safety, and we want to be able to come in and make a positive difference,” said Goodloe. But ultimately, doctors want to go home safely to their families, and they want their patients to be able to do that, too.
A version of this article appeared on Medscape.com.
, and one third of those assaults resulted in an injury.
The attacks included verbal assaults with threats of violence (64%), hits/slaps (40%), being spit on (31%), kicked (26%), and punched (25%). Nearly one in four physicians said they were assaulted multiple times.
The same survey found that 85% of emergency physicians believe that violence in the emergency department (ED) has increased over the past 5 years; nearly half (45%) say it has greatly increased.
To offset this disturbing trend, healthcare systems are trying new measures to reduce or prevent violence in the ED and protect staff and patients. EDs have security cameras, metal detectors, panic alarms, and security guards. And now more security guards are using body-worn cameras (bodycams) like police wear, to protect ED doctors and staff.
Bodycams in the ED
Scott Hill, EdD, CPP, CHPA, a board member of the International Association for Healthcare Security and Safety Foundation, recently published a study on the impact of hospital security officers using bodycams. The study surveyed more than 100 hospitals. Fifty-three had security guards wearing bodycams; 57 had security guards without them.
The study supported the benefits of implementing body-worn cameras in a healthcare environment, said Hill, the former director of safety and security for King’s Daughters Health System in Ashland, Kentucky. “We were a little surprised by the data, but basically there was a positive impact…on the safety of hospital staff. There was higher officer confidence, better record-keeping, improved customer service, better training ... and better protection from false allegations.”
Hill told Medscape Medical News that bodycams can make for a safer ED. “The body-worn camera group believed that the cameras would have a positive impact that would make patients and staff feel safer,” said Hill. The idea is that security guards will use force more appropriately because the bodycam provides protection from false accusations.
Appropriate, timely intervention with disruptive or violent patients (or their family members) creates a safer environment for doctors, nurses, and other staff. (While the study found that hospital personnel felt safer with security guards wearing bodycams, physicians and healthcare staff were not surveyed in the study.)
Should Doctors Wear Bodycams?
While the idea of ED physicians or nurses wearing bodycams has been suggested, it’s a novel one to Jeffrey Goodloe, MD, an emergency physician in Tulsa, Oklahoma, and member of the American College of Emergency Physicians’ board of directors. “Even with a very vast network of colleagues, both professional and personal friends…I am personally not aware of an ER physician who wears a bodycam,” said Goodloe.
However, there’s no consensus on whether they should, he added. “If you ask 10 emergency room physicians [about wearing bodycams], some will say, ‘That would help’; some would say, ‘from an academic perspective, we don’t have the data,’ some would say, ‘what about privacy concerns?’ You’ll get all those responses,” said Goodloe.
“If a doctor is wearing a bodycam, one of the risks is that patients may not want to be upfront because they see a camera and they see it’s being recorded,” said Edward Wright, MD, a board-certified emergency medicine physician who owns freestanding emergency rooms (ERs) in San Antonio. “It could cause a lot of damage to the relationship between the patient and the physician.”
Other Potential Drawbacks of Bodycams
When it comes to patient care, “the presence of a body camera [on a security guard] in and of itself is not intrusive or detrimental to the professional actions of an emergency physician,” said Goodloe, who often sees police officers and security guards with bodycams in the ED. “However, when we are in the process of treating patients, there is a certain amount of privacy that patients and their families and loved ones have a reasonable right to expect in an ED.”
Wright has seen police officers wearing body cameras as well as patients using their phones to record in the ED. “That happens a lot…in Texas, anyone in a public place can record audio and video,” said Wright. “I would say the concern with anyone recording things is patient’s privacy and HIPPA concerns.” While some areas, like the waiting room and hallways, are considered public, the patient care areas are typically private — and a bodycam could violate that privacy.
“The biggest concern we have with bodycams is the unintended violation of someone else’s privacy,” said Goodloe. In an ER setting, he explains that it’s difficult for someone wearing a bodycam to prevent unintentionally recording multiple other patients in the footage. “How do we provide care and protect patient privacy?”
And while a camera makes a record, it may not always be an accurate reflection of what happened. “You might have a video record of what I said and what the patient said, but the camera doesn’t tell the whole story,” said Wright. “There are nuances, and there may be family members who are not on camera…you have to consider that something may be missing.”
Most EDs have wall-mounted security cameras; a 2023 study found that 94.7% had security cameras in key locations. Signage alerting patients of cameras is also common; more than half of EDs have signage warning patients and visitors that they are being recorded, so there may be little expectation of privacy in public areas. But unfortunately, sometimes, patients must be triaged, and treated, in the hallways of busy EDs, where they are subject to being recorded either on stationary or body-worn cameras.
Keeping the ED Safe
Putting aside privacy concerns, security measures meant to protect staff and patients can only do so much. “Some security measures are more of a feel-good measure,” said Wright. “We have automatic door locks for our doors, but the whole side of the building is glass…my personal feeling is that cameras can be a deterrent, but if someone has an intention to hurt someone else or is psychotic, you aren’t going to be able to stop them from what they’re going to do.”
Regardless, we’re likely to see more security measures like body-worn cameras in the ED. “Workplace safety is very much on the minds of ER physicians and ER nurses,” said Goodloe. “EDs have become sites of workplace violence with unacceptable increasing frequency…how do we solve this, [while] simultaneously not discouraging or preventing access to emergency care when and where people need it most?”
“We truly care about patient safety and our colleagues’ safety, and we want to be able to come in and make a positive difference,” said Goodloe. But ultimately, doctors want to go home safely to their families, and they want their patients to be able to do that, too.
A version of this article appeared on Medscape.com.
New Cancer Drugs: Do Patients Prefer Faster Access or Clinical Benefit?
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
When the Food and Drug Administration (FDA) grants cancer drugs accelerated approval, a key aim is to provide patients faster access to therapies that can benefit them.
The downside of a speedier approval timeline, however, is that it’s often not yet clear whether the new drugs will actually allow a patient to live longer or better. Information on overall survival and quality of life typically comes years later, after drugs undergo confirmatory trials, or sometimes not at all, if companies fail to conduct these trials.
During this waiting period, patients may be receiving a cancer drug that provides no real clinical benefit but comes with a host of toxicities.
In fact, the odds are about as good as a coin flip. For cancer drugs that have confirmatory trial data, more than half don’t ultimately provide an overall survival or quality of life benefit.
Inherent to the accelerated approval process is the assumption that patients are willing to accept this uncertainty in exchange for faster access.
But is that really the case?
The researchers asked about 870 adults with experience of cancer challenges — either their own cancer diagnosis or that of family or a close friend — whether they valued faster access or certainty that a drug really works.
In the study, participants imagined they had been diagnosed with cancer and could choose between two cancer drugs under investigation in clinical trials but with uncertain effectiveness, and a current standard treatment. Participants had to make a series of choices based on five scenarios.
The first two scenarios were based on the impact of the current standard treatment: A patient’s life expectancy on the standard treatment (6 months up to 3 years), and a patient’s physical health on the standard treatment (functional status restricted only during strenuous activities up to completely disabled).
The remaining three scenarios dealt with the two new drugs: The effect of the new drugs on a surrogate endpoint, progression-free survival (whether the drugs slowed tumor growth for an extra month or 5 additional months compared with the standard treatment), certainty that slowing tumor growth will improve survival (very low to high), and the wait time to access the drugs (immediately to as long as 2 years).
The researchers assessed the relative importance of survival benefit certainty vs wait time and how that balance shifted depending on the different scenarios.
Overall, the researchers found that, if there was no evidence linking the surrogate endpoint (progression-free survival) to overall survival, patients were willing to wait about 8 months for weak evidence of an overall survival benefit (ie, low certainty the drug will extend survival by 1-5 months), about 16 months for moderate certainty, and almost 22 months for high certainty.
Despite a willingness to wait for greater certainty, participants did value speed as well. Overall, respondents showed a strong preference against a 1-year delay in FDA approval time. People who were aged 55 years or more and were non-White individuals made less than $40,000 year as well as those with the lowest life expectancy on a current standard treatment were most sensitive to wait times while those with better functional status and longer life expectancies on a current treatment were less sensitive to longer wait times.
“Our results indicate that some patients (except those with the poorest prognoses) would find the additional time required to generate evidence on the survival benefit of new cancer drugs an acceptable tradeoff,” the study authors concluded.
Although people do place high value on timely access to new cancer drugs, especially if there are limited treatment options, many are willing to wait for greater certainty that a new drug provides an overall survival benefit, lead author Robin Forrest, MSc, with the Department of Health Policy, London School of Economics in England, said in an interview.
In the study, respondents also did not place significant value on whether the drug substantially slowed cancer growth. “In other words, substantial progression-free survival benefit of a drug did not compensate for lack of certainty about a drug’s benefit on survival in respondents’ drug choices,” the authors explained.
“In an effort to move quickly, we have accepted progression-free survival [as a surrogate endpoint],” Jyoti D. Patel, MD, oncologist with Northwestern Memorial Hospital, Chicago, Illinois, who wasn’t involved in the study. But a growing body of evidence indicates that progression-free survival is often a poor surrogate for overall survival. And what this study suggests is that “patients uniformly care about improvements in overall survival and the quality of that survival,” Patel said.
Bishal Gyawali, MD, PhD, was not surprised by the findings.
“I always thought this was the real-world scenario, but the problem is the voices of ordinary patients are not heard,” Gyawali, with Queen’s University, Kingston, Ontario, Canada, who also wasn’t involved in the study, said in an interview.
“What is heard is the loud noise of ‘we need access now, today, yesterday’ — ‘we don’t care if the drug doesn’t improve overall survival, we just need a drug, any drug’ — ‘we don’t care how much it costs, we need access today,’ ” Gyawali said. “Not saying this is wrong, but this is not the representation of all patients.”
However, the voices of patients who are more cautious and want evidence of benefit before accepting toxicities don’t make headlines, he added.
What this survey means from a policy perspective, said Gyawali, is that accelerated approvals that do not mandate survival endpoint in confirmatory trials are ignoring the need of many patients who prioritize certainty of benefit over speed of access.
The study was funded by the London School of Economics and Political Science Phelan United States Centre. Forrest had no relevant disclosures. Gyawali has received consulting fees from Vivio Health. Patel has various relationships with AbbVie, Anheart, AstraZeneca, Bristol-Myers Squibb, Guardant, Tempus, Sanofi, BluePrint, Takeda, and Gilead.
A version of this article first appeared on Medscape.com.
FROM THE LANCET ONCOLOGY
Do GLP-1s Lower VTE Risk in People With Type 2 Diabetes?
Overall, GLP-1 agonist use was associated with a 20% reduction in VTE, compared with DPP-4 inhibitor use, in those with type 2 diabetes, and this benefit held regardless of people’s obesity status, said study investigator Cho-Han Chiang, MD, a medical resident at Mount Auburn Hospital, Cambridge, Massachusetts, who presented the findings at the American Society of Hematology (ASH) 2024 Annual Meeting.
The incidence of VTE has increased by 20% over the past 10 years, and obesity is a risk factor for VTE, Chiang explained. A growing body of evidence demonstrated that GLP-1s provide a variety of cardiovascular benefits in people with type 2 diabetes, but data on VTE benefits remain more limited.
In the retrospective study, the researchers combed electronic health records from the TriNetX global database, which includes more than 250 million patients, and identified adults with type 2 diabetes who were taking a GLP-1 agonist or a DPP-4 inhibitor.
After excluding anyone with prior VTE or atrial fibrillation as well as those treated with both drugs or with oral anticoagulants, patients on GLP-1s were matched with those on DPP-4 inhibitors based on predetermined variables, including age, sex, race, body mass index (BMI), hemoglobin A1c, use of other antidiabetic agents, and underlying comorbidities. VTE was a composite of pulmonary embolism and deep vein thrombosis.
The researchers also performed a subgroup analysis that stratified patients by obesity status, defined as a BMI ≥ 30.
Within 1 year of GLP-1 or DPP-4 prescription, VTE occurred at a rate of 6.5 cases/1000 person-years in the GLP-1 group vs 7.9 cases/1000 person years in the DPP-4 inhibitor group (hazard ratio [HR], 0.80; P < .001).
The 20% risk reduction in VTE held across various subgroups of BMI, including among those with obesity, Chiang reported.
Among patients with the highest BMI (≥ 40), VTE occurred at a rate of 7.2 cases/1000 person years in the GLP-1 group vs 9.6 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.74). Among patients with the next highest BMI (30-34.9), VTE occurred at a rate of 4.8 cases/1000 person years in the GLP-1 group vs 7.9 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.60). Among those with the lowest BMI (18.5-24.9), VTE occurred significantly less frequently among those in the GLP-1 group — 4.7 cases/1000 person years vs 7.4 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.61).
The lower risk for VTE associated with GLP-1s also held across the individual components of the composite VTE. Pulmonary embolism occurred at a rate of 3.1 cases/1000 person years in the GLP-1 group vs 3.9 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.78), and deep vein thrombosis occurred in 4.2 cases/1000 person years in the GLP-1 group vs 5.0 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.82).
Interestingly, the GLP-1 and DPP-4 curves started diverging within the first 30 days of the index prescription date, said Chiang.
Session moderator Ghadeer Dawwas, PhD, said in an interview that patients with type 2 diabetes are increasingly using GLP-1 agonists because of the cardiovascular benefits associated with the agents, which include lower risks for stroke and heart failure, but the antithrombotic benefits are still debated.
“The current study indicates that GLP-1 agonists may help lower the risk of VTE in patients with type 2 diabetes, irrespective of their baseline body weight,” said Dawwas, a pharmacoepidemiologist and assistant professor of medicine at Vanderbilt University Medical Center in Nashville, Tennessee. “However, given the current landscape of evidence and the existence of conflicting data on VTE risk, clinicians should proceed with caution and await further studies to validate these findings before making clinical decisions.”
This study was funded by the National Blood Clot Alliance and Conquer Cancer Foundation. Chiang and Dawwas had no disclosures.
A version of this article appeared on Medscape.com.
Overall, GLP-1 agonist use was associated with a 20% reduction in VTE, compared with DPP-4 inhibitor use, in those with type 2 diabetes, and this benefit held regardless of people’s obesity status, said study investigator Cho-Han Chiang, MD, a medical resident at Mount Auburn Hospital, Cambridge, Massachusetts, who presented the findings at the American Society of Hematology (ASH) 2024 Annual Meeting.
The incidence of VTE has increased by 20% over the past 10 years, and obesity is a risk factor for VTE, Chiang explained. A growing body of evidence demonstrated that GLP-1s provide a variety of cardiovascular benefits in people with type 2 diabetes, but data on VTE benefits remain more limited.
In the retrospective study, the researchers combed electronic health records from the TriNetX global database, which includes more than 250 million patients, and identified adults with type 2 diabetes who were taking a GLP-1 agonist or a DPP-4 inhibitor.
After excluding anyone with prior VTE or atrial fibrillation as well as those treated with both drugs or with oral anticoagulants, patients on GLP-1s were matched with those on DPP-4 inhibitors based on predetermined variables, including age, sex, race, body mass index (BMI), hemoglobin A1c, use of other antidiabetic agents, and underlying comorbidities. VTE was a composite of pulmonary embolism and deep vein thrombosis.
The researchers also performed a subgroup analysis that stratified patients by obesity status, defined as a BMI ≥ 30.
Within 1 year of GLP-1 or DPP-4 prescription, VTE occurred at a rate of 6.5 cases/1000 person-years in the GLP-1 group vs 7.9 cases/1000 person years in the DPP-4 inhibitor group (hazard ratio [HR], 0.80; P < .001).
The 20% risk reduction in VTE held across various subgroups of BMI, including among those with obesity, Chiang reported.
Among patients with the highest BMI (≥ 40), VTE occurred at a rate of 7.2 cases/1000 person years in the GLP-1 group vs 9.6 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.74). Among patients with the next highest BMI (30-34.9), VTE occurred at a rate of 4.8 cases/1000 person years in the GLP-1 group vs 7.9 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.60). Among those with the lowest BMI (18.5-24.9), VTE occurred significantly less frequently among those in the GLP-1 group — 4.7 cases/1000 person years vs 7.4 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.61).
The lower risk for VTE associated with GLP-1s also held across the individual components of the composite VTE. Pulmonary embolism occurred at a rate of 3.1 cases/1000 person years in the GLP-1 group vs 3.9 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.78), and deep vein thrombosis occurred in 4.2 cases/1000 person years in the GLP-1 group vs 5.0 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.82).
Interestingly, the GLP-1 and DPP-4 curves started diverging within the first 30 days of the index prescription date, said Chiang.
Session moderator Ghadeer Dawwas, PhD, said in an interview that patients with type 2 diabetes are increasingly using GLP-1 agonists because of the cardiovascular benefits associated with the agents, which include lower risks for stroke and heart failure, but the antithrombotic benefits are still debated.
“The current study indicates that GLP-1 agonists may help lower the risk of VTE in patients with type 2 diabetes, irrespective of their baseline body weight,” said Dawwas, a pharmacoepidemiologist and assistant professor of medicine at Vanderbilt University Medical Center in Nashville, Tennessee. “However, given the current landscape of evidence and the existence of conflicting data on VTE risk, clinicians should proceed with caution and await further studies to validate these findings before making clinical decisions.”
This study was funded by the National Blood Clot Alliance and Conquer Cancer Foundation. Chiang and Dawwas had no disclosures.
A version of this article appeared on Medscape.com.
Overall, GLP-1 agonist use was associated with a 20% reduction in VTE, compared with DPP-4 inhibitor use, in those with type 2 diabetes, and this benefit held regardless of people’s obesity status, said study investigator Cho-Han Chiang, MD, a medical resident at Mount Auburn Hospital, Cambridge, Massachusetts, who presented the findings at the American Society of Hematology (ASH) 2024 Annual Meeting.
The incidence of VTE has increased by 20% over the past 10 years, and obesity is a risk factor for VTE, Chiang explained. A growing body of evidence demonstrated that GLP-1s provide a variety of cardiovascular benefits in people with type 2 diabetes, but data on VTE benefits remain more limited.
In the retrospective study, the researchers combed electronic health records from the TriNetX global database, which includes more than 250 million patients, and identified adults with type 2 diabetes who were taking a GLP-1 agonist or a DPP-4 inhibitor.
After excluding anyone with prior VTE or atrial fibrillation as well as those treated with both drugs or with oral anticoagulants, patients on GLP-1s were matched with those on DPP-4 inhibitors based on predetermined variables, including age, sex, race, body mass index (BMI), hemoglobin A1c, use of other antidiabetic agents, and underlying comorbidities. VTE was a composite of pulmonary embolism and deep vein thrombosis.
The researchers also performed a subgroup analysis that stratified patients by obesity status, defined as a BMI ≥ 30.
Within 1 year of GLP-1 or DPP-4 prescription, VTE occurred at a rate of 6.5 cases/1000 person-years in the GLP-1 group vs 7.9 cases/1000 person years in the DPP-4 inhibitor group (hazard ratio [HR], 0.80; P < .001).
The 20% risk reduction in VTE held across various subgroups of BMI, including among those with obesity, Chiang reported.
Among patients with the highest BMI (≥ 40), VTE occurred at a rate of 7.2 cases/1000 person years in the GLP-1 group vs 9.6 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.74). Among patients with the next highest BMI (30-34.9), VTE occurred at a rate of 4.8 cases/1000 person years in the GLP-1 group vs 7.9 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.60). Among those with the lowest BMI (18.5-24.9), VTE occurred significantly less frequently among those in the GLP-1 group — 4.7 cases/1000 person years vs 7.4 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.61).
The lower risk for VTE associated with GLP-1s also held across the individual components of the composite VTE. Pulmonary embolism occurred at a rate of 3.1 cases/1000 person years in the GLP-1 group vs 3.9 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.78), and deep vein thrombosis occurred in 4.2 cases/1000 person years in the GLP-1 group vs 5.0 cases/1000 person years in the DPP-4 inhibitor group (HR, 0.82).
Interestingly, the GLP-1 and DPP-4 curves started diverging within the first 30 days of the index prescription date, said Chiang.
Session moderator Ghadeer Dawwas, PhD, said in an interview that patients with type 2 diabetes are increasingly using GLP-1 agonists because of the cardiovascular benefits associated with the agents, which include lower risks for stroke and heart failure, but the antithrombotic benefits are still debated.
“The current study indicates that GLP-1 agonists may help lower the risk of VTE in patients with type 2 diabetes, irrespective of their baseline body weight,” said Dawwas, a pharmacoepidemiologist and assistant professor of medicine at Vanderbilt University Medical Center in Nashville, Tennessee. “However, given the current landscape of evidence and the existence of conflicting data on VTE risk, clinicians should proceed with caution and await further studies to validate these findings before making clinical decisions.”
This study was funded by the National Blood Clot Alliance and Conquer Cancer Foundation. Chiang and Dawwas had no disclosures.
A version of this article appeared on Medscape.com.
FROM ASH 2024